Abstract
Background
Acute myeloid leukemia (AML) is a myeloid neoplasm accounts for 7.6% of hematopoietic malignancies. AML is a complex disease, and understanding its pathophysiology is contributing to the improvement in the treatment and prognosis of AML. In this study, we assessed the expression profile and molecular functions of CCAAT enhancer binding protein gamma (CEBPG), a gene implicated in myeloid differentiation and AML progression.
Methods
shRNA mediated gene interference was used to down-regulate the expression of CEBPG in AML cell lines, and knockdown efficiency was detected by RT-qPCR and western blotting. The effect of knockdown on the growth of AML cell lines was evaluated by CCK-8. Western blotting was used to detect PARP cleavage, and flow cytometry were used to determine the effect of knockdown on apoptosis of AML cells. Genes and pathways affected by knockdown of CEBPG were identified by gene expression analysis using RNA-seq. One of the genes affected by knockdown of CEBPG was Eukaryotic translation initiation factor 4E binding protein 1 (EIF4EBP1), a known repressor of translation. Knockdown of EIF4EBP1 was used to assess its potential role in AML progression downstream of CEBPG.
Results
We explored the ChIP-Seq data of AML cell lines and non-AML hematopoietic cells, and found CEBPG was activated through its distal enhancer in AML cell lines. Using the public transcriptomic dataset, the Cancer Cell Line Encyclopedia (CCLE) and western blotting, we also found CEBPG was overexpressed in AML. Moreover, we observed that CEBPG promotes AML cell proliferation by activating EIF4EBP1, thus contributing to the progression of AML. These findings indicate that CEBPG could act as a potential therapeutic target for AML patients.
Conclusion
In summary, we systematically explored the molecular characteristics of CEBPG in AML and identified CEBPG as a potential therapeutic target for AML patients. Our findings provide novel insights into the pathophysiology of AML and indicate a key role for CEBPG in promoting AML progression.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12935-021-02305-z.
Keywords: CEBPG, EIF4EBP1, Acute myeloid leukemia, Proliferation, Apoptosis
Introduction
Acute myeloid leukemia (AML) is a myeloid neoplasm that accounts for 7.6% of hematopoietic malignancies. It is caused by the oncogenic transformation of hematopoietic progenitors in the bone marrow (BM), which results in the destruction of blood tissue. AML is reported to have a long-term survival of less than 20% [1–3]. Every year there are about 18,000 new cases AML in Europe [4]. AML is a complex disease, and understanding its pathophysiology will contribute to improving the treatment and prognosis of AML [5–8].
CCAAT enhancer binding proteins (CEBPs) including CEBPA, CEBPB, CEBPD, CEBPE, CEBPG and CEBPZ, are suggested as potential biomarkers for cancer prognosis [9–14]. CEBPB plays a role in gastric cancer progression [15], and is involved in breast cancer cell migration and invasion [16]. Both CEBPB and CEBPD function in cancer cell survival [17]. CEBPD is also reported to participate in papillary thyroid carcinoma progression [18]. CEBPE is suggested as a prognostic factor for AML [19], and CEBPZ is also reported to be mutated in AML [20].
Among CEBPs, CEBPA, CEBPE and CEBPZ have been reported to function in AML development [9, 19, 20], however the role of CCAAT enhancer binding protein gamma (CEBPG) in AML is unclear. CEBPG is a member of leucine-zipper transcription factor family that plays a role in many biological processes [21–24]. Knockdown of CEBPG suppressed tumor growth [25]. CEBPG is suggested as a biomarker for lung cancer risk [26]. It is also involved in the differentiation arrest in AML [27, 28]. Although the roles of CEBPG in several types of cancer have been revealed, its expression profile and molecular functions in AML remain unresolved. Therefore, in this study we assess the role of CEBPG in AML progression.
In the present study, shRNA mediated gene interference was used to down-regulate the expression of CEBPG in AML cell lines, and the knockdown efficiency was detected by RT-qPCR and western blotting. The effect of CEBPG knockdown on the growth of AML cell lines was evaluated by Cell Counting Kit-8 (CCK-8) assays. Western blotting was used to detect poly(ADP-ribose) polymerase (PARP) cleavage, and flow cytometry was used to determine the effect of CEBPG knockdown on apoptosis of AML cells. Genes and pathways affected by knockdown of CEBPG were identified by gene expression analysis using RNA-seq.
One of the genes affected by knockdown of CEBPG was Eukaryotic translation initiation factor 4E binding protein 1 (EIF4EBP1). EIF4EBP1 is a translation repressor protein [29] that plays a role in multiple types of cancer, including lung, breast, and liver cancer [30–33]. For example, EIF4EBP1 is reported to be significantly overexpressed in hepatocellular carcinoma (HCC) tissues and is related to poor survival of patients with HCC [33]. However, the biological effect and underlying mechanism of EIF4EBP1 in AML has not been explored. Therefore, knockdown of EIF4EBP1 was used to assess its potential role in AML progression downstream of CEBPG.
In the present study, we explored the ChIP-Seq data of AML cell lines and non-AML hematopoietic cells and found CEBPG was activated through its distal enhancer in AML cell lines. Using the public transcriptomic dataset, the Cancer Cell Line Encyclopedia (CCLE) and western blotting, we also found that CEBPG was overexpressed in AML. Moreover, CEBPG promotes AML cell proliferation by activating EIF4EBP1, thus contributing to the progression of AML. These findings indicate that CEBPG could act as a potential therapeutic target for AML patients.
Materials and methods
Cell lines and culture
Human AML cell lines, including NB4,THP-1, MV4-11, and K562 which was from blastic crisis of chronic myelogenous leukemia were obtained from the cell bank of the American type culture collection and cultured in RPMI medium (Termo Fisher Scientifc) containing 10% fetal bovine serum (Biological Industries, CT, USA), and 1% penicillin–streptomycin (Beyotime Biotechnology, Shanghai, China) at 37 °C in a humidified incubator with an atmosphere of 5% CO2 and tested routinely for mycoplasma.
Lentivirus preparation and infection
Short hairpin RNA (shRNA) targeting CEBPG and EIF4EBP1 (Table 1) were constructed in the pLKO.1-puro lentiviral vector (IGE BIOTECHNOLOGY LTD, Guangzhou, China). For lentivirus preparation, the envelope plasmid and packaging plasmid were purchased from Addgene (pMD2.G: #12,259; psPAX2:#12,260; Cambridge, MA, USA). pMD2.G, psPAX2 and the transfer plasmid were cotransfected into 293FT cells using polyethylenimine (linear MW 25,000 Da, 5 mg/mL, pH7.0) (cat. No. 23966–1; Polysciences, Warrington, PA, USA) according to the manufacturer’s instructions. After 6 h, the culture medium was completely replaced with fresh medium. The viral supernatant was harvested at 48 h post-transfection and filtered through a 0.22 μm filter.The leukemia cells were then infected with lentivirus in the presence of 10 μg/mL Polybrene (Sigma–Aldrich) for 24 h. Stable cell lines were selected with puromycin (Sigma-Aldrich).
Table 1.
shRNAs used to knockdown CEBPG and EIF4EBP1
| Name | Sequence |
|---|---|
| Homo-CEBPG -sh1 | CCGGGATTTGTTTCTTGAGCATGCACTCGAG |
| TGCATGCTCAAGAAACAAATCTTTTTGAATT | |
| Homo-CEBPG -sh2 | CCGGTGGCGACAATGCAGGACAGTACTCGA |
| GTACTGTCCTGCATTGTCGCCATTTTTGAATT | |
| Homo-CEBPG -sh3 | CCGGGCAACGCCGAGAGAGGAACAACTCGA |
| GTTGTTCCTCTCTCGGCGTTGCTTTTTGAATT | |
| Homo-EIF4EBP1-sh1 | CCGGGCCAGAGCCACCTGCGCAATACTCGA |
| GTATTGCGCAGGTGGCTCTGGCTTTTTGAATT | |
| Homo-EIF4EBP1-sh2 | CCGGGCAATAGCCCAGAAGATAAGCCTCGA |
| GGCTTATCTTCTGGGCTATTGCTTTTTGAATT | |
| Homo-EIF4EBP1-sh3 | CCGGGCGGTGAAGAGTCACAGTTTGCTCGA |
| GCAAACTGTGACTCTTCACCGCTTTTTGAATT |
Cell viability assay
Leukemia cells were seeded in 96-well plates at a density of 1 × 103 cells per well. The cell viability was determined by Cell Counting kit-8 (CCK8) assay (Dojindo Molecular Technologies, Tokyo, Japan) according to the manufacturer’s instructions. Cell proliferation was calculated as a percentage of that in cells in control medium. Each concentration was tested in triplicate and repeated in at least three independent experiments. The calculation was performed by Graph Prism software 7.0 (GraphPad Software Inc., San Diego, CA, USA).
RNA preparation and real-time PCR expression analysis
Total RNA was extracted from cell pellets using TRIzol®reagent (Invitrogen, CA, USA), according to the manufacturer’s protocol. For cDNA synthesis, 1 µg of total RNA was converted to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA). Quantitative real-time PCR analysis was carried out using LightCycler® 480 SYBR Green I Master mix (cat. No. 04707516001; Roche, Penzberg, Germany) with a LightCycler 480 Real Time System (Roche), according to the manufacturer’s protocol. mRNA expression levels were calculated using the Ct method with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression as an internal reference. Primer sequences are listed in Table 2.
Table 2.
Primers used for qRT-PCR analyses
| Name | Sequence (5’- > 3’) |
|---|---|
| CEBPG Forward | GAAAAAGAGCCGGTTGAAAAGC |
| CEBPG Reverse | ACTGTACGTTGTCTGCAAGGT |
| EIF4EBP1 Forward | CTATGACCGGAAATTCCTGATGG |
| EIF4EBP1 Reverse | CCCGCTTATCTTCTGGGCTA |
| GAPDH Forward | TGCACCACCAACTGCTTAG |
| GAPDH Reverse | GATGCAGGGATGATGTTC |
| PDGFB Forward | CTCGATCCGCTCCTTTGATGA |
| PDGFB Reverse | CGTTGGTGCGGTCTATGAG |
| SRC Forward | TGGCAAGATCACCAGACGG |
| SRC Reverse | GGCACCTTTCGTGGTCTCAC |
| PLCG1 Forward | GGAAGACCTCACGGGACTTTG |
| PLCG1 Reverse | GCGTTTTCAGGCGAAATTCCA |
| EIF4E Forward | ATGTGGCGCTGTTGTTAATGT |
| EIF4E Reverse | CTGCGTGGGACTGATAACCAA |
| AXL Forward | GTGGGCAACCCAGGGAATATC |
| AXL Reverse | GTACTGTCCCGTGTCGGAAAG |
| PIK3R2 Forward | TCACCTTCTGCTCCGTTGTG |
| PIK3R2 Reverse | GGAGGTCCGTGTGTACTCTTC |
| MET Forward | AGCGTCAACAGAGGGACCT |
| MET Reverse | GCAGTGAACCTCCGACTGTATG |
Western blotting analysis
Western blotting analysis was conducted using the following primary antibodies: CEBPG (cat. sc-517003; 1:500; Santa Cruz Biotechnology, Inc. Dallas, Texas,USA), EIF4EBP1 (cat. #9644,1:1000; Cell Signaling Technology, Boston, MA, USA), and PARP (cat. No. 9542; 1:1000; Cell Signaling Technology), with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (cat. No. MA3374; 1:1000; Millipore) as a reference protein. Peroxidase-conjugated Afniure goat anti-rabbit IgG (H + L) (cat.111-035-003; 1:5000) and goat anti-mouse IgG (H + L) (cat. No. 115-035-003; 1:5000) secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). ImageJ software was used for band quantifcation. Then, protein levels were determined using a GAPDH antibody for normalization.
Cell apoptosis assay
Leukemia cells (MV4-11, NB4, and K562 cell lines) were infected with lentivirus in the presence of 10 μg/mL Polybrene (Sigma-Aldrich) for 24 h. Stable cell lines were selected with puromycin (Sigma-Aldrich). Following 4 days incubation, leukemia cells were harvested and washed with cold PBS, suspended in 1 × binding bufer, and stained with fuorescein isothiocyanate (FITC)-Annexin V antibody and PI solution using an FITC-Annexin V apoptosis kit (cat. No.556420; BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer’s instructions. Cell apoptosis was analyzed by flow cytometry (Beckman Gallios™ Flow Cytometer; Beckman).
RNA-seq and data processing
RNA-seq was carried out according to the protocols suggested by Novogene, Beijing, China. First, total RNA was reverse transcribed to cDNA for library construction, and the cDNA library was then sequenced. The raw reads were filtered and clean reads were mapped according to HISAT. The gene expression level (as fragments per kilobase of exon model per million reads mapped) was then calculated. Differentially expressed genes (P < 0.05 and fold-change > 2 or fold-change < 0.5) were identified using DESeq2 analysis. For enrichment analysis, differentially expressed genes were analyzed using the DAVID Bioinformatics Resources v6.8 online server (https://david.ncifcrf.gov).
Chromatin immunoprecipitation (ChIP)
3–5 × 107 cells were crosslinked with 1% formaldehyde for 10 min and neutralized with 1.25 M glycine for 5 min at room temperature. Fixed cells were harvested, lysed, and sonicated using a Bioruptor (Diagenode, Liège, Belgium). Sonicated chromatin was incubated with anti-histone H3 (acetyl K27) antibody (cat. No. ab4729; Abcam, Cambridge, UK) overnight at 4 °C. DNA was eluted and purified using a QIAquick PCR purification kit (cat. No. 208106; Qiagen, Hilden, Germany). Samples were sequenced on a novaseq 6000 platform (Novogene Bioinformatics Technology Co., Ltd. Beijing, China). Raw data of ChIP-Seq H3K27ac analysis for NB4 cell line was aligned to the reference genome (UCSC hg38) using Bowtie2 (v 2.3.5) [34], with alignment parameters -p 4 -q -x. Peaks were identified using MACS2 (v2.0.9) [35], with parameters -g hs -n test -B -q 0.01. The bedgraph files generated by MACS2 were converted to bigwig files using the UCSC bedGraphToBigWig tool, and then bigwig files were visualized by Integrative Genomics Viewer (IGV) [36].
Public ChIP-Seq data collection and analysis
In this study, we searched public ChIP-Seq H3K27ac datasets of AML cell lines and non-AML hematopoietic cells in the Cistrome database (http://www.cistrome.org/). The ChIP-Seq datasets of H3K27ac and CEBPG in K562 cell line were also obtained in the Cistrome database. The bigwig files of those datasets obtained (GSE113040, GSE80779, GSE76783, GSE79899, GSE71809, GSE107147, GSE70660, GSE93372, GSE105532, GSE70482) were further visualized by Integrative Genomics Viewer (IGV) [36].
Statistical analysis
The association between EIF4EBP1 expression and overall survival of AML patients were assessed using the Kaplan–Meier analysis. Comparison between two groups was carried out using the Student’s t-test or the Mann–Whitney u test. Statistical analysis was carried out by GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistically significant P values are indicated as *P < 0.05, ** P < 0.01, ***P < 0.001, and ****P < 0.0001.
Results
CEBPG is activated through its distal enhancer and is overexpressed in AML cell lines
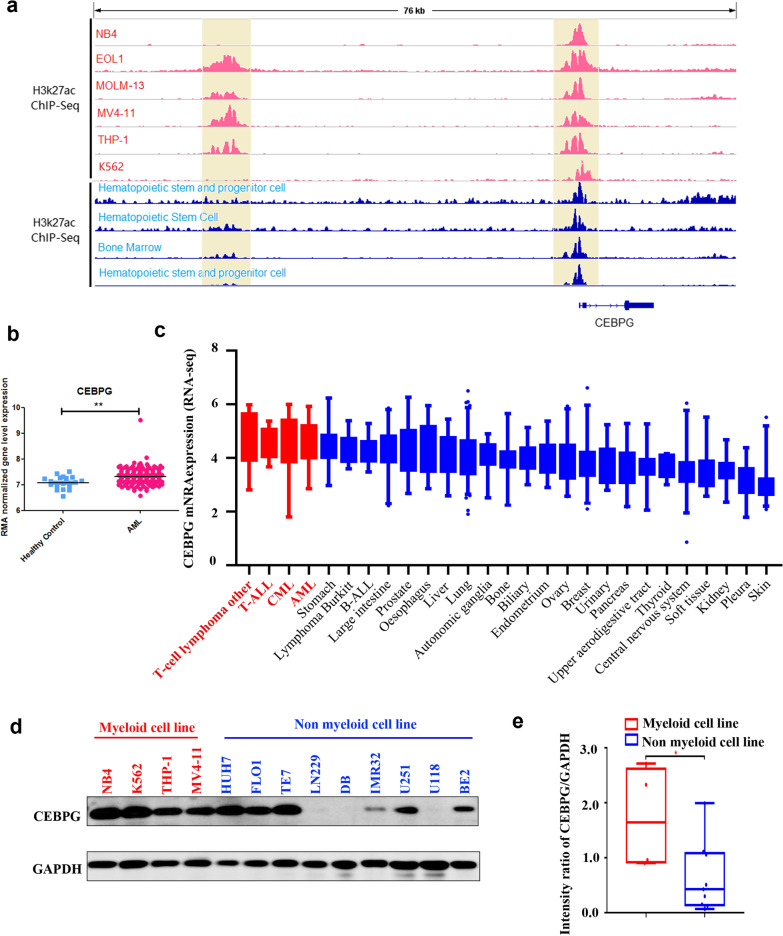
By interrogating ChIP-Seq data of AML cell lines (Fig, 1a, tracks 1–6, K562 cell line also included) and non-AML hematopoietic cells (Fig. 1a, tracks 7–10), we found that the enhancer region of CEBPG in AML cell lines showed coincident H3K27ac signals that were not present in non-AML hematopoietic cells, suggesting a potential role in transcription regulation. Then, we assessed the expression pattern of CEBPG between AML patients and healthy controls in a public transcriptomic dataset (GSE114868) [37], and found that CEBPG was more highly expressed in AML samples (Fig. 1b) relative to that in healthy control samples (the differentially expressed genes between AML and control samples in dataset GSE114868 are listed in Additional file 1: Table S1). Moreover, the Cancer Cell Line Encyclopedia (CCLE; https://portals.broadinstitute.org/ccle) which includes CEBPG mRNA expression profiles for multiple cancer cell lines, showed that CEBPG was highly expressed in hematologic malignancies including AML (Fig. 1c). We also assessed the levels of CEBPG in AML and non-AML cell lines using western blotting, and found higher levels of CEBPG in AML cell lines than in non-AML cell lines (Fig. 1d and e). Collectively, these data suggested that CEBPG is activated through its distal enhancer and overexpressed in AML.
Fig. 1.
a ChIP-Seq data analysis results for CEBPG of AML cell lines (K562 cell line included, tracks 1–6) and non-AML hematopoietic cells (tracks 7–10); b expression pattern of CEBPG between AML patients and healthy controls in public transcriptomic dataset (GSE114868); c CEBPG was highly expressed in hematologic tumors including AML according to the Cancer Cell Line Encyclopedia (CCLE; https://portals.broadinstitute.org/ccle); d western blotting results of the expression levels of CEBPG in AML/non-AML cell lines; e CEBPG markedly upregulated in AML cell lines compared with non-AML cell lines by western blotting
CEBPG is oncogenic and promotes AML cell proliferation
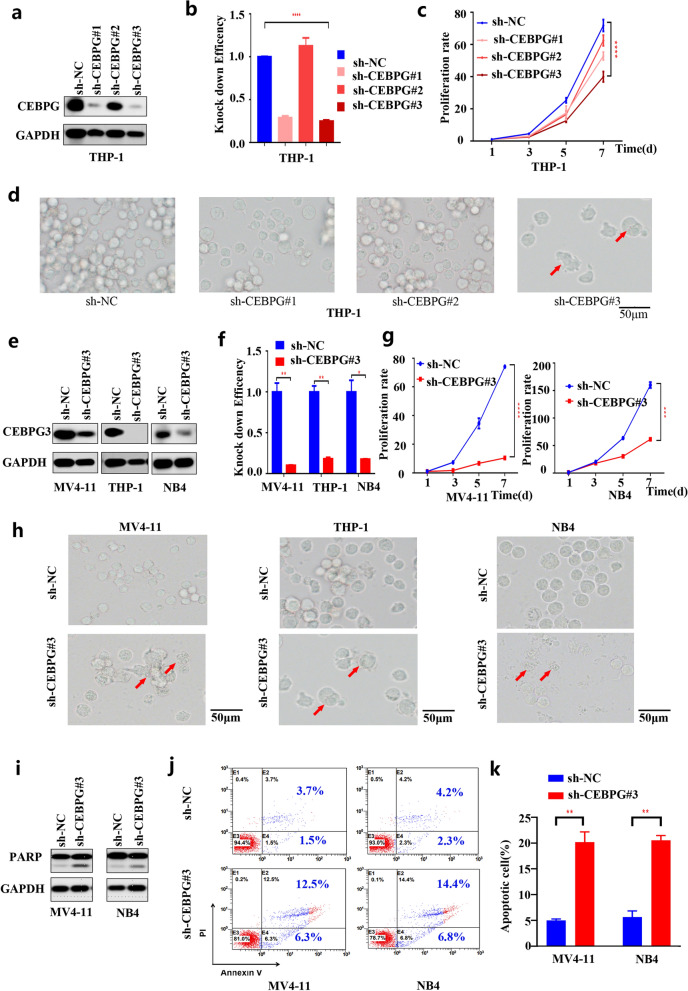
To address the biological significance of CEBPG, we selected three AML cell lines with high CEBPG protein levels shown in Fig. 1d (THP-1, NB4 and MV4-11) and performed shRNA-mediated knockdown of CEBPG using three independent shRNAs (Table 1). Knockdown efficiency of CEBPG was evaluated using western blotting and qPCR (Fig. 2a, b, e and f). Notably, knockdown of CEBPG significantly inhibited the proliferation rates of all 3 AML cell lines (Fig. 2c, d, g and h). We also assessed the level of the apoptotic protein PARP using western blotting and found that PARP levels were increased in both MV4-11 and NB4 cell lines upon knockdown of CEBPG (Fig. 2i). Knockdown of CEBPG also increased the apoptotic rates of MV4-11 and NB4 cell lines (Fig. 2j and k). Altogether, these data suggested that CEBPG is oncogenic and contributes to the proliferation of AML cells.
Fig. 2.
a Knockdown efficiency of CEBPG was evaluated in THP-1 cell line by western blotting. b Knockdown efficiency of CEBPG was evaluated in THP-1 cell line by qPCR. c Knockdown of CEBPG significantly inhibited the proliferation rates of THP-1 cell line. d Knockdown of CEBPG significantly inhibited the proliferation rates of THP-1 cell line. e Knockdown efficiency of CEBPG was evaluated in MV4-11, THP-1, and NB4 cell lines by western blotting. f Knockdown efficiency of CEBPG was evaluated in MV4-11, THP-1, and NB4 cell lines by qPCR. g Knockdown of CEBPG significantly inhibited the proliferation rates of MV4-11 and NB4 cell lines. h Knockdown of CEBPG significantly inhibited the proliferation rates of MV4-11, THP-1, and NB4 cell lines. i PARP was increased in both MV4-11 and NB4 cell lines upon knockdown of CEBPG. j Flow cytometry showed that knockdown of CEBPG increased the apoptotic rates of MV4-11 and NB4 cell lines. k Knockdown of CEBPG increased the apoptotic rates of MV4-11 and NB4 cell lines
CEBPG activates EIF4EBP1 in AML cell lines
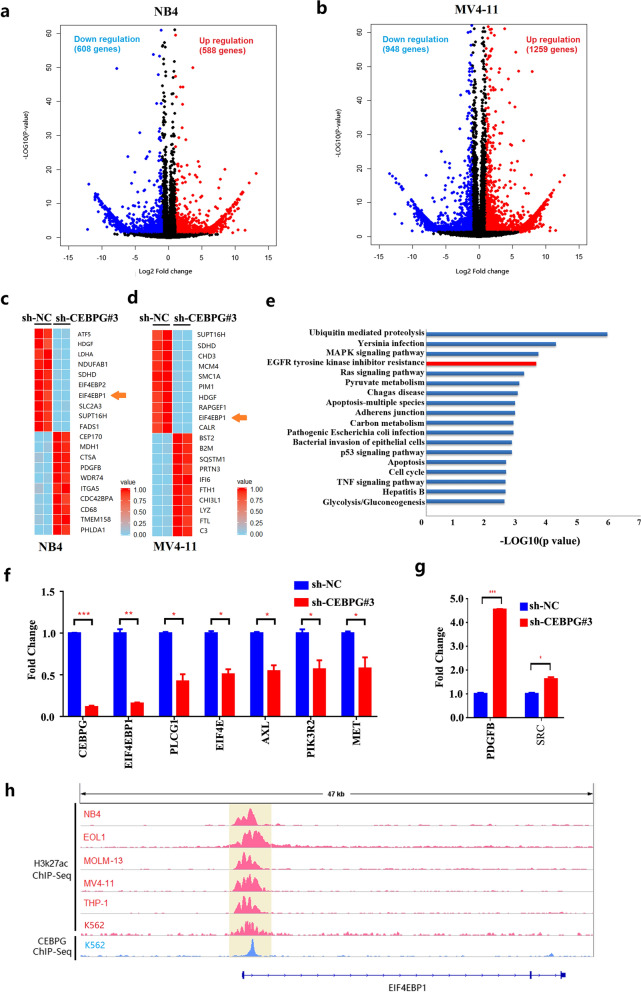
To reveal potential targets responsible for CEBPG-promoted AML cell proliferation, RNA-seq analyses were performed on NB4 and MV4-11 cell lines comparing shRNA control cells with CEBPG knockdown cells. A total of 1196 and 2207 differently expressed genes (DEGs) were identified upon CEBPG knockdown, in NB4 and MV4-11 cell lines respectively (Log2 |fold change|> 1, P < 0.05, Fig. 3a and b). EIF4EBP1 was included in the top 10 downregulated genes upon CEBPG knockdown in both NB4 and MV4-11 cell lines (Fig. 3c and d). Next, we conducted a functional enrichment analysis of all DEGs using the KEGG Pathway Database. The results showed a significant enrichment for EGFR tyrosine kinase inhibitor resistance signaling (ranking 4th), which involves EIF4EBP1 (Fig. 3e). Therefore, EIF4EBP1 was selected for in-depth investigation. To further determine the regulation of CEBPG on EGFR tyrosine kinase inhibitor resistance signaling and EIF4EBP1, a total of 8 genes (EIF4EBP1, PLCG1, EIF4E, AXL, PIK3R2, MET, PDGFB and SRC) from the EGFR tyrosine kinase inhibitor resistance signaling pathway was selected for qRT-PCR validation. In accordance with the RNA-Seq results, the mRNA levels of 6 of these genes, including EIF4EBP1, were downregulated while 2 genes were upregulated in NB4 cells in response to CEBPG silencing (Fig. 3f and g). Additionally, ChIP-Seq data of AML cell lines and K562 cell line showed that the promoter region of EIF4EBP1 had coincident H3K27ac signals (Fig. 3h, tracks 1–6), while the ChIP-Seq data from K562 cells further indicated that EIF4EBP1 was bound by CEBPG at its TSS-proximal regions (Fig. 3h, track 7), suggesting a potential role for CEBPG in the transcriptional regulation of EIF4EBP1. Therefore, we next investigated the role of EIF4EBP1 in NB4 and K562 cells.
Fig. 3.
a Volcano Plot of RNA-seq results for NB4 cell line in either the absence or presence of CEBPG. b Volcano Plot of RNA-seq results for MV4-11 cell line in either the absence or presence of CEBPG. c Top 10 downregulated and top 10 upregulated genes upon CEBPG knockdown in NB4 cell line. d Top 10 downregulated and top 10 upregulated genes upon CEBPG knockdown in MV4-11 cell line. e Enrichment analysis results of all DEGs by using the KEGG Pathway Database. f qRT-PCR results of 6 genes (EIF4EBP1, PLCG1, EIF4E, AXL, PIK3R2 and MET) from EGFR tyrosine kinase inhibitor resistance signaling pathway in NB4 cell line when silencing CEBPG. g qRT-PCR results of 2 genes (PDGFB and SRC) from EGFR tyrosine kinase inhibitor resistance signaling pathway in NB4 cell line when silencing CEBPG. h ChIP-Seq data of AML cell lines and K562 cell line showed that the promoter region of EIF4EBP1 had coincident H3K27ac signals (tracks 1–6), ChIP-Seq data of K562 cell line further indicated that EIF4EBP1 was bound by CEBPG at its TSS-proximal regions (track 7)
EIF4EBP1 knockdown interferes with AML cell proliferation and increases apoptosis
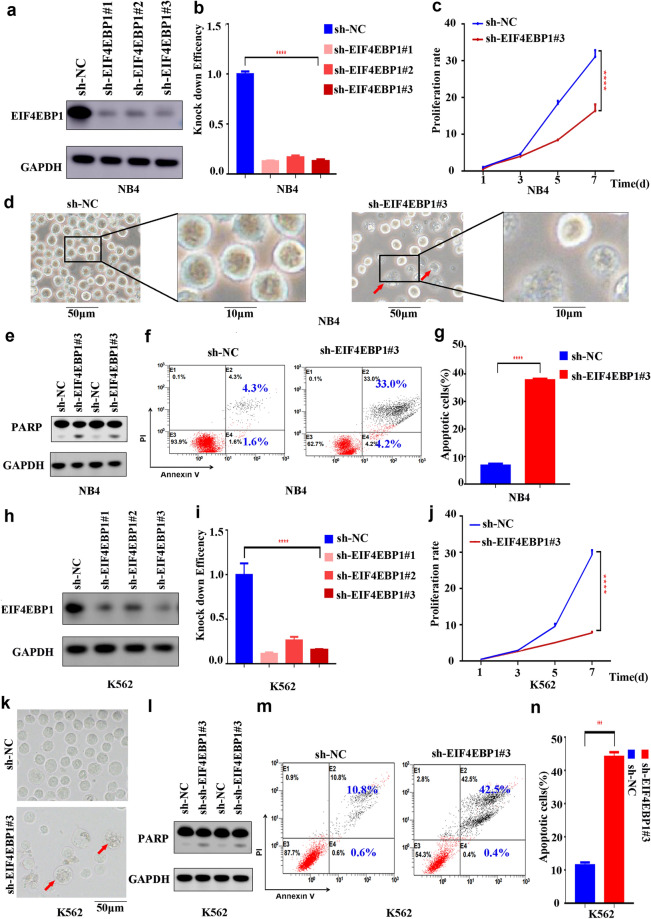
To evaluate the biological significance of EIF4EBP1, we selected 2 cell lines (NB4 and K562) and knocked down EIF4EBP1 in both cell lines using three independent shRNAs (Table 1). Knockdown efficiency of EIF4EBP1 was evaluated using western blotting and qPCR (Fig. 4a, b, h and i). Notably, knockdown of EIF4EBP1 significantly inhibited the proliferation rates of both cell lines (Fig. 4c, d, j and k). We also assessed the expression level of the apoptotic protein PARP using western blotting and found that PARP levels increased in both NB4 and K562 cell lines upon knockdown of EIF4EBP1 (Fig. 4e and l). Knockdown of EIF4EBP1 also increased the apoptotic rates of NB4 and K562 cell lines (Fig. 2f, g, 4m and n). Collectively, these data suggested that EIF4EBP1 is required to sustain proliferation and survival of AML cells.
Fig. 4.
a Knockdown efficiency of EIF4EBP1 was evaluated in NB4 cell line by western blotting. b Knockdown efficiency of EIF4EBP1 was evaluated in NB4 cell line by qPCR. c Knockdown of EIF4EBP1 significantly inhibited the proliferation rates of NB4 cell line. d Knockdown of EIF4EBP1 significantly inhibited the proliferation rates of NB4 cell line. e PARP was increased in NB4 cell line upon knockdown of EIF4EBP1. f Flow cytometry showed that knockdown of EIF4EBP1 increased the apoptotic rates of NB4 cell line. g Knockdown of EIF4EBP1 increased the apoptotic rates of NB4 cell line. h Knockdown efficiency of EIF4EBP1 was evaluated in K562 cell line by western blotting. i Knockdown efficiency of EIF4EBP1 was evaluated in K562 cell line by qPCR. j Knockdown of EIF4EBP1 significantly inhibited the proliferation rates of K562 cell line. k Knockdown of EIF4EBP1 significantly inhibited the proliferation rates of K562 cell line. l PARP was increased in K562 cell line upon knockdown of EIF4EBP1. m Flow cytometry showed that knockdown of EIF4EBP1 increased the apoptotic rates of K562 cell line. n Knockdown of EIF4EBP1 increased the apoptotic rates of K562 cell line
Identification of EIF4EBP1 as an unfavorable prognostic factor for AML patients
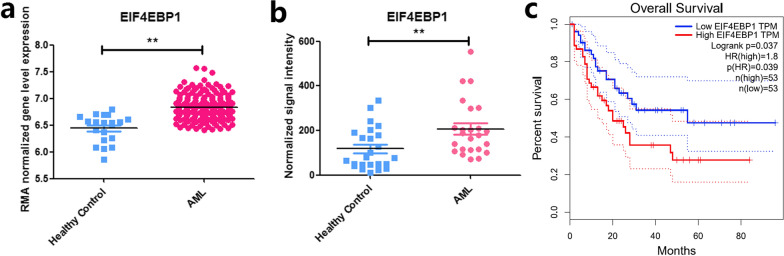
We assessed the expression pattern of EIF4EBP1 between AML patients and healthy controls in two public transcriptomic datasets (GSE114868 and GSE142700) [37]. The results showed that EIF4EBP1 was significantly overexpressed in AML samples in both datasets (Fig. 5a and b). To further explore the prognostic value of EIF4EBP1, we used the online tool http://gepia.cancer-pku.cn/ and the result showed that the overall survival of AML patients with higher EIF4EBP1 expression was significantly poorer than those with lower EIF4EBP1 expression (Fig. 5c). These results suggested that EIF4EBP1 represents a negative prognostic factor for AML patients.
Fig. 5.
a EIF4EBP1 was significantly overexpressed in AML samples in public transcriptomic dataset GSE114868. b EIF4EBP1 was significantly overexpressed in AML samples in public transcriptomic dataset GSE142700. c Base on the online tool http://gepia.cancer-pku.cn/, the overall survival of AML patients with higher EIF4EBP1 expression was significantly poorer than those with lower EIF4EBP1 expression
Discussion
AML is an aggressive malignancy with poor prognosis [8]. It is a complex disease and a detailed understanding of its pathophysiology is required to improve the treatment and prognosis of AML [5–8].
CCAAT enhancer binding proteins (CEBPs) including CEBPA, CEBPB, CEBPD, CEBPE, CEBPG and CEBPZ, are suggested as potential biomarkers for cancer prognosis [9–14]. Among CEBPs, CCAAT enhancer binding protein gamma (CEBPG), a member of leucine-zipper transcription factor family, has been implicated in multiple cancers [25–28]. For example, it is reported that CEBPG significantly promotes the proliferation and migration of esophageal squamous cell carcinoma (ESCC) cells, and is thus suggested as a prognostic factor for patients with ESCC [21].
Although a role for CEBPG in myeloid differentiation has been demonstrated [27, 28], if and how it contributes to the pathogenesis of AML is unclear. Here, we explored the function of CEBPG in AML and found that CEBPG is upregulated in AML and contributes to the proliferation of AML cells. We also demonstrated that CEBPG promotes AML cell proliferation by activating EIF4EBP1 in AML cell lines.
Eukaryotic translation initiation factor 4E binding protein 1 (EIF4EBP1) gene encodes a translation repressor protein [29]. This protein plays a role in multiple cancer types, including lung, breast, and liver cancer [30–33]. For example, EIF4EBP1 is reported to be significantly overexpressed in hepatocellular carcinoma (HCC) tissues and is related to poor survival of HCC patients [33]. However, the biological effect and underlying mechanism of EIF4EBP1 in AML has not been explored. In this study, we found the knockdown of EIF4EBP1 significantly inhibited proliferation and increases apoptosis in NB4 and K562 cells. Furthermore, in two public transcriptomic datasets (GSE114868 and GSE142700) [37], EIF4EBP1 was observed to be significantly overexpressed in AML samples. EIF4EBP1 was also identified as an unfavorable prognostic factor for AML patients using the online tool http://gepia.cancer-pku.cn/. Taken together, these results suggested that EIF4EBP1 is involved in the pathogenesis of AML and represents a negative prognostic factor for AML patients.
In summary, we explored the function of CEBPG in AML and identified CEBPG as a potential therapeutic target for AML patients. Our findings provide novel insights into the pathophysiology of AML and elucidated a crucial role of CEBPG in promoting AML progression.
Supplementary Information
Additional file 1: Table S1. Differentially expressed genes between AML and control samples in dataset GSE114868.
Acknowledgements
We would like to thank the department of Hematology, Children’s Hospital of Soochow University, for the support in this study.
Abbreviations
- AML
Acute myeloid leukemia
- ChIP
Chromatin immunoprecipitation
- CEBPG
CCAAT enhancer binding protein gamma
- EIF4EBP1
Eukaryotic translation initiation factor 4E binding protein 1
Authors’ contributions
FF, JL, S-YH and JP designed and directed the study; YJ, S-YW, Y-LC, Z-MZ performed most of the experiments, analyzed the data, and wrote the paper; Y-FT, YX and X-ML helped with statistical analysis; H-RW, RZ and H-BC performed part of the experiments; J-JP, J-JY, S-QJ, ZZ, X-RC performed lentivirus preparation and transfection; Y-PZ, and J-WW participated in western blotting, PCR, and in vitro experiments; C-xF and FF collected clinical data; YX and X-LL supported the design of primers for real-time PCR; CF, XC and YZ helped with the apoptosis and cell cycle analysis; Z-HL, DW and GL participated in plasmid construction. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation (81971867, 81802499, 81872845, 81902534, 82072767, 52003183); Natural Science Foundation of Jiangsu Province (BK20180206, BK20180207, SBK2019021442, BK20190185, BK20190186, BK20191175); the Universities Natural Science Foundation of Jiangsu Province (No.16KJB310014); Jiangsu province’s science and technology support program (Social Development) project (BE2021657); Department of Pediatrics Clinical Center of Suzhou (Szzx201504); Gusu Health Talents program of Soochow city (2020-104); the Applied Foundational Research of Medical and Health Care of Suzhou City (SYS2018075, SYS2018074, SYS2019080, SYS2019082, SYS2019078, SYS2020150, SYS2020151); The Science and Technology Project of Soochow(SS201709).
Availability of data and materials
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request (GSE178287).
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
The consents for publication from all authors were obtained.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
You Jiang, Shui-Yan Wu, Yan-Ling Chen, and Zi-Mu Zhang contributed equally to this work
Contributor Information
Jian Pan, Email: panjian2008@163.com.
Fang Fang, Email: fangf@suda.edu.cn.
Jun Lu, Email: drlujun_sz@163.com.
References
- 1.Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Récher C. Clinical Implications of Inflammation in Acute Myeloid Leukemia. Front Oncol. 2021;11:623952. doi: 10.3389/fonc.2021.623952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquer H, Tostain M, Kaci N, Roux B, Benajiba L. Descriptive and functional genomics in acute myeloid leukemia (AML): paving the road for a cure. Cancers (Basel) 2021;13(4):748. doi: 10.3390/cancers13040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser O, Trama A, Maynadié M, Stiller C, Marcos-Gragera R, De Angelis R, Mallone S, Tereanu C, Allemani C, Ricardi U, Schouten HC, RARECARE Working Group Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012;48(17):3257–3266. doi: 10.1016/j.ejca.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H. Acute myeloid leukemia—major progress over four decades and glimpses into the future. Am J Hematol. 2016;91:131–145. doi: 10.1002/ajh.24246. [DOI] [PubMed] [Google Scholar]
- 6.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392:593–606. doi: 10.1016/S0140-6736(18)31041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadia TM, Ravandi F, Cortes J, Kantarjian H. Toward individualized therapy in acute myeloid leukemia: a contemporary review. JAMA Oncol. 2015;1:820–828. doi: 10.1001/jamaoncol.2015.0617. [DOI] [PubMed] [Google Scholar]
- 8.Doucette K, Karp J, Lai C. Advances in therapeutic options for newly diagnosed, high-risk AML patients. Ther Adv Hematol. 2021;12:20406207211001138. doi: 10.1177/20406207211001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstandin NP, Pastore F, Herold T, Dufour A, Rothenberg-Thurley M, Hinrichsen T, Ksienzyk B, Tschuri S, Schneider S, Hoster E, Berdel WE, Woermann BJ, Sauerland MC, Braess J, Bohlander SK, Klein HG, Hiddemann W, Metzeler KH, Spiekermann K. Genetic heterogeneity of cytogenetically normal AML with mutations of CEBPA. Blood Adv. 2018;2(20):2724–2731. doi: 10.1182/bloodadvances.2018016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Reebye V, Hitchen P, Fan J, Jiang H, Sætrom P, Rossi J, Habib NA, Huang KW. Mechanisms involved in the activation of C/EBPα by small activating RNA in hepatocellular carcinoma. Oncogene. 2019;38:3446–3457. doi: 10.1038/s41388-018-0665-6. [DOI] [PubMed] [Google Scholar]
- 11.Akasaka T, Balasas T, Russell LJ, Sugimoto KJ, Majid A, Walewska R, Karran EL, Brown DG, Cain K, Harder L, Gesk S, Martin-Subero JI, Atherton MG, Brüggemann M, Calasanz MJ, Davies T, Haas OA, Hagemeijer A, Kempski H, Lessard M, Lillington DM, Moore S, Nguyen-Khac F, Radford-Weiss I, Schoch C, Struski S, Talley P, Welham MJ, Worley H, Strefford JC, Harrison CJ, Siebert R, Dyer MJ. Five members of the CEBP transcription factor family are targeted by recurrent IGH translocations in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) Blood. 2007;109:3451–3461. doi: 10.1182/blood-2006-08-041012. [DOI] [PubMed] [Google Scholar]
- 12.Avellino R, Havermans M, Erpelinck C, Sanders MA, Hoogenboezem R, van de Werken HJ, Rombouts E, van Lom K, van Strien PM, Gebhard C, Rehli M, Pimanda J, Beck D, Erkeland S, Kuiken T, de Looper H, Gröschel S, Touw I, Bindels E, Delwel R. An autonomous CEBPA enhancer specific for myeloid-lineage priming and neutrophilic differentiation. Blood. 2016;127:2991–3003. doi: 10.1182/blood-2016-01-695759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyamsunder P, Shanmugasundaram M, Mayakonda A, Dakle P, Teoh WW, Han L, Kanojia D, Lim MC, Fullwood M, An O, Yang H, Shi J, Hossain MZ, Madan V, Koeffler HP. Identification of a novel enhancer of CEBPE essential for granulocytic differentiation. Blood. 2019;133:2507–2517. doi: 10.1182/blood.2018886077. [DOI] [PubMed] [Google Scholar]
- 14.Lourenço AR, Coffer PJ. A tumor suppressor role for C/EBPα in solid tumors: more than fat and blood. Oncogene. 2017;36:5221–5230. doi: 10.1038/onc.2017.151. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Liu B, Chen Z, Li G, Zhang Z. MSC-induced lncRNA HCP5 drove fatty acid oxidation through miR-3619-5p/AMPK/PGC1alpha/CEBPB axis to promote stemness and chemo-resistance of gastric cancer. Cell Death Dis. 2020;11(4):233. doi: 10.1038/s41419-020-2426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Gao Y, Tang L, Ning K, Geng N, Zhang H, Li Y, Li Y, Liu F, Li F. A novel PAK4-CEBPB-CLDN4 axis involving in breast cancer cell migration and invasion. Biochem Biophys Res Commun. 2019;511(2):404–408. doi: 10.1016/j.bbrc.2019.02.070. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Jefferson P, Zhou Q, Angelastro JM, Greene LA. Dominant-negative ATF5 compromises cancer cell survival by targeting CEBPB and CEBPD. Mol Cancer Res. 2020;18(2):216–228. doi: 10.1158/1541-7786.MCR-19-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Xia S, Zhang L, Wang W, Chen L, Zhan W. MiR-324-5p/PTPRD/CEBPD axis promotes papillary thyroid carcinoma progression via microenvironment alteration. Cancer Biol Ther. 2020;21(6):522–532. doi: 10.1080/15384047.2020.1736465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K, Du Y, Wei DQ, Zhang F. CEBPE expression is an independent prognostic factor for acute myeloid leukemia. J Transl Med. 2019;17(1):188. doi: 10.1186/s12967-019-1944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold T, Metzeler KH, Vosberg S, Hartmann L, Röllig C, Stölzel F, Schneider S, Hubmann M, Zellmeier E, Ksienzyk B, Jurinovic V, Pasalic Z, Kakadia PM, Dufour A, Graf A, Krebs S, Blum H, Sauerland MC, Büchner T, Berdel WE, Woermann BJ, Bornhäuser M, Ehninger G, Mansmann U, Hiddemann W, Bohlander SK, Spiekermann K, Greif PA. Isolated trisomy 13 defines a homogeneous AML subgroup with high frequency of mutations in spliceosome genes and poor prognosis. Blood. 2014;124(8):1304–1311. doi: 10.1182/blood-2013-12-540716. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Lin L, Shen Z, Li Y, Cao H, Peng L, Qiu Y, Cheng X, Meng M, Lu D, Yin D. CEBPG promotes esophageal squamous cell carcinoma progression by enhancing PI3K-AKT signaling. Am J Cancer Res. 2020;10(10):3328–3344. [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukada J, Yoshida Y, Kominato Y, Auron PE. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine. 2011;54:6–19. doi: 10.1016/j.cyto.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Fong Y, Shen KH, Chen LJ, Cheng JT. Changes of CCAAT enhancer-binding proteins (CEBPs) in the lung of streptozotocin-induced diabetic rats. Horm Metab Res. 2011;43:261–267. doi: 10.1055/s-0030-1270452. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Croniger CM, Lekstrom-Himes J, Zhang P, Fenyus M, Tenen DG, Darlington GJ, Hanson RW. Metabolic response of mice to a postnatal ablation of CCAAT/enhancer-binding protein alpha. J Biol Chem. 2005;280:38689–38699. doi: 10.1074/jbc.M503486200. [DOI] [PubMed] [Google Scholar]
- 25.Atkins M, Potier D, Romanelli L, Jacobs J, Mach J, Hamaratoglu F, Aerts S, Halder G. An ectopic network of transcription factors regulated by hippo signaling drives growth and invasion of a malignant tumor model. Curr Biol. 2016;26(16):2101–2113. doi: 10.1016/j.cub.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Blomquist T, Crawford EL, Mullins D, Yoon Y, Hernandez DA, Khuder S, Ruppel PL, Peters E, Oldfield DJ, Austermiller B, Anders JC, Willey JC. Pattern of antioxidant and DNA repair gene expression in normal airway epithelium associated with lung cancer diagnosis. Cancer Res. 2009;69(22):8629–8635. doi: 10.1158/0008-5472.CAN-09-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown AL, de Smith AJ, Gant VU, Yang W, Scheurer ME, Walsh KM, Chernus JM, Kallsen NA, Peyton SA, Davies GE, Ehli EA, Winick N, Heerema NA, Carroll AJ, Borowitz MJ, Wood BL, Carroll WL, Raetz EA, Feingold E, Devidas M, Barcellos LF, Hansen HM, Morimoto L, Kang AY, Smirnov I, Healy J, Laverdière C, Sinnett D, Taub JW, Birch JM, Thompson P, Spector LG, Pombo-de-Oliveira MS, DeWan AT, Mullighan CG, Hunger SP, Pui CH, Loh ML, Zwick ME, Metayer C, Ma X, Mueller BA, Sherman SL, Wiemels JL, Relling MV, Yang JJ, Lupo PJ, Rabin KR. Inherited genetic susceptibility to acute lymphoblastic leukemia in down syndrome. Blood. 2019;134:1227–1237. doi: 10.1182/blood.2018890764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberich-Jordà M, Wouters B, Balastik M, Shapiro-Koss C, Zhang H, Di Ruscio A, Radomska HS, Ebralidze AK, Amabile G, Ye M, Zhang J, Lowers I, Avellino R, Melnick A, Figueroa ME, Valk PJ, Delwel R, Tenen DG. C/EBPγ deregulation results in differentiation arrest in acute myeloid leukemia. J Clin Invest. 2012;122:4490–4504. doi: 10.1172/JCI65102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Ma X, Liu J, Wan Y, Jiang Y, Xia Y, Cheng W. Prognostic value of an autophagy-related gene expression signature for endometrial cancer patients. Cancer Cell Int. 2020;20:306. doi: 10.1186/s12935-020-01413-6.eCollection2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li SQ, Feng J, Yang M, Ai XP, He M, Liu F. Sauchinone: a prospective therapeutic agent-mediated EIF4EBP1 down-regulation suppresses proliferation, invasion and migration of lung adenocarcinoma cells. J Nat Med. 2020;74(4):777–787. doi: 10.1007/s11418-020-01435-4. [DOI] [PubMed] [Google Scholar]
- 31.Qin X, Jiang B, Zhang Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle. 2016;15(6):781–786. doi: 10.1080/15384101.2016.1151581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan B, Liu B, Yu G, Huang Y, Lv C. Differentially expressed autophagy-related genes are potential prognostic and diagnostic biomarkers in clear-cell renal cell carcinoma. Aging (Albany NY) 2019;11(20):9025–9042. doi: 10.18632/aging.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha YL, Li PD, Yuan LJ, Zhang MY, Zhang YJ, Rao HL, Zhang HZ, Zheng XF, Wang HY. EIF4EBP1 overexpression is associated with poor survival and disease progression in patients with hepatocellular carcinoma. PLoS ONE. 2015;10:e0117493. doi: 10.1371/journal.pone.0117493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langmead B, Salzberg S. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HH, Chen FY, Chou WC, Hou HA, Ko BS, Lin CT, Tang JL, Li CC, Yao M, Tsay W, Hsu SC, Wu SJ, Chen CY, Huang SY, Tseng MH, Tien HF, Chen RH. Long non-coding RNA HOXB-AS3 promotes myeloid cell proliferation and its higher expression is an adverse prognostic marker in patients with acute myeloid leukemia and myelodysplastic syndrome. BMC Cancer. 2019;19(1):617. doi: 10.1186/s12885-019-5822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Differentially expressed genes between AML and control samples in dataset GSE114868.
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request (GSE178287).