Abstract
Eukaryotic cells respond to stress via adaptive programs that include reversible shutdown of key cellular processes, the formation of stress granules, and a global increase in ubiquitination. The primary function of this ubiquitination is thought to be for tagging damaged or misfolded proteins for degradation. Here, working in mammalian cultured cells, we found that different stresses elicited distinct ubiquitination patterns. For heat stress, ubiquitination targeted specific proteins associated with cellular activities downregulated during stress, including nucleocytoplasmic transport and translation, as well as stress granule constituents. Ubiquitination was not required for the shutdown of these processes or for stress granule formation but was essential for the resumption of cellular activities and for stress granule disassembly. Thus, stress-induced ubiquitination primes the cell for recovery following heat stress.
One Sentence Summary:
Stress-induced ubiquitination is essential for recovery of cellular activities following heat stress.
Eukaryotic cells have a stereotypical response to a variety of stresses that aids in their survival until normal growth conditions are restored (1, 2). This stress response includes the inhibition of global translation along with the upregulation of expression of select stress response factors (1–7). Other biological pathways are also shut down or perturbed, including nucleocytoplasmic transport (8–13), RNA splicing (14, 15), and cell cycle activities (16–18). Inhibition of translation and subsequent polysome disassembly leads a rise in the cytoplasmic concentration of ribosome-free mRNA that culminates in the formation of cytoplasmic condensates known as stress granules (19). These stress-induced cellular changes are adaptive in the short term but require coordinated reversal after stress is removed in order to resume cellular activities and reestablish homeostasis. Accordingly, upon the removal of stress, stress granules disassemble while translation and other biological pathways return to normal activity on a time scale of minutes to a few hours (2, 20–22). However, the mechanisms that facilitate this recovery are not well understood.
One hallmark of the cellular response to many types of stress is a global increase in poly-ubiquitin conjugation, which has largely been attributed to increased protein quality control (PQC) activity in response to stress-induced protein damage and translation arrest (1–3). This increased activity is required for the ubiquitin-dependent degradation of both defective ribosomal products (DRiPs), the primary source of misfolded proteins in cells under normal conditions (4), and proteins susceptible to stress-induced misfolding, such as thermolabile proteins or proteins with intrinsically disordered domains (2, 5–7). Ubiquitin and some ubiquitin pathway proteins are found in stress granules (23–28), and several deubiquitinating enzymes can help to regulate stress granule dynamics (23, 27). Furthermore, when PQC function is disrupted, ubiquitinated DRiPs can accumulate in stress granules and impair stress granule function (29–31).
The ubiquitin-selective segregase p97/valosin-containing protein (VCP) facilitates stress granule disassembly (29, 32, 33), which may suggest a role for ubiquitination in this process. However, recent studies investigating ubiquitin conjugation have produced conflicting results. Whereas one study found that nuclear SUMO-primed ubiquitination was important for stress granule disassembly (34), another group reported minimal ubiquitination of stress granule proteins and showed that ubiquitin conjugation was entirely dispensable for stress granule dynamics (35). Thus, the role of ubiquitination in regulating stress granule dynamics remains unresolved, as does any potential role of ubiquitination in regulating other cellular activities that are altered by stress.
Analysis of stress-dependent changes to the global protein ubiquitin modification landscape, or “ubiquitinome,” can provide unbiased insights into the stress response by connecting differentially ubiquitinated proteins with specific biological pathways. To this end, several proteomic techniques allow for quantitative comparisons of ubiquitinomes from differentially treated cell or tissue samples (36–39). Analysis of the ubiquitinome in yeast has suggested that heat shock-induced ubiquitination primarily targets cytosolic misfolded proteins (40, 41), consistent with the presumption that ubiquitination in this setting relates mainly to PQC. However, comprehensive analyses in human cells or directly comparing changes in ubiquitination in response to various stresses have not been undertaken, and it remains unclear to what extent ubiquitination patterns may differ in these different contexts.
Results
Different cellular stresses induce distinct patterns of ubiquitination
To investigate whether cells deploy different patterns of ubiquitination to cope with different types of stress, we performed proteomic analysis following tandem ubiquitin binding entity (TUBE)-based capture of the ubiquitinome in HEK293T cells exposed to heat stress (42°C), oxidative stress (sodium arsenite), osmotic stress (sorbitol), UV stress, or proteasomal inhibition (bortezomib) as well as unstressed cells (Fig. 1, A and B). Following exposure to each treatment, lysates were incubated with Halo-linked TUBE resin to capture ubiquitin conjugates, followed by label-free liquid chromatography with tandem mass spectrometry (LC-MS/MS) (Fig. 1, A and B). Immunoblotting indicated that all stress types except UV led to an increase in total ubiquitin conjugates (input blot, Fig. 1C). While the TUBEs likely have higher baseline affinity for binding poly-ubiquitinated over mono-ubiquitinated proteins, conditions were optimized such that the TUBEs captured the vast majority of all ubiquitinated material (compare bound and unbound blots, Fig. 1C). We used stringent buffer conditions (1% NP-40) and a high salt wash to minimize capture of non-ubiquitinated proteins by the TUBEs. We detected ~200–300 ubiquitinated proteins per stress condition and 500 unique proteins across all five stress conditions (Fig. 1D, table S1).
Fig. 1. Different cellular stresses induce different patterns of ubiquitination.
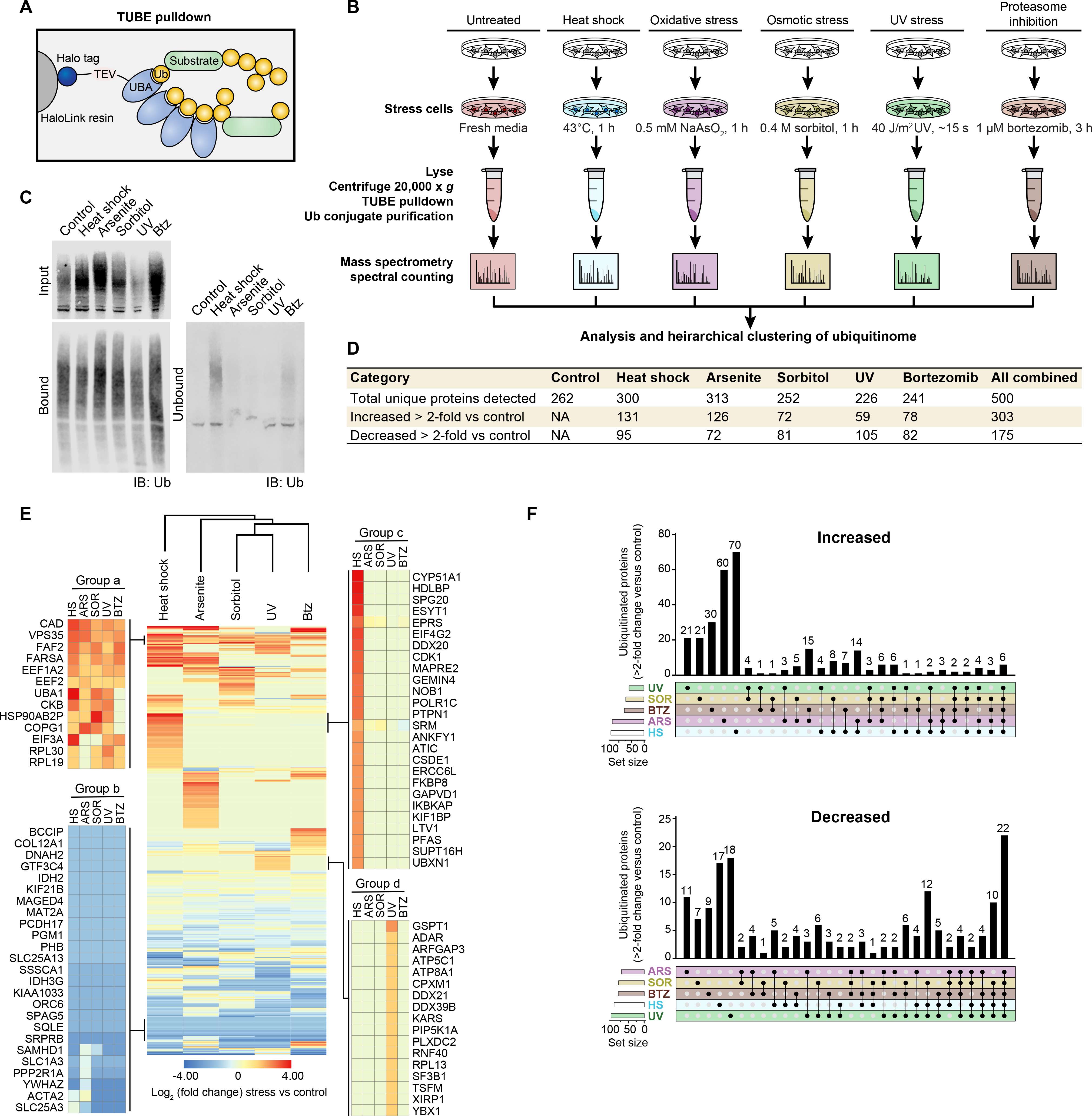
(A) Schematic illustrating HALO-linked TUBE resin and ubiquitinated protein capture from HEK293T cells. (B) Workflow for sample preparation and TUBE proteomics. HEK293T cells were treated with the indicated stress or incubated with fresh media for 1 h as a control prior to lysis. For UV treatment, media was removed and cells were briefly exposed to UV light (40 J/m2) followed by the addition of fresh media 1 h prior to lysis. (C) Immunoblot for ubiquitin (P4D1 antibody) showing TUBE capture of ubiquitinated proteins from cells stressed as in (B). (D) Summary of statistics from proteomic analysis. (E) Heatmap illustrating global stress-induced changes to the ubiquitinome. Colors indicate log2 fold change in spectral counts of stressed samples versus control samples. Example groups of proteins with a shared pattern are shown in the blow-ups. (F) UpSet plot indicating proteins changed > 2-fold versus control for each stress.
Unsupervised hierarchical clustering revealed that each type of stress was associated with a distinct pattern of ubiquitination relative to control cells (Fig. 1E). Indeed, the majority of proteins with increased or decreased ubiquitination were associated with a single type of stress (Fig. 1F). Several patterns emerged from this analysis. Some proteins showed increased or decreased ubiquitination in response to all 5 stresses, suggesting that certain ubiquitination events were part of a non-specific, generalized stress response (e.g., Groups a and b, Fig. 1E). Other proteins showed altered ubiquitination in a stress-specific manner. For example, ubiquitination of some proteins was increased exclusively in response to heat shock (Group c, Fig. 1E) or UV exposure (Group d, Fig. 1E). The emergence of these distinct protein groups suggested stress-specific patterns of ubiquitination that represented distinct adaptive responses to stressors. To test this hypothesis, we investigated ubiquitination in response to heat and oxidative stress more deeply.
Heat stress leads to rapid increases in total ubiquitination and a time-dependent shift in solubility of ubiquitin conjugates
We first examined the kinetics of ubiquitin conjugate accumulation during heat shock and recovery. Heat stress-induced ubiquitination occurred rapidly, with increased ubiquitin conjugates apparent as early as 15 min and peaking within 30 min following heat shock (Fig. 2A). The accumulated ubiquitin conjugates remained at a constant elevated level during prolonged stress for at least 150 min (Fig. 2A). Immunoblotting using antibodies specific for poly-ubiquitin (FK2) and K48-linked or K63-linked poly-ubiquitin chains showed similar stress-dependent increases in signal as those observed using the P4D1 antibody, which recognizes unconjugated ubiquitin as well as mono-ubiquitinated and poly-ubiquitinated proteins (fig. S1A). Thus, the observed stress-dependent increase in ubiquitin conjugation consisted largely of poly-ubiquitination and was not highly chain type-specific.
Fig. 2. Heat shock induces significant changes to the global ubiquitinome.
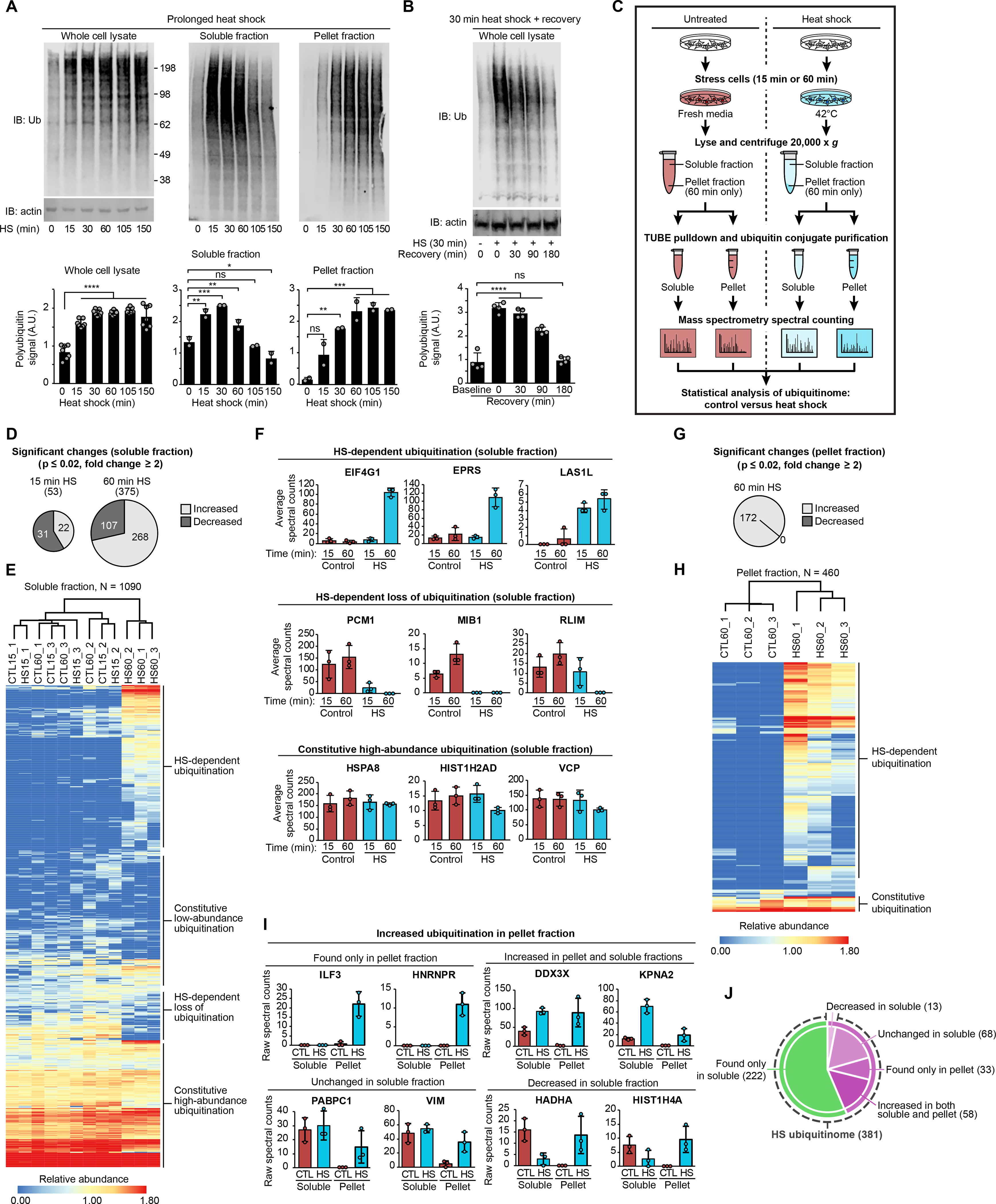
(A-B) Immunoblots showing ubiquitin conjugation levels at time points following heat shock (A) and during recovery from 30 min heat shock (B) in HEK293T whole cell lysates, soluble fractions, and pellet fractions after centrifugation (14,000 x g). Bar graphs indicate mean + s.d. and individual values for quantification of Western blots from ≥ 3 replicates for each experiment. ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ANOVA with Dunnett’s test. (C) Workflow for samples from HEK293T cells. (D) Charts indicating the number of significantly changed proteins in soluble fractions for heat-shocked samples (15 min or 60 min) compared to control. (E) Heatmap illustrating heat shock-induced changes to the global ubiquitinome in soluble fractions. Colors indicate relative abundance as quantified by spectral counting. (F) Spectral count values for representative proteins of indicated categories; individual values and mean (± s.d.) are shown from three replicates of each sample condition in soluble fractions. (G) Chart indicating the number of significantly changed proteins in pellet fractions for heat-shock samples compared to control. (H) Heatmap illustrating heat shock-induced changes to the global ubiquitinome in pellet fractions. (I) Spectral count values for representative proteins of indicated categories; individual values and mean (± s.d.) are shown from three replicates of each sample condition. CTL, control; HS, 60 min heat shock. (J) Chart indicating the number of proteins with heat shock-dependent increase in ubiquitination. Proteins detected in the pellet fraction are shown in purple, proteins detected only in the soluble fraction are shown in green. Dashed line indicates categories included in the heat shock ubiquitinome.
Prolonged heat shock results in progressive loss of solubility in a fraction of the proteome (42–45), although the relationship to ubiquitination is not clear. Here we observed a time-dependent dynamic shift in the ubiquitinome between soluble and insoluble fractions. Accumulation of poly-ubiquitin conjugates in the soluble fraction peaked at about 30 min and then declined over the course of the experiment. Poly-ubiquitin conjugates accumulated more slowly in the insoluble fraction, plateauing after 60 min (Fig. 2A). The accumulation of poly-ubiquitin conjugates after heat stress was temporary, returning to baseline within 3 hours after a 30-min heat shock (Fig. 2B).
With this kinetic profile in mind, we next performed further proteomic analysis of the heat stress-associated ubiquitinome by TUBE-LC-MS/MS. We examined this ubiquitinome in the soluble fraction at an early time point (15 min) corresponding to the initial increase in ubiquitination, and at a later time point (60 min) (Fig. 2C). To recover ubiquitinated proteins accumulated in the pellet fraction at a later time point (Fig. 2A), we resolubilized the pellet fractions from samples at the 60-min time point in a urea buffer prior to incubation with TUBEs. Combined data from three replicates for both fractions yielded quantification of >1,000 ubiquitinated proteins in the TUBE-recovered samples, indicating good depth of coverage and reproducibility (fig. S1, B and C, table S2), indicating that our TUBE-based method can detect ubiquitin conjugates at least as efficiently other methods of ubiquitin capture (35). We also verified heat stress-induced increase in poly-ubiquitination of seven representative proteins by immunoblotting (fig. S1D). Replicating the 60-min time point experiment in U2OS cells gave results very similar to those in HEK293T cells (fig. S1E and table S3). Similarly, heat shock led to an accumulation of ubiquitin conjugation in both induced pluripotent stem cell (iPSC)-derived neurons and mouse cortical neurons (fig. S1F) and TUBE proteomic analysis revealed substantial overlap between the heat shock ubiquitinome of HEK293T cells and these neuronal cell types (fig. S1, G–I and table S3). Indeed, many of the proteins with the largest increase in abundance following heat stress were shared between these three cell types (fig. S1, G–I). Thus, stress-dependent changes to the ubiquitinome were not highly cell type-specific.
The temporal profile of changes in the ubiquitinome in the soluble fraction revealed only modest changes at 15 min but more substantial changes at 60 min following heat stress (Fig. 2, D–F). Thus, despite an increase in a poly-ubiquitin smear by immunoblotting at 15 min (Fig. 2A), the global shift in the ubiquitinated protein landscape was better reflected later in the stress response. We therefore directed the majority of our subsequent analyses to examining changes to the ubiquitinome after 60 min of heat shock.
We also detected significant changes to the ubiquitinome in the pellet fraction. Whereas there was no loss of ubiquitinated proteins in the pellet fraction, 172 ubiquitinated proteins were significantly increased after 60 min of heat stress (Fig. 2, G and H). Roughly 80% of these proteins had also been detected in the soluble fraction, where these proteins were enriched, depleted, or unchanged in response to heat stress (Fig. 2, I and J). While some ubiquitinated proteins did accumulate in the pellet fraction following heat shock, an individual protein’s ubiquitination status was not predictive of its solubility. Because detailed descriptions of heat-dependent changes in solubility have been published elsewhere (42–45), in subsequent analyses we assessed the significance of heat stress-induced ubiquitination of all proteins whether they appeared in the soluble fraction, pellet fraction, or both. Heat stress induced a global shift in the ubiquitin landscape, resulting in a “heat shock ubiquitinome” of 381 proteins, defined as all proteins showing a predicted net total increase in ubiquitination in response to heat stress (Fig. 2J and table S4).
Heat and arsenite stress induce different changes to the ubiquitinome
The pattern of ubiquitination following arsenite stress was most similar to that generated by heat stress (Fig. 1E). To examine this more deeply, we characterized arsenite stress-induced ubiquitination at 60 min following the same approach that we used to define heat stress-induced ubiquitination. After identifying proteins whose ubiquitination was significantly altered relative to control (tables S4 and S5), we compared the responses to arsenite stress versus heat stress. This analysis confirmed significant overlap in the arsenite stress- and heat stress-induced ubiquitinomes (fig. S1J). This overlap was evident for both proteins that showed increased ubiquitination and those that showed decreased ubiquitination (fig. S1K). Nevertheless, this analysis also confirmed stress-specific patterns in ubiquitination. Indeed, more than 400 significant changes in ubiquitination were unique to either arsenite or heat stress (fig. S1, J and K, tables S2 and S5).
Stress-dependent ubiquitination is not reflected by altered abundance
We next sought to determine the extent to which changes in the stress-induced ubiquitinomes reflected changes in total protein levels owing to altered rates of synthesis or ubiquitin-dependent degradation. To investigate changes in expression at the protein level, we performed multiplexed tandem mass tagging (TMT) MS quantification of the whole proteome from unstressed, heat-stressed, or arsenite-stressed cells in the presence or absence of proteasome inhibition (0.5 μM bortezomib) (Fig. 3A). Lysates were digested and labeled by TMT in 11-plex mode, followed by nanoscale LC-high resolution MS/MS. Of the 12,586 proteins quantified, the vast majority of proteins showed no significant stress-dependent change in total abundance using 1.5-fold and P ≤ 0.05 as a cut-off (Fig. 3B, fig. S2A, and table S6). Nevertheless, we detected several expected changes in response to both stress types, including the accumulation of stress response transcription factors ATF4 and XBP1, and depletion of BTG1, a regulator of ATF4 (46) (fig. S2B and table S6). No proteins with a stress-dependent increase in ubiquitination showed a significant increase in total protein levels. Thus, increased capture in the TUBE experiments reflected increased ubiquitination rather than increased levels of total protein. Additionally, only eight proteins with heat shock-dependent increases in ubiquitination showed a significant decrease of ≥ 1.25-fold in total protein levels in response to stress, of which only three proteins (EPPK1, GCN1, CYP51A1) decreased ≥ 1.5-fold (Fig. 3B, left panel, blue dots, and fig. S2B). The levels of these three proteins were stabilized during heat shock by the addition of bortezomib, indicating that they were targets of heat stress-dependent proteasomal degradation (Fig. 3B, right panel, and fig. S2B). Nonetheless, for nearly all cases with heat shock (Fig. 3B) and all cases with arsenite (fig. S2A), a stress-dependent increase in ubiquitination did not result in an overall downregulation of total protein levels. Treatment with bortezomib during the 1-hour heat shock did not significantly alter ubiquitinated protein enrichment based on TUBE pulldown (fig. S2, C and D), indicating that proteasomal degradation did not significantly alter the composition of the heat shock ubiquitinome during this time frame. Thus, either the observed ubiquitination was partially non-degradative or, more likely, only a fraction of each protein was ubiquitinated in response to stress, such that ubiquitin-dependent degradation did not substantially reduce the overall abundance of most proteins. Similarly, RNA-seq analysis revealed that, although we observed many significant heat shock-dependent changes in transcription, including HSPs and other stress response genes (47), most heat shock ubiquitinome genes were not regulated at the level of transcription, with a few exceptions (Fig. 3C, fig. S2E, table S7). Furthermore, upregulation of heat shock response genes at the mRNA level (e.g., HSPA6, DNAJB1, table S7) was not yet apparent at the protein level at the 1-hour time point used here (table S6). This difference in protein level versus transcript level changes is consistent with the expected lag time between stress-dependent induction at the transcriptional and translational levels (48–50). Thus, while it is likely that the transcriptional response to heat shock has a significant contribution to recovery from stress over longer time scales, it does not appear to be directly involved in the short-term reversal of stress response activities investigated here.
Fig. 3. Proteomic and transcriptomic analyses reveal additional details of the heat shock ubiquitinome.
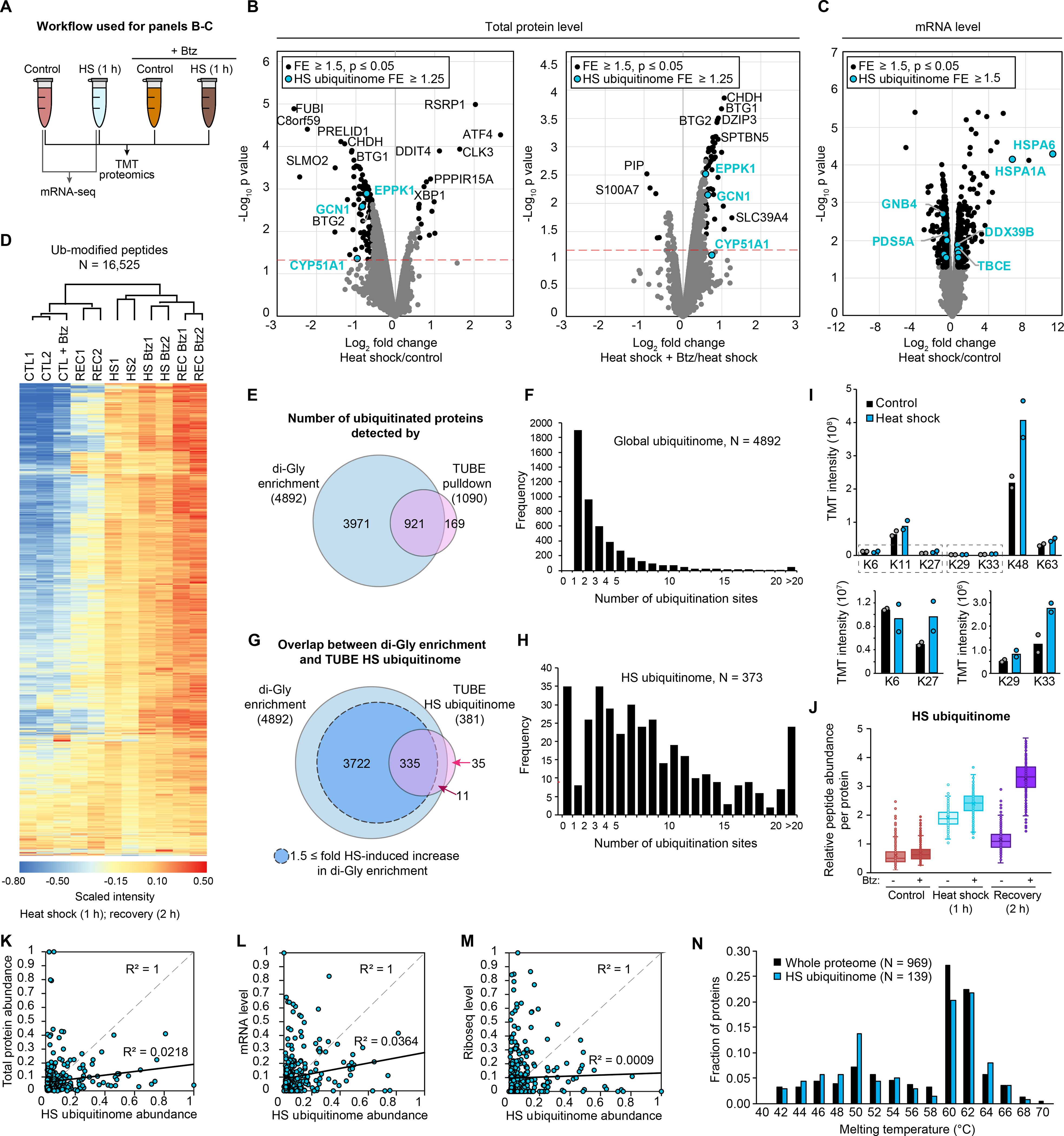
(A) Workflow for analysis in HEK293T cells. (B-C) Volcano plots indicating (B) changes in protein abundance for heat shock versus control samples (left) and versus heat shock with proteasome inhibitor bortezomib (Btz) (right) and (C) changes in mRNA levels in response to heat shock (C). Statistically significant changes are shown for the whole proteome or transcriptome (black dots) and heat shock ubiquitinome (blue dots). Red dashed lines indicate P = 0.05. (D) Heatmap of changes to the ubiquitinome in HEK293T cells following 1 h heat shock (HS) and 1 h heat shock followed by a 2 h recovery (REC) in the presence or absence of bortezomib as determined by di-GLY profiling. Color indicates scaled TMT intensity. (E) Venn diagram showing overlap of ubiquitinated proteins detected in di-GLY and TUBE experiments. (F) Histogram showing the number of ubiquitination sites per protein detected for all proteins in di-GLY experiments. (G) Venn diagram showing overlap of heat shock-dependent increases in ubiquitination as determined by di-GLY and TUBE experiments. (H) Histogram showing the number of ubiquitination sites per protein for the heat shock ubiquitinome. (I) Bar graphs indicating TMT intensity for each poly-ubiquitin linkage type. Mean and individual values are shown. (J) Box-and-whisker plots showing relative abundance of ubiquitinated proteins for heat shock ubiquitinome proteins. (K-M) Correlation between abundance of heat shock ubiquitinome proteins in TUBE experiments and total protein abundance (K), transcript abundance (L), and ribosome occupancy (M) as determined by Ribo-seq, with R2 values displayed. (N) Histogram showing melting temperatures as determined in reference (54) for all proteins measured (black bars) and measured heat shock ubiquitinome proteins (blue bars).
Paired di-GLY and TMT quantitative proteomics indicate that heat stress-induced ubiquitination leads to degradation during the recovery phase
Di-GLY proteomics, which uses enrichment of the Lys-ϵ-Gly-Gly (di-GLY) remnant generated on ubiquitinated peptides following trypsin digestion, is an alternative approach to identify and quantify the ubiquitin-modified proteome (51, 52). To complement and validate our TUBE proteomics, we paired di-GLY proteomics with TMT labeling in the presence or absence of proteasome inhibition to quantify changes in ubiquitination in response to a 60-min heat stress and following 2 hours of recovery. Across all samples, we quantified 16,525 unique ubiquitin-modified peptides, corresponding to 4,892 unique proteins (Fig. 3D and table S8). The heat stress-induced ubiquitinomes as determined by di-GLY/TMT proteomics were highly correlated with our TUBE-based proteomics, detecting at least one ubiquitin-modified peptide for most proteins identified by TUBE pulldown (Fig. 3, E and F, and tables S2, S8). Of the 381 proteins in the heat shock ubiquitinome by TUBE pulldown, 335 had at least one peptide increased by ≥ 1.5-fold in response to heat shock in the di-GLY analysis (Fig. 3G and tables S4 and S8). Of the 46 that did not, 35 were not detected in the di-GLY experiment, 8 had overall reduced ubiquitinated peptide levels in response to heat shock (HS/CTL = 0.53–0.95), and 3 had modestly increased levels (HS/CTL = 1.29–1.33) (Fig. 3G and tables S4 and S8). The amplitudes of the observed changes were often lower in the di-GLY profiling experiment, likely owing to ratio suppression commonly observed in TMT-based quantification (tables S2 and S8) (52).The heat shock ubiquitinome had much higher numbers of ubiquitination sites per protein (8.6) than the global ubiquitinome (3.4) (Fig. 3, F and H), suggesting concerted ubiquitination of a subset of proteins that define the heat shock ubiquitinome. We also identified 3,722 proteins not detected in the TUBE spectral counting for which ubiquitin-modified peptide levels were increased by at least 1.5-fold in response to heat shock (Fig. 3G). Comparing these results with the total proteome (table S6), > 8,000 proteins had no detectable increase in heat shock-dependent ubiquitination, representing the majority of the expressed proteome in HEK293T cells. Thus, this ubiquitination does not represent a global increase in ubiquitination across the entire proteome. While the additional heat shock-dependent ubiquitination events detected only by di-GLY profiling represent interesting biology that warrants further investigation, the TUBE-based experiments identified a critical subset of the heat shock ubiquitinome that allowed for comparison between more sample conditions (e.g., soluble vs pellet, arsenite vs other stresses) owing to the reduced time and resources required. We thus adjusted the definition of the “heat shock ubiquitinome” as those proteins detected in the TUBE experiments but excluding the 8 proteins that showed a heat shock-dependent decrease in the di-GLY experiment (373 proteins, table S4).
Analysis of the di-GLY data and quantification of modification sites on the internal lysines of ubiquitin revealed a ~1.5–2-fold heat shock-dependent increase in all poly-ubiquitin linkage types except K6 (Fig. 3I). This finding was consistent with our immunoblotting results (fig. S1A) that suggested that the heat shock-dependent increase in poly-ubiquitination was not highly chain type-specific. K48 was the most abundant linkage type in control conditions and exhibited a further 2-fold increase following heat shock, suggesting a significant portion of the heat shock ubiquitinome is targeted for proteasomal degradation. We therefore analyzed the di-GLY data to determine the effect of proteasome inhibition on the levels of ubiquitin-modified peptides (Fig. 3J). In accordance with our TUBE experiments and whole-proteome analysis, treatment with bortezomib had a modest effect on peptide abundance in heat-shocked samples for most heat shock ubiquitinome proteins. While most heat shock-dependent ubiquitination recovered to baseline levels after 2 hours in the absence of proteasomal inhibition, the levels remained elevated during recovery in the presence of bortezomib. This result suggests that many proteins ubiquitinated during continued heat stress are ultimately targeted for proteasomal degradation upon recovery.
The heat shock ubiquitinome is not defined by the most abundant, highly translated, or thermo-labile proteins
We next examined general features of the heat shock ubiquitinome by analyzing whole-proteome TMT and RNA-seq datasets along with several proteome-wide datasets. We note that the label-free MS approach used in our TUBE experiments can be biased toward identifying abundant proteins. However, proteins detected in the TUBE pulldown experiment spanned five orders of magnitude in TMT intensity and included proteins represented in the bottom 5th percentile of abundance in the proteome (tables S2 and S6), indicating that the heat shock ubiquitinome included low abundance proteins. The heat shock ubiquitinome was not biased by protein or mRNA abundance, and low abundance proteins were well represented (Fig. 3, K and L). This observation, along with the high degree of correlation with the results of the di-GLY profiling, indicated that the quantification of TUBE pulldown samples reflected levels of ubiquitinated protein rather than total protein or transcript levels. Similarly, based on analysis of ribosome profiling data from unstressed cells, we found no correlation between the TUBE-based spectral counting quantification of heat shock ubiquitinome proteins and density of ribosome occupancy for the corresponding mRNA (Fig. 3M). Thus, the heat shock ubiquitinome was not composed of the most actively translated proteins. Finally, we compared the heat shock ubiquitinome to a previous proteome-wide investigation of protein thermostability (54). This published dataset included 139 proteins from the heat shock ubiquitinome, which showed a similar bimodal distribution of melting temperatures as compared to all 969 proteins analyzed in the study (Fig. 3N). Thus, it is unlikely that the heat shock ubiquitinome represents the proteins most likely to misfold as a result of temperature increase. Taken together it seemed that the heat shock ubiquitinome was not primarily defined by total protein abundance, translation activity, or thermostability.
The functional landscape of the heat shock ubiquitinome reflects cellular activities perturbed by stress
To investigate the functional significance of the heat shock ubiquitinome, we performed gene ontology (GO) analysis (DAVID (55) and PANTHER (56)) along with literature curation (Fig. 4, A–C). The heat shock ubiquitinome was highly enriched in biological pathways that were downregulated or shut down during cellular stress. Furthermore, we detected enrichment of proteins involved in stress granule formation, including translation initiation factors and other components of the translational machinery (Fig. 4C). The heat shock ubiquitinome also included nucleocytoplasmic transport proteins (e.g., 11 of the 18 importin α/β superfamily members, nuclear pore complex components, and RNA transporters). Indeed, stress disrupts nucleocytoplasmic transport through localization of transport factors into stress granules (8), further linking ubiquitination with downregulated cellular activities and stress granules (Fig. 4C).
Fig. 4. The heat shock ubiquitinome consists of proteins from biological pathways associated with the stress response.
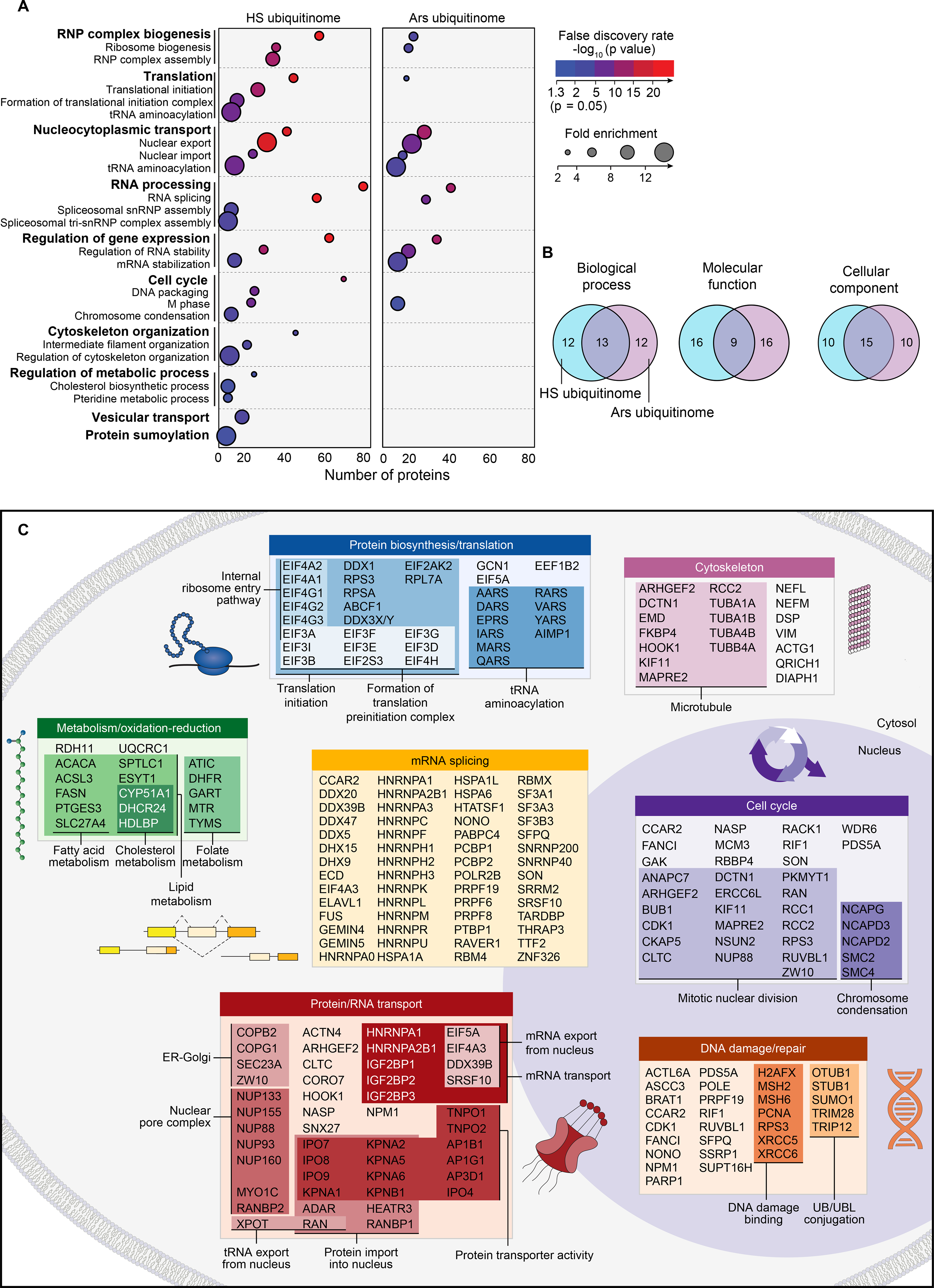
(A) Dot plot of gene ontology (GO) enrichment showing significantly overrepresented GO terms for heat shock and arsenite ubiquitinomes in HEK293T cells. Color indicates FDR P value and dot size indicates overrepresentation fold enrichment compared to the whole genome. Dots are not shown for terms with no statistically significant (P < 0.05) enrichment. (B) Overlap of top 25 GO as terms ranked by statistical significance (FDR P value) for heat shock and arsenite ubiquitinomes. (C) DAVID functional clustering of the heat shock ubiquitinome along with literature curation was used to identify pathways targeted by heat shock-induced ubiquitination.
Under non-stress conditions, PQC proteins, such as HSP chaperones and VCP, are engaged with misfolded proteins and nascent translation products that are targets for proteasomal degradation, and in some cases HSPs themselves can be directly ubiquitinated as a result (57–59). These PQC-related proteins were not over-represented in the GO analysis but were captured by TUBEs at high levels in both control and heat shock conditions in many cases (Fig. 2F, bottom row and table S2). Thus, heat shock-induced ubiquitination occurs both as a response to increased PQC demands and plays a role in regulating activities that comprise the cellular stress response, including stress granule assembly. Furthermore, ubiquitination may contribute to underexplored aspects of the heat stress response such as folate and cholesterol metabolism (Fig. 4C).
Comparisons of the GO analysis of the heat shock- and arsenite-induced ubiquitinomes showed overlap of many GO terms (e.g., splicing, nucleocytoplasmic transport) and others that were unique to heat shock (e.g., translation, metabolic process) (Fig. 4, A and B), suggesting that ubiquitination may play distinct roles in the response to each stress type. As expected, owing to the greater number of unique proteins identified in the heat shock ubiquitinome than for the arsenite ubiquitinome, the heat shock ubiquitinome generally included both a higher number of proteins and higher levels of enrichment over control samples within the GO categories shared by both ubiquitinomes (Fig. 4A and tables S2, S4 and S5). However, the stress-dependent increase in total ubiquitin conjugation was actually higher for arsenite than for heat shock (Fig. 1C and fig. S3A). Thus, the more prominent GO pattern observed for the heat shock ubiquitinome was not simply because of a stronger induction of stress responsive ubiquitination compared to arsenite stress. Rather, different stress types could lead to different patterns of ubiquitination.
mRNPs are ubiquitinated and undergo compositional remodeling upon heat shock
A large fraction of the heat shock ubiquitinome was mRNA-binding proteins (Fig. 4), suggesting that some heat shock-dependent ubiquitination might occur in the context of mRNP complexes. To examine this possibility, we first captured mRNPs using magnetic beads conjugated with oligo(dT)25, which binds to the poly(A) tail of mRNAs, and performed immunoblotting to assess the presence of mRNA bound to poly-ubiquitinated proteins (Fig. 5, A and B). Ubiquitin conjugates co-purified with polyadenylated mRNA to a much greater extent after heat stress. Poly(A) binding protein (PABP)-mediated 3’ end retrieval is an orthologous method for capturing polyadenylated mRNAs (60). When we isolated mRNPs by immunoprecipitation (IP) of endogenous PABPC1, we similarly observed heat stress-induced ubiquitin conjugates that co-purified with mRNPs (Fig. 5, A and B). Arsenite stress did not produce a similar level of increased ubiquitin conjugates associated with PABPC1, nor did treatment with the translation inhibitors cycloheximide, puromycin, or cephaeline in the absence of heat stress, indicating that this phenomenon was specific to heat stress (fig. S3A).
Fig. 5. Heat shock induces ubiquitination of mRNP complexes.
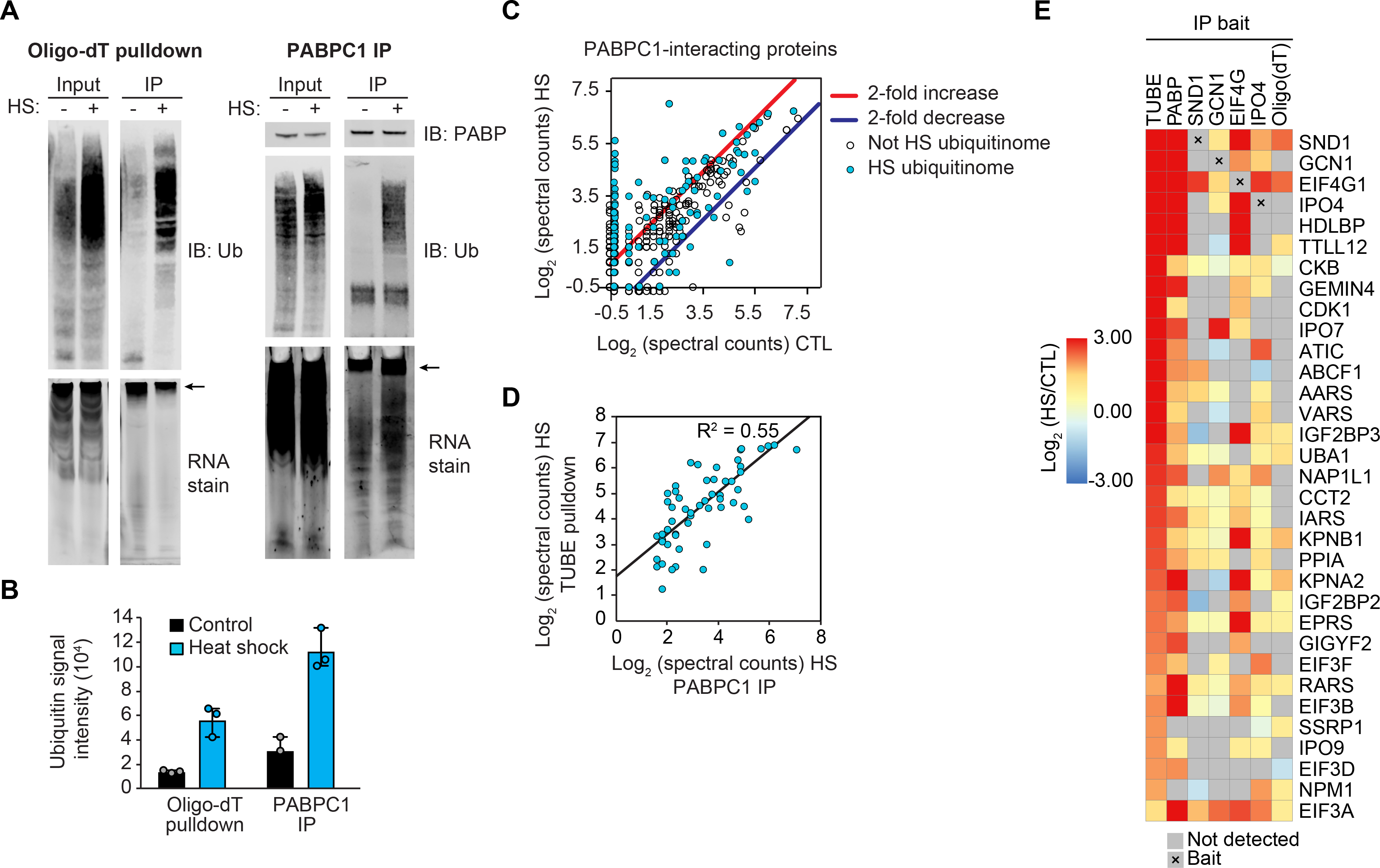
(A) Immunoblots and RNA gels showing polyadenylated mRNA isolated by oligo(dT) resin (left) or PABPC1 immunoprecipitation (right) pulling down poly-ubiquitinated proteins from HEK293T cells after 60 min heat shock. Isolated mRNA was visualized by SYBR orange RNA stain; arrow indicates high molecular weight RNA. (B) Quantification of immunoblot analysis for ubiquitin conjugation shown in (A). Results represent mean and individual values of 3 replicate experiments. Error bars indicate data range. (C) Scatter plot showing abundance (log2 [spectral counts]) of PABPC1-interacting proteins in heat shock versus control conditions. Results represent averages of 2 replicates. Lines indicate 2-fold increase (red) or decrease (blue) with heat shock; blue dots indicate heat shock ubiquitinome proteins. (D) Correlation between abundance of heat shock ubiquitinome proteins detected in TUBE pulldown and PABPC1 IP in 60 min heat-shocked samples. (E) Heatmap illustrating changes in abundance in 60 min heat shock versus control samples for proteins detected in TUBE pulldown, endogenous protein IP (PABP, SND1, GCN1, EIF4G, IPO4), or oligo(dT) pulldown.
The observed heat shock-dependent increase in ubiquitination associated with captured mRNPs suggests that ubiquitination may occur on assembled complexes, rather than on individual RNA-binding proteins. To examine this possibility, we used proteomics to investigate the link between heat shock-dependent changes to the composition of mRNPs and the heat shock ubiquitinome. Direct analysis of the PABPC1 protein-protein interaction network from IP of the endogenous protein revealed substantial remodeling in response to heat stress. Indeed, 105 proteins showed increased interaction with PABPC1 after heat shock, whereas 18 proteins showed decreased interaction (P ≤ 0.02, fold enrichment/depletion ≥ 2, detected in both replicates) (Fig. 5C and table S9). More than half of the proteins that exhibited a heat stress-dependent increase in co-IP with PABPC1 (59 of 103) were also components of the heat shock ubiquitinome (Fig. 5C). Moreover, the relative amount of protein retrieved in PABPC1 IP after heat stress correlated with abundance in the TUBE pulldown experiment (Fig. 5, D and E, tables S2 and S9). Consistent results were obtained with 4 additional bait proteins and with oligo(dT) capture, substantiating the idea that heat shock-induced ubiquitination of mRNPs coincides with a remodeling of their composition (Fig. 5E and table S9). Some of the interactions in the co-IP experiments were observed at low levels in control samples but increased with heat shock (e.g., GCN1 with EIF4G), whereas others were only detected after heat shock (e.g., TTLL12 with PABP and EIF4G) (Fig. 5E and table S9). One interpretation of these experiments is that PABPC1 complexes were stabilized through direct, poly-ubiquitin-mediated interactions. However, when we repeated the PABPC1 IP with the addition of the nonspecific deubiquitinating enzyme USP2, which was sufficient to eliminate poly-ubiquitin, we saw no change in the PABPC1 interactome (fig. S3B). Thus, heat shock-induced ubiquitination correlated with a remodeling of the protein components of mRNPs, with some pre-existing interactions that became stabilized upon heat shock as well as some novel heat shock-dependent interactions.
Ubiquitination is required for the disassembly of heat shock-induced stress granules
We next pursued our observation that the heat shock ubiquitinome was strongly enriched for protein constituents of stress granules. We began by assembling a list of 726 protein constituents of stress granules as reported in three studies (25, 26, 28) (table S10). Many more stress granule proteins were represented in the heat shock ubiquitinome than the arsenite ubiquitinome (Fig. 6, A and B). For a high-confidence stress granule proteome (those detected in at least 2 of the 3 studies mentioned above) (tables S2, S5, and S10), ubiquitination of stress granule proteins clearly increased in response to heat shock (Fig. 6C). As expected (35), this increase in ubiquitination was not apparent in response to arsenite stress (Fig. 6D). Indeed, poly-ubiquitin robustly co-localized with stress granule markers in nearly all cells exposed to heat shock, but not in those exposed to arsenite (Fig. 6, E–G). The group of stress granule proteins with increased ubiquitination in response specifically to heat stress included the key stress granule regulator G3BP1, the central node in the network of interactions that underlie stress granule assembly (61). In our companion study, we define the lysine residues in G3BP1 that are ubiquitinated in response to heat shock, and we define the mechanism whereby G3BP1 ubiquitination mediates stress granule disassembly during recovery from heat stress (62).
Fig. 6. Heat shock-induced stress granules contain ubiquitinated proteins and require active ubiquitination for disassembly.
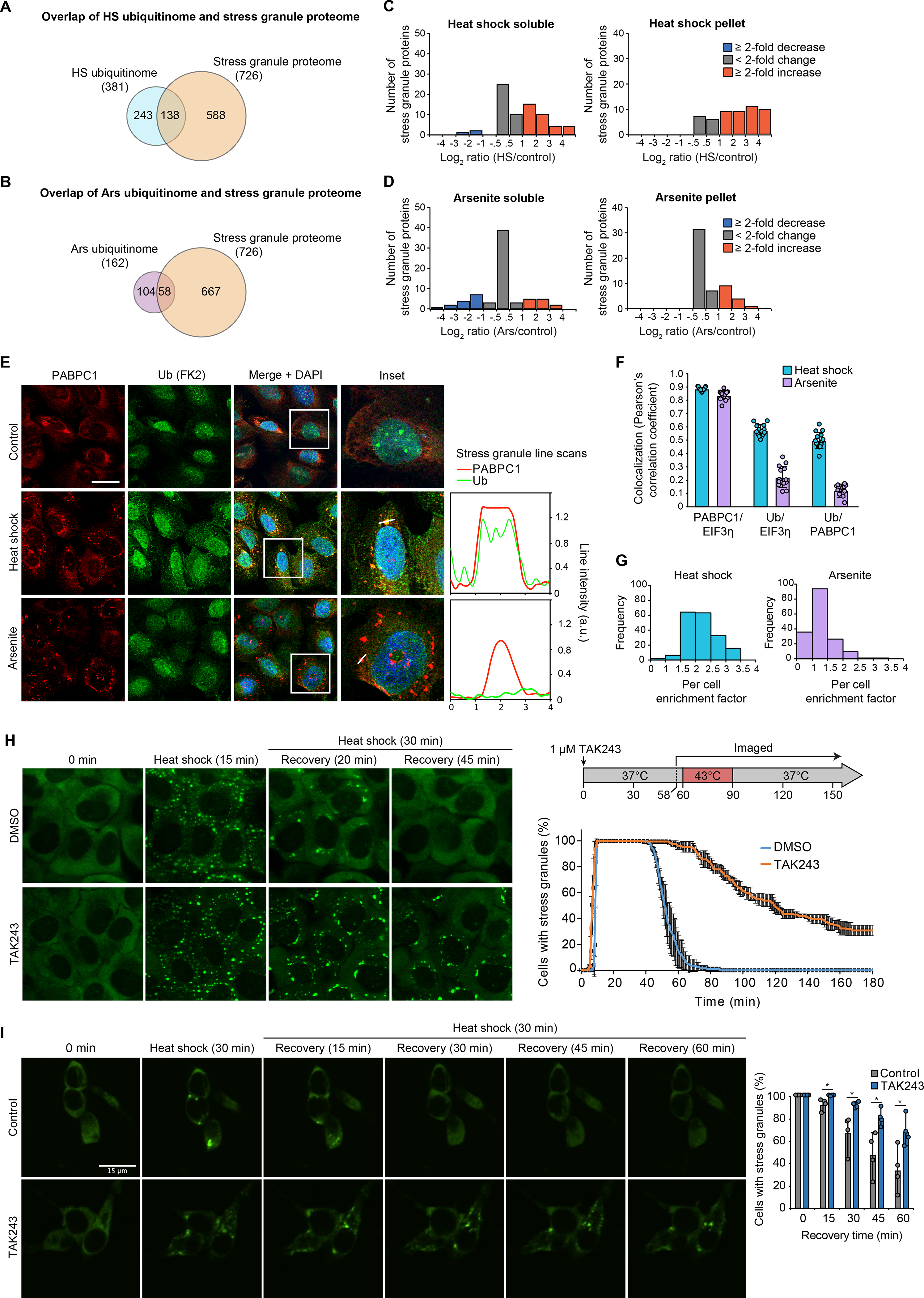
(A-B) Overlap of heat shock (A) and arsenite (B) ubiquitinomes of HEK293T cells with the stress granule proteome. (C-D) Histograms showing changes in ubiquitinated protein abundance for high-confidence stress granule proteins in response to 60 min heat shock (C) or 60 min arsenite (D) as determined by TUBE (tables S2 and S5). (E) U2OS cells were exposed to 90 min of sodium arsenite or heat shock, fixed, and stained for stress granule markers PABPC1 (red), EIF3η (not shown), and poly-ubiquitin (FK2, green). Graphs represent a line scan of signal intensity for PABPC1 and FK2 channels across the indicated stress granule. Scale bar, 50 μm. (F) Co-localization between immunofluorescent signal for PABPC1 and EIF3η and poly-ubiquitin as determined images collected as in (E). Bar graphs represent mean (± s.d.) and individual Pearson’s correlation coefficient values for > 10 images (total of > 100 cells for each condition). (G) Histogram of enrichment factor of ubiquitin in stress granules (ratio of the mean intensity of FK2-ubiquitin signal in granules to the mean intensity of the total cell area, excluding granules) for > 100 cells for each condition. (H-I) Live cell imaging of cells stably expressing GFP-G3BP1 during heat stress and recovery in the presence or absence of TAK243 for U2OS cells (H) or iPSC-derived neurons (I). Plots show the percentage of cells with ≥ 2 stress granules at the indicated time, with (H, line graph) solid lines and error bars representing average values and s.d. for three biological replicates with 30–50 cells each and (I, bar graph) mean, data range (error bars in I) and individual values shown for four biological replicates with 48–120 neurons each.
To further investigate the relationship between poly-ubiquitin conjugation and stress granule dynamics, we used the E1 ubiquitin activating enzyme (UBA1) inhibitor, TAK243 (63). First, we verified that TAK243 treatment led to dose-dependent and time-dependent depletion of cellular ubiquitin conjugates (fig. S4A). Whereas high concentrations or prolonged exposure to TAK243 was toxic to cells (fig. S4B), incubation with 1 μM TAK243 was sufficient to block most cellular ubiquitination within 20 min while causing no detectable loss of viability for up to 4 hours (fig. S4, A and B). Furthermore, ubiquitination did not impact the dynamics of stress granules induced by arsenite (fig. S4C and movies S1, S2), as expected (35). However, given the differences between the ubiquitin response to heat shock and arsenite, we next assessed the role of ubiquitination in regulating the assembly and disassembly of stress granules induced by heat shock. To our surprise, elimination of ubiquitination with TAK243 did not prevent stress granule formation in response to heat shock, but impaired stress granule disassembly after the removal of stress (Fig. 6H and movies S3, S4). We confirmed this observation with imaging of 10 additional stress granule markers, which showed persistence in stress granules for up to two hours after restoration of normal growth temperature (fig. S4, D–F). We also observed a similar stress granule disassembly defect in cells genetically depleted for UBA1 (fig. S4, G–I). We next repeated the live cell imaging analysis of stress granule dynamics in iPSC-derived neurons. Here too we observed a significant delay in stress granule disassembly during recovery from heat shock in response to TAK243 treatment (Fig. 6I). Thus, across a wide variety of cell types, ubiquitination is essential for normal disassembly of stress granules during recovery from heat stress.
Given that VCP is involved in stress granule disassembly (29, 32, 33), we next sought to compare the effects of inhibition of UBA1 and VCP on stress granule disassembly kinetics. Treatment with the VCP inhibitor CB5083 prior to heat shock led to delayed stress granule disassembly with nearly identical kinetics as those observed for cells treated with TAK243 (fig. S4J). Thus, ubiquitination and VCP likely act on stress granule disassembly through the same pathway.
We also examined how mitigating stress granule assembly impacted the heat shock ubiquitinome. Blocking stress granule assembly either pharmacologically (using cycloheximide (64)) or genetically (using G3BP1/2 double KO cells (65)) had no detectable effect on the heat shock ubiquitinome, consistent with the idea that ubiquitination of mRNPs occurs upstream and independent from stress granule formation, (fig. S5, A–D and table S3). Thus, whereas stress-dependent ubiquitination can occur in the absence of heat shock-induced stress granules and is not required for their assembly, it is essential for their rapid disassembly during recovery.
Reversal of mRNP remodeling following heat shock requires ubiquitination
Given our finding that disassembly of heat shock-induced stress granules was dependent on ubiquitination, we next investigated the importance of ubiquitination in the reversibility of the heat shock-dependent interactions we observed in the remodeled mRNPs. We added 1 μM TAK243 to the media prior to and during heat shock and confirmed elimination of the poly-ubiquitin signal associated with either PABPC1 or oligo(dT) retrieval (Fig. 7A and fig. S6). TAK243 treatment did not block the heat shock-dependent remodeling of the mRNP interactome; however, similar to what we observed with stress granules, elimination of ubiquitination prevented the reversal of this stress-dependent remodeling during recovery (Fig. 7A and fig. S6). Thus, ubiquitination is required for disassembly of stress granules and for the reversibility of mRNP remodeling following restoration of normal growth conditions after heat shock.
Fig. 7. Ubiquitination is required for the recovery of nucleocytoplasmic transport and translation following heat shock.
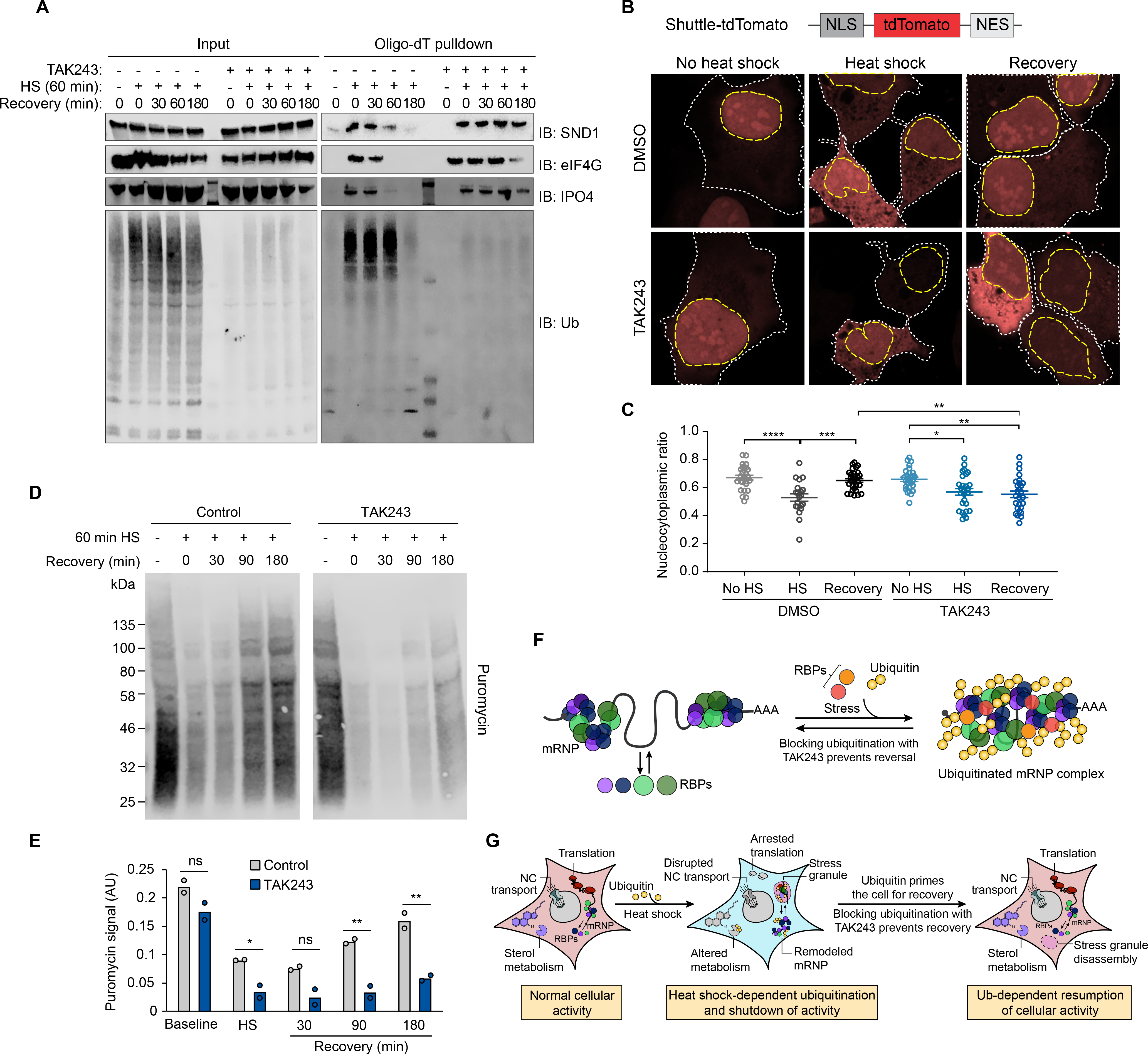
(A) Immunoblot showing formation and dissolution of poly-ubiquitinated protein-mRNA complex during heat shock and isolated by oligo(dT) resin. Where indicated, TAK243 was added to media 30 min prior to heat shock and maintained for the duration of the experiment. In drug-treated unstressed samples, HEK293T cells were incubated with TAK243 for 180 min prior to lysis. (B) HEK293T cells expressing nucleocytoplasmic transport reporter NLS-tdTomato-NES were fixed and imaged after no stress, 60 min heat shock, or 60 min heat shock and 120 min recovery. Cells were treated with TAK243 or DMSO for 30 min prior to heat shock or for a total of 120 min in non-heat-shocked cells. Nuclear and cytoplasmic boundaries are indicated with dashed yellow and white lines respectively. (C) Quantification of nucleocytoplasmic ratio of tdTomato intensity from cell images described in (B) from 20–30 cells for each condition. Error bars indicate s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ANOVA with Tukey’s test. (D) Immunoblotting of HEK293T cells treated with puromycin to label nascent transcripts for 30 min prior to heat shock and recovery in the presence or absence of TAK243. Cells were lysed at indicated times and translational activity was analyzed by immunoblotting for puromycin. (E) Quantification of immunoblots shown in (D). Average and individual values for puromycin signal are shown for two replicate experiments. n.s., not significant, *P < 0.05, **P < 0.01, student’s t-test. (F-G) Proposed model illustrating heat shock-induced ubiquitination associated with (F) mRNP remodeling and (G) stress granule formation and shutdown of cellular activities, and the requirement for active ubiquitination for reversal of these processes during recovery.
Ubiquitination is required for recovery of heat shock-induced impairment of nucleocytoplasmic transport
To investigate the role of ubiquitination in stress-induced perturbation of nucleocytoplasmic transport, we used a Shuttle-tdTomato nucleocytoplasmic reporter assay in which tdTomato was conjugated to both a nuclear localization signal (NLS) and nuclear export signal (NES) that mediate its shuttling between the nucleus and cytoplasm (8). Upon heat shock, we observed a significant redistribution of the reporter from the nucleus (ratio of nuclear to total fluorescence (N/W) ~ 0.75 at steady state) to the cytoplasm (N/W ~0.5 following heat shock) (Fig. 7, B and C). This result is consistent with a previous report that stress-dependent re-localization of this reporter was due to a stress-induced defect in exportin-1-mediated nuclear export (8). Under normal growth conditions, inhibition of ubiquitination with TAK243 had no significant impact on the ratio of nuclear versus cytoplasmic reporter signal, indicating that ubiquitination was not a critical determinant of canonical nuclear import or export. Moreover, following elimination of ubiquitination with TAK243, cells exposed to heat shock still showed a defect in nuclear import activity, indicating that ubiquitination was not required for this inhibition. However, cells treated with TAK243 failed to recover from impaired nucleocytoplasmic shuttling even upon restoration of normal growth conditions for 2 hours. Thus, similar to disassembly of stress granules and reversal of mRNP remodeling, ubiquitination was essential for recovery of nucleocytoplasmic shuttling following heat stress (Fig. 7, B and C).
Ubiquitination is required for reinitiating translation following heat shock
Protein synthesis is also strongly perturbed by heat stress (66) owing to broad shutdown of most translation, with the exception of heat shock proteins whose translation is preserved (67). To investigate the role of ubiquitination in stress-induced perturbation of translation, we used a metabolic labeling assay (68). As expected, upon heat shock, translation activity was dramatically decreased as measured by puromycin incorporation into nascent translation products, followed by a time-dependent recovery after restoration of normal growth conditions (Fig. 7, D and E). Whereas TAK243 treatment had no effect on basal translation levels, heat-shocked cells treated with TAK243 showed a profound failure to recover protein synthesis following restoration of normal growth conditions (Fig. 7, D and E). Thus, ubiquitination was essential for the recovery of translation during the recovery phrase from heat shock.
Together, our results demonstrate that heat stress induces the targeted ubiquitination of a specific set of proteins and that this ubiquitination is required for recovery from multiple heat stress-induced cellular perturbations, including reversal of mRNP remodeling, disassembly of stress granules, and resumption of nucleocytoplasmic transport and protein synthesis (Fig. 7, F and G). Furthermore, the role of stress-induced ubiquitination is context-dependent; indeed, several of these observations did not hold true for arsenite stress. Our extensive proteomics datasets describing the heat shock ubiquitinome also suggest that ubiquitination facilitates other aspects of the stress response that have yet to be elucidated. Indeed, in preliminary studies exploring the role of ubiquitination on sterol metabolism, we observed that TAK243 treatment had significant effects on the cholesterol biosynthesis pathway during both heat shock and recovery in a pattern entirely different than that observed for the other cellular activities investigated above (Fig. 7G and fig. S7).
Discussion
The results presented here provide several insights into the mechanisms by which ubiquitination facilitates recovery from heat stress. Our proteomic and biochemical results reveal a heat shock-dependent increase in ubiquitination of mRNPs and stress granule resident proteins, and we propose that the presence of these ubiquitin conjugates within stress granules facilitates an interaction with VCP to promote their timely disassembly during recovery (62). This disassembly paradigm is apparently not used during recovery from arsenite stress (35), despite previous evidence suggesting the involvement of VCP in this process VCP can function in a ubiquitin-independent manner to disassemble some protein complexes (69), which may explain this apparent contradiction. Alternatively, VCP may not actively disassemble arsenite-induced stress granules, but may act to prevent the localization of ubiquitinated DRiPs into aberrant stress granules. These DRiPs would then accumulate when VCP is inhibited, resulting in a loss of dynamism and resistance to disassembly or clearance by a ubiquitin-independent mechanism. This scenario would account for the observation of a disassembly defect upon VCP inhibition but not UBA1 inhibition.
Inhibited ubiquitination also prevented the recovery of nucleocytoplasmic and translation activity. Perhaps critical transport and translation factors are sequestered in the persistent stress granules and thus unable to perform their normal functions upon the removal of stress. This has been proposed as a mechanism by which cellular stress disrupts nucleocytoplasmic transport (8), and our results further suggest a role for ubiquitination in liberating proteins from stress granules following the removal of heat stress.
While the ubiquitin conjugation substrates identified here provide insight into the stress response, identification of the E3 ligases responsible for this heat shock-dependent ubiquitination is an important next step. Our total proteome analysis did not reveal any statistically significant stress-dependent increase in E3 ligases, suggesting that the increased ubiquitination was not likely influenced by upregulation of specific E3s. However, several E3 ligases were highly enriched in the TUBE pulldown experiments, including TRIP12, TRIM25, and TRIM28 which warrant future investigation as candidate E3s. Nedd4, an E3 ligase critical for heat shock-dependent ubiquitination in HeLa cells and mouse embryonic fibroblasts, was highly enriched after heat shock in our TUBE pulldown from mouse cortical neurons but not in any of the human cells used here. Thus, despite a commonality of ubiquitination substrates between cell lines, the E3 ligases involved may have some cell type specificity. Finally, in accord with a recent investigation identifying a role for SUMO-targeted ubiquitination in the stress response (34), we found that SUMO was among the most enriched heat shock ubiquitinome proteins in both our TUBE and di-GLY analyses, suggesting that SUMO-targeted E3 ligases are likely to contribute to heat shock-dependent ubiquitin conjugation.
Certain aspects of the adaptive nature of stress-induced ubiquitination have long been recognized. Particularly with proteotoxic insults such as heat shock, stress induces several types of potentially hazardous proteins (e.g., DRiPs, thermolabile misfolded proteins, and other damaged proteins) that must be cleared to prevent the formation of toxic species (29–31, 42–44). This need is largely met by ubiquitination and subsequent proteasomal degradation of the offending species. A substantial portion of the proteome becomes transiently insoluble in response to heat stress (42–45), which suggested a second mode whereby the cell can temporarily reshape the proteome to survive a stressful period. Our results add a third dimension to these concepts, indicating that changes to the ubiquitinome are tailored to specific stressors and, in the case of heat shock stress, ubiquitination serves to prime the cell for recovery. Furthermore, our direct comparisons of the relationship between ubiquitination and heat shock- or arsenite-induced stress granules highlight important differences in context-dependent mechanisms regulating what otherwise appear to be very similar assemblies. Finally, our combined proteome and ubiquitinome datasets represent a vast repository of potentially interesting findings. Our di-GLY-TMT analysis is especially rich, representing a deep and comprehensive ubiquitinome, including many heat shock-dependent ubiquitination events that were not reflected in our TUBE results. These datasets therefore provide a valuable community resource providing insight into many unexplored aspects of the roles of ubiquitination in response to stress.
Methods Summary
All experiments were performed in HEK293T and/or U2OS cells unless otherwise indicated. Primary cultures of cortical neurons were prepared from embryo day 15 ICR mice (Charles River Laboratories) as previously described (70). iPS neurons were differentiated with a two-step protocol (pre-differentiation and maturation) as previously described (71). For heat shock treatment, cell culture media was replaced with fresh media pre-warmed to 42°C and placed at 42°C for the indicated time. In recovery experiments, the cells were returned to the 37°C incubator for the indicated time. For matched control-treated cells, media was replaced with fresh media warmed to 37°C and maintained at 37°C for the indicated time prior to harvest. For other stresses, media was replaced with fresh media containing 0.5 mM sodium arsenite, 0.4 M sorbitol, or 1 μM bortezomib for the indicated time. For UV stress, media was removed and replaced with 2 ml PBS and 40 J/m2 UV radiation was delivered. When indicated, 1 μM TAK243 was added directly to cell culture media 30 min prior to stress treatment and maintained in the media until lysis.
Cell pellets were collected by centrifugation followed by removal of media by aspiration, and the cell pellet was washed twice with PBS. Cell pellets were then lysed in the indicated buffers supplemented with deubiquitinase and protease inhibitors. Lysates were centrifuged and the supernatant fraction was collected as the “soluble fraction” for TUBE or IP lysis buffers or “whole cell lysate” for urea lysis buffer. To collect pellet fractions, the insoluble pellet from TUBE lysis buffer was washed and resolubilized in urea lysis buffer.
For TUBE pulldowns, soluble or pellet cell lysate fractions were incubated with His-Halo-TUBE beads overnight at 4°C. Beads with captured proteins were then washed and captured proteins were then released from the beads by denaturation in 1x Laemmli sample buffer with reducing agent and heating at 75°C for 5 min.
For immunoprecipitation and Oligo(dT) pulldown, cells were lysed in IP lysis buffer and lysates were incubated overnight at 4°C with Protein G Dynabeads (Invitrogen) conjugated to antibodies against endogenous proteins according to the manufacturer’s protocol. For Oligo(dT) pulldown, lysate was incubated with Oligo d(T)25 magnetic bead resin (New England Biolabs) overnight at 4°C. Following overnight incubation, beads were washed and proteins were eluted in 1x LDS sample buffer with reducing agent for analysis by immunoblot and MS.
Mass spectrometric (MS) analysis was performed according to an optimized platform as previously reported (72). Eluates from the TUBE pulldown or IP experiments were run on an SDS gel and visualized by Coomassie staining (Thermo Fisher Scientific). The whole gel lanes were excised into gel bands. The digested peptides were extracted from the gel bands, dried and reconstituted in loading buffer. Peptides were then fractionated by HPLC system (and detected by an in-line mass spectrometer. MS/MS spectra were searched against UniProt human protein database and filtered by mass accuracy and matching scores to reduce protein false discovery rate (FDR) to about 1%.
Proteomic profiling of whole proteome was carried out with a previously reported protocol (73). Briefly, for each sample, HEK293T cells from one 10-cm dish were harvested, washed with ice-cold PBS and collected by centrifugation. Approximately 100 μg of protein per sample of cell lysate was digested, followed by Cys reduction, alkylation, and quenching. The samples were further digested with trypsin and differentially labeled with 11-plex Tandem Mass Tag (TMT) reagents (Thermo Fisher Scientific) according to the manufacturer’s protocol.
The TMT-labeled samples were mixed equally, desalted, and fractionated on an offline HPLC (Agilent 1220) using basic pH reverse phase liquid chromatography. The fractions were dried, reconstituted and analyzed by acidic pH reverse phase LC-MS/MS on an Ultimate 3000 UPLC system (Thermo Fisher Scientific). Peptides were eluted, ionized by electrospray ionization and detected by an in-line Orbitrap Fusion mass spectrometer. MS/MS spectra were searched against UniProt human protein database and filtered by mass accuracy and matching scores to reduce protein FDR to about 1%.
For di-GLY TMT ubiquitinome analysis, proteomic profiling of the ubiquitinome was performed with a previously reported protocol (52) with modification. Briefly, for each sample, HEK293T cells from five 15-cm dish were harvested, washed with ice-cold PBS and collected by centrifugation. Cells were lysed, immediately digested with LysC (Wako), quenched with DTT (30 mM for 30 min) and subjected to trypsin digestion and desalting. The desalted peptides were resuspended, centrifuged, and incubated with di-GLY antibody beads (Cell Signaling Technology) for 2 h at 4°C. The di-GLY peptides were eluted at room temperature, dried, and resuspended for 11-plex TMT labeling. The TMT labeled peptides were combined equally among replicates, desalted, and analyzed by LC/LC-MS/MS using a similar protocol descried above.
For proteomics statistics, summed spectral counts of replicate experiments was compared among different treatments with missing values replaced by a constant low value to calculate fold change and P values. Stress-induced changes were considered statistically significant with P ≤ 0.02 and fold change ≥ 2, excluding proteins detected in only one of three replicates for experiments performed in triplicate. In TMT-based quantification experiments (total proteome and di-GLY profiling), stress-induced changes were considered statistically significant with P ≤ 0.05 and fold change ≥ 1.5. The heat shock ubiquitinome was defined from the TUBE experiment as proteins with statistically significant increase in the soluble fraction and proteins with statistically significant increase in the pellet fraction but not decreased in the soluble fraction (Fig. 2J), excluding 8 proteins for which peptides were measured to have decreased abundance in the di-GLY TMT experiment.
Supplementary Material
Acknowledgments:
We thank A. Ordureau (Harvard) and W. Harper (Harvard) for providing the plasmid encoding His-HALO-TUBE and for suggesting protocols for purification and use of the TUBEs; R. Parker (University of Colorado) for helpful discussions; and J. Messing (St. Jude) and J. Temirov (St. Jude) for assistance with microscopy.
Funding: This work was supported by a St. Jude George Mitchell Fellowship (B.A.M.); NIH grants 5F32GM117815 (B.A.M.), R01GM114260 (J.P.), and R35NS097074 (J.P.T.); and HHMI (J.P.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests: J.P.T. is a consultant for 5AM and Third Rock Ventures.
Data and materials availability: All data is available in the main text or the supplementary materials.
References and Notes:
- 1.Harding HP et al. , An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Pakos-Zebrucka K et al. , The integrated stress response. EMBO Rep 17, 1374–1395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brostrom CO, Prostko CR, Kaufman RJ, Brostrom MA, Inhibition of translational initiation by activators of the glucose-regulated stress protein and heat shock protein stress response systems. Role of the interferon-inducible double-stranded RNA-activated eukaryotic initiation factor 2alpha kinase. J Biol Chem 271, 24995–25002 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Dever TE et al. , Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68, 585–596 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Ron D, Translational control in the endoplasmic reticulum stress response. J Clin Invest 110, 1383–1388 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wek RC, Jiang HY, Anthony TG, Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34, 7–11 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Lu PD, Harding HP, Ron D, Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 167, 27–33 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K et al. , Stress Granule Assembly Disrupts Nucleocytoplasmic Transport. Cell 173, 958–971 e917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuta M et al. , Heat-shock induced nuclear retention and recycling inhibition of importin alpha. Genes Cells 9, 429–441 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Kelley JB, Paschal BM, Hyperosmotic stress signaling to the nucleus disrupts the Ran gradient and the production of RanGTP. Mol Biol Cell 18, 4365–4376 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodiha M, Chu A, Matusiewicz N, Stochaj U, Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Differ 11, 862–874 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Kose S, Imamoto N, Nucleocytoplasmic transport under stress conditions and its role in HSP70 chaperone systems. Biochim Biophys Acta 1840, 2953–2960 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto Y et al. , Cellular stresses induce the nuclear accumulation of importin alpha and cause a conventional nuclear import block. J Cell Biol 165, 617–623 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalgi R, Hurt JA, Lindquist S, Burge CB, Widespread inhibition of posttranscriptional splicing shapes the cellular transcriptome following heat shock. Cell Rep 7, 1362–1370 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto K et al. , Control of the heat stress-induced alternative splicing of a subset of genes by hnRNP K. Genes Cells 21, 1006–1014 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Pearce AK, Humphrey TC, Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol 11, 426–433 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Radmaneshfar E et al. , From START to FINISH: the influence of osmotic stress on the cell cycle. PLoS One 8, e68067 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhl NM, Rensing L, Heat shock effects on cell cycle progression. Cell Mol Life Sci 57, 450–463 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balagopal V, Parker R, Polysomes P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr Opin Cell Biol 21, 403–408 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jousse C et al. , Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol 163, 767–775 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novoa I, Zeng H, Harding HP, Ron D, Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153, 1011–1022 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R, Distinct stages in stress granule assembly and disassembly. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie X et al. , Deubiquitylases USP5 and USP13 are recruited to and regulate heat-induced stress granules through their deubiquitylating activities. J Cell Sci 131, (2018). [DOI] [PubMed] [Google Scholar]
- 24.Kwon S, Zhang Y, Matthias P, The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev 21, 3381–3394 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markmiller S et al. , Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell 172, 590–604 e513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youn JY et al. , High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies. Mol Cell 69, 517–532 e511 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Kedersha N et al. , G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol 212, 845–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain S et al. , ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 164, 487–498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turakhiya A et al. , ZFAND1 Recruits p97 and the 26S Proteasome to Promote the Clearance of Arsenite-Induced Stress Granules. Mol Cell 70, 906–919 e907 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Alberti S, Mateju D, Mediani L, Carra S, Granulostasis: Protein Quality Control of RNP Granules. Front Mol Neurosci 10, 84 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganassi M et al. , A Surveillance Function of the HSPB8-BAG3-HSP70 Chaperone Complex Ensures Stress Granule Integrity and Dynamism. Mol Cell 63, 796–810 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Buchan JR, Kolaitis RM, Taylor JP, Parker R, Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153, 1461–1474 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B et al. , ULK1 and ULK2 Regulate Stress Granule Disassembly Through Phosphorylation and Activation of VCP/p97. Mol Cell 74, 742–757 e748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keiten-Schmitz J et al. , The Nuclear SUMO-Targeted Ubiquitin Quality Control Network Regulates the Dynamics of Cytoplasmic Stress Granules. Mol Cell 79, 54–67 e57 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Markmiller S et al. , Active Protein Neddylation or Ubiquitylation Is Dispensable for Stress Granule Dynamics. Cell Rep 27, 1356–1363 e1353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaudette P, Popp O, Dittmar G, Proteomic techniques to probe the ubiquitin landscape. Proteomics 16, 273–287 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Ordureau A, Munch C, Harper JW, Quantifying ubiquitin signaling. Mol Cell 58, 660–676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopitz-Otsoa F et al. , Integrative analysis of the ubiquitin proteome isolated using Tandem Ubiquitin Binding Entities (TUBEs). J Proteomics 75, 2998–3014 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Fulzele A, Bennett EJ, Ubiquitin diGLY Proteomics as an Approach to Identify and Quantify the Ubiquitin-Modified Proteome. Methods Mol Biol 1844, 363–384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang NN et al. , Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nat Cell Biol 16, 1227–1237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang NN, Zhu M, Rose A, Wu KP, Mayor T, Deubiquitinase activity is required for the proteasomal degradation of misfolded cytosolic proteins upon heat-stress. Nat Commun 7, 12907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu G et al. , Identification of proteins sensitive to thermal stress in human neuroblastoma and glioma cell lines. PLoS One 7, e49021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace EW et al. , Reversible, Specific, Active Aggregates of Endogenous Proteins Assemble upon Heat Stress. Cell 162, 1286–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sui X et al. , Widespread remodeling of proteome solubility in response to different protein homeostasis stresses. Proc Natl Acad Sci U S A 117, 2422–2431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng AH, Fang NN, Comyn SA, Gsponer J, Mayor T, System-wide analysis reveals intrinsically disordered proteins are prone to ubiquitylation after misfolding stress. Mol Cell Proteomics 12, 2456–2467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuniati L et al. , Tumor suppressor BTG1 promotes PRMT1-mediated ATF4 function in response to cellular stress. Oncotarget 7, 3128–3143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vihervaara A, Duarte FM, Lis JT, Molecular mechanisms driving transcriptional stress responses. Nat Rev Genet 19, 385–397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diller KR, Stress protein expression kinetics. Annu Rev BiomedEng 8, 403–424 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Subjeck JR, Sciandra JJ, Johnson RJ, Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol 55, 579–584 (1982). [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y et al. , The role of heat shock factors in stress-induced transcription. Methods Mol Biol 787, 21–32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu G, Paige JS, Jaffrey SR, Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat Biotechnol 28, 868–873 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rose CM et al. , Highly Multiplexed Quantitative Mass Spectrometry Analysis of Ubiquitylomes. Cell Syst 3, 395–403 e394 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rauniyar N, Yates JR 3rd, Isobaric labeling-based relative quantification in shotgun proteomics. JProteome Res 13, 5293–5309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leuenberger P et al. , Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability. Science 355, (2017). [DOI] [PubMed] [Google Scholar]
- 55.Huang da W, Sherman BT, Lempicki RA, Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Mi H et al. , PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45, D183–D189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang J et al. , CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem 276, 42938–42944 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Taipale M et al. , A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell 158, 434–448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paul I, Ghosh MK, A CHIPotle in physiology and disease. Int J Biochem Cell Biol 58, 37–52 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Hwang HW et al. , PAPERCLIP Identifies MicroRNA Targets and a Role of CstF64/64tau in Promoting Non-canonical poly(A) Site Usage. Cell Rep 15, 423–435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang P et al. , G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 181, 325–345 e328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gwon Y et al. , Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyer ML et al. , A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat Med 24, 186–193 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Kedersha N et al. , Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151, 1257–1268 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang P et al. , G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell, (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panniers R, Translational control during heat shock. Biochimie 76, 737–747 (1994). [DOI] [PubMed] [Google Scholar]
- 67.Panniers R, Henshaw EC, Mechanism of inhibition of polypeptide chain initiation in heat-shocked Ehrlich ascites tumour cells. Eur J Biochem 140, 209–214 (1984). [DOI] [PubMed] [Google Scholar]
- 68.Schmidt EK, Clavarino G, Ceppi M, Pierre P, SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6, 275–277 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Weith M et al. , Ubiquitin-Independent Disassembly by a p97 AAA-ATPase Complex Drives PP1 Holoenzyme Formation. Mol Cell 72, 766–777 e766 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Yamashita N et al. , Regulation of spine development by semaphorin3A through cyclin-dependent kinase 5 phosphorylation of collapsin response mediator protein 1. J Neurosci 27, 12546–12554 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C et al. , Scalable Production of iPSC-Derived Human Neurons to Identify Tau-Lowering Compounds by High-Content Screening. Stem Cell Reports 9, 1221–1233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu P, Duong DM, Peng J, Systematical optimization of reverse-phase chromatography for shotgun proteomics. J Proteome Res 8, 3944–3950 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai etal B., Deep Profiling of Proteome and Phosphoproteome by Isobaric Labeling, Extensive Liquid Chromatography, and Mass Spectrometry. Methods Enzymol 585, 377–395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Figley MD, Bieri G, Kolaitis RM, Taylor JP, Gitler AD, Profilin 1 associates with stress granules and ALS-linked mutations alter stress granule dynamics. J Neurosci 34, 8083–8097 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berg S et al. , ilastik: interactive machine learning for (bio)image analysis. Nat Methods 16, 1226–1232 (2019). [DOI] [PubMed] [Google Scholar]
- 76.McQuin C et al. , CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol 16, e2005970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X et al. , JUMP: a tag-based database search tool for peptide identification with high sensitivity and accuracy. Mol Cell Proteomics 13, 3663–3673 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng J, Elias JE, Thoreen CC, Licklider LJ, Gygi SP, Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J Proteome Res 2, 43–50 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Xu P et al. , Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bustos D, Bakalarski CE, Yang Y, Peng J, Kirkpatrick DS, Characterizing ubiquitination sites by peptide-based immunoaffinity enrichment. Mol Cell Proteomics 11, 1529–1540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eng JK et al. , A deeper look into Comet--implementation and features. J Am Soc Mass Spectrom 26, 1865–1874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou JY et al. , Galectin-3 is a candidate biomarker for amyotrophic lateral sclerosis: discovery by a proteomics approach. J Proteome Res 9, 5133–5141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smyth GK, Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3, Article3 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H, UpSet: Visualization of Intersecting Sets. IEEE Trans Vis Comput Graph 20, 1983–1992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valentin-Vega YA et al. , Cancer-associated DDX3X mutations drive stress granule assembly and impair global translation. Sci Rep 6, 25996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


