ABSTRACT
Background
The pattern of change in maternal bone turnover throughout pregnancy is poorly characterized.
Objectives
We investigated changes across pregnancy in a marker of maternal bone resorption, urinary C-terminal telopeptide of type I collagen (CTX), the influence of gestational vitamin D supplementation, and associations between CTX and maternal postnatal bone indices.
Methods
MAVIDOS (the Maternal Vitamin D Osteoporosis Study) is a randomized, double-blind, placebo-controlled trial of 1000 IU cholecalciferol/d compared with placebo from 14 weeks of gestation to birth. Maternal second-void urinary α- and β-CTX were measured (ELISA) at 14 and 34 weeks of gestation; DXA was performed within 2 wk postpartum. The Mann–Whitney Rank Sum test, Spearman's rank correlation, and linear regression were used to compare median CTX values within and between groups from early to late pregnancy, and associations with maternal bone outcomes.
Results
In total, 372 women had CTX and 25-hydroxyvitamin D [25(OH)D] measured in early and late pregnancy. CTX at 14 and 34 weeks of gestation were correlated in both placebo (r = 0.31) and cholecalciferol (r = 0.45) groups (P < 0.0001). Median CTX increased from 14 to 34 weeks of gestation in both groups (n = 372 total) [placebo (n = 188): from 223.6 to 449.7 μg/mmol creatinine; cholecalciferol (n = 184): from 222.3 to 419.3 μg/mmol creatinine; P = 0.03 for placebo compared with cholecalciferol difference in CTX at 34 weeks of gestation]. The conditional mean ± SD increase in CTX [z-score (SD)] from early to late pregnancy was greater in the placebo group (n = 188) than in the cholecalciferol group (n = 184) (placebo: 0.16 ± 0.92; cholecalciferol: −0.16 ± 1.06; P-difference < 0.01). Higher CTX at 34 weeks of gestation was associated, similarly in both groups, with lower maternal total hip and lumbar spine bone mineral content and bone mineral density (BMD) (e.g., lumbar spine BMD: β = −0.02 g · cm−2 · SD−1 increase in CTX; 95% CI: −0.027, −0.002 g · cm−2 · SD−1; P = 0.02, n = 283).
Conclusions
Maternal urinary CTX, a bone resorption marker, rises through pregnancy, although to a lesser degree with gestational cholecalciferol supplementation, and is inversely associated with maternal bone mass postpartum.
This trial was registered at www.isrctn.com as ISRCTN 82927713 and eudract.ema.europa.eu as EudraCT 2007-001716-23.
Keywords: osteoporosis, epidemiology, vitamin D, bone turnover, pregnancy, CTX, DXA
Introduction
The human fetal skeleton begins to mineralize between 8 and 12 weeks of gestation when primary ossification centers form in the vertebrae and long bones (1). Approximately 30 g calcium are required in total for its development, 80% of which is accrued in the third trimester. Maternal calcium homeostasis adapts to meet the calcium demands of the developing fetus (1–3), particularly through increased intestinal absorption and renal reabsorption of calcium. The maternal skeleton's role in supplying the fetus, and how it might respond to interventions such as vitamin D supplementation, are not well defined, because of the difficulty in using DXA, which involves ionizing radiation, in pregnancy. Biochemical markers of bone turnover offer a noninvasive method of monitoring changes in bone resorption or formation during pregnancy (4), and provide some insight into the impact of pregnancy on maternal bone and its contribution to fetal development. There are 2 groups of bone turnover markers: markers of bone formation [such as N-terminal collagen extension propeptide (P1NP), bone alkaline phosphatase] and markers of resorption [such as C-terminal telopeptide of type I collagen (CTX), N-terminal telopeptide of type I collagen (NTX), deoxypyridinoline, hydroxyproline]. In the vitamin D–deficient state, plasma calcium concentrations may be reduced with a consequent rise in parathyroid hormone (PTH), leading to increased bone resorption (5). Indeed, at the population level, there is evidence of inverse associations between 25-hydroxyvitamin D [25(OH)D] concentrations and markers of bone resorption such as CTX (6–9). However, few studies have investigated these relations across normal pregnancies, or the influence of vitamin D supplementation (10–17).
The MAVIDOS (Maternal Vitamin D Osteoporosis Study) trial of vitamin D supplementation in pregnancy (of which the primary aim was to investigate the effect of gestational cholecalciferol supplementation on offspring bone mass) provides an opportunity to study the impact of this supplement on a marker of maternal bone resorption (CTX) and maternal postpartum bone indices (18, 19). As the basis for this post hoc analysis, we hypothesized that vitamin D supplementation in pregnancy would result in reduced osteoclastic bone resorption, and thus that we would observe 1) an inverse association between vitamin D supplementation [or 25(OH)D concentration] and CTX concentrations, and 2) an inverse association between maternal CTX concentrations and measures of maternal postpartum bone mass.
Methods
MAVIDOS
MAVIDOS (ISRCTN 82927713; EudraCT 2007-001716-23) was a multicenter, double-blind, randomized, placebo-controlled trial of vitamin D supplementation in pregnancy. The primary outcome was neonatal bone mass. A detailed description of the study methods (18) and primary findings have been published previously (19).
Briefly, women attending 1 of 3 UK hospitals [University Hospital Southampton National Health Service (NHS) Foundation Trust, Southampton, United Kingdom; Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom; Sheffield Teaching Hospitals NHS Foundation Trust (University of Sheffield), Sheffield, United Kingdom] for early pregnancy ultrasound screening (11–14 weeks of gestation) between 6 October, 2008 and 11 February, 2014 were invited to participate in the study (19, 20). Inclusion criteria were age > 18 y, singleton pregnancy, and gestation < 17 wk based on last menstrual period and ultrasound measurements. Women with known metabolic bone disease, renal stones, hyperparathyroidism, or hypercalciuria; those taking medication known to interfere with fetal growth; those with fetal anomalies on ultrasonography; and those already using >400 IU vitamin D/d supplementation were excluded. A screening blood sample was obtained and analyzed on the local NHS platform [all 3 laboratories (Southampton, Oxford, and Sheffield) participate in the Vitamin D External Quality Assessment Scheme (DEQAS) vitamin D quality assurance system (http://www.deqas.org/)]. Women with 25(OH)D between 25 and 100 nmol/L and serum calcium < 2.75 mmol/L were eligible to enroll fully in the study. Participants were randomly assigned in a double-blind design to either 1000 IU cholecalciferol/d or matched placebo (Merck KGaA/Sharp Clinical Services; previously DHP-Bilcare), which was commenced before 17 weeks of gestation. All participants received standard antenatal care and could continue self-administration of dietary supplements containing ≤400 IU vitamin D/d. Data from Southampton participants were used in the current analysis because CTX was not measured in Oxford and Sheffield participants.
Maternal assessments during pregnancy
Before commencing the study medication, and again at 34 weeks of gestation, the women attended their hospital for a detailed assessment of diet (including supplement use), lifestyle (smoking, physical activity, employment), and health (past medical history, current medication use) using interviewer-led questionnaires. Ethnic group was self-reported by the participant as “White, Black Caribbean, Black African, Black Other, Indian, Pakistani, Bangladeshi, Chinese, Other Asian group or Other (specify).” This was then categorized as white or nonwhite. Anthropometric measurements included height, measured to the nearest 0.1 cm using a stadiometer (Seca), and weight, assessed to the nearest 0.1 kg using calibrated electronic scales (Seca).
Assessment of 25(OH)D and CTX
On the day that the study medication was dispensed and at 34 weeks of gestation, a nonfasted venous blood sample was obtained, and serum stored at −80°C. 25(OH)D was assessed by chemiluminescent immunoassay (Liaison automated platform, Diasorin). All samples were analyzed in a single batch at the end of the study at Medical Research Council (MRC) Human Nutrition Research, Cambridge, United Kingdom. Details of assay performance and quality control through participation in DEQAS, National Institute of Standards and Technology (NIST), and the UK National External Quality Assessment Service (NEQAS) are given elsewhere (21, 22).
Participants were asked to provide a second-void early-morning urine sample to measure urine α- and β-CTX on the day the study medication was started (∼14 weeks of gestation) and again at 34 weeks of gestation. Owing to the diurnal variation in CTX, in addition to the practical difficulties of obtaining a fasted blood sample from pregnant women, second-void early-morning urine collection by the participant at home was selected as the most appropriate measure of bone resorption. All samples were stored at −70°C and those from Southampton participants were sent in a batch for measurement by ELISA (Immunodiagnostic Systems) at the Academic Unit of Bone Metabolism, University of Sheffield, Sheffield, United Kingdom. Total CTX (sum of the α and β forms) is expressed as a ratio to urine creatinine (µg/mmol) (23). The assay had an interassay CV of 6% (24).
Maternal and neonatal DXA
Mothers and neonates underwent DXA assessment; mothers underwent DXA at the lumbar spine and hip sites, neonates at the whole-body and lumbar spine sites (instrument at the Southampton center: Hologic Discovery, Hologic Inc.) within 2 wk of delivery. The instrument underwent daily quality control. To maximize the scan quality, infants were undressed, clothed in a standard towel, fed, and pacified before the assessment. The total radiation dose was estimated to be 0.04 mSv, equivalent to ∼7 d exposure to background radiation in the United Kingdom. All DXA images were reviewed for movement artefacts and quality by 2 operators who were blinded to treatment allocation.
Ethics
The trial was approved by the Southampton and South West Hampshire Research Ethics Committee (07/H0502/113). MAVIDOS was registered prospectively (ISRCTN: 82927713; EUDRACT: 2007-001716-23); full approval from the UK Medicines & Healthcare products Regulatory Agency (MHRA) was granted, and written informed consent was obtained from all participants.
Statistics
Women who had urinary CTX and 25(OH)D measured at either 14 or 34 weeks of gestation, had not taken cholecalciferol supplements of >400 IU daily in addition to the study intervention, and who delivered a live-born infant were included in the analysis. All outcomes were assessed for normality using visual inspection. Maternal characteristics in the placebo and vitamin D–supplemented groups were documented. Comparisons between median CTX concentrations in the placebo and cholecalciferol-supplemented groups in early pregnancy (∼14 weeks of gestation) and late pregnancy (34 weeks of gestation) were made using the Mann–Whitney Rank Sum test. In order to avoid problems from regression to the mean and correlation between baseline value and change, early- and late-pregnancy CTX were summarized into a change in CTX variable, calculated as the residual from the regression of the later value on the earlier; differences between groups were tested using a t test (25). This change in CTX variable was then used as an outcome variable in linear regression to explore maternal factors associated with change. Change in CTX was also used as a predictor in regression analyses examining associations between maternal CTX and neonatal bone indices. Spearman's correlation was used to test associations between CTX in early pregnancy and late pregnancy, and between CTX (in early or late pregnancy) and maternal serum 25(OH)D measurements (in early or late pregnancy).
The distribution of CTX in early and late pregnancy was positively skewed and was therefore transformed and standardized using a Fisher-Yates transformation for inclusion in regression analyses. Fisher-Yates transformed CTX values were used as a predictor in regression analysis with DXA-assessed bone outcomes; β coefficients represent the change in bone outcome per 1-SD increase in CTX. In order to remove the additional influence of season on 25(OH)D concentrations, further analyses included adjustment for season of birth, using UK Meteorological Office classifications: winter (December–February), spring (March–May), summer (June–August), and autumn (September–November). Covariates identified as associated with maternal CTX concentrations and known to be associated with bone mass were included in CTX–bone regression models. All analyses were performed in Stata version 14 (Statacorp).
Results
Characteristics of the participants
In total, 965 women recruited to the MAVIDOS trial remained in the study until delivery, and of these 630 had CTX measured in either early (n = 493) or late pregnancy (n = 498). A total of 372 women had a CTX measurement at both time points (placebo group, n = 188; cholecalciferol-supplemented group, n = 184), as shown in Supplemental Figure 1. Table 1 summarizes the characteristics of the mothers with a measurement of urine CTX in early or late pregnancy, demonstrating a mean age of 30.4 y, 95.0% being of self-reported white ethnicity, and 44.5% being nulliparous. There was no evidence of selective dropout (as shown in Supplemental Table 1, comparing the participants of this substudy with the overall MAVIDOS trial) and thus in this subgroup analysis baseline characteristics appeared comparable between the mothers randomly assigned to placebo or cholecalciferol. Baseline maternal 25(OH)D concentration (measured at recruitment, 14 weeks of gestation) was similar in both groups. At 34 weeks of gestation mean ± SD maternal 25(OH)D concentration was significantly higher in the cholecalciferol-supplemented group (66.0 ± 20.4 nmol/L) than in those who received placebo (42.5 ± 20.6 nmol/L; P < 0.0001).
TABLE 1.
Characteristics of MAVIDOS (Maternal Vitamin D Osteoporosis Study) mothers with a measurement of C-terminal telopeptide of type I collagen available in early or late pregnancy, in the study overall, and in the placebo group and cholecalciferol-supplemented group separately1
| All | Placebo | 1000 IU cholecalciferol/d | ||||
|---|---|---|---|---|---|---|
| Baseline characteristic | Total n | Value | Total n | Value | Total n | Value |
| Age,2 y | 607 | 30.4 ± 5.2 | 296 | 30.4 ± 5.3 | 311 | 30.3 ± 5.3 |
| Height,2 cm | 602 | 165.4 ± 6.5 | 292 | 165.5 ± 6.7 | 310 | 165.2 ± 6.4 |
| Weight,2 kg | 607 | 71.8 ± 15.0 | 296 | 72.8 ± 14.4 | 311 | 70.8 ± 15.5 |
| Pregnancy weight gain,3 kg | 536 | 9.5 ± 3.6 | 264 | 9.3 ± 3.7 | 272 | 9.7 ± 3.4 |
| 25(OH)D at 14 wk, nmol/L | 630 | 44.6 ± 16.2 | 312 | 44.1 ± 16.1 | 318 | 45.0 ± 16.3 |
| 25(OH)D at 34 wk, nmol/L | 561 | 54.2 ± 23.6 | 282 | 42.5 ± 20.6 | 279 | 66.0 ± 20.4 |
| Adjusted calcium at 14 wk, mmol/L | 630 | 2.38 ± 0.08 | 312 | 2.38 ± 0.09 | 318 | 2.37 ± 0.07 |
| Adjusted calcium at 34 wk, mmol/L | 560 | 2.34 ± 0.11 | 280 | 2.34 ± 0.12 | 280 | 2.34 ± 0.10 |
| BMI,2 kg/m2 | 602 | 24.9 [22.5–28.6] | 292 | 25.5 [22.9–29.5] | 310 | 24.4 [22.3–28.1] |
| First pregnancy | 604 | 269 (44.5) | 294 | 134 (45.6) | 310 | 135 (43.5) |
| White (Caucasian) ethnicity | 604 | 574 (95.0) | 296 | 285 (96.3) | 308 | 289 (93.8) |
| A Level or higher qualification | 602 | 450 (74.8) | 293 | 214 (73.0) | 309 | 236 (76.4) |
| Smoker2 | 605 | 54 (8.9) | 295 | 27 (9.2) | 310 | 27 (8.7) |
| More than 1 h strenuous exercise per week2 | 553 | 79 (14.3) | 270 | 33 (12.2) | 283 | 46 (16.3) |
Values are mean ± SD, median [IQR], or n (%) unless otherwise indicated. 25(OH)D, 25-hydroxyvitamin D.
Characteristics at early-pregnancy study visit.
Pregnancy weight gain = 34-wk maternal weight minus 14-wk maternal weight.
Maternal urinary CTX in early and late pregnancy
As demonstrated graphically in Figure 1, both early- and late-pregnancy urinary CTX were in a positively skewed distribution: a few women had very high CTX measures (>1500 μg/mmol creatinine). In both the placebo and 1000 IU cholecalciferol/d–supplemented mothers, urinary CTX increased markedly from 14 to 34 weeks of gestation (median 14- and 34-wk CTX: placebo group, 223.8 and 445.3 μg/mmol creatinine, respectively; cholecalciferol-supplemented group, 217.5 and 420.0 μg/mmol creatinine, respectively; both P-difference < 0.0001), as shown in Table 2. Median CTX at 34 weeks of gestation tended to be greater in the placebo group than in the cholecalciferol-supplemented group (mean difference: 25.3 μg/mmol; P = 0.06). Restricting the analysis to women with CTX measures in early and late pregnancy (n = 372) led to greater observed differences in median CTX at 34 weeks of gestation (30.4 μg/mmol greater in the placebo group than in the cholecalciferol-supplemented group, P = 0.03), as also shown in Table 2. The conditional increase in mean ± SD [z-score (SD)] from early to late pregnancy was greater in the placebo group than in the cholecalciferol group (placebo: 0.16 ± 0.92; cholecalciferol: −0.16 ± 1.06; P-difference < 0.01). Note that the negative conditional change in CTX in the cholecalciferol group is relative to the overall distribution of standardized conditional change in CTX so still represents a rise: both groups demonstrated a large, positive absolute increase in CTX (median percentage change from early to late pregnancy, placebo: 111.1%; IQR: 47.1%–210.9%; cholecalciferol-supplemented: 88.8%; IQR: 25.9%–183.1%). When stratified by baseline 25(OH)D status in early pregnancy (sufficient, ≥50 nmol/L; or insufficient, <50 nmol/L), classified according to the 2011 report on dietary requirements for calcium and vitamin D from the Institute of Medicine (26), a threshold effect was observed. Women with vitamin D insufficiency at baseline in the placebo group had a greater conditional increase in CTX (0.28 SD, n = 126) than their counterparts in the cholecalciferol-supplemented group (−0.15 SD, n = 123; P-difference < 0.01). In women with vitamin D sufficiency, similar conditional increases were observed in the placebo and cholecalciferol-supplemented groups, as shown in Table 2.
FIGURE 1.
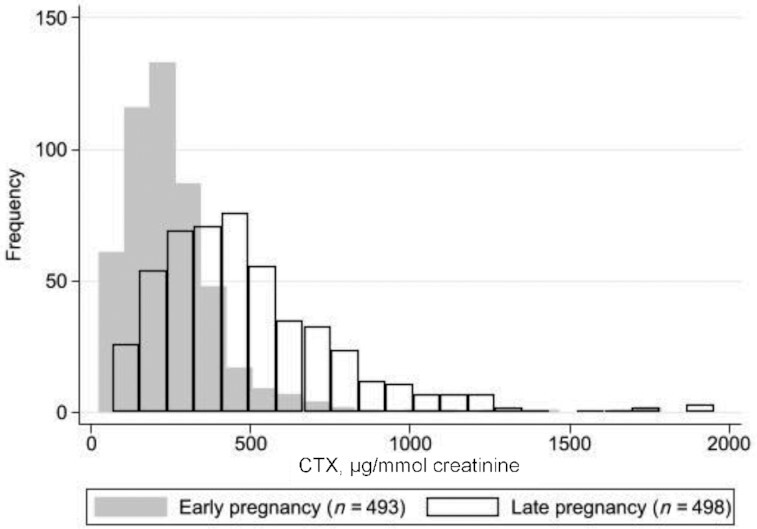
Frequency distribution of maternal urinary CTX (μg/mmol creatinine) in early (14 weeks of gestation) (median: 219.4; IQR: 154.7–306.4; n = 493) and late pregnancy (34 weeks of gestation) (median: 437.4; IQR: 292.3–633.0; n = 498) in all mothers with a measurement of CTX. CTX, C-terminal telopeptide of type I collagen.
TABLE 2.
Maternal urinary CTX in EP and LP and conditional change in CTX from EP to LP, in all mothers in the MAVIDOS (Maternal Vitamin D Osteoporosis Study) trial CTX substudy with a measure of EP or LP CTX, and in the placebo group and 1000 IU cholecalciferol–supplemented groups separately; and in all mothers with a measure of both EP and LP CTX, and in the placebo group and 1000 IU cholecalciferol–supplemented groups separately1
| All | Placebo | 1000 IU cholecalciferol/d | |||||
|---|---|---|---|---|---|---|---|
| n | Value | n | Value | n | Value | P value2 | |
| Participants with EP or LP CTX measures | |||||||
| EP CTX, µg/mmol creatinine | 493 | 219.4 [154.7–306.4] | 242 | 223.8 [155.0–308.6] | 251 | 217.5 [154.5–305.2] | N/A |
| LP CTX, µg/mmol creatinine | 498 | 437.4 [292.3–633.0] | 252 | 445.3 [317.6–657.3] | 246 | 420.0 [252.2–608.5] | 0.06 |
| Participants with both EP and LP CTX measures | |||||||
| EP CTX, µg/mmol creatinine | 372 | 223.0 [159.5–316.4] | 188 | 223.6 [153.6–311.8] | 184 | 222.3 [169.2–319.8] | N/A |
| LP CTX, µg/mmol creatinine | 372 | 429.6 [291.0–660.6] | 188 | 449.7 [327.3–687.2] | 184 | 419.3 [238.9–629.9] | 0.03 |
| Δ CTX, z score | 188 | 0.16 ± 0.92 | 184 | −0.16 ± 1.06 | <0.01 | ||
| Δ CTX (absolute) | 188 | 258.1 ± 322.1 | 184 | 214.5 ± 316.7 | 0.19 | ||
| Vitamin D–sufficient women, baseline 25(OH)D ≥ 50 nmol/L | |||||||
| EP CTX, µg/mmol creatinine | 168 | 232.8 [167.0–317.6] | 81 | 239.3 [175.2–326.9] | 87 | 225.3 [163.4–303.4] | N/A |
| LP CTX, µg/mmol creatinine | 161 | 409.1 [286.3–573.2] | 75 | 398.7 [300.0–573.2] | 86 | 414.4 [265.8–604.8] | 0.96 |
| Δ CTX, z score | 61 | −0.14 ± 0.92 | 61 | −0.17 ± 1.11 | 0.85 | ||
| Vitamin D–insufficient women, baseline 25(OH)D < 50 nmol/L | |||||||
| EP CTX, µg/mmol creatinine | 320 | 215.8 [142.7–303.2] | 159 | 216.0 [148.0–299.6] | 161 | 215.7 [139.7–307.5] | N/A |
| LP CTX, µg/mmol creatinine | 335 | 444.5 [293.2–660.4] | 176 | 459.7 [333.8–675.9] | 159 | 422.1 [249.5–633.0] | <0.01 |
| Δ CTX, z score | 126 | 0.28 ± 0.89 | 123 | −0.15 ± 1.04 | <0.01 | ||
1Values are median [IQR] or mean ± SD unless otherwise indicated. Change in CTX was calculated with a regression model with 34-wk CTX as the dependent variable and adjusting for 14-wk CTX; absolute change in CTX is also shown. Participants were further stratified according to vitamin D sufficiency (≥50 nmol/L or insufficiency < 50 nmol/L) in EP. EP = 14 weeks of gestation; LP = 34 weeks of gestation; ΔCTX = conditional change in CTX (LP CTX adjusted for EP CTX), in SD. N/A, not applicable; CTX, C-terminal telopeptide of type I collagen; EP, early pregnancy; LP, late pregnancy; 25(OH)D, 25-hydroxyvitamin D.
2 P values represent outcomes of tests for differences between the placebo group and vitamin D–supplemented group [Rank Sum tests for LP CTX, t tests for Δ CTX (z score)].
Maternal factors associated with CTX in early and late pregnancy
Table 3 documents the univariate associations between maternal characteristics and CTX concentrations in early or late pregnancy and with conditional change in CTX. Greater maternal age, parity, and height were associated with lower CTX in early and late pregnancy. Cholecalciferol supplementation (1000 IU/d) from 14 weeks of gestation was associated with −0.18 SD lower late pregnancy CTX than in those who received placebo (95% CI: −0.35, −0.001 SD; P = 0.05). There was evidence of associations between lower conditional change in CTX from early to late pregnancy and lower BMI, greater baseline 25(OH)D, and cholecalciferol supplementation (−0.32 SD conditional change in CTX compared with those who received placebo; 95% CI: −0.52, −0.11 SD; P = 0.002).
TABLE 3.
Univariate maternal determinants of CTX in EP and LP (Fisher-Yates scores), SD increase per unit change in maternal predictor in those with both an EP and an LP measure1
| EP CTX (14 weeks of gestation) | LP CTX2 (34 weeks of gestation) | Δ CTX2,3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure | n | β4 (95% CI) | P value | n | β4 (95% CI) | P value | n | β (95% CI) | P value |
| Maternal age, y | 493 | −0.03 (−0.05, −0.01) | <0.001 | 469 | −0.03 (−0.04, −0.01) | 0.01 | 372 | −0.02 (−0.037, 0.002) | 0.07 |
| Strenuous exercise >1 h/wk (Y/N) | 440 | −0.10 (−0.36, 0.16) | 0.47 | 425 | −0.11 (−0.40, 0.18) | 0.47 | 329 | −0.17 (−0.50, 0.16) | 0.31 |
| Smoking in early pregnancy (Y/N) | 491 | 0.36 (0.05, 0.68) | 0.03 | 467 | −0.20 (−0.53, 0.13) | 0.24 | 370 | −0.20 (−0.57, 0.17) | 0.30 |
| Parity ≥1 (Y/N) | 491 | −0.48 (−0.66, −0.31) | <0.001 | 467 | −0.33 (−0.52, −0.15) | <0.001 | 371 | −0.11 (−0.32, 0.09) | 0.28 |
| Caucasian ethnicity (Y/N) | 490 | 0.17 (−0.25, 0.58) | 0.44 | 466 | 0.23 (−0.21, 0.67) | 0.31 | 369 | 0.33 (−0.20, 0.86) | 0.22 |
| Weight, kg | 493 | −0.01 (−0.01, 0.00) | 0.07 | 469 | 0.002 (−0.004, 0.008) | 0.50 | 372 | 0.01 (−0.001, 0.013) | 0.12 |
| Height, cm | 488 | −0.01 (−0.03, 0.00) | 0.05 | 465 | −0.02 (−0.03, −0.01) | 0.004 | 368 | −0.01 (−0.029, 0.004) | 0.13 |
| BMI, kg/cm2 | 488 | −0.01 (−0.03, 0.01) | 0.21 | 465 | 0.02 (−0.001, 0.034) | 0.07 | 368 | 0.02 (0.001, 0.041) | 0.04 |
| 14-wk 25(OH)D, nmol/L | 488 | 0.004 (−0.001, 0.009) | 0.14 | 496 | −0.004 (−0.010, 0.001) | 0.13 | 371 | −0.01 (−0.014, −0.001) | 0.03 |
| 14-wk calcium, mmol/L | 488 | 0.53 (−0.56, 1.62) | 0.34 | 496 | 0.67 (−0.36, 1.70) | 0.20 | 371 | 1.14 (−0.09, 2.38) | 0.07 |
| 34-wk 25(OH)D, nmol/L | 489 | −0.001 (−0.016, 0.003) | 0.55 | 364 | −0.003 (−0.009, 0.002) | 0.19 | |||
| 34-wk calcium, mmol/L | 488 | 0.15 (−0.63, 0.92) | 0.71 | 363 | 0.19 (−0.74, 1.11) | 0.70 | |||
| Change in 25(OH)D from 14 to 34 wk, nmol/L | 487 | 0.001 (−0.003, 0.005) | 0.60 | 363 | 0.001 (−0.004, 0.006) | 0.77 | |||
| Vitamin D–supplemented group (Y/N) | 498 | −0.18 (−0.35, −0.00) | 0.05 | 372 | −0.32 (−0.52, −0.11) | 0.002 | |||
| Season of delivery, summer (proxy for season of measurement) | 438 | 0.02 (−0.16, 0.21) | 0.81 | 488 | 0.07 (−0.11, 0.25) | 0.44 | 366 | 0.07 (−0.14, 0.27) | 0.52 |
CTX, C-terminal telopeptide of type I collagen; EP, early pregnancy; LP, late pregnancy; 25(OH)D, 25-hydroxyvitamin D.
SD change in urinary CTX (Fisher-Yates score) per unit exposure.
Adjusted for treatment group, except when associations between the vitamin D–supplemented group and CTX were being tested.
Conditional change in CTX (LP CTX adjusted for EP CTX), as z score.
Associations between early- and late-pregnancy urinary CTX and maternal serum 25(OH)D
There were modest correlations between early- and late-pregnancy CTX in both the placebo and treatment groups (placebo group, r = 0.31; treatment group, r = 0.45, both P < 0.0001); see Supplemental Table 2. Indeed, both early- and late-pregnancy 25(OH)D were also correlated, with evidence of a greater magnitude of correlation in the placebo group (r = 0.36, P < 0.0001) than in the cholecalciferol-supplemented group (r = 0.22, P = 0.0003). No major correlations were demonstrated between early- or late-pregnancy serum 25(OH)D measurements and CTX measures. In the United Kingdom, season is strongly associated with maternal 25(OH)D in pregnancy (19). Supplemental Table 3 presents CTX concentrations by season, demonstrating little evidence for any seasonal effect.
In multivariate regression analyses adjusting for season of delivery (Table 4) we observed a trend toward modest inverse associations between late-pregnancy maternal 25(OH)D and CTX in late pregnancy (in both groups combined, β = −0.004 SD · nmol−1 · L−1 greater 25(OH)D; 95% CI: −0.008, −0.001 SD · nmol−1 · L−1; P = 0.056) and also conditional change in CTX (β = −0.008 SD · nmol−1 · L−1 greater 25(OH)D; 95% CI: −0.012, −0.003 SD · nmol−1 · L−1, P = 0.001). Associations between early-pregnancy 25(OH)D and conditional change in CTX were observed, but were less robust (P = 0.049). Adjustment for maternal age, smoking, parity, BMI, and height (factors which were associated with CTX measures) did not materially alter the observed trends.
TABLE 4.
Associations between EP or LP 25(OH)D, and LP CTX (Fisher-Yates score) or conditional change in CTX1
| Exposure | EP 25(OH)D | LP 25(OH)D | |||
|---|---|---|---|---|---|
| Outcome | LP CTX2 | Δ CTX3 | LP CTX2 | Δ CTX3 | |
| Adjusted for season | n | 486 | 365 | 479 | 358 |
| β | −0.004 | −0.007 | −0.004 | −0.008 | |
| 95% CI | (−0.010, 0.002) | (−0.013, −0.001) | (−0.008, −0.001) | (−0.012, −0.003) | |
| P value | 0.156 | 0.049 | 0.056 | 0.001 | |
| Adjusted for season of delivery, maternal age, parity, BMI, smoking, and height | n | 450 | 358 | 443 | 351 |
| β | −0.003 | −0.006 | −0.004 | −0.008 | |
| 95% CI | (−0.009, 0.003) | (−0.013, 0.001) | (−0.008, −0.001) | (−0.012, −0.003) | |
| P value | 0.396 | 0.097 | 0.054 | 0.002 | |
CTX, C-terminal telopeptide of type I collagen; EP, early pregnancy; LP, late pregnancy; 25(OH)D, 25-hydroxyvitamin D.
Difference in urinary CTX per unit exposure (Fisher-Yates score), SD.
Conditional change in CTX (LP CTX adjusted for EP CTX), SD.
Maternal CTX in late pregnancy, conditional change in CTX, and maternal postpartum bone health
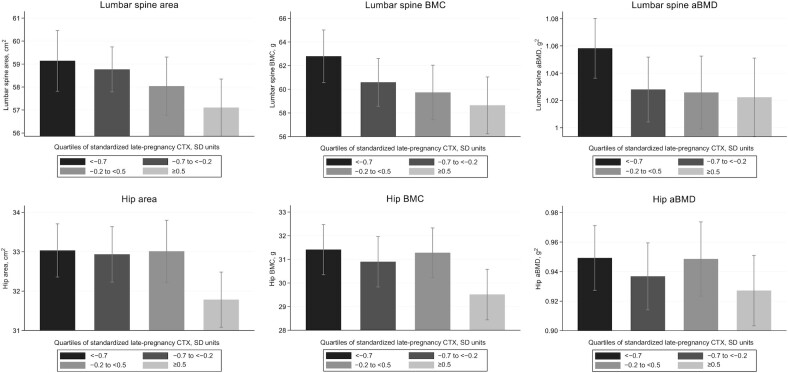
In the 283 mothers with a late-pregnancy measure of urine CTX and postpartum DXA, greater maternal urinary CTX at 34 weeks of gestation was associated with lower maternal postpartum lumbar spine and total hip bone mineral content (BMC) and areal bone mineral density (aBMD), after adjustment for maternal age, parity, smoking, and BMI in early pregnancy (in the groups combined). These associations appeared more robust in the cholecalciferol than in the placebo group and CTX–bone associations are summarized in Table 5 and Figure 2 (groups combined). The direction of associations with conditional change in CTX (Table 5) followed a similar but nonsignificant pattern and were substantially attenuated compared with those for late-pregnancy CTX. Additional adjustment for maternal height attenuated the observed relations (Supplemental Table 4A, B).
TABLE 5.
Associations between LP urine CTX or conditional change in CTX, in the vitamin D–supplemented group, placebo group, or combined, and maternal lumbar spine and total hip area, BMC, and aBMD1
| Total population | Placebo | 1000 IU cholecalciferol/d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | β (95% CI) | P value | n | β (95% CI) | P value | n | β (95% CI) | P value | |
| LP CTX2 | |||||||||
| Lumbar spine | |||||||||
| Bone area, cm2 | 283 | −0.34 (−0.96, 0.29) | 0.29 | 135 | −0.73 (−1.697, 0.235) | 0.14 | 148 | −0.08 (−0.92, 0.80) | 0.86 |
| BMC, g | 283 | −1.17 (−2.29, −0.04) | 0.04 | 135 | −1.30 (−2.972, 0.377) | 0.13 | 148 | −1.11 (−2.69, 0.46) | 0.17 |
| aBMD, g/cm2 | 283 | −0.015 (−0.027, −0.003) | 0.02 | 135 | −0.009 (−0.028, 0.010) | 0.34 | 148 | −0.019 (−0.036, −0.003) | 0.03 |
| Total hip | |||||||||
| Bone area, cm2 | 279 | −0.32 (−0.70, 0.06) | 0.10 | 133 | −0.32 (−0.94, 0.29) | 0.30 | 146 | −0.37 (−0.85, 0.11) | 0.13 |
| BMC, g | 279 | −0.68 (−1.21, −0.14) | 0.01 | 133 | −0.51 (−1.31, 0.30) | 0.22 | 146 | −0.87 (−1.62, −0.13) | 0.02 |
| aBMD, g/cm2 | 279 | −0.012 (−0.02, −0.00) | 0.05 | 133 | −0.004 (−0.022, 0.013) | 0.61 | 146 | −0.017 (−0.033, −0.002) | 0.03 |
| Δ CTX3 | |||||||||
| Lumbar spine | |||||||||
| Bone area, cm2 | 226 | −0.23 (−0.92, 0.45) | 0.50 | 109 | −0.86 (−1.93, 0.22) | 0.12 | 117 | 0.22 (−0.72, 1.17) | 0.64 |
| BMC, g | 226 | −0.72 (−1.98, 0.54) | 0.26 | 109 | −1.07 (−2.98, 0.84) | 0.27 | 117 | −0.66 (−2.49, 1.16) | 0.47 |
| aBMD, g/cm2 | 226 | −0.01 (−0.02, 0.01) | 0.22 | 109 | 0.00 (−0.03, 0.02) | 0.78 | 117 | −0.02 (−0.037, 0.003) | 0.10 |
| Total hip | |||||||||
| Bone area, cm2 | 223 | −0.29 (−0.70, 0.12) | 0.16 | 108 | −0.32 (−0.97, 0.33) | 0.33 | 115 | −0.34 (−0.87, 0.20) | 0.21 |
| BMC, g | 223 | −0.32 (−0.92, 0.29) | 0.31 | 108 | −0.24 (−1.15, 0.67) | 0.60 | 115 | −0.48 (−1.35, 0.38) | 0.27 |
| aBMD, g/cm2 | 223 | 0.00 (−0.01, 0.01) | 0.83 | 108 | 0.003 (−0.02, 0.02) | 0.74 | 115 | −0.01 (−0.03, 0.01) | 0.47 |
Models adjusted for maternal age, parity, smoking, and BMI at baseline. aBMD, areal bone mineral density; BMC, bone mineral content; CTX, C-terminal telopeptide of type I collagen; LP, late pregnancy.
Fisher-Yates transformed, SD.
Conditional change in CTX (LP CTX adjusted for early-pregnancy CTX), z score.
FIGURE 2.
Late-pregnancy urinary CTX (shown in quarters of the distribution, SD) and maternal postpartum lumbar spine area, BMC, and aBMD (n = 283) (A) and total hip area, BMC, and aBMD (n = 279) (B) assessed by DXA (in both groups combined). CTX divided into quarters of the distribution: −1.5 SD to <−0.7 SD; −0.7 SD to <−0.2 SD; −0.2 SD to <0.5 SD; and ≥0.5 SD. aBMD, areal bone mineral density; BMC, bone mineral content; CTX, C-terminal telopeptide of type I collagen.
Conditional change in CTX, and neonatal bone indices
A total of 321 mother–neonate pairs had a measure of early- or late-pregnancy maternal CTX and neonatal whole-body or lumbar spine DXA. Supplemental Table 5 presents the characteristics of the neonates. No differences in neonatal anthropometry or DXA characteristics were observed between the placebo and cholecalciferol-supplemented groups. On regression analyses (Table 6) greater maternal conditional change in CTX was associated with greater neonatal total-body and total spine area, BMC, and aBMD (adjusted for neonatal sex, age at DXA scan, and gestational age at delivery). The magnitude and direction of associations were similar between the placebo and cholecalciferol-supplemented groups; no interaction was observed between conditional change in CTX and randomization group.
TABLE 6.
Associations between conditional change in pregnancy urine CTX (Δ CTX) in the vitamin D–supplemented and placebo groups combined (total population), and neonatal DXA outcomes for the total body and total spine after adjustment for sex, infant age at DXA scan, and gestational age at birth1
| Total population | |||
|---|---|---|---|
| Δ CTX | n | β (95% CI) | P value |
| Neonatal total-body DXA | |||
| Total area, cm | 321 | 4.781 (1.567, 7.995) | 0.004 |
| Total BMC, g | 321 | 1.754 (0.798, 2.711) | <0.001 |
| Total BMD, g/cm2 | 321 | 0.003 (0.001, 0.005) | 0.002 |
| Neonatal total spine DXA | |||
| Total spine area, cm | 290 | 0.140 (0.027, 0.252) | 0.015 |
| Total spine BMC, g | 290 | 0.071 (0.022, 0.120) | 0.005 |
| Total spine BMD, g/cm2 | 290 | 0.004 (0.000, 0.008) | 0.038 |
Δ CTX = conditional change in CTX (late-pregnancy CTX adjusted for early-pregnancy CTX), SD. BMC, bone mineral content; BMD, bone mineral density; CTX, C-terminal telopeptide of type I collagen.
Discussion
In this post hoc analysis of the MAVIDOS randomized controlled trial (RCT), we have demonstrated that maternal gestational cholecalciferol supplementation is associated with a smaller gestational increase in bone resorption markers than placebo is. Although maternal urinary CTX almost doubled from 14 weeks to 34 weeks of gestation in both vitamin D–supplemented and placebo groups, the conditional increase in CTX from early to late pregnancy was lower in the cholecalciferol-supplemented than in the placebo group, with greater differences in conditional increase in CTX between the groups observed in vitamin D–insufficient women. Furthermore, late-pregnancy CTX was inversely associated with postpartum measures of maternal bone from DXA.
Our observation of increasing markers of bone resorption throughout pregnancy is replicated in other smaller studies, in which bone resorption markers have been shown to rise markedly through gestation, whereas markers of bone formation tend to be low in early pregnancy, reaching normal values by term, such that there is a net resorptive state in late pregnancy (10–17, 27). Studies of maternal bone mass throughout pregnancy and the postpartum period support this, showing that bone mineral density (BMD) declines by a small amount [between 2% and 5% at both trabecular (28) and cortical sites in the axial and peripheral skeleton (29)], but importantly, pregnancy does not have a negative impact on bone health in later life (30). Both circadian (peaking in the early morning) and seasonal (peaking during winter months) variations in bone resorption markers have been observed (7, 31). Vitamin D has been proposed to play a role in this seasonal variation. Again, in keeping with our findings, several studies have demonstrated a negative association between 25(OH)D status and markers of bone resorption, in both nonpregnant and pregnant women (6–9). Our observation that greater conditional increases in CTX were associated with greater neonatal bone mass is in keeping with a previous study from our group using the Southampton Women's Survey cohort. Greater maternal bone loss in pregnancy (as measured by calcaneal ultrasound) was shown to be associated with increased BMD in their infants (32), consistent with associations between maternal bone resorption and transplacental calcium transfer to the developing fetus.
The link, however, between maternal vitamin D and its modulation of calcium transfer to the fetus is less clear. Circulating 1,25-dihydroxycholecalciferol [1,25(OH)2D (calcitriol)] increases during pregnancy with (most importantly) the maternal kidney and also possibly the placenta providing the necessary 1-α-hydroxylase activity (11, 16, 33). Increased 1,25(OH)2D is thought to contribute to greater maternal intestinal calcium absorption (34). Several factors are known to contribute to the regulation of the balance between maternal and fetal bone formation and resorption (osteoblast and osteoclast activity), including PTH and parathyroid hormone–related peptide (PTHrp) (35–37), receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG), Sclerostin and fibroblast growth factor-23 (FGF-23), the Wnt pathway, oestrogens, and prolactin (38). It is, therefore, unlikely that the study of 1 potential mediating factor [maternal serum 25(OH)D concentrations] and 1 measure of bone resorption (urinary CTX) is likely to provide conclusive information about the system, although it does provide an important step toward our understanding of this complex process. Indeed, the European Food Safety Authority has identified the associations between vitamin D, bone turnover markers, and bone health as a current knowledge gap, as outlined in their 2016 scientific opinion article (39).
To our knowledge, MAVIDOS represents the first opportunity to study vitamin D and pregnancy bone markers in an interventional setting. The mechanism for the observed inverse relationship between maternal cholecalciferol supplementation vs. placebo and CTX concentrations could be related to greater calcium availability from maternal intestinal absorption for the developing fetus in mothers with improved 25(OH)D status, reducing the requirement for the release of calcium by maternal bone resorption. Our findings that higher maternal serum 25(OH)D concentration in late pregnancy is associated with lower late-pregnancy CTX, after adjustment for season, and that women with vitamin D insufficiency in early pregnancy had greater conditional increases in CTX throughout pregnancy, support this hypothesis. Studies of calcium and vitamin D in pregnant women provide supportive evidence for the resulting notion that maternal 25(OH)D status in late pregnancy is important in regulating maternal bone turnover to ensure calcium availability for the developing fetus. One previous RCT of calcium supplementation in pregnancy showed that daily consumption of a 1100-mg Ca supplement led to a 15% reduction in urinary NTX during the second and third trimesters (40). A prospective cohort showed that higher calcium intake in late pregnancy was associated with reduced bone resorption, with greater effects in winter, when vitamin D–mediated calcium absorption is less (9). Other longitudinal studies of maternal pregnancy 25(OH)D status demonstrated that vitamin D repletion was associated with lower markers of bone resorption. In 2 small studies (n = 47 and n = 60) of pregnant women and healthy nonpregnant controls, greater 25(OH)D status, and greater concentrations of the vitamin D metabolites [1,25(OH)2D, 24,25(OH)2D] tended to be associated with lower bone resorption markers (serum NTX and CTX, respectively) in the second and third trimesters and postpartum period, whereas no correlation between 25(OH)D and bone resorption markers was seen in the nonpregnant controls (6, 8). In nonpregnant women, a high bone turnover in those who are vitamin D–deficient has been observed, with vitamin D supplementation attenuating this effect in some studies (41) but not others (42, 43).
CTX, released by osteoclastic resorption of bone, is significantly correlated with lower total hip BMD, distal radius BMD, lumbar spine BMD, and poorer bone indices assessed by peripheral quantitative computed tomography and histology (44–46). Our finding that greater urinary CTX in late pregnancy was associated with lower lumbar spine and hip BMC and BMD is in keeping with this observation. Interestingly, the associations were of consistent direction but attenuated with conditional change in CTX, which may reflect lower statistical power given the smaller cohort with measures at both time points or potentially that the absolute value in late pregnancy rather than change in concentrations is the more important factor. Notably, although the magnitude of the associations between CTX and bone measures was attenuated after adjustment for maternal height, this was a modest effect on aBMD, suggesting CTX–aBMD relations at least partly independent of linear skeletal size.
Limitations
Our study had some limitations, the first being that, as a result of ethical stipulations, participants with 25(OH)D concentrations <25 mmol/L at screening at 12 weeks of gestation could not be included. However, vitamin D deficiency had developed in a proportion of women by gestational week 34, particularly in the placebo group and those with winter deliveries. Our findings may also not be relevant to nonwhite populations, because our study did not include many women who were not of self-reported white ethnicity. In addition, we could not exclude the possibility that some participants were taking vitamin D in addition to the study drug, although reported supplement use did not differ between groups at baseline interview. Furthermore, as a result of the primary trial design, we were unable to characterize dietary calcium intake in detail, and assays of vitamin D metabolites [1,25(OH)2D, 24,25(OH)2D], PTH, and PTHrp were not available, meaning a comprehensive assessment of maternal factors determining bone turnover could not be made. Limitations regarding the use of CTX as a marker of bone resorption should also be recognized including its circadian rhythm and relation with food intake (although early-morning, second-void urine was used to minimize this variation). We were not able to adjust fully for hemodilution in pregnancy [which may also affect serum 25(OH)D concentrations] (47) and individual changes in maternal glomerular filtration rate, but did use the CTX value relative to creatinine to account for renal function (13). The assay used in this study does not distinguish between the different isoforms of CTX. α-CTX in particular is predominantly released from growing fetal bone and may cross the placenta. One study estimated that ∼9% of α-CTX and 2% of β-CTX in the mother's urine in late pregnancy may be coming from the fetal skeleton (48); therefore, it is not possible to quantify the fetal contribution to the mother's urinary CTX. Because the participants were recruited in early pregnancy, prepregnancy DXA scans could not be performed. Therefore, we did not have the opportunity to explore changes in BMC and BMD, and their relation with CTX, from prepregnancy to postpartum. Maternal breastfeeding is known to play a large role in determining amounts of bone resorption and postpartum BMD, particularly at trabecular sites (49); lumbar spine BMD has been shown to decline by a mean of 5%–10% during exclusive breastfeeding (49). Although mothers underwent DXA within 2 wk postpartum, the number of days spent breastfeeding before DXA assessment may be important, but these data were not available. Finally, it should be acknowledged that this is a subanalysis of an RCT, so although the differences in CTX between groups and associations with bone indices are biologically plausible and consistent with existing medical literature, they should be recognized as post hoc and require replication.
Conclusion
In conclusion, we have shown that maternal urinary CTX rises from early to late pregnancy, with the magnitude of this rise appearing to be reduced by gestational cholecalciferol supplementation, and is inversely associated with maternal bone mass postpartum. These findings are consistent with a protective effect of gestational vitamin D supplementation on maternal bone health and inform our understanding of bone resorption in pregnancy and the potential relation with vitamin D metabolism. Long-term follow-up of both mothers and offspring, with repeat assessments of bone indices, will enable us to determine better the lasting impact of cholecalciferol supplementation in pregnancy.
Supplementary Material
Acknowledgments
The MAVIDOS Trial Group comprises NK Arden, A Carr, M Clynes, EM Dennison, MZ Mughal, and SJ Woolford.
The authors’ responsibilities were as follows—EMC and NCH: designed the research; EMC, KM, RJM, and NCH: wrote the paper; FG and RE, alongside IS and AP: undertook or advised upon the laboratory analyses; CP, SD, SRC, and HMI: performed the statistical analysis; NJB, SHK, ATP, RF, SVG, KMG, and MKJ: oversaw the MAVIDOS trial at the Oxford, Sheffield, and Southampton sites; NCH and CC: supervised the study and the preparation of the manuscript; CC: had primary responsibility for the final content; and all authors: contributed to manuscript preparation and read and approved the final manuscript. EMC reports honoraria/travel support from UCB, Eli Lilly, Pfizer, and Amgen outside the submitted work. NJB reports remuneration from Internis Pharmaceuticals Ltd. outside the submitted work. ATP reports grants from the Arthritis Research Council during the conduct of the study. HMI reports grants from Medical Research Council, Arthritis Research UK (ARUK), and the European Union's Seventh Framework Programme during the conduct of the study; and although not directly receiving funding from other bodies, members of her team have received funding from the following companies from other work: Danone, Nestec, Abbott Nutrition. KMG reports reimbursement for speaking at Nestlé Nutrition Institute conferences, and grants from Abbott Nutrition & Nestec, outside the submitted work; in addition, KMG has a patent Phenotype Prediction pending, a patent Predictive Use of CpG Methylation pending, and a patent Maternal Nutrition Composition pending, not directly related to this work. MKJ reports personal fees from Stirling Anglia, Consilient Health, and Internis outside the submitted work. RE reports grants and personal fees from Amgen; grants from the Department of Health; grants from AstraZeneca (AZ); grants, personal fees, and nonfinancial support from Immunodiagnostic Systems; grants from the ARUK/Medical Research Council (MRC) Center for Excellence in Musculoskeletal Ageing Research; grants from the National Institute for Health Research; grants from MRC/AZ Mechanisms of Diseases Call; grants from the MRC; grants and personal fees from Alexion; grants and other from the National Osteoporosis Society; grants, personal fees, and other from Roche; personal fees from Otsuka, Novartis, Merck, Bayer, Johnson & Johnson, Fonterra Brands, and Janssen Research; personal fees and other from Eli Lilly; personal fees from Ono Pharma, Alere (Unipath), and Chronos; personal fees and other from CL Biosystems; other from European Calcified Tissue Society and the International Osteoporosis Foundation Committee of Scientific Advisors; personal fees from Teijin Pham Limited; other from the American Society for Bone and Mineral Research, personal fees from D-STAR; and personal fees from GSK Nutrition, outside the submitted work. NK Arden has received honoraria, held advisory board positions (which involved receipt of fees), and received consortium research grants from Merck; grants from Roche, Bioiberica, and Novartis; personal fees from Smith & Nephew, Nicox, and Flexion; and personal fees from Bioventus and Freshfields, outside the submitted work. CC reports personal fees from ABBH, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, and Takeda, outside the submitted work. NCH reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare, Kyowa Kirin, and Internis Pharma, outside the submitted work. All other authors report no conflicts of interest.
Notes
Supported by Arthritis Research UK and Medical Research Council (MRC) grant 4050502589 (to CC), Bupa Foundation, UK Royal Osteoporosis Society, National Institute for Health Research (NIHR) Southampton Biomedical Research Center grant IS-BRC-1215-20004 (to CC), University of Southampton and University Hospital Southampton National Health Service Foundation Trust, and NIHR Oxford Biomedical Research Centre, University of Oxford. EMC is supported by Wellcome Trust grant 201268/Z/16/Z. AP is funded by the MRC (programme code U105960371). KMG is supported by NIHR Senior Investigator grant NF-SI-0515-10042, NIHR Southampton 1000 Days Plus Global Nutrition Research Group grant 17/63/154, the European Union (Erasmus+ Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP and ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP), US National Institute on Aging of the NIH award U24AG047867, and UK Economic and Social Research Council and Biotechnology and Biological Sciences Research Council (BBSRC) award nES/M00919X/1. The work leading to these results was supported by the European Union's Seventh Framework Programme (FP7/2007–2013), projects EarlyNutrition and ODIN under grants 289346 and 613977, and by the BBSRC (HDHL-Biomarkers, BB/P028179/1), as part of the ALPHABET project, supported by an award made through the ERA-Net on Biomarkers for Nutrition and Health (ERA HDHL), Horizon 2020 Framework Programme grant 696295 (to NCH). Members of the authorship contribute to the European Reference Network on Rare Bone Diseases. We are extremely grateful to Merck GmbH for the kind provision of the Vigantoletten supplement. Merck GmbH had no role in the trial execution, data collection, analysis, or manuscript preparation. The authors had full access to all study data.
Supplemental Tables 1–5 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: aBMD, areal bone mineral density; CTX, C-terminal telopeptide of type I collagen; DEQAS, Vitamin D External Quality Assessment Scheme; MAVIDOS, the Maternal Vitamin D Osteoporosis Study; NHS, National Health Service; NTX, N-terminal telopeptide of type I collagen; PTH, parathyroid hormone; PTHrp, parathyroid hormone–related peptide; RCT, randomized controlled trial; 1,25(OH)2D, 1,25-dihydroxycholecalciferol, calcitriol; 24,25(OH)2D, 24,25-dihydroxycholecalciferol; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Elizabeth M Curtis, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Camille Parsons, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Kate Maslin, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom; School of Nursing and Midwifery, University of Plymouth, Plymouth, United Kingdom.
Stefania D'Angelo, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Rebecca J Moon, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom; Paediatric Endocrinology, University Hospitals Southampton National Health Service Foundation Trust, Southampton, United Kingdom.
Sarah R Crozier, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom.
Fatma Gossiel, Academic Unit of Bone Metabolism, University of Sheffield, Sheffield, United Kingdom.
Nicholas J Bishop, Academic Unit of Child Health, Sheffield Children's Hospital, University of Sheffield, Sheffield, United Kingdom.
Stephen H Kennedy, Nuffield Department of Women's & Reproductive Health, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom.
Aris T Papageorghiou, Nuffield Department of Women's & Reproductive Health, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom.
Robert Fraser, Department of Obstetrics and Gynaecology, Sheffield Hospitals National Health Service Trust, University of Sheffield, Sheffield, United Kingdom.
Saurabh V Gandhi, Department of Obstetrics and Gynaecology, Sheffield Hospitals National Health Service Trust, University of Sheffield, Sheffield, United Kingdom.
Ann Prentice, Medical Research Council Nutrition and Bone Health, University of Cambridge, Cambridge, United Kingdom.
Hazel M Inskip, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom; National Institute for Health Research Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton National Health Service Foundation Trust, Southampton, United Kingdom.
Keith M Godfrey, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom; National Institute for Health Research Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton National Health Service Foundation Trust, Southampton, United Kingdom.
Inez Schoenmakers, Department of Medicine, Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, United Kingdom.
M Kassim Javaid, National Institute for Health Research Oxford Biomedical Research Centre, University of Oxford, Oxford, United Kingdom.
Richard Eastell, Academic Unit of Bone Metabolism, University of Sheffield, Sheffield, United Kingdom.
Cyrus Cooper, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom; National Institute for Health Research Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton National Health Service Foundation Trust, Southampton, United Kingdom; National Institute for Health Research Oxford Biomedical Research Centre, University of Oxford, Oxford, United Kingdom.
Nicholas C Harvey, Medical Research Council Lifecourse Epidemiology Centre, University of Southampton, Southampton, United Kingdom; National Institute for Health Research Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton National Health Service Foundation Trust, Southampton, United Kingdom.
MAVIDOS Trial Group:
N K Arden, A Carr, M Clynes, E M Dennison, M Z Mughal, and S J Woolford
Data availability
Data described in the article (de-identified), code book, and analytic code will be made available upon request pending application to and approval of the corresponding author.
References
- 1. Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab. 2001;86(6):2344–8. [DOI] [PubMed] [Google Scholar]
- 2. Givens MH, Macy IG. The chemical composition of the human fetus. J Biol Chem. 1933;102(1):7–17. [Google Scholar]
- 3. Kumar R, Cohen WR, Silva P, Epstein FH. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest. 1979;63(2):342–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robins SP. Collagen crosslinks in metabolic bone disease. Acta Orthop Scand Suppl. 1995;266:171–5. [PubMed] [Google Scholar]
- 5. Heaney RP. Vitamin D: how much do we need, and how much is too much?. Osteoporos Int. 2000;11(7):553–5. [DOI] [PubMed] [Google Scholar]
- 6. Park H, Brannon PM, West AA, Yan J, Jiang X, Perry CA, Malysheva O, Mehta S, Caudill MA. Maternal vitamin D biomarkers are associated with maternal and fetal bone turnover among pregnant women consuming controlled amounts of vitamin D, calcium, and phosphorus. Bone. 2017;95:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thiering E, Bruske I, Kratzsch J, Hofbauer LC, Berdel D, von Berg A, Lehmann I, Hoffmann B, Bauer CP, Koletzko Set al. . Associations between serum 25-hydroxyvitamin D and bone turnover markers in a population based sample of German children. Sci Rep. 2015;5:18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haliloglu B, Ilter E, Aksungar FB, Celik A, Coksuer H, Gunduz T, Yucel E, Ozekici U. Bone turnover and maternal 25(OH) vitamin D3 levels during pregnancy and the postpartum period: should routine vitamin D supplementation be increased in pregnant women?. Eur J Obstet Gynecol Reprod Biol. 2011;158(1):24–7. [DOI] [PubMed] [Google Scholar]
- 9. O'Brien EC, Kilbane MT, McKenna MJ, Segurado R, Geraghty AA, McAuliffe FM. Calcium intake in winter pregnancy attenuates impact of vitamin D inadequacy on urine NTX, a marker of bone resorption. Eur J Nutr. 2018;57(3):1015–23. [DOI] [PubMed] [Google Scholar]
- 10. Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15(3):557–63. [DOI] [PubMed] [Google Scholar]
- 11. Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61(3):514–23. [DOI] [PubMed] [Google Scholar]
- 12. Gallacher SJ, Fraser WD, Owens OJ, Dryburgh FJ, Logue FC, Jenkins A, Kennedy J, Boyle IT. Changes in calciotrophic hormones and biochemical markers of bone turnover in normal human pregnancy. Eur J Endocrinol. 1994;131(4):369–74. [DOI] [PubMed] [Google Scholar]
- 13. Kaur M, Godber IM, Lawson N, Baker PN, Pearson D, Hosking DJ. Changes in serum markers of bone turnover during normal pregnancy. Ann Clin Biochem. 2003;40(Pt 5):508–13. [DOI] [PubMed] [Google Scholar]
- 14. Møller UK, Streym S, Mosekilde L, Heickendorff L, Flyvbjerg A, Frystyk J, Jensen LT, Rejnmark L. Changes in calcitropic hormones, bone markers and insulin-like growth factor I (IGF-I) during pregnancy and postpartum: a controlled cohort study. Osteoporos Int. 2013;24(4):1307–20. [DOI] [PubMed] [Google Scholar]
- 15. Paoletti AM, Orru M, Floris L, Guerriero S, Ajossa S, Romagnino S, Melis GB. Pattern of bone markers during pregnancy and their changes after delivery. Horm Res. 2003;59(1):21–9. [DOI] [PubMed] [Google Scholar]
- 16. Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67(4):693–701. [DOI] [PubMed] [Google Scholar]
- 17. Yamaga A, Taga M, Minaguchi H, Sato K. Changes in bone mass as determined by ultrasound and biochemical markers of bone turnover during pregnancy and puerperium: a longitudinal study. J Clin Endocrinol Metab. 1996;81(2):752–6. [DOI] [PubMed] [Google Scholar]
- 18. Harvey NC, Javaid K, Bishop N, Kennedy S, Papageorghiou AT, Fraser R, Gandhi SV, Schoenmakers I, Prentice A, Cooper C. MAVIDOS Maternal Vitamin D Osteoporosis Study: study protocol for a randomized controlled trial. The MAVIDOS Study Group. Trials. 2012;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D'Angelo Set al. . Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4(5):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curtis EM, Krstic N, Cook E, D'Angelo S, Crozier SR, Moon RJ, Murray R, Garratt E, Costello P, Cleal Jet al. . Gestational vitamin D supplementation leads to reduced perinatal RXRA DNA methylation: results from the MAVIDOS Trial. J Bone Miner Res. 2019;34(2):231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32–40. [DOI] [PubMed] [Google Scholar]
- 23. Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908–23. [DOI] [PubMed] [Google Scholar]
- 24. Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NF, McCloskey EV, Walsh JS, Eastell R. Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int. 2016;27(1):21–31. [DOI] [PubMed] [Google Scholar]
- 25. Aickin M. Dealing with change: using the conditional change model for clinical research. Permanente J. 2009;13(2):80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones Get al. . The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ó Breasail M, Ward KA, Schoenbuchner SM, Ceesay M, Mendy M, Jarjou LM, Moore SE, Prentice A. Pregnancy-related change in pQCT and bone biochemistry in a population with a habitually low calcium intake. J Bone Miner Res. 2021;36(7):1269–80. [DOI] [PubMed] [Google Scholar]
- 28. Møller UK, Við Streym S, Mosekilde L, Rejnmark L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int. 2012;23(4):1213–23. [DOI] [PubMed] [Google Scholar]
- 29. Ó Breasail M, Prentice A, Ward K. Pregnancy-related bone mineral and microarchitecture changes in women aged 30 to 45 years. J Bone Miner Res. 2020;35(7):1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song SY, Kim Y, Park H, Kim YJ, Kang W, Kim EY. Effect of parity on bone mineral density: a systematic review and meta-analysis. Bone. 2017;101:70–6. [DOI] [PubMed] [Google Scholar]
- 31. Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31(1):57–61. [DOI] [PubMed] [Google Scholar]
- 32. Javaid MK, Crozier SR, Harvey NC, Taylor P, Inskip HM, Godfrey KM, Cooper C. Maternal and seasonal predictors of change in calcaneal quantitative ultrasound during pregnancy. J Clin Endocrinol Metab. 2005;90(9):5182–7. [DOI] [PubMed] [Google Scholar]
- 33. Turner M, Barré PE, Benjamin A, Goltzman D, Gascon-Barré M. Does the maternal kidney contribute to the increased circulating 1,25-dihydroxyvitamin D concentrations during pregnancy?. Miner Electrolyte Metab. 1988;14(4):246–52. [PubMed] [Google Scholar]
- 34. Lieben L, Stockmans I, Moermans K, Carmeliet G. Maternal hypervitaminosis D reduces fetal bone mass and mineral acquisition and leads to neonatal lethality. Bone. 2013;57(1):123–31. [DOI] [PubMed] [Google Scholar]
- 35. Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal–placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci U S A. 1996;93(26):15233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simmonds CS, Karsenty G, Karaplis AC, Kovacs CS. Parathyroid hormone regulates fetal-placental mineral homeostasis. J Bone Miner Res. 2010;25(3):594–605. [DOI] [PubMed] [Google Scholar]
- 37. Riccardi D, Brennan SC, Chang W. The extracellular calcium-sensing receptor, CaSR, in fetal development. Best Pract Res Clin Endocrinol Metab. 2013;27(3):443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanz-Salvador L, García-Pérez MÁ, Tarin JJ, Cano A. Bone metabolic changes during pregnancy: a period of vulnerability to osteoporosis and fracture. Eur J Endocrinol. 2015;172(2):R53–65. [DOI] [PubMed] [Google Scholar]
- 39. European Food Safety Authority. Scientific Opinion on Dietary Reference Values for vitamin D. Parma, Italy: European Food Safety Authority; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ettinger AS, Lamadrid-Figueroa H, Mercado-García A, Kordas K, Wood RJ, Peterson KE, Hu H, Hernández-Avila M, Téllez-Rojo MM. Effect of calcium supplementation on bone resorption in pregnancy and the early postpartum: a randomized controlled trial in Mexican women. Nutr J. 2014;13(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nahas-Neto J, Cangussu LM, Orsatti CL, Bueloni-Dias FN, Poloni PF, Schmitt EB, Nahas EAP. Effect of isolated vitamin D supplementation on bone turnover markers in younger postmenopausal women: a randomized, double-blind, placebo-controlled trial. Osteoporos Int. 2018;29(5):1125–33. [DOI] [PubMed] [Google Scholar]
- 42. Madar AA, Knutsen KV, Stene LC, Brekke M, Lagerlov P, Meyer HE, Macdonald HM. Effect of vitamin D3-supplementation on bone markers (serum P1NP and CTX): a randomized, double blinded, placebo controlled trial among healthy immigrants living in Norway. Bone Rep. 2015;2:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macdonald HM, Wood AD, Aucott LS, Black AJ, Fraser WD, Mavroeidi A, Reid DM, Secombes KR, Simpson WG, Thies F. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1-year double-blind RCT in postmenopausal women. J Bone Miner Res. 2013;28(10):2202–13. [DOI] [PubMed] [Google Scholar]
- 44. Marques EA, Gudnason V, Lang T, Sigurdsson G, Sigurdsson S, Aspelund T, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris TB. Association of bone turnover markers with volumetric bone loss, periosteal apposition, and fracture risk in older men and women: the AGES-Reykjavik longitudinal study. Osteoporos Int. 2016;27(12):3485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chavassieux P, Portero-Muzy N, Roux JP, Garnero P, Chapurlat R. Are biochemical markers of bone turnover representative of bone histomorphometry in 370 postmenopausal women?. J Clin Endocrinol Metab. 2015;100(12):4662–8. [DOI] [PubMed] [Google Scholar]
- 46. Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11(3):337–49. [DOI] [PubMed] [Google Scholar]
- 47. Takaoka N, Nishida K, Sairenchi T, Umesawa M, Noguchi R, Someya K, Kobashi G. Changes in vitamin D status considering hemodilution factors in Japanese pregnant women according to trimester: a longitudinal survey. PLoS One. 2020;15(10):e0239954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15(1):129–37. [DOI] [PubMed] [Google Scholar]
- 49. Kovacs CS. The skeleton is a storehouse of mineral that is plundered during lactation and (fully?) replenished afterwards. J Bone Miner Res. 2017;32(4):676–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article (de-identified), code book, and analytic code will be made available upon request pending application to and approval of the corresponding author.