Abstract
Context
Cyproterone acetate (CPA) is a competitive inhibitor of the androgen receptor and exerts negative hypothalamic feedback. It is often used in combination with estrogens in trans women to achieve feminization. However, CPA has been associated with side effects such as changes in liver enzyme concentrations and increases in prolactin concentrations. The question is whether the testosterone-lowering effect, as well as these side effects, are dose dependent.
Objective
To assess the lowest effective dose of CPA in trans women to prevent side effects.
Methods
This longitudinal study, conducted at gender identity centers in Amsterdam, Ghent, and Florence, is part of the European Network for the Investigation of Gender Incongruence (ENIGI), a multicenter prospective cohort study. Participants were trans women (n = 882) using estrogens only or in combination with 10, 25, 50, or 100 mg CPA daily. The primary outcome measure was the concentration of testosterone at 3 and/or 12 months of hormone therapy.
Results
Using estrogens only (without CPA) led to testosterone concentrations of 5.5 nmol/L (standard error of the mean [SEM] 0.3). All doses of CPA resulted in testosterone concentrations below the predefined threshold of suppression of 2 nmol/L (10 mg, 0.9 nmol/L, SEM 0.7; 25 mg, 0.9 nmol/L, SEM 0.1; 50mg, 1.1 nmol/L, SEM 0.1; 100 mg, 0.9 nmol/L, SEM 0.7). Higher prolactin and lower high-density lipoprotein concentrations were observed with increasing doses of CPA. No differences in liver enzyme concentrations were found between the doses.
Conclusion
Compared with higher doses of CPA, a daily dose of 10 mg is equally effective in lowering testosterone concentrations in trans women, while showing fewer side effects.
Keywords: trans people, hormone therapy, anti-androgen, cyproterone acetate, testosterone
Gender dysphoria is defined as an incongruence between the sex assigned to a person at birth and that person’s gender identity for a period of at least 6 months, leading to significant psychological distress (1). The prevalence of gender dysphoria has increased rapidly over the last 20 years (2). People with gender dysphoria often receive hormone therapy (HT) and gender-affirming surgery (2). Trans women (birth-assigned male, female gender identity) can be treated with estrogens, usually in combination with adjunct anti-androgens. While estrogens are considered a lifelong therapy, anti-androgens are generally discontinued after genital surgery (eg, orchiectomy) (3).
A widely used anti-androgen in Europe is cyproterone acetate (CPA). The anti-androgenic effects of CPA are mainly caused by competitively blocking the androgen receptor. CPA inhibits the influence of androgens on organs such as the skin and prostate. It also influences sexual desire and spermatogenesis. Furthermore, CPA has an anti-gonadotropic effect causing a decrease in the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). As CPA is a progestogen, it also shows progestogen-like effects, such as changes in lipid metabolism (4).
Historically, CPA has been used in the treatment of prostate cancer, hypersexuality, and hirsutism. The first studies on effects of CPA in trans women were published in the 1980s by Asscheman et al (5) and Gooren et al (6). After CPA was introduced in trans women, serious health concerns were raised regarding increases in prolactin concentrations (7), changes in liver enzymes (8), changes in lipid concentrations (4), and increases in the prevalence of thrombosis (9). Recently, concerns have been raised about the development of meningiomas in long-term use of high cumulative doses of CPA (10-12).
Besides treatment with CPA, other anti-androgen treatment options for trans women include the use of spironolactone and gonadotropin-releasing hormone (GnRH) agonists (13). Spironolactone is an antagonist of aldosterone, but it also acts as a moderate antagonist of the androgen receptor, leading to a decline in testosterone concentrations (14, 15). Testosterone concentrations usually do not decline to female reference ranges, but the use of spironolactone does cause feminization (16). A recent study by Pappas et al (17) showed that spironolactone in combination with high doses (ie, median dose of 6 mg/day) of estrogens resulted in suppressed testosterone concentrations. However, no data have been presented on the side effects of these high doses of estrogens. At initiation of spironolactone use and with subsequent dose alterations potassium checks are recommended (18). Therefore, use of spironolactone needs to be monitored more closely (14). However, opposite to CPA, spironolactone as an adjunct anti-androgen agent has not been associated with increases in prolactin concentrations (19, 20).
GnRH agonists initially raise the concentrations of LH and FSH. Over time, however, the overstimulation of the hypothalamic-pituitary-gonadal axis leads to decreases in LH and FSH concentrations, subsequently leading to a decline in testosterone concentrations (13, 14, 21). Unfortunately, unlike CPA and spironolactone, the route of administration of GnRH agonists is through subcutaneous or intramuscular injection, and data on the long-term safety of their use are limited (13). However long-term safety in patients with precocious puberty or prostate cancer has been researched (22, 23).
Worldwide, the choice of anti-androgen treatment appears to be depending on the preference of trans women, availability, regional experiences, comorbidity, and costs. Because of the raised concerns, centers are increasingly choosing to switch from CPA to GnRH agonists as the first treatment choice. In the United States, CPA has never been licensed for any indication (24). In the United Kingdom, GnRH agonists are treatment of first choice and are widely reimbursed (25). At the beginning of 2020, a safety report on CPA was published by the European Medicines Agency (EMA) on the risk of meningiomas with use of CPA (11), and the restriction of CPA was suggested in situations where alternative treatment options are available. The EMA additionally called for the lowest possible effective dose of CPA to be used if CPA treatment was maintained. Aside from the raised concerns and reported side effects, CPA has been shown to be a very effective anti-androgen that leads to adequately suppressed testosterone concentrations in trans women. Therefore, in cases where other anti-androgen agents are unavailable, contraindicated, or not reimbursed, it is important to find the lowest effective dose of CPA.
Over the years, in included participating centers of the European Network for the Investigation of Gender Incongruence (ENIGI) study, CPA doses have been successively lowered from 100 mg to 10 mg due to the concerns described above. Whether lower doses of CPA are as effective in lowering testosterone and whether the presence and severity of the side effects decrease with lower doses remains unclear. As such, the aim of this study is to assess the effects and side effects of lower doses of CPA and to identify the lowest effective dose of CPA while attempting to minimize the occurrence or magnitude of side effects.
Methods
Study Population
This study is part of the ENIGI, a multicenter prospective cohort study. This study represents a collaboration between different European gender identity centers that use comparable treatment protocols. In this study, the centers included were the Amsterdam University medical center, location VUmc, Amsterdam, the Netherlands; Ghent University Hospital, Ghent, Belgium; and the University of Florence, Florence, Italy. Data collection started in 2010 and is ongoing (3). The study was set up to describe the clinical effects and side effects of specific forms of HT. Participants were included the moment they started HT.
In the current study, trans women of the ENIGI study were eligible for inclusion if they had started with estrogen treatment (either with or without CPA) and had at least a 3-month period of follow-up registered. Participants were included from 2010 to March 2020. Individuals who had undergone an orchiectomy or had previously used HT prior to the initiation of HT were excluded from this study. Additionally, individuals with suppressed serum testosterone concentrations at baseline (defined as testosterone concentrations below 2 nmol/L) were excluded. All participants with missing baseline testosterone concentrations were excluded. Finally, participants were additionally excluded if the specific form and dose of HT at the time of a laboratory measurement was unavailable. The study protocol was approved by all the ethical committees of participating hospitals, and informed consent was obtained following institutional guidelines.
Treatment Protocol
At baseline, the participants’ demographic characteristics were assessed. All participants included in this study were above the age of 16 years. The treatment protocol for trans women consisted of an estradiol agent in combination with CPA. Estradiol therapy consisted of either oral (2-6 mg daily), transdermal (50-150 mcg/24 hours), or gel 17-β estradiol. It was recommended for participants over 40 years of age to use transdermal estradiol because of the possible higher risk of thrombosis with the oral use of estradiol (9). At the beginning of this study, the starting dose of CPA was 100 mg. This dose was reduced over time in steps to, respectively, 50 mg, 25 mg, and 10 mg. If the preferred dose of a participant was lower than the standard dose dictated by the protocol used at that time, the preferred dose was used. The follow-up consisted of clinical visits every 3 months in the first year.
Data Collection
Blood was drawn at start of the HT, as well as after 3 months and after 12 months of HT. The serum measurements taken included testosterone, estradiol, prolactin, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), low-density lipoprotein cholesterol (LDL-C), high-density lipoproteins cholesterol (HDL-C), total cholesterol, and triglyceride concentrations. The samples were analyzed at local laboratories.
In Amsterdam, testosterone was measured using a radioimmunoassay (Coat-A-Count; Siemens, Los Angeles, CA, USA) with an interassay coefficient of variation (CV) of 7% to 20% and a lower limit of quantification (LOQ) of 1.0 nmol/L. From January 2013, testosterone was measured using competitive immunoassay (Architect; Abbott, Abbott Park, IL, USA) with an interassay CV range of 6% to 16% and an LOQ of 0.1 nmol/L. From October 2018, testosterone was measured using liquid chromatography with tandem mass spectrometry (LC-MS/MS) with an interassay CV of 4% to 9% and an LOQ of 0.1 nmol/L. No conversion formula was needed. In Ghent, testosterone was measured using an E170 Modular (Gen II; Roche Diagnostics, Mannheim, Germany). After March 2015, testosterone was measured using an E170 Modular (Gen II; Roche Diagnostics) with an interassay CV of 2.6% and an LOQ of 0.4 nmol/L. Concentrations before the change of assay were converted (26). In Florence, testosterone was measured using electrochemiluminescence immunoassay (Roche Cobas E801; Roche Diagnostics) with an interassay CV of 6.8% and an LOQ of 0.4 nmol/L.
Information related to the occurrence of thrombosis and meningiomas in the first 12 months of HT was collected. Each participant’s body mass index (BMI) was calculated using measured height and weight with light indoor clothes but no shoes at clinical visits.
Primary and Secondary Outcomes
The primary outcome of this study was the concentration of testosterone with different CPA doses, measured at 3 and/or 12 months. The secondary outcome was a comparison of the CPA doses in relation to percentages of participants with suppressed testosterone and hyperprolactinemia. Furthermore, a comparison was made between the different doses of CPA and participants’ prolactin concentrations, liver enzyme concentrations, lipid concentrations, and the occurrence of thrombosis and meningiomas.
Statistical Analysis
In this study, the baseline characteristics are described as the mean (SD), the median (interquartile range, [IQR]), or percentages. The primary and secondary results are given as the mean with standard error of the mean (SEM) or confidence intervals (CI).
Primary analyses
Linear mixed-model analyses with measurements clustered within participants were used to analyze the concentration of testosterone with different CPA doses (ie, no CPA, 10 mg, 25 mg, 50 mg, or 100 mg). Analyses were repeated per center because of possible differences in testosterone values with use of nonidentical assays between centers.
Secondary analyses
Logistic mixed-model analyses with measurements clustered within participants were performed to analyze testosterone suppression with different doses of CPA. As a cutoff level for suppressed testosterone, 2 nmol/L was used (14). This is the in-hospital reference concentrations used for cis women (birth-assigned women, female gender identity). The percentages of participants with suppressed testosterone at 3 and/or 12 months were given. To determine the association between CPA doses and prolactin concentrations, linear mixed-model analyses were performed, with measurements clustered within participants. The percentages of participants with hyperprolactinemia at 3 and/or 12 months were also given. To define hyperprolactinemia, the in-hospital reference ranges for cis women were used (>0.6 U/L). Participants who used medication known for increasing prolactin concentrations (eg, antipsychotics and antidepressants (27)) were excluded from these particular analyses.
To study whether different CPA doses were associated with changes in liver enzymes or lipid concentrations, linear mixed-model analyses were used, with measurements clustered within participants.
Last, the occurrence of thrombosis and meningiomas was studied by searching the database for complications related to HT. However, no analysis could be performed because of the low incidence rate. Statistical analyses were performed using Stata version 15.1 (StataCorp, College Station, TX, USA).
Results
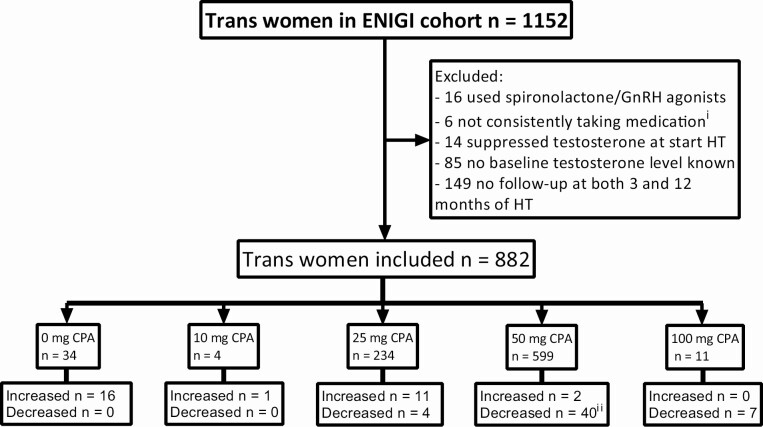
The total ENIGI cohort consisted of 1152 trans women. After the exclusion of participants who did not fit the study criteria, 882 participants remained eligible for analysis (Fig. 1).
Figure 1.
Flowchart of inclusion and exclusion of participants with number of participants per starting dose. Participants that changed dose after 3 months of hormone therapy are given per starting dose. Suppressed testosterone is defined as testosterone concentrations <2.0 nmol/L. Abbreviations: CPA, cyproterone acetate; ENIGI, European Network for the Investigation of Gender Incongruence; HT, hormone therapy. iPatient files of participants with outlying testosterone concentrations were searched. If appeared from consults, medication was not taken consistently, participants were excluded. iiMostly due to a change in protocol in which the dose of CPA was lowered from 50 mg to 25 mg daily.
Baseline Characteristics
The baseline characteristics of the participants at the time of the initial dose of CPA are presented in Table 1. On average, participants using 50 mg of CPA were slightly older than participants using 25 mg of CPA. From the recorded ethnicities, 97% was white. No participants using 10 mg of CPA used tobacco or consumed more than 7 units of alcohol (approximately 10 g of pure alcohol per unit) per week. There were no differences in the baseline testosterone concentrations among participants. Furthermore, there were no clinically relevant differences between the participants’ concentrations of prolactin, AST, ALT, GGT, ALP, LDL-C, HDL-C, total cholesterol, or triglycerides at baseline.
Table 1.
Baseline characteristics of all participants by starting dose of CPA
| No CPA | 10 mg CPA | 25 mg CPA | 50 mg CPA | 100 mg CPA | |
|---|---|---|---|---|---|
| Number of participants | 34 | 4 | 234 | 599 | 11 |
| - Amsterdam University medical center | 32 | 4 | 206 | 378 | 9 |
| - Ghent University Hospital | 2 | 0 | 27 | 195 | 2 |
| - University Hospital Florence | 0 | 0 | 1 | 26 | 0 |
| Age at initiation HT, year (IQR) | 27 [23-31] | 30 [19-57] | 25 [21-35] | 28 [23-42] | 34 [26-53] |
| Active smoking, %yes | 20.0 | 0.0 | 20.4 | 15.1 | 0.0 |
| Alcohol use >7 units/week, %yes | 9.1 | 0.0 | 5.9 | 7.3 | 27.3 |
| BMI, kg/m2 (IQR) | 22.7 [19.7-26.5] | 20.5 [17.6-20.6] | 22.7 [19.6-25.7] | 22.7 [20.5-25.9] | 23.0 [21.1-28.1] |
| Testosterone at initiation of HT, nmol/L (IQR) | 17.5 [14.0-26.0] | 20.0 [17.0-26.5] | 18.0 [13.0-22.0] | 19.0 [14.0-24.0] | 19.8 [16.5-25.2] |
| Prolactin at initiation of HT, U/L (IQR) | 0.1 [0.1-0.1] | 0.1 [0.1-0.2] | 0.1 [0.1-0.2] | 0.1 [0.1-0.2] | 0.1 [0.11-0.2] |
| Liver enzymes (IQR) | |||||
| AST, U/L | 21 [19-25] | 23 [19-24] | 21 [18-25] | 22 [18-26] | 21 [15-27] |
| ALT, U/L | 22 [16-29] | 15 [14-22] | 21 [16-28] | 21 [17-30] | 16 [13-25] |
| GGT, U/L | 18 [13-23] | 13 [11-19] | 17 [14-26] | 20 [15-28] | 16 [13-18] |
| ALP, U/L | 72 [54-82] | 63 [52-73] | 75 [63-89] | 70 [58-83] | 100 [100-100] |
| Lipid profile (IQR) | |||||
| LDL-C, mmol/L | 2.4 [1.8-3.5] | 1.8 [1.6-2.2] | 2.3 [1.8-2.9] | 2.5 [2.0-3.2] | 2.2 [1.8-3.0] |
| HDL-C, mmol/L | 1.2 [1.1-1.5] | 1.7 [1.6-1.8] | 1.3 [1.1-1.5] | 1.4 [1.1-1.6] | 1.4 [1.2-1.7] |
| Total cholesterol, mmol/L | 4.2 [3.5-5.5] | 3.8 [3.6-4.3] | 4.2 [3.6-4.9] | 4.4 [3.8-5.2] | 3.9 [3.7-4.8] |
| Triglycerides, mmol/L | 1.0 [0.9 -1.4] | 0.6 [0.6-0.9] | 1.0 [0.8-1.5] | 0.9 [0.7-1.3] | 0.7 [0.7-0.9] |
Values presented as median with 25-75 interquartile ranges (IQR). Active smoking (yes/no) and alcohol use >7 units (approximately 10 g of pure alcohol per unit) per week (yes/no) presented as percentages of corresponding dose.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CPA, cyproterone acetate; GGT, gamma-glutamyl transferase; HDL-C, high-density lipoprotein cholesterol; HT, hormone therapy; LDL-C, low-density lipoprotein cholesterol.
Changes in CPA Doses
At 3 months, 16 of 36 participants using no CPA (ie, taking only estrogen) started using CPA. Of the participants using 10 mg of CPA, 1 had a dose increase to 50 mg because of unsuppressed testosterone at 3 months. At 12 months of HT, this participant still had unsuppressed testosterone, despite using 50 mg of CPA. Of the participants using 25 mg of CPA, 11 had a dose increase after 3 months, among these, 4 had unsuppressed testosterone. After the increase to 50 mg of CPA, 2 of these participants had testosterone concentrations that remained above the reference concentrations. Furthermore, 2 participants had a dose increase from 50 mg to 100 mg of CPA even though their testosterone concentrations were already suppressed.
Regarding dose decreases, 11 participants stopped using CPA after 3 months of HT. After a reduction in CPA dose from 50 mg to 25 mg, 1 participant had an increase in testosterone concentration to 19.1 nmol/L. The remaining 39 participants still had suppressed testosterone concentrations after dose reduction. The high number of participants who had a dose reduction from 50 mg to 25 mg can be explained by a change in protocol in 2017.
Primary Results
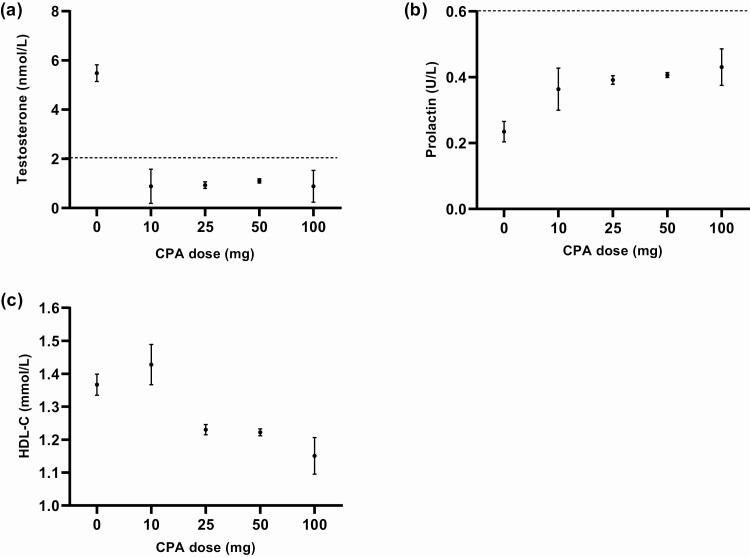
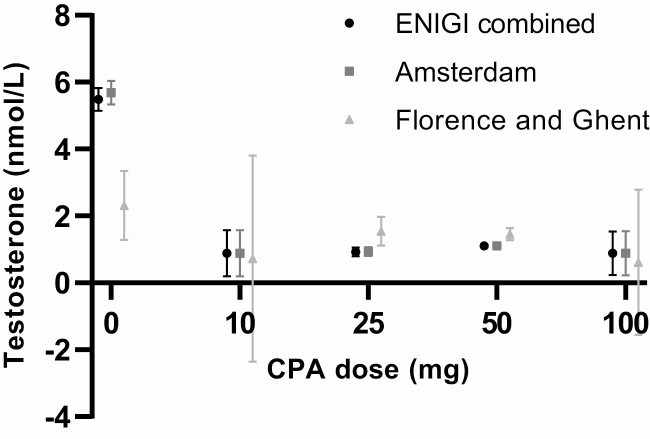
The use of only estrogens resulted in a testosterone concentration of 5.5 nmol/L (SEM = 0.3). All doses of CPA resulted in testosterone concentrations below the predefined threshold of suppression of 2 nmol/L (10 mg, 0.9 nmol/L, SEM = 0.7; 25 mg, 0.9 nmol/L, SEM = 0.1; 50 mg, 1.1 nmol/L, SEM = 0.1; 100 mg, 0.9 nmol/L, SEM = 0.7). No difference in testosterone concentrations between the different CPA doses was found (Fig. 2A). Furthermore, no relevant differences in testosterone concentrations were found when repeating analysis per center (Fig. 3).
Figure 2.
Testosterone (a), prolactin (b), and HDL-C concentrations (c) after 3 and 12 months of estrogen treatment in combination with different doses of CPA. The effects are based on mixed-model analysis at 3 and 12 months of hormone therapy. Participants using antidepressants or antipsychotics were excluded in analyses of prolactin concentrations. All results are presented as mean with standard error of the mean (SEM). Adequate suppression of testosterone is defined as testosterone concentrations < 2.0 nmol/L. Hyperprolactinemia is defined as prolactin concentrations >0.6 U/L. Abbreviations: CPA, cyproterone acetate; HDL-C, high-density lipoprotein cholesterol.
Figure 3.
Testosterone concentrations after 3 and 12 months of estrogen treatment per center in combination with different doses of CPA. The effects are based on mixed-model analysis at 3 and 12 months of hormone therapy. Results are presented as mean with standard error of the mean (SEM). Adequate suppression of testosterone is defined as testosterone concentrations <2.0 nmol/L. Abbreviation: CPA, cyproterone acetate.
Secondary Results
Testosterone suppression
After start of HT, the odds of having a suppressed testosterone concentration were higher with all doses of CPA compared with use of no CPA (ie, only estrogens). No difference was observed among different doses of CPA. At 3 and 12 months of HT, 46.3% of participants using no CPA had suppressed testosterone, whereas this percentage was higher in participants using 10 mg (92.3%), 25 mg (96.2%), 50 mg (93.4%), and 100 mg (100.0%) of CPA.
Prolactin concentrations
In participants without use of CPA, the mean concentration of prolactin during HT was 0.2 U/L (SEM = 0.0). CPA caused higher concentrations of prolactin in participants using 25 mg (+0.2 U/L; 95% CI, 0.1 to 0.2), 50 mg (+0.2 U/L; 95% CI, 0.1 to 0.2), and 100 mg (+0.2 U/L; 95% CI, 0.1 to 0.3) of CPA compared with those in participants using no CPA. A dose of 10 mg of CPA did not cause an increase to the same extent as did the other doses (+0.1 U/L; 95% CI −0.01 to 0.3) (Fig. 2B). At 3 and 12 months of HT, none of the participants who did not use CPA had prolactin concentrations above the reference ranges. These percentages were higher in participants using 10 mg (9.1%), 25 mg (11.7%), 50 mg (13.8%), and 100 mg (14.3%) of CPA.
Liver enzymes
The results of analyses of all liver enzymes are shown in Table 2. In participants using no CPA, the mean ALT concentrations during HT were 28 U/L (SEM = 2). The ALT concentrations were lower in all participants using CPA (10 mg, −7 U/L, [95% CI, −14 to 0.2]; 25 mg, −6 U/L, [95% CI, −9 to -3]; 50 mg, −6 U/L, [95% CI, −9 to −2]; 100 mg, −7 U/L, [95% CI, −14 to −0.6]). No relevant differences were found between the doses of CPA and the concentrations of AST, GGT, and ALP. Mean concentrations of liver enzymes remained within the reference range among all different doses of CPA.
Table 2.
Liver enzymes and lipid concentrations after start of hormone therapy
| No CPA | 10 mg CPA | 25 mg CPA | 50 mg CPA | 100 mg CPA | |
|---|---|---|---|---|---|
| Liver enzymes (SEM) | |||||
| AST, U/L | 21 (1) | 17 (2) | 19 (0) | 19 (0) | 18 (2) |
| ALT, U/L | 28 (2) | 21 (3) | 22 (0) | 22 (0) | 20 (3) |
| GGT, U/L | 22 (5) | 20 (10) | 27 (2) | 24 (1) | 22 (8) |
| ALP, U/L | 67 (2) | 61 (5) | 63 (1) | 61 (1) | 81 (18) |
| Lipid profile (SEM) | |||||
| LDL-C, mmol/L | 2.5 (0.1) | 2.2 (0.1) | 2.2 (0.0) | 2.4 (0.0) | 2.4 (0.1) |
| HDL-C, mmol/L | 1.4 (0.0) | 1.4 (0.1) | 1.2 (0.0) | 1.2 (0.0) | 1.2 (0.1) |
| Total cholesterol, mmol/L | 4.4 (0.1) | 4.0 (0.2) | 4.0 (0.0) | 4.0 (0.0) | 4.0 (0.1) |
| Triglycerides, mmol/L | 1.2 (0.0) | 0.9 (0.1) | 1.0 (0.0) | 1.0 (0.0) | 1.0 (0.1) |
Measurements are clustered within participants at 3 and 12 months of hormone therapy. Concentrations are presented as mean with standard error of the mean (SEM).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CPA, cyproterone acetate; GGT, gamma-glutamyl transferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Lipid concentrations
The results of analyses of all lipid concentrations are shown in Table 2. The HDL-C concentrations in participants using no CPA were 1.4 mmol/L (SEM = 0.0). No difference was seen in those taking 10 mg of CPA (+0.1 mmol/L; 95% CI, −0.1 to 0.2) compared with no CPA. Lower lipid concentrations were seen in participants using 25 mg (−0.1 mmol/L; 95% CI, −0.2 to −0.1), 50 mg (−0.1 mmol/L; 95% CI, −0.2 to −0.1), or 100 mg (−0.2 mmol/L; 95% CI, −0.3 to −0.1) of CPA compared to participants using no CPA (Fig. 2C). No relevant differences were found between doses of CPA and concentrations of LDL-C, total cholesterol, or triglycerides. Mean lipid concentrations remained within the reference range among all different doses of CPA
Thrombosis and meningiomas
In this cohort, 1 participant had a superficial venous thrombosis between 9 and 12 months of follow-up. No underlying clotting deficiencies could be found. This participant used 50 mg of CPA in combination with 4 mg of estradiol valerate per day. Furthermore, 1 participant was diagnosed with a pulmonary embolism between 3 and 12 months of follow-up. This 62-year-old participant with an extensive medical history used 25 mg of CPA and 50 mcg of transdermal estradiol at 3 months. HT was ceased at 3 months for unknown reason. None of the participants were diagnosed with a meningioma.
Discussion
In this cohort study of 882 trans women using estrogens, we investigated testosterone concentrations when successively tapering the dose of CPA over the last decade. In the current study, serum testosterone concentrations were equally suppressed among all different doses of CPA. With regard to side effects, CPA doses above 10 mg resulted in higher prolactin concentrations and lower HDL-C concentrations. Among different doses of CPA, no differences were observed in concentrations of liver enzymes or blood lipids.
This study is part of the ENIGI initiative, a multicenter prospective cohort study. The main treatment protocol for trans women in this study was 50 mg of CPA daily combined with estrogens. In the first year of study inclusion, a few participants received a dose of 100 mg of CPA. Shortly thereafter, in-hospital protocol changed to 50 mg of CPA. As more health concerns related to CPA use were raised over the years, the dose was further lowered from 50 mg to 25 mg and, finally, to 10 mg. However, due to the coronavirus (COVID-19) pandemic, limited results from participants with 10 mg of CPA were available for analysis.
The successive tapering of CPA doses led to participants being allocated to different CPA doses independent of patient characteristics but based on the time frame that HT was started in, which limits bias by indication. This is illustrated in Table 1, which reveals no key differences between participants’ characteristics.
The documented effects on testosterone concentrations are in line with those of an earlier study by Fung et al (28), which was performed in a smaller cohort on participants using 25 mg and 50 mg of CPA. They showed that 25 mg of CPA was just as effective as 50 mg of CPA in lowering testosterone concentrations. Our study showed that the same accounts for the testosterone-lowering effects of 10 mg of CPA.
Higher prolactin concentrations were found in participants using CPA doses of more than 10 mg. However, the results were not in the range of hyperprolactinemia. In an earlier study by Defreyne et al (29), an increase in prolactin was found after the start of CPA, which declined to the baseline after an orchiectomy was performed and anti-androgens were ceased. However, as some trans women choose not to have an orchiectomy and to instead keep using CPA for prolonged periods, questions arise about whether these elevated prolactin concentrations can lead to long-term complications. Our results suggest that lower doses of CPA have less influence on prolactin concentrations, reducing the risk of possible concomitant long-term side effects.
Adverse effects of CPA on liver enzymes were previously described for CPA doses over 100 mg, usually in elderly patients with prostate cancer (8, 30). An elevation of liver enzyme concentrations was not found in the current study in a healthy population using lower doses of CPA. The different doses of CPA did not lead to clinically relevant differences in liver enzymes, and the results remained below the reference ranges used in our hospitals. These results suggest that lower doses of CPA in a healthy population do not lead to changes in liver enzymes.
The effects of different doses of CPA on lipid metabolism were analyzed using changes in serum concentrations of LDL-C, HDL-C, total cholesterol, and triglycerides. Doses of CPA above 10 mg resulted in lower concentrations of HDL-C compared with participants not using CPA (ie, only estrogens). Other lipids remained either stable or lower with the use of CPA. No clinically important differences were found between the doses. The changes in lipid concentrations in feminizing HT have previously been described in the same cohort (26). That study showed that 50 mg of CPA in combination with estradiol led to a decrease in HDL-C concentrations. This study also showed that estrogens appear to have a beneficial effect on lipid concentrations; however, high doses of CPA counteract this beneficial effect. In a study conducted by Gava et al (31), the effectiveness of CPA and GnRH agonists were compared. The study found that HDL-C concentrations were reduced in participants using CPA but increased with the use of GnRH agonists. Progestogens with more anti-androgenic activity seem to reverse the beneficial effect of estrogens (4). The lowering of LDL-C, total cholesterol, and triglyceride concentrations could be interpreted as beneficial. However, decreases in HDL-C concentrations could lead to cardiovascular problems in the long term (32). Our results suggest that CPA decreases HDL-C concentrations in a dose-dependent manner. As a 10 mg dose of CPA did not meaningfully alter HDL-C concentrations, it may therefore lead to fewer long-term complications than higher doses of CPA.
During our follow-up period, 1 participant was diagnosed with a superficial thrombosis between 9 and 12 months of follow-up. In addition, 1 participant was diagnosed with a pulmonary embolism after ceasing HT. Earlier studies have shown that the incidence of venous thromboembolism decreases with decreasing doses and increasing duration of estradiol use (33). CPA in combination with estradiol has been shown to result in an increased risk of venous thromboembolism (9, 34-36).
Furthermore, in the current study, no participants were diagnosed with a meningioma during the first 12 months of HT. Nota et al (10) indicated that, in a large cohort of trans women using CPA, 8 participants over the age of 45 years were diagnosed with a meningioma; all of these participants had used CPA for extensive periods of more than 59 months in doses above 25 mg. In a study by Weill et al (12), it appeared that the risk of developing a meningioma increased with the cumulative dose of CPA. As the duration of follow-up was relatively short in our study, we were unable to assess whether different doses of CPA resulted in more cases of thrombosis or meningiomas in the long term.
Strengths and Limitations
The current study compares the effects of different doses of CPA in a large prospective cohort study, including 882 trans women, on transgender individuals during HT. The current study is also the first to report on the anti-androgenic potency of 10 mg CPA. Furthermore, this study analyzed important secondary outcomes providing a broader view of the topic.
The first and most important limitation of this study is the possible risk of indication bias in participants using 10 mg of CPA. The results showed that some participants received the dose of 10 mg at arbitrary times in the study period; that is, if the participants preferred to use the lower dose of 10 mg of CPA even before it became the standard dose (eg, they chose to use 10 mg even though 25 mg was the starting dose). The other reductions in dose of CPA happened at specific points in time, when the reduced dose became the standard dose administered to participants; these dose reductions could therefore be attributed to a quasi-randomization principle. Furthermore, there is a possibility that differences between doses could not be detected because of the lack of power in the group of participants using 10 mg. A third limitation was the missing data on testosterone concentrations and CPA dose used, which caused the exclusion of a number of participants. Last, the participants’ motives for changing their dose were often unknown; for example, some participants received an increased dose of CPA even though their testosterone concentrations were already suppressed, and vice versa.
Conclusion
In conclusion, in this cohort of trans women, 10 mg of CPA was found to be effective in lowering testosterone concentrations to the range observed in cis women. A dose of 10 mg was equally effective as higher doses, was found to have less influence on prolactin concentrations, and allowed higher HDL-C concentrations to be maintained. While GnRH agonists are preferred over CPA due to the fewer associated long-term side effects, this study shows that CPA at a low dose is a viable option when GnRH agonists are contraindicated, not available, or not reimbursed. Future research should focus on assessing the effectiveness of an even lower dose of CPA (eg, 5 mg) and the potential long-term side effects.
Acknowledgments
Financial Support: This research was not part of a grant or fellowship.
Glossary
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AST
aspartate transaminase
- CPA
cyproterone acetate
- CV
coefficient of variation
- ENIGI
European Network for the Investigation of Gender Incongruence
- FSH
follicle-stimulating hormone
- GGT
gamma-glutamyl transferase
- GnRH
gonadotropin-releasing hormone
- HDL-C
high-density lipoprotein cholesterol
- HT
hormone therapy
- LDL-C
low-density lipoprotein cholesterol
- LH
luteinizing hormone
- LOQ
lower limit of quantification
Additional Information
Disclosures: The authors have nothing to declare.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Beek TF, Cohen-Kettenis PT, Kreukels BP. Gender incongruence/gender dysphoria and its classification history. Int Rev Psychiatry. 2016;28(1):5-12. [DOI] [PubMed] [Google Scholar]
- 2. Wiepjes CM, Nota NM, de Blok CJM, et al. The Amsterdam cohort of gender dysphoria study (1972-2015): trends in prevalence, treatment, and regrets. J Sex Med. 2018;15(4):582-590. [DOI] [PubMed] [Google Scholar]
- 3. Dekker MJ, Wierckx K, Van Caenegem E, et al. A European network for the investigation of gender incongruence: endocrine part. J Sex Med. 2016;13(6):994-999. [DOI] [PubMed] [Google Scholar]
- 4. Jiang Y, Tian W. The effects of progesterones on blood lipids in hormone replacement therapy. Lipids Health Dis. 2017;16(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism. 1989;38(9):869-873. [DOI] [PubMed] [Google Scholar]
- 6. Gooren LJ, Assies J, Asscheman H, de Slegte R, van Kessel H. Estrogen-induced prolactinoma in a man. J Clin Endocrinol Metab. 1988;66(2):444-446. [DOI] [PubMed] [Google Scholar]
- 7. Nota NM, Dekker M, Klaver M, et al. Prolactin levels during short- and long-term cross-sex hormone treatment: an observational study in transgender persons. Andrologia. Published online August 25, 2017;49(6). doi:10.1111/and.12666 [DOI] [PubMed] [Google Scholar]
- 8. Bessone F, Lucena MI, Roma MG, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver Int. 2016;36(2):302-310. [DOI] [PubMed] [Google Scholar]
- 9. Asscheman H, T’Sjoen G, Lemaire A, et al. Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review. Andrologia. 2014;46(7):791-795. [DOI] [PubMed] [Google Scholar]
- 10. Nota NM, Wiepjes CM, de Blok CJM, et al. The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment. Brain. 2018;141(7):2047-2054. [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency. Restrictions in use of cyproterone due to meningioma risk. European Medicines Agency. Published February 14, 2020. Accessed October 20, 2020. https://www.ema.europa.eu/en/news/restrictions-use-cyproterone-due-meningioma-risk [Google Scholar]
- 12. Weill A, Nguyen P, Labidi M, et al. Use of high dose cyproterone acetate and risk of intracranial meningioma in women: cohort study. BMJ. 2021;372:n37. [DOI] [PubMed] [Google Scholar]
- 13. Tangpricha V, den Heijer M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017;5(4):291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903. [DOI] [PubMed] [Google Scholar]
- 15. Prior JC, Vigna YM, Watson D. Spironolactone with physiological female steroids for presurgical therapy of male-to-female transsexualism. Arch Sex Behav. 1989;18(1):49-57. [DOI] [PubMed] [Google Scholar]
- 16. Angus L, Leemaqz S, Ooi O, et al. Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy. Endocr Connect. 2019;8(7):935-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappas II, Craig WY, Spratt LV, Spratt DI. Efficacy of sex steroid therapy without progestin or GnRH agonist for gonadal suppression in adult transgender patients. J Clin Endocrinol Metab. 2021;106(3):e1290-e1300. [DOI] [PubMed] [Google Scholar]
- 18. Safer JD, Tangpricha V. Care of Transgender Persons. N Engl J Med. 2019;381(25):2451-2460. [DOI] [PubMed] [Google Scholar]
- 19. Bisson JR, Chan KJ, Safer JD. Prolactin levels do not rise among transgender women treated with estradiol and spironolactone. Endocr Pract. 2018;24(7):646-651. [DOI] [PubMed] [Google Scholar]
- 20. Sofer Y, Yaish I, Yaron M, Bach MY, Stern N, Greenman Y. Differential endocrine and metabolic effects of testosterone suppressive agents in transgender women. Endocr Pract. 2020;26(8):883-890. [DOI] [PubMed] [Google Scholar]
- 21. Angus LM, Nolan BJ, Zajac JD, Cheung AS. A systematic review of antiandrogens and feminization in transgender women. Clin Endocrinol (Oxf). 2021;94(5):743-752. [DOI] [PubMed] [Google Scholar]
- 22. Mul D, Hughes IA. The use of GnRH agonists in precocious puberty. Eur J Endocrinol. 2008;159 Suppl 1:S3-S8. [DOI] [PubMed] [Google Scholar]
- 23. Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306(21):2359-2366. [DOI] [PubMed] [Google Scholar]
- 24. Mamoojee Y, Seal LJ, Quinton R. Transgender hormone therapy: understanding international variation in practice. Lancet Diabetes Endocrinol. 2017;5(4):243-246. [DOI] [PubMed] [Google Scholar]
- 25. Wylie K, Barrett J, Besser M, et al. Good practice guidelines for the assessment and treatment of adults with gender dysphoria. Sex Relationsh Ther. 2014;29(2):154-214. [Google Scholar]
- 26. van Velzen DM, Paldino A, Klaver M, et al. Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J Clin Endocrinol Metab. 2019;104(6):1937-1947. [DOI] [PubMed] [Google Scholar]
- 27. Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007;3(5):929-951. [PMC free article] [PubMed] [Google Scholar]
- 28. Fung R, Hellstern-Layefsky M, Lega I. Is a lower dose of cyproterone acetate as effective at testosterone suppression in transgender women as higher doses? Int J Transgenderism. 2017;18:123-128. [Google Scholar]
- 29. Defreyne J, Nota N, Pereira C, et al. Transient elevated serum prolactin in trans women is caused by cyproterone acetate treatment. LGBT Health. 2017;4(5):328-336. [DOI] [PubMed] [Google Scholar]
- 30. Kim JH, Yoo BW, Yang WJ. Hepatic failure induced by cyproterone acetate: a case report and literature review. Can Urol Assoc J. 2014;8(5-6):E458-E461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gava G, Cerpolini S, Martelli V, Battista G, Seracchioli R, Meriggiola MC. Cyproterone acetate vs leuprolide acetate in combination with transdermal oestradiol in transwomen: a comparison of safety and effectiveness. Clin Endocrinol (Oxf). 2016;85(2):239-246. [DOI] [PubMed] [Google Scholar]
- 32. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The framingham study. Am J Med. 1977;62(5):707-714. [DOI] [PubMed] [Google Scholar]
- 33. Lidegaard Ø, Løkkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lidegaard Ø, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65(3):187-196. [DOI] [PubMed] [Google Scholar]
- 35. Vasilakis-Scaramozza C, Jick H. Risk of venous thromboembolism with cyproterone or levonorgestrel contraceptives. Lancet. 2001;358(9291):1427-1429. [DOI] [PubMed] [Google Scholar]
- 36. Toorians AW, Thomassen MC, Zweegman S, et al. Venous thrombosis and changes of hemostatic variables during cross-sex hormone treatment in transsexual people. J Clin Endocrinol Metab. 2003;88(12):5723-5729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.