Abstract
The ability to generate new hippocampal neurons throughout adulthood and successfully integrate them into existing neural networks is critical to cognitive function, while disordered regulation of this process results in neurodegenerative or psychiatric disease. Consequently, identifying the molecular mechanisms promoting homeostatic hippocampal neurogenesis in adults is essential to understanding the etiologies of these disorders and developing therapeutic interventions. For example, recent evidence identifies a strong association between metabolic function and adult hippocampal neurogenesis. Hippocampal neural stem cell (NSC) fate dynamically fluctuates with changes in substrate availability and energy status (AMP/ATP and NAD+/NADH ratios). Furthermore, many metabolic hormones, such as insulin, insulin-like growth factors, and leptin exhibit dual functions also modulating hippocampal neurogenesis and neuron survivability. These diverse metabolic inputs to NSC’s from various tissues seemingly suggest the existence of a system in which energy status can finely modulate hippocampal neurogenesis. Supporting this hypothesis, interventions promoting energy balance, such as caloric restriction, intermittent fasting, and exercise, have shown encouraging potential enhancing hippocampal neurogenesis and cognitive function. Overall, there is a clear relationship between whole body energy status, adult hippocampal neurogenesis, and neuron survival; however, the molecular mechanisms underlying this phenomenon are multifaceted. Thus, the aim of this review is to analyze the literature investigating energy status-mediated regulation of adult neurogenesis in the hippocampus, highlight the neurocircuitry and intracellular signaling involved, and propose impactful future directions in the field.
Keywords: hippocampus, neurogenesis, metabolism, energy balance, metabolic disease, neurodegenerative disease
1. Introduction:
Adult neurogenesis (AN) is the complex process through which neural stem cells (NSC’s) differentiate into newborn neurons and are integrated into existing neural networks [1]. The extent of AN in humans throughout the lifespan is still somewhat debated [2-4]; however, accumulating evidence indicates AN is critical to learning, memory, stress response, and mood regulation [5-7]. AN occurs primarily in two neurogenic niches, the subgranular zone (SGZ) of the dentate gyrus (DG), and the subventricular zone (SVZ) of the lateral ventricles [8]. Furthermore, NSC’s are subject to multifaceted regulation determining their fate, including inputs from presynaptic signals, hormones, and neuropeptides, while disturbed function of this neurocircuitry is associated with epilepsy, depression, and neurodegenerative disease [5,9]. Considering the importance of AN to healthy brain function, many studies have focused on deciphering the physiological mechanisms underlying homeostatic AN regulation.
Many circulating factors have dual functions regulating both metabolism and AN, including liver-derived insulin-like growth factor 1 (IGF-1) [10], ghrelin from the stomach [11], leptin from adipose [12], and irisin from skeletal muscle [13]. These diverse signals to NSC’s from various metabolically active tissues seemingly provide an ideal system in which energy status can dynamically and finely regulate neurogenesis. Supporting this hypothesis, epidemiological studies have revealed a strong association between metabolic disorders, cognitive impairment, and neurodegenerative diseases [14,15]. Recently, caloric restriction, intermittent fasting, and physical activity have been shown to stimulate hippocampal AN and improve neuronal survival by increasing circulating neurotrophic factors, bolstering stress resistance, improving autophagy, and modulating the transcriptome [16-18]. As a result, these approaches have demonstrated promising potential in improving cognitive function and reducing the risk/severity of psychiatric and neurodegenerative disorders [16-18].
Excessive neuronal differentiation can also be detrimental due to inadequate NSC self-renewal and NSC exhaustion. For example, the benefits of energy deficit to AN exhibit a negative parabolic relationship, where excessive caloric restriction or chronic energy surplus both impair AN and neuronal survivability (Figure 1) [16-18]. This phenomenon is referred to as hormesis; during which, a moderate amount of something, such as caloric restriction, produces favorable effects; however, excessive amounts have deleterious effects. Hormesis is a recurring theme when examining the connections between energy status and AN.
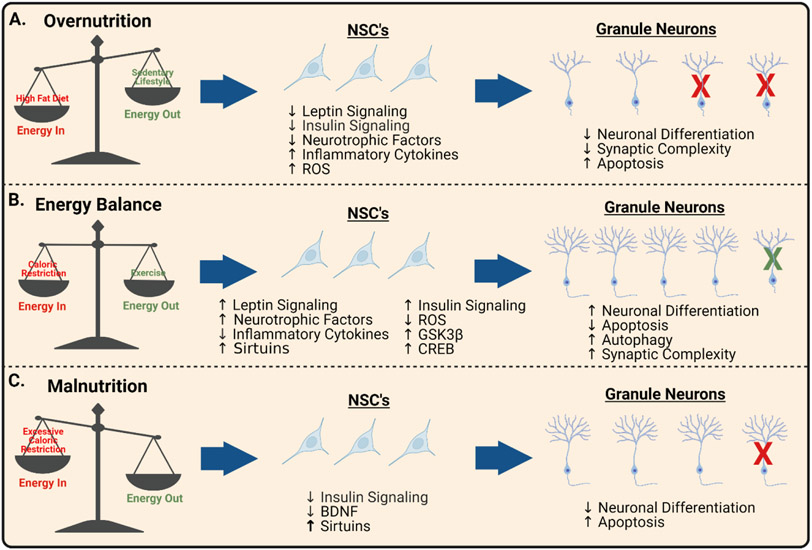
Figure 1. Energy Balance and Hippocampal Neurogenesis.
(A) Overnutrition due to excess caloric intake, lipotoxicity, and/or sedentary lifestyle results in decreased neuronal differentiation, reduced synaptic complexity, and increased apoptosis (red X’s) in hippocampal NSC’s. These impairments in AN are, in part, due to leptin and insulin resistance, decreased neurotrophic factors, increased inflammatory cytokines, and increased oxidative damage in the neurogenic niche. (B) Energy balance is promoted by moderate caloric restriction and physical exercise, resulting in augmented neuronal differentiation, increased autophagy (green X’s), improved synaptic complexity, and reduced apoptosis in newborn hippocampal neurons. These neurogenic benefits are mediated, in part, due to increased leptin and insulin signaling, neurotrophic factors, stress resistance, sirtuins, GSK3β signaling, and CREB signaling. (C) Malnutrition due to excessive caloric restriction or poor nutrient intake results in inadequate substrate availability, reduced neuronal differentiation, and increased apoptosis (red X’s) in the hippocampus. These impairments in AN are, in part, due to reduced insulin signaling, decreased BDNF, overactive sirtuins, and excessive cellular stress. Created with BioRender.com.
Overall, there is a clear relationship between whole body energy status, AN, and newborn neuron survival, although the molecular mechanisms underlying this phenomenon are incompletely understood. The following review analyzes the literature investigating energy status-mediated regulation of AN in the hippocampus, highlights the neurocircuitry and intracellular signaling involved, and proposes impactful future directions in the field.
2. Adult Hippocampal Neurogenesis and Circuitry:
In adult humans, 700 new neurons are formed in each hippocampus daily, accounting for an annual turnover of approximately 1.75% [3]. These newborn neurons originate from self-renewing and multipotent NSC’s in the SGZ between the granule cell (GC) layer and the hilus. Briefly, when radial NSC’s transition from the quiescent to activated state, they commit to self-renewal [19], gliogenesis [20], or neurogenesis [4,20]. Rodent studies reveal that within the first week of neuronal fate specification, radial NSC’s become Type II intermediate progenitors and then Type III neuroblasts, which are transition cells to postmitotic immature neurons [21]. In week 2, immature granule cells extend dendrites to the molecular layer and migrate to the GC layer [21]. During these first couple weeks, gamma aminobutyric acid (GABA) uniquely elicits depolarizing effects on NSC’s and immature neurons due to high expression of the NKCC1 Na+/K+/Cl− cotransporter maintaining high intracellular Cl− concentrations [22]. Consequently, tonic GABA released from local parvalbumin interneurons and synaptic GABAergic inputs have been shown to be driving factors to progenitor differentiation and neuroblast integration [22,23].
Two to three weeks after activation, newborn neurons undergo glutamatergic and synaptic integration [24,25]. While approximately 50% of the newborn neurons die during integration, the remaining send primarily glutamatergic outputs to the CA3 region and the hilus to form synapses with hilar interneurons, mossy cells, and CA3 pyramidal cells [26]. Additionally, the immature neurons begin to receive presynaptic afferents from the cortex and are capable of generating action potentials [1]. During week 3, GABA begins to elicit hyperpolarizing effects on granule cells due to reduced NKCC1 expression and increased KCC2 K+/Cl− cotransporter expression maintaining low intracellular Cl− concentrations [27]. Finally, weeks 4-6 of newborn neuron are characterized by increased excitability and plasticity [28,29], during which, optogenetic inhibition of these neurons impairs spatial and contextual learning [28,29].
Ultimately, it takes approximately 8 weeks for newborn hippocampal neurons to reach maturity in rodents [30]. Functional DG neurons receive sensory input from the perforant path of the entorhinal cortex and deliver excitatory signals to the CA3 hippocampal region via non-myelinated mossy fibers [31]. This intra-hippocampal circuitry, termed the “trisynaptic circuit” then sends long-distance projections to a plethora of brain regions including the medial septum [32], entorhinal cortex [32], prefrontal cortex [33], striatum [34], septum [35], amygdala [36], and insular cortex [36]. Interference with these connections significantly impairs performance in a multitude of learning-related tasks [37,38].
3. Metabolic Characteristics Drive NSC Fate:
Quiescent NSC’s rely primarily on glycolysis [39] and fatty acid oxidation [40], while active NSC’s are characterized by increased mitochondrial oxidative phosphorylation and lipogenesis (Figure 2) [41]. These distinct characteristics are largely dictated by metabolic gene signatures, which are critical to their fate [42]. For example, disturbed lipogenesis in mice via NSC-specific fatty acid synthase deletion promotes NSC quiescence and impairs AN [43]. Furthermore, NSC quiescence appears to be regulated by genes related to fat metabolism, such as Spot14, which reduces malonyl CoA availability, and subsequently reduces lipogenic substrate and promotes fatty acid oxidation [43]. On the contrary, dysfunction of fatty acid oxidation genes, such as Trimethyllysine Hydroxylase Epsilon, Carnitine Palmitoyltransferase I, or PPARδ, promotes NSC differentiation, but sacrifices renewal and results in NSC pool depletion [44,45]. These studies demonstrate the importance of homeostatic lipid metabolism in NSC’s to healthy AN and may provide insight into the relationship between dyslipidemia observed in metabolic disease states and neurodegenerative disorders.
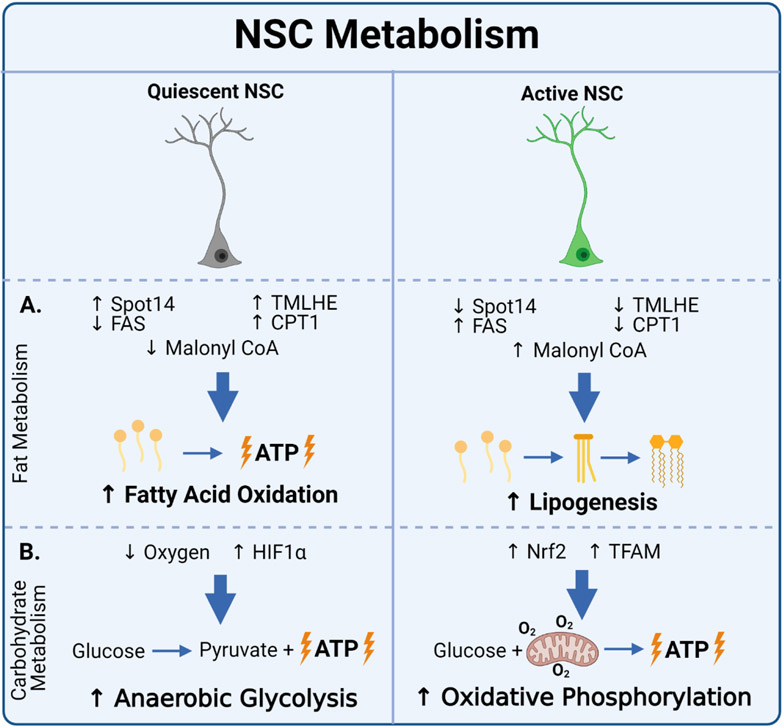
Figure 2. NSC Metabolism.
(A) Fat metabolism: Quiescent NSC’s exhibit increased fatty acid oxidation (characterized by increased Spot14 expression, TMLHE expression, and CPT1 activity, and decreased FAS expression and malonyl CoA levels). Active NSC’s exhibit increased lipogenesis (characterized by decreased Spot14 expression, TMLHE expression, and CPT1 activity, and increased FAS expression and malonyl CoA levels). Spot14 = thyroid hormone response protein 14; TMLHE = trimethyllysine hydroxylase epsilon; CPT1 = Carnitine palmitoyltransferase I; FAS = fatty acid synthase;. (B) Carbohydrate metabolism: Quiescent NSC’s regulated by HIF1α rely primarily on anaerobic glycolysis. Active NSC’s exhibit primarily oxidative phosphorylation, characterized by elevated TFAM and NRF2 expression. HIF1α = hypoxia-inducible factor 1α; Nrf2 = nuclear response factor 2; TFAM = mitochondrial transcription factor A. Created with BioRender.com.
Mitochondrial function in NSC’s is also critical to their differentiation and survival [46]. Ablation of mitochondrial transcription factor A in NSC’s impairs AN, while treatment with the mitochondrial function enhancer piracetam has opposite effects [46]. Mechanistically, disrupted mitochondrial function in the hippocampus increases reactive oxygen species (ROS), which increases activity of the transcription factor nuclear factor erythroid 2–related factor 2 (nrf2) [47]. Nrf2 then inhibits the self-renewal-favoring Notch signaling pathway, resulting in aberrant differentiation and NSC pool depletion [47]. Overall, both mitochondrial function and substrate metabolism are key driving factors in NSC fate, and manipulation of these metabolic phenomena has been a promising focus of research into homeostatic AN. The following sections overview how changes in energy status, through dietary interventions or physical activity, can alter the metabolic environment and subsequently modulate AN.
4. Overnutrition and AN:
4.1. Metabolic disorders are associated with impaired AN
Epidemiological studies have established a strong relationship between metabolic disease states, such as obesity and diabetes, and neurodegenerative pathologies [14,15]. Additionally, many studies have used high-fat-diet (HFD), leptin-deficient, or other genetic rodent models of metabolic disease and observed impaired AN and accelerated age-related cognitive deficits [48-62]. Even short term high fat or palmitic acid rich diets (2-4 weeks) impair AN, independent from changes in body weight, implicating lipotoxicity as an important factor in HFD-mediated hippocampal pathologies [58,63]. Particularly, saturated fats have been shown to have greater detrimental effects than unsaturated [61,64].
Despite the clear relationship between metabolic disease states and impaired AN, the mechanisms underlying this phenomenon are complex. While total NSC’s remain unchanged, most studies observe diminished proliferative capacity in obese/diabetic rodents [48,54,57,62,63]; however, in some cases hyperproliferation is observed [50,51,53]. The discrepancies in these findings may be due to differences in metabolic disease models used, which vary in onset and cause of metabolic dysfunction, as well as animal age during observations. More consistently, models of metabolic disease exhibit increased apoptosis [49,53,59], decreased neurotrophic factors [55,56], and decreased synaptic complexity in the DG and CA1 regions of the hippocampus [52,60].
4.2. Hormonal resistances likely contribute to metabolic disease related impairments in AN
Considering metabolic disease states often are accompanied by insulin resistance [14], insensitivity to insulin may play a causal role in metabolic disease-related AN impairments. As a result, focus has been put into the connection between insulin resistance and neurodegenerative diseases, even sometimes referring to Alzheimer’s Disease as a “Type 3 Diabetes” [14]. For example, studies in cell culture and rodent models have demonstrated that insulin and insulin-like growth factors 1 and 2 (IGF1 and IGF2) are critical to hippocampal AN [10,65-68], memory [69,70], learning [69,70], and long term potentiation [70]. Insulin, IGF1, and IGF2 directly stimulate hippocampal NSC proliferation and neuronal differentiation via multiple downstream signaling pathways including PI3kinase→AKT and MAPKK signaling [10,65-70]. For example, insulin-mediated PI3kinase→AKT signaling inhibits quiescence-inducing forkhead box O (FOXO) transcriptional activity [71]. Additionally, insulin activates mammalian target of rapamycin (mTOR) to negatively regulate NSC autophagy and stimulate AN [72-75]. Interestingly, glycogen synthase kinase 3 (GSK3) is inhibited by insulin, but GSK3α and GSK3β both promote AN by negatively regulating β catenin and Notch1 transcription of hairy and enhancer of Split1 (hes-1). Despite being susceptible to negative regulation by insulin, C/EBPβ and GSK3β are activated specifically by IGF2 and IGFR2 signaling to stimulate AN [70], while genetic knockdown of GSK3 results in hyperproliferation and reduced neuronal differentiation [70]. These findings possibly demonstrate the existence of GSK3 hormesis, during which, a balance between insulin-mediated GSK3 inhibition and IGF2-mediated GSK3 activation is critical to homeostatic AN.
Insensitivity to the anorexigenic adipokine leptin also develops in metabolic disease states [76], and leptin protects hippocampal neurons from neurotrophic factor withdrawal, excitotoxic damage, and oxidative stress [77]. Via PI3kinase→AKT and janus kinase (JAK)→signal transducer and activator of transcription 3 (STAT3) signaling, leptin directly stimulates NSC proliferation and AN [12]. Through similar pathways, leptin increases expression of antioxidative and antiapoptotic genes superoxide dismutase (SOD) and bclxl, respectively, and stabilizes mitochondrial membrane potential [77]. Lastly, leptin increases NMDA receptor expression and calcium conductance in the hippocampus via MAPK, PI3K, and src kinase signaling mechanisms [78].
4.3. Other contributors to metabolic-disease related impairments in AN
Increased ROS and inflammatory cytokines also contribute to many metabolic-disease related hippocampal pathologies [59,63,79]. Overnutrition leads to increases in tumor-necrosis factor α (TNFα) and IκB/NF-κB -mediated hippocampal apoptosis [59,80], while increases in ROS result in oxidative damage, reduced proliferative capacity, and depleted NSC pool [47,55,81]. There are also likely many other factors contributing to impaired AN in metabolic disease states. Considering the increasing prevalence of metabolic disease today, and the clear connection to disordered hippocampal AN, determining the causal relationship between the two is critical. For example, perturbations in gut microbiota and subsequent effects on inflammation and AN is an emerging topic of research [82]. Additionally, since NSC fate is driven by specific substrate metabolism characteristics, hyperglycemia and dyslipidemia observed in metabolic disease states likely have direct impacts on AN [59,83,84]. Altering substrate availability in the neurogenic niche likely has drastic effects on metabolic flux, NSC fate, and hippocampal cell health, providing an interesting direction for future research.
4.4. Summary of overnutrition and AN
Those with diabetes or obesity have a greater risk of neurodegenerative disease and cognitive decline [14,15]; however, there remain many questions regarding the causal relationship between metabolic disease and disordered AN. There are mixed reports on the effects of energy surplus and metabolic disease on NSC proliferation, but studies consistently observe even acute overnutrition to elicit increased apoptosis [49,53,59], decreased neurotrophic factors [55,56], decreased synaptic complexity [52,60], and impaired AN in the hippocampus [48,54,57,62,63]. Mechanistically, diminished function of pro-neurogenic hormones such as insulin [71-75], IGF1/2 [70], and leptin [12] likely are directly related to the metabolic disease-related hippocampal pathologies (Figure 3). Increased cell damage from ROS and inflammation are also likely involved [59,63,79]; however, etiology of AN pathologies is multifaceted and more mechanistic insights are needed. Overall, despite increased awareness and scientific advancements, diabetes and obesity remain unrelenting epidemics. As a result, continued research into interventions promoting energy homeostasis, such as through diet and physical activity, is increasingly imperative.
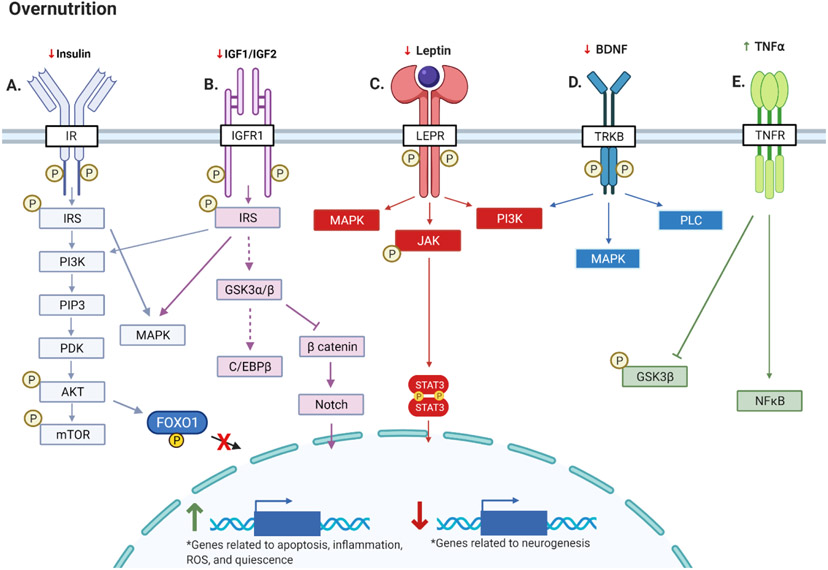
Figure 3. Overnutrition and AN.
Chronic energy surplus: (A) Results in resistance to insulin, which decreases mTOR activity and increases FOXO1 activity to reduce AN and promote quiescence, respectively; (B) Results in resistance to IGF1 and IGF2, which decreases GSK3β signaling and increases Notch signaling, to decrease AN and promote NSC renewal, respectively; (C) Results in resistance to leptin and decreased downstream neurogenic, anti oxidative, and antiapoptotic JAK→STAT3 signaling; (D) Decreases concentrations and activity of BDNF; (E) Increases TNFα, inflammatory pathways, and inhibition of GSK3β. Created with BioRender.com.
5. Caloric Restriction (CR) and AN:
5.1. Moderate CR improves AN and cognitive function
In humans, lifelong caloric intake positively correlates with incidence of neurodegenerative disease, and accumulating evidence suggests CR can improve cognitive function [85]. For example, one study demonstrated 30% CR for 3 months in older adults improves verbal memory [64], while another observed improvements in pattern separation after only 4 weeks CR (500kcal/day) [86]. These encouraging findings suggest a potential ability of CR to promote homeostatic AN in humans, a hypothesis that has been supported by many studies using animal models.
Briefly, 10-40% CR improves hippocampal AN [87,88], cognitive function [56,64,89,90], neuronal survivability [91,92], ROS buffering [93], autophagy [72], and anti-inflammatory capacity [87] in rodents and primates. Moreover, studies utilizing IF (alternating days of fasting and ad libitum food intake) demonstrate that even isocaloric intermittent CR elicits neurogenic effects independent from changes in total caloric intake or weight loss [86,94-98]. While the mechanisms underlying CR/IF-mediated neurogenesis are incompletely understood, in most cases increases in brain-derived neurotrophic factor (BDNF) [91,92,97,99-101], glial-derived neurotrophic factor (GDNF) [99], neurotrophin-3 [101], and/or fibroblast growth factor 2 [97] are observed, which all directly stimulate hippocampal AN. Particularly, the beneficial effects of CR on NSC proliferation are blunted in BDNF knockdown mice, highlighting the importance of this neurotrophic factor [100].
5.2. CR improves stress-resistance in hippocampal NSC’s via mTOR, sirtuins, and CREB
CR/IF and low substrate availability may pose a minor hormetic stress to NSC’s, and subsequently prime the neurogenic niche to better respond to future stressors [93]. For example, stress response mediators like heat shock protein 70 and glucose regulated protein 78 are elevated in response to CR, which protect NSC’s from excitotoxic and oxidative damage [93]. Low energy status is also characterized by low ATP and high AMP levels, and high intracellular AMP:ATP ratios improve autophagy by stimulating AMPK activity, subsequently inhibiting mTOR, and ultimately relieving inhibition of autophagy related genes [72,74]. Although homeostatic mTOR activity is important to AN (Section 4.2), overactive mTOR sacrifices NSC renewal, results in NSC pool depletion, increases ROS production, and impairs autophagy [74].
Intracellular reductions in NADH and subsequent increases in NAD+ are also consequences of low energy status. Sirtuins are deacetylases that sense these elevations in NAD+ and exhibit multiple pro-neurogenic functions [102-104], providing another mechanism through which the hippocampus can finely couple energy status to AN. Hippocampal SIRT1 expression enhances stress-resistance by deacetylating stress-related p53, NFκB, and FOXO proteins, which improves cell survival, inflammation, and microglia development [102-104]. Consequently, CR-mediated SIRT1 activation promotes dendritic development, NSC activation, and neuronal differentiation [102-104]. Mechanistically, SIRT1 activates cyclic AMP response element binding protein (CREB) signaling to induce stress resistance and hippocampal AN [105,106]. CREB is also activated by AMPK, calcium/calmodulin dependent protein kinases, protein kinase A, and protein kinase C, further coupling energy status to hippocampal AN regulation. Interestingly, the popular anti-diabetic drug metformin targets AMPK→PKC→CREB signaling and has been shown to promote both AN and NSC renewal in adult mice [107,108]. Despite this, long term metformin treatment in humans is associated with cognitive impairment and increased neurodegenerative disease risk, possibly demonstrating metformin hormesis [109,110]. These findings are further complicated by another study observing CREB-deficient mice have enhanced hippocampal AN [111].
The molecular mechanisms facilitating SIRT1 and CREB function regarding AN are dynamic in response to energy status and substrate availability, possibly explaining mixed findings in the literature [112]. While studies demonstrating the AN-inducing effects of SIRT1 are encouraging [102-104], other studies have observed SIRT1 activation to have opposite effects [113,114] or to promote glial differentiation [115]. These discrepancies are likely due to fluctuating functions of SIRT1 and CREB with changes in substrate availability. SIRT1 and CREB increase hes-1 expression to facilitate NSC self-renewal in hypoglycemic environments, but drive AN in hyperglycemic environments [112]. While these regulatory phenomena remain incompletely understood, they highlight how subtle changes in energy status and substrate availability in the neurogenic niche drastically change NSC fate and hippocampal health.
5.4. Malnutrition has detrimental effects on AN
While modest CR/IF elicits encouraging effects on AN and cognitive function, adequate energy and nutrient availability is essential to homeostatic AN. Some studies have shown CR/IF can lead to overactive SIRT1, anxiety, reduced BDNF levels, and impaired DG neurogenesis, especially in younger rodent models [56,116,117]. In extreme cases, IF even can result in premature death due to hypoglycemia in rodent amyloid precursor protein mutant models of Alzheimer’s Disease [118]. These studies highlight the existence of CR/IF hormesis, by underscoring the detrimental effects of insufficient energy intake and excessive cellular stress to hippocampal AN. They also suggest that energy balance, perhaps with energy deficit in moderation to prime stress responses, is likely the optimal strategy for homeostatic AN. For example, while excessive fat intake is harmful to AN (Section 4.1), inadequate intake of certain fats, such as omega 3 fatty acids, is equally as detrimental [119]. In fact, increased intake of omega 3 fatty acids is associated with attenuated cognitive decline in older populations, suggesting quality and nutrient content of energy intake is also important [120]. Overall, while IF/CR studies observe promising evidence promoting AN, caution should be taken to ensure adequate baseline intake of critical nutrients.
5.5. Summary of CR and AN
In summary, studies utilizing 10-40% CR, and even isocaloric IF, promote AN and cell survival in the hippocampus. While these dietary strategies may have indirect benefits due to weight loss, they also directly enhance ROS buffering capacities, autophagy, anti-inflammatory mechanisms, and neurotrophic factor activity. Additionally, they may elicit many of their beneficial effects by providing an acute stress to NSC’s, and subsequently improving responses to future stressors. Predominant molecular mechanisms underlying CR/IF-mediated AN include increased BDNF activity, sirtuin expression, and CREB signaling, as well as negative regulation of mTOR (Figure 4). Notably, excessive CR/IF can have deleterious effects on AN, likely due to inadequate energy intake, and increased cellular stress.
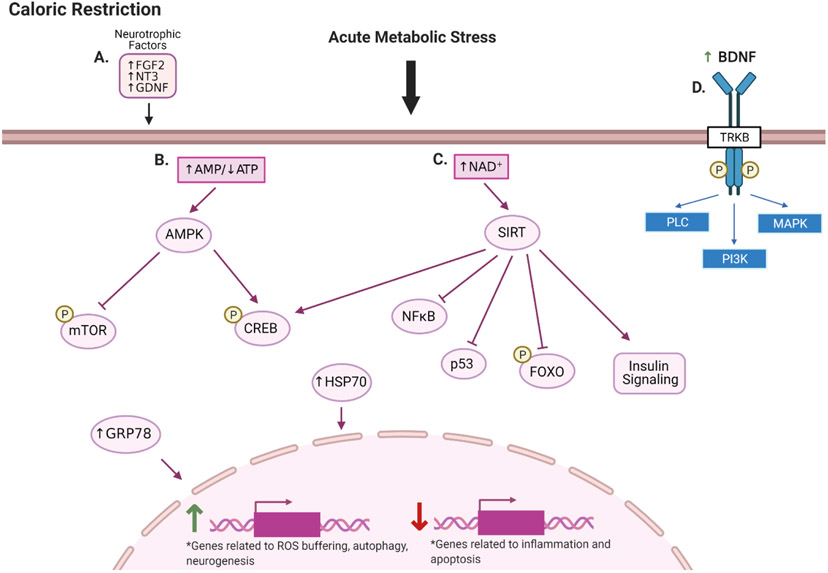
Figure 4. CR and AN.
Moderate caloric restriction: (A) Increases expression of neurotrophic factors; (B) Provides acute metabolic stress increasing the AMP/ATP ratio and activating AMPK. AMPK inhibits mTOR to promote autophagy and preserve the NSC pool and activates CREB which elicits neurogenic and neuroprotective effects. (C) Increases NAD+ levels and activates sirtuins. Sirtuins deacetylate and inhibit inflammatory, apoptotic, and quiescent signaling, and improve insulin signaling. (D) BDNF and its receptor TrkB are particularly important to CR-mediated AN. Created with BioRender.com.
6. Exercise and AN:
6.1. Aerobic exercise is neuroprotective and promotes AN
Aerobic exercise is another common method of creating energy deficit that has potent health benefits involving diverse physiological functions. For example, overwhelming evidence indicates aerobic exercise improves cognitive function, while decreasing risk of neurodegenerative disease [121-125]. Furthermore, aerobic exercise increases NSC proliferation, neuronal differentiation, newborn neuron survival, synaptic plasticity, and dendritic spine growth in healthy [126-129], aged [126,130,131], obese/diabetic [121], Alzheimer’s Disease [132-134], Parkinson’s [135], and Schizophrenia animal models [125]. While the benefits of exercise are well-documented, the mechanisms through which it promotes AN and hippocampal health are diverse. Aerobic exercise improves delivery of nutrients and energy substrates to the neurogenic niche via enhanced angiogenesis and increased blood-brain-barrier (BBB) permeability [136-138]. Particularly, the improved delivery of oxygen in response to exercise training prevents activation of anti-neurogenic hypoxia-inducible factors (HIF’s) [139]. Inadequate oxygen delivery stimulates HIF’s, which increase glycolytic genes to favor NSC quiescence and stimulate Notch→hes signaling to favor NSC renewal rather than neurogenesis [139].
Similar to CR hormesis, the acute stress of exercise also elicits a plethora of neuroprotective effects improving health of NSC’s and survivability of newborn neurons. Anti-inflammatory effects and improved immune function are demonstrated by reductions in interleukin 1β and TNF-α, as well as increases in IL6, IL10, interferon gamma, and macrophage inflammatory protein 1-alpha [124,140,141]. Anti-oxidative effects include increased anti-oxidative proteins like thioredoxin, superoxide dismutase, glutathione reductase, glutathione peroxidase, and glutathione transferase [121]. Additionally, one study determined β2 adrenergic signaling was essential to exercise-mediated upregulation of some of these anti oxidative proteins [121].
6.2. Aerobic exercise increases activity of neurotrophic factors in the hippocampus
Aerobic exercise also directly promotes hippocampal AN and synaptic plasticity through expression of various factors like BDNF, GDNF, synaptophysin, and postsynaptic density protein 95 [121,135,142]. Acute increases in core temperature after exercise may also directly stimulate VEGF and hippocampal AN [143]. Specifically, an important hippocampal signaling axis involving peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α)-mediated transcription of fibronectin type III domain-containing protein 5 (FNDC5) and subsequent BDNF activity has been identified in the process of exercise-induced AN [142,144]. Knockout or pharmacological inhibition of PGC1α or the BDNF receptor tropomyosin receptor kinase B (TrkB), abolishes the hippocampal benefits of exercise [142,144]. Furthermore, FNDC5 facilitates release of the myokine irisin during exercise, which, through unknown receptors, promotes synaptic plasticity, increases AN, and improves cognitive function [13,145].
As mentioned previously (Section 4.2), impaired leptin and insulin sensitivity are hallmarks of metabolic disease, and preservation of these hormones’ functions is critical to hippocampal AN. Aerobic exercise training increases leptin concentrations and leptin receptor expression in the hippocampus, while leptin-deficient mice experience resistances to exercise’s cognitive benefits [146]. Aerobic exercise also improves insulin/IGF1 signaling, which has been shown to directly stimulate DG AN, increase BDNF, and improve cognitive function [147,148]. However, the exercise-mediated insulin signaling mechanisms are complicated and have produced mixed findings in the DG. For example, 10 days voluntary wheel running increases phosphorylation of AKT which results in inhibitory phosphorylation of GSK3β and FOXO1 to promote NSC self-renewal and neuron survivability [149]. Conversely, another study demonstrates 30 days voluntary wheel running stimulates DG AN by decreasing inhibitory phosphorylation of GSK3β, independent from insulin signaling and possibly via dopamine→cAMP→PKA signaling [148]. Notably, these two studies utilized different experimental timelines, and insulin-dependent and insulin-independent effects of exercise on GSK3β activity are intriguing. These results support the hypothesis of GSK3β hormesis and suggest the existence of an evolutionarily beneficial mechanism promoting homeostatic AN post-exercise. More specifically, insulin mediated inhibition of GSK3β post-exercise may be an essential negative feedback mechanism to prevent aberrant GSK3β-mediated AN and NSC depletion. Overall, these studies reveal exercise simultaneously promotes insulin-dependent hippocampal NSC survival and insulin-independent, GSK3β-mediated, AN.
6.3. Aerobic exercise modulates diverse micro-RNA’s (miRNA’s)
Recent evidence suggests a potent ability of aerobic exercise to modulate expression of diverse miRNA’s to alter hippocampal gene expression. These miRNA-induced gene expression changes favor AN, NSC proliferation, and cell survival through regulation of various pathways including IP3, insulin, and NFκB signaling [150-152]. Improta-Caria et al. reviews this emerging research focus in detail, and highlights commonalities among physiological functions of miRNA’s subject to regulation by exercise [153]. Prominent physiological functions associated with exercise-mediated miRNA changes include: fatty acid metabolism, ubiquitin-mediated proteolysis, endocytosis, cell growth and division, apoptosis, p53 signaling, insulin signaling, endoplasmic reticulum protein processing, and transforming growth factor β signaling [153]. Moreover, that review identifies many specific miRNA’s that are pathologically altered in Alzheimer’s Disease, but may be rescued in response to exercise, therefore, demonstrating promising therapeutic potential for exercise in Alzheimer’s Disease at the post-transcriptional level [153].
6.4. Summary of exercise and AN
Convincing evidence demonstrates exercise promotes hippocampal AN and neuron survivability to enhance cognitive function. In response to exercise, increased angiogenesis and BBB permeability facilitate adequate nutrient and energy substrate availability for the neurogenic niche, while improved antioxidative, immune, and anti-inflammatory capacities enhance newborn neuron health (Figure 5). Aerobic exercise also increases function of many neurotrophic factors and other neurogenic proteins including BDNF, GDNF, irisin, insulin/IGF’s, leptin, and GSK3β. Additionally, studies have identified a potent ability of exercise to modulate gene expression through regulation of various miRNA’s.
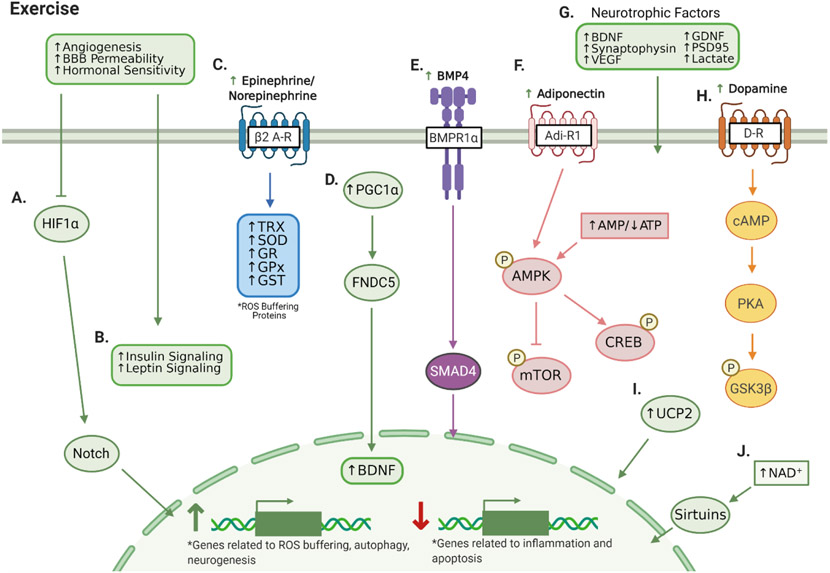
Figure 5. Exercise and AN.
Aerobic exercise improves angiogenesis, BBB permeability, and hormonal sensitivity, (A) Enhancing oxygen availability which inhibits HIF1α and downstream Notch signaling to reduce glycolytic gene expression and promote AN. (B) Improved angiogenesis and hormonal sensitivity also increases insulin and leptin signaling. (C) Exercise-mediated β2 adrenergic receptor signaling increases expression of ROS buffering proteins thioredoxin (TRX), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx), and glutathione-S-transferase (GST). (D) Exercise-induced PGC1α stimulates FNDC5, which is critical to increasing BDNF. (E) Exercise increases circulating bone morphogenic protein 4, which binds to hippocampal bone morphogenic protein receptor 1α to stimulate neurogenic transcriptional activity of SMAD4. (F) Exercise increases adiponectin levels and AMP/ATP ratios to activate AMPK. AMPK inhibits mTOR to promote NSC renewal and autophagy and activates CREB to promote stress resistance and AN. (G) Exercise increases expression and activity of a plethora of neurotrophic factors. (H) Exercise stimulates dopamine→cAMP→PKA signaling to increase GSK3β activity. (I) The neuroprotective effects of exercise are greatly blunted in UCP2 knockout mice. (J) Exercise increases NAD+ levels, which stimulates the neurogenic and neuroprotective effects of sirtuins. Created with BioRender.com.
Overall, the neurogenic effects of exercise are well-documented; however, further research is necessary to comprehensively map out the complex, multifaceted physiological mechanisms involved. Many additional molecular mediators have been identified (Figure 5) including: 1) vascular endothelial growth factor [154], 2) Uncoupling protein 2 [155], 3) Bone morphogenic protein 4 (BMP4)→SMAD1/5/8 transcriptional activity [156,157], 4) Adiponectin→AMPK signaling [158], and 5) Lactate [159]. Moreover, while most studies have focused solely on aerobic exercise, recent evidence indicates resistance exercise may elicit similar hippocampal benefits [160,161]. In summary, there are clear benefits of exercise to hippocampal AN, and further identification of its many complicated mechanisms of action could be valuable to designing exercise mimetics in the future.
7. Future Directions: Hippocampal – Hypothalamic Connections:
Considering the accumulating evidence suggesting hippocampal AN dynamically fluctuates with energy status, the hypothalamus may be an intriguing brain region for future research. The hypothalamus is a critical control center regulating energy balance and substrate metabolism [162], and many hormones/neuropeptides involved in hippocampal AN exhibit dual functions regulating modulating hypothalamic neuron activity to regulate metabolism. For example, while BDNF, insulin, leptin, and cholecystokinin, stimulate hippocampal AN, they also regulate hypothalamic neurons to decrease food intake, increase energy expenditure, and reduce circulating glucose levels [163-165]. Surprisingly, the orexigenic hormone ghrelin also stimulates hippocampal AN via PI3kinase→AKT, ERK, and STAT3 signaling [166]. These studies imply the existence of a regulatory mechanism between energy status and hippocampal AN, in which both hunger and satiety hormones promote AN.
The synaptic connections between hypothalamic and hippocampal neurons remain relatively underexplored, although prominent neuron populations are co-expressed in these regions. For example, neuropeptide Y/agouti-related peptide (NPY/AgRP) -expressing neurons in the arcuate nucleus (ARC) of the hypothalamus stimulate food intake and reduce energy expenditure, while NPY receptors (Y1 receptors) are also expressed in the hippocampus and promote DG AN [167,168]. A recent report even observed optogenic stimulation of ARC NPY/AgRP neurons to improve memory in mice [169]. Furthermore in the hypothalamus, pro-opiomelanocortin-expressing (POMC) neurons synapse with melanocortin 4 receptor (MC4R)-expressing neurons to induce satiety, while intra-hippocampal POMC→MC4R circuitry regulates synaptic plasticity and cognitive function in mouse models of Alzheimer’s Disease [170].
Overall, commonalities in regulatory hormones/neuropeptides in the hippocampus and the hypothalamus suggest the existence of a complex circuitry between these regions. Synaptic connections between the hypothalamus and the hippocampus could allow for nutrient/energy sensing by hypothalamic neurons bordering the CSF in the third ventricle and subsequent communication with the hippocampus to finely regulate neurogenesis. Further complicating this system is the recently discovered prevalence of neurogenesis in the hypothalamus, which is critical to homeostatic metabolic regulation and therefore indirectly important to hippocampal AN [171]. Regulation of hypothalamic neurogenesis is a controversial topic, but studies suggest, similar to in the hippocampus, BDNF and exercise promote this process, while chronic overnutrition is detrimental [172-174]. Thus, the hippocampus and hypothalamus are likely intricately connected regarding function, regulation, and neurocircuitry; however, this concept remains relatively unexplored.
8. Closing Remarks:
Hippocampal AN is finely modulated by changes in substrate availability, nutrient access, and metabolic hormones, establishing a connection between metabolic and cognitive health. Consequently, metabolic disease and chronic overnutrition are highly associated with increased neurodegenerative disease risk, highlighting the value of metabolic interventions promoting homeostatic hippocampal AN. Promising dietary interventions include moderate CR and IF, which not only improve metabolic function, but directly promote homeostatic hippocampal AN. Exercise has also demonstrated a potent capacity to promote health AN, suggesting a combination between dietary and exercise interventions could yield optimal results. Overall, these interventions yield encouraging neurogenic effects; however, the mechanisms underlying their effects remain incompletely understood. Continued research into the complex neurocircuitry connecting energy status and hippocampal AN is critical to establishing exercise and diet mimetics as therapeutic tools for metabolic and neurodegenerative diseases.
HIGHLIGHTS:
Specific metabolic characteristics drive neural stem cell fate
Circulating metabolic factors couple energy status with hippocampal neurogenesis
Overnutrition perturbs hippocampal neurogenesis and exacerbates apoptosis
Caloric restriction and exercise promote homeostatic neurogenesis
ACKNOWLEDGEMENTS
Funding: The funding for this project was provided by East Carolina University start up and the National Institute of Diabetes and Digestive and Kidney Disease (DK121215) to HH.
ABBREVIATIONS:
- AN
adult neurogenesis
- NSC
neural stem cell
- GC
granule cell
- SGZ
subgranular zone
- SVZ
subventricular zone
- BBB
blood-brain barrier
- IGF
insulin-like growth factor
- GABA
gamma aminobutyric acid
- DG
dentate gyrus
- nrf2
nuclear factor erythroid 2–related factor 2
- HFD
high fat diet
- MAPKK
mitogen-activated protein kinase kinase
- GSK3
glycogen synthase kinase 3
- C/EBP
CCAAT-enhancer-binding proteins
- FOXO
forkhead box O
- SOD
superoxide dismutase
- CR
caloric restriction
- IF
intermittent fasting
- BDNF
brain-derived neurotrophic factor
- GDNF
glial-derived neurotrophic factor
- TNFα
tumor necrosis factor α
- ROS
reactive oxygen species
- CREB
cyclic AMP response element binding protein
- HIF1α
hypoxia-inducible factor α
- PGC1α
proliferator-activated receptor gamma coactivator 1-alpha
- FNDC5
fibronectin type III domain-containing protein 5
- TrkB
tropomyosin receptor kinase B
- NPY/AgRP
neuropeptide Y/agouti-related peptide
- POMC
proopiomelanocortin
- ARC
arcuate nucleus
- ERK
extracellular-regulate kinase
- JAK
janus kinase
- STAT3
signal transducer and activator of transcription 3
- hes-1
hairy and enhancer of Split1
- mTOR
mammalian target of rapamycin
- miRNA
micro RNA
- MC4R
melanocortin 4 receptor
- CSF
cerebrospinal fluid
Footnotes
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Van Praag H, Schinder AF, Christle BR, Toni N, Palmer TD, Gage FH, Functional neurogenesis in the adult hippocampus, Nature. 415 (2002) 1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee H, Thuret S, Adult Human Hippocampal Neurogenesis: Controversy and Evidence, Trends Mol. Med 24 (2018) 521–522. doi: 10.1016/j.molmed.2018.04.002. [DOI] [PubMed] [Google Scholar]
- [3].Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J, Dynamics of hippocampal neurogenesis in adult humans, Cell. 153 (2013) 1219. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH, Neurogenesis in the adult human hippocampus, Nat. Med 4 (1998) 1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [5].Christian KM, Song H, Ming GL, Functions and dysfunctions of adult hippocampal neurogenesis, Annu. Rev. Neurosci 37 (2014) 243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Deng W, Aimone JB, Gage FH, New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory?, Nat. Rev. Neurosci 11 (2010) 339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Aimone JB, Deng W, Gage FH, Resolving New Memories: A Critical Look at the Dentate Gyrus, Adult Neurogenesis, and Pattern Separation, Neuron. 70 (2011) 589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Song J, Olsen RHJ, Sun J, Ming GL, Song H, Neuronal circuitry mechanisms regulating adult mammalian neurogenesis, Cold Spring Harb. Perspect. Biol 8 (2016). doi: 10.1101/cshperspect.a018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kang E, Wen Z, Song H, Christian KM, Ming GL, Adult neurogenesis and psychiatric disorders, Cold Spring Harb. Perspect. Biol 8 (2016). doi: 10.1101/cshperspect.a019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Åberg MAI, Åberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS, IGF-I has a direct proliferative effect in adult hippocampal progenitor cells, Mol. Cell. Neurosci 24 (2003) 23–40. doi: 10.1016/S1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- [11].Li E, Chung H, Kim Y, Kim DH, Ryu JH, Sato T, Kojima M, Park S, Ghrelin directly stimulates adult hippocampal neurogenesis: Implications for learning and memory, Endocr. J 60 (2013) 781–789. doi: 10.1507/endocrj.EJ13-0008. [DOI] [PubMed] [Google Scholar]
- [12].Garza JC, Guo M, Zhang W, Lu XY, Leptin increases adult hippocampal neurogenesis in vivo and in vitro, J. Biol. Chem 283 (2008) 18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, Gonçalves RA, Clarke JR, Beckman D, Staniszewski A, Berman H, Guerra LA, Forny-Germano L, Meier S, Wilcock DM, de Souza JM, Alves-Leon S, Prado VF, Prado MAM, Abisambra JF, Tovar-Moll F, Mattos P, Arancio O, Ferreira ST, De Felice FG, Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models, Nat. Med 25 (2019) 165–175. doi: 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kandimalla R, Thirumala V, Reddy PH, Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal, Biochim. Biophys. Acta - Mol. Basis Dis 1863 (2017) 1078–1089. doi: 10.1016/j.bbadis.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Craft S, Watson GS, Insulin and neurodegenerative disease: Shared and specific mechanisms, Lancet Neurol. 3 (2004) 169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- [16].Zainuddin MSA, Thuret S, Nutrition, adult hippocampal neurogenesis and mental health, Br. Med. Bull 103 (2012) 89–114. doi: 10.1093/bmb/lds021. [DOI] [PubMed] [Google Scholar]
- [17].Heberden C, Modulating adult neurogenesis through dietary interventions, Nutr. Res. Rev 29 (2016) 163–171. doi: 10.1017/S0954422416000081. [DOI] [PubMed] [Google Scholar]
- [18].Saraulli D, Costanzi M, Mastrorilli V, Farioli-Vecchioli S, The Long Run: Neuroprotective Effects of Physical Exercise on Adult Neurogenesis from Youth to Old Age, Curr. Neuropharmacol 15 (2017) 519–533. doi: 10.2174/1570159x14666160412150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H, In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics, Cell. 145 (2011) 1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J, Identification of a neural stem cell in the adult mammalian central nervous system, Cell. 96 (1999) 25–34. doi: 10.1016/S0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- [21].Zhao C, Deng W, Gage FH, Mechanisms and Functional Implications of Adult Neurogenesis, Cell. 132 (2008) 645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- [22].Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming GL, Song H, GABA regulates synaptic integration of newly generated neurons in the adult brain, Nature. 439 (2006) 589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, Ming GL, Song H, Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus, Nat. Neurosci 16 (2013) 1728–1730. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA, Short-term and long-term survival of new neurons in the rat dentate gyrus, J. Comp. Neurol 460 (2003) 563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- [25].Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH, NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus, Nature. 442 (2006) 929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- [26].Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF, Neurons born in the adult dentate gyrus form functional synapses with target cells, Nat. Neurosci 11 (2008) 901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K, The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system, Neuroscience. 104 (2001) 933–946. doi: 10.1016/S0306-4522(01)00149-X. [DOI] [PubMed] [Google Scholar]
- [28].Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S, Optical controlling reveals time-dependent roles for adult-born dentate granule cells, Nat. Neurosci 15 (2012) 1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ge S, hao Yang C, sen Hsu K, li Ming G, Song H, A Critical Period for Enhanced Synaptic Plasticity in Newly Generated Neurons of the Adult Brain, Neuron. 54 (2007) 559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhao C, Teng EM, Summers RG, Ming GL, Gage FH, Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus, J. Neurosci 26 (2006) 3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Amaral DG, Scharfman HE, Lavenex P, The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies), Prog. Brain Res 163 (2007) 3–790. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yuan M, Meyer T, Benkowitz C, Savanthrapadian S, Ansel-Bollepalli L, Foggetti A, Wulff P, Alcami P, Elgueta C, Bartos M, Somatostatin-positive interneurons in the dentate gyrus of mice provide local- and long-range septal synaptic inhibition, Elife. 6 (2017). doi: 10.7554/elife.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thierry AM, Gioanni Y, Dégénétais E, Glowinski J, Hippocampo-prefrontal cortex pathway: Anatomical and electrophysiological characteristics, Hippocampus. 10 (2000) 411–419. doi:. [DOI] [PubMed] [Google Scholar]
- [34].Albouy G, King BR, Maquet P, Doyon J, Hippocampus and striatum: Dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation, Hippocampus. 23 (2013) 985–1004. doi: 10.1002/hipo.22183. [DOI] [PubMed] [Google Scholar]
- [35].Müller C, Remy S, Septo–hippocampal interaction, Cell Tissue Res. 373 (2018) 565–575. doi: 10.1007/s00441-017-2745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ghaziri J, Tucholka A, Girard G, Boucher O, Houde JC, Descoteaux M, Obaid S, Gilbert G, Rouleau I, Nguyen DK, Subcortical structural connectivity of insular subregions, Sci. Rep 8 (2018). doi: 10.1038/s41598-018-26995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang GW, Cai JX, Reversible disconnection of the hippocampal-prelimbic cortical circuit impairs spatial learning but not passive avoidance learning in rats, Neurobiol. Learn. Mem 90 (2008) 365–373. doi: 10.1016/j.nlm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- [38].Wang GW, Cai JX, Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats, Behav. Brain Res 175 (2006) 329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- [39].Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M, Hypoxia requires Notch signaling to maintain the undifferentiated cell state, Dev. Cell 9 (2005) 617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [40].Stoll EA, Makin R, Sweet IR, Trevelyan AJ, Miwa S, Horner PJ, Turnbull DM, Neural stem cells in the adult subventricular zone oxidize fatty acids to produce energy and support neurogenic activity, Stem Cells. 33 (2015) 2306–2319. doi: 10.1002/stem.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cavallucci V, Fidaleo M, Pani G, Neural Stem Cells and Nutrients: Poised Between Quiescence and Exhaustion, Trends Endocrinol. Metab 27 (2016) 756–769. doi: 10.1016/j.tem.2016.06.007. [DOI] [PubMed] [Google Scholar]
- [42].Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, Song H, Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis, Cell Stem Cell. 17 (2015) 360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Knobloch M, Braun SMG, Zurkirchen L, Von Schoultz C, Zamboni N, Araúzo-Bravo MJ, Kovacs WJ, Karalay Ö, Suter U, MacHado RAC, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S, Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis, Nature. 493 (2013)226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xie Z, Jones A, Deeney JT, Hur SK, Bankaitis VA, Inborn Errors of Long-Chain Fatty Acid β-Oxidation Link Neural Stem Cell Self-Renewal to Autism, Cell Rep. 14 (2016) 991–999. doi: 10.1016/j.celrep.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP, A PML-PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance, Nat. Med 18 (2012) 1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Beckervordersandforth R, Ebert B, Schäffner I Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, Friedland K, Steib K, von Wittgenstein J, Keiner S, Redecker C, Hölter SM, Xiang W, Wurst W, Jagasia R, Schinder AF, li Ming G, Toni N, Jessberger S, Song H, Lie DC, Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis, Neuron. 93 (2017) 560–573.e6. doi: 10.1016/j.neuron.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, Sesaki H, Lagace DC, Germain M, Harper ME, Park DS, Slack RS, Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program, Cell Stem Cell. 19 (2016) 232–247. doi: 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- [48].Bracke A, Domanska G, Bracke K, Harzsch S, van den Brandt J, Bröker B, von Bohlen Und Halbach O, Obesity Impairs Mobility and Adult Hippocampal Neurogenesis., J. Exp. Neurosci 13 (2019) 1179069519883580. doi: 10.1177/1179069519883580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rotermund C, Truckenmüller FM, Schell H, Kahle PJ, Diet-induced obesity accelerates the onset of terminal phenotypes in α-synuclein transgenic mice, J. Neurochem 131 (2014) 848–858. doi: 10.1111/jnc.12813. [DOI] [PubMed] [Google Scholar]
- [50].Hamilton A, Patterson S, Porter D, Gault VA, Holscher C, Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain, J. Neurosci. Res 89 (2011) 481–489. doi: 10.1002/jnr.22565. [DOI] [PubMed] [Google Scholar]
- [51].Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F, Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats, Exp. Neurol 222 (2010) 125–134. doi: 10.1016/j.expneurol.2009.12.022. [DOI] [PubMed] [Google Scholar]
- [52].Beauquis J, Roig P, De Nicola AF, Saravia F, Short-Term Environmental Enrichment Enhances Adult Neurogenesis, Vascular Network and Dendritic Complexity in the Hippocampus of Type 1 Diabetic Mice, PLoS One. 5 (2010) e13993. doi: 10.1371/journal.pone.0013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lang BT, Yan Y, Dempsey RJ, Vemuganti R, Impaired neurogenesis in adult type-2 diabetic rats, Brain Res. 1258 (2009) 25–33. doi: 10.1016/j.brainres.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W, Lee IS, Won MH, Seong JK, Yoon YS, Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type ii diabetes, Neurochem. Res 34 (2009) 1039–1046. doi: 10.1007/s11064-008-9870-y. [DOI] [PubMed] [Google Scholar]
- [55].Park HR, Park M, Choi J, Park KY, Chung HY, Lee J, A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor, Neurosci. Lett 482 (2010) 235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- [56].Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet J-L, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP, Sex-Dependent Metabolic, Neuroendocrine, and Cognitive Responses to Dietary Energy Restriction and Excess, Endocrinology. 148 (2007) 4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Robison LS, Albert NM, Camargo LA, Anderson BM, Salinero AE, Riccio DA, Abi-Ghanem C, Gannon OJ, Zuloaga KL, High-fat diet-induced obesity causes sex-specific deficits in adult hippocampal neurogenesis in mice, ENeuro. 7 (2020). doi: 10.1523/ENEURO.0391-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C, High-fat diet impairs hippocampal neurogenesis in male rats, Eur. J. Neurol 13 (2006) 1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- [59].van der Borght K, Köhnke R, Göransson N, Deierborg T, Brundin P, Erlanson-Albertsson C, Lindqvist A, Reduced neurogenesis in the rat hippocampus following high fructose consumption, Regul. Pept 167 (2011) 26–30. doi: 10.1016/j.regpep.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [60].Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP, Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats, Hippocampus. 18 (2008) 1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Winocur G, Greenwood CE, Studies of the effects of high fat diets on cognitive function in a rat model, in: Neurobiol. Aging, Elsevier Inc., 2005: pp. 46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [62].Bonds JA, Shetti A, Stephen TKL, Bonini MG, Minshall RD, Lazarov O, Deficits in hippocampal neurogenesis in obesity-dependent and -independent type-2 diabetes mellitus mouse models, Sci. Rep 10 (2020) 16368. doi: 10.1038/s41598-020-73401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Park HR, Kim JY, Park KY, Lee J, Lipotoxicity of palmitic acid on neural progenitor cells and hippocampal neurogenesis, Toxicol. Res 27 (2011) 103–110. doi: 10.5487/TR.2011.27.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Witte AV, Fobker M, Gellner R, Knecht S, Flöel A, Caloric restriction improves memory in elderly humans, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rafalski VA, Brunet A, Energy metabolism in adult neural stem cell fate, Prog. Neurobiol 93 (2011) 182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [66].Bracko O, Singer T, Aigner S, Knobloch M, Winner B, Ray J, Clemenson GD, Suh H, Couillard-Despres S, Aigner L, Gage FH, Jessberger S, Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis, J. Neurosci 32 (2012) 3376–3387. doi: 10.1523/JNEUROSCI.4248-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ziegler AN, Schneider JS, Qin M, Tyler WA, Pintar JE, Fraidenraich D, Wood TL, Levison SW, IGF-II promotes stemness of neural restricted precursors, Stem Cells. 30 (2012) 1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Åberg MAI, Åberg ND, Hedbäcker H, Oscarsson J, Eriksson PS, Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus, J. Neurosci 20 (2000) 2896–2903. doi: 10.1523/jneurosci.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Soto M, Cai W, Konishi M, Kahn CR, Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 6379–6384. doi: 10.1073/pnas.1817391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM, A critical role for IGF-II in memory consolidation and enhancement, Nature. 469 (2011) 491–499. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Renault VM, Rafalski VA, Morgan AA, Salih DAM, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A, FoxO3 Regulates Neural Stem Cell Homeostasis, Cell Stem Cell. 5 (2009) 527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yu S-W, Baek S-H, Brennan RT, Bradley CJ, Park SK, Lee YS, Jun EJ, Lookingland KJ, Kim E-K, Lee H, Goudreau JL, Kim SW, Autophagic Death of Adult Hippocampal Neural Stem Cells Following Insulin Withdrawal, Stem Cells. 26 (2008) 2602–2610. doi: 10.1634/stemcells.2008-0153. [DOI] [PubMed] [Google Scholar]
- [73].Bai X, Wey MC-Y, Fernandez E, Hart MJ, Gelfond J, Bokov AF, Rani S, Strong R, Rapamycin improves motor function, reduces 4-hydroxynonenal adducted protein in brain, and attenuates synaptic injury in a mouse model of synucleinopathy, Pathobiol. Aging Age-Related Dis 5 (2015) 28743. doi: 10.3402/pba.v5.28743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Licausi F, Hartman NW, Role of mTOR complexes in neurogenesis, Int. J. Mol. Sci 19 (2018). doi: 10.3390/ijms19051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang J, Ji F, Liu Y, Lei X, Li H, Ji G, Yuan Z, Jiao J, Ezh2 regulates adult hippocampal neurogenesis and memory, J. Neurosci 34 (2014) 5184–5199. doi: 10.1523/JNEUROSCI.4129-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS, Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action, Nat. Med 1 (1995) 1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- [77].Guo Z, Jiang H, Xu X, Duan W, Mattson MP, Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization, J. Biol. Chem 283 (2008) 1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- [78].Shanley LJ, Irving AJ, Harvey J, Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity., J. Neurosci 21 (2001) RC186–RC186. doi: 10.1523/jneurosci.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D, Hypothalamic IKKβ/NF-κB and ER Stress Link Overnutrition to Energy Imbalance and Obesity, Cell. 135 (2008) 61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira ALR, Saad MJ, Velloso LA, High-Fat Diet Induces Apoptosis of Hypothalamic Neurons, PLoS One. 4 (2009) e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Limoli CL, Giedzinski E, Baure J, Rola R, Fike JR, Altered growth and radiosensitivity in neural precursor cells subjected to oxidative stress, Int. J. Radiat. Biol 82 (2006) 640–647. doi: 10.1080/09553000600887816. [DOI] [PubMed] [Google Scholar]
- [82].Cox AJ, West NP, Cripps AW, Obesity, inflammation, and the gut microbiota, Lancet Diabetes Endocrinol. 3 (2015)207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- [83].Gold PE, Glucose and age-related changes in memory, in: Neurobiol. Aging, Elsevier Inc., 2005: pp. 60–64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [84].Ferreiro E, Lanzillo M, Canhoto D, Carvalho da Silva AM, Mota SI, Dias IS, Ferreira IL, Fontes AR, Mastrella G, Pinheiro P, Valero J, Rego AC, Chronic hyperglycemia impairs hippocampal neurogenesis and memory in an Alzheimer’s disease mouse model, Neurobiol. Aging 92 (2020) 98–113. doi: 10.1016/j.neurobiolaging.2020.04.003. [DOI] [PubMed] [Google Scholar]
- [85].Luchsinger JA, Tang MX, Shea S, Mayeux R, Caloric intake and the risk of Alzheimer disease, Arch. Neurol 59 (2002) 1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- [86].Kim C, Pinto AM, Bordoli C, Buckner LP, Kaplan PC, Del Arenal IM, Jeffcock EJ, Hall WL, Thuret S, Energy restriction enhances adult hippocampal neurogenesis-associated memory after four weeks in an adult human population with central obesity; a randomized controlled trial, Nutrients. 12 (2020) 638. doi: 10.3390/nu12030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wu P, Shen Q, Dong S, Xu Z, Tsien JZ, Hu Y, Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice, Neurobiol. Aging 29 (2008) 1502–1511. doi: 10.1016/j.neurobiolaging.2007.03.028. [DOI] [PubMed] [Google Scholar]
- [88].Kitamura T, Mishina M, Sugiyama H, Dietary restriction increases hippocampal neurogenesis by molecular mechanisms independent of NMDA receptors., Neurosci. Lett 393 (2006) 94–96. doi: 10.1016/j.neulet.2005.08.073. [DOI] [PubMed] [Google Scholar]
- [89].Halagappa VKM, Guo Z, Pearson M, Matsuoka Y, Cutler RG, LaFerla FM, Mattson MP, Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease, Neurobiol. Dis 26 (2007) 212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- [90].Stewart J, Mitchell J, Kalant N, The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes, Neurobiol. Aging 10 (1989) 669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- [91].Lee J, Duan W, Long JM, Ingram DK, Mattson MP, Dietary restriction increases the number of newly generated neural cells, and BDNF expression, in the dentate gyrus of rats, J. Mol. Neurosci 15 (2000) 99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- [92].Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M, Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice, Neurobiol. Aging 25 (2004) 333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- [93].Mattson MP, Duan W, Wan R, Guo Z, Prophylactic Activation of Neuroprotective Stress Response Pathways by Dietary and Behavioral Manipulations, NeuroRx. 1 (2004) 111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, Diwan A, Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury, Autophagy. 11 (2015) 1537–1560. doi: 10.1080/15548627.2015.1063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Fontán-Lozano Á, Sáez-Cassanelli JL, Inda MC, De Los Santos-Arteaga M, Sierra-Domínguez SA, López-Lluch G, Delgado-García JM, Carrión ÁM, Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor, J. Neurosci 27 (2007) 10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Michael Anson R, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP, Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R, Age and energy intake interact to modify cell stress pathways and stroke outcome, Ann. Neurol 67 (2010) 41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Baik S, Rajeev V, Fann DY, Jo D, Arumugam TV, Intermittent fasting increases adult hippocampal neurogenesis, Brain Behav. 10 (2020) e01444. doi: 10.1002/brb3.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK, Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lee J, Duan W, Mattson MP, Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice, J. Neurochem 82 (2002) 1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- [101].Lee J, Seroogy KB, Mattson MP, Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice, J. Neurochem 80 (2002) 539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- [102].Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM, Neuronal SIRT1 activation as a novel mechanism underlying the prevention of alzheimer disease amyloid neuropathology by calorie restriction, J. Biol. Chem 281 (2006) 21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- [103].Codocedo JF, Allard C, Godoy JA, Varela-Nallar L, Inestrosa NC, SIRT1 Regulates Dendritic Development in Hippocampal Neurons, PLoS One. 7 (2012) e47073. doi: 10.1371/journal.pone.0047073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y, Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS, Regulation of Neurogenesis in Adult Mouse Hippocampus by cAMP and the cAMP Response Element-Binding Protein, J. Neurosci 22 (2002) 3673–3682. doi: 10.1523/jneurosci.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kumar A, Singh N, Inhibitor of Phosphodiestearse-4 improves memory deficits, oxidative stress, neuroinflammation and neuropathological alterations in mouse models of dementia of Alzheimer’s Type, Biomed. Pharmacother 88 (2017) 698–707. doi: 10.1016/j.biopha.2017.01.059. [DOI] [PubMed] [Google Scholar]
- [107].Wang J, Gallagher D, Devito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD, Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation, Cell Stem Cell. 11 (2012) 23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- [108].Fatt M, Hsu K, He L, Wondisford F, Miller FD, Kaplan DR, Wang J, Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation, Stem Cell Reports. 5 (2015) 988–995. doi: 10.1016/j.stemcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Imfeld P, Bodmer M, Jick SS, Meier CR, Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: A population-based case-control study, J. Am. Geriatr. Soc 60 (2012) 916–921. doi: 10.1111/j.1532-5415.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- [110].Moore EM, Mander AG, Ames D, Kotowicz MA, Carne RP, Brodaty H, Woodward M, Boundy K, Ellis KA, Bush AI, Faux NG, Martins R, Szoeke C, Rowe C, Watters DA, Increased risk of cognitive impairment in patients with diabetes is associated with metformin, Diabetes Care. 36 (2013) 2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA, cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response, J. Neurosci 27 (2007) 7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fusco S, Leone L, Barbati SA, Samengo D, Piacentini R, Maulucci G, Toietta G, Spinelli M, McBurney M, Pani G, Grassi C, A CREB-Sirt1-Hes1 Circuitry Mediates Neural Stem Cell Response to Glucose Availability, Cell Rep. 14 (2016) 1195–1205. doi: 10.1016/j.celrep.2015.12.092. [DOI] [PubMed] [Google Scholar]
- [113].Saharan S, Jhaveri DJ, Bartlett PF, SIRT1 regulates the neurogenic potential of neural precursors in the adult subventricular zone and hippocampus, J. Neurosci. Res 91 (2013) 642–659. doi: 10.1002/jnr.23199. [DOI] [PubMed] [Google Scholar]
- [114].Park HR, Kong KH, Yu BP, Mattson MP, Lee J, Resveratrol inhibits the proliferation of neural progenitor cells and hippocampal neurogenesis, J. Biol. Chem 287 (2012) 42588–42600. doi: 10.1074/jbc.M112.406413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schröter F, Ninnemann O, Siegert E, Bendix I, Brüstle O, Nitsch R, Zipp F, Aktas O, Sirt1 contributes critically to the redox-dependent fate of neural progenitors, Nat. Cell Biol 10 (2008) 385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- [116].Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, Kendler KS, Chen X, Hettema JM, Van Den Oord EJ, Rubio JP, Guarente L, SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive, Cell. 147 (2011) 1459–1472. doi: 10.1016/jcell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cardoso A, Marrana F, Andrade JP, Caloric restriction in young rats disturbs hippocampal neurogenesis and spatial learning, Neurobiol. Learn. Mem 133 (2016) 214–224. doi: 10.1016/j.nlm.2016.07.013. [DOI] [PubMed] [Google Scholar]
- [118].Pedersen WA, Culmsee C, Ziegler D, Herman JP, Mattson MP, Aberrant stress response associated with severe hypoglycemia in a transgenic mouse model of Alzheimer’s disease, J. Mol. Neurosci 13 (1999) 159–165. doi: 10.1385/jmn:13:1-2:159. [DOI] [PubMed] [Google Scholar]
- [119].Freemantle E, Vandal M, Tremblay-Mercier J, Tremblay S, Blachère JC, Bégin ME, Thomas Brenna J, Windust A, Cunnane SC, Omega-3 fatty acids, energy substrates, and brain function during aging, Prostaglandins Leukot. Essent. Fat. Acids 75 (2006) 213–220. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [120].Van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D, Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study, Am. J. Clin. Nutr 85 (2007) 1142–1147. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- [121].Han TK, Leem YH, Kim HS, Treadmill exercise restores high fat diet-induced disturbance of hippocampal neurogenesis through β2-adrenergic receptor-dependent induction of thioredoxin-1 and brain-derived neurotrophic factor, Brain Res. 1707 (2019) 154–163. doi: 10.1016/j.brainres.2018.11.035. [DOI] [PubMed] [Google Scholar]
- [122].Hillman CH, Erickson KI, Kramer AF, Be smart, exercise your heart: Exercise effects on brain and cognition, Nat. Rev. Neurosci 9 (2008) 58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- [123].Adlard PA, Perreau VM, Pop V, Cotman CW, Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease, J. Neurosci 25 (2005) 4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Parachikova A, Nichol KE, Cotman CW, Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition, Neurobiol. Dis 30 (2008) 121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wolf SA, Melnik A, Kempermann G, Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia, Brain. Behav. Immun 25 (2011) 971–980. doi: 10.1016/j.bbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- [126].Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A, Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials, Psychosom. Med 72 (2010) 239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gomes da Silva S, Unsain N, Mascó DH, Toscano-Silva M, De Amorim HA, Silva Araújo BH, Simões PSR, Da Graça Naffah-Mazzacoratti M, Mortara RA, Scorza FA, Cavalheiro EA, Arida RM, Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats, Hippocampus. 22 (2012) 347–358. doi: 10.1002/hipo.20903. [DOI] [PubMed] [Google Scholar]
- [128].Van Praag H, Kempermann G, Gage FH, Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus, Nat. Neurosci 2 (1999) 266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- [129].Van Praag H, Christie BR, Sejnowski TJ, Gage FH, Running enhances neurogenesis, learning, and long-term potentiation in mice, Proc. Natl. Acad. Sci. U. S. A 96 (1999) 13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G, Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus, Neurobiol. Aging 27 (2006) 1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- [131].Trinchero MF, Herrero M, Schinder AF, Rejuvenating the Brain With Chronic Exercise Through Adult Neurogenesis, Front. Neurosci 13 (2019) 1000. doi: 10.3389/fnins.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mirochnic S, Wolf S, Staufenbiel M, Kempermann G, Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease, Hippocampus. 19 (2009) 1008–1018. doi: 10.1002/hipo.20560. [DOI] [PubMed] [Google Scholar]
- [133].Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G, Cognitive and Physical Activity Differently Modulate Disease Progression in the Amyloid Precursor Protein (APP)-23 Model of Alzheimer’s Disease, Biol. Psychiatry 60 (2006) 1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [134].Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, Van Praag H, Spiegelman BM, Gage FH, Tanzi RE, Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model, Science (80-. ) 361 (2018). doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I, Exercise exerts neuroprotective effects on Parkinson’s disease model of rats, Brain Res. 1310 (2010) 200–207. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- [136].Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA, An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Shanker Sharma H, Cervós-Navarro J, Kumar Dey P, Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats, Neurosci. Res 10 (1991) 211–221. doi: 10.1016/0168-0102(91)90058-7. [DOI] [PubMed] [Google Scholar]
- [138].Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT, Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats, Proc. Natl. Acad. Sci. U. S. A 87 (1990) 5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, Quaegebeur A, Boon R, Zhao H, Boeckx B, Chang J, Wu C, Le Noble F, Lambrechts D, Dewerchin M, Kuo CJ, Huttner WB, Carmeliet P, Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis, EMBO J. 35 (2016) 924–941. doi: 10.15252/embj.201592372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Liu Y, Chu JMT, Yan T, Zhang Y, Chen Y, Chang RCC, Wong GTC, Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer’s disease mice, J. Neuroinflammation 17 (2020) 4. doi: 10.1186/s12974-019-1653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW, Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid, J. Neuroinflammation 5 (2008) 13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF, TrkB Regulates Hippocampal Neurogenesis and Governs Sensitivity to Antidepressive Treatment, Neuron. 59 (2008) 399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Koyama Y, Mukuda T, Hamasaki S, Nakane H, Kaidoh T, Short-term Heat Exposure Promotes Hippocampal Neurogenesis via Activation of Angiotensin II Type 1 Receptor in Adult Rats, Neuroscience. 385 (2018) 121–132. doi: 10.1016/j.neuroscience.2018.05.045. [DOI] [PubMed] [Google Scholar]
- [144].Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM, Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway, Cell Metab. 18 (2013) 649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS, FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise, Metabolism. 61 (2012) 1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yook JS, Rakwal R, Shibato J, Takahashi K, Koizumi H, Shima T, Ikemoto MJ, Oharomari LK, McEwen BS, Soya H, Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory, Proc. Natl. Acad. Sci. U. S. A 166 (2019) 10988–10993. doi: 10.1073/pnas.1815197116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Trejo JL, Carro E, Torres-Alemán I, Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus, J. Neurosci 21 (2001) 1628–1634. doi: 10.1523/jneurosci.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zang J, Liu Y, Li W, Xiao D, Zhang Y, Luo Y, Liang W, Liu F, Wei W, Voluntary exercise increases adult hippocampal neurogenesis by increasing GSK-3β activity in mice, Neuroscience. 354 (2017) 122–135. doi: 10.1016/j.neuroscience.2017.04.024. [DOI] [PubMed] [Google Scholar]
- [149].Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, Davis S, Inhibition of PI3K-Akt Signaling Blocks Exercise-Mediated Enhancement of Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus, PLoS One. 4 (2009) e7901. doi: 10.1371/journal.pone.0007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Moon HY, Yoon KJ, Lee WS, Cho HS, Kim DY, Kim JS, Neural maturation enhanced by exercise-induced extracellular derivatives, Sci. Rep 10 (2020) 1–11. doi: 10.1038/s41598-020-60930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Li J, Wang H, MiR-15b reduces amyloid-β accumulation in SH-SY5Y cell line through targetting NF-κB signaling and BACE1, Biosci. Rep 38 (2018). doi : 10.1042/BSR20180051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Pons-Espinal M, Gasperini C, Marzi MJ, Braccia C, Armirotti A, Pötzsch A, Walker TL, Fabel K, Nicassio F, Kempermann G, De Pietri Tonelli D, MiR-135a-5p Is Critical for Exercise-Induced Adult Neurogenesis, Stem Cell Reports. 12 (2019) 1298–1312. doi: 10.1016/j.stemcr.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Improta-Caria AC, Nonaka CKV, Cavalcante BRR, De Sousa RAL, Júnior RA, de F. Souza BS, Modulation of microRNAs as a potential molecular mechanism involved in the beneficial actions of physical exercise in Alzheimer disease, Int. J. Mol. Sci 21 (2020) 1–35. doi: 10.3390/ijms21144977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD, VEGF is necessary for exercise-induced adult hippocampal neurogenesis, Eur. J. Neurosci 18 (2003)2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- [155].Dietrich MO, Andrews ZB, Horvath TL, Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation, J. Neurosci 28 (2008) 10766–10771. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Derynck R, Zhang YE, Smad-dependent and Smad-independent pathways in TGF-β family signalling, Nature. 425 (2003) 577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- [157].Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P, The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways, J. Biol. Chem 277 (2002) 5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- [158].Yaua SY, Lia A, Hooc RLC, Chingb YP, Christied BR, Leea TMC, Xuc A, Soa KF, Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 15810–15815. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A, Feldman N, Roichman A, Illouz T, Varvak A, Nicola R, Madar R, Okun E, L-lactate promotes adult hippocampal neurogenesis, Front. Neurosci 13 (2019). doi: 10.3389/fnins.2019.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Codina-Martínez H, Fernández-García B, Díez-Planelles C, Fernández ÁF, Higarza SG, Fernández-Sanjurjo M, Díez-Robles S, Iglesias-Gutiérrez E, Tomás-Zapico C, Autophagy is required for performance adaptive response to resistance training and exercise-induced adult neurogenesis, Scand. J. Med. Sci. Sports 30 (2020) 238–253. doi: 10.1111/sms.13586. [DOI] [PubMed] [Google Scholar]
- [161].Marston KJ, Brown BM, Rainey-Smith SR, Peiffer JJ, Resistance Exercise-Induced Responses in Physiological Factors Linked with Cognitive Health, J. Alzheimer’s Dis 68 (2019) 39–64. doi: 10.3233/JAD-181079. [DOI] [PubMed] [Google Scholar]
- [162].Andermann ML, Lowell BB, Toward a Wiring Diagram Understanding of Appetite Control., Neuron. 95 (2017) 757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Elmquist JK, Elias CF, Saper CB, From Lesions to Leptin: Review Hypothalamic Control of Food Intake and Body Weight, Neuron. 22 (1999) 221–232. [DOI] [PubMed] [Google Scholar]
- [164].Asrican B, Wooten J, Li YD, Quintanilla L, Zhang F, Wander C, Bao H, Yeh CY, Luo YJ, Olsen R, Lim SA, Hu J, Jin P, Song J, Neuropeptides Modulate Local Astrocytes to Regulate Adult Hippocampal Neural Stem Cells, Neuron. 108 (2020) 349–366.e6. doi: 10.1016/j.neuron.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Marosi K, Mattson MP, BDNF mediates adaptive brain and body responses to energetic challenges, Trends Endocrinol. Metab 25 (2014) 89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chung H, Li E, Kim Y, Kim S, Park S, Multiple signaling pathways mediate ghrelin-induced proliferation of hippocampal neural stem cells, J. Endocrinol 218 (2013) 49–59. doi: 10.1530/JOE-13-0045. [DOI] [PubMed] [Google Scholar]
- [167].Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB, Rapid, reversible activation of AgRP neurons drives feeding behavior in mice., J. Clin. Invest 121 (2011) 1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger AG, Gray WP, Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus, J. Neurochem 93 (2005) 560–570. doi: 10.1111/J.1471-4159.2005.03057.X. [DOI] [PubMed] [Google Scholar]
- [169].Zimmer MR, Schmitz AE, Dietrich MO, Activation of Agrp neurons modulates memory-related cognitive processes in mice, Pharmacol. Res 141 (2019) 303–309. doi: 10.1016/j.phrs.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Varela L, Horvath TL, Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis., EMBO Rep. 13 (2012) 1079–86. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Yoo S, Blackshaw S, Regulation and function of neurogenesis in the adult mammalian hypothalamus, Prog. Neurobiol 170 (2018) 53–66. doi: 10.1016/j.pneurobio.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Engel DF, Bobbo VCD, Solon CS, Nogueira GA, Moura-Assis A, Mendes NF, Zanesco AM, Papangelis A, Ulven T, Velloso LA, Activation of GPR40 induces hypothalamic neurogenesis through p38- and BDNF-dependent mechanisms, Sci. Rep 10 (2020) 11047. doi: 10.1038/s41598-020-68110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M, α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors, Nat. Commun 4 (2013) 1–13. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- [174].Laing BT, Huang H, Exercise improves hypothalamic function induced by high-fat diet, Immunoendocrinology. 3 (2016). doi: 10.14800/ie.1446. [DOI] [Google Scholar]