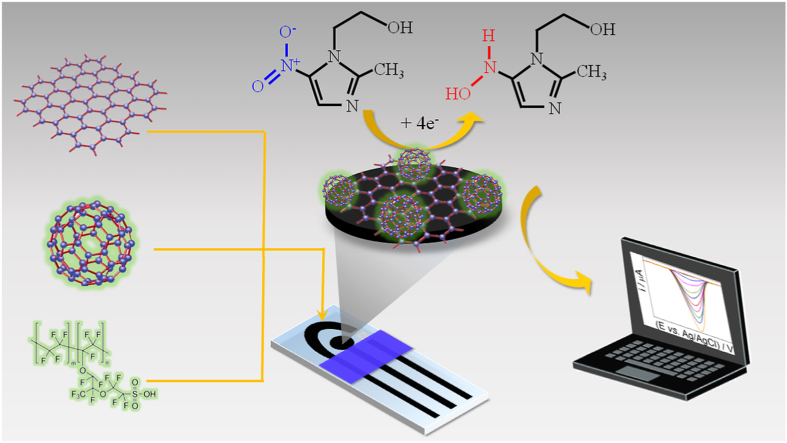
Abstract
Monitoring the concentration of antibiotics in body fluids is essential to optimizing the therapy and minimizing the risk of bacteria resistance, which can be made with electrochemical sensors tailored with appropriate materials. In this paper, we report on sensors made with screen-printed electrodes (SPE) coated with fullerene (C60), reduced graphene oxide (rGO) and Nafion (NF) (C60-rGO-NF/SPE) to determine the antibiotic metronidazole (MTZ). Under optimized conditions, the C60-rGO-NF/SPE sensor exhibited a linear response in square wave voltammetry for MTZ concentrations from 2.5 × 10−7 to 34 × 10−6 mol/L, with a detection limit of 2.1 × 10−7 mol/L. This sensor was also capable of detecting MTZ in serum and urine, with recovery between 94% and 100%, which are similar to those of the standard chromatographic method (HPLC-UV). Because the C60-rGO-NF/SPE sensor is amenable to mass production and allows for MTZ determination with simple principles of detection, it fulfills the requirements of therapeutic drug monitoring programs.
Keywords: Metronidazole, Fullerene, Reduced graphene oxide, Screen-printed electrodes, Antibiotic
Graphical abstract
Highlights
-
•
Determination of metronidazole (MTZ) in urine and synthetic serum sample.
-
•
The MTZ is a synthetic antibiotic widely used for surgical prophylaxis, in patients with burn wounds and other pathologies.
-
•
The detection of MTZ is relevant to minimize its side effects and avoid acquired resistance.
-
•
We employed fullerene C60-rGO and nafion in low-cost screen-printed electrode (SPE).
-
•
Electrochemical sensor is amenable to mass production.
1. Introduction
The alarming increase in bacteria resistance has brought failure to many treatments requiring antibiotics [1], which is especially worrying because introducing new drugs into the market is expensive and takes a long time. New approaches are therefore needed to preserve the efficacy of currently approved antibiotics [2], as exemplified by the therapeutic drug monitoring (TDM) program used in clinical practice to quantify concentrations of antibiotics and other drugs in body fluids [2,3]. With TDM combined with knowledge from pharmacokinetics, one may identify situations where an unnecessary amount of drug has been administered, and optimize the concentration which would inhibit bacterial growth [4,5]. An important requirement for the success of TDM is to develop low-cost, easy-to-use tests to quantify the drugs in body fluids. Today, this type of test is performed with expensive, time-consuming methods, including radioimmunoassays, high-performance liquid chromatography (HPLC), fluorescence polarization immunoassay, enzyme immunoassay, and enzyme-linked immunosorbant assay [[6], [7], [8], [9]]. In this context, electrochemical sensors and biosensors are strong candidates to fulfill the TDM requirements, for they have been proven excellent in the monitoring of antimicrobial drugs [[10], [11], [12]], in addition to the detection of antibiotics in water [10,13], food [14,15], and biological samples [16,17].
The development of efficient electrochemical sensors for TDM which are also of low cost, demands a judicious choice of materials, both for the electrodes and coating layers used in functionalization. From the large library of materials for this purpose, carbon nanomaterials (e.g., graphite, nanohorns, fullerenes, carbon nanotubes, graphene, carbon nanoparticles, and nanodiamonds) should be highlighted for their reproducible electrocatalytic responses, biocompatibility and enhanced electron transport [18,19]. In this study, we chose carbon ink to produce screen-printed electrode (SPE) amenable for mass production [20], which was modified by two other types of carbon nanomaterials, namely, reduced graphene oxide (rGO) and fullerene (C60) in order to leverage their intrinsic properties such as increased active area, suitability for immobilization of electrocatalytic compounds and easy fabrication [21]. Fullerenes have been widely used as nanomediator for sensors since they allow for operation at lower potentials, thus reducing the interference from electroactive compounds [[22], [23], [24], [25], [26], [27]].
We tested the suitability of the electrochemical sensors by determining the concentration of metronidazole (MTZ), a synthetic antibiotic to treat trichomoniasis, dysentery, liver abscesses, rosacea and anaerobically infected burn wounds, in addition to surgical prophylaxis [28,29]. For patients with burn wounds, in particular, sepsis is a major cause of morbidity and mortality due to the inability to maintain a sterile environment in the hospital and avoid contamination with microorganisms and their ensuing antibiotic resistance [2,30,31]. Detection of MTZ is also relevant to minimizing its side effects, which include nauseas, diarrhea, neurotoxicity, optic neuropathy, peripheral neurophaty and ancephatopathy. Furthermore, it has shown genotoxic effects in animal models [28]. The electrochemical sensors reported to determine MTZ were made with molecularly imprinted polymers [[32], [33], [34], [35], [36]], β-cyclodextrin-functionalized gold nanoparticles/poly(l-cysteine) [37], 3D hierarchical porous graphene/polythionine [38], Ni/Fe-layered double hydroxides [39], modified glassy carbon electrodes, multi-walled carbon nanotubes [40], composite film derived from cysteic acid, poly(diallydimethylammonium chloride)-functionalized graphene [41], and carbon paste electrode [[42], [43], [44]] in pharmaceutical drug tablets and fish tissue [32,33,40], and human blood serum [35]. In this work, we employed C60-rGO and Nafion, for the first time to the best of our knowledge, to modify a low-cost SPE.
2. Materials and methods
2.1. Reagents, materials, and apparatus
MTZ, clindamycin, dipyrone, tetracycline, diclofenac, ranitidine, uric acid, caffeine and C60 were purchased from Sigma-Aldrich (São Paulo, Brazil). Graphene was obtained from Graphene Supermarket (Calverton, MD, USA). A stock solution of MTZ at 1.0 × 10−2 mol/L was prepared by diluting 17.1 mg in a 10 mL capacity flask. The electrochemical measurements were performed with an Autolab potentiostat/galvanostat (model PGSTAT-30, Eco Chemie, Utrecht, The Netherlands) controlled by NOVA 2.1 software. The electrochemical system had a screen-printed sensor with three-electrodes: pseudo-reference electrode (Ag/AgCl), auxiliary electrode made of carbon ink and the working electrode with functionalized carbon ink (r=0.15 cm) connected with an auxiliary cable.
HPLC-UV analysis was made with a Shimadzu model 10ATvp LC system (San Francisco, CA, USA), consisting of two pumps (LC-10AT), column oven (CTO10A), and UV detector (SPD-10A). The mobile phase consisted of a 1.0 × 10−3 mol/L phosphate buffer (pH 7.0) solution and acetonitrile at the ratio of 95:5 (V/V) (filtered through a 0.22 μm pore membrane filter) at the temperature of 20 °C. The flow rate was 1 mL/min and detection was made at 320 nm. This analytical method was used for the comparison with the electrochemical sensor [45].
2.2. Fabrication and preparation of C60-rGO-NF/SPE sensors
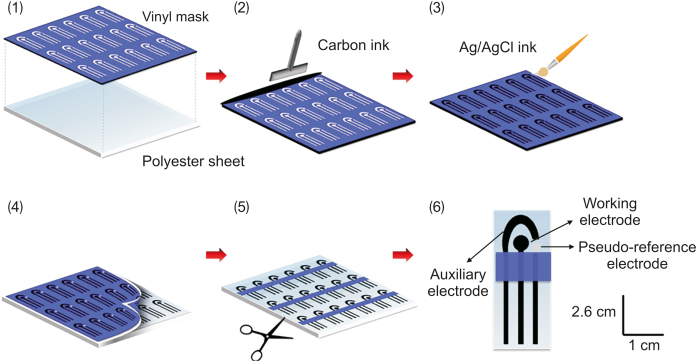
SPE were made as reported in the literature [46,47]. A negative mask with the SPE model was prepared in an adhesive vinyl polymeric material using Silhouette Studio version 2.7.4 software and an electronic craft cutter from Silhouette Cameo (Silhouette America, São Paulo, Brazil). Fig. 1 shows the main steps for preparing the electrode: (1) the vinyl mask was fixed on a polyester sheet (USA Folien Laserjet Clear A4 transparency film); (2) the carbon ink (C2160602D2 from Gwent Electronic Materials Ltd., São Paulo, Brazil) was deposited on the support with a plastic spatula and cured at 90 °C for 30 min; (3) the Ag/AgCl ink (C2051014P10, Gwent Electronic Materials Ltd., São Paulo, Brazil) was applied to the part corresponding to the pseudo-reference electrode, and then the ink was cured at 60 °C for 30 min; (4) removal of the vinyl mask; (5) delimitation of the geometric area of the working electrodes with a rectangular vinyl mask, followed by a heater press and (6) SPE for use.
Fig. 1.
Schematic representation of the preparation of screen-printed electrode (SPE).
To obtain an active sensor, the SPE was placed in an acid bath (0.5 mol/L H2SO4) under stirring for 1 min (for partial functionalization of the electrode surface). A suspension (2 mL) containing rGO (3 mg) and NF (50 μL, 0.5% V/V) was prepared and subjected to an ultrasonic bath for 20 min to yield a homogeneous dispersion. An aliquot of 3 μL of C60 solution prepared in CH2Cl2 was cast onto the electrode surface, and dried for 1 h. In the pretreatment process cyclic voltammetry (2 cycles) was used with the potential scanning from 0 to −1.5 V at a scan rate of 20 mV/s in a 1 mol/L KOH solution. Another potential scanning was carried out (550 to −50 mV) at a scan rate of 50 mV/s using the phosphate buffer solution (pH 7.0) with the goal of stabilizing the electrochemical sensor response [48].
2.3. Preparation of synthetic urine and serum samples
The synthetic urine sample was prepared by following the procedure reported by Laube et al. [49] using the compounds found in real samples: 49, 20, 10, 15, 18, 18 mmol/L of NaCl, KCl, CaCl2, KH2PO4, NH4Cl, and urea were added. The remaining volume of the flask was filled with ultrapure water. The synthetic serum sample was prepared as described by Parham and Zargar [50]. The flask volume was completed with ultrapure water. The total volume of the samples was 25 mL. After this step, the samples were spiked with two levels of concentration of MTZ and an aliquot of 250 μL was added separately in 10 mL of supporting electrolyte solution.
3. Results and discussion
3.1. Morphological characterization of the C60-rGO-NF/SPE sensor
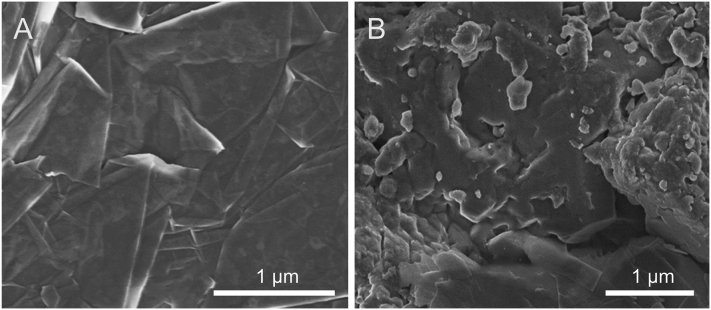
The scanning electron microscopy image in Fig. 2A is typical of rGO, with smooth multilayers in overlapping sheets with crumpled and wrinkled sheets. Fig. 2B shows a dense, uniform C60 film deposited on rGO, resulting from an evenly dispersed C60-rGO mixture which had been subjected to an ultrasound bath before modifying the surface electrode. NF is very diluted (0.01% V/V) and therefore it could not be visualized in a straightforward manner, though it may be responsible for some lighter shadows on the image.
Fig. 2.
Morphological characterization by scanning electron microscopy images of (A) reduced graphene oxide (rGO) and (B) C60-rGO.
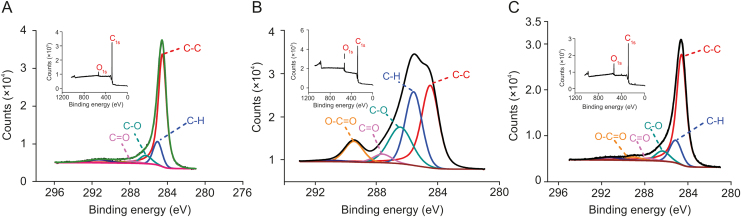
The chemical composition of graphene, GO and rGO was assessed with X-ray photoelectron spectroscopy (XPS). Figs. 3A and B display two well-defined binding energy peaks for graphene and GO at 285.4 eV assigned to C1s (graphene = 96.8% and GO = 87.3%) and at 530.9 eV due to O1s (graphene = 3.2% and GO = 12.7%, insets in Figs. 3A and B). With deconvolution of the XPS spectra of C1s, one may determine the degree of oxidation and the binding of carbon atoms: aromatic C–C bonds (carbon sp2, 284.5 eV); C–H (285.6 eV); C–O (ether/alcohol, 286.5 eV), C O (carbonyl, 287.6 eV), O–C O (carboxyl, 289.5 eV). There was a large difference in peak intensity between graphene and GO. For graphene the aromatic bond (C–C) peak was large, indicating the expected high degree of order of the hexagonal carbon structure. On the other hand, for GO there was an increase in the peaks related to carbon bonds with oxygenated groups (CO, C O and O–C O) and CH, with a consequent decrease in the peak related to aromatic C–C. This confirmed that oxygenated groups were inserted in the carbon structure of graphene. When GO was reduced using NaBH4, these groups were removed, and their corresponding peaks decreased significantly, as shown in Fig. 3C for rGO.
Fig. 3.
Evaluation of graphene functionalization by X-ray photoelectron spectroscopy analysis: (A) graphene, (B) GO and (C) rGO, and respective insets.
3.2. Catalytic activity from synergic effect between C60 and rGO
The analytic response of the C60-rGO-NF/SPE sensor was determined using cyclic voltammetry for a concentration of MTZ of 1.0 × 10−4 mol/L. Fig. 4 shows the cathodic peak due to reduction of MTZ on the electrode surface at −0.9 V vs. Ag/AgCl (3.0 mol/L KCl). This peak increased when the SPE electrode was modified with C60 and rGO, with the current for C60-rGO-NF/SPE being 5 times the value for SPE and 2.1 times the value for rGO/SPE. The increased current can be attributed to an increase in the porosity of these nanomaterials which increases the surface area of the electrode. The electrochemical reduction of MTZ involves four electrons according to reaction below [42].
| R–NO2+ 4e− + 4H+ → R–NHOH + H2O |
where 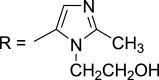 .
.
Fig. 4.
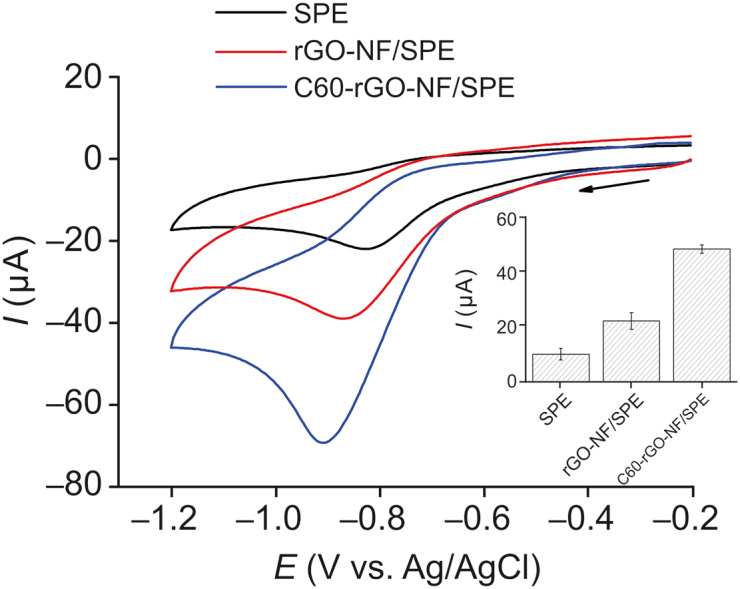
Cyclic voltammograms of 1.0 × 10−4 mol/L metronidazole (MTZ) in 0.1 mol/L phosphate buffer (pH 7.0) for bare SPE, rGO-NF/SPE and C60-rGO-NF/SPE. Scan rate=25 mV/s. NF: Nafion.
3.3. Effect of potential scan rate and pH
The presence of a cathodic peak and absence of anodic peaks in Fig. 5 indicate an irreversible redox process for MTZ on the C60-rGO-NF/SPE sensor. The cathodic peak shifted to a more negative potential and increased with the scan rate. The insets show that the cathodic peak current decreased linearly with the scan rate, with a correlation coefficient of 0.998. We also tried the v1/2 dependence but fitting was poorer, with a correlation coefficient of 0.967. Therefore, the MTX redox reaction was controlled by an adsorption process (rather than a diffusion process).
Fig. 5.
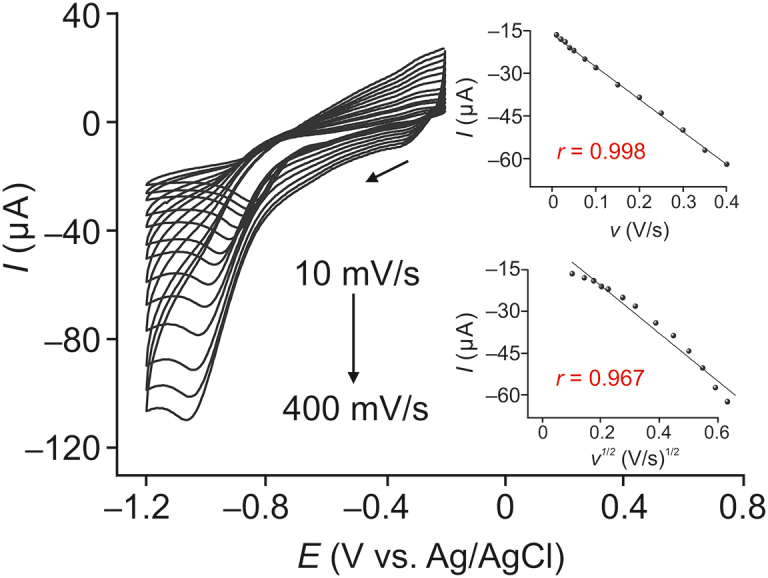
Effect of different scan rates on cyclic voltammograms at the C60-rGO-NF/SPE sensor in the presence of 5.0 × 10−5 mol/L MTZ in 0.1 mol/L phosphate buffer (pH 7.0).
Fig. S1 shows that the cathodic peak current for MTZ increased with the pH up to pH 7, above which it leveled off. This is why we selected pH 7 for subsequent experiments to determine MTZ with the C60-rGO-NF/SPE sensor. The potential at which the cathodic peak occurred increased with pH, as is typical of an irreversible electrochemical process. The equation for the Ep vs. pH in Fig. S1 is Ep=−0.4–0.062pH, where the slope of −0.062 mV/pH corresponds to an equal number of protons and electrons in the electrochemical reduction of MTZ [51].
3.4. Determination of MTZ using the C60-rGO-NF/SPE sensor
First, a comparison was made between differential pulse voltammetry and square wave voltammetry (SWV). From the analysis of peak current intensity and stability in the electrochemical signal, we inferred that SWV presented the best response. Thus, this technique was chosen for the detection and quantification studies of MTZ. The quantitative determination of MTZ was performed under optimized conditions for the C60-rGO-NF/SPE sensor. Fig. 6 shows a linear increase in the peak current with MTZ concentration in the range between 2.5 × 10−7 and 34 × 10−6 mol/L, with a regression equation Ip (μA) = 0.15 + 0.1CMTZ (μmol/L) (r=0.998). The limit of detection (LOD) was 2.1 × 10−7 mol/L, calculated using the statistical method described by da Silva and Machado [52], LOD = yB + 3SB, where yB is the intercept of the calibration plot used as the blank signal and SB is the standard deviation (obtained directly from the analytical curve). The concentrations for which the sensor works are clinically relevant as they correspond to serum concentrations from patients reported in the literature. For instance, after administering a single dose of 200 mg MTZ, the blood concentration varied from 5.8 × 10−7 mol/L to 2.8 × 10−6 mol/L within 24 h and with a renal excretion of 13%–46% [53]. In subsidiary experiments we verified that the sensor displayed a linear behavior for MTZ concentrations up to 10−6 mol/L (not shown). We also tested the accumulation potential (Eacc) at potentials 0, −0.2, and −0.4 V with an accumulation time of 30 s, and did not observe an appreciable increase in current. This is favorable for the electrochemical analysis, and Eacc was not used for determining MTZ in the SWV experiments.
Fig. 6.
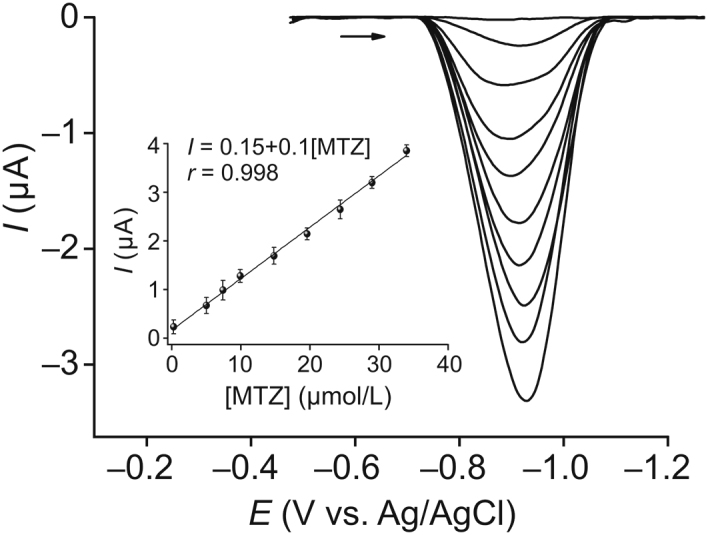
SWV at C60-rGO-NF/SPE sensor for different concentrations of MTZ in a phosphate buffer (pH=7.0) and analytical curve (inset). Parameters square wave voltammetry (SWV): f=15 Hz, A=75 mV, and ΔE=5 mV.
Table 1 shows that LOD and linear range for the C60-rGO-NF/SPE sensor are competitive with other electrochemical devices to determine MTZ in the literature [36,[38], [39], [40],[42], [43], [44],54]. Furthermore, this sensor is highly stable, providing repeatable results with small sample volumes. The whole methodology is also promising because it is amenable to mass production of electrodes at a low cost, which is essential for disposable devices.
Table 1.
Comparison of analytical results for the C60-rGO-NF/SPE sensor with other methods to determine metronidazole in the literature.
| Electrode sensor | Method | Linear range (μmol/L) | LOD (μmol/L) | Refs. |
|---|---|---|---|---|
| MIP/MWCNT/GCEa | CV | 1.2–20 | 0.00029 | [36] |
| 3D-HPG/PTH/GCEb | CV | 0.05–70 | 0.001 | [38] |
| Ni/Fe-LDH electrodec | Amperometry | 5.0–1610 | 58 | [39] |
| Polydopamine/MWCNTs–COOH nanocomposites/GCEd | DPV | 5–5000 | 0.25 | [40] |
| LDH/CQDs@CPEe | DPV | 1.5–300 | 0.2 | [42] |
| CPEf | SWV | 1–100 | 0.99 | [43] |
| CPE-CDg | DPVi | 0.5–103 | 0.28 | [44] |
| Carbon fibre microdisk electrode | SWVh | 1.0–2.2 | 0.5 | [54] |
| C60-rGO-NF/SPE | SWV | 0.25–34.0 | 0.21 | This work |
Molecularly imprinted polymer (MIP) and multi-walled carbon nanotubes (MWCNT) modified glassy carbon electrode (GCE).
Graphene-like carbon architecture and polythionine modified GCE.
Ni/Fe-layered double hydroxides (Ni/Fe-LDH) on the GCE.
Polydopamine and carboxylic MWCNT modified GCE.
LDH and carbon quantum dots (CQD)@carbon paste electrode.
Carbon paste electrode.
Electropolymerization of a-cyclodextrin on carbon paste electrode.
Square wave voltammetry.
Differential pulse voltammetry.
3.5. Interference and repeatability studies
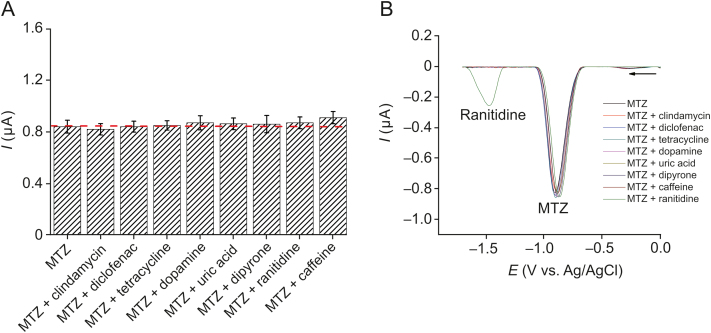
The repeatability of the electrochemical signal for MTZ using C60-rGO-NF/SPE sensor was evaluated in 0.1 mol/L phosphate buffer solution containing 5.0 × 10−6 mol/L MTZ in 14 measurements. The relative standard deviation (RSD) of the cathodic peak current was 3.6% (Fig. S2). When different electrodes were employed, RSD was 4.9% (for seven electrodes, i.e., n=7). Hence, the proposed sensor had a good repeatability. The influence of possible interferents in plasma and urine for MTZ determination was found to be negligible, as demonstrated in Fig. 7, when several drugs and substances were added to MTZ. There was no significant change in the MTZ analytical signal, when clindamycine, diclofenac, tetracycline, dopamine, uric acid, dipyrone, ranitidine and caffeine were tested in SWV experiments using a 1:1 concentration ratio (analyte:interference). In addition, no change in analytical signal was observed for a 1:10 concentration ratio (analyte:interference). The MTZ concentration used was 5.0 × 10−7 mol/L, while the interferent concentration was 5.0 × 10−6 mol/L.
Fig. 7.
(A) Study of interference with different drugs and (B) SWV graph.
3.6. Analytical applications to urine and serum samples
Proof-of-concept experiments were performed to determine MTZ in synthetic serum and urine samples. Table 2 shows the SWV results for the C60-rGO-NF/SPE sensor under the same conditions identified in the optimization process. Recovery of MTZ ranged from 94% to 100% with RSD of 3.3% in triplicate experiments for serum and urine samples. Significantly, the relative error in these results was within 10% of the values obtained with the standard HPLC method (Fig. S3).
Table 2.
Results from the analysis of synthetic urine and serum samples using C60-rGO-NF/SPE sensor under optimized conditions. The last column shows the relative error compared with the standard HPLC method.
| Matrices | Added (mol/L) | FoundProposed methoda (mol/L) | FoundComparative methoda (mol/L) | Recoveryb (sensor, %) | Relative errorc |
|---|---|---|---|---|---|
| Urine | 1.0 × 10−6 | (9.6 ± 0.1) × 10−7 | (9.5 ± 0.1) × 10−7 | 96 | −3.2 |
| 1.0 × 10−5 | (9.4 ± 0.2) × 10−6 | (9.4 ± 0.1) × 10−6 | 94 | 0 | |
| Serum | 1.0 × 10−6 | (1.0 ± 0.2) × 10−6 | (1.1 ± 0.1) × 10−6 | 100 | −9.1 |
| 1.0 × 10−5 | (9.5 ± 0.3) × 10−6 | (9.9 ± 0.1) × 10−6 | 95 | 4.0 |
Average of 3 concentrations.
Recovery percentage = (FoundProposed method/added) × 100.
Relative error = [(FoundProposed method − FoundComparative method)/FoundComparative method] × 100.
4. Conclusion
We have designed an electrochemical sensor that can be employed in therapeutic drug monitoring, for which distinct carbon nanomaterials were combined. The suitability of the methodology was demonstrated with the antibiotic MTZ, which could be determined with an LOD of 2.1 × 10−7 mol/L using the C60-rGO-NF/SPE sensor. Furthermore, this sensor could be applied to determine MTZ in urine and serum samples, with recoveries similar to those of the standard HPLC-UV technique. The method exhibits high stability, repeatability and reproducibility. The high performance of the sensor may be attributed to the synergy in electrocatalytic activity of C60 and rGO, as indicated by the results with cyclic voltammetry and SWV. The fast response and low cost of the electrochemical sensors require a small expenditure of materials and reagents. The materials, concepts, and methodology are generic and may be extended to other antibiotics and drugs, and this is promising for drug monitoring to fight bacteria resistance.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the financial support granted by CNPq, INEO, CAPES and FAPESP (Grant Nos.: 2018/22214-6, 2017/24053-7 and 2016/0991-5).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.03.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ventola C.L. The antibiotic resistance crisis Part 1 : causes and threats. Pharmacol. Ther. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 2.Fournier A., Eggimann P., Pantet O. Impact of real-time therapeutic drug monitoring on the prescription of antibiotics in burn patients requiring admission to the intensive care unit. Clin. Therapeut. 2018;62:1–17. doi: 10.1128/AAC.01818-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn D., Klaas C., Fobker M. Handbook of Analytical Separations. Vol. 7. Elsevier; Amsterdam: 2020. Therapeutic Drug Monitoring of Antibiotics in Critically Ill Patients; pp. 169–183. [Google Scholar]
- 4.Roberts J.A., Norris R., Paterson D.L. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2011;73:27–36. doi: 10.1111/j.1365-2125.2011.04080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabilat C., Gros M.F., Nicolau D. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:791–797. doi: 10.1007/s10096-019-03769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang J., Lee M.H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2019;24:1–10. doi: 10.3904/kjim.2009.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Companyó R., Granados M., Guiteras J. Antibiotics in food : legislation and validation of analytical methodologies. Anal. Bioanal. Chem. 2009;395:877–891. doi: 10.1007/s00216-009-2969-4. [DOI] [PubMed] [Google Scholar]
- 8.Darwish I.A. Immunoassay Methods and their applications in pharmaceutical analysis : basic methodology and recent advances. Int. J. Biomed. Sci. 2006;2:217–235. [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez-Fernández V., Dominguez-Vega E., Crego A.L. Recent advances in the analysis of antibiotics by CE and CEC. Electrophoresis. 2012;33:127–146. doi: 10.1002/elps.201100409. [DOI] [PubMed] [Google Scholar]
- 10.Pollap A., Kochana J. Electrochemical immunosensors for antibiotic detection. Biosensors. 2019;9:4325–4329. doi: 10.3390/bios9020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zacco E., Adrian J., Galve R. Electrochemical magneto immunosensing of antibiotic residues in milk. Biosens. Bioelectron. 2007;22:2184–2191. doi: 10.1016/j.bios.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Munteanu F.-D., Titoiu A.M., Marty J.-L. Detection of antibiotics and evaluation of antibacterial activity with screen-printed electrodes. Sensors. 2018;18:901–925. doi: 10.3390/s18030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Online V.A., Jacobs M., Nagaraj V.J. Analytical Methods an electrochemical sensor for the detection of antibiotic. Anal. Methods. 2013;5:4325–4329. [Google Scholar]
- 14.Stevenson H.S., Shetty S.S., Thomas N.J. Ultrasensitive and rapid-response sensor for the electrochemical detection of antibiotic residues within meat samples. ACS Omega. 2019;4:6324–6330. doi: 10.1021/acsomega.8b03534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Liu C., Sarpong V. Multisegment nanowire/nanoparticle hybrid arrays as electrochemical biosensors for simultaneous detection of antibiotics. Biosens. Bioelectron. 2019;126:632–639. doi: 10.1016/j.bios.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Elfiky M., Salahuddin N., Hassanein A. Detection of antibiotic Ofloxacin drug in urine using electrochemical sensor based on synergistic effect of different morphological carbon materials. Microchem. J. 2019;146:170–177. [Google Scholar]
- 17.Liu X., Huang D., Lai C. Recent advances in sensors for tetracycline antibiotics and their applications. Trac-trend. Anal. Chem. 2018;109:260–274. [Google Scholar]
- 18.Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014;43:744–764. doi: 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- 19.Zhang A., Lieber C.M. Nano-bioelectronics. Chem. Eng. Sci. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegrist J., Gorkin R., Bastien M. Validation of a centrifugal microfluidic sample lysis and homogenization platform for nucleic acidextraction with clinical samples. Lab Chip. 2010;10:363–371. doi: 10.1039/b913219h. [DOI] [PubMed] [Google Scholar]
- 21.Rowley-Neale S.J., Randviir E.P., Abo A.S. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today. 2018;10:218–226. [Google Scholar]
- 22.Kurbanoglu S., Ozkan S.A. Electrochemical carbon based nanosensors : a promising tool in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2018;147:439–457. doi: 10.1016/j.jpba.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 23.Yáñez-Sedeño P., Campuzano S., Pingarrón J.M. Fullerenes in electrochemical catalytic and affinity biosensing: a review. C-J. Carbon Res. 2017;3:21. [Google Scholar]
- 24.Afreen S., Muthoosamy K., Manickam S. Functionalized fullerene ( C 60 ) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 2015;63:354–364. doi: 10.1016/j.bios.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 25.Pilehvar S., De Wael K. Recent advances in electrochemical biosensors based on fullerene-C60 nano-structured platforms. Biosensors (Basel) 2015;5:712–735. doi: 10.3390/bios5040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shetti N.P., Malode S.J., Nandibewoor S.T. Electrochemical behavior of an antiviral drug acyclovir at fullerene-C60-modified glassy carbon electrode. Bioelectrochemistry. 2012;88:76–83. doi: 10.1016/j.bioelechem.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Goyal R.N., Gupta V.K., Bachheti N. Fullerene-C60-modified electrode as a sensitive voltammetric sensor for detection of nandrolone—an anabolic steroid used in doping. Anal. Chim. Acta. 2007;597:82–89. doi: 10.1016/j.aca.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Carlos J., Ledezma R. Therapeutic uses of metronidazole and its side effects : an update. Eur. Rev. Med. Pharmacol. Sci. 2019;23:397–401. doi: 10.26355/eurrev_201901_16788. [DOI] [PubMed] [Google Scholar]
- 29.Rao C.M., Ghosh A., Raghothama C. Does metronidazole reduce lipid peroxidation in burn injuries to promote healing? Burns. 2002;28:427–429. doi: 10.1016/s0305-4179(01)00126-7. [DOI] [PubMed] [Google Scholar]
- 30.Bracco D., Eggimann P. Prophylaxis with systemic antibiotics in patients with severe burns. Br. Med. J. 2010;340:10–12. doi: 10.1136/bmj.c208. [DOI] [PubMed] [Google Scholar]
- 31.Azimi L., Alaghehbandan R., Asadian M. Multi-drug resistant Pseudomonas aeruginosa and Klebsiella pneumoniae circulation in a burn hospital, Tehran, Iran. GMS Hyg. Infect. Control. 2019;14:1–5. doi: 10.3205/dgkh000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Liu Y., Yang Y. Novel electrochemical sensing platform based on a molecularly imprinted polymer decorated 3D nanoporous nickel skeleton for ultrasensitive and selective determination of metronidazole. ACS Appl. Mater. Interfaces. 2015;7:15474–15480. doi: 10.1021/acsami.5b03755. [DOI] [PubMed] [Google Scholar]
- 33.Song H., Zhang L., Yu F. Molecularly imprinted polymer functionalized nanoporous Au-Ag alloy microrod : novel supportless electrochemical platform for ultrasensitive and selective sensing of metronidazole. Electrochim. Acta. 2016;208:10–16. [Google Scholar]
- 34.Gu Y., Yan X., Li C. Biomimetic sensor based on molecularly imprinted polymer with nitroreductase-like activity for metronidazole detection. Biosens. Bioelectron. 2016;77:393–399. doi: 10.1016/j.bios.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 35.Ensafi A.A., Nasr-Esfahani P., Rezaei B. Metronidazole determination with an extremely sensitive and selective electrochemical sensor based on graphene nanoplatelets and molecularly imprinted polymers on graphene quantum dots. Sensor. Actuator. B Chem. 2018;270:192–199. [Google Scholar]
- 36.Liu Y., Liu J., Tang H. Fabrication of highly sensitive and selective electrochemical sensor by using optimized molecularly imprinted polymers on multi-walled carbon nanotubes for metronidazole measurement. Sensor. Actuator. B Chem. 2015;206:647–652. [Google Scholar]
- 37.Gu Y., Liu W., Chen R. β-cyclodextrin-functionalized gold nanoparticles/poly (L-cysteine) modified glassy carbon electrode for sensitive determination of metronidazole. Electroanalysis. 2013;25:1209–1216. [Google Scholar]
- 38.Yang M., Guo M., Yu Y. Sensitive voltammetric detection of metronidazole based on three-dimensional graphene-like carbon architecture/polythionine modified glassy carbon electrode. J. Electrochem. Soc. 2018;165:530–535. [Google Scholar]
- 39.Nejati K., Asadpour-Zeynali K. Electrochemical synthesis of nickel–iron layered double hydroxide: application as a novel modified electrode in electrocatalytic reduction of metronidazole. Mater. Sci. Eng. C. Mat. Bio. Appl. 2014;35:179–184. doi: 10.1016/j.msec.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Tursynbolat S., Bakytkarim Y., Huang J. Ultrasensitive electrochemical determination of metronidazole based on polydopamine/carboxylic multi-walled carbon nanotubes nanocomposites modified GCE. J. Pharm. Anal. 2018;8:124–130. doi: 10.1016/j.jpha.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W., Zhang J., Li C. A novel composite film derived from cysteic acid and PDDA-functionalized graphene: enhanced sensing material for electrochemical determination of metronidazole. Talanta. 2013;104:204–211. doi: 10.1016/j.talanta.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Alizadeh S., Hasanzadeh M. A novel electrochemical sensor based on LDH/CQD @ Carbon paste electrode for voltammetric determination of metronidazole in real Samples. Int. J. Sci. Eng. Res. 2018;9:466–475. [Google Scholar]
- 43.Nikodimos Y., Amare M. Electrochemical determination of metronidazole in tablet samples using carbon paste electrode. J. Anal. Methods Chem. 2016;2016:3612943. doi: 10.1155/2016/3612943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernández-Jiménez A., Roa-Morales G., Reyes-Pérez H. Voltammetric determination of metronidazole using a sensor based on electropolymerization of α-cyclodextrin over a carbon paste electrode. Electroanalysis. 2016;28:704–710. [Google Scholar]
- 45.Tashtoush B.M., Jacobson E.L., Jacobson M.K. Validation of a simple and rapid hplc method for determination of metronidazole in Dermatological. Drug Dev. Ind. Pharm. 2008;34:840–844. doi: 10.1080/03639040801928598. [DOI] [PubMed] [Google Scholar]
- 46.Afonso A.S., Uliana C.V., Martucci D.H. Simple and rapid fabrication of disposable carbon-based electrochemical cells using an electronic craft cutter for sensor and biosensor applications. Talanta. 2016;146:381–387. doi: 10.1016/j.talanta.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 47.De Oliveira R.A.G., Materon E.M., Melendez M.E. Disposable microfluidic immunoarray device for sensitive breast cancer Biomarker Detection. ACS Appl. Mater. Interfaces. 2017;9:27433–27440. doi: 10.1021/acsami.7b03350. [DOI] [PubMed] [Google Scholar]
- 48.Goyal R.N., Singh S.P. Voltammetric determination of paracetamol at C60 -modified glassy carbon electrode. Electrochim. Acta. 2006;51:3008–3012. [Google Scholar]
- 49.Laube N., Mohr B., Hesse A. Laser-probe-based investigation of the evolution of particle size distributions of calcium oxalate particles formed in artificial urines. J. Cryst. Growth. 2001;233:367–374. [Google Scholar]
- 50.Parham H., Zargar B. Determination of isosorbide dinitrate in arterial plasma , synthetic serum and pharmaceutical formulations by linear sweep voltammetry on a gold electrode. Talanta. 2001;55:255–262. doi: 10.1016/s0039-9140(01)00416-7. [DOI] [PubMed] [Google Scholar]
- 51.Bard A.J., Faulkner L.R. John Wiley & Sons, Inc; New York: 1980. Electrochemical Methods, Fundamentals and Applications; p. 98. [Google Scholar]
- 52.da Silva O.B., Machado S.A.S. Evaluation of the detection and quantification limits in electroanalysis using two popular methods: application in the case study of paraquat determination. Anal. Methods. 2012;4:2348–2354. [Google Scholar]
- 53.Kane P., Mcfadzean J.A., Squires S. Absorption and excretion of metronidazole.Part I. Serum concentration and unrinary excretion after oral administration. Br. J. Vener. Dis. 1961;37:273–275. doi: 10.1136/sti.37.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartlett P.N., Ghoneim E., El-Hefnawy G. Voltammetry and determination of metronidazole at a carbon fiber microdisk electrode. Talanta. 2005;66:869–874. doi: 10.1016/j.talanta.2004.12.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.