Abstract
Background
Chemotherapy‐induced peripheral neuropathy (CIPN) is a common, debilitating adverse effect of neurotoxic chemotherapy that significantly worsens the quality of life of cancer survivors.
Materials and Methods
Survivors of solid tumors with persistent moderate‐to‐severe CIPN defined as numbness, tingling, or pain rated ≥4 on an 11‐point numeric rating scale (NRS) were randomized in a 1:1:1 ratio to 8 weeks of real acupuncture (RA) versus sham acupuncture (SA) versus usual care (UC). We previously reported the primary endpoint (NRS); here we report the following health‐related quality of life endpoints: Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity (FACT/GOG‐Ntx), Hospital Anxiety and Depression Scale (HADS), Insomnia Severity Index (ISI), and Brief Fatigue Inventory (BFI). For each endpoint, the mean changes from baseline and 95% confidence intervals were estimated within each arm and compared between arms using linear mixed models.
Results
We enrolled 75 survivors of solid tumors with moderate‐to‐severe CIPN into the study. Compared with baseline, at week 8, FACT/GOG‐Ntx, HADS anxiety, and ISI scores significantly improved in RA and SA, but not in UC. Compared with UC, at week 8, FACT/GOG‐Ntx scores significantly increased in RA and SA arms indicating improved CIPN‐related symptoms and quality of life (p = .001 and p = .01). There was no statistically significant difference between RA and SA. There was no difference in HADS depression or BFI among RA, SA, and UC at weeks 8 and 12.
Conclusion
Acupuncture may improve CIPN‐related symptoms and quality of life in cancer survivors with persistent CIPN. Further large sample size studies are needed to delineate placebo effects.
Implications for Practice
The authors conducted a randomized sham acupuncture‐ and usual care‐controlled clinical trial to evaluate the impact of acupuncture on health‐related quality of life outcomes in patients with solid tumors with chemotherapy‐induced peripheral neuropathy (CIPN). Statistically significant improvements in quality of life, anxiety, insomnia, and fatigue were achieved with 8 weeks of real acupuncture when compared with baseline, without statistically significant differences between real and sham acupuncture. These findings suggest that acupuncture may be effective for improving CIPN‐related symptoms and quality of life and reducing anxiety and insomnia in cancer survivors with persistent CIPN, with further study needed to delineate placebo effects.
Keywords: Acupuncture, Neuropathy, Quality of life, Cancer, Anxiety, Insomnia
Short abstract
Acupuncture is a promising and safe nonpharmacological approach to potentially address the unmet need for an effective treatment of chemotherapy‐induced peripheral neuropathy. This article reports the results of a randomized sham acupuncture‐ and usual care‐controlled clinical trial to evaluate the effect of acupuncture on health‐related quality of life outcomes in patients with solid tumors dealing with chemotherapy‐induced peripheral neuropathy.
Introduction
Efforts to prolong survival through early detection and advancements in therapies have significantly increased the number of cancer survivors in the U.S., with an estimated 18 million survivors expected by 2022 [1]. Many survivors experience lasting effects from cancer and its treatments. Chemotherapy‐induced peripheral neuropathy (CIPN) is one of the most common chemotherapy‐induced toxicities; it can manifest in symptoms such as paresthesia, hyperalgesia, and allodynia [2, 3]. CIPN can persist long after chemotherapy completion with patients experiencing pain, sensory loss, poor dexterity, and gait disturbance that significantly worsen quality of life, impact daily functions, and increase fall risks [4, 5, 6, 7]. Currently, there are very limited treatment options for CIPN; additional treatments are needed [8].
Acupuncture is a promising and safe nonpharmacological approach to potentially address the unmet need for an effective CIPN treatment. Researchers have conducted a number of pilot studies that are either single arm or compare acupuncture with usual care alone in treating persistent CIPN after chemotherapy completion. Study results indicate that acupuncture significantly reduces CIPN symptoms and improves quality of life compared with baseline or usual care alone [9, 10, 11]. Our recently completed study (n = 75) was the first randomized sham acupuncture‐ (placebo) and usual care‐controlled clinical acupuncture trial that found acupuncture reduced the CIPN symptoms of pain, numbness, and tingling [12]. Within the trial, we further investigated the impact of acupuncture on health‐related quality of life (HRQoL) outcomes that reflect patient satisfaction and perceived benefits of acupuncture not captured by objective indicators. In this manuscript, we report on these HRQoL outcomes including the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity (FACT/GOG‐Ntx), Hospital Anxiety and Depression Scale (HADS), Insomnia Severity Index (ISI), and Brief Fatigue Inventory (BFI).
Materials and Methods
Study Participants
We conducted a three‐arm, randomized controlled trial comparing acupuncture, sham acupuncture, and usual care at Memorial Sloan Kettering Cancer Center (MSK) in New York City. MSK's Institutional Review Board approved the study protocol and we registered the trial at ClinicalTrials.gov (NCT03183037). We recruited patients from July 2017 to June 2018. Eligible patients were survivors of solid tumors who had completed chemotherapy at least 3 months prior to study enrollment, demonstrated persistent moderate‐to‐severe CIPN (defined as numbness, tingling, or pain rated ≥4 on a numeric rating scale [NRS]), and were not taking any neuropathy medication or were on stable neuropathic medication for at least 3 months. We excluded patients with pacemakers or those who had received acupuncture treatments within 5 years of enrollment. We obtained informed consent from all patients prior to enrollment.
Study Design
We stratified subjects based on the most bothersome CIPN symptom (tingling vs. numbness vs. pain) and symptom severity (moderate 4–6 vs. severe 7–10 on the NRS). Patients were then allocated at a 1:1:1 ratio to real acupuncture (RA), sham acupuncture (SA), or usual care (UC) groups through computer‐generated randomization. Randomization was conducted by the Clinical Research Database at MSK in randomly permuted blocks.
Interventions
In both RA and SA groups, participants received a total of 10 treatments over 8 weeks, with biweekly treatments for the first 2 weeks and weekly treatments thereafter. Patients wore blindfolds so they could not see throughout the treatments.
Real Acupuncture
We developed a standardized acupuncture protocol based on prior literature [13, 14] and input from experienced acupuncturists. Four licensed acupuncturists, each with more than 10 years of experience, performed acupuncture at the following points on bilateral ears: Shen Men, point zero, and a third electrodermal active point [9], and bilateral body: LI‐4, PC‐6, SI‐3, LR‐3, GB‐43, ST‐40, Bafeng 2, and Bafeng 3. After disinfecting the skin surface, a licensed acupuncturist inserted sterile filiform acupuncture needles in auricular (0.16 mm × 15 mm) and body (0.25 mm × 30 mm or 40 mm) acupoints. Needles remained for 30 minutes after the patient experienced the de qi sensation (feeling of soreness, numbness, or distention) at LI‐4, SI‐3, and ST‐40 points. The acupuncturist also applied electrical acupuncture bilaterally as follows: from LR‐3 (negative) to GB‐43 (positive) at 2–5 Hz for 30 minutes. Electrical current varied within the range of 0–40 mA to establish consistent stimulation. The acupuncturist spoke with the patient to assess response to prior treatments. All patients received a core point protocol, but acupuncturists did not place additional hand points if the patients did not have symptoms in their hands. Additionally, acupuncturists could select up to two points of their choice based on the patient's presentation.
Sham Acupuncture
Acupuncturists performed a previously validated noninsertion procedure on nonacupoints [15, 16]. After disinfecting the skin surface, an acupuncturist tapped empty needle guiding tubes adjacent to each of the eight acupoints in the arm and leg (LI‐4, SJ‐5, LI‐11, ST‐40) to produce a discernible sensation and then immediately applied a needle to the dermal surface with a piece of adhesive tape; needles remained for 30 minutes. The acupuncturist attached an electric acupuncture device to the needles but did not turn it on.
Usual Care
Patients in the usual care group received no acupuncture treatments or any other interventions throughout the study period.
Patient‐Reported Outcome Measures
Participants completed FACT/GOG‐Ntx‐11 at week 4 (middle of treatment) and week 8 (end of treatment) and HADS, ISI, and BFI questionnaires at week 8 (end of treatment). Research staff also administered assessments at week 12 following completion of the intervention to assess the duration of effect.
FACT/GOG‐Ntx
The FACT/GOG‐Ntx is an 11‐item questionnaire assessing sensory, motor, and hearing dysfunctions associated with neuropathy. The assessment provides clinical meaning to the changes in neuropathic symptoms. A lower score indicates more functional disability and more severe neurotoxicity with a 0–44 score range. This questionnaire has been validated with its reliability assessed in multiple oncology group studies of chemotherapy‐induced neuropathy [17, 18].
HADS
The HADS is a self‐rated instrument for anxiety and depression symptoms in the past week. It has seven items for anxiety and seven items for depression (14 total items, with each score ranging from 0 to 3) and has been widely used for people with cancer [19]. The scores for these seven items are then added for a single total score with a range of 0–21 for HADS anxiety and HADS depression. HADS has demonstrated good reliability with a Cronbach's α ranging from 0.68 to 0.93, with an average of 0.83 for anxiety and a range from 0.67 to 0.9, and an average of 0.83 for depression and a good correlation with other similar questionnaires with a range 0.49–0.83 [19]. A HADS score of 0–7 is considered not significant, 8–10 is subclinically significant, and 11–21 is clinically significant depression or anxiety.
ISI
Patient‐reported insomnia severity was measured by the ISI as a secondary objective measurement. The ISI has a Cronbach's α = 0.9 and good validity, especially among patient‐reported outcome measures created to retrospectively evaluate the effect on daytime functioning and level of associated distress [20]. It uses seven items that are assessed on a five‐point scale from 0 to 4, with a larger score indicating more severe insomnia symptoms. The scores for these seven items are then added for a single total score with a range of 0–28. The cutoff scores are 0–7 (showing no clinically significant sleep difficulties), 8–14 (sleep difficulties require more consideration), and 15+ (there is clinically significant insomnia) [20]. In a representative sample of 1,670 patients with cancer, the ISI has also shown internal consistency, specificity, construct validity, and sensitivity. It has established the minimum significant change in value to ensure that the difference is statistically and clinically relevant to patients. An eight‐point decrease is considered to be a clinically significant improvement [21]. The ISI has also been used in several insomnia trials with cancer survivors and has shown sensitivity to change in reactions to interventions in addition to differing effects between interventions.
BFI
The BFI is often used to measure the fatigue of patients with cancer with good reliability with a Cronbach's α of 0.96 and good correlation with other measurements of fatigue (r = −.88, p < .001) [22]. The BFI items rate the current amount of fatigue a person is experiencing and includes the worst and typical fatigue experienced in the prior 24 hours. Each item is rated on a scale that ranges from 0 (no fatigue) to 10 (as bad as possible).
Mao Expectancy of Treatment Effects
Prerandomization treatment expectancy was assessed using the Mao Expectancy of Treatment Effects (METE), a validated measure. This treatment expectancy scale is a four‐item instrument; each item is scored from 1 (“Not at all agree”) to 5 (“Completely agree”). Outcome expectancy has long been considered an important predictor of treatment outcomes and has gained increasing recognition in clinical trials. The METE total score is the sum of the four item scores, linearly rescaled to range from 0 to 100, with higher scores indicating greater expectancy [23]. Baseline expectancy has been shown to predict treatment outcomes of acupuncture [24].
Masking
The patients, investigators, research study coordinators, and statistician were blinded to the treatment assignments between RA and SA. Acupuncturists were not blinded to RA or SA.
Statistical Analysis
To estimate potential treatment effects and provide insight into symptom trajectories over time while also including patients with missing follow‐up scores in the analysis per the intention‐to‐treat principle, we analyzed each outcome measure using a constrained linear mixed model. We constrained the treatment arms to have a common baseline mean [25], reflecting the prerandomization timing of the baseline assessment. The dependent variable vector included the prerandomization baseline (week 0) assessment, as well as all postrandomization assessments at weeks 4 (if applicable), 8, and 12. The independent variables were treatment arm, week (categorical), and arm‐by‐week interaction. A patient‐level random intercept was included in the model to account for the repeated within‐patient outcome measurements over time. All randomized patients with at least one outcome assessment were included in the model. Results are reported as least‐squares means, mean differences, and confidence intervals, with inferences regarding differences between arms and within‐arm change based on model coefficients from the arm‐by‐week interaction and contrasts of model‐adjusted means.
Results
From July 2017 to June 2018, we enrolled and randomized 75 patients to RA (n = 27), SA (n = 24), or UC (n = 24) groups. Seven patients (9%) withdrew from the trial because of withdrawing consent (n = 3), noncompliance (n = 2), unstable medical condition (n = 1), and lost to follow‐up (n = 1). This left 68 evaluable participants at week 8 (primary endpoint), with 24 patients in RA, 23 in SA, and 21 in UC. The consort diagram (Fig. 1) was published in the primary paper [12].
Figure 1.
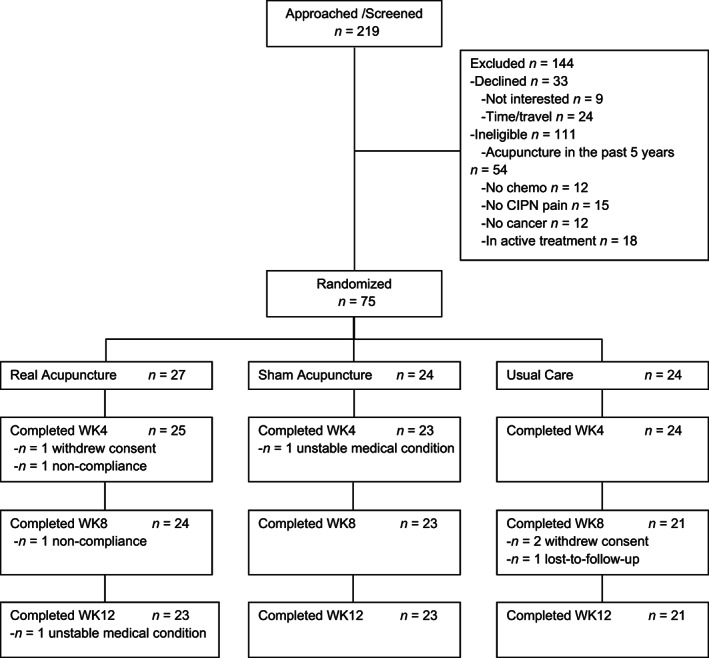
CONSORT diagram of study. CONSORT diagram depicting flow of included participants through each stage of the randomized controlled trial.Abbreviations: CIPN, chemotherapy‐induced peripheral neuropathy; WK, week.
Baseline Patient Characteristics
Baseline patient characteristics are shown in Table 1. Patient characteristics were well balanced among the three groups in most areas. There was no significant difference in drop‐off rates among the three groups. The treatment compliance rate was high, with 89% (24/27) of patients in the RA group, 96% (23/24) in the SA group, and 88% (21/24) in the UC group completing treatment and evaluation.
Table 1.
Patient characteristics
| Characteristics | All patients, n = 75 | Real acupuncture, n = 27 | Sham acupuncture, n = 24 | Usual care, n = 24 |
|---|---|---|---|---|
| Age, median (range) | 59.7 (36.3–85.9) | 60.3 (51.0–79.7) | 62.7 (43.0–86.0) | 57.3 (36.3–70.6) |
| Gender | ||||
| Male | 15 (20) | 7 (26) | 5 (21) | 3 (12) |
| Female | 60 (80) | 20 (74) | 19 (79) | 21 (88) |
| BMI, median (range) | 28.7 (19.8–46) | 30.2 (20.2–46) | 27.9 (19.8–39.5) | 27.8 (21.1–35.1) |
| Race | ||||
| White | 55 (73) | 22 (81) | 22 (92) | 11 (46) |
| Black | 13 (17) | 4 (15) | 0 | 9 (38) |
| Asian | 3 (4) | 0 | 2 (8) | 1 (4) |
| Unknown | 4 (5) | 1 (4) | 0 | 3 (12) |
| Hispanic ethnicity | 7 (9) | 3 (11) | 1 (4) | 3 (12) |
| Cancer type | ||||
| Breast | 40 (53) | 13 (48) | 11 (46) | 16 (67) |
| Lung | 1 (1) | 0 | 0 | 1 (4) |
| Colon/rectal | 12 (16) | 5 (18) | 6 (25) | 1 (4) |
| Testicular | 5 (7) | 4 (15) | 0 | 1 (4) |
| Melanoma | 1 (1) | 0 | 0 | 1 (4) |
| Head/neck | 1 (1) | 0 | 1 (4) | 0 |
| Ovarian | 4 (5) | 2 (7) | 2 (8) | 0 |
| Cervical | 1 (1) | 0 | 0 | 1 (4) |
| Pancreatic | 1 (1) | 0 | 1 (4) | 0 |
| Endometrial | 6 (8) | 3 (11) | 2 (8) | 1 (4) |
| Squamous cell | 1 (1) | 0 | 0 | 1 (4) |
| Stomach | 1 (1) | 0 | 1 (4) | 0 |
| Uterine | 1 (1) | 0 | 0 | 1 (4) |
| Cancer stage | ||||
| Stage I | 14 (19) | 7 (26) | 1 (4) | 6 (25) |
| Stage II | 35 (47) | 12 (44) | 13 (54) | 10 (42) |
| Stage III | 22 (29) | 7 (26) | 9 (38) | 6 (25) |
| Stage IV | 4 (5) | 1 (4) | 1 (4) | 2 (8) |
| Chemo type | ||||
| Taxane‐based only | 39 (52) | 13 (48) | 11 (46) | 15 (62) |
| Platinum‐based only | 20 (27) | 8 (30) | 9 (38) | 3 (12) |
| Taxane and platinum combined | 16 (21) | 6 (22) | 4 (17) | 6 (25) |
| Years since chemo, median (range) | 3.8 (0.3–40.8) | 5.4 (0.5–40.8) | 3.0 (0.3–12.2) | 3.3 (0.3–11.4) |
Values are reported as no. (%) unless otherwise indicated.
Abbreviation: BMI, body mass index.
Health‐Related Quality of Life Outcomes
Within‐Group Comparison
Compared with baseline, at week 8, FACT/GOG‐Ntx and ISI scores significantly improved in both RA and SA arms, but not in the UC arm (Table 2). At week 8, we observed a 4.02 (95% confidence interval [CI] 2.26–5.77) point increase in FACT‐GOG/Ntx in the RA arm, a 3.24 (95% CI 1.44–5.04) point increase in the SA arm and 0.15 (95% CI −2.05 to 1.75) point decrease in the UC arm.
Table 2.
Health‐related quality of life changes from baseline
| Outcome, week | Real acupuncture (n = 27) | Sham acupuncture (n = 24) | Usual care (n = 24) | |||
|---|---|---|---|---|---|---|
| Mean (95% CI) | Change from baseline, mean (95% CI) | Mean (95% CI) | Change from baseline, mean (95% CI) | Mean (95% CI) | Change from baseline, mean (95% CI) | |
| FACT/GOG‐Ntx | ||||||
| 0 | 25.47 (23.66–27.28) | NA | 25.47 (23.66–27.28) | NA | 25.47 (23.66–27.28) | NA |
| 4 | 27.41 (25.07–29.74) | 1.93 (0.12 to 3.75) a | 27.51 (25.18–29.84) | 2.04 (0.24 to 3.84) a | 26.71 (24.31–29.11) | 1.24 (−0.65 to 3.13) |
| 8 | 29.49 (27.19– 31.78) | 4.02 (2.26 to 5.77) b | 28.71 (26.38–31.04) | 3.24 (1.44 to 5.04) b | 25.32 (22.91–27.72) | −0.15 (−2.05 to 1.75) |
| 12 | 29.17 (26.85– 31.48) | 3.69 (1.91 to 5.48) b | 29.68 (27.35–32.01) | 4.21 (2.41 to 6.01) b | 27.30 (24.92–29.68) | 1.83 (−0.03 to 3.70) c |
| HADS anxiety | ||||||
| 0 | 6.92 (5.94–7.89) | NA | 6.92 (5.94–7.89) | NA | 6.92 (5.94–7.89) | NA |
| 8 | 5.87 (4.62–7.11) | −1.05 (−2.01 to −0.09) a | 6.02 (4.76–7.28) | −0.90 (−1.88 to 0.08) c | 7.09 (5.80–8.38) | 0.17 (−0.85 to 1.20) |
| 12 | 5.70 (4.44–6.95) | −1.22 (−2.19 to −0.24) a | 5.86 (4.60–7.12) | −1.06 (−2.04 to −0.08) a | 6.48 (5.19–7.77) | −0.44 (−1.46 to 0.59) |
| HADS depression | ||||||
| 0 | 4.78 (3.92–5.63) | NA | 4.78 (3.92–5.63) | NA | 4.78 (3.92–5.63) | NA |
| 8 | 4.64 (3.52–5.77) | −0.13 (−1.05 to 0.79) | 3.43 (2.28–4.57) | −1.35 (−2.29 to −0.41) d | 5.13 (3.95–6.31) | 0.36 (−0.62 to 1.34) |
| 12 | 4.47 (3.33–5.61) | −0.30 (−1.24 to 0.63) | 4.42 (3.28–5.57) | −0.35 (−1.29 to 0.59) | 4.89 (3.71–6.07) | 0.11 (−0.87 to 1.09) |
| ISI | ||||||
| 0 | 11.89 (10.28–13.51) | NA | 11.89 (10.28–13.51) | NA | 11.89 (10.28–13.51) | NA |
| 8 | 9.78 (7.69–11.86) | −2.12 (−3.76 to −0.47) a | 9.73 (7.62–11.85) | −2.16 (−3.84 to −0.48) a | 10.38 (8.20–12.55) | −1.51 (−3.27 to 0.24) c |
| 12 | 9.71 (7.60–11.82) | −2.18 (−3.85 to −0.51) a | 9.69 (7.58–11.80) | −2.20 (−3.88 to −0.52) a | 10.52 (8.35–12.69) | −1.37 (−3.13 to 0.38) |
| BFI severity | ||||||
| 0 | 4.87 (4.30–5.44) | NA | 4.87 (4.30–5.44) | NA | 4.87 (4.30–5.44) | NA |
| 8 | 3.75 (2.87–4.63) | −1.12 (−1.98 to −0.27) a | 4.45 (3.55–5.35) | −0.42 (−1.29 to 0.45) | 4.68 (3.74–5.61) | −0.19 (−1.10 to 0.71) |
| 12 | 4.45 (3.56–5.35) | −0.42 (−1.29 to 0.45) | 4.22 (3.32–5.12) | −0.65 (−1.52 to 0.22) | 3.26 (2.33–4.20) | −1.61 (−2.52 to −0.70) b |
p < .05.
p < .001.
p < .10.
p < .01.
Abbreviations: BFI, Brief Fatigue Inventory; CI, confidence interval; FACT/GOG‐Ntx, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity; HADS, Hospital Anxiety and Depression Scale; ISI, Insomnia Severity Index; NA, not applicable.
HADS anxiety reduced by −1.05 (95% CI −2.01 to −0.09) points in the RA group (p = .032) and − 0.90 (95% CI −1.88 to 0.08) in the SA group (p = .072) and increased by 0.17 (95% CI −0.85 to 1.20) in the UC group (p = .74) on a 0–21‐point scale at week 8. HADS depression scores significantly improved from baseline to week 8 in the SA arm by −1.35 (95% CI −2.29 to −0.41) points (p = .005) but did not improve in the other two arms. At week 8, there was statistically significant improvement in ISI scores in both RA and SA groups, but not in the UC group (Table 2). There was also significant reduction in the BFI in the RA group at week 8, but not in the SA and UC groups.
Between‐Group Comparison
Compared with UC, improvement in FACT/GOG‐Ntx scores at week 8 was significantly larger in both RA and SA groups (p = .001 and .01). There was no statistically significant difference between RA and SA (p = .54). Compared with UC, the improvement in HADS depression scores at week 8 was significantly larger in the SA group (p = .013) but not in the RA group (p = .47), and the difference between RA and SA was not statistically significant (p = .07). There were no statistically significant differences between groups at weeks 8 and 12 in HADS anxiety, ISI, and BFI scores (Table 3).
Table 3.
Between‐arm differences in changes from baseline
| Outcome, week | RA‐UC, mean (95% CI) | SA‐UC, mean (95% CI) | RA‐SA, mean (95% CI) |
|---|---|---|---|
| FACT/GOG‐Ntx | |||
| 4 | 0.69 (−1.89 to 3.28) | 0.80 (−1.77 to 3.38) | 0.11 (−2.41 to 2.63) |
| 8 | 4.17 (1.62 to 6.72) a | 3.40 (0.81 to 5.98) b | −0.77 (−3.25 to 1.71) |
| 12 | 1.86 (−0.68 to 4.41) | 2.38 (−0.18 to 4.93) c | 0.51 (−1.99 to 3.01) |
| HADS anxiety | |||
| 8 | −1.23 (−2.61 to 0.16) c | −1.07 (−2.47 to 0.33) | 0.15 (−1.20 to 1.50) |
| 12 | −0.78 (−2.18 to 0.62) | −0.62 (−2.02 to 0.78) | 0.16 (−1.21 to 1.52) |
| HADS depression | |||
| 8 | −0.49 (−1.81 to 0.83) | −1.70 (−3.04 to −0.37) b | −1.22 (−2.51 to 0.08) c |
| 12 | −0.42 (−1.75 to 0.92) | −0.46 (−1.80 to 0.87) | −0.05 (−1.35 to 1.25) |
| ISI | |||
| 8 | −0.60 (−2.97 to 1.77) | −0.64 (−3.04 to 1.75) | −0.04 (−2.36 to 2.27) |
| 12 | −0.81 (−3.20 to 1.58) | −0.83 (−3.23 to 1.56) | −0.02 (−2.36 to 2.31) |
| BFI severity | |||
| 8 | −0.93 (−2.13 to 0.27) | −0.22 (−1.44 to 0.99) | 0.70 (−0.47 to 1.88) |
| 12 | 1.19 (−0.02 to 2.40) c | 0.96 (−0.26 to 2.17) | −0.23 (−1.41 to 0.95) |
p < .01.
p < .05.
p < .10.
Abbreviations: BFI, Brief Fatigue Inventory; CI, confidence interval; FACT/GOG‐Ntx, Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity; HADS, Hospital Anxiety and Depression Scale; ISI, Insomnia Severity Index; RA, real acupuncture; SA, sham acupuncture; UC, usual care.
Adverse Effects
No severe adverse events associated with acupuncture treatment were reported. Six patients in the RA group reported grade 1 adverse events such as pain at the needling site (3), bruising (2), and feeling claustrophobic with the eye mask on (1). The patients in the SA group did not report any adverse events.
METE
METE scores at baseline were 53.29 (SD: 24.31) in all participants, 45.19 (20.86) in RA, 53.39 (24.17) in SA, and 61.98 (25.80) in UC, respectively, with p = .037. There was no interaction between baseline METE scores and treatment response in any of the HRQoL outcomes.
Blinding
Of the patients randomized to RA, 56% (13/27) guessed that they received real acupuncture compared with 26% (6/23) of patients in SA; 13 patients were not sure about their treatment assignment (p = .091).
Medication Changes
The majority (68%) of patients were not on any antineuropathy or pain medications throughout the trial. The 22 patients on pain medication at baseline were asked not to change it during the study. One patient in the RA group reported increased pain medicine usage and one patient in the SA group reported decreased pain medication usage. Two patients in the UC group reported increased pain medication usage.
Discussion
In this randomized sham acupuncture‐ and usual care‐controlled clinical trial of patients with solid tumors with moderate‐to‐severe CIPN symptoms, statistically significant improvements in function, quality of life, anxiety, insomnia, and fatigue were achieved with eight weeks of real acupuncture compared with baseline. However, there were no statistically significant differences between real and sham acupuncture in these HRQoL outcomes.
At week 8, we observed an average 4.02‐point increase in FACT‐GOG/Ntx in the RA group, a 3.24‐point increase in the SA group, and a − 0.15 decrease in the UC group, suggesting improved function and quality of life in RA and SA but not in UC. This result is in line with our primary endpoint result, which showed significant reduction in CIPN symptoms in RA (tingling, numbness, and pain) compared with UC. The magnitude of the increase in FACT/GOG‐Ntx in RA is not only statistically significant, but also clinically significant because it meets the recommended minimal clinically important difference of 3.3–4.4 on the FACT/GOG‐Ntx subscale [26]. These findings are also consistent with a previous pilot acupuncture CIPN trial that found acupuncture significantly improved the FACT/GOG‐Ntx subscale by 8.7 ± 8.9 points in the acupuncture arm compared with usual care (1.2 ± 5.4) [11]. We did not observe a significant group difference between RA and SA, possibly due to the small sample size and relatively broad, multidimensional nature of FACT/GoG‐Ntx.
We found a 1‐point reduction in HADS measured anxiety as well as a > 2‐point reduction compared with baseline in ISI scores in both RA and SA at week 8, versus a 0.17‐point increase in HADS anxiety and 1.51‐point reduction in ISI in UC. Although the reduction achieved by RA was statistically significant in HADS anxiety, it was not considered clinically significant in reference to the minimally important difference found in the current literature, which is suggested to be 1.5–1.7 points [27, 28, 29]. This could be due to the lack of power of our small study as well as to the low preintervention anxiety and depression levels seen in our sample group, which leave little room for improvement. Furthermore, our acupuncture protocol focused on reducing CIPN symptoms, and we preselected acupoints for each patient prior to their treatment. We did not tailor the treatment to respond to participant HRQoL needs either through interviews or patient‐reported outcomes during the study. This suggests a need for further studies that include more targeted acupoints to examine the benefits of acupuncture in improving psychogenic symptoms. Our results indicate that baseline METE scores were highest in UC, followed by SA and lowest in RA, which did not predict treatment response in HRQoL outcomes. This suggests that treatment expectancy did not cause the treatment response in the RA group.
This is the first acupuncture clinical trial to incorporate a sham acupuncture control and assess the effects of RA and SA in improving HRQoL compared with usual care. These preliminary improvements of HRQoL factors are worth reporting as they reveal the perceived value and benefits of acupuncture from the patient perspective. Furthermore, because CIPN can significantly impact quality of life, it is important to capture measures beyond symptomatic improvement, such as the impact of an intervention on improving physical and psychosocial disabilities. Because psychogenic symptoms are often concomitant or may masquerade as somatic symptoms of pain and sensory loss [30, 31, 32], using these measures to evaluate the effectiveness of an intervention in treating CIPN is important.
The theoretical framework that supports the benefits of acupuncture in improving CIPN symptoms involves targeting specific detoxification points: Ba Feng, Ba Xie points; pain reduction points: LI‐4; as well as anxiety reduction point: PC‐6, all of which we included in our study. Our preliminary results support the benefits of acupuncture, particularly for pain reduction and CIPN‐related quality of life symptoms as measured by FACT/GOG‐Ntx. The effects are less pronounced with anxiety, depression, insomnia, and fatigue. Interestingly, our study did not show a significant difference between RA and SA in improving HRQoL, suggesting the possible benefits of the placebo effect or of a provider patient care component in improving psychogenic symptoms. These findings are promising and warrant further investigation to reveal the true benefits of acupuncture in improving various HRQoL measures.
Our study has several limitations. First, as a single center study with a small sample size, we are restricted by the generalizability of our results and power to delineate the placebo effect. Second, our study had a relatively short 4‐week follow‐up period and we are unable to speak to the long‐term durability of acupuncture on HRQoL.
Conclusion
Despite these limitations, our study was the first acupuncture CIPN trial incorporating a placebo control with preliminary data supporting the benefits of acupuncture in improving CIPN‐related HRQoL outcomes. These findings set the stage for larger scale studies to further investigate the efficacy of acupuncture in improving symptom burden and quality of life in patients with CIPN.
Author Contributions
Conception/design: Ting Bao, W. Iris Zhi
Provision of study material or patients: Ting Bao, Lauren Piulson
Collection and/or assembly of data: Connie Chen, Matthew Weitzman, Yi Lily Zhang, Lauren Piulson
Data analysis and interpretation: Raymond Baser, Qing Susan Li, Connie Chen, W. Iris Zhi,
Manuscript writing: Ting Bao, Connie Chen, Christina Seluzicki, W. Iris Zhi
Final approval of manuscript: Ting Bao, Raymond Baser, Connie Chen, Matthew Weitzman, Yi Lily Zhang, Christina Seluzicki, Qing Susan Li, Lauren Piulson, W. Iris Zhi
Disclosures
The authors indicated no financial relationships.
Acknowledgments
The authors thank Patricia Chen for her assistance conducting this study. The authors also thank all the cancer survivors in the study for their participation. This work was supported in part by a National Institutes of Health/National Cancer Institute Cancer Center grant (grant number P30 CA008748), the Translational and Integrative Medicine Research Fund at Memorial Sloan Kettering Cancer Center, and the Frueauff Foundation. The funding sources were not involved in the study design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication. Ting Bao is supported by the National Cancer Institute (grant numbers R37CA248563, R01CA251470, and R01CA240417).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Fuentes AC, Lambird JE, George TJ, Jr et al. Cancer survivor's history and physical. South Med J 2017;110:37–44. [DOI] [PubMed] [Google Scholar]
- 2. Schneider BP, Hershman DL, Loprinzi C. Symptoms: Chemotherapy‐induced peripheral neuropathy. Adv Exp Med Biol 2015;862:77–87. [DOI] [PubMed] [Google Scholar]
- 3. Ewertz M, Qvortrup C, Eckhoff L. Chemotherapy‐induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol 2015;54:587–591. [DOI] [PubMed] [Google Scholar]
- 4. Seretny M, Currie GL, Sena ES et al. Incidence, prevalence, and predictors of chemotherapy‐induced peripheral neuropathy: A systematic review and meta‐analysis. Pain 2014;155:2461–2470. [DOI] [PubMed] [Google Scholar]
- 5. Bao T, Basal C, Seluzicki C et al. Long‐term chemotherapy‐induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016;159:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Argyriou AA, Bruna J, Anastopoulou GG et al. Assessing risk factors of falls in cancer patients with chemotherapy‐induced peripheral neurotoxicity. Support Care Cancer 2020;28:1991–1995. [DOI] [PubMed] [Google Scholar]
- 7. Kolb NA, Smith AG, Singleton JR et al. The association of chemotherapy‐induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol 2016;73:860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loprinzi CL, Lacchetti C, Bleeker J et al. Prevention and management of chemotherapy‐induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol 2020;38:3325–3348. [DOI] [PubMed] [Google Scholar]
- 9. Bao T, Goloubeva O, Pelser C et al. A pilot study of acupuncture in treating bortezomib‐induced peripheral neuropathy in patients with multiple myeloma. Integr Cancer Ther 2014;13:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia MK, Cohen L, Guo Y et al. Electroacupuncture for thalidomide/bortezomib‐induced peripheral neuropathy in multiple myeloma: A feasibility study. J Hematol Oncol 2014;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu W, Giobbie‐Hurder A, Freedman RA et al. Acupuncture for chemotherapy‐induced peripheral neuropathy in breast cancer survivors: A randomized controlled pilot trial. The Oncologist 2020;25:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bao T, Patil S, Chen C et al. Effect of acupuncture vs sham procedure on chemotherapy‐induced peripheral neuropathy symptoms: A randomized clinical trial. JAMA Netw Open 2020;3:e200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vincent C. The safety of acupuncture. BMJ 2001;323:467–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alimi D, Rubino C, Pichard‐Leandri E et al. Analgesic effect of auricular acupuncture for cancer pain: A randomized, blinded, controlled trial. J Clin Oncol 2003;21:4120–4126. [DOI] [PubMed] [Google Scholar]
- 15. Lao L, Bergman S, Hamilton GR et al. Evaluation of acupuncture for pain control after oral surgery: A placebo‐controlled trial. Arch Otolaryngol Head Neck Surg 1999;125:567–572. [DOI] [PubMed] [Google Scholar]
- 16. Berman BM, Lao L, Langenberg P et al. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: A randomized, controlled trial. Ann Intern Med 2004;141:901–910. [DOI] [PubMed] [Google Scholar]
- 17. Calhoun EA, Welshman EE, Chang CH et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group‐Neurotoxicity (Fact/GOG‐Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 2003;13:741–748. [DOI] [PubMed] [Google Scholar]
- 18. Huang HQ, Brady MF, Cella D et al. Validation and reduction of FACT/GOG‐Ntx subscale for platinum/paclitaxel‐induced neurologic symptoms: A gynecologic oncology group study. Int J Gynecol Cancer 2007;17:387–393. [DOI] [PubMed] [Google Scholar]
- 19. Bjelland I, Dahl AA, Haug TT et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 20. Morin CM, Belleville G, Belanger L et al. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savard MH, Savard J, Simard S et al. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology 2005;14:429–441. [DOI] [PubMed] [Google Scholar]
- 22. Mendoza TR, Wang XS, Cleeland CS et al. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer 1999;85:1186–1196. [DOI] [PubMed] [Google Scholar]
- 23. Cohen P, Cohen J, Aiken LS et al. The problem of units and the circumstance for POMP. Multivariate Behav Res 1999;34:315–346. [Google Scholar]
- 24. Bauml J, Xie SX, Farrar JT et al. Expectancy in real and sham electroacupuncture: Does believing make it so? J Natl Cancer Inst Monogr 2014;2014:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenward MG, White IR, Carpenter JR. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? by G. F. Liu, K. Lu, R. Mogg, M. Mallick and D. V. Mehrotra, Statistics in Medicine 2009; 28:2509–2530. Stat Med. 2010;29:1455–1456; author reply 1457. [DOI] [PubMed] [Google Scholar]
- 26. Yost KJ, Eton DT. Combining distribution‐ and anchor‐based approaches to determine minimally important differences: The FACIT experience. Eval Health Prof 2005;28:172–191. [DOI] [PubMed] [Google Scholar]
- 27. Lemay KR, Tulloch HE, Pipe AL et al. Establishing the minimal clinically important difference for the Hospital Anxiety and Depression Scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev 2019;39:E6–E11. [DOI] [PubMed] [Google Scholar]
- 28. Puhan MA, Frey M, Buchi S et al. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smid DE, Franssen FM, Houben‐Wilke S et al. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: A prospective analysis. J Am Med Dir Assoc 2017;18:53–58. [DOI] [PubMed] [Google Scholar]
- 30. de Heer EW, Gerrits MM, Beekman AT et al. The association of depression and anxiety with pain: A study from NESDA. PLoS One 2014;9:e106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jaracz J, Gattner K, Jaracz K et al. Unexplained painful physical symptoms in patients with major depressive disorder: Prevalence, pathophysiology and management. CNS Drugs 2016;30:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerrits MM, van Oppen P, Leone SS et al. Pain, not chronic disease, is associated with the recurrence of depressive and anxiety disorders. BMC Psychiatry 2014;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]


