Abstract
Background:
Supplemental breast cancer screening with breast MRI is recommended for women at high breast cancer risk. Recent national trends in breast MRI utilization are unknown.
Methods:
We used claims data from a large national insurer to calculate screening breast MRI rates from 2006 to 2016 in a U.S. cohort of 10 million women aged 20–64 years. Utilization was stratified by subgroups of women with a BRCA mutation, family history of breast cancer and prior breast cancer history and stratified by age. Joinpoint regression evaluated annual changes in trends.
Results:
Total sample included 37,447 screening breast MRIs in 25,617 women. Overall screening breast MRI rates were low and increased from 2.9 to 12.1 exams/10,000 women from 2006 to 2016. MRI use in women with a BRCA mutation increased 21% on average annually from 210.8 to 1562.0/10,000 from 2006 to 2016. By 2016, women aged 50–64 years with a BRCA mutation had the highest breast MRI use (1669.6/10,000) compared with younger women (1198.4, 1519.1, and 1567.2/10000 in 20–29, 30–39, and 40–49 year-old, respectively). Women with a BRCA mutation comprised <1% of our study population but received 9% of screening breast MRI exams. Breast MRI rates among women with a family history or prior breast cancer history initially increased from 2006–2008, but then stabilized or decreased.
Conclusion:
Observed increases in breast MRI use indicate improvement in concordance with breast imaging guidelines. However, women with BRCA mutations remain under-screened, particularly younger women, identifying a clear gap to enhance access.
Keywords: Female, breast cancer, early detection of cancer, magnetic resonance imaging, cohort studies, algorithms
INTRODUCTION
Use of breast cancer risk assessment to identify women at elevated breast cancer risk has modified the “one-size fits all” breast cancer screening guidelines to include supplemental advanced imaging for high risk women. As an example, since 2007, leading professional and advocacy organizations have recommended screening breast MRI as an adjunct to routine mammography beginning at age 25–30 years among women with high familial breast cancer risk (>20–25%), genetic mutation carriers (i.e., BRCA 1/2 carriers) and a personal history with chest irradiation treatment.1–3 Screening breast MRI has improved sensitivity and cancer detection for these subgroups of women;4 however, use of breast MRI to screen for breast cancer can lead to more false positive results requiring biopsy.5,6
Early studies of the changes in clinical practice as a result of these guidelines detected a rapid rise in the use of breast MRI prior to initial guideline recommendations by the American Cancer Society (ACS) in 2007 with a plateau in use through 2012.2,5,7 In national data from the Breast Cancer Surveillance Consortium, screening breast MRI rates increased more than 4-fold between 2005 and 2007 from 8 to 34 per 10,000 women and then remained fairly stable at 43 breast MRI examinations per 10,000 women until 2009.5 Similarly, in a New England regional area insurer, by 2011 screening breast MRI accounted for more than half of breast MRIs conducted, but nearly 80% of screening MRIs performed were done in women who did not meet the ACS criteria.7 Among women with BRCA mutation who did meet the ACS criteria, less than half had received a MRI for breast cancer screening.7 Since these studies were published, limited contemporary data are available on screening MRI use, particularly in this high-risk subgroup of women with known genetic mutations.
In the current analysis, we extend this prior work to examine national screening breast MRI trends in both recommended (i.e., BRCA mutation) and non-recommended subgroups of women (i.e., family history of breast cancer or prior breast cancer). Using claims data from a large national insurer, we present temporal trends in screening breast MRI use between 2006 and 2016. We evaluate national screening MRI rates overall and by age in three risk subgroups of women: 1) diagnosed BRCA mutations; 2) family history of breast cancer; and 3) prior breast cancer diagnosis.
METHODS
Study design and population
We conducted a retrospective observational cohort study using health insurance claims data from Optum’s de-identified Clinformatics® Data Mart Database (Eden Prairie, MN). This database includes enrollment information and all medical, pharmacy, and hospitalization claims for approximately 40 million members (20 million women) of a large national health insurance plan enrolled from January 1, 2006 to December 31, 2016. The study was approved by the Harvard Pilgrim Institutional Review Board with a waiver of informed consent.
We restricted our analytic cohort to women aged 20 to 64 with at least one year of continuous enrollment between 2006–2016. We included younger women aged 20–40 years in our sample, who are recommended to receive breast MRI with mammography if they have a BRCA mutation.3
Identification of risk subgroups
We categorized eligible women into one of three breast cancer risk groups based on the occurrence and timing of International Classification of Disease (ICD) 9/10 diagnosis codes8,9 in the medical claims: 1) BRCA mutation carrier; 2) family history of breast cancer, and 3) prior breast cancer diagnosis. Women entered as BRCA mutation carriers subgroup if they had any diagnosis code indicating a genetic susceptibility to malignant neoplasm of breast (ICD9/10 V84.01 and Z15.01, respectively). Women without BRCA mutations entered the family history of breast cancer subgroup on the date of their first diagnosis for family history of malignant breast neoplasm (ICD9 V16.3 or ICD10 Z80.3). Women entered the prior breast cancer diagnosis subgroup if at least 6 months had elapsed since the first of at least two diagnoses for personal history of breast cancer (ICD9 V10.3 or ICD10 Z85.3), carcinoma in situ (ICD9 233 or ICD10 D05) or malignant breast neoplasm (ICD9 174 or ICD10 C50).
For women meeting the criteria for multiple risk groups, we imposed the following order to ensure a woman was in only one risk subgroup at any point: prior breast cancer diagnosis > BRCA mutation > family history. For example, a woman with a BRCA mutation who was subsequently diagnosed with breast cancer would be categorized into the BRCA mutation carrier group until 6 months after her breast cancer diagnosis, after which she would enter the prior breast cancer group.
Patient Covariates
Observed patient covariates from membership files included age (categorized as 20–29, 30–39, 40–49, 50–64 years) and U.S. region (West, South, Midwest, Northeast). We used similar methods to Wharam and colleagues to assign neighborhood-level race and poverty,10,11 using census tract data.
Identification of screening breast MRI
Because billing codes are not specific to the indication or purpose for breast MRI, we developed a claims-based algorithm that used procedure, diagnosis, and pharmacy codes to conservatively identify screening breast MRI exams (Figure 1). We first identified all breast MRI exams in the study cohort using Healthcare Common Procedure Coding System (HCPCS)12 and Current Procedure Terminology (CPT)13 codes. To restrict to breast MRIs billed for screening purposes, we only included exams where a woman: a) did not have a diagnosis for a complication of a breast implant in the 9 months prior; b) had no documented breast cancer diagnostic workup in the 3 months prior; and c) had no prior diagnosis for personal history of breast cancer if she was not in our prior breast cancer subgroup. Because this algorithm used look-backs in the medical claims, we also required that each woman have at least 12 months continuous enrollment before her screening MRI (Figure 1 and Appendix Table 1).
FIGURE 1.
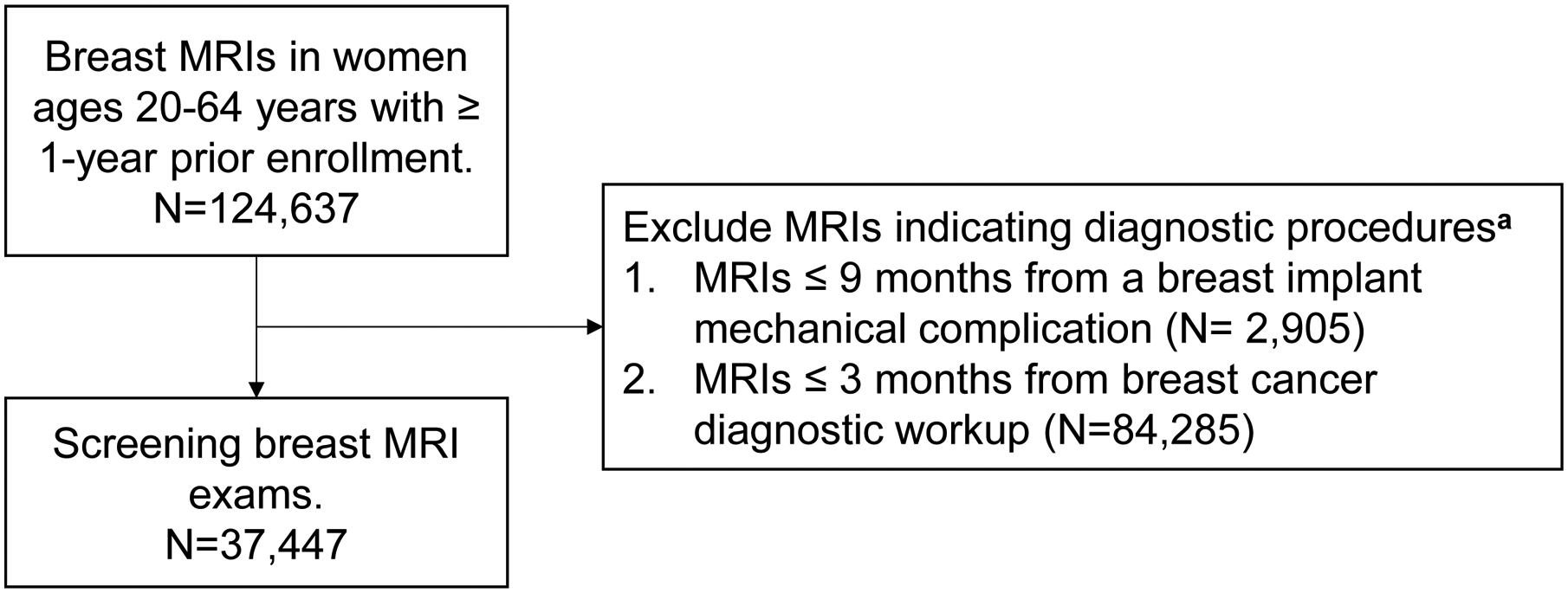
Algorithm to identify screening MRI exams in medical claims.
a See Appendix Table 1 for full list of procedure, diagnosis and pharmacy codes.
Statistical analysis
We calculated annual screening breast MRI rates per 10,000 women overall and by age (20–29, 30–39, 40–49, 50–64 years) for the entire cohort and for each risk subgroup. Rates were calculated across all ages groups, except for women aged 20–29 years in the prior breast cancer group, who were too few to accurately calculate MRI trends. Women were censored when they turned 65, because the majority of women become Medicare-eligible and exit the commercial insurer.
To control for demographic changes over time, we standardized all rates to the age group, U.S. region, race, and neighborhood poverty levels of women enrolled in 2016. Because women in different risk strata were clinically distinct and not comparable to each other in terms of breast MRI use, we performed this standardization separately for each of our three risk subgroups. We also calculated rates for women not meeting our three risk subgroups as Other. Further, among women with prior breast cancer, we also calculated screening breast MRI rates stratified by BRCA mutation status. We used SAS Studio (SAS® 9.4M6) for data extraction and all rate calculations.14
We applied the National Cancer Institute’s Joinpoint regression software (versions 4.7.0.0 and 4.8.0.1)15,16 to analyze changes in screening MRI rates across the study period. We summarized average annual percent changes within three specified time periods: 1) 2006–2016; 2) 2006–2008; and 3) 2008–2016, based on the most common joinpoints set automatically by the regression software.
RESULTS
Study Population
From 2006 to 2016, we identified 10.1 million women meeting inclusion criteria. The three risk groups represented the following proportions: women with BRCA mutations (N=10,715; 0.1% of total); women with family history of breast cancer (N=480,552; 5% of total); and women with prior breast cancer (N=148,011; 2% of total). Each woman contributed an average (standard deviation) of 2.5 (2.4) years to our denominator.
In the sample of women enrolled in 2016, demographics differed by risk subgroup; women with BRCA mutations were more likely to be less than 40 years old (32%) compared to women with a family history (17%) or prior breast cancer (3%) and more likely to live in the Northeast (13%) relative to women with a family history (10%) or prior breast cancer (11%). Consistent with the overall characteristics of this insured population, women in all three risk groups were more likely to live in the South, in predominantly white neighborhoods, and in neighborhoods with lower poverty levels (Table 1).
Table 1.
Characteristics of Women Enrolled in 2016a
| Subgroups | |||||
|---|---|---|---|---|---|
| All women | BRCA mutation | Family history | Prior breast cancer | Otherd | |
| Characteristics | N (%) | N (%) | N (%) | N (%) | N(%) |
| Total population | 2,976,004 | 4,565 | 187,196 | 45,535 | 2,776,129 |
| Age, years | |||||
| 20–29 | 597,537 (20%) | 397 (9%) | 6,969 (4%) | 112 (1%) | 591,948 (21%) |
| 30–39 | 685,970 (23%) | 1,064 (23%) | 23,702 (13%) | 1,472 (3%) | 665,341 (24%) |
| 40–49 | 691,793 (23%) | 1,405 (31%) | 56,200 (30%) | 8,689 (19%) | 637,066 (23%) |
| 50–64 | 1,000,704 (34%) | 1,699 (37%) | 100,325 (53%) | 35,262 (77%) | 881,774 (32%) |
| Race/ethnicity b | |||||
| White neighborhood | 1,598,152 (54%) | 2,804 (61%) | 114,638 (61%) | 27,539 (60%) | 1,475,265 (53%) |
| Black neighborhood | 57,013 (2%) | 34 (1%) | 3,114 (2%) | 849 (2%) | 53,722 (2%) |
| Mixed neighborhood | 662,551 (22%) | 960 (21%) | 39,947 (21%) | 9,848 (22%) | 619,959 (22%) |
| Hispanic surname | 297,101 (10%) | 328 (7%) | 12,703 (7%) | 3,233 (7%) | 283,686 (10%) |
| Asian surname | 144,205 (5%) | 118 (3%) | 4,345 (2%) | 1,431 (3%) | 139,332 (5%) |
| Missing | 216,982 (7%) | 321 (7%) | 12,449 (7%) | 2,635 (6%) | 204,165 (8%) |
| Region of US | |||||
| West | 639,789 (21%) | 1,045 (23%) | 41,074 (22%) | 9,486 (21%) | 596,302 (22%) |
| South | 1,280,231 (43%) | 1,731 (38%) | 75,839 (41%) | 19,031 (42%) | 1,198,708 (43%) |
| Midwest | 759,500 (26%) | 1,200 (26%) | 50,541 (27%) | 12,050 (26%) | 705,236 (25%) |
| Northeast | 286,378 (10%) | 582 (13%) | 19,457 (10%) | 4,853 (11%) | 266,137 (10%) |
| Missing | 10,106 (<1%) | 7 (<1%) | 285 (<1%) | 115 (<1%) | 9,746 (<1%) |
| Neighborhood poverty c | |||||
| <10% | 1,435,028 (48%) | 2,611 (57%) | 104,208 (56%) | 24,896 (55%) | 1,323,190 (48%) |
| ≥10% | 1,282,638 (43%) | 1,583 (35%) | 69,234 (37%) | 17,688 (39%) | 1,208,775 (43%) |
| Missing | 258 338 (9%) | 371 (8%) | 13,754 (7%) | 2,951 (6%) | 244,164 (9%) |
Optum’s de-identified Clinformatics® Data Mart Database (Eden Prairie, MN)
White and Black neighborhood defined using the 2008–2012 American Community Survey as a census tract with ≥75% of residents being one race; Mixed neighborhoods defined as census tracts with no majority race; Hispanic and Asian defined using surname analysis.
Poverty levels defined using the 2008–2012 American Community Survey at the census tract level.
Includes months prior to BRCA, family history, or breast cancer diagnosis
Overall MRI Use
Using our claims-based algorithm, we identified a total of 37,447 screening breast MRIs in 25,617 women that met inclusion criteria and were aged 20–64 from 2006 to 2016. Overall, screening breast MRI use was low (Figure 2). However, rate of use did increase four-fold from 2.9 to 12.1 exams/10,000, at an average increase of 14% per year (Table 2). The rate increase was greatest from 2006 to 2008, changing on average by 45% annually and then by 7% annually from 2008 to 2016. Similar trends were seen across all age groups, although absolute rates of use were highest among women aged 40–64 years compared with younger women. We did not observe any significant increasing trends among the 97% of women who did not belong to any of our three risk sub-groups; in 2016, rates in this group were comparable to the overall rates observed in 2006 (1.9/10,000 compared to 2006 overall rates of 2.9/10,000).
FIGURE 2.
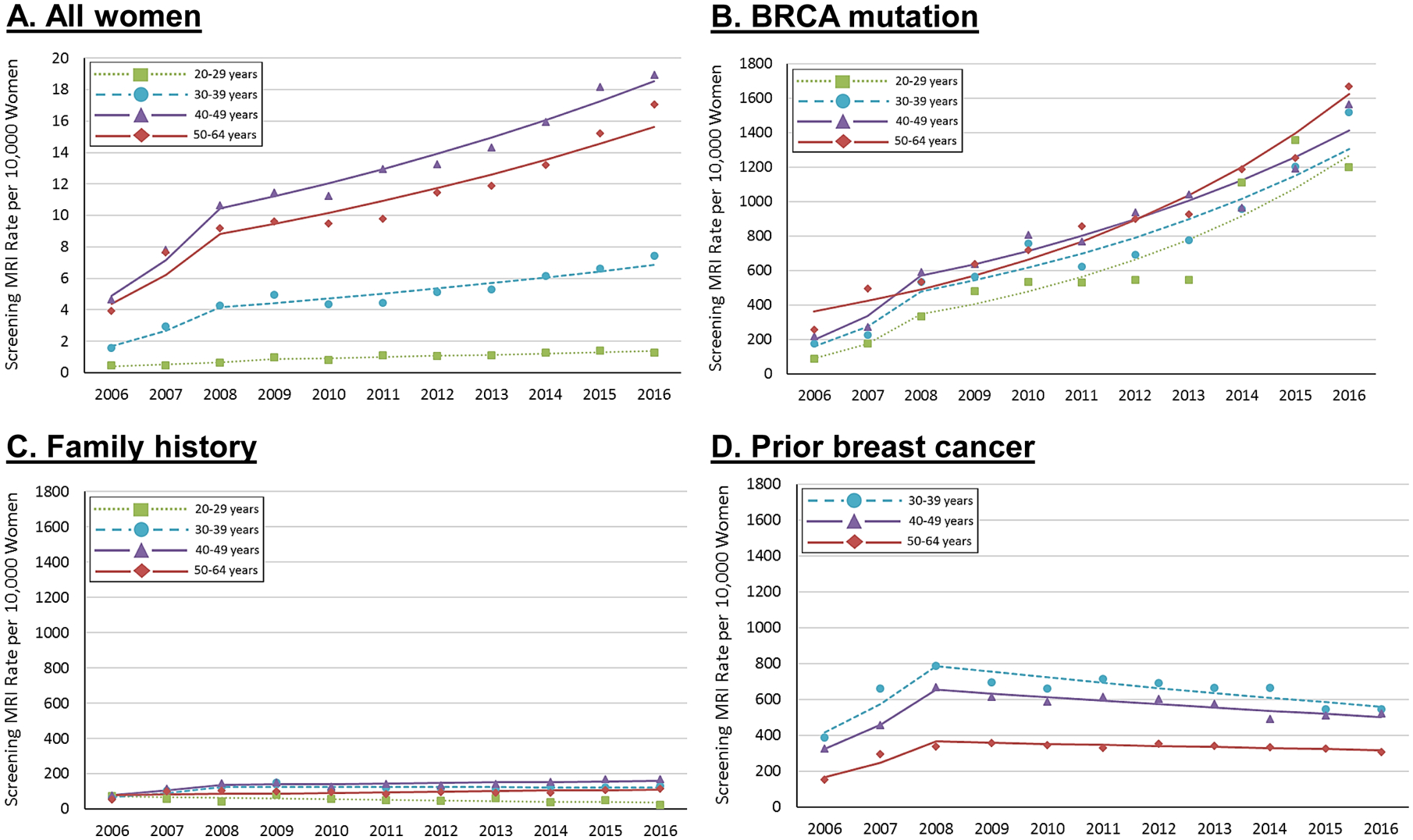
Screening breast MRI rates per 10,000 women, 2006–2016.a
a To account for changing populations across the study period, all rates are standardized, by subgroup, to the age group, US region, race, and neighborhood poverty levels of women enrolled in 2016.
Table 2.
Screening MRI Trends in Women 20–64 years old, by Age and Risk Subgroup
| % Populationa | % with MRI | % all MRIs | MRI Rate per 10,000 | Average Percent Change Per Year (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2008 | 2016 | 2006– 2016 | 2006 −2008 | 2008–2016 | ||||
| 100% | <1% | 100% | 2.9 | 6.7 | 12.1 | 14% (11,18%) | 45% (23,72%) | 7% (5,9%) | |
| 20–29 years | 26% | <1% | 2% | 0.5 | 0.6 | 1.3 | 13% (7,19%) | 28% (8,52%) | 9% (5,13%) |
| 30–39 years | 29% | <1% | 10% | 1.6 | 4.3 | 7.4 | 15% (9,21%) | 58% (17,112%) | 6% (3,10%) |
| 40–49 years | 29% | <1% | 35% | 4.7 | 10.7 | 19.0 | 14% (12,17%) | 46% (27,68%) | 7% (6,9%) |
| 50–64 years | 33% | <1% | 53% | 3.9 | 9.2 | 17.1 | 14% (10,18%) | 42% (17,73%) | 7% (5,10%) |
| BRCA Mutation | <1% | 20% | 9% | 210.8 | 534.7 | 1562.0 | 21% (16,26%) | 59% (24,103%) | 13% (10,16%) |
| 20–29 years | <1% | 12% | <1% | 89.2 | 333.5 | 1198.4 | 30% (14,49%) | 97% (−9,328%)b | 18% (8,28%) |
| 30–39 years | <1% | 14% | 2% | 175.0 | 532.6 | 1519.1 | 24% (13,35%) | 74% (5,186%) | 13% (7,20%) |
| 40–49 years | <1% | 17% | 3% | 216.7 | 592.0 | 1567.2 | 22% (15,29%) | 70% (22,137%) | 12% (8,16%) |
| 50–64 years | <1% | 20% | 4% | 256.7 | 535.8 | 1669.6 | 16% (12,20%) | 16% (12,20%) | 16% (12,20%) |
| Family History | 5% | 2% | 38% | 62.3 | 116.6 | 131.0 | 6% (1,11%) | 29% (0,66%) | 1% (−2,4%) b |
| 20–29 years | <1% | 1% | 1% | 71.8 | 43.9 | 24.4 | −6% (−11,−1%) | −6% (−11,−1%) | −6% (−11,−1%) |
| 30–39 years | <1% | 2% | 5% | 63.4 | 124.4 | 131.7 | 6% (0,13%)b | 37% (−5,98%)b | 0% (−4,4%)b |
| 40–49 years | 2% | 2% | 15% | 76.8 | 145.8 | 167.9 | 7% (2,12%) | 31% (−1,72%)b | 2% (−1,5%)b |
| 50–64 years | 3% | 2% | 17% | 53.3 | 103.5 | 117.5 | 4% (−1,8%)b | 4% (−1,8%)b | 4% (−1,8%)b |
| Prior Breast Cancer | 2% | 6% | 40% | 191.8 | 415.6 | 355.3 | 6% (4,8%) | 46% (33,59%) | −2% (−3,−1%) |
| 30–39 years | <1% | 8% | 2% | 387.1 | 786.4 | 543.6 | 3% (−1,8%)b | 38% (8,75%) | −4% (−7,−2%) |
| 40–49 years | <1% | 7% | 12% | 323.7 | 668.1 | 519.5 | 5% (1,8%) | 42% (18,71%) | −3% (−5,−1%) |
| 50–64 years | 1% | 5% | 26% | 151.2 | 337.8 | 307.0 | 7% (5,9%) | 49% (34,65%) | −2% (−3,−1%) |
| Other | 97% | <1% | 13% | 1.3 | 2.4 | 1.9 | 1% (−2, 5%) b | 1% (−2,5%) b | 1% (−2,5%) b |
| 20–29 years | 26% | <1% | <1% | 0.1 | 0.2 | 0.2 | −1% (−11, 10%)b | −1% (−11, 10%)b | −1% (−11, 10%)b |
| 30–39 years | 28% | <1% | 1% | 0.6 | 1.2 | 0.7 | −1%(−6,4%)b | −1% (−6,4%)b | −1% (−6,4%)b |
| 40–49 years | 28% | <1% | 5% | 2.2 | 3.8 | 3.0 | 2%(−2,7)b | 22% (−4,57%)b | −2% (−5,0.4%)b |
| 50–64 years | 31% | <1% | 7% | 1.9 | 3.7 | 3.2 | 2% −2,6%)b | 2% (−2,6%)b | 2% (−5,6%)b |
Age group percentages do not add up to totals since this is a rolling cohort of women.
Indicates a non-statistically significant change in rate, with the 95% confidence interval including zero.
BRCA mutation carriers
Although women with diagnosed BRCA mutations and no history of breast cancer represented <1% enrollees, this high-risk subgroup received 9% (N=3,440) of all screening MRIs. Across our study period, 20% (N=2,147) of women with BRCA mutations ever received a screening MRI (Table 2).
Women with BRCA mutation had the highest rates of screening breast MRI use across our three risk subgroups (Figure 2). Screening breast MRI rates increased from 210.8 exams/10,000 women in 2006 to 1,562.0/10,000 in 2016, representing an average increase of 21% per year (Table 2). Rates increased most dramatically from 2006–2008 at 59% per year, and this rate of increase slowed to 13% per year from 2008–2016. Rates of increase in use from 2006–2008 were highest among women aged 30–39 and 40–49 years compared with women aged 50–64 years. However, overall rates were highest among women 50–64 years, relative to younger women.
Additional details on the full cohort of women with BRCA mutations by demographics and MRI rates are available in Appendix Tables 2 and 3.
Family History of Breast Cancer
Women with family history of breast cancer represented 5% of our total denominator and received 38% (N=14,097) of screening MRIs. Only 2% (N=10,133) of women with a family history of breast cancer ever received an MRI during her enrollment (Table 2).
Rates of screening breast MRI in women with a family history increased from 62.3 to 131.0/10,000 during our study period, at an average yearly change of 6%. The rate increased from 2006–2008 by 29% per year. Women aged 40–49 years had the highest rate of breast MRI use in 2016 (167.9/10 000). Rates were slightly lower in women aged 30–39 and 50–64 years (131.7 and 117.5 /10,000, respectively) and lowest in women under 30 (24.4/10,000). MRI use among women aged 30–49 years initially demonstrated non-significant increases from 2006–2008 and then stabilized to no increasing rate from 2008–2016. We observed a consistent decreasing trend of 6% per year among women aged 20–29 years (Table 2).
Prior Breast Cancer Diagnosis
Women 30–64 years old with a prior breast cancer diagnosis (N=147,648) received 40% (N=14,909) of all screening breast MRI exams observed. Six percent (N=9,359) of women with prior breast cancer received at least one screening MRI (Table 2).
Among women with prior breast aged 30–64 years, rates of screening breast MRI increased from 191.8 to 355.3/10,000 from 2006 to 2016 at an increase of 6% per year. Like the other risk subgroups, we observed large increases in screening rates from 2006 to 2008 at an average annual change of 46%. However, unlike the other groups, breast MRI use decreased by 2% per year after 2008 and decreased across all age groups (Table 2). Women with prior breast cancer aged 30–39 years had higher rates of screening breast MRI than older survivors, with 2016 rates of 543.6/10,000 compared with 519.5 and 307.0/10,000 in women aged 40–49 and 50–64 years, respectively.
When we further stratified women with prior breast cancer by BRCA mutation status, 16% of women with BRCA mutation had received a breast MRI compared 6% of women without BRCA mutation (Table 3). Across all time points, women with BRCA mutation had higher screening breast MRI rates compared with women without BRCA mutation. However, compared with women with BRCA mutation and no prior breast cancer diagnosis, screening breast MRI rates were more than half lower ( 2016 rates of 1562.0 / 10,000 in Table 2 versus 702.5/10,000 in Table 3, respectively). Further, similar to patterns in all women with prior breast cancer, we observed in women with both BRCA mutation and prior breast cancer a large but non-significant increasing trend from 2006 to 2008 (23%), followed by a slight significant decline until 2016 (−4%, Table 3).
Table 3.
Screening MRI Trends in Women 20–64 years old with prior breast cancer by BRCA status
| % Populationa | % with MRI | % all MRIs | MRI Rate per 10,000 | Average Percent Change Per Year (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2008 | 2016 | 2006– 2016 | 2006 −2008 | 2008–2016 | ||||
| Women with BRCA Mutation | <1% | 16% | 4% | 675.0 | 1056.6 | 702.5 | 0.8% (−4,6%) b | 23% (−6,62%) b | −4% (−7,−1%) |
| 30–39 years | <1% | 11% | 1% | 863.5 | 1379.0 | 242.8 | −6% (−15,3%)b | −6% (−15,3%)b | −6% (−15,3%)b |
| 40–49 years | <1% | 12% | 1% | 300.9 | 887.8 | 607.6 | 7% (−2,17%)b | 82% (8,206%) | −6% (−12,−1%) |
| 50–64 years | <1% | 18% | 2% | 862.0 | 989.6 | 1073.7 | 0.2% (−2,3%)b | 0.2% (−2,3%)b | 0.2% (−2,3%)b |
| Women without BRCA Mutation | 1% | 6% | 36% | 181.3 | 396.3 | 322.9 | 5% (4,7%) | 46% (33, 61%) | −3% (−4,−2%) |
| 30–39 years | <1% | 8% | 2% | 351.9 | 687.0 | 590.2 | 4% (−0.1,7%)b | 35% (10,66%) | −3% (−5,−0.9%) |
| 40–49 years | <1% | 7% | 10% | 315.7 | 662.5 | 504.6 | 4% (0.2,9%) | 42% (13,78%) | −4% (−6,−1%) |
| 50–64 years | <1% | 5% | 24% | 144.8 | 325.5 | 272.2 | 6% (4,9%) | 49% (30,72%) | −3% (−4.−1%) |
Age group percentages do not add up to totals since this is a rolling cohort of women.
Indicates a non-statistically significant change in rate, with the 95% confidence interval including zero.
DISCUSSION
In our large study of commercially-insured women representing a recent decade of breast imaging, we found screening breast MRI rates were low on a population-level, with a 14% annual change from 3 to 12 MRIs per 10,000 women from 2006 to 2016. Women with BRCA mutations were the major contributor to the rise in screening breast MRI use, with the highest utilization and largest changes in screening rates. Rates of screening MRI were lower among women with family history of breast cancer or prior breast cancer diagnosis and had stabilized or declined by 2016. Nonetheless, the absolute number of screening breast MRIs in the U.S. are done in women with a family history or prior breast cancer diagnosis.
Imaging referrals and orders from clinicians need to be better aligned to breast MRI screening guidelines. The steady and increasing rate of screening breast MRI use in women with BRCA mutations indicates improving adherence to these guidelines, which has not been well-documented in previous studies. However, almost 4 out of every 5 observed screening breast MRIs were performed in our two non-recommended risk subgroups and rate increases have slowed since the initial rise in 2006–2008. Screening breast MRI rates were lowest in younger women relative to women 50–64 years with BRCA mutations - even though younger women may benefit most from breast MRI.3,17 Breast cancer incidence in BRCA-positive women rapidly increases during their 30s and 40s, before rates stabilize in women’s 50s.18 Further, women of reproductive age face the dual conflict of family planning while mitigating cancer risk with prophylactic mastectomy and oophorectomy. Screening for breast cancer early with mammography and breast MRI delays decisions for prophylactic mastectomy for younger women until family planning decisions are complete. Nonetheless, our findings indicate a clear opportunity for improved clinical breast cancer management among BRCA mutation carriers at all ages.
Few studies to date have evaluated barriers to breast MRI imaging in women with BRCA mutations. In the ACRIN 6666 study, women with elevated lifetime risk were recommended to receive breast MRI to screen for breast cancer; 42% of women declined participation.19 Women’s reported barriers included claustrophobia; time constraints; financial concerns; their physician would not provide a referral and/or did not believe MRI was indicated; or they were not interested. Further studies should qualitatively assess women’s perspectives on breast MRI use and barriers to adoption in breast health.
Previous research has shown that >80% of breast MRIs occur in women who do not meet professional and clinical guidelines,7 and >35% among women considered at low-to-average risk of breast cancer.20 In our data, we were unable to ascertain whether women met all recommended criteria for screening breast MRI. However, women identified with a family history of breast cancer did not have concurrent coding for BRCA mutation. Some of these women might have a lifetime risk >20%, indicating screening breast MRI may be considered. However, previous studies indicate that a family history alone might be driving breast MRI use; women with only one first degree relative have 8-fold increased likelihood to receive screening breast MRI compared with women with no family history.20 A larger proportion of women with only a family history of breast cancer receive breast MRI compared with women at high lifetime risk meeting clinical guidelines.20 Our results support these previous findings, and indicate a continued need for clinical breast cancer risk assessment to more fully ascertain family history and determine personal risk for breast cancer before a recommendation for breast MRI.
Women with a prior breast cancer continue to receive a substantial proportion of screening breast MRIs, despite limited guidance or recommendation for advanced imaging screening in this population. Not covered in our study time period, in 2018, the American College of Radiology (ACR) recommended the use of breast MRI among women diagnosed with breast cancer at <50 years or if they had dense breast tissue,21 based on observational studies indicating a potential benefit.22,23 However, studies have also shown that breast MRI does not have improved accuracy over mammography among women with prior breast cancer.24 The 2018 ACR guideline did not specifically mention women with BRCA mutation and prior breast cancer, a special population with elevated rates of breast MRI that may benefit from additional screening and the subgroup with among the highest rates historically. In our subgroup of women with prior breast cancer, while a higher proportion of women with BRCA mutation had received a breast MRI compared to women without, women without BRCA mutation represented 36% of all breast MRIs received. Nonetheless, further research will need to evaluate how the new 2018 recommendation influences current patterns of care, which might be dependent on the adoption of insurance reimbursement, specifically from Centers for Medicare and Medicaid Services and consideration of women’s BRCA mutation status in guidelines..
Strengths of our analysis included use of a large national dataset of medical claims, which provided sufficient power to assess historical and contemporary trends in a geographically-diverse sample across 11 years. This dataset captures all medical encounters for enrolled members regardless of healthcare system, which enabled us to identify screening breast MRIs and to stratify by breast cancer risk subgroups. We do have a few limitations to consider. Our dataset only included information on women with employer-based insurance, so our results may not be generalizable to other populations. Additionally, because breast MRI billing codes do not specify clinical indication, we used available health care utilizations to exclude breast MRIs used for non-screening purposes (e.g., MRIs performed for diagnostic workup or to assess the extent of disease among newly-diagnosed breast cancer patients). Our results therefore likely underestimate actual screening breast MRI rates, but do not differentially affect trends over time or by risk strata. Further, our algorithm has not yet been validated to confirm these assumptions. Additionally, we did not have self-report information from women about potential risk groups, including self-report of family history or prior atypia, and were not able to calculate lifetime breast cancer risk. Instead we relied on diagnosis information from claims to identify subgroups of women. We observed increased coding for BRCA mutation and family history of breast cancer in claims in more recent years. Reasons for increasing prevalence of these conditions could be due to increased clinical awareness to document and bill for related-services. Further, non-discrimination legislation passed in 2008 (Genetic Information Nondiscrimation Act),25 may have mitigated previous concerns for genetic privacy. There is the possibility that the coverage use of breast MRI is dependent on identification of BRCA mutation status. Nonetheless, our results do not indicate wide adoption of breast MRI even in women with these identified BRCA mutations.
In conclusion, clinical practice should continue to mitigate use of breast MRI in women not currently recommended due to breast cancer risk or subsequent cancer risk. Further, women with BRCA mutations remain under-screened, particularly younger women, identifying a clear gap to enhance access to screening breast MRI.
Funding Support:
This research was supported by the U.S. National Cancer Institute of the National Institutes of Health under award number R01 CA207373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- MRI
magnetic breast imaging
- BRCA
BReast CAncer gene 1 or 2
Appendix
Appendix Table 1.
Diagnosis, Procedure, and Pharmacy Codes Used to Define Screening MRI
| Identify breast MRI procedures in medical claims using the following: | |
|
CPT: 76093, 77058; HCPCS: C8903–05 (breast MRI, unilateral) CPT: 76094, 77059; HCPCS: C8906–08 (breast MRI, bilateral) Note: Exclude from screening numerator if there is another MRI procedure within 30–90 days before (2000–2005) or 1–90 days before (after 2006) |
|
| Exclude as workup for breast implant complication if within 270 days after any of the following: | |
| Mechanical Complication of Breast Implant |
ICD-9 Diagnosis codes: 99654 (mechanical complications due to breast prosthesis), 99679 (other complications of internal prosthetic device, implant, and graft) ICD-10 Diagnosis codes: T854*XA (mechanical complication of breast prosthesis and implant, initial), T858*XA (other complications of internal prosthetic devices, implants and grafts, not elsewhere classified, initial) |
| Exclude as a diagnostic MRI if within 90 days after any of the following: | |
| Breast Surgery |
CPT: 19160, 19180, 19182, 19200, 19220, 19240, 19301–7, 19162 (mastectomy); 19361, 19364, 19366–9 (breast reconstruction); 19260, 19271, 19272 (excision of chest wall tumor); 38525 (biopsy or excision of deep axillary lymph nodes); 38740, 38745 (axillary lymphadenectomy); 19340 (insertion of breast prosthesis) ICD-9 Procedure Codes: 403, 4022, 4023, 4051 (excision of regional, axillary or mammary lymph nodes); 8521 (lumpectomy); 8522 (resection of quadrant of breast); 8524 (excision of ectopic breast tissue); 8525 (excision of nipple); 8523, 8541–8 (mastectomy) ICD-10 Procedure Codes: 0H5(W or X)ZZ (destruction of nipple); 0HB(T, U, or V)ZZ (excision of breast); 0HB(W,X, or Y)ZZ (excision of nipple); 0HT(U or V)ZZ (resection of breast); 0HT(W or X)ZZ (resection of nipple); 0HTY0ZZ (resection of supermumerary breast) |
| Radiation Therapy |
CPT: 77261–3, 77280, 77285, 77290, 77295, 77299 (therapeutic radiology treatment planning); 77300, 77301, 77305, 77310, 77315, 77321, 77326, 77327, 77328, 77331 (dosimetry); 77332–4 (design of treatment devices); 77336, 77370, 77399 (medical physics consultation); 77401–9, 77411–8 (radiation treatment delivery); 77427, 77431, 77432, 77499 (radiation treatment management); 77470 (special treatment procedure) HCPCS: Q3001 (brachytherapy radioelements) |
| Exclude as a diagnostic MRI if within 90 days after any of the following: | |
| Breast Biopsy |
CPT: 10021–2, 88170–1 (fine needle aspiration); 19081–6, 19100–3, 77031 (breast biopsy); 76095–6, 77032,19295 (guidance for breast biopsy or needle placement); 88173 (cytopathology) ICD-9 Procedure Codes: 8511–2 (breast biopsy), 8520 (excision or destruction of breast tissue, not specified) ICD-10 Procedure Codes: 0H9(T, U, or V)ZX (drainage of breast); 0H9(W or X)ZX (drainage of nipple); 0HB(T, U, or V)ZX (excision of breast, diagnostic); 0HB(W or X)ZX (excision of nipple, diagnostic); 0HBY*ZX (excision of supernumerary breast, diagnostic) |
| Sign or Symptom of Breast Problem |
ICD-9 Diagnosis Codes: 6117* (signs and symptoms of breast problem) ICD-10 Diagnosis Codes: N63** (unspecified lump in breast); N645*(other symptoms in breast) |
| Abnormal Breast Image |
ICD-9 Diagnosis Codes: 79380, 79381, 79389 (nonspecific abnormal findings on radiological and other examination of breast) Note: DO NOT use 79382, as this corresponds to dense breasts ICD-10 Diagnosis Codes: R920, R921, R928 (abnormal and inconclusive findings on diagnostic imaging of breast) Note: DO NOT use R922, as this corresponds to dense breasts |
| Diagnostic Mammogram |
CPT: 76082, 76090, 76091, 77055, 77056, 77065, 77066, 77051 HCPCS: G0204, G0206, G0205, G0207, G0236, G0205, G0207, G0236 |
| Chemotherapy for Breast Cancer |
CPT: 96400, 96405, 96406, 96413, 96415, 96520, 96408, 96409, 96410, 96411, 96412, 96414, 96416, 96417, 96420, 96422, 96423, 96425, 96440, 96445, 96450, 96530, 96542, 96545, 96549 (chemotherapy administration) HCPCS: C9280, J8520, J8521, J8530, J8540, J9000, J9001, J9025, J9035, J9045, J9060, J9062, J9070, J9080, J9090–7, J9170, J9171, J9190, J9201, J9207, J9208, J9211, J9260, J9264, J9265, J9355, J9390, J9395, Q0083–5, Q0163, Q0164, Q0166, Q0167, Q0169, Q0173, Q0174, Q0175, Q0177, Q0180, Q0181, X7052, X7624, X7646 (various chemotherapeutic agents) ICD-9 Procedure codes: 8592, 9925 (injection or infusion of cancer chemotherapeutic substance) |
| Chemotherapy for Breast Cancer (continued) |
ICD-10 Procedure codes: 3E00XGC, 3E0(3, 4, 5, 6, 9, B, C, D, R, S, or U)** 05 (antineoplastic), XW03351, XW033B3, XW033C3, XW04351, XW043B3, XW043C3 (new antineoplastic technologies) NDC Codes: available from authors upon request. Note: Require active breast cancer diagnosis (carcinoma in situ ICD9/10 233.**; D05.** or malignant breast neoplasm ICD9/10 174.**; C50.**) within 7 days to exclude treatment for other cancers |
Appendix Table 2.
Characteristics of BRCA-positive Women and Women with Prior Breast Cancer Enrolled in 2016a
| Subgroup | ||||
|---|---|---|---|---|
| All Women with BRCA Mutation | Women with BRCA Mutation and without Prior Breast Cancer | Women with BRCA Mutation and with Prior Breast Cancer | Women without BRCA Mutation and with Prior Breast Cancer | |
| Characteristics | N(%) | N (%) | N (%) | N (%) |
| Total population | 6,953 | 4,565 | 2,745 | 42,790 |
| Age, years(1%) | ||||
| 20–29 | 413 (6%) | 397 (9%) | 25 (1%) | 87 (<1%) |
| 30–39 | 1,320 (19%) | 1,064 (23%) | 313 (11%) | 1,159 (3%) |
| 40–49 | 2,234 (32%) | 1,405 (31%) | 968 (35%) | 7,721 (18%) |
| 50–64 | 2,986 (43%) | 1,699 (37%) | 1,439 (53%) | 33,823 (79%) |
| Race/ethnicity b | ||||
| White neighborhood | 4,301 (62%) | 2,804 (61%) | 1,703 (62%) | 25,836 (60%) |
| Black neighborhood | 62 (<1%) | 34 (1%) | 30 (1%) | 819 (2%) |
| Mixed neighborhood | 1,460 (21%) | 960 (21%) | 583 (21%) | 9,265 (22%) |
| Hispanic surname | 462 (7%) | 328 (7%) | 157 (6%) | 3,076 (7%) |
| Asian surname | 192 (3%) | 118 (3%) | 86 (3%) | 1,345 (3%) |
| Missing | 476 (7%) | 321 (7%) | 186 (7%) | 2,449 (6%) |
| Region of US | ||||
| West | 1,560 (22%) | 1,045 (23%) | 600 (22%) | 8,886 (21%) |
| South | 2,636 (38%) | 1,731 (38%) | 1,035 (38%) | 17,996 (42%) |
| Midwest | 1,860 (27%) | 1,200 (26%) | 766 (28%) | 11,284 (26%) |
| Northeast | 888 (13%) | 582 (13%) | 342 (12%) | 4,511 (11%) |
| Missing | 9 (<1%) | 7 (<1%) | 2 (<1%) | 113 (<1%) |
| Neighborhood poverty c | ||||
| <10% | 4,048 (58%) | 2,611 (57%) | 1,630 (59%) | 23,266 (55%) |
| ≥10% | 2,365 (34%) | 1,583 (35%) | 909 (33%) | 16,779 (39%) |
| Missing | 540 (8%) | 371 (8%) | 206 (8%) | 2,745 (6%) |
Optum’s de-identified Clinformatics® Data Mart Database (Eden Prairie, MN)
White and Black neighborhood defined using the 2008–2012 American Community Survey as a census tract with ≥75% of residents being one race; Mixed neighborhoods defined as census tracts with no majority race; Hispanic and Asian defined using surname analysis.
Poverty levels defined using the 2008–2012 American Community Survey at the census tract level.
Appendix Table 3.
Screening MRI Trends in Women 20–64 years old in women with BRCA mutation overall.
| % Populationa | % with MRI | % all MRIs | MRI Rate per 10,000 | Average Percent Change Per Year (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2008 | 2016 | 2006– 2016 | 2006 –2008 | 2008–2016 | ||||
| All Women with BRCA Mutation | <1% | 23% | 13% | 313.5 | 663.8 | 1307.0 | 14% (10,19%) | 46% (17,82%) | 8% (5,10%) |
| 20–29 years | <1% | 13% | 1% | 88.4 | 351.2 | 1099.0 | 31% (16, 47%) | 101% (2, 295%) | 17% (9,26%) |
| 30–39 years | <1% | 15% | 2% | 253.0 | 681.1 | 1278.2 | 18% (9,28%) | 64% (3,159%) | 9% (4,15%) |
| 40–49 years | <1% | 18% | 4% | 261.1 | 657.7 | 1201.2 | 17% (11, 23%) | 68 (25, 126%) | 6% (3, 10%) |
| 50–64 years | <1% | 23% | 6% | 410.6 | 704.0 | 1427.7 | 10% (7, 13%) | 10% (7, 13%) | 10% (7, 13%) |
Age group percentages do not add up to totals since this is a rolling cohort of women.
Indicates a non-statistically significant change in rate, with the 95% confidence interval including zero.
Footnotes
Conflict of Interest Disclosures: Dr. Karla Kerlikowske reports being an unpaid consultant for the STRIVE study conducted by GRAIL. Dr. Janie Lee reports research funding and consulting fees from GE Healthcare. All other authors have no conflicts of interest to declare.
Data Accessibility:
The data that support the findings of this study are not available due limitations with contracting. Additional inquiries should be directed to senior author, NKS.
REFERENCES
- 1.Bevers TB, Helvie M, Bonaccio E, et al. NCCN Guidelines Version 1.2019: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network; 17 May 2019. [Google Scholar]
- 2.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 3.Daly MB, Pilarski R, Berry MP, et al. NCCN Guidelines Version 3.2019 Genetic/Familial High-Risk Assessment: Breast and Ovarian. National Comprehensive Cancer Network; 18 January 2019. [Google Scholar]
- 4.Warner E Screening BRCA1 and BRCA2 Mutation Carriers for Breast Cancer. Cancers. 2018;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wernli KJ, DeMartini WB, Ichikawa L, et al. Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med. 2014;174(1):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buist DSM, Abraham L, Lee CI, et al. Breast biopsy intensity and findings following breast cancer screening in women with and without a personal history of breast cancer. JAMA Intern Med. 2018;178(4):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA Intern Med. 2014;174(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz A, Percy C, Jack A, et al. , eds. International Classification of Diseases for Oncology (ICD-O), Third Edition. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 9.World Health Organization. International Classification of Diseases (ICD-10). 1994; http://www.who.int/classifications/en/. Accessed January 18, 2013.
- 10.Wharam JF, Zhang F, Wallace J, et al. Vulnerable and less vulnerable women in high-deductible health plans experienced delayed breast cancer care. Health Aff (Millwood). 2019;38(3):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leopold C, Wagner AK, Zhang F, et al. Total and out-of-pocket expenditures among women with metastatic breast cancer in low-deductible versus high-deductible health plans. Breast Cancer Res Treat. 2018;171(2):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HCPCS: Medicare’s National Level II Codes. Chicago: American Medical Association; 2008. [Google Scholar]
- 13.Beebe M CPT 2009: Current Procedural Terminology. Chicago: American Medical Association; 2008. [Google Scholar]
- 14.Statistical Analysis System (SAS) OnlineDoc, Version 9.4 [computer program]. Cary, NC: SAS Institute Inc. [Google Scholar]
- 15.Statistical Methodology and Applications Branch Surveillance Research Program National Cancer Institute. Joinpoint Regression Program, Version 4.7.0.0. 2019. [Google Scholar]
- 16.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 17.Chiarelli AM, Blackmore KM, Muradali D, et al. Performance measures of magnetic resonance imaging plus mammography in the High Risk Ontario Breast Screening Program. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. [DOI] [PubMed] [Google Scholar]
- 19.Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill DA, Haas JS, Wellman R, et al. Utilization of breast cancer screening with magnetic resonance imaging in community practice. J Gen Intern Med. 2018;33(3):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breat cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408–414. [DOI] [PubMed] [Google Scholar]
- 22.Cho N, Han W, Han BK, et al. Breast cancer screening with mammography plus ultrasonography or Magnetic Resonance Imaging in women 50 years or younger at diagnosis and treated with breast conservation therapy. JAMA Oncol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sippo DA, Burk KS, Mercaldo SF, et al. Performance of screening breast MRI across women with different elevated breast cancer risk indications. Radiology. 2019;292(1):51–59. [DOI] [PubMed] [Google Scholar]
- 24.Wernli K, Ichikawa L, Kerlikowske K, et al. Surveillance breast MRI and mammography performance measures in women with a personal history of breast cancer. Radiology. 2019;292(2):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Genetic Information Nondiscrimination Act. United States of America: U.S. Equal Employment Opportunity Commission; 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not available due limitations with contracting. Additional inquiries should be directed to senior author, NKS.


