Abstract
Understanding the spread of SARS-CoV-2, how and when evidence emerged, and the timing of local, national, regional, and global responses is essential to establish how an outbreak became a pandemic and to prepare for future health threats. With that aim, the Independent Panel for Pandemic Preparedness and Response has developed a chronology of events, actions, and recommendations, from December, 2019, when the first cases of COVID-19 were identified in China, to the end of March, 2020, by which time the outbreak had spread extensively worldwide and had been characterised as a pandemic. Datapoints are based on two literature reviews, WHO documents and correspondence, submissions to the Panel, and an expert verification process. The retrospective analysis of the chronology shows a dedicated initial response by WHO and some national governments, but also aspects of the response that could have been quicker, including outbreak notifications under the International Health Regulations (IHR), presumption and confirmation of human-to-human transmission of SARS-CoV-2, declaration of a Public Health Emergency of International Concern, and, most importantly, the public health response of many national governments. The chronology also shows that some countries, largely those with previous experience with similar outbreaks, reacted quickly, even ahead of WHO alerts, and were more successful in initially containing the virus. Mapping actions against IHR obligations, the chronology shows where efficiency and accountability could be improved at local, national, and international levels to more quickly alert and contain health threats in the future. In particular, these improvements include necessary reforms to international law and governance for pandemic preparedness and response, including the IHR and a potential framework convention on pandemic preparedness and response.
Introduction
The COVID-19 pandemic is the result of the rapid international spread of SARS-CoV-2, a coronavirus that causes COVID-19 disease. As of Oct 22, 2021, more than 242·3 million infections and 4·9 million deaths have been documented, making it one of the most extensive pandemics in history,1 which occurred despite evaluations showing that many countries were reportedly prepared to respond to an emerging infectious disease.2 To understand how SARS-CoV-2 caused a global pandemic and to prepare for future health threats, establishing a clear and accurate understanding of the sequence of key early epidemiological events and of authorities' responses to the emergence of SARS-CoV-2 is essential. With that aim, the Independent Panel for Pandemic Preparedness and Response (henceforth called the Independent Panel) has developed a chronology of events and actions from December, 2019, when cases were first identified in China, to the end of March, 2020, when COVID-19 cases were found in nearly every country in the world and the outbreak had been characterised as a pandemic. On the basis of these findings, the Independent Panel developed a set of recommendations to improve the speed and efficiency of disease detection and alerts to help countries to respond more effectively to future global health emergencies.3 By identifying the timeline of key events, the chronology also enables assessment of adherence to international obligations, in particular, under the International Health Regulations (IHR) 2005, which are currently the only binding international legal instrument dedicated to the prevention, detection, and response to cross-border health threats.4 The IHR create obligations for States Parties and set out clear duties and responsibilities for WHO, WHO's Director-General, and specialised committees in the event of a health threat with potential international consequences.
Using a conceptual framework for key stages from outbreak to pandemic, we map the chronology of documented actions against the obligations under the IHR to establish how systems functioned and to identify potential areas for improvement and further clarity in early outbreak alert and response. From this analysis, we propose a series of corresponding objectives to correct weaknesses in global preparedness for future potential high-impact respiratory pathogens with primary features similar to those of SARS-CoV-2.5 These objectives inform and assist in applying the lessons from COVID-19 to reform the IHR and to initiate a potential framework convention on pandemic preparedness and response.
Methods
Three complementary methods were adopted to analyse the timing of events and responses to COVID-19. First, we conducted a systematic review to identify peer-reviewed articles and public reports that examined the scientifically documented origins and the early spread of SARS-CoV-2 (see appendix p 1 for a full overview of search strategy and methodology). Experts, including from China and WHO, were identified to contribute to validation of the findings. Two systematic searches were performed: one on the origins of SARS-CoV-2 and another on the early spread of the virus. The search strategy on the origins was designed to retrieve all eligible articles on the earliest scientific evidence concerning the appearance or spread of SARS-CoV-2, published between Dec 1, 2019, and March 31, 2021, and that met inclusion criteria. The electronic databases MEDLINE, Embase, Global Health, Latin American and Caribbean Center on Health Sciences Information, Western Pacific Region Index Medicus, and Africa Wide Information were searched using the search terms “coronavirus”, “novel virus”, “2019-nCOV”, “COVID-19”, “SARS-CoV-2”, or “Wuhan virus”, following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. 6133 articles were identified and a total of 53 publications were included in the final review. The search strategy on early spread was designed to retrieve all eligible articles focusing on the epidemiology of COVID-19 spread published between Dec 1, 2019, and March 31, 2021, in the electronic databases MEDLINE, Embase, Global Health, Latin American and Caribbean Center on Health Sciences Information, Western Pacific Region Index Medicus, and Africa Wide Information. Search terms included “spread”, “transmission”, or “seroprevalence”, and “COVID-19”, “SARS-CoV-2”, “coronavirus”, “novel virus”, or “Wuhan virus”. The results were narrowed to articles that examined, either in real time or retrospectively, the spread of the virus both in China and outside China within the early months of the pandemic (December, 2019, to March, 2020). In both systematic reviews, researchers in relevant fields were contacted to identify additional published and preprint studies. To identify further relevant studies, reference lists of included articles were searched and a forward citation search was performed on included studies using Web of Science. There were no language restrictions in the search strategy. Four authors independently assessed papers for inclusion, extracted data, and assessed the quality of evidence using a metric adapted from the Confidence in the Evidence from Reviews of Qualitative Research approach. A narrative synthesis of the findings was conducted. A meta-analysis was not done because of the substantial methodological heterogeneity in the included studies. 6494 articles were identified in this search, and 57 articles met inclusion criteria for the final review.
Second, as per the Independent Panel's mandate (appendix p 3), we systematically reviewed internal documents and correspondence from WHO, with a focus on the crucial correspondence that followed the initial detection of the outbreak and activities related to the functioning of the IHR. Specific questions were posed to the WHO Secretariat and responses were provided through a COVID-19 document repository and through discussions with WHO staff members as part of the Independent Panel's programme of work. In addition, we reviewed 350 technical guidance documents produced by WHO and its Regional Offices and analysed these outputs for their timeliness and evidence base. The timing and nature of key correspondence and guidance documents were mapped onto the chronology.
Finally, to supplement the systematic review, we established an open call for public submissions to share accounts of the timing of the key events during the outbreak. Submissions were then compared and validated against existing public records, with a particular focus on detection, assessment, and reporting activities in line with the IHR.
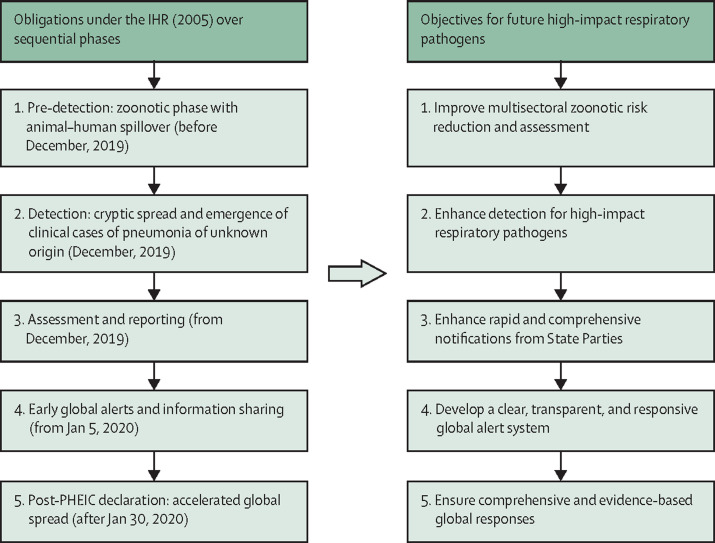
To guide the analysis of the chronology and find areas for improvement for future pandemic preparedness and response, we developed a conceptual framework (figure ) for consideration of the steps taken in relation to COVID-19 and associated IHR obligations and potential measures to improve alert in future outbreaks. The relevant IHR provisions include obligations on States Parties to meet minimum core public health capacities, including surveillance, response, and reporting capabilities, assessment and notification of events that might constitute a potential Public Health Emergency of International Concern (PHEIC) within specific timeframes, and ongoing information sharing obligations. The IHR set out the duties and responsibilities of WHO, WHO's Director-General, and specialised committees, including the power to request verification of reports of events and duties of confidentiality and information sharing, to determine that an event constitutes a PHEIC and to issue temporary recommendations to all States Parties. The IHR also establish the specific criteria for the declaration of a PHEIC. Because the IHR govern country preparedness for potential international health threats and rapid notification and international mobilisation, compliance with these obligations is vital for global health. Establishing how the IHR functioned during the COVID-19 pandemic can inform potential technical, governance, and law reform, including by identifying potential gaps that can guide IHR reform or constitute elements of a new international instrument, such as a pandemic treaty.
Figure.
Conceptual framework for analysis of obligations and informing objectives for future threats
IHR=International Health Regulations. PHEIC=Public Health Emergency of International Concern.
Using the structure of IHR obligations and outbreak timelines, the conceptual framework guides examination over the phases of, first, pre-detection (ie, zoonotic phase with animal–human spillover, before December, 2019); second, detection (ie, cryptic spread, emergence of clinical cases of pneumonia of unknown origin, and subsequent characterisation of the virus, between December, 2019, and early January, 2020); third, assessment and reporting (from States Parties to WHO, from Dec 30, 2019); fourth, global alert and information sharing (from WHO and States Parties to States Parties, from Jan 5, 2020); and finally, post-PHEIC declaration (ie, the accelerated global spread phase, from Jan 30 to March 31, 2020). These periods are examined in the Results.
From the application of the conceptual framework to the chronology, we identified a series of corresponding objectives for international governance reforms to prepare for and respond to future high-impact respiratory pathogens, which share similar features and issues identified in the analysis of SARS-CoV-2 and address particular weaknesses in global preparedness.5 These objectives include: first, improving multisectoral zoonotic risk reduction and assessment; second, enhancing detection for potentially high-impact respiratory pathogens; third, enhancing rapid and comprehensive notifications from States Parties; fourth, establishing a clear, transparent, and responsive global alert system; and lastly, supporting comprehensive and evidence-based global responses.
Results
We describe findings and observations under the thematic subheadings developed in the conceptual framework (figure). The full Independent Panel chronology is available in the appendix (pp 4–51) and on the Independent Panel's website.
Pre-detection: zoonotic phase with animal–human spillover
In-depth study of the pre-detection zoonotic phase (ie, before December, 2019) was not part of the Independent Panel's mandate. However, given its importance to future surveillance and detection of pathogens with pandemic potential and implementation of One Health strategies, the Independent Panel conducted a literature review current as of March 31, 2021, in parallel with the work of the Joint WHO-China Origins Investigation, whose results were reviewed for this Health Policy.6 The evidence suggests that SARS-CoV-2 has zoonotic origins.7 Although various animal species can become infected and some can transmit SARS-CoV-2,8 the evidence suggests a bat species as the most probable reservoir host; the intermediate host remains unknown.9, 10, 11 At this point, definitively confirming zoonotic progenitors and the exact transmission cycle is not possible. Data from phylogenetic studies suggest that human-to-human transmission was probably occurring in China from at least November, 2019, or possibly earlier.6, 7 This timeline is also concordant with estimated epidemic growth rates and doubling times from early laboratory-confirmed case data.12, 13, 14 Whereas some studies have claimed to find evidence of SARS-CoV-2 in clinical and environmental samples taken in several countries outside China before December, 2019, most of that evidence is preliminary and not yet independently verified.15, 16, 17, 18, 19 Heterogeneous and, at times, unvalidated diagnostic methods were used, and the occurrence of false positives due to sample cross-contamination, contamination of reagents, and cross-reactivity with known coronaviruses was a possibility.20, 21 Despite these notable study limitations, spread of the virus outside of China in late 2019 is possible, particularly given the probable human-to-human transmission in Wuhan, China and considerable international air travel from the city.
Detection: cryptic spread and emergence of clinical cases of pneumonia of unknown origin
Clinicians in Wuhan hospitals began treating patients with pneumonia of unknown origin in December, 2019, with the earliest patient documented to have laboratory-confirmed COVID-19 developing symptoms on Dec 8.6 Concerned about a patient with pneumonia not responding to usual treatments, doctors sent a lung fluid sample to a private laboratory (Vision Medicals, Guangzhou, China) on Dec 24.22, 23 Clinicians became suspicious about the possibility of human-to-human transmission potentially as the result of infection with a novel pathogen in late December on the basis of the identification of a cluster of cases of pneumonia of unknown origin, and sent further samples to different laboratories.10, 24, 25, 26 On Dec 26, a physician at the Hubei Hospital of Integrated Chinese and Western Medicine in Wuhan treated a woman with fever and cough, whose thoracic CT scans findings were consistent with pneumonia of unknown origin.25 Her husband was initially admitted to a different hospital department with shortness of breath and was transferred to the same department as his wife on Dec 27, when hospital staff realised they were a couple. Their son, who was asymptomatic, was also tested and was shown to have similar CT chest findings.25 Although the woman reported close contact with the Huanan Seafood Market, her husband reported no contact.6 The clinician overseeing these patients reported these cases to the Jianghan District Center for Disease Control on Dec 27, and Wuhan Center for Disease Control representatives took samples from all three on the same day.25, 26 According to later press reports, a private laboratory that had received samples from a Wuhan hospital on Dec 27 also shared next-generation sequencing data of a potential new severe acute respiratory syndrome (SARS)-like coronavirus with the Chinese Academy of Medical Sciences and Wuhan officials.23, 27 On Dec 30, the Wuhan Institute of Virology received lung fluid samples from seven patients with pneumonia of unknown origin from the Jinyintan Hospital in Wuhan.28 Some patients reported frequenting the Huanan Seafood Market in Wuhan, which became the initial site of epidemiological investigation. Two studies of the first laboratory-confirmed cases retrospectively concluded that, considering only 55–66% of cases had been exposed to the seafood market, human-to-human transmission might have been occurring beyond the market, and the market might have been a site of initial amplification of the virus rather than its origin.6, 29, 30
Assessment and reporting
The Wuhan Municipal Health Commission issued its first notices to hospitals about cases of pneumonia of unknown origin on Dec 30.31 There were instructions in the notices not to share, but the information was posted on social media sites.32 Reporting on the notices by the Chinese business publication Finance Sina on Dec 31 triggered a chain of events that signalled the emergence of the outbreak to WHO, including through queries directed to WHO by Taiwan and the media. A machine-translated account of the Finance Sina article was published on the website of the International Society for Infectious Diseases' Program for Monitoring Emerging Diseases, which was picked up by WHO's Epidemic Intelligence from Open Sources.26, 33, 34 Near-immediate media attention given to the notices, which alerted neighbouring areas to the potential threat and prompted IHR queries,35 shows the speed and cross-border nature of today's digital environment for outbreak alerts and the importance of open sharing platforms. Various regions, starting with Taiwan and Hong Kong, began preparing interventions on Dec 31, including screening travellers from Wuhan.36, 37, 38 The Huanan Seafood Market was shut down for disinfection on Jan 1, 2020.29, 39
In the early afternoon of Dec 31 (China Standard Time), the Wuhan Municipal Health Commission issued a public bulletin describing 27 cases of pneumonia of unknown origin.40 This bulletin was not sent to WHO, but the WHO Country Office in China took note of it shortly after it was posted.41 Once WHO identified reports about the outbreak on Dec 31, rapid, escalating efforts from various levels of the organisation (the headquarters, the Western Pacific Regional Office, and the WHO Country Office in China) took place to obtain more information from the Chinese authorities,41 in the form of emails, letters, and formal requests under the IHR (appendix pp 11–14).
WHO initiated a series of actions during the first days of 2020, both informally and through official IHR reporting systems. On Jan 1, the WHO Western Pacific Regional Office IHR Focal Point made a request to the Chinese authorities for information.26 On the same day, WHO activated its Incident Management System.41 On the evening (Central European Time) of Jan 1, WHO Headquarters requested WHO Western Pacific Regional Office to repeat the information request formally under Article 10 of the IHR, which includes specific time requirements for health information verification (appendix p 12). On Jan 3, the Western Pacific Regional Office contacted the Chinese National IHR Focal Point to formally invoke Article 10 of the IHR and, on the same day, the Chinese National Health Commission officially responded to WHO's request by providing brief information about the first set of 44 reported cases during a technical briefing to the WHO Country Office.26 This briefing was the first of three in-person meetings; the other two were held on Jan 11 and Jan 16 (appendix pp 13, 18, 20). WHO's Western Pacific Regional Office provided the first public announcement by WHO via a Twitter thread on Jan 4: “#China has reported to WHO regarding a cluster of pneumonia cases in Wuhan, Hubei Province. The Govt has also met with our country office, and updated @WHO on the situation. Govt actions to control the incident have been instituted and investigations into the cause are ongoing.”42
Early global alerts and information sharing
On Jan 5, 2020, WHO notified all country governments about the cases through the IHR Event Information System.26, 41 On the same day, it issued its first Disease Outbreak News notice on the cluster.43 Chinese scientists acted quickly to sequence the virus. Scientists at the Wuhan Institute of Virology completed a partial sequence on Jan 2, and had fully sequenced the genome by Jan 7.28 In parallel, a Shanghai Public Health Clinical Center group completed sequencing by Jan 5 and submitted the sequence to GenBank on that day.28, 44, 45 Scientists affiliated with the China Center for Disease Control and Prevention were able to isolate the virus by Jan 7.31 The genetic sequence from the Shanghai group was released publicly on Jan 11 on open-access websites.44, 46 A laboratory PCR test to detect SARS-CoV-2 was developed by Jan 10.31 PCR diagnostic reagents were made available to Wuhan hospitals from Jan 11.30, 31
In the meantime, possible COVID-19 cases were being detected in countries outside China, starting with a suspected case identified in Thailand on Jan 8 (through airport screening), an infection that was confirmed on Jan 12 and reported to WHO on Jan 13.47, 48 Travellers continued to leave Wuhan in large numbers (an estimated 4·3 million) during Chūnyùn, the pre-Lunar New Year travel period, between Jan 11 and Jan 23—the day Wuhan was placed under lockdown.49 Infections were reported to WHO under the IHR by other countries, including Japan (Jan 15), South Korea (Jan 20), and the USA (Jan 21), all in incoming travellers from Wuhan.48, 50 On Jan 15, the WHO Country Office in China reached an agreement with Chinese authorities to visit Wuhan (appendix p 19). The first WHO mission to Wuhan took place on Jan 20–21.26, 41
Evolving and, at times, ambiguous and contradictory messages were circulated about the suspected mode of transmission of the virus. For example, on Jan 14, a WHO headquarters tweet indicated that Chinese authorities had found “no clear evidence of human-to-human transmission,” but indicated at a press conference earlier the same day that it was “possible”.26, 41, 51 Although the absence of clear evidence was consistent with human-to-human transmission still being possible, the nuance was not sufficiently clear. WHO's Western Pacific Regional Office indicated there was possibly limited human-to-human transmission on Jan 19.52 Nanshan Zhong, a pulmonologist who had a prominent role in China's SARS response in 2003, led a mission to Wuhan that, on Jan 20, confirmed human-to-human transmission on Chinese state television, noting that hospital staff in Wuhan were being infected.26, 53
On Jan 22–23, the IHR Emergency Committee was convened to consider whether to advise the WHO Director-General that the outbreak constituted a PHEIC.54 On the first day of the meeting, WHO had reported 314 cases, including four confirmed cases outside of China in three countries, all with direct connections to Wuhan.55 By that time, 18 areas, countries, and regions had implemented border controls (mostly screening of airline passengers) applicable to travellers from Wuhan.54 By the second day of the Emergency Committee meeting on Jan 23, WHO reported 581 cases, including ten cases outside of China in four countries, all connected to Wuhan.56 On the same day, WHO reported that “there is now more evidence that 2019-nCoV spreads from human-to-human and also across generations of cases. Moreover, family clusters involving persons with no reported travel to Wuhan have been reported from Guangdong Province”.56 Although confirming “human-to-human transmission is occurring”, the Emergency Committee said several members considered that it was too early to declare a PHEIC and did not recommend that determination. The committee said it stood ready to reconvene as the situation evolved.54
Wuhan introduced important public health measures on Jan 23, including a suspension of public transport, mandatory mask wearing, and a ban on travelling outside the Hubei province.47, 57, 58 Retrospective analyses estimate that the vast majority (86%) of infections in China were undetected before that date, and that there had been broad, rapid spread before Jan 23.59 Such analyses also estimate the basic reproduction number (R 0) to have been as high as 5·7, suggesting rapid spread until the implementation of public health measures such as cordon sanitaire on Jan 23.30, 60, 61, 62
On Jan 24, WHO updated its advice on international traffic and trade, advising that countries institute exit screening and consider implementing entry screening; however, it continued to recommend against restrictions on international travel.63 That same day, the first case reports strongly suggesting human-to-human transmission and asymptomatic infection were published, as well as common symptoms and clinical characteristics of COVID-19, confirming the severity of the disease.29, 64
WHO's Director-General Tedros Adhanom Ghebreyesus visited China on Jan 27–28 and met with President Xi Jinping.41 After returning to Geneva, Switzerland, the Director-General reconvened the IHR Emergency Committee. On Jan 30, 2020, the Emergency Committee advised that the event constituted a PHEIC under the IHR and the Director-General declared the outbreak a PHEIC.65 At this stage, the Director-General announced 98 known cases outside of China in 18 countries and no known deaths.65 In keeping with the IHR's aims of preventing international spread while preserving international travel and trade, when issuing temporary recommendations with the PHEIC declaration, the Emergency Committee expressly noted that “there is no reason for measures that unnecessarily interfere with international travel and trade. WHO doesn't recommend limiting trade and movement”.65
Post-PHEIC: accelerated global spread
Laboratory-confirmed cases of SARS-CoV-2 increased from 132 cases in 23 countries in all regions except the African continent, on Feb 1, to 5304 cases in 53 countries by Feb 27 (excluding 705 cases on the Diamond Princess cruise ship).66 During this period, most countries appear to have taken only modest public health measures.67 In the meantime, there was a marked decrease in transmission within China, indicating the likely success of the lockdown and public health measures taken.62 The measures introduced in China on Jan 23 caused a dramatic reduction in R 0: from over 2·0 before the measures, to 1·2 between Jan 23 and Feb 1, and to 0·5 for the period of Feb 2–16.61
Phylogenetic analyses from Europe and the USA provide evidence that the virus had been cryptically circulating in the community in these countries weeks before its initial detection, suggesting that a global spread occurred early.68, 69, 70 Computational models and artificial intelligence were able to predict the trajectory of the virus' spread along international air routes.71, 72 As in China, seroprevalence studies consistently showed that most cases in early epicentres were undetected.19, 73, 74 Border closures and other mitigation efforts appear to have occurred in reaction to index cases rather than prophylactically. This delayed reaction might have been influenced by the screening protocols and country case definitions that were recommended, which focused heavily on travel history from Wuhan and symptomatology, and by poor knowledge and communication about the degree of asymptomatic transmission.75
The WHO Executive Board was briefed on the outbreak at its regular session held on Feb 3–8, 2020. The opening speech of the Director-General to the Executive Board, on Feb 3, devoted 14 paragraphs to the PHEIC, beginning at paragraph 53 of his remarks.76 A technical briefing on COVID-19 took place with the Executive Board on Feb 4.77
By March 11, when WHO's Director-General used the term global pandemic to characterise the COVID-19 outbreak, 118 000 cases had been reported in 114 countries.78 That same day, Italy became the first European country to enact a national lockdown, followed shortly by Spain on March 14 and by France on March 17.79 On March 20, Europe recorded more cases (104 591) than the Western Pacific Region (93 349), which includes China.67 By March 28, both Italy and the USA had surpassed China in number of reported cases.67
The Independent Panel's chronology of SARS-CoV-2 spread and national and international actions against the obligations under the IHR is summarised in the table . It lists the criteria for obligations arising under the IHR, the relevant IHR article, the applicable intervenient, the required response, and any relevant time periods stipulated under the IHR, compares these aspects against the COVID-19 chronology, and provides more information about the event itself.
Table.
Comparison of IHR time periods with the COVID-19 chronology by phase and action criteria
| Relevant IHR article | Applicable intervenient | Response required by IHR | IHR-stipulated timeline | Timeline of COVID-19 actions | COVID-19 event | |
|---|---|---|---|---|---|---|
| Pre-detection (assess capacities) | Article 59 and Annex 1 | State | Assess capacity to meet minimum surveillance requirements | No later than June, 2009 | NA | NA |
| Pre-detection (meet capacities) | Article 5 and Annex 1 | State | Ensure capacity to detect, assess, notify, and report events | June, 2012 | Self-reported full compliance with surveillance core capacity | China reported 100% surveillance capacity in 2019, but no Joint External Evaluation has been done and published for China |
| Event detected by local surveillance system (t1) | Article 4 and Annex 1 | State (local community level, primary public health response level, or both) | Detect events involving disease or death above expected rates and report essential information immediately to the subnational or national public health authority | Dec 27, 2019 (ie, immediately upon detection at local level) | Dec 30, 2019 (t1 + 72 h) | The Wuhan CDC received reported cases of pneumonia of unknown origin from the Hubei Provincial Hospital, Wuhan on Dec 27, 2019; China's internal timeline indicates that the National Health Commission did not receive reports until the WMHC released an urgent notice on treatment of patients on Dec 30 |
| Event reported to the subnational surveillance system | Article 5 and Annex 1 | Subnational level (eg, state or province) | Confirm and assess urgent events and report immediately; in case of an urgent event, report all essential information to national level public health authority (eg, China Center for Disease Control and Prevention) | Dec 27, 2019 (ie, immediately upon detection at the subnational level [Hubei province]) | Unclear | Unclear if this action occurred, but direct national reporting from local to national levels, bypassing subnational level, is permitted; unclear when and if the Hubei Provincial CDC reported to the Chinese National Government |
| Event reported to national level (t2) | Article 6 and Annex 1 | State | Assess all reports of urgent events within 48 h; China NFP to notify WHO (per Articles 6 or 7) if conditions are met | Jan 1, 2020 (t2 + 48 h at most) | Jan 3, 2020 (t1 + 168 h, or t2 + 96 h) | Upon learning of the cases (Dec 30, 2019), the NHC commenced investigation; cases probably satisfied the IHR definition of urgent,4 requiring assessment within 48 h, but it is not necessarily evident during the early stages of an outbreak whether an event meets this definition; no action appears to have been taken by Jan 1, 2020 (no statements exist that Annex 2 was used by national authorities to assess if an Article 6 notification was required) |
| Event is a potential PHEIC | Article 6.1 and Annex 2 | State | Notify WHO of potential PHEIC within 24 h of assessment, via NFP | Dec 31, 2019 (t2 + 24 h at most) | Jan 3, 2020 (t1 + 168 h, or t2 + 96 h), or Jan 4, 2020 (t1 + 192 h, or t2 + 144 h) | No statements exist that the Annex 2 assessment process was followed, or of a clear Article 6 notification; a potential PHEIC includes a serious public health impact and an unusual or unexpected event (these were probably already met at this stage; however, whether a novel pathogen meets the criteria for constituting a potential PHEIC requires a degree of subjective assessment); China NFP confirmed information on Jan 3, 2020; although States must confirm if Article 6 is triggered following an Article 10 request, whether China's response included this is unclear; on Jan 4, WPRO stated “China has reported to WHO regarding a cluster of pneumonia cases“42 |
| Event is a potential PHEIC | Article 6.2 and Annex 2 | State | Provide accurate and sufficiently detailed public health information | Ongoing (ie, in a “timely” manner, as stated in Article 6.2) | Information actively sought by WHO | WPRO requested further information (Jan 4, 2020); WHO awaiting further information (Jan 5);similar statements about the provision of information from WHO's Director-General in January; viral genetic sequence first shared |
| Event is unexpected or unusual and is a potential PHEIC | Article 7 | State | Provide all relevant public health information | Ongoing (ie, in a “timely” manner”, as stated in Article 7) | Information actively sought by WHO | As above |
| Event is not a potential PHEIC, including if information to assess is insufficient | Article 8 | State | Advise and consult WHO on event and appropriate control measures, via NFP | Ongoing | NA | Article 8 consultation request does not appear to have occurred |
| Event is not a potential PHEIC, and more information becomes available | Annex 2 | State | Reassess when more information becomes available to determine if a notification under Article 6 is required | Ongoing | None | No indications Annex 2 assessments occurred; might have justified Article 6 notification delays |
| Reports of an event from other sources (on receipt of t3) | Article 9.1 | WHO | Assess reports from other sources, communicate to the State where the event is allegedly occurring | Ongoing (t3) | WHO obligation | First media report (Dec 31, 2019); Taiwan contacts the WHO headquarters (Dec 31); New York Times contacts WHO Country Office with a news request; WHO headquarters detects ProMED reports; WHO Country Office sees local bulletin from WMHC |
| In case of evidence of a public health risk to another State from exported or imported cases (t4) | Article 9.2 | Other States | Inform WHO within 24 h of receipt of evidence | t4 + 24 h at most | NA | NA |
| Reports of an event from other sources (verification) | Article 10.1 | WHO | Request verification from State of other reports | Ongoing | Jan 3, 2020 (0415 h CET; t3 + 72 h) | WHO Country Office seeks information from the Chinese Government (Dec 31, 2019); WPRO IHR Focal Point requests information from China's NFP (Jan 1, 2020); WHO headquarters requests WPRO to use Article 10 (Jan 1); WPRO sends formal verification request under Article 10 (Jan 3; this action appears to have been the only one meeting Article 10 verification steps) |
| If WHO seeks verification of reports from other sources (t5) | Article 10.2 | State | Upon request, verify and provide, within 24 h, acknowledgment of request and available public health information on the events and assessment of events under Article 6 (and relevant public health information) | t5 + 24 h at most | Jan 3, 2020 (0915 h CET; t5 + 5 h) | China NFP acknowledges verification request received and verifies information within 5 h; no detail on whether China then made an official Article 6 notification, or if Annex 2 was used; no further public health information appears to have been provided (WPRO requests more info on Jan 4, 2020) |
| WHO can share information with other States | Articles 10.4, 11.1, and 11.3 | WHO | If State doesn't accept offer of collaboration (Article 10.3), WHO may share information with other states if justified by the magnitude of the public health risk (Article 10.4); similarly, WHO must share information it receives that is necessary to enable a public health response to the risk with other states, if there is evidence confirming international spread, if control measures are unlikely to succeed, or if international measures are immediately needed (Articles 11.1 and 11.2); in such cases, WHO must consult with the affected State (Article 11.3) | No stipulated time for notifying other states; decision based on whether there is a public health risk to other states | Jan 4, 2020 (0422 h CET) | WHO informed China it would notify all IHR Focal Points; unclear which provision WHO was sharing information under; if China did not reject assistance under Article 10.3, Article 11 might apply to the disclosure of information (which would allow public sharing of information, consistent with WPRO's tweet and DONS on Jan 4, 2020) |
Time markers under the IHR are t1 for cases first detected, t2 for event assessed by State, t3 for WHO receiving report from other sources, t4 for the timepoint at which other states must report if they receive evidence of event in another state (not relevant in this scenario), and t5 for verification requested. CDC=Center for Disease Control. CET=Central European Time. DONS=Disease Outbreak News. IHR=International Health Regulations. NA=not applicable. NFP=National Focal Point. NHC=China's National Health Commission. PHEIC=Public Health Emergency of International Concern. WMHC=Wuhan Municipal Health Commission. WPRO=WHO's Western Pacific Regional Office.
Discussion
This analysis of events and early responses to the COVID-19 pandemic and the existing IHR obligations distils vital lessons and potential objectives for improving future pandemic preparedness and response. These objectives reflect necessary considerations for the potential emergence of high-impact respiratory pathogens, which we believe, on the basis of the experience documented from the outbreak of SARS-CoV-2, warrant reform of existing international laws and governance systems such as the IHR. The analysis has some caveats. First, the COVID-19 pandemic was unprecedented in its speed and breadth. Second, it arose in early winter, when the incidence of viral pneumonia is typically increasing, which made it more difficult to distinguish a dangerous novel pathogen among seasonal spikes in respiratory illness. Third, although SARS-CoV-2 evolved from a family of viruses familiar to humans, it did not behave in the same way, including by transmitting between people who exhibited no symptoms. Finally, the virus also emerged during a time of heavy travel within and from China. This combination of circumstances allowed SARS-CoV-2 to spread stealthily and quickly throughout the world. We appreciate the uncertainties around this and other disease outbreaks, and acknowledge the response of clinicians and public health officials. Nonetheless, only by critically analysing the early stages of the pandemic can systemic weaknesses be identified and addressed. Building on such analyses, the reform of global governance arrangements for pandemics—such as revisions of the IHR, the negotiation and adoption of a pandemic treaty, and the establishment of a leader-level accountability and oversight mechanism—can provide the framework for improved global systems for preparedness and response.
Functioning of the IHR during the outbreak of SARS-CoV-2
As has been the case in two previous PHEICs (namely, the H1N1 influenza pandemic and the Ebola epidemic in west Africa), the World Health Assembly established on May 19, 2020, a review of the functioning of the IHR in the COVID-19 response. The IHR review committee presented its report in May, 2021, at the same time as the Independent Panel.26 Although the two processes benefited from an exchange of information and perspectives as they conducted their respective inquiries and their recommendations are considerably consistent, their mandates were different in scope. The IHR review committee examined specifically the functioning of the IHR, including with reference to its respective provisions, whereas the Independent Panel was concerned with the broader review of the totality of the response to COVID-19 to date, including whether the IHR are fit for the purpose of strengthening the alert and response to a potential pandemic event.
Setting the chronology of events in the COVID-19 response against the obligations under the IHR suggests that, first, there is little clarity inherent to detection, assessment, and notification early in outbreaks that the IHR are not sufficiently tailored to consider; and second, that the IHR are less proactive and time-sensitive than optimal in responding to a potential highly transmissible respiratory pathogen. The chronology is not intended to indicate or conclude that any relevant party acted in bad faith or sought deliberately to flout their IHR obligations, but it does suggest that the IHR are insufficiently precise in several areas, or fail to encourage sufficiently proactive response to outbreaks with pandemic potential. Furthermore, concerns about the potential appearance of delayed reporting or mismatched obligations might undermine transparency, information sharing, and notification in future pandemics (panel ).
Panel. Assessing the IHR against the chronology of the COVID-19 response.
Our chronological map has highlighted the following key areas in which the experience with the COVID-19 pandemic points to the need for reform of the International Health Regulations (IHR):
-
•
Inclusion of the assessment of governance and leadership capacities for rapid assessment, notification, and early outbreak response
-
•
Removal of constraints in WHO reporting publicly on events with pandemic potential in advance of or alongside verification
-
•
Greater specificity on the scope of information to be shared with WHO, and that WHO can share with States Parties and other entities, including viral genetic sequence data (where available)
-
•
Closer definition of the nature of events to be reported from local and subnational levels to national level and national level to WHO including in the case of a potentially high-impact respiratory disease
-
•
Streamlined processes to facilitate WHO verification of events detected through conventional and open-intelligence surveillance within 24 h of the first signals being received
-
•
More open and transparent proceedings of the IHR Emergency Committee, and clearer and more objective criteria for determining a Public Health Emergency of International Concern
-
•
Presumption that assistance to, and cooperation of, States Parties will be required in outbreak response
Improving multisectoral zoonotic risk reduction and assessment
COVID-19 has again shown the global implications of zoonotic spillover events and the potential scale of risk that zoonoses pose to human health, underpinning the importance of integrated approaches that frame preparedness and response activities through the interactions between (and at the interfaces of) animal, human, and environmental health, such as the One Health and Planetary Health movements. Whereas the IHR set out the core capacities for preparedness and the procedures and principles to enact between outbreak detection and notification, it does not include sufficient detail on assessing potential zoonotic risks before human cases or obligations to address the upstream drivers of spillover. Efforts have taken place to coordinate between relevant international organisations, such as WHO, the World Organisation for Animal Health, and the Food and Agriculture Organization, in a tripartite collaboration, and to improve surveillance using One Health approaches through the voluntary Joint External Evaluation tool for assessing public health capabilities related to the IHR. This tool expressly includes One Health surveillance targets designed to link early warning surveillance systems under both IHR and World Organisation for Animal Health obligations. However, international governance and individual national obligations remain largely fragmented among sectors and legal instruments, and stop short of the integration necessary for the increased zoonotic risk posed by the Anthropocene.80
In addition to commitments to mitigate the increasing pressures on zoonotic drivers, such as land use change and climate change,81, 82 special urgency exists for coordinated, evidence-based, and international governance for zoonotic risk.83, 84 This governance should precede the moment when cases are detected in humans (with a view to preventing spillovers) and include cross-sectoral shared systems,82 as well as obligations for local capacity building, regular and transparent surveillance and notifications, data sharing, and risk assessment.85 The establishment of the One Health High Level Expert Panel in May, 2021, by WHO, the World Organisation for Animal Health, the Food and Agriculture Organization, and the UN Environment Programme intends to build processes for the development of the scientific evidence base and for advancing policy advice for One Health. In addition, increasing recognition of planetary health approaches, which expressly address equity, sustainable development, and economic, social, and political drivers of health risks, serves to expand consideration of zoonotic risk in a wider context.86 Evolving surveillance technology through artificial intelligence, serology surveillance, and whole-genome sequencing offer promising and innovative ways to enhance the efficiency and speed of future outbreak detection. However, fragmented governance between human and animal health and inefficiently cohesive efforts in public health science and equity, access to pathogen samples and sequence data, and equitable sharing of benefits from their use are hindering progress.81 To address legal gaps, the international community should support the tripartite collaboration and translate the future work of the One Health High Level Expert Panel into national obligations and policy making.
Enhancing detection for high-impact respiratory pathogens
Recognising the threat of novel emerging diseases, the 2005 revision of the IHR (in the wake of the SARS outbreak) broadened the scope of surveillance obligations on States Parties to capture a range of public health threats, rather than setting a list of specific notifiable diseases. Articles 5 and 6 and Annexes 1 and 2 of the IHR outline surveillance and notification principles and guidance, but specificity on implementation is insufficient, such as nuances for types of events and the scope of domestic laws, policies, and processes that could serve as both enablers and barriers to rapid and comprehensive detection and notification. As shown during the COVID-19 pandemic, concerns regarding the operation and implementation of these articles were a particular concern. More specific and rigorous translational activities, including the use of epidemic simulations that test clinical, technical, legal, and governance capabilities, should be tested on the grounds such that deficiencies can be identified and corrected.
In addition to the formal requirements of the IHR, a process of voluntary Joint External Evaluation has been developed to support IHR capacity building. Although the Joint External Evaluation addresses countries' capacities that include both event-based and indicator-based surveillance, the events at the early stages of the emergence of SARS-CoV-2 cases in humans prove a crucial need for States Parties to rapidly adjust syndromic surveillance and switch to proactive surveillance, especially when initial cases indicate the possibility of a respiratory pathogen.
In the period immediately after the genetic sequencing of the new virus, broader syndromic case definitions and active surveillance might have identified what appears to have been a substantially greater number of COVID-19 cases and a greater extent of spread in all geographies than was recorded in the early phase of the pandemic. Estimating the true extent of infection was not possible until diagnostic testing was developed and case fatality rates estimated. Even after the development of diagnostic tests, estimates of the true extent of national and international spread appear to have been limited by narrow early screening criteria that focused primarily on direct travel history from Wuhan. This approach did not adequately take into account the speed and scale of globalised international travel, particularly during the Lunar New Year period. A more inclusive case definition of confirmed and suspected cases—for example, not restricting the case definition to travellers from Wuhan—might have resulted in earlier detection and response in countries, and in potentially more accurate estimates of the size of the outbreak. Early definitions and surveillance also did not appear to adequately factor in the possibility of human-to-human transmission, despite known mechanisms of transmission for high-impact respiratory pathogens such as influenza viruses and coronaviruses (eg, SARS-CoV and MERS-CoV). Given its quick spread and propensity for silent transmission, COVID-19 demands global disease detection adjustments that promptly consider possible asymptomatic transmission and cryptic viral circulation outside from original epicentres.
Future outbreaks of novel diseases with respiratory features should be approached with a rebuttable presumption of the risk of sustained human-to-human and asymptomatic transmission, which is consistent with a precautionary approach warranted by the potential scale of harm in not doing so. Rebutting this presumption should be investigated with urgency to avoid unnecessary disruption, and the full suite of clinical, epidemiological, and laboratory data about early possible cases should be actively collected.
Enhancing rapid and comprehensive notifications from States Parties
A rebuttable presumption of the risk of a high-impact respiratory pathogen should also extend to States Parties' obligations to notify WHO and to immediately and transparently share the full suite of clinical, epidemiological, and laboratory data about early possible cases. Although the sharing of these data is already a legally binding obligation under the IHR, as the chronology notes, waiting for a State Party to make (or be able to make) an Article 6 notification of a potential PHEIC might not be sufficiently timely to prevent the spread of potential high-impact respiratory viruses. In the absence of evidence and a specific presumption of the risk of a potential high-impact respiratory pathogen, the delays needed to gather this information to complete the Annex 2 questions can have substantial consequences. Whereas Annex 2 includes the immediate notification of SARS as a potential PHEIC, there is scope for Annex 2 to be amended, or Article 6 to be interpreted, to always include immediate notification of respiratory illness clusters as potential PHEICs, similarly to the requirement that any novel human influenza is reported as a potential PHEIC. Although flexibility under the IHR would enable a State Party to notify or seek consultation from WHO in such cases, this conceptual change might shift the current norms towards a presumption of cross-border risk. A similar purpose would be served if States Parties adopted new procedures for immediate notification of potential high-impact respiratory pathogens under a new pandemic treaty.
The chronology shows the complexity of the early stages of an outbreak, including WHO's limited power to act on other reports received. Other international legal regimes, in particular nuclear and chemical arms control regimes, have verification and investigatory powers. However, these powers largely serve as compliance and verification of breached obligations, and are also set to be used in the biological arms control space. Taking lessons from these regimes but focusing on promoting greater public health capacity to respond to an outbreak rather than on a punitive response, WHO should have full authority to investigate pathogens with cross-border potential within, for example, 3 days of an event being reported in any State Party, which could include the ability to deploy a public health outbreak investigation team, with a focus on rapid public health validation and response, and to publish initial findings. Any investigation mechanism must also consider the public health implications from potential unintended consequences of tightened domestic information controls, and the importance of protecting health-care workers' rights to share information about potential outbreaks.87
Developing a clear, transparent, and responsive global alert system
Earlier warnings and better information sharing about the risk posed by the outbreak might have had a substantial effect on the eventual extent of the pandemic. Considering the events detailed in the chronology, including the alarm given by some areas and governments in early January and the international spread confirmed by mid-January, an IHR Emergency Committee could have been convened earlier. Furthermore, the PHEIC declaration should have been made sooner, supported by the rapid growth in the number of cases in China and elsewhere, even over the course of the Emergency Committee's first 2-day meeting.
The procedures for PHEIC declarations should be clearer and more transparent. The delay in the PHEIC declaration can be partly attributed to the IHR themselves, which focus WHO's deliberations on whether the affected country has the capacity to contain the threat and whether the pathogen is likely to spread internationally. With better understanding of the situation through Emergency Committee deliberations that are more open than the current post-meeting summary statements, governments would be better able to assess their capabilities and vulnerability even if a PHEIC is not declared. PHEIC declarations are ultimately made by WHO's Director-General, who might take into account a broad range of factors in addition to the advice of an Emergency Committee. Among other considerations, the Emergency Committee should give greater weight to the global health consequences of not declaring a PHEIC, especially if there is a risk that the event involves a high-impact respiratory pathogen. PHEIC criteria as they have been interpreted by the Emergency Committee set a threshold that requires both the risk of the outbreak to be high, and a judgement that the State Party where the outbreak first occurs will not have the capacity to contain it. In this context, States Parties will naturally want to defend their capacity for response and might delay reporting outbreaks that might potentially be declared a PHEIC. However, the goal of pandemic containment would be better served by changing the incentives so that seeking international cooperation is rewarded as a sign of good global citizenship, rather than as an admission of poor capacity to deal with a novel public health threat.
The chronology also shows inconsistent messaging on the part of WHO over which countries were at risk. When WHO did recommend a PHEIC declaration on Jan 30, 2020, the Emergency Committee stated that a global coordinated effort was needed to enhance preparedness in other regions of the world that might need support. It also noted that further international spread could occur in any country and that all countries should be prepared to identify and contain it. In a news conference on the same day, WHO's Director-General said: “the main reason for this declaration is not because of what is happening in China, but because of what is happening in other countries. Our greatest concern is the potential for the virus to spread to countries with weaker health systems, and which are ill-prepared to deal with it”.65 With the focus on weaker health systems, high-income countries without previous experience with outbreaks of novel coronaviruses and with advanced health systems and higher preparedness scores might have been lulled into a false sense of security. Without adequate simulation exercises to test the systems in place, many countries were caught off-guard, without a clear understanding of what they were and were not capable of achieving. COVID-19 has shown that assumptions about disease emergence and transmission and capabilities to respond to infectious disease threats are not only incorrect but pose a risk to global health security.88
Promoting comprehensive and evidence-based global responses
Because many countries delayed public health responses beyond border screening—even after the PHEIC declaration—until COVID-19 had infected communities domestically, no guarantee exists that an earlier declaration alone would have substantially changed the international response. Although national responses varied substantially in the crucial first 6 weeks after the PHEIC declaration, they were mostly too slow and inappropriately tailored to prevent the pandemic. There are many reasons for this delayed response, including insufficient capacities, capabilities, and previous experience in some countries, imprecise evaluations of readiness, ambiguous communication of risk to high-income countries, distrust in scientific evidence, and insufficient political will in some of the most affected States Parties.3
Existing international governance and law give insufficient guidance and defined obligations for what measures States Parties should take after a PHEIC declaration. As per the IHR, WHO's Director-General must issue Temporary Recommendations to States Parties, but these are, by definition, non-binding, and no enforcement mechanism exists beyond WHO requesting States Parties to provide a public health justification where additional health measures substantially interfere with international traffic. Whether through IHR reform or in a new pandemic treaty, there is a compelling rationale for States Parties to be required to conduct immediate national risk assessments after a PHEIC declaration.
Furthermore, the Temporary Recommendations against travel restrictions were promptly ignored by many countries, which might also have given some States Parties a false sense of security and delayed appropriate national public health measures. The nuance and evidence base concerning travel restrictions for a high-impact respiratory pathogen has changed as a result of this pandemic,89 which can have implications for future outbreaks. States Parties might be more willing to delay notifying WHO of disease outbreaks to avoid the now more widely accepted necessity of travel restrictions, for example. The injunction of the IHR against unnecessary trade and travel restrictions are designed to prevent the use of public health emergencies as a tool to gain trade advantages. However, as the experiences of COVID-19 and other pandemics show, in some contexts the prompt restriction of travel can greatly reduce the growth of an epidemic, both within or between national borders. These experiences must be factored into any consideration of a potential pandemic treaty.
Before the COVID-19 pandemic, experts identified the insufficient evidence base for vital non-pharmaceutical interventions for a high-impact respiratory pathogen (eg, travel restrictions) as a global health security risk.5 With COVID-19 proving the crucial nature of non-pharmaceutical interventions as the first line of defence against a novel pathogen, all efforts must be made to establish which interventions are effective and under what circumstances. Given how the delays seen in many countries contributed to the exponential increase in global transmission, particularly during February and March, 2020, we recognise that a limitation of this Health Policy is the scarcity of systemic analysis of the timing and nature of national government actions after international alerts. However, a precise understanding of the communication and actions during the earliest stages of the pandemic has allowed the assessment of detection, alert, and notification systems in detail.
The case for a global pandemic treaty
Our analysis is of particular relevance to reforms to the international systems for pandemic preparedness, including consideration of a potential Framework Convention on Pandemic Preparedness and Response, which will be the topic of a Special Session of the World Health Assembly in November, 2021. The Independent Panel, the IHR Review Committee tasked with considering potential reforms to the IHR, and various States Parties are recommending that the international community considers a pandemic treaty.26 Our mapping of the chronology against IHR requirements shows a range of areas beyond the mandate or interpretation of current WHO instruments that should be considered for improvement of global pandemic governance. Different proposals have varying levels of political feasibility. However, given the worldwide impact of the COVID-19 pandemic and current political momentum towards a pandemic treaty, the international community has a vital and time-limited opportunity to reset the international system for pandemic preparedness and response. The importance of this international reset could not be clearer, and, once implemented, must not be left to languish: any reforms or new instruments that establish new States Parties obligations, mechanisms, or international organisation powers should be supported by an ongoing multilateral process, such as regular conferences of parties. Such regular meetings of parties to the IHR or a new pandemic treaty will be essential to build the norms and international trust needed for an international system capable of preparing for, and responding to, future novel health threats.26
A pandemic treaty presents the opportunity to enact comprehensive reform in pandemic preparedness and response. Building on the gaps identified in this analysis, we argue that four considerations exist that support the case for—and provide the foundation for items to include in—a global pandemic treaty.
First, a pandemic treaty centred on the principle of equity would be an important signal of international commitment to guard against the entrenchment of global division and injustice. The differing effects of public health measures between countries with different income levels and inequitable global vaccine access and distribution has resulted in a divergent response to COVID-19, wherein the pandemic threat recedes in wealthy countries with high vaccine coverage, but persists in resource-constrained settings, with the potential of becoming an endemic disease with frequently fatal consequences. A pandemic treaty is an opportunity not to only move beyond the prioritisation of notifications and national sovereignty under the IHR, but to develop and instil norms of equity, justice, and global public goods of pandemic preparedness and response. Second, a pandemic treaty could provide high-level complementarity to the IHR and any potential post-pandemic reforms and proactive multidisciplinary approaches to zoonotic risk. Third, the treaty would be an opportunity to establish greater accountability, outbreak support, and global access to vital public health information. Under Article 2(d) of the WHO Constitution, WHO technical assistance and emergency aid is limited to where it is requested or accepted by WHO Member States. The IHR is consistent with these procedures, where WHO's investigative powers are limited to offering consultations (Article 8). In addition, in drafting the IHR, WHO Member States carefully limited WHO powers, requiring that the organisation verify any reports from other sources, such as other States Parties and the media, with the affected State, and imposing confidentiality requirements unless the event is determined to be a PHEIC or evidence exists of risk of international spread (Article 11), pointedly preserving state sovereignty.90 In addition, no express requirement that States Parties share genetic sequence information for a pathogen exists under the obligation to share public health information established by Article 6. Although this gap could be addressed through interpretive guidance on Article 6 of the IHR by the World Health Assembly, a pandemic treaty would create a wider opportunity to endorse the goal of enhancing rapid, open, and ongoing sharing of sequence data for pathogen identification, genomic epidemiology, tracking of variants, and the development of diagnostics, therapeutics, and vaccines. Finally, development of a solid evidence base for non-pharmaceutical interventions must be factored into any consideration of an international systems reset. The pandemic treaty might serve as an opportunity to establish processes and entities—similar to the processes of the Intergovernmental Panel on Climate Change or Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services—tasked with building an evidence base for future global health risks and response measures,83 which recognises that although spillover events can be reduced, they are unlikely to be completely prevented. Nevertheless, transparent early alerts, building strong health systems, clear public health protocols, universal access to health care, coordinated and evidence-based global responses, and constant testing and revisions of systems and protocols might prevent the next outbreak from becoming a pandemic.
Conclusion
Several actions taken early in the COVID-19 pandemic merit recognition. Clinicians and laboratory staff in Wuhan were quick to investigate suspicious cases and collect and analyse samples. Researchers sequenced the genome of the novel virus rapidly, enabling its sharing with the international community and allowing a reliable test to become available quickly. Once WHO was alerted to the outbreak, the organisation took immediate steps to activate requests for more information and initiate a response. Although they caused severe hardship, the public health measures introduced in Wuhan from Jan 23, 2020, helped to drastically contain transmission in China, showing that public health measures could rapidly reduce transmission.
The collected evidence of the global devastation caused by SARS-CoV-2 clearly indicates much room for improvement exists to ensure a more rapid and efficient response in the future, particularly for potentially high-impact respiratory pathogens, which are most likely to cause the next pandemic. Information about cases and their characteristics could have been shared faster. There was insufficient clarity around the interpretation of IHR requests from WHO and other countries, and a complex interplay among the three levels of WHO (country, regional, and headquarters) and their interaction with the Chinese authorities. Few restrictions were in place in Wuhan before Jan 23, despite evidence that transmission was already occurring in other parts of the country and internationally. Most countries instituted containment measures only after substantial intra-country spread was evident. With the benefit of hindsight, the precautionary principle was not sufficiently exercised in assessing human-to-human and asymptomatic transmission or in the declaration of a PHEIC, despite the obvious concern of many health jurisdictions, especially those with recent memories of the consequences of recent epidemics, as they began to rapidly institute initial containment measures. Instituting movement restrictions earlier, before millions began travelling for Lunar New Year celebrations and other activities nationally and internationally, would have helped to curb transmission, as shown by the rapid reduction in transmission after these measures were implemented. When the outbreak was ultimately designated a PHEIC, many countries still did not respond adequately. In addition, the evidence base for control measures was insufficient, and guidance around control measures were frequently inconsistent. These are important lessons for future new international governance arrangements. Enhanced requirements should seek to ensure that all countries respond to PHEICs and protect their populations, as a bare minimum, by conducting urgent and comprehensive risk assessments. These efforts, alongside a commitment to open science and the rapid deployment of the best of scientific responses, are essential to reduce the spread, morbidity, and mortality of future emerging outbreaks.
The present world is highly interconnected, and deadly pathogens can spark potential pandemics in a matter of days. The COVID-19 pandemic not only revealed and exploited gaps in current disease detection, alert, and response mechanisms, but also plainly showed that a reset of the global health and health security system as a whole is required. A global pandemic treaty might be a key opportunity to secure these changes. The IHR can be interpreted as being based on an outdated and unfounded assumption that low-income countries are the weak links in global health security. COVID-19 disproved that assumption, wreaking havoc in some of the wealthiest and reportedly best-prepared countries. The pandemic has shown the immeasurable value of leadership and rapid, evidence-based decision making to activate national capacities, and the dire consequences when they are undervalued. This pandemic also provides a clear argument for rules and norms that enhance transparency, information sharing, and collaboration among countries for all international collective health and security. Responses to COVID-19 can be seen as a test for a future emerging infectious pathogen that could spread even more rapidly and be many times more devastating in health and economic effects. Collectively, the international system and countries have failed this test, proving the need for a new framework that assumes risk in all countries and demands universal commitment to rapid detection and fully transparent and timely communications and responses.
Declaration of interests
AP has worked as a consultant with WHO on unrelated projects; was a member of a WHO Technical Advisory Group related to the IHR and COVID-19; is principal investigator in a project funded by the Carnegie Corporation to examine legal elements of a potential pandemic treaty (grant number G-21-58414); and is principal investigator of a project funded by the US Centers for Disease Control to examine national responses to COVID-19 (grant number NU2HGH2021000469: Component 3). HL-Q has worked as a consultant with WHO on unrelated projects and is a member of the WHO Europe High Level Expert Consultation Group on COVID-19 strategies. MBa has worked as a consultant with WHO on projects concerning gaps in pandemic preparedness, a global hub for pandemic and epidemic intelligence, and investment in WHO. RMO has previously worked as a Consultant for WHO with the Violence Against Women Team on unrelated topics. MBo has worked as a consultant with WHO on unrelated projects. CM has worked as a consultant with WHO on unrelated projects and worked full time at the WHO Headquarters from 2000 to 2008. SMA has worked as a consultant with WHO on unrelated projects and is the Lead Project Director of a grant from The Rockefeller Foundation to examine the intersection of data, determinants of health, and decision making. NB has worked as a consultant to WHO through the Global Preparedness Monitoring Board, on a project related to polio assets and COVID-19, and on unrelated topics; and is consulting with the Nuclear Threat Initiative and Johns Hopkins University as they develop the Global Health Security Index (2021). SM has worked with the World Bank to help accelerate their COVID-19 rapid response, and as a Senior Advisor with the Bill and Melinda Gates Foundation. AN has worked as a staff member at WHO, including as Acting Director General and as a Country Representative; and worked as the Ambassador for Global Health for the Government of Sweden before being appointed to lead the Independent Panel secretariat. AK has done unpaid work for the Biden-Harris campaign on COVID-19 related school reopenings, and worked as an unpaid medical fellow on the Massachusetts Department of Public Health COVID-19 response. RP is the US Global Malaria Coordinator at the US Agency for International Development, but his views in this paper are based on his work with the Independent Panel, in which he served until Jan 31, 2021. GKW has served on the UN's High-Level Commission on Health Employment and Economic Growth. The views expressed herein do not necessarily reflect the views of the US Government or its departments and agencies. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
Data for the study was collected under the auspices of the Independent Panel for Pandemic Preparedness and Response. The analysis of this paper is separate from the Independent Panel's final report and has been facilitated by the Independent Panel Secretariat. The Secretariat of the Independent Panel for Pandemic Preparedness and Response is independent and impartial.
Contributors
SS, AP, HL-Q, AN, CM, and NB conceived and designed this Health Policy paper. SS, CM, RMO, NB, CN, EB, SM, RP, AK, SMA, MBo, MJ, and HL-Q collected the data. SS, CM, RMO, NB, CN, EB, MBo, MBa, and AP analysed the data and drafted the manuscript with input from all authors. All authors contributed to revising the manuscript.
Supplementary Material
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Inf Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nuclear Threat Initiative. Johns Hopkins Bloomberg School of Public Health Center for Health Security Global Health Security Index. 2019. https://www.ghsindex.org/wp-content/uploads/2019/10/2019-Global-Health-Security-Index.pdf
- 3.Independent Panel on Pandemic Preparedness and Response Final report of the Independent Panel on Pandemic Preparedness and Response. 2021. https://theindependentpanel.org/wp-content/uploads/2021/05/COVID-19-Make-it-the-Last-Pandemic_final.pdf [DOI] [PMC free article] [PubMed]
- 4.WHO . 3rd edn. World Health Organization; Geneva: 2008. International health regulations (2005) [Google Scholar]
- 5.Nuzzo J, Mullen L, Snyder M, Cicero A, Inglesby TV. Johns Hopkins Center for Health Security; Baltimore, MD: 2019. Preparedness for a high-impact respiratory pathogen pandemic. [Google Scholar]
- 6.WHO . World Health Organization; Geneva: 2021. WHO-convened global study of origins of SARS-CoV-2: China part. [Google Scholar]
- 7.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–e00220. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79 doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dorp L, Acman M, Richard D, et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020;83 doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isabel S, Graña-Miraglia L, Gutierrez JM, et al. Evolutionary and structural analyses of SARS-CoV-2 D614G spike protein mutation now documented worldwide. Sci Rep. 2020;10 doi: 10.1038/s41598-020-70827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Zai J, Zhao Q, et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020;92:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deslandes A, Berti V, Tandjaoui-Lambotte Y, et al. SARS-CoV-2 was already spreading in France in late December 2019. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amendola A, Bianchi S, Gori M, et al. Evidence of SARS-CoV-2 RNA in an oropharyngeal swab specimen, Milan, Italy, early December 2019. Emerg Infect Dis. 2021;27:648–650. doi: 10.3201/eid2702.204632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Rosa G, Mancini P, Bonanno Ferraro G, et al. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apolone G, Montomoli E, Manenti A, et al. Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy. Tumori. 2020 doi: 10.1177/0300891620974755. published online Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basavaraju SV, Patton ME, Grimm K, et al. Serologic testing of US blood donations to identify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–reactive antibodies: December 2019–January 2020. Clin Infect Dis. 2020;72:e1004–e1009. doi: 10.1093/cid/ciaa1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surkova E, Nikolayevskyy V, Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Respir Med. 2020;8:1167–1168. doi: 10.1016/S2213-2600(20)30453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren L-L, Wang Y-M, Wu Z-Q, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Huang Y. Exclusive: traceability of new coronavirus gene sequencing: when did the alarm sound? Caixin. Feb 26, 2020. https://web.archive.org/web/20200227094018/http:/china.caixin.com/2020-02-26/101520972.html in Chinese.
- 24.Petersen E, Hui D, Hamer DH, et al. Li Wenliang, a face to the frontline healthcare worker. The first doctor to notify the emergence of the SARS-CoV-2, (COVID-19), outbreak. Int J Infect Dis. 2020;93:205–207. doi: 10.1016/j.ijid.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Cui W, Zhang F. Who was the first doctor to report the COVID-19 outbreak in Wuhan, China? J Nucl Med. 2020;61:782–783. doi: 10.2967/jnumed.120.247262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO . World Health Organization; Geneva: 2021. WHO's work in health emergencies. [Google Scholar]
- 27.Associated Press . Associated Press; June 6, 2020. China delayed releasing coronavirus info, frustrating WHO.https://apnews.com/article/united-nations-health-ap-top-news-virus-outbreak-public-health-3c061794970661042b18d5aeaaed9fae [Google Scholar]
- 28.Wuhan Institute of Virology . Wuhan Institute of Virology, CAS; Jan 29, 2020. Wuhan Institute of Virology fully launches scientific research on novel coronavirus pneumonia.https://web.archive.org/web/20200414083649/http://www.whiov.ac.cn/xwdt_105286/zhxw/202001/t20200129_5494574.html in Chinese. [Google Scholar]
- 29.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The State Council Information Office of the People's Republic of China Fighting COVID-19: China in action. July 6, 2020. http://www.scio.gov.cn/zfbps/32832/Document/1681809/1681809.htm in Chinese.
- 32.Green A. Li Wenliang. Lancet. 2020;395:682. [Google Scholar]
- 33.Finance Sina Wuhan pneumonia of unknown cause has been isolated and tested, and the results will be announced as soon as possible. Finance Sina. Dec 31, 2020. https://finance.sina.cn/2019-12-31/detail-iihnzahk1074832.d.html?from=wap in Chinese.
- 34.ProMED Undiagnosed pneumonia—China (Hubei): request for information. Dec 30, 2019. https://scholar.harvard.edu/files/kleelerner/files/20191230_promed_-_undiagnosed_pneumonia_-_china_hu-_rfi_archive_number-_20191230.6864153.pdf
- 35.Taiwan Centers for Disease Controls The facts regarding Taiwan's email to alert WHO to possible danger of COVID-19. April 11, 2020. https://www.cdc.gov.tw/En/Bulletin/Detail/PAD-lbwDHeN_bLa-viBOuw?typeid=158
- 36.Wang CJ, Ng CY, Brook RH. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 37.Taiwan Today CDC implements extra inspection measures for Wuhan flights. Taiwan Today. Jan 2, 2020. https://taiwantoday.tw/news.php?unit=2,6,10,15,18&post=168773
- 38.The Government of the Hong Kong Special Administrative Region CHP closely monitors cluster of pneumonia cases on Mainland. Dec 31, 2019. https://www.info.gov.hk/gia/general/201912/31/P2019123100667.htm
- 39.WHO . World Health Organization; Geneva: 2020. Origin of SARS-CoV-2.https://apps.who.int/iris/bitstream/handle/10665/332197/WHO-2019-nCoV-FAQ-Virus_origin-2020.1-eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- 40.Lawrence SV. COVID-19 and China: a chronology of events (December 2019-January 2020) May 13, 2020. https://crsreports.congress.gov/product/pdf/r/r46354
- 41.WHO . World Health Organization; Geneva: 2020. Listings of WHO's response to COVID-19.https://www.who.int/news/item/29-06-2020-covidtimeline [Google Scholar]
- 42.@WHOWPRO Jan 4, 2020. https://twitter.com/whowpro/status/1213331054313885702
- 43.WHO . World Health Organization; Geneva: 2020. COVID-19—China.https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ [Google Scholar]
- 44.Zhang Y-Z. Novel 2019 coronavirus genome. January, 2020. https://virological.org/t/novel-2019-coronavirus-genome/319
- 45.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J. Chinese researchers reveal draft genome of virus implicated in Wuhan pneumonia outbreak. Science Magazine. Jan 11, 2020. https://www.sciencemag.org/news/2020/01/chinese-researchers-reveal-draft-genome-virus-implicated-wuhan-pneumonia-outbreak
- 47.Okada P, Buathong R, Phuygun S, et al. Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.8.2000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO . World Health Organization; Geneva: 2020. Novel coronavirus (2019-nCoV) situation report—1.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 [Google Scholar]
- 49.Tian H, Liu Y, Li Y, et al. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368:638–642. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Jan 21, 2020. First travel-related Case of 2019 novel coronavirus detected in United States.https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html [Google Scholar]
- 51.@WHO Jan 14, 2020. https://twitter.com/WHO/status/1217043229427761152?s=20
- 52.@WHOWPRO Jan 19, 2020. https://twitter.com/WHOWPRO/status/1218741294291308545
- 53.National Health Commission of the People's Republic of China The high-level expert group of the National Health Commission answers reporters' questions on the pneumonia epidemic caused by the new coronavirus. Jan 21, 2020. http://www.nhc.gov.cn/xcs/fkdt/202001/8d735f0bb50b45af928d9944d16950c8.shtml
- 54.WHO . World Health Organization; Geneva: 2020. Statement on the first meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)https://www.who.int/news/item/23-01-2020-statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- 55.WHO . World Health Organization; Geneva: 2020. Novel coronavirus (2019-nCoV) situation report—2.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200122-sitrep-2-2019-ncov.pdf?sfvrsn=4d5bcbca_2 [Google Scholar]
- 56.WHO . World Health Organization; Geneva: 2020. Novel coronavirus (2019-nCoV) situation report—3.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200123-sitrep-3-2019-ncov.pdf?sfvrsn=d6d23643_8 [Google Scholar]
- 57.Wang L, Yan B, Boasson V. A national fight against COVID-19: lessons and experiences from China. Aust N Z J Public Health. 2020;44:502–507. doi: 10.1111/1753-6405.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allam Z. Elsevier; Amsterdam: 2020. Surveying the Covid-19 pandemic and its implications. [Google Scholar]
- 59.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao X, Cheng S, Wu D, Wu T, Lin X, Wang C. Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nature. 2020;584:420–424. doi: 10.1038/s41586-020-2554-8. [DOI] [PubMed] [Google Scholar]
- 62.Kucharski AJ, Russell TW, Diamond C, et al. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. 2020;20:553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO . World Health Organization; Geneva: 2020. Updated WHO advice for international traffic in relation to the outbreak of the novel coronavirus 2019-nCoV.https://www.who.int/news-room/articles-detail/updated-who-advice-for-international-traffic-in-relation-to-the-outbreak-of-the-novel-coronavirus-2019-ncov-24-jan [Google Scholar]
- 64.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghebreyesus T. World Health Organization; Geneva: 2020. WHO Director-General's statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV)https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov) [Google Scholar]
- 66.WHO . World Health Organization; Geneva: 2020. Novel coronavirus (2019-nCoV) situation report—12.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200201-sitrep-12-ncov.pdf [Google Scholar]
- 67.Ritchie H, Mathieu E, Rodés-Guirao L, et al. COVID-19: international and domestic travel. Our World in Data. https://ourworldindata.org/covid-international-domestic-travel
- 68.Gámbaro F, Behillil S, Baidaliuk A, et al. Introductions and early spread of SARS-CoV-2 in France, 24 January to 23 March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.26.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alteri C, Cento V, Piralla A, et al. Genomic epidemiology of SARS-CoV-2 reveals multiple lineages and early spread of SARS-CoV-2 infections in Lombardy, Italy. Nat Commun. 2021;12:434. doi: 10.1038/s41467-020-20688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bedford T, Greninger AL, Roychoudhury P, et al. Cryptic transmission of SARS-CoV-2 in Washington state. Science. 2020;370:571–575. doi: 10.1126/science.abc0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilbert M, Pullano G, Pinotti F, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395:871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haider N, Yavlinsky A, Simons D, et al. Passengers' destinations from China: low risk of novel coronavirus (2019-nCoV) transmission into Africa and South America. Epidemiol Infect. 2020;148:e41. doi: 10.1017/S0950268820000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rostami A, Sepidarkish M, Leeflang MMG, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2020;27:331–340. doi: 10.1016/j.cmi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernard Stoecklin S, Rolland P, Silue Y, et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghebreyesus T. World Health Organization; Geneva: 2020. Report by the Director-General.https://apps.who.int/gb/ebwha/pdf_files/EB146/B146_2-en.pdf [Google Scholar]
- 77.WHO . World Health Organization; Geneva: 2020. WHO-AUDIO Executive Board EB146 coronavirus briefing.https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-executive-board-eb146-coronavirus-briefing-script-04feb2020-final.pdf?sfvrsn=70a66dfc_2 [Google Scholar]
- 78.Ghebreyesus T. World Health Organization; Geneva: 2020. WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 [Google Scholar]
- 79.European Centre for Disease Prevention and Control Timeline of ECDC's response to COVID-19. https://www.ecdc.europa.eu/en/covid-19/timeline-ecdc-response
- 80.Carlson CJ, Albery GF, Phelan A. Preparing international cooperation on pandemic prevention for the Anthropocene. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2020-004254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daszak P, das Neves C, Amuasi J, et al. Workshop report on biodiversity and pandemics of the intergovernmental platform on biodiversity and ecosystem services. Oct 29, 2020. https://zenodo.org/record/4158500#.YUoSvY70mUk
- 82.Dobson AP, Pimm SL, Hannah L, et al. Ecology and economics for pandemic prevention. Science. 2020;369:379–381. doi: 10.1126/science.abc3189. [DOI] [PubMed] [Google Scholar]
- 83.Carlson CJ, Phelan AL. A choice between two futures for pandemic recovery. Lancet Planet Health. 2020;4:e545–e546. doi: 10.1016/S2542-5196(20)30245-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daszak P, Keusch GT, Phelan AL, Johnson CK, Osterholm MT. Infectious disease threats: a rebound to resilience. Health Aff (Millwood) 2021;40:204–211. doi: 10.1377/hlthaff.2020.01544. [DOI] [PubMed] [Google Scholar]
- 85.Kreuder Johnson C, Hitchens PL, Smiley Evans T, et al. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci Rep. 2015;5 doi: 10.1038/srep14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation-Lancet Commission on planetary health. Lancet. 2015;386:1973–2028. doi: 10.1016/S0140-6736(15)60901-1. [DOI] [PubMed] [Google Scholar]
- 87.Davies SE. Infectious disease outbreak response: mind the rights gap. Med Law Rev. 2017;25:270–292. doi: 10.1093/medlaw/fwx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.WHO Global Preparedness Monitoring Board . World Health Organization; Geneva: 2020. A world in disorder: Global Preparedness Monitoring Board annual report 2020.https://www.preventionweb.net/publication/world-disorder-global-preparedness-monitoring-board-annual-report-2020 [Google Scholar]
- 89.Grépin KA, Ho T-L, Liu Z, et al. Evidence of the effectiveness of travel-related measures during the early phase of the COVID-19 pandemic: a rapid systematic review. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2020-004537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burci GL. Shifting norms in international health law. Am Soc Intl L Proc. 2004;98:16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.