ABSTRACT
An ability of plants memorizing past heat exposure to modulate the expression of stress response transcripts during recovery is essential for efficient acquired thermotolerance. In this study, we demonstrated that expression of heat response transcripts spiked at 30 min or 1 h, but dramatically declined at 3 h during recoveries following exposure to 5-min heat stress in Arabidopsis. In contrast, expression of transcripts up-regulated by 45-min heat stress was sustained for 30 min or 1 h then declined during recovery. These results suggest that heat memory can be differently modulated depending on the duration of heat exposure, and indicate that plants can memorize even 5-min heat stress to regulate acclimatory responses during recovery. Later hypothesis can be supported by the finding that accumulation of heat response proteins was also modulated during recovery following 5-min heat stress. In addition, 5-min heat stress followed by 3 h recovery was efficient to activate acquired thermotolerance of plants, although spike of transcript expression was observed at 1 h during recovery. These results suggest that plants possess the ability to quickly memorize heat stress and reset cellular states during recovery to adapt to subsequent severe heat stress.
KEYWORDS: Acquired thermotolerance, heat memory, heat stress, recovery
As sessile organisms, plants had evolved various strategies to acclimate to heat stress. For instance, plants possess the ability to adapt to an abrupt temperature increase, referred to as basal thermotolerance.1–4 In addition, plants are able to cope with lethal heat stress when pre-exposed to sublethal heat stimuli.1–4 This type of heat acclimatory response, referred to as acquired thermotolerance, might be essential to survive under the natural environment in which plants are always subjected to fluctuating temperatures. Successful acquisition of tolerance to lethal heat stress is associated with enhanced expression of stress response transcripts during pre-exposure to sublethal heat stimuli.3 Many of these transcripts required for acquired thermotolerance encode different HEAT SHOCK TRANSCRIPTION FACTORs (HSFs), molecular chaperones such as HEAT SHOCK PROTEINs (HSP), and reactive oxygen species (ROS) scavenging enzymes.3 Previous studies clearly demonstrated the significance of HSFs in the regulation of acquired thermotolerance.4–6 Indeed, network of HSFs might function as a hub to integrate various signaling pathways which are involved in the regulation of acquired thermotolerance, such as unfolded protein responses in the endoplasmic reticulum and ROS regulatory systems, as well as expression of HSPs.4,6−,9 In addition, significance of HSFs, as well as ROS regulatory systems in acquired thermotolerance can be also supported by the finding that deficiency in cytosolic ASCORBATE PEROXIDASE 2 (APX2) or HSFA7a resulted in impairment of acquired thermotolerance.10 Furthermore, HSP70 and 90 that are regulated via the functions of HSFs were shown to be required for the regulation of DNA-binding activity of HSFs during pre-exposure of plants to sublethal heat stress.11
Plants possess the ability to remember a past heat exposure to prepare for the subsequent and otherwise, lethal heat stress, referred to as “heat memory.”6,12 It has been demonstrated that recovery phase following the exposure to sublethal heat stimuli is also essential for reprogramming of the cellular state in the regulation of acquired thermotolerance.5,13 Expression of certain transcripts including several Hsps and Hsfs that are upregulated by sublethal heat stimuli can be maintained at high level for several hours or even days during recovery.6,14 Recent studies identified key regulators required for the sustained expression of these memory transcripts during recovery following the application of sublethal heat stimuli. For example, HSFA2 was shown to recruit methyltransferase to heat response genes, leading to sustained expression of these genes.6 Transcripts regulated by HSFA2 during recovery following the application of sublethal heat stimuli include small Hsps as well as Apx2.6 In addition, sustained expression of memory transcripts was also associated with accumulation of the histone H3 lysine 4 trimethylation and dimethylation that persisted even after active transcription from the loci had subsided.6,15,16 Furthermore, a transposable element ONSEN was also shown to play key roles in the regulation of heat memory.17 ONSEN insertions enhance heat responsiveness of multiple genes, resulting in re-arrangement of heat response signaling networks.17 HSFA1s and HSFA2 might act as activators of ONSEN under heat stress. These HSFs possess a highly conserved N-terminal DNA-binding domain that is required for the binding to heat shock element (HSE) that exists in ONSEN promoter.18 These results suggest that network of HSFs and other integrated pathways play pivotal roles to modulate expression of heat response transcripts during recovery following the exposure to sublethal heat stimuli.
Previous studies demonstrated the ability of plants to rapidly respond to abiotic stresses within several minutes or even seconds.19 For example, more than 700 transcripts were up-regulated within 60 s following the application of high light stress.20 In addition, long-distance signals can be propagated through whole plant within minutes when a small part of a plant is exposed to abiotic stresses including heat stress.19,21 Despite these findings, hitherto studies focusing on heat memory analyzed the plants exposed to hours of heat stress followed by recovery. In this study, to characterize memory of short heat stress in Arabidopsis, we analyzed the expression of heat response transcripts and proteins during recovery following the exposure to 5-min heat stress. In addition, we also tested whether 5-min heat stress followed by recovery is efficient to activate acquired thermotolerance in Arabidopsis.
To study early responses of plants to heat stress, we analyzed expression of heat response transcripts in Arabidopsis plants exposed to heat stress for 20–60 s and 5–60 min heat stress (Figure 1). Transcripts tested in this study have been shown to be efficient heat stress markers that are highly responsive to temperature increase.2,9,13,14 Expression of heat response transcripts tested in this study was not clearly up- or down-regulated in response to heat stress within 60 s. Expression of these transcripts, however, started to be up-regulated at 5 min following the application of heat stress. In addition, the highest expression of these transcripts was observed at 15, 45, or 60 min following the application of heat stress.
Figure 1.
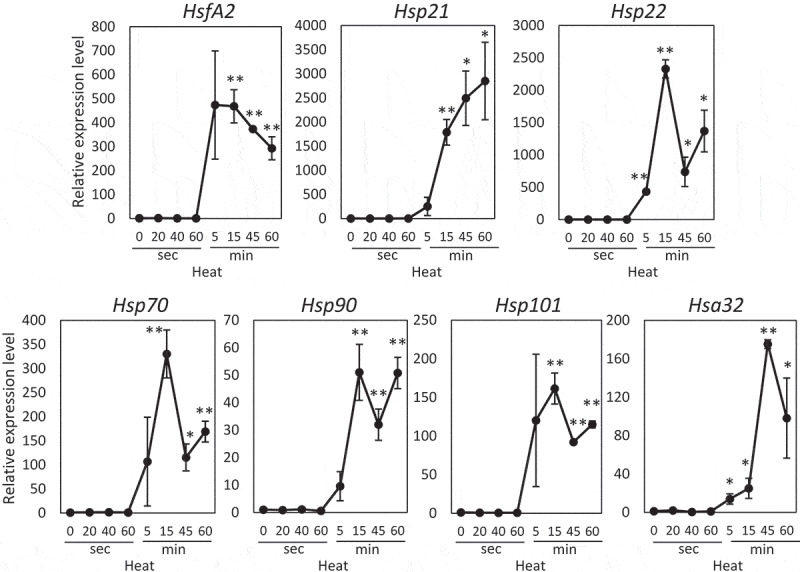
Accumulation of transcripts encoding different acclimatory proteins during heat stress. Plants were sampled immediately after the exposure to 20–60 s or 5–60 min heat stress (40°C). Steady-state level of transcripts was then determined by quantitative real-time PCR (qPCR) analysis as described in the method part. Relative expression (Y-axis) was determined based on the threshold cycle values for target transcripts that were calculated with the CT of Ef1a as an internal control. * and **; Student’s t-test significant at p < .05 and p < .01, respectively, compared to control. Bars indicate standard error (n = 3).
It has been demonstrated that expression of transcripts up-regulated in response to hours of heat stress can be maintained at high level for hours or even days during recovery.6 We therefore tested if plants possess the ability to maintain or modulate expression of transcripts during recovery following exposure to even short period of heat stress (Figure 2). In this study, we focused on two different durations of heat stress; 5-min heat stress in which heat response transcripts started to be up-regulated and 45-min heat stress in which expression of heat response transcripts reached to high level. When plants were exposed to 45-min heat stress, expression of HsfA2, Hsp22, Hsp70, Hsp90, and Hsp101 was sustained at high level for 30 min or 1 h, then, declined during recovery (Figure 2a). On the other hand, expression of Hsp21 and Hsa32 just gradually declined during recovery. Different patterns of expressions were observed in these heat response transcripts when plants were exposed to 5-min heat stress followed by recovery (Figure 2b). Expression of HsfA2 transcript upregulated in response to 5-min heat stress was sustained for 30 min and started to be declined at 1 h during recovery. In contrast, expression of other heat response transcripts dramatically upregulated during recovery following exposure to 5-min heat stress. Interestingly, expression of these transcripts spiked at 30 min or 1 h, then, dramatically declined at 3 h following the onset of recovery. These results indicate that expression of heat response transcripts during recovery is differently modulated depending on the durations of heat stress. Previous studies demonstrated that the expression of small Hsp transcripts tended to be sustained for longer duration during recovery compared to that of large Hsp transcripts, when plants were exposed to hours of heat stress.6,14 However, in this study, almost similar trends were observed in the expression of transcripts encoding small and large HSPs under the conditions employing short periods of heat stress. We also analyzed the accumulation of HSP70, HSP101, and APX1 proteins during recovery following exposure to heat stress. In this study, we included APX1, a cytosolic H2O2 scavenging enzyme, because the accumulation of this protein was shown to be associated with tolerance of plants to abiotic stresses including heat stress.22,23 When plants were exposed to 45-min heat stress, accumulation of these proteins tended to be upregulated (Supplemental Figure 1). Accumulation of HSP70 and HSP101 was sustained at high level for longer duration compared to the expression of transcripts during recovery (Supplemental Figure 1). In addition, repetition of up- and down-regulation was observed in the accumulation of HSP101 and APX1 during recovery. When plants were exposed to 5-min heat stress followed by recovery, repetition of up- and down-regulation was observed in the accumulation of all proteins we tested during recovery (Supplemental Figure 2). Taken together, these results suggest that plants possess the ability to memorize short heat stress and regulate the heat response mechanisms during recovery.
Figure 2.
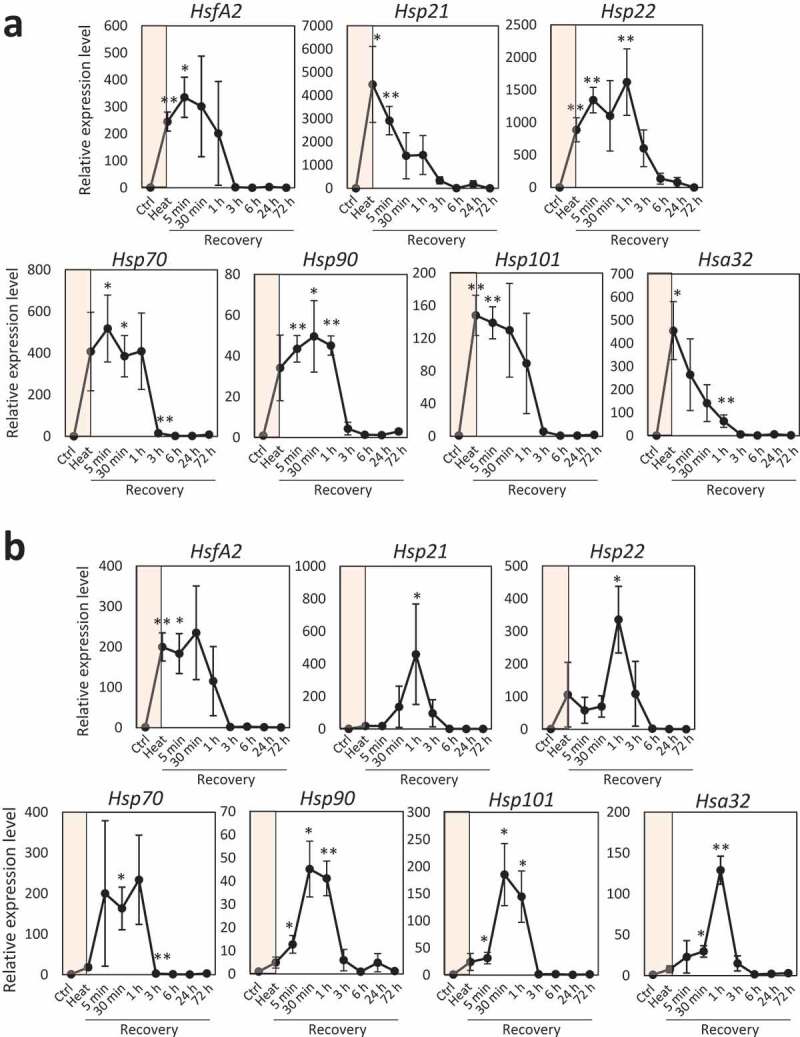
Accumulation of stress response transcripts during the recovery phase following the application of heat stress. (a) Accumulation of transcripts during recovery following 5-min heat stress. (b) Accumulation of transcripts during recovery following 45-min heat stress. Steady-state level of transcripts was determined by quantitative real-time PCR (qPCR) analysis as described in the method part. Relative expression (Y-axis) was determined based on the threshold cycle values for target transcripts that were calculated with the CT of Ef1a as an internal control. * and **; Student’s t-test significant at p < .05 and p < .01, respectively, compared to control. Bars indicate standard error (n = 3). Shaded color on the graphs indicates heat stress treatment.
The results obtained from the analyses of transcripts and proteins indicated that plants possess the ability to modulate the level of transcripts and proteins during recovery following exposure to short period of heat stress. These findings prompted us to test if 5-min heat stress followed by recovery can enhance acquired thermotolerance in plants. We therefore tested the heat tolerance of plants that were exposed to 5-min heat stress followed by different durations of recovery (Figure 3a, Supplemental Figure 3). Surprisingly, enhanced acquired thermotolerance was observed when plants were exposed to 5-min heat stress followed by 3 h recovery (Figure 3a, Supplemental Figure 3). In contrast, 5-min heat stress followed by 1 h recovery did not enhance heat tolerance of plants, although the spike of expression of heat response transcripts was detected at 1 h during recovery following the application of 5-min heat stress (Figure 2b). These results suggest that reset of expression of heat response transcripts during recovery following 5-min heat stress might be important for the regulation of acquired thermotolerance. In addition, when plants were pre-exposed to 45-min heat stress, enhanced acquired thermotolerance was observed following 1 h recovery, but, not 3 h recovery (Figure 3b), suggesting that acquired thermotolerance might be differently regulated depending on the duration of pre-treatment with heat stress and following recovery phase.
Figure 3.
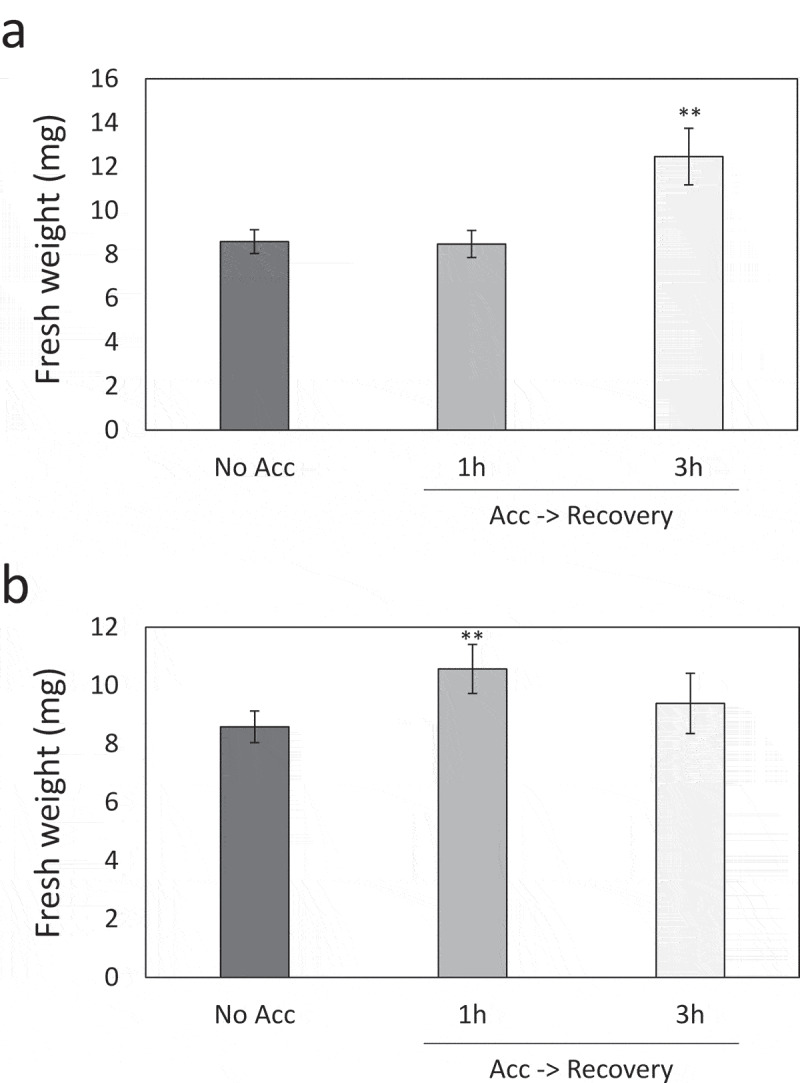
Acquired thermotolerance of plants induced by 5- or 45-min heat stress followed by different durations of recovery. (a) Acquired thermotolerance of plants acclimated with 5-min heat stress. (b) Acquired thermotolerance of plants acclimated with 45-min heat stress. Plants were pre-exposed to 5 (A) or 45 (B) min heat stress followed by 0–3 h recovery. Then, plants were subjected to lethal heat stress. Heat tolerance was evaluated by fresh weight. **; Student’s t-test significant at p < .01 (n = 15–35) compared to plants subjected to lethal heat stress without pre-treatment with 5-min heat stress (no ACC). Bars indicate standard error.
In this study, we showed that heat memory represented by the expression of heat response transcripts is differently modulated during recovery depending on the durations of heat stress. When plants were exposed to 45-min heat stress, heat response transcripts maintained for 30 min or 1 h and declined at 3 h following the onset of recovery (Figure 2a). This sustained period of the transcript expressions observed in this study seems to be shorter than that observed in previous studies in which plants were exposed to hours of heat stress.6,14 On the other hand, expression of heat response transcripts was spiked at 30 min or 1 h following the onset of recovery, when plants were exposed to 5-min heat stress (Figure 2a). This spiked expression of heat response transcripts indicates that plants possess the ability to memorize 5-min heat stress and regulate heat response mechanisms duringrecovery. This hypothesis can be also supported by the repetitive up- and down-regulation of protein accumulations during recovery following 5-min heat stress (Supplemental Figures 1 and 2). In addition, different temporal patterns of the accumulation were observed between transcripts and proteins, suggesting that mechanisms regulating transcription and translation might function in different timing during recovery following the application of short period of heat stress. Furthermore, mechanisms to stabilize transcripts or proteins might also function in different timing. In future studies, it is necessary to identify key factors that regulate the level of transcripts and proteins during recovery following the application of short period of heat stress.
We cannot ignore the possibility that it may take more than 5 min to achieve equal temperature throughout the whole plant. However, it has been demonstrated that certain sets of proteins and transcripts can be significantly up- or down-regulated in response to 5-min heat stress,24,25 suggesting that plants have the ability to respond to short period of heat stress. In addition, previous studies demonstrated that when a small part of a plant was exposed to abiotic stimuli including heat, long-distance signaling was rapidly spread through the whole plant.26,27 Thus, short period of heat stress within 5 min might be efficient to activate heat response mechanisms, even if it does not achieve equal temperature throughout the whole plant.
Different from other transcripts, expression of HsfA2 transcript was highly up-regulated in response to 5-min heat stress and maintained at high level for 30 min. Although expression of HsfA2 transcript started to decline at 1 h during recovery, it tended to be still higher than that under-controlled conditions (Figure 2a). These results indicate that HSFA2 could be a candidate factor that mediates fast response to heat stress within 5 min and regulation of heat memory during recovery. Indeed, it has been proposed that HSFA2 might govern various key processes underlying acquired thermotolerance and heat memory, such as sustained expression of transcripts encoding small HSPs, modulation of ROS regulatory systems, recruitment of methyltransferase to heat response genes to sustain their expression and activation of transposable element.4,6,18 Thus, HSFA2 could be required for the spike of expression of other heat response transcripts during recovery following 5-min heat stress. To confirm this hypothesis, it should be necessary to investigate the expression of heat memory transcripts and proteins during recovery following 5-min heat stress in the mutants deficient in HSFA2.
Although the spike of the expression of heat response transcripts was observed at 1 h during recovery following the application of 5-min heat stress, efficient activation of acquired thermotolerance was observed when plants were pre-treated with 5-min heat stress followed by 3 h recovery (Figure 3). These results suggest that reset of expression of transcripts during recovery might be also important for the re-programming of the cellular state. A recent review suggested that reset (i.e., down-regulation) of the certain memory transcripts and signaling molecules during recovery might be also essential for acquired thermotolerance.6,12 Indeed, certain sets of transcripts that were declined during the recovery phase exhibited enhanced re-induction upon re-exposure to second severe stress.14,28 Thus, it should be necessary to elucidate how the expression of transcripts can be re-induced during exposure to second heat stress, following the exposure to 5-min heat stress accompanied by different durations of recovery. In addition, it is still not clear why the efficiency of heat treatment to activate acquired thermotolerance is different depending on duration of recovery. To address these questions, changes in transcripts, proteins, and metabolites during recovery and subsequent severe heat stress should be comprehensively analyzed. Furthermore, it should be interesting to address whether the spike of expression of transcripts at 1 h is necessary for the re-programming of cellular signals at 3 h during recovery following 5-min heat stress. To address this question, it is necessary to analyze mutants deficient in the transcript(s) that can be up-regulated at 1 h during the recovery phase.
In this study, Arabidopsis thaliana ecotype Col-0 were grown in soil mixture (MetroMix 200, SUN GRO) in 9 × 9 x 6 cm3 pots under controlled conditions: 21°C, 16/8 h light/dark cycle, 50 μmol m–2 s–1 in a growth chamber (LPH-241 S, NK System, Tokyo, Japan). Twenty-one to twenty-four-day-old plants grown as described above were used in this study.
To analyze the expression of transcripts and proteins, three biological replicates each containing 15–20 plants were obtained from independent experiments. To analyze the fast response of plants to heat stress, plants grown as above were exposed to 40°C heat stress for 0, 20, 40 and 60 s, and 5, 15, 45, and 60 min. To analyze expression patterns of transcripts and proteins during recovery, plants grown as above were exposed to 5- or 45-min heat stress followed by recovery for 0, 5, and 30 min and 1, 3, 6, 24, and 72 h under controlled conditions as above. Following these heat treatments or recovery, entire rosettes that were directly exposed to temperature changes were sampled and immediately frozen in liquid nitrogen.
RNA extraction was performed as previously described.9 First-strand complementary DNAs (cDNAs) were produced after DNaseI treatment from 1 µg of total RNA using M-MuLV reverse transcriptase (New England Biolabs). qRT-PCR was performed in an optical 96-well plate with the ABI Prism 7000 system and the Thunderbird qPCR Master Mix (Toyobo, Tokyo, Japan). Threshold cycle values for target transcripts were calculated with the CT of Ef1a as an internal control. Primers used in this study are listed in Supplemental Table.1. Protein extraction and Western blot analysis were performed as previously described.9 Antibodies that react with APX129 were used for protein gel blot analysis. Antibodies to detect HSP70 and HSP101 were purchased from Funakoshi Inc. (Tokyo, Japan).
To evaluate the acquired thermotolerance, three independent experiments employing 5–12 plants for each treatment were performed; 21- to 24-day-old plants grown as above were treated with 40°C heat stress for 5 min followed by recovery at 21°C for 0, 1, or 3 h. Plants were then subjected to 44°C heat stress for 36 h with continuous light condition. Following the application of heat stress at 44°C, plants were recovered for 7 days under-controlled conditions as above and measured in fresh weight. In the growth chamber used for the heat treatment in this study, we are not able to set 44°C during the dark cycle because of the mechanical feature of heating systems. Thus, to prevent the effects of lowered temperature during the dark cycle on acquired thermotolerance, plants were exposed to 44°C heat stress with continuous light.
Supplementary Material
Funding Statement
This paper was supported by funding from Grant-in-Aid for Young Scientists (B) (17K15403) and Sophia University in Japan.
Abbreviations
- APX
ascorbate peroxidase
- HSE
heat shock element
- HSF
heat shock transcription factor
- HSP
heat shock protein
- ROS
reactive oxygen species
Disclosure of potential conflicts of interest
The author has declared that no competing interests exist.
Supplementary material
Supplemental material for this article can be accessed on the publisher’s website.
References
- 1.Larkindale J, Hall JD, Knight MR, Vierling E.. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138(2):1–6. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem. 2008;283(14):9269–9275. doi: 10.1074/jbc.M709187200. [DOI] [PubMed] [Google Scholar]
- 3.Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biochem Sci. 2012;37(3):118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Katano K, Honda K, Suzuki N. Integration between ROS regulatory systems and other signals in the regulation of various types of heat responses in plants. Int J Mol Sci. 2018;19(11):E3370. doi: 10.3390/ijms19113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007;143(1):251–262. doi: 10.1104/pp.106.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich T, Faivre L, Bäurle I, Schubert D. Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ. 2019;42(3):762–770. doi: 10.1111/pce.13373. [DOI] [PubMed] [Google Scholar]
- 7.Miller G, Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot. 2006;98(2):279–288. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driedonks N, Xu J, Peters JL, Park S, Rieu I. Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front Plant Sci. 2015;6:999. doi: 10.3389/fpls.2015.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataoka R, Takahashi M, Suzuki N. Coordination between bZIP28 and HSFA2 in the regulation of heat response signals in Arabidopsis. Plant Signal Behav. 2017;12(11):e1376159. doi: 10.1080/15592324.2017.1376159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008;146(2):748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn A, Bublak D, Schleiff E, Scharf KD. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell. 2011;23(2):741–755. doi: 10.1105/tpc.110.076018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crisp PA, Ganguly D, Eichten SR, Borevitz JO, Pogson BJ. Reconsidering plant memory: intersections between stress recovery, RNA turnover, and epigenetics. Sci Adv. 2016;2(2):e1501340. doi: 10.1126/sciadv.1501340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol. 2006;140(4):1297–1305. doi: 10.1104/pp.105.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lämke J, Brzezinka K, Altmann S, Bäurle I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. Embo J. 2016;35(2):162–175. doi: 10.15252/embj.201592593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 2009;10(6):R62. doi: 10.1186/gb-2009-10-6-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brzezinka K, Altmann S, Czesnick H, Nicolas P, Gorka M, Benke E, Kabelitz T, Jähne F, Graf A, Kappel C, et al. Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. Elife. 2016;5:pii: e17061. doi: 10.7554/eLife.17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito H, Gaubert H, Bucher E, Mirouze M, Vaillant I, Paszkowski J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature. 2011;472(7341):115–119. doi: 10.1038/nature09861. [DOI] [PubMed] [Google Scholar]
- 18.Cavrak VV, Lettner N, Jamge S, Kosarewicz A, Bayer LM, Mittelsten Scheid O. How a retrotransposon exploits the plant’s heat stress response for its activation. PLoS Genet. 2014;10(1):e1004115. doi: 10.1371/journal.pgen.1004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R. Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci. 2019;24(1):25–37. doi: 10.1016/j.tplants.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki N, Devireddy AR, Inupakutika MA, Baxter A, Miller G, Song L, Shulaev E, Azad RK, Shulaev V, Mittler R. Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. Plant J. 2015;84(4):760–772. doi: 10.1111/tpj.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhury FK, Devireddy AR, Azad RK, Shulaev V, Mittler R. Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol. 2018;178(4):1461–1472. doi: 10.1104/pp.18.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A, Inupakutika MA, Mittler R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot. 2016;67(18):5381–5390. doi: 10.1093/jxb/erw299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katano K, Kataoka R, Fujii M, Suzuki N. Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physio Biochem. 2018;123:288–296. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Ma X, Wang H, Li B, Clark G, Guo Y, Roux S, Sun D, Tang W. Proteomic study of microsomal proteins reveals a key role for arabidopsis annexin 1 in mediating heat stress-induced increase in intracellular calcium levels. Mol Cell Proteomics. 2015;14(3):686–694. doi: 10.1074/mcp.M114.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Chen S, Shi X, Liu D, Zhao P, Lu Y, Cheng Y, Liu Z, Nie X, Song W, et al. Hybrid sequencing reveals insight into heat sensing and signaling of bread wheat. Plant J. 2019;98(6):1015–1032. doi: 10.1111/tpj.14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell. 2013;25(9):3553–3569. doi: 10.1105/tpc.113.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. J Exp Bot. 2014;65(5):1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 28.Liu HC, Lämke J, Lin SY, Hung MJ, Liu KM, Charng YY, Bäurle I. Distinct heat shock factors and chromatin modifications mediate the organ autonomous transcriptional memory of heat stress. Plant J. 2018;95(3):401–413. doi: 10.1111/tpj.13958. [DOI] [PubMed] [Google Scholar]
- 29.Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17(1):268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


