Abstract
Lessons Learned
Inhibition of glycoprotein fucosylation, as monotherapy and in combination with immune checkpoint blockade, is a promising therapeutic strategy for treating a broad range of cancers.
In this first‐in‐human, first‐in‐class, phase I study in advanced solid tumors, SGN‐2FF demonstrated dose‐proportional pharmacokinetics, evidence of pharmacodynamic target inhibition of glycoprotein fucosylation, and preliminary antitumor activity.
SGN‐2FF was associated with thromboembolic events that led to study termination.
Background
We conducted a first‐in‐human, first‐in‐class, phase I study of SGN‐2FF, a potent small‐molecule inhibitor of glycoprotein fucosylation, in patients with advanced solid tumors.
Methods
The study consisted of four parts: SGN‐2FF monotherapy dose‐escalation (part A) and expansion (part B), and SGN‐2FF + pembrolizumab dose‐escalation (part C) and expansion (part D). The objectives were to evaluate safety and tolerability, maximum tolerated dose (MTD), pharmacokinetics (PK), pharmacodynamics (PD), and antitumor activity of SGN‐2FF monotherapy and SGN‐2FF + pembrolizumab.
Results
Forty‐six patients were enrolled (part A, n = 33; part B, n = 6; part C, n = 7; part D did not enroll any patients). During part A (n = 32) exploring 1–15 g once daily (QD) and 2–5 g twice daily (b.i.d.), grade 3 dose‐limiting toxicities were diarrhea (2 g and 15 g QD) and increased lipase (2 g QD). The MTD was 10 g daily. In part A, common toxicities were grades 1–2 diarrhea, fatigue, and nausea (each 47%); thromboembolic events (grades 2–5) occurred in 5 of 32 patients (16%). Safety measures included concurrent prophylactic anticoagulation with low‐molecular weight heparin (LMWH). In part C, despite the safety measures implemented, a thromboembolic event occurred in one of seven patients (14%) during the SGN‐2FF lead‐in period. Of 28 evaluable patients in part A, 1 patient with advanced head and neck squamous cell carcinoma achieved Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 complete response (CR) and 10 (36%) had RECIST v1.1 stable disease, including 1 patient with advanced triple‐negative breast cancer with 51% tumor burden reduction. SGN‐2FF administration led to dose‐proportional increases in exposure and PD reduction in protein fucosylation.
Conclusion
SGN‐2FF demonstrated proof‐of‐mechanism and preliminary antitumor activity but was associated with thromboembolic events leading to study termination.
Keywords: Immunotherapy, Small molecule agents, Clinical trials, Fucosylation inhibitor, SGN‐2FF
Discussion
Changes in the pattern of carbohydrate expression on glycoproteins can affect cell‐cell interactions within the tumor microenvironment, allowing for tumor proliferation, invasion, and metastasis; aberrant fucosylation has emerged as a central player in tumor microenvironment signaling—a hallmark of cancer [1]. Fucosylation inhibition has the potential to enhance immune‐mediated antitumor activity through modulation of both cell‐mediated antitumor immunity and antibody‐dependent cell‐mediated cytotoxic response across a broad range of cancers. SGN‐2FF is an orally bioavailable small‐molecule inhibitor of glycoprotein fucosylation that demonstrated encouraging preclinical antitumor activity in mouse models with multiple suggested mechanisms of action, including direct and indirect effects on immune cells, tumor cells, and the tumor microenvironment. Based on these promising preclinical results, we conducted this phase I study of SGN‐2FF in patients with advanced solid tumors.
SGN‐2FF demonstrated evidence of PD target inhibition based on results showing decreasing levels of immunoglobulin G (IgG) fucosylation with increasing SGN‐2FF dose and time on treatment (Fig. 1), with rates consistent with the half‐life of IgG. We observed preliminary antitumor activity with a best response of CR per RECIST v1.1 in a patient with squamous cell carcinoma of the head and neck who had a 100% reduction in tumor burden after 10 cycles. One patient with triple‐negative breast cancer who had received eight prior lines of systemic therapy, including PD‐L1 therapy, achieved a best response of stable disease (SD) based on RECIST v.1.1 criteria and a partial response (PR) based on immune‐related RECIST (irRECIST) criteria, with a 51% reduction in tumor burden after four cycles (Fig. 2). The PR was unconfirmed because of the patient's hospital admission during cycle 6.
Figure 1.
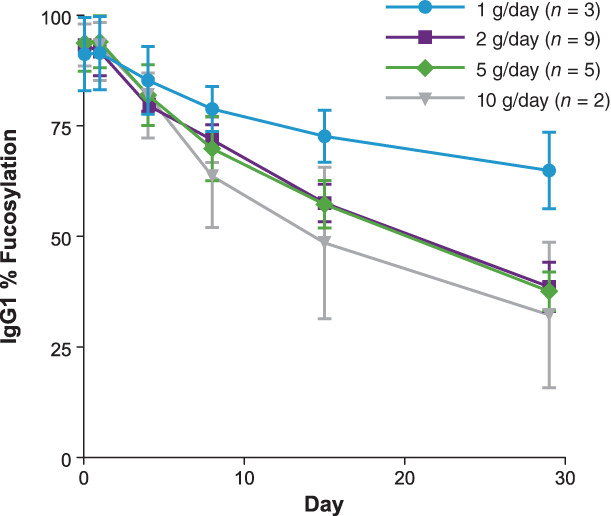
Levels of IgG fucosylation (%) by SGN‐2FF dose after oral administration.Abbreviation: IgG, immunoglobulin G.
Figure 2.
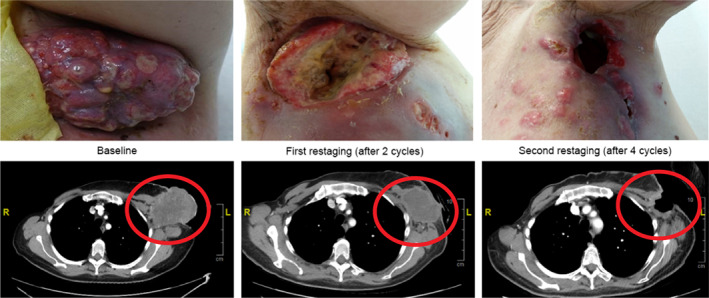
Summary of response in patient with breast cancer. Patient with metastatic triple‐negative breast cancer received 8 prior lines of systemic therapy before enrolling in the 10 g once daily monotherapy dose cohort. Best responses of stable disease per RECIST version 1.1 and of partial response (PR) per immune‐related RECIST as evidenced by a 51% reduction in axillary mass after 4 cycles were documented. The response was progressive disease per RECIST version 1.1 criteria because of the appearance of a pulmonary nodule. The PR per immune‐related RECIST was unconfirmed because of patient hospitalization during cycle 6 for progressive disease and leptomeningeal metastases.
Common treatment‐emergent adverse events (TEAEs) were grade 1–2 diarrhea, fatigue, and nausea, with each occurring in 47% of patients. Thromboembolic events (grade 2–5) occurred that were independent of dose in 5 of 32 patients (16%) in part A and 1 of 7 patients (14%) during the SGN‐2FF lead‐in period before pembrolizumab administration in part C. Based on these safety findings, the study was terminated. Clinical studies of second generation fucosylation inhibitors will likely require a more tumor‐specific targeted approach to fucosyltransferase inhibition.
Trial Information
| Disease | Advanced cancer/solid tumor only |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of Study | Phase I, 3+3 |
| Primary Endpoint | Safety |
| Secondary Endpoint | Pharmacodynamics, other |
| Additional Details of Endpoints or Study Design | |
| This multicenter, open‐label, phase I dose‐escalation study enrolled 46 patients with advanced solid tumors from February 23, 2017 to March 8, 2019. It was designed to evaluate the safety and tolerability of SGN‐2FF, identify the MTD or optimal biological dose of SGN‐2FF, and characterize the PK, PD, and antitumor activity of SGN‐2FF as monotherapy and in combination with pembrolizumab. | |
| This study consisted of four parts including dose‐escalation, dose finding, and biopsy (part A) and expansion (part B) cohorts for SGN‐2FF monotherapy, and dose‐escalation (part C) and expansion (part D) cohorts for SGN‐2FF in combination with pembrolizumab. The monotherapy and combination therapy dose‐escalation parts were conducted using a standard 3+3 design to determine a dose that demonstrated a dose‐limiting toxicity (DLT) rate less than 33%. | |
| Patients enrolled in part A and part B had histologically or cytologically confirmed, locally advanced, or metastatic solid malignancy that was relapsed, refractory, or progressing after prior systemic therapy, and for which no standard therapy was available. Eligible diagnoses for patients in parts A and B were non‐small cell lung cancer, squamous cell carcinoma of the head and neck, breast carcinoma, urothelial carcinoma, colorectal carcinoma, and renal cell carcinoma. Patients enrolled in part C had a histologically confirmed, advanced solid malignancy for which pembrolizumab treatment is approved. Part D did not enroll any patients. | |
| Key inclusion criteria were age ≥ 18 years, Eastern Cooperative Oncology Group performance status 0–1, adequate hematologic and end organ function, and measurable disease. After thromboembolic events occurred in five patients (16%) in part A of the trial, the eligibility criteria were updated on September 17, 2018, to require that patients with a history of prior deep vein thrombosis (DVT) but no evidence of clot per screening ultrasound, as well as those with a history of pulmonary embolism (PE) ≥4 weeks prior to the first dose of SGN‐2FF, be receiving therapeutic anticoagulation to decrease the risk of a recurrent thromboembolic event (TE). Subsequently, to further protect patient safety, all patients except for those who were already receiving therapeutic anticoagulation were mandated as of September 17, 2018, to receive prophylaxis with LMWH. At the same time, D‐Dimer testing and reflexive Doppler ultrasound were added as required baseline screening procedures for all newly enrolled patients and the presence of DVT was added as an exclusion criterion. | |
| Adverse events (AEs) were graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.03. The DLT evaluation period for part A was 28 days from the first dose of SGN‐2FF. A DLT was defined as any of the following: grade 5 toxicity; any grade 3 or grade 4 nonhematologic toxicity (excluding nausea, vomiting, and/or diarrhea lasting ≤3 days and reversible with medical intervention); any grade 3 or grade 4 nonhematologic laboratory value that did not downgrade to grade 2 within 3 days after onset despite optimal medical management; grade 4 hematologic toxicity lasting ≥7 days; or grade ≥ 3 febrile neutropenia. | |
| Antitumor activity was assessed by radiographic imaging at protocol‐specified time points and based on objective response assessments as defined by RECIST version 1.1 and irRECIST [2, 3]. | |
| Blood samples for PK assessments were collected at 12 selected time points in cycle 1 on days 1, 4, 8, and 15. PD biomarker assessments included complete blood count, IgG fucosylation, and lectin binding of peripheral blood leukocytes. Fucosylated and nonfucosylated IgG1 and IgG2 were quantified using liquid chromatography‐tandem mass spectrometry to evaluate IgG fucosylation levels. | |
| Investigator's Analysis | Demonstration of PD target inhibition of fucosylation and preliminary antitumor activity. |
Drug Information: Part A
| Generic Name | SGN‐2FF |
| Company Name | Seagen Inc. |
| Drug Type | Small molecule |
| Drug Class | Fucosylation inhibitor |
| Dose | 1, 2, 5, 10, 15 g QD; 2 and 5 g b.i.d. grams (g) per flat dose |
| Route | oral (po) |
Dose‐Escalation: Part A
| Dose level | Dose of drug: SGN‐2FF | Number enrolled | Number evaluable for toxicity |
|---|---|---|---|
| 1 | 1 g QD | 3 | 3 |
| 2 | 2 g QD | 8 | 7 |
| 3 | 5 g QD | 8 | 8 |
| 4 | 10 g QD | 4 | 4 |
| 5 | 15 g QD | 3 | 3 |
| 6 | 2 g b.i.d. | 3 | 3 |
| 7 | 5 g b.i.d. | 4 | 4 |
Drug Information: Part B
| Generic Name | SGN‐2FF |
| Company Name | Seagen Inc. |
| Drug Type | Small molecule |
| Drug Class | Fucosylation inhibitor |
| Dose | 5 g BID grams (g) per flat dose |
| Route | oral (po) |
Dose‐Expansion: Part B
| Dose level | Dose of drug: SGN‐2FF | Number enrolled | Number evaluable for toxicity |
|---|---|---|---|
| 1 | 5 g QD | 6 | 6 |
Drug Information: Part C
| SGN‐2FF | |
| Generic Name | SGN‐2FF |
| Company Name | Seagen Inc. |
| Drug Type | Small molecule |
| Drug Class | fucosylation inhibitor |
| Dose | 2 and 5 g QD grams (g) per flat dose |
| Route | oral (po) |
| Pembrolizumab | |
| Generic Name | Pembrolizumab |
| Trade Name | Keytruda |
| Company Name | Merck |
| Drug Type | Antibody |
| Drug Class | Immune therapy |
| Dose | 200 mg per flat dose |
| Route | IV |
Dose‐Escalation: Part C
| Dose level | Dose of drug: SGN‐2FF | Dose of drug: Pembrolizumab | Number enrolled | Number evaluable for toxicity |
|---|---|---|---|---|
| 1 | 2 g QD | 200 mg every 21 days | 4 | 4 |
| 2 | 5 g QD | 200 mg every 21 days | 3 | 3 |
Patient Characteristics: Part A
| Number of Patients, Male | 14 |
| Number of Patients, Female | 18 |
| Stage | Metastatic, 30; locally advanced, 2 |
| Age | Median (range): 62 (34–81) years |
| Number of Prior Systemic Therapies | Median: null |
| Performance Status: ECOG |
0 — 15 1 — 17 2 — 0 3 — 0 Unknown — 0 |
| Other | We enrolled 46 patients with advanced solid tumors at 12 sites in the U.S. between February 23, 2017, and March 8, 2019. Forty‐five patients were treated with SGN‐2FF (32 patients in part A, 6 patients in part B, and 7 patients in part C; Fig. 3). The median duration of treatment in part A was 9.1 weeks (range, 0–57). The majority of patients in part A were White (75%) and female (56%); 27% of patients had received prior therapy with checkpoint inhibitors. Patient characteristics for the 32 treated patients in part A are displayed in Table 1. |
| Cancer Types or Histologic Subtypes |
Breast carcinoma, 13 Colorectal carcinoma, 8 Non‐small cell lung cancer, 7 Squamous cell carcinoma of head and neck, 2 Urothelial carcinoma, 2 Gastric/gastroesophageal adenocarcinoma, 0 |
Figure 3.
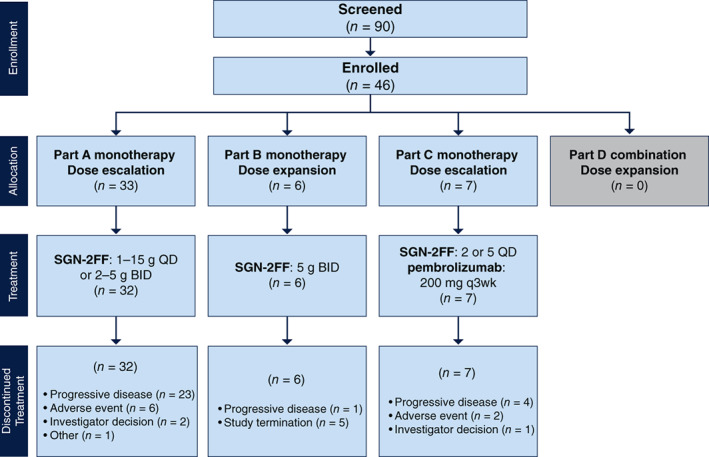
Patient flow diagram (Parts A–D) SGN‐2FF was administered orally; pembrolizumab was administered intravenously on day 1 of each cycle. Abbreviations: BID, twice daily; q, every; QD, once daily.
Table 1.
Baseline demographic and disease characteristics (part A)
| Characteristics | Value |
|---|---|
| Age, yr | |
| n | 32 |
| Median (range) | 62.0 (34–81) |
| Gender, n (%) | |
| Female | 18 (56) |
| Male | 14 (44) |
| Race, n (%) | |
| White | 24 (75) |
| Black or African American | 3 (9) |
| Asian | 3 (9) |
| Unknown | 1 (3) |
| Other | 1 (3) |
| ECOG performance status, n (%) | |
| 0 | 15 (47) |
| 1 | 17 (53) |
| Disease diagnosis, n (%) | |
| Breast carcinoma | 13 (41) |
| Colorectal carcinoma | 8 (25) |
| Non‐small cell lung cancer | 7 (22) |
| Squamous cell carcinoma of head and neck | 2 (6) |
| Urothelial carcinoma | 2 (6) |
| Gastric/gastroesophageal adenocarcinoma | 0 |
| Time since diagnosis, mo | |
| n | 30 |
| Median (range) | 54.4 (9–168) |
| Tumor spread, n (%) | |
| Metastatic | 30 (94) |
| Locally advanced | 2 (6) |
| Disease status | |
| Refractory | 29 (91) |
| Relapsed | 3 (9) |
| Prior therapies a | |
| Systemic b | 32 (100) |
| Radiation | 26 (81) |
| Surgery | 22 (69) |
| Checkpoint inhibitor | 8 (25) |
Subjects who received the same type of therapy multiple times were counted once for that type of therapy.
Checkpoint inhibitors were excluded.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Patient Characteristics: Part B
| Number of Patients, Male | 0 |
| Number of Patients, Female | 6 |
| Stage | Metastatic, 6 |
| Age | Median (range): 69.5 (62–81) years |
| Number of Prior Systemic Therapies | Median: null |
| Performance Status: ECOG |
0 — 2 1 — 4 2 — 0 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes | Breast carcinoma, 6 |
Patient Characteristics: Part C
| Number of Patients, Male | 6 |
| Number of Patients, Female | 1 |
| Stage | Metastatic, 6 |
| Age | Median (range): 65 (48–78) years |
| Number of Prior Systemic Therapies | Median: null |
| Performance Status: ECOG |
0 — 1 1 — 6 2 — 0 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes |
Squamous cell carcinoma of head and neck, 4 Gastric/gastroesophageal adenocarcinoma, 1 Non‐small cell lung cancer, 1 Urothelial cancer, 1 |
Primary Assessment Method: Part A
| Title | Response Assessment |
| Number of Patients Screened | 40 |
| Number of Patients Enrolled | 33 |
| Number of Patients Evaluable for Toxicity | 32 |
| Number of Patients Evaluated for Efficacy | 28 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 1 (4%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 10 (36%) |
| Response Assessment PD | n = 16 (57%) |
| Response Assessment OTHER | n = 1 (4%) |
| Outcome Notes |
Of the 28 efficacy‐evaluable patients treated with SGN‐2FF in part A, a best response of CR was observed in 1 (4%) patient in the 5 g QD dose cohort who had squamous cell carcinoma of the head and neck. The patient had a 100% reduction in tumor burden after 10 cycles (Fig. 4). The objective response rate was 4% (95% confidence interval [CI], 2.5–100), the disease control rate was 39% (95% CI, 71.5–100), and the clinical benefit rate was 18% (95% CI, 47.8–100).
Primary Assessment Method: Part B
| Title | Response |
| Number of Patients Screened | 21 |
| Number of Patients Enrolled | 6 |
| Number of Patients Evaluable for Toxicity | 6 |
| Number of Patients Evaluated for Efficacy | 2 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 0 (0%) |
| Response Assessment PD | n = 2 |
| Response Assessment OTHER | n = 0 (0%) |
Primary Assessment Method: Part C
| Title | Response assessment |
| Number of Patients Screened | 17 |
| Number of Patients Enrolled | 7 |
| Number of Patients Evaluable for Toxicity | 7 |
| Number of Patients Evaluated for Efficacy | 4 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 1 (25%) |
| Response Assessment PD | n = 3 (75%) |
Adverse Events: Part A
| The most common TEAEs in part A were diarrhea (47%), fatigue (47%), and nausea (47%) (Tables 2, 3, 4). |
| Six patients (19%) in part A had TEAEs that led to permanent treatment discontinuation (grade 3–4 events of hypercalcemia and increased lipase [treatment‐related], and grade 5 events of breast cancer [progression], hyponatremia [treatment‐related], hypoxia, and PE). Eighteen patients (56%) in part A had one or more serious adverse event (SAE), with four patients (13%) having any treatment‐related SAE and with PE events (PE or embolism [16%]), pleural effusion (6%), and pneumonia (6%) the most common. There were 14 deaths (44%) that occurred during part A before study termination. The causes of death were breast, non‐small cell lung, and colon or colorectal cancer in 10 patients and hyponatremia (treatment‐related), hypoxia, pneumonia, and PE each in 1 patient. Six deaths occurred within 30 days of the last dose of SGN‐2FF, but none were related to SGN‐2FF treatment. Thromboembolic events (grade 2–5) occurred in 5 (16%) of 32 patients in the monotherapy dose escalation and dose finding cohorts in part A and in 1 (14%) of 7 patients during the SGN‐2FF lead‐in period for combination therapy dose escalation in part C. In the patient in part C who experienced a thromboembolic event, the event occurred after the eligibility and screening criteria were updated on November 17, 2018. There was no evidence of the patient having DVT based on the results of screening Doppler ultrasound, and the patient had been receiving mandatory thromboprophylaxis with LMWH. There was a grade 5 event of PE in one patient in part A. The median time to first TEAE of PE or embolism was 56.5 days, (range, 23–169). The dose levels at which PE occurred with SGN‐2FF study treatment were 2, 5, and 15 g QD and 5 g b.i.d. |
Table 2.
TEAEs occurring in ≥20% of patients (part A)
| TEAE | 1 g QD (n = 3), n (%) | 2 g QD (n = 7), n (%) | 5 g QD (n = 8), n (%) | 10 g QD (n = 4), n (%) | 15 g QD (n = 3), n (%) | 2 g BID (n = 3), n (%) | 5 g BID (n = 4), n (%) | Total (n = 32), n (%) |
|---|---|---|---|---|---|---|---|---|
| Any event | 3 (100) | 7 (100) | 8 (100) | 4 (100) | 3 (100) | 3 (100) | 4 (100) | 32 (100) |
| Diarrhea | 0 | 3 (43) | 3 (38) | 4 (100) | 3 (100) | 1 (33) | 1 (25) | 15 (47) |
| Fatigue | 0 | 4 (57) | 2 (25) | 1 (25) | 3 (100) | 2 (67) | 3 (75) | 15 (47) |
| Nausea | 1 (33) | 4 (57) | 4 (50) | 1 (25) | 2 (67) | 2 (67) | 1 (25) | 15 (47) |
| Vomiting | 0 | 3 (43) | 3 (38) | 3 (75) | 2 (67) | 1 (33) | 2 (50) | 14 (44) |
| Dyspnea | 1 (33) | 4 (57) | 4 (50) | 1 (25) | 1 (33) | 1 (33) | 1 (25) | 13 (41) |
| Decreased appetite | 0 | 3 (43) | 2 (25) | 1 (25) | 2 (67) | 1 (33) | 2 (50) | 11 (34) |
| Cough | 1 (33) | 2 (29) | 3 (38) | 1 (25) | 0 | 0 | 1 (25) | 8 (25) |
| Hypernatremia | 0 | 2 (29) | 2 (25) | 2 (50) | 0 | 1 (33) | 1 (25) | 8 (25) |
| Pyrexia | 0 | 2 (29) | 3 (38) | 0 | 1 (33) | 0 | 2 (50) | 8 (25) |
| Constipation | 0 | 2 (29) | 2 (25) | 0 | 1 (33) | 1 (33) | 1 (25) | 7 (22) |
BID, twice‐daily; QD, once daily; TEAE, treatment‐emergent adverse event.
Table 3.
TEAEs occurring in ≥20% of patients (part B)
| Preferred term | (n = 6), n (%) |
|---|---|
| Any event | 5 (83) |
| Diarrhea | 4 (67) |
| Fatigue | 3 (50) |
| Nausea | 3 (50) |
Abbreviation: TEAE, treatment‐emergent adverse event.
Table 4.
TEAEs occurring in ≥20% of patients (part C)
| Preferred Term | 2g QD (n = 4), n (%) | 5g QD (n = 3), n (%) | Total (n = 7), n (%) |
|---|---|---|---|
| Any event | 4 (100) | 3 (100) | 7 (100) |
| Decreased appetite | 2 (50) | 2 (67) | 4 (57) |
| Fatigue | 1 (25) | 3 (100) | 4 (57) |
| Back pain | 1 (25) | 2 (67) | 3 (43) |
| Constipation | 0 | 2 (67) | 2 (29) |
| Diarrhea | 1 (25) | 1 (33) | 2 (29) |
| Early satiety | 1 (25) | 1 (33) | 2 (29) |
Abbreviations: QD, once daily; TEAE, treatment‐emergent adverse event.
Serious Adverse Events: Part A
| Name | Grade | Attribution |
|---|---|---|
| Pulmonary embolism (2 g QD) | 4 | Unrelated |
| Pulmonary embolism | 3 | Unrelated |
| Abdominal pain | 3 | Unrelated |
| Decreased appetite | 3 | Unrelated |
| Dehydration | 3 | Unrelated |
| Colon cancer | 3 | Unrelated |
| Colon cancer | 5 | Unrelated |
| Pulmonary embolism | 4 | Unrelated |
| Pulmonary embolism | 5 | Unrelated |
| Dyspnea | 2 | Unrelated |
| Arterial thrombosis (5 g QD) | 3 | Unrelated |
| Cellulitis | 3 | Unrelated |
| Cellulitis | 3 | Unrelated |
| Hypercalcemia | 3 | Unrelated |
| Pulmonary embolism | 4 | Unrelated |
| Pneumonia | 3 | Unrelated |
| Influenza | 2 | Unrelated |
| Gastrostomy tube site complication | 1 | Unrelated |
| Hyponatremia | 3 | Related |
| Hyponatremia | 5 | Related |
| Confusional state (10 g QD) | 3 | Unrelated |
| Sepsis | 4 | Unrelated |
| Pleural effusion | 3 | Unrelated |
| Pneumonia | 3 | Unrelated |
| Non‐small cell lung cancer | 5 | Unrelated |
| Localized edema | 1 | Unrelated |
| Delirium | 3 | Unrelated |
| Breast cancer | 5 | Unrelated |
| Seizure | 3 | Unrelated |
| Diarrhea (15 g QD) | 3 | Related |
| Colitis | 3 | Related |
| Embolism | 3 | Related |
| Hypoxia (2 g b.i.d.) | 3 | Unrelated |
| Hypoxia | 3 | Unrelated |
| Pyrexia (5 g b.i.d.) | 2 | Unrelated |
| Pleural effusion | 2 | Unrelated |
| Pleural effusion | 3 | Unrelated |
| Embolism | 3 | Related |
| Tumor hemorrhage | 3 | Related |
Dose‐Limiting Toxicities: Part A
| Dose level | Dose of drug: SGN‐2FF | Number enrolled | Number evaluable for toxicity | Number with a dose‐limiting toxicity | Dose‐limiting toxicity information |
|---|---|---|---|---|---|
| 2 | 2 g QD | 8 | 7 | 1 | Grade 3 diarrhea and grade 3 increased lipase |
| 5 | 15 g QD | 3 | 3 | 1 | Grade 3 increased lipase |
Adverse Events: Part B
| The most common TEAEs overall in the 6 patients treated with SGN‐2FF in part B were diarrhea (67%), fatigue (50%), and nausea (50%) and in the seven patients treated with SGN‐2FF + pembrolizumab were decreased appetite (57%), fatigue (57%), and back pain (57%). During part B, there were no SAEs reported. |
Adverse Events: Part C
| The most common TEAEs reported in the seven patients treated with SGN‐2FF + pembrolizumab were decreased appetite (57%), fatigue (57%), and back pain (57%). |
Serious Adverse Events: Part C
| Name | Grade | Attribution |
|---|---|---|
| Gastrointestinal hemorrhage (2 g QD) | 5 | Unrelated |
| Back pain (5 g QD) | 3 | Unrelated |
| Metastases to central nervous system | 3 | Unrelated |
| Hydrocephalus | 2 | Unrelated |
| Corona virus infection | 3 | Unrelated |
| Sepsis | 4 | Unrelated |
| Deep vein thrombosis | 2 | Unrelated |
| Thrombocytopenia | 4 | Related |
Assessment, Analysis, and Discussion
| Completion | Toxicity; study terminated before completion |
| Investigator's Assessment | Demonstration of PD target inhibition of fucosylation and preliminary antitumor activity |
This study represents the first‐in‐human, first‐in‐class study of a fucosylation inhibitor. Fucosylation, a central process in mammalian cells, involves the assembly of fucose‐containing glycans from guanosine diphosphate (GDP)‐fucose by fucosyltransferase enzymes [4]. Aberrant fucosylation is central to several hallmarks of cancer including tumor progression, invasion, and metastasis [5]. Aberrant fucosylation has also been implicated in immune evasion based on the central role fucose plays in selectin‐dependent leukocyte adhesion and lymphocyte homing [6]. Natural killer cell‐mediated tumor immune and antibody‐dependent cell‐mediated cytotoxic (ADCC) response is also heavily regulated by fucosylation of IgG antibodies [7, 8]. More recent data indicate that core fucosylation of N‐linked oligosaccharides is critical for the stable expression of programmed cell death 1 and binding to ligands on exhausted T cells [9]. Afucosylation of the core fucose within the Fc N‐glycan domain has also been shown to enhance ADCC [10, 11]. Accordingly, the development of fucosylation inhibitors as both monotherapy and in combination with immune checkpoint blockade presents a novel therapeutic strategy to enhance cell‐mediated antitumor immunity and ADCC across a broad range of cancers.
SGN‐2FF is a synthetic monosaccharide analog of L‐fucose. On entry into cells, SGN‐2FF is converted into the active component GDP‐2‐fluoro‐fucose (GDP‐2FF). GDP‐2FF directly inhibits fucosyltransferase enzymes. In preclinical studies, SGN‐2FF reduced selectin‐dependent tumor cell adhesion and T‐regulatory populations in cultured T cells while increasing antigen‐dependent T‐cell responses through increased T‐cell receptor signaling and enhanced dendritic cell maturation in T cell/dendritic cell cocultures [12]. Mouse xenograft and syngeneic models of renal, hepatocellular, colon, and breast carcinoma demonstrated that SGN‐2FF delayed tumor growth across a broad range of cancers.
In this phase I study, we report increases in plasma SGN‐2FF concentrations following oral administration that were dose‐proportional up to 15 g once daily and evidence of pharmacodynamic target inhibition with decreasing levels of IgG fucosylation corresponding to increasing dosing of SGN‐2FF administration and time on treatment, and with rates consistent with the half‐life of IgG (Fig. 5) [13]. We additionally report preliminary antitumor activity in patients with metastatic squamous cell carcinoma of the head and neck (1 complete response) and breast cancer (1 stable disease with a 51% reduction in tumor burden). Dose‐limiting toxicities included grade 3 diarrhea and grade 3 lipase increased. Common side effects such as nausea, fatigue, and diarrhea were generally low grade and manageable. A higher‐than‐expected rate of thromboembolic events was observed (16% in monotherapy dose escalation and dose finding and 14% in the SGN‐2FF lead‐in portion of the combination dose‐escalation) that was independent of dose. Baseline screening for DVT and the exclusion of patients with DVT present at baseline were implemented near the end of monotherapy dose escalation and dose finding (part A). Subsequent patients enrolled were mandated to receive concurrent prophylactic dose anticoagulation with low‐molecular weight heparin if not already receiving therapeutic anticoagulation. Unfortunately, even with implementation of these preventive safety measures, a subsequent pulmonary embolism event occurred in a patient during the SGN‐2FF lead‐in portion of the combination dose‐escalation, prior to initiation of pembrolizumab. Therefore, despite evidence of antitumor activity in metastatic squamous cell carcinoma of the head and neck and breast cancer, the study sponsor made an internal decision to close the study based on the higher‐than‐expected number of thromboembolic events despite prophylactic anticoagulation. The study was terminated on June 24, 2019, with no patients remaining in long term follow‐up as of July 25, 2019.
Figure 5.
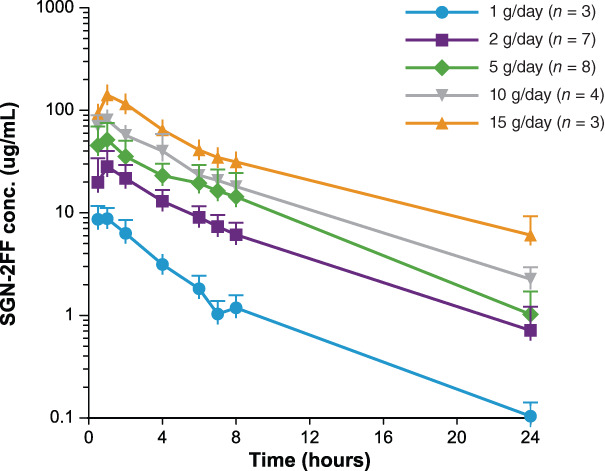
SGN‐2FF plasma concentration time profiles for patients during dose‐escalation after oral administration on day 1 of cycle 1.
Abbreviation: conc., concentration.
No evidence of hypercoagulable state or thromboembolic events was seen during the nonhuman toxicology studies of SGN‐2FF. Thromboembolic events and hypercoagulability have not been reported to date. We hypothesize there may be a relationship between the role fucosylation plays in leukocyte adhesion and binding to cellular adhesion molecules on activated endothelial cells. Lectin pathway activation and complement activation have also been implicated in playing a role in coagulation and venous thromboembolism [14]. Studies in transgenic sickle cell mice show that inhibition of 2‐fluorofucose can decrease microvascular stasis and vaso‐occlusion after hemoglobin infusion to precipitate an occlusive crisis [15]. Additional preclinical modeling may be indicated before embarking on additional studies of this agent to assess the mechanism of afucosylation and thromboembolism. Based upon the mechanism of fucosylation in lectin binding and cell‐cell adhesion, the possibility of tumor thromboembolism could not be ruled out.
In conclusion, in this phase I first‐in‐human, first‐in‐class trial, SGN‐2FF demonstrated evidence of pharmacodynamic target inhibition of fucosylation and preliminary antitumor activity in patients with advanced solid tumors. Development of second generation fucosylation inhibitors that provide a more tumor‐specific targeted approach to fucosyltransferase inhibition may offer an improved therapeutic window.
Disclosures
Laura Quan Man Chow: Cullinan‐Apollo, Daiichi Sankyo, Elicio Therapeutics, Merck, Alkermes, Gilead, Novartis, Regeneron, Sanofi‐Genzyme, Nanobiotix, AstraZeneca, Bluprint Therapeutics, Beigene, Ipsen (C/A), Alkermes, Oncorus (RF); Karen Reckamp: Seagen, Amgen, AstraZeneca, Blueprint, Boehringer Ingelheim, Daiichi Sankyo, EMD Soreno, Genentech, GSK, Janssen, Lilly, Merck KGA, Mirati, Takeda (C/A), Seagen, Calithera, Daiichi Sankyo, Elevation Oncology (RF–institutional); Peter Haughney: Seagen (E, OI); Rachel E.Sanborn: AstraZeneca, Janssen Oncology, Macrogenics, Blueprint Medicines, Daiichi Sankyo, Lilly Oncology (C/A), AstraZeneca (H—panel expert, educational moderator), Amgen (H—Educational presentation), Merck, AstraZeneca (RF—investigator‐sponsored trials), BMS (RF—institutional funding); Timothy A. Yap: Almac, Aduro, AstraZeneca, Atrin, Axiom, Bayer, Bristol Myers Squibb, Calithera, Clovis, Cybrexa, EMD Serono, F‐Star, Guidepoint, Ignyta, I‐Mab, Jansen, Merck, Pfizer, Repare, Roche, Rubius, Schrodinger, Seagen, Varian and Zai Labs (C/A), Artios, AstraZeneca, Bayer, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F‐Star, GlaxoSmithKline, Genentech, ImmuneSensor, Ipsen, Jounce, Karyopharm, Kyowa, Merck, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Repare, Sanofi, Scholar Rock, Seagen, Tesaro, Vertex Pharmaceuticals (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
Figure 4.
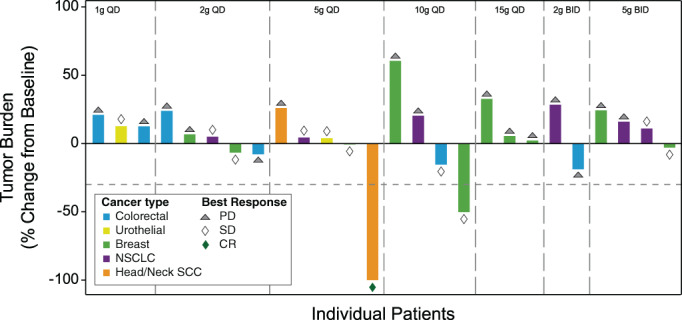
Change in maximum tumor burden based on RECIST v1.1. Abbreviations: CR, complete response; NSCLC, non small‐cell lung cancer; PD, progressive disease; QD, once daily; SCC, squamous cell cancer; SD, stable disease.
Acknowledgments
The authors acknowledge Nan Wang for statistical guidance as an employee of Seagen Inc. Medical writing assistance was funded by Seagen Inc. and provided by Gary Dorrell as an employee of Seagen Inc. and Ranjana Sundara of MMS Holdings, Inc. L.Q.M. Chow is currently affiliated with the University of Texas at Austin, Austin, TX, USA.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this articlemay be reproduced, stored, or transmitted in any form or for any means without the prior permission inwriting from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02952989
- Sponsors: Seagen Inc.
- Principal Investigator: Khanh T. Do
- IRB Approved: Yes
Contributor Information
Khanh T. Do, Email: khanhdomd@gmail.com.
Timothy A. Yap, Email: tyap@mdanderson.org.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 2. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 3. Bohnsack O, Ludajic K, Hoos A. Adaptation of the immune related response criteria: Irrecist. Ann Oncol 2014;25(suppl 4):1070Pa. [Google Scholar]
- 4. Becker DJ, Lowe JB. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003;13:41R–53R. [DOI] [PubMed] [Google Scholar]
- 5. Pinho SS, Reis CA. Glycosylation in cancer: Mechanisms and clinical implications. Nat Rev Cancer 2015;15:540–555. [DOI] [PubMed] [Google Scholar]
- 6. Homeister JW, Thall AD, Petryniak B et al. The alpha(1,3)fucosyltransferases Fuct‐IV and Fuct‐VII exert collaborative control over selectin‐dependent leukocyte recruitment and lymphocyte homing. Immunity 2001;15:115–126. [DOI] [PubMed] [Google Scholar]
- 7. McQueen KL, Parham P. Variable receptors controlling activation and inhibition of Nk cells. Curr Opin Immunol 2002;14:615–621. [DOI] [PubMed] [Google Scholar]
- 8. Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res 2003;90:127–156. [DOI] [PubMed] [Google Scholar]
- 9. Okada M, Chikuma S, Kondo T et al. Blockage of core fucosylation reduces cell‐surface expression of PD‐1 and promotes anti‐tumor immune responses of T cells. Cell Rep 2017;20:1017–1028. [DOI] [PubMed] [Google Scholar]
- 10. Shields RL, Lai J, Keck R et al. Lack of fucose on human GgG1 N‐linked oligosaccharide improves binding to human fcgamma RIII and antibody‐dependent cellular toxicity. J Biol Chem 2002;277:26733–26740. [DOI] [PubMed] [Google Scholar]
- 11. Shinkawa T, Nakamura K, Yamane N et al. The absence of fucose but not the presence of galactose or bisecting N‐acetylglucosamine of human IgG1 complex‐type oligosaccharides shows the critical role of enhancing antibody‐dependent cellular cytotoxicity. J Biol Chem 2003;278:3466–3473. [DOI] [PubMed] [Google Scholar]
- 12. Field JJ, Zeng W, Shih VFS, et al. The fucosylation inhibitor 2‐fluorofucose exhibits anti‐tumor activity and modulates immune cell activity both in vitro and in vivo. J Immunother Cancer 2016;4:P298a. [Google Scholar]
- 13. Cohen S, Freeman T. Metabolic heterogeneity of human gamma‐globulin. Biochem J 1960;76:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Høiland, II , Liang RA, Hindberg K et al. Associations between complement pathways activity, mannose‐binding lectin, and odds of unprovoked venous thromboembolism. Thromb Res 2018;169:50–56. [DOI] [PubMed] [Google Scholar]
- 15. Belcher JD, Chen C, Nguyen J et al. The fucosylation inhibitor, 2‐fluorofucose, inhibits vaso‐occlusion, leukocyte‐endothelium interactions and NF‐kB activation in transgenic sickle mice. PloS One 2015;10:e0117772. [DOI] [PMC free article] [PubMed] [Google Scholar]


