Abstract
Objective:
Sugar-sweetened beverage (SSB) consumption had substantially increased across successive US birth cohorts until 2000, and adolescents and young adults under age 50 have the highest consumption. However, the link between SSBs and early-onset colorectal cancer (EO-CRC) remains unexamined.
Design:
In the Nurses’ Health Study II (1991–2015), we prospectively investigated the association of SSB intake in adulthood and adolescence with EO-CRC risk among 95,464 women who had reported adulthood beverage intake using validated food frequency questionnaires (FFQs) every 4 years. A subset of 41,272 participants reported beverage intake at age 13–18 using a validated high school-FFQ in 1998. Cox proportional hazards models were used to estimate RRs with 95% CIs.
Results:
We documented 109 EO-CRC cases. Compared with individuals who consumed <1 serving/wk of SSBs in adulthood, women who consumed ≥2 servings/d had a more than doubled risk of EO-CRC (RR, 2.18; 95% CI, 1.10–4.35; Ptrend=0.02), with a 16% higher risk (RR, 1.16; 95% CI, 1.00–1.36) per serving/d increase. Each serving/d increment of SSB intake at age 13–18 was associated with a 32% higher risk of EO-CRC (RR, 1.32; 95% CI, 1.00–1.75). Replacing each serving/d of adulthood SSB intake with that of artificially sweetened beverages, coffee, reduced fat milk, or total milk was associated with a 17–36% lower risk of EO-CRC.
Conclusion:
Higher SSB intake in adulthood and adolescence was associated with a higher risk of EO-CRC among women. Reduction of SSB consumption among adolescents and young adults may serve as a potential strategy to alleviate the growing burden of EO-CRC.
Keywords: early-onset colorectal cancer, sugar-sweetened beverages
INTRODUCTION
The incidence of early-onset colorectal cancer (EO-CRC, age <50 at diagnosis) has been on the rise in many high income countries over the past two decades.1–3 In the US population born after 1950, the EO-CRC incidence has increased across subsequent birth cohorts.4,5 Compared to adults born around 1950, those born around 1990 had two times the risk of colon cancer and four times the risk of rectal cancer.4 This postulates that increasingly prevalent exposures across birth cohorts may be driving the incidence upward; yet, such etiologic factors remain largely unidentified.
Sugar-sweetened beverages (SSBs, e.g. soft drinks, fruit drinks, sports drinks, and energy drinks) comprise the leading (39%) source of added sugar in US diets,6 and 12% of the population consume more than 3 servings per day.7 Coinciding with the rising EO-CRC incidence that showed a birth cohort effect,4,5 age- and birth cohort-specific SSB consumption considerably increased from 1977 to 2001.8 For example, caloric contribution from SSBs more than doubled from 5.1% to 12.3% among individuals aged 19–39 and from 4.8% to 10.3% among those aged 2–18.8 SSBs can exert adverse metabolic repercussions throughout the life course.9–11 In adulthood, each additional daily serving of SSBs was associated with a 12% higher risk of obesity12 and a 18% higher risk of type 2 diabetes.13 SSBs are also considered a major contributor to childhood obesity14–16 and insulin resistance.17 Interestingly, childhood obesity is known to exacerbate insulin resistance during puberty18,19 and interrupt recovery of glucose homeostasis afterwards, which can leave lasting adverse metabolic consequences.20
As growing evidence supports a plausible link of metabolic conditions, including adulthood and adolescent obesity and early-onset type 2 diabetes with EO-CRC,21–23 investigation of SSB intake throughout the life course with EO-CRC risk is a logical step and immediate need due to limited actionable strategies to reduce the growing burden of EO-CRC. Although existing epidemiologic evidence linking SSBs and colorectal cancer (CRC) risk is inconclusive;24–28 emerging experimental studies strongly support such a link.29,30 Artificially sweetened beverages (ASBs) and 100% fruit juice have been considered alternatives to SSBs and increasingly consumed in adolescents and adults,31–33 but much remains to be answered regarding their long-term health implications.
To address these knowledge gaps, we investigated the association between intake of sweetened beverages in adulthood and adolescence, with a particular emphasis on SSBs, and risk of EO-CRC. We leveraged data from the Nurses’ Health Study II (NHSII), a large, prospective US cohort of young women, which utilizes a validated assessment of both adulthood and adolescent beverage intake.
METHODS
Study population
The NHSII is an ongoing prospective cohort study of 116,429 US female registered nurses aged 25–42 at enrollment in 1989. Biennially, participants self-reported detailed information on demographics, lifestyle, and medical history. Dietary intake was assessed using validated, semiquantitative food frequency questionnaires (FFQs) approximately every four years. Return of the completed questionnaire implied informed consent to study participation. The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital and that of participating state cancer registries as required.
Ascertainment of colorectal cancer
The primary endpoint was the diagnosis of incident invasive EO-CRC. We requested permission to review medical records or pathology reports for CRC diagnoses reported on a biennial questionnaire or lethal CRC cases identified from the National Death Index, tumor registries, or death certificates. Study physicians were masked to participant information, reviewed the records, and confirmed diagnosis, date, anatomic location, histology, and stage.
Assessment of adulthood and adolescent beverage intake
In 1991 and every four years thereafter, beverage intake was assessed via validated semiquantitative FFQs34,35 where participants reported how often, on average, they consumed each of ~130 food or beverage items during the past 12 months. Nine response options were available, ranging from “never or less than once per month (referred to never)” to “≥6 times per day”. SSBs were defined as carbonated or noncarbonated beverages with sugar (e.g. soft drinks, fruit drinks, sports drinks, and sweetened tea beverages). ASBs were defined as low-calorie carbonated beverages. One standard serving size for SSBs and ASBs was equivalent to 12 ounces (oz). Fruit juice included apple juice, orange juice, grapefruit juice, prune juice, and other fruit juices, with 4–6 oz as the standard portion size. We also assessed consumption of other beverages including tap or bottled water, tea, coffee, reduced fat milk, and whole milk with the serving size of 8 oz. In the current analysis, we converted the portion size of any beverages into 8 oz for consistency. As a measure of validity, the Pearson correlation coefficients between the FFQ and multiple dietary records were 0.84 for soft drinks and fruit juice, 0.93 for tea, 0.78 for coffee, 0.81 for reduced fat milk, and 0.62 for whole milk.36
In 1998, we additionally assessed dietary intake in adolescence among a subset of participants through a supplementary, high school (HS)-FFQ, comprising 124 food items typically consumed between 1960 and 1982 during which participants were at age 13–18. Adolescent beverage consumption was categorized into SSBs, ASBs, and fruit juice, having 8 oz as one serving size. The validity and reproducibility of adolescent diet recalled in adulthood have been reported.37,38 The Pearson correlation coefficient was 0.58 for energy-adjusted nutrient intake among two FFQs that 80 young women completed in high school and the HS-FFQ administered 10 years later.37 In randomly selected 333 NHSII participants who also completed a second HS-FFQ in 2002, the Spearman’s rank correlation coefficient for adolescent beverage intake was 0.70 between the two HS-FFQs.38
Assessment of covariates
Detailed information on potential CRC risk factors was obtained at baseline with updates during follow-up. This included weight, menopausal status and menopausal hormone use, family history of CRC in one or more first-degree relatives at any age at first diagnosis, smoking habits, physical activity in metabolic equivalent of task (MET)-hours, regular use (≥2 times/wk) of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), and current use of multivitamins. Using FFQs, intake of total calories, alcohol, red and processed meat, dietary fiber, total folate (from foods and supplements), and total calcium was updated every four years. Diet quality was assessed by the Alternative Healthy Eating Index (AHEI)-2010.39 Biennially, participants reported history of colonoscopy/sigmoidoscopy during the previous two years with the corresponding indications.
In 1989, we asked participants to recall their health status and lifestyle in adolescence. Adolescent body mass index (BMI) was calculated using recalled weight at age 18 and height. Pack-years of smoking before age 20 were obtained using the duration and average number of cigarettes smoked per day. Alcohol intake at age 15–17 was estimated from the usual number of alcoholic drinks consumed per week. Dietary intake and multivitamin use at age 13–18 was assessed via a HS-FFQ. In 1997, physical activity in MET-hours/wk at grade 9–12 was assessed using a questionnaire with reasonable reliability.40
Statistical analysis
We set the analytic baseline to 1991 when beverage intake was first reported. We excluded women who had died or had CRC or inflammatory bowel disease prior to baseline, who reported implausible energy intake (<600 or >3,500 kcal/d), and who had missing information on beverage intake. A total of 95,464 women were included in the final analysis.
As our primary analysis, we investigated the associations of adulthood and adolescent beverage intake with risk of EO-CRC. Person-years of follow-up accrued from the date of the 1991 questionnaire return to the diagnosis of EO-CRC, 50th birthday, death, or the end of follow-up (June 2015), whichever came first. To deal with the time-varying nature of adulthood beverage intake over the long duration of follow-up, we calculated the cumulative average of beverage intake collected across all available FFQs from the study baseline up to each questionnaire cycle to better represent long-term beverage intake reflecting true changes and minimizing the extent of measurement error and within-person variation. Intake of SSBs, ASBs, and fruit juice was categorized a priori according to a modification of the categories used in the prior studies (<1 serving/wk, 1 serving/wk to <1 serving/d, 1 to <2 servings/d, ≥2 servings/d).27,41 Cox proportional hazards models were used to compute hazard ratios (HRs), as estimates of relative risks (RRs), and 95% confidence intervals (CIs). Models were stratified by age in months and biennial questionnaire cycle and adjusted for total caloric intake. We adjusted for the following putative CRC risk factors: race, height, BMI, menopausal status and menopausal hormone use, family history of CRC, pack-years of smoking, physical activity, regular use of aspirin or NSAIDs, current use of multivitamins, intake of alcohol, red and processed meat, dietary fiber, total folate, and total calcium, AHEI-2010 score without SSBs and alcohol, and lower endoscopy due to screening or for other indications within the past 10 years. In additional analyses, we mutually adjusted for consumption of SSBs, ASBs, and fruit juice. Test for trend was performed using the median of each category of beverage intake as a continuous variable.
For adolescent SSBs (<1 serving/wk, 1 serving/wk to <2 servings/d, ≥2 servings/d), we restricted the analyses to 41,272 participants who completed and returned the HS-FFQ in 1998. In addition to age- and energy-adjusted models, we adjusted for the following putative CRC risk factors in adolescence: race, height, BMI at age 18, pack-years of smoking before age 20, intake of alcohol at age 15–17, red and processed meat, dietary fiber, total folate, and total calcium at age 13–18, multivitamin use at age 13–18, and physical activity at grade 9–12.
We estimated risk of EO-CRC associated with substitution of other beverages including ASBs, fruit juice, water, tea, coffee, reduced fat milk, or total milk for SSB intake in adulthood, by adding SSBs and the alternative beverage in the same multivariable model. RR was calculated using the difference in the two β coefficients, with 95% CI using the corresponding variances and covariance.42 Further, we performed stratified analyses for the association of SSB intake (per each serving/d increment) with risk of EO-CRC, according to family history of CRC (yes, no), BMI (<25, ≥25 kg/m2), alcohol use (never, ever), cigarette use (never, ever), and adolescent SSB intake (<1, ≥1 serving/d). Test for interaction was performed using the Wald test with a cross-product term of SSB intake, modeled as a continuous variable, and each potential effect modifier. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc.), with a 2-sided ɑ=0.05.
Patient and public involvement
No patients and the public were involved in the design, conduct, reporting, and dissemination plans of this research.
RESULTS
Among 95,464 women studied, we documented a total of 109 incident EO-CRC cases over up to 24 years of follow-up (1,358,142 person-years). Participant characteristics of person-years according to beverage intake are presented in Table 1. Individuals with higher SSB intakes in adulthood tended to be less physically active and more likely to have a lower endoscopy history due to indications, use NSAIDs, and consume red and processed meat. Also, they were less likely to take multivitamins and had lower intake of alcohol, fiber, folate, and calcium, along with poorer diet quality. Individuals with higher ASB intakes were more likely to be overweight, and those with higher fruit juice intakes were less likely to be overweight. Similarly, women with greater SSB consumption at age 13–18 were more likely to engage in unhealthy diet and lifestyle in adolescence (Supplementary Table 1). Baseline characteristics of the study population according to beverage intake in adulthood is shown in Supplementary Table 2.
Table 1.
Age-standardized participant characteristics of person-years according to average beverage intake in adulthood among women younger than age 50 in the NHSII, 1991–2015a
| Sugar-sweetened beverages | Artificially sweetened beverages | Fruit juice | ||||
|---|---|---|---|---|---|---|
| Characteristic | <1 serving/wk | ≥2 servings/d | <1 serving/wk | ≥2 servings/d | <1 serving/wk | ≥2 servings/d |
| Person-years, No. | 536 446 | 138 469 | 424 283 | 353 780 | 450 890 | 14 825 |
| Age, y | 42.0 (5.2) | 41.5 (5.5) | 41.8 (5.3) | 42.0 (5.2) | 41.9 (5.2) | 40.1 (5.6) |
| Race, white, % | 94 | 90 | 91 | 95 | 93 | 85 |
| Height, cm | 165 (6.6) | 165 (6.7) | 165 (6.7) | 165 (6.6) | 165 (6.6) | 165 (6.8) |
| BMI, kg/m² | 25.2 (5.3) | 25.5 (6.2) | 23.7 (4.8) | 26.7 (5.9) | 25.5 (5.6) | 23.6 (4.8) |
| Postmenopausal, % | 8.8 | 11 | 8.9 | 9.8 | 9.9 | 8.1 |
| Current menopausal hormone use among postmenopausal women, % | 66 | 63 | 62 | 65 | 66 | 63 |
| Family history of colorectal cancer, % | 5.8 | 5.6 | 5.7 | 6.0 | 5.8 | 4.8 |
| Screening lower endoscopy within the past 10 y, % | 3.3 | 3.1 | 3.2 | 3.1 | 3.2 | 3.5 |
| Lower endoscopy due to other indications within the past 10 y, % | 6.5 | 8.3 | 6.8 | 7.5 | 6.9 | 6.6 |
| Ever smokers, % | 36 | 33 | 32 | 36 | 38 | 29 |
| Pack-years among ever smokers | 12.3 (9.4) | 15.5 (11.3) | 13.7 (10.5) | 13.1 (9.8) | 13.9 (10.3) | 11.6 (9.4) |
| Physical activity, MET-h/wk | 24.5 (26.4) | 19.9 (24.6) | 21.3 (24.8) | 23.2 (25.4) | 20.9 (24.1) | 29.8 (33.1) |
| Regular use of aspirin, % | 11 | 12 | 10 | 12 | 11 | 10 |
| Regular use of non-aspirin NSAIDs, % | 26 | 31 | 22 | 33 | 28 | 22 |
| Current use of multivitamins, % | 47 | 42 | 47 | 45 | 41 | 52 |
| Dietary intake | ||||||
| Alcohol, g/d | 3.7 (6.3) | 2.3 (5.2) | 2.8 (5.6) | 3.6 (6.3) | 3.2 (6.1) | 3.1 (6.4) |
| Red and processed meat, servings/wk | 5.5 (3.7) | 8.1 (4.5) | 6.3 (4.1) | 6.8 (4.1) | 6.1 (3.9) | 6.1 (4.1) |
| Dietary fiber, g/d | 20.1 (5.6) | 14.6 (3.9) | 17.9 (5.6) | 18.4 (4.9) | 18.2 (5.7) | 18.7 (5.7) |
| Total folate, μg/d | 523 (267) | 410 (213) | 497 (267) | 478 (234) | 455 (258) | 597 (252) |
| Total calcium, mg/d | 1119 (438) | 837 (329) | 1018 (417) | 1042 (395) | 1022 (439) | 1039 (377) |
| Alternative Healthy Eating Index-2010 scoreb,c | 42.4 (8.9) | 35.3 (7.5) | 40.3 (9.4) | 38.9 (8.1) | 39.2 (8.9) | 43.2 (9.5) |
Abbreviations: BMI, body mass index; MET, metabolic equivalent of task; NHSII, Nurses’ Health Study II; NSAID, nonsteroidal anti-inflammatory drug.
Data are presented as mean (standard deviation) of person-years unless otherwise indicated. All values other than age were directly standardized to the age distribution (in 5-year intervals) of all participants.
Without sugar-sweetened beverages and alcohol.
According to the predefined intake criteria for 11 dietary components (e.g. fruits, vegetables, whole grains, nuts and legumes, red and processed meat, sugar-sweetened beverages, sodium, alcohol, polyunsaturated fatty acids, omega-3 fatty acids, and trans fatty acids), a score ranging from 0 to 10 was given to each component, yielding a total score ranging from 0 to 110. A higher score reflects better diet quality.
Higher SSB intake in adulthood was associated with a higher risk of EO-CRC after adjusting for a list of putative CRC risk factors. Compared with individuals who consumed <1 serving/wk, women who consumed ≥2 servings/d had a 2.2-fold higher risk of EO-CRC (RR, 2.18; 95% CI, 1.10–4.35; Ptrend=0.02), with a 16% higher risk per each additional serving/d of SSB intake (RR, 1.16; 95% CI, 1.00–1.36) (Table 2). Additional adjustment for adulthood intake of ASBs and fruit juice slightly attenuated the magnitude of this association (Supplementary Table 3). In contrast, each serving/d increase in ASBs or fruit juice consumption in adulthood was not associated with risk of EO-CRC (ASBs: RR, 0.93; 95% CI, 0.83–1.04; fruit juice: RR, 1.20; 95% CI, 0.74–1.94) (Table 2).
Table 2.
Sweetened beverage intake in adulthood and risk of early-onset colorectal cancer
| Exposure | <1 serving/wk | 1 serving/wk to <1 serving/d | 1 serving/d to <2 servings/d | ≥2 servings/d | Ptrenda | Each serving/d increase |
|---|---|---|---|---|---|---|
| Sugar-sweetened beverages | ||||||
| Person-years | 536 446 | 504 341 | 178 886 | 138 469 | ||
| No. of cases | 45 | 34 | 14 | 16 | ||
| Age- and energy-adjusted RR (95% CI) | 1 [Reference] | 0.89 (0.56–1.41) | 1.03 (0.55–1.92) | 1.72 (0.93–3.20) | 0.06 | 1.11 (0.96–1.29) |
| Multivariable RR (95% CI)b | 1 [Reference] | 0.97 (0.61–1.55) | 1.24 (0.65–2.39) | 2.18 (1.10–4.35) | 0.02 | 1.16 (1.00–1.36) |
| Artificially sweetened beverages | ||||||
| Person-years | 424 283 | 321 864 | 258 215 | 353 780 | ||
| No. of cases | 32 | 33 | 19 | 25 | ||
| Age- and energy-adjusted RR (95% CI) | 1 [Reference] | 1.25 (0.76–2.04) | 0.95 (0.54–1.68) | 0.86 (0.50–1.46) | 0.32 | 0.96 (0.86–1.07) |
| Multivariable RR (95% CI)b | 1 [Reference] | 1.20 (0.73–1.98) | 0.86 (0.48–1.54) | 0.73 (0.42–1.27) | 0.11 | 0.93 (0.83–1.04) |
| Fruit juice | ||||||
| Person-years | 450 890 | 799 663 | 92 765 | 14 825 | ||
| No. of cases | 44 | 59 | 5 | 1 | ||
| Age- and energy-adjusted RR (95% CI) | 1 [Reference] | 0.81 (0.53–1.22) | 0.66 (0.25–1.71) | 0.90 (0.12–6.76) | 0.41 | 1.04 (0.64–1.67) |
| Multivariable RR (95% CI)b | 1 [Reference] | 0.86 (0.56–1.31) | 0.77 (0.29–2.05) | 1.20 (0.16–9.11) | 0.69 | 1.20 (0.74–1.94) |
Abbreviations: CI, confidence interval; RR, relative risk.
One beverage serving is 8 oz.
Calculated using the median of each category of beverage intake as a continuous variable.
Additionally adjusted for race (white, nonwhite), height (continuous), body mass index (continuous), menopausal status and menopausal hormone use (premenopausal, postmenopausal never user, postmenopausal ever user, unknown menopausal status or hormone use), family history of colorectal cancer (yes, no), pack-years of smoking (continuous), physical activity (continuous), regular use of aspirin (yes, no), regular use of nonsteroidal anti-inflammatory drugs (yes, no), current use of multivitamins (yes, no), intake of alcohol, red and processed meat, dietary fiber, total folate [from foods and supplements], and total calcium (all continuous), Alternative Healthy Eating Index-2010 score without sugar-sweetened beverages and alcohol (continuous), and lower endoscopy due to screening (yes, no) or for other indications within the past 10 years (yes, no).
Among a subset of participants, adolescent SSB consumption was also associated with a higher risk of EO-CRC after adjusting for potential confounding factors in adolescence (Table 3). Each serving/d increment of SSB intake at age 13–18 was associated with a 32% higher risk of subsequently developing EO-CRC (RR, 1.32; 95% CI, 1.00–1.75). Further adjustment for adulthood intake of SSBs and total calories slightly attenuated the effect estimates (data not shown).
Table 3.
Sugar-sweetened beverage intake at age 13–18 and risk of early-onset colorectal cancer
| <1 serving/wk | 1 serving/wk to <2 servings/d | ≥2 servings/d | Ptrenda | Each serving/d increase | |
|---|---|---|---|---|---|
| Person-years | 113 475 | 218 172 | 25 788 | ||
| No. of cases | 12 | 17 | 6 | ||
| Age- and energy-adjusted RR (95% CI) | 1 [Reference] | 0.73 (0.34–1.58) | 2.43 (0.83–7.05) | 0.05 | 1.19 (0.92–1.54) |
| Multivariable RR (95% CI)b | 1 [Reference] | 0.78 (0.36–1.73) | 3.41 (1.08–10.8) | 0.01 | 1.32 (1.00–1.75) |
Abbreviations: CI, confidence interval; RR, relative risk.
One beverage serving is 8 oz.
Calculated using the median of each category of beverage intake as a continuous variable.
Additionally adjusted for race (white, nonwhite), height (continuous), body mass index at age 18 (continuous), pack-years of smoking before age 20 (continuous), intake of alcohol at age 15–17, red and processed meat, dietary fiber, total folate [from foods and supplements], and total calcium at age 13–18 (all continuous), multivitamin use at age 13–18 (yes, no), and physical activity at grade 9–12 (continuous).
For adulthood SSB intake, we further evaluated the association of replacing 1 serving/d of SSBs with an equivalent amount of other beverages. This was associated with a 17–36% lower risk of EO-CRC (ASBs: RR, 0.83; 95% CI, 0.69–0.99; coffee: RR, 0.82; 95% CI, 0.68–0.99; reduced fat milk: RR, 0.65; 95% CI, 0.47–0.90; and total milk: RR, 0.64; 95% CI, 0.46–0.89) (Figure 1). Substituting water or tea for SSBs appeared to be associated with a lower EO-CRC risk but did not reach statistical significance. Fruit juice substitution did not appear to reduce risk of EO-CRC. In a stratified analysis for adulthood SSB intake and risk of EO-CRC, the positive association did not substantially vary by family history of CRC, BMI, alcohol use, cigarette use, and adolescent SSB intake (all Pinteraction ≥0.23) (Supplementary Figure 1).
Figure 1. Substitution of other beverages for sugar-sweetened beverages with risk of early-onset colorectal cancer.
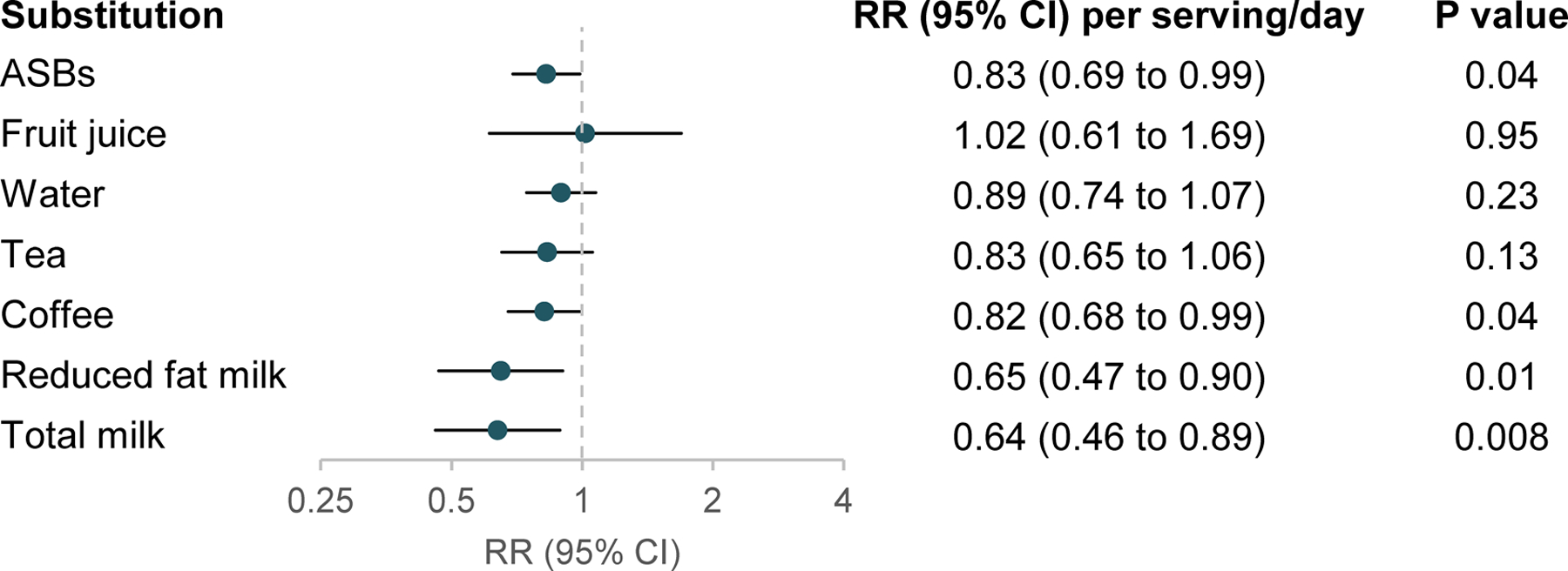
Abbreviations: ASB, artificially sweetened beverage; CI, confidence interval; RR, relative risk.
One beverage serving is 8 oz. The models were adjusted for covariates listed in the footnote of Table 2.
DISCUSSION
In this large prospective US cohort study of younger women, higher SSB consumption in adulthood and adolescence was associated with a substantially higher risk of EO-CRC; no associations were observed for ASBs or fruit juice consumption in adulthood. Replacing SSB intake in adulthood with ASBs, coffee, reduced fat milk, or total milk was associated with a lower risk of EO-CRC. Our findings add unique epidemiologic evidence that SSB intake may partly contribute to the rapid increase of CRC in younger adults.
Although US adults aged 20–34 have had the highest level of SSB consumption (1.7 servings/d on average) across all age groups over the past several decades,43,44 epidemiologic evidence on the role of SSBs in colorectal carcinogenesis remains inconclusive. In a pooled analysis of 13 prospective cohort studies, soft drink consumption was not associated with colon cancer.24 Recent cohort studies from France and the US reported no association between total SSBs and CRC;25–27 however, these studies were subject to a limited number of cases consuming >1 serving/d of SSBs, and some used baseline SSB intake only.25,27 In contrast, a recent Australian cohort study with 112 cases consuming ≥1 time/d of soft drinks showed a 28% (HR, 1.28; 95% CI, 1.04–1.57) higher CRC risk among these individuals, compared with those who consumed <1 time/mo.28 During the second half of the 20th century, average US per capita soft drink consumption has dramatically increased by ~500%.45 More importantly, compared to older generations, those born after the 1950s started to drink SSBs earlier in life and had higher intake across successive cohorts.8 This age- and birth cohort-specific SSB consumption coinciding with the rising incidence of EO-CRC4,5 lends support to the link between SSBs and risk of EO-CRC and may help explain the discrepancy between prior studies and ours. As accumulating evidence supports early- versus late-onset CRC may have different proportions of genetic, pathologic, and molecular characteristics,46 established or putative CRC risk factors need to be examined in the context of EO-CRC, as strengths of the association for the same risk factor may differ. Our study thus addressed these knowledge gaps by leveraging long-term SSB intake assessed repeatedly via validated FFQs and demonstrated a positive association of SSB intake in adulthood with risk of EO-CRC. This association was independent of a list of confounding factors and did not appreciably vary by CRC family history, obesity, and alcohol intake. We also reported a 32% higher risk of EO-CRC per serving/d increment of SSBs in adolescence. While our sample size was limited and this finding requires further validation, it is important to note that 30% of US children and adolescents consume >1.5 servings of SSBs daily.7 Taken together, our study provides preliminary evidence linking SSB intake across different life stages with risk of EO-CRC. Further studies with larger sample sizes in more diverse populations are needed to validate these findings.
In line with prior studies among older adults,25,26,28 we found that intake of ASBs or fruit juice in adulthood was not associated with risk of EO-CRC. However, considering the emerging link between these beverages and diabetes,13 as well as overall and obesity-related cancers,25,26 future research is warranted. Of note, substitution of reduced fat milk or total milk for SSBs appeared to be especially beneficial in lowering EO-CRC risk, which may point to the protective role of milk and calcium against EO-CRC, as with that in CRC among older adults.47 From 1989 to 2008, along with a 63% increase in SSB consumption among US school-aged children, there was a 22% reduction in milk consumption,48 which may have exacerbated EO-CRC risk among younger adults.
Various biological mechanisms support the plausibility of our findings on SSBs and EO-CRC. Compared to intake of isocaloric solid foods, energy-containing beverages that lack dietary compensation suppress feeling satiety and promote excess energy intake, which can ultimately result in weight gain.9,49 As indicated by their high glycemic index,50 SSBs initiate rapid blood glucose response and insulin secretion, which in the long-term can induce insulin resistance, inflammation, obesity, and type 2 diabetes,51 all of which are metabolic conditions tied to increased CRC risk.52 Specifically, fructose, a major component of sucrose and high fructose corn syrup (HFCS), which are primary sweeteners of SSBs, has been postulated to exert these adverse metabolic effects.9,53 In addition to this classic, obesity-related pathway, emerging data have uncovered some novel mechanisms. Excess fructose surpassing the small intestinal absorption capacity reaches the colon.29,54 By causing dysbiosis and endotoxemia,55 fructose can impair gut barrier function and increase gut permeability,56 which could promote colorectal carcinogenesis. A recent experimental study demonstrated that HFCS-treated mice had substantial colon tumor growth with aggressive tumor grade, independent of obesity and metabolic syndrome,30 which lends additional support to the link between SSBs and CRC risk.
Strengths of our study include a long-term follow-up and rigorous dietary data spanning various life stages from adolescence, which have been considered important aspects in studying the etiology of EO-CRC.46 Specifically, our analyses included 95,464 young women followed for up to 24 years. Prospective and repeated assessment of SSB intake via validated FFQs allowed the capture of long-term intake, reflecting true changes and minimizing the extent of measurement error and recall bias. Additional use of validated data on adolescent dietary intake enabled us to examine the association between SSBs and EO-CRC across different life stages. Several limitations need to be considered while interpreting our findings. First, as with every observational study, residual or unmeasured confounding cannot be ruled out completely. Nonetheless, we have collected an extensive list of putative CRC risk factors both in adulthood and adolescence and thus were able to account for a wide variety of potential confounding factors. Second, despite detailed SSB intake information collected across various life stages, we were not able to conclusively identify the etiologically relevant time window of exposure due to the limited number of EO-CRC cases (n=109). Third, the low proportion (<2%) of diabetic individuals in our study population, which was similar to the prevalence among US adults under age 45,57 allowed limited power and feasibility to account for or stratify by a personal history of diabetes when testing our hypothesis. Fourth, we were not able to probe whether the similar association would be observed among individuals carrying pathogenic germline mutations, which have been identified among ~20% of EO-CRC cases.58 Fifth, the vast majority of our study population is comprised of white female nurses. The generalizability of our findings to men or other racial/ethnic groups remains to be explored.
In conclusion, in this large prospective cohort study of US women, higher SSB intake in adulthood and adolescence was associated with a substantially higher risk of EO-CRC. Considering the well-established, adverse health consequences of SSBs and the highest consumption being characterized in adolescents and young adults under age 50, our findings reinforce the public health importance of limiting SSB intake for better health outcomes. With recent downward trends,43,44 limiting SSB intake may serve as an effective and actionable strategy to curb the rising incidence of EO-CRC.
Supplementary Material
SIGNIFICANCE OF THIS STUDY.
What is already known about this subject?
Incidence of early-onset colorectal cancer (EO-CRC, diagnosed under age 50) has been on the rise in many high income countries over the past two decades.
Sugar-sweetened beverages (SSBs) can exert adverse metabolic repercussions throughout the life course, including childhood and adulthood obesity and type 2 diabetes.
Despite the highest level of SSB consumption being characterized among adolescents and young adults, the association between SSBs and EO-CRC has not been investigated.
What are the new findings?
Compared to <1 serving/wk of SSB consumption, higher intake (i.e. ≥2 servings/d) in adulthood was associated with a 2.2-fold higher risk of EO-CRC.
Each serving/d increment of SSB intake at age 13–18 was associated with a 32% higher relative risk of EO-CRC.
Replacing adulthood intake of SSBs with that of artificially sweetened beverages, coffee, reduced fat milk, or total milk was associated with a lower risk of EO-CRC.
How might it impact on clinical practice in the foreseeable future?
SSB consumption may contribute to the rising incidence of EO-CRC.
Reducing SSB intake and/or replacing SSBs with other healthier beverages among adolescents and young adults may serve as a potential actionable strategy to alleviate the growing burden of EO-CRC.
Acknowledgments:
We would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Funding:
This work was supported by grants U01 CA176726 (Willett), R01 CA205406 (Ng), R21 CA230873 (Ogino, Wu), R01 CA151993 (Ogino), R35 CA197735 (Ogino), R35 CA253185 (Chan), R03 CA197879 (Wu), R21 CA222940 (Wu), R37 CA246175 (Cao), and K07 CA218377 (Cao) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Otegbeye is supported by T32 CA009621. Dr. Ng is supported in part by Department of Defense CA160344 and the Project P Fund. Dr. Chan is the Stuart and Suzanne Steele MGH Research Scholar. Dr. Wu is supported in part by an Investigator Initiated Grant from the American Institute for Cancer Research.
Abbreviations:
- AHEI
Alternative Healthy Eating Index
- ASB
artificially sweetened beverage
- BMI
body mass index
- CI
confidence interval
- CRC
colorectal cancer
- EO-CRC
early-onset colorectal cancer
- FFQ
food frequency questionnaire
- HFCS
high fructose corn syrup
- HR
hazard ratio
- HS
high school
- MET
metabolic equivalent of task
- NHSII
Nurses’ Health Study II
- NSAID
nonsteroidal anti-inflammatory drug
- RR
relative risk
- SSB
sugar-sweetened beverage
Footnotes
Competing interests: Dr. Ng has received institutional research funding from Pharmavite, Revolution Medicines, and Evergrande Group, has served on an advisory board for Seattle Genetics and Array BioPharma, and served as a consultant to X-Biotix Therapeutics. Dr. Meyerhardt has received institutional research funding from Boston Biomedical, has served as an advisor/consultant to Ignyta and COTA Healthcare, and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. Dr. Chan previously served as a consultant for Bayer Pharma AG, Pfizer Inc. and Boehringer Ingelheim for topics unrelated to this work. No other conflicts are reported.
Ethics approval: The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital (1999-P-003389) and that of participating state cancer registries as required.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145–64. [DOI] [PubMed] [Google Scholar]
- 2.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68:1820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68:2179–85. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy CC, Singal AG, Baron JA, et al. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology 2018;155:1716–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 2015.
- 7.Marriott BP, Hunt KJ, Malek AM, et al. Trends in intake of energy and total sugar from sugar-sweetened beverages in the United States among children and adults, NHANES 2003–2016. Nutrients 2019;11:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med 2004;27:205–10. [DOI] [PubMed] [Google Scholar]
- 9.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients 2019;11:1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullee A, Romaguera D, Pearson-Stuttard J, et al. Association between soft drink consumption and mortality in 10 European countries. JAMA Intern Med 2019;179:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin P, Li Q, Zhao Y, et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 2020;35:655–71. [DOI] [PubMed] [Google Scholar]
- 13.Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik VS, Pan A, Willett WC, et al. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller A, Bucher Della Torre S. Sugar-sweetened beverages and obesity among children and adolescents: a review of systematic literature reviews. Child Obes 2015;11:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luger M, Lafontan M, Bes-Rastrollo M, et al. Sugar-sweetened beverages and weight gain in children and adults: a systematic review from 2013 to 2015 and a comparison with previous studies. Obes Facts 2017;10:674–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Light K, Henderson M, et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J Nutr 2014;144:81–6. [DOI] [PubMed] [Google Scholar]
- 18.Kelly LA, Lane CJ, Weigensberg MJ, et al. Pubertal changes of insulin sensitivity, acute insulin response, and beta-cell function in overweight Latino youth. J Pediatr 2011;158:442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinehr T, Wolters B, Knop C, et al. Strong effect of pubertal status on metabolic health in obese children: a longitudinal study. J Clin Endocrinol Metab 2015;100:301–8. [DOI] [PubMed] [Google Scholar]
- 20.Kelsey MM, Zeitler PS. Insulin resistance of puberty. Curr Diab Rep 2016;16:64. [DOI] [PubMed] [Google Scholar]
- 21.Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali Khan U, Fallah M, Tian Y, et al. Personal history of diabetes as important as family history of colorectal cancer for risk of colorectal cancer: a nationwide cohort study. Am J Gastroenterol 2020. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Zheng X, Zong X, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Albanes D, Beeson WL, et al. Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: pooled analysis of prospective cohort studies. J Natl Cancer Inst 2010;102:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarem N, Bandera EV, Lin Y, et al. Consumption of sugars, sugary foods, and sugary beverages in relation to adiposity-related cancer risk in the Framingham Offspring cohort (1991–2013). Cancer Prev Res 2018;11:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chazelas E, Srour B, Desmetz E, et al. Sugary drink consumption and risk of cancer: results from NutriNet-Santé prospective cohort. BMJ 2019;366:l2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacheco LS, Anderson CAM, Lacey JV Jr., et al. Sugar-sweetened beverages and colorectal cancer risk in the California Teachers Study. PLoS One 2019;14:e0223638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodge AM, Bassett JK, Milne RL, et al. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr 2018;21:1618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang C, Hui S, Lu W, et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab 2018;27:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019;363:1345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity 2007;15:2739–47. [DOI] [PubMed] [Google Scholar]
- 32.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2008;121:e1604–14. [DOI] [PubMed] [Google Scholar]
- 33.Fakhouri TH, Kit BK, Ogden CL. Consumption of diet drinks in the United States, 2009–2010. NCHS Data Brief 2012:1–8. [PubMed] [Google Scholar]
- 34.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol 2018;187:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 37.Maruti SS, Feskanich D, Rockett HR, et al. Validation of adolescent diet recalled by adults. Epidemiology 2006;17:226–9. [DOI] [PubMed] [Google Scholar]
- 38.Maruti SS, Feskanich D, Colditz GA, et al. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol 2005;161:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baer HJ, Schnitt SJ, Connolly JL, et al. Early life factors and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev 2005;14:2889–97. [DOI] [PubMed] [Google Scholar]
- 41.Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019;139:2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller A, O’Reilly EJ, Malik V, et al. Substitution of sugar-sweetened beverages for other beverages and the risk of developing coronary heart disease: results from the Harvard Pooling Project of Diet and Coronary Disease. Prev Med 2020;131:105970. [DOI] [PubMed] [Google Scholar]
- 43.Rehm CD, Penalvo JL, Afshin A, et al. Dietary intake among US adults, 1999–2012. JAMA 2016;315:2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Rehm CD, Onopa J, et al. Trends in diet quality among youth in the United States, 1999–2016. JAMA 2020;323:1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putnam JJ, Allshouse JE. Food consumption, prices, and expenditures, 1970–97: U.S. Department of Agriculture, Economic Research Service, 1999.
- 46.Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and colorectal cancer. Continuous Update Project Expert Report 2018. [Google Scholar]
- 48.Lasater G, Piernas C, Popkin BM. Beverage patterns and trends among school-aged children in the US, 1989–2008. Nutr J 2011;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 50.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23. [DOI] [PubMed] [Google Scholar]
- 52.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanhope KL. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med 2012;63:329–43. [DOI] [PubMed] [Google Scholar]
- 54.Zhao S, Jang C, Liu J, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020;579:586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todoric J, Di Caro G, Reibe S, et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Do MH, Lee E, Oh MJ, et al. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018;10:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015;314:1021–9. [DOI] [PubMed] [Google Scholar]
- 58.Stoffel EM, Koeppe E, Everett J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology 2018;154:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


