Abstract
Objectives
Existing evidence produces conflicting findings regarding the effect of sesame intake on inflammatory biomarkers; this knowledge gap has yet to be met through systematic review and meta-analysis. This meta-analysis of randomized, controlled clinical trials (RCTs) was conducted to evaluate the effects of sesame consumption on markers of inflammation in humans.
Methods
PubMed, Scopus, and the Cochrane Database of Systematic Reviews were searched through August 2020 to identify relevant papers for inclusion. Using the random-effects model, data were evaluated as weighted mean differences (WMD) with 95% confidence intervals (CI). Cochrane's Q and I-squared (I2) tests were used to identify within-studies heterogeneity.
Results
Seven RCTs with 310 participants (157 intervention and 153 control) were included in the meta-analysis. Sesame consumption reduced serum level interleukin-6 (IL-6) (WMD − 0.90; 95% CI (−1.71, −0.09), I2 = 80.4%) compared to the control group. However, sesame intake had no significant effects on C-reactive protein (CRP) and tumor necrosis factor-α (TNF-α) compared to the control group. Subgroup analysis identified a reduction in serum CRP, TNF-α, and IL-6 concentration among studies with participants who had a higher level of these biomarkers at baseline, those which used sesamin capsules, and those with a bigger sample size, those conducted in Asia, and studies on females.
Conclusion
Sesame consumption reduced serum levels of IL-6 but did not affect CRP and TNF-α in humans. Additional trials should be conducted utilizing a larger and longer treatment duration, along with studies using different sesame formulations (capsule, oil, and seed) and conducting on participants with varied health conditions.
1. Introduction
Low-grade chronic inflammation, characterized by the chronically low levels of inflammatory biomarkers, is associated with type 2 diabetes, cardiovascular disease, aging, obesity, and different kinds of cancer [1–3]. Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) are among the most commonly studied inflammatory biomarkers [4]. IL-6, with both anti-inflammatory and pro-inflammatory properties, is produced by macrophages and T-cells [5, 6]. TNF-α is a pro-inflammatory cytokine produced by macrophages following inflammatory stimuli [7]. CRP, an acute-phase protein, is secreted from the liver [8] as a response to elevated concentrations of IL-6 and TNF-α [9]. Given the role of inflammation in the pathogenesis of chronic illness, anti-inflammatory agents such as exercise, antioxidants, and nonsteroidal drugs are practical preventive measures for chronic diseases [10, 11]. Dietary factors are also considered the main alternative treatments for low-grade inflammation [12, 13]. Among dietary factors, nutraceuticals and plant oils have garnered great attention due to the adverse effects and high cost of many medications [14, 15]. Several studies indicated anti-inflammatory effects of oil crop intake including flaxseed, olive, and canola oils both in Asian [16, 17] and Western countries [18, 19].
Sesame (Sesamum indicum) is a culinary ingredient that has been used in food preparation for thousands of years, especially in Asian countries [20]. The seeds contain an abundance of polyunsaturated fatty acids (PUFA), vitamin E, phytosterol, fiber, and bioactive lignans (sesamin, sesamol, sesamolin, and episesamin), which produce antioxidant and anti-inflammatory activity [21–23]. One of the most abundant lignans in sesame (sesamin) has been found to exhibit beneficial effects on inflammatory markers [24], hyperglycemia [25], hypertension [26], and hyperlipidemia [27] in humans. Sesamin is found in many sesame-containing products, including sesame oil, sesame seeds, sesame meals, and sesame-based supplements [28].
Evidence from animal studies reveals that sesamin improves hyperglycemia, reduces inflammation, and improves insulin resistance in diabetic mice [29]. However, human studies have produced conflicting results [24, 30–35]. While some studies found that sesame consumption reduces inflammatory biomarkers levels [33, 35], others did not find the same benefits [24, 34]. This study is the first systematic review and meta-analysis of randomized controlled trials, with the purpose of quantifying the overall effect of sesame consumption on inflammatory biomarkers in humans.
2. Materials and Methods
This systematic review and meta-analysis utilize the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [36]. The protocol was registered at the Center for Open Science Framework (OSF) database (https://www.osf.io, DOI: 10.17605/OSF.IO/TJ4N8).
2.1. Search Strategy
The search included online databases of PubMed, Scopus, and the Cochrane Database of Systematic Reviews continued until August 2020. It was conducted using medical subject headings (MESH) and relevant keywords of the following terms: (“Sesame∗” OR “Sesame Oil” OR “Sesame Seed” OR “Sesamum” OR “Sesamin supplement”) AND (“adiponectin” OR “leptin” OR “TGF-β” OR “high sensitive CRP” OR “IL-10” OR “IL-8” OR “TNF” OR “IL-6” OR “hs-CRP” OR “CRP” OR “Biological marker” OR “Systemic inflammation” OR “Interleukin” OR “Interleukin-1β” OR “adipokines” OR “myokine” OR “Neurogenic Inflammation” OR “Inflammation Mediator” OR “Monocyte chemotactic protein 1” OR “Intercellular adhesion molecule-1” OR “p-selectin” OR” eselectin” OR “Matrix metalloproteinase” OR “Acute phase reactant” OR “Cytokine” OR “Transforming growth factor beta” OR “C- Reactive protein” OR “Tumor necrosis factor” OR “Interleukin-6” OR “Interleukin-8” OR “Interleukin-10” OR “Inflammatory biomarker” OR “inflame”∗). Database search restrictions were not utilized. The Google Scholar, reference lists of the eligible RCTs, and related review articles were also assessed to identify RCTs that were not found in the online database search.
2.2. Eligibility Criteria
Randomized controlled clinical trials were included if they met the following criteria: (1) studies which evaluated the effects of consuming sesame or its products (sesame seed, sesame oil, or sesamin supplement) on serum concentrations of inflammatory biomarkers, including CRP, TNF-α, and IL-6; (2) studies which reported mean or median, standard deviation (SD), and 95% confidence interval on inflammatory biomarkers. Both parallel and crossover designs were included. It was excluded if a study did not evaluate serum inflammatory markers or included sesame in a combination of active ingredients. Two separate investigators (SR and AY) evaluated each article's title and abstract based on the inclusion/exclusion criteria. Discrepancies in data extraction were resolved by the principal investigator (LS).
2.3. Data Extraction
Data were extracted independently by two researchers (MAZ and RF) and included the following information: first author's surname, publication year, study location, participants health status, age (mean/range) and sex, number of participants, study design, duration of intervention, sesame product and dosage, characteristics of the placebo/control group, mean and standard deviation (SD) both at baseline and post-intervention. Data from studies that did not report inflammatory measures as SI units were converted to these measures.
2.4. Risk of Bias Assessment
The potential risk of bias for each included study was assessed using the Cochrane Collaboration's tool [37]. This tool evaluates factors such as blinding, loss of follow-up, random sequence generation, selective reporting of outcomes, and other potential sources of bias. Studies were ranked as having a high, unclear, or low risk of bias based on those criteria.
2.5. Statistical Analysis
Effect sizes, which were calculated from the mean and standard deviations of the change in inflammatory markers, were pooled using a random-effects model and reported as weighted mean difference (WMD) and 95% confidence interval. Mean changes were calculated if the study did not provide those data. The formula used for calculating mean changes was as follows: final mean value minus baseline mean value. Standard deviations were calculated as SDChange = [38]. A correlation coefficient equal to 0.8 was considered as R-value when calculating SD change [38]. Data that was reported as median and interquartile ranges was converted to means and standard deviations with the method described by Hozo et al. [39]. If required, confidence intervals were converted to standard deviations using the formula [38]. To identify heterogeneity, we calculated I2, and values above 50% were interpreted as evidence of heterogeneity. We also conducted subgroup analysis using predefined factors. These included the type of sesame product (i.e., seed, oil, and supplement), baseline serum values, study size, duration, geographic region, sex, and baseline BMI. Sensitivity analyses were conducted to assess the role of each study in the final effect size. Egger's test and visual inspection of funnel plots were used to identify the potential for publication bias [40]. All statistical procedures were conducted using STATA (Version 12.0, Stata Corp, College Station, TX, USA), and statistical significance was defined as P < 0.05.
3. Results
3.1. Study Selection
The online database search produced 632 relevant manuscripts, and no additional documents were identified through the evaluation of reference lists. Removal of 210 duplicate manuscripts left 422 abstracts to screen. After screening, 28 studies remained for full-text evaluation. This process eliminated an additional 21 studies: irrelevant outcomes (n = 14); review articles (n = 2); animal research (n = 3); a combination of sesame with other substances (n = 1); and failure to evaluate serum biomarkers (n = 1). A total of seven studies remained for this analysis (Figure 1).
Figure 1.
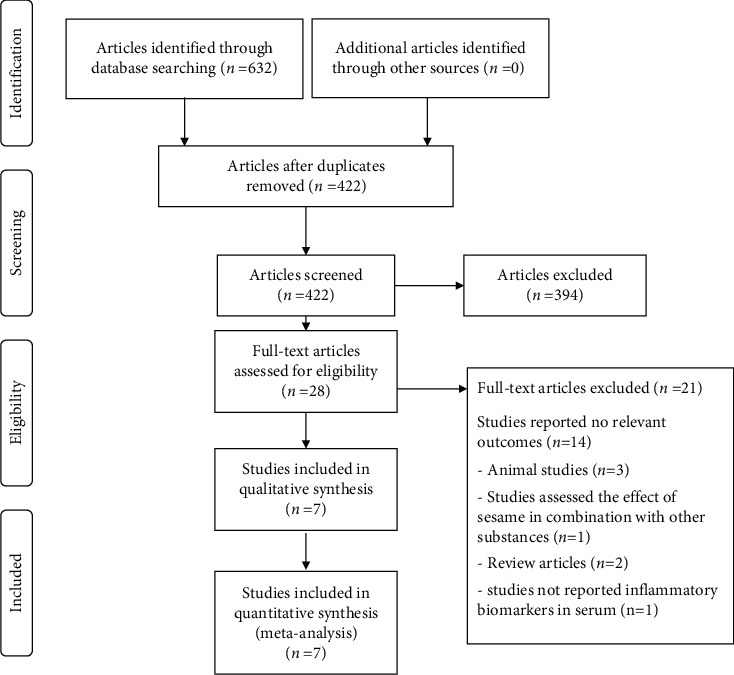
Study selection diagram.
3.2. Study Characteristics
All the identified studies were RCTs published between 2009 and 2019 (see Table 1). The studies reflected 157 participants in treatment groups and 153 participants in control groups and had interventions ranging from 4 weeks to 8 weeks. The 310 participants were aged 16 to 70 years. Two of the seven trials only included males [30, 34], one only included females [33], and the other four trials included both sexes [24, 31, 32, 35]. Four studies were conducted in Iran [31, 33, 35], one in Brazil [30], one in Greece [34], and one study in Australia [24]. Different sesame products were used for the intervention: sesame oil was used in two studies [31, 34], three studies used sesame seeds [24, 30, 32], and two studies used sesamin-based supplement [33, 35]. Placebo materials also varied; options included starch capsules [33, 35], sunflower oil [31], a combination of honey, maltodextrin, milk, and caramel coloring [30], and glucosamine [32]. One study did not identify which placebo materials were used [24], and another study did not use the standard of care (regular diet) rather than a placebo [34]. None of the studies reported any significant side effects. Patient's health among the studies varied, one study used patients with type 2 diabetes [35], one used patients with hypertension [34], one used patients with metabolic syndrome [31], one used overweight patients [24], one used semiprofessional athletes [30], one used patients with rheumatoid arthritis [33], and one used patients with knee osteoarthritis [32]. Six of the trials used a parallel design [30–35], and the other used a crossover design [24].
Table 1.
Characteristics of randomized trials on the effects of sesame consumption on inflammatory biomarkers in humans included in the meta-analysis.
| Reference | Location | Subjects and gender | Age, y1 | Design | Intervention type | Duration (wk) | Outcomes | Outcome2 | Notes about subjects | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control (name and composition) | Intervention | Control | ||||||||
| Helli et al. [33] | Iran | F : 44 | S + C: | Parallel | Sesamin capsule (200 mg/d) | Placebo capsules (starch) | 6 | hs-CRP | hs-CRP (mg/L) : before: 22.28 ± 5.85; after: 17.33 ± 4.05 | hs-CRP (mg/L) : before: 20.74 ± 7.43; after: 21.91 ± 7.81 | 44 patients with rheumatoid arthritis |
| S : 22 | 55.49 ± 5.98 | TNF-α | TNF (ng/L) : before: 2.17 ± 0.71; after: 1.67 ± 0.58 | TNF (ng/L) : before: 2.03 ± 0.82; after: 2.06 ± 0.36 | |||||||
| C : 22 | IL-6 | IL-6 (ng/L) : before: 4.84 ± 3.67 after: 3.45 ± 1.69 | IL-6 (ng/L) : before: 4.38 ± 2.67; after: 4.58 ± 1.85 | ||||||||
|
| |||||||||||
| Farajbaksh et al. [31] | Iran | M : 16 | 30–70 yrs | Parallel | Sesame oil (30 mL/d) | Placebo (sunflower oil) | 8 | hs-CRP | hs-CRP (mg/L) : before: 43.8 ± 13.4; after: 39.6 ± 17 | Hs-CRP (mg/L) : before: 43.5 ± 17.4; after: 43.9 ± 20.1 | 47 patients with metabolic syndrome |
| F : 31 | S : 48.04 ± 7.67 | ||||||||||
| S : 24 (7/17) | C : 50.17 ± 7.6 | ||||||||||
| C : 23 (9/14) | |||||||||||
|
| |||||||||||
| Da silva barbosa et al. [30] | Brazil | M : 20 | 16–18 yrs | Sesame seed (40 g/d) | Placebo (honey, maltodextrin, cow milk, and artificial caramel food coloring) | 4 | hs-CRP | hs-CRP (mg/L) : before: 3.66 ± 1.4; after: 0.8 ± 1.0 | hs-CRP (mg/L) : before: 3.31 ± 1.8; after: 0.6 ± 0.4 | 20 semiprofessional soccer players | |
| S : 10 | |||||||||||
| C : 10 | |||||||||||
|
| |||||||||||
| Mohammad Shahi et al. [35] | Iran | F + M: 48 | 30–60 yrs | Parallel | Sesamin capsule (200 mg/d) | Placebo capsules (starch) | 8 | hs-CRP | hs-crp (mg/L) : before: 2.83 ± 1.35; after: 2.53 ± 1.44 | Hs-CRP (mg/L) : before: 2.80 ± 1.25; after: 2.92 ± 1.3 | 44 type 2 diabetes mellitus |
| S : 24 | S : 50 ± 12.3 | TNF-α | TNF (ng/L) before: 1.92 ± 0.76; after: 1.3 ± 0.27 | TNF (ng/L) : before: 1.66 ± 0.76; after: 1.86 ± 0.9 | |||||||
| C : 24 | C : 51.72 ± 12.24 | IL-6 | IL-6 (ng/L) : before: 20.19 ± 12.1; after: 17.2 ± 9.13 | IL-6 (ng/L) : before: 21.26 ± 10.73 after: 22.19 ± 11.14 | |||||||
| IL-6 | Adiponectin (µg/mL) : before: 6.21 ± 1.33; after: 7.34 ± 2.88 | Adiponectin (µg/L) : before: 6.60 ± 1.62; after: 6.19 ± 1.10 | |||||||||
|
| |||||||||||
| Haghighian et al. [32] | Iran | F + M: 45 | 50–70 yrs | Parallel | Sesame seed (40 g/d) + acetaminophen | Placebo (glucosamine) + acetaminophen | 8 | hs-CRP | hs-CRP (mg/L) : before: 1.45 ± 1.12; after: 1.42 ± 1.32 | hs-CRP (mg/L) : before: 1.64 ± 1.19; after: 1.68 ± 0.87 | 45 patients with knee osteoarthritis |
| S : 22 | S : 56.90 ± 6.39 | IL-6 | IL-6 (ng/L) : before: 2.29 ± 0.82; after: 0.38 ± 0.05 | IL-6 (ng/L) : before: 2.43 ± 0.68; after: 1.53 ± 0.04 | |||||||
| C : 23 | C : 58.27 ± 7.84 | ||||||||||
|
| |||||||||||
| Karatzi et al. [34] | Greece | M : 30 | S : 49.8 ± 8.46 | Parallel | Sesame oil (35 g/d) | Control | 8 | CRP | CRP (mg/L) : before: 1.8 ± 1.8; after: 2.0 ± 2.3 | CRP (mg/L) : before: 1.9 ± 2.3; after: 2.0 ± 2.5 | 30 hypertension men |
| S : 17 | C : 56.8 ± 12 | TNF-α | TNF (ng/L) : before: 0.77 ± 1.0; after: 0.96 ± 1.5 | TNF (ng/L) : before: 0.31 ± 0.51; after: 0.54 ± 0.61 | |||||||
| C : 13 | |||||||||||
|
| |||||||||||
| Wu et al. [24] | Australia | F + M: 3 | S: 54.7 ± 8.6 | Crossover | Sesame seed (25 g/d) | Placebo | 5 | hs-CRP | hs-CRP (mg/L) : before: 2.1 ± 2.5; after: 1.89 ± 2.06 | hs-CRP (mg/L) : before: 1.98 ± 2.29; after: 2.02 ± 2.42 | 38 overweight men and women |
| S : 38 | C : 54.7 ± 8.6 | TNF-α | TNF-α (ng/L) : before: 1.85 ± 0.59; after: 1.83 ± 0.58 | TNF-α (ng/L) : before: 1.83 ± 0.61; after: 1.8 ± 0.59 | |||||||
| C : 38 | IL-6 | IL-6 (ng/L) : before: 2.08 ± 1.16; after: 2.05 ± 1.03 | IL-6 (ng/L) : before: 2.14 ± 1.3; after: 2.25 ± 1.33 | ||||||||
1Values are overall ranges and means ± SDs in each group. 2Values are means ± SDs. C: control; CRP: C-reactive protein; F: female; hs-CRP: highly sensitive C-reactive protein; IL-6: interleukin-6; M: male; S: sesame; and TNF-α: tumor necrosis factor-α.
3.3. Assessment of the Risk of Bias
After assessing the quality of studies using the six domains of the Cochrane Collaboration's tool, five studies were found to be of high quality, while the others were classified as fair. All studies described which method was used for randomization. Two studies had poor allocation concealment, and five studies were single- or double-blinding (Table 2).
Table 2.
Cochrane's risk of bias assessment for randomized controlled trials on the effect of sesame consumption on inflammatory biomarkers in human1.
| Reference | Random sequence generation | Allocation concealment | Blinding of participants, personnel, and outcome assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Helli et al. [33] | L | L | L | L | L | L |
| Farajbakhsh et al. [31] | L | H | L | L | L | L |
| Da Silva Barbosa et al. [30] | L | H | L | L | L | L |
| Mohammad Shahi al. [35] | L | H | L | L | L | L |
| Haghighian et al. [32] | L | H | H | L | L | L |
| Karatzi et al. [34] | L | H | H | L | L | L |
| Wu et al. [24] | L | L | L | L | L | L |
1H: high risk of bias; L: low risk of bias; and U: unclear risk of bias.
3.4. Meta-Analysis
3.4.1. Findings on the Effect of Sesame on CRP
Combining the effect sizes from 7 studies, we did not find a significant reduction in serum CRP concentrations after sesame consumption, as compared to control group [weighted mean difference [Weighted Mean Difference (WMD) − 0.55; 95% CI (−1.22, 0.12), I2 = 75.3%] (Figure 2). The subgroup analysis found that baseline serum CRP levels, baseline BMI, intervention type, duration of treatment, geographic region, sample size, and sex are all sources of heterogeneity. Subgroup analysis also found that CRP was significantly reduced in studies which were conducted in Iran [WMD – 1.38; 95% CI (−2.70, −0.06), P=0.039], studies in which participants had higher baseline serum CRP (≥10 mg/L) [WMD – 5.91; 95% CI (−8.25, −3.58), P < 0.01], studies conducted on females [WMD – 6.12; 95% CI (−8.63, −3.61), P < 0.001], and studies with a larger sample size (≥40) [WMD – 1.38; 95% CI (−2.70, −0.06), P=0.039] (Table 3).
Figure 2.
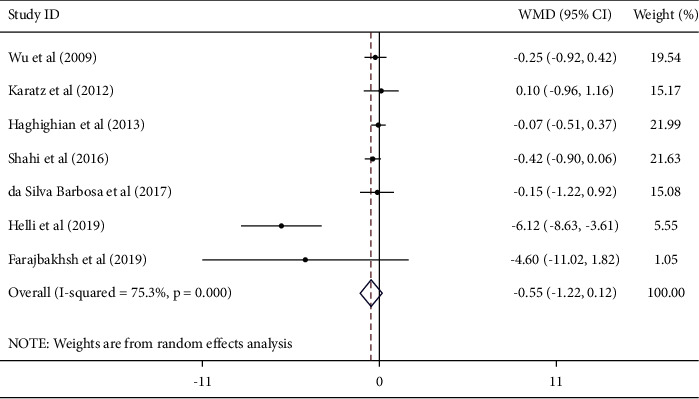
Forest plot showing the effects of sesame consumption on circulating CRP (WMDs and 95% CIs) in humans using the random-effects model. CI: confidence interval; CRP: C-reactive protein; and WMD: weighted mean difference.
Table 3.
Pooled estimates of the effects of sesame consumption on inflammatory biomarkers within different subgroups3.
| Number of trials | WMD (95% CI) | P value | P-heterogeneity | I 2 (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | TNF | IL-6 | CRP | TNF | IL-6 | CRP | TNF | IL-6 | CRP | TNF | IL-6 | CRP | TNF | IL-6 | |
| Total | 7 | 4 | 4 | −0.55 (−1.22, 0.12) | −0.34 (−0.77, 0.08) | −0.90 (−1.71, −0.09) | 0.109 | 0.111 | 0.029 | <0.001 | <0.001 | 0.002 | 75.1 | 88.4 | 80.4 |
|
| |||||||||||||||
| Baseline serum biomarker¹ | |||||||||||||||
| <10 mg/L | 5 | — | 2 | −0.20 (−0.48, 0.06) | — | −0.56 (−1.41, 0.28) | 0.138 | — | 0.193 | 0.831 | — | 0.158 | 0 | — | 49.8 |
| ≥10 mg/L | 2 | — | 2 | −5.91 (−8.25, −3.58) | — | −1.94 (−3.57, −0.30) | <0.001 | — | 0.020 | 0.666 | — | 0.029 | 0 | — | 78.9 |
|
| |||||||||||||||
| Baseline BMI | |||||||||||||||
| ≥30 kg/m2 | 3 | 2 | 1 | −3.40 (−8.16, 1.35) | −0.24 (−0.77, 0.28) | −0.73 (−2.13, 0.66) | 0.161 | 0.363 | 0.303 | <0.001 | 0.002 | — | 90.5 | 89.5 | — |
| <30 kg/m2 | 4 | 2 | 3 | −0.19 (−0.49, 0.10) | −0.45 (−1.21, 0.31) | −1.75 (−4.23, 0.73) | 0.193 | 0.245 | 0.167 | 0.693 | 0.008 | 0.266 | 0 | 85.9 | 24.5 |
|
| |||||||||||||||
| Type of intervention | |||||||||||||||
| Sesame capsule | 2 | 2 | 2 | −0.62 (−1.09, −0.15) | −0.67 (−0.95, −0.38) | −1.94 (−3.57, −0.30) | 0.271 | <0.001 | 0.020 | <0.001 | 0.190 | 0.020 | 94.8 | 41.9 | 14.9 |
| Sesame seed | 3 | 1 | 2 | −0.12 (−0.47, 0.22) | 0.01 (−0.15, 0.17) | −0.56 (−1.41, 0.28) | 0.476 | 0.907 | 0.193 | 0.908 | — | 0.193 | 0 | — | 89.8 |
| Sesame oil | 2 | 1 | — | −0.02 (−1.07, 1.02) | −0.04 (−0.52, 0.44) | — | 0.582 | 0.871 | — | 0.157 | — | — | 50.1 | — | — |
|
| |||||||||||||||
| Duration of treatment | |||||||||||||||
| <8 wk | 3 | 2 | 2 | −1.73 (−3.92, 0.45) | −0.24 (−0.77, 0.28) | −0.73 (−2.13, 0.66) | 0.120 | 0.363 | 0.303 | <0.001 | 0.002 | 0.029 | 90.1 | 89.5 | 78.9 |
| =8 wk | 4 | 2 | 2 | −0.21 (−0.55, 0.12) | −0.45 (−1.21, 0.31) | −1.75 (−4.23, 0.73) | 0.216 | 0.245 | 0.167 | 0.356 | 0.008 | 0.158 | 7.5 | 85.9 | 49.8 |
|
| |||||||||||||||
| Geographic region | |||||||||||||||
| Asia | 4 | 2 | 3 | −1.38 (−2.70, −0.06) | −0.67 (−0.95, −0.38) | −1.23 (−1.95, −0.52) | 0.039 | <0.001 | 0.001 | <0.001 | 0.190 | 0.266 | 87.3 | 41.9 | 24.5 |
| Non-Asia | 3 | 2 | 1 | −0.14 (−0.65, 0.35) | 0.005 (−0.15, 0.16) | −0.14 (−0.48, 0.20) | 0.559 | 0.955 | 0.428 | 0.861 | 0.848 | — | 0 | 0 | — |
|
| |||||||||||||||
| Sample size | |||||||||||||||
| <40 | 3 | 2 | 1 | −0.14 (−0.65, 0.35) | 0.005 (−0.15, 0.16) | −0.14 (−0.48, 0.20) | 0.559 | 0.955 | 0.428 | 0.861 | 0.848 | 0.428 | 0 | 0 | — |
| ≥40 | 4 | 2 | 3 | −1.38 (−2.70, −0.06) | −0.67 (−0.95, −0.38) | −1.23 (−1.95, −0.52) | 0.039 | <0.001 | <0.001 | <0.001 | 0.190 | 0.001 | 87.3 | 41.9 | 24.5 |
|
| |||||||||||||||
| Sex | |||||||||||||||
| Men | 2 | 1 | − | −0.02 (−0.77, 0.72) | −0.04 (−0.52, 0.44) | — | 0.950 | 0.871 | — | — | — | — | 0 | — | — |
| Women | 1 | 1 | 1 | -6.12 (−8.63, −3.61) | −0.53 (−0.82, −0.23) | −1.59 (−2.84, −0.33) | <0.001 | 0.001 | 0.013 | 0.411 | — | — | — | — | — |
| Both | 4 | 2 | 3 | −0.24 (−0.53, 0.04) | −0.39 (−1.20, 0.41) | −0.72 (−1.62, 0.17) | 0.103 | 0.342 | 0.116 | 0.745 | <0.001 | 0.002 | 0 | 95.2 | 84.1 |
1<10 mg/L, ≥10 mg/L for CRP, <2 ng/L, ≥2 ng/L for TNF, <3 ng/L, ≥3 ng/L for IL-6, 2BMI: body mass index; CI: confidence interval; CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; and WMD: weighted mean difference.
3.4.2. Effect of Sesame on IL-6
Based on the findings of 4 studies, a significant reduction in serum IL-6 was found after sesame consumption [WMD − 0.90; 95% CI (−1.71, −0.09), I2 = 80.4%] (Figure 3). According to subgroup analyses, intervention type, baseline serum values of IL-6, geographic region, baseline BMI, duration of treatment, and sample size are all sources of heterogeneity. Subgroup analysis also found that sesame consumption decreased serum IL-6 in studies which used sesamin capsule and in studies of participants with higher baseline serum IL-6 (≥2 ng/L) [WMD – 1.94; 95% CI (−3.57, −0.30), P=0.020], studies conducted in Iran, studies with larger sample sizes (≥40) [WMD – 1.23; 95% CI (−1.95, −0.52), P < 0.001], and studies conducted on females [WMD – 1.59; 95% CI (−2.84, −0.33), P=0.013] (Table 3).
Figure 3.
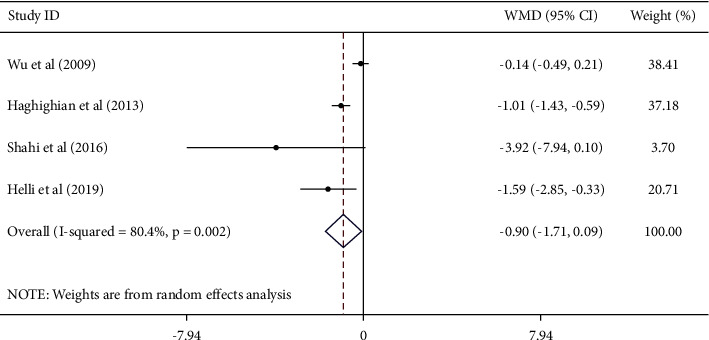
Forest plot showing the effects of garlic supplementation on circulating IL-6 (WMDs and 95% CIs) in adults using the random-effects model. CI: confidence interval; IL-6: interleukin-6; and WMD: weighted mean difference.
3.4.3. Effect of Sesame on TNF-α
Pooling the effect sizes from 4 studies, we failed to find a significant reduction in serum TNF-α concentrations after sesame consumption [WMD − 0.35; 95% CI (−0.78, 0.08), I2 = 88.4%] (Figure 4). Subgroup analysis identified intervention type, geographic region, and sample size as the sources of heterogeneity. Subgroup analysis also found that sesame consumption had a significant effect on TNF-α in studies which were conducted in Iran, used sesamin supplementation, had larger samples (≥40) [WMD − 0.67; 95% CI (−0.95, −0.38), P < 0.001], and studies conducted on females [(WMD) – 0.53; 95% CI (−0.82, −0.23), P=0.001] (Table 3).
Figure 4.
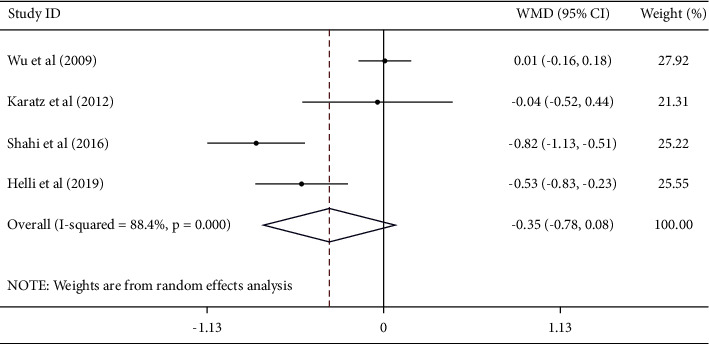
Forest plot showing the effects of sesame consumption on circulating TNF-α (WMDs and 95% CIs) in humans using the random-effects model. CI: confidence interval; TNF-α: tumor necrosis factor-α; and WMD: weighted mean difference.
3.5. Sensitivity Analysis
A sensitivity analysis was conducted to identify the size of the influence of each study on the overall effect size produced. This analysis found that none of the studies significantly affected the outcome (Supplementary Figures 1–3).
3.6. Publication Bias
Publication bias was assessed using visual inspection of funnel plots and the Egger test. Inspection of the funnel plots did not provide any evidence of publication bias (Supplementary Figures 4–6). These observations were confirmed by Egger's linear regression for CRP (P=0.12), IL-6 (P=0.31), and TNF-α (P=0.44).
4. Discussion
The present meta‐analysis showed that sesame and the consumption of its products could lower serum IL‐6 levels but did not affect serum concentrations of CRP and TNF-α in humans. Besides, a significant reduction was identified in serum CRP, TNF-α, and IL-6 concentration among studies with participants who had a higher level of these biomarkers at baseline, used sesamin capsules, had a larger sample size, were conducted in Asia, and studies with female participants. This is the first systematic review and meta‐analysis to identify the effects of sesame supplementation on serum inflammatory biomarkers.
We found that serum IL-6 decreased followed by sesame consumption. In line with our result, Hsu et al. [41] revealed that administration of different dosages of sesame oil (0, 1, 2, or 4 mL/kg, orally) decreased IL-6 and TNF-α in the rat. Another study in microglia cells showed neuroprotective influences of sesamin supplementation by its effects on lowering mRNA levels of IL-6 [42]. Chaval and Forse indicated that mice fed the diet that contained 1% sesamol had lower serum levels of IL-6 and PGES2 in comparison to the control mice [43]. One randomized double-blind, placebo-controlled showed daily consumption of a low-fat muffin plus flaxseed lignan complex (500 mg/d of secoisolariciresinol diglucoside) for 6 weeks did not decrease IL-6 compared to a low-fat muffin in 22 healthy postmenopausal women [44]. The level of lignans in sesame is even higher than in flaxseed, which was previously considered the richest source of lignans [45]. One animal study indicated more beneficial effects of the sesame seed lignan in lowering breast tumor growth compared to flaxseed lignan [46].
We did not find significant effects of total sesame consumption on serum CRP and TNF-α concentrations. In consistency with our results, an in vitro study showed supplementation for 12 weeks with 18 g/d of sesame oil did not have a significant effect on TNF-α and PGE-2 in cultured blood of healthy male volunteers [47]. In addition, supplementation with 25 g/day sesame seeds for 5 weeks had no beneficial effects on CRP, TNF-α, and IL-6 in overweight or obese men and women [24]. In contrast to our findings, one RCT study indicated that 0.5 mL/kg/day sesame oil consumption along with interferon beta-1a decreased TNF-α measured in supernatants of peripheral blood mononuclear cells in patients with multiple sclerosis compared to patients who only received interferon beta-1a [48]. In one randomized clinical trial study, supplementation with 360 mg/d flaxseed-derived lignin for twelve weeks decreased C-reactive protein in 39 diabetic women [49]. In an animal study conducted by Chiang et al., sesamin significantly reduced the serum CRP, TNF-α, and IL-6 levels in the rat with liver injury received 10 mg/kg sesamin orally [50]. These inconsistent findings might be partially due to different sources of sesame, geographic region, sex, and study sample size.
Using sesame as sesamin capsule decreases CRP, IL-6, and TNF-α significantly. This may be due to the high bioavailability of sesame lignan in sesamin capsules compared to sesame seed or sesame oil. In addition, our study also showed that sesame intake lowered CRP, IL-6, and TNF-α in studies in which participants had higher baseline serum levels of these markers. Rheumatoid arthritis, knee osteoarthritis, lupus erythematosus, and multiple sclerosis are chronic inflammatory systemic diseases (CIDs) with high serum inflammatory biomarkers [51].
The participants of most included studies had normal serum levels of inflammatory markers at the baseline. Therefore, this may be a reason for observing no significant effects in the meta-analysis. Besides, this study also indicated that sesame intake lowers CRP and IL-6 among participants with high baseline serum levels of these markers. Because nearly all participants had normal serum TNF-α, we could not perform subgroup analysis for this biomarker. Although obese individuals have higher concentrations of inflammatory biomarkers than normal weight subjects, this study did not show any anti-inflammatory effects of sesame in both obese (BMI ≥ 30 Kg/m2) and non-obese (BMI < 30 Kg/m2) persons due to a limited number of studies in each subgroup. Furthermore, the duration of eligible studies (≤8 weeks) might not be long enough to see the possible effects on inflammatory markers. Moreover, sesame is a commonly used oil, especially in Asian countries [20]. The studies conducted in Iran show that sesame consumption declined inflammatory biomarkers. Another reason for failing to find a significant association may be a low number of participants.
Several mechanisms of action have been identified as responsible for the anti-inflammatory effects. Sesamol (3–100 μM) was found to lessen the production of nitric oxide (NO) and pro-inflammatory cytokines, suppress the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and promote Nrf2, an antioxidant pathway. It was also found to block the mitogen-activated protein kinase (MAPK) and NF-κB pathways and decrease reactive oxygen species production, which decreased the inflammatory response [52]. In a study by Chu and colleagues, one subcutaneous injection of sesamol (10 mg/kg, s.c.) was found to significantly reduce the levels of NF-κB, iNOS, and interleukin 1β (IL-1β) [53]. Another study revealed that sesamol (5–20 μM) significantly inhibits the expression of several matrix metalloproteinases (MMPs), TNFα- and IL-1β-induced gelatinolysis of MMP-9, MMP-1, and MMP-13 expression, IL-1β- and phosphorylation of ERK1/2 or p38 MAPKs in SW1353 cells, a human chondrosarcoma cell line [54]. The same study revealed that oral administration of sesamol (30 mg/kg, p.o.) for 2 weeks attenuated MMP-1 and MMP-9 expression in the cartilage of monosodium iodoacetate (MIA)-induced osteoarthritis in male Wistar rats [54]. Sesamol (0.1–1.0 mg/kg, p.o.) also inhibited the activation of mucosal NF-κB and attenuated the recruitment of inflammatory cells, including CD68 + Kupffer cells and neutrophils [55]. Noteworthy, Chang and colleagues demonstrated that sesamol (2.5–25 μM) treatment in platelets significantly decreased collagen-induced phosphorylation of IκB kinase β (IKKβ) [56] (Figure 5).
Figure 5.
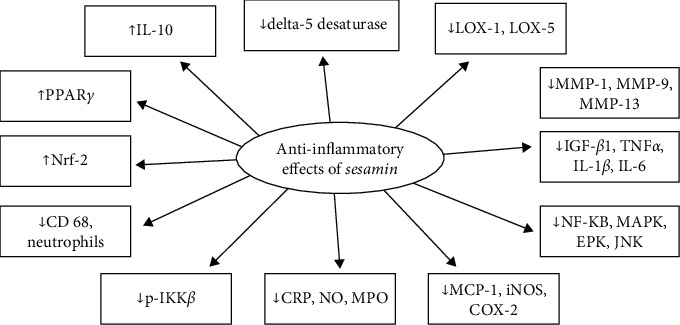
The mechanism of sesame reducing inflammation.
This study has several limitations. The total number of clinical trials that qualified for this analysis was small, and more studies are required to deepen our understanding. Additionally, this analysis includes studies conducted on a wide range of dosing protocols, participant ages, underlying health status, and duration. We tried to detect the source of heterogeneity by subgroup analysis. However, because studies were performed in participants with a different health condition and limited number of studies for each subgroup, we could not perform subgroup analysis according to health status. The overall result may have been influenced by the differences in dosing and sourcing of sesame. Subgroup analysis to identify dose-dependence or contrast groups by dosing was not feasible due to variations in the sesame preparations and the difficulty of obtaining accurate dose conversions. In addition, most of the trials included in this analysis were conducted in Asian countries and were small-scale studies assessing effects elsewhere. Finally, the high risk of random allocation and blinding biases found in some studies resulted in lower scientific evidence.
5. Conclusion
In conclusion, the consumption of sesame can significantly reduce serum IL-6 levels without any effects on CRP and IL-6. The small numbers of related RCTs and high amounts of heterogeneity among these studies limit generalizability. We recommend that additional high-quality RCTs be conducted on participants with different health conditions, varying the duration of intervention, and multiple forms and dosages of sesame consumption to confirm our findings.
Data Availability
Data will be made available if deemed necessary upon request to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Shabnam Rafiee and Laila Shirbeigi conceptualized and designed the study. Shabnam Rafiee, Roghaye Faryabi, and Alireza Yargholi drafted the manuscript and collected data. Mohammad Ali Zareian analyzed the data. Laila Shirbeigi and Shabnam Rafiee reviewed the protocol for important intellectual content. Jessie Hawkins and Nitin Shivappa as natives did English language editing. All authors read and approved the final manuscript.
Supplementary Materials
Supplementary Figure 1. Analysis of the influence of sesame consumption on serum CRP concentrations in humans. CI: confidence interval; CRP: C-reactive protein. Supplementary Figure 2. Analysis of the influence of sesame consumption on serum IL-6 concentrations in humans. CI: confidence interval; IL-6: interleukin-6. Supplementary Figure 3. Analysis of the influence of sesame consumption on serum TNF concentrations in humans. CI: confidence interval; TNF: tumor necrosis factor. Supplementary Figure 4. Funnel plot for assessing publication bias in the studies reporting the effects of sesame consumption on serum CRP concentrations in humans. CRP: C-reactive protein; SE: standard error; WMD: weighted mean difference. Supplementary Figure 5. Funnel plot for assessing publication bias in the studies reporting the effects of sesame consumption on serum IL-6 concentrations in humans. IL-6: interleukin-6; SE: standard error; and WMD: weighted mean difference. Supplementary Figure 6. Funnel plot for assessing publication bias in the studies reporting the effects of sesame consumption on serum TNF concentrations in humans. TNF: tumor necrosis factor; SE: standard error; and WMD: weighted mean difference.
References
- 1.Guarner V., Rubio-Ruiz M. E. Aging and Health-A Systems Biology Perspective . Vol. 40. Karger Publishers; 2015. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease; pp. 99–106. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutrition Reviews . 2007;65(suppl_3):S152–S156. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B., Shu B., Yang J., Liu J., Xi T., Xing Y. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes & Control . 2014;25(10):1397–1405. doi: 10.1007/s10552-014-0445-8. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil G. S. Inflammation and metabolic disorders. Nature . 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson-Smith A. C., Chen Y.-F., Newman M. S., May L. T., Sehgal P. B., Ruddle F. H. Regional localization of the interferon-β2B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21. Genomics . 1988;2(3):203–208. doi: 10.1016/0888-7543(88)90003-1. [DOI] [PubMed] [Google Scholar]
- 6.Van Der Poll T., Keogh C. V., Guirao X., Buurman W. A., Kopf M., Lowry S. F. Interleukin‐6 gene‐deficient mice show impaired defense against pneumococcal pneumonia. The Journal of Infectious Diseases . 1997;176(2):439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 7.Cesari M., Penninx B. W. J. H., Newman A. B., et al. Inflammatory markers and onset of cardiovascular events. Circulation . 2003;108(19):2317–2322. doi: 10.1161/01.cir.0000097109.90783.fc. [DOI] [PubMed] [Google Scholar]
- 8.Pepys M. The acute phase response and C-reactive protein. Oxford textbook of medicine . 1995;2:1527–1533. [Google Scholar]
- 9.Pepys M. B., Hirschfield G. M. C-reactive protein: a critical update. Journal of Clinical Investigation . 2003;111(12):1805–1812. doi: 10.1172/jci200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esser N., Paquot N., Scheen A. J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opinion on Investigational Drugs . 2015;24(3):283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- 11.Askari M., Faryabi R., Mozaffari H., Darooghegi Mofrad M. The effects of N-Acetylcysteine on serum level of inflammatory biomarkers in adults. Findings from a systematic review and meta-analysis of randomized clinical trials. Cytokine . 2020;135 doi: 10.1016/j.cyto.2020.155239.155239 [DOI] [PubMed] [Google Scholar]
- 12.Galland L. Diet and inflammation. Nutrition in Clinical Practice . 2010;25(6):634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 13.Eichelmann F., Schwingshackl L., Fedirko V., Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obesity Reviews . 2016;17(11):1067–1079. doi: 10.1111/obr.12439. [DOI] [PubMed] [Google Scholar]
- 14.Darooghegi Mofrad M., Milajerdi A., Koohdani F., Surkan P. J., Azadbakht L. Garlic supplementation reduces circulating C-reactive protein, tumor necrosis factor, and Interleukin-6 in adults: a systematic review and meta-analysis of randomized controlled trials. Journal of Nutrition . 2019;149(4):605–618. doi: 10.1093/jn/nxy310. [DOI] [PubMed] [Google Scholar]
- 15.Rahimlou M., Jahromi N. B., Hasanyani N., Ahmadi A. R. Effects of flaxseed interventions on circulating inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Advances in Nutrition . 2019;10(6):1108–1119. doi: 10.1093/advances/nmz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morshedzadeh N., Shahrokh S., Aghdaei H. A., et al. Effects of flaxseed and flaxseed oil supplement on serum levels of inflammatory markers, metabolic parameters and severity of disease in patients with ulcerative colitis. Complementary Therapies in Medicine . 2019;46:36–43. doi: 10.1016/j.ctim.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Zong G., Demark-Wahnefried W., Wu H., Lin X. Effects of flaxseed supplementation on erythrocyte fatty acids and multiple cardiometabolic biomarkers among Chinese with risk factors of metabolic syndrome. European Journal of Nutrition . 2013;52(5):1547–1551. doi: 10.1007/s00394-012-0461-y. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Rodriguez E., Biel-Glesson S., Fernandez-Navarro J. R., et al. Effects of virgin olive oils differing in their bioactive compound contents on biomarkers of oxidative stress and inflammation in healthy adults: a randomized double-blind controlled trial. Nutrients . 2019;11(3) doi: 10.3390/nu11030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse M., Von Loeffelholz C., Hoffmann D., et al. Dietary rapeseed/canola-oil supplementation reduces serum lipids and liver enzymes and alters postprandial inflammatory responses in adipose tissue compared to olive-oil supplementation in obese men. Molecular Nutrition & Food Research . 2015;59(3):507–519. doi: 10.1002/mnfr.201400446. [DOI] [PubMed] [Google Scholar]
- 20.Namiki M. Nutraceutical functions of sesame: a review. Critical Reviews in Food Science and Nutrition . 2007;47(7):651–673. doi: 10.1080/10408390600919114. [DOI] [PubMed] [Google Scholar]
- 21.Jin Q., Liu Y., Wang X., Dai H. Sesamin as a natural antioxidant. Journal of the Chinese Cereals and Oils Association . 2005;20:89–93. [Google Scholar]
- 22.Kamal-Eldin A., Moazzami A., Washi S. Sesame seed lignans: potent physiological modulators and possible ingredients in functional foods & nutraceuticals. Recent Patents on Food, Nutrition & Agriculture . 2011;3(1):17–29. doi: 10.2174/2212798411103010017. [DOI] [PubMed] [Google Scholar]
- 23.Elleuch M., Besbes S., Roiseux O., Blecker C., Attia H. Quality characteristics of sesame seeds and by-products. Food Chemistry . 2007;103(2):641–650. doi: 10.1016/j.foodchem.2006.09.008. [DOI] [Google Scholar]
- 24.Wu J. H. Y., Hodgson J. M., Puddey I. B., Belski R., Burke V., Croft K. D. Sesame supplementation does not improve cardiovascular disease risk markers in overweight men and women. Nutrition, Metabolism, and Cardiovascular Diseases . 2009;19(11):774–780. doi: 10.1016/j.numecd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Sankar D., Ali A., Sambandam G., Rao R. Sesame oil exhibits synergistic effect with anti-diabetic medication in patients with type 2 diabetes mellitus. Clinical Nutrition . 2011;30(3):351–358. doi: 10.1016/j.clnu.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Khosravi‐Boroujeni H., Nikbakht E., Natanelov E., Khalesi S. Can sesame consumption improve blood pressure? A systematic review and meta‐analysis of controlled trials. Journal of the Science of Food and Agriculture . 2017;97(10):3087–3094. doi: 10.1002/jsfa.8361. [DOI] [PubMed] [Google Scholar]
- 27.Khalesi S., Paukste E., Nikbakht E., Khosravi-Boroujeni H. Sesame fractions and lipid profiles: a systematic review and meta-analysis of controlled trials. British Journal of Nutrition . 2016;115(5):764–773. doi: 10.1017/s0007114515005012. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J.-C., Feng D.-W., Zheng G.-S. Extraction of sesamin from sesame oil using macroporous resin. Journal of Food Engineering . 2010;100(2):289–293. doi: 10.1016/j.jfoodeng.2010.04.011. [DOI] [Google Scholar]
- 29.Hong L., Yi W., Liangliang C., Juncheng H., Qin W., Xiaoxiang Z. Hypoglycaemic and hypolipidaemic activities of sesamin from sesame meal and its ability to ameliorate insulin resistance in KK-Ay mice. Journal of the Science of Food and Agriculture . 2013;93(8):1833–1838. doi: 10.1002/jsfa.5974. [DOI] [PubMed] [Google Scholar]
- 30.Barbosa C. V. d. S., Silva A. S., de Oliveira C. V. C., et al. Effects of sesame (Sesamum indicum L.) supplementation on creatine kinase, lactate dehydrogenase, oxidative stress markers, and aerobic capacity in semi-professional soccer players. Frontiers in Physiology . 2017;8:p. 196. doi: 10.3389/fphys.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farajbakhsh A., Mazloomi S. M., Mazidi M., et al. Sesame oil and vitamin E co-administration may improve cardio metabolic risk factors in patients with metabolic syndrome: a randomized clinical trial. European Journal of Clinical Nutrition . 2019;73(10):1403–1411. doi: 10.1038/s41430-019-0438-5. [DOI] [PubMed] [Google Scholar]
- 32.Haghighian M. K., Alipoor B., Mahdavi A. M., Sadat B. E., Jafarabadi M. A., Moghaddam A. Effects of sesame seed supplementation on inflammatory factors and oxidative stress biomarkers in patients with knee osteoarthritis. Acta Medica Iranica . 2015;53(4):207–213. [PubMed] [Google Scholar]
- 33.Helli B., Shahi M. M., Mowla K., Jalali M. T., Haghighian H. K. A randomized, triple‐blind, placebo‐controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytotherapy Research . 2019;33(9):2421–2428. doi: 10.1002/ptr.6433. [DOI] [PubMed] [Google Scholar]
- 34.Karatzi K., Stamatelopoulos K., Lykka M., et al. Acute and long-term hemodynamic effects of sesame oil consumption in hypertensive men. Journal of Clinical Hypertension . 2012;14(9):630–636. doi: 10.1111/j.1751-7176.2012.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammad Shahi M., Zakerzadeh M., Zakerkish M., Zarei M., Saki A. Effect of sesamin supplementation on glycemic status, inflammatory markers, and adiponectin levels in patients with type 2 diabetes mellitus. Journal of Dietary Supplements . 2017;14(1):65–75. doi: 10.1080/19390211.2016.1204404. [DOI] [PubMed] [Google Scholar]
- 36.Moher D., Shamseer L., Shamseer L., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews . 2015;4(1):p. 1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins J. P., Thomas J., Chandler J., et al. Cochrane Handbook for Systematic Reviews of Interventions . New Jersey, USA: John Wiley & Sons; 2019. [Google Scholar]
- 38.Borenstein M., Hedges L. V., Higgins J. P., Rothstein H. R. Introduction to Meta-Analysis . New Jersey, USA: John Wiley & Sons; 2011. [Google Scholar]
- 39.Hozo S. P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology . 2005;5(1):p. 13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ . 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu D.-Z., Chen S.-J., Chu P.-Y., Liu M.-Y. Therapeutic effects of sesame oil on monosodium urate crystal-induced acute inflammatory response in rats. Springer Plus . 2013;2(1):p. 659. doi: 10.1186/2193-1801-2-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeng K.-C. G., Hou R. C. W., Wang J.-C., Ping L.-I. Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-activated protein kinase and nuclear factor-κB. Immunology Letters . 2005;97(1):101–106. doi: 10.1016/j.imlet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Chavali S. R., Forse R. A. Decreased production of interleukin-6 and prostaglandin E2 associated with inhibition of delta5 desaturation of ω6 fatty acids in mice fed safflower oil diets supplemented with sesamol. Prostaglandins, Leukotrienes and Essential Fatty Acids . 1999;61(6):347–352. doi: 10.1054/plef.1999.0112. [DOI] [PubMed] [Google Scholar]
- 44.Hallund J., Tetens I., Bügel S., Tholstrup T., Bruun J. M. The effect of a lignan complex isolated from flaxseed on inflammation markers in healthy postmenopausal women. Nutrition, Metabolism, and Cardiovascular Diseases . 2008;18(7):497–502. doi: 10.1016/j.numecd.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Milder I. E. J., Arts I. C. W., Putte B. V. D., Venema D. P., Hollman P. C. H. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. British Journal of Nutrition . 2005;93(3):393–402. doi: 10.1079/bjn20051371. [DOI] [PubMed] [Google Scholar]
- 46.Truan J. S., Chen J.-M., Thompson L. U. Comparative effects of sesame seed lignan and flaxseed lignan in reducing the growth of human breast tumors (MCF-7) at high levels of circulating estrogen in athymic mice. Nutrition and Cancer . 2012;64(1):65–71. doi: 10.1080/01635581.2012.630165. [DOI] [PubMed] [Google Scholar]
- 47.Ten Wolde S., Engels F., Miltenburg A. M., Kuijpers E. A., Struijk-Wielinga G. I., Dijkmans B. A. Sesame oil in injectable gold: two drugs in one? Rheumatology . 1997;36(9):1012–1015. doi: 10.1093/rheumatology/36.9.1012. [DOI] [PubMed] [Google Scholar]
- 48.Faraji F., Hashemi M., Ghiasabadi A., et al. Combination therapy with interferon beta-1a and sesame oil in multiple sclerosis. Complementary Therapies in Medicine . 2019;45:275–279. doi: 10.1016/j.ctim.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Pan A., Demark-Wahnefried W., Ye X., et al. Effects of a flaxseed-derived lignan supplement on C-reactive protein, IL-6 and retinol-binding protein 4 in type 2 diabetic patients. British Journal of Nutrition . 2009;101(8):1145–1149. doi: 10.1017/S0007114508061527. [DOI] [PubMed] [Google Scholar]
- 50.Chiang H.-M., Chang H., Yao P.-W., et al. Sesamin reduces acute hepatic injury induced by lead coupled with lipopolysaccharide. Journal of the Chinese Medical Association . 2014;77(5):227–233. doi: 10.1016/j.jcma.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Straub R. H., Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evolution, Medicine, and Public Health . 2016;2016(1):37–51. doi: 10.1093/emph/eow001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X.-L., Liou C.-J., Li Z.-Y., Lai X.-Y., Fang L.-W., Huang W.-C. Sesamol suppresses the inflammatory response by inhibiting NF-κB/MAPK activation and upregulating AMP kinase signaling in RAW 264.7 macrophages. Inflammation Research . 2015;64(8):577–588. doi: 10.1007/s00011-015-0836-7. [DOI] [PubMed] [Google Scholar]
- 53.Chu P.-Y., Hsu D.-Z., Hsu P.-Y., Liu M.-Y. Sesamol down-regulates the lipopolysaccharide-induced inflammatory response by inhibiting nuclear factor-kappa B activation. Innate Immunity . 2010;16(5):333–339. doi: 10.1177/1753425909351880. [DOI] [PubMed] [Google Scholar]
- 54.Lu Y.-C., Jayakumar T., Duann Y.-F., et al. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. Journal of Agricultural and Food Chemistry . 2011;59(9):4969–4978. doi: 10.1021/jf1046738. [DOI] [PubMed] [Google Scholar]
- 55.Hsu D. Z., Chen Y. W., Chu P. Y., Periasamy S., Liu M. Y. Protective effect of 3, 4-methylenedioxyphenol (sesamol) on stress-related mucosal disease in rats. BioMed Research International . 2013;2013:8. doi: 10.1155/2013/481827.481827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang C. C., Lu W. J., Ong E. T., et al. A novel role of sesamol in inhibiting NF-κB-mediated signaling in platelet activation. Journal of Biomedical Science . 2011;18(1):93–10. doi: 10.1186/1423-0127-18-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Analysis of the influence of sesame consumption on serum CRP concentrations in humans. CI: confidence interval; CRP: C-reactive protein. Supplementary Figure 2. Analysis of the influence of sesame consumption on serum IL-6 concentrations in humans. CI: confidence interval; IL-6: interleukin-6. Supplementary Figure 3. Analysis of the influence of sesame consumption on serum TNF concentrations in humans. CI: confidence interval; TNF: tumor necrosis factor. Supplementary Figure 4. Funnel plot for assessing publication bias in the studies reporting the effects of sesame consumption on serum CRP concentrations in humans. CRP: C-reactive protein; SE: standard error; WMD: weighted mean difference. Supplementary Figure 5. Funnel plot for assessing publication bias in the studies reporting the effects of sesame consumption on serum IL-6 concentrations in humans. IL-6: interleukin-6; SE: standard error; and WMD: weighted mean difference. Supplementary Figure 6. Funnel plot for assessing publication bias in the studies reporting the effects of sesame consumption on serum TNF concentrations in humans. TNF: tumor necrosis factor; SE: standard error; and WMD: weighted mean difference.
Data Availability Statement
Data will be made available if deemed necessary upon request to the corresponding author.


