Abstract
Background and Aims:
Data on the relation of egg consumption with risk of type 2 diabetes (T2D) and coronary heart disease (CHD) are limited and inconsistent. Few studies have controlled for overall dietary patterns in egg-T2D or egg-CHD analyses, and it is unclear whether any observed elevated risks of T2D and CHD with frequent egg consumption is real or due to confounding by dietary habits. We tested the hypothesis that frequent egg consumption is associated with a higher risk of T2D and CHD risk after adjustment for overall dietary patterns among adults.
Design:
We used prospective cohort design to complete time-to-event analyses.
Methods:
We pooled de novo, harmonized, individual-level analyses from nine US cohorts (n=103,811). Cox regression was used to estimate hazard ratios separately in each cohort adjusting for age, ethnicity, body mass index (BMI), exercise, smoking, alcohol intake, and dietary patterns. We pooled cohort-specific results using an inverse-variance weighted method to estimate summary relative risks.
Results:
Median age ranged from 25 to 72 years. Median egg consumption was 1 egg per week in most of the cohorts. While egg consumption up to one per week was not associated with T2D risk, consumption of ≥2 eggs per week was associated with elevated risk [27% elevated risk of T2D comparing 7+ eggs/week with none (95% CI: 16% to 37%)]. There was little evidence for heterogeneity across cohorts and we observed similar conclusions when stratified by BMI. Overall, egg consumption was not associated with the risk of CHD. However, in a sensitivity analysis, there was a 30% higher risk of CHD (95% CI: 3%-56%) restricted to older adults consuming 5-6 eggs/week.
Conclusions:
Our data showed an elevated risk of T2D with egg consumption of ≥2 eggs per week but not with <2 eggs/week. While there was no overall association of egg consumption with CHD risk, the elevated CHD observed with consumption of 5-6 eggs/week in older cohorts merits further investigation.
Keywords: Nutrition, epidemiology, type 2 diabetes, CHD, diet quality
Introduction
Coronary heart disease (CHD) remains one of the leading causes of morbidity and mortality in the US.1 While there has been a decline in some CHD risk factors over time (smoking, hypertension, and dyslipidemia), the prevalence of type 2 diabetes mellitus (T2D) has been on the rise worldwide.2, 3 Currently, about one in twenty Americans is affected by T2D,4 and its prevalence has increased by almost 3-fold5 in the past 50 years. T2D is associated with a two- to three-fold increased risk of cardiovascular events including atrial fibrillation,1, 6 heart failure,7 CHD,1 and total mortality.8, 9 Data on the association of egg consumption with T2D or CHD risk have been limited and inconsistent. Results from the Zutphen Study10 indicated a positive association between egg consumption and fasting glucose. While the China Health and Nutrition Survey reported a higher odds of T2D with egg consumption in adults11, prospective cohort studies from France, Finland, Spain, and Japan did not show an association between egg consumption and risk of T2D (pooled RR comparing extreme categories of 0.85 (95% CI: 0.65-1.07)).12 Furthermore, some but not all US prospective cohorts12 reported an elevated risk of T2D with more frequent consumption of eggs. Our earlier meta-analysis showed an elevated risk of T2D when comparing highest with lowest category of egg consumption among US cohorts but not in non-US cohorts.12 Moreover, with regard to CHD, while prospective studies have not observed a higher risk of CHD with egg consumption,13, 14 stratified analysis suggested an elevated risk of CHD with egg intake among people with T2D.15 The inconsistency of the findings in the literature could be partially attributable to a lack of consideration of overall diet quality in previous analyses of egg consumption and health outcomes. Thus, the current study sought to examine the association of egg consumption with incidence of (a) T2D and (b) CHD with a comprehensive adjustment for potential confounding factors including overall diet quality and BMI in prospective US cohorts.
Methods
Study population
Eligible cohorts were required to be prospective large US-based studies with data on food frequency questionnaire, T2D, and/or CHD. Two of the nine cohorts identified were excluded from egg-CHD analyses because of a lack of adequate number of CHD (CARDIA) or overlap with an existing and approved manuscript (REGARDS). A brief description of the nine prospective US cohorts is provided below.
The Atherosclerosis Risk in Communities (ARIC) Study:
The ARIC Study is an ongoing community-based prospective cohort study of 15,792 participants aged 45–64 years at baseline and recruited from four communities in the United States starting in 1987-1989. The goal of ARIC is to investigate the etiology of atherosclerosis and its consequences and variation in cardiovascular risk factors, medical care, and disease by race, sex, place, and time. After the first examination (1987–1989), participants attended 5 follow-up exams as well as followed for CVD events for an average of 20.8 years. A detailed description of ARIC has been published previously.16
Cardiovascular Health Study (CHS):
The CHS is an ongoing prospective cohort of 5,888 adults aged 65+ years who were selected from randomly generated Medicare-eligibility lists in 4 US communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA). After an initial recruitment of 5,201 participants between 1989 and 1990, a total of 687 mostly African-American subjects were recruited between 1992 and 1993. Participants were followed up every 6 months, alternating between telephone calls and clinic visits through 1999 and by telephone only through the present (with the exception of a clinic visit in 2005-2006). A detailed description of the CHS has been published previously.17
The Coronary Artery Risk Development in Young Adults (CARDIA) Study:
The CARDIA study is an ongoing multicenter longitudinal study of risk factors for coronary artery disease in 5,115 black and white adult men and women aged 18 to 30 years at baseline examination (1985–1986) and free of CVD. CARDIA participants have been followed up for an average of 12.5 years. A detailed description of CARDIA has been published previously.18
The Jackson Heart Study (JHS):
The JHS is an ongoing prospective cohort of 5,301 African-American adult men and women aged 35-84 years at baseline examination in 2000 to 2004 and designed to study determinants of chronic diseases among African-Americans. The third clinic examination has been completed with data collection for an average of seven years of follow up. Ongoing annual telephone calls have been used to collect information on comorbidity. A detailed description of the JHS has been previously published.19
The Multi-Ethnic Study of Atherosclerosis (MESA):
The MESA is an ongoing multi-ethnic cohort study of 6,814 adults aged 45 to 84 years at baseline designed to investigate the prevalence and progression of subclinical cardiovascular disease. MESA participants were recruited from six U.S. geographical areas (Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York City, New York; and St. Paul, Minnesota). For current analyses, MESA participants have been followed up for a median of 13.2 years. A detailed description of MESA has been published previously.20
The Physicians’ Health Study (PHS):
The PHS I was a randomized, double-blind, placebo-controlled trial designed to study low-dose aspirin and beta-carotene for the primary prevention of cardiovascular disease and cancer in 22,071 US male physicians between 1982 and 1995. The PHS II was a completed randomized trial designed to assess the effects of vitamin supplements on cardiovascular disease and cancer between 1997 and 2011. PHS II enrolled 7641 members of the PHS I as well as 7000 newly recruited male physicians. Current analyses used dietary questionnaires collected between 1997 and 2001 and an average of 9 years of follow up. Detailed descriptions of PHS I and II have been published previously.21, 22
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study:
The REGARDS study is an ongoing prospective cohort study of 30,239 black and white community-dwelling residents aged 45+ years at baseline examination (2003 to 2007) designed to understand determinants of racial and geographic disparities in stroke. REGARDS participants have been followed up by ongoing telephone assisted interview every six months. Fifty-six percent of the cohort includes residents of the Stroke Belt states (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Louisiana, and Arkansas) and the remainder from the rest of the 40 contiguous US states. Also, the study oversampled African-Americans. A detailed description of REGARDS has been published previously.23
Women’s Antioxidant Cardiovascular Study (WACS):
The WACS was a randomized, double-blind, placebo-controlled, 2x2x2x2 factorial trial of vitamin C, vitamin E, ß-carotene, and folic acid/vitamin B6/vitamin B12 in the prevention of cardiovascular events among 8,711 women aged 40+ years with preexisting cardiovascular disease or 3+ coronary risk factors (5442 women in the B vitamin component). Participants were followed up for a mean duration of 9.4 years, from 1995-1996 to 2005. A detailed description of WACS has been published.24
Women’s Health Study (WHS):
The WHS was a randomized trial designed to assess low-dose aspirin and vitamin E for preventing cancer and cardiovascular disease among 39,876 healthy female professionals aged 45+ years, enrolling participants between 1992 and 1995. After completion of the trial in 2004, 33,682 women (89% of surviving participants) consented to continue observational follow up via annual questionnaires. The ongoing questionnaires ascertain information on lifestyle risk factors and development of medical outcomes, including T2D and CHD. Self-reports of T2D are confirmed with a follow-up questionnaire, and CHD self-reports are confirmed with review of medical records. A detailed description of the WHS has been published previously.25
All participants provided informed consent for the parent studies, and the meta-analysis was approved by the Institutional Review Board of Brigham and Women Hospital. Overall, we analyzed data on 103,811 subjects across 9 US prospective cohorts. We used a pre-specified protocol with standardized definitions of exposures, outcomes, covariates, and statistical analysis for all cohorts.
Assessment of egg consumption
Egg consumption was assessed at baseline in each cohort using a food frequency questionnaire (FFQ) or diet history.26 We used the reported frequency of eggs consumed per week [PHS, WHS, WACS, ARIC, CHS, and REGARDS] or number of times eggs were consumed per week multiplied by portion size (1, 2, or 3 eggs) [JHS and CARDIA] to define the number of eggs consumed per week within each cohort. In MESA where frequency of intake of “eggs, omelettes, huevos rancheros” was reported without portion size, we assumed one egg per occasion. Subjects with extreme values of energy intake were excluded from cohort-specific analyses. For restricted cubic spline analysis, we used the midpoint as average intake of eggs per week in each category. If the highest category of egg consumption had an open upper boundary, we multiplied the lower boundary by 1.5 to obtain an estimate of average egg consumption in that category as previously described.26
Assessment of T2D during follow up
Incident T2D was defined using fasting glucose ≥ 126 mg/dl, non-fasting glucose ≥ 200 mg/dl, hemoglobin A1C ≥ 6.5%, use of hypoglycemic medications, Centers for Medicare & Medicaid Services (CMS) claim codes for diabetes (ICD-9-CM 250.XX), or self-report of a diagnosis of diabetes, depending on the availability of such information in the respective cohort.
Assessment of coronary heart disease
Coronary heart disease (CHD) was defined as a composite of fatal or non-fatal myocardial infarction, coronary death, coronary angioplasty or revascularization (myocardial infarction or CHD death in PHS). CHD events were adjudicated by cohort-specific endpoint committees.
Other relevant variables
Each cohort collected baseline information on race-ethnicity, age, sex, body mass index, smoking, alcohol intake, education, physical activity, comorbidity, and dietary intake. Dietary variables of interest include fruit and vegetables, sugar-sweetened beverages, legumes, nuts, whole grains, sodium intake, low-fat dairy products, fish intake, fried foods, fresh red meat, processed meat (bacon, sausage, salami, hot dog, etc.), trans-fatty acids, and saturated fatty acids. We used information from the food frequency questionnaire (FFQ) or cohort-specific information on consumption of fruits, vegetables, nuts, legumes, dairy products, whole grain, meats, and beverages to compute the Dietary Approach to Stop Hypertension (DASH) score for adjustment of overall diet quality. Within each cohort, DASH score was created using quintiles of 8 DASH components (fruits, vegetables, nuts and legumes, low-fat dairy products, whole grain, sweetened beverages, dietary sodium, and red and processed meats); quintile ranking was assigned for healthy food and six minus quintile ranking was assigned for unhealthy foods (sodium, red/processed meats, and sweetened beverages).27 DASH score was computed as sum of assigned values (range: 8 to 40). The rationale for selecting DASH over other indices for diet quality such as alternate healthy eating index (aHEI), alternate Mediterranean diet (aMED), and recommended food score (RFS) is supported by the paper by de Koning et al.28 showing a lower risk of T2D with DASH after adjustment for aHEI/aMED and not the reverse. Dyslipidemia was defined as history of hypercholesterolemia, total cholesterol of 200+ mg/dl, or use of lipid-lowering drugs.
Statistical analysis
Person-time of follow up was computed as the time from egg assessment to the first occurrence of outcome (T2D or CHD), death, or last follow-up. Sex-specific analyses were performed within each cohort and the results were centrally meta-analyzed. For cohorts with person-time data, a Cox proportional hazard model was used to estimate hazard ratios (95% CI) using the lowest frequency of egg consumption as reference; otherwise, logistic regression was used (REGARDS study did not have person-time of follow up) to estimate the relative risk. Adjustment was made for age, field center (if applicable), body mass index, smoking, alcohol intake, education, physical activity, dyslipidemia for CHD outcome, and DASH score (continuous). In sensitivity analyses, we controlled for DASH score (modeled continuously and as quintiles in separate models) as well as eight individual DASH components. We also stratified by body mass index (<25 vs. ≥25 kg/m2) and diet quality (high quality using top 2 quintiles of DASH score vs. lower quality diet consisting of bottom 3 quintiles of DASH score). Hazard ratios obtained from each cohort were used to perform fixed effect meta-analysis using inverse weighted variance (DerSimonian Laird method).29 We initially pooled relative risks and their 95% confidence intervals comparing the highest to the lowest category of egg consumption in each study. For studies that stratified analyses by sex, we considered each sex as an independent study. We assessed heterogeneity using the Q statistic, I-squared, and p value <0.05. To assess dose-response relation and evaluate the shape of the egg-T2D or egg-CHD relationship, we used generalized least squares regression described by Greenland and Longnecker30 and fit cubic splines with knots at 5, 35, 65, and 95 percentile of the egg distribution. For our CHD analyses, we also stratified by prevalent T2D and restricted to high-risk group (age 70+ or prevalent T2D). Lastly, we conducted a sensitivity analysis restricted to cohorts whose participants were ≥60 years on average at baseline and had the opportunity to reach age 70 or older at the end of follow up. The rationale for such sensitivity analysis is that CHD is rare in young adults and inclusion of low-risk young adults could dilute and/or mask the true association of egg intake with CHD. Two-sided p values were used with alpha level of 0.05.
Results
Of the total 103,811 subjects included in current analyses, 35% were men. The mean age at the time of egg consumption assessment ranged from 24.9 years in the CARDIA cohort to 73.0 years in CHS. Median egg consumption was 3 eggs per week in CARDIA, 2 per week in JHS, MESA, and REGARDS; and about 1 egg per week in the remaining five cohorts (Table 1). Egg consumption was associated with higher body mass index, male sex, and current smoking.
Table 1.
Characteristics of nine cohorts meta-analyzed *
| Characteristics | Cohort Name |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ARIC (n=15412) |
CARDIAƗ (n=5094) |
CHS (n=2317) |
JHS (n=3908) |
MESA (n=6531) |
PHS (n=18266) |
REGARDSƗ (n=9030) |
WACS (n=6164) |
WHS (n=37089) |
|
| Age (y) | 54.2 ±5.8 | 24.9±3.7 | 77.8±4.5 | 53.4±13.0 | 62.2±10.2 | 66.3±9.2 | 64.9±8.4 | 60.8±8.9 | 54.6±7.0 |
| Body mass (kg/m2) | 27.7 ±5.4 | 24.5 ±5.1 | 26.5 ±4.5 | 31.1 ±7.1 | 28.3 ± 5.4 | 25.7 ±3.3 | 29.1±5.6 | 29.6±6.4 | 25.9±4.9 |
| Men (%) | 44.0% | 45.6% | 37.0% | 38.0% | 47.4% | 100.0% | 43.6% | 0.0% | 0.0% |
| White (%) | 73.0% | 48.5% | 84.0% | 0.0% | 39.0% | 93.0% | 72.8% | 95.0% | 94.0% |
| Black (%) | 26.7% | 51.5% | 15.0% | 100.0% | 26.8% | 0.7% | 27.2% | 2.6% | 2.0% |
| Current smoking (%) | 26.1% | 13.3% | 7.0% | 14.0% | 12.6% | 3.3% | 10.7% | 16.0% | 13.0% |
| Alcohol intake (%) | 38.6% | 13.5% | 46.0% | 49.0% | 69.1% | 82.0% | 46.7% | 49.6% | 56.0% |
| Fruits (serv/d) | 2.5 ±1.9 | 3.6 ±3.5 | 3.4 ±1.9 | 2.6 ±2.5 | 1.9 ±1.6 | 2.5 ±1.7 | 3.4 ±2.7 | 2.3 ±1.5 | 2.2 ±1.4 |
| Vegetables (serv/d) | 1.5 ±1.1 | 3.1±2.7 | 4.0 ±2.4 | 3.2 ±2.4 | 2.3 ±1.6 | 2.3 ±1.4 | 2.8 ±1.8 | 4.2 ±2.4 | 4.0 ±2.3 |
| Nut/legume (serv/d) | 0.2±0.3 | 0.8±1.8 | 0.7 ±0.7 | 0.8 ±0.8 | 0.5 ±0.7 | 1.3 ±1.2 | 0.6 ±0.8 | 0.8 ±0.6 | 0.7 ±0.6 |
| Dairy products (serv/d) | 1.0±1.1 | 1.2±1.8 | 1.0 ±0.9 | 0.2 ±0.4 | 0.8 ±1.2 | 0.9 ±1.0 | 2.5 ±2.4 | 1.2 ±1.1 | 1.2 ±1.0 |
| Whole grain (serv/d) | 1.2 ±1.2 | 1.5±1.6 | 1.5 ±1.3 | 1.0±1.2 | 0.8 ±0.7 | 1.4 ±1.6 | 1.6 ±1.0 | 1.4 ±1.2 | 1.5 ±1.2 |
| Sodium intake (g/d) | 1.5±0.6 | 4.4±2.7 | 2.6±1.1 | 3.4±1.6 | 2.3±4.7 | 1.5±0.6 | 2.3±1.0 | 1.7±0.3 | 1.9±0.3 |
| Red/processed meat (serv/d) | 1.1 ±0.8 | 1.0±0.9 | 0.8±0.7 | 1.2±1.3 | 0.6±0.6 | 0.7±0.6 | 1.9±1.2 | 0.8±0.6 | 0.8±0.5 |
| Sweetened beverage (serv/d) | 0.5±1.0 | 0.6±1.1 | 0.4±0.6 | 0.3±0.5 | 0.4±0.9 | 0.3±0.6 | NA | 1.0±1.3 | 1.0±1.2 |
| Egg intake (median/wk) | 1.0 | 3.0 | 1.0 | 2.0 | 2.0 | 1.0 | 1.8 | 1.0 | 1.0 |
| DASH score | 23.8 ±4.1 | 23.9±5.2 | 24.4±4.7 | 24.0±5.0 | 25.1±4.7 | 24.2±4.9 | 24.4±4.4 | 24.2±5.2 | 24.0±5.4 |
ARIC: Atherosclerosis Risk in Community
CARDIA: The Coronary Artery Risk Development in Young Adults Study
CHS: Cardiovascular Health Study
JHS: Jackson Heart Study
MESA: Multi-Ethnic Study of Atherosclerosis
PHS: Physicians’ Health Study
REGARDS: The Reasons for Geographic and Racial Differences in Stroke Study
WACS: The Women’s Antioxidant Cardiovascular Study
WHS: Women’s Health Study
DASH: Dietary approach to stop hypertension
Study that did not contribute to CHD analyses
Egg consumption and risk of T2D
During follow up, a total of 11,734 new cases of T2D occurred. While consumption of up to 1 egg per week was not associated with T2D risk, intake of 2 or more eggs per week was associated with about 11% to 27% higher risk of T2D in a pooled analysis controlling for age, sex, smoking, body mass index, alcohol intake, DASH score, and physical activity (Table 2 and supplemental Fig 1). There was minimal evidence for heterogeneity across egg categories (all p values for heterogeneity > 0.05 and all I2 of 0% in the first three egg categories and 30% - 66% in the top egg categories -- Supplemental Fig 1). Adjustment for DASH components instead of a single DASH score did not alter the results (Table 2). Additional adjustment for energy intake had no influence on the results (corresponding pooled HRs (95% CI) were: 1.00 (ref), 1.01 (0.94-1.08); 0.96 (0.88-1.03); 1.12 (1.05-1.18); 1.15 (1.05-1.24); and 1.27 (1.16-1.37). Restricted cubic splines were consistent for a higher risk of T2D with higher frequency of egg consumption (Fig 1). When stratified by diet quality, we observed elevated risk of T2D with frequent egg consumption in subjects with poorer diet (bottom 3 quintiles of DASH score) [adjusted HR (95% CI): 1.00 (ref), 1.06 (0.97-1.15), 1.00 (0.90-1.09), 1.15 (1.06-1.23), 1.21 (1.07-1.34), and 1.53 (1.39-1.67) across consecutive categories of egg consumption, respectively]. Corresponding values for subjects in the top 2 quintiles of DASH score were 1.00 (ref), 0.97 (0.87-1.07), 1.00 (0.88-1.111), 1.15 (1.06-1.25), 1.18 (1.05-1.32), and 1.08 (0.92-1.23), respectively. When stratified by body mass index, we observed positive associations between high consumption of eggs with incidence of T2D in people with normal and abnormal body mass index (Table 4).
Table 2.
Pooled RR (95% CI) of T2D by egg intake in nine US cohorts
| Pooled RR (95%)* |
||||
|---|---|---|---|---|
| Frequency of egg consumption | Model 1 | Model 2 | Model 3 | Model 4 |
| <1 egg/month | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1-3 eggs/month | 1.04 (0.98-1.11) | 1.01 (0.95-1.08) | 1.02 (0.95-1.09) | 1.01 (0.94-1.08) |
| 1 egg/week | 1.01 (0.94-1.08) | 0.98 (0.91-1.06) | 0.98 (0.91-1.06) | 0.96 (0.89-1.03) |
| 2-4 eggs/week | 1.25 (1.19-1.31) | 1.16 (1.09-1.22) | 1.14 (1.08-1.21) | 1.11 (1.05-1.18) |
| 5-6 eggs/week | 1.19 (1.11-1.27) | 1.14 (1.04-1.24) | 1.17 (1.08-1.27) | 1.13 (1.04-1.23) |
| ≥7 eggs/week | 1.50 (1.40-1.59) | 1.23 (1.14-1.33) | 1.28 (1.18-1.38) | 1.27 (1.16-1.37) |
Model 1 adjusted for age and sex
Model 2 adjusted for age, sex, body mass index, smoking, alcohol intake, exercise, and DASH score (continuous)
Model 3 controlled for age, sex, body mass index, smoking, alcohol intake, exercise, and DASH score quintiles
Model 4 controlled for age, sex, body mass index, smoking, alcohol intake, exercise, and components of DASH score
Figure 1.
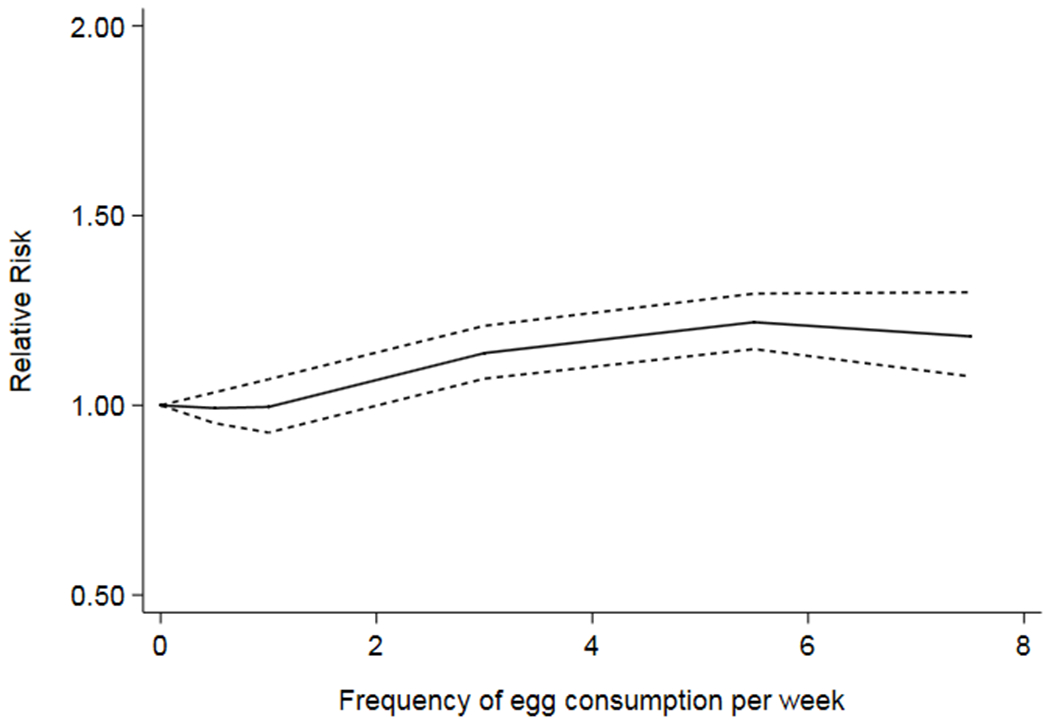
Restricted cubic spline depicting the relation of egg consumption with risk of T2D
Table 4.
Pooled RR (95% CI) of T2D and coronary heart disease (CHD) by egg intake stratified by body mass index
| Pooled RR (95% CI) for T2D |
Pooled RR (95% CI) for CHD |
|||
|---|---|---|---|---|
| Egg consumption | BMI <25 kg/m2 | BMI ≥25 kg/m2 | BMI <25 kg/m2 | BMI ≥25 kg/m2 |
| <1 egg/month | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1-3 eggs/month | 1.03 (0.88-1.18) | 1.00 (0.92-1.08) | 1.00 (0.85-1.14) | 0.94 (0.84-1.04) |
| 1 egg/week | 1.14 (0.98-1.31) | 0.94 (0.86-1.03) | 1.03 (0.89-1.18) | 0.86 (0.77-0.96) |
| 2-4 eggs/week | 1.05 (0.91-1.20) | 1.16 (1.08-1.23) | 1.03 (0.89-1.18) | 0.90 (0.81-1.00) |
| 5-6 eggs/week | 1.29 (1.03-1.55) | 1.19 (1.08-1.29) | 1.18 (0.96-1.39) | 0.88 (0.76-1.00) |
| ≥7 eggs/week | 1.23 (0.98-1.48) | 1.34 (1.23-1.46) | 1.05 (0.76-1.35) | 1.13 (0.98-1.28) |
Adjusted for age, sex, body mass index, smoking, alcohol intake, exercise, and DASH score (continuous)
Egg consumption and risk of CHD
We meta-analyzed seven cohorts with a total of 5,867 new cases of CHD during follow up. Egg consumption was not associated with a higher risk of CHD in multivariable adjusted model (Table 3). Adjustment for DASH quintiles and DASH components (Table 3) and stratification by body mass index (Table 4) yielded similar conclusions. Additional adjustment for dyslipidemia did not alter the results [HR (95% CI): 1.00 (ref), 1.00 (0.92-1.07), 0.94 (0.86-1.02), 1.01 (0.93-1.08), 0.95 (0.84-1.05), and 1.10 (0.98-1.22) from the lowest to the highest category of egg consumption, respectively]. Furthermore, egg consumption was not associated with the incidence of CHD among people with prevalent T2D [HR (95% CI): 1.00 (ref), 0.92 (0.75-1.08), 0.74 (0.56-0.92), 0.90 (0.74-1.06), 1.00 (0.81-1.18), and 1.05 (0.80-1.29) for egg intake of <1/month, 1-3/month, 1/week, 2-3/week. 4-6/week, and 7+/week, respectively]. Corresponding values for people free of T2D were 1.00 (ref), 1.02 (0.93-1.12), 0.99 (0.89-1.08), 0.98 (0.89-1.07), 0.92 (0.78-1.07), and 1.14 (0.97-1.30). Finally, restriction to subjects aged 70+ years or those with prevalent T2D at baseline did not alter the results [HR (95% CI): 1.00 (ref), 0.99 (0.86-1.11), 0.91 (0.77-1.04), 0.98 (0.86-1.09), 1.01 (0.78-1.24), and 1.05 (0.86-1.24) from the lowest to the highest category of egg intake, respectively]. Finally, in a sensitivity analysis restricted to cohorts (i) whose participants’ mean age at baseline was 60+y and (ii) with adequate follow up to allow participants to reach age 70+ at the end of follow up (CHS, MESA, and PHS), multivariable adjusted pooled hazard ratios for CHD were 1.00 (ref), 1.02 (0.88-1.16), 0.99 (0.84-1.13), 1.06 (0.93-1.19), 1.30 (1.03-1.56), and 1.00 (0.80-1.20) across consecutive categories of egg consumption, respectively.
Table 3.
Pooled RR (95% CI) of coronary heart disease by egg intake in seven US cohorts
| Pooled RR (95%)* |
||||
|---|---|---|---|---|
| Frequency of egg consumption | Model 1 | Model 2 | Model 3 | Model 4 |
| <1 egg/month | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1-3 eggs/month | 0.98 (0.91-1.06) | 0.98 (0.90-1.06) | 0.97 (0.89-1.05) | 1.00 (0.92-1.08) |
| 1 egg/week | 0.93 (0.85-1.00) | 0.92 (0.84-1.00) | 0.91 (0.83-1.00) | 0.93 (0.85-1.02) |
| 2-4 eggs/week | 1.03 (0.96-1.10) | 0.96 (0.89-1.04) | 0.97 (0.89-1.04) | 0.97 (0.89-1.05) |
| 5-6 eggs/week | 0.96 (0.86-1.07) | 0.94 (0.82-1.05) | 0.93 (0.82-1.04) | 0.94 (0.82-1.06) |
| ≥7 eggs/week | 1.24 (1.12-1.36) | 1.11 (0.98-1.25) | 1.12 (0.99-1.25) | 1.06 (0.92-1.19) |
Model 1 adjusted for age and sex
Model 2 adjusted for age, sex, body mass index, smoking, alcohol intake, exercise, and DASH score (continuous)
Model 3 controlled for age, sex, body mass index, smoking, alcohol intake, exercise, and DASH score quintiles
Model 4 controlled for age, sex, body mass index, smoking, alcohol intake, exercise, and components of DASH score
Discussion
Main findings
In this pooled analysis of nine US cohorts, we found that consumption of 2 or more eggs per week was associated with an elevated risk of T2D after adjustment for potential confounding factors including dietary patterns, whereas infrequent consumption of eggs (up to 1 egg per week) was not related with T2D risk. The elevated risk of T2D was more apparent in those who were in the 2 bottom tertiles of DASH score. Furthermore, egg consumption was not associated with elevated risk of CHD, irrespective of BMI in overall analysis. However, in a sensitivity analysis restricted to cohorts with older ages, there was a 30% higher risk of CHD (95% CI: 3% to 56%) in people consuming 5-6 eggs/week and no meaningful association in other categories of egg consumption.
Egg consumption and risk of T2D
Previous meta-analyses showed an elevated risk of T2D with egg consumption in US cohorts but not in non-US studies,12, 31 thereby raising the possibility of confounding by modifiable factors inherent to US populations. For example, in the US, eggs are often consumed with food items known to be associated with a higher risk of T2D such as bacon, fried potatoes and other fried foods, sausages, and red meats.32 To minimize confounding by dietary habits in our analyses, we used a standardized approach to control for DASH score as a surrogate of dietary habits within each cohort before meta-analyzing study-specific estimates of effects. Such adjustment did not eliminate a positive association of frequent egg consumption with T2D risk and the conclusion was similar when we used DASH components or DASH quintiles instead of DASH score (continuous) to control for confounding by dietary habits. Furthermore, the egg-T2D relation persisted when stratified by healthy dietary patterns (using top 2 quintiles of DASH score) and unhealthy dietary patterns (bottom 3 quintiles of DASH score).
Potential biologic mechanisms underlying egg-T2D relation
It has been suggested that dietary cholesterol (from eggs) might play a role in the observed egg-T2D relation; but there is limited evidence to support a causal role of dietary cholesterol and T2D. While an average egg may contain about 200 mg of dietary cholesterol,33 randomized controlled trials in humans do not support the hypothesis that egg consumption raises LDL-cholesterol. Among subjects with metabolic syndrome, a 12-week intervention with 3 eggs/d vs an egg substitute did not alter LDL-cholesterol but decreased triglycerides and increased HDL-cholesterol.34, 35 A 4-week intervention with 1, 2, or 3 eggs per day among healthy adults led to increased HDL-cholesterol and decreased LDL-cholesterol compared to no egg consumption.36 In another cross-over trial of healthy subjects, intake of 2 eggs/d for four weeks led to increased LDL- and HDL-cholesterol, but no change in LDL-to-HDL ratio compared to oatmeal breakfast.37 In contrast, Ballesteros found no effect of one egg/d on plasma lipids after 5 weeks of intervention compared with oatmeal-based breakfast among diabetic subjects.38 Overall, these data do not support a detrimental role of eggs on LDL-cholesterol. To the contrary, eggs may improve lipoprotein profile by raising HDL-cholesterol.
Regarding biomarkers of glucose metabolism, a five-week intervention with one egg/d did not alter plasma glucose compared 34 to oatmeal-based breakfast in diabetic subjects.38 Other clinical trials showed no effects of an intervention with eggs on plasma glucose.34, 36, 38 Fried eggs may increase energy density of eggs and lead to weight gain with subsequent development of T2D. This hypothesis is not supported by our data where secondary analyses did not reveal a change in body mass index over time when comparing various frequencies of egg consumption (data not shown). Such findings are consistent with short-term clinical trial data.34, 39
Egg consumption and CHD risk
In our pooled analysis of 7 US cohorts, we found no overall association between egg consumption and incidence of CHD (overall and among people with prevalent T2D or aged 70+). In a sensitivity analysis restricted to high-risk older participants, an elevated risk of CHD was restricted to consumption of 5-6 eggs/week. Our findings are consistent with results of a recent meta-analysis of prospective studies showing no association of egg consumption with CHD and cardiovascular disease.14 In contrast, a pooled analysis of six US prospective cohorts reported an 8% higher risk of CHD with each additional consumption of half egg (95% CI: 3% to 14%) in a multivariable model controlling for age, sex, race/ethnicity, and education.40 Furthermore, analysis of nearly 200,000 participants of the Million Veteran Program showed about 12% higher risk of CHD comparing consumption of one or more eggs per day with no intake while infrequent consumption of eggs was not related with incidence of CHD.41
In contrast, a prospective study of half a million Chinese showed 12% lower risk of CHD (95% CI: 7% to 16%) comparing daily intake of 0.8 egg with none; this association was stronger in people with normal body mass index (p interaction BMI*egg = 0.046).42 Of note is that the China Kadoorie Biobank study42 did not have information on frequent consumption of eggs (1+/d) to compare with our results. In MVP data, neither adiposity nor prevalent diabetes modified the relation of egg consumption with incident CHD.41
Previous studies have suggested that trimethylamine N-Oxide (TMAO) – a metabolite of choline – might be the missing link between egg consumption, choline, and incident CHD. It has been suggested that TMAO might increase the risk of death43, 44 and predict major adverse cardiac events.44 Although eggs are good source of choline, it is important to note that choline can also be provided by other foods including meats, milk, grain, and fish.45
Dietary choline might be more bioavailable through egg consumption than with choline supplements. In a crossover trial of 30 healthy adults, intake of 3 eggs/d led to a 20% increase in plasma choline after 4 weeks of intervention whereas no change in plasma choline was seen with choline supplement (400 mg/d).46 Despite an increase in plasma choline with egg intervention, no change in plasma TMAO was seen with either egg intervention or choline supplementation.46 It appears unlikely that TMAO might explain earlier reports of elevated risk of CHD with egg consumption in diabetic subjects.
As discussed above, trial data showed favorable lipoprotein profile with egg intervention including lower LDL, triglycerides, higher HDL, and lower LDL/HDL ratio. Furthermore, other clinical trials have demonstrated that intervention with eggs did not alter blood pressure, plasma C-reactive protein, apolipoprotein B, and weight.39 To the contrary, egg intervention resulted in decreased plasma serum amyloid A and tumor necrosis-alfa, increase in large HDL particles, and reduction of VLDL and medium VLDL particles.34 Taken together, a lack of an adverse association with cardiometabolic risk factors is in line with our findings of no increased risk of CHD with egg consumption.
Limitations and strengths
The current study has several limitations. Assessment of egg consumption was completed only at one point for most cohorts thereby precluding evaluation of change in egg consumption over time. However, correlation coefficient between egg consumption at visit 1 and visit 3 in ARIC was 0.37, suggesting reasonable level of consistency about dietary habits over time. Furthermore, we did not have information on the mode of egg preparation (fried, boiled, and scrambled eggs) for further evaluation. Since most cohorts did not screen for diabetes or use the lower fasting glucose thresholds endorsed more recently by the American Diabetic Association, we might have missed some cases of T2D. However, such under-ascertainment of T2D is less likely to be differential across egg consumption categories because cohort-specific endpoint committees were not aware of the egg consumption status of participants. A major limitation remains unmeasured and/or residual confounding that may explain the observed findings. Finally, many cohorts included in our CHD meta-analysis enrolled younger adults with mean age <60 at baseline. Because CHD risk is low in younger adults, it is possible that younger age might have diluted the egg-CHD association. The observed elevated risk of CHD with consumption of 5-6 eggs/week when our analysis was restricted to older cohorts merits further investigation. Despite these shortcomings, our study has several strengths including a large sample size with adequate number of incident T2D for sub-analyses; a use of standardized protocol for all cohorts; prospective design; adjustment for dietary patterns using DASH score and its components in a sensitivity analysis; and the availability of data from men and women from different ethnic groups.
In conclusion, data from this large pooled study provide evidence in support of a modest and graded association of egg consumption with T2D risk. The elevated risk of CHD restricted to consumption of 5-6 eggs per week observed in older cohorts merits confirmation.
Supplementary Material
Supplemental Online Figure 1. Forest plot depicting relation of egg consumption with incidence of T2D using fixed effect model
Supplemental Online Figure 2. Forest plot depicting relation of egg consumption with incidence of coronary heart disease using fixed effect model
Acknowledgements
We are indebted to study participants of all participating cohorts.
Source of funding
The current data analysis was supported by an investigator-initiated grant from the American Egg Board (Luc Djoussé, PI).
The Multi-Ethnic Study of Atherosclerosis (MESA) was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS.
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
The Coronary Artery Risk Development in Young Adults Study (CARDIA) was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content.
The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
The Physicians’ Health Study was supported by grants CA-34944 and CA-40360, and CA-097193 from the National Cancer Institute and grants HL-26490, HL088081, and HL-34595 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
JHS acknowledgement.
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The authors also wish to thank the staffs and participants of the JHS.
JHS disclaimer.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
The parent REGARDS study was supported by cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service
The Women’s Health Study was supported by grants CA047988, UM1 CA182913, HL043851, HL080467, and HL099355 from the National Institutes of Health.
The Women’s Antioxidant Cardiovascular Study (WACS) was supported by investigator-initiated grant HL47959 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr. Djousse received investigator-initiated grant from American Egg Board for current analyses; sponsor had no influence on design, conduct, and interpretation of the results.
Other co-authors have no disclosures.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Wirehn AB, Andersson A, Ostgren CJ and Carstensen J. Age-specific direct healthcare costs attributable to diabetes in a Swedish population: a register-based analysis. Diabet Med. 2008;25:732–7. [DOI] [PubMed] [Google Scholar]
- 3.Marchant K. Diabetes and chronic kidney disease: a complex combination. Br J Nurs. 2008;17:356–61. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS and Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. [DOI] [PubMed] [Google Scholar]
- 5.Howard BV, Rodriguez BL, Bennett PH, Harris MI, Hamman R, Kuller LH, Pearson TA and Wylie-Rosett J. Prevention Conference VI: Diabetes and Cardiovascular disease: Writing Group I: epidemiology. Circulation. 2002;105:e132–7. [DOI] [PubMed] [Google Scholar]
- 6.Huxley RR, Filion KB, Konety S and Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erqou S, Lee CT, Suffoletto M, Echouffo-Tcheugui JB, de Boer RA, van Melle JP and Adler AI. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. Eur J Heart Fail. 2013;15:185–93. [DOI] [PubMed] [Google Scholar]
- 8.Yano Y, Kario K, Ishikawa S, Ojima T, Gotoh T, Kayaba K, Tsutsumi A, Shimada K, Nakamura Y, Kajii E and Group JMSCS. Associations between diabetes, leanness, and the risk of death in the Japanese general population: the Jichi Medical School Cohort Study. Diabetes Care. 2013;36:1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monesi L, Tettamanti M, Cortesi L, Baviera M, Marzona I, Avanzini F, Monesi G, Nobili A, Riva E, Fortino I, Bortolotti A, Fontana G, Merlino L, Trevisan R and Roncaglioni MC. Elevated risk of death and major cardiovascular events in subjects with newly diagnosed diabetes: findings from an administrative database. Nutr Metab Cardiovasc Dis. 2014;24:263–70. [DOI] [PubMed] [Google Scholar]
- 10.Feskens EJ and Kromhout D. Habitual dietary intake and glucose tolerance in euglycaemic men: the Zutphen Study. Int J Epidemiol. 1990;19:953–9. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Li M and Shi Z. Higher egg consumption associated with increased risk of diabetes in Chinese adults-China Health and Nutrition Survey. Br J Nutr. 2020:1–8. [DOI] [PubMed] [Google Scholar]
- 12.Djousse L, Khawaja OA and Gaziano JM. Egg consumption and risk of type 2 diabetes: a meta-analysis of prospective studies. Am J Clin Nutr. 2016;103:474–80. [DOI] [PubMed] [Google Scholar]
- 13.Drouin-Chartier JP, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks FM, Rosner B, Willett WC, Hu FB and Bhupathiraju SN. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ. 2020;368:m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godos J, Micek A, Brzostek T, Toledo E, Iacoviello L, Astrup A, Franco OH, Galvano F, Martinez-Gonzalez MA and Grosso G. Egg consumption and cardiovascular risk: a dose-response meta-analysis of prospective cohort studies. Eur J Nutr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu FB, Stampfer MJ, Rimm EB, Manson JE, Ascherio A, Colditz GA, Rosner BA, Spiegelman D, Speizer FE, Sacks FM, Hennekens CH and Willett WC. A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA. 1999;281:1387–94. [DOI] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A and et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K and Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 19.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF and Taylor HA Jr. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6-18–29. [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 21.Steering Committee of the Physicians’ Health Study Research G. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. [DOI] [PubMed] [Google Scholar]
- 22.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ and Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS and Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE and Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH and Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Gan Y, Wu C, Qu X, Sun G and Lu Z. Coffee consumption and the risk of gastric cancer: a meta-analysis of prospective cohort studies. BMC Cancer. 2015;15:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, Tinker LF, Van Horn L, Waring ME, Li W, Shikany JM and Eaton CB. Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr. 2014;99:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB and Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R and Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S and Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 31.Drouin-Chartier JP, Schwab AL, Chen S, Li Y, Sacks FM, Rosner B, Manson JE, Willett WC, Stampfer MJ, Hu FB and Bhupathiraju SN. Egg consumption and risk of type 2 diabetes: findings from 3 large US cohort studies of men and women and a systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr. 2020;112:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cahill LE, Pan A, Chiuve SE, Sun Q, Willett WC, Hu FB and Rimm EB. Fried-food consumption and risk of type 2 diabetes and coronary artery disease: a prospective study in 2 cohorts of US women and men. Am J Clin Nutr. 2014;100:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song WO and Kerver JM. Nutritional contribution of eggs to American diets. J Am Coll Nutr. 2000;19:556S–562S. [DOI] [PubMed] [Google Scholar]
- 34.Blesso CN, Andersen CJ, Barona J, Volk B, Volek JS and Fernandez ML. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors in metabolic syndrome. J Clin Lipidol. 2013;7:463–71. [DOI] [PubMed] [Google Scholar]
- 35.Blesso CN, Andersen CJ, Barona J, Volek JS and Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism. 2013;62:400–10. [DOI] [PubMed] [Google Scholar]
- 36.DiMarco DM, Missimer A, Murillo AG, Lemos BS, Malysheva OV, Caudill MA, Blesso CN and Fernandez ML. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids. 2017;52:255–263. [DOI] [PubMed] [Google Scholar]
- 37.Missimer A, DiMarco DM, Andersen CJ, Murillo AG, Vergara-Jimenez M and Fernandez ML. Consuming Two Eggs per Day, as Compared to an Oatmeal Breakfast, Decreases Plasma Ghrelin while Maintaining the LDL/HDL Ratio. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballesteros MN, Valenzuela F, Robles AE, Artalejo E, Aguilar D, Andersen CJ, Valdez H and Fernandez ML. One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients. Nutrients. 2015;7:3449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuller NR, Caterson ID, Sainsbury A, Denyer G, Fong M, Gerofi J, Baqleh K, Williams KH, Lau NS and Markovic TP. The effect of a high-egg diet on cardiovascular risk factors in people with type 2 diabetes: the Diabetes and Egg (DIABEGG) study-a 3-mo randomized controlled trial. Am J Clin Nutr. 2015;101:705–13. [DOI] [PubMed] [Google Scholar]
- 40.Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM and Allen NB. Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality. JAMA. 2019;321:1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djousse L, Ho YL, Nguyen XT, Quaden RM, Gagnon DR, Gaziano JM and Cho K. Egg consumption and risk of coronary artery disease in the Million Veteran Program. Clin Nutr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin C, Lv J, Guo Y, Bian Z, Si J, Yang L, Chen Y, Zhou Y, Zhang H, Liu J, Chen J, Chen Z, Yu C, Li L and China Kadoorie Biobank Collaborative G. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart. 2018;104:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WH and Hazen SL. Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide and 5-Year Mortality Risk in Stable Coronary Artery Disease: The Contributory Role of Intestinal Microbiota in a COURAGE-Like Patient Cohort. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL and Luscher TF. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vennemann FB, Ioannidou S, Valsta LM, Dumas C, Ocke MC, Mensink GB, Lindtner O, Virtanen SM, Tlustos C, D’Addezio L, Mattison I, Dubuisson C, Siksna I and Heraud F. Dietary intake and food sources of choline in European populations. Br J Nutr. 2015;114:2046–55. [DOI] [PubMed] [Google Scholar]
- 46.Lemos BS, Medina-Vera I, Malysheva OV, Caudill MA and Fernandez ML. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J Am Coll Nutr. 2018;37:716–723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Online Figure 1. Forest plot depicting relation of egg consumption with incidence of T2D using fixed effect model
Supplemental Online Figure 2. Forest plot depicting relation of egg consumption with incidence of coronary heart disease using fixed effect model


