Abstract
Hypertension is a potent cardiovascular risk factor with deleterious end-organ effects and is especially prevalent among patients with chronic kidney disease. The Systolic Blood Pressure Intervention Trial enrolled patients at an elevated cardiac risk including patients with mild to moderate chronic kidney disease and found that an intensive systolic blood pressure goal of < 120 mm Hg significantly reduced the rates of adverse cardiovascular events and all-cause mortality and non-significantly reduced the rates of probable dementia; these results were consistent whether one had chronic kidney disease or not. On the other hand, results of intensive blood pressure therapy on chronic kidney disease progression were inconclusive and there was an increased risk of incident chronic kidney disease and acute kidney injury, but the declines in kidney function appear to be hemodynamically driven and reversible. Overall, an intensive blood pressure target is effective in reducing cardiovascular disease and all-cause mortality and may reduce the risk of probable dementia in patients with mild to moderate CKD. More studies are needed to determine its long-term effects on kidney function.
Introduction
High blood pressure (BP) is one of the leading causes of preventable cardiovascular (CV) disease and death. Chronic kidney disease (CKD) is a risk factor for CV disease and many patients have concomitant high BP. High BP, in turn, is a risk-factor for kidney disease1. Therefore, successful management of high BP is a top priority for optimizing CV health2. The Systolic Blood Pressure Intervention Trial (SPRINT)3 continues to generate a wealth of information regarding how an intensive BP target affects a wide spectrum of health outcomes. The primary goal of SPRINT was to test whether an intensive systolic blood pressure (SBP) goal of < 120 mm Hg compared to a standard SBP goal of < 140 mm Hg improved CV outcomes in patients at high risk for CV disease. SPRINT pre-specified patients with CKD as a subgroup of interest, and SPRINT remains the largest randomized trial of BP targets in CKD to date. This paper provides a narrative review of what we have learned from SPRINT through a kidney-centric lens.
Cohort characteristics by baseline CKD status
SPRINT enrolled patients aged 50 years or older with high BP and an elevated risk of CV events. SPRINT had a particular focus on recruiting patients with CKD, defined as an estimated glomerular filtration rate (eGFR) of 20 to less than 60ml/min per 1.73m2, calculated using the four-variable Modification of Diet in Renal Disease (MDRD) equation4. Notable exclusions included patients with type 2 diabetes mellitus (DM), as they were recently studied in the recent Action to Control Cardiovascular Risk in Diabetes BP trial (ACCORD BP) trial5; patients with polycystic kidney disease, as they were recently studied in the Halt Progression of Polycystic Kidney Disease (HALT-PKD) trial6; patients with glomerulonephritis requiring immunosuppressive medications; and patients with proteinuria >1g per day, as prior post hoc analyses suggested slower CKD progression with more intensive BP targets in this subgroup3, 7, 8.
SPRINT enrolled 9361 participants, of which 2646 (28.3%) had baseline CKD. Patients in the CKD subgroup compared to the non-CKD subgroup were generally older, and had a larger proportion of women, participants of non-Hispanic white race and a higher prevalence of clinical cardiovascular disease (Table 1)3, 9, 10. The average eGFR in the CKD subgroup was 47.9 ml/min per 1.73m2 and had relatively low levels of albuminuria (mean urinary albumin-to-creatinine ratio [UACR] 80.6 mg/g). Baseline mean BPs between the CKD and non-CKD cohort were similar (139.2/74.9 vs. 139.2 / 79.4 mmHg), although patients with CKD required more medications to achieve the same BP than patients without CKD (2.1 vs. 1.7 agents)9, 10.
Table 1–
| Characteristic |
Overall Cohort (N – 9361) |
CKD (N - 2646) |
Non-CKD (N – 6715) |
|---|---|---|---|
| Age, y, mean ± SD |
67.9 ± 9.4 | 71.9 ± 9.3 | 66.3 ± 9.0 |
| - Age ≥ 75yr, % | 28.2 | 43.9 | 21.9 |
|
| |||
| Women, % | 35.6 | 40.0 | 33.7 |
|
| |||
| Race, % | |||
| - non-Hispanic White | 57.7 | 67.2 | 54.0 |
| - Black Race | 29.9 | 24.1 | 32.2 |
| - Hispanic Race | 10.5 | 7.2 | 11.8 |
| - Other | 1.9 | 1.6 | 2.0 |
|
| |||
| Clinical Cardiovascular Disease*, % | 16.7 | 19.7 | 14.0 |
|
| |||
| Never Smoked, % | 44 | 45.6 | 43.5 |
|
| |||
| Serum Creatinine, mg/dL, mean ± SD | 1.07 ± 0.34 | 1.43 ± 0.39 | 0.93 ± 0.17 |
|
| |||
| eGFR, ml/min per 1.73m2, mean ± SD | 71.8 ± 20.6 | 47.9 ± 9.5 | 81.2 ± 15.5 |
| - eGFR 45 – 59, % | 18.8 | 66.0 | n/a |
| - eGFR < 45, % | 9.5 | 34.0 | n/a |
|
| |||
| Urinary ACR, mg/g | |||
| - mean (SD) | 42.6 ± 166.3 | 80.6 ± 243.4 | |
| - median (IQR) | 13.3 (6.4 – 43.1) | 8.6 (5.5 – 17.1) | |
|
| |||
| BP, mm Hg, mean ± SD | |||
| - Systolic | 139.7 ± 15.6 | 139.2 ± 16.1 | 139.9 ± 15.4 |
| - Diastolic | 78.1 ± 11.9 | 74.9 ± 12.2 | 79.4 ± 11.6 |
|
| |||
| No. of antihypertensives agents, mean ± SD | 1.8 ± 1.0 | 2.1 ± 1.0 | 1.7 ± 1.0 |
Clinical CV Disease:
- Previous MI, percutaneous coronary intervention, coronary artery bypass, carotid endarterectomy, carotid stenting.
- Peripheral arterial disease with revascularization.
- Acute coronary syndrome with or without resting ECG changes, ECG changes on a graded exercise test, or positive cardiac imaging study.
- ≥ 50% diameter stenosis of a coronary, carotid, or lower extremity artery.
- Abdominal aortic aneurysm (AAA) ≥ 5cm with or without repair.
CKD – chronic kidney disease, SD – standard deviation, eGFR – estimated glomerular filtration rate, ACR – albumin to creatinine ratio, BP – blood pressure.
During the course of the trial, considerable separation was achieved between the two BP treatments arms. In the CKD subgroup, the mean achieved SBP was 123 mm Hg and 135 mm Hg in the intensive and standard treatment arms, respectively9; in the non-CKD subgroup the achieved SBP was 119 and 135 mm Hg, respectively10. Patients in the intensive arm required an average of 1 additional BP medication (3 versus 2) compared to the standard arm, and more patients used angiotensin converting enzyme inhibitors /angiotensin receptor blockers (ACEI/ARBs) and diuretics in the intensive arm (76.7%, 67.0%, respectively) than in the standard arm (55.2%, 42.9%, respectively)3. These patterns were similar among patients with and without baseline CKD9, 10.
Cardiovascular and death outcomes by baseline CKD status
The primary CV outcome was a composite of non-fatal myocardial infarction (MI), other acute coronary syndromes (ACS), non-fatal stroke, non-fatal acute decompensated congestive heart failure, and CV death3. SPRINT was stopped early after a median of 3.26 of the planned mean 5 years of follow-up when an interim analysis showed an overwhelming benefit of the intensive versus standard BP target on the primary CV outcome: 1.65% per year versus 2.19% per year, HR 0.75, 95% CI: 0.64 – 0.89 (p < 0.001). The benefit of the intensive BP target was also seen for several key secondary outcomes, including death from any cause and the composite of the primary CV outcome or death from any cause (Figure 1)3. Outcomes occurred more frequently in participants with baseline CKD, but rates were lower in the intensive versus the standard BP arm and the benefits of the intensive BP target did not vary by baseline CKD status (Figure 1)9, 10. Yet, the separation in the cumulative event curves for the primary CV outcome occurred approximately 2 years into follow-up among participants with CKD, compared with < 6 months among participants without CKD9, 10. Reasons for this discrepancy are unclear.
Figure 1.
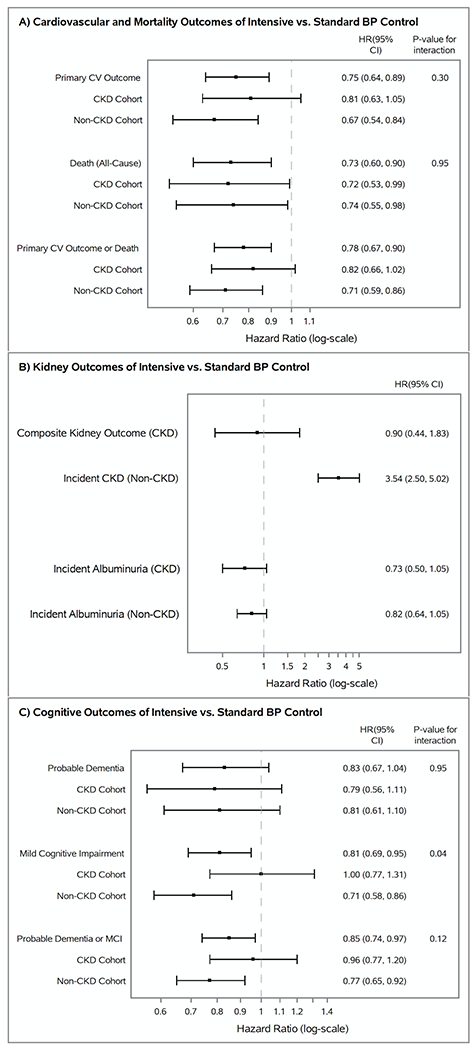
Forest plots of hazard ratios and 95% confidence intervals of intensive vs. standard blood pressure control and their outcomes in SPRINT, stratified by baseline CKD status. Panel A depicts the primary cardiovascular outcome (defined as the composite of myocardial infarction, acute coronary syndrome, stroke, acute decompensated heart failure, and cardiovascular death), all-cause mortality, and the composite of the primary cardiovascular outcome and all-cause mortality. Panel B shows the composite kidney outcome for the CKD cohort (defined as a ≥ 50% decline in eGFR from baseline confirmed on testing 90 days later or reaching end-stage kidney disease), the risk of developing incident chronic kidney disease for the non-CKD cohort, and the risk of developing incident albuminuria. Panel C displays the cognitive outcomes assessed in SPRINT.3, 9–10, 33–34
BP – blood pressure, CV – cardiovascular, HR – hazard ratio, CI – confidence interval, CKD – chronic kidney disease, MCI – mild cognitive impairment.
While the benefits of the intensive BP target did not vary by baseline CKD status when it was modeled as a dichotomous variable (i.e. eGFR < 60 vs. eGFR ≥ 60 ml/min per 1.73m2), a post-hoc analysis stratified the SPRINT cohort into four categories of kidney function: ≥ 90ml/min per 1.73m2, 60 - 89 ml/min per 1.73m2, 45 – 59 ml/min per 1.73m2, and < 45 ml/min per 1.73m2 11. The authors found a progressive attenuation in the benefit of intensive BP lowering on the primary CV outcome with each successive lower category of baseline eGFR; an interaction term between the intervention group and eGFR suggested a potential interaction of the intervention by eGFR modeled as continuous variable (Pint = 0.019). Given the post hoc nature of the analysis and relatively small number of patients in the lowest eGFR category, these results should be considered hypothesis-generating12. To date, the benefits of intensive BP lowering among patients with more advanced CKD are uncertain and underscores the need for a future trial to specifically target this population.
Patients with CKD also have increased vascular stiffness13 which can lead to lower diastolic blood pressures (DBP); aggressively lowering BP could reduce coronary perfusion and lead to an increase in adverse events. A recent post hoc analysis of baseline DBP in the SPRINT CKD subgroup found that patients with lower DBP had higher risks for the primary CV outcome and all-cause mortality than those with higher DBP14. Yet the beneficial effect of the intensive versus standard BP target was still observed across tertiles of baseline DBP (Pint > 0.2), suggesting that lower DBP should not necessarily deter physicians from pursuing more intensive SBP lowering in these patients.
Beyond eGFR considerations, albuminuria is also an independent risk factor for CV disease15. A post hoc analysis by Chang et. al.16 stratified SPRINT participants by baseline albuminuria (UACR < or ≥ 30 mg/g). A UACR of 30mg/g is a well-established cut-off above which increases in albuminuria is directly correlated with an increase in the risk of cardiovascular death and all-cause mortality15. The SPRINT cohort had 1723 participants with baseline albuminuria (median UACR 70, inter-quartile range (IQR) 42 – 162 mg/g); at baseline, they were generally older, had a lower eGFR, higher SBP, and a higher prevalence of CV disease than participants without baseline albuminuria (median UACR 8, IQR 5 – 13 mg/g). While patients with baseline albuminuria had higher rates of the primary CV outcome and all-cause mortality, the benefit of an intensive versus standard BP target remained consistent regardless of baseline albuminuria status (Pint > 0.05)16. The consistent benefit of the intensive BP target by baseline albuminuria status was also similar among the CKD and non-CKD subgroups.
SPRINT and CV / death outcomes in context
In contrast to SPRINT, prior studies in CKD did not detect a benefit of more intensive BP lowering on CV outcomes or death. These studies, which included the MDRD17, African American Study in Kidney Disease (AASK)18, and the Ramipril Efficacy in Nephropathy – 2 (REIN-2)19, differed from SPRINT with regard to baseline characteristics, severity of CKD and degree of albuminuria or proteinuria (Table 2). Importantly, these prior trials were all primarily designed to assess the effect of more intensive BP lowering on CKD progression, and therefore were not powered to detect differences in CV outcomes17–19. There has been longer-term follow-up examining the risk of death for two of these trials. In an extended observational follow-up analysis of AASK (median follow-up 14.4 years) participants in the intensive BP arm had lower adjusted risk of death than participants in the standard BP arm (HR 0.81, 95% CI 0.68 to 0.98; p - 0.03)20. Similarly, an observational long-term follow-up of the MDRD (median follow-up 19.3 years) showed lower risk of death in the intensive versus standard BP arm (HR 0.82, 95% CI 0.68 to 0.98, P - 0.03)21.
Table 2–
| Characteristic | MDRD | AASK | REIN-2 | ACCORD (CKD cohort) | SPRINT (CKD cohort) |
|---|---|---|---|---|---|
| Sample Size, n | 840 - Mod. CKD Cohort – 585 - Severe CKD Cohort – 255 |
1094 - UPCR ≤ 0.22g/g – 733 - UPCR ≥ 0.22g/g – 357 |
335 | CKD – 1726 - CKD I – 693 - CKD II – 632 - CKD III – 401 |
CKD* – 2646 - eGFR 45 - <60 – 1759 - eGFR <45 – 891 |
| Follow-up, y | Mean – 2.2 (0 to 3.7) | Median – 3.8 (3 to 6.4) | Med. (IQR) – 1.6 (1.0 – 2.9) | Mean – 4.7 | Med. (IQR) – 3.3 (2.8 – 3.8) |
| Study Pop. | Non-Diabetic‡ | Hypertensive CKD†† | Non-Diabetic | Diabetic | Non-Diabetic |
| Age, y (mean ± SD) | Int. BP arm – 51.8 ± 12.4 Std. BP arm – 52.0 ± 12.2 |
Int. BP arm – 54.5 ± 10.9 Std. BP arm – 54.7 ± 10.4 |
Int. BP arm – 54.6 ± 14.7 Std. BP arm – 53.1 ± 15.8 |
Overall - 63.2 ± 7.3 | Overall - 71.9 ± 9.3 |
| GFR or eGFR Criteria† | Mod. CKD Cohort – 25 – 55 Severe CKD Cohort – 13 – < 25 |
20 – 65 | < 75 | N/A | 20 - < 60 |
| GFR, eGFR†, mean ± SD | Int. BP arm – 32.7 ± 12.1 Std. BP arm – 32.3 ± 11.9 Mod. CKD Cohort – 38.6 ± 8.9 Severe CKD Cohort – 18.5 ± 3.4 |
Int. BP arm – 46.0 ± 12.9 Std. BP arm – 45.3 ± 13.2 |
Int. BP arm – 38.6 ± 17.9 Std. BP arm – 38.9 ± 21.7 |
91.6 ± 28.8 (overall cohort) |
Overall - 47.9 ± 9.5 |
| sCr, mg/dL, mean ± SD |
Mod. CKD Cohort – 1.9 ± 0.5 Severe CKD Cohort – 3.4 ± 0.9 |
2.00 ± 0.70 | 2.7 ± 1.1 | 1.0 ± 0.3 | 1.43 ± 0.39 |
| Proteinuria Criteria | > 10g/d excluded | > 2.5g/d excluded | < 1g/d excluded | > 1g/d excluded | > 1g/d excluded |
| Proteinuria, median (IQR) |
Overall: (g/d) 0.32 (0.07 – 1.5) Mod. CKD Cohort - 0.2 (0.0 – 2.9) Severe CKD Cohort – 0.8 (0.1 – 3.9) |
UPCR, Overall: (g/g) 0.08 (0.03–0.37) UPCR ≥ 0.22g/g subgroup: (g/g) - Int. BP arm – 0.96 (0.35–1.99) - Std. BP arm – 0.73 (0.42–1.37) |
(Mean ± SD, g/d) Int. BP arm – 2.8 ± 1.9 Std. BP arm – 2.9 ± 1.9 |
UACR (mg/g) 14.3 (6.9 – 44.8) Data for entire cohort |
UACR - 12.8 (6.5 – 42.6) mg/g UACR, mean ± SD - 80.9 ± 236.2 mg/g |
| Target BP, mmHg | Int. BP arm – MAP ≤ 92 (~125/75) - Age >60 - MAP ≤ 98 (~135/80) Std. BP arm – MAP ≤ 107 (~140/90) - Age >60 - MAP ≤ 113 (~150/95) |
Int. BP arm – MAP ≤ 92 (~125/75) Std. BP arm – MAP 102–107 (~135/85 –140/90) |
Int. BP arm - < 130/80 Std. BP arm – DBP < 90 |
Int. BP arm – SBP < 120 Std. BP arm – SBP < 140 |
Int. BP arm – SBP < 120 Std. BP arm – SBP < 140 |
| Achieved BP, mmHg, mean ± SD | Int. BP arm – MAP 93.0 ± 7.3 (~125/77) Std. BP arm – MAP 97.7 ± 7.7 (~133/80) Diff, mean – MAP 4.7 (~8/3) |
Int. BP arm – MAP 95 (~128/78) Std. BP arm – MAP 104 (~141/85) Diff, mean – MAP 9 (~13/7) |
Int. BP arm – 130/80 Std. BP – 134/82 Diff, mean – 4/2 |
Int. BP arm – SBP - 122 Std. BP arm – SBP - 134 SBP Diff, mean – 12 |
Int. BP arm – 123/66 Std. BP arm – 135.3/72.4 SBP Diff, mean – 12.3 |
| ACEI / ARB use (Int. vs. Std.) | 51 vs. 32% | Similar in both arms | 100% in both arms | 41 vs. 30% (Data for entire cohort) |
71.7% vs. 57% |
eGFR < 60ml/min per 1.73m2
All GFR, CrCl units are ml/min, and eGFR units are ml/min per 1.73m2
The study population was mostly non-diabetic. Diabetics that did not require insulin therapy were allowed but only 26 of 840 participants had diabetic nephropathy.
Presumed hypertensive CKD. Patients were only included if they had hypertension, were non-diabetic, and had no other identified causes of renal dysfunction.
sCr – Serum Creatinine Concentration, UPCR – Urine protein-creatinine ratio, BP – blood pressure. SBP – systolic blood pressure, Int. – Intensive, Std. – Standard, Diff. – Difference. Mod. – Moderate, Med. – Median, IQR – inter-quartile range
The BP intervention in SPRINT mirrors that of ACCORD-BP, which also randomized patients to an intensive SBP goal of <120mmHg or a standard SBP goal of < 140mmHg5. The primary difference is that ACCORD-BP included only patients with type 2 DM, which was an exclusion criterion for SPRINT. In contrast to SPRINT, ACCORD-BP failed to find a significant benefit of intensive BP lowering on the primary CV outcome (HR 0.88, 95% CI: 0.73 – 1.06, P - 0.20) and even a non-significant increase in the risk for all-cause mortality (HR 1.07, 95% CI: 0.85 – 1.35, P - 0.55). Given that ACCORD-BP excluded participants with a serum creatinine concentration > 1.5 mg/dL, the cohort had a mean eGFR of 91ml/min per 1.73m2 and few participants had an eGFR < 60 ml/min per 1.73m2 (Table 2)5, 22. Despite the milder phenotype, the ACCORD BP CKD subgroup experienced 1.5 to 2 times the number of CV events as compared to the non-CKD subgroup, and no differences in the effect (or lack thereof) of intensive BP control were observed among participants with and without baseline CKD22.
One postulated reason for ACCORD-BP’s discordant results with SPRINT is that ACCORD-BP had less statistical power23 given its smaller sample size and lower than expected primary event rate [ACCORD 2010]. Second, ACCORD had a 2x2 factorial design that also randomized participants to intensive versus standard glycemic control. A clever post hoc analysis24 found evidence of interaction between intensive glycemic control and the intensive BP target: taking only participants in the standard glycemic arm, intensive BP control did reduce the risk of the primary CV outcome, with an effect size similar to that which was seen in SPRINT (HR 0.77, 95% CI: 0.63 – 0.95)3, 24.
Summary: SPRINT and CV / death outcomes in CKD
Targeting a lower SBP is beneficial for patients with mild to moderate CKD for reducing the risk of CV events and death. These conclusions are reflected in the recent 2021 KDIGO Clinical Practice Guidelines for the Management of BP in CKD, which recommends that adults with high BP and CKD be treated with a target SBP of < 120 mm Hg, when tolerated, and using standardized office BP measurement25. However, there are certain subgroups where the benefit is less certain, including in patients with heavy proteinuria, more advanced CKD, and any other population that was not well represented in SPRINT.
Kidney outcomes by baseline CKD status
Kidney outcomes in the CKD cohort
SPRINT pre-specified a composite kidney outcome as one of its secondary outcomes, and its definition depended on baseline CKD status3. For participants with baseline CKD, the composite kidney outcome was defined as a sustained ≥50% decrease in eGFR from baseline (confirmed with follow-up testing 90 days later) or end-stage kidney disease (ESKD) requiring dialysis or kidney transplant. Participants in the intensive BP arm had a decline in eGFR observed by the first month of follow-up that stabilized by the sixth month, while participants in the standard BP arm had an increase in eGFR and subsequent stabilization during this same time period9. Despite this rapid and early divergence in eGFR of the two treatment arms, analyses of eGFR decline from the sixth month and onwards did not show any clinically significant differences between the two arms, and there was no significant difference in the composite kidney outcome (Figure 1)9. However, SPRINT participants in the CKD subgroup had relatively mild CKD and minimal albuminuria and therefore had relatively slow CKD progression. Adding in the early trial termination, it is not surprising that only 31 participants (1.2%) in the CKD subgroup experienced the composite renal outcome, with 16 requiring long-term dialysis and none receiving kidney transplantation9.
Incident albuminuria within the CKD cohort tended to be lower in the intensive BP arm, although the difference did not quite reach statistical significance (HR 0.72, 95% CI: 0.48 – 1.07, P = 0.11). Yet, levels of urinary albuminuria were significantly lower in the intensive BP group throughout the entire trial9.
Kidney outcomes in the non-CKD cohort
For participants without baseline CKD, the kidney outcome, incident CKD, was defined as a confirmed ≥ 30% decrease in eGFR to < 60 ml/min per 1.73m2. In this subgroup, a similar rapid early divergence in the eGFR followed by stabilization at month 6 was seen between the two treatment arms, with an average eGFR difference of 4-5ml/min per 1.73m2 after 6 months10. In contrast to the CKD subgroup, however, in this non-CKD subgroup, 140 participants (4.2%) in the intensive BP arm and 40 (1.2%) in the standard BP arm developed incident CKD (HR 3.54, 95% CI 2.50-5.02; Figure 1)10. While the majority of the extra kidney outcomes in the intensive arm were due to the acute eGFR decline in the first 6 months, rates of a subsequent 30% eGFR decline from 6 months to the end of the trial were still significantly higher in the intensive arm (HR 2.50, 95% CI: 1.57 – 4.11; P < 0.001)10. Yet among the 140 participants in the intensive BP arm with incident CKD, by the end of the trial, 40% improved to a <30% decline in eGFR. Among the 40 participants in the standard BP arm with incident CKD, 25% improved to a <30% decline in eGFR10. No patients in the non-CKD cohort developed ESKD requiring dialysis or kidney transplant. Again, this suggests that the decrease in eGFR was predominantly hemodynamically related and reversible, as incident albuminuria, a marker of kidney damage, was actually lower in the intensive arm, although the results did not quite reach statistical significance (Figure 1).
Kidney outcomes and baseline albuminuria
Just as Chang et. al.’s stratified analysis by baseline albuminuria status showed higher rates of the primary CV outcome and death among patients with albuminuria at baseline, patients with pre-existing albuminuria also experienced higher rates of the SPRINT kidney outcome16. While the absolute number of kidney events were low, patients with prior albuminuria played an out-sized role contributing to the respective kidney outcomes, especially within the CKD subgroup.
When stratified by baseline albuminuria status, intensive BP control significantly increased the relative risk of a ≥ 40% decline in eGFR in patients without baseline albuminuria (HR 4.55, 95% CI: 2.37 – 8.75), but this relative effect was significantly attenuated in patients with albuminuria (HR 1.48, 95% CI: 0.91 – 2.39) (Pint < 0.001)16. This pattern was observed even more so in the CKD subgroup as the number of patients with baseline albuminuria who experienced a ≥ 40% eGFR decline was similar between the intensive and standard BP arms at 25 and 22 patients respectively (HR 1.07, 95% CI: 0.60 – 1.90)16. Given that patients in the intensive arm experienced an acute drop in eGFR while patients in the standard BP arm did not, the fact that rates of eGFR decline were similar between the two arms suggest that patients with baseline albuminuria experience faster rates of CKD progression with the standard BP target and derive a relatively greater benefit with intensive BP control.
Acute Kidney Injury events in SPRINT
Related to the secondary kidney outcomes is the occurrence of acute kidney injury (AKI), a serious adverse event in SPRINT. While rates of serious adverse events overall were similar among the intensive and standard BP arms (38.3% versus 37.1%, P=0.25), AKI occurred more often in the intensive compared with the standard BP arm (4.4% versus 2.6%, P<0.001)3. A subsequent analysis of AKI in SPRINT examined whether there was recovery of kidney function after an AKI event, which was determined by comparing the peak recorded serum creatinine value with the lowest outpatient SPRINT creatinine value obtained in the subsequent 365 days. Kidney recovery was categorized as complete (within 20% of pre-AKI values) or partial (recovery to within 30% of pre-AKI values)26. Reassuringly, they found complete resolution of the AKI for 90.4% events in the intensive arm and 86.9% of events in the standard arm.
SPRINT and kidney outcomes in context
The results of the kidney outcomes in SPRINT were largely inconclusive within the CKD cohort and even showed a possible harm with an intensive BP target in patients without CKD due to the rapid initial drop in eGFR. Yet, the rapid initial fall in eGFR with intensive BP lowering mirrors the results seen in other trials testing interventions that ultimately proved to be beneficial, including the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study, one of the major trials that led to ARBs becoming the standard or care for treatment of proteinuric kidney disease27, and the more recent sodium-glucose cotransporter 2 inhibitors (SGLT2is) trials28, 29. These results highlight the inherent challenges of conducting a CKD progression study population that is at a lower risk of CKD progression such as in SPRINT, with only mildly reduced eGFR and amounts of albuminuria, where a prohibitively long follow-up time would be required to make a more definitive conclusion about the effect of intensive BP control on kidney outcomes.
As noted above, the MDRD, AASK and REIN-2 studies were designed to examine the effect of more intensive BP control on CKD progression. Like SPRINT, all three trials enrolled participants with non-diabetic kidney disease. Yet, they also had notable differences in their baseline characteristics, severity of kidney disease, and study-design (Table 2)8, 17, 19. Compared to the SPRINT CKD subgroup, the AASK population was younger, all self-identified as African American, and only included patients with presumed hypertensive CKD18 Like SPRINT, AASK enrolled patients with mild to moderate CKD, with mean eGFR of 45 to 50 ml/min per 1.73m2 and low levels of albuminuria. The MDRD and REIN-2 participants were younger than the SPRINT CKD cohort but had more advanced CKD, with mean eGFR of 30 to 40ml/min per 1.73m2 and heavier albuminuria.17, 19 Not surprisingly, 13 percent of MDRD participants developed ESKD during the trial while only 0.6% of the SPRINT CKD cohort reached ESKD.9, 17 The MDRD and AASK studies also used mean arterial pressure (MAP) targets while more recent BP targeting trials including SPRINT focused on SBP targets.
Despite these differences, like SPRINT, none of these previous trials showed a significant benefit of a more intensive BP target on their respective kidney outcomes. However, observational follow-up studies of the MDRD and AASK trials showed a benefit of intensive BP lowering for reducing the rate of CKD progression8, 30. A subsequent meta-analysis of the MDRD and AASK studies with a median follow-up of 14.9yrs continued to find a marginal benefit of intensive BP lowering on the outcome of ESKD requiring dialysis or kidney transplantation (HR 0.88, 95% CI 0.78-1.00)20.
Furthermore, both the MDRD and AASK trials and subsequent follow-up studies demonstrated that patients with higher levels of proteinuria experienced fasters rates of kidney function decline and derived a greater benefit from an intensive BP target7, 8, 18, 30, 31. While the overall low kidney event rates in SPRINT preclude making a definitive verdict, there was also a trend within SPRINT towards a similar conclusion.
Summary: SPRINT and kidney outcomes
Taken together, these results suggest that the initial decline in eGFR with more intensive BP lowering observed in SPRINT is likely due to the reversible effects of hemodynamic changes rather than true intrinsic kidney damage. Further support for a hemodynamic etiology is provided by a longitudinal urinary biomarker study32. A random sample of 978 SPRINT participants with baseline CKD had stored urine samples analyzed for biomarkers of tubular injury at baseline, year 1 and year 4 of follow-up. The results showed no increase for any of the urine biomarkers in the intensive versus standard BP arm for the duration of the study, despite having a lower eGFR. While the aggregate data favors a lower BP target in patients with CKD and proteinuria, it remains to be seen whether intensive BP lowering overall will ultimately prove beneficial, detrimental, or neutral with regard to kidney outcomes.
Cognitive outcomes by baseline CKD status
A key secondary outcome in SPRINT assessed whether intensive vs. standard BP lowering reduced the incidence of probable dementia, mild cognitive impairment (MCI) or the composite of both outcomes33. After a median follow-up of 5.1 years, the results showed that an intensive BP target non-significantly lowered the risk of probable dementia and significantly reduced the incidence of MCI and the composite of probable dementia and MCI (Figure 1)33. As with the other outcomes, the incidence of probable dementia, MCI, and their composite were higher in patients with CKD compared with the non-CKD subgroup. Yet, results were generally similar for participants with and without baseline CKD defined by eGFR criteria, although intensive vs. standard BP control may have a larger benefit on reducing rates of MCI in patients without CKD than with CKD (Pinteraction = 0.04) (Figure 1). Exploratory modeling of eGFR as a continuous variable suggested that the benefit of intensive vs. standard BP control may be reduced or even harmful as eGFR declines, although these analyses are only hypothesis-generating34. Tests for treatment effect heterogeneity between patients with and without albuminuria were not significant for cognitive outcomes, although the effect estimates were more attenuated among participants with baseline albuminuria.
Summary: SPRINT and cognitive outcomes
Because the trial was terminated early and cognitive events occurred less frequently than anticipated, the trial was likely underpowered to detect significant treatment effects and treatment heterogeneity between patients with CKD and non-CKD for dementia as ascertaining cognitive outcomes usually requires longer periods of follow-up34. Yet, the aggregate data brought forth by SPRINT33 and the kidney-focused secondary analysis34 highlights the following: patients with baseline CKD and albuminuria have higher crude rates of adverse cognitive outcomes than those without baseline CKD and albuminuria; an intensive vs. standard BP target tends to reduce the rates of dementia and MCI in patients with and without CKD. Questions remain about the benefits of intensive BP control in patients with more advanced CKD and higher degrees of albuminuria. Importantly, concerns of cerebral hypoperfusion that could potentially arise from a lower BP target and lead to faster cognitive decline35 were not observed in SPRINT.
Conclusions
Since the publication of the main trial results in 20153, SPRINT has continued to provide a wealth of information to help guide BP management and advance our understanding of how intensive BP targets affect a wide variety of important clinical outcomes. In this kidney-centric narrative review of SPRINT, we conclude that the data support more intensive SBP targets in patients with CKD to reduce the occurrence of CV events and death and possibly to reduce the occurrence of cognitive events. Intensive BP lowering appears to confer short-term reductions in eGFR, particularly in patients without CKD, but its effect on longer-term kidney outcomes remains to be determined in future trials. As with all things in medicine, treatment decisions must be individualized for each particular patient.
Sources of Funding:
AHH is supported by the T32DK007357 from the NIH/NIDDK.
TIC:
Disclosures:
AHH: nothing to disclose
TIC: Served as site co-investigator for SPRINT; served on KDIGO guideline committee for Management of BP in CKD. Received funding unrelated to the current work paid by Janssen Pharmaceuticals to Stanford University and has served as a consultant for Bayer, Janssen Pharmaceuticals, Novo Nordisk, Fresenius Medical Care, Tricida, Gilead and AstraZeneca.
References
- 1.Townsend RR and Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol. 2015;11:555–63. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 3.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA, Simons-Morton DG, Basile JN, Corson MA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG and Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–62. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Wright JT Jr., Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, Cushman WC, Hawfield AT, Johnson KC, Lewis CE, et al. Effects of Intensive BP Control in CKD. J Am Soc Nephrol. 2017;28:2812–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beddhu S, Rocco MV, Toto R, Craven TE, Greene T, Bhatt U, Cheung AK, Cohen D, Freedman BI, Hawfield AT, et al. Effects of Intensive Systolic Blood Pressure Control on Kidney and Cardiovascular Outcomes in Persons Without Kidney Disease: A Secondary Analysis of a Randomized Trial. Ann Intern Med. 2017;167:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obi Y, Kalantar-Zadeh K, Shintani A, Kovesdy CP and Hamano T. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med. 2018;283:314–327. [DOI] [PubMed] [Google Scholar]
- 12.Cheung AK, Chertow GM, Greene T, Kimmel PL, Rahman M, Reboussin D, Rocco M and Group SR. Benefits and risks of intensive blood-pressure lowering in advanced chronic kidney disease. J Intern Med. 2018;284:106–107. [DOI] [PubMed] [Google Scholar]
- 13.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P and Boutouyrie P. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–7. [DOI] [PubMed] [Google Scholar]
- 14.Chang TI, Wei G, Boucher R, Kramer H, Chertow GM, Cheung AK, Greene T, Whelton PK and Beddhu S. Baseline Diastolic Blood Pressure and Cardiovascular Outcomes in SPRINT Participants with Chronic Kidney Disease. Kidney360. 2020;1:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, Chronic Kidney Disease Prognosis C, van der Velde M, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–52. [DOI] [PubMed] [Google Scholar]
- 16.Chang AR, Kramer H, Wei G, Boucher R, Grams ME, Berlowitz D, Bhatt U, Cohen DL, Drawz P, Punzi H, et al. Effects of Intensive Blood Pressure Control in Patients with and without Albuminuria: Post Hoc Analyses from SPRINT. Clin J Am Soc Nephrol. 2020;15:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW and Striker G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–84. [DOI] [PubMed] [Google Scholar]
- 18.Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31. [DOI] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–46. [DOI] [PubMed] [Google Scholar]
- 20.Ku E, Gassman J, Appel LJ, Smogorzewski M, Sarnak MJ, Glidden DV, Bakris G, Gutierrez OM, Hebert LA, Ix JH, et al. BP Control and Long-Term Risk of ESRD and Mortality. J Am Soc Nephrol. 2017;28:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku E, Glidden DV, Johansen KL, Sarnak M, Tighiouart H, Grimes B and Hsu CY. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int. 2015;87:1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papademetriou V, Zaheer M, Doumas M, Lovato L, Applegate WB, Tsioufis C, Mottle A, Punthakee Z, Cushman WC and Group AS. Cardiovascular Outcomes in Action to Control Cardiovascular Risk in Diabetes: Impact of Blood Pressure Level and Presence of Kidney Disease. Am J Nephrol. 2016;43:271–80. [DOI] [PubMed] [Google Scholar]
- 23.Perkovic V and Rodgers A. Redefining Blood-Pressure Targets--SPRINT Starts the Marathon. N Engl J Med. 2015;373:2175–8. [DOI] [PubMed] [Google Scholar]
- 24.Beddhu S, Chertow GM, Greene T, Whelton PK, Ambrosius WT, Cheung AK, Cutler J, Fine L, Boucher R, Wei G, et al. Effects of Intensive Systolic Blood Pressure Lowering on Cardiovascular Events and Mortality in Patients With Type 2 Diabetes Mellitus on Standard Glycemic Control and in Those Without Diabetes Mellitus: Reconciling Results From ACCORD BP and SPRINT. J Am Heart Assoc. 2018;7:e009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes Blood Pressure Work G. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:S1–S87. [DOI] [PubMed] [Google Scholar]
- 26.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, Umanath K, Rahbari-Oskoui F, Porter AC, Pisoni R, Lewis CE, et al. Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. 2018;71:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. [DOI] [PubMed] [Google Scholar]
- 28.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 29.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 30.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ and Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–51. [DOI] [PubMed] [Google Scholar]
- 31.Upadhyay A, Earley A, Haynes SM and Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154:541–8. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra R, Craven T, Ambrosius WT, Killeen AA, Haley WE, Cheung AK, Chonchol M, Sarnak M, Parikh CR, Shlipak MG, et al. Effects of Intensive Blood Pressure Lowering on Kidney Tubule Injury in CKD: A Longitudinal Subgroup Analysis in SPRINT. Am J Kidney Dis. 2019;73:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Group SMIftSR, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurella Tamura M, Gaussoin SA, Pajewski NM, Chelune GJ, Freedman BI, Gure TR, Haley WE, Killeen AA, Oparil S, Rapp SR, et al. Kidney Disease, Intensive Hypertension Treatment, and Risk for Dementia and Mild Cognitive Impairment: The Systolic Blood Pressure Intervention Trial. J Am Soc Nephrol. 2020;31:2122–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saper CB. How low can you go? Ann Neurol. 2015;78:665–6. [DOI] [PubMed] [Google Scholar]


