Abstract
The present study demonstrated the protective effects of low-molecular-weight adipose-derived stem cell-conditioned medium (LADSC-CM) in a mouse model of dry eye syndrome. Mice subjected to desiccating stress and benzalkonium chloride had decreased tear secretion, impaired corneal epithelial tight junction with microvilli, and decreased conjunctival goblet cells. Topical application of adipose-derived stem cell-conditioned medium (ADSC-CM) stimulated lacrimal tear secretion, preserved tight junction and microvilli of the corneal epithelium, and increased the density of goblet cells and MUC16 expression in the conjunctiva. The low-molecular-weight fractions (< 10 kDa and < 3 kDa) of ADSC-CM (LADSC-CM) provided better protections than the > 10 kDa or > 3 kDa fractions of ADSC-CM. In the in vitro study, desiccation for 10 min or hyperosmolarity (490 osmols) for 24 h caused decreased viability of human corneal epithelial cells, which were reversed by LADSC-CM. The active ingredients in the LADSC-CM were lipophobic and stable after heating and lyophilization. Our study demonstrated that LADSC-CM had beneficial effects on experimental dry eye. It is worthy of further exploration for the active ingredient(s) and the mechanism.
Subject terms: Medical research, Eye diseases
Introduction
Dry eye is a failure of homeostasis of the tear film due to inadequate production, malfunction, or excessive loss of tear components, including mucin, aqueous, and lipid1. The central mechanism is lack of hydration and hyperosmolar tissue damage2, but also involves inflammation and neurosensory abnormalities3,4. Reduced tear secretion leads to inflammation and peripheral nerve damage4, while neural degeneration or injury leads to further decreased tear production, forming a vicious cycle. The prevalence of dry eye is increasing worldwide5–7. Common associated factors include age5–14, female gender7,12–15, extended visual display terminal use8,16–20, sleep disorder21,22, environmental factors9,12,17,23,24, seasonality24, etc., among which age is the most important and universal10.
Current treatments for dry eyes include lubricants or tear supplements for lacking components such as sodium hyaluronate and diquafosol, anti-inflammatory drugs such as corticosteroids or cyclosporine, epithelial growth factor, autologous serum, platelet lysate (or platelet-rich plasma), or/and punctal occlusion, etc25. Among these treatments, only diquafosol, a P2Y2 agonist, claims to increase aqueous, lipid, and mucin components of tear production25. Autologous serum provides lubrication and some biochemical features mimicking natural tears but has only limited conclusions about its effects on symptoms and signs of dry eye26. Punctal occlusion decreases tear outflow but increases the concentration of inflammatory mediators in the tear film27, hence its role in dry eye treatment is inconclusive28. Holland et al. reviewed twenty-six trials investigating thirteen ophthalmic drugs for dry eye and described “None of the large (N > 100) studies demonstrated statistical significance of primary endpoints for both a sign and a symptom endpoint versus a control treatment in the same published trial”29. Therefore, further investigation for better treatment is warranted for this unmet need.
Stem cells are thought promising in degenerative disorders, and their roles in the dry eye have been investigated. Intravenous injection of bone marrow-derived mesenchymal stem cells (BMSC) was reported beneficial for the clinical symptoms in patients with refractory dry eye secondary to GVHD30. Topical application of BMSC showed some advantages in a rat benzalkonium chloride-induced dry eye syndrome31. Periorbital administration of BMSC induced aqueous tear production and increased the number of conjunctival goblet cells in a mice concanavalin A-induced inflammatory dry eye model32. Topical application of adipose-derived mesenchymal stem cells was reported to reduce the inflammatory markers CD4, IL-1, IL-6, and TNFα in dogs with keratoconjunctivitis sicca33.
Apart from the stem cells, the paracrine factors released from stem cells also enhance tissue regeneration and alleviate inflammation34–37. Human uterine cervical stem cells-conditioned medium has been reported to help rat corneal epithelial cells regeneration38,39. In contrast, adipose-derived stem cells (ADSCs)-conditioned medium (ADSC-CM) was found to contain growth factors such as VEGF, FGF-2, HGF, G-CSF, GM-CSF, IL-6, KGF, VEGF, TGF-β3, SDF-1a, etc40,41. ADSC-CM has been reported to speed recovery from liver diseases42,43, protect photoaging of the skin44, promote hair growth45, etc46. Human ADSC derived extracellular vesicles (size about 100 nm) eye drops have recently been shown to alleviate ocular surface damage in a mouse model of dry eye disease47. In this study, we demonstrated the protective effects of ADSC-CM on a mouse model of dry eye, which were attributed to the low-molecular-weight components (< 3 kD) in ADSC-CM (LADSC-CM) containing small-sized molecules (< 2 nm) that are far smaller than extracellular vesicles such as exosomes (with a size range 30–150 nm).
Results
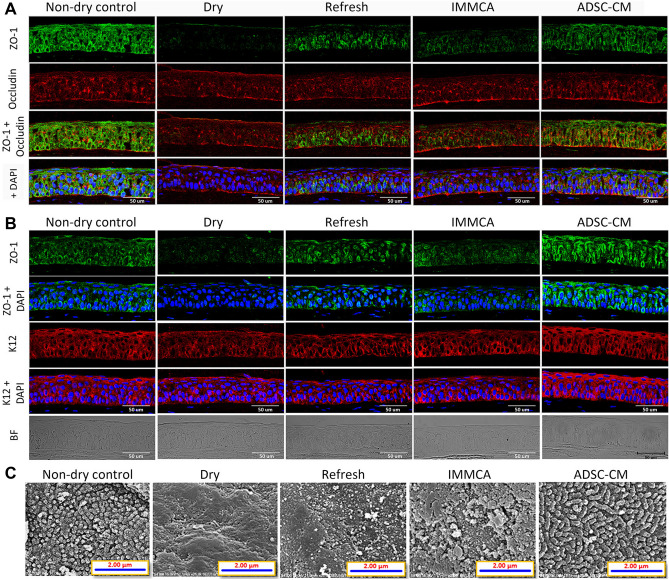
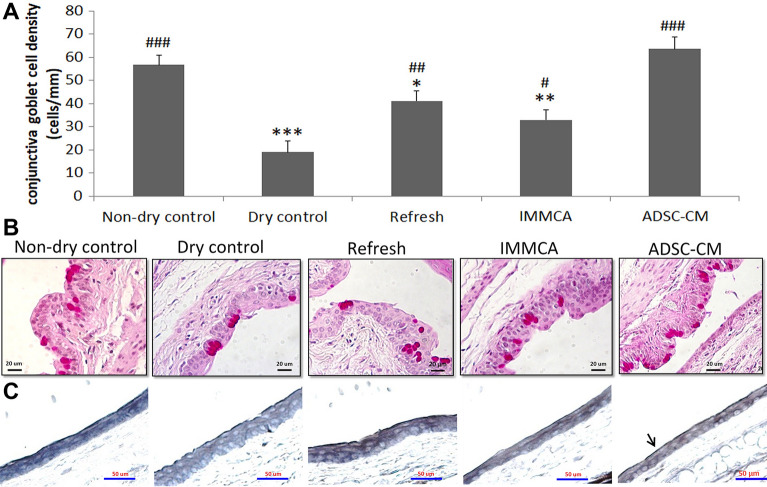
In a controlled-environment chamber (CEC)48–50 combined with benzalkonium chloride (BAC)51–53 BALB/c mice dry eye model, topical application of ADSC-CM showed better protection than Refresh Plus lubricant eye drops (Allergan, Westport, Ireland), Iscove’s modified Dulbecco’s medium supplemented with glutamine, 10% fetal bovine serum, and mesenchymal stem cells culture adjuvant (abbreviated as IMMCA, which was the same medium as ADSC-CM but not conditioned by ADSCs). The confocal microscopy study of corneal epithelium revealed a decreased expression of zonula occludens-1 (ZO-1), occludin, and keratin 12 (K12) in the CEC-induced dry eye. The impaired expression was partially reversed by Refresh Plus and IMMCA, but the best expression was noted in the ADSC-CM group (Fig. 1A,B). The scanning electron microscopy (SEM) study demonstrated that the microvilli of corneal epithelium were lost in the CEC-induced dry eye mice. There were some preserved microvilli but also with bare area in the group treated with Refresh Plus and IMMCA, while most microvilli were well maintained in the group treated with ADSC-CM (Fig. 1C). Periodic acid-Schiff (PAS) staining showed that conjunctival goblet cells were reduced in the CEC-induced dry eye. Refresh Plus and IMMCA partially reversed the reduction, while ADSC-CM best preserved the density of goblet cells (Fig. 2A,B). The immunohistochemical study of MUC16 also showed that the ADSC-CM group had the best expression of MUC16 (Fig. 2C).
Figure 1.
Confocal microscopic examination of the tight junction and scanning electron microscopy of corneal epithelium of BALB/c mice in the controlled-environment chamber (CEC)-induced dry eye model. (A) ZO-1 and occludin expression was suppressed in the BALB/c mice from CEC. Although topical application of Refresh Plus lubricant eye drops or IMMCA alleviated the suppressed expression caused by dry stress, ADSC-CM showed the best rescue. (B) In another experiment, ZO-1 and K12 expressions were also suppressed by dry stress, and the best expression was demonstrated in the ADSC-CM group. (C) Scanning electron microscopy of cornea demonstrated that the microvilli of corneal epithelium were lost in the dry eye mice. Topical application of Refresh Plus lubricant eye drops or IMMCA partially preserved the microvilli of corneal epithelium, while ADSC-CM protected the microvilli best from dry damage. Magnification: ×25,000.
Figure 2.
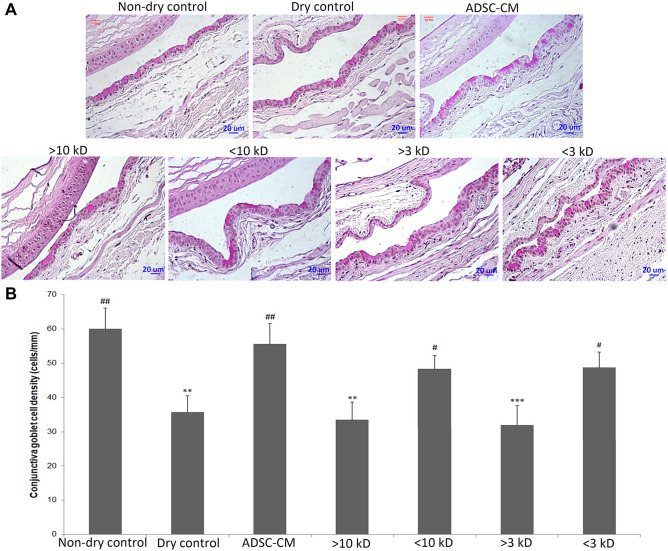
Conjunctival goblet cell density and MUC16 expression of the BALB/c mice in the controlled-environment chamber (CEC)-induced dry eye model. (A,B) Periodic acid–Schiff staining showed that conjunctival goblet cells were reduced in the CEC-induced dry eye. Refresh Plus lubricant eye drops and Iscove’s modified Dulbecco’s medium supplemented with mesenchymal stem cells culture adjuvant (IMMCA) partially reversed the reduction, while ADSC-CM best preserved the density of goblet cells. Data were presented as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 compared with non-dry control. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with dry control group. (N = 5). (C) Immunohistochemical analysis of MUC16 showed that conjunctival MUC16 expression was continuous in the non-dry group (99% continuity) but disrupted in the dry group (89% continuity), Refresh Plus group (90% continuity), and IMMCA group (90% continuity). Topical application of ADSC-CM in the CEC-dry eye mice helped keep the continuous expression of MUC16 (97% continuity).
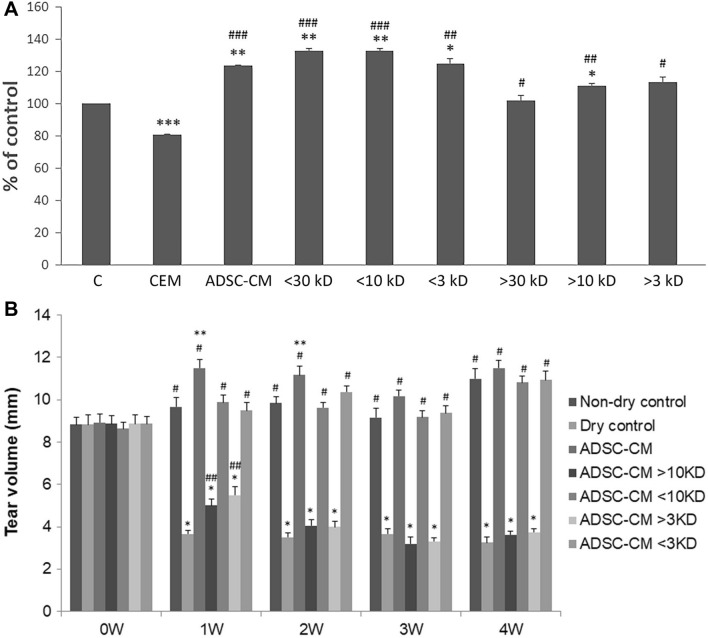
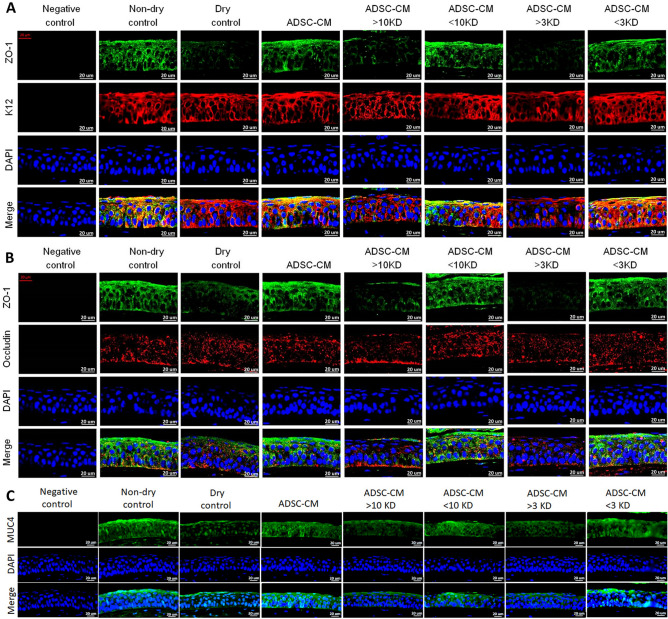
The second part of our study examined the effects of different size fractions of ADSC-CM in the dry eye via the in vitro human corneal epithelial cells (HCEC) desiccation stress study54–58 and the in vivo CEC mice dry eye model. ADSC-CM were fractionated into > 30 kD, < 30 kD, > 10 kD, < 10 kD, > 3 kD, and < 3 kD. The fractions of < 30 kD, < 10 kD, and < 3 kD provided better cell viabilities than those of > 30 kD, > 10 kD, and > 3 kD in the HCEC desiccation stress study (Fig. 3A). In the in vivo CEC mice dry eye experiment, mice treated with the fraction of < 10 kD and < 3 kD had higher tear secretion level (Fig. 3B), stronger expression of ZO-1 and MUC4 (Fig. 4), higher goblet cell density (Fig. 5), and better-preserved microvilli (Fig. 6).
Figure 3.
Different size fractions of ADSC-CM showed different protective effects in the HCEC desiccation stress experiment and tear stimulation effects in the controlled-environment chamber (CEC) mice. (A) HCECs treated with fractions of molecular size < 30 kD, < 10 kD, < 3kD after desiccation showed better viability than thosed treated with > 30 kD, > 10 kD, and > 3 kD. (N = 3) *p < 0.05, **p < 0.01, ***p < 0.001 compared with the non-dry control. #p < 0.05, ##p < 0.01, ### p < 0.001 compared with the corneal epithelial cell basal medium (CEM). (N = 5) (B) In the CEC-induced dry eye study, mice reated with fractions ADSC-CM with molecular size < 10 kD or < 3kD had more tear secretion than thosed treated with > 10 kD or > 3 kD. (N = 4). *p < 0.05, **p < 0.01, *** p < 0.001, compared with Non-dry control. #p < 0.05, # # p < 0.01, # # # p < 0.001, compared with Dry control. The values were expressed as the means ± SD. (N = 5).
Figure 4.
Confocal examination of tight junction and MUC4 expression of the corneal epithelium in the CEC mice. Mice treated with fractions of ADSC-CM with molecular size < 10 kD or < 3kD showed better expression of (A) ZO-1 and K12, (B) ZO-1 and occludin, and (C) MUC4 than those treated with fractions > 10 kD or > 3 kD. (N = 3).
Figure 5.
The conjunctival goblet cell density of the BALB/c mice in the controlled-environment chamber (CEC)-induced dry eye model. (A) The conjunctival goblet cells demonstrated by Periodic acid-Schiff stain were reduced in the controlled-environment chamber (CEC)-induced dry eye. Mice treated with ADSC-CM, fractions of < 10 kD, or < 3 kD, had higher goblet cell densities than those treated with > 10 kD and > 3 kD. The analytical data of the above were presented in (B). Data were in means ± SD. *p < 0.01, compared with Non-dry control. #p < 0.05, ##p < 0.01 compared with Dry control. (N = 3).
Figure 6.
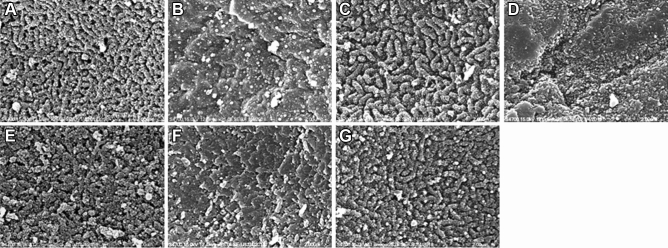
Scanning electron microscopy of cornea demonstrated that the microvilli of corneal epithelium were well-preserved in the non-dry control (A) but were mostly lost in the dry eye mice (B). Topical application of ADSC-CM (C), < 10 kD fraction of ADSC-CM (E), or < 3 kD fraction of ADSC-CM (G) preserved the microvilli best from dry damage. In contrast, those treated with topical > 10 kD fraction of ADSC-CM (D) or > 3 kD (F) did not have good surfaces covered by microvilli. Magnification: ×25,000 (N = 3).
Further characterization of the active components in the LADSC-CM showed that heating to 56 °C for 30 min or 100 °C for 3 min did not reduce the defensive effects of LADSC-CM on HCECs against desiccating stress or hyperosmolarity stress. The protective capabilities did not lose after lyophilization, storage, and reconstitution. Neither did 1:1 hexane extraction for three times change the protective effects of LADSC-CM (Fig. 7).
Figure 7.
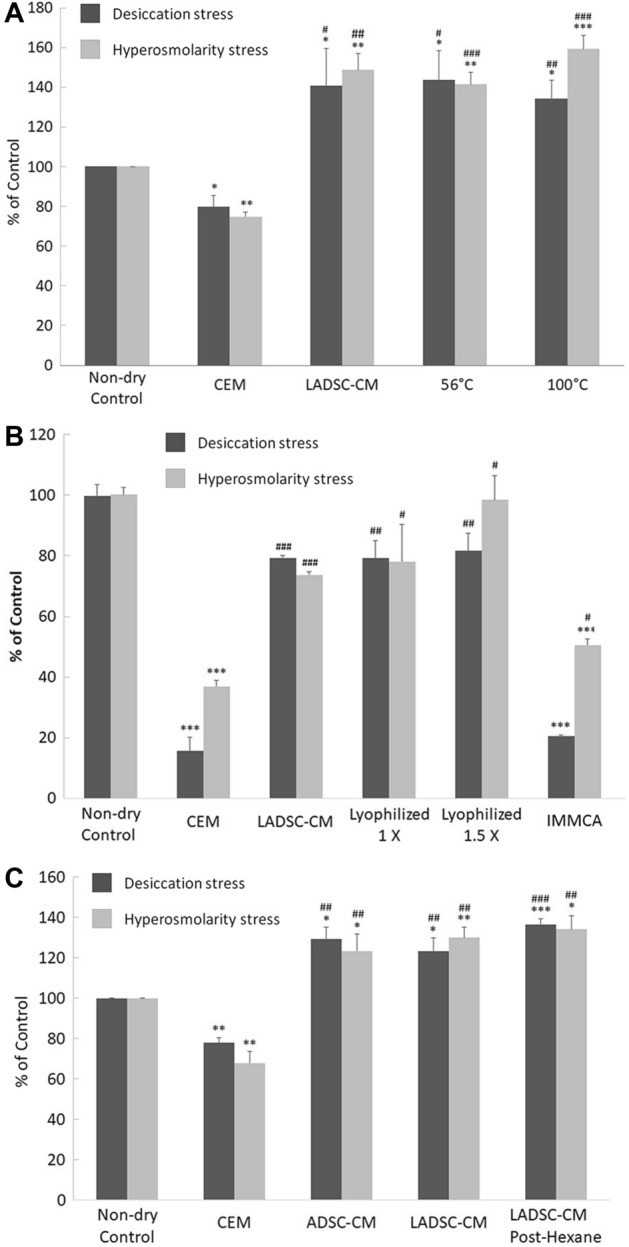
Protective effects of LADSC-CM on HCECs in desiccation stress or hyperosmolarity stress. (A) Heating to 56 °C for 30 min or 100 °C for 3 min did not reduce the protective effects of LADSC-CM. (B) The defensive capabilities after lyophilization, storage, and reconstitution were similar to those before the process. (C) An equal volume of hexane extraction three times did not change the protective effects of LADSC-CM (N = 5 in each experiment).
Discussion
Aging is one of the most critical factors in dry eye59,60, and antiaging approaches have been suggested for dry eye treatment61,62. Stem cells play essential roles against aging, but the application of stem cells might provoke the concerns of tumorigenesis or cellular rejection. In contrast, trophic factors in the conditioned medium secreted by stem cells also help tissue repair but minimize rejection problems as the conditioned medium is devoid of cells63–67. Besides, trophic factors in the conditioned medium might be freeze-dried or manufactured, packaged, and transported more easily. Therefore, stem cell-derived condition medium is promising as a pharmaceutical for regenerative medicine.
BAC has been used to induce dry eye in animals51,52, and the BAC-induced dry eye model was proved stable and widely used for research31,53,68–75. CEC-induced dry eye is another widely used model48,76. Disruption of tight junction, loss of conjunctival goblet cell, and impairment of membrane-associated mucin have been described in dry syndrome77–79. Previous studies described a similar finding in the BAC- or CEC-dry eye model48,80–86. In our study, the mice in the CEC treated with BAC concomitantly had significantly decreased expression of ZO-1 and occludin in the corneal epithelium. Topical application of ADSC-CM reversed or even increased the reduced expression. The corneal epithelium in the ADSC-CM group also showed the best presentation of K12, which meant preserving the characteristics of corneal epithelium87. The preservation of tight junction by ADSC-CM might help the corneal epithelium carry out housekeeping functions that border the external environment, including providing a barrier to fluid loss, toxin irritation, and pathogen entrance. Microvilli injury indicated by the surface covered by microvilli was suggested as the best determining indicator of progressive corneal exposure to dry eye conditions88. In our study, desiccation stress caused the loss of microvilli. The surface covered by microvilli was about 50 to 60% in the groups treated with Refresh Plus lubricant eye drops or IMMCA, while that in the ADSC-CM group was similar to that in the non-dry control group.
ADSC-CM protected not only the tight junction of corneal epithelial cells but also the conjunctival goblet cells and the membrane-associated mucin Muc16 expression. Conjunctival goblet cell density was an ocular biomarker of dry eye89. Muc16 was one of the major membrane-associated mucins expressed on the ocular surface epithelium90. Although Muc16 was expressed only in the conjunctival epithelia in mice91, in contrast to both the corneal and conjunctival epithelia in humans92, the loss of Muc16 in the conjunctiva affected the homeostasis of the corneal epithelium and stroma and upregulated the inflammatory signaling cascade93. In our study, the goblet cell density was decreased in the CEC mice, which was alleviated by Refresh Plus lubricant eye drops, IMMCA, or ADSC-CM. ADSC-CM protected the goblet cells density best to the level similar to that of non-dry mice. The Muc16 expression was continuous in the non-dry group and interrupted in the dry control group. ADSC-CM preserved the integrity of Muc16 expression from dry injury.
HCEC desiccation stress and hyperosmolarity stress are two widely used in vitro models to simulate dry eye conditions55,94–100. In the HCEC desiccation stress study, ADSC-CM showed better protective or regenerative effect than the commercial corneal epithelial basal medium CEM, with the fractions of < 30 kD, < 10 kD, and < 3 kD providing better cell viabilities than those of > 30 kD, > 10 kD, and > 3 kD. From above, we believe something in LADSC-CM (< 3 kD) beneficial for dry eye and are exploring the possible underlying mechanism.
Topical application of human ADSC-derived exosomes (size about 100 nM) has recently been reported to alleviate ocular surface damage in a mouse model of dry eye disease47. In contrast, our study showed better protective effects of the low-molecule-weight (< 3 kD, less than 0.6 nM; < 10 kD, less than 1.5 nM ) fractions of ADSC-CM (LADSC-CM). Mice treated with LADSC-CM had more tear secretion, well-preserved tight junction and MUC16 expression of the cornea, higher conjunctival goblet cell density, and less damaged microvilli of corneal epithelium. LADSC-CM contained only molecules smaller than the known smallest virus that is in the size of about 15 nM, making its clinical application free from infection risk. The protective capabilities remain similar after heating to 56 °C for 30 min or 100 °C for 3 min, or lyophilization and reconstitution, which meant the active ingredients were relatively stable and might be easier for transportation. The active components in LADSC-CM were resistant to hexane extractions and were probably polar in characters.
In conclusion, our study demonstrated the beneficial effects of LADSC-CM in both the in vitro HCEC desiccation stress study and in vivo mice dry-induced ocular surface injury. The active ingredients might be stable and polar molecules. Further investigation of the exact active ingredient(s) and the underlying mechanism is needed.
Methods
Isolation of ADSCs and preparation of ADSC-CM
This study was approved by the Buddhist Tzu Chi General Hospital Internal Review Board (IRB102-130). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants. Human adipose tissue was harvested during cosmetic liposuction from abdominal subcutaneous fat of three women (age: 23, 28, and 30). Stromal-vascular fraction (SVF) cells were isolated using a modified method described by Griesche and colleagues101. Collagenase type I (final concentration: 0.4 mg/mL; Sigma) was added for enzymatic digestion in a hybridization oven (37 °C, 30° angle, 15 rpm, 45 min). Digested adipose tissue was centrifuged at 400 × g for 10 min to generate the SVF pellets for subsequent ADSCs culture. The stemness of the ADSCs was confirmed by their osteogenesis, chondrogenesis, and adipogenesis after induction. ADSCs at passages 2 to 5 were cultured in non-Phenol Red Iscove’s modified Dulbecco’s medium (Gibco™) supplemented with glutamine (200 mM; Gibco™), 10% FBS (HyClone™) and mesenchymal stem cells culture adjuvant (FGF2, 10 ng/ml, R&D Systems; N-acetyl-L-cysteine, 2 mM, Sigma; L-ascorbic acid-2-phosphate, 0.2 mM, Sigma) (The medium was abbreviated as IMMCA). Conditioned mediums were collected after 72 h of culture and mixed, centrifuged at 300 × g for 5 min, filtered through 0.22 μm syringe filter, aliquoted, and frozen for experimental use.
Preparation of different size fractions of ADSC-CM
Collected ADSC-CM was added to 30 kDa, 10 kDa, or 3 kDa Amicon ultra-15 centrifugal filter tube (Millipore, Billerica, USA) or Spectrum® hollow fiber filter (Repligen, Boston, USA and) and centrifuged at 4000 g. The supernatants (> 30 kDa, > 10 kDa, > 3 kDa, respectively) were diluted with IMDM to their initial concentration. The filtered fluid (< 30 kDa, < 10 kDa, < 3 kDa) were also collected for experiments.
Characterization of active components in the LADSC-CM
For the heating test, LADSC-CM was incubated at either 56 °C for 30 min or 100 °C for 3 min and was tested for activity. For the lyophilization test, aliquots of the dialyzed LADSC-CM samples (1 ml) were prepared in 5 ml lyophilized vials followed by lyophilization in a programmable freeze dryer. The lyophilized products were stored at 4 °C for one week and were then reconstituted with water for injection for activity test. For the lipophilicity test, equal volumes of LADSC-CM and hexane (3 mL for each) were mixed and vortexed for 20 min and centrifuged at 2000 rpm for 5 min, and the hexane fraction was discarded. The extraction was repeated three times, the lower layer (aqueous phase) was collected for test.
Human corneal epithelial cells (HCECs) culture
Normal primary HCECs from American Type Culture Collection (ATCC®, Manassas, VA, USA) were maintained according to the instructions. The HCECs were grown in a corneal epithelial cell basal medium supplemented with corneal epithelial cell growth kit components (CEM, ATCC®). The cells were cultured at 37 °C in a moist atmosphere with 5% carbon dioxide. The culture medium was changed every 2 or 3 days. In this study, only sub-confluent HCECs at passage three were used.
Desiccating stress
A modified in vitro desiccation stress on HCECs was used in our study55,57,96. Briefly, 2 × 104 HCECs were seeded in 96-well dishes and cultivated for 24 h to attach to the dishes (about 80% confluence). The medium was aspirated, and the dishes were left dry for 10 min at 37 °C. After desiccation, the testing culture mediums were replenished to the respective culture dishes. The HCECs that did not undergo the desiccation stress were deemed as control. After incubation for four hours, the cells were counted using the Cell Counting Kit-8 (CCK-8 assay).
Hyperosmolarity stress
1.5 × 104 HCECs were seeded in 96 well-dishes and cultivated overnight to attach to the dishes (approximately 60% confluence) and were then treated for 24 h with fresh medium (311 mOsm/kg, normal control) or the medium containing another 90 mM NaCl (490 mOsm/kg, hypertonic groups). After Hypertonic treatment, the cells were cultured in respective testing culture mediums. The cells were estimated using a CCK-8 assay after 4 h of incubation.
CCK-8 assay
Cell viability was measured using Cell Counting Kit-8 (CCK-8; Enzo Life Sciences, Farmingdale, NY, USA). 10 μl CCK-8 reagent was added to cells grown on a 96-well culture plate containing 100 μl culture media. After incubation at 37 °C for 3 h, the cells were estimated via absorbance at 450 nm using a microplate reader (MicroQuant, BioTek Instruments, Inc., Winooski, VT, USA).
Induced murine dry eye model
The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). All experimental procedures were approved by the Laboratory Animal Care and Use Committee at Tzu Chi University. All methods were performed in accordance with the relevant guidelines and regulations. Dry eye-related ocular surface signs of BALB/c mice were induced in a controlled-environment chamber (CEC)48–50 combined with topical BAC51–53. Briefly, 12-week-old female BALB/c mice were housed in CEC with a relative humidity of 10 ± 3%, temperatures of 21–25℃, and airflows of 10–15 L/min. Each experimental and control group consisted of 5 mice. Mice of dry control and experimental groups were housed in the CECs and received topical 0.2% BAC daily. Mice of the non-dry control group were in a chamber of humidity of 75 ± 3%. The experimental groups received the respective testing eye drops twice a day for 28 days. In the first experiment, the testing eye drops were Refresh Plus lubricant eye drops, IMMCA, and ADSC-CM. In the second part experiment, the testing eye drops were the original ADSC-CM, ADSC-CM with a molecular weight > 10 KD, < 10 KD, > 3 KD, and < 3 KD, respectively. Tear secretion assay was performed weekly. The mice were sacrificed with overdoses of pentobarbital at the end of the experiments (28th day), and the eyeballs were harvested for histological and immunohistochemical study and scanning electric microscopic examination.
Tear secretion assay
Tear secretion was estimated by the length of the tear-absorbed, color-changed region on Zone-Quick phenol red thread (Showa Yakuhin Kako Co., LTD., Japan). Briefly, the excess tears were removed for a standard time of 4 s, and the Zone-Quick phenol red threads were then held with jeweler forceps and placed in the lateral fornix for 30 s. The left eyes were measured first and then the right eyes. The average of both eyes was used for analysis.
Histological analysis
The eyes and ocular adnexa were fixed in 10% formaldehyde and embedded in paraffin. Central vertical plane sections of 3 μm thickness were stained with hematoxylin–eosin or Periodic acid-Schiff. The densities of conjunctival goblet cells were calculated using the ImageJ assay.
Immunohistochemistry
The eyes were fixed in 10% formaldehyde. After paraffin embedding, 3-μm-thick sections were dewaxed in xylene, rehydrated in a series of ethanol solutions, and washed twice in distilled water. Antigen retrieval was performed with Dako Target Retrieval Solution pH 9 (Dako, Glostrup, Denmark) for 15 min at 90–95 ˚C. Sections were blocked with 1% BSA in PBS with 0.3% Triton X-100 for at least 1 h at room temperature. The slides were incubated with the rabbit anti-ZO-1(Mid) (1:100; Invitrogen, Camarillo, CA, USA), mouse anti-occludin (1:50; Thermo Scientific, Rockford, IL, USA), or goat anti-cytokeratin 12 (1:50; Santa Cruz, Santa Cruz, CA, USA) overnight at 4 °C, followed by Alexa Fluor 488 donkey anti-rabbit IgG (H + L) (1:800; Jackson ImmunoResearch, West Grove, PA, USA), Dylight 550-conjugated goat anti-mouse IgG (H + L) or Dylight 550-conjugated donkey anti-goat IgG (H + L) (1:500; Bethyl Laboratories, Montgomery, TX, USA) for 1 h at room temperature. The nucleus was counterstained with 4’,6’-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR, USA). The slides were mounted and examined with a Zeiss LSM 510 META confocal microscope. In negative controls, the primary antibody was substituted with the blocking buffer.
MUC16 staining was performed on 8-μm-thick sections using Histofine Mouse Stain Kit (Nichirei, Tokyo, Japan). The sections were incubated with mouse anti-MUC16 (1:50; Santa Cruz, Santa Cruz, CA, USA) overnight at 4 °C, and finally with Histofine Simple Stain Max PO for 10 min. The horseradish peroxidase reaction was developed with 3,3′-diaminobenzidine tetrahydrochloride w/Co (D-0426, Sigma, Saint Louise, Missouri, USA). Negative control studies were also performed without using the primary antibodies. After dehydration in graded ethanol and xylene, sections were mounted in Histokit (Hecht Assistent, Sondheim, Germany) and analyzed.
Scanning electron microscopy analysis
Fresh corneas were first fixed in 2% paraformaldehyde for 24 h, and then in 2.5% glutaraldehyde solution in 0.2 M cacodylate buffer and 1% tannic acid at pH 7.0–7.3 for another 24 h, followed by postfixation with 1% osmium tetroxide solution in 0.2 M cacodylate buffer solution for 1 h. Samples were then dehydrated by a critical point dryer (Hitachi Ltd., Japan) and coated with platinum in an ion sputter coater (Hitachi Ltd., Japan). Finally, the samples were observed and photographed with the scanning electron microscope (Hitachi Ltd., Japan).
Statistical analysis
Data were expressed as means ± SD. Only one sample from each mouse was used for the analysis of each examination result. In the tear secretion assay, the average of estimates from both eyes was used. For goblet cell density, only the left eye of each mouse was sectioned for Periodic acid-Schiff stain and calculation. For CCK viability assay, each sample in the same group was from different rounds of experiments. One-way ANOVA and two-sample t-test were used to compare CCK assay, tear secretion assay, and conjunctival goblet cell density. A p < 0.05 was considered statistically significant.
Acknowledgements
This study was supported by Tzu Chi Foundation and Ministry of Science and Technology, R.O.C. (Grant No.105-2314-B-303-012). The funding sources had no involvement in study design, data collection and analysis, and writing and submission.
Author contributions
Y.C.L. designed the study, Y.C.L. and L.Y.S. analyzed the data, Y.C.L. wrote the main manuscript text, and Y.C.L. and J.R.Z. prepared the figures. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Craig JP, et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Bron AJ, et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017;15:438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Research in dry eye Report of the research subcommittee of the international dry eye workshop (2007) Ocul. Surf. 2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte C, et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017;15:404–437. doi: 10.1016/j.jtos.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu NN, Liu L, Li J, Sun YZ. Prevalence of and risk factors for dry eye symptom in mainland china: A systematic review and meta-analysis. J. Ophthalmol. 2014;2014:748654. doi: 10.1155/2014/748654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo YK, et al. Dry eye disease: A review of epidemiology in taiwan, and its clinical treatment and merits. J. Clin. Med. 2019 doi: 10.3390/jcm8081227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dana R, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age united states health care system. Am. J. Ophthalmol. 2019;202:47–54. doi: 10.1016/j.ajo.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Courtin R, et al. Prevalence of dry eye disease in visual display terminal workers: A systematic review and meta-analysis. BMJ Open. 2016;6:e009675. doi: 10.1136/bmjopen-2015-009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouen PA, White ML. Dry eye disease: Prevalence, assessment, and management. Home Healthc. Now. 2018;36:74–83. doi: 10.1097/NHH.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton F, et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Mashaghi A, Hong J, Chauhan SK, Dana R. Ageing and ocular surface immunity. Br. J. Ophthalmol. 2017;101:1–5. doi: 10.1136/bjophthalmol-2015-307848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tandon R, et al. Association of dry eye disease and sun exposure in geographically diverse adult (>/=40 years) populations of India: The SEED (sun exposure, environment and dry eye disease) study—Second report of the ICMR-EYE SEE study group. Ocul. Surf. 2020;18:718–730. doi: 10.1016/j.jtos.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am. J. Ophthalmol. 2017;182:90–98. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Caffery B, et al. Prevalence of dry eye disease in Ontario, Canada: A population-based survey. Ocul. Surf. 2019;17:526–531. doi: 10.1016/j.jtos.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Vehof J, Snieder H, Jansonius N, Hammond CJ. Prevalence and risk factors of dry eye in 79,866 participants of the population-based lifelines cohort study in the Netherlands. Ocul. Surf. 2020 doi: 10.1016/j.jtos.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard AL, Wolffsohn JS. Digital eye strain: Prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018;3:e000146. doi: 10.1136/bmjophth-2018-000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talens-Estarelles C, Garcia-Marques JV, Cervino A, Garcia-Lazaro S. Use of digital displays and ocular surface alterations: A review. Ocul. Surf. 2020 doi: 10.1016/j.jtos.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Titiyal JS, Falera RC, Kaur M, Sharma V, Sharma N. Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study. Indian J. Ophthalmol. 2018;66:207–211. doi: 10.4103/ijo.IJO_698_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanyuda A, et al. Physical inactivity, prolonged sedentary behaviors, and use of visual display terminals as potential risk factors for dry eye disease: JPHC-NEXT study. Ocul. Surf. 2020;18:56–63. doi: 10.1016/j.jtos.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Yamanishi R, et al. Changes in distribution of dry eye diagnostic status among visual display terminal workers according to the revised criteria of the Asia Dry Eye Society. Cornea. 2020;39:578–583. doi: 10.1097/ICO.0000000000002218. [DOI] [PubMed] [Google Scholar]
- 21.Ayaki M, et al. Sleep disorders are a prevalent and serious comorbidity in dry eye. Invest. Ophthalmol. Vis. Sci. 2018;59:143–150. doi: 10.1167/iovs.17-23467. [DOI] [PubMed] [Google Scholar]
- 22.Matossian C, Song X, Chopra I, Sainski-Nguyen A, Ogundele A. The prevalence and incidence of dry eye disease among patients using continuous positive airway pressure or other nasal mask therapy devices to treat sleep apnea. Clin. Ophthalmol. 2020;14:3371–3379. doi: 10.2147/OPTH.S274949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong JY, Lee YC, Hsieh CJ, Tseng CC, Yiin LM. Association between dry eye disease, air pollution and weather changes in Taiwan. Int. J. Environ. Res. Public Health. 2018 doi: 10.3390/ijerph15102269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzam DB, et al. A novel epidemiological approach to geographically mapping population dry eye disease in the United States through Google Trends. Cornea. 2020 doi: 10.1097/ICO.0000000000002579. [DOI] [PubMed] [Google Scholar]
- 25.Kojima T, Dogru M, Kawashima M, Nakamura S, Tsubota K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020 doi: 10.1016/j.preteyeres.2020.100842. [DOI] [PubMed] [Google Scholar]
- 26.Shtein RM, et al. Autologous serum-based eye drops for treatment of ocular surface disease: A report by the American Academy of Ophthalmology. Ophthalmology. 2020;127:128–133. doi: 10.1016/j.ophtha.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Coursey TG, de Paiva CS. Managing Sjogren's syndrome and non-Sjogren syndrome dry eye with anti-inflammatory therapy. Clin. Ophthalmol. 2014;8:1447–1458. doi: 10.2147/OPTH.S35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ervin AM, Law A, Pucker AD. Punctal occlusion for dry eye syndrome: summary of a Cochrane systematic review. Br. J. Ophthalmol. 2019;103:301–306. doi: 10.1136/bjophthalmol-2018-313267. [DOI] [PubMed] [Google Scholar]
- 29.Holland EJ, Darvish M, Nichols KK, Jones L, Karpecki PM. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019;17:412–423. doi: 10.1016/j.jtos.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Weng J, et al. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol. Ther. 2012;20:2347–2354. doi: 10.1038/mt.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyazyildiz E, et al. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014;2014:250230. doi: 10.1155/2014/250230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MJ, et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 2015;23:139–146. doi: 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sgrignoli MR, et al. Reduction in the inflammatory markers CD4, IL-1, IL-6 and TNFalpha in dogs with keratoconjunctivitis sicca treated topically with mesenchymal stem cells. Stem Cell Res. 2019;39:101525. doi: 10.1016/j.scr.2019.101525. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osugi M, et al. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A. 2012;18:1479–1489. doi: 10.1089/ten.TEA.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon SH, Bhang SH, Jang HK, Rhim T, Kim BS. Conditioned medium of adipose-derived stromal cell culture in three-dimensional bioreactors for enhanced wound healing. J. Surg. Res. 2015;194:8–17. doi: 10.1016/j.jss.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 37.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res. Int. 2014;2014:965849. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sendon-Lago J, et al. Corneal regeneration by conditioned medium of human uterine cervical stem cells is mediated by TIMP-1 and TIMP-2. Exp. Eye Res. 2019;180:110–121. doi: 10.1016/j.exer.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Bermudez MA, et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest. Ophthalmol. Vis. Sci. 2015;56:983–992. doi: 10.1167/iovs.14-15859. [DOI] [PubMed] [Google Scholar]
- 40.Kim WS, et al. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Park BS, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol. Surg. 2008;34:1323–1326. doi: 10.1111/j.1524-4725.2008.34283.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu T, Wu S, Ouyang G. Periostin: A new extracellular regulator of obesity-induced hepatosteatosis. Cell Metab. 2014;20:562–564. doi: 10.1016/j.cmet.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Nahar S, et al. Cytokines in adipose-derived mesenchymal stem cells promote the healing of liver disease. World J. Stem Cells. 2018;10:146–159. doi: 10.4252/wjsc.v10.i11.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim WS, Park BS, Sung JH. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch. Dermatol. Res. 2009;301:329–336. doi: 10.1007/s00403-009-0951-9. [DOI] [PubMed] [Google Scholar]
- 45.Fukuoka H, Narita K, Suga H. Hair regeneration therapy: Application of adipose-derived stem cells. Curr Stem Cell Res. Ther. 2017;12:531–534. doi: 10.2174/1574888X12666170522114307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: A review. Stem Cell Res. Ther. 2020;11:312. doi: 10.1186/s13287-020-01831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu C, et al. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci. Rep. 2020;10:14521. doi: 10.1038/s41598-020-71337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barabino S, et al. The controlled-environment chamber: A new mouse model of dry eye. Invest. Ophthalmol. Vis. Sci. 2005;46:2766–2771. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 49.Kim CE, et al. RGN-259 (thymosin beta4) improves clinically important dry eye efficacies in comparison with prescription drugs in a dry eye model. Sci. Rep. 2018;8:10500. doi: 10.1038/s41598-018-28861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim CE, Kim YJ, Hwang MW, Park YJ, Yang J. Cevimeline-induced anti-inflammatory effect through upregulations of mucins in the ocular surface of a dry eye mouse model. Biomed. Pharmacother. 2021;139:111571. doi: 10.1016/j.biopha.2021.111571. [DOI] [PubMed] [Google Scholar]
- 51.Xiong C, et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Invest. Ophthalmol. Vis. Sci. 2008;49:1850–1856. doi: 10.1167/iovs.07-0720. [DOI] [PubMed] [Google Scholar]
- 52.Lin Z, et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol. Vis. 2011;17:257–264. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang R, et al. Dose-dependent benzalkonium chloride toxicity imparts ocular surface epithelial changes with features of dry eye disease. Ocul. Surf. 2020;18:158–169. doi: 10.1016/j.jtos.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Paulsen K, Maile S, Giebel J, Tost FH. Lubricating agents differ in their protection of cultured human epithelial cells against desiccation. Med. Sci. Monit. 2008;14:PI12–16. [PubMed] [Google Scholar]
- 55.Higuchi A, Kawakita T, Tsubota K. IL-6 induction in desiccated corneal epithelium in vitro and in vivo. Mol. Vis. 2011;17:2400–2406. [PMC free article] [PubMed] [Google Scholar]
- 56.Tost F, Keiss R, Grossjohann R, Jurgens C, Giebel J. Effect of different artificial tears against desiccation in cultured human epithelial cells. Med. Sci. Monit. 2012;18:BR188–192. doi: 10.12659/msm.882728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng X, Goto T, Shiraishi A, Ohashi Y. In vitro efficacy of ocular surface lubricants against dehydration. Cornea. 2013;32:1260–1264. doi: 10.1097/ICO.0b013e31829cfd44. [DOI] [PubMed] [Google Scholar]
- 58.Hwang SB, et al. Protective effects of cyclosporine a emulsion versus cyclosporine a cationic emulsion against desiccation stress in human corneal epithelial cells. Cornea. 2020;39:508–513. doi: 10.1097/ICO.0000000000002244. [DOI] [PubMed] [Google Scholar]
- 59.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003;136:318–326. doi: 10.1016/S0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 60.Bian F, et al. Age-associated antigen-presenting cell alterations promote dry-eye inducing Th1 cells. Mucosal Immunol. 2019;12:897–908. doi: 10.1038/s41385-018-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsubota K, et al. The era of antiaging ophthalmology comes of age: Antiaging approach for dry eye treatment. Ophthalmic Res. 2010;44:146–154. doi: 10.1159/000316594. [DOI] [PubMed] [Google Scholar]
- 62.Tsubota K, et al. The antiaging approach for the treatment of dry eye. Cornea. 2012;31(Suppl 1):S3–8. doi: 10.1097/ICO.0b013e31826a05a8. [DOI] [PubMed] [Google Scholar]
- 63.Yang D, et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS ONE. 2013;8:e59020. doi: 10.1371/journal.pone.0059020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burlacu A, Grigorescu G, Rosca AM, Preda MB, Simionescu M. Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev. 2013;22:643–653. doi: 10.1089/scd.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bian S, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. (Berl) 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 66.Lopez-Verrilli MA, et al. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi: 10.1016/j.neuroscience.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 67.Monsel A, Zhu YG, Gudapati V, Lim H, Lee JW. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin. Biol. Ther. 2016;16:859–871. doi: 10.1517/14712598.2016.1170804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C, et al. Research on the stability of a rabbit dry eye model induced by topical application of the preservative benzalkonium chloride. PLoS ONE. 2012;7:e33688. doi: 10.1371/journal.pone.0033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao X, et al. Therapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse model. Invest. Ophthalmol. Vis. Sci. 2012;53:191–197. doi: 10.1167/iovs.11-8553. [DOI] [PubMed] [Google Scholar]
- 70.Xiao X, et al. Amniotic membrane extract ameliorates benzalkonium chloride-induced dry eye in a murine model. Exp. Eye Res. 2013;115:31–40. doi: 10.1016/j.exer.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z, et al. Therapeutic effects of topical doxycycline in a benzalkonium chloride-induced mouse dry eye model. Invest. Ophthalmol. Vis. Sci. 2014;55:2963–2974. doi: 10.1167/iovs.13-13577. [DOI] [PubMed] [Google Scholar]
- 72.Lin Z, et al. Serine protease inhibitor A3K suppressed the formation of ocular surface squamous metaplasia in a mouse model of experimental dry eye. Invest. Ophthalmol. Vis. Sci. 2014;55:5813–5820. doi: 10.1167/iovs.13-13546. [DOI] [PubMed] [Google Scholar]
- 73.Ehrenberg M, Zolotariov E, Loeb E, Poliansky V, Levy A. Combining sodium hyaluronate and polyvinylpyrrolidone therapies for the rabbit cornea: A new approach to relief of the human dry eye syndrome. Curr. Eye Res. 2015;40:913–922. doi: 10.3109/02713683.2014.969810. [DOI] [PubMed] [Google Scholar]
- 74.Choi JH, et al. The efficiency of cyclosporine a-eluting contact lenses for the treatment of dry eye. Curr Eye Res. 2019;44:486–496. doi: 10.1080/02713683.2018.1563702. [DOI] [PubMed] [Google Scholar]
- 75.Qu M, et al. Therapeutic effects of STAT3 inhibition on experimental murine dry eye. Invest. Ophthalmol. Vis. Sci. 2019;60:3776–3785. doi: 10.1167/iovs.19-26928. [DOI] [PubMed] [Google Scholar]
- 76.Starr CE, et al. Dry eye disease flares: A rapid evidence assessment. Ocul. Surf. 2021;22:51–59. doi: 10.1016/j.jtos.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Shoari A, Kanavi MR, Rasaee MJ. Inhibition of matrix metalloproteinase-9 for the treatment of dry eye syndrome; A review study. Exp. Eye Res. 2021;205:108523. doi: 10.1016/j.exer.2021.108523. [DOI] [PubMed] [Google Scholar]
- 78.Fahim MM, Haji S, Koonapareddy CV, Fan VC, Asbell PA. Fluorophotometry as a diagnostic tool for the evaluation of dry eye disease. BMC Ophthalmol. 2006;6:20. doi: 10.1186/1471-2415-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul. Surf. 2004;2:124–130. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 80.Chen W, et al. A murine model of dry eye induced by an intelligently controlled environmental system. Invest. Ophthalmol. Vis. Sci. 2008;49:1386–1391. doi: 10.1167/iovs.07-0744. [DOI] [PubMed] [Google Scholar]
- 81.Pelegrino FS, Pflugfelder SC, De Paiva CS. Low humidity environmental challenge causes barrier disruption and cornification of the mouse corneal epithelium via a c-jun N-terminal kinase 2 (JNK2) pathway. Exp. Eye. Res. 2012;94:150–156. doi: 10.1016/j.exer.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Droy-Lefaix MT, Bueno L, Caron P, Belot E, Roche O. Ocular inflammation and corneal permeability alteration by benzalkonium chloride in rats: A protective effect of a myosin light chain kinase inhibitor. Invest. Ophthalmol. Vis. Sci. 2013;54:2705–2710. doi: 10.1167/iovs.12-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Portal C, Gouyer V, Gottrand F, Desseyn JL. Preclinical mouse model to monitor live Muc5b-producing conjunctival goblet cell density under pharmacological treatments. PLoS ONE. 2017;12:e0174764. doi: 10.1371/journal.pone.0174764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barabino S, Rolando M, Chen L, Dana MR. Exposure to a dry environment induces strain-specific responses in mice. Exp. Eye Res. 2007;84:973–977. doi: 10.1016/j.exer.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Marko CK, Tisdale AS, Spurr-Michaud S, Evans C, Gipson IK. The ocular surface phenotype of Muc5ac and Muc5b null mice. Invest. Ophthalmol. Vis. Sci. 2014;55:291–300. doi: 10.1167/iovs.13-13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barabino S, Antonelli S, Cimbolini N, Mauro V, Bouzin M. The effect of preservatives and antiglaucoma treatments on the ocular surface of mice with dry eye. Invest. Ophthalmol. Vis. Sci. 2014;55:6499–6504. doi: 10.1167/iovs.14-14548. [DOI] [PubMed] [Google Scholar]
- 87.Liu CY, et al. Cornea-specific expression of K12 keratin during mouse development. Curr. Eye Res. 1993;12:963–974. doi: 10.3109/02713689309029222. [DOI] [PubMed] [Google Scholar]
- 88.Julio G, Merindano MD, Canals M, Caum C, Rallo M. Indicators of progressive corneal exposure to dry eye conditions. Optom. Vis. Sci. 2012;89:1042–1049. doi: 10.1097/OPX.0b013e31825da352. [DOI] [PubMed] [Google Scholar]
- 89.Usuba FS, et al. Dry eye in rheumatoid arthritis patients under TNF-inhibitors: Conjunctival goblet cell as an early ocular biomarker. Sci. Rep. 2020;10:14054. doi: 10.1038/s41598-020-70944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008;8:477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, et al. MUC16 expression during embryogenesis, in adult tissues, and ovarian cancer in the mouse. Differentiation. 2008;76:1081–1092. doi: 10.1111/j.1432-0436.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gipson IK. Distribution of mucins at the ocular surface. Exp. Eye Res. 2004;78:379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 93.Shirai K, et al. Effects of the loss of conjunctival Muc16 on corneal epithelium and stroma in mice. Invest. Ophthalmol. Vis. Sci. 2014;55:3626–3637. doi: 10.1167/iovs.13-12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shivakumar S, et al. Chloroquine protects human corneal epithelial cells from desiccation stress induced inflammation without altering the autophagy flux. Biomed. Res. Int. 2018;2018:7627329. doi: 10.1155/2018/7627329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Redfern RL, Barabino S, Baxter J, Lema C, McDermott AM. Dry eye modulates the expression of toll-like receptors on the ocular surface. Exp. Eye Res. 2015;134:80–89. doi: 10.1016/j.exer.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsuo T. Trehalose protects corneal epithelial cells from death by drying. Br. J. Ophthalmol. 2001;85:610–612. doi: 10.1136/bjo.85.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Narayanan S, Manning J, Proske R, McDermott AM. Effect of hyperosmolality on beta-defensin gene expression by human corneal epithelial cells. Cornea. 2006;25:1063–1068. doi: 10.1097/01.ico.0000228785.84581.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schulze U, et al. Trefoil factor family peptide 3 (TFF3) is upregulated under experimental conditions similar to dry eye disease and supports corneal wound healing effects in vitro. Invest. Ophthalmol. Vis. Sci. 2014;55:3037–3042. doi: 10.1167/iovs.13-13423. [DOI] [PubMed] [Google Scholar]
- 99.Igarashi T, et al. Short-time exposure of hyperosmolarity triggers interleukin-6 expression in corneal epithelial cells. Cornea. 2014;33:1342–1347. doi: 10.1097/ICO.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 100.Lema C, Reins RY, Redfern RL. High-mobility group box 1 in dry eye inflammation. Invest. Ophthalmol. Vis. Sci. 2018;59:1741–1750. doi: 10.1167/iovs.17-23363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Griesche N, et al. A simple modification of the separation method reduces heterogeneity of adipose-derived stem cells. Cells Tissues Organs. 2010;192:106–115. doi: 10.1159/000289586. [DOI] [PubMed] [Google Scholar]