Abstract
Coral microbiomes are critical to holobiont functioning, but much remains to be understood about how prevailing environment and host genotype affect microbial communities in ecosystems. Resembling human identical twin studies, we examined bacterial community differences of naturally occurring fire coral clones within and between contrasting reef habitats to assess the relative contribution of host genotype and environment to microbiome structure. Bacterial community composition of coral clones differed between reef habitats, highlighting the contribution of the environment. Similarly, but to a lesser extent, microbiomes varied across different genotypes in identical habitats, denoting the influence of host genotype. Predictions of genomic function based on taxonomic profiles suggest that environmentally determined taxa supported a functional restructuring of the microbial metabolic network. In contrast, bacteria determined by host genotype seemed to be functionally redundant. Our study suggests microbiome flexibility as a mechanism of environmental adaptation with association of different bacterial taxa partially dependent on host genotype.
Subject terms: Evolutionary ecology, Bacterial genetics
The microbiomes associated with reef corals are complex and diverse. Here, the authors investigate fire coral clones naturally occurring in distinct habitats as a model system to disentangle the contribution of host genotype and environment on their microbiome, and predict genomic functions based on taxonomic profiles.
Introduction
Microbial communities of eukaryotic organisms play a critical role in the ecological success and health of their hosts1,2 as they provide a broad set of functions related to host metabolism, immunity, and stress tolerance within the so-called metaorganism3–7. Consequently, changes in microbial community composition are increasingly hypothesized to contribute to acclimatization and holobiont adaptation1,8,9. Previous studies have demonstrated that host-associated microbial community compositions are not stochastic, but determined by host species and habitat10–16. Consistently, transplant experiments have revealed intraspecific variation of microbial community composition across disparate environments, which may serve as a potential source of adaptive variation6,17–19. Yet, empirical studies differentiating the relative contribution from the host genetic background and surrounding environment on microbiome structure in natural systems remain scarce and are largely limited to the biomedical field and human microbiome studies20–22. However, such information is critical to assess how flexible microbial associations are and to what degree they contribute to the physiology of their host organisms1,5,9,23.
Reef-building corals are a prime example for organisms that critically depend on their microbial communities with regard to both host physiology and ecosystem functioning24,25. Accordingly, coral health is dependent on the structure and composition of the coral metaorganism primarily comprised of the coral animal host, its endosymbiotic dinoflagellate algae (Symbiodiniaceae family)26, and a suite of other microbes (bacteria, archaea, fungi, viruses), collectively termed the coral holobiont7,27–29. Corals depend on Symbiodiniaceae satisfying their energy requirements via the transfer of photosynthetically fixed carbon30 and the assimilation of dissolved inorganic nitrogen and phosphorus31, while the association with bacteria may serve a wide variety of functional roles, including nitrogen fixation, sulfur cycling, protection against pathogens, and stress tolerance6,32–37. The microbiome associated with reef corals has been reported as one of the most complex and diverse studied to date24. The complexity of coral holobiont structure and the variable coral reef environment can induce a high degree of variability in the bacterial community composition25,38,39, and have together contributed to uncertainties with regard to the role and significance of bacterial symbionts in aiding ecological adaptation of corals. Previous transplant and aquarium-based experiments studying the combined influence of host genotype and environment on coral microbial communities have revealed contrasting outcomes, from high host-genotype specificity of coral microbiomes16 to flexible environmental associations6,12,19. Disentangling the influence of host genetic background (genotype) and environment on coral-microbiome structure thus requires robust inferences based on in situ surveys that avoid the influence of manipulation through collection or rearing40.
Fire corals of the genus Millepora (Cnidaria, Hydrozoa), similar to stony corals (Cnidaria, Anthozoa), are an important component of reef communities worldwide that are associated with symbiotic algae and microbes41,42 and build calcareous skeletons, thus contributing to reef accretion and community dynamics43. A recent study of Millepora cf. platyphylla (see ref. 44,45 for synonymy reasons), a conspicuous reef-builder that inhabits a wide range of reef environments, identified several clonally replicated genotypes across distinct environments on a barrier reef ecosystem in Moorea, French Polynesia46. These clones were produced naturally through asexual fragmentation (i.e., likely wave-induced breakage), while dispersed across adjacent habitats (<210 m apart) via cross-reef transport. Specific environmental gradients across spatially adjacent reef habitats, such as light incidence, temperature, nutrients, and water flow (among others)47, have been reported as underlying factors of substantial variation in the occurrence and persistence of bacterial symbionts11,12,48,49. Similar to studying microbiome structure and function employing identical twin type designs (commonly used in human studies)20–22, fire coral clones naturally occurring in distinct habitats provide an ideal study system to tease apart the contributions of host genotype and environment on bacterial association (‘nature versus nurture’).
Here we sought to investigate bacterial communities of clonal genotypes of M. cf. platyphylla across distinct reef habitats to determine microbial association of different genotypes in the same environment (genetic basis) and of the same genotype(s) in different environments (environmental basis). To do this, samples were collected from three environmentally disparate, but spatially adjacent reef habitats on the north shore of Moorea: the mid slope, upper slope, and back reef. A total of six distinct clonal genotypes were selected to assess the effects of host genotype and reef habitat on bacterial community composition. Bacterial communities of M. cf. platyphylla were characterized using 16S ribosomal RNA gene amplicon sequencing with subsequent prediction of genomic function based on taxonomic profiles50. The design of our surveys enabled the discrimination of bacterial community members that align with host genotypes (irrespective of environment) and those that align with environmental differences (irrespective of host genotype) to decipher the relative contribution of both factors on shaping coral microbiomes. Our study shows that host genotype, but mostly reef habitat contribute to bacterial community composition of fire corals. The presence of taxonomically and presumably functionally diverse guilds of bacteria in distinct reef habitats suggests a functional restructuring of the microbial metabolic network in response to environmental changes. In contrast, bacteria determined by host genotype appear functionally redundant as revealed by the lack of discriminant predicted functions between genotypes.
Results
Composition of the fire coral microbiome
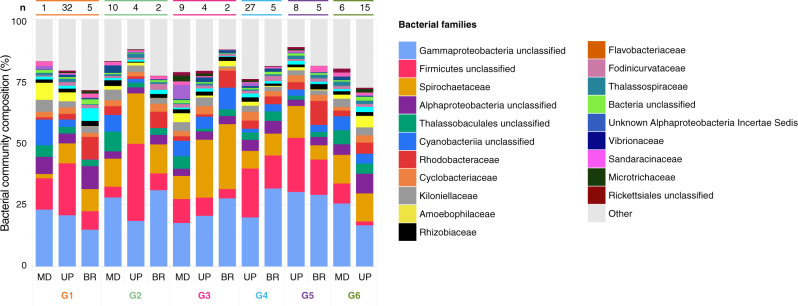
To discriminate the relative contribution of host genetic background and surrounding environment on coral-microbiome composition, we determined bacterial communities of six clonal genotypes of M. cf. platyphylla from three adjacent, but environmentally distinct reef habitats at Moorea, French Polynesia (Fig. 1 and Supplementary Data 1) using 16S rRNA gene amplicon sequencing. After quality trimming and removal of chimeric, undesired (e.g., chloroplasts and mitochondria), and rare sequences, 16S rRNA gene sequencing from 135 colonies of the fire coral M. cf. platyphylla, yielded 1 236 195 sequences that were further clustered into 20 144 amplicon sequencing variants (ASVs) (Supplementary Data 2). M. cf. platyphylla associated with 45 unique bacterial phyla, 107 classes, and 265 orders. The most abundant phylum across all fire coral samples was the Proteobacteria (51%), followed by the Firmicutes (15%), Spirochaetes (10%), and Bacteroidetes (9%). Bacterial communities were dominated by ASVs belonging to members of the families Spirochaetaceae and Rhodobacteraceae, as well as other unclassified Gammaproteobacteria, Firmicutes, Alphaproteobacteria, Thalassobaculales, and Cyanobacteria families (Fig. 2). Notably, 14 ASVs (of the 20 144) belonged to the well-known coral symbiont Endozoicomonas13, together representing 0.2% of the relative abundance of the fire coral bacterial community (Supplementary Data 2). Although no ASV could be identified that was present across all fire coral samples, we found 16 bacterial ASVs that were present in at least 80% of samples (n ≥ 108) and that we defined as putative members of a core microbiome, following the threshold used by Hernandez-Agreda et al.51. These bacterial taxa were from 3 phyla, 3 classes, 4 orders, and 3 families (Table 1) and comprised on average 41% of the relative abundance of the bacterial community across all samples. Gammaproteobacteria and unclassified Firmicutes were the most dominant groups, representing 38% (6 ASVs out of 16, accounting for 17% of the relative abundance of the total bacterial community) and 19% (3 ASVs, 14%) of the core microbiome, respectively.
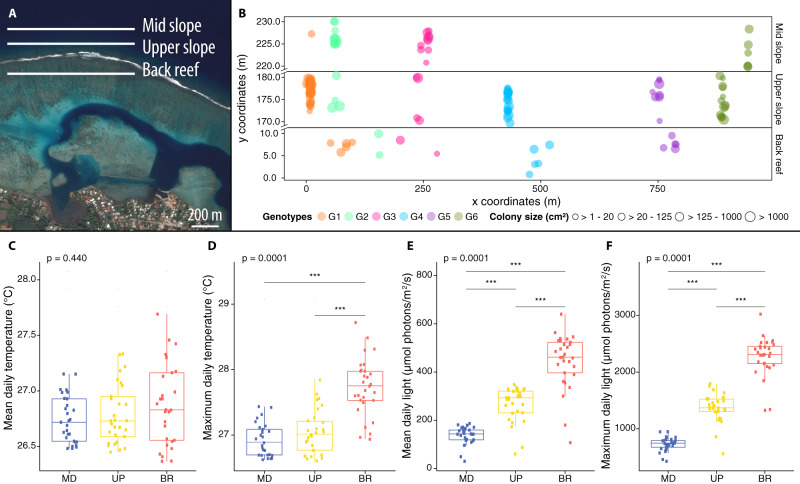
Fig. 1. Design of in situ surveys using fire coral clones to resolve the contribution of host genotype and environment on microbiome structure.
A Map of the location of each transect surveyed in the mid slope (MD: 12 meters depth), upper slope (UP: 6 meters), and back reef (BR: < 1 meter) habitats on the north shore of Moorea, French Polynesia. B Spatial distribution of six clonal genotypes with clones found in at least two of these habitats. Clonal genotypes are represented by a unique color and numbered from G1 to G6. C Mean temperature, (D) maximum temperature, (E) mean light, and (F) maximum light estimated at each of the habitat. For C to F the boxes represent the 25th to 75th percentile, lines show medians, and error bars depict 1.5X IQR. Mean and maximum temperature at each of the three habitats were based on 1 440 data points collected daily for a period of 29 days, while mean and maximum light measurements were based on 960 data points collected daily for a period of 29 days. One-way Kruskal–Wallis test significance is shown on the top of each box plot (P-values), while post hoc pairwise comparison level *** refers to P < 0.001.
Fig. 2. Bacterial community composition of clonal genotypes.
ASV-based microbial community composition of clonal genotypes among reef habitats at the bacterial family level. The number of clonal replicates for each genotype in each of the three habitats, mid slope (MD), upper slope (UP), and back reef (BR), is shown on the top of the stacked column plot (n). A total of 135 samples were examined.
Table 1.
Summary of bacterial ASVs corresponding to the putative core microbiome (presence in >80% of samples) of Millepora cf. platyphylla.
| ASV | Number of samples | Number of sequences | Relative abundance (%) | Lowest taxonomic level from SILVA classification | Closest relative (GenBank accession number; Sequence identity; Host or environment) |
|---|---|---|---|---|---|
| ASV00001 | 126 | 102 894 | 8.14 | Firmicutes unclassified | Uncultured Mollicutes (AY166838; 91%; Ascidian Ecteinascidia turbinata) |
| ASV00002 | 134 | 63 393 | 5.02 | Gammaproteobacteria unclassified | Uncultured Gammaproteobacteria (HM474931; 95%; Seawater) |
| ASV00003 | 134 | 52 392 | 4.15 | Spirochaetaceae (Spirochaeta sp.) | Uncultured bacteria (EU420442; 95%; Pristine mangrove sediments) |
| ASV00004 | 128 | 48 548 | 3.84 | Gammaproteobacteria unclassified | Uncultured bacteria (KY373376; 97%; Coral Acropora hyacinthus) |
| ASV00005 | 122 | 38 273 | 3.03 | Firmicutes unclassified | Uncultured Mollicutes (AY166838; 91%; Ascidian Ecteinascidia turbinata) |
| ASV00006 | 132 | 35 171 | 2.78 | Gammaproteobacteria unclassified | Uncultured Gammaproteobacteria (KY373292; 99%; Coral Acropora hyacinthus) |
| ASV00007 | 120 | 34 193 | 2.71 | Firmicutes unclassified | Uncultured Mollicutes (AY166838; 91%; Ascidian Ecteinascidia turbinata) |
| ASV00008 | 118 | 27 486 | 2.18 | Gammaproteobacteria unclassified | Uncultured bacteria (KY373376; 97%; Coral Acropora hyacinthus) |
| ASV00009 | 129 | 23 996 | 1.90 | Gammaproteobacteria unclassified | Uncultured Gammaproteobacteria (HM474931; 95%; Seawater) |
| ASV00011 | 128 | 20 389 | 1.61 | Gammaproteobacteria unclassified | Uncultured Gammaproteobacteria (HM474931; 95%; Seawater) |
| ASV00012 | 110 | 19 609 | 1.55 | Spirochaetaceae (Spirochaeta sp.) | Uncultured bacteria (EU420442; 95%; Pristine mangrove sediments) |
| ASV00013 | 109 | 17 035 | 1.35 | Spirochaetaceae (Spirochaeta sp.) | Uncultured bacteria (KY376315; 94%; Coral Acropora hyacinthus) |
| ASV00014 | 132 | 16 451 | 1.30 | Spirochaetaceae (Spirochaeta sp.) | Uncultured bacteria (GU118677; 98%; Coral Montastraea faveolata) |
| ASV00018 | 123 | 9 471 | 0.75 | Kiloniellaceae (Tistlia sp.) | Uncultured bacteria (KY373349; 100%; Coral Acropora hyacinthus) |
| ASV00029 | 110 | 4 263 | 0.34 | Thalassospiraceae (Thalassospira sp.) | Uncultured bacteria (KY377249; 97%; Coral Acropora hyacinthus) |
| ASV00032 | 108 | 3 935 | 0.31 | Thalassobaculales unclassified | Uncultured bacteria (GU118225; 100%; Coral Diploria strigosa) |
Bacterial community composition differs between host genotypes
To test for a possible effect of host genotype on microbial association, we assessed the assemblage of bacterial ASVs across different clonal host genotypes for each habitat. Our data revealed that bacterial communities differed significantly between fire coral genotypes present on the mid slope (PERMANOVA, F = 1.23, P < 0.05; genotypes G2 and G6, pairwise test, P < 0.05) and upper slope (PERMANOVA, F = 1.83, P < 0.001; all genotypes, pairwise test, P < 0.05, with the exception of G1 and G2 that are genetically very similar, see Supplementary Data 1) (Supplementary Table 1). In contrast, no differences were observed between host genotypes on the back reef. These results suggest a host genotype effect on microbiome composition for fire coral colonies inhabiting the mid and upper slope habitats (Fig. 3A). Accordingly, bacterial communities of fire coral clones from the upper slope were characterized by distinct bacterial families (PERMANOVA, F = 3.65, P < 0.001, Supplementary Data 3). Similarity percentage (SIMPER) analysis showed that presence and abundance of ASVs related to members of the bacterial families Spirochaetaceae, Rhodobacteraceae, and Sandaracinaceae, and unclassified Firmicutes (among others) explained between 45 and 57% of the differences in bacterial communities associated with the different genotypes in the upper slope (SIMPER and Kruskal–Wallis tests, P < 0.05, Supplementary Data 3). In the mid slope habitat, significant variation between genotypes G2 and G6 was only detected at the ASV level, but no clear pattern was detected when ASVs were grouped by bacterial family.
Fig. 3. Genetic and environmental contribution to variation in bacterial communities.
A Non-metric multidimensional scaling (nMDS) of bacterial community composition for each reef habitat depicts host genotype contribution to microbial community composition. Each plot shows one of the three habitats (mid slope, upper slope, and back reef), each symbol represents a colony, symbol colors denote the genotype (G1 = orange (n = 38), G2 = light green (n = 16), G3 = pink (n = 15), G4 = blue (n = 32), G5 = purple (n = 13), G6 = dark green (n = 21)), ellipses are drawn around each group’s centroid (95%) for genotypes with n ≥ 3 samples. B Non-metric multidimensional scaling (nMDS) of bacterial community composition for each host clonal genotype depicts habitat contribution to microbial community composition. Each plot shows one of the six genotypes (G1 to G6), each symbol represents a clone, symbol colors denote the habitat in which the clone was found (mid slope = blue (n = 26), upper slope = yellow (n = 90), back reef = red (n = 19)), ellipses are drawn around each group’s centroid (95%) for habitats with n ≥ 3 samples. One-way PERMANOVA test significance is shown for each analysis (P-values).
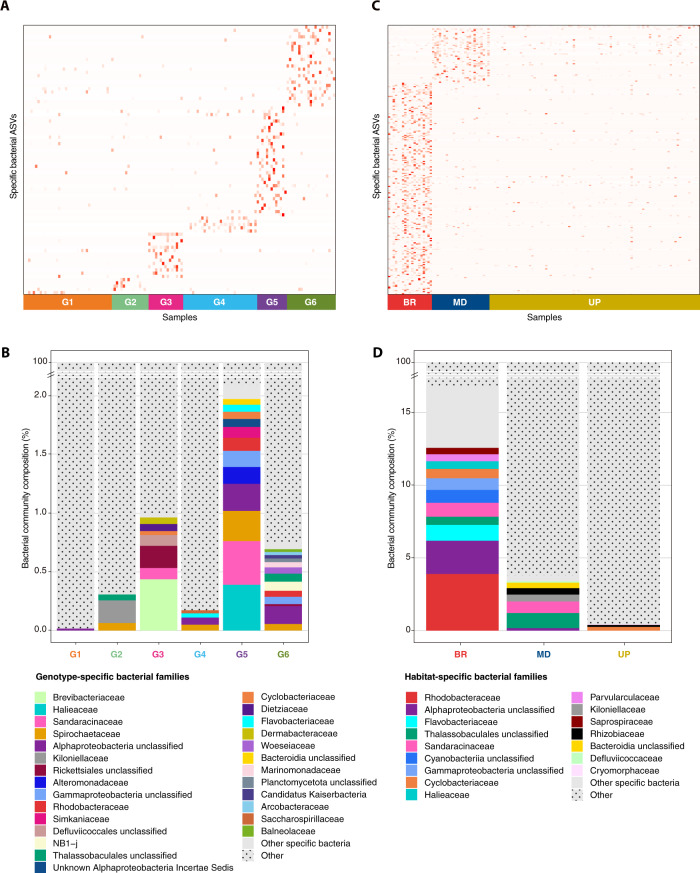
To identify specific bacterial ASVs that characterized microbiome variations between fire coral genotypes, we further analyzed our data for the presence of candidate indicator taxa. Each host genotype was associated with a specific set of bacterial taxa, which were each detected in low abundances (<2% of the bacterial community per genotype) (Fig. 4A). The number of indicator taxa ranged from 1 to 34 (mean: 13.8 and median: 9.5) and their phylogenetic membership varied between genotypes (Fig. 4B and Supplementary Data 4). Indicator taxa for specific genotypes were dominated by members of the bacterial classes Alpha- and Gammaproteobacteria, Actinobacteria, and Spirochaetes (among others) (Supplementary Data 4).
Fig. 4. Bacterial ASVs representative of host genotype and reef habitat.
Indicator bacterial ASVs associated with contribution of host genotype (A, B) and environment (C, D) to bacterial community structure. Heatmap based on IndicSpecies analysis shows specific bacterial ASVs that characterize (A) each host genotype, numbered from G1 to G6 and (C) fire corals in each of the three habitats, mid slope (MD), upper slope (UP), and back reef (BR). Each cell represents the standard transformation of the counts for each specific ASVs per genotype and habitat. B Specific bacterial families associated with host genotype and (D) reef habitat. Bar plots represent the relative abundance of the counts for each identified specific bacterial ASV at the family level, represented by a unique color; less common specific bacterial families were grouped as ‘Other specific bacteria’ and are shown in light gray; dotted bars are ASVs that are not specific to one of the six genotypes and to one of the three habitats, note different scales in B and D. See Supplementary Data 4 for details on IndicSpecies analysis.
Bacterial community composition differs between reef habitats
To test for an environmental effect on microbial community composition of M. cf. platyphylla, three environmentally disparate but spatially adjacent reef habitats were selected. These habitats were characterized by distinct in situ temperature and light conditions (Fig. 1 and Supplementary Fig. 1). Temperature profiles showed a similar daily mean water temperature across the three habitats (BR: 26.89 ± 0.07 °C, UP: 26.79 ± 0.05 °C, MD: 26.74 ± 0.04 °C), but a three- to four-fold greater diel amplitude at the back reef (1.37 ± 0.43 °C) compared to both fore reef habitats (UP: 0.45 ± 0.18 °C, MD: 0.34 ± 0.15 °C). Consequently, daily maximum temperatures were significantly higher at the back reef (27.73 ± 0.08 °C) compared to the upper slope (27.05 ± 0.06 °C) and the mid slope (26.92 ± 0.05 °C, Kruskal–Wallis, P < 0.001). Light intensity profiles revealed a significantly higher daily mean and maximum light levels at the back reef (446.28 ± 20.99 and 2 271.69 ± 63.80 µmol photons/m2/s, respectively) compared to the upper slope (266.57 ± 13.36 and 1371.83 ± 46.54 µmol photons/m2/s) and the mid slope (137.50 ± 6.82 and 726.38 ± 22.55 µmol photons/m2/s, Kruskal–Wallis, P < 0.001). Fire corals on the back reef were therefore exposed to a much more variable and extreme environment, as commonly found on barrier reef systems52,53.
Accordingly, microbial community composition demonstrated a strong environmental component, as detected by separate analyses of each of the six genotypes across the three distinct reef habitats (Fig. 3B). In fact, differences in bacterial community composition were predominantly affiliated with the habitat (two-way PERMANOVA, F = 2.86, P < 0.001), but also with the interaction of host genotype and reef habitat (F = 1.18, P < 0.01) (Supplementary Table 1). Several bacterial families contributed to differences in bacterial community composition between reef habitats, but only for one host genotype per habitat combination (Supplementary Data 3). For instance, prevalent members of Rhodobacteraceae, Flavobacteriaceae, and Sandaracinaceae, and unclassified Alphaproteobacteria were significantly more abundant on the back reef habitat, in addition to members of the rare families Halieaceae, Desulfovibrionaceae, and Pirellulaceae (among others) (G1; SIMPER and Kruskal–Wallis tests, P < 0.05). The abundant Thalassospiraceae family was significantly more abundant on the upper slope (G2; SIMPER and Kruskal–Wallis tests, P < 0.01), while members of the rare families Endozoicomonadaceae and Caulobacteraceae (among others) were significantly more abundant on the mid slope (G6, SIMPER and Kruskal–Wallis tests, P < 0.01).
To further explore this, a second analysis focusing on indicator taxa associated with particular reef habitats revealed that several bacterial taxa responded to differences in environmental conditions (Fig. 4C). Generally, the indicator taxa for reef habitats had higher relative abundances than the genotype-specific taxa. Nonetheless, all of these reef habitat bacterial indicator taxa together represented less than 20% of the bacterial community (Fig. 4D). Of the 195 bacterial indicator taxa characteristic of the back reef habitat, 58 belonged to the class Alphaproteobacteria (including 19 of the Rhodobacteraceae representing a quarter of the relative abundance of the back reef specific bacterial taxa) and 21 to the Bacteroidetes (6 of the Flavobacteriaceae and 6 Cyclobacteriaceae) (Fig. 4D and Supplementary Data 4). Fifty bacterial indicator taxa were identified for the mid slope, of which approximately three quarters belonged to the Alphaproteobacteria (13 unclassified Thalassobaculales, 3 Rhizobiaceae, and 6 Kiloniellaceae, including 4 of the genus Pelagibius) and 1 to the Polyangia (ASV00030 Sandaracinaceae). Only three indicator ASVs were identified for the upper slope habitat, two of which belonged to the classes Cytophagales (Cyclobacteriaceae of the genus Fulvivirga) and Flavobacteriales (Cryomorphaceae) of the phylum Bacteroidetes, and one Alphaproteobacteria of the family Rhizobiaceae.
To assess whether time since fragmentation contributed to microbiome structuring, we used colony size as a proxy. We did not find any patterns of bacterial community composition associated with distinct colony size classes among clonal replicates that were found in distinct reef habitats (PERMANOVA based on the ASV composition of four genotypes; G1, G4, G5, G6, P > 0.05, Supplementary Table 2). The richness of bacterial ASVs was also found to have no significant correlation with colony size (r = –0.158, P > 0.05).
Inferred functional predictions of bacterial indicator taxa for host genotype and environment
Among all functional traits identified using predictive metagenomic analysis (MetaCyc data for prokaryotes), 24 functions distinguished the microbial communities associated with distinct reef habitats (LDA > 2.5; Supplementary Data 5), while no discriminant functional traits were identified that differentiated host genotype microbiomes. Functional predictions of bacterial taxa associated with the mid slope habitat were distinct mostly through enrichment of functions related to the biosynthesis of nucleotides and co-factors, while the upper slope included enriched functions related to metabolism (aromatic compounds, amino and nucleic acids) and the biosynthesis of diverse organic molecules (nucleotides and amino acids) (Supplementary Fig. 2A). Microbiomes of fire corals inhabiting the back reef habitat included enriched functional predictions related to the TCA cycle and nitrogen and sulfur compound metabolism, as well as the biosynthesis of carbohydrates, vitamins, and electron carriers (Supplementary Fig. 2A).
Discussion
To assess the relative contribution of host genotype and environment to microbiome structure, we explored bacterial community composition among genetically identical fire coral colonies that inhabit contrasting reef environments. We found bacterial taxa specific to both host genotype and reef habitat. This suggests that genetic and environmental factors play a role in the capacity of corals to form bacterial associations, although the habitat seems to have a stronger effect compared to the host genetic background. Interestingly, environmentally determined taxa suggest a functional restructuring of the microbial metabolic network, while bacteria determined by host genotype appear functionally redundant.
Similar to the microbiome of scleractinian corals11,25,54, we found that the microbial community of the hydrocoral M. cf. platyphylla was dominated by members of Alpha- and Gammaproteobacteria. Despite this high-level similarity with other cnidarian reef species, members of the Endozoicomonadaceae family were comparably rare in fire corals, with increased abundance in colonies of the mid slope and more specifically in one of the six genotypes (G6). Although highly variable within and between coral species, bacteria of the genus Endozoicomonas have been reported as one of the most abundant members of the coral microbiome13,24,55. In fact, Endozoicomonas dominate the microbiome of many stony coral species (including Porites astreoides56, Stylophora pistillata and Pocillopora verrucosa13,57, Acropora hemprichii58, Acropora millepora59, as well as other marine animals55,60). It is thought that Endozoicomonas play a significant role in nutrient acquisition and cycling of organic compounds61 because of their ability to metabolize dimethylsulfoniopropionate (DMSP)62,63. The capacity to degrade DMSP appears to be present in a variety of bacterial taxa that were found in the fire coral microbiome, including Cytophagales, Flavobacteriales, Desulfovibrionales, and other Alphaproteobacteria of the families Rhodobacteraceae and Rhizobiaceae63–66. These bacterial taxa found in fire corals could play a similar role as Endozoicomonas in scleractinian corals.
Intrinsic (host-induced) and extrinsic (environment-induced) factors contribute to the diversity of coral-associated microbiomes48,67,68. Durante et al.68 showed that the abundance of some bacterial taxa (i.e., Methylobacterium and Alteromonas) were highly variable between genotypes of Acropora corals, while other studies have demonstrated environmental-induced variation in several bacterial taxa6,11,19. Yet, our surveys provided us with a great opportunity to tease apart the contribution of host genotype and environment to microbial community structure in natural marine populations (but see ref. 69 for an example in plants). In this study, we identified several bacterial taxa that were specifically associated with host genotype (from 11 abundant bacterial families and many other rarer families, Fig. 4B). Importantly, these distinct taxa were not associated with any discriminant predicted functional traits between coral host genotypes, suggesting that differences in bacterial community composition between host genotypes within the same environment are likely functionally redundant. In other words, the same function is putatively conveyed by different bacterial taxa pending host genotype. For instance, different taxa known to play roles in nutrient cycling by providing diazotrophically derived nitrogen (DDN) to the coral host and photosynthetic symbionts27,70–72 were specific to distinct genotypes (Actinobacteria (G3), Spirochaetes (G5), and Planctomycetes (G6)). Similarly, distinct bacterial taxa involved in the cycling of sulfur32,73–75, through degradation of dimethylsulfide (DMS)76 and DMSP32,77, were also specifically associated with distinct genotypes (Brevibacteriaceae (G3), Rhodobacteraceae (G5, G6), Woeseiaceae (G6), and Alteromonadaceae families, comprising Alteromonas (G2, G5)). Another functional group of genotype-specific bacterial taxa was comprised of heterotrophic consumers27,78–81 (Brevibacterium (G3), Flavobacterium (G4), and Sandaracinaceae family (G5)). The presence of taxonomically diverse, but presumably functionally similar guilds of bacteria in distinct host genotypes, suggests that the bacterial community might be structured by functional redundancy rather than by specific taxa82,83. Thus, bacterial signatures may differ taxonomically between coral host genotypes16,25, while occupying similar functional niches. Further investigations based on metatranscriptomic analysis will help to decipher whether these bacteria play similar functions between different host genotypes.
The restructuring of microbial communities has been suggested as an important mechanism of coral host plasticity and adaptation6,9,19,29,84. Our data provide evidence of flexible microbiomes in fire coral clones between reef habitats, most likely pointing to a functional restructuring of the microbial metabolic network in response to environmental cues9,19. Specifically, our data show that there are significant differences among the microbiomes of genetically identical colonies that were found in distinct reef habitats, supporting microbiome flexibility as a mechanism of environmental adaptation (sensu9,19). Although the specific biological benefit to the host remains to be shown, this environmental flexibility represents a promising characteristic for the manipulation of Beneficial Microorganisms for Corals (BMCs)85–88. In this context, we identified several habitat-specific bacterial taxa that varied to a great extent between environments (including 10 abundant bacterial families, Fig. 4D). The number of indicator bacterial taxa was between 4 to 65 times higher on the back reef habitat compared to the mid and upper slope, with a similar pattern with regard to taxonomic diversity. Notably, the back reef environment was more variable and more extreme in terms of light and temperature conditions than the more stable mid and upper slope. This may constrain flexibility of bacterial associations, and thus result in lower genotype-associated variation in bacterial community composition in the back reef habitat (as shown by high microbiome similarity between host genotypes in this particular habitat). Fire corals inhabiting the back reef habitat were also exposed to varying levels of disturbance (sewage and pollution)89 and extreme conditions with regard to partial pressure of carbon dioxide and tide range52,53. These observations agree with the notion that environmental disturbances lead to specialization of bacterial communities11,48,90,91.
In fire corals, mixotrophic members of the Rhodobacteraceae were identified as the most representative indicator bacteria of the variable back reef habitat. This bacterial taxon is known to be involved in carbon, nitrogen, and sulfur cycling92,93, and can therefore confer additional sources of nutrients to corals exposed to elevated temperatures (as in Santos et al.94). Because of their mixotrophy, Rhodobacteraceae are metabolically more flexible than specialist bacterial species (i.e., exclusively autotrophic or heterotrophic taxa)92,95,96. Cyanobacteria also play an important role in nitrogen cycling, while other members of the Rhodobacteraceae and Halieaceae (including Rubribacterium and Pseudohalia genera) are capable of aerobic photoheterotrophy, utilizing light as a source of energy97. Predicted functional profiles of indicator bacterial taxa associated with the back reef habitat were also related to the bacterial secretion system and specifically to its contribution to membrane transport, suggesting a role in bacteria–host symbiosis98 (Supplementary Fig. 2B). Terpenoid backbone synthesis was identified as another discriminant trait, which is often binned with the reduced forms of coenzyme Q (CoQH2; ubiquinol) that plays an integral role in respiratory electron transport during thermal stress in corals99. The elevated temperature and light incidence observed in the back reef habitat, along with the accumulation of ROS when corals are exposed to such stressors100,101, may disrupt the coral-Symbiodiniaceae symbiosis and lead to bleaching. The presence of specific bacteria that may be involved in nutrient cycling (nitrogen and sulfur metabolism and TCA cycle), together with early decarboxylation processes involved in respiratory electron transport (Supplementary Fig. 2A), might contribute to holobiont resilience in variable and extreme environments such as the back reef habitat. Although the putative role of these bacteria has rarely been studied in coral reef environments, they may have a role in the thermal tolerance of corals.
Conversely, Rhizobiales and Thalassobaculales were associated with the mid slope habitat. Members of the Rhizobiales may provide additional sources of fixed nitrogen to corals inhabiting mid-shelf reefs33,102, and more specifically to corals that are highly autotrophic103, including Millepora species104. Thalassobaculales are known to establish partnership with Symbiodiniaceae in juvenile Acropora corals105, which aligns with the higher abundance of fire coral juveniles previously observed on the mid slope at Moorea106. Bacteroidetes of the Cytophagia-Flavobacteria group (Cyclobacteriaceae and Cryomorphaceae families) were involved in the coral response to environmental conditions characterizing the upper slope. Their presence aligned with an enrichment of predicted functions related to amino acid metabolism, which corresponds with their previously reported contribution to coral nutrient uptake via the production and/or remineralization of organic matter ingested or produced by the coral host107.
Despite previous studies proposing that the variability of coral microbiomes is correlated with the age of the coral colony108,109, we found no apparent changes in bacterial community composition of clonal fragments that were linked to their size. Thus, our data suggest that environmental adaptation of bacterial communities may occur rapidly upon fragmentation and reattachment in a new environment, aligning with fast microbial shifts recorded in juvenile corals (2 weeks102) and transplanted corals (<24 h6).
Our study shows that host genotype, but mostly environmental setting contribute to fire coral bacterial associations. The associated bacterial functional predictions suggest that two processes shape these coral-microbiome associations. While genotype-bacterial associations seem to be less specific taxonomically and rather determined by functional redundancy of the present taxa, distinct functional profiles of habitat-specific bacterial taxa suggest environmental adaptation of the microbial community. Further studies are needed to identify and quantify genetic factors and environmental variables, as well as spatiotemporal dynamics, that contribute to coral bacterial community structure and determine how they influence coral health. Such information is critical as the underlying molecular mechanisms by which the microbiome may shape coral host phenotype, ecology, and evolution are still poorly understood.
Methods
Sampling design
Our sampling design is described in detail in Dubé et al.46,110, where fire coral colonies were sampled to investigate the clonal structure and dispersal of sexual propagules between habitats on a barrier reef system. Briefly, between May to September 2013, 3 160 fragments of the fire coral M. cf. platyphylla were collected from three adjacent reef habitats located on the north shore of Moorea Island, French Polynesia (17.5267 S, 149.8348 W): the mid slope (13 m depth), upper slope (6 m depth), and back reef (< 1 m depth) (Fig. 1A). Within each habitat, three 300 m-long by 10 m-wide belt transects were laid over the reef, parallel to the shore. All colonies of M. cf. platyphylla were georeferenced by determining their position along the transect-line (0–300 m) and straight-line distance from both sides of the transect (0–10 m). From these measures, each colony was mapped with x and y coordinates. The colony size (projected surface) of each colony was estimated (in cm2) from 2D photographs using ImageJ 1.4f111. Small fragments of tissue-covered skeleton (<2 cm3) were also collected from each colony using a hammer and a chisel and placed in 2 ml tubes. Prior to transfer and preservation of the samples in 80% ethanol for further molecular analysis, each fragment was rinsed with 70% ethanol to reduce the possibility of contamination from bacteria present in seawater. Field experiments were approved by the Ethical Committee from the Presidency of French Polynesia (#0085) and performed in accordance with relevant Polynesian regulations.
Environmental conditions
The temperature and light intensity were monitored over a one-month period (i.e., from August 23 to September 26, 2019) to assess the environmental differences between the three surveyed reef habitats. Temperature was recorded in 60-sec intervals using in situ deployed HOBO Pendant Temperature Data Loggers (Onset, USA), while the light conditions were recorded in 90-sec intervals using two 2π PAR Loggers (Odyssey, New Zealand). Differences in daily temperature and light intensity between reef habitats were assessed using Kruskal–Wallis tests (because assumptions of normality and homoscedasticity were not satisfied) with the R package ‘stats’, and the complementary post hoc pairwise comparisons were also conducted.
DNA extraction and clonal genotypes
From our previous surveys46,106,110, 3 160 colonies of M. cf. platyphylla were sampled and genotyped using microsatellite loci (as described in Dubé et al.46) to identify clone mates (i.e., genetically identical colonies produced through asexual fragmentation). Briefly, all colony fragments were incubated at 55 °C for 1 hour in 450 µL of lysis buffer with proteinase K (QIAGEN, Hilden, Germany) and DNA was extracted using a QIAxtractor automated genomic DNA extraction instrument, according to manufacturer’s instructions. Each colony was amplified at twelve polymorphic microsatellite loci (for locus information refer to Dubé et al.112) in four multiplex polymerase chain reactions (PCRs) using the Type-it Multiplex Master Mix (QIAGEN, Hilden, Germany). Samples were sent to the GenoScreen platform (Lille, France) for fragment analysis on an Applied Biosystems 3730 Sequencer with the GeneScan 500 LIZ size standard. All alleles were scored and checked manually using GENEMAPPER v.4.0 (Applied Biosystems, Foster City CA, USA). Further details on the microsatellites loci and genotyping procedure are described in Dubé et al.46. Multilocus genotypes (MLGs) were identified in GENCLONE v.2.0113. Colonies with the same alleles at all loci were assigned to the same MLG (genet) and were considered as clone mates due to fragmentation when the genotype probability (GP) was < 0.001. GP was computed in GENALEX v.6.5114. We selected six genotypes with at least four clonal replicates in at least two of the surveyed habitats (n = 135 samples) to examine variation in bacterial communities among fire coral clones across distinct reef habitats (Fig. 1B and Supplementary Data 1 for MLGs of selected samples). A map of the locations of each clonal genotype was produced using the package ‘ggplot2’115 as implemented in R software v.3.1.3116.
PCR amplification and sequencing conditions
The V5 and V6 region of the 16S rRNA gene were amplified using the primers 784F and 1061R117 with added sequencing adapters (forward: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGAGGATTAGATACCCTGGTA–3′; reverse: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCRRCACGAGCTGACGAC-3′; Illumina over-hang adaptor sequences are underlined). Ten μl PCRs containing 1 μl of template DNA and 0.25 μM of each primer were run in triplicate per sample using the Multiplex PCR Kit (QIAGEN, Hilden, Germany). PCR cycling conditions were 95 °C for 15 min, followed by 30 cycles of 95 °C for 30 s, 55 °C for 90 s, and 72 °C for 30 s, with a final extension time of 10 min at 72 °C. Amplification success was verified on a 1% agarose gel, and successful triplicate reactions were pooled and cleaned using the illustra ExoProStar PCR and Sequence Reaction Clean-Up Kit (GE Healthcare Life Sciences, Pittsburgh PA, USA). Indexing adaptors were added via PCR (8 cycles) according to the Nextera XT DNA library preparation protocol using the Multiplex PCR Kit. Indexed PCR products were purified and normalized using the SequalPrep Normalization Plate Kit (Invitrogen, Carlsbad CA, USA), subsequently quantified using a Qubit dsDNA HS Kit (Invitrogen, Carlsbad CA, USA), and run on the Bioanalyzer 2100 (Agilent Technologies, Santa Clara CA, USA) to confirm amplicon length and purity. The 16 S rRNA gene amplicon library was sequenced at the KAUST BioScience Core Laboratory on the Illumina HiSeq 2500 platform using the rapid-run mode with 2 × 250 bp overlapping paired-end reads with a 10% phiX control. Determined sequencing data for this project are available under NCBI BioProject PRJNA610240.
Sequence data processing
Demultiplexed paired-end sequencing reads were processed with QIIME 2 pipeline (version 2020.6) for quality control, error correction, and taxonomical classification118. Briefly, a total of 25 829 809 reads were obtained from the 135 samples after demultiplexing. DADA2119 was used for denoising, filtering, merging, and chimera removal from these sequences and to generate amplicon sequence variants (ASVs). Sequencing results were subsequently rarefied based on the sample having the smallest number of sequences, i.e. to 9 357 sequences (as described in the MiSeq SOP protocol120). Taxonomic identification of ASVs was performed using the classify‐sklearn method121 via the q2-feature-classifier plugin122 against (99% of clustering) the 16 rRNA (full length) Silva SSU 138 database123.
16S rRNA gene-based microbial community analysis
Taxonomically annotated 16S sequences were used to create bacterial community composition stacked column plots at the family level using the means of relative abundances from samples grouped by genotype and habitat (Fig. 2). Plots were drawn using the package ‘ggplot2’ in R115. To assess the contribution of host genotype and environment to microbiome community structuring, differences in the assemblage of bacterial ASVs were tested using a two-way permutational multivariate analysis of variance (PERMANOVA) with the adonis function in the ‘vegan’ R package124. The effect of host genotype and environment were also investigated separately using one-way PERMANOVA tests and the results were visualized in non-metric multidimensional scaling (nMDS) ordination plots with ellipses drawn around each group’s centroid using the package ‘ggplot2’ in R115 for groups with n ≥ 3 samples. All statistical analyses were performed on Bray Curtis distances of log (x + 1) transformed ASV counts using R116 including only groups with sufficient replication (i.e., n ≥ 3), which resulted in the exclusion of G2 and G3 in the back reef habitat and of G1 in the mid slope. Similarity percentage (SIMPER) analyses, combined with Kruskall–Wallis tests, were conducted using the R package ‘vegan’124 to determine the degree of dissimilarity in bacterial communities between host genotypes as well as between habitats, and to determine which bacterial families were responsible for the largest portion of those dissimilarities. To confirm that the data fulfilled the requirements for SIMPER testing, multivariate tests calculating the dispersion of samples between genotypes and habitats were performed using the ‘betadisper’ function in ‘vegan’124. Homogeneity of multivariate dispersion between groups (i.e., genotypes and habitats) was tested with ANOVAs (P > 0.05). ASVs that were consistently present in at least 80% of samples were considered members of the core microbiome51. These ASVs were therefore designated as putative core microbiome members and their sequences were BLASTed against the GenBank database [nr/nt and 16S rRNA sequences (Bacteria and Archaea)] to identify closely related matches.
We also analyzed our data to characterize changes in microbial communities related to colony size differences at the genotype level. In particular, we assessed colonies of the same genotype that were produced naturally in different habitats through wave-induced breakage, i.e. microbiomes of small recently fragmented clones versus larger clones that have been most likely fragmented earlier. To do so, fire coral colonies were first categorized into size classes (cm²) based on the previsously reported growth rate of M. platyphylla, i.e. 2 cm in diameter per year125 (a putative indicator of the fragmentation time scale): 3–13, 13–28, 28–50, 50–79, 79–113, 113–154, 154–201, 201–254, 254–314, 314–380, 380–452, 452–531, 531–615, 615–707, 707–804, 804–907, 907–1017, 1017–1134, 1134–1256, 1256–2826, 2826–5024, and >5024 cm². Pearson’s correlation coefficient was used to determine whether the number of ASVs increases with increasing colony size, whereas differences in bacterial composition between size classes were tested using a one-way PERMANOVA. Of note is the intra-genotype morphological plasticity previously observed within each of the six genotypes of M. cf platyphylla, where clones were mostly encrusting in the mid slope and back reef habitats, but characterized by the sheet-tree morphology in the upper slope46 (See Supplementary Data 1). Because coral morphology aligned with environmental differences, it was not possible to determine whether microbiomes were different between growth forms.
Bacterial species representative of host genotype and reef habitat
We employed the R statistical package IndicSpecies126 to identify ASVs that were significantly associated with distinct fire coral host genotypes and/or reef habitats (Supplementary Data 4). The analysis was conducted on ASV count data. All samples were assigned to one of the six host genotypes using the command ‘groups’. IndicSpecies was run using the command ‘multipatt’ with the function Indval.g for corrections of unequal sample sizes and 9 999 permutations to assess statistical significance. Significant ASVs were summarized (command ‘summary’) for each genotype separately and for all genotype combinations. This analysis was also performed for the three habitats. Only ASVs that were highly significantly (P <0.01) associated with one or several groups were considered. Heatmaps showing bacterial ASVs associated with a specific host genotype and/or a reef habitat were compiled using ‘ggplot2’115.
Taxonomically inferred functional profiling of host genotype and reef habitat bacterial species
To better understand the potential functional profiles of specific bacterial taxa in host genotype and/or reef habitats, we applied a computational approach using the program PICRUSt2 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States)50. PICRUSt2 predicts metagenomic functional content from the 16S rRNA marker gene by estimating the genomic copy numbers of each gene family, based on the strain’s phylogenetic relationship with regard to all bacteria and archaea for which sequenced genomes are available50. KEGG orthology (KO) metagenomes, enzyme commission (EC) metagenomes, and MetaCyc pathway abundances were predicted through the QIIME 2 implementation of PICRUSt2 in the module called q2-picrust2. Briefly, QIIME 2-compatible ASV tables for both host genotype- and reef habitat-specific bacterial ASVs (i.e., bacterial indicator taxa) were imported in QIIME 2 format. The 16S ASVs were aligned (NSTI cutoff value of 2) to a reference phylogenetic tree of 16S sequence variants from sequenced prokaryotes. Then, the software predicted functional gene families and copy numbers for each specific ASV. During the process, the ASVs were normalized for their 16S copy number in the corresponding bacteria. Individual KEGG Ortholog groups (KOs) were summarized at KEGG-Pathway levels 1, 2, and 3 and with the MetaCyc pathway. A weighted Nearest Sequenced Taxon Index (NSTI) score was calculated for each sample to confirm the accuracy of this computational approach, which mostly depends on the availability of reasonably related reference genomes127. This score is the average branch length between each ASV and its closest sequenced relative, weighted by abundance. In this study, mean weighted NSTI scores for the host genotype analysis were 0.16 (SD ± 0.10) and 0.20 (SD ± 0.04) for the reef habitat analysis (Supplementary Data 6). These values were within the range of soil and mammal microbiomes that have been previously predicted with reasonable accuracy127. The count tables of metagenome predictions were further analyzed using the Galaxy web application (https://huttenhower.sph.harvard.edu/galaxy/) and the LEfSe method128 to identify significantly different metagenome functions of microbial communities among host genotypes and reef habitats, respectively (LDA > 2.0 for levels 1–3 for individual KOs and LDA > 2.5 for MetaCyc pathways, Supplementary Data 5). Of note, genomic content inference based on taxonomic profiles only enables the prediction of functions associated with given bacterial taxa, while metagenomic and/or metatranscriptomic data are needed to confirm these predicted metabolic functions.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank KAUST Bioscience Core Lab for sequencing and Sidki Bouslama (University Laval) for his help with the PICRUSt2 analysis. This work was funded by the Laboratoire d’Excellence “CORAIL” project COMIC and KAUST baseline funds to CRV.
Author contributions
C.E.D., E.B., S.P. and C.A.-F.B. conceived and designed research. C.E.D. and A.M. collected field data. C.E.D, M.Z., A.M. and C.R.V analyzed and interpreted data. C.E.D. generated molecular data and drafted the manuscript. C.E.D., M.Z., and C.R.V. wrote the manuscript. All authors revised drafts of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL, University of Konstanz.
Data availability
The sequencing data generated in this study are available under NCBI BioProject ID PRJNA610240. Bacterial ASV reference sequences corresponding to the putative core microbiome are available under GenBank Accession numbers MZ045394-MZ045409. Other data generated in this study are provided in the Supplementary Data files.
Code availability
Codes and scripts used for this study are available at: https://github.com/CarolineDUBE/Bacteria_NatCommun.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Tyler Carrier, Bettina Glasl, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. E. Dubé, Email: caroline.dube.qc@gmail.com
C. R. Voolstra, Email: christian.voolstra@uni-konstanz.de
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-26543-x.
References
- 1.McFall-Ngai M, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA. 2013;110:3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang C, et al. Metaorganisms in extreme environments: do microbes play a role in organismal adaptation? Zoology. 2018;127:1–9. doi: 10.1016/j.zool.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Mueller UG, Sachs JL. Engineering microbiomes to improve plant and animal health. Trends Microbiol. 2015;23:606–617. doi: 10.1016/j.tim.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Theis, K. R., Whittaker, D. J. & Rojas, C. A. A hologenomic approach to animal behavior. In Evolution in Action: Past, Present and Future 247–263 (Springer, 2020).
- 5.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler M, Seneca FO, Yum LK, Palumbi SR, Voolstra CR. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 2017;8:1–8. doi: 10.1038/ncomms14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins SJ, et al. A genomic view of the reef-building coral Porites lutea and its microbial symbionts. Nat. Microbiol. 2019;4:2090–2100. doi: 10.1038/s41564-019-0532-4. [DOI] [PubMed] [Google Scholar]
- 8.Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Voolstra CR, Ziegler M. Adapting with microbial help: Microbiome flexibility facilitates rapid responses to environmental change. BioEssays. 2020;2:2000004. doi: 10.1002/bies.202000004. [DOI] [PubMed] [Google Scholar]
- 10.Cárdenas CA, Bell JJ, Davy SK, Hoggard M, Taylor MW. Influence of environmental variation on symbiotic bacterial communities of two temperate sponges. FEMS Microbiol. Ecol. 2014;88:516–527. doi: 10.1111/1574-6941.12317. [DOI] [PubMed] [Google Scholar]
- 11.Pantos O, Bongaerts P, Dennis PG, Tyson GW, Hoegh-Guldberg O. Habitat-specific environmental conditions primarily control the microbiomes of the coral Seriatopora hystrix. ISME J. 2015;9:1916–1927. doi: 10.1038/ismej.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roder C, Bayer T, Aranda M, Kruse M, Voolstra CR. Microbiome structure of the fungid coral Ctenactis echinata aligns with environmental differences. Mol. Ecol. 2015;24:3501–3511. doi: 10.1111/mec.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neave MJ, et al. Differential specificity between closely related corals and abundant Endozoicomonas endosymbionts across global scales. ISME J. 2017;11:186–200. doi: 10.1038/ismej.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrier TJ, Reitzel AM. Convergent shifts in host-associated microbial communities across environmentally elicited phenotypes. Nat. Commun. 2018;9:1–9. doi: 10.1038/s41467-018-03383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollock FJ, et al. Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-07275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasl B, Smith CE, Bourne DG, Webster NS. Disentangling the effect of host-genotype and environment on the microbiome of the coral Acropora tenuis. PeerJ. 2019;7:e6377. doi: 10.7717/peerj.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macke E, Callens M, De Meester L, Decaestecker E. Host-genotype dependent gut microbiota drives zooplankton tolerance to toxic cyanobacteria. Nat. Commun. 2017;8:1–13. doi: 10.1038/s41467-017-01714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casey JM, Connolly SR, Ainsworth TD. Coral transplantation triggers shift in microbiome and promotion of coral disease associated potential pathogens. Sci. Rep. 2015;5:11903. doi: 10.1038/srep11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler M, et al. Coral bacterial community structure responds to environmental change in a host-specific manner. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 22.Rothschild D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 23.Jaspers C, et al. Resolving structure and function of metaorganisms through a holistic framework combining reductionist and integrative approaches. Zoology. 2019;113:81–87. doi: 10.1016/j.zool.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Blackall LL, Wilson B, van Oppen MJH. Coral—the world’s most diverse symbiotic ecosystem. Mol. Ecol. 2015;24:5330–5347. doi: 10.1111/mec.13400. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Agreda A, Gates RD, Ainsworth TD. Defining the core microbiome in corals’ microbial soup. Trends Microbiol. 2017;25:125–140. doi: 10.1016/j.tim.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 26.LaJeunesse TC, et al. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018;28:2570–2580. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Rohwer F, Seguritan V, Azam F, Knowlton N. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 2002;243:1–10. doi: 10.3354/meps243001. [DOI] [Google Scholar]
- 28.Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- 29.Bourne DG, Morrow KM, Webster NS. Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 2016;70:317–340. doi: 10.1146/annurev-micro-102215-095440. [DOI] [PubMed] [Google Scholar]
- 30.Muscatine L, Porter JW, Kaplan IR. Resource partitioning by reef corals as determined from stable isotope composition. Mar. Biol. 1989;100:185–193. doi: 10.1007/BF00391957. [DOI] [Google Scholar]
- 31.Rädecker N, Pogoreutz C, Voolstra CR, Wiedenmann J, Wild C. Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol. 2015;23:490–497. doi: 10.1016/j.tim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Wegley L, Edwards R, Rodriguez‐Brito B, Liu H, Rohwer F. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environ. Microbiol. 2007;9:2707–2719. doi: 10.1111/j.1462-2920.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 33.Raina JB, Tapiolas D, Willis BL, Bourne DG. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl. Environ. Microbiol. 2009;75:3492–3501. doi: 10.1128/AEM.02567-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lema KA, Willis BL, Bourne DG. Corals form characteristic associations with symbiotic nitrogen-fixing bacteria. Appl. Environ. Microbiol. 2012;78:3136–3144. doi: 10.1128/AEM.07800-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krediet CJ, Ritchie KB, Paul VJ, Teplitski M. Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proc. R. Soc. B. 2013;280:20122328. doi: 10.1098/rspb.2012.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasl B, Herndl GJ, Frade PR. The microbiome of coral surface mucus has a key role in mediating holobiont health and survival upon disturbance. ISME J. 2016;10:2280–2292. doi: 10.1038/ismej.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boilard A, et al. Defining coral bleaching as a microbial dysbiosis within the coral holobiont. Microorganisms. 2020;8:1682. doi: 10.3390/microorganisms8111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apprill A, Weber LG, Santoro AE. Distinguishing between microbial habitats unravels ecological complexity in coral microbiomes. mSystems. 2016;1:e00143–16. doi: 10.1128/mSystems.00143-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasl B, et al. Microbial indicators of environmental perturbations in coral reef ecosystems. Microbiome. 2019;7:1–13. doi: 10.1186/s40168-019-0705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damjanovic K, Blackall LL, Peplow LM, van Oppen MJH. Assessment of bacterial community composition within and among Acropora loripes colonies in the wild and in captivity. Coral Reefs. 2020;39:1245–1255. doi: 10.1007/s00338-020-01958-y. [DOI] [Google Scholar]
- 41.Dubé, E. B. et al. Ecology, biology and genetics of Millepora hydrocorals on coral reefs. In Invertebrates - Ecophysiology and Management (eds. Ray, S., Diarte-Plata, G. & Escamilla-Montes, R.), (IntechOpen, 2019).
- 42.Rodríguez L, et al. Genetic relationships of the hydrocoral Millepora alcicornis and its symbionts within and between locations across the Atlantic. Coral Reefs. 2019;38:255–268. doi: 10.1007/s00338-019-01772-1. [DOI] [Google Scholar]
- 43.Lewis JB. Biology and ecology of the hydrocoral Millepora on coral reefs. Adv. Mar. Biol. 2006;50:1–55. doi: 10.1016/S0065-2881(05)50001-4. [DOI] [PubMed] [Google Scholar]
- 44.Arrigoni R, et al. An integrated morpho-molecular approach to delineate species boundaries of Millepora from the Red Sea. Coral Reefs. 2018;37:967–984. doi: 10.1007/s00338-018-01739-8. [DOI] [Google Scholar]
- 45.Boissin E, Leung JK, Denis V, Bourmaud CA, Gravier-Bonnet N. Morpho-molecular delineation of structurally important reef species, the fire corals, Millepora spp., at Réunion Island, Southwestern Indian Ocean. Hydrobiologia. 2020;847:1237–1255. doi: 10.1007/s10750-020-04179-0. [DOI] [Google Scholar]
- 46.Dubé CE, Boissin E, Maynard JA, Planes S. Fire coral clones demonstrate phenotypic plasticity among reef habitats. Mol. Ecol. 2017;26:3860–3869. doi: 10.1111/mec.14165. [DOI] [PubMed] [Google Scholar]
- 47.Schwartzman JA, Ruby EG. Stress as a normal cue in the symbiotic environment. Trends Microbiol. 2016;24:414–424. doi: 10.1016/j.tim.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Oppen MJH, et al. Adaptation to reef habitats through selection on the coral animal and its associated microbiome. Mol. Ecol. 2018;27:2956–2971. doi: 10.1111/mec.14763. [DOI] [PubMed] [Google Scholar]
- 49.Sunagawa S, et al. Structure and function of the global ocean microbiome. Science. 2015;348:6237. doi: 10.1126/science.1261359. [DOI] [PubMed] [Google Scholar]
- 50.Douglas GM, et al. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020;38:685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez-Agreda A, Leggat W, Bongaerts P, Herrera C, Ainsworth TD. Rethinking the coral microbiome: simplicity exists within a diverse microbial biosphere. MBio. 2018;9:e00812–18. doi: 10.1128/mBio.00812-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bongaerts P, et al. Adaptive divergence in a scleractinian coral: physiological adaptation of Seriatopora hystrix to shallow and deep reef habitats. BMC Evol. Biol. 2011;11:303. doi: 10.1186/1471-2148-11-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albright R, Benthuysen J, Cantin N, Caldeira K, Anthony K. Coral reef metabolism and carbon chemistry dynamics of a coral reef flat. Geophys. Res. Lett. 2015;42:3980–3988. doi: 10.1002/2015GL063488. [DOI] [Google Scholar]
- 54.Pootakham W, et al. Dynamics of coral‐associated microbiomes during a thermal bleaching event. MicrobiologyOpen. 2018;7:e00604. doi: 10.1002/mbo3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neave MJ, Apprill A, Ferrier-Pagès C, Voolstra CR. Diversity and function of prevalent symbiotic marine bacteria in the genus. Endozoicomonas. Appl. Microbiol. Biotechnol. 2016;100:8315–8324. doi: 10.1007/s00253-016-7777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer JL, Paul VJ, Teplitski M. Community shifts in the surface microbiomes of the coral Porites astreoides with unusual lesions. PLoS ONE. 2014;9:e100316. doi: 10.1371/journal.pone.0100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayer T, et al. The microbiome of the Red Sea coral Stylophora pistillata is dominated by tissue-associated Endozoicomonas bacteria. Appl. Environ. Microbiol. 2013;79:4759–4762. doi: 10.1128/AEM.00695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jessen C, et al. In-situ effects of eutrophication and overfishing on physiology and bacterial diversity of the Red Sea coral Acropora hemprichii. PLoS ONE. 2013;8:e62091. doi: 10.1371/journal.pone.0062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrow KM, et al. Natural volcanic CO2 seeps reveal future trajectories for host–microbial associations in corals and sponges. ISME J. 2015;9:894–908. doi: 10.1038/ismej.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dubé CE, Ky CL, Planes S. Microbiome of the black-lipped pearl oyster Pinctada margaritifera, a multi-tissue description with functional profiling. Front. Microbiol. 2019;10:1548. doi: 10.3389/fmicb.2019.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neave MJ, Michell CT, Apprill A, Voolstra CR. Endozoicomonas genomes reveal functional adaptation and plasticity in bacterial strains symbiotically associated with diverse marine hosts. Sci. Rep. 2017;7:40579. doi: 10.1038/srep40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tandon K, et al. Comparative genomics: dominant coral-bacterium Endozoicomonas acroporae metabolizes dimethylsulfoniopropionate (DMSP) ISME J. 2020;14:1290–1303. doi: 10.1038/s41396-020-0610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ngugi DK, Ziegler M, Duarte CM, Voolstra CR. Genomic blueprint of glycine betaine metabolism in coral metaorganisms and their contribution to reef nitrogen budgets. iScience. 2020;23:101120. doi: 10.1016/j.isci.2020.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.González JM, Kiene RP, Moran MA. Transformation of sulfur compounds by an abundant lineage of marine bacteria in the α-subclass of the class Proteobacteria. Appl. Environ. Microbiol. 1999;65:3810–3819. doi: 10.1128/AEM.65.9.3810-3819.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Curson ARJ, Rogers R, Todd JD, Brearley CA, Johnston AWB. Molecular genetic analysis of a dimethylsulfoniopropionate lyase that liberates the climate-changing gas dimethylsulfide in several marine α-proteobacteria and Rhodobacter spharoides. Environ. Microbiol. 2008;10:757–767. doi: 10.1111/j.1462-2920.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 66.Reisch CR, Moran MA, Whitman WB. Bacterial catabolism of dimethylsulfoniopropionate (DMSP) Front. Microbiol. 2011;2:172. doi: 10.3389/fmicb.2011.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson JR, Rivera HE, Closek CJ, Medina M. Microbes in the coral holobiont: partners through evolution, development, and ecological interactions. Front. Cell. Infect. Microbiol. 2015;4:176. doi: 10.3389/fcimb.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Durante MK, Baums IB, Williams DE, Vohsen S, Kemp DW. What drives phenotypic divergence among coral clonemates of Acropora palmata? Mol. Ecol. 2019;28:3208–3224. doi: 10.1111/mec.15140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner MR, et al. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016;7:1–5. doi: 10.1038/ncomms12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuerst J, Sagulenko E. Beyond the bacterium: planctomycetes challenge our concepts of microbial structure and function. Nat. Rev. Microbiol. 2011;9:403–413. doi: 10.1038/nrmicro2578. [DOI] [PubMed] [Google Scholar]
- 71.Forquin-Gomez, M. P. et al. The family Brevibacteriaceae. In Prokaryotes Actinobacteria. 4th edn., (eds. Rosenberg E. et al.), 141–153 (Springer, 2014).
- 72.Baker BJ, Lazar CS, Teske AP, Dick GJ. Genomic resolution of linkages in carbon, nitrogen, and sulfur cycling among widespread estuary sediment bacteria. Microbiome. 2015;3:14. doi: 10.1186/s40168-015-0077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian RM, et al. Genomic analysis reveals versatile heterotrophic capacity of a potentially symbiotic sulfur‐oxidizing bacterium in sponge. Environ. Microbiol. 2014;16:3548–3561. doi: 10.1111/1462-2920.12586. [DOI] [PubMed] [Google Scholar]
- 74.Gauthier ME, Watson JR, Degnan SM. Draft genomes shed light on the dual bacterial symbiosis that dominates the microbiome of the coral reef sponge Amphimedon queenslandica. Front. Mar. Sci. 2016;3:196. doi: 10.3389/fmars.2016.00196. [DOI] [Google Scholar]
- 75.Dyksma S, et al. Ubiquitous Gammaproteo-bacteria dominate dark carbon fixation in coastal sediments. ISME J. 2016;8:1939–1953. doi: 10.1038/ismej.2015.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raina JB, Dinsdale EA, Willis BL, Bourne DG. Do the organic sulfur compounds DMSP and DMS drive coral microbial associations? Trends Microbiol. 2010;18:101–108. doi: 10.1016/j.tim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Morrow KM, Moss AG, Chadwick NE, Liles MR. Bacterial associates of two Caribbean coral species reveal species-specific distribution and geographic variability. Appl. Environ. Microbiol. 2012;78:6438–6449. doi: 10.1128/AEM.01162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabdono A, Radjasa OK. Phylogenetic diversity of organophosphorous pesticide-degrading coral bacteria from mid-west coast of Indonesia. Biotechnology. 2008;7:694–701. doi: 10.3923/biotech.2008.694.701. [DOI] [Google Scholar]
- 79.Kannapiran E, Ravindran J. Dynamics and diversity of phosphate mineralizing bacteria in the coral reefs of Gulf of Mannar. J. Basic Microbiol. 2012;52:91–98. doi: 10.1002/jobm.201100095. [DOI] [PubMed] [Google Scholar]
- 80.Mahmoud HM, Kalendar AA. Coral-associated actinobacteria: diversity, abundance, and biotechnological potentials. Front. Microbiol. 2016;7:204. doi: 10.3389/fmicb.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Probandt D, et al. Permeability shapes bacterial communities in sublittoral surface sediments. Environ. Microbiol. 2017;19:1584–1599. doi: 10.1111/1462-2920.13676. [DOI] [PubMed] [Google Scholar]
- 82.Doolittle WF, Booth A. It’s the song, not the singer: an exploration of holobiosis and evolutionary theory. Biol. Philos. 2017;32:5–24. doi: 10.1007/s10539-016-9542-2. [DOI] [Google Scholar]
- 83.Louca S, et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018;2:936–943. doi: 10.1038/s41559-018-0519-1. [DOI] [PubMed] [Google Scholar]
- 84.Kelly LW, et al. Local genomic adaptation of coral reef-associated microbiomes to gradients of natural variability and anthropogenic stressors. Proc. Natl Acad. Sci. USA. 2014;111:10227–10232. doi: 10.1073/pnas.1403319111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peixoto RS, Rosado PM, Leite DCDA, Rosado AS, Bourne DG. Beneficial microorganisms for corals (BMC): proposed mechanisms for coral health and resilience. Front. Microbiol. 2017;8:341. doi: 10.3389/fmicb.2017.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peixoto RS, et al. Coral probiotics: premise, promise, prospects. Annu. Rev. Anim. Biosci. 2021;9:265–288. doi: 10.1146/annurev-animal-090120-115444. [DOI] [PubMed] [Google Scholar]
- 87.Voolstra, C. R. et al. Extending the natural adaptive capacity of coral holobionts. Nat Rev Earth Environ. 1–16 (2021). 10.1038/s43017-021-00214-3.
- 88.Santoro, E. P. et al. Coral microbiome manipulation elicits metabolic and genetic restructuring to mitigate heat stress and evade mortality. Sci Adv.7 (2021). 10.1126/sciadv.abg3088. [DOI] [PMC free article] [PubMed]
- 89.Adam TC, et al. Landscape‐scale patterns of nutrient enrichment in a coral reef ecosystem: implications for coral to algae phase shifts. Ecol. Appl. 2021;31:e2227. doi: 10.1002/eap.2227. [DOI] [PubMed] [Google Scholar]
- 90.Buckling A, Kassen R, Bell G, Rainey PB. Disturbance and diversity in experimental microcosms. Nature. 2000;408:961–964. doi: 10.1038/35050080. [DOI] [PubMed] [Google Scholar]
- 91.Berga M, Szekely AJ, Langenheder S. Effects of disturbance intensity and frequency on bacterial community composition and function. PLoS ONE. 2012;7:e36959. doi: 10.1371/journal.pone.0036959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neulinger SC, Järnegren J, Ludvigsen M, Lochte K, Dullo WC. Phenotype-specific bacterial communities in the cold-water coral Lophelia pertusa (Scleractinia) and their implications for the coral’s nutrition, health, and distribution. Appl. Environ. Microbiol. 2008;74:7272–7285. doi: 10.1128/AEM.01777-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kanukollu S, et al. Distinct compositions of free-living, particle-associated and benthic communities of the Roseobacter group in the North Sea. FEMS Microbiol. Ecol. 2016;92:1. doi: 10.1093/femsec/fiv145. [DOI] [PubMed] [Google Scholar]
- 94.Santos HF, et al. Climate change affects key nitrogen-fixing bacterial populations on coral reefs. ISME J. 2014;8:2272–2279. doi: 10.1038/ismej.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sorokin DY, Tourova TP, Muyzer G. Citreicella thiooxidans gen. nov., sp. nov., a novel lithoheterotrophic sulfur-oxidizing bacterium from the Black Sea. Syst. Appl. Microbiol. 2005;28:679–687. doi: 10.1016/j.syapm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Chen, Y. J. et al. Metabolic flexibility allows generalist bacteria to become dominant in a frequently disturbed ecosystem. bioRxiv (2020). Preprint at 10.1101/2020.02.12.945220
- 97.Spring S, Scheuner C, Göker M, Klenk HP. A taxonomic framework for emerging groups of ecologically important marine gammaproteobacteria based on the reconstruction of evolutionary relationships using genome-scale data. Front. Microbiol. 2015;9:281. doi: 10.3389/fmicb.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Preston GM. Metropolitan microbes: type III secretion in multi-host symbionts. Cell Host Microbe. 2007;2:291–294. doi: 10.1016/j.chom.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 99.Lutz A, Raina J-B, Motti CA, Miller DJ, van Oppen MJH. Host coenzyme Q redox state is an early biomarker of thermal stress in the coral Acropora millepora. PLoS ONE. 2015;10:e0139290. doi: 10.1371/journal.pone.0139290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith DJ, Suggett DJ, Baker NR. Is photoinhibition of zooxanthellae photosynthesis the primary cause of thermal bleaching in corals? Glob. Chang. Biol. 2005;11:1–11. doi: 10.1111/j.1529-8817.2003.00895.x. [DOI] [Google Scholar]
- 101.Gardner SG, et al. A multi-trait systems approach reveals a response cascade to bleaching in corals. BMC Biol. 2017;15:1–14. doi: 10.1186/s12915-017-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lema KA, Bourne DG, Willis BL. Onset and establishment of diazotrophs and other bacterial associates in the early life history stages of the coral Acropora millepora. Mol. Ecol. 2014;23:4682–4695. doi: 10.1111/mec.12899. [DOI] [PubMed] [Google Scholar]
- 103.Pogoreutz C, et al. Nitrogen fixation aligns with nifH abundance and expression in two coral trophic functional groups. Front. Microbiol. 2017;8:1187. doi: 10.3389/fmicb.2017.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marangoni LF, et al. Peroxynitrite generation and increased heterotrophic capacity are linked to the disruption of the coral–dinoflagellate symbiosis in a scleractinian and hydrocoral species. Microorganisms. 2019;7:426. doi: 10.3390/microorganisms7100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quigley KM, Alvarez Roa C, Torda G, Bourne DG, Willis BL. Co‐dynamics of Symbiodiniaceae and bacterial populations during the first year of symbiosis with Acropora tenuis juveniles. MicrobiologyOpen. 2020;9:e959. doi: 10.1002/mbo3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dubé CE, Mercière A, Vermeij MJA, Planes S. Population structure of the hydrocoral Millepora platyphylla in habitats experiencing different flow regimes in Moorea, French Polynesia. PLoS ONE. 2017;12:e0173513. doi: 10.1371/journal.pone.0173513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agostini S, et al. Biological and chemical characteristics of the coral gastric cavity. Coral Reefs. 2012;31:147–156. doi: 10.1007/s00338-011-0831-6. [DOI] [Google Scholar]
- 108.Williams AD, Brown BE, Putchim L, Sweet MJ. Age-related shifts in bacterial diversity in a reef coral. PLoS ONE. 2015;10:e0144902. doi: 10.1371/journal.pone.0144902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sweet MJ, Brown BE, Dunne RP, Singleton I, Bulling M. Evidence for rapid, tide-related shifts in the microbiome of the coral Coelastrea aspera. Coral Reefs. 2017;36:815–828. doi: 10.1007/s00338-017-1572-y. [DOI] [Google Scholar]
- 110.Dubé CE, Boissin E, Mercière A, Planes S. Parentage analyses identify local dispersal events and sibling aggregations in a natural population of Millepora hydrocorals, a free‐spawning marine invertebrate. Mol. Ecol. 2020;29:1508–1522. doi: 10.1111/mec.15418. [DOI] [PubMed] [Google Scholar]
- 111.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 112.Dubé CE, Planes S, Zhou Y, Berteaux-Lecellier V, Boissin E. Genetic diversity and differentiation in reef-building Millepora species, as revealed by cross-species amplification of fifteen novel microsatellite loci. PeerJ. 2017;5:e2936. doi: 10.7717/peerj.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arnaud-Haond S, Belkhir K. GENCLONE: A computer pro- gram to analyze genotypic data, test for clonality and describe spatial clonal organization. Mol. Ecol. Notes. 2007;7:15–17. doi: 10.1111/j.1471-8286.2006.01522.x. [DOI] [Google Scholar]
- 114.Peakall R, Smouse PE. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes. 2006;6:288–295. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer, 2016).
- 116.R Development Core Team. R: A language and environment for statistical computing (ISBN 3-900051-07-0, http://www.R-project.org/ (R Foundation for Statistical Computing, 2020).
- 117.Andersson AF, et al. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PloS ONE. 2008;3:e2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Callahan BJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pedregosa F, et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- 122.Bokulich NA, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6:1–17. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yilmaz P, et al. The SILVA and "All-species Living Tree Project (LTP)" taxonomic frameworks. Nucl. Acids Res. 2014;42:D643–D648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oksanen, J. et al. vegan: Community Ecology Package (2018).
- 125.Weerdt WH. Transplantation experiments with Caribbean Millepora species (Hydrozoa, Coelenterata), including some ecological observations on growth forms. Bijdr. Dierkd. 1981;51:1–19. doi: 10.1163/26660644-05101001. [DOI] [Google Scholar]
- 126.Cáceres MD, Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90:3566–3574. doi: 10.1890/08-1823.1. [DOI] [PubMed] [Google Scholar]
- 127.Langille MG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The sequencing data generated in this study are available under NCBI BioProject ID PRJNA610240. Bacterial ASV reference sequences corresponding to the putative core microbiome are available under GenBank Accession numbers MZ045394-MZ045409. Other data generated in this study are provided in the Supplementary Data files.
Codes and scripts used for this study are available at: https://github.com/CarolineDUBE/Bacteria_NatCommun.