Abstract
Asthma is a chronic inflammatory airway disease characterized by airway hyperresponsiveness, inflammation, and remodeling. Asthma often develops during childhood and causes lifelong decrements in lung function and quality of life. Risk factors for childhood asthma are numerous and include genetic, epigenetic, developmental, and environmental factors. Uncontrolled maternal asthma during pregnancy exposes the developing fetus to inflammatory insults, which further increase the risk of childhood asthma independent of genetic predisposition. This review focuses on the role of maternal asthma in the development of asthma in offspring. We will present maternal asthma as a targetable and modifiable risk factor for childhood asthma and discuss the mechanisms by which maternal inflammation increases childhood asthma risk. Topics include how exposure to maternal asthma in utero shapes structural lung development with a special emphasis on airway nerves, how maternal type-2 cytokines such as IL-5 activate the fetal immune system, and how changes in lung and immune cell development inform responses to aero-allergens later in life. Finally, we highlight emerging evidence that maternal asthma establishes a unique “asthma signature” in the airways of children, leading to novel mechanisms of airway hyperreactivity and inflammatory cell responses.
Keywords: asthma, childhood, developmental origins, eosinophil, inflammation, maternal, nerves
1 |. INTRODUCTION
Asthma is a chronic inflammatory airway disease characterized by excessive bronchoconstriction and airway hyperresponsiveness.1 The resulting airflow obstruction manifests with wheezing, shortness of breath, chest tightness, and cough that significantly affects quality of life.2 Over 300 million people worldwide suffer from asthma,3 which often begins in childhood and can lead to lifelong reductions in pulmonary function.4–10 Parental asthma and atopy are clearly linked to development of asthma in children11,12; however genetic inheritance and environmental exposures explain only part of this risk. Maternal asthma confers more risk than paternal asthma13 and improved asthma control during pregnancy reduces asthma in offspring,14 suggesting exposure to maternal factors in utero uniquely impacts fetal development and asthma risk. This review focuses on the role of maternal asthma in the development of asthma in offspring, with an emphasis on maternal cytokines and effects of eosinophils on airway development and function.
2 |. DEVELOPMENTAL ORIGINS OF CHILDHOOD ASTHMA
Asthma that develops in childhood has a profound impact on lifelong lung health.10 Reduced lung function in childhood strongly predicts reduced lung function in adulthood4,5 and is associated with lifelong asthma risk.5–9 Risk factors for development of childhood asthma are numerous and include genetic, developmental, and environmental factors. For example, parental atopy,11,12 smoking,15 maternal obesity,16 and lower socioeconomic status15 are parental factors that increase childhood asthma risk, whereas male sex,15 low birth weight,17 and prematurity18 are fetal factors that increase disease risk. Furthermore, airway hyperreactivity at birth predicts persistent wheezing during childhood and childhood asthma,19,20 suggesting in utero programming of airway responses has a central role in childhood asthma risk. Children with risk factors for asthma are particularly susceptible to early life environmental exposures to respiratory virus infections from respiratory syncytial virus21 and rhinovirus,22 and early allergen sensitization to house dust mites,23 which trigger wheezing that often persists.24 These risk factors are representative of the complex interactions between genetics and environmental exposures that influence fetal lung development and immunologic responses, which lead to asthma later in life.
3 |. MATERNAL ASTHMA UNIQUELY INCREASES CHILDHOOD ASTHMA RISK
Parental asthma is a clear risk factor for childhood asthma11,12; however, genetics and shared environments explain only part of this risk. Maternal asthma is a greater risk factor than paternal asthma for childhood asthma13 and children of mothers with uncontrolled asthma during pregnancy have greater risk of developing asthma than children of mothers with well-controlled asthma.25,26 Furthermore, mothers who had their asthma intensively managed during pregnancy had children with a lower risk of respiratory illnesses27 and asthma14 compared to mothers with symptom-guided asthma care. Thus, maternal factors, which are potentially modifiable, influence childhood asthma risk by affecting fetal programming in utero. Fetal programming can have long-term consequences on airway function. Airway hyperreactivity can be detected at birth in newborn animals28 and neonatal airway hyperreactivity in humans is associated with increased risk of asthma in adolescence19,20 Maternal atopy and asthma are associated with impaired infant lung function29 and airway hyperreactivity,30 independent of allergen sensitization at birth. Postnatal allergen sensitization further augments airway hyperreactivity in children exposed to maternal asthma and allergen sensitization predicts the persistence of wheeze.23
Augmented airway hyperreactivity in children exposed to maternal asthma in utero may occur secondary to structural changes in airways. Using a transgenic mouse model of asthma, we found that dams with elevated eosinophils and IL-5, markers of type-2 high asthma (discussed in the following text) give birth to offspring with increased sensory nerve density in airway epithelium31 (Fig. 1). Airway sensory nerves respond to inhaled stimuli and provoke bronchoconstriction in response to a variety of chemical and mechanical stimuli.32,33 Correspondingly, offspring with increased airway epithelial innervation exhibited airway hyperreactivity at baseline and severe airway hyperreactivity after allergen sensitization and challenge.31 Innervation in these offspring did not increase further with postnatal exposure to house dust mite allergen or IL-5, demonstrating that in utero development of airway nerves and airway hyperreactivity is a uniquely sensitive time period to the influence of maternal asthma. These changes are reminiscent of changes in adults with asthma.34 Bronchoscopic biopsies collected from adults with type 2-high asthma had increased sensory innervation, which correlated with worse lung function.34 It is possible that hyperinnervation in adults with asthma occurred during prenatal development and predisposed to asthma development and worse disease later in life.
FIGURE 1. Mechanisms of increased childhood asthma risk in offspring born to mothers with asthma.
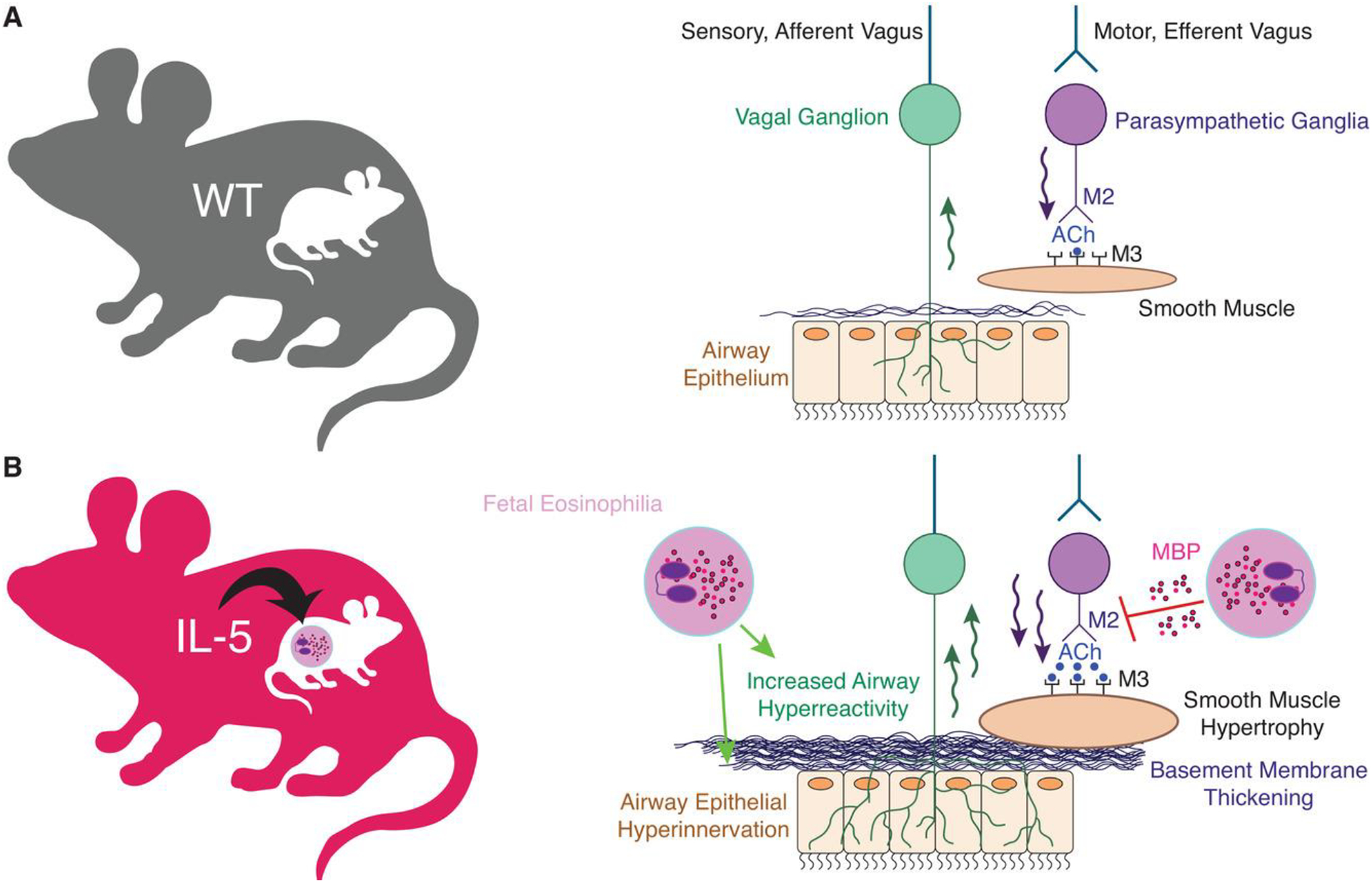
(A) Under homeostatic conditions, afferent sensory nerves detect external stimuli and activate efferent parasympathetic nerves through a central nerve-reflex pathway. Parasympathetic nerves release acetylcholine (ACh) onto airway smooth muscle M3-muscarinic receptors (M3) to induce airway contraction. M2-muscarinic receptors (M2) located on presynaptic postganglionic nerves inhibit further acetylcholine release. (B) Maternal asthma increases airway hyperreactivity and bronchoconstriction in offspring by inducing airway sensory hyperinnervation, basement membrane thickening, and smooth muscle hypertrophy. In mice, developmental reprogramming is mediated by maternal IL-5 and fetal eosinophils. Eosinophils also increase bronchoconstriction by releasing cationic proteins that inhibit M2-muscarinic receptor function and potentiate acetylcholine release
Airway nerves are not the only cell type that undergoes reprogramming when exposed to maternal asthma. Bronchial biopsies from children of asthmatics demonstrate epithelial basement membrane thickening.35 Evidence of subepithelial remodeling precedes the clinical diagnosis of asthma in some children,36 suggesting that tissue remodeling may be an inciting factor for asthma as opposed to simply a response to chronic airway inflammation. Airway epithelium also undergoes remodeling in response to maternal asthma. Mice born to dams sensitized to ovalbumin demonstrate increased goblet cell hyperplasia in response to allergen exposure compared to mice born to non-allergic dams.37 Offspring born to allergic sheep have reduced type II alveolar cells38 and surfactant B production,39 which combined with potential goblet cell hyperplasia, would presumably increase airway narrowing and sputum production. These effects are not unique to maternal asthma, however, as maternal smoking can increase smooth muscle hypertrophy and subepithelial collagen deposition.40
Maternal asthma also reprograms fetal immune cells. Murine offspring exposed to maternal asthma have potentiated inflammatory cell recruitment and cytokine secretion after allergen exposure later in life.41 We similarly found in a transgenic model of asthma that wild-type offspring born to IL-5 transgenic mice have potentiated inflammatory cell recruitment to lungs after allergen exposure.31 Inflammatory cell populations in the lungs of offspring born to IL-5 transgenic dams were not different before allergen exposure, suggesting fetal cells undergo reprogramming that may increase sensitivity to recruitment signals or enhance hematopoietic potential. In humans this is evidenced by infants born to parents with asthma who have potentiated immune proliferative responses to allergens without prior sensitization.42 Epigenetic changes may underlie these enhanced immune responses. Immune cells collected from children of asthmatic mothers harbor many differentially methylated regions compared to immune cells collected from children of nonasthmatic mothers.43 Immune cell epigenetic profiles at birth also identify children who will later develop asthma, a trajectory that is modified by the presence of maternal asthma.44 In mice, exposure to maternal asthma alters the epigenetic landscape in dendritic cells, which corresponds with enhanced antigen uptake and presentation.45
4 |. EFFECTS OF MATERNAL INFLAMMATORY CYTOKINES ON FETAL LUNG DEVELOPMENT
Asthma is a heterogeneous disease with many underlying mechanisms. In order to group asthmatics who share a common mechanism, phenotypes have been developed that categorize asthmatics based on observable characteristics (e.g., severity, age of onset), environmental factors (e.g., allergen sensitivity), and airway inflammatory leukocytes profiles.46 The most common phenotype, termed “type 2-high” asthma, accounts for two-thirds of asthmatics and is characterized by cytokines classically released from CD4+ Th2 T helper cells and type 2 innate lymphoid cells, such as IL-4, IL-5, and IL-13.47 Type 2 cytokines stimulate eosinophil hematopoiesis, migration, and activation48 and as a result, an abundance of airway and peripheral blood eosinophils is a hallmark of this phenotype.49 Eosinophils contribute to pathogenic features of asthma such as airway remodeling50 and hyperreactivity,51 and their levels in asthma correlate with disease severity,52,53 exacerbation frequency,54–56 and progressive decline in lung function.57
Several human studies suggest maternal type 2 cytokines mediate increased childhood asthma risk. In pregnant mothers with asthma, higher maternal serum IL-5 levels correlates with increased infant asthma risk58 whereas increased ratios of maternal IFN-γ to IL-13 or IL-4 were associated with lower rates of asthma in children.59 Similarly, increased IFN-γ to IL-4 ratios in pregnant mothers were associated with reduced childhood atopy.60 These studies suggest excessive maternal type-2 inflammation or an imbalance between type-2 and type-1 inflammation may increase childhood asthma risk.
The effects of maternal cytokines on fetal development are also observed in mice. Blocking maternal IL-4 in pregnant mice exposed to allergen throughout pregnancy reduces offspring airway hyperreactivity and inflammation,37 as does exposing pregnant mice to type 1 IFNs61 or LPS.62 We recently demonstrated that maternal IL-5 crosses the murine placenta and affects fetal lung development in wild-type offspring born to IL-5 transgenic mice (IL-5tg)(31). IL-5tg mice express an IL-5 transgene in airway CC10 club cells, resulting in airway eosinophilia and elevated circulating IL-563 that expose the developing fetus to IL-5 in utero as well. Wild-type mice exposed to IL-5 in utero had significantly increased airway hyperreactivity and airway inflammation compared to wild-type offspring of wild-type dams.31
Whether maternal cytokines affect a developing fetus by passing through the placenta from maternal to fetal circulation or by modulating placental cytokine release is unclear. Cytokine passage across the placenta varies by species and by cytokine.64–67 In humans, IL-6, but not IL-8, TNF-α, or IL-1 crossed the placenta, whereas neither IL-4 or IL-13 cross the murine placenta.68 We found elevated levels of IL-5 in amniotic fluid of wild-type offspring born to IL-5 transgenic dams, suggesting IL-5 crosses the placenta, albeit at highly variable rates.31 The placenta also secretes cytokines directly into fetal circulation69–72 and augments expression after maternal exposures, such as to LPS.73 Furthermore, placentas from woman with asthma have increased expression of TNF-α, IL-1β, IL-6, IL-8, and IL-5, particularly when the developing fetus was female,74 and also have decreased vascularity75 compared to placentas from woman without asthma. Thus, the placenta may serve a key regulatory role in the transfer of maternal inflammation to the developing fetus.
5 |. EFFECTS OF EOSINOPHILS ON AIRWAY NERVE FUNCTION
Whether cytokines pass from maternal circulation to the fetus or are derived from the placenta, the question remains as to how they change fetal development. We found that the effects of IL-5 on the developing lung were mediated by fetal eosinophils and fetuses with congenital eosinophil deficiency were protected from IL-5-induced nerve remodeling, airway hyperreactivity, and airway inflammation dams.31 These data suggest that activation of the fetal immune system by maternal cytokines is required for transmission of disease risk, as opposed to cytokines directly affecting lung cell development.
How eosinophils function during development and cause increased airway innervation is less clear. Lung eosinophils are minimal at birth, increase rapidly during postnatal development, and then decline to adult levels.76,77 These lung resident eosinophils participate in immune homeostasis, generally exerting anti-inflammatory actions and suppressing Th2 sensitization.78 Most studies have focused on eosinophils recruited in response to inflammatory stimuli and their effects in fully developed lungs, a population that is functionally and transcriptionally distinct from lung resident eosinophils.31 In animal models of asthma, inflammatory eosinophils are recruited and increase in the airways after exposure to allergen (e.g., house dust mite),79 antigen (e.g., ovalbumin),80,81 and ozone.82,83 Once activated, eosinophils degran ulate, releasing highly charged cationic proteins such as major basic protein and eosinophil peroxidase, as well as chemokines, cytokines, and growth factors84–86 that promote airway hyperreactivity and airway remodeling. Similarly, eosinophil accumulation and degranulation are provoked in transgenic mice that over-express eosinophil hematopoietic and chemotactic factors IL-5 and eotaxin.63,87 Eliminating eosinophils using eosinophil-specific cre-recombinase knockout technology (PHIL mice) protects against airway hyperreactivity and remodeling after allergen exposure,90,91 whereas adoptive transfer of eosinophils restores airway hyperreactivity in allergen-exposed IL-5−/− knockout mice,88 demonstrating that eosinophils contribute to airway hyperreactivity. At this time it is unclear whether fetal eosinophils mediating airway hyperinnervation after exposure to maternal IL-5 are “resident” or “inflammatory” in nature.
In developed lungs, eosinophils physically interact with nerves and affect nerve function. For example, eosinophils release the granule protein major basic protein, which blocks parasympathetic M2 muscarinic receptors, resulting in loss of M2’s inhibitory feedback.89 As a consequence, parasympathetic nerves release excessive amounts of acetylcholine, which potentiates bronchoconstriction in humans with asthma90,91 and in allergen-exposed animals.80,81,92–101 Eosinophil major basic protein and another granule protein eosinophil peroxidase also directly activate pulmonary sensory nerves102 and increase neuronal responsiveness capsaicin, ATP, and electrical stimulation.103,104 Similarly, these effects occur on sensory nerves in the skin in atopic dermatitis, where eosinophils increase sensory nerve density and exacerbate itch (a nerve-mediated reflex similar to reflex bronchoconstriction in the lung).105,106 How these individual eosinophil-derived mediators increase airway innervation is an active area of research and recent development of an eosinophil-specific selective knockout mouse using cre-lox recombination will greatly aid future investigations.107
Airway nerves actively recruit eosinophils by releasing chemotactic factors such as eoxtaxin-1. Parasympathetic and sensory nerves express eotaxin-1 and increase expression after antigen challenge.100,105 Eotaxin-1 then binds eosinophil CCR3 receptors to promote eosinophil migration to nerves. As a result, eosinophils are clustered around nerve axons and ganglia in humans who died of fatal asthma exacerbations and after antigen and ozone exposure in animals.80,97,98,101,108,109 Eosinophils’ proximity to nerves is crucial to their effects on nerve function96 and preventing eosinophil migration by blocking CCR3 specifically,100 or by reducing eosinophils generally using corticosteroids,99 prevents development of nerve-mediated airway hyperreactivity. Once eosinophils arrive at nerves, they physically adhere to neuronally expressed adhesion molecules VCAM-1 and ICAM-1.110 Binding triggers eosinophil degranulation and release of granule proteins that mediate eosinophils effects.111,112 Nerves up-regulate expression of adhesion molecules in response to antigen sensitization,113 TNF-α, IFN-γ,114 and nerve growth factor.105 In turn, blocking eosinophil binding to VCAM-1 or ICAM-1 prevents airway hyperreactivity in antigen challenged guinea pigs95,114 and monkeys in vivo.115
Whether these mechanisms of eosinophil-nerve interactions in fully developed lungs after allergen exposures translate to prenatal development remain to be tested. However, it is clear that the effects of eosinophils during lung development have particularly severe consequences for airway function later in life. For example, mice with increased sensory innervation that are subsequently sensitized to house dust mite allergen in adulthood, develop fatal bronchoconstriction in response to inhaled serotonin.91 Airway hyperreactivity in these animals was far worse than airway hyperreactivity due to either increased airway sensory innervation or house dust mite sensitization alone. Animals that lacked eosinophils were protected from these effects, underscoring the significance of eosinophil-nerve interactions when airway nerve development is dysregulated. Vagotomy prevented lethal bronchoconstriction after house dust mite, further reinforcing the central role of nerve reflexes in severe bronchoconstriction. In a separate study, maternal house dust mite exposure similarly potentiated offspring airway responses after offspring were exposed to house dust mite.41 This study did not specifically address the role of eosinophils, but did show that maternal immunoglobulins were not required for vertical transmission of asthma risk. Thus, humans with type 2-high asthma and increased airway innervation may suffer more severe outcomes after concurrent sensitization to aero-allergens.
6 |. TARGETING MATERNAL CYTOKINES TO MODIFY CHILDHOOD ASTHMA RISK
The advent of tailored treatments based on asthma phenotype holds great promise for maternal transmission of asthma risk, both for the mother and child. Efforts to target type 2-high asthma by depleting airway eosinophils recently led to the development of anti-IL-5 monoclonal antibodies mepolizumab and reszilumab,116–120 followed more recently by the anti-IL-5 receptor antibody benralizumab.121,122 Animal studies demonstrated that pharmacologic neutralization of IL-5 reduced airway eosinophilia, remodeling, and hyperreactivity.123–128 Subsequent human trials with patients selected for type 2-high asthma based on elevated peripheral blood eosinophil counts showed improvements in exacerbations, lung function, and quality of life.116–122 Because IL-5 is a key mediator in the transmission of asthma risk, both mothers with type 2-high asthma and their children may benefit from these targeted therapies. However, this must be balanced with the duality of eosinophil functions in the lung. Like other immune cells, subsets of eosinophils exist in the lungs. In healthy fully developed lungs, “resident” eosinophils are present, which are IL-5 independent, don’t expand after allergen exposure, and express genes involved in tissue homeostasis and suppression of inflammation.78 In contrast, IL-5 dependent “inflammatory” eosinophils are recruited to lungs after exposure to environmental allergens.78,129,130 Inflammatory eosinophils express proinflammatory genes78 and are suppressed by steroids131 and by blocking IL-5.132 The discovery of tissue resident eosinophils coupled the recently identified immune regulatory roles of eosinophils (reviewed in Lee et al., 2010133) suggests eosinophils play important roles in maintaining tissue homeostasis and healthy immune responses. Whether blocking IL-5 during development could selectively prevent the harmful effector functions of eosinophils while preserving beneficial regulatory functions remains to be tested.
Approximately one-third of asthmatics do not have eosinophilia in blood or lungs and perhaps unsurprisingly, these patients respond poorly to type 2 cytokine targeted therapies. This group is classified as a type 2-low asthma phenotype46 and its inflammatory mechanisms are not well understood, which has hampered discovery of biomarkers and effective treatments for this phenotype. In type 2 low asthma, elevated levels of IL-17A, IL17F, IL-21, and IL-22 are present and neutrophilic inflammation is common.134 Whether children born to woman with type 2-low asthma have the same asthma risk as children born to woman with type 2-high asthma remains to be tested. Furthermore, how cytokines identified in type 2 low asthma contribute to asthma pathogenesis remains to be fully clarified. For example, data are conflicting as to whether IL-17A is beneficial or harmful in asthma. Airway IL-17A increases in animals after allergen exposure, and in children, serum levels correlate with disease severity.135 Neutralizing IL-17A reduces airway inflammation and airway remodeling in allergic animals,136 whereas exogenous IL-17A potentiates airway smooth muscle proliferation,137 secretion of proinflammatory cytokines,137,138 and methacholine-induced smooth muscle contraction.139 Conversely, others have found that neutralizing IL-17A in allergen-sensitized animals increases allergic inflammation140 and blocking IL-17 receptor alpha in humans with moderate to severe asthma showed no improvement in asthma control or pulmonary function.141
Maternal asthma may also change the underlying mechanism(s) of asthma in children and thus the efficacy of different treatment modalities. Immune cells collected from neonates born to mothers with asthma contain hundreds of differentially methylated regions compared to neonates born to mother without asthma.44 Monitoring these neonates for development of asthma later in life identified SMAD3 hypermethylation and secretion of IL-1β as a unique signature in children born to asthmatic mothers who would then go on to develop asthma themselves.44 In mice, we found that airway hyperreactivity in wild-type offspring born to IL-5 transgenic dams could be blocked with an antagonist of neurokinin-1 receptors, which are the target of the sensory neuropeptide substance P (unpublished observation). In contrast, neurokinin-1 antagonists worsened airway hyperreactivity in wild-type offspring born to wild-type dams. How exposure to maternal IL-5 during development changed the therapeutic efficacy of neurokinin-1 antagonists is currently unclear, but it suggests fetal programming from maternal asthma influences response to neurokinin antagonists or other therapeutics may be selectively beneficial in children born to mothers with asthma.
7 |. FUTURE DIRECTIONS
Maternal asthma represents a unique and potentially modifiable risk factor for childhood asthma. Crucial next steps need to focus on treatment modalities that reduce maternal inflammation and improve disease control during pregnancy, while testing whether targeted therapies against type-2 cytokines like IL-5 are superior to indiscriminate suppression of inflammation (i.e., steroids). It is unknown whether all children born to mothers with asthma have an increased risk of developing asthma themselves via the mechanisms discussed in this review or whether these findings are restricted to children born to mothers with allergic or type 2-high phenotypes. If maternal inflammatory phenotypes during pregnancy inform those in the developing fetus, and targeted therapies are either not available or without proven efficacy in controlling maternal disease, alternative strategies could focus on reversing lung changes in offspring after birth. Investigating reversibility of hyperinnervation and immune signatures in the early postnatal period could be highly valuable as results may apply to both adults and children with asthma. Finally, several fundamental questions remain regarding eosinophil biology. Specifically, how and which eosinophil-derived products increase airway innervation, and whether this occurs in all tissues with resident eosinophil populations or is somehow restricted to developing lungs, is of interest. Although there is scant knowledge on how eosinophils function during in utero development, recent studies indicate they have an active role in shaping airway physiology and immune responses that impact lung function throughout life.
ACKNOWLEDGMENTS
Funded by the National Institutes of Health: F30HL132414, K08HL121254, R01HL124165, R01HL144008.
Abbreviations:
- IL-5tg
IL-5 transgenic mice
Footnotes
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138:4S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming L, Murray C, Bansal AT, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J. 2015;46:1322–1333. [DOI] [PubMed] [Google Scholar]
- 3.Holguin F, Cardet JC, Chung KF, et al. Management of Severe Asthma: a European Respiratory Society/American Thoracic Society Guideline. Eur Respir J. 2019;44:1377–1378. [Google Scholar]
- 4.Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haland G, Carlsen KC, Sandvik L, et al. Oraacle. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. [DOI] [PubMed] [Google Scholar]
- 6.Owens L, Laing IA, Zhang G, Le Souef PN. Infant lung function predicts asthma persistence and remission in young adults. Respirology. 2017;22:289–294. [DOI] [PubMed] [Google Scholar]
- 7.Tai A, Tran H, Roberts M, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014;133:1572–1578. e1573. [DOI] [PubMed] [Google Scholar]
- 8.McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paaso EM, Jaakkola MS, Rantala AK, Hugg TT, Jaakkola JJ. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: a prospective cohort study. Respir Res. 2014;15:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly YJ, Brabin BJ, Milligan P, Heaf DP, Reid J, Pearson MG. Maternal asthma, premature birth, and the risk of respiratory morbidity in schoolchildren in Merseyside. Thorax. 1995;50:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010;5:e10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morten M, Collison A, Murphy VE, et al. Managing Asthma in Pregnancy (MAP) trial: fENO levels and childhood asthma. J Allergy Clin Immunol. 2018;142:1765–1772. e1764. [DOI] [PubMed] [Google Scholar]
- 15.Arshad SH, Stevens M, Hide DW. The effect of genetic and environmental factors on the prevalence of allergic disorders at the age of two years. Clin Exp Allergy. 1993;23:504–511. [DOI] [PubMed] [Google Scholar]
- 16.Harpsoe MC, Basit S, Bager P, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol. 2013;131:1033–1040. [DOI] [PubMed] [Google Scholar]
- 17.Barker DJ, Osmond C, Forsen TJ, Thornburg KL, Kajantie E, Eriksson JG. Foetal and childhood growth and asthma in adult life. Acta Paediatr. 2013;102:732–738. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133:1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisgaard H, Jensen SM, Bonnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. [DOI] [PubMed] [Google Scholar]
- 20.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163:37–42. [DOI] [PubMed] [Google Scholar]
- 21.Mejias A, Wu B, Tandon N, et al. Risk of childhood wheeze and asthma after respiratory syncytial virus infection in full-term infants. Pediatr Allergy Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 22.Bergroth E, Aakula M, Elenius V, et al. Rhinovirus Type in Severe Bronchiolitis and the Development of Asthma. J Allergy Clin Immunol Pract. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. [DOI] [PubMed] [Google Scholar]
- 24.Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martel MJ, Rey E, Beauchesne MF, et al. Control and severity of asthma during pregnancy are associated with asthma incidence in offspring: two-stage case-control study. Eur Respir J. 2009;34: 579–587. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Agerbo E, Schlunssen V, Wright RJ, Li J, Munk-Olsen T. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J Allergy Clin Immunol. 2018;141:886–892. e883. [DOI] [PubMed] [Google Scholar]
- 27.Mattes J, Murphy VE, Powell H, Gibson PG. Prenatal origins of bronchiolitis: protective effect of optimised asthma management during pregnancy. Thorax. 2014;69:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher JT, Brundage KL, Waldron MA, Connelly BJ. Vagal cholinergic innervation of the airways in newborn cat and dog. J Appl Physiol. 1985;69:1525–1531. [DOI] [PubMed] [Google Scholar]
- 29.Lowe L, Murray CS, Custovic A, et al. Specific airway resistance in 3-year-old children: a prospective cohort study. Lancet. 2002;359:1904–1908. [DOI] [PubMed] [Google Scholar]
- 30.Young S, Le Souef PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324: 1168–1173. [DOI] [PubMed] [Google Scholar]
- 31.Lebold KM, Drake MG, Hales-Beck L, Fryer AD, Jacoby DB. IL-5 exposure in utero increases lung nerve density and causes airway reactivity in adult offspring. Am J Resp Cell Mol Biol. 2019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol. 2002;2:211–215. [DOI] [PubMed] [Google Scholar]
- 33.Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–1948. [DOI] [PubMed] [Google Scholar]
- 34.Drake MG, Scott GD, Blum ED, et al. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci Transl Med. 2018;10:eaar8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berankova K, Uhlik J, Honkova L, Pohunek P. Structural changes in the bronchial mucosa of young children at risk of developing asthma. Pediatr Allergy Immunol. 2014;25:136–142. [DOI] [PubMed] [Google Scholar]
- 36.Pohunek P, Warner JO, Turzikova J, Kudrmann J, Roche WR. Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol. 2005;16:43–51. [DOI] [PubMed] [Google Scholar]
- 37.Hamada K, Suzaki Y, Goldman A, et al. Allergen-independent maternal transmission of asthma susceptibility. J Immunol. 2003;170: 1683–1689. [DOI] [PubMed] [Google Scholar]
- 38.Wooldridge AL, Clifton VL, Moss TJM, et al. Maternal allergic asthma during pregnancy alters fetal lung and immune development in sheep: potential mechanisms for programming asthma and allergy. J Physiol. 2019;597:4251–4262. [DOI] [PubMed] [Google Scholar]
- 39.Clifton VL, Moss TJ, Wooldridge AL, et al. Development of an experimental model of maternal allergic asthma during pregnancy. J Physiol. 2016;594:1311–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blacquiere MJ, Timens W, Melgert BN, Geerlings M, Postma DS, Hylkema MN. Maternal smoking during pregnancy induces airway remodelling in mice offspring. Eur Respir J. 2009;33: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 41.Richgels PK, Yamani A, Chougnet CA, Lewkowich IP. Maternal house dust mite exposure during pregnancy enhances severity of house dust mite-induced asthma in murine offspring. J Allergy Clin Immunol. 2017;140:1404–1415. e1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43–50. [DOI] [PubMed] [Google Scholar]
- 43.Gunawardhana LP, Baines KJ, Mattes J, Murphy VE, Simpson JL, Gibson PG. Differential DNA methylation profiles of infants exposed to maternal asthma during pregnancy. Pediatr Pulmonol. 2014;49: 852–862. [DOI] [PubMed] [Google Scholar]
- 44.DeVries A, Wlasiuk G, Miller SJ, et al. Epigenome-wide analysis links SMAD3 methylation at birth to asthma in children of asthmatic mothers. J Allergy Clin Immunol. 2017;140:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fedulov AV, Kobzik L. Allergy risk is mediated by dendritic cells with congenital epigenetic changes. Am J Respir Cell Mol Biol. 2011;44: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy BD, Noel PJ, Freemer MM, et al. Future Research Directions in Asthma. An NHLBI Working Group Report. Am J Respir Crit Care Med. 2015;192:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McBrien CN, Menzies-Gow A. The Biology of Eosinophils and Their Role in Asthma. Front Med. 2017;4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobsen EA, Ochkur SI, Doyle AD, et al. Lung Pathologies in a Chronic Inflammation Mouse Model Are Independent of Eosinophil Degranulation. Am J Respir Crit Care Med. 2017;195:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. [DOI] [PubMed] [Google Scholar]
- 52.Cianchetti S, Bacci E, Ruocco L, et al. Are sputum eosinophil cationic protein and eosinophils differently associated with clinical and functional findings of asthma. Clin Exp Allergy. 2014;44:673–680. [DOI] [PubMed] [Google Scholar]
- 53.Duncan CJ, Lawrie A, Blaylock MG, Douglas JG, Walsh GM. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur Respir J. 2003;22:484–490. [DOI] [PubMed] [Google Scholar]
- 54.Denlinger LC, Phillips BR, Ramratnam S, et al. National Heart L, Blood Institute’s Severe Asthma Research Program I. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med. 2017;195:302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerkhof M, Tran TN, van den Berge M, et al. Association between blood eosinophil count and risk of readmission for patients with asthma: historical cohort study. PLoS One. 2018;13:e0201143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3:849–858. [DOI] [PubMed] [Google Scholar]
- 57.Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51:1702536. [DOI] [PubMed] [Google Scholar]
- 58.Soto-Ramirez N, Karmaus W, Yousefi M, Zhang H, Liu J, Gangur V. Maternal immune markers in serum during gestation and in breast milk and the risk of asthma-like symptoms at ages 6 and 12 months: a longitudinal study. Allergy Asthma Clin Immunol. 2012;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothers J, Stern DA, Lohman IC, et al. Maternal cytokine profiles during pregnancy predict asthma in children of mothers without asthma. Am J Respir Cell Mol Biol. 2018;59:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JH, Kim KH, Woo HY, Shim JY. Maternal cytokine production during pregnancy and the development of childhood wheezing and allergic disease in offspring three years of age. J Asthma. 2008;45:948–952. [DOI] [PubMed] [Google Scholar]
- 61.Lima C, Souza VM, Faquim-Mauro EL, et al. Modulation of the induction of lung and airway allergy in the offspring of IFN-gamma-treated mother mice. J Immunol. 2005;175:3554–3559. [DOI] [PubMed] [Google Scholar]
- 62.Blumer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin Exp Allergy. 2005;35:397–402. [DOI] [PubMed] [Google Scholar]
- 63.Lee JJ, McGarry MP, Farmer SC, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106:802–807. [DOI] [PubMed] [Google Scholar]
- 65.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103: 546–550. [DOI] [PubMed] [Google Scholar]
- 66.Reisenberger K, Egarter C, Vogl S, Sternberger B, Kiss H, Husslein P. The transfer of interleukin-8 across the human placenta perfused in vitro. Obstet Gynecol. 1996;87:613–616. [DOI] [PubMed] [Google Scholar]
- 67.Dilworth MR, Sibley CP. Review: Transport across the placenta of mice and women. Placenta. 2013;34:S34–39. Suppl. [DOI] [PubMed] [Google Scholar]
- 68.Lim RH, Kobzik L. Transplacental passage of interleukins 4 and 13. PLoS One. 2009;4:e4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Topping V, Romero R, Than NG, et al. Interleukin-33 in the human placenta. J Matern Fetal Neonatal Med. 2013;26:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181:1530–1536. [DOI] [PubMed] [Google Scholar]
- 71.Steinborn A, von Gall C, Hildenbrand R, Stutte HJ, Kaufmann M. Identification of placental cytokine-producing cells in term and preterm labor. Obstet Gynecol. 1998;91:329–335. [DOI] [PubMed] [Google Scholar]
- 72.Abelius MS, Janefjord C, Ernerudh J, et al. The placental immune milieu is characterized by a Th2- and anti-inflammatory transcription profile, regardless of maternal allergy, and associates with neonatal immunity. Am J Reprod Immunol. 2015;73:445–459. [DOI] [PubMed] [Google Scholar]
- 73.Gayle DA, Beloosesky R, Desai M, Amidi F, Nunez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1024–1029. [DOI] [PubMed] [Google Scholar]
- 74.Scott NM, Hodyl NA, Murphy VE, et al. Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol. 2009;182:1411–1420. [DOI] [PubMed] [Google Scholar]
- 75.Mayhew TM, Jenkins H, Todd B, Clifton VL. Maternal asthma and placental morphometry: effects of severity, treatment and fetal sex. Placenta. 2008;29:366–373. [DOI] [PubMed] [Google Scholar]
- 76.Steer CA, Martinez-Gonzalez I, Ghaedi M, Allinger P, Matha L, Takei F. Group 2 innate lymphoid cell activation in the neonatal lung drives type 2 immunity and allergen sensitization. J Allergy Clin Immunol. 2017;140:593–595. e593. [DOI] [PubMed] [Google Scholar]
- 77.de Kleer IM, Kool M, de Bruijn MJ, et al. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity. 2016;45:1285–1298. [DOI] [PubMed] [Google Scholar]
- 78.Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aalbers R, de Monchy JG, Kauffman HF, et al. Dynamics of eosinophil infiltration in the bronchial mucosa before and after the late asthmatic reaction. Eur Respir J. 1993;6:840–847. [PubMed] [Google Scholar]
- 80.Verbout NG, Jacoby DB, Gleich GJ, Fryer AD. Atropine-enhanced, antigen challenge-induced airway hyperreactivity in guinea pigs is mediated by eosinophils and nerve growth factor. Am J Physiol Lung Cell Mol Physiol. 2009;297:L228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costello RW, Evans CM, Yost BL, et al. Antigen-induced hyperreactivity to histamine: role of the vagus nerves and eosinophils. Am J Physiol. 1999;276:L709–714. [DOI] [PubMed] [Google Scholar]
- 82.Wicher SA, Jacoby DB, Fryer AD. Newly divided eosinophils limit ozone-induced airway hyperreactivity in nonsensitized guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2017;312:L969–L982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wicher SA, Lawson KL, Jacoby DB, Fryer AD, Drake MG. Ozone-induced eosinophil recruitment to airways is altered by antigen sensitization and tumor necrosis factor-alpha blockade. Physiol Rep. 2017;5:e13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spencer LA, Szela CT, Perez SA, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ochkur SI, Jacobsen EA, Protheroe CA, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–7889. [DOI] [PubMed] [Google Scholar]
- 88.Shen HH, Ochkur SI, McGarry MP, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. [DOI] [PubMed] [Google Scholar]
- 89.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ayala LE, Ahmed T. Is there loss of protective muscarinic receptor mechanism in asthma?. Chest. 1989;96:1285–1291. [DOI] [PubMed] [Google Scholar]
- 91.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol. 1989;67:2461–2465. [DOI] [PubMed] [Google Scholar]
- 92.Fryer AD, Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol. 1991;71:2255–2261. [DOI] [PubMed] [Google Scholar]
- 93.Fryer AD, Jacoby DB. Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J Clin Invest. 1992;90:2292–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elbon CL, Jacoby DB, Fryer AD. Pretreatment with an antibody to interleukin-5 prevents loss of pulmonary M2 muscarinic receptor function in antigen-challenged guinea pigs. Am J Respir Cell Mol Biol. 1995;12:320–328. [DOI] [PubMed] [Google Scholar]
- 95.Fryer AD, Costello RW, Yost BL, et al. Antibody to VLA-4, but not to L-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J Clin Invest. 1997;99:2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol. 1997;273: L93–103. [DOI] [PubMed] [Google Scholar]
- 97.Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW. Pretreatment with antibody to eosinophil major basic protein prevents hyper-responsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest. 1997;100:2254–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Costello RW, Fryer AD, Belmonte KE, Jacoby DB. Effects of tachykinin NK1 receptor antagonists on vagal hyperreactivity and neuronal M2 muscarinic receptor function in antigen challenged guinea-pigs. Br J Pharmacol. 1998;124:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans CM, Jacoby DB, Fryer AD. Effects of dexamethasone on antigen-induced airway eosinophilia and M(2) receptor dysfunction. Am J Respir Crit Care Med. 2001;163:1484–1492. [DOI] [PubMed] [Google Scholar]
- 100.Fryer AD, Stein LH, Nie Z, et al. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006;116:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nie Z, Jacoby DB, Fryer AD. Etanercept prevents airway hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. Br J Pharmacol. 2009;156:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol. 1985;91:1318–1326. [DOI] [PubMed] [Google Scholar]
- 103.Gu Q, Lim ME, Gleich GJ, Lee LY. Mechanisms of eosinophil major basic protein-induced hyperexcitability of vagal pulmonary chemosensitive neurons. Am J Physiol Lung Cell Mol Physiol. 2009;296:L453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu Q, Wiggers ME, Gleich GJ, Lee LY. Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am J Physiol Lung Cell Mol Physiol. 2008;294:L544–552. [DOI] [PubMed] [Google Scholar]
- 105.Foster EL, Simpson EL, Fredrikson LJ, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011;6:e22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee JJ, Protheroe CA, Luo H, et al. Eosinophil-dependent skin innervation and itching following contact toxicant exposure in mice. J Allergy Clin Immunol. 2015;135:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doyle AD, Jacobsen EA, Ochkur SI, et al. Homologous recombination into the eosinophil peroxidase locus generates a strain of mice expressing Cre recombinase exclusively in eosinophils. J Leukoc Biol. 2013;94:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yost BL, Gleich GJ, Jacoby DB, Fryer AD. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L627–635. [DOI] [PubMed] [Google Scholar]
- 109.Verhein KC, Jacoby DB, Fryer AD. IL-1 receptors mediate persistent, but not acute, airway hyperreactivity to ozone in guinea pigs. Am J Respir Cell Mol Biol. 2008;39:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sawatzky DA, Kingham PJ, Court E, et al. Eosinophil adhesion to cholinergic nerves via ICAM-1 and VCAM-1 and associated eosinophil degranulation. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1279–1288. [DOI] [PubMed] [Google Scholar]
- 111.Walsh MT, Curran DR, Kingham PJ, et al. Effect of eosinophil adhesion on intracellular signaling in cholinergic nerve cells. Am J Respir Cell Mol Biol. 2004;30:333–341. [DOI] [PubMed] [Google Scholar]
- 112.Kingham PJ, McLean WG, Sawatzky DA, Walsh MT, Costello RW. Adhesion-dependent interactions between eosinophils and cholinergic nerves. Am J Physiol Lung Cell Mol Physiol. 2002;282: L1229–1238. [DOI] [PubMed] [Google Scholar]
- 113.Miao H, Xue QF, Hu QH, et al. In situ expression of ICAM-1 and its mRNA in the lung tissue of asthmatic rats. Clin Hemorheol Microcirc. 1997;17:325–331. [PubMed] [Google Scholar]
- 114.Nie Z, Nelson CS, Jacoby DB, Fryer AD. Expression and regulation of intercellular adhesion molecule-1 on airway parasympathetic nerves. J Allergy Clin Immunol. 2007;119:1415–1422. [DOI] [PubMed] [Google Scholar]
- 115.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990;247:456–459. [DOI] [PubMed] [Google Scholar]
- 116.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. [DOI] [PubMed] [Google Scholar]
- 117.Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–556. [DOI] [PubMed] [Google Scholar]
- 118.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. [DOI] [PubMed] [Google Scholar]
- 119.Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38: 2058–2070. e2051. [DOI] [PubMed] [Google Scholar]
- 120.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. [DOI] [PubMed] [Google Scholar]
- 121.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376: 2448–2458. [DOI] [PubMed] [Google Scholar]
- 122.FitzGerald JM, Bleecker ER, Nair P, et al. Investigators Cs. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. [DOI] [PubMed] [Google Scholar]
- 123.Trifilieff A, Fujitani Y, Coyle AJ, Kopf M, Bertrand C. IL-5 deficiency abolishes aspects of airway remodelling in a murine model of lung inflammation. Clin Exp Allergy. 2001;31:934–942. [DOI] [PubMed] [Google Scholar]
- 124.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kung TT, Stelts DM, Zurcher JA, et al. Involvement of IL-5 in a murine model of allergic pulmonary inflammation: prophylactic and therapeutic effect of an anti-IL-5 antibody. Am J Respir Cell Mol Biol. 1995;13:360–365. [DOI] [PubMed] [Google Scholar]
- 126.Brusselle GG, Kips JC, Tavernier JH, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24: 73–80. [DOI] [PubMed] [Google Scholar]
- 127.Coyle AJ, Le Gros G, Bertrand C, et al. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–59. [DOI] [PubMed] [Google Scholar]
- 128.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol. 1995;12:254–259. [DOI] [PubMed] [Google Scholar]
- 129.Tomaki M, Zhao LL, Lundahl J, et al. Eosinophilopoiesis in a murine model of allergic airway eosinophilia: involvement of bone marrow IL-5 and IL-5 receptor alpha. J Immunol. 2000;165:4040–4050. [DOI] [PubMed] [Google Scholar]
- 130.Inman MD, Ellis R, Wattie J, Denburg JA, O’Byrne PM. Allergen-induced increase in airway responsiveness, airway eosinophilia, and bone-marrow eosinophil progenitors in mice. Am J Respir Cell Mol Biol. 1999;21:473–479. [DOI] [PubMed] [Google Scholar]
- 131.Shen H, O’Byrne PM, Ellis R, Wattie J, Tang C, Inman MD. The effects of intranasal budesonide on allergen-induced production of interleukin-5 and eotaxin, airways, blood, and bone marrow eosinophilia, and eosinophil progenitor expansion in sensitized mice. Am J Respir Crit Care Med. 2002;166:146–153. [DOI] [PubMed] [Google Scholar]
- 132.Kelly EA, Esnault S, Liu LY, et al. Mepolizumab Attenuates Airway Eosinophil Numbers, but Not Their Functional Phenotype, in Asthma. Am J Respir Crit Care Med. 2017;196:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Samitas K, Zervas E, Gaga M. T2-low asthma: current approach to diagnosis and therapy. Curr Opin Pulm Med. 2017;23:48–55. [DOI] [PubMed] [Google Scholar]
- 135.Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. 2013;43:1018–1026. [DOI] [PubMed] [Google Scholar]
- 136.Camargo LDN, Righetti RF, Aristoteles L, et al. Effects of anti-IL-17 on inflammation, remodeling, and oxidative stress in an experimental model of asthma exacerbated by LPS. Front Immunol. 2017;8:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012;5:140–149. [DOI] [PubMed] [Google Scholar]
- 138.Peters M, Kohler-Bachmann S, Lenz-Habijan T, Bufe A. Influence of an allergen-specific Th17 response on remodeling of the airways. Am J Respir Cell Mol Biol. 2016;54:350–358. [DOI] [PubMed] [Google Scholar]
- 139.Kudo M, Melton AC, Chen C, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schnyder-Candrian S, Togbe D, Couillin I, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–1302. [DOI] [PubMed] [Google Scholar]


