Abstract
Metastatic breast cancer is one of the deadliest forms of malignancy, primarily driven by its characteristic microenvironment comprising of cancer cells interacting with stromal components. These interactions induce genetic and metabolic alterations creating a conducive environment for tumor growth. In this study, we developed a physiologically-relevant three dimensional (3D) vascularized breast cancer microenvironment comprising of metastatic MDA-MB-231 cells and human umbilical vein endothelial cells (HUVEC) loaded in human dermal fibroblasts (HDF) laden fibrin, representing the tumor stroma. The matrix as well as stromal cell density impacted the transcriptional profile of genes involved in tumor angiogenesis and cancer invasion, which are hallmarks of cancer. Cancer-specific canonical pathways and activated upstream regulators were also identified by the differential gene expression signatures of these composite cultures. Additionally, a tumor associated vascular bed of capillaries is established exhibiting dilated vessel diameters, representative of in vivo tumor physiology. Further, employing aspiration-assisted bioprinting, we identified cancer-endothelial crosstalk, in the form of collective angiogenesis of tumor spheroids bioprinted at close proximity. Overall, this bottom-up approach of tumor microenvironment fabrication provides an insight into the potential of in vitro tumor models and enables identification of novel therapeutic targets as a pre-clinical drug screening platform.
Keywords: Tumor microenvironment, tumor angiogenesis, metastatic breast cancer, in vitro tumor model, aspiration-assisted bioprinting
Graphical Abstract
A vascularized metastatic breast tumor model was fabricated to study two of the most important hallmarks of cancer, tumor angiogenesis, and cancer invasion. The role of matrix and stromal cell density on a tumor microenvironment was demonstrated and the expression of genes closely related to tumor development was highlighted. Further, employing aspiration-assisted bioprinting, 3D bioprinting of tumor spheroids reveal cancer-endothelial interaction in the form of collective angiogenesis.

1. Introduction
Breast cancer, a global health concern, is the most frequently diagnosed form of malignancy in women.[1] It usually metastasizes to distant organs of the body such as bone,[2] lung,[3] liver[4] and brain[5], [6] through vascular and lymphatic dissemination, which further heightens its mortality rate. The breast tumor microenvironment, composed of extracellular matrix (ECM), fibroblasts, adipocytes, endothelial and immune cells, has proven to play an astounding role in tumor invasion, angiogenesis, genetic alterations in bystander stromal cells, and immune cell recruitment.[7],[8] All of these processes, in tandem, constitute the dynamic microenvironment, and their interactions are critical to understand breast cancer pathogenesis for developing novel and effective therapies. Although, two-dimensional (2D) cell cultures have shed considerable light on genetic and epigenetic changes in breast cancer progression[9],[10], this reductionist view of understanding cancer fails to account for the complex ‘tumor microenvironment’. While use of in vivo tumor models have increased over the years, disseminating each individual step to gain mechanistic insights into the metastatic cascade is far too complex in in vivo settings.[11] This ushers in the importance of three-dimensional (3D) in vitro culture systems in dissecting and studying the various hallmarks of cancer.[8]
Various kinds of 3D models, such as hydrogel constructs,[12] polymeric scaffolds,[13],[14] microcarrier beads,[15] hanging droplets[16] etc., have been increasingly used in studying different aspects of tumor growth. Such 3D models preserve the cell-cell and cell-matrix interactions that dictate the entire metastatic cascade, including formation of primary neoplasm, initiation of tumor vasculature, tumor cell invasion into the surrounding matrix, and intravasation into surrounding blood vessels to eventually form secondary malignancies. This dynamic process is primarily driven by changes in the tumor microenvironment such as variations in ECM stiffness at various stages of cancer progression, abnormal tumor angiogenesis involving leaky and hyperpermeable vessels [17],[18] and cancer-fibroblast interaction giving rise to cancer-associated fibroblasts (CAFs).[19] These cell-cell contact signaling or paracrine signaling mechanisms gradually transform a tumor-suppressive environment to a tumor-supportive one. Thus, it is imperative to understand the cellular crosstalk behind these mechanisms by fabricating a physiologically-relevant 3D tumor model.
Although, 3D breast tumor models have been fabricated to study various aspects of cancer progression,[20], [21], [22], [23] none of these studies have provided a fundamental understanding of how varying the density of the stromal and matrix components could shed light on tumor angiogenesis and cancer invasion. Moreover, extracting meaningful information from transcriptomic variations in these complex hetero-culture systems have not been explored yet. In this study, an in vitro platform was developed which restores cell-cell and cell-ECM interactions through an architecturally-relevant 3D model. The intricacies of this model were introduced in a hierarchical manner elaborating the significance, impact and interaction between different cellular and acellular components in a tumor microenvironment. Employing metastatic triple-negative breast cancer cells (MDA-MB-231), human umbilical vein endothelial cells (HUVECs) and human dermal fibroblasts (HDFs) as the cellular components and fibrin as the matrix, this vascularized tumor model exhibits some of the major hallmarks of cancer including, cancer invasion and tumor angiogenesis as well as effect of stromal cells on these biological functions. Using RNA sequencing on these hetero-culture systems, transcriptomic variations between tumors co-cultured in the presence and absence of fibroblasts, have been identified. Subsequent pathway analysis on these cultures also revealed the activation of signaling pathways indicating the transformation of fibroblasts to a ‘CAF-like’ state. Additionally, various upstream angiogenic and metastatic regulators were determined to be involved in cancer progression and tumor angiogenesis. This study also enumerates the establishment of a vascular tumor bed, which closely mimics the abnormal tumor vasculature, including tumor cell intravasation into surrounding capillaries. Furthermore, this study demonstrates the potential of a recently developed aspiration-assisted bioprinting technique in understanding cancer-endothelial cell interactions, which highlights the immense potential of using 3D bioprinting techniques to fabricate relevant tumor models for cancer research.[24]
2. Results
2.1. Fabrication and characterization of in-vitro tumor spheroids
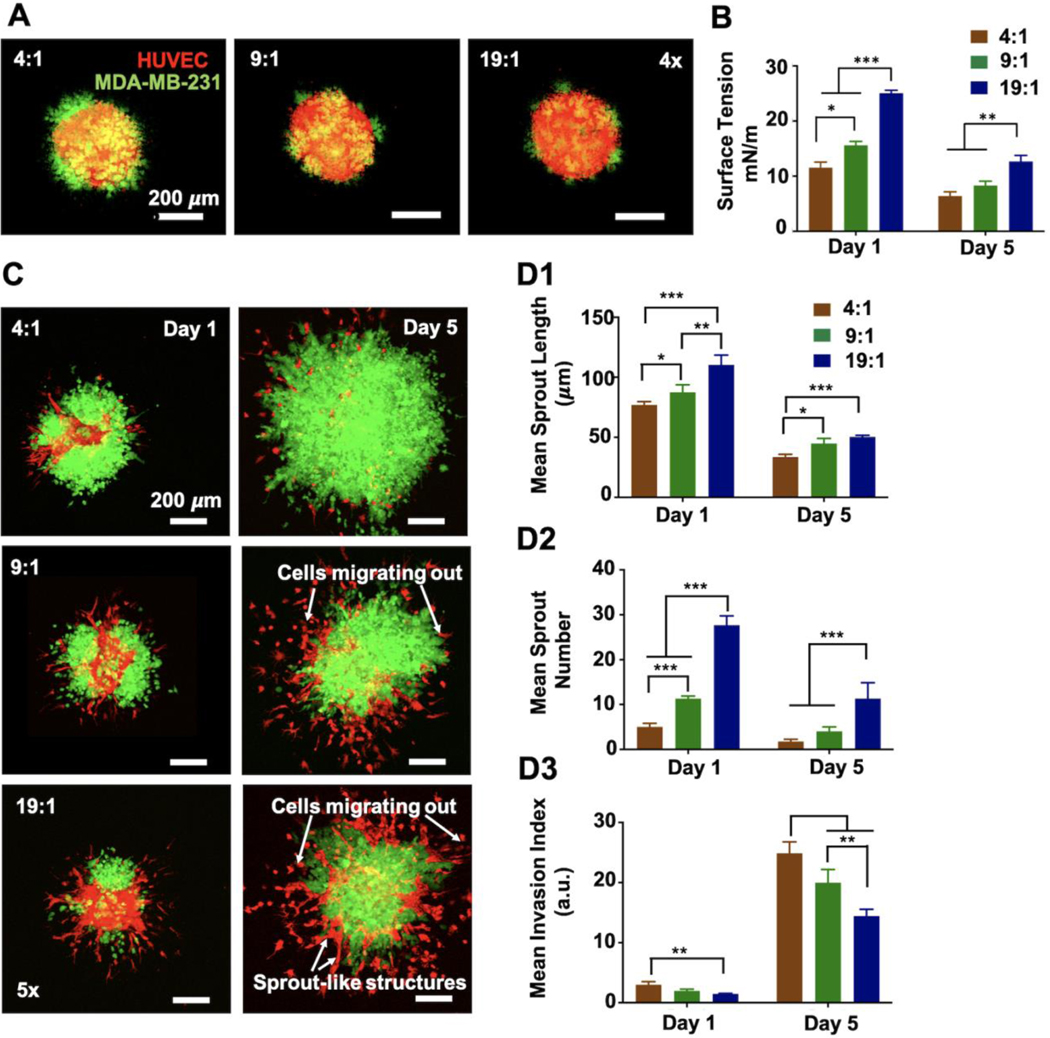
Using 96-well U bottom cell repellant plates, tumor spheroids were fabricated in three different HUVEC:MDA-MB-231 ratios, namely, 4:1, 9:1 and 19:1. Over 24 h of culture, all three ratios formed compact 3D tumor spheroids (Figure 1A). Fluorescent images on Day 1, revealed that the periphery of spheroids was more dominated by MDA-MB-231 cells while HUVECs were distributed more uniformly. Tumor spheroids were cultured for a period of five days and the changes in their growth and proliferation behavior as well as mechanical properties were assessed on Days 1 and 5. Tumor spheroids acquired a mean dimeter of 270–330 μm for all three ratios on Day 1, which increased up to an average of ~480 μm on Day 5 for 4:1, ~431 μm for 9:1 and ~350 μm for 19:1 (Figures S1A-B). The percent increase in tumor spheroid diameter over five days was the highest for 4:1 (31%) as compared to 9:1 (28%) and 19:1 (20%). CCK-8 activity was measured for all three ratios for Days 1 and 5 to assess cellular proliferation (Figure S1C). It was observed that the normalized CCK-8 activity at Day 5 decreased with decreasing MDA-MB-231 ratio. To evaluate their mechanical properties, surface tension of spheroids was measured on Days 1 and 5. On Day 1, 4:1 exhibited the lowest surface tension, 11.53 mN/m, as compared to both 9:1, 15.6 mN/m, and 19:1, 25.05 mN/m (Figure 1B). A similar trend was also observed for Day 5; however, surface tension for all groups decreased on Day 5 as compared to Day 1. Overall, 19:1 exhibited the highest surface tension indicating its enhanced compactness with respect to other groups.
Figure 1.
Tumor spheroids, comprising of td-Tomato+ HUVECs and GFP+ metastatic MDA-MB-231 cells, were prepared in three different ratios, 4:1, 9:1 and 19:1, respectively. Spheroids were analyzed before and after fibrin encapsulation to determine the optimal ratio for tumor model. (A) Fluorescent images of fabricated tumor spheroids showing the distribution of both cell types for all mixing ratios. (B) Graphical representation of surface tension of 4:1, 9:1 and 19:1 tumor spheroids at Days 1 and 5. (C) Fluorescent images of tumor spheroids encapsulated in fibrin at Days 1 and 5. Graphical representation of (D1) the mean sprout length and (D2) mean sprout number and (D3) mean invasion index at Days 1 and 5. (n=5, *p< 0.05; **p<0.01; ***p<0.001)
It is important to determine a co-culture ratio where the high metabolism of cancer cells would not suppress the growth and proliferation of other cell types or hinder any critical tumor-stroma interactions. Thus, all groups were compared to determine an optimal ratio based on their propensity to exhibit tumor angiogenesis as well as cancer invasion in fibrin. Specifically, tumor spheroids encapsulated in fibrin were observed over a period of five days to deduce which ratio among the three exhibited sprouting angiogenesis without getting dominated or overcrowded by the aggressive cancer cells. After 24 h of culture (Day 1), small sprout-like structures were observed originating from the spheroids for all groups (Figure 1C). 19:1 exhibited the highest average sprout length as well as the highest average sprout number on Day 1 (Figures 1D1-D2). As shown in Figure 1C, these sprouts gradually disintegrated and HUVECs were mostly observed to be migrating into the fibrin matrix by Day 5. This observation was consistent for all three ratios; however, a few organized sprout-like structures were observed for 19:1 even on Day 5. Overall, both the average sprout length and number decreased significantly on Day 5 for all groups (Figures 1D1-D2). Moreover, MDA-MB-231 cells, being highly metastatic, migrated out from the tumor periphery, invaded into the surrounding fibrin matrix and even dominated the sprout formation (Figure 1C). As the invasion index was the highest for 4:1 on both Days 1 and 5 (Figure 1D3), sprout formation was severely affected by rapid cancer cell proliferation. Interestingly, 19:1 displayed a balanced behavior as compared to the other groups, where the cancer cells invaded into fibrin without hindering sprout formation (Figure 1C). Additionally, as previously mentioned, 19:1 tumor spheroid being structurally compact, rendered it easier to handle causing minimal spheroid damage during fibrin encapsulation or even 3D bioprinting (discussed later in this study). Thus, 19:1 ratio was chosen as the optimal HUVEC:MDA-MB-231 combination for the rest of the study.
2.2. The effect of matrix density on angiogenesis and tumor invasion
Under in-vivo conditions, the early steps of angiogenesis include degradation of the endothelial basement membrane and surrounding ECM, followed by migration of endothelial cells into surrounding stroma driven towards angiogenic stimuli.[25] Hence, ECM density plays an important role in guiding angiogenesis as well as cancer invasion and makes it essential to evaluate the effect of matrix composition on these biological functions to better mimic the tumor microenvironment. Six different combinations of fibrinogen (F) and thrombin (T) concentrations were selected for this study. The first three involved varying the fibrinogen concentration as 1.5 mg/ml (F1.5), 3 mg/ml (F3) and 6 mg/ml (F6) while keeping the thrombin concentration constant at 1.2 U/ml (T1.2). Thereafter, the thrombin concentration was varied as 1.2 U/ml (T1.2), 2.4 U/ml (T2.4) and 4.8 U/ml (T4.8) while keeping the fibrinogen concentration constant at F3. On mixing fibrinogen and thrombin, the onset of turbidity in the gel clot was visually monitored, which indicated onset of fibrin crosslinking. It was observed that the crosslinking time increased from F1.5 to F6 (Figure S2A). Additionally, increasing the thrombin concentration decreased the crosslinking time (Figure S2B). Pre-vascularized 19:1 co-culture tumor spheroids were encapsulated in all above-mentioned combinations to observe how fibrin network architecture affected the growth and angiogenic behavior of spheroids (Figure S3). These fibrin encapsulated tumor spheroids were cultured for up to a period of 5 days. On Day 1, tumor spheroids exhibited sprout-like structures for all fibrin combinations (Figures 2A1-B1). F1.5T1.2, probably being the least dense or most porous, resulted in the highest average sprout length as compared to F3T1.2 and F6T1.2 on both Days 1 and 5. The mean sprout length at Day 1 was ~ 181 μm for F1.5T1.2, ~ 99 μm for F3T1.2 and ~ 40 μm for F6T1.2, which decreased to ~ 67 μm, ~ 48 μm, and ~ 33 μm, respectively, at Day 5, for all groups (Figure 2A2). The average number of sprouts also followed a similar trend with a higher mean sprout number on Day 1 as compared to Day 5 (Figure 2A3). HUVECs could not maintain stable sprouts beyond Day 3 and hence these sprouts disintegrated, tip cells responsible for leading the sprout regressed, and instead, HUVECs migrated out from spheroids. Interestingly, varying the thrombin concentration also affected the angiogenic sprouting behavior. The mean sprout length as well as the mean sprout numbers were higher for F3T1.2 as compared to F3T2.4 and F3T4.8 (Figures 2B2-B3). Sprout length increased to an average of ~ 68 μm for F3T2.4 and ~ 57 μm for F3T4.8 as compared to ~ 99 μm for F3T1.2, on Day 1. However, for Day 5, the mean sprout length decreased to ~ 48 and ~ 45 μm for F3T1.2 and F3T2.4, respectively, while remaining similar (~ 40 μm) for F3T4.8.
Figure 2:
Impact of fibrin density on angiogenic sprouting and cancer invasion. (A1) Representative fluorescent images of tumor spheroids (19:1 ratio) encapsulated in fibrin with varying fibrinogen concentrations at a constant thrombin concentration. Images represent the initiation of sprouting and tumor invasion on Day 1 and subsequent disintegration of spouts and increased invasion on Day 5. Graphical representation of (A2) the mean sprout length, (A3) mean sprout number, and (A4) mean invasion index of cancer cells at Days 1 and 5. (B1) Representative fluorescent images of tumor spheroids (19:1 ratio) encapsulated in fibrin with varying thrombin concentrations at constant fibrinogen concentration at Days 1 and 5. Graphical representation of (B2) the mean sprout length, (B3) mean sprout number, and (B4) mean invasion index of cancer cells at Days 1 and 5 for constant fibrinogen concentrations (n=5, *p< 0.05; **p<0.01; ***p<0.001).
Varying matrix density also affected tumor growth. Cancer cells were determined to be ~1.75- and 1.78-folds more invasive at Days 1 and 5, respectively, for F1.5T1.2, when compared to F3T1.2 (Figure 2A4). Similarly, invasion index of F1.5T1.2 was determined to be 3–4 folds higher than that of F6T1.2 for both time points. For the groups with varying thrombin concentration, the invasion index was ~ 1.1- and 1.8-folds higher for F3T1.2 as compared to F3T2.4 and F3T4.8, respectively, at Day 1. At Day 5, the difference in the growth behavior became more apparent with ~ 1.5 and 3-fold increase for F3T1.2 with respect to F3T2.4 and F3T4.8, respectively (Figure 2B4).
To comprehend this observed difference in angiogenic and invasive behavior with varying fibrin precursor concentrations, ultrastructural changes in fibrin architecture was also investigated for all matrix densities at Days 1 and 5. Largely, all fibrinogen and thrombin concentrations resulted in fibrin with highly porous network with interconnected macro and micro-pores (Figure 3A1, B1). On further investigation, it was observed that on Day 1, F1.5T1.2 formed thicker fibers with a mean diameter of 95 nm as compared to F3T1.2 and F6T1.2, which were 75 and 57 nm, respectively (Figure 3A2). Increasing fibrinogen concentration resulted in decreased protofibril diameter, increased fibrin network density, and reduced pore size (Figures 3A1-A2). When increasing thrombin concentration from T1.2 to T4.8 while keeping the fibrinogen concentration constant, mean fiber diameter decreased from 75 nm for F3T1.2 to 34 nm for F3T4.8, on Day 1 (Figure 3B2). Low fibrinogen and thrombin concentrations (F1.5T1.2 or F3T1.2) produced fibrin clots that were composed of thick, loosely woven fibrin strands as compared to high precursor concentrations (F6T1.2 or F3T4.8). However, a significant change in network organization was observed after 5 days of culture. Fibrin appeared to have undergone extensive degradation by Day 5, leading to decreased fiber diameters (Figures 3A1, B1, second row). F1.5T1.2, probably being the least dense, underwent extensive fiber reorganization and degradation with mean fiber diameter decreased to 30.4 nm (Figures 3A1-A2, Day 5). Among the groups with constant fibrinogen concentration, more degradation was observed for F3T1.2 than others (Figure 3B1, Day 5). The mean fiber diameter obtained was 22.8 nm for F3T1.2 and 29.4 nm and 28.7 nm for F3T2.4 and F3T4.8, respectively (Figure 3B2, Day 5). Both F1.5T1.2 and F3T1.2 formed interconnected macro-pores dotted with several micropores making it conducive for cells to invade, sprout and migrate through easily. Interestingly, even though F3T2.4 and F3T4.8 had lower mean fiber diameters than F3T1.2 on Day 1, after five days of culture, invading cells seemed to have restructured the matrix by pushing the fibers together to form thicker bundles (Figure 3B1, second row).
Figure 3:
Ultrastructural and mechanical properties of different fibrin concentrations. (A1) SEM images of fibrin architecture (12000x) formulated with a constant thrombin concentration (1.2 U/ml) and varying fibrinogen concentrations (1.5, 3, and 6 mg/ml) at Days 1 and 5. (A2) Graphical representation of mean fiber diameter of fibrin observed at Days 1 and 5. (B1) SEM images of fibrin architecture formulated with a constant fibrinogen concentration (3 mg/ml) and varying thrombin concentrations (1.2, 2.4, and 4.8 U/ml) at Days 1 and 5 of culture. (B2) Graphical representation of mean fiber diameter of fibrin observed at Days 1 and 5. (C) Plot of storage modulus versus shear strain for all fibrin concentrations. (D) Graphical representation of relative stiffness obtained from shear modulus at 1% shear strain for all fibrin concentrations. Inset SEM images represent higher magnification (120,000x)view of fibers formed (n=5 for all, *p< 0.05; **p<0.01; ***p<0.001).
This difference in ultrastructure of fibrin architecture was also expected to affect fibrin gel stiffness. Thus, rheological study was performed for all fibrin samples to determine the stiffness of the gel clots formed (Figure 3C). As the fibrinogen concentration was varied, F6T1.2 formed stiffer gels (~ 53 Pa) as compared to F3T1.2 (~14 Pa) and F1.5T1.2 (~7 Pa) (Figure 3D). This supported our previous observation, where sprouting and cell invasion were hindered in F6T1.2, indicating the stiff nature of this fibrin combination. When stiffness among the groups with varying thrombin concentrations were compared, the gel with the lowest thrombin concentration (F3T1.2) resulted in a stiffer gel (~14 Pa) than the gel with the highest thrombin concentration, F3T4.8 (~10 Pa).
These studies suggested that, both F1.5T1.2 and F3T1.2 groups could be used for fabrication of tumor models; however, as F1.5T1.2 exhibited increased crosslinking times, weaker mechanical properties, and faster degradation, all further experiments were performed using F3T1.2.
2.3. The impact of HDF density on tumor angiogenesis
Fibroblasts are known to be the most abundant cell type in the native breast tumor microenvironment.[26] Recent studies have shown cancer cells secrete cytokines to transform normal resident fibroblasts to ‘activated’ or cancer-associated fibroblasts (CAFs) by epigenetic modulation.[27],[28] These activated fibroblasts secrete growth factors and signaling molecules to eventually aid in tumor angiogenesis as well as cancer invasion. Moreover, fibroblasts are also known to support and strengthen capillary formation during angiogenesis.[29] Thus, to recapitulate a native tumor niche, pre-vascularized tumor spheroids (19:1) were encapsulated in fibrin preloaded with human dermal fibroblasts (HDFs). HDF population in fibrin, representing the tumor stroma, was varied from 0.25 million cells/ml (25k) to 2 million cells/ml (200k) in order to analyze the effect of fibroblast density on angiogenic sprouting as well as cancer invasion. Sprouting angiogenesis was initiated within 24 h of culture, for all HDF densities. These sprouts were guided by the ‘tip cell’ following chemokine gradients in the matrix.[[30]] ‘Stalk cells’ followed behind the tip cell and arranged themselves in a capillary like structure, supported by the surrounding HDFs. Over a period of five days, these sprouts grew radially outwards from the spheroid forming a primary vessel structure, which then branched into secondary vessels or capillaries (Figures 4A-B). These capillaries developed a hollow lumen, mimicking native physiology (Figure 4B). Thick capillaries were also observed growing through the tumor center, and the capillary sprouting extended through different Z planes as well. As discussed in previous sections, HUVEC:MDA-MB-231 co-cultured tumor spheroids could not maintain stable sprouts over time in the absence of HDFs (control).
Figure 4:
Impact of HDF density on angiogenic sprouting. (A) Fluorescent images of tumor spheroids encapsulated in fibrin loaded with varying densities of HDFs (0.25 million cells/ml (25k), 0.50 million cells/ml (50k), 1 million cells/ml (100k) and 2 million cells/ml (200k)). The control group was fabricated without any HDFs in the matrix. (B) A fluorescent image of capillary branching into secondary vessels. Graphical representation of all (C1-C4) sprouting properties and (C5) normalized invasion index at Day 5 (n=5 for all, *p< 0.05; **p<0.01; ***p<0.001).
The angiogenic sprouting for all HDF densities was analyzed using Angiotool. The total vessel length (TVL), which was defined as the sum of all the euclidean distance between the pixels of all vessel elements present, was dependent on HDF density. As the HDF density increased from 25k to 100k there was an increase in TVL from 35 to 55 mm (Figure 4C1). However, further increase in HDF density from 100k to 200k resulted in shorter vessels, thus lowering TVL to 50 mm. Although, sprouts did not grow long for the 200k group, they were observed to be thicker in morphology. With a low density of HDFs (25k-50k), tip cells guiding the sprouts invaded a longer distance. As the primary sprout grew longer, the main vessel branched into secondary vessels, giving rise to junctions or branching points. Varying HDF density also affected the vessel branching and density. Branching index (BI), defined as the number of vessel junctions normalized per unit area and vessels density (VD), the percentage of area occupied by vessels, followed a similar trend like TVL (Figures 4C2-C3). Furthermore, it was construed that VD and mean lacunarity (ML) were inverse properties. A higher VD was expected to result in lesser availability of free space or lacuna around the tumor spheroid, as shown in Figure 4C4. It is important to note here that all the above sprouting properties increased up until HDF concentration of 50k, remained almost similar from 50k to 100k and then decreased beyond 100k. Using 200k HDFs for the tumor stroma resulted in decreased sprouting length, branching index as well as vessel density. Thus, a range of 50k-100k HDFs served as the critical range of stromal cell density, which would suffice fabrication of an in-vitro tumor model with stable vascularization.
The invasive nature of MDA-MB-231 cells was also influenced by changing the HDF density (Figure 4C5). As the HDF density increased from 25k to 200k, the mean invasion index at Day 5 of the growing tumor decreased by ~ 2 folds (from 17.8 to 8.7 ). Comparing the invasion index of all HDF containing groups with the control group, mean invasion index for 25k, 50k and 100k groups were found to be higher than the control group. As the HDF density increased to 200k, the mean invasion index was found to be similar to that for the control group.
2.4. RNA sequencing study
In order to gain insights into the molecular mechanisms triggered by different microenvironmental conditions in the tumor models, a transcriptomic analysis was performed with biological duplicates of six experimental groups, cultured for three days. The co-culture time for the RNA sequencing was defined as three days instead of five because in the presence of HDFs there was extensive degradation of fibrin matrix which might result in loss of angiogenic sprouts. Thus, three days was considered an optimal time point to evaluate mRNA expression for all experimental groups. Three of these groups were cultured in fibrin (F3T1.2) devoid of HDFs or any support cells and comprised of tumor spheroids containing only MDA-MB-231 cells (MDA231-only), only HUVECs (HUVEC-only), and containing both HUVEC and MDA-MB-231 cells combined at a ratio of 19:1 (H231). The remaining three groups comprised of spheroids cultured in a HDF-laden matrix and included spheroids containing only HUVECs (HUVEC-HDF), tumor spheroids made up of 19:1 combination of HUVECs and MDA-MB-231 cells (H231-HDF) and HDFs alone cultured in fibrin (HDF-only). HDF concentration in these cultures were all maintained at 50k as this was ascertained to be the critical concentration of HDFs from our previous experiments.
Principal component analysis (PCA) showed high transcriptional homogeneity between the biological duplicates of each group. Additionally, more than 90% variability among the groups was captured by the first two axes, PC1 and PC2 (Figure 5A). A distinct variation was observed between the groups containing HDFs (clustered towards the top-right) and the groups with no HDFs (clustered towards the top left, probably dominated by MDA-MB-231 cells). However, HUVEC-only group was the most different from all other groups as it did not contain any HDFs or MDA-MB-231 cells, and clustered towards the bottom of the PC2 vs PC1 plot.
Figure 5:
Comparison of gene expression profiles and pathway analysis of H231 with MDA231-only and HUVEC-only spheroid cultures. (A) Principle component (PC) analysis of all groups used in RNA sequencing study. Transcriptional variations between the groups denoted by the first two PC axes, PC1 and PC2. (B1) Heatmap of all 173 differentially expressed genes in H231 as compared to MDA231-only and HUVEC-only monoculture spheroids. (B2) Ten most enriched and significant canonical pathways in H231 co-culture tumor spheroid (p-value <0.05). (C) Heatmap of all genes expressed in H231 due to the presence of MDA-MB-231. (D) Heatmap of all genes expressed in H231 co-culture due to the presence of HUVECs. Genes were annotated pink for angiogenesis, blue for metastasis and orange if regulating both angiogenesis and metastasis based on the literature Ten most enriched and significant canonical pathways in H231 co-culture tumor spheroid due to the presence of (E1) MDA-MB-231 and (E2) HUVECs (p-value <0.05).
Gene expression signature of the homocellular cultures, MDA231-only and HUVEC-only, were compared with the co-culture H231. 173 Differentially expressed genes (DEGs) were found in H231, among which 129 genes were upregulated and 44 were downregulated in H231 with respect to both MDA231- and HUVEC-only groups. Gene expression of all 173 genes were depicted on a heatmap (Figure 5B1) and the individual genes along with their functions were tabulated in Table S1. As mentioned in Table S1, among the 173 genes, 10 long noncoding RNAs (lncRNA), and 8 pseudogenes were differentially expressed in H231 co-culture as compared to the MDA231- and HUVEC-only groups. Ingenuity pathway analysis (IPA) of the 173 DEGs revealed oxidative phosphorylation, mitochondrial dysfunction, EIF2 signaling, and sirtuin signaling pathway as some of the most enriched canonical pathways (Figure 5B2, Table S2). Interestingly, almost all of the 173 DEGs were found to be involved in cancer progression, tumor growth, recurrence or distant organ metastasis, and tumor angiogenesis. This indicated that H231, being a combination of HUVEC and MDA-MB-231 cells, exhibited variations in transcriptomic profiles that were otherwise not obvious in the homocellular MDA231- and HUVEC-only groups. Further, to ascertain the effect of MDA-MB-231 cells and HUVECs individually on the H231 co-culture, genes differentially expressed between HUVEC- and MDA231-only were overlapped with genes differentially expressed between H231 versus HUVEC-only and H231 versus MDA231-only, respectively. 2736 genes were found in H231, influenced by the presence of MDA-MB-231 cells (Figure S4A, Table S3) and only an overlap of 76 genes, influenced by HUVECs in the H231 co-culture (Figure S4B, Table S3). To determine how MDA-MB-231 and HUVECs individually affect downstream biological functions of angiogenesis and cancer invasion, when mixed together in H231, genes regulating angiogenesis and metastasis were functionally annotated based on their predicted functions (by IPA) and depicted on a heatmap (Figure 5C-D). It was observed that MDA-MB-231 cells caused upregulation of genes associated with metastasis and downregulation of most genes associated with angiogenesis pathways in H231 (Figure 5C). Whereas, HUVECs caused upregulation of a few angiogenesis- (MGP, MMP2, ACVRL1, IL1RL1, LRRC32, etc.), and metastasis-related genes (ANKRD1, MYO10, MTUS1, MEG3, etc.) in H231 (Figure 5D). Further, most enriched pathways in H231 due to the presence of MDA-MB-231 cells correspondeded to pathways regulating cell division, metabolism (sirtuin signaling pathway, superpathway of cholesterol biosynthesis), cell survival (EIF2 signaling pathway) and cancer progression (hereditary breast cancer signaling) (Figure 5E1, Table S4). Similarly, pathways regulating tumor initiation and vascularization (hepatic stellate cell activation, HIF1α signaling, inhibition of matrix metalloproteases) were enriched due to the presence of HUVECs in H231 (Figure 5E2, Table S5).
As presented previously, the introduction of HDFs in fibrin had significantly impacted the tumor microenvironment by enhancing tumor angiogenesis as well as cancer invasion up until a critical concentration of 0.5 million HDFs/ml (50k). Therefore, we sought to compare the transcriptional variations as well as activated pathways in H231-HDF versus H231. 212 Canonical pathways were enriched in H231-HDF, majority of which were involved in cancer progression (integrin signaling, IL-8 signaling, GP6 signaling pathway, regulation of the epithelial-mesenchymal transition pathway, etc.), tumor angiogenesis (HIF1α, CXCR4, and PDGF signaling, etc.) as well as fibroblast activation (hepatic stellate cell activation, IL-17A signaling in fibroblasts, etc.) (Figure 6A, Table S6). Genes from the most enriched pathway, hepatic stellate cell activation pathway, belonged to the collagen family, SMAD family, MMPs, plasminogen activator inhibitor-1 as well as several growth factors, all of which were upregulated in H231-HDF (Figure S5A). Further, when H231-HDF was compared with HUVEC-HDF and HDF-only groups, genes associated with the ‘transformation of fibroblast cell line’ function, namely, CLUL1, EEF1A1, FH, FEN1, AURKB and HSP90AA1 were all upregulated and IRF1, SERPINA3, BMI1 and CLU were downregulated in H231-HDF (Figure S5B). Additionally, expression of various ‘activated fibroblast’ or ‘CAF’ markers, such as, FN1, FAP, RPS6KA1, EGLN1, ARHGAP26, POSTN, CXCR4, CCT6A, TGFBI, SERPINE1, PLOD2, COL1A1, NME2, VIM, COL3A1, P4HB and CAV1, were investigated in H231-HDF vs HUVEC-HDF and HDF-only groups (Figure S5C). All of these genes were upregulated with the exception of CAV1 and P4HB. P4HB was also downregulated in the H231 group as compared to its homocellular counterparts, MDA231- and HUVEC-only groups.
Figure 6:
Comparison of gene expression profiles and activated pathways in H231-HDF versus H231. (A) Significant (p-value <0.05) and most enriched canonical pathways in H231-HDF versus H231. Heatmap of genes involved in (B) tumor invasion and (C) development of vasculature, two major hallmarks of cancer, were differentially expressed in H231-HDF as compared to H231.
Genes involved in regulation of the epithelial-mesenchymal transition (EMT) pathway (Figure S5D), HIF1α signaling pathway (Figure S5E), GP6 signaling pathway (Figure S5F), all of which play a significant role in cancer progression and angiogenesis, were all up-regulated in H231-HDF. Nearly, 70% of the ‘tumor invasion’ associated genes and ~80% of the angiogenesis or ‘development of vasculature’ associated genes were upregulated in H231-HDF (Figures 6B-C). The regulator effects analysis in IPA predicts how activated or inhibited transcription regulators might cause an increase or decrease in a downstream functional outcome. Using this feature, lysophosphatidic acid (LPA), HMGB1, and DEF6 were determined to be some of the prominent regulators predicted to activate downstream function of tumor invasion (Figure S6). BSG and CCL11 were also predicted to be activated and involved in downstream function of angiogenesis (Figures S7). IPA also predicts upstream regulators, including transcription factors, cytokines, growth factors, enzymes, receptors, kinases, and pharmacological agents which are all capable of regulating gene expression. Based on the DEGs in H231-HDF microenvironment, 2716 upstream regulators were identified (Table S7). Among these, some of the highly enriched and activated upstream regulators were identified as TP53, TGFB1, TWIST1, PDGFBB, etc., indicating the importance of these transcription regulators in a complex breast tumor microenvironment.
2.5. Fabrication of a tumor-associated vascular bed
The native tumor microenvironment is crowded by the presence of capillaries or leaky blood vessels, which help cancer cells to metastasize to secondary locations.[[31]] In order to accurately replicate a vascularized tumor microenvironment, analogous to a native tumor niche, another level of complexity was added to our in-vitro tumor model. To fabricate a vascular bed of capillaries encompassing a growing tumor, HUVECs were introduced in fibrin along with HDFs. The ratio of HUVECs to HDFs were maintained at an optimal ratio of 2:1. A ratio of 1:1 or 1:2 did not produce a capillary bed since, in both cases, the concentration of HUVECs was not sufficient as compared to that of the HDFs (data not shown). The total number of both cell types combined in the tumor stroma, was varied from 0.25 million cells/ml (25k) to 2 million cells/ml (200k), similar to the previous case with HDFs (presented in Section 2.3.). These samples were cultured for a slightly longer time (seven days) as compared the previous cases (five days) as it was observed that a culture period of five days was not sufficient for complete vascular network formation. When comparing all the groups, it was observed that 25k did not facilitate complete connection of vascular network, even after a 7-day culture period. As the cell density in the tumor stroma increased from 25k to 50k, 100k, and 200k, sprouting capillaries from tumor spheroids and those in the matrix anastomosed to arrange into thick capillaries. The capillaries gradually arranged themselves into a complex vascular network and were also observed to be growing internally through the tumor core (Figure 7A). Furthermore, some cancer cells also intravasated into the capillaries growing internally through the tumor, as shown in Figure 7B.
Figure 7:
Fabrication of a vascular bed. (A) Representative fluorescent images of tumor spheroids encapsulated in fibrin loaded with varying densities of HDFs and HUVECs. The combined HDF and HUVEC density was varied as 0.25 million cells/ml (25k), 0.50 million cells/ml (50k), 1 million cells/ml (100k), and 2 million cells/ml (200k). The first row shows 4x magnification images of the tumor and the second row shows higher magnification images (10x) of the selected region. (B) Confocal images of a capillary growing internally through the tumor exhibiting cancer cell intravasation. Orthogonal projection of the stack shows a cancer cell residing inside a hollow capillary. (C) Graphical representation of all sprouting properties and normalized invasion index at Day 7. (n=5, *p< 0.05; **p<0.01; ***p<0.001)
It was interesting to note that capillaries with different diameters were formed mimicking in-vivo physiology (Figure S8A).[32] As shown in Figure S8B, capillaries within tumor spheroids had an average diameter of ~ 40 μm for the 50k, ~ 50 μm for the 100k and ~ 70 μm for the 200k case. Capillaries surrounding the tumor, which formed via anastomosis with neighboring HUVECs preloaded in fibrin, had an average diameter of ~ 20 to 40 μm while capillaries formed away from the tumor site were ~ 10 to 17 μm on average, for all cases. However, for 25k case, capillaries had an average diameter of only 10 to 14 μm, irrespective of their proximity to the primary tumor site.
The capillary network was further analyzed using Angiotool to obtain quantitative information (Figure 7C). The mean TVL was found to be significantly low for 25k, ~53 mm, while 200k had the highest mean TVL of ~160 mm. As smaller or disconnected capillaries formed for 25k as compared to a well-connected network of capillaries for 200k, these values qualitatively aligned well with our observations. Meanwhile, TVL for 50k and 100k were determined to be similar, ~ 92 mm. All other parameters, such as, BI and VD were similarly the lowest for 25k and the highest for 200k. As mentioned before, mean lacunarity showed an inverse behavior of VD, being the highest for 25k and the lowest for 200k. The extent of cancer cell invasion into fibrin was dependent on the stromal cell density. Invasion index at Day 7 was the highest for 25k having a value of ~ 13 and significantly decreased to ~ 9, ~ 5 and ~ 4 for 50k, 100k and 200k, respectively.
2.6. Effect of proximity of tumor spheroids on cancer invasion and tumor angiogenesis
Physiological changes in tumor behavior is a direct result of the tumor microenvironment, where cancer-endothelial cell crosstalk is equally important as the cancer-fibroblast crosstalk. This cancer-endothelial crosstalk could be modulated via paracrine signaling based on the chemokine and growth factor gradients in the surrounding matrix.[[33]] Thus, we sought to enquire if pre-vascularized tumors, when placed in close proximity, influenced angiogenesis and metastasis. In this regard, aspiration-assisted bioprinting[[24]] was employed to bioprint pre-vascularized tumor spheroids in order to determine proximity induced effects on their collective angiogenic as well as metastatic behavior. The tumor spheroids were bioprinted in a triangle pattern to resemble a group of tumors growing near each other and thus equally capable of influencing each other (Figure 8A). The mean distances between tumor spheroids were maintained at ~700 (Near-700), ~1200 (Mid-1200), ~1700 (Mid-1700) and ~2500 μm (Far-2500, isolated spheroids used as a control group). The general idea was to bioprint four different model groups, one being in a very close proximity, two in the mid-range, and then the last being the one with the lowest proximity. While culturing these tumor spheroids for five days, it was observed that all tumor spheroids exhibited sprouting angiogenesis but the sprouting properties were dependent on the proximity of tumors to each other. The combined length of sprouts progressively increased from ~21 mm for Near-700 μm to ~23 mm for Mid-1200 group, reaching a peak of ~35 mm for Mid-1700 and then the value decreased to ~22 mm for the control group (Far-2500 μm, Figure 8B). However, an opposite trend was observed for branching index and vessel density for the first three groups. Since vessels density was calculated based on the percentage of vessels occupying the entire vascularized area, as the distance between spheroids increased, the vessel density decreased. However this decrease was only significant for the control group as compared to the Near-700 and Mid-1200 μm groups. Overall, tumor spheroids in the control group had significantly lower sprouting length, and branching index as well as vessel density. These results clearly indicated that the proximity between vascularized tumor spheroids influenced angiogenic sprouting. Irrespective of the distance between tumors, it was also observed that the invasion index/spheroid was similar for all four groups (Figure 8C).
Figure 8:
Aspiration-assisted bioprinting of tumor spheroids. (A) Representative fluorescent images of spheroids bioprinted at 700 μm (Near-700), 1200 μm (Mid-1200), 1700 μm (Mid-1700) and 2500 μm (Far-2500, control) distances. Graphical representation of (B) sprouting properties and (C) invasion index for all distances at Day 5. (Note: invasion index was normalized to Day 1; n=5, *p< 0.05; **p<0.01; ***p<0.001).
3. Discussion
Tumor spheroids serve as essential tools for understanding the intricate tumor-stroma crosstalk, identify therapeutic targets and utilize the platform for pre-clinical anti-cancer drug screening. Overcoming the drawbacks of 2D culture systems, spheroid cultures have gained importance as they tend to preserve cellular interactions as observed in vivo.[34] Unlike most cell types, metastatic MDA-MB-231 does not undergo Cadherin-induced spheroid formation and is usually unable to form compact spheroids. Thus, co-culturing these cells with HUVECs probably upregulates ECM deposition and enhances their integrin ß1-ECM interactions, forming compact spheroids within a day of culture.[35] Addition of HUVECs with MDA-MB-231 cells pre-vascularizes the tumor and exhibits a suitable co-culture model for studying cancer-endothelial interaction in vitro. Three different ratios of HUVEC and MDA-MB-231 cells were compared in this study namely, 4:1, 9:1 and 19:1. We started with a 4:1 ratio of HUVEC and MDA-MB-cells because of the highly metastatic nature of MDA-MB-231 cells. A 1:1 co-culture ratio of both cells resulted in MDA-MB-231 dominance over HUVEC growth and proliferation in vitro, as observed from even Day 5 cultures of the 4:1 ratio. Thus, starting with the 4:1 ratio, HUVEC population was gradually increased to 9:1 and then 19:1 ratio until a balance was achieved between capillary sprouting as well as tumor invasion. Additionally, the 19:1 co-culture was representative of native tumor physiology as suggested by their transcriptomic profiles. Genes differentially expressed in the presented co-culture models as compared to the homocellular were found to be involved in cancer progression, metabolism and tumor growth. Interestingly, the differential expression of several long noncoding RNAs (lncRNAs) and pseudogenes, some of which have previously been studied in relation to cancer, highlights the importance of using such 3D co-culture systems for in-vitro tumor modeling.[36]
These pre-vascularized co-culture tumor spheroids, when encapsulated in varying fibrin concentrations, exhibited sprout-like structures, even in the absence of any support cells, such as fibroblasts, which are known to support angiogenesis.[29] This could be due the fibrin’s inherent angiogenic properties, such as integrin binding domains[37] along with the presence of pro-angiogenic factors in culture media and cytokines secreted by the metastatic breast cancer cells. MDA-MB-231s are known to secrete vascular endothelial growth factor (VEGF), an important ‘tip cell’ activator, thus promoting angiogenesis.[38] Tip cells respond to chemotaxis, extending multiple filopodia towards the biochemical stimuli. Stalk cells, on the other hand, proliferate in response to ECM cues, including mechanical and static stretch.[39] However, without the presence of support cells, these sprouts gradually disintegrate even before maturation. Tip cells probably lose their phenotype, transform into proliferating stalk cells, and eventually undergo apoptosis after being dominated by rapidly multiplying cancer cells in fibrin.[40],[29]
Varying fibrinogen and thrombin concentrations affected fibrin network architecture and clot rigidity leading to altered cell migration and angiogenic sprouting. Angiogenesis as well as cancer invasion was significantly impaired in higher fibrinogen concentrations probably due to slower rate of tissue plasminogen activator (tPA) -mediated plasmin generation leading to reduced rate of fibrinolysis.[41] Moreover, it has been previously observed that even though there is a small degree of change in fiber branching at higher fibrinogen concentrations, increased fiber density from greater total mass of protein produces stiffer clots.[42],[43] However, higher thrombin concentrations produced lower clot stiffness probably due to lower protofibril packing per fiber,[44] resulting in complex networks with lower porosity, thus hindering cancer invasion as well as endothelial sprouting.[42] Fibrin clot containing co-culture HUVEC:MDA-MB-231 spheroids underwent degradation resulting in reduced fiber diameters for all fibrin concentrations, after five days of culture. This could be due to an increased proteolytic activity of MDA-MB-231 cells,[45] whereby secreted serine proteases could further induce endothelial cells to adopt a mesenchymal phenotype, eventually undergoing an endothelial to mesenchymal transition.[46],[47]
Fibroblasts, an integral component of tumor stroma, play a prominent role in cancer progression, by initiating ECM remodeling, secreting chemokines, and facilitating angiogenic recruitment of endothelial cells and pericytes.[28], [48] Incorporation of fibroblasts in fibrin enhanced tumor angiogenesis as well as cancer invasion but only up to a critical concentration range of 0.5–1 million cells/ml. This positive regulation was brought about by cellular crosstalk between MDA-MB-231 cells, HUVECs and HDFs, which was evident from the enrichment of several canonical pathways which are known to play indispensable role in cancer metastasis, invasion and tumor angiogenesis. Additionally, metastatic regulator LPA, predicted to be activated in HUVEC-MDA-MB-231 co-cultures in HDF-laden fibrin, is a naturally occurring bioactive phospholipid involved in multiple cellular events associated with tumor initiation and metastasis.[49],[50] It is also known to promote trans-differentiation of myofibroblasts by paracrine mechanism and is considered to be a therapeutic target.[51] Similarly, DEF6, HMGB1, BSG, CCL11, activated regulators in our tumor microenvironment, could all be potentially explored as possible targets for developing breast cancer therapies. TP53, TGF-β1 and TWIST1 were among the top activated upstream regulators identified by IPA. TP53 (tumor protein p53) is a tumor suppressor gene frequently mutated in cancer, involved in cell-cycle regulation, apoptosis, and angiogenesis,[52] while TGF-β1 and TWIST1 are known for their role in regulating EMT and enhancing metastasis.[53] Previous studies have also identified TWIST1 as a key regulator of CAFs.[54]
It is known that activated fibroblasts in a tumor microenvironment act differently as compared to fibroblasts from non-tumorigenic sources. Since, in our study, fibroblasts were distributed throughout the entire fibrin clot, some were in close contact with cancer cells while the rest, although not in direct contact, were in presence of the same conditioned media.[[55]] In our tumor model, IPA revealed ‘Hepatic Stellate Cell Activation Pathway’ as the most enriched pathway, which is indicative of fibroblast activation.[56] Upregulation of several genes from the collagen family, involved in this pathway, shed light on the collagen types which might play a critical role in activating fibroblasts.[57] Similarly, upregulation of matrix metalloproteinase (MMP1, MMP2) and tissue inhibitor of metalloproteinase (TIMP1, TIMP2) involved in this pathway, highlighted the specific ECM remodeling ability of HDFs in this microenvironment.[58] Additionally, upregulation of activated fibroblast markers, indicated that HDFs that are in close association with cancer cells were probably undergoing transcriptomic changes to a more ‘CAF-like’ state. Furthermore, downregulation of gene CAV1 also suggested an ‘activated state’ of fibroblasts and loss of CAV1 has previously shown to enhance MDA-MB-231 tumor growth.[59] Absence of CAV1 from tumor stroma is also considered an important predictor of poor clinical outcome in human breast cancer patients. Beyond 1 million HDF cells/ml, vascular network formation and cancer metastasis was diminished, which could be due to cell-cell contact inhibition caused by crowding of stromal cells and nutrient deprivation resulting in shorter vessels and reduced invasion.[60], [61], [62] Even though a combination of MDA-MB-231 cells and HDFs was not considered as a group, comparing H231-HDF and HUVEC-HDF groups could provide some insight or predict MDA-MB-231 interaction with HDFs. In the future, we would consider studying MDA-MB-231 spheroids alone with HDFs for a deeper understanding of the influence of cancer cells on HDFs.
The co-culture spheroids sprout capillaries from the tumor, which in an in-vivo setting, anastomose with existing capillaries around the tumor.[63] This results in a vascular bed of tumor associated capillaries as represented by our tumor model as well. Anastomosis of HUVECs from the sprouting tumor spheroid with the vascular network formed in the fibrin matrix resulted in a wide range of capillaries, the largest being 70 μm and smallest being 10 μm, similar to in-vivo tumor microenvironment.[32],[64] Formation of dilated capillaries through the tumor core could be probably attributed to the upregulation of VEGFA production, induced by hypoxia or hypoxia-inducible factor-1, which is known to perpetuate unregulated angiogenesis.[65] Furthermore, metastatic MDA-MB-231 cells intravasated into these dilated capillaries.[66], [67], [68]
Aspiration-assisted bioprinting was employed to 3D bioprint a representative tumor model in fibrin highlighting the effect of proximity of multiple tumors on collective tumor angiogenesis and cancer invasion.[69],[70],[71],[72] Tumor spheroids were aspirated from media by applying a critical lifting pressure, which was determined by the surface tension of spheroids. Surface tension correlates well with the mechanical properties and compactness of spheroids.[24] Cell-cell adhesion molecules, such as N-cadherin, play an important role in driving tissue surface tension and has proven to be instrumental in dictating tissue cohesivity and hence in-vitro tumor invasiveness.[73] By controlling the distance between tumor spheroids, cell-cell signaling could be regulated which directly affected vessel length and vessel density as well as the branching index. Tumor spheroids bioprinted in close proximity sprouted capillaries in a confined space leading to shorter but highly branched vessels. Being close to each other in the presence of activated fibroblasts probably upregulated the combined secretion of several angiogenic growth factors, such as VEGF, Angpt-1, PDGF, in the vicinity of spheroids.[74] This localized concentration of angiogenic growth factors might have led to enhanced sprouting properties, which gradually fused to form a vascular network. Increasing the distance between tumor spheroids (from 700 to 1700 μm), allowed the tip cells leading the sprout to travel longer distances probably following chemokine gradients. However, morphological properties of sprouts decreased for tumor spheroids bioprinted far from each other (beyond 1700 μm distance), indicating that tumor proximity influenced angiogenesis and overall cell-cell signaling. Cancer invasion was however not affected by changing tumor proximity, which corroborated that invasion is more closely related to fibroblast density. Since the HDF density was constant for all bioprinted groups, there was no difference in cancer cell migration behavior from the tumor spheroids.
4. Conclusion
3D Tumor models serve as a platform for recapitulating a native tumor niche and gaining a deeper understanding of the cancer mechanistic pathways. This study provides a bottom-up approach to fabricating a breast tumor microenvironment necessary for understanding the hallmarks of cancer such as tumor angiogenesis and cancer invasion. A co-culture of endothelial and cancer cells was determined to be more representative of native conditions as compared to the homocellular spheroid model. Further, varying the fibroblast density in the tumor model not only established a relevant tumor stroma, but also unraveled a critical range of fibroblast density required to study metastasis and angiogenesis in vitro. Enrichment of fibroblast activation pathways, upregulation of genes involved in cancer progression, and tumor angiogenesis as well as activation of critical upstream regulators indicated that cancer cell-fibroblast interactions play an integral role in elucidating key mechanisms in a tumor microenvironment. Additionally, incorporation of endothelial cells in the tumor stroma along with fibroblasts established a tumor vascular bed with varying capillary diameters. Capillaries as thick as 70 μm developed through the tumor core and cancer cells intravasated into these thick hollow capillaries, exhibiting their metastatic signature. Finally, by employing 3D bioprinting, this study also revealed that proximity between spheroids influenced angiogenesis but did not affect cancer invasion. Overall, fabrication of a physiologically-relevant in vitro tumor model is not only essential for understanding critical cancer-stroma crosstalk but also identifying novel therapeutic targets and developing targeted therapies against cancer.
5. Materials and Methods
Cells and reagents:
GFP+ MDA-MB-231 breast cancer cells were donated by Dr. Danny Welch, from University of Kansas, Kansas City, USA. They were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Corning Cellgro, Manassas, VA) supplemented with 5% fetal bovine serum (FBS) (Life Technologies, Grand Island, NY), 1 mM Glutamine (Life Technologies, Carlsbad, CA) 1 mM penicillin- streptomycin (Life Technologies, Carlsbad, CA). Human umbilical vein endothelial cells (HUVECs, Lonza, Walkersville, MD) were cultured in MCDB 131 media (Corning, NY) supplemented with 10% FBS, 1mM Glutamine, 1 mM penicillin-streptomycin, 0.5 mM bovine brain extract (BBE, Lonza, Walkersville, MD), 1200 U/ml heparin (Sigma-Aldrich, St. Louis, MO) and 0.25 mM endothelial cell growth supplement (ECGS, Sigma-Aldrich). HUVECs were used at passages 3 through 8. Human dermal fibroblasts (HDFs), obtained from Dr. Nicolas Zavazava’s laboratory (The University of Iowa, Iowa City, IA), were cultured in DMEM supplemented with 10% FBS, 1% glutamine, 1% sodium pyruvate, and 1% penicillin-streptomycin. HDFs were used at passages 7 through 12. Cells were maintained at 37 °C with 5% CO2 in an air-humidified atmosphere. Cell culture medium was changed every 2–3 days. Sub-confluent cultures were detached from the flasks using a 0.25% trypsin-0.1% EDTA solution (Life Technologies) and split to maintain cell growth. HUVECs were transduced with tdTomato lentiviral vector to ease visualization for all experiments (see Supporting Information).
Fabrication of tumor spheroids, measuring tumor growth and proliferation:
Cells were individually trypsinized and suspended in calculated volumes of cell culture media. Cells were seeded in a 96-well U-bottom cell repellant plate at a density of 5,000 cells/spheroid in 75 μl of EGM-2MV media, and cultured for 24 h to form spheroids (Figure S3). HUVECs and MDA-MB-231 cells were mixed in three different ratios namely, 4:1, 9:1 and 19:1, to determine the optimum working ratio for further experiments. Tumor growth and proliferation was measured for all above-mentioned groups (4:1, 9:1 and 19:1) using a standard CCK8 protocol. Briefly, CCK8 solution was diluted in EGM-2MV media at a ratio of 1:20. Around 10 spheroids were used for each group and spheroids were exposed to 350 μL of CCK8 solution, followed by 3 h incubation.
Surface tension measurements:
In order to measure the surface tension of tumor spheroids, a micropipette aspiration technique was performed according to our previous studies.[24],[75] Briefly, the customized straight micropipette tips were fabricated from a borosilicate glass Pasteur pipet (VWR, 14673–043, Radnor, PA) using a P97 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA). Aspirated spheroids were monitored via a STC-MC33USB monochromatic camera (Sentech, Japan) equipped with 1–61448 and 1–61449 adaptor tubes (Navitar, Rochester, NY). Surface tension of 4:1, 9:1, and 19:1 tumor spheroids were measured at Days 1 and 5.
Fabrication of the 3D in vitro tumor model in fibrin:
Fibrinogen (F) and thrombin (T) were mixed in different ratios to understand the effect of matrix density on angiogenic sprouting behavior of tumor spheroids. By maintaining the thrombin concentration constant at 1.2 U/ml, the fibrinogen concentration was varied as 1.5 mg/ml (F1.5T1.2), 3 mg/ml (F3T1.2), and 6 mg/ml (F6T1.2). In addition, while the fibrinogen concentration was maintained constant at 3mg/ml, the thrombin concentration was varied from 1.2 U/ml (F3T1.2), 2.4 U/ml (F3T2.4), and 4.8 U/ml (F3T4.8). To fabricate a fibrin construct, 100 μL of say, 3 mg/ml fibrinogen was mixed with 2.4 μL of 50 U/ml thrombin to achieve F3T1.2. The onset of turbidity in fibrin clots was observed visually and time was recorded using a stopwatch. Tumor spheroids were individually pipetted out from the U bottom 96-well plate and suspended in fibrinogen in an Eppendorf tube. After adding the required volume of thrombin, the spheroid containing gel was cast on a coverslip. These 3D constructs were then cultured in EGM-2MV (Lonza) for 5 or 7 days as necessary.
Fabrication of fibrin constructs for vascularization experiments:
Upon fabrication, tumor spheroids were suspended in 100 μL of 3 mg/ml fibrinogen and then 2.4 μL of 50 U/ml thrombin was added (Figure S3). After thorough and careful mixing, fibrin gel was cast on a coverslip, incubated at the room temperatures for 10 min followed by a 20-min incubation at 37 °C. After complete crosslinking, about 400 μl of EGM-2MV media (Lonza) was added to each construct and constructs were cultured for 5 days. For fibrin constructs containing HDFs as support cells, the HDF density was varied as 0.25 million cells/ml (25k), 0.5 million cells/ml (50k), 1 million cells/ml (100k), and 2 million cells/ml (200k). To create a vascular bed, HUVECs were mixed with HDFs at a ratio of 2:1. The total number of both cell types combined were similarly varied from 0.25 million cells/ml (25k) to 2 million cells/ml (200k). For both these above mentioned cases, cells (HDFs, HUVECs) were suspended in 3 mg/ml fibrinogen prior to introducing the tumor spheroids. Fibrin constructs with only HDFs were cultured for 5 days; on the other hand, the constructs with both HUVECs and HDFs were cultured for 7 days.
Analysis of vascular network and cancer cell migration:
Images of sprouting HUVEC spheroids were taken on an Axiozoom (Zeiss, NY) and EVOS microscope (Life Technologies, CA). Sprouting properties, such as total vessels length (TVL), branching index (BI), vessels density (VD) and mean lacunarity (ML), were measured using Angiotool.[76] In order to analyze sprouting properties of tumor spheroids cultured in fibrin without HDFs on Day 5, the sprout detection threshold in the Angiotool software was adjusted to select only the ‘sprout-like’ structures. Since most of the sprouts were disintegrated, the number as well as the length of sprouts on Day 5 were calculated based on the sprout-like structures originating from the spheroid edge. All other randomly migrating HUVECs were not taken into consideration as they did not contribute to sprout formation. Cancer cell migration was measured by calculating the invasion index of a tumor spheroid at a certain time point using the below equation:
| (2) |
Area occupied by the spheroid at Day 0 and metastasized tumor area at Day n was measured in Fiji ImageJ. As MDA-MB-231 cells used in this study were GFP+, fluorescent images of tumor spheroids were converted to 16 bits, followed by Otsu thresholding, binarization and then area measurement in ImageJ.
RNA sequencing:
Total RNA was extracted from fibrin constructs using the TRIzol method.[77] Fibrin constructs containing spheroids were gradually lysed in TRIzol using a tissue homogenizer (Argos Technologies, Cole-Parmer, IL), allowing for the dissolution of RNA while keeping RNAse contaminants away. The lysed samples were incubated in room temperature for 5 min and then 200 μL of chloroform was added for every 1 ml initial homogenization solution. The samples were then carefully mixed and centrifuged at 4 °C, 12000xg for 15 min. Following chloroform extraction, the aqueous layer from each sample was collected in a separate Eppendorf tube and carefully mixed with 500 μL of isopropanol for every 1 mL TRIzol. The samples were further incubated at room temperature for 10 min and then centrifuged at 12000xg, 4 °C for 10 min. RNA appeared as a gel-like pellet at the corner of the Eppendorf tube. The RNA pellet was carefully washed with ethanol three times. Nanodrop (NanoDrop 1000 Spectrophotometer, Thermo Fisher, Waltham, MA) was used to check RNA yield and quality and then the quality was further analyzed using the Agilent Bioanalyser to generate a RNA integrity number (RIN). All samples with RIN > 7 were used for RNA sequencing. Two independent experiments were performed for each type of sample. The groups chosen for RNA sequencing were as follows: i) MDA-MB-231 spheroids, ii) HUVEC spheroids, iii) HUVEC – MDA-MB-231 spheroids (19:1), iv) HUVEC spheroids in fibrin pre-loaded with HDFs, v) HUVEC-MDA-MB-231 spheroids (19:1) in fibrin pre-loaded with HDFs, and vi) HDFs in fibrin. RNA-seq experiments were conducted at the Genomic Core Facility (Penn State, University Park) according to an in-house pipeline. Library preparation followed the published protocol for the QuantSeq 3’mRNA FWD library prep.[[78]] End point PCR library amplification was optimized by qPCR. Libraries were sequenced on the Illumina NextSeq 550 using the 150nt Mid-Output flow cell generating single end reads of 100 bps. Fragments were trimmed using Trim Galore software (https://github.com/FelixKrueger/TrimGalore) and reads with quality <20 were filtered out. Fragments were quasi-mapped to the human transcriptome hg38 using salmon (version 0.7.2)[[79]] at the gene level and differential expression analysis was performed using DESeq2 package in R (R version 3.6.1).[[80]] Differentially expressed genes were filtered on the adjusted p-values and fold changes were used for Ingenuity pathway analysis (IPA QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
Aspiration assisted bioprinting of tumor spheroids:
To study the difference in proximity induced collective angiogenic sprouting of tumor spheroids, droplets of fibrinogen (6 mg/ml, pre-loaded with 1 million HDFs/ml) and thrombin (2.4 U/ml), were deposited in a layer by layer fashion, onto circular glass by using micro-valves (INKX0517500A, Lee Company, Bashville, TN) with 250 μm nozzles (INZA3100914K, Lee Company). Tumor spheroids were bioprinted by picking each tumor out from media using a customized glass pipette with a diameter of ~80 μm and vacuum pressure of ~25 mmHg. Tumor spheroids were then printed into a semi-crosslinked fibrin matrix using a printing speed of 600 mm/s.[24] The first tumor was bioprinted within 30 sec of fibrin deposition, followed by the next two within the next minute (Figure 8A). Spheroids were bioprinted in a triangle pattern where the distance between tumor centers were maintained at ~700, 1200, 1700, and 2500 μm (control).
Statistics:
All data were presented as the mean ± standard deviation and analyzed by Minitab 17.3 (Minitab Inc., State College, PA, USA) using one-way analysis of variance (ANOVA) followed by the Posthoc Tukey’s multiple comparison test. When comparing multiple groups with a single control group, a Dunnett Multiple Comparisons test was used. Statistical differences were considered significant at *p < 0.05, **p <0.01, ***p < 0.001.
Supplementary Material
Acknowledgement
We are grateful to late Dr. N. Zavazava (Department of Internal Medicine at the University of Iowa) for providing HDFs and Dr. Danny Welch (University of Kansas, Kansas City, USA) for providing MDA-MB-231 cells. We acknowledge Brianna Frederick, an undergraduate student at Penn State, for her assistance in culturing cells and imaging bioprinted constructs. We also acknowledge the support from The Huck Institutes of Life Sciences and Materials Research Institute for providing facilities for characterization of experiments and Penn State Genomics Core Facility, University Park, PA, for helping with RNA sequencing. This work was supported by NSF awards 1914885 (I.T.O.) and 1624515 (I.T.O.) and NIH award R21 CA224422 01A1 (I.T.O. and D.U.)
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Madhuri Dey, Department of Chemistry, Penn State University, University Park, PA, 16802, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA 16802, USA.
Bugra Ayan, Engineering Science and Mechanics Department, Penn State University, University Park, PA 16802, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA 16802, USA.
Dr. Marina Yurieva, The Jackson Laboratory for Genomic Medicine and University of Connecticut Health Center, Farmington, CT 06032, USA
Dr. Derya Unutmaz, The Jackson Laboratory for Genomic Medicine and University of Connecticut Health Center, Farmington, CT 06032, USA
Prof. Ibrahim T Ozbolat, Engineering Science and Mechanics Department, Penn State University, University Park, PA 16802, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA 16802, USA; Biomedical Engineering Department, Penn State University, University Park, PA 16802, USA; Materials Research Institute, Penn State University, University Park, PA 16802, USA.
References
- [1].N. N. C. Institute, 2020. [Google Scholar]
- [2].Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordó N-Cardo C, Guise TA, Massagué J, A Multigenic Program Mediating Breast Cancer Metastasis to Bone, n.d. [DOI] [PubMed] [Google Scholar]
- [3].A. J.; Minn GP; Gupta PM; Siegel P. D. Bos, Genes That Mediate Breast Cancer Metastasis to Lung, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien P-A, 2000, DOI 10.1067/msy.2000.103883. [DOI] [PubMed] [Google Scholar]
- [5].Bos PD, Zhang XHF, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, Van De Vijver MJ, Gerald WL, Foekens JA, Massagué J, Nature 2009, 459, 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS, Breast Cancer Metastasis to the Central Nervous System, n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hanahan D, Weinberg RA, Cell 2011, 144, 646. [DOI] [PubMed] [Google Scholar]
- [8].Hanahan D, Weinberg RA, Cell 2000, 100, 57. [DOI] [PubMed] [Google Scholar]
- [9].Huang Y, Nayak S, Jankowitz R, Davidson NE, Oesterreich S, Breast Cancer Res. 2011, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Berdasco M, Esteller M, n.d, DOI 10.1016/j.devcel.2010.10.005. [DOI] [Google Scholar]
- [11].Holen I, Speirs V, Morrissey B, Blyth K, DMM Dis. Model. Mech 2017, 10, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang Y, Mirza S, Wu S, Zeng J, Shi W, Band H, Band V, Duan B, Oncotarget 2018, 9, 32191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dhiman HK, Ray AR, Panda AK, Biomaterials 2004, 25, 5147. [DOI] [PubMed] [Google Scholar]
- [14].Rijal G, Bathula C, Li W, 2017, DOI 10.1155/2017/8074890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Becker JL, Blanchard DK, J. Surg. Res 2007, 142, 256. [DOI] [PubMed] [Google Scholar]
- [16].Djomehri SI, Burman B, Gonzalez ME, Takayama S, Kleer CG, Cell Commun J. Signal. 2019, 13, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mcdonald DM, Baluk P, Significance of Blood Vessel Leakiness in Cancer 1, 2002. [PubMed] [Google Scholar]
- [18].Oraevsky AA, Savateeva EV, Solomatin SV, Karabutov AA, Andreev VG, Gatalica Z, Khamapirad T, Henrichs PM, Biomed. Optoacoustics III 2002, 4618, 81. [Google Scholar]
- [19].Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM, Br. J. Cancer 2014, 110, 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Peela N, Sam FS, Christenson W, Truong D, Watson AW, Mouneimne G, Ros R, Nikkhah M, Biomaterials 2016, 81, 72. [DOI] [PubMed] [Google Scholar]
- [21].Jaganathan H, Gage J, Leonard F, Srinivasan S, Souza GR, Dave B, Godin B, Sci. Rep 2014, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C, PLoS One 2010, 5, DOI 10.1371/journal.pone.0008849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pickl M, Ries CH, Oncogene 2009, 28, 461. [DOI] [PubMed] [Google Scholar]
- [24].Ayan B, Heo DN, Zhang Z, Dey M, Povilianskas A, Drapaca C, Ozbolat IT, Sci. Adv 2020, 6, eaaw5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D, Biomed Res. Int 2014, 2014, DOI 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Folgueira MAAK, Maistro S, Katayama MLH, Roela RA, Mundim FGL, Nanogaki S, De Bock GH, Brentani MM, Biosci. Rep 2013, 33, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hu M, Yao J, Cai L, Bachman KE, Van Den Brûle F, Velculescu V, Polyak K, Nat. Genet 2005, 37, 899. [DOI] [PubMed] [Google Scholar]
- [28].Kalluri R, Zeisberg M, Nat. Rev. Cancer 2006, 6, 392. [DOI] [PubMed] [Google Scholar]
- [29].Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CCW, Mol. Biol. Cell 2011, DOI 10.1091/mbc.E11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blanco R, Gerhardt H, Cold Spring Harb. Perspect. Med 2013, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Paduch R, Cell. Oncol 2016, DOI 10.1007/s13402-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Forster J, Harriss-Phillips W, Douglass M, Bezak E, Hypoxia 2017, Volume 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Choi H, Moon A, Arch. Pharm. Res 2018, 41, 711. [DOI] [PubMed] [Google Scholar]
- [34].Peng W, Unutmaz D, Ozbolat IT, Trends Biotechnol. 2016, 34, 722. [DOI] [PubMed] [Google Scholar]
- [35].Ivascu A, Kubbies M, Int. J. Oncol 2007, 31, 1403. [PubMed] [Google Scholar]
- [36].Schmitt AM, Chang HY, Cancer Cell 2016, DOI 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Hinsbergh VW, a Collen P. Koolwijk, Ann. N. Y. Acad. Sci 2001, 936, 426. [DOI] [PubMed] [Google Scholar]
- [38].Szot CS, Buchanan CF, Freeman JW, Rylander MN, Tissue Eng. - Part C Methods 2013, 19, 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Perfahl H, Hughes BD, Alarcón T, Maini PK, Lloyd MC, Reuss M, Byrne HM, J. Theor. Biol 2017, 414, 254. [DOI] [PubMed] [Google Scholar]
- [40].Chen W, Xia P, Wang H, Tu J, Liang X, Zhang X, Li L, Cell Commun J. Signal. 2019, 13, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gabriel$ DA, Muga K, Boothroyd EM, THE JOURNAL OF BIOLOGICAL CHEMISTRY The Effect of Fibrin Structure on Fibrinolysis*, 1992. [PubMed] [Google Scholar]
- [42].Ryan EA, Mockros LF, Weisel JW, Lorand L, Biophys. J 1999, 77, 2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li W, Sigley J, Pieters M, Helms CC, Nagaswami C, Weisel JW, Guthold M, Biophys. J 2016, 110, 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Domingues MM, Macrae FL, Duval C, Mcpherson HR, Bridge KI, Ajjan RA, Ridger VC, Connell SD, Philippou H, Arï Ens RAS, 2016, 127, 487. [DOI] [PubMed] [Google Scholar]
- [45].Jessani N, Humphrey M, McDonald WH, Niessen S, Masuda K, Gangadharant B, Yates JR, Mueller BM, Cravatt BF, Proc. Natl. Acad. Sci. U. S. A 2004, DOI 10.1073/pnas.0404727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yao J, Guihard PJ, Blazquez-Medela AM, Guo Y, Moon JH, Jumabay M, Boström KI, Yao Y, Circ. Res 2015, 117, 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Piera-Velazquez S, Jimenez SA, Physiol. Rev 2019, 99, 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davis GE, Stratman AN, Sacharidou A, Koh W, n.d, DOI 10.1016/B978-0-12-386041-5.00003-0. [DOI] [Google Scholar]
- [49].Panupinthu N, Lee HY, Mills GB, Br. J. Cancer 2010, 102, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mills GB, Moolenaar WH, Nat. Rev. Cancer 2003, 3, 582. [DOI] [PubMed] [Google Scholar]
- [51].Mazzocca A, Dituri F, Lupo L, Quaranta M, Antonaci S, Giannelli G, Hepatology 2011, 54, 920. [DOI] [PubMed] [Google Scholar]
- [52].Mantovani F, Collavin L, Del Sal G, Cell Death Differ. 2019, 26, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu GL, Yang HJ, Liu T, Lin YZ, Asian Pac. J. Trop. Med 2014, 7, 76. [DOI] [PubMed] [Google Scholar]
- [54].Lee KW, Yeo SY, Sung CO, Kim SH, Cancer Res. 2015, 75, 73. [DOI] [PubMed] [Google Scholar]
- [55].Kalluri R, Nat. Rev. Cancer 2016, 16, 582. [DOI] [PubMed] [Google Scholar]
- [56].Barrera LN, Evans A, Lane B, Brumskill S, Oldfield FE, Campbell F, Andrews T, Lu Z, Perez-Mancera PA, Liloglou T, Ashworth M, Jalali M, Dawson R, Nunes Q, Phillips PA, Timms JF, Halloran C, Greenhalf W, Neoptolemos JP, Costello E, Cancer Res. 2020, 80, 2861. [DOI] [PubMed] [Google Scholar]
- [57].Nissen NI, Karsdal M, Willumsen N, J. Exp. Clin. Cancer Res 2019, 38, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bonnans C, Chou J, Werb Z, Nat. Rev. Mol. Cell Biol 2014, DOI 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Castello-Cros R, Bonuccelli G, Molchansky A, Capozza F, Witkiewicz AK, Birbe RC, Howell A, Pestell RG, Whitaker-Menezes D, Sotgia F, Lisanti MP, Cell Cycle 2011, 10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Alkasalias T, Moyano-Galceran L, Arsenian-Henriksson M, Lehti K, Int. J. Mol. Sci 2018, 19, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Alkasalias T, Flaberg E, Kashuba V, Alexeyenko A, Pavlova T, Savchenko A, Szekely L, Klein G, Guven H, Lawrence EO Berkeley National Laboratory, 2014, 2, DOI 10.1073/pnas.1419554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Leong MC, Vedula SRK, Lim CT, Ladoux B, Commun. Integr. Biol 2013, 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zuazo-Gaztelu I, Casanovas O, Front. Oncol 2018, DOI 10.3389/fonc.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sevick EM, Jain RK, Cancer Res. 1991. [Google Scholar]
- [65].Farnsworth RH, Lackmann M, Achen MG, Stacker SA, Oncogene 2014, 33, 3496. [DOI] [PubMed] [Google Scholar]
- [66].Chiang SPH, Cabrera RM, Segall JE, Am. J. Physiol. - Cell Physiol 2016, 311, C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yamamura T, Tsukikawa S, Yamada K, Yamaguchi S, J. Surg. Oncol 2001, 78, 259. [DOI] [PubMed] [Google Scholar]
- [68].Ehsan SM, Welch-reardon KM, Waterman ML, Christopher CW, George SC, Genetics M, Integr. Biol 2015, 6, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Heo DN, Ayan B, Dey M, Banerjee D, Wee H, Lewis GS, Ozbolat IT, Biofabrication 2021, DOI 10.1088/1758-5090/abc1bf. [DOI] [PubMed] [Google Scholar]
- [70].Wu Y, Ayan B, Moncal KK, Kang Y, Dhawan A, Koduru SV, Ravnic DJ, Kamal F, Ozbolat IT, Adv. Healthc. Mater 2020, DOI 10.1002/adhm.202001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ayan B, Celik N, Zhang Z, Zhou K, Kim MH, Banerjee D, Wu Y, Costanzo F, Ozbolat IT, Commun. Phys 2020, DOI 10.1038/s42005-020-00449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ayan B, Wu Y, Karuppagounder V, Kamal F, Ozbolat IT, Sci. Rep 2020, DOI 10.1038/s41598-020-69960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Winters BS, Shepard SR, Foty RA, Int. J. Cancer 2005, 114, 371. [DOI] [PubMed] [Google Scholar]
- [74].Fukumura D, Xavier R, Sugiura T, Chen Y, Park E-C, Lu N, Selig M, Nielsen G, Tumor Induction of VEGF Promoter Activity in Stromal Cells Culture Express Significant Levels of at Least One Polypep-Tide Mitogenic for Endothelial Cells, Including Vascular Endothelial Growth Factor/Vascular Permeability Factor, 1998. [Google Scholar]
- [75].Guevorkian K, Colbert M-J, Durth M, Dufour S, Brochard-Wyart F, Aspiration of Biological Viscoelastic Drops, 2010. [DOI] [PubMed] [Google Scholar]
- [76].Zudaire E, Gambardella L, Kurcz C, Vermeren S, PLoS One 2011, 6, e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, Xu Y, Wu YS, Hu XM, Ping BH, Wang Q, Int. J. Mol. Med 2017, 40, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Moll P, Ante M, Seitz A, Reda T, QuantSeq 3’ MRNA Sequencing for RNA Quantification, 2014. [Google Scholar]
- [79].Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C, Nat. Methods 2017, 14, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Love MI, Huber W, Anders S, Genome Biol. 2014, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.