Key Points
Question
For children with community-acquired pneumonia discharged from an emergency department, observational unit, or inpatient ward (within 48 hours), is subsequent outpatient treatment with oral amoxicillin at a dose of 35 to 50 mg/kg per day noninferior to 70 to 90 mg/kg per day, and is a 3-day course noninferior to 7 days, with regard to the need for antibiotic re-treatment?
Findings
In this 2 × 2 factorial randomized clinical trial of 814 children requiring amoxicillin for community-acquired pneumonia at hospital discharge, antibiotic re-treatment within 28 days occurred in 12.6% vs 12.4% of those randomized to lower vs higher doses, and in 12.5% vs 12.5% of those randomized to 3-day vs 7-day amoxicillin duration. Both comparisons met the prespecified 8% noninferiority margin.
Meaning
Among children with community-acquired pneumonia discharged from an emergency department, observational unit, or inpatient ward, further outpatient treatment with oral amoxicillin at a dose of 35 to 50 mg/kg per day was noninferior to a dose of 70 to 90 mg/kg per day and 3 days was noninferior to 7 days with regard to the need for later antibiotic re-treatment.
Abstract
Importance
The optimal dose and duration of oral amoxicillin for children with community-acquired pneumonia (CAP) are unclear.
Objective
To determine whether lower-dose amoxicillin is noninferior to higher dose and whether 3-day treatment is noninferior to 7 days.
Design, Setting, and Participants
Multicenter, randomized, 2 × 2 factorial noninferiority trial enrolling 824 children, aged 6 months and older, with clinically diagnosed CAP, treated with amoxicillin on discharge from emergency departments and inpatient wards of 28 hospitals in the UK and 1 in Ireland between February 2017 and April 2019, with last trial visit on May 21, 2019.
Interventions
Children were randomized 1:1 to receive oral amoxicillin at a lower dose (35-50 mg/kg/d; n = 410) or higher dose (70-90 mg/kg/d; n = 404), for a shorter duration (3 days; n = 413) or a longer duration (7 days; n = 401).
Main Outcomes and Measures
The primary outcome was clinically indicated antibiotic re-treatment for respiratory infection within 28 days after randomization. The noninferiority margin was 8%. Secondary outcomes included severity/duration of 9 parent-reported CAP symptoms, 3 antibiotic-related adverse events, and phenotypic resistance in colonizing Streptococcus pneumoniae isolates.
Results
Of 824 participants randomized into 1 of the 4 groups, 814 received at least 1 dose of trial medication (median [IQR] age, 2.5 years [1.6-2.7]; 421 [52%] males and 393 [48%] females), and the primary outcome was available for 789 (97%). For lower vs higher dose, the primary outcome occurred in 12.6% with lower dose vs 12.4% with higher dose (difference, 0.2% [1-sided 95% CI –∞ to 4.0%]), and in 12.5% with 3-day treatment vs 12.5% with 7-day treatment (difference, 0.1% [1-sided 95% CI –∞ to 3.9]). Both groups demonstrated noninferiority with no significant interaction between dose and duration (P = .63). Of the 14 prespecified secondary end points, the only significant differences were 3-day vs 7-day treatment for cough duration (median 12 days vs 10 days; hazard ratio [HR], 1.2 [95% CI, 1.0 to 1.4]; P = .04) and sleep disturbed by cough (median, 4 days vs 4 days; HR, 1.2 [95% CI, 1.0 to 1.4]; P = .03). Among the subgroup of children with severe CAP, the primary end point occurred in 17.3% of lower-dose recipients vs 13.5% of higher-dose recipients (difference, 3.8% [1-sided 95% CI, –∞ to10%]; P value for interaction = .18) and in 16.0% with 3-day treatment vs 14.8% with 7-day treatment (difference, 1.2% [1-sided 95% CI, –∞ to 7.4%]; P value for interaction = .73).
Conclusions and Relevance
Among children with CAP discharged from an emergency department or hospital ward (within 48 hours), lower-dose outpatient oral amoxicillin was noninferior to higher dose, and 3-day duration was noninferior to 7 days, with regard to need for antibiotic re-treatment. However, disease severity, treatment setting, prior antibiotics received, and acceptability of the noninferiority margin require consideration when interpreting the findings.
Trial Registration
ISRCTN Identifier: ISRCTN76888927
This randomized clinical trial compares the need for re-treatment of respiratory infection following hospitalization for community-acquired pneumonia among children receiving amoxicillin at high doses vs low doses and at 3-days vs 7-days duration.
Introduction
Children younger than 5 years commonly receive oral antibiotics, mainly for respiratory infections.1,2 In a retrospective cohort study from the UK, the Netherlands, and Belgium, and repeated point-prevalence surveys conducted in 28 European emergency departments (EDs) between 2014 and 2016, 10% to 40% of children with infection symptoms were diagnosed with possible serious bacterial infections requiring antibiotics, compared with less than 5% in primary care, and the lower respiratory tract was the second most common focus.3,4
Bacteria have been causally implicated in approximately one-third of community-acquired pneumonia (CAP) cases among children younger than 5 years admitted to the hospital, with codetection of viruses and bacteria being common in symptomatic and asymptomatic young children.5,6,7 Neither chest radiographs nor inflammatory biomarkers differentiate which children with CAP require antibiotics.8,9,10 The lack of predictive diagnostic tests to rule out or confirm the need for antibiotics means that young children with clinical signs of CAP are likely to continue to be prescribed antibiotics, especially in hospitals. Optimizing antibiotic treatment to minimize drug exposure while achieving high rates of clinical cure would inform essential antibiotic stewardship interventions.
Amoxicillin is widely recommended as the first-line antibiotic for CAP in young children.11,12,13 Randomized clinical trial evidence from low- and middle-income countries supports treatment duration of 3 to 5 days in mild or moderate disease.14,15 However, the most appropriate total daily dose of oral amoxicillin treatment has not been investigated in any trial, and it is unclear whether evidence supporting 3-day treatment can be generalized from low- and middle-income countries to high-income secondary care settings with differing diagnostic criteria.11,12,13 The CAP-IT trial (Community-Acquired Pneumonia: a randomized controlled trial) aimed to evaluate whether lower dose and shorter amoxicillin treatment were noninferior to higher dose and longer treatment, with regard to the need for antibiotic re-treatment within 28 days.
Methods
Study Design
This was a multicenter, randomized, blinded, placebo-controlled, 2 × 2 factorial, noninferiority trial conducted in 28 hospitals in the UK and 1 in Ireland, comparing total daily amoxicillin dose (35-50 mg/kg or 70-90 mg/kg) and duration (3 or 7 days) for treatment of childhood CAP. The trial protocol was approved by the West London and GTAC (Gene Therapy Advisory Committee) research ethics committee (16/LO/0831) (Supplement 1).16 Parents or legal guardians of participating children provided written informed consent prior to any study procedures.
Participants
Children were eligible if they were older than 6 months of age, weighed 6 to 24 kg, were clinically diagnosed with CAP, and treatment with amoxicillin monotherapy on discharge from hospital ED, observational unit, or inpatient ward was planned. Consistent with British Thoracic Society guidelines, CAP was defined as (1) parent- or guardian-reported cough within the previous 96 hours; (2) measured temperature of 38 °C or parent- or guardian-reported fever within previous 48 hours; and (3) signs of labored or difficult breathing or focal chest sign(s) (eTable 1 in Supplement 2).12 Enrollment took place at discharge if inclusion and exclusion criteria were met (eMethods 2 in Supplement 2). Exclusion criteria were (1) uninterrupted prior β-lactam antibiotic treatment for more than 48 hours or any prior non-β-lactam treatment; (2) severe underlying chronic disease; (3) any contraindications to amoxicillin, including allergy; (4) complicated pneumonia (defined as signs of sepsis or local parenchymal or pleural complications); or (5) bilateral wheezing without focal chest signs.
Information on race and ethnicity was collected based on UK Census options through participant self-identification. The reason for collecting this information is because outcomes for acute infections and respiratory disease in the UK and US have been reported to be poorer among children from racial and ethnic backgrounds other than White.17,18
Randomization and Blinding
A computer-generated randomization list was produced by the trial statistician based on blocks of 8 and containing an equal number of the 4 possible combinations of dose and duration in random order. Participants were randomized simultaneously to each of the 2 factorial randomizations in a 1:1 ratio by dispensing the next sequentially numbered set of trial drug bottles. Randomization was stratified by study site and whether or not patients had received any nontrial antibiotics in the hospital before being enrolled.
Blinding was achieved by independent rebottling, packaging, and labeling of 2 amoxicillin brands, and trial kits were assigned sequential numbers based on the randomization list and delivered ready to dispense to site pharmacies. Lower and higher drug doses were achieved by administering the same volume according to a weight-banded dosing chart (eTable 2 in Supplement 2) using 125 mg/5mL and 250 mg/5mL amoxicillin suspension, which were otherwise of identical appearance, smell, and taste. In an effort to ensure blinding for the duration comparison, a single amoxicillin brand was used for the first 3 days, followed by a different amoxicillin-containing suspension (of the same concentration) or a matching placebo suspension for days 4 to 7.
Procedures
Children were screened against eligibility criteria during ED or hospital admission by trained staff assessing the parent- or guardian-reported history and physical examination. No radiological or laboratory diagnostic tests were mandated, but results were collected if done as part of routine care. A nasopharyngeal swab for Streptococcus pneumoniae carriage and resistance was taken at enrollment prior to administration of the study drug.
Follow-up data were collected during scheduled telephone calls 3, 7, 14, and 21 days after discharge and by face-to-face visit (or telephone call if a visit was not possible) on day 28 and in case of unplanned reattendances or readmissions. At all follow-up contacts, information was collected regarding CAP symptoms, adverse events, trial medication adherence, and any nontrial antibiotic prescriptions. Parents and guardians were provided with a diary (paper or electronic) to be completed during the first 14 days in which they recorded CAP symptom data plus information on health service utilization. At the 28-day visit, a repeat nasopharyngeal swab was collected. Primary care physicians were asked about nontrial antibiotic prescriptions if the 28-day visit was missed, provided written consent had been given.
Nasopharyngeal swabs were frozen at below −20 °C within 6 hours of being obtained. Samples were batched and sent to the Children’s Vaccine Centre, Bristol University, for screening culture. All S pneumoniae isolates were then transferred to the University of Antwerp for confirmatory analysis and for penicillin and amoxicillin susceptibility testing, interpreted according to EUCAST Clinical Breakpoint Tables version 10.0 as sensitive, nonsusceptible, or resistant (eMethods 2 in Supplement 2).19
Outcomes
The primary end point was clinically indicated treatment with systemic antibiotics (other than trial medication) for a respiratory tract infection, including CAP, within 28 days of randomization. All primary end points were reviewed by an end point review committee, blinded to treatment allocation, to adjudicate whether treatment was clinically indicated and prescribed for respiratory tract infection.
The secondary end points were as follows: (1) severity (graded as not present, slight/little, moderate, bad, severe/very bad) and duration (with the first day the symptom is reported not present defined as resolved) of 9 parent-reported CAP symptoms (fever, cough, phlegm, fast breathing, wheezing, disturbed sleep, eating/drinking less, interference with normal activity, vomiting); (2) potential amoxicillin-related clinical adverse events (diarrhea, thrush, skin rash); (3) adherence to trial medication (eMethods 2 in Supplement 2); and (4) phenotypic penicillin nonsusceptibility or resistance at 28 days in nasopharyngeal S pneumoniae isolates (eMethods 3 in Supplement 2). The prespecified analysis also included serious adverse events.
Sample Size Calculation
The trial was designed to demonstrate noninferiority of lower dose compared with higher dose, and shorter duration compared with longer duration, in terms of the primary end point. The noninferiority margin was defined as a risk difference of 8% assessed against a 1-sided 95% CI.20 Given a 15% antibiotic re-treatment rate based on internal pilot data, 15% loss to follow-up, and assuming no interaction between the dose and duration interventions, the sample size of 800 participants was estimated to achieve 90% power.
As it was unclear at trial initiation what the primary end point rate would be, data from a preplanned internal pilot phase were reviewed by the independent data monitoring committee (eMethods 4 in Supplement 2). After 227 children were enrolled (160 from the ED, 67 after inpatient stay), it was noted that disease severity at enrollment was not significantly different among children from each clinical pathway (eMethods 5 in Supplement 2), and the re-treatment end point rate of 15% was higher than the 5% rate originally assumed. The data and safety monitoring committee, with support from the trial steering committee, recommended the following amendments: (1) joint analysis of children immediately discharged from the ED and discharged after an inpatient stay (eMethods 5 in Supplement 2); and (2) revision of the noninferiority margin from 4% to 8% to be closer to the most conservative 10% noninferiority margin recommended by the Infectious Diseases Society of America for noninferiority trials in CAP with a mortality end point (eMethods 6 in Supplement 2). For binary clinical end points, a noninferiority margin of up to 20% could be acceptable per the Infectious Diseases Society of America.21
Statistical Design and Analysis
The primary analysis included only participants who received the trial drug, and patients were analyzed in the groups to which they were randomized. The proportion of children meeting the primary end point was obtained from the cumulative incidence at day 28 as estimated by Kaplan-Meier methods accounting for loss to follow-up. The main effect of each randomization was estimated by collapsing across levels of the other randomization factor, after checking for the absence of statistical interaction between the 2 randomizations. Other tests for additive interaction were also prespecified for each randomization group with previous systemic antibacterial exposure.
Prespecified sensitivity analyses included the following: (1) re-treatment regardless of reason or indication; (2) re-treatment specifically for CAP or chest infection; and (3) for duration, considering only re-treatments after 3 days from randomization. To provide support that a null result was not due to the inclusion of children with mild infection less likely to benefit from antibiotics, another prespecified analysis was limited to children with at least 2 abnormal physiological parameters at enrollment, considered the severe group (eMethods 7 in Supplement 2). In addition, 2 post hoc analyses were undertaken: (1) ontreatment analysis with nonadherence defined as taking less than 80% of the trial medication (all trial medication including placebo and active drug only) (eMethods 8 in Supplement 2); and (2) subgroup analysis of children who had not received antibiotics in the hospital (most discharged immediately from the ED) and those who had received up to 48 hours of β-lactam treatment in the hospital before enrollment (eMethods 9 in Supplement 2).
Analyses of secondary end points were not adjusted for multiple comparisons. Because of the potential for type 1 error due to multiple comparisons, findings for secondary end points and analyses should be interpreted as exploratory. Binary outcomes were compared between groups using the χ2 or Fisher exact test and logistic regression. Ordered outcomes were compared using rank tests. Duration of CAP symptoms was analyzed using time-to-event methods, restricted to children with the particular symptom at enrollment, until the first day the symptom was reported as absent. For all Cox models, the proportional hazards assumption was tested on the basis of Schoenfeld residuals. In none of these tests was the proportionality assumption violated. For secondary end points, all significance tests were performed under the standard null hypothesis of no difference.
Analyses of primary and secondary end points were to be based on observed data only taking into account information across all visits, with multiple imputation to be considered if data were missing for more than 10% of participants.
Data were analyzed using Stata software, version 15 (StataCorp). Differences in the primary end point are presented with 1-sided 95% CIs for the noninferiority analyses, and differences in secondary end points are presented with 2-sided 95% CIs. All statistical tests had a significance threshold of .05. See Supplement 3 for the statistical analysis plan.
The data and safety monitoring committee provided oversight of the study and reviewed unblinded data 3 times during the trial.
Results
Between February 1, 2017, and April 23, 2019, 2642 children were assessed for eligibility, and 824 were randomized (Figure 1). Ten children received no trial medication and were excluded from the analysis, resulting in an analysis population of 814.
Figure 1. Patient Recruitment, Randomization, and Follow-up in the CAP-IT Trial.
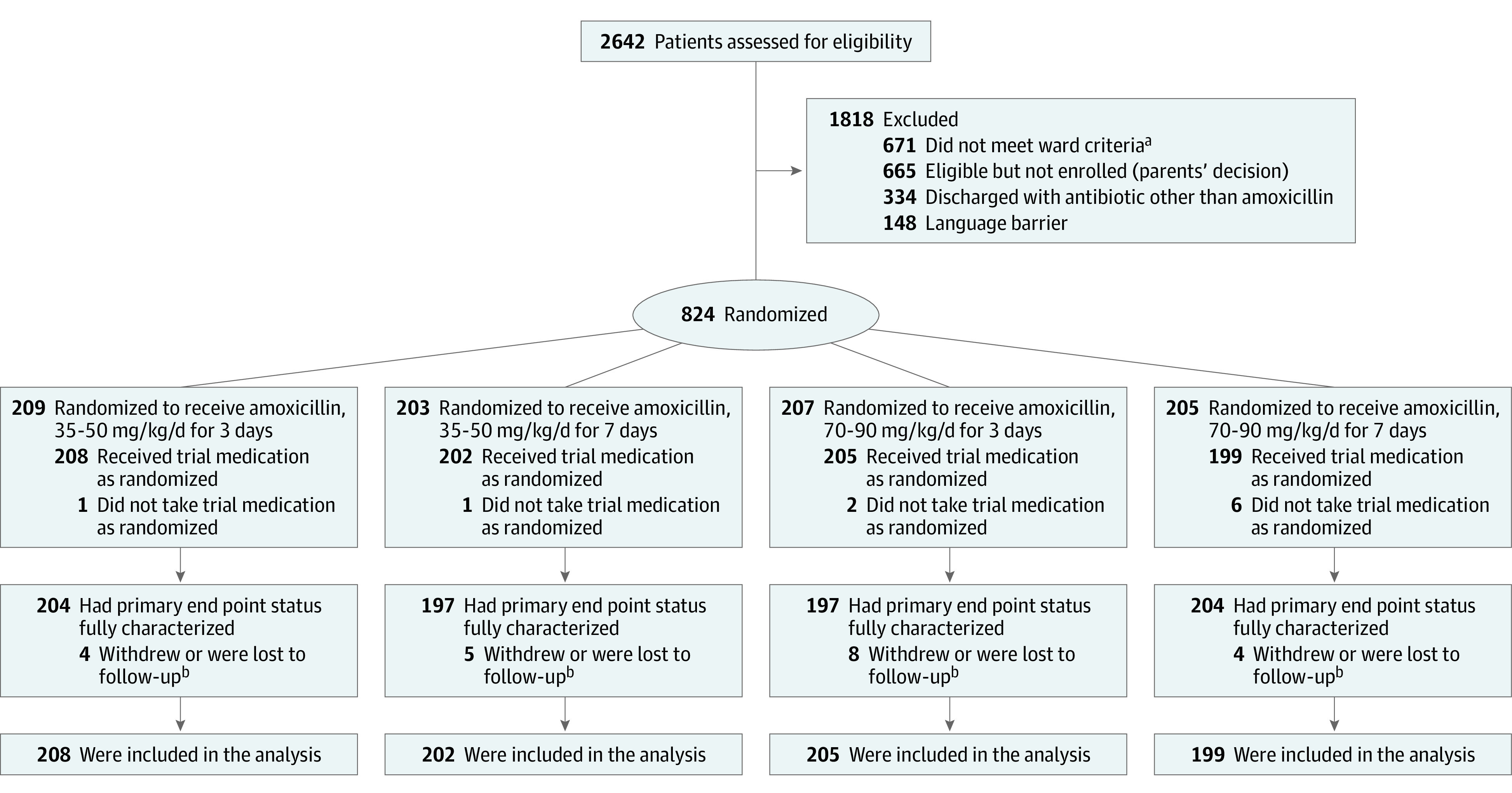
aWard criteria indicates children recruited from inhospital pediatric wards or units with an inpatient stay longer than 48 hours and treated with non–β-lactam antibiotics as inpatients.
bFollow-up included time up to withdrawal.
Of these, 421 (52%) children were male, 393 were female (48%), and median (IQR) age was 2.5 years (1.6-3.7) (Table 1). At presentation, 441 (54%) were febrile, 578 (71%) had tachycardia, and 528 (65%) had tachypnea. At randomization, 591 (73%) children were discharged directly from the ED, and 223 (27%) had an inpatient stay of less than 48 hours (eFigure 1, eTable 3, and eTable 4 in Supplement 2). Two hundred eighteen (98%) children who were inpatients and 24 (4%) who were discharged directly from the ED had received β-lactam antibiotics (100% treated for <48 hours and 185 (76%) <24 hours; eTable 5 in Supplement 2).
Table 1. Participant Characteristics at Baseline or Presentation (for Inpatients).
| Amoxicillin dosing and durationa | ||||
|---|---|---|---|---|
| 35-50 mg/kg/d for 3 days (n = 208) | 35-50 mg/kg/d for 7 days (n = 202) | 70-90 mg/kg/d for 3 Days (n = 205) | 70-90 mg/kg/d for 7 Days (n = 199) | |
| Demographics | ||||
| Age, median (IQR), y | 2.5 (1.7-3.7) | 2.6 (1.6-3.9) | 2.5 (1.7-3.8) | 2.3 (1.4-3.6) |
| Male sex | 110 (53) | 100 (50) | 107 (52) | 104 (52) |
| Female sex | 98 (47) | 102 (50) | 98 (48) | 95 (48) |
| Race and ethnicity | ||||
| Asian or British Asian | 32 (15) | 23 (11) | 21 (10) | 30 (15) |
| Black or Black British | 20 (10) | 20 (10) | 20 (10) | 16 (8) |
| Multiracial | 15 (7) | 17 (8) | 14 (7) | 14 (7) |
| White | 139 (67) | 136 (67) | 144 (70) | 135 (68) |
| Otherb | 2 (1) | 6 (3) | 6 (3) | 4 (2) |
| Medical history | ||||
| Asthma or inhaler use within past month | 54 (26) | 65 (32) | 71 (35) | 65 (33) |
| Allergy or eczema | 52 (25) | 63 (31) | 56 (27) | 58 (29) |
| Prematurity | 26 (13) | 17 (8) | 25 (12) | 18 (9) |
| Other underlying disease | 16 (8) | 21 (10) | 5 (2) | 14 (7) |
| Routine vaccinations | ||||
| Yes | 198 (95) | 190 (94) | 196 (96) | 189 (95) |
| No | 8 (4) | 6 (3) | 7 (3) | 5 (3) |
| Unknown | 2 (1) | 6 (3) | 2 (1) | 5 (3) |
| History of current concern | ||||
| Duration of cough, median (IQR), d | 4 (2-7) | 4 (2-6) | 4 (3-7) | 4 (2-7) |
| Duration of fever, median (IQR), d | 2 (2-4) | 3 (1-4) | 3 (2-4) | 2 (1-4) |
| Systemic antibiotics in last 3 mo | 30 (14) | 34 (17) | 36 (18) | 29 (15) |
| Systemic antibiotics in last 48 h | 61 (29) | 58 (29) | 62 (30) | 61 (31) |
| <12 h | 34 (56) | 33 (57) | 34 (55) | 32 (52) |
| 12-<24 h | 15 (25) | 12 (21) | 18 (29) | 15 (25) |
| ≥24 h | 12 (19) | 13 (23) | 10 (16) | 14 (23) |
| Clinical examination | ||||
| Weight, median (IQR), kg | 13.9 (11.5-16.5) | 13.4 (11.2-17.0) | 13.8 (11.5-16.4) | 13.0 (10.7-15.9) |
| Temperature, median (IQR), °C | 38.2 (37.3-38.8) | 38.0 (37.2-38.9) | 37.9 (37.0-38.6) | 38.1 (37.4-38.7) |
| Abnormal temperaturec | 121 (58) | 106 (52) | 100 (49) | 114 (57) |
| Heart rate, median (IQR), beats/min | 146 (133-160) | 146 (130-161) | 140 (129-153) | 146 (131-162) |
| Abnormal heart ratec | 154 (74) | 153 (76) | 128 (62) | 143 (72) |
| Respiratory rate, median (IQR), breaths/min | 38 (30-44) | 37 (30-44) | 36 (30-42) | 40 (32-46) |
| Abnormal respiratory ratec | 138 (66) | 132 (65) | 124 (61) | 134 (68) |
| Oxygen saturation, median (IQR), % | 96 (95-98) | 96 (95-98) | 97 (95-98) | 96 (94-98) |
| Abnormal oxygen saturationc | 7 (3) | 11 (5) | 11 (5) | 14 (7) |
| Nasal flaring | 18 (9) | 15 (7) | 17 (8) | 25 (13) |
| Chest retractions | 117 (57) | 122 (60) | 122 (60) | 122 (61) |
| Pallor | 48 (23) | 34 (17) | 45 (22) | 42 (21) |
| Dullness to percussion | ||||
| Absent | 105 (85) | 89 (86) | 93 (87) | 93 (85) |
| Unilateral | 18 (15) | 14 (14) | 13 (12) | 14 (13) |
| Bilateral | 0 | 0 | 1 (1) | 2 (2) |
| Bronchial breathing | ||||
| Absent | 146 (83) | 137 (80) | 130 (82) | 133 (82) |
| Unilateral | 23 (13) | 30 (18) | 26 (16) | 24 (15) |
| Bilateral | 6 (3) | 4 (2) | 2 (1) | 5 (3) |
| Reduced breath sounds | ||||
| Absent | 108 (54) | 94 (49) | 94 (48) | 93 (50) |
| Unilateral | 82 (41) | 86 (45) | 92 (47) | 76 (41) |
| Bilateral | 10 (5) | 10 (5) | 10 (5) | 16 (9) |
| Crackles/crepitations | ||||
| Absent | 37 (18) | 32 (16) | 34 (17) | 31 (16) |
| Unilateral | 147 (72) | 140 (70) | 143 (72) | 132 (68) |
| Bilateral | 20 (10) | 28 (14) | 22 (11) | 30 (16) |
Numeric values are presented as No. (%) unless othwise indicated.
For race and ethnicity, other includes Middle Eastern/North African (n = 12), Latin American (n = 3), and children with missing data (n = 3).
Abnormal parameters are reported for the following clinical measures: temperature (≥38 °C), heart rate (>140/min for age 1-2 years; >120/min for age ≥3 years), respiratory rate (>37/min for age 1-2 years; >28/min for age ≥3 years), and oxygen saturation (<92%).
Follow-up data were available for 757 (93%) participants at day 3, 716 (88%) at day 7, 676 (83%) at day 14, and 619 (76%) at day 21. Final 28-day follow-up was face to face for 484 (59%) participants, and 158 (19%) families were contacted by telephone. Including additional information from family physicians regarding any subsequent antibiotic prescriptions (n = 147), the primary end point was evaluable for 789 (97%) children, with the remaining 25 providing data up to the point of last contact.
Primary Outcome
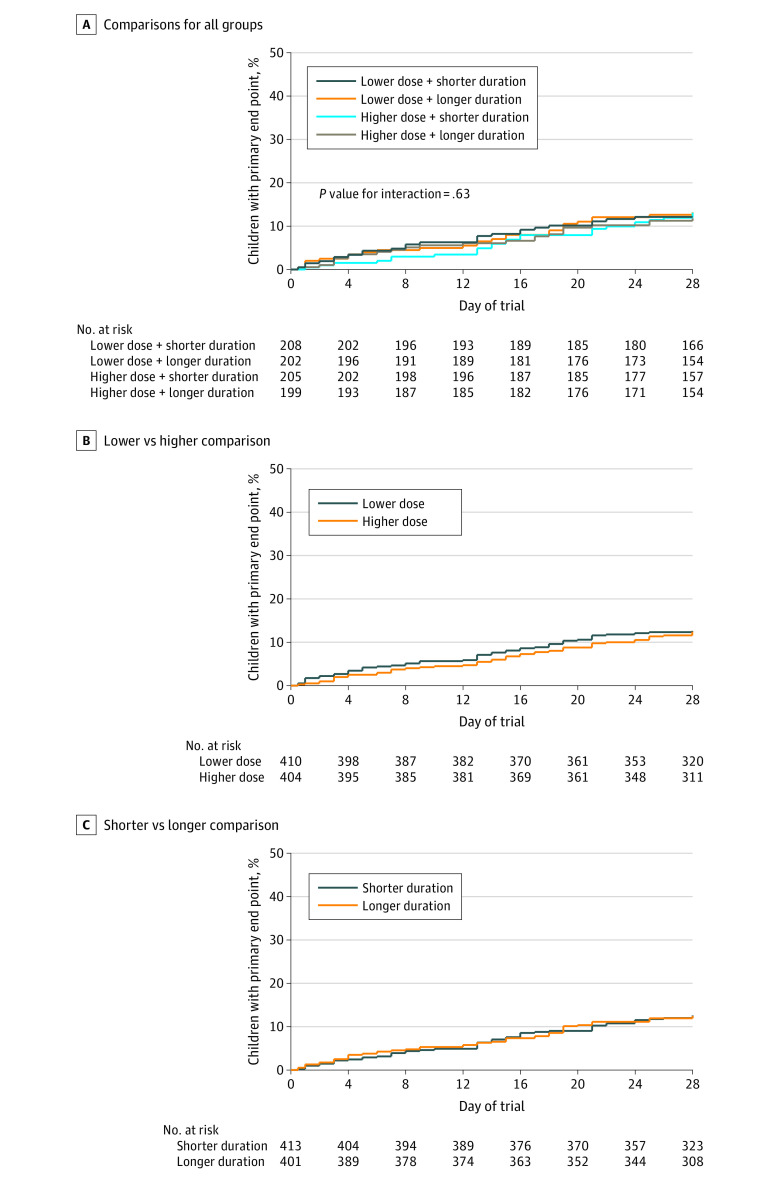
For the primary outcome, 139 children received nontrial systemic antibiotic treatment by day 28, with criteria for the primary end point met in 100 (12.5% [90% CI, 10.7% to 14.6%]) (Figure 2A; eTable 6, eTable 7, and eTable 8 in Supplement 2). There was no significant interaction between randomized factorial groups (P = .63; Figure 2A). The proportions meeting the primary end point were 12.6% (51/410) in the lower-dose group vs 12.4% (49/404) in the higher-dose group (difference, 0.2% [1-sided 95% CI, –∞ to 4.0%]; Figure 2B), and 12.5% (51/413) in the shorter-duration group vs 12.5% (49/401) in the longer-duration group (difference, 0.1% [1-sided 95% CI, –∞ to 3.9%]; Figure 2C). Both comparisons satisfied the noninferiority criterion (Figure 3). There were no significant interactions between use of antibiotics in the preceding 48 hours and either dose (P = .46) or duration randomizations (P = .59) (eFigure 2 in Supplement 2).
Figure 2. Kaplan-Meier Curves Indicating Time to Experiencing the Primary End Point.
The primary end point is clinically indicated treatment with systemic antibiotics (other than trial medication) for a respiratory tract infection within 4 weeks of randomization. Median observation time was not reported since more than 75% of participants were observed for the entire 28-day period. Lower dose indicates 35 to 50 mg/kg/d; higher dose, 70 to 90 mg/kg/d; shorter duration, 3-day course; longer duration, 7-day course.
A, No. (%) with primary end point by day 28: lower + shorter, 25 (12.1 [90% CI, 8.9-16.4]); lower + longer, 26 (13.1 [90% CI, 9.7-17.7]); higher + shorter, 26 (13.1 [90% CI, 9.6-17.6]); and higher + longer, 23 (11.8 [90% CI, 8.5-16.2]).
B, No. (%) with primary end point by day 28: lower, 51 (12.6 [90% CI, 10.1-15.6]); higher, 49 (12.4 [90% CI, 10.0-15.5]). Difference, 0.2% (upper bound of 1-sided 95% CI, 4.0%).
C, No. (%) with primary end point by day 28: shorter, 51 (12.5 [90% CI, 10.1-15.5]); longer, 49 (12.5 [90% CI, 10.0-15.5]). Difference, 0.1% (upper bound of 1-sided 95% CI, 3.9%).
Figure 3. Noninferiority Sensitivity and Subgroup Analyses for the Primary End Point for the Amoxicillin Dose and Dose Duration Randomizations.
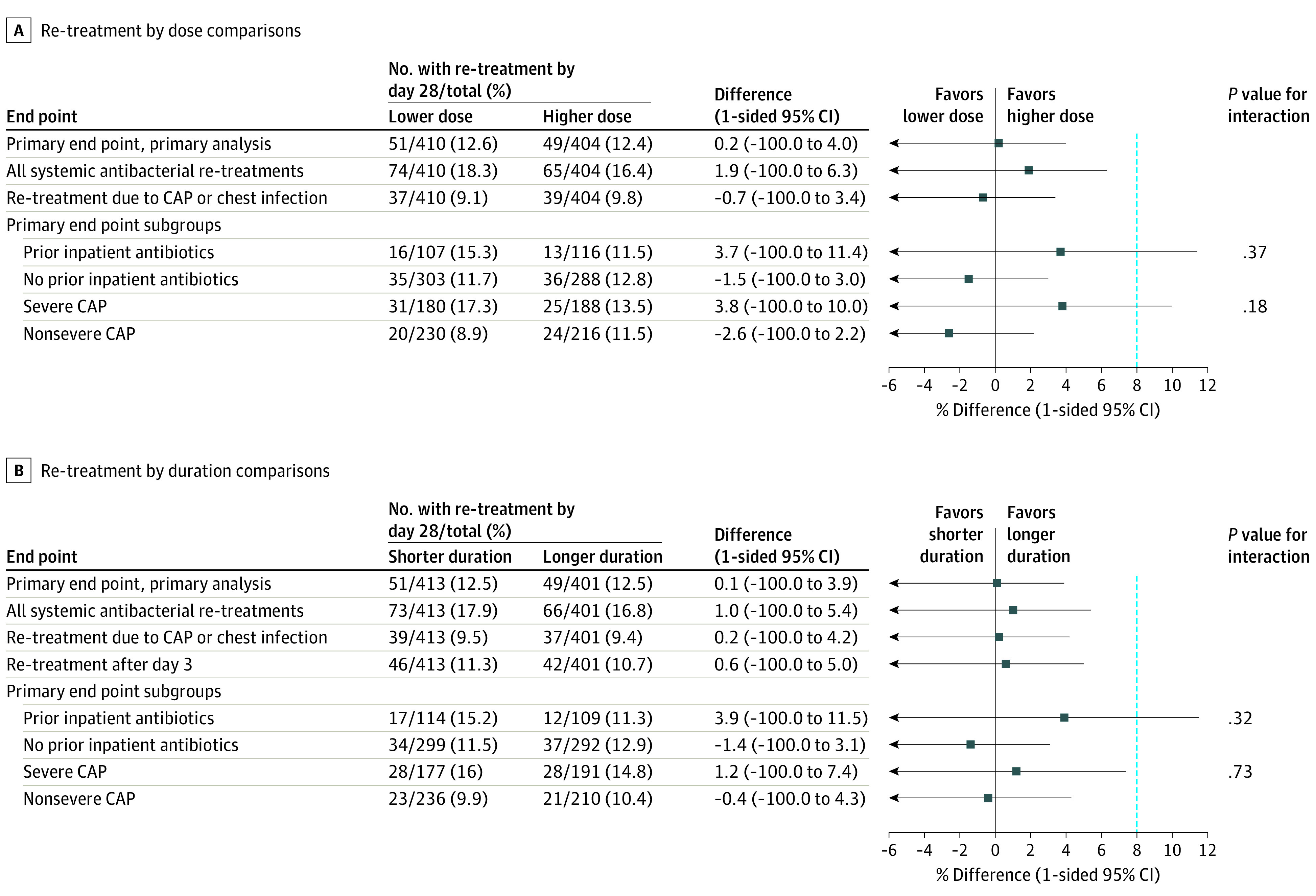
The primary analysis and 3 prespecified analyses are shown for both randomizations including all systemic antibacterial re-treatments, only re-treatments for community-acquired pneumonia (CAP) or chest infection, and by severe CAP subgroups. In addition, a post hoc subgroup analysis by prior inpatient antibiotic exposure is shown. A sensitivity analysis including only re-treatments after day 3 is shown for the duration randomization. One-sided 95% CIs are shown with the lower bound extending to −100%. The blue dashed vertical line at 8% indicates the noninferiority margin.
For the prespecified subgroup analysis among children with severe CAP, the primary end point occurred in 31/180 (17.3%) in the lower-dose group vs 25/188 (13.5%) in the higher-dose group (difference, 3.8% [1-sided 95% CI, –∞ to 10%]; P value for interaction, .18) and in 28/177 (16.0%) in the 3-day group vs 28/191 (14.8%) in the 7-day group (difference, 1.2% [1-sided 95% CI, –∞ to 7.4%]; P value for interaction, .73) (Figure 3).
Post hoc ontreatment analysis of 693 children who took 80% or more doses showed noninferiority for lower dose (lower vs higher, 9.5% vs 10.2%; difference, –0.7% [1-sided 95% CI, –∞ to 3.1%]) and shorter duration (shorter vs longer, 10.5% vs 9.2%; difference, 1.3% [1-sided 95% CI, –∞ to 5.1%]) (eFigure 3 and eFigure 4 in Supplement 2). In addition, in the subgroup of 591 children without prior inpatient antibiotics, the primary end point occurred in 11.7% in the lower-dose group vs 12.8% in the higher-dose group (difference, –1.5% [1-sided 95% CI, –∞ to 3.0%]) and in 11.5% in the shorter-duration group vs 12.9% in the longer-duration group (difference, –1.4% [1-sided 95% CI, –∞ to 3.1%]). Among the 223 children enrolled following inpatient antibiotic treatment, the corresponding rates were 15.3% in the lower-dose group vs 11.5% in the higher-dose group (difference, 3.7% [1-sided 95% CI, –∞ to 11.4%]) and 15.2% in the shorter-duration group vs 11.3% in the longer-duration group (difference, 3.9% [1-sided 95% CI, –∞ to 11.5%]) (eFigure 5, eFigure 6, eFigure 7, and eFigure 8 in Supplement 2); neither comparison met the noninferiority criterion. Post hoc interaction tests for these subgroups were not statistically significant (P = .37 with dose randomization; P = .32 with duration randomization).
Secondary Outcomes
Resolution of vomiting, fever, fast breathing, wheezing, interference with normal activity, reduced appetite, and phlegm production was not significantly different between groups by dose or duration. Cough persisted for longer in the shorter- vs longer-duration groups (median, 12 days vs 10 days; hazard ratio 1.2 [90% CI, 1.0 to 1.4]; P = .04), as did sleep disturbed by cough (median, 4 days vs 4 days; hazard ratio 1.2 [90% CI, 1.0 to 1.3]; P = .03; eFigure 9 and eFigure 11 in Supplement 2). There was no significant association between dose or duration of amoxicillin and severity of cough symptoms (eFigure 10 and eFigure 12 in Supplement 2).
A baseline nasopharyngeal sample was obtained from 647 participants, of which 272 (42%) were colonized by S pneumoniae with penicillin nonsusceptibility identified in 46 (16.9%) samples. At the final visit, 437 children provided a sample, of which 129 (29.5%) were positive for S pneumoniae, and penicillin nonsusceptibility was identified in 21 samples. No penicillin-resistant pneumococci were identified, and there was no significant difference in day 28 pneumococcal carriage or penicillin nonsusceptibility according to the dose or duration of amoxicillin (Table 2; eTable 11, eTable 12, eTable 13, and eTable 14 in Supplement 2).
Table 2. Streptococcus pneumoniae and Antimicrobial Resistance on Day 28 in Lower (35-50 mg/kg per Day) and Higher (70-90 mg/kg per Day) Dose and Shorter (3-Day) and Longer (7-Day) Duration Groups.
| Outcome | Amoxicillin dose | Amoxicillin duration | ||||||
|---|---|---|---|---|---|---|---|---|
| 35-50 mg/kg per Day (n = 410) | 70-90 mg/kg per Day (n = 404) | Difference, % (95% CI) | P value | 3 Days (n = 413) | 7 Days (n = 401) | Difference, % (95% CI) | P value | |
| Culture sample available | 224/410 (55) | 213/404 (53) | 2 (–5 to 9) | .58 | 205/413 (50) | 232/401 (58) | –8 (–15 to –1) | .02 |
| Streptococcus pneumoniae colonization | 66/224 (29) | 63/213 (30) | 0 (–9 to 8) | .98 | 65/205 (32) | 64/232 (28) | 4 (–4 to 13) | .35 |
| Penicillin MIC, mg/L | ||||||||
| 0.016 | 18 (27) | 10 (16) | .49 | 15 (23) | 13 (20) | .56 | ||
| 0.032 | 35 (53) | 44 (70) | 36 (55) | 43 (67) | ||||
| 0.064 | 1 (2) | 0 | 0 | 1 (2) | ||||
| 0.125 | 4 (6) | 1 (2) | 3 (5) | 2 (3) | ||||
| 0.25 | 6 (9) | 5 (8) | 8 (12) | 3 (5) | ||||
| 0.5 | 0 | 1 (2) | 1 (2) | 0 | ||||
| 1 | 2 (3) | 1 (2) | 1 (2) | 2 (3) | ||||
| 2 | 0 | 1 (2) | 1 (2) | 0 | ||||
| Penicillin nonsusceptibilitya | ||||||||
| Including all samples | 12/224 (5) | 9/213 (4) | 1 (–3 to 5) | .58 | 14/205 (7) | 7/232 (3) | 4 (–0 to 8) | .06 |
| In positive samples | 12/66 (18) | 9/63 (14) | 4 (–9 to 17) | .55 | 14/65 (22) | 7/64 (11) | 11 (–2 to 23) | .10 |
| Amoxicillin MIC | ||||||||
| 0.016 | 42 (64) | 43 (68) | .61 | 40 (62) | 45 (70) | .21 | ||
| 0.032 | 14 (21) | 11 (17) | 12 (18) | 13 (20) | ||||
| 0.064 | 4 (6) | 5 (8) | 7 (11) | 2 (3) | ||||
| 0.125 | 2 (3) | 0 | 1 (2) | 1 (2) | ||||
| 0.25 | 2 (3) | 2 (3) | 3 (5) | 1 (2) | ||||
| 0.5 | 0 | 0 | 0 | 0 | ||||
| 1 | 2 (3) | 1 (2) | 1 (2) | 2 (3) | ||||
| 2 | 0 | 1 (2) | 1 (2) | 0 | ||||
| Amoxicillin resistance/nonsusceptibilityb | ||||||||
| a) including all samples | 2/224 (1) | 2/213 (1) | 0 (–2 to 2) | >.99 | 2/205 (1) | 2/232 (1) | 0 (–2 to 2) | >.99 |
| b) in positive samples | 2/66 (3) | 2/63 (3) | 0 (–6 to 6) | >.99 | 2/65 (3) | 2/64 (3) | 0 (–6 to 6) | >.99 |
Abbreviation: MIC, minimal inhibitory concentration.
Break points for penicillin MIC: less than or equal to 0.064 mg/L indicates sensitive, 0.125 to 2 mg/L indicates nonsusceptible, and greater than 2 mg/L indicates resistant.
Break points for amoxicillin MIC: less than or equal to 0.5 mg/L indicates sensitive, greater than 0.5 to 1 mg/L indicates nonsusceptible, and greaterh than 1 mg/L indicates resistant. The data stratified by randomization groups can be found in eTable 11 in Supplement2.
Adverse Events
Of potentially amoxicillin-related clinical adverse events, diarrhea was reported in 345 (44%) children after baseline, skin rash in 193 (24%), and oral thrush in 57 (7%). Rash occurred in 106 (27%) children allocated to longer treatment compared with 87 (22%) children allocated to shorter treatment (Table 3; eTable 9 in Supplement 2). Active trial medication was discontinued early by 47 (6%) participants, while 112 (14%) took fewer doses or a lower volume than prescribed (Table 3; eTable 9 in Supplement 2). The main reasons for early discontinuation were clinical deterioration (n = 23), gagging or spitting out (n = 7), adverse events (n = 6), and clinical improvement (n = 3). Children randomized to 3 days of amoxicillin were more likely to complete their full treatment course compared with those randomized to a 7-day course (98% vs 91%).
Table 3. Adherence and Adverse Events in Lower (35-50 mg/kg per Day) and Higher (70-90 mg/kg per Day) Dose and Shorter (3-Day) and Longer (7-Day) Duration Groups.
| Outcome | Amoxicillin dose | Amoxicillin duration | ||||||
|---|---|---|---|---|---|---|---|---|
| 35-50 mg/kg per Day (n = 410) | 70-90 mg/kg per Day (n = 404) | Difference, % (95% CI) | P value | 3 Days (n = 413)a | 7 Days (n = 401)a | Difference, % (95% CI) | P value | |
| Adherence: complete course taken | ||||||||
| All treatmenta | 355 (87) | 366 (91) | –4 (–8 to –0) | .07 | 358 (87) | 363 (91) | –4 (–8 to 1) | .09 |
| Active treatment onlyb | 383 (93) | 384 (95) | –2 (–5 to 2) | .32 | 404 (98) | 363 (91) | 7 (4 to 10) | <.001 |
| Adherence: all doses taken and all volumes as prescribed | ||||||||
| All treatmentb | 306 (75) | 309 (76) | –2 (–8 to 4) | .54 | 300 (73) | 315 (79) | –6 (–12 to –0) | .05 |
| Active treatment onlyc | 352 (86) | 350 (87) | –1 (–6 to 4) | .75 | 387 (94) | 315 (79) | 15 (11 to 20) | <.001 |
| Clinical possibly drug-related adverse events post enrollment | ||||||||
| Diarrhea | 168 (42) | 177 (45) | –4 (–10 to 3) | .31 | 187 (46) | 158 (41) | 6 (–1 to 12) | .11 |
| Oral thrush | 27 (7) | 30 (8) | –1 (–5 to 3) | .60 | 25 (6) | 32 (8) | –2 (–6 to 2) | .26 |
| Rash | 94 (23) | 99 (25) | –2 (–8 to 4) | .52 | 87 (22) | 106 (27) | –6 (–12 to –0) | .06 |
| Serious adverse event, any d | 23 (6) | 20 (5) | 1 (–2 to 4) | .67 | 25 (6) | 18 (4) | 2 (–2 to 5) | .32 |
Courses were considered complete when trial drug was taken on all 7 days.
Including nonadherence to placebo.
Ignoring nonadherence to placebo.
No participant had more than 1 serious adverse event, all serious adverse events were hospitalizations (most for respiratory distress), no deaths. The data stratified by randomization groups can be found in eTable 10 in Supplement 2.
In total, 43 (5%) children experienced a serious adverse event; all were hospitalizations, and most (37 [86%]) were due to respiratory illness (Table 3; and eTable 9 in Supplement 2). One serious adverse event (hospital admission for intravenous treatment because of vomiting on day 2 in a patient randomized to the higher-dose, shorter-duration group) was classified as related to trial medication. There were no deaths.
Discussion
In this pragmatic trial that evaluated dose and duration of amoxicillin for treatment of childhood CAP on discharge from the ED or an inpatient ward, antibiotic re-treatment rates for respiratory tract infection within 4 weeks were noninferior among those randomized to lower- vs higher-dose amoxicillin and among those randomized to a 3-day vs a 7-day course of treatment.
Noninferiority was confirmed in all prespecified sensitivity analyses. For the prespecified subgroup of children with severe disease at baseline, the CI was within the noninferiority margin for the duration comparison; however, for the dose comparison, it did not meet the noninferiority criterion, although the test for interaction by CAP severity at baseline was not statistically significant. The results were consistent with noninferiority in all post hoc ontreatment analyses, including only children taking more than 80% of the trial drug. In a post hoc subgroup analysis separating children discharged from the ED and those requiring inpatient hospitalization, the CI was within the noninferiority margin only for the larger ED group; it did not meet the noninferiority criterion for the children discharged after inpatient treatment, although the test for interaction by previous receipt of antibiotics were not statistically significant.
Few trials have compared different durations of the same antibiotic for treatment of CAP in adults or children, and none to our knowledge have compared both dose and duration in the same trial for childhood CAP.15,22,23,24,25,26,27,28 The recently completed Canadian SAFER trial comparing 5-day with 10-day high-dose oral amoxicillin treatment for childhood CAP on discharge from the ED found comparable clinical cure rates in both groups (89% in the 5-day group and 84% in 10-day group) at 2 to 3 weeks.27 Similarly, 3-day β-lactam therapy was recently reported to be noninferior to 8-day treatment in adults hospitalized with CAP in non–critical care wards.28 As in this trial, re-treatment with nontrial antibiotics was part of the composite primary end point in the SAFER trial and provides a reasonable and important end point for high-resource settings where mortality and critical illness from childhood CAP are low.29 Re-treatment rates in both the current trial and the SAFER trial are similar to the 10% to 11% previously observed for amoxicillin-treated lower respiratory tract infection in UK general practice.27,30,31
In this trial, amoxicillin was prescribed in 2 instead of 3 divided daily doses, an approach endorsed by patient representatives in the design phase and consistent with international guidance.11,32,33,34 The trial findings suggest that a lower total daily amoxicillin dose may be used in twice-daily dosing regimens, especially when prevalence of penicillin-resistant pneumococci is low. Observations of saturability of amoxicillin gut absorption limiting the achievement of desired amoxicillin exposure when using high oral doses at low administration frequency require further investigation.35
Limitations
This trial has several limitations. First, it is not possible to unequivocally identify children likely to benefit from antibiotics. Biomarkers and chest radiographs have been shown to have questionable discriminatory ability and are discouraged by some guidelines.8,9,10,11,12 Although children with a mixed picture of CAP and obstructive airway disease were included, those with wheezing but without clinical signs of CAP were not included, and only 16% of children received bronchodilators or steroids compared with the 48% bronchodilator use observed in the most recent UK pediatric pneumonia audit.36 Children commonly show a mixed pattern of disease (bacterial, viral with or without airway obstruction), and some antibiotic re-treatment may have been for self-limiting disease unlikely to respond to antibiotics.
Second, the trial findings do not inform total treatment duration for children initially admitted to the hospital. Optimal total treatment duration may differ for children requiring prolonged intravenous treatment as inpatients. Only 13% of children receiving inpatient treatment in this trial received antibiotics intravenously, consistent with UK recommendations.12
Third, the trial was not powered to investigate noninferiority of lower dose and shorter duration of home-based oral amoxicillin treatment in the subgroup of children discharged after an inpatient stay, and the tests for interaction may have been similarly underpowered.
Fourth, these findings should not be considered generalizable to children with very severe disease, including those with underlying comorbidities who may benefit from higher dose or longer treatment.
Conclusions
Among children with CAP discharged from an ED or hospital ward (within 48 hours), low-dose outpatient oral amoxicillin was noninferior to high dose, and 3-day duration was noninferior to 7 days, with regard to need for further antibiotic re-treatment. However, disease severity, treatment setting, prior antibiotics, and acceptability of the noninferiority margin require consideration when interpreting the findings.
Trial Protocol
eTable 1. Features Defined as Indicating Presence of Complicated Pneumonia
eMethods 1. Full Inclusion and Exclusion Criteria
eTable 2. Weight Bands for Dosing of Trial Medication
eMethods 2. Details of Adherence Assessment
eMethods 3. Details of Microbiological Analysis
eMethods 4. Details of Main Protocol Amendment
eMethods 5. Stratification by PED and WARD Pathways
eMethods 6. Rationale for Change to Noninferiority Margin
eMethods 7. Prespecified Sensitivity and Subgroup Analyses
eMethods 8. Post Hoc Ontreatment Analysis
eMethods 9. Post Hoc Subgroup Analysis for PED and WARD Pathways
eFigure 1 A and B. CAP Symptoms at Pretrial Entry in WARD, and at Trial Entry in PED and WARD
eTable 3. Participant Characteristics at Presentation, by Dose and Duration Randomisations
eTable 4. Chest X-ray Results at Trial Entry as Reported by Sites
eTable 5. Inpatient Management for Children in the WARD Group
eTable 6. Prior Exposure to Antibiotics
eTable 7. Summary of ERC Review
eTable 8. Reasons for Starting Nontrial Systemic Antibacterials, as Adjudicated by the ERC
eTable 9. Description of the Primary End Point
eFigure 2 A and B. Primary End Point, Analysis of Interactions
eFigure 3 A and B. Ontreatment Analysis of Dose Randomisation
eFigure 4 A and B. Ontreatment Analysis of Duration Randomisation
eFigure 5. Primary End Point Analysis for Dose Randomisation in PED Pathway
eFigure 6. Primary End Point Analysis for Duration Randomisation in PED Pathway
eFigure 7. Primary End Point Analysis for Dose Randomisation in WARD Pathway
eFigure 8. Primary End Point Analysis for Duration Randomisation in WARD Pathway
eFigure 9 A and B. Time to Resolution of Cough by Randomisation Group
eFigure 10 A and B. Cough Prevalence and Severity by Randomisation Group and Time Point
eFigure 11 A and B. Time to Resolution of Sleep disturbed by Cough by Randomisation Group
eFigure 12 A and B. Prevalence and Severity of Sleep Disturbed by Cough by Randomisation Group and Time Point
eTable 10. Adherence and Adverse Events, by 4 Randomized Groups
eTable 11. S. pneumoniae and Antimicrobial Resistance on Day 28, by 4 Randomized Groups
eTable 12. S. pneumoniae Carriage
eTable 13. Penicillin Nonsusceptibility in Patients With Available Culture Result (Positive or Negative)
eTable 14. Penicillin Nonsusceptibility in Patients With a Culture Positive for S. pneumoniae
Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Jackson C, Hsia Y, Bielicki JA, et al. Estimating global trends in total and childhood antibiotic consumption, 2011-2015. BMJ Glob Health. 2019;4(1):e001241. doi: 10.1136/bmjgh-2018-001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994-2007. Pediatrics. 2011;127(3):411-418. doi: 10.1542/peds.2010-2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Maat J, van de Voort E, Mintegi S, et al. ; Research in European Pediatric Emergency Medicine study group . Antibiotic prescription for febrile children in European emergency departments: a cross-sectional, observational study. Lancet Infect Dis. 2019;19(4):382-391. doi: 10.1016/S1473-3099(18)30672-8 [DOI] [PubMed] [Google Scholar]
- 4.Verbakel JY, Van den Bruel A, Thompson M, et al. ; European Research Network on Recognising Serious Infection (ERNIE) . How well do clinical prediction rules perform in identifying serious infections in acutely ill children across an international network of ambulatory care datasets? BMC Med. 2013;11(1):10. doi: 10.1186/1741-7015-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among US children. N Engl J Med. 2015;372(9):835-845. doi: 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien KL, Baggett HC, Brooks WA, et al. ; Pneumonia Etiology Research for Child Health (PERCH) Study Group . Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394(10200):757-779. doi: 10.1016/S0140-6736(19)30721-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMuri GP, Gern JE, Eickhoff JC, Lynch SV, Wald ER. Dynamics of bacterial colonization with Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis during symptomatic and asymptomatic viral upper respiratory tract infection. Clin Infect Dis. 2018;66(7):1045-1053. doi: 10.1093/cid/cix941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fancourt N, Deloria Knoll M, Baggett HC, et al. Chest radiograph findings in childhood pneumonia cases from the multisite PERCH study. Clin Infect Dis. 2017;64(suppl_3):S262-S270. doi: 10.1093/cid/cix089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florin TA, Ambroggio L, Brokamp C, et al. Biomarkers and disease severity in children with community-acquired pneumonia. Pediatrics. 2020;145(6):e20193728. doi: 10.1542/peds.2019-3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higdon MM, Le T, O’Brien KL, et al. Association of c-reactive protein with bacterial and respiratory syncytial virus–associated pneumonia among children aged <5 years in the PERCH study. Clin Infect Dis. 2017;64(suppl_3):S378-S386. doi: 10.1093/cid/cix150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley JS, Byington CL, Shah SS, et al. ; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America . The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. doi: 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris M, Clark J, Coote N, et al. ; British Thoracic Society Standards of Care Committee . British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1-ii23. doi: 10.1136/thoraxjnl-2011-200598 [DOI] [PubMed] [Google Scholar]
- 13.Le Saux N, Robinson JL; Canadian Paediatric Society, Infectious Diseases and Immunization Committee . Uncomplicated pneumonia in healthy Canadian children and youth: practice points for management. Paediatr Child Health. 2015;20(8):441-450. doi: 10.1093/pch/20.8.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsburg A-S, Mvalo T, Nkwopara E, et al. Amoxicillin for 3 or 5 Days for chest-indrawing pneumonia in Malawian children. N Engl J Med. 2020;383(1):13-23. doi: 10.1056/NEJMoa1912400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodha R, Kabra SK, Pandey RM. Antibiotics for community-acquired pneumonia in children. Cochrane Database Syst Rev. 2013(6):CD004874. doi: 10.1002/14651858.CD004874.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyttle MD, Bielicki JA, Barratt S, et al. ; PERUKI, GAPRUKI and the CAP-IT trial team . Efficacy, safety and impact on antimicrobial resistance of duration and dose of amoxicillin treatment for young children with Community-Acquired Pneumonia: a protocol for a randomised controlled trial (CAP-IT). BMJ Open. 2019;9(5):e029875. doi: 10.1136/bmjopen-2019-029875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126(2):204-213. doi: 10.1542/peds.2009-3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netuveli G, Hurwitz B, Levy M, et al. Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005;365(9456):312-317. doi: 10.1016/S0140-6736(05)17785-X [DOI] [PubMed] [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing . Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. Accessed September 21, 2021. https://www.eucast.org.
- 20.US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research . Community-Acquired Bacterial Pneumonia: Developing Drugs for Treatment. Guidance for Industry. 2020. Accessed September 21, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/community-acquired-bacterial-pneumonia-developing-drugs-treatment
- 21.Spellberg B, Talbot GH, Brass EP, et al. ; Infectious Diseases Society of America . Position paper: recommended design features of future clinical trials of antibacterial agents for community-acquired pneumonia. Clin Infect Dis. 2008;47(suppl_3):S249-S265. doi: 10.1086/591391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haider BA, Lassi ZS, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;16(2):CD005976. doi: 10.1002/14651858.CD005976.pub2 [DOI] [PubMed] [Google Scholar]
- 23.López-Alcalde J, Rodriguez-Barrientos R, Redondo-Sánchez J, et al. . Short-course versus long-course therapy of the same antibiotic for community-acquired pneumonia in adolescent and adult outpatients. Cochrane Database Syst Rev. 2018;9(9):CD009070. doi: 10.1002/14651858.CD009070.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakhale S, Mulpuru S, Verheij TJM, Kochen MM, Rohde GGU, Bjerre LM. Antibiotics for community-acquired pneumonia in adult outpatients. Cochrane Database Syst Rev. 2014;(10):CD002109. doi: 10.1002/14651858.CD002109.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tansarli GS, Mylonakis E. Systematic review and meta-analysis of the efficacy of short-course antibiotic treatments for community-acquired pneumonia in adults. Antimicrob Agents Chemother. 2018;62(9):e00635-18. doi: 10.1128/AAC.00635-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. doi: 10.1097/INF.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 27.Pernica JM, Harman S, Kam AJ, et al. Short-course antimicrobial therapy for pediatric community-acquired pneumonia: the SAFER randomized clinical trial. JAMA Pediatr. 2021;175(5):475-482. doi: 10.1001/jamapediatrics.2020.6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinh A, Ropers J, Duran C, et al. ; Pneumonia Short Treatment (PTC) Study Group . Discontinuing β-lactam treatment after 3 days for patients with community-acquired pneumonia in non-critical care wards (PTC): a double-blind, randomised, placebo-controlled, non-inferiority trial. Lancet. 2021;397(10280):1195-1203. doi: 10.1016/S0140-6736(21)00313-5 [DOI] [PubMed] [Google Scholar]
- 29.Madhi SA, De Wals P, Grijalva CG, et al. The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr Infect Dis J. 2013;32(3):e119-e127. doi: 10.1097/INF.0b013e3182784b26 [DOI] [PubMed] [Google Scholar]
- 30.Berni E, Scott LA, Jenkins-Jones S, et al. Non-response to antibiotic treatment in adolescents for four common infections in UK primary care 1991-2012: a retrospective, longitudinal study. Antibiotics (Basel). 2016;5(3):25. doi: 10.3390/antibiotics5030025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Currie CJ, Berni E, Jenkins-Jones S, et al. Antibiotic treatment failure in four common infections in UK primary care 1991-2012: longitudinal analysis. BMJ. 2014;349:g5493. doi: 10.1136/bmj.g5493 [DOI] [PubMed] [Google Scholar]
- 32.Esposito S, Cohen R, Domingo JD, et al. Antibiotic therapy for pediatric community-acquired pneumonia: do we know when, what and for how long to treat? Pediatr Infect Dis J. 2012;31(6):e78-e85. doi: 10.1097/INF.0b013e318255dc5b [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization . Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities: Evidence Summaries. WHO Press; 2014. Accessed September 21, 2021. https://apps.who.int/iris/bitstream/handle/10665/137319/9789241507813_eng.pdf [PubMed]
- 34.Llor C, Hernández S, Bayona C, et al. A study of adherence to antibiotic treatment in ambulatory respiratory infections. Int J Infect Dis. 2013;17(3):e168-e172. doi: 10.1016/j.ijid.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 35.de Velde F, de Winter BCM, Koch BCP, van Gelder T, Mouton JW; COMBACTE-NET Consortium . Non-linear absorption pharmacokinetics of amoxicillin: consequences for dosing regimens and clinical breakpoints. J Antimicrob Chemother. 2016;71(10):2909-2917. doi: 10.1093/jac/dkw226 [DOI] [PubMed] [Google Scholar]
- 36.Legg J, Rampton C.. British Thoracic Society Paediatric Pneumonia Audit 2016/2017 Report. British Thoracic Society;2018. Accessed September 21, 2021. https://www.brit-thoracic.org.uk/quality-improvement/clinical-audit/bts-national-audit-reports/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Features Defined as Indicating Presence of Complicated Pneumonia
eMethods 1. Full Inclusion and Exclusion Criteria
eTable 2. Weight Bands for Dosing of Trial Medication
eMethods 2. Details of Adherence Assessment
eMethods 3. Details of Microbiological Analysis
eMethods 4. Details of Main Protocol Amendment
eMethods 5. Stratification by PED and WARD Pathways
eMethods 6. Rationale for Change to Noninferiority Margin
eMethods 7. Prespecified Sensitivity and Subgroup Analyses
eMethods 8. Post Hoc Ontreatment Analysis
eMethods 9. Post Hoc Subgroup Analysis for PED and WARD Pathways
eFigure 1 A and B. CAP Symptoms at Pretrial Entry in WARD, and at Trial Entry in PED and WARD
eTable 3. Participant Characteristics at Presentation, by Dose and Duration Randomisations
eTable 4. Chest X-ray Results at Trial Entry as Reported by Sites
eTable 5. Inpatient Management for Children in the WARD Group
eTable 6. Prior Exposure to Antibiotics
eTable 7. Summary of ERC Review
eTable 8. Reasons for Starting Nontrial Systemic Antibacterials, as Adjudicated by the ERC
eTable 9. Description of the Primary End Point
eFigure 2 A and B. Primary End Point, Analysis of Interactions
eFigure 3 A and B. Ontreatment Analysis of Dose Randomisation
eFigure 4 A and B. Ontreatment Analysis of Duration Randomisation
eFigure 5. Primary End Point Analysis for Dose Randomisation in PED Pathway
eFigure 6. Primary End Point Analysis for Duration Randomisation in PED Pathway
eFigure 7. Primary End Point Analysis for Dose Randomisation in WARD Pathway
eFigure 8. Primary End Point Analysis for Duration Randomisation in WARD Pathway
eFigure 9 A and B. Time to Resolution of Cough by Randomisation Group
eFigure 10 A and B. Cough Prevalence and Severity by Randomisation Group and Time Point
eFigure 11 A and B. Time to Resolution of Sleep disturbed by Cough by Randomisation Group
eFigure 12 A and B. Prevalence and Severity of Sleep Disturbed by Cough by Randomisation Group and Time Point
eTable 10. Adherence and Adverse Events, by 4 Randomized Groups
eTable 11. S. pneumoniae and Antimicrobial Resistance on Day 28, by 4 Randomized Groups
eTable 12. S. pneumoniae Carriage
eTable 13. Penicillin Nonsusceptibility in Patients With Available Culture Result (Positive or Negative)
eTable 14. Penicillin Nonsusceptibility in Patients With a Culture Positive for S. pneumoniae
Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement