Abstract
Background
Today, a suitable vaccine has not yet been discovered to prevent Toxoplasma gondii infection. Therefore, prophylaxis can be suggested as the preferred approach to prevent toxoplasmosis. This study aims to evaluate the prophylactic effects of synthesized zinc nanoparticles (ZnNPs) using Lavandula angustifolia Vera., by microwave method on chronic toxoplasmosis in mice.
Methods
BALB/c Mice orally administrated with ZnNPs the doses of 32.5, 75, 150 mg/kg/day for two weeks. On the 15th day, the mice were intraperitoneally infected with the Tehran strain of T. gondii (25 tissue cysts). The mean diameter and the numbers of brain tissue cysts, as well as the mRNA levels of inducible nitric oxide synthesize (iNOs), and interferon-gamma (IFN-γ) in mice of each experimental group were evaluated.
Results
The synthesized ZnNPs represent a spherical form with a size ranging from 30 to 80 nm. The results revealed that oral administration of Zn NPs at the doses of 32.5 (p < 0.001) and 75 mg/kg/day (p < 0.001) for 14 days significantly reduced the mean number and diameter of the brain tissue cysts in tested mice. No T. gondii tissue cyst was observed after oral administration of Zn NPs at the doses of 150 mg/kg. Based on the results of Real-time PCR analysis, the expression level of IFN-γ and iNOs was significantly increased (p < 0.001) in mice treated with 32.5, 75, 150 mg/kg/day for two weeks.
Conclusion
The obtained findings of the current investigation exhibit the significant prophylactic effects of ZnNPs against chronic toxoplasmosis in mice; so that oral administration of ZnNPs the doses 32.5, 75, 150 mg/kg reduced the parasite load and even completely controlled the infection in mice. The results show that the ZnNPs had strengthened the innate immune system which could be the reason for its strong prophylactic effects. However, further in vivo and clinical investigations are required to confirm these results as well as other possible mechanisms that can trigger these pharmacological properties.
Keywords: Latent toxoplasmosis, Nanomedicine, Toxoplasmosis, Mice, mRNA, In vivo
1. Introduction
Toxoplasmosis is one of the most common parasitic diseases in the world that has high medical and veterinary importance. The infection caused by Toxoplasma gondii (T. gondii), an intracellular parasite, which has infected about a third of the world's population and approximately all warm-blooded animals (Saadatnia and Golkar, 2012). Routinely, human become infected through eating not well-cooked meat infected with tissue cysts of T. gondii, (ii) eating and drinking of food and water contaminated with sporulated oocysts expelled in feces of the cat as the definitive host, (iii) fetal infection through congenital transmission during maternal pregnancy with one of the above routs, and (iv) transmission of infection through miscellaneous routes such as organ transplantation and blood transfusion (Dubey, 1998, Attias et al., 2020). Although, toxoplasmosis commonly causes no apparent clinical signs in immunocompetent people; nonetheless, acute and deadly clinical forms may be reported in immunocompromised people (such as organ recipients, patients, patients with immunodeficiency, etc.) and infants who are congenitally infected (Weiss and Dubey, 2009).
Although there is limited chemotherapy for toxoplasmosis, however, the combination of pyrimethamine and sulfadiazine as well as other synthetic drugs such as spiramycin, atovaquone, clindamycin, azithromycin, etc., are recommended as the desired options for the treatment of toxoplasmosis (Innes, 2010 Feb, Wei et al., 2015). Recent reports have revealed that existing chemotherapy is accompanying by unwanted side effects such as teratogenic effects, osteoporosis, impaired liver function, and suppression of bone marrow activity especially in patients with defective immune systems (Pearson et al., 1999, Montazeri et al., 2018). Since a suitable vaccine has not yet been discovered to prevent this infection, prophylaxis can thus be suggested as the preferred approach to prevent the toxoplasmosis, particularly in pregnant women who were not formerly found to be seronegative for toxoplasmosis and immunocompromised patients with a CD4 < 100 cells/μL (Rajapakse et al., 2017, Derouin and Pelloux, 2008).
Nanotechnology is the recognition of the properties of the smallest components of materials and the use of these properties to create new technologies or improve the quality of previous technologies (Bhattacharyya et al., 2009 Sep). Nanotechnology has had a significant impact on prevention (medical technology), high stability and compatibility, as well as low toxicity of nanoparticles, leading to their use as drug delivery systems, bio-systems, and biosensors (Keskinbora and Jameel, 2018).
Nanomedicine is the application of nanoparticle technology in the prevention and treatment of diseases in the human body. This science has the ability to completely transform medical science. Nanomedical applications include diagnostic tests, chemotherapy, insulin pumps, needleless injections, hearing aids, various medical sensors, and drug delivery systems in body tissues (Emerich and Thanos, 2003, Mirza and Siddiqui, 2014).
In material sciences, “green synthesis” has been considered as a reliable, sustainable, and environmentally friendly method that is used to make a wide range of materials including nanomaterials, metal oxides, composites, and biological materials. Sequence, green synthesis is an important tool to reduce the destructive effects associated with synthesis methods for nanoparticles that are commonly used in laboratories and industry (Jahangirian et al., 2017). Plants are widely used in synthesis due to their compatibility with the environment and due to their characteristics such as abundance and lack of special conditions and nutrients for growth, they are a suitable option for the production of nanoparticles biologically (Sathishkumar et al., 2018).
Microwaves are a region of electromagnetic waves that propagate in the frequency range of 300 MHz to 30 GHz. These waves are most used in the field of the heating process (Heating). In 1946, the effective interaction of microwave waves with various materials and melting chocolate using these waves was known and was known as microwave heating (Microwave Heating). Today, providing safe, fast, easy, and cost-effective methods for the synthesis of various nanomaterials with specific and controllable size and structure is of particular importance (Mohammadnezhad and Dizaji, 2013). One of the most important of these methods, which has been broadly applied in the production of some nanomaterials, is the microwave heating technique, which according to some features such as uniform and specific heat spreading, superheating, selective or selective heating, elevating reaction speed and accordingly, decreasing the time and energy needed to perform synthesis has become progressively applied in the production of a number of nanomaterials (Xiang and Chen, 2019).
After iron, zinc (Zn) is the second most common element in the human body, which is involved in the activation of more than 300 enzymes that regulate macromolecule biosynthesis in DNA, RNA, proteins, as well as cell growth and proliferation, and other types of metabolism (Roohani et al., 2013). Zn deficiency leads to reduced growth, anorexia, immune system disorders, increased oxidative stress, skin reactions, delayed ulcers and decreased reproductive capacity (Hambidge, 2000). Zn affects the production of anti-inflammatory cytokines such as interleukins of IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α). It is also involved in the identification of natural killer cells (NK cells) from major histocompatibility complex (MHC) class 1 primary cells. Zinc (Zn) is also important for the balance between different T cell subsets (Hojyo and Fukada, 2016). Zn-nanoparticles (ZnNPs) depending on some properties such as preparation method, size of the particle, concentration, pH, time, and biocompatibility represents a wide range of biological and pharmacological properties such as improving the immune system, use in drug delivery, antimicrobial effects, etc (Agarwal et al., 2017).
Considering the importance of toxoplasmosis in the field of human infectious diseases and conducting numerous researches in the field of preparation of drugs for prevention and treatment of toxoplasmosis, especially in immunocompromised patients, mothers during pregnancy and infants, we decided to evaluate the prophylactic effects of synthesized ZnNPs using Lavandula angustifolia (Lamiaceae family), flowering herbs with a wide range of pharmacological possessions such as anti-oxidant, anticonvulsant, anti-inflammatory, anxiolytic, and antimicrobial activities (Prusinowska and Śmigielski, 2014), by microwave method on chronic toxoplasmosis in mice.
2. Materials and methods
2.1. Preparation of biogenic ZnNPs
Aerial parts of L. angustifolia were was collected from the rural areas of Kerman, Iran during September 2019. The materials were extracted and prepared by means of a percolation system using 80% methanol for 72 h at the 21 °C. Preparation of biogenic ZnNPs performed based on the method explained elsewhere (Salari et al., 2017). In brief, 10 ml of L. angustifolia methanolic extract (LAME) at the concentration of 5 mg/ml was added to 40 ml of ZnSO4 solution (Merck Chemicals, Darmstadt, Germany). In the next step, this combination was exposed to microwave in a microwave oven (850 W, 60 s) to decrease the ions of metal; whereas the creation of a dark gray color indicated the production of Zn NPs. After washing the produced NPs by chloroform, ethyl alcohol, and distilled water, respectively, it was characterized by means of various approaches.
2.2. Characterization of biogenic ZnNPs
2.2.1. UV–Vis spectrum analysis
The UV–visible spectrum of Zn NPs was determined (200–600 nm) by means of a spectrophotometer (JENWAY 6405). By means of an X-ray diffractometer (Philips, PW1710) equipped with CuK radiation in the 2θ ranging from 0 to 80o we determined the crystalline structure of the synthesized Zn NPs.
2.2.2. Scanning electron microscope (SEM) analysis
By an SEM apparatus (KYKY-EM3200) armed with an energy dispersive X-ray (EDX) microanalyzer, the surface and elemental structure of the synthesized Zn NPs were determined. The pattern of the related size scattering of synthesized NPs was measured via calculating 400 single particles from various SEM pictures.
2.2.3. Fourier transform infrared spectroscopy
We also used an FTIR spectrophotometer (Shimadzu IR-470, Japan) at a resolution of 40 mm in the potassium bromide disks to evaluate the surface chemistry of biogenic Zn NPs.
2.3. Prophylactic effects of chronic toxoplasmosis
2.3.1. Animals
In this study, we used 48 male BALB/c mice (6–8 weeks old) weighing from 20 to 25 g. Animals were kept in a colony room with a 12:12 h light/ dark cycle at 21 ± 2 °C and handled based on the standard protocols for the use of laboratory animals.
3. Ethics
This study was carried out in strict accordance with the recommendations of the Guide for Care and Use of Lab-oratory Animals of National Institutes of Health. The protocol was approved by the Committee on Ethics of Animal Experiments, Lorestan University of Medical Sciences (LUMS.REC.1399.309). The study complied with the Helsinki Declaration.
3.0.1. Parasite
Tehran strain of T. gondii (type II) was used in this study to establish chronic toxoplasmosis. The parasite was prepared by intraperitoneal inoculation of tissue cysts of T. gondii (15–20 cysts) from brain tissue of infected BALB/c mice every 3 months into new mice.
3.0.2. Animal model of chronic toxoplasmosis
The mice model of latent toxoplasmosis was induced based on the process reported elsewhere (Mahmoudvand et al., 2015). For this purpose, a homogenized suspension (0.5 ml) from the brains of infected mice containing 25 to 30 T. gondii tissue cysts with penicillin and streptomycin antibiotics was injected intraperitoneally into mice of any test group.
3.0.3. Confirmation of infection
Confirmation of toxoplasmosis was checked by the determination of anti-T. gondii IgG antibody in the serum samples of tested mice using a modified agglutination test (MAT) kit (Toxo screen DA, Biomérieux, Lyon, France). The agglutination titer of 1/20 ≤ was positive and was end-titrated by 2 fold dilutions.
3.0.4. Study design
Fig. 1 shows the study design of the present study, animals were divided into two main groups (control and experimental group) with six sub-groups include: C1 (non-treated non infected); C2 (treated with normal saline); C3 (infected mice treated with Atovaquone 100 mg/kg/day); Ex1 (treated with ZnNPs 32.5 mg/kg/day); Ex2 (treated with ZnNPs 75 mg/kg/day); and Ex3 (treated with ZnNPs 150 mg/kg/day). After 14 days of oral administration, the mice in all groups except the C1 group were infected with the T. gondii.
Fig. 1.
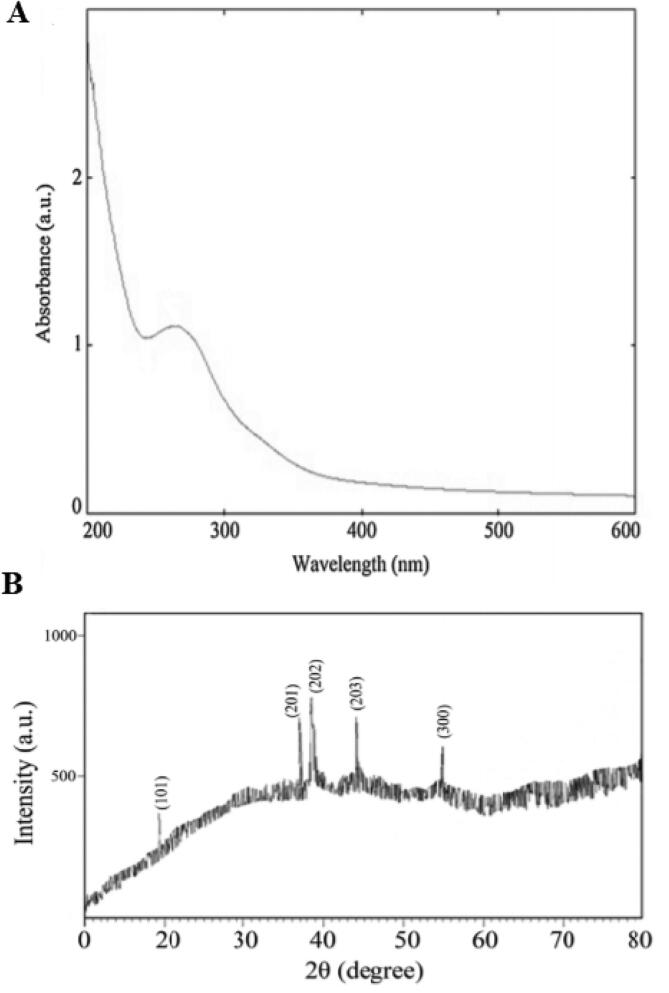
(A) UV–visible spectrum and (B) X-ray diffraction patterns of the Zn NPs synthesized using L. angustifolia Vera. extract by microwave method.
3.0.5. Sample collection
Primarily, animals were completely anesthetized by intraperitoneal administration of ketamine (150 mg/kg) and xylazine (10 mg/kg). Immediately after decapitation of animals, whole-brain tissues of mice were aseptically collected. The right brain hemisphere was used for parasitological investigations; while the left hemisphere was kept at −80 °C to use for the molecular tests.
3.0.6. Anti-parasitic effects of ZnNPs
Anti-parasitic effects of ZnNPs were evaluated by assessment of the unstained smears provided from the right brain hemisphere of each mouse. Then, the diameter and the number of tissue cysts were recorded at two magnifications of 100 × and 400 × by means of light microscopy (Shaapan et al., 2021).
3.0.7. Induction of innate immune system
In this survey, we evaluate the mRNA levels of some effective immune mediators (inducible nitric oxide synthesize, iNOs) and cytokines (interferon-gamma, IFN-γ) in controlling T. gondii infection in all tested mice by means of quantitative real-time PCR. After extraction of the total brain RNA by the RNA-easy kits (Qiagen, Hilden, Germany); they were reverse transcribed based on the manufacturer’s instructions. In the next step, the obtained complementary DNA (cDNA) was used for conventional PCR amplification or real-time PCR. All amplification products were determined by SYBR green (Ha et al., 2010). The reaction conditions of real-time PCR were included initial denaturation at 95 °C for 10 min, 40 amplification cycles [denaturation at 95 °C for 10 s, annealing at 56 °C for 30 s, and elongation at 72 °C for 30 s], followed by one cycle at 72 °C for 5 min. The iQTM5 optical system software (Bio-Rad, Hercules, CA) was applied to data analysis. β-actin was used as a housekeeping gene and normalization control. Table 1 exhibits oligonucleotide primers applied for real-time PCR.
3.1. Statistical analysis
By means of SPSS statistical package version, 22.0 (SPSS Inc., Chicago, IL, USA) data analysis was performed. One-way ANOVA with Turkey’s potshot test was used to assess differences between experimental groups.
4. Results
4.1. UV–Vis spectrum analysis
Fig. 1A shows the confirmation of the Zn NPs synthesis using UV–vis spectral analysis; which demonstrated an absorption peak in the range of 230–330 nm. Fig. 3 exhibited the analysis of the EDX of NPs; indicating that Zn absorption peaks including ZnL˛1, ZnK˛1, and ZnKˇ1 at 1.01, 8.64, and 9.57 keV, respectively. The XRD pattern was shown in Fig. 1B, the results indicated the 101, 201, 202, 203, and 300 refraction peaks at 19.6◦, 36.9◦, 39, 43.9◦, and 54.8◦, respectively.
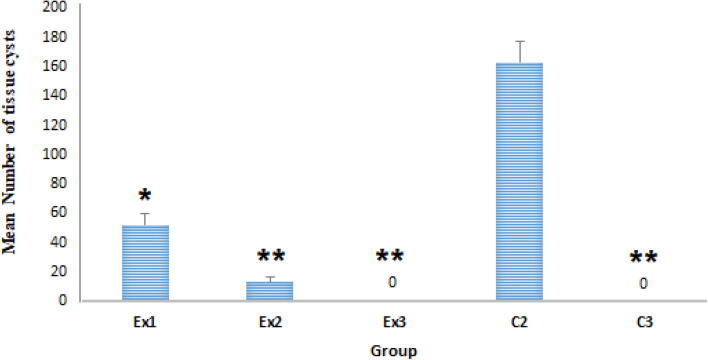
Fig. 3.
The results revealed that oral administration of Zn NPs at the doses of 32.5 and 75 mg/kg/day for 14 days reduced the mean number of the brain tissue cysts in tested mice. No T. gondii tissue cyst was observed after oral administration of Zn NPs at the doses of 150 mg/kg. Ex1 (32.5 mg/kg), Ex2 (75 mg/kg), and Ex3 (150 mg/kg), C2 (treated with normal saline), C3 (treated with atovaquone 100 mg/kg/day), * p < 0.05, ** p < 0.001.
4.2. SEM and FTIR analysis
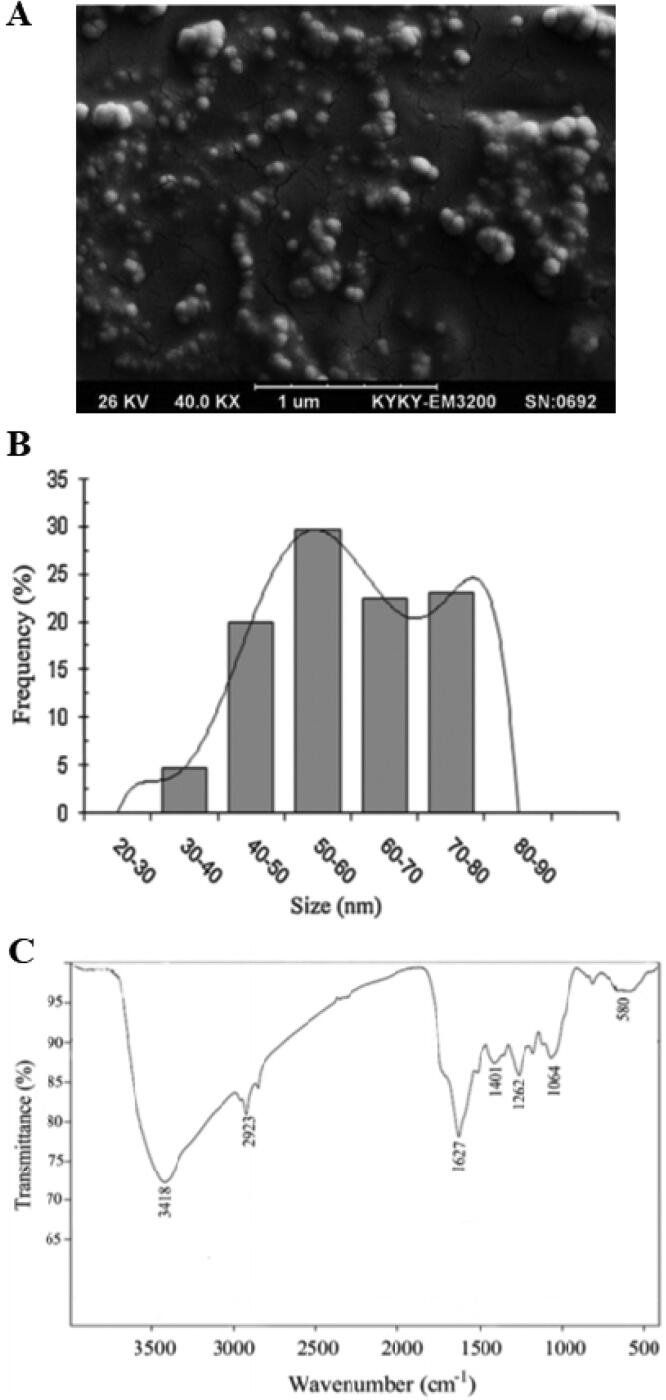
Based on the results of SEM analysis, the synthesized ZnNPs represent a spherical form with some aggregates of different lengths (Fig. 2A). Considering the size of the synthesized NPs, the results showed that the size of Zn NPs ranged from 30 to 80 nm; while the majority of NPs were between 50 and 60 nm (Fig. 2B). Based on the results of the FTIR analysis, the synthesized Zn NPs demonstrated the peaks at 1407, 1262, 1064, and 580 cm−1 that could be related to C C, C N, C N, and C– (F, Cl, or Br) moieties, respectively (Fig. 2C). The highest absorption peaks at 1627 cm-1, 3418 cm − 1, and 2923 cm-1 could cause by the polypeptide amide bond-1, O H stretching of the phenolic bond, and O H stretching of the carboxylic acid, respectively.
Fig. 2.
(A) SEM micrograph, (B) particle size distribution, and (C) the FTIR spectrum of the Zn NPs synthesized using L. angustifolia Vera. extract by microwave method.
4.3. The mean number of T. Gondii tissue cysts
The mean number of the T. gondii tissue cysts in mice treated with Zn NPs was shown in Fig. 3. The results revealed that oral administration of Zn NPs at the doses of 32.5 (p < 0.001) and 75 mg/kg/day (p < 0.001) for 14 days significantly reduced the mean number of the brain tissue cysts in tested mice when compared with the control group (C2). The findings also exhibited that similar to a positive control (C3), no T. gondii tissue cyst was observed after oral administration of Zn NPs at the doses of 150 mg/kg for 14 days in mice of the tested group of Ex3.
4.4. The mean diameter of T. Gondii tissue cysts
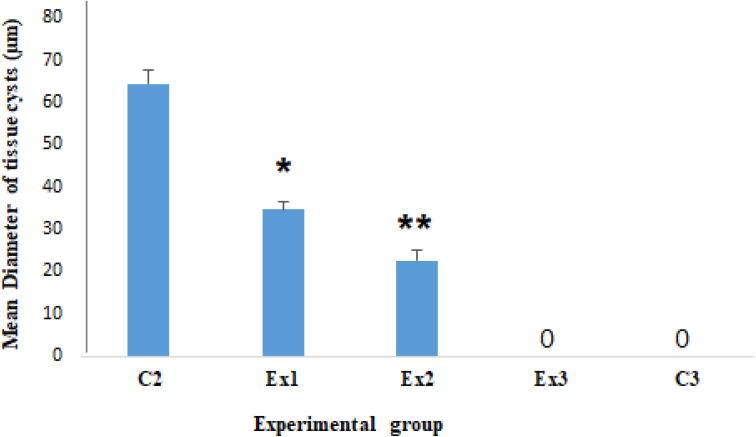
Fig. 4 shows the mean diameter of T. gondii tissue cysts in mice of all tested groups. The findings revealed that after oral administration of Zn NPs at the doses of 32.5 and 75 mg/kg/day for 14 days the mean diameter of T. gondii tissue cysts significantly (p < 0.001) reduced 46.3% and 64.9% in mice of tested groups of Ex1 and Ex2, respectively.
Fig. 4.
The findings revealed that after oral administration of Zn NPs at the doses of 32.5 and 75 mg/kg/day for 14 days the mean diameter of T. gondii tissue cysts reduced 46.3% and 64.9% in mice of tested groups, respectively. Ex1 (32.5 mg/kg), Ex2 (75 mg/kg), and Ex3 (150 mg/kg), C2 (treated with normal saline), C3 (treated with atovaquone 100 mg/kg/day). * p < 0.05, ** p < 0.001.
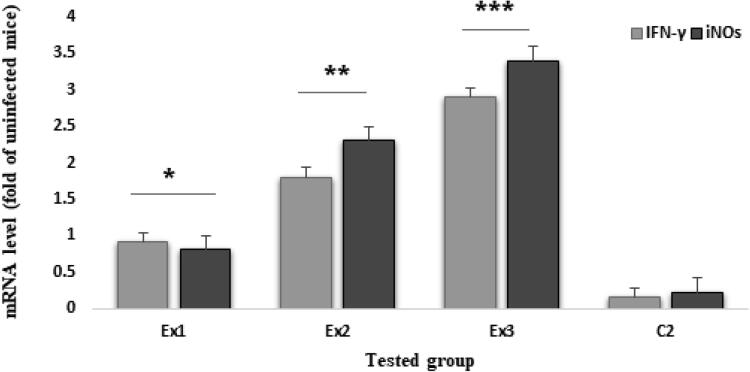
4.5. The expression level of cytokines by Real-Time PCR
Fig. 5 exhibited the expression level of IFN-γ and iNOs in the tested mice after oral administration of Zn NPs at the doses of 32.5, 75, and 150 mg/kg for 14 days. Based on the results of Real-time PCR analysis, the expression level of IFN-γ and iNOs was significantly increased (p < 0.001) in mice of the tested groups Ex1, Ex2, and Ex3 in comparison with control groups.
Fig. 5.
Based on the results of Real-time PCR analysis, the expression level of IFN-γ and iNOs was significantly increased (p < 0.001) in mice of the tested groups Ex1 (32.5 mg/kg), Ex2 (75 mg/kg), and Ex3 (150 mg/kg) in comparison with control groups.
5. Discussion
Today, the considerable worldwide prevalence of human toxoplasmosis and the broad spectrum of clinical symptoms linked to this infection make toxoplasmosis one of the major health problems with an expected underestimated impact (Saadatnia and Golkar, 2012, Dubey, 1998). In recent years, despite many efforts by researchers to develop an effective vaccine to prevent and interrupt the parasite transmission cycle in humans and animals, however, no highly effective vaccine has been discovered. Moreover, reviews have demonstrated that a wide range of medicinal herbs have been evaluated in the prevention and treatment of T. gondii both in vitro and in vivo; whereas the most of the investigations were performed on Artemisia annua and Glycyrrhiza glabra extracts (Sharif et al., 2016). Based on what was said, prophylactic strategies are strongly recommended especially for high-risk individuals such as transplant recipients, HIV-infected patients, pregnant women, whose symptoms of toxoplasmosis can be very dangerous and even fatal (Zhang et al., 2013). Nowadays, it has been proven that the existing chemotherapy regimens for treatment and prophylaxis toxoplasmosis are associated with adverse side effects and responses such as teratogenic effects, hematological disorders, myelosuppression, and gastrointestinal effects, etc (Innes, 2010 Feb, Wei et al., 2015, Pearson et al., 1999, Montazeri et al., 2018); hence, the discovery of novel effective agents especially with immune-boosting mechanisms seems very necessary.
The obtained results demonstrated that oral administration of Zn NPs at the doses of 32.5 (p < 0.001) and 75 (p < 0.001) for 21 days significantly reduced the mean number of the brain tissue cysts in tested mice when compared with the control group (C2). The findings also exhibited that similar to a positive control (C3), no T. gondii tissue cyst was observed after oral administration of Zn NPs at the doses of 150 mg/kg for 14 days in mice of the tested group of E3. The findings revealed that after oral administration of Zn NPs at the doses of 32.5 and 75 mg/kg for 21 days significantly (p < 0.001) reduced the mean diameter of T. gondii tissue cysts 46.3% and 64.9% in mice of tested groups of Ex1 and Ex2, respectively.
Considering the antimicrobial effects, previous studies demonstrated the high potent antimicrobial activity of these NPs against both Gram-positive and Gram-negative bacteria (e.g. Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and Enterococcus faecalis, Escherichia coli, Campylobacter jejuni), H1N1 influenza virus, Candida spp., Aspergillus spp., Fusarium spp., Trichophyton mentagrophytes, Microsporum canis, etc (Sirelkhatim et al., 2015, Xie et al., 2011, Ghaffari et al., 2019, Sun et al., 2018).
By anti-parasitic effects of ZnNPs, Dkhil et al (Dkhil et al., 2015) have demonstrated that oral administration of 10 mg/kg/day ZNPs for 5 days, significantly reduced the oocyst shedding and inflammatory injury in the jejunum of mice infected with Eimeria papillata infection (Dkhil et al., 2015). (Nazir et al., 2019) have revealed that ZnO-based nano-formulations significantly inhibited the growth rate of Leishmania tropica promastigotes with IC50 values ranging from 0.012 to 0.084 µg/mL; they also reported that the inhibition of the parasite growth was because of the production of reactive oxygen species (ROS) by these NPs (Nazir et al., 2019). Recently, Jan et al (Jan et al., 2020) have shown that biogenic synthesis ZnO NPs in a dose-dependent manner significantly decreased the growth rate promastigote and amastigote forms of L. tropica with IC50 value of 48 µg/mL and 51 µg/mL, respectively. In a study conducted by Esmaeilnejad et al (Esmaeilnejad et al., 2018), the results demonstrated the wormicidal effect of ZnO NPs at the dose of 16 ppm; so that entirely killed the Haemonchus contortus worm after 16 h exposure through oxidative/nitrosative damages to biomolecules of the worm (Esmaeilnejad et al., 2018). Previous studies exhibited that these nanoparticles probably show their antimicrobial effects through two main mechanisms including (i) direct effects by impaired cell permeability, inhibit cell growth, and induction of apoptosis; (ii) indirect effects by producing oxidative stress via the H2O2 production and the zinc ions release in the environment which enables their penetration of the cell wall and acting their toxicity effects (Khashan et al., 2020, Sasai et al., 2018).
Interferon-gamma is considered as a key factor involved in both innate and adaptive immunity (Yarovinsky, 2014). Increased production of this cytokine in various ways, including increased expression level of iNOs, ROS, and NO production, and inhibition of intracellular tryptophan can lead to potential antimicrobial responses especially against toxoplasmosis (Chandrasekar et al., 2015). Previous studies revealed that these cytokine and mediators such as NO are considered as the valuable tools for evaluation of immune system in mice (Shaapan et al., 2021, Salimi et al., 2019).
Here, we evaluate the expression level of iNOs and IFN-γ in all tested mice treated by various doses of ZnNPs by means of quantitative real-time PCR. Based on the results of real-time PCR analysis, the expression level of IFN-γ and iNOs was significantly increased (p < 0.001) in mice of receiving 32.5, 75, and 150 mg/kg of ZnNPs for 14 days in comparison with control groups. These results suggested that the decrease in parasite load (tissue cysts) in the infected mice treated with ZnNPs for 14 days may be attributed to the improvement of the immune system, mainly the innate immune system, which causes the control of toxoplasmosis.
With respect to the toxicity of ZnNPs, previously Salimi et al [44] have demonstrated that no significant toxicity on hematological and serum biochemical parameters was observed after oral administration of biogenic ZnNPs especially at the doses 1, and 2 g/kg/day for 14 days; representing that oral administration of ZnNPs for 14 days at the lower doses such as the doses 325, 750, 1500 mg/kg has no toxicity in BALB/c mice.
6. Conclusion
The obtained findings of the current investigation revealed the considering prophylactic effects of ZnNPs against mice model chronic toxoplasmosis; so that oral administration of ZnNPs the doses 32.5, 75, 150 mg/kg reduced the parasite load and even completely controlled the infection in mice. We also found that the ZnNPs had strengthened the innate immune system which could be the reason for its strong prophylactic effects. However, further investigations are required to confirm these results as well as other possible mechanisms that can trigger these pharmacological properties.
Funding
This research received no external funding.
8. Data availability statement
All data generated or analyzed during this study are included in this published article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Asghar Sepahvand, Email: fungimed44@yahoo.com.
Hossein Mahmoudvand, Email: dmahmodvand@gmail.com.
References
- Saadatnia G., Golkar M. A review on human toxoplasmosis. Scand. J. Infect. Dis. 2012;44(11):805–814. doi: 10.3109/00365548.2012.693197. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 1998;28(7):1019–1024. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Attias M., Teixeira D.E., Benchimol M., Vommaro R.C., Crepaldi P.H., De Souza W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors. 2020;13(1) doi: 10.1186/s13071-020-04445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L.M., Dubey J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009;39(8):895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes E.A. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health. 2010;57(1) doi: 10.1111/j.1863-2378.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- Wei H.X., Wei S.S., Lindsay D.S., Peng H.J. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0138204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson P.A., Piracha A.R., Sen H.A., Jaffe G.J. Atovaquone for the treatment of toxoplasma retinochoroiditis in immunocompetent patients. Ophthalmology. 1999;106(1):148–153. doi: 10.1016/S0161-6420(99)90021-0. [DOI] [PubMed] [Google Scholar]
- Montazeri M., Mehrzadi S., Sharif M., Sarvi S., Tanzifi A., Aghayan S.A., Daryani A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018;9:2587. doi: 10.3389/fmicb.2018.02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse S, Weeratunga P, Rodrigo C, de Silva NL, Fernando SD. Prophylaxis of human toxoplasmosis: a systematic review. Pathog Glob Health. 2017 Oct;111(7):333-342. doi: 10.1080/20477724.2017.1370528. Epub 2017. PMID: 28948861; PMCID: PMC5694886. [DOI] [PMC free article] [PubMed]
- Derouin F., Pelloux H. ESCMID Study Group on Clinical Parasitology. Prevention of toxoplasmosis in transplant patients. Clin. Microbiol. Infect. 2008;14(12):1089–1101. doi: 10.1111/j.1469-0691.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya D., Singh S., Satnalika N., Khandelwal A., Jeon S.H. Nanotechnology, big things from a tiny world: a review. Int. J. u-and e-Service Sci. Technol. 2009;2(3):29–38. [Google Scholar]
- Keskinbora K.H., Jameel M.A. Nanotechnology applications and approaches in medicine: a review. J. Nanosci. Nanotechnol. Res. 2018;2(2):6. [Google Scholar]
- Emerich D.F., Thanos C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003;3(4):655–663. doi: 10.1517/14712598.3.4.655. [DOI] [PubMed] [Google Scholar]
- Mirza A.Z., Siddiqui F.A. Nanomedicine and drug delivery: a mini review. Int. Nano Letters. 2014;4(1):94. [Google Scholar]
- Jahangirian H., Lemraski E.G., Webster T.J., Rafiee-Moghaddam R., Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int. J. Nanomed. 2017;12:2957. doi: 10.2147/IJN.S127683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar P., Gu F.L., Zhan Q., Palvannan T., Yusoff A.R. Flavonoids mediated ‘Green’nanomaterials: A novel nanomedicine system to treat various diseases–Current trends and future perspective. Mater. Lett. 2018;1(210):26–30. [Google Scholar]
- Mohammadnezhad A., Dizaji H.R. Synthesis and characterization of CdS: Zn nanoparticles by microwave irradiation method. J. Appl. Chem. 2013;7(25) [Google Scholar]
- Xiang H., Chen Y. Energy-converting nanomedicine. Small. 2019;15(13):1805339. doi: 10.1002/smll.201805339. [DOI] [PubMed] [Google Scholar]
- Roohani N., Hurrell R., Kelishadi R., Schulin R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013;18(2):144. [PMC free article] [PubMed] [Google Scholar]
- Hambidge M. Human zinc deficiency. J. Nutrition. 2000;130(5):1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- Hojyo S., Fukada T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016;31:2016. doi: 10.1155/2016/6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal H., Kumar S.V., Rajeshkumar S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour.-Effic. Technol. 2017;3(4):406–413. [Google Scholar]
- Prusinowska R., Śmigielski K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review. Herba polonica. 2014;60(2):56–66. [Google Scholar]
- Salari Z., Ameri A., Forootanfar H. Microwave-assisted biosynthesis of zinc nanoparticles and their cytotoxic and antioxidant activity. J. Trace Elem. Med. Biol. 2017;39:116–123. doi: 10.1016/j.jtemb.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H., Ziaali N., Ghazvini H., Shojaee S., Keshavarz H., Esmaeilpour K., Vahid S. Toxoplasma gondii Infection Promotes Neuroinflammation Through Cytokine Networks and Induced Hyperalgesia in BALB/c Mice. Inflammation. 2015;39:405–412. doi: 10.1007/s10753-015-0262-6. [DOI] [PubMed] [Google Scholar]
- Shaapan R.M., Al-Abodi H.R., Alanazi A.D., Abdel-Shafy S., Rashidipour M., Shater A.F., Mahmoudvand H. Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection. Molecules. 2021;26(4):819. doi: 10.3390/molecules26040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S., Hamamura M.J., Nalcioglu O., Muftuler L.T. A PIN diode controlled dual-tuned MRI RF coil and phased array for multi nuclear imaging. Phys. Med. Biol. 2010;55:2589–2600. doi: 10.1088/0031-9155/55/9/011. [DOI] [PubMed] [Google Scholar]
- Sharif M., Sarvi S., Pagheh A.S., Asfaram S., Rahimi M.T., Mehrzadi S., Ahmadpour E., Gholami S., Daryani A. The efficacy of herbal medicines against Toxoplasma gondii during the last 3 decades: a systematic review. Can. J. Physiol. Pharmacol. 2016;94(12):1237–1248. doi: 10.1139/cjpp-2016-0039. [DOI] [PubMed] [Google Scholar]
- Zhang N.Z., Chen J., Wang M., Petersen E., Zhu X.Q. Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev. Vaccines. 2013;12(11):1287–1299. doi: 10.1586/14760584.2013.844652. [DOI] [PubMed] [Google Scholar]
- Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015;7(3):219-242. doi: 10.1007/s40820-015-0040-x. Epub 2015 Apr 19. PMID: 30464967; PMCID: PMC6223899. [DOI] [PMC free article] [PubMed]
- Xie Y., He Y., Irwin P.L., Jin T., Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011;77(7):2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., Pirhajati-Mahabadi V. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26(1):1. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Li J., Le T. Zinc oxide nanoparticle as a novel class of antifungal agents: current advances and future perspectives. J. Agric. Food. Chem. 2018;66(43):11209–11220. doi: 10.1021/acs.jafc.8b03210. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A., Al-Quraishy S., Wahab R. Anticoccidial and antioxidant activities of zinc oxide nanoparticles on Eimeria papillata-induced infection in the jejunum. Int. J. Nanomed. 2015;10:1961. doi: 10.2147/IJN.S79944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir S., Rabbani A., Mehmood K., Maqbool F., Shah G.M., Khan M.F., Sajid M. Antileishmanial activity and cytotoxicity of ZnO-based nano-formulations. Int. J. Nanomed. 2019;14:7809. doi: 10.2147/IJN.S203351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan H., Shah M., Usman H., Khan M.A., Zia M., Hano C., Abbasi B.H. Biogenic Synthesis and Characterization of Antimicrobial and Antiparasitic Zinc Oxide (ZnO) Nanoparticles Using Aqueous Extracts of the Himalayan Columbine (Aquilegia pubiflora) Front. Mater. 2020;7:249. doi: 10.3389/fmats.2020.00249. [DOI] [Google Scholar]
- Esmaeilnejad B., Samiei A., Mirzaei Y., Farhang-Pajuh F. Assessment of oxidative/nitrosative stress biomarkers and DNA damage in Haemonchus contortus, following exposure to zinc oxide nanoparticles. Acta Parasitologica. 2018;63(3):563–571. doi: 10.1515/ap-2018-0065. [DOI] [PubMed] [Google Scholar]
- Khashan K.S., Sulaiman G.M., Hussain S.A. Synthesis, Characterization and Evaluation of Anti-bacterial, Anti-parasitic and Anti-cancer Activities of Aluminum-Doped Zinc Oxide Nanoparticles. J. Inorg. Organomet. Polym. 2020;30:3677–3693. doi: 10.1007/s10904-020-01522-9. [DOI] [Google Scholar]
- Sasai M., Pradipta A., Yamamoto M. Host immune responses to Toxoplasma gondii. Int. Immunol. 2018;30(3):113–119. doi: 10.1093/intimm/dxy004. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014;14(2):109–121. doi: 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B.S., Yadav S., Victor E.S., Majumdar S., Deobagkar-Lele M., Wadhwa N., Podder S., Das M., Nandi D. Interferon-gamma and nitric oxide synthase 2 mediate the aggregation of resident adherent peritoneal exudate cells: implications for the host response to pathogens. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0128301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaapan RM, Al-Abodi HR, Alanazi AD, Abdel-Shafy S, Rashidipour M, Shater AF, Mahmoudvand H. Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection. molecules. 2021 Jan;26(4):819. [DOI] [PMC free article] [PubMed]
- Salimi A., Rahimi H.R., Forootanfar H., Jafari E., Ameri A., Shakibaie M. Toxicity of microwave-assisted biosynthesized zinc nanoparticles in mice: a preliminary study. Artif. Cells Nanomed. Biotechnol. 2019;47(1):1846–1858. doi: 10.1080/21691401.2019.1611592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.