Abstract
Cardiovascular disease (CVD) is the leading cause of mortality worldwide yet, despite advances in treatment, CVD remains an underestimated and undermanaged condition, with an even greater risk in Type 2 Diabetes Mellitus (T2DM). Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2i) are a promising novel drug class reported to improve Cardiovascular (CV) and renal outcomes in T2DM. Recent large-scale trials have assessed their CV safety with unexpected findings of multiple systemic benefits that could potentially reverse CVD. In this systematic review, we examined ten Randomized Controlled Trials (RCTs) that looked at cardiovascular outcomes in Type 2 diabetics and SGLT-2i. The RCTs were appropriately screened, looking for clear primary or secondary outcomes on CV events, and compared with placebo or other antidiabetic drugs. The RCTs had an average sample population studied of 5,549 participants with a mean follow-up time of 2.66 years. Three of the studies focused on CV parameters and risk factors. The remaining had defined CV composite events, and all consistently observed at least one CV benefit when using SGLT-2i. Our review of SGLT-2i in Type 2 diabetics showed the greatest benefit in reducing Heart Failure (HF) exacerbation and modest lowering of CV complications in high CV risk participants. Overall, there is still uncertainty about the exact mechanisms of SGLT-2i in their CV benefit, and whether they would favor pre-diabetic populations and those at earlier stages of CVD.
Keywords: sodium-glucose cotransporter 2 inhibitor, diabetes type 2, cardiovascular disease, cardiovascular benefit, cardiovascular outcome trials
Introduction and background
Every 17 seconds, a new diabetes diagnosis is made in the United States (US) [1]. Worldwide, it is one of the most prevalent chronic conditions, forecasted to affect 700 million adults by 2045 [2]. Diabetes, notably Type 2 Diabetes Mellitus (T2DM), is a polygenic disease that has a range of clinical severity. Its pathogenesis is closely linked to environmental factors such as obesity, diet, and smoking - with serious implications for accelerating cardiovascular disease (CVD). T2DM and CVD are part of the ‘‘common soil hypothesis’’ in that they share many risk factors - both environmental and genetic [3]. It is known that the most common cause of morbidity in T2DM are CVD sequelae such as myocardial infarction (MI), heart failure (HF), and stroke with T2DM individuals at equal risk of MI and stroke when compared to an individual with a prior MI or stroke history [4]. Obesity is the leading risk factor for developing T2DM and is an independent contributor to CVD through other conditions including hypertension, obstructive sleep apnoea, and dyslipidemia [5]. It can be anticipated that the need to address CVD will increase as obesity is projected to reach 50% of the adult population in the US by 2030 [6]. Our limitation of treatment is evident by CVD conditions like chronic HF, and accelerated coronary artery disease (CAD), being two to four times more likely to develop in individuals with diabetes [4].
The introduction of Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2i), licensed in 2013 for T2DM, has often been used as an adjunct alongside other diabetic medication in severe cases. However, several new suggested mechanisms of SGLT-2i may be the key for optimizing glycemic control, earlier on, and improving CVD outcomes. SGLT-2i works by blocking an important sodium-coupled glucose channel in the proximal tubule of the kidney. This promotes natriuresis with glucose loss that has multiple benefits - as seen in randomized controlled trials (RCTs) - including weight loss, lowering blood pressure, urate, and plasma volume. It has been observed that SGLT-2i has a unique role in reducing cardiovascular (CV) events, atherosclerosis, and exacerbation of heart failure (HF) [7]. This has been demonstrated further in a recent large-international trial of T2DM patients (the Asia Pacific, the Middle East, and North America) that SGLT-2i, even in patients without a CVD diagnosis, had a significantly lower risk of atherothrombotic events and death overall [8].
However, despite the promising effects of SGLT-2i and their cardiorenal protection, there is still disparity about their mechanisms for reducing CVD risk. We do not yet understand the systemic effects and the extent that SGLT-2i benefit diabetic patients - especially in the context of co-morbidities. Ongoing Cardiovascular Outcome Trials (CVOTs) have shown mixed data with degrees of significance [9]. It has prompted debate as to whether SGLT-2i cardiorenal protection is relevant in pre-diabetic populations, who would benefit most, and what are the reasons for their clinical benefit in the CVOTs [10].
Review
In an aging population, diabetes will grow as one of the greatest global challenges. It already represents a major economic burden on healthcare systems with 9.4 times greater per capita spent on healthcare for diabetic complications [11]. Our systematic review aims to analyse the largest clinical trials to date to establish the level of known cardiovascular benefit from SGLT-2i. We will also evaluate the mechanisms proposed, and provide clarity on vulnerable subgroups that would benefit most from SGLT-2i intervention. The articles in this review were systematically screened and their methods and results are outlined.
Method
Our methods and results for systematic review are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines following our screening selection [12].
Search Strategy
We used electronic databases PubMed and Cochrane Library to look for articles using Medical Subject Headings (MeSH) and keywords to highlight the most relevant reviews and studies for analysis. The keywords included: ‘‘Diabetes Mellitus Type 2’’, ‘‘Sodium-Glucose Transporter 2 Inhibitors’’, ‘‘Cardiovascular Disease’’ and ‘‘Outcomes’’. We used the Boolean method to put together the keywords to an algorithm to use in PubMed. The articles were screened to highlight those most relevant to the search question and selected according to the inclusion/exclusion criteria.
Inclusion and Exclusion Criteria
The selection choice was purely from randomized control trials (RCTs) published from 2016-2021. All selected articles were peer-reviewed and published in the English language. Grey Literature was excluded. Our selection for eligibility followed the Population, Intervention, Comparison, Outcomes (PICO) model.
Data Extraction
The data retrieval and review were completed by two separate researchers independently. In the case of any disagreements, the researchers would discuss the data for its relevance and design to eligibility criteria to reach an accord. A third researcher was counseled for objectivity if a decision could not be met.
Critical Appraisal of Studies
We critically appraised our screened articles using the Cochrane risk of bias tool [13]. The bias risk assessment looked at seven causes of potential bias, and a summary was given for each clinical trial in this review in Table 1.
Table 1. A summary of the RCTs bias using the Cochrane assessment tool.
RCT=Randomized Controlled Trial
| COCHRANE APPRAISAL | Random Sequence Generation - Selection bias | Allocation of concealment - Selection bias | Blinding of both participants and evaluators - Performance bias | Blinding of assessment during outcome collection - Detection bias | Incomplete outcome data - Attrition bias | Selective reporting - Reporting bias | Other bias / Comments |
| Wanner et al. 2016 [14] EMPA-REG | LOW RISK | HIGH RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | Small sample size to generalise to the broader population |
| Neal et al. 2017 [10] CANVAS | LOW RISK | LOW RISK | HIGH RISK | LOW RISK | LOW RISK | LOW RISK | Few clinical events, small proportion of participants to generalise |
| Wiviott et al. 2018 [7] DECLARE TIMI-58 | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | Premature discontinuation of thousands of patients from each study group - statistical data possibly affected |
| Perkovic et al. 2019 [15] CREDENCE | LOW RISK | LOW RISK | LOW RISK | LOW RISK | HIGH RISK | LOW RISK | Trial stopped early - potential limited power |
| Phrommintikul et al. 2019 [16] | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | Small sample size |
| Bonora et al. 2019 [17] | LOW RISK | LOW RISK | LOW RISK | LOW RISK | UNCLEAR | LOW RISK | Small sample size |
| Petrie et al. 2020 [18] DAPA-HF | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | N/A |
| Fuchigami et al. 2020 [19] | LOW RISK | LOW RISK | LOW RISK | HIGH RISK | LOW RISK | LOW RISK | Small sample size, poor genetic diversity (all Japanese), short follow up |
| Packer et al. 2020 [20] EMPEROR-REDUCED | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | LOW RISK | N/A |
| Cannon et al. 2020 [21] VERTIS-CV | LOW RISK | LOW RISK | LOW RISK | HIGH RISK | LOW RISK | LOW RISK | N/A |
Results
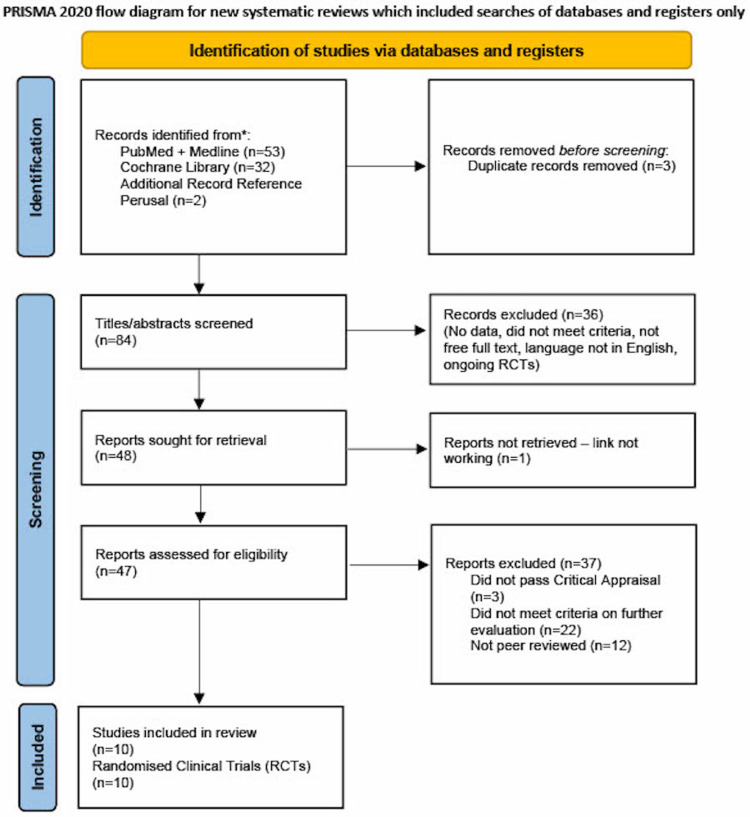
A total of 87 articles were generated from keywords, eligibility criteria, and databases. Of the 87 articles, 53 were from PubMed, 32 from Cochrane Library, and two articles were obtained from references to relevant journals. Duplicates were removed and 84 were screened from their titles and abstracts. A further 36 were discarded due to topic irrelevance. Of the remaining 48, one was removed for the link not working, three for not passing the critical appraisal, 22 for not meeting the criteria, and 12 for not being peer-reviewed. 10 articles met the criteria and were only RCTs. Our PRISMA flow diagram is shown below in Figure 1.
Figure 1. PRISMA 2020 flow diagram .
PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analyses [12]
All the reviewed clinical trials differed in design, population, and primary endpoints. However, the study of SGLT-2i and Major Adverse Cardiovascular Events (MACE) was a common part of each RCT. This is shown in a summarized table below in Table 2.
Table 2. An outline summary of the clinical trials (n = number of patients in RCT).
RCT=Randomized Controlled Trial, MACE=Major Adverse Cardiovascular Events, CVD=Cardiovascular Disease, RF=Risk Factors, MI= Myocardial Infarction, HF= Heart Failure, eGFR=Estimated Glomerular Filtration Rate, ESRD=End Stage Renal Disease, BMI=Body Mass Index, ACEi=Angiotensin Converting Enzyme Inhibitors, ARB=Angiotensin Receptor Blockers, T2DM=Type 2 Diabetes Mellitus, HbA1c=Glycosylated Hemoglobin Type A1C, HDL=High Density Lipoprotein, SBP=Systolic Blood Pressure, HFrEF=Heart Failure with Ejection Failure, NYHA=New York Heart Association, LVEF=Left ventricular ejection fraction, NT-proBNP=N-Terminal pro B-type Natriuretic Peptide, ICD=Implantable cardiac defibrillator.
| COCHRANE APPRAISAL | Study Design | Inclusion criteria | Intervention | Primary Outcome | Secondary Outcome | Conclusions |
| Wanner et al. 2016 [14] EMPA-REG | Multicentre randomized double-blind placebo-controlled trial | Insufficient glycemic control and High risk of CV events N = 7020 | Empagliflozin 10mg, Empagliflozin 25mg or Placebo | First occurrence of MACE (3-point) which included death from CV causes, non-fatal MI, or nonfatal stroke. | Expanded occurrence of MACE to include unstable angina as well as HF exacerbation, renal events and transient ischemic attack | Primary outcome - significant lower risk of MACE in the empagliflozin group than in the placebo group |
| Neal et al. 2017 [10] CANVAS | Randomized double-blind placebo-controlled | Male or female T2DM ≥ 30yrs with symptomatic CVD or 50yrs or older with two or more RF for CVD N = 9734 | Canagliflozin 100mg, Canagliflozin 300mg or Placebo | Composite of death from CV causes, nonfatal MI or nonfatal stroke | Death from any cause, from CV cause, progression of albuminuria and composite of death from hospitalization for HF | Pt. with T2DM with Canagliflozin had lower risk of death from CV cases, nonfatal myocardial infarction, nonfatal stroke than placebo |
| Wiviott et al. 2018 [7] DECLARE TIMI-58 | Randomized double-blind placebo-controlled | Male or female T2DM ≥ 40 yrs with T2DM and High risk for CV Events N = 17160 | Dapagliflozin 10mg or Placebo | Composite endpoint of CV death, MI, Ischemic stroke or Hospitalization due to HF | Renal Composite endpoint - ≥40% decrease in eGFR to eGFR <60 ml/min/1.73m2 and/or ESRD and/or Renal or CV death | Pt. with T2DM with treatment with dapagliflozin did not result in higher or lower MACE rate than placebo but did result in lower rate of CV death or hospitalization for HF. |
| Perkovic et al. 2019 [15] CREDENCE | Randomized double-blind placebo-controlled | T2DM with HbA1c ≥6.5 and ≤12% with eGFR ≥30 and ≤90, Pt. need to be on maximum tolerated dose of ACEi or ARB 4 weeks prior to randomization, urine albumin to creatinine ratio >300mg/g and <5000mg/g N = 4401 | Canagliflozin 100mg or Placebo once daily | Composite of doubling of serum creatinine, ESRD and renal or CV death | Composite Endpoint of CV death and Hospitalized HF | Pt. with T2DM and kidney disease, the risk of CV events was lower in the canagliflozin group than placebo |
| Phrommintikul et al. 2019 [16] | Prospective randomized double-blinded study | Adults ≥21 or non-childbearing potential female, inadequately controlled T2DM with at least half maximum dose of metformin. Stable documented coronary artery disease N = 49 | Dapagliflozin 10mg or Vildagliptin 50-100mg | Individuals who have ST segment depression, average SBP, myocardial dysfunction, myocardial injury, oxidative stress, ventricular wall stretch and inflammation event. | N/A | Cardiovascular benefits observed only in SGLT-2i |
| Bonora et al. 2019 [17] | Randomized single blind placebo control | Male and Female aged 18-75yrs, T2DM on oral agents +/- insulin, with T2DM duration > 6 months with HbA1c 7-10% N = 33 | Dapagliflozin 10mg or Placebo | Change from baseline in reverse cholesterol transport, measured as cholesterol efflux capacity of patient's plasma | Change from baseline in HDL cholesterol levels, Changes from baseline in the distribution in HDL subclasses, and adverse events | Observed no changes in cardiac function parameters estimated by impedance cardiography in T2DM. |
| Petrie et al. 2020 [18] DAPA-HF | Randomized quadruple blinded placebo-controlled | Male or Female aged ≥18yrs, diagnosis of symptomatic HFrEF (NYHA class II-IV) present for 2 months, LVEF ≤40%, elevated NT-proBNP eGFR ≥30mL/min/1.73m2 N = 4742 | Dapagliflozin 5mg, 10mg or Placebo | Composite endpoint of CV death, hospitalization due to heart failure or due to HF | Recurrent hospitalizations due to HF and CV death, composite endpoint of ≥50% sustained decline in eGFR, ESRD or Renal Death, endpoint of all-cause mortality | Dapagliflozin when added to recommended therapy significantly reduced the risk of worsening HF or CV death independent of diabetes status |
| Fuchigami et al. 2020 [19] | Randomized parallel open blinded study | Male and female pt. who are ≥20yrs but ≤80yrs, T2DM, no antidiabetic medication or only using beguanide, HbA1c ≥7.1%, BMI ≥23kg/m2 N = 340 | Dapagliflozin 5mg or Sitagliptin 50mg | Achieving HbA1c ≤ 7%, Body weight loss of 3% and avoidance of hypoglycaemia | Reduction in fasting blood glucose, body weight, BMI, lipid metabolism marker and other cardiovascular parameters | Compared to sitagliptin, dapagliflozin was more effective at improving cardiometabolic RF |
| Packer et al. 2020 [20] EMPEROR-REDUCED | Randomized double blinded placebo-controlled | Male or female ≥18yrs, pt. with chronic HF and elevated NT-proBNP, use of medical devices such as ICDs N = 3730 | Empagliflozin 10mg or Placebo | Time to first event of CV death or hospitalization for HF | Total number of HF events, eGFR slope of change from baseline, all-cause mortality, all-cause hospitalization | Empagliflozin group had lower risk of CV or HF hospitalization than placebo group whether diabetic or not |
| Cannon et al. 2020 [21] VERTIS-CV | Randomized double-blind placebo-controlled | T2DM diagnosis, HbA1c 7-10.5%, on stable anti-hyperglycaemic agents, BMI ≥18, hx of atherosclerosis or cerebro/peripheral vascular disease N = 8238 | Ertugliflozin 5mg, 15mg or Placebo | Time to first MACE, change from baseline in HbA1c% at week 18, change from Haemoglobin baseline at week 18 | Time to occurrence of CV death or HF hospitalization, composite of renal death, renal dialysis/transplant, or doubling serum creatinine | Pt. with T2Dm and atherosclerotic CV disease, ertugliflozin was not significantly different for MACE when compared to placebo |
Out of the 10 RCTs chosen, seven were double-blinded, one was quadruple blinded, with the remaining two RCTs open [19] and single-blinded [17]. The primary outcomes were a mixture of the timing of MACE, a composite endpoint of CV death, hospitalization due to HF, renal function, HbA1c reduction, and changes in cholesterol baseline. The majority of the large-scale trials also investigated for secondary outcomes that included a reduction in a range of renal, stroke, and hospitalization events. These secondary outcomes varied from mild to moderate beneficial changes that were statistically significant alongside the primary outcomes [20].
The populations being studied included adult males and females with set and defined CV risk factors or already established CVD [18]. The majority of RCTs were composed of T2DM participants with poor glycemic control that were screened with different parameters according to their renal function, HbA1c, medication status, BMI, CV risk or due to a combination of these. The SGLT-2i used in RCTs differed in dose and type and included Empagliflozin, Dapagliflozin, Canagliflozin and Ertugliflozin.
Three of the RCTs had significantly smaller population studies (less than 400), which is important to consider with their respective study conclusions. In terms of primary outcomes, from Table 2, it is evident that the RCTs have yielded varying results when assessing cardioprotective effects of SGLT-2i. Bonora et al. 2019 [17], did not show any change in cardiac function, and the DECLARE-TIMI [7] trial showed cardiovascular benefit only in reduction of hospitalization from HF and CV death. The VERTIS-CV trial [21] also showed no significant change in MACE events or CV death in participants taking the SGLT-2i relative to placebo.
SGLT-2i across all RCTs did not demonstrate poorer primary CV outcomes when compared to the placebo.
However, with the exception of the VERTIS-CV trial [21], the other six larger scale RCTs (population size greater than 3000) observed a primary outcome beneficial reduction in MACE events or CV hospitalization. The reduction was noted particularly for HF exacerbation, including RCTs that included diabetic and non-diabetic individuals [20]. The RCTs also had relatively few participants lost to follow-up from the administration and tolerability of the drug. The mean time for follow-up of the seven large-scale RCTs was 2.66 years. They show SGLT-2i are non-inferior to placebos. The most significant cardioprotective effects were seen in patients already at a high risk of cardiovascular events and severe HF - noted in EMPEROR-REDUCED by the percentage of ejection fraction and those who had a history in the last 12 months of HF exacerbation and level of N-terminal brain natriuretic peptide (BNP) [20].
Discussion
In order to bring new questions and conclusions in this systematic review, we will analyze the differences in RCT outcomes, the true cardiovascular benefit in T2DM, as well as the study limitations and potential mechanisms of SGLT-2i.
Cardiovascular Benefits of SGLT-2i and Limitations
The RCTs in this review have shown cardiorenal protection in T2DM patients with SGLT-2i not currently seen in other anti-diabetic drugs. EMPA-REG 2016 trial, 7028 patients, all at high risk of CVD, reported a reduction in death due to CV causes in the SGLT-2i group (empagliflozin) vs placebo. This finding is reinforced by the CANVAS 2017 trial, with 9734 subjects reporting fewer rates of Major Adverse Cardiovascular Event (MACE) in the SGLT-2i group (canagliflozin) than placebo [10].
Across the large scale RCTs, the most significant cardiovascular benefit seen collectively in these RCTs has been a reduction in the effect on HF hospitalization - most notably in the CREDENCE [15] and EMPEROR-REDUCED [20] with CREDENCE specifically for HF hospitalization reporting a hazard ratio of 0.61; 95% CI, 0.47-0.8 P <0.001. The EMPEROR-REDUCED [20] has confirmed the DAPA-HF [18] findings where, in a cohort of 3730, lower heart failure hospitalization was considerable in the SGLT-2i group with a CV history (i.e., individuals with the previous MACE). It is evident that this benefit is amplified in T2DM and patients with chronic kidney disease (CKD).
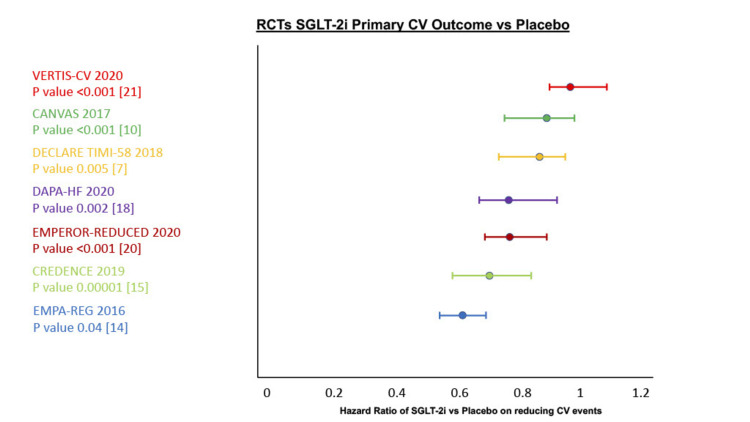
However, with such differing RCTs in study design and criteria, we decided to review the large-scale RCTs in isolation from the smaller scale homogenous trials to establish the true cardiovascular benefit from SGLT-2i vs placebo outlined below in Table 3 and Figure 2.
Table 3. A summary of cardiovascular outcomes in SGLT-2i vs Placebo.
RCT=Randomized Controlled Trials, CI=Confidence Interval, MACE=Major Adverse Cardiovascular Events, CV=Cardiovascular, MI=Myocardial Infarction, HF=Heart Failure
| RCT vs Placebo | Population studied | Intervention | Median follow up | Primary Outcome measured | Hazard Ratio for Primary Outcome | 95% Confidence Interval (CI) and P value |
| EMPA-REG 2016 [14] | 7020 | Empagliflozin 10mg, 20mg vs Placebo | 3.1 years | Composite of Cardiovascular death | 0.61 | 95% CI 0.55-0.69 P value <0.001 |
| CANVAS 2017 [10] | 9734 | Canagliflozin 100mg, 300mg vs Placebo | 2.43 years | MACE | 0.86 | 95% CI 0.75-0.97 P value <0.001 |
| DECLARE TIMI-58 2018 [7] | 17160 | Dapagliflozin 10mg vs Placebo | 4.2 years | Composite of CV death or HF hospitalization | 0.83 (lower HF hospitalizations) | 95% CI 0.73-0.95 P value = 0.005 |
| CREDENCE 2019 [15] | 4401 | Canagliflozin 100mg vs Placebo | 2.62 years | Composite of end-stage kidney disease or death from renal or CV causes | 0.7 | 95% CI 0.59-0.82 P value = 0.00001 |
| DAPA-HF 2020 [18] | 4742 | Dapagliflozin 5mg, 10mg vs Placebo | 1.5 years | Composite of worsening HF or CV death | 0.75 | 95% CI 0.63-0.9 P value 0.002 |
| EMPEROR-REDUCED 2020 [20] | 3730 | Empagliflozin 10mg vs Placebo | 1.3 years | Composite of CV death or HF hospitalization | 0.75 | 95% CI 0.65 to 0.86 P value <0.001 |
| VERTIS-CV 2020 [21] | 8238 | Ertugliflozin 5mg, 15mg vs Placebo | 3.5 years | MACE | 0.97 | 95% CI 0.85-1.11 P value <0.001 |
Figure 2. Cardiovascular Outcomes Hazard Ratio vs Placebo.
RCT=Randomized Clinical Trial, CV=Cardiovascular, SGLT-2i=Sodium-Glucose Cotransporter-2 Inhibitors
RCTs - VERTIS-CV [21], EMPA-REG [14], CANVAS [10], DECLARE TIMI-58 [7], DAPA-HF [18], CREDENCE [15], EMPEROR-REDUCED [20]
It is evident, from Figure 2, that the true cardiovascular benefit is overall promising with statistically significant reductions in primary CV outcomes in six of the seven large-scale RCTs. The most recent VERTIS-CV trial did not show a cardiovascular benefit in SGLT-2i, and the difference is likely multifactorial.
Firstly, there is a degree of unknown as to how SGLT-2i exert their systemic beneficial effects and whether this is merely due to chance. This is a crucial debate on SGLT-2i mechanisms that is masking the true long-term benefits of this drug class. Secondly, there is a dissimilarity among the CVOTs due to the population profile, the inclusion and exclusion criteria, and the specified endpoints that did not all statistically measure HF hospitalization like in the VERTIS-CV trial [21]. Thirdly, the difference in primary outcomes can be partly attributed to distortion, especially in recent studies where patient cohorts have potentially been receiving more rigorous CVD prevention therapies. The increasing use of medications such as anticoagulants, biguanides, and statins means that patients generally have been experiencing secondary prevention for a longer period - thereby underestimating the true benefit of SGLT-2i in isolation [22,23].
We decided to not include the three smaller trials for true CV benefit evaluation because their data was founded on sample sizes of less than 50 patients, with short follow-up times, that may have underestimated the long-term effects of SGLT-2i on cardiovascular function and remodeling [16,17]. The third smaller trial showed promising findings of SGLT-2i vs sitagliptin but the data stemmed from patients only in Japan [19]. This is important to consider, given the large genetics at play in CVD, when conducting future research to recruit a large heterogeneous sample.
The CVOTs primary and secondary outcome data indicate that the renal system plays a significant role in SGLT-2i beneficial outcomes. This can underestimate the benefit of SGLT-2i when only small proportions of patients with established kidney disease are included or when patients with severe kidney disease are excluded [7,10]. At the same time, this is where new research needs to expand on the impact SGLT-2i have in patients without established CV or renal disease, as many patients in RCTs benefitted despite not having a history of CVD or CKD. To optimize our understanding of SGLT-2i on CVD, the relative proportion and severity of individuals with established CVD should be more closely outlined in future RCTs, and a thorough CV history from participants is pivotal to minimize the potential for any confounders [20].
Despite the positive findings from six of the CVOTs for reducing CV events, there is insufficient data to extrapolate these findings to the general population [10,14]. The mean follow-up time across the seven CVOTs was 2.66 years. In order to ascertain the long-term benefits of SGLT-2i, subsequent research should either follow up T2DM participants over a longer period of time, or would need to ensure a large enough and relevant population (CV and T2DM risk) pool to extrapolate to the general population. The long-term benefits of SGLT-2i should be aimed at comparing the rates of micro- and macrovascular complications in T2DM.
New research should focus on minorities more at risk of CVD e.g., African Americans and Hispanics. These cohorts with T2DM should be further evaluated to see the effects of SGLT-2i and would provide more data to help represent the true population. Other significant cardiovascular outcomes have included a reduction in cardiac risk of death in patients without a diagnosis of diabetes [20]. This drives a need for future research to explore and evaluate the true benefit of SGLT-2i in individuals at risk of CVD or individuals who are pre-diabetic. It was estimated that 88 million adults had pre-diabetes in the US alone in 2018 with an estimated 18.2 million adults having a degree of coronary heart disease - the most common type of CVD and closely linked with diabetes [24,25].
Our limitations in this review were being unable to comment on the long-term CV benefits of SGLT-2i as the majority of data from the CVOTs are not applicable to the general population, based on patient demographic and strict T2DM status, and more ongoing data from current trials are warranted before a wide conclusion can be drawn. We also did not fully evaluate the adverse side effects of SGLT-2i as the safety profile of SGLT-2i has been well documented, and there was minimal to add from the CVOTs. The majority of adverse side effects in the CVOTs commented on genital and urinary infections, being greater in the SGLT-2i group than placebo, and levels of diabetic ketoacidosis being slightly higher in patients on SGLT-2i. There was data conflict as to whether SGLT-2i increased the risk of amputations or bone fractures [7,10,15].
We would hope that in a longer-scale trial with a larger population that these safety concerns could be better assessed. In general, it has been regarded as a relatively safe drug to use, and close monitoring can be easily achieved.
Overall, SGLT-2i have shown significant CV benefit, the greatest benefit of reducing hospitalizations, CV death, and HF exacerbations compared to MACE. This could mean earlier use of SGLT-2i in patients with recent T2DM diagnosis or HF as these are progressive diseases. The use in non-diabetics or pre-diabetics with risk factors for CVD or CKD should also be strongly considered, and should be a focal point for future trials.
Mechanism of Action of SGLT-2i
There are many proposed SGLT-2i mechanisms though what is less known are the primary mechanisms SGLT-2i exert their CV benefits. Certain accompanying factors have been proposed for explaining how they exert protective effects for specific CVD like HF exacerbation [26].
It is agreed upon that SGLT-2i in T2DM works by inhibiting the sodium-glucose transporter in the proximal tubule of the kidneys, reducing the amount of sodium and glucose reabsorbed, and promoting natriuresis and glycosuria. However, hyperglycemia has not been shown to be a strong or a reliable risk factor for CVD and would not explain the CV benefit seen acutely in both diabetic and non-diabetic patients [27].
The natriuresis effect has been shown to increase sodium excretion by up to 60% and this is thought to reduce the afterload on the heart and improve ventricular cardiac function [28]. However, the blood pressure effect shown from the CVOTs is modest and is unlikely to be the main reason for lower CV risk [14,20]. Clinical trials have speculated on the diuretic effect of SGLT-2i, and it has been shown to decrease albuminuria, plasma volume, and this has been thought to be the main driver in reducing HF exacerbation [10]. A suggested explanation for SGLT-2i has been that a reduction in plasma volume, intraglomerular pressure, interstitial volume, and an increase in erythrocyte mass are key to SGLT-2i systemic effects - not seen in loop and thiazide diuretics [29].
The CVOTs have seen a consistent reduction in cardiovascular HF hospitalization, the most notable in the DAPA-HF and the EMPEROR-REDUCED trial that had a reduction in HF Hazard Ratio of 0.7; 95% CI 0.58-0.85 P<0.001 [20]. Poor cardiac function is thought to lead to dysfunctional energy metabolism such as mitochondrial oxidative phosphorylation, making the heart more dependent on glycolysis [30]. SGLT-2i have demonstrated an increase in ketogenesis from adipose tissue thereby providing alternative energy for the heart and restoring levels of adenosine triphosphate (ATP). This suggested mechanism has yet to be evaluated in humans and should be a point of reference for future research [31].
While other CV events are less clear in terms of inflammation, HF severity is correlated with increased inflammatory biomarkers. There is not a clinically proven SGLT-2i drug class as superior to one another however, empagliflozin, canagliflozin, and dapagliflozin have shown benefit in regulating the immune system by downregulating extracellular matrix processes and levels of fibrosis [32]. This likely helps reduce Reactive Oxygen Species (ROS) and improve contractile function. The SGLT-2i cardioprotective effects are also suggested to be chronic inflammatory modulation of many processes such as macrophage response and specifically the nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 3 (NLRP3) inflammasome that is likely to help prevent cardiac remodeling [33].
Looking at the EMPA-REG outcome trial which raises the possibility of myocardium effects, further analysis showed a decrease in the Left Ventricular (LV) mass index of participants on empagliflozin vs placebo. This raises new research opportunities to assess the extent of reversibility, and the impact this has on cardiac function in the long term [14,34,35]. Much of the renal cardiorenal benefits in this trial continued to improve after discontinuation, indicating that SGLT-2i may have greater potency and reversibility than previous thought.
The DAPA-HF data demonstrates this observation of SGLT-2i independent of diabetes and the potential increase in erythropoietin (EPO) that could be behind improving renal function [18]. Key areas of study need to look further into SGLT-2i on sympathetic nervous system reduction, the extent to which failing hearts use ketones as secondary fuel, and the inflammatory process that raises the CV risk profile.
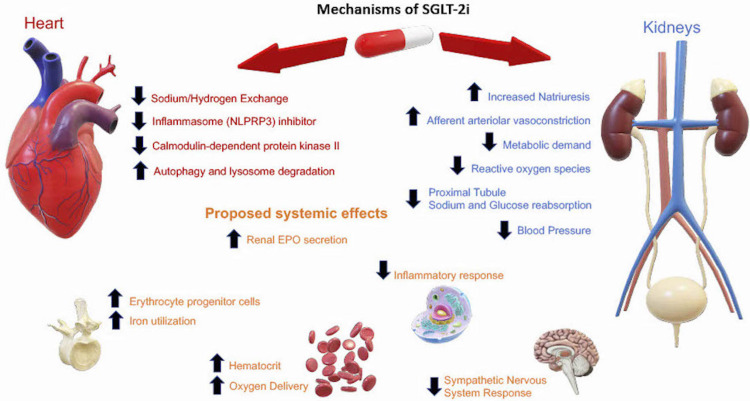
Looking at the data from CVOTs, as well as experimental data, we can infer that the CV benefits are due to direct prevention of pathologic cardiac remodeling and indirectly by renal protection. SGLT-2i likely exert their indirect systemic effects by promoting afferent arteriolar constriction, reducing renal stress and intraglomerular pressure, and this lowers metabolic demand on the cardiorenal system and ultimately helps to maintain contractility. The proposed mechanisms known so far are summarised in Figure 3.
Figure 3. SGLT-2i Mechanisms of systematic and cardiorenal effects.
SGLT-2i=Sodium-Glucose Cotransporter-2 Inhibitors, NLPRP3=Nucleotide-binding Oligomerization Domain, Leucine-rich Repeat and Pyrin Domain-containing 3, EPO=Erythropoietin
Conclusions
SGLT-2i have proven to be a relatively well tolerated and safe drug - in the doses studied in the CVOTs - with the majority reducing the risk of HF exacerbation and hospitalization in T2DM. There was a moderate effect in lowering the risk of composite death from all CV causes, although there were mixed data likely due to limited homogenous sample size populations, and cohorts in recent trials already receiving extensive CV prevention therapy. A more interesting observation was the same cardiovascular benefit seen in patients with and without diabetes, raising the notion of SGLT-2i mechanisms being more independent regarding glycemic control.
Our systematic review brings in a wider scope of data focused on cardiovascular benefits. It shows that the favorable mechanisms of SGLT-2i are still unclear, and this should be a point for future research to study the extent that SGLT-2i prevent cardiac remodeling, the direct and indirect effects they have on the heart, and the relationship to whether the clinical benefits are largely from renal protection. The main limitation of our study is not being able to evaluate the true long-term adverse side effects of SGLT-2i, and the long-term benefit-risk in vulnerable individuals. This is vital to address in future interventional studies with longer follow-ups for participants. We should aim to examine a larger sub-group population to assess SGLT-2i in genetically higher risk CV groups, individuals with recent T2DM or HF diagnosis, and the effects in pre-diabetics. This will help us analyze more clearly the likely beneficial mechanisms of SGLT-2i and its application to the general population.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Increasing awareness about peripheral artery disease can save limbs and lives. [ Jun; 2021 ];Fakorede FA. https://www.ajmc.com/view/contributor-increasing-awareness-about-peripheral-artery-disease-can-save-limbs-and-lives. Am J Manag Care. 2018 24:0. [PubMed] [Google Scholar]
- 2.Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. [ Jun; 2021 ];Saeedi P, Petersohn I, Salpea P, et al. Diabetes Res Clin Pract. 2019 157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Metabolic syndrome and cancer: "The common soil hypothesis". Bellastella G, Scappaticcio L, Esposito K, Giugliano D, Maiorino MI. Diabetes Res Clin Pract. 2018;143:389–397. doi: 10.1016/j.diabres.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Front Endocrinol (Lausanne) 2018;9:2. doi: 10.3389/fendo.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardiovascular risk and obesity. Cercato C, Fonseca FA. Diabetol Metab Syndr. 2019;11:74. doi: 10.1186/s13098-019-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Projected U.S. state-level prevalence of adult obesity and severe obesity. Ward ZJ, Bleich SN, Cradock AL, et al. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 7.Dapagliflozin and cardiovascular outcomes in type 2 diabetes. Wiviott SD, Raz I, Bonaca MP, et al. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 8.Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. Kosiborod M, Lam CS, Kohsaka S, et al. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 9.The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PA, Rangaswami J. Cardiorenal Med. 2020;10:1–10. doi: 10.1159/000503919. [DOI] [PubMed] [Google Scholar]
- 10.Canagliflozin and cardiovascular and renal events in type 2 diabetes. Neal B, Perkovic V, Mahaffey KW, et al. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 11.Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. Khan MA, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. J Epidemiol Glob Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. https://journals.plos.org/plosmedicine/article. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RoB 2: A revised Cochrane risk-of-bias tool for randomized trials. [ Jul; 2021 ];https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. 2021 doi: 10.1016/j.jclinepi.2020.06.015. [DOI] [PubMed]
- 14.Empagliflozin and progression of kidney disease in type 2 diabetes. Wanner C, Inzucchi SE, Lachin JM, et al. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 15.Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. Perkovic V, Jardine MJ, Neal B, et al. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 16.Effects of dapagliflozin vs vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: a randomised study. Phrommintikul A, Wongcharoen W, Kumfu S, Jaiwongkam T, Gunaparn S, Chattipakorn S, Chattipakorn N. Br J Clin Pharmacol. 2019;85:1337–1347. doi: 10.1111/bcp.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Effects of the SGLT2 inhibitor dapagliflozin on cardiac function evaluated by impedance cardiography in patients with type 2 diabetes. Secondary analysis of a randomized placebo-controlled trial. Bonora BM, Vigili de Kreutzenberg S, Avogaro A, Fadini GP. Cardiovasc Diabetol. 2019;18:106. doi: 10.1186/s12933-019-0910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. Petrie MC, Verma S, Docherty KF, et al. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: a prospective, randomized study (DIVERSITY-CVR) Fuchigami A, Shigiyama F, Kitazawa T, et al. Cardiovasc Diabetol. 2020;19:1. doi: 10.1186/s12933-019-0977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardiovascular and renal outcomes with empagliflozin in heart failure. Packer M, Anker SD, Butler J, et al. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 21.Cardiovascular outcomes with ertugliflozin in type 2 diabetes. Cannon CP, Pratley R, Dagogo-Jack S, et al. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 22.Type 2 diabetes Therapies: a STEPS approach. [ Jul; 2021 ];Steinberg J, Carlson L. https://www.aafp.org/afp/2019/0215/p237.html. Am Fam Physician. 2019 99:237–243. [PubMed] [Google Scholar]
- 23.Time trends in statin use and incidence of recurrent cardiovascular events in secondary prevention between 1999 and 2013: a registry-based study. Laleman N, Henrard S, van den Akker M, Goderis G, Buntinx F, Van Pottelbergh G, Vaes B. BMC Cardiovasc Disord. 2018;18:209. doi: 10.1186/s12872-018-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. [ Jul; 2021 ];https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf 2020 10:2021. [Google Scholar]
- 25.Heart Disease Facts . [ Jul; 2021 ];https://www.cdc.gov/heartdisease/facts.htm 2020 10:2021. [Google Scholar]
- 26.Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) Inhibitors: a state-of-the-art review. Lopaschuk GD, Verma S. JACC Basic Transl Sci. 2020;5:632–644. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revisiting the links between glycaemia, diabetes and cardiovascular disease. Sattar N. Diabetologia. 2013;56:686–695. doi: 10.1007/s00125-012-2817-5. [DOI] [PubMed] [Google Scholar]
- 28.Renal handling of ketones in response to sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Barsotti E, Clerico A, Muscelli E. Diabetes Care. 2017;40:771–776. doi: 10.2337/dc16-2724. [DOI] [PubMed] [Google Scholar]
- 29.Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJ, Boulton DW. Diabetes Obes Metab. 2018;20:479–487. doi: 10.1111/dom.13126. [DOI] [PubMed] [Google Scholar]
- 30.Myocardial energetics in heart failure with preserved ejection fraction. AbouEzzeddine OF, Kemp BJ, Borlaug BA, et al. Circ Heart Fail. 2019;12:0. doi: 10.1161/CIRCHEARTFAILURE.119.006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Bedi KC Jr, Snyder NW, Brandimarto J, et al. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Lee TM, Chang NC, Lin SZ. Free Radic Biol Med. 2017;104:298–310. doi: 10.1016/j.freeradbiomed.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Empagliflozin blunts worsening cardiac dysfunction associated with reduced nlrp3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Byrne NJ, Matsumura N, Maayah ZH, et al. Circ Heart Fail. 2020;13:0. doi: 10.1161/CIRCHEARTFAILURE.119.006277. [DOI] [PubMed] [Google Scholar]
- 34.SGLT2 inhibitor, canagliflozin, attenuates myocardial infarction in the diabetic and nondiabetic heart. Lim VG, Bell RM, Arjun S, Kolatsi-Joannou M, Long DA, Yellon DM. JACC Basic Transl Sci. 2019;4:15–26. doi: 10.1016/j.jacbts.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Verma S, Garg A, Yan AT, et al. Diabetes Care. 2016;39:0–3. doi: 10.2337/dc16-1312. [DOI] [PubMed] [Google Scholar]