Abstract
As the world endures a viral pandemic superimposed on a diabetes pandemic, the latter incorporates most of the comorbidities associated with the former, thereby exacerbating risk of death in both. An essential approach to both pandemics is prevention and unrealized earlier treatment. Thus, in this Perspective relating to diabetes, we emphasize a paradigm of, first, reversible β-cell organ dysfunction and then irreversible β-cell organ failure, which directly indicate the potential for earlier prevention, also unrealized in current guidelines. Four pillars support this paradigm: epidemiology, pathophysiology, molecular pathology, and genetics. A substantial worldwide knowledge base defines each pillar and informs a more aggressive preventive approach to most forms of the disorder. This analysis seeks to clarify the temporal and therapeutic relationships between lost β-cell function and content, illuminating the potential for earlier diagnoses and, thus, prevention. We also propose that myriad pathways leading to most forms of diabetes converge at the endoplasmic reticulum, where stress can result in β-cell death and content loss. Finally, genetic and nongenetic origins common to major types of diabetes can inform earlier diagnosis and, potentially, prevention, with the aim of preserving β-cell mass.
Introduction
Diabetes has been historically dichotomized (type 2 diabetes [T2D] and type 1 diabetes [T1D]), resulting in distinct advocacy, even with distinct academic entities, creating cognitive dissonance at many healthcare and research levels. As a result, the best outcome for affected individuals has been compromised, both in terms of public health initiatives and data-driven, preventive-centered therapies, as recently emphasized by the Lancet Commission on diabetes (1). Distinct from that duality concept (splitting) and the recent move to identify heterogeneity of diabetes, in this Perspective we primarily chose an integrated approach (lumping) to rotate thinking toward prevention to improve clinical care. Our review of the diabetes literature focuses on the β-cell organ, thereby exposing the unrealized need and potential benefits of earlier screening and diagnoses for prevention and treatment of most forms of diabetes. Realizing the potential of personalized medicine should improve patient care, while the identification of pathways common to different forms of diabetes should aid that process through cross-fertilization of ideas.
The purpose of this Perspective is to outline this preventive approach, how it can be realized scientifically, and to what clinical end. Our scientific approach conceptualizes diabetes as a consequence of β-cell dysfunction and failure and postulates that all β-cells conceptually comprise an organ, the β-cell organ, which is a paradigm supported by four data-driven pillars: epidemiology, pathophysiology, molecular pathology, and genetics. Thus, it is the continuum of β-cell organ dysfunction (BCOD) and potential failure (BCOF) that requires greater clarity at each of the four pillars to better realize diabetes prevention. In brief, epidemiology indicates that more than half of the population in industrialized countries is at diabetes risk with a high frequency of diabetes-related gene risk variants. Pathophysiological data support these epidemiological findings using β-cell studies, which imply that BCOD/F can develop substantially earlier than currently described in clinical guidelines. Importantly, these studies also indicate that initially, BCOD is related to reversible functional loss, and only later does BCOF occur due to irreversible content loss, illuminating the need for earlier preventive approaches to both T2D and T1D. Molecular mechanisms of reduced β-cell dysfunction and failure in both forms of the disorder indicate that excessive proinsulin production is a compensatory attempt to maintain fuel-related metabolic homeostasis, which results in endoplasmic reticulum (ER) stress. Virtually all non-β-cell pathways leading to diabetes molecularly converge at the β-cell ER, which critically must adapt; if it does not, then β-cell apoptosis can follow (BCOF). These ER processes are, in part, also regulated by common gene variants associated with altered risk for the two major types of diabetes. Given that the β-cell organ is inherently fragile, when proinsulin synthesis approaches or exceeds maximal capacity, then ER stress, β-cell death, and, finally, organ content loss can occur. Whether for T2D or T1D, these changes point toward the need for earlier screening to better prevent diabetes.
Considering BCOD/F as a β-cell organ paradigm illustrates the scientific rationale for earlier detection of all common forms of diabetes. Such an approach could improve our effectiveness to diagnose, prevent, and treat forms of diabetes using uncomplicated screening measures within current public health infrastructures as explicitly prescribed by the Lancet Commission on diabetes in 2021 (1).
Epidemiology
Epidemiology indicates a high proportion of the population has the disease and, by implication, a high frequency of diabetes-related gene risk variants. Chronic disorders, e.g., dysglycemias, are at pandemic proportions, with worldwide diabetes prevalence doubling from 1980 to 2014. In California ∼60% of the population over age 55 has prediabetes, and 55% of all adults are dysglycemic (2). The U.S. Centers for Disease Control and Prevention (CDC) indicated that diabetes prevalence in 2015 was ∼32% in individuals over 65 years old, while prediabetes in 2012 was ∼50% at the same age, with a resulting dysglycemic prevalence of 82% (3). We now suggest that even these figures are underestimates based on histological and functional data presented below. By implication, >60% of individuals of all ages have diabetes-related common gene variants and, thus, a large fraction of “control cohorts” in diabetes research have high disease risk. It follows that genome-wide association studies (GWAS) underestimate genetic risk; thus, only ∼20% of diabetes heritability is currently explained by genetic variants when median heritability estimates are ∼40% (4). These control subjects could also confound other studies and organ donor consortium data (see below).
An epidemiological reanalysis of U.S. diabetes mortality indicates that the population-attributable fraction (PAF) of diabetes deaths using self-reports and/or hemoglobin A1c (HbA1c) levels in the National Health Interview Survey and National Health and Nutrition Examination Survey was ∼11.5%, whereas U.S. CDC analyses (death certificates) were substantially lower at ∼3.5%, indicating diabetes in 2010–2011 represented the third leading cause of U.S. deaths (5). Further, prediabetes deaths contributed an additional PAF of 2.2%, which is nearly two-thirds the CDC’s diabetes death estimate. Given the need for earlier disease detection and prevention, we examined the pathophysiology of diabetes from the perspective of this BCOD/F paradigm.
Pathophysiology
Pathophysiological data, using β-cell organ studies, imply that BCOD/F can develop substantially earlier than described using current worldwide clinical guidelines. These studies imply BCOD is related to reversible functional loss, which only later becomes irreversible due to β-cell content loss via β-cell death, and then, if sufficiently severe at the organ level, i.e., BCOF, illuminating the need for earlier preventive approaches to both T2D and T1D. The development of international organ procurement programs for diabetes, e.g., the Network for Pancreatic Organ Donors with Diabetes, and diligent use of surgical and autopsy specimens, has transformed the histological landscape of diabetes. Data on β-cell function provide a composite output of all β-cells, although notably not all β-cells are behaving in unison, since they show substantial heterogeneity, including differential vascular supply, neural innervation, and local environmental changes, e.g., pancreatic exocrine alterations. While β-cell content loss and function could be due to dedifferentiation (6), such cell numbers are too low to account for the β-cell functional and content loss reported in diabetes (7–10). Given limitations to interrogating individual β-cell function, we focused here on the overall function of β-cells as a composite.
T2D and β-Cell Organ Dysfunction and Failure
Pathophysiological studies derived from over 3,000 subjects from eight countries imply that T2D BCOD/F can develop earlier than widely appreciated.
Functional
β-Cell function in 460 subjects (ranging from normal to T2D) using the disposition index (importantly linked to 2-h oral glucose tolerance test [OGTT] data) showed that a “normal” 2-h glucose post-OGTT of 120 mg/dL (6.7 mmol/L) reflected 50–60% functional loss (lower yellow circle), while a 2-h glucose of 180–200 mg/dL (10–11.1 mmol/L) was associated with ∼80% reduction (lower orange circle) (Fig. 1A) (11). Similarly, in 1,601 obese adolescents, those with a 2-h blood glucose of 100–119 mg/dL (5.6–6.6 mmol/L) or 120–139 mg/dL (6.7–7.7 mmol/L) showed striking 20% (upper yellow) and 50–70% (lower yellow) functional reductions from normal (green circle) (12).
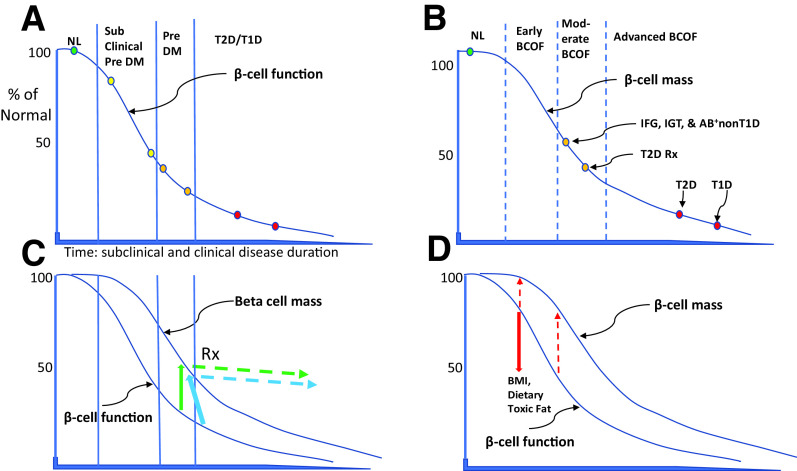
Figure 1.
Natural history of BCOD and BCOF depicted as percentage of normal on y-axis. The x-axis depicts duration for T1D (years) and T2D (decades). Two sigmoid curves approximate referenced, data-specific BCOD (A) and β-cell mass (B). A: Solid vertical lines depict currently defined normal (NL, green circle), subclinical prediabetes (subclinical Pre DM, yellow circles), prediabetes (Pre DM, orange circles), and T2D and T1D (red circles). B: Dashed vertical lines define normal (NL, green circle), early BCOF (no data available), moderate BCOF (orange circles), and advanced BCOF (red circles). C: Green solid arrow depicts ∼75% BCOD but only ∼50% BCOF content loss. BCOD can be rescued from the natural history or inadequate treatment with preserved β-cell content in some individuals (green dashed arrow, Rx [treatment]). Even some T2D cases can be rescued, with treatment returning them to prediabetes (blue arrows). D: Red solid arrow depicts impairment of BCOD, e.g., by environmental factors, from ∼25% to ∼50%, with less impairment of β-cell mass from ∼5% to ∼20% (red dashed arrows).
Histological
In the last 20 years, T2D studies (n = 11) of surgical, autopsy, and organ donors (n = 672) from eight countries reported β-cell content (mass, volume, or area) reductions of 30–60% in cases with impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or T2D (Fig. 1B, orange circles, Table 1, and Supplementary Material, part 1 and references therein). Moreover, β-cell neogenesis and apoptosis data were elevated severalfold (Table 1). Although there is concern over the validity of β-cell mass measurements in adults due to individual variation, it is possible to control for these variations, e.g., by BMI and age matching (Table 1). By data mining, we discovered additional variation in a small proportion of control subjects with dysglycemias in the range of IFG or IGT, but these variations implied that we had underestimated content reductions. Finally, it is now recognized that treatment can rescue reduced T2D-associated BCOD (Fig. 1C, green and blue arrows), potentially explaining why β-cell content loss varies and, indeed, overlaps between untreated prediabetes and treated T2D (Fig. 1B) (13,14).
Table 1.
Literature of BCOD/F in T2D and T1D
| Year (reference*) | Pancreas source, subject number (N) | Control β-cell mass (mg), area or volume (%)/pancreas | β-Cell content, reduced versus control (%) | β-Cell neogenesis‡ | β-Cell apoptosis‡ | Country of study | ||
|---|---|---|---|---|---|---|---|---|
| T2D | IFG/IGT | T2D (short term)†† | T2D (long term)†† | |||||
| 2002 (1) | Autopsy (29) | 30 (x = 13) | Lean: none | Japan | ||||
| 2003 (2) | Autopsy (124) | Obese, 2.6%; lean, 1.7% | 40 (obese) | 63 (obese), 41 (lean) | All T2D+ | All T2D+ | U.S. | |
| 2003 (3) | OD (9); surgery (35) | 1,300 mg and 1.94% | 30 (6.2) | South Korea | ||||
| 2008 (4) | Autopsy (109) | Obese, 950 mg; lean, 800 mg | 24 (1–5) | 61 (>15) | Belgium | |||
| 2009 (5) | Surgery (33) | 1.22% | 7 | 65 | Germany | |||
| 2010 (6) | OD (60) | Obese, 2,100 mg; lean, 950 mg | 0 (lean) (<4) | 50 (obese) (x = 6) | All T2D+ | Obese + lean: none | Canada | |
| 2011 (7) | Autopsy (68) | 3,900§ | 2 (x = 10) | T2D+ | U.S./Sweden | |||
| 2013 (8) | Surgery (42) | 1.60% | 61 | 58 | 69 (x = 15) | All T2D+ | AllT2D+ | Japan |
| 2016 (9) | OD (30) | 77% Syn+Ins+ | 26 | U.S./Italy | ||||
| 2016 (10) | Autopsy (27) | 30 | All T2D+ | U.S. | ||||
| 2016 (11) | Surgery (99) | 1.48% | 54 | Japan | ||||
| 2019 (12) | OD (7) | All T2D+ | U.S. | |||||
| T1D | Pre-T1D | T1D (long term) | β-Cell proliferation | |||||
|---|---|---|---|---|---|---|---|---|
| 2005 (13) | Autopsy (56) | 1.140% | 98 | None | + | + | U.S. | |
| 2012 (14) | OD and autopsy (28) | + | Belgium | |||||
| 2016 (15) | OD (159) | 800 mg | 50 | 95 | U.S. | |||
| 2016 (16) | OD (38) | 660 mg | 30 | 98 | France | |||
| 2017 (17) | OD (106) | 1,150 mg | 95 | + | + | None | U.S. |
Open cells, no data. For recent T1D and proliferation, see the text. OD, organ donors; Syn, synaptophysin.
Supplementary Material, part 1, references 1–17.
Statistically significant <0.05.
Area expressed as μm2.
Short- and long-term durations are in years, and x is mean in years.
Summary
These studies illustrate how fasting glucose or 2-h OGTT functional results can be semiquantitatively compared with β-cell histological content using subjects tested at defined levels of dysglycemia (Fig. 1A and B). Further, functional parameters describe the natural history of progression from early dysglycemia (subclinical prediabetes) to prediabetes and T2D glucose levels (Fig. 1A). Remarkably, prediabetes histological studies show similar reductions of β-cell content loss (Fig. 1B, upper orange circle) compared with functional loss (Fig. 1A, orange circles), whether in living, organ donor, or autopsied lean or obese adults. Moreover, any level of β-cell dysfunction that exceeds content loss (Fig. 1C and D) implies the former can antedate the latter (see below). These T2D processes consistently involve histologically demonstrable apoptosis (Table 1).
Treatment of prediabetes and earlier stages of T2D can rescue reduced BCOD (Fig. 1C, solid and dashed green and blue arrows, Rx). For example, a controlled, randomized, and interventional trial of obese T2D patients showed that after only 8 weeks of a very-low-calorie diet, ∼43% reverted from T2D to prediabetes 6 months and even 2 years later (13,14). Interestingly, those subjects who responded, compared with the remainder, had shorter diabetes duration (∼4 vs. ∼10 years), lower baseline HbA1c (7.1% vs. 8.4% [52 vs. 62 mmol/mol]), higher fasting insulin (20 vs. 9 mU/L), higher ALT (43 vs. 22 units/L), higher triglyceride levels (175 vs. 115 mg/dL [1.97 vs. 1.30 mmol/L]), and higher hepatic triglyceride content (13% vs. 8%). This functional reversal of T2D, due to treatment-improved β-cell function, was likely due to triglyceride reduction via improved liver and exocrine pancreas function. By implication, the fragility or robustness of the unfolded protein response (UPR), as discussed under molecular pathology (Fig. 2), determines the compensatory insulin secretion, itself determined by the differential response to diet, including palmitate, and the variable genetic background.
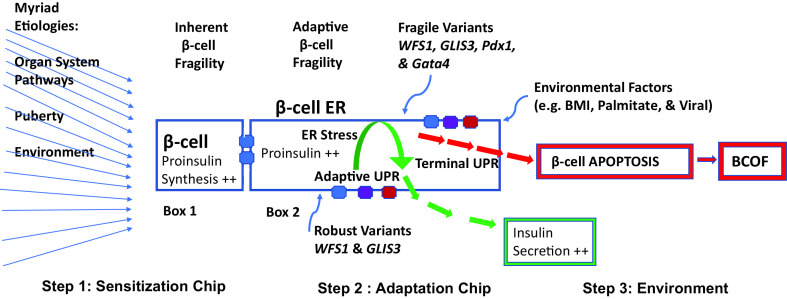
Figure 2.
Key elements of the β-cell molecular machine that create intra- and interindividual variability of BCOD/F in both T2D and T1D. For myriad etiologies, the blue arrows (left side of figure) depict enormous numbers of genetic and nongenetic pathways affecting β-cells, e.g., immunogenic (autoimmunity) or nonimmunogenic (insulin resistance, developmental, and environmental), which can singly or in combination converge to initiate step 1, β-cell sensitization (box 1). Thus, from myriad etiologies there is one major pathway to β-cell death, apoptosis. In box 1, the ER space is depicted where the sensitization step of excessive proinsulin synthesis sensitizes β-cells to damage, e.g., from compensatory insulin secretion or common or rare gene variants, and each person has a relatively unique gene chip. This step 1 sensitization chip represents the composite genetic outcomes from different organs, tissues, and cells leading directly or indirectly to increased proinsulin synthesis. However the β-cell organ is inherently fragile (text above box 1) due to small size, low threshold for proinsulin synthesis to create sensitization, and a relative lack of regenerative capacity. In box 2, despite myriad etiologies and inherent fragility, the adaptive UPR machine (step 2) leads to sensing and degradation of excessive amounts of unfolded proinsulin in the healthy ER (green arrows) mediated by regulatory ER membrane-resident proteins (blue, purple, and red structures on edges of box 2; see the text for details), and robust gene variants (blue arrow to lower ER edge of box 2). However, if the individual is genetically fragile (step 2, blue arrow to upper ER edge of box 2; see the text for details) or environmentally impaired (step 3), adaptive fragility results in the terminal UPR, leading to cell death and BCOF (box 2, red arrows). These composite genetic events at this level are represented by the adaptation chip and modulated by step 3, environmental factors. The functional outcome of an individual’s sensitization and adaptation chips to given environmental exposures results in highly variable phenotypes, be they normal glucose homeostasis or, more commonly, degrees of BCOD—the dysglycemic continuum.
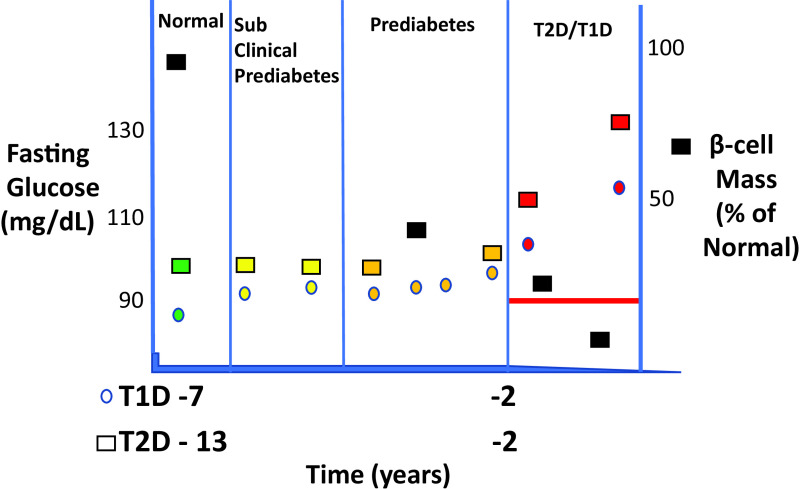
We therefore propose that persistently increasing fasting glucose levels to >90 mg/dL (5 mmol/L) is potentially abnormal, as previously suggested (Fig. 3) (15–18). It follows that the β-cell organ paradigm can be used to better define lower glucose thresholds for disease prevention (Supplementary Material, part 1, recommendations).
Figure 3.
Diabetes progression is similar during the seven years prior to T1D or 13 years prior to T2D, as depicted by “normal,” but slightly increasing, fasting glucose levels up to 1 to 2 years prior to diabetes onset. Both disorders have a rapid escalation of glucose levels during the 1 to 2 years prior to disease onset. The mean age for T1D progressors >5 years was 15 years at baseline. Colored squares indicate various periods before and after T2D, colored circles indicate periods before and after T1D, and black squares indicate data for β-cell mass estimates. The red line depicts the estimated, conceptual threshold level of β-cell mass below which lifestyle and tablets no longer suffice for treatment, i.e., exogenous insulin requiring.
T1D and BCOD/F
Recent reviews highlight the need for reassessing the pathophysiology of T1D (19–23). The BCOD/F paradigm we propose reflects a broader drive for earlier T1D diagnosis and treatment (24,25). Multiple autoantibody-positive children without diabetes (relatives of T1D patients) can have a detrimental Index60 >1, “normal” glucose tolerance, yet 61% progress to T1D in 5 years (22), suggesting early to moderate BCOF due to β-cell loss, similar to the case for pre-T2D (Fig. 1A and B). Further, older children and adults without T1D, but at risk for T1D, can have limited and patchy, not generalized, islet inflammation, so-called vitiligo of the islets, not dissimilar to adults with new-onset or established T2D (26–28). Thus, both T1D and T2D cases can show similar metabolic changes, even islet inflammation and circulating β-cell-specific T cells, notably in adults with T1D in whom islet infiltrates can be similar to those of some T2D subjects in whom islet CD68+ macrophages predominate (28,29). In contrast, T1D children <7 years old are different, with comparatively rapid loss of β-cell function, often with multiple autoantibodies and islet infiltrates showing larger numbers of infiltrating B-cells and CD8+ T cells (27).
Functional
T1D β-cell content studies cannot be linked to defined earlier dysglycemias. However, autoantibody-positive children and adults without diabetes can have early glucose and insulin secretory abnormalities, similar to those in T2D (Fig. 3) (22,23).
Histological
To test the validity of β-cell content vis-à-vis autoimmunity, we identified five relevant studies (n = 387 subjects) (Table 1 and Supplementary Material, part 1), each with comparable results; here, we focus on two of them illustrating the potential of deep data mining (26,30). Our conclusions, after recalculating published data, are that single-autoantibody-positive subjects without diabetes >20 years old show substantial 30–50% reductions in β-cell content versus autoantibody-negative control subjects (Fig. 1B, upper orange circle, and Supplementary Material, part 2). Moreover, the 1- or 2-h OGTT is comparable to autoantibody positivity in detecting subclinical prediabetes in adults at T1D risk with early to moderate BCOF (Fig. 1B, upper orange circle). There is ∼95% β-cell content reduction in long-standing T1D (Fig. 1B, red circle). Further, and to be confirmed, we found apparent similarities of pre-T1D and T2D in β-cell content (Fig. 1B, upper orange), neogenesis, and apoptosis, emphasizing the potential of defining BCOD before BCOF irrespective of the clinical status. Additionally, four β-cell content studies showed >90% β-cell content loss in adult T1D, and additionally with evidence of proliferation in children and neogenesis in adults, likely explaining residual, though low, C-peptide values. Further, apoptosis was described in 2 of 3 studies, with the caveat that the single negative study only examined islet β-cells rather than including isolated and periductal β-cell clusters of one to three cells (Table 1) (31). Of note, enhanced HIF1α/PFKFB3 signaling with reduced β-cell apoptotic rates, as observed in T1D organ donors, could explain lower but demonstrable apoptosis (32). Thus, using multiple pancreas sources and methodologies, cases of T1D with BCOF show β-cell content loss, cell death, and limited regeneration (Supplementary Material, part 3).
The BCOD/F paradigm we describe is in line with the striking similarities among T1D and T2D (Fig. 1A and B). By determining minimal dysglycemia, intervention aimed at diabetes prevention in both conditions could start earlier. Functional screening and follow-up studies of early BCOD are relatively easy to perform, whereas diagnostics and treatments become more complicated later (Supplementary Material, part 1, recommendations) (1). As investigators focus on earlier dysglycemia, these concepts can be confirmed, refuted, or refined.
Compensatory Proinsulin Synthesis and Insulin Secretion
Although extensively described for T2D, especially during insulin resistance (33), we posit that T1D-related BCOD also follows periods of compensatory, excessive proinsulin synthesis, as suggested by both rodent and human studies. After extensive human pancreatectomy, remaining normal β-cells increase both proinsulin synthesis and insulin secretion to compensate for hyperglycemia antedating subsequent BCOF and insulin requiring T1D due to severe β-cell loss. The BB rat T1D model also reveals diabetes-prone (prediabetes) animals, before immune-mediated spontaneous diabetes onset, with mild hyperglycemia, yet we noted in vitro–perifused, isolated noninflamed or inflamed islets, both showing substantially elevated, biphasic glucose-stimulated insulin secretion, consistent with compensatory insulin production and secretion in vivo (34). Imaging mass cytometry data from human β-cells (35) and prospective studies show progressively increasing fasting C-peptide values in autoantibody-positive children without diabetes during the 5 years before T1D, both demonstrating changes consistent with β-cell compensation (23).
The β-cell organ paradigm provides a potential functional explanation for T1D remissions, which mirror those observations described above for T2D. If new-onset T1D subjects are treated for 1 month with intensive insulin in an observational trial, there is a likely reduction of compensatory proinsulin production (Fig. 2, box 1). We found that this strategy is associated with improved β-cell function after termination of exogenous insulin using only sulfonylurea treatment, which, in 54% of subjects, resulted in insulin-free remissions, whereas without sulfonylurea 22% went into remission (Fig. 1C, blue arrows) (36). By implication, a certain resilience of the β-cell organ exists, likely due to the degree of β-cell content (Fig. 3). β-Cell apoptosis/regeneration (neogenesis) dynamics will be crucial to better understand such β-cell compensation in both T2D and T1D (Supplementary Material, part 3).
Summary
There is a potential to identify both T2D and T1D at stages well before the onset of currently recognized clinical disease. Even T1D guidelines under discussion for earlier intervention may not optimize the best time for intervention (24,25). The aim of preventive strategies should be to preserve β-cell mass and, therefore, function. We then considered molecular mechanisms associated with BCOD/F and whether they showed similarities between T2D and T1D.
Molecular Pathology
Comprehensive reviews describe stressed β-cell characteristics, including compensatory insulin secretion impacting the UPR, apoptosis, and organ failure by increased proinsulin synthesis (37,38). β-Cell death involves multiple intracellular organelles in integrated stress responses. We restrict our focus here to pathways primarily related to the ER and apoptotic cell death and for those less related to the ER, e.g., immunogenic cell death and emerging forms of β-cell death (refer to Supplementary Material, part 4). Molecular mechanisms during reduced β-cell function in both T2D and T1D indicate that excessive proinsulin production results in ER stress attempting to maintain fuel-related metabolic homeostasis. Virtually all pathways leading to diabetes converge at the ER. β-Cell characteristics associated with increased proinsulin synthesis include three tumblers, namely, tumbler 1, sensitization and compensatory insulin secretion; tumbler 2, ER stress and the UPR; and tumbler 3, apoptotic alterations resulting in individual β-cell death. In addition, and largely unrealized, the β-cell organ is inherently fragile due to very small organ mass, normally functioning at a critically high level and having minimal regenerative properties. Thus, when proinsulin synthesis approaches or exceeds maximal capacity, it can create sufficient ER stress to induce β-cell death and organ failure (BCOF). By implication, limiting ER stress earlier could be beneficial in virtually all forms of diabetes.
Tumbler 1: β-Cell Sensitization and Compensatory Insulin Secretion
The NODk nonimmune mouse model uses the Ins2 gene promotor-driven hen egg lysozyme (HEL) transgene’s product to create β-cell sensitization, the first tumbler in the active ER process (Fig. 2, box 1). Hence, HEL is excessively expressed in the ER, simulating excess proinsulin production and subclinically sensitizing β-cells to ER stress and limited apoptosis without causing clinical diabetes (39). From these observations, we derived the clinically relevant conceptual bridge linking myriad initiating pathways of BCOD to converge at the ER (Fig. 2, thin blue arrows on left, and box 1). In effect, this process could create BCOF by reducing β-cell mass, which itself creates a new cycle of proinsulin demands affecting remaining healthy β-cells, i.e., compensatory proinsulin production, as identified in human imaging mass cytometry (35,40), pre-T1D (23,41), and T2D (33).
Explaining the sensitization event at the molecular level, rare human cases, i.e., INS gene mutation–induced diabetes of youth (MIDY), illustrate that the sensitization tumbler alone is sufficient to generate both apoptosis and diabetes (42). An analogous process is seen in the Akita mouse, which has a proinsulin cysteine mutation leading to proinsulin misfolding, aggregation, excessive oxidation due to futile oxidative folding, and progressive β-cell loss (38,43). Thus, a single-allele mutation not only distorts the mutant molecule, but it also binds to and affects folding, aggregation, trafficking, and secretion of wild-type proinsulin molecules. It follows that when wild-type proinsulin is produced in excess in normal human β-cells (∼20% misfolding), as observed with compensatory proinsulin synthesis, the excess misfolded proinsulin can lead to increased aggregation and oxidation qualitatively similar to that in MIDY.
Importantly, given the role of genetic and nongenetic factors in the origins of human diabetes, the nonimmune NODk sensitization model also requires genetic and/or environmental enhancers to create clinical diabetes via β-cell apoptosis. Remarkably, using genetic inbreeding of HEL sensitized mice (39), one of three transgene (HEL)-inducible diabetes-associated genes was Glis3, implicated in human T1D and T2D. The Glis3 gene product is a transcription factor that mediates the antiapoptotic adaptive response by upregulation of Manf, enhancing the adaptive UPR, making it more robust (Fig. 2, box 2, green arrows). Loss of function of just one Glis3 allele limits Manf upregulation, resulting in greater fragility of the β-cell adaptive step, leading to apoptosis and diabetes (Fig. 2, box 2, red arrows and BCOF). Human T2D islets share some of these abnormalities (39). Finally, increasing dietary palmitate in this NODk model can also result in diabetes, linking sensitization to environmental factors that are clinically important in humans (Fig. 2) (39,44).
Tumbler 2: ER Stress in β-Cells
Molecular processes involving common and disease-related genetic variants focus attention on the β-cell organ’s mechanical and regulatory features that likely determine whether the stressed β-cell can survive. We posit that myriad pathways leading to diabetes converge at the β-cell ER (Fig. 2, thin blue arrows on left). Supporting such convergence, both rodent studies and clinical trials have targeted multiple ER molecular mechanisms (38,45,46). Increased proinsulin synthetic demands (Fig. 2, box 1) can create ER stress activating UPR adaptation (Fig. 2, box 2, green arrows). It follows that ER regulatory proteins, including the PKR-like ER kinase (PERK), ATF6α, IREα, and other ER proteins regulating luminal calcium levels all assist UPR adaption and can limit ER stress and the risk of apoptosis (Fig. 2, box 2, colored structures on edges). These regulatory proteins are responsible for enlarging ER physical capacity through increasing lipid membrane synthesis, reducing protein synthetic demands (including proinsulin synthesis), and creating more chaperone proteins, thereby improving UPR efficiency. Also determining UPR efficiency are a variety of genes having common, diabetes-associated variants that can enhance (Fig. 2, box 2, robust variants) or reduce (Fig. 2, box 2, fragile variants) adaptive UPR efficiency (see below). Emphasizing this complexity is the artist’s pallet analogy, which provides the genetic architecture illustrating the fine resolution within the sensitization and adaptation gene variant chips in Fig. 2 (47).
If the β-cells as an organ are healthy, then normal function can be restored. On the other hand, if the β-cell machine is too fragile and/or the demands are great, the insulin secretory pool may decrease. Nevertheless, these dysfunctional and potentially dysglycemic processes can be reversible if the adaptive UPR succeeds in preventing the β-cell ER machine from entering the terminal UPR (Fig. 2, box 2, red arrows). β-Cell dysfunction and dysglycemia can also be rescued by treatments including reducing proinsulin synthetic demands at both the cellular and organ levels (Fig. 1C, green and blue arrows). From a practical point of view, the earlier the dysfunction is diagnosed, the more effective prevention is likely to be, given that it is the overall β-cell organ that defines islet secretory function, itself an integrated composite of heterogeneity in intraindividual β-cell function and interindividual β-cell mass.
Tumbler 3: β-Cell Death
However, if adaptation fails, then the terminal UPR occurs, leading to proapoptotic responses and cell death (Fig. 2, box 2, red arrows). When sufficiently severe, clinical BCOF would develop in both common forms of diabetes. A genetically determined, robust UPR helps sustain insulin secretion in healthy individuals. One of the above-described regulatory proteins, IREα, having a role in the human adaptive UPR, also ironically mediates the terminal UPR through higher levels of autophosphorylation and oligomeric mechanisms during excessive ER stress. These adverse circumstances, e.g., excess proinsulin synthesis and allosterically modulated ER regulatory proteins, can create fragile β-cells and deviate antiapoptotic properties to proapoptotic changes, resulting in ER maladaptation, BCOD, and, ultimately, β-cell death, which, if sufficiently severe and widespread, can cause BCOF (Fig. 2, box 2, red arrows, and β-cell apoptosis box).
Additionally, β-cell stressors, including H2O2, palmitate, and cytokines (interleukin-1β, tumor necrosis factor-α, and interferon-γ) can cause ER calcium depletion, thereby linking ER calcium levels to both genetic and environmental mechanisms of human apoptosis and BCOD/F (39,46,48). Further, cytokines can mediate both apoptotic and immunogenic β-cell death in that they can alter β-cell prohormone processing and upregulate β-cell HLA class I and II proteins (46,49–52). In T1D, cytokines mediate human β-cell death by increasing transcripts associated with induced regulatory elements (IRE) (50). To understand the function of one common T1D gene variant, cytokines can stimulate the human islet IRE enhancer region of TNFSF18, while the G allele of variant rs78037977 disrupts IRE activation, likely explaining its association with reduced T1D risk (odds ratio 0.78). On the other hand, T2D common risk variants overlap stable regulatory elements in these cytokine-inducible enhancer regions. Further, proinflammatory cytokines (intereleukin-1β and interferon-γ) elicit activation of cis-regulatory element enhancers in human β-cells. Finally, β-cell IRE enhancers are activated after cytokine exposure, consistent with a stimulus response to protect the β-cell (50). Thus, several lines of evidence associate diabetes-related genetic variants with human β-cell function, survival, or death. Such molecular genetic processes focus attention on the β-cell organ’s mechanical and regulatory environment, which, taken in the round, could have a role in both disease induction and disease prevention.
Importantly, at least two related mechanisms can determine β-cell death, apoptotic, also called homeostatic, physiologic, or tolerogenic apoptosis, occurring in the β-cell as described above, and immunogenic, or immune-mediated cytotoxicity from outside the β-cell. These processes involve phagocytic mechanisms after apoptosis occurs, as well described in cancer (53) and, to a lesser extent, in T1D, as detailed in Supplementary Material, part 4 (54).
Evidence Linking BCOD/F With Human β-Cell Apoptosis
Evidence from autopsy, surgical, and organ donor specimens document β-cell apoptosis in BCOD/F in both T2D and T1D (Table 1). Further, apoptotic-related gene variants include common GLIS3 variants in both T1D and T2D plus low-frequency, high-impact variants causing neonatal diabetes (4). GLIS3 variants may result in gain or loss of function, potentially enabling more robust or more fragile ER adaptation responses, respectively (4,44,55). Low-frequency, high-impact WFS1 variants are also related to apoptosis in both T2D and T1D (4,56). T2D risk is reduced by two common, low-impact WFS1 variants (57), while that risk is increased by a common, low-impact SPRY2 apoptotic variant (4). Apoptotic events are also implicated by transcriptomic data in a human β-cell line, in that knockdown of a long noncoding RNA (lncRNA) (HI-LNC25) results in decreased GLIS3 transcription, potentially enabling apoptosis, and this same lncRNA is also reduced in islets from T2D (58). Further, increased proinsulin production alone can cause diabetes, as evidenced by the human apoptotic prototype, the Akita mouse mutation, itself analogous to the INS gene mutation in MIDY (42). Further, ER dysfunction is critical to the human Wolcott-Rallison syndrome, which is caused by rare mutations in PERK, a key regulator of the UPR (46,59), while Wolfram 1 and 2 dysglycemic syndromes emphasize the important role of ER calcium and redox homeostasis in human β-cell apoptosis (48,56,57). Finally, a recent study has evaluated whole pancreatic apoptotic transcripts from organ donor control subjects, autoantibody-positive individuals without diabetes, and T1D and T2D subjects (41). Transcripts of genes associated with monogenic forms of diabetes caused by nonredundant single-gene mutations included elevated EIF2AK3 (PERK transcript) pancreatic transcripts across the spectrum of disorders as well as changes in pre-T1D before dysglycemia. Interrogating apoptotic integrated stress response genes identified transcripts associated with both T1D and autoantibody-positive subjects without diabetes in these heterogeneous cell populations. Using routine antibody immunofluorescence studies coupled with in situ hybridization, the study related these pancreatic transcripts to pancreatic exocrine and islet tissues, illustrating how disease-associated pancreatic apoptotic transcripts can be common to clinical heterogeneity.
Summary
To gain novel mechanistic insights into BCOD/F and the potential reversal of β-cell dysfunction, we linked apoptosis to both altered expression of human gene variants and dysglycemic disorders associated with these variants, as well as with β-cell content loss. These proposed convergent pathways of sensitization and adaptation gene responses and gene variants (Fig. 2, sensitization and adaptation chips) form a composite to β-cell function (Fig. 2). Such integrated responses are further modulated by the environment, which together determine an individual's position on the BCOD/F curves of Fig. 1A and B. Appreciating the importance of these converging ER pathways in diabetes has led to studies to identify novel treatments. Critical to the success of such drugs, clinical thresholds need to be redefined so that interventions can be initiated at the earliest stage.
Genetic Implications
Genetic and epigenetic studies, as described above, extend and strengthen the proposal that the earlier introduction of therapy can reverse BCOD or limit BCOF. In this section, we continue to emphasize common features of T2D and T1D while appreciating the pressing clinical imperative to identify distinct heterogeneity between and within them both.
Clinical and Genetic Features Common to T2D and T1D
Common clinical features of diabetes, irrespective of its causes, include slowly progressive deterioration of fasting glucose levels (Fig. 3) and altered protein and lipid glycation, each leading to both macrovascular and microvascular complications. Indeed, the existence of metabolic memory for macrovascular diseases in diabetes is predicated on earlier diagnosis and treatment in both T2D and T1D (60,61). As set out in this Perspective, we identify common features that could be targets for earlier disease prevention or modification, specifically age (Fig. 4), obesity (Supplementary Material, part 5), and T2D gene variants linked to T1D (see hypothesis section).
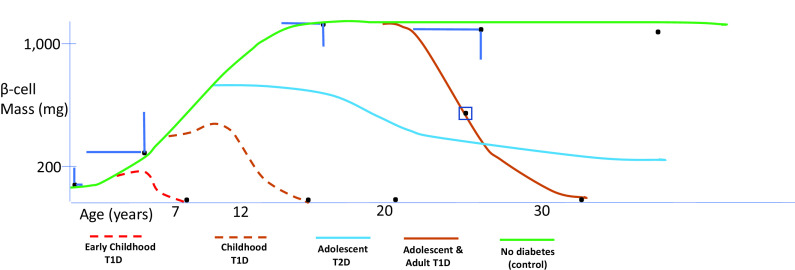
Figure 4.
Clustering acute BCOF is illustrated using β-cell mass (y-axis) and cohort age (x-axis). β-Cell mass data are derived from subjects without diabetes (black dots ±1 SD, green curve; references 26 and 31; see the main text). For those with diabetes, black dots near the x-axis (except for the black dot in the square box; see the main text) are derived from the main text. Various phenotypic biomarkers used to cluster cases are derived from references in the main text. Other colored lines indicate possible courses to massive β-cell content loss, with stylized dashed lines due to little information. These clinical severity clusters below match the color codes in the figure and suggest more careful attention addressing population screening, especially in young children (Table 2 and Supplementary Material, part 1, recommendations).
Age at Diabetes Onset
In both T2D and T1D, the genetic risk load impacts age at onset in the context of growth. The earlier the diagnosis, the greater the genetic load. Certainly, in young-onset (<7 years of age) T1D the concordance rate between identical twins, the risk of HLA DR3 and DR4 heterozygosity, the association with both MHC class I and class II genes, and the Gene Risk Score are all much greater than those in adult-onset disease (19,52,62–64). Further, maturity-onset diabetes of the young (MODY) and common T2D gene variants are associated with β-cell growth and development (NEUROG3, HNF1B, NKX2.2, GLIS3, etc.), environmental effects (TCF7L2), and β-cell survival (CDKN2A/B, GLIS3, BCL11A, and WFS1) and primarily operate before full β-cell mass is achieved (4,65,66). That reduced mass may account, in part, for at-risk children having a more rapid progression to clinical diabetes (Fig. 4 and Table 2). Recognizing that there is substantial variability in β-cell mass in normal children renders comparisons of β-cell mass estimates difficult in children with diabetes (26,31). Compared with older-onset T1D, children diagnosed before age 7 years also have an increased frequency of 6 non-HLA alleles found either specifically in β-cells (GLIS3), likely involved in T-cell function (IL2RA, IL10, IKZF3, and THEMIS), thymus (THEMIS), β-cell development/functions (IKZF3 and IL10), or both immune cells and β-cells (CTSH) (67). Finally, in those 2 months before and after birth, an apoptosis wave, followed by replication, replenishes β-cells. This apoptosis event could create immune events in those with appropriate gene variants, explaining the very early onset of T1D autoantibody positivity (Supplementary Material, part 4).
Table 2.
Clusters for childhood, adolescent, and adult T1D and adolescent T2D
| Parameter | Early childhood T1D | Adolescent T1D | Adolescent T2D | Adolescent and adult T1D |
|---|---|---|---|---|
| Clinical result | Very severe | Severe | Severe | Less severe |
| Genes | ||||
| HLA | Class I and II++ | I and II+ | Nil | I & II |
| Gene Risk Score | High++ | High+ | Nil | + |
| Variants | Some T2D | Some T2D | T2D | Some T2D |
| Insulitis | +++ | ++ | + | + |
| AutoAB | Multiple | Single/multiple | Nil | Single/multiple |
| Environment | Viral? BMI | Viral? BMI | Puberty | Viral? BMI |
| Immunogenotype | Proinsulin-DR4 | GAD-DR3 |
Plus signs indicate relative magnitude.
High T1D risk is characterized by a cluster of risk factors, each predictive of T1D yet complementary in that, taken together, they create a high risk of disease progression. These factors include genetic risk variants; age, especially when <7 years; metabolic changes, e.g., altered Index60 >1; and immune alterations, e.g., multiple autoantibodies (Fig. 4), as well as nongenetic risk, including stochastic and epigenetic effects (19,22,27). By implication, any one of these factors may predict diabetes, but taken in isolation they carry less disease risk and, in some cases, that risk is negligible, e.g., common genetic risk variants. Since β-cell loss is less severe in T1D with increasing age at diagnosis, it follows that autoimmune diabetes in adults may be metabolically nearly indistinguishable from T2D (28,29). Moreover, as we have shown, only a fraction of T1D adults with genetic risk ever develop the disease; witness low T1D concordance rates even in cotwins of T1D adult-onset monozygotic twins (68). By implication, in adult-onset T1D, the nongenetic effect must be substantial, potentially due to environmental, epigenetic, and/or stochastic events, e.g., even identical twins show random intrinsic gene penetrance affecting different MHC susceptibility genes (69).
Hypothesis
We postulate that pathways initially leading to T1D in our BCOD/F paradigm can involve, in part, T2D-associated and/or BMI-associated common gene variants in the context of nongenetic events. Supporting this concept are:
Initial T1D β-cell apoptosis is followed by antigen exposure and recognition via immunogenic phagocytosis and presentation to the genetically predisposed immune system (e.g., HLA) to create an autoimmune response (Supplementary Material, part 4) (20,46).
Some 19 of 94 common T2D gene variants, e.g., TCF7L2, GLIS3, and WFS1, are linked to T1D, while both T1D and T2D common risk variants are linked to transcription factors causing MODY, many potentially initiating apoptosis (65,66,70). Given that 60–80% of children will have T2D gene variants, as inferred in the Epidemiology section, there is likely a cumulative effect of common, low-impact, coding and noncoding gene variants associated with both T1D and T2D, as described in human embryonic and adult tissues (46,65,66). For example, risk variants cluster in regulatory DNA regions, which are recognition sites for each of several MODY transcription factors (HNF4A, etc.) affecting β-cell development and function. In this way, the impact of common or low-frequency MODY coding variants could be amplified by common, noncoding gene variants clustered in regulatory regions.
Consistent with nonautoimmune initiation of T1D, both subjects with MODY and subjects with gestational diabetes mellitus, during growth phases, with common detrimental HLA haplotypes or other T1D risk variants, can first develop apoptotic and then immune forms of cell death associated with autoantibodies secondary to nonimmunogenic MODY mutations. Early apoptotic cell death near puberty or during gestation could lead to clinical phenotypes similar to autoimmune diabetes, even mistaken for T1D (71,72).
T2D risk variants of TCF7L2 are associated with accelerated conversion from single (insulin autoantibody or IA-2 antigen) to multiple autoantibodies in the context of increased BMI in children, thereby increasing T1D risk (Supplementary Material, part 5) (73).
Given that higher-impact but common T2D risk variants, e.g., TCF7L2 and GLIS3, associate with T1D, we may be underestimating lower-impact, common T2D gene variant risk loads in T1D GWAS cohorts, which are of much smaller size (62,70).
Both T1D and T2D are associated with increased proinsulin levels, in excess of C-peptide, notably in individuals with T1D aged <7 years at diagnosis and associated with evidence of aberrant proinsulin processing (63,74).
Both T1D and T2D have a slowly progressing disease process with mildly elevated glucose levels until ∼2 years prior to disease onset, when both disorders show more marked glycemic deterioration (Fig. 3 and Supplementary Material, part 6) (15,23,75). For T1D, adult deaths from diabetic ketoacidosis, ∼800 in the U.S. in 2017, emphasize the importance of earlier diagnosis and prevention (76,77).
Summary
By understanding what is common to BCOD/F in T2D and T1D, we can better appreciate what features are distinct. Diabetes risk is a continuum, much as blood glucose, insulin secretion, and glucose disposition are continua. The nature of both T2D and T1D will depend on environmental insults and nongenetic effects, attained β-cell mass, cumulative T2D and T1D coding and noncoding gene variants, and developmental factors, including growth and pregnancy, autoimmunity, and BMI. However, irrespective of the disease origins and pathogenesis, central to the nature of dysglycemia is inappropriate insulin secretion from remaining viable β-cells, consequent β-cell ER stress, the UPR, and, eventually, β-cell attrition.
Conclusions
This Perspective has attempted to interpret recent T2D and T1D research, highlighting features common to both. Thus, 60–80% of the general population has diabetes-related common gene variants. Low-impact but common gene variants can behave cumulatively, resulting in broad phenotypic alterations. Additionally, some gene variants influence trans-acting transcription factors, which can amplify their effects by binding to enormous numbers of distant DNA targets, creating distinct transcriptomic phenotypes. Critically related to these observations are phenotypes of reversibly reduced BCOD and irreversible BCOF, both occurring much earlier than previously recognized. By inference, earlier approaches might prevent or modulate these clinical phenotypes. Moreover, although T2D and T1D show many similarities mechanistically, there are important differences that can be leveraged for earlier screening, diagnosis, and prevention to protect β-cell mass. Finally, integrating the four pillars we outline into clinical phenotypes could also be valuable for earlier diagnosis and prevention (Supplementary Material, part 6).
Article Information
Funding. R.D.L. has been recently funded by the European Foundation for the Study of Diabetes (MMBP1C3R), EU (EU-FP7: 282510), Bart’s Charity (OGA011582), British Twin Trust, and Diabetes UK (MMBG1K9R/S).
Duality of Interest. R.D.L. is a special board member for Diamyd and ProventionBio. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.15141837.
References
- 1. Chan JCN, Lim LL, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021;396:2019–2082 [DOI] [PubMed] [Google Scholar]
- 2. Babey SH, Wolstein J, Diamant AL, Goldstein H. Prediabetes in California: nearly half of California adults on path to diabetes. Policy Brief UCLA Cent Health Policy Res 2016;(PB2016-1):1–8 [PubMed] [Google Scholar]
- 3. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314: 1021–1029 [DOI] [PubMed] [Google Scholar]
- 4. Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 2018;50:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stokes A, Preston SH. Deaths attributable to diabetes in the United States: comparison of data sources and estimation approaches. PLoS One 2017;12:e0170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of beta-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 2016;101:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler AE, Dhawan S, Hoang J, et al. Beta-cell deficit in obese type 2 diabetes, a minor role of beta-cell dedifferentiation and degranulation. J Clin Endocrinol Metab 2016;101:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Md Moin AS, Dhawan S, Cory M, Butler PC, Rizza RA, Butler AE. Increased frequency of hormone negative and polyhormonal endocrine cells in lean individuals with type 2 diabetes. J Clin Endocrinol Metab 2016;101:3628–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Md Moin AS, Cory M, Ong A, et al. Pancreatic nonhormone expressing endocrine cells in children with type 1 diabetes. J Endocr Soc 2017;1:385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Md Moin AS, Dhawan S, Shieh C, Butler PC, Cory M, Butler AE. Increased hormone-negative endocrine cells in the pancreas in type 1 diabetes. J Clin Endocrinol Metab 2016;101:3487–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giannini C, Weiss R, Cali A, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes 2012;61:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steven S, Hollingsworth KG, Al-Mrabeh A, et al. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care 2016;39:808–815 [DOI] [PubMed] [Google Scholar]
- 14. Al-Mrabeh A, Hollingsworth KG, Shaw JAM, et al. 2-Year remission of type 2 diabetes and pancreas morphology: a post-hoc analysis of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2020;8:939–948 [DOI] [PubMed] [Google Scholar]
- 15. Sagesaka H, Sato Y, Someya Y, et al. Type 2 diabetes: when does it start? J Endocr Soc 2018;2:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee CMY, Colagiuri S, Woodward M, et al. Comparing different definitions of prediabetes with subsequent risk of diabetes: an individual participant data meta-analysis involving 76,513 individuals and 8208 cases of incident diabetes. BMJ Open Diabetes Res Care 2019;7:e000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu T, Jacobs DR Jr, Sinaiko AR, et al. Childhood BMI and fasting glucose and insulin predict adult type 2 diabetes: the International Childhood Cardiovascular Cohort (i3C) Consortium. Diabetes Care 2020;43:2821–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tirosh A, Shai I, Tekes-Manova D, et al.; Israeli Diabetes Research Group . Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005;353:1454–1462 [DOI] [PubMed] [Google Scholar]
- 19. Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 2020;43:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linsley PS, Greenbaum CJ, Nepom GT. Uncovering pathways to personalized therapies in type 1 diabetes. Diabetes 2021;70:831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludvigsson J. Time to leave rigid traditions in type 1 diabetes research. Immunotherapy 2017;9:619–621 [DOI] [PubMed] [Google Scholar]
- 22. Jacobsen LM, Bocchino L, Evans-Molina C, et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia 2020;63:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evans-Molina C, Sims EK, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group . β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 2018;3:e120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Insel R, Dutta S, Hedrick J. Type 1 diabetes: disease stratification. Biomed Hub 2017;2(Suppl. 1):111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of beta-cell destruction and the age at onset of type 1 diabetes. Diabetes 2016;65:1362–1369 [DOI] [PubMed] [Google Scholar]
- 28. Lundberg M, Seiron P, Ingvast S, Korsgren O, Skog O. Insulitis in human diabetes: a histological evaluation of donor pancreases. Diabetologia 2017;60: 346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care 2014;37:3286–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diedisheim M, Mallone R, Boitard C, Larger E. β-cell mass in nondiabetic autoantibody-positive subjects: an analysis based on the Network for Pancreatic Organ Donors Database. J Clin Endocrinol Metab 2016;101:1390–1397 [DOI] [PubMed] [Google Scholar]
- 31. Lam CJ, Jacobson DR, Rankin MM, Cox AR, Kushner JA. Beta cells persist in T1D pancreata without evidence of ongoing beta-cell turnover or neogenesis. J Clin Endocrinol Metab 2017;102:2647–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nomoto H, Pei L, Montemurro C, et al. Activation of the HIF1α/PFKFB3 stress response pathway in beta cells in type 1 diabetes. Diabetologia 2020;63:149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dirice E, De Jesus DF, Kahraman S, et al. Human duct cells contribute to β cell compensation in insulin resistance. JCI Insight 2019;4:e99576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teruya M, Takei S, Forrest LE, Grunewald A, Chan EK, Charles MA. Pancreatic islet function in nondiabetic and diabetic BB rats. Diabetes 1993;42:1310–1317 [DOI] [PubMed] [Google Scholar]
- 35. Damond N, Engler S, Zanotelli VRT, et al. A map of human type 1 diabetes progression by imaging mass cytometry. Cell Metab 2019;29:755–768.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Selam JL, Woertz L, Lozano J, Robinson M, Chan E, Charles MA. The use of glipizide combined with intensive insulin treatment for the induction of remissions in new onset adult type I diabetes. Autoimmunity 1993;16:281–288 [DOI] [PubMed] [Google Scholar]
- 37. Papa FR. Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb Perspect Med 2012;2:a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghosh R, Colon-Negron K, Papa FR. Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes. Mol Metab 2019;27S:S60–S68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dooley J, Tian L, Schonefeldt S, et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet 2016;48:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang YJ, Traum D, Schug J, et al.; HPAP Consortium . Multiplexed in situ imaging mass cytometry analysis of the human endocrine pancreas and immune system in type 1 diabetes. Cell Metab 2019;29:769–783.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hiller H, Beachy DE, Lebowitz JJ, et al. Monogenic diabetes and integrated stress response genes display altered gene expression in type 1 diabetes. Diabetes 2021;70:1885–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu M, Haataja L, Wright J, et al. Mutant INS-gene induced diabetes of youth: proinsulin cysteine residues impose dominant-negative inhibition on wild-type proinsulin transport. PLoS One 2010;5:e13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arunagiri A, Haataja L, Pottekat A, et al. Proinsulin misfolding is an early event in the progression to type 2 diabetes. eLife 2019;8:e44532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liston A, Todd JA, Lagou V. Beta-cell fragility as a common underlying risk factor in type 1 and type 2 diabetes. Trends Mol Med 2017;23:181–194 [DOI] [PubMed] [Google Scholar]
- 45. Morita S, Villalta SA, Feldman HC, et al. Targeting ABL-IRE1α signaling spares ER-stressed pancreatic β cells to reverse autoimmune diabetes. Cell Metab 2017;25:883–897.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 2020;16: 349–362 [DOI] [PubMed] [Google Scholar]
- 47. McCarthy MI. Painting a new picture of personalised medicine for diabetes. Diabetologia 2017;60:793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hara T, Mahadevan J, Kanekura K, Hara M, Lu S, Urano F. Calcium efflux from the endoplasmic reticulum leads to β-cell death. Endocrinology 2014;155:758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lautenschlager I, Inkinen K, Taskinen E, Charles MA, Hayry P. Major histocompatibility complex protein expression on pancreas and pancreatic islet endocrine cell subsets. Am J Pathol 1989;135:1129–1137 [PMC free article] [PubMed] [Google Scholar]
- 50. Ramos-Rodríguez M, Raurell-Vila H, Colli ML, et al. The impact of proinflammatory cytokines on the β-cell regulatory landscape provides insights into the genetics of type 1 diabetes. Nat Genet 2019;51:1588–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sims EK, Syed F, Nyalwidhe J, et al. Abnormalities in proinsulin processing in islets from individuals with longstanding T1D. Transl Res 2019; 213:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colli ML, Ramos-Rodríguez M, Nakayasu ES, et al. An integrated multi-omics approach identifies the landscape of interferon-α-mediated responses of human pancreatic beta cells. Nat Commun 2020;11:2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garg AD, Romano E, Rufo N, Agostinis P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. Cell Death Differ 2016;23:938–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rojas J, Bermudez V, Palmar J, et al. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res 2018;2018:9601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nogueira TC, Paula FM, Villate O, et al. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet 2013;9:e1003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu S, Kanekura K, Hara T, et al. A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc Natl Acad Sci U S A 2014;111:E5292–E5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sandhu MS, Weedon MN, Fawcett KA, et al. Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet 2007;39:951–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morán I, Akerman I, van de Bunt M, et al. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab 2012;16:435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Julier C, Nicolino M. Wolcott-Rallison syndrome. Orphanet J Rare Dis 2010;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lind M, Imberg H, Coleman RL, Nerman O, Holman RR. Historical HbA1c values may explain the type 2 diabetes legacy effect: UKPDS 88. Diabetes Care 2021;44:2231–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lachin JM, Bebu I, Nathan DM.; DCCT/EDIC Research Group . The beneficial effects of earlier versus later implementation of intensive therapy in type 1 diabetes. Diabetes Care 2021; 44:2225–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mishra R, Åkerlund M, Cousminer DL, et al. Genetic discrimination between LADA and type 1 Diabetes within the MHC. Diabetes Care 2020;43: 418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leete P, Oram RA, McDonald TJ, et al.; TIGI study team . Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia 2020;63:1258–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grubb AL, McDonald TJ, Rutters F, et al. A type 1 diabetes genetic risk score can identify patients with GAD65 autoantibody-positive type 2 diabetes who rapidly progress to insulin therapy. Diabetes Care 2019;42:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 2012;337: 1190–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maurano MT, Haugen E, Sandstrom R, et al. Large-scale identification of sequence variants influencing human transcription factor occupancy in vivo. Nat Genet 2015;47:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Inshaw JRJ, Cutler AJ, Crouch DJM, Wicker LS, Todd JA. Genetic variants predisposing most strongly to type 1 diabetes diagnosed under age 7 years lie near candidate genes that function in the immune system and in pancreatic β-cells. Diabetes Care 2020;43:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Redondo MJ, Yu L, Hawa M, et al. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia 2001;44:354–362 [DOI] [PubMed] [Google Scholar]
- 69. Alper CA, Husain Z, Larsen CE, et al. Incomplete penetrance of susceptibility genes for MHC-determined immunoglobulin deficiencies in monozygotic twins discordant for type 1 diabetes. J Autoimmun 2006;27:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Aylward A, Chiou J, Okino ML, Kadakia N, Gaulton KJ. Shared genetic risk contributes to type 1 and type 2 diabetes etiology. 7 November 2018 [Epub ahead of print] Hum Mol Genet. DOI: 10.1093/hmg/ddy314 [DOI] [PubMed] [Google Scholar]
- 71. Yu MG, Keenan HA, Shah HS, et al. Residual β cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest 2019;129:3252–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Incani M, Baroni MG, Cossu E. Testing for type 1 diabetes autoantibodies in gestational diabetes mellitus (GDM): is it clinically useful? BMC Endocr Disord 2019;19:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Redondo MJ, Geyer S, Steck AK, et al.; Type 1 Diabetes TrialNet Study Group . TCF7L2 genetic variants contribute to phenotypic heterogeneity of type 1 diabetes. Diabetes Care 2018;41:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heaton DA, Millward BA, Gray P, et al. Evidence of beta cell dysfunction which does not lead on to diabetes: a study of identical twins of insulin dependent diabetics. Br Med J (Clin Res Ed) 1987;294:145–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ramphul K, Joynauth J. An update on the incidence and burden of diabetic ketoacidosis in the U.S. Diabetes Care 2020;43:e196–e197 [DOI] [PubMed] [Google Scholar]
- 77. Jensen ET, Stafford JM, Saydah S, et al. Increase in prevalence of diabetic ketoacidosis at diagnosis among youth with type 1 diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Care 2021;44:1573–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]