Abstract
Aflatoxin B1 (AFB1) is a secondary metabolite produced by Aspergillus flavus and parasitic aspergillus, mainly existing in cereals, peanuts, corn, and other crops, which seriously endanger poultry, human health, and environment. Morin, a flavonoid compound extracted from moraceae plants, possess antioxidant, antibacterial, and anti-inflammatory effects. However, whether morin has a protective effect on AFB1-induced liver and kidney damage in chicks has not been specifically reported. In this study, we mainly confirmed the protective effect of morin on AFB1-induced liver and kidney damage in chicks and clarified its mechanism. It was found that morin can significantly reduce the liver biochemical indicators of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and kidney indicators of creatinine (CRE) and urea nitrogen (BUN) levels. Meanwhile, histopathological examination showed that morin effectively relieved AFB1-caused liver damage, including hepatocyte disruption, swelling, and inflammatory cell infiltration, and effectively relieved kidney damage, including renal cell necrosis, exfoliation, and vacuolization. Further investigation of its mechanism demonstrated that morin significantly inhibited AFB1-induced heterophil extracellular traps (HETs) release, and decreased the level of malondialdehyde (MDA) but increased the levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in vivo. Moreover, quantitative real-time PCR (qRT-PCR) analysis showed that morin also significantly decreased AFB1-induced mRNA expressions of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), caspase-1, caspase-3, and caspase-11. In conclusion, all results confirmed that morin could protect AFB1-caused liver and kidney damage by inhibiting HETs release, regulating oxidative stress, and inhibiting inflammatory response, suggesting that morin can be utilized as a potential drug for prevention and treatment of aflatoxicosis in poultry breeding industry.
Key words: morin, aflatoxin B1, heterophil extracellular traps, inflammatory response, oxidative stress
INTRODUCTION
Aflatoxin is a type of secondary metabolite with high toxicity and low molecular weight produced by fungi such as Aspergillus flavus, Parasitic aspergillus and specific aspergillus, which have strong toxicity, mutagenicity and carcinogenicity, among which aflatoxin B1 (AFB1) has the strongest toxicity (Rawal et al., 2010). AFB1 pollution was related to feed safety, animal production and food safety, and even threatens human health. Hence, it is an importance to solve the contamination of AFB1 in feed and the residual problems in animals and livestock products. Exposure to aflatoxins during pregnancy poses a threat to the health of the fetus, which can be passed from mother to fetus through umbilical cord blood and breast milk (Denning et al., 1990). It has been reported that AFB1 level in serum of jaundice with 6-phosphoglucose dehydrogenase deficiency in newborns, and found that 58% of newborns and 75% of mothers tested positive (Raafat et al., 2021). As the most important hepatocellular carcinogen, AFB1 is the main cause of hepatocellular carcinoma (HCC) in the world and studies have found that AFB1 and hepatitis B virus (HBV) work in conjunction to further enhance the incidence of HCC (Rushing and Selim, 2019). AFB1-induced oxidative stress is one of the important ways in which its toxicity is exerted (Abdel-Wahhab and Aly, 2003). But deeper mechanism underlying aflatoxicosis and more efficient drugs for prevention and treatment still remain to be investigated.
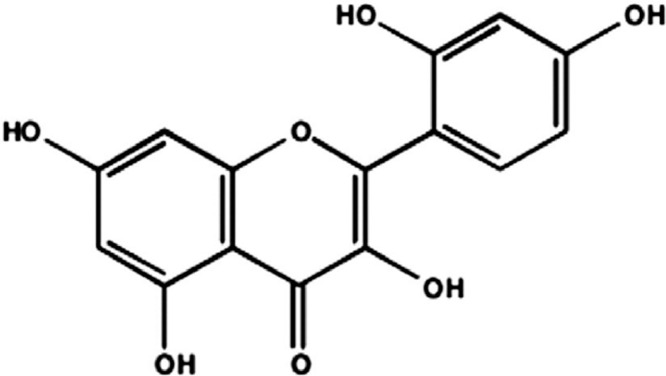
Morin (the chemical structure is shown in Figure 1), is a kind of natural pigment extracted from moriaceae plants, which belongs to flavonoids and has biological properties, including anti-inflammatory (Wei et al., 2015), antibacterial (Li et al., 2020), and antioxidant (Wei et al., 2015), antiatherosclerosis (Zhou et al., 2017), lower blood sugar and antistress, and has been widely used in clinical treatment of coronary heart disease, diabetes, and cancer. It is reported that that morin can exert antibacterial effects on Staphylococcus aureus, Bacillus and Micrococcus flavus by interfering with bacterial DNA synthesis and inhibiting the activity of adenosine triphosphate (ATPase) (Xu et al., 2001). Some studies show that morin can inhibit TLR4/ NF-κB and activate Nrf2 and HO-signaling pathways to protect LPS-induced acute liver injury in mice (Tian et al., 2017). Morin also can effectively improve diethyl ammonium nitrate-induced the liver fibrosis in rats by moderating Hippo/YAP and TGF-β1/Smad signal transduction (Perumal et al., 2017). At present, it is reported that a variety of compounds such as vitamins A, C and E (Alpsoy and Yalvac, 2011), and eugenol, can resist the oxidative stress response of AFB1. However, as a traditional antioxidant drug, whether morin could also play a protective role in liver and kidney injury in chicks with aflatoxicosis has not been reported.
Figure 1.
Chemical structure of morin.
Neutrophil extracellular traps (NETs) are the body's first line of defense against external pathogens found in recent years. NETs are a network structure with DNA as the skeleton and modified by citrullinated histone 3 (citH3) and elastase (Wei et al., 2019b), with antibacterial (Brinkmann et al., 2004), antiviral (Saitoh et al., 2012), and antiparasitic (Wei et al., 2016) effects. Heterophil extracellular traps (HETs) have a similar function to NETs, except that HETs do not have the expression of myeloperoxidase (MPO) (Chuammitri et al., 2009). It has been demonstrated that N. caninum can induce the release of canine NETs in vitro to resist infection (Wei et al., 2016). Study also been confirmed that abamectin (AVM) can reduce respiratory burst in carp by inhibiting the activation of PI3K-ERK signaling pathway, thus inhibiting the release of NETs in carp (Zheng et al., 2020). Similar to abamectin, morin is a recognized therapeutic drug, whether its therapeutic effect is related to the release of NETs, this requires us to further explore.
Therefore, the mechanism of morin on AFB1-induced liver and kidney injury in chicks was investigated in our study, and its protective effects were explored. It is expected to supply experimental foundation for the veterinary clinical implementation of morin and provide experimental data and reference for the prevention and control of mycotoxin poisoning.
MATERIALS AND METHODS
Reagents and Materials
AFB1 (A6636, purity ≥ 98%, Sigma-Aldrich [Shanghai] Trading Co., Ltd., Shanghai, China). Morin (purity ≥ 98%) was purchased from Chengdu Pusi Biological Co., Ltd. (Chengdu, China). Pico Green dsDNA Assay kit (Thermo Fisher Scientific, Waltham, MA). The kit of AST, ALT, BUN, and CRE was obtained from the Institute of Biological Engineering of Nanjing Jiancheng (Nanjing, China). The kit of GSH-Px SOD, CAT, and MDA was obtained from the Institute of Biological Engineering of Nanjing Jiancheng (Nanjing, China). RevertAid First Strand cDNA Synthesis kit was obtained from Thermo. qPCR master mix was obtained from Roche (Mannheim, Germany).
Establishment of Animal Models
Twenty-five one-day-old chicks were purchased from Foshan breeding farm (Foshan, Guangdong), and the chicks were fed adaptively for one day. The establishment of the infected chick model was started on the second day. The test chicks were randomly assigned into 5 groups: control group, AFB1 group (5 mg/kg), morin treatment group (20 mg/kg, 40 mg/kg, 80 mg/kg). On the first day of modeling, the treatment group was intraperitoneally injected with morin for prevention, and was injected again 16 h later. Blood was collected 24 h later, the chicks were killed and liver and kidney tissues were taken. All implementation of the experiment was conducted in compliance with the Foshan University Animal Research Ethics Committee guidelines (FSUeae2019112501).
Detection of HETs in Serum
The collected blood was stationary, centrifuged at 12,000 rpm for 10 min, and the upper serum was extracted, diluted 5-fold with TE dye, and then the same amount of Pico Green was added. Finally, the experimental results were observed with fluorescence microplate reader.
Detection of ALT and AST in Serum
Measurement of levels of ALT and AST in serum with the kit, and the experimental results were detected at the wavelength of 510 nm by the microplate reader.
Detection of BUN and CRE in Serum
CRE and BUN levels in serum were examined with corresponding kits and the experimental results were detected at the wavelength of 510 nm by the microplate reader.
Histopathological Assessment of Liver and Kidney Tissue
The collected liver and kidney tissue samples were fixed in 10% formalin. Then, the tissue was dehydrated, embedded, and stained with hematoxylin and eosin (Wu et al., 2021). Finally, a revolve's hybrid microscope was used to observe pathological changes.
Detection of Oxidase Activity and Antioxidant Enzyme Activity
Oxidase MDA and antioxidant oxidase GSH-Px, SOD and CAT levels were detected examined with corresponding kits (Guo et al., 2021). Among, SOD and CAT were measured with serum, and MDA and GSH-Px were measured with liver tissue. In addition, we need to use TBD reagents for quantitative analysis of protein concentration in samples (Cao et al., 2020).
Quantitative Real-Time PCR Analysis
QRT-PCR was used to detect the mRNA expression of inflammatory factors TNF-a, IL-1β, IL-6, inflammatory mediator COX-2, iNOS and caspase signaling pathway key factors caspase-1, caspase-3, and caspase-11. CFX Connect Real-Time System instrument was used to real-time PCR fluorescence quantification. The primers of the target gene are in Table 1. qRT-PCR detection adopts Roche 25 μL system, the condition was 50°C, 2 min; 95°C, 10 min; 95°C, 15 s; 60°C, 60 s, a total of 40 cycles. GAPDH as the internal reference, and was used the 2−ΔΔCT method to process the data (Qianru et al., 2021).
Table 1.
The sequence of primers used in the experiment (chicks).
| Gene | Primer | Sequence 5′ > 3′ | bp (size) |
|---|---|---|---|
| GAPDH | Sense | GGCACTGTCAAGGCTGAGAA | 23 |
| Antisense | CACCTGCATCTGCCCATTTG | ||
| IL-1β | Sense | ACTGGGCATCAAGGGCTA | 20 |
| Antisense | GGTAGAAGATGAAGCGGGTC | ||
| TNF-α | Sense | CAGGACAGCCTATGCCAACA | 20 |
| Antisense | ACAGCCAAGTCAACGCTCCT | ||
| IL-6 | Sense | GCAGGACGAGATGTGCAAGA | 20 |
| Antisense | ATTTCTCCTCGTCGAAGCCG | ||
| COX-2 | Sense | TGTCCTTTCACTGCTTTCCAT | 21 |
| Antisense | TTCCATTGCTGTGTTTGAGGT | ||
| iNOS | Sense | CCTGGAGGTCCTGGAAGAGT | 20 |
| Antisense | CCTGGGTTTCAGAAGTGGC | ||
| caspase-1 | Sense | GATACGTGACTCCATCGACCC | 21 |
| Antisense | CTTCTTCAGCATTGTAGTCC | ||
| caspase-3 | Sense | AAGGCTCCTGGTTTATTC | 18 |
| Antisense | CTGCCACTCTGCGATTTAC | ||
| caspase-11 | Sense | CCCCCACCATCTCAACAAGT | 20 |
| Antisense | GTCCCTGAACAGTTCCCACA |
Statistical Analysis
All date were calculated by GraphPad Prism 5.0 software using one-way analysis of variance (ANOVA) with Tukey multiple comparison test. Differences among groups were regarded statistically significant at levels of P < 0.05.
RESULTS
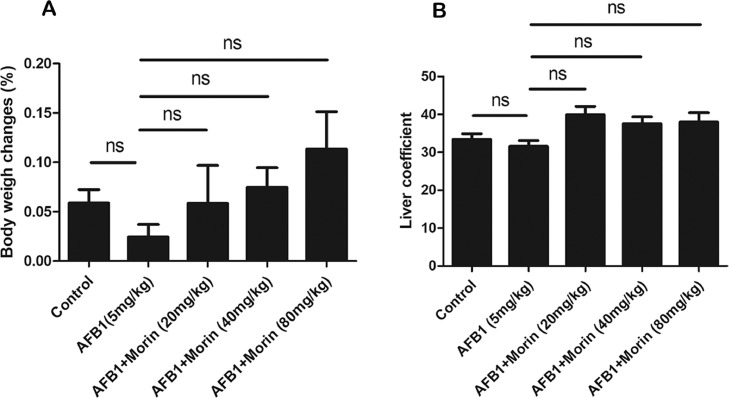
Change of Body Weight and Liver Coefficient in Chicks
Body weight of each chick was recorded and calculated liver coefficient (liver coefficient = [liver weight/body weight] × 100). The results manifested that the growth rate of body weight in the morin treatment group (20 mg/kg, 80 mg/kg) was significantly increase compared with the AFB1 group (Figure 2A), confirming that morin has a certain effect on the weight of chicks. However, the liver coefficient of each group of chicks did not change significantly (Figure 2B).
Figure 2.
Changes of body weight and liver coefficient in chicks. (A) Changes of body weight. (B) Changes of liver coefficient. The date were expressed with mean ± SEM. (*P < 0.05, **P < 0.01).
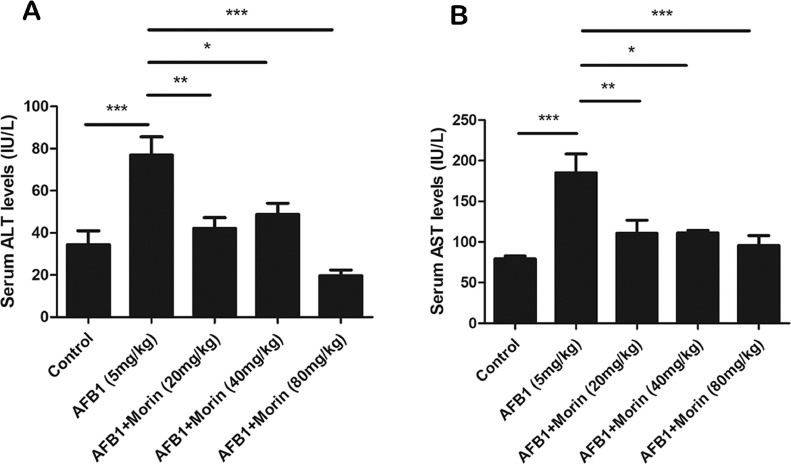
Morin Significantly Reduces the Levels of ALT and AST in Serum
ALT and AST are indicators of liver damage. The date exhibited that AFB1 significantly boost ALT and AST levels, while morin showed a completely opposite trend compared with AFB1 (Figure 3A and B), which preliminarily suggests that morin has a beneficial effect on AFB1-induced liver injury in chicks.
Figure 3.
Detection of liver index. (A) ALT level in serum of chicks. (B) AST level in serum of chicks. The date were expressed with mean ± SEM. (*P < 0.05, **P < 0.01, ***P < 0.001).
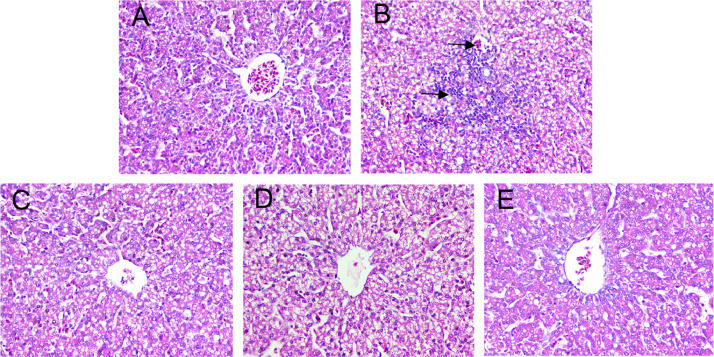
Morin Significantly Alleviates AFB1-Induced the Liver Damage in Chicks
To further confirm the effects of morin on AFB1-caused liver injury, histopathological examination was carried out. As showed in Figure 4, AFB1 treatment induced hepatocyte swelling, fragmentation, and inflammatory cell infiltration in liver, but the morin (20, 40, 80 mg/kg) effectively alleviated the liver tissue damage, which further confirmed that morin has a mitigating effect on AFB1-induced liver damage in chicks.
Figure 4.
Histopathological changes of liver tissues. (A) Control. (B) AFB1 (5 mg/kg). (C) AFB1 + morin (20 mg/kg). (D) AFB1 + morin (40 mg/kg). (E) AFB1 + morin (80 mg/kg). The black arrows indicate cells rupture and inflammatory cell infiltration.
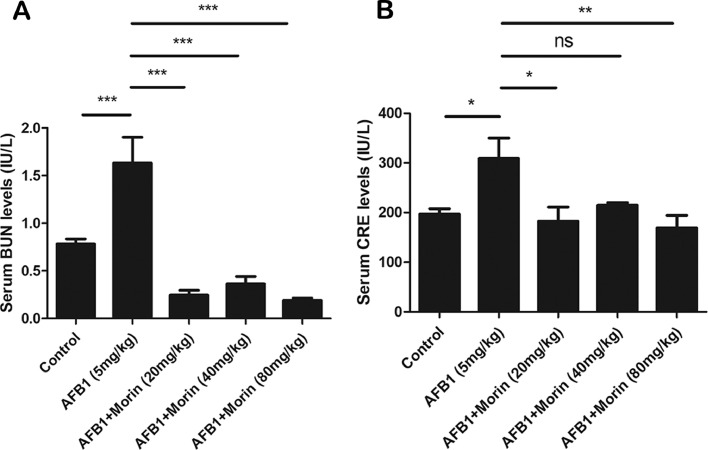
Morin Significantly Reduces the Levels of BUN and CRE in Serum
BUN and CRE as important indicators of kidney injury, we found that AFB1 significantly increased BUN and CRE levels compared with the control group, while morin showed a completely opposite trend compared with AFB1 (Figure 5A and B), which also preliminarily suggests that morin has a beneficial effect on AFB1-caused kidney injury in chicks.
Figure 5.
Changes of kidney index. (A) BUN level in serum. (B) CRE level in serum. The date were expressed with mean ± SEM. (*P < 0.05, **P < 0.01, ***P < 0.001).
Morin Significantly Alleviates AFB1-Induced the Kidney Injury in Chicks
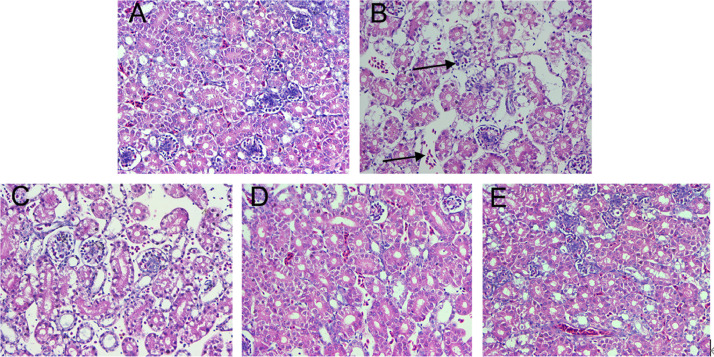
To further confirm the effects of morin on AFB1-caused kidney injury, histopathological examination was carried out. The kidney of the AFB1 treatment group showed renal cell necrosis, exfoliation and vacuolization, while the morin treatment group, with the increase of the morin concentration (20, 40, 80 mg/kg), effectively alleviated the kidney tissue injury (Figure 6), which further confirmed morin has a mitigating effect on AFB1-caused kidney injury in chicks.
Figure 6.
Histopathological changes of kidney tissues. (A) Control. (B) AFB1 (5 mg/kg). (C) AFB1 + morin (20 mg/kg). (D) AFB1 + morin (40 mg/kg). (E) AFB1 + Morin (80 mg/kg). The black arrows indicate cells necrosis, exfoliation, and vacuolization.
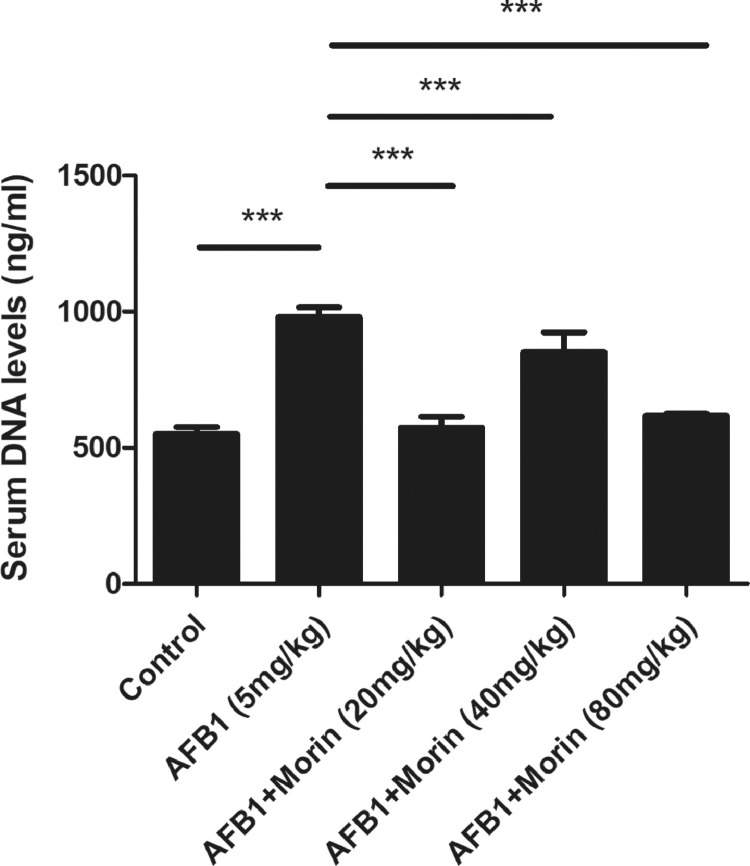
Morin Significantly Reduced the Level of AFB1-Induced HETs in Serum
To investigate the mechanism underlying the protective of morin on AFB1-induced tissue injury, HETs released in serum were detected by Pico Green. Blood samples were collected to detect the level of HETs in serum. The experimental result indicated that AFB1 markedly induced the generation of HETs in serum, while morin significantly inhibited the generation of HETs compared with the AFB1 group (Figure 7). Thus, we first confirmed that morin alleviated AFB1-caused liver and kidney injury by inhibiting the release of HETs.
Figure 7.
The release of HETs in serum. Pico Green was used to detect the release of HETs. The date were expressed with mean ± SEM. (***P < 0.001).
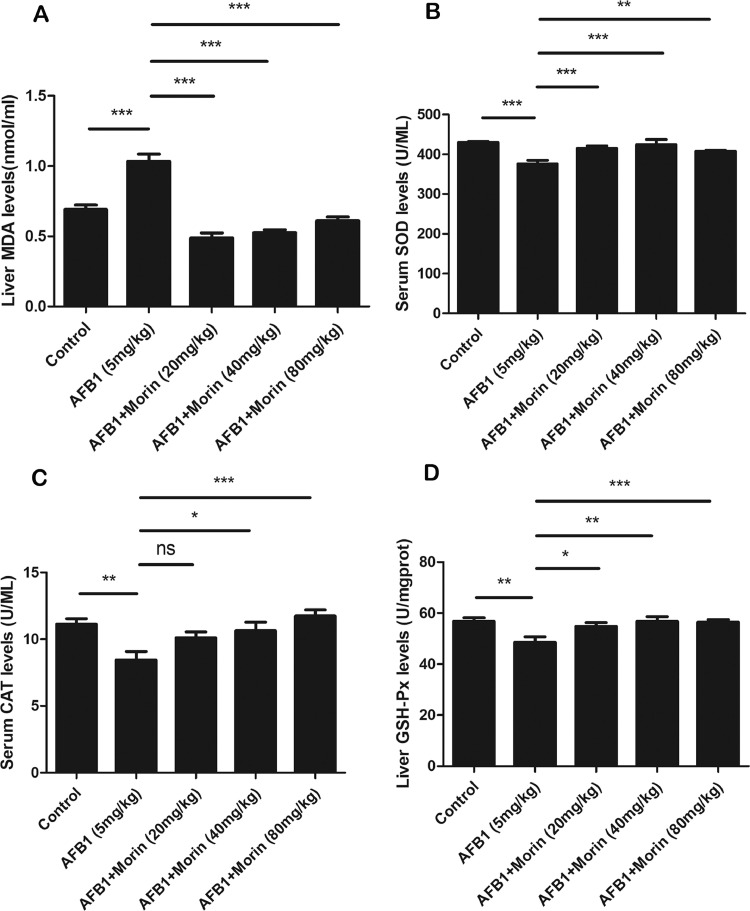
Morin Significantly Alleviates AFB1-Caused Oxidative Stress in Chicks
It is reported that oxidative stress is a crucial mechanism in the toxic effects of AFB1, the function of morin on AFB1-caused oxidative stress was detected. The result indicated that AFB1 markedly added the level of oxidase MDA, while markedly reduced the levels of antioxidant enzyme GSH-Px, SOD and CAT. However, compared with AFB1, morin effectively alleviated the imbalance between oxidase and antioxidant enzyme (Figure 8).
Figure 8.
Changes of oxidative stress. (A) Level of MDA in liver. (A) Level of SOD in serum. (A) Level of CAT in serum. (A) Level of GSH-Px in liver. The date were expressed with mean ± SEM. (*P < 0.05, **P < 0.01, ***P < 0.001).
Morin Significantly Inhibited the mRNA Levels of AFB1-Induced Inflammatory Mediators and Inflammatory Factors in Liver
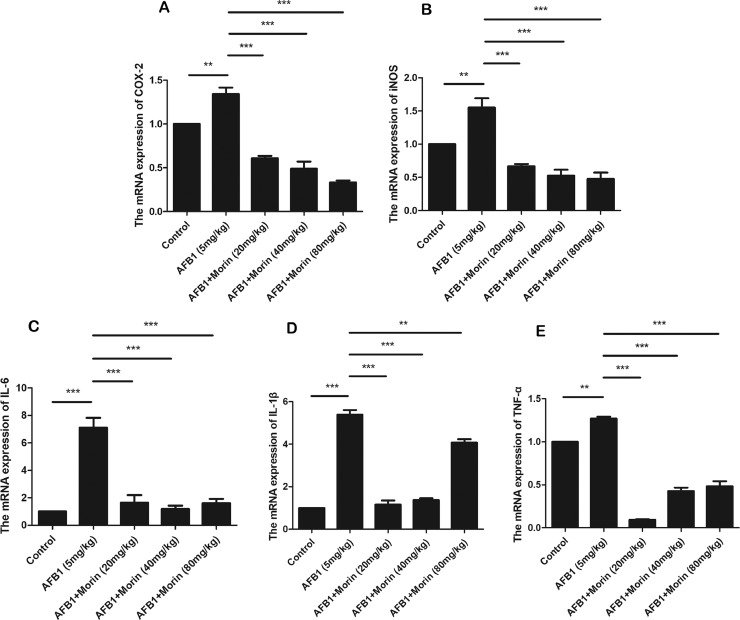
Inflammatory response is an important mechanism in the toxic effects of AFB1; the effect of morin on AFB1-caused inflammatory responses was examined. qRT-PCR analysis was utilized to detect the expression of inflammatory mediators COX-2 and iNOS, and inflammatory factors TNF-α, IL-1β, IL-6. The experimental results indicated that AFB1 notably improved the expression levels of these cytokines, while morin markedly inhibited the expression of these cytokines (Figure 9).
Figure 9.
The expression of inflammatory mediators COX-2 and iNOS, and inflammatory factors TNF-α, IL-1β and IL-6 in liver. Extracted liver tissue, homogenized, and analyzed by real-time quantitative PCR. The date were expressed with mean ± SEM. (**P < 0.01, ***P < 0.001).
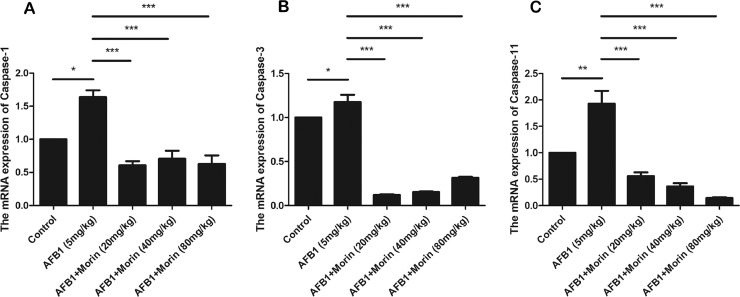
Morin Significantly Inhibited AFB1-Induced the mRNA Expression of Caspase-1, Caspase-3, and Caspase-11
Caspase signaling pathway has also been reported to be involving in the process of oxidative stress and inflammatory responses. ROS can induce damage to the mitochondrial membrane, leading to cytochrome c efflux, and activating the caspase signaling pathway. Therefore, we detected the mRNA expression of key factors of the caspase signaling pathway. The result confirmed that AFB1 markedly increased the mRNA expression of caspase-1, caspase-3 and caspase-11, while morin markedly decreased the expression of these factors (Figure 10).
Figure 10.
The expression of caspase-1, caspase-3, and caspase-11 in liver. The date were expressed with mean ± SEM. (*P < 0.05, **P < 0.01, ***P < 0.001).
DISCUSSION
AFB1, as the most toxic mycotoxin, which is the main cause of aspergillosis. There have been lots of studies on the toxicity of AFB1, including hepatotoxicity, neurotoxicity, and carcinogenicity. Liver, as the main detoxification organ and the main site of substance metabolism transformation in animals, is extremely vulnerable to AFB1 damage, which is mainly mediated by 4 mechanisms: cytochrome P450 (CYP450) enzyme system, mitochondria, immune system, and free radicals. After AFB1 enters the body, it is absorbed from the duodenum of the digestive tract and enters the liver through the portal venous, where it is transformed into AFBO by CYP1A2 and CYP3A4 in the phase I metabolism enzyme CYP450 enzyme system in hepatocytes (Yang et al., 2013). The above process is the most significant reason why AFB1 causes body disorders, and produces toxicity and carcinogenic effects. AFB1 also can induce hepatocyte apoptosis through up-regulating the expression of death receptors FAS and TNFR1, and down-regulating the expression of antiapoptotic receptors XIAP and Bcl-2 (Mughal et al., 2017). In the previous experiments, we have confirmed that AFB1 can cause liver and kidney damage in chicks and preliminary elucidated the mechanism.
Morin is a kind of natural flavonoids, which have a natural antioxidant structure. The quinone formed by its oxidation has a strong antioxidant activity, which can protect the unsaturated fatty acids on the cell membrane from oxidation (Xie et al., 2003). Studies have confirmed that morin can play a certain protective role on IFOS-caused liver damage in mice by inhibiting oxidative stress, inflammation as well as cell apoptosis (Özdemir et al., 2020). Morin also can alleviate LPS-caused mastitis by inhibiting PI3K/ AKT, MAPK, NF-κB, and NLRP3 signaling pathways (Jiang et al., 2020). However, related to protective effects of morin on AFB1-caused damage remain unknown. Therefore, in this experimental, mainly explored the protective effects of morin on AFB1-caused liver and kidney damage and elucidated its mechanism.
Based on more accurate assessment of the dosage and effects of morin, intraperitoneal injection was selected. Moreover, the method of administration with morin was consistent with that in mice and rats (Jiang et al., 2020; Khamchai et al., 2020). First, the levels of AST, ALT, BUN, and CRE in serum were examined to confirm that AFB1 toxicity model was successfully established. The liver and kidney damage indexes of AFB1 group were significantly increased, while morin groups markedly inhibited the expression of these enzymes, which preliminarily confirmed the role of morin in protecting against AFB1-caused liver and kidney damage in chicks. At the same time, we found that although there was no remarkable difference in liver index between the groups, the growth rate of weight was markedly improved in the morin treatment group (20 mg/kg, 80 mg/kg), confirming the effect of morin on the weight of chicks. Furthermore, histopathological examination confirmed that morin effectively relieved AFB1-caused hepatocyte disruption, swelling, and inflammatory cell infiltration, and renal cell necrosis, exfoliation, and vacuolization, which further confirmed the protective effects of morin on liver and kidney damage in chicks. Next, we researched the protective mechanism of morin against AFB1-caused liver and kidney injury in chicks.
NETs, as the first line of defense for innate immunity, are a double-edged sword for the organism. On the one hand, it plays an important role in the body's defense against pathogens. On the other hand, it will cause tissue damage and participate in the formation of some diseases, such as atherosclerosis and thrombosis (Moschonas and Tselepis, 2019). Study confirmed that NETs and histones play an important role in the injury of bovine mammary epithelial cells (BMEC), and inhibiting the formation of excessive NETs can alleviate mastities-related injury (Wei et al., 2019a). We also have found that AFB1 could cause liver and kidney damage in chicks through inducing the release of HETs. Whether the protective effect of morin on AFB1 caused injury in chicks is related to HETs is also an important aim of our experiment. HETs work as the vital innate immune responses of chicken, and the best time to detect HETs in serum is within 24 h, thus the timing of the study was selected. It is found that morin significantly reduced the formation of HETs in serum, and it was first confirmed that morin alleviated AFB1-caused injury in chicks by inhibiting the release of HETs.
In the normal metabolic process of the body, redox is in dynamic equilibrium, but when the production of reactive oxygen species (ROS) in the body exceeds the amounts of antioxidants, oxidative stress will occur. Oxidative stress is involved in many liver diseases, such as hepatic fibroproliferative disease, alcoholic liver disease, and nonalcoholic fatty liver disease (Cichoż-Lach and Michalak, 2014). MDA is the final products of lipid peroxidation produced by ROS, and can independently reflect the degree of damage to the membrane system and the degree of attack by free radicals on cells, so it is considered a good marker for evaluating oxidative stress (Wang et al., 2019). SOD is a specific antioxidant enzyme that scavenges superoxide anion in ROS free radicals, which indirectly view of the body's ability to remove oxygen free radicals, and has an extremely crucial function in the study of liver injury (Zhang et al., 2018). GSH-Px as an important indicator to measure the redox state of cells, which can counteract the toxicity of oxygen radicals by directly supplying H+, and reducing oxidative damage to the liver. CAT, exist in various tissues of animals, especially in the liver at high concentrations, which can effectively break down hydrogen peroxide into carbon dioxide and water to protect cells from oxidative damage (Alfonso-Prieto et al., 2009). The result indicated that AFB1 markedly reduced the levels of antioxidant enzymes GSH-Px, SOD and CAT, also increased the level of antioxidant enzymes MDA, while the morin group showed the opposite trend, effectively alleviating the imbalance between oxidase and antioxidant enzymes, indicating that morin can effectively inhibit AFB1-caused oxidative stress.
It is reported that ROS can cause the expression of inflammatory cytokines and the activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways (Li et al., 2014). NF-κB protein usually forms homo/heterodimers from p65 and p50, and is in an inactive state in the cytoplasm due to the formation of a trimer complex with the inhibitory protein IκB (Pantano et al., 2006). After being stimulated by the pathogen, IκB is dissociated from the trimer, and the NF-κB dimer is exposed to nuclear localization sequences (NLS), which rapidly enter the nucleus from the cytoplasm and bind to specific sequences on the nuclear DNA, and promoting the transcription of related genes (Zhang and Ghosh, 2001). TNF-α is the most important proinflammatory cytokine involved in the activation of NF-κB, inducing the expression of IL-1β, IL-6, COX-2, iNOS, and other downstream inflammatory mediators (Rajendran et al., 2018). Our results exhibited that AFB1 significantly promoted the mRNA expression of inflammatory factors TNF-α, IL-1β, IL-6 and inflammatory mediators COX-2 and iNOS, but the morin group significantly inhibited the expression of these factors, indicating that morin could effectively inhibit AFB1-induced inflammatory response in chicks.
Studies have shown that oxidative stress is one of the apoptosis-inducing factors (Chandra et al., 2000), and many substances that induce apoptosis are generally oxidants or stimuli in cell oxidative metabolism. The activated of NF-κB may drive the transcription of proapoptotic factor genes or inhibit the expression of antiapoptotic proteins. Meanwhile, caspase signaling pathway and inflammatory response are in tandem. Caspase-1 plays an important role in Il-1β secretion and caspase-11 is a homology of human inflammatory caspase-family members caspase-4 and caspase-5 (Van Opdenbosch and Lamkanfi, 2019). We confirmed that AFB1 markedly boost the expression of caspase-1, caspase-3, and caspase-11, while morin significantly decreased the expression of these factors, confirming that morin can effectively inhibit the activation of AFB1-induced caspase signaling pathway in chicks.
Based on the above results, we confirmed that morin can effectively alleviate AFB1-caused liver and kidney damage in chicks, which can provide experimental basis and theoretical reference for the practical application of morin. In next research, the feeding mode with morin should be choose, such as drinking water, and more chicks and older chicken are need to confirm the effect of morin in poultry farming.
CONCLUSION
Morin, as a natural compound commonly found in Chinese herbal medicines and dietary supplements, has been fully researched and confirmed for its safety and has received satisfactory results. Our experiments further demonstrated that morin could effectively alleviate AFB1-caused liver and kidney damage by inhibiting the release of HETs, oxidative stress, inflammatory response. Therefore, this study shows that morin has the potential as a feed additive to protect the liver and kidney of poultry, prevent of harmful substances in fodder and improve the performance of poultry.
ACKNOWLEDGMENTS
The work was funded by the Pearl River Talent Plan in Guangdong Province of China (2019CX01N111), National Natural Science Foundation of China (No. 32002309), and Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515110524).
DISCLOSURES
The authors have no conflict of interest.
REFERENCES
- Abdel-Wahhab M.A., Aly S.E. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J. Agric. Food Chem. 2003;51:2409–2414. doi: 10.1021/jf0209185. [DOI] [PubMed] [Google Scholar]
- Alfonso-Prieto M., Biarnés X., Vidossich P., Rovira C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009;131:11751–11761. doi: 10.1021/ja9018572. [DOI] [PubMed] [Google Scholar]
- Alpsoy L., Yalvac M.E. Key roles of vitamins A, C, and E in aflatoxin B1-induced oxidative stress. Vitam. Horm. 2011;86:287–305. doi: 10.1016/B978-0-12-386960-9.00012-5. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Cao C., Zhang H., Wang K., Li X. Selenium-rich yeast mitigates aluminum-mediated testicular toxicity by blocking oxidative stress, inhibiting NO production, and disturbing ionic Homeostasis. Biol. Trace Elem. Res. 2020;195:170–177. doi: 10.1007/s12011-019-01820-5. [DOI] [PubMed] [Google Scholar]
- Chandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- Chuammitri P., Ostojić J., Andreasen C.B., Redmond S.B., Lamont S.J., Palić D. Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet. Immunol. Immunopathol. 2009;129:126–131. doi: 10.1016/j.vetimm.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D.W., Allen R., Wilkinson A.P., Morgan M.R. Transplacental transfer of aflatoxin in humans. Carcinogenesis. 1990;11:1033–1035. doi: 10.1093/carcin/11.6.1033. [DOI] [PubMed] [Google Scholar]
- Guo J.M., Xing H.J., Cai J.Z., Zhang H.F., Xu S.W. H(2)S exposure-induced oxidative stress promotes LPS-mediated hepatocyte autophagy through the PI3K/AKT/TOR pathway. Ecotoxicol. Environ. Saf. 2021;209 doi: 10.1016/j.ecoenv.2020.111801. [DOI] [PubMed] [Google Scholar]
- Jiang A., Zhang Y., Zhang X., Wu D., Liu Z., Li S., Liu X., Han Z., Wang C., Wang J., Wei Z., Guo C., Yang Z. Morin alleviates LPS-induced mastitis by inhibiting the PI3K/AKT, MAPK, NF-κB and NLRP3 signaling pathway and protecting the integrity of blood-milk barrier. Int. Immunopharmacol. 2020;78 doi: 10.1016/j.intimp.2019.105972. [DOI] [PubMed] [Google Scholar]
- Khamchai S., Chumboatong W., Hata J., Tocharus C., Suksamrarn A., Tocharus J. Morin protects the blood-brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci Rep. 2020;710:13379. doi: 10.1038/s41598-020-70214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Wang G., Li M., Li L., Liu H., Sun M., Wen Z. Morin inhibits Listeria monocytogenes virulence in vivo and in vitro by targeting listeriolysin O and inflammation. BMC Microbiol. 2020;20:112. doi: 10.1186/s12866-020-01807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Huang K., Liu X., Liu J., Lu X., Tao K., Wang G., Wang J. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK-3β/NF-κB signaling pathway. Oxid. Med. Cell Longev. 2014;2014 doi: 10.1155/2014/241864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschonas I.C., Tselepis A.D. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. 2019;288:9–16. doi: 10.1016/j.atherosclerosis.2019.06.919. [DOI] [PubMed] [Google Scholar]
- Mughal M.J., Xi P., Yi Z., Jing F. Aflatoxin B1 invokes apoptosis via death receptor pathway in hepatocytes. Oncotarget. 2017;8:8239–8249. doi: 10.18632/oncotarget.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir S., Kucukler S., Comakli S., Kandemir F.M. The protective effect of morin against ifosfamide-induced acute liver injury in rats associated with the inhibition of DNA damage and apoptosis. Drug. Chem. Toxicol. 2020:1–10. doi: 10.1080/01480545.2020.1822390. [DOI] [PubMed] [Google Scholar]
- Pantano C., Reynaert N.L., van der Vliet A., Janssen-Heininger Y.M. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid. Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- Perumal N., Perumal M., Halagowder D., Sivasithamparam N. Morin attenuates diethylnitrosamine-induced rat liver fibrosis and hepatic stellate cell activation by co-ordinated regulation of Hippo/Yap and TGF-beta1/Smad signaling. Biochimie. 2017;140:10–19. doi: 10.1016/j.biochi.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Qianru C., Xueyuan H., Bing Z., Qing Z., Kaixin Z., Shu L. Regulation of H(2)S-induced necroptosis and inflammation in broiler bursa of Fabricius by the miR-15b-5p/TGFBR3 axis and the involvement of oxidative stress in this process. J. Hazard Mater. 2021;406 doi: 10.1016/j.jhazmat.2020.124682. [DOI] [PubMed] [Google Scholar]
- Raafat N., Emam W.A., Gharib A.F., Nafea O.E., Zakaria M. Assessment of serum aflatoxin B(1) levels in neonatal jaundice with glucose-6-phosphate dehydrogenase deficiency: a preliminary study. Mycotoxin. Res. 2021;37:109–116. doi: 10.1007/s12550-020-00421-9. [DOI] [PubMed] [Google Scholar]
- Rajendran P., Chen Y.F., Chen Y.F., Chung L.C., Tamilselvi S., Shen C.Y., Day C.H., Chen R.J., Viswanadha V.P., Kuo W.W., Huang C.Y. The multifaceted link between inflammation and human diseases. J. Cell Physiol. 2018;233:6458–6471. doi: 10.1002/jcp.26479. [DOI] [PubMed] [Google Scholar]
- Rawal S., Kim J.E., Coulombe R., Jr. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res. Vet. Sci. 2010;89:325–331. doi: 10.1016/j.rvsc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Rushing B.R., Selim M.I. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., Yamamoto N., Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host. Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Tian Y., Li Z., Shen B., Zhang Q., Feng H. Protective effects of morin on lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting TLR4/NF-kappaB and activating Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2017;45:148–155. doi: 10.1016/j.intimp.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ruan X., Li Y., Cheng J., Mueck A.O. Oxidative stress indicators in Chinese women with PCOS and correlation with features of metabolic syndrome and dependency on lipid patterns. Arch. Gynecol. Obstet. 2019;300:1413–1421. doi: 10.1007/s00404-019-05305-7. [DOI] [PubMed] [Google Scholar]
- Wei Z., He X., Kou J., Wang J., Chen L., Yao M., Zhou E., Fu Y., Guo C., Yang Z. Renoprotective mechanisms of morin in cisplatin-induced kidney injury. Int. Immunopharmacol. 2015;28:500–506. doi: 10.1016/j.intimp.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Wei Z., Hermosilla C., Taubert A., He X., Wang X., Gong P., Li J., Yang Z., Zhang X. Canine neutrophil extracellular traps release induced by the apicomplexan parasite neospora caninum in vitro. Front. Immunol. 2016;7:436. doi: 10.3389/fimmu.2016.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Wang J., Wang Y., Wang C., Liu X., Han Z., Fu Y., Yang Z. Effects of neutrophil extracellular traps on bovine mammary epithelial cells in vitro. Front. Immunol. 2019;10:1003. doi: 10.3389/fimmu.2019.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Zhao Y., Zhang N., Han Z., Liu X., Jiang A., Zhang Y., Wang C., Gong P., Li J., Zhang X., Yang Z. Eimeria tenella induces the release of chicken heterophil extracellular traps. Vet. Parasitol. 2019;275 doi: 10.1016/j.vetpar.2019.108931. [DOI] [PubMed] [Google Scholar]
- Wu D., Li S., Li P., Jiang A., Liu Z., Zhang Y., Wang J., Yang Z., Wei Z. Diacetoxyscirpenol-induced heterophil extracellular traps contribute to the immune toxicity of liver injury in chickens. Food Chem. Toxicol. 2021;148 doi: 10.1016/j.fct.2020.111926. [DOI] [PubMed] [Google Scholar]
- Xie Y., Yang Q., Nelson B.D., DePierre J.W. The relationship between liver peroxisome proliferation and adipose tissue atrophy induced by peroxisome proliferator exposure and withdrawal in mice. Biochem. Pharmacol. 2003;66:749–756. doi: 10.1016/s0006-2952(03)00386-1. [DOI] [PubMed] [Google Scholar]
- Xu H., Ziegelin G., Schröder W., Frank J., Ayora S., Alonso J.C., Lanka E., Saenger W. Flavones inhibit the hexameric replicative helicase RepA. Nucleic Acids Res. 2001;29:5058–5066. doi: 10.1093/nar/29.24.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zhang Z., Wang X., Wang Y., Zhang X., Lu H., Wang S.L. Cytochrome P450 2A13 enhances the sensitivity of human bronchial epithelial cells to aflatoxin B1-induced DNA damage. Toxicol. Appl. Pharmacol. 2013;270:114–121. doi: 10.1016/j.taap.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang N., Xu Y., Tan H.Y., Li S., Feng Y. Molecular mechanisms involved in oxidative stress-associated liver injury induced by chinese herbal medicine: an experimental evidence-based literature review and network pharmacology study. Int. J. Mol. Sci. 2018;19:2745. doi: 10.3390/ijms19092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Wang S., Zhang Q., Zhang Z., Xu S. Avermectin inhibits neutrophil extracellular traps release by activating PTEN demethylation to negatively regulate the PI3K-ERK pathway and reducing respiratory burst in carp. J. Hazard Mater. 2020;389 doi: 10.1016/j.jhazmat.2019.121885. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Cao Z.Q., Wang H.Y., Cheng Y.N., Yu L.G., Zhang X.K., Sun Y., Guo X.L. The anti-inflammatory effects of Morin hydrate in atherosclerosis is associated with autophagy induction through cAMP signaling. Mol. Nutr. Food Res. 2017;61:1–10. doi: 10.1002/mnfr.201600966. [DOI] [PubMed] [Google Scholar]