Abstract
The obesity epidemic continues its relentless advance and is paralleled by an increase in the incidence of AF. Several epidemiological studies have highlighted obesity as an independent risk factor for the development of AF. This relationship is likely multifactorial through a number of interacting mechanisms. Weight loss through lifestyle changes or surgery has been associated with reverse remodelling of the atrial substrate and subsequent reduction in AF, making it an essential pillar in the management of AF in obese patients. In this review, the epidemiological data that support the obesity–AF relationship, the current insights into the underlying pathophysiological mechanism, the impact of weight loss on reverse remodelling and AF reduction, and the strategies to achieve weight loss in patients with AF are discussed.
Keywords: AF, obesity, atrial substrate, epicardial atrial adipose tissue, reverse remodelling, weight loss, risk factor management
The obesity epidemic continues its relentless advance, currently affecting 2 billion people.[1] Obesity is associated with an increased burden of a spectrum of diseases, with the focus in this article being on AF. The health-related burden associated with obesity is estimated to have a substantial economic impact.[2] In alignment with this epidemic, the prevalence of AF is increasing. In the US, a large observational study showed a significant increase in the incidence of new AF between 1980 and 2000, and estimated that the projected number of persons with AF in the US would exceed 10 million by 2050.[3] Similar trends have been seen in Europe and Australia.[4,5] The pathophysiological mechanisms that underline the AF–obesity relationship remain incompletely understood. There are, however, consistent data that support weight management in improving AF outcomes. In this review, we discuss the AF–obesity relationship, the proposed underlying mechanisms, and the impact of weight loss on AF and its arrhythmogenic substrate.
The Obesity–AF Association
A number of observational cohort studies have highlighted obesity as an independent risk factor for the development of AF.[6–11] In the Framingham Heart Study, 526 out of 5,282 participants developed AF over a mean follow-up of 13.7 years.[11] Obesity was found to be an independent predictor for incident AF after adjusting for traditional risk factors. Similar findings were found in the Women’s Heart Study, where incident AF was confirmed in 2.4% of the cohort over a 12.9-year follow-up period.[9] BMI was linearly associated with AF risk, with a 4.7% increase in risk with each kg/m[2].
To examine the impact of the obesity-associated traditional risk factors on the AF risk, a recent retrospective Korean study studied the AF risk among a cohort of metabolically healthy obese individuals (no diabetes, hypertension and dyslipidaemia) compared with metabolically unhealthy obese and non-obese individuals.[8] Compared with non-obese individuals, the metabolically healthy obese cohort had a 20% increased AF risk, whereas metabolic unhealthiness increased the AF risk by 40%. In addition, elevated BMI has been shown to be associated with AF clinical phenotype progression from paroxysmal to persistent.[10] In the Olmsted County study, where 3,248 patients with paroxysmal AF were followed up, 17% progressed to persistent AF. Obesity was independently associated with progression to persistent AF, even after adjusting for traditional risk factors, signalling its impact on the arrhythmogenic substrate of AF.
However, while the aforementioned evidence supports the obesity–AF relationship, the mechanism underpinning the impact of obesity on the incidence of AF remains incompletely understood and is likely multifactorial (Figure 1). In the next section, we discuss the potential mechanisms that underpin the obesity–AF relationship, highlighting the recent insights from both animal and human studies.
Figure 1: Obesity, AF, Weight Loss and Reverse Remodelling.
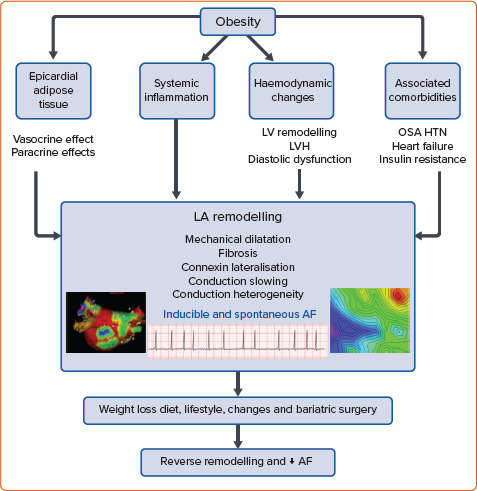
LA = left atrium; LV = left ventricle; LVH = left ventricular hypertrophy; OSA = obstructive sleep apnoea; HTN = hypertension.
Pathophysiological Changes in Obesity and AF
Haemodynamic Changes
Obesity leads to left atrial and ventricular remodelling through a number of haemodynamic mechanisms. Obesity is associated with an increase in both cardiac output and systolic blood pressure with little change in the heart rate.[12] This is related to both activation of the renin–angiotensin–aldosterone system and enhancement of the sympathetic nervous system.[13] This increase in cardiac output will increase venous return to the heart, leading to enhanced atrial and ventricular wall stretch, and eventually dilatation.[14] Moreover, obesity has a haemodynamic impact on the heart through the associated medical comorbidities, such as hypertension, sleep apnoea and insulin resistance. Hypertension increases left ventricular afterload, leading to further structural remodelling in the form of left ventricular hypertrophy, diastolic dysfunction and left atrial dilatation.[15] Obesity-associated sleep-disordered breathing is associated with surges in symptomatic nervous system activity through intermittent episodes of hypoxia, which has been shown to be associated with a significant increase in the prevalence of both atrial and ventricular arrhythmia, even after adjusting for traditional risk factors.[16]
Left Atrial Remodelling and Left Atrial Substrate
Animal studies have consistently demonstrated the presence of an arrhythmogenic substrate in association with obesity. In an ovine model, progressive changes in electrical and structural atrial remodelling were seen in a cohort of 30 sheep being fed a high-calorie diet over an 8-month period.[17] Increasing weight was associated with increasing left atrial (LA) volume, LA fibrosis and upregulation of inflammatory markers. The net effect was decreased conduction velocity and an increase in conduction heterogeneity, with an associated increase in both inducible and spontaneous AF. Other animal studies noted that a high-fat diet could increase AF duration due to slow atrial conduction and reduced pulmonary vein refractoriness. These changes could occur without necessarily being accompanied by development of obesity.[18,19]
Similar abnormal substrate has been seen in human studies. A large observational longitudinal study found that obesity was a strong predictor of LA enlargement, after adjusting for age and sex.[5] Moreover, Mahajan et al. characterised the electroanatomic atrial remodelling and epicardial adipose tissue in a cohort of obese patients and compared it with a non-obese cohort.[20] Obesity was associated with an increase in all measures of epicardial adipose tissue, with a predominant distribution adjacent to the posterior left atrium and the atrioventricular groove. Obese patients had reduced global conduction velocity, increased fractionation and increased low-voltage areas. Low-voltage areas were predominantly seen in the posterior and/or inferior LA, matching the location of epicardial adipose tissue (EAT) on cardiovascular MRI.
Role of Epicardial Adipose Tissue
The availability of cross-sectional imaging modalities such as cardiac computed tomography has enabled us to examine the relationship between the quantity and quality of the EAT and AF. This layer of adipose tissue is located between the visceral pericardial and the epicardial surface, and there is no fascial layer that separates the EAT and the myocardium. Epidemiological studies utilising non-invasive imaging with a focus on the abundance of EAT have consistently demonstrated an independent association between EAT volume and incident AF, even after adjusting for AF risk factors, including BMI and LA enlargement.[21,22] Beyond predicting AF, this association appears to impact on the outcome of AF ablation.[23] However, the underlying electrophysiological, cellular and molecular mechanisms that link epicardial adipose tissue with AF progression remain poorly defined, and a number of mechanistic theories have been proposed.
The proximity of the EAT layer to the myocardium means the EAT can exert important paracrine and vasocrine effects on neighbouring cardiomyocytes.[24,25] In chronic inflammatory disorders, the epicardium becomes a site of deranged adipogenesis, leading to secretion of pro-inflammatory adipokines, such as interleukin-1β, interleukin-6, activin-A and tumour necrosis factor-alpha, which may play an important role in the development of atrial fibrosis.[26] In an ovine model, Mahajan et al. demonstrated that sustained obesity resulted in global biatrial endocardial electrical and structural remodelling, and associated EAT infiltration in the posterior LA wall.[27] A cohort of 10 sheep fed a calorie-dense diet to induce obesity were compared with 10 lean sheep, and all 20 sheep underwent invasive and non-invasive assessments of their atrial substrate. Compared with the lean sheep cohort, the obese sheep demonstrated both abnormal structural (increased LA volume and pressure) and electrical (reduced atrial conduction velocity, increased conduction heterogeneity, increased fractionated electrograms and decreased posterior LA voltage) remodelling. This was associated with more frequent, prolonged and greater cumulative duration of AF. Epicardial fat was seen to infiltrate the posterior LA in the obese group, and was associated with reduced endocardial voltage in this region.
Prior tissue culture studies have shown that epicardial adipose tissue is capable of releasing adipo-fibrokines, promoting atrial fibrosis and its associated electrical substrate.[28,29] The critical role local EAT has on the atrial substrate was elegantly demonstrated in a recent study.[30] In a cohort of patients undergoing cardiac surgery without AF, higher local epicardial adipose tissue volume on imaging correlated with slowed conduction, greater electrogram fractionation, increased fibrosis and lateralisation of cardiomyocyte connexin-40 (Figure 2). Moreover, atrial conduction heterogeneity was increased with more extensive myocardial adipose infiltration. Cardiomyocyte culture studies using multielectrode arrays showed that cardiac adipose tissue-secreted factors slowed conduction velocity and contained proteins with capacity to disrupt intermyocyte electromechanical integrity.
Figure 2: Right Atrial Conduction is Dependent on Anterior Right Atrial Adiposity.
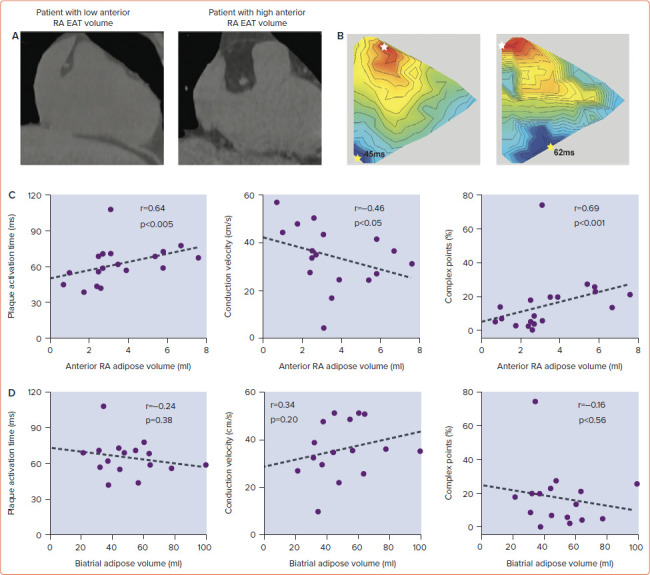
A: Exemplar CT images of patients with low and high anterior RA EAT volumes. B: Exemplar isochronal (3-ms steps) maps at 600-ms pacing rate from patients with either low or high anterior RA EAT volumes. White star indicates the earliest plaque activation; the yellow star indicates latest plaque activation. C: Significant correlations existed between the RA EAT volume and plaque activation time, conduction velocity and number of complex points. D: In contrast, these electrophysiological parameters did not correlate with the biatrial adipose tissue volume. EAT = epicardial adipose tissue; RA = right atrial. Source: Nalliah et al. 2020.[30] Reproduced with permission from Elsevier.
Recently, non-invasive assessment of the EAT activity on CT has gained significant interest. As inflammation increases, a significant change in the degree of epicardial fat attenuation values represented by Hounsfield units can be detected.[31] This suggests that EAT attenuation may serve as a novel non-invasive radiographic sensor of inflammation. Adipose tissue activity on CT has been shown to be an independent predictor of high-risk coronary artery plaques and major adverse cardiovascular events.[32] El Mahdiui et al. demonstrated that a higher attenuation of the EAT was associated with higher AF recurrent rates.[33] In a cohort of 460 patients undergoing first AF catheter ablation for symptomatic AF, the total EAT and posterior LA adipose tissue was traced, and the attenuation value of the posterior LA adipose tissue was assessed. Following a mean follow-up of 18 months, 37% patients had AF recurrence, and patients with higher attenuation (>–96.4 Hounsfield units) were more likely to have AF recurrence than lower attenuation on both univariate and multivariate analyses. Similar findings were demonstrated in another study that examined attenuation of the entire LA periatrial EAT.[34] The ability of cardiac CT to provide both quantitative and qualitative assessments of the EAT provides a promising tool that may assist in individualising therapy for patients with AF.
Role of Inflammation
In addition to the local inflammatory role of EAT on the arrhythmogenic substrate of AF, obesity is associated with elevated systemic inflammatory markers, which have been linked to AF. Inflammation has been linked to various pathological processes, such as oxidative stress, apoptosis and fibrosis, which may promote AF substrate formation.[35]
Impact of Weight Loss on the Atrial Substrate and AF
Observational retrospective studies have suggested a role for weight loss in reducing AF.[36] A number of prospective studies, both observational and randomised, have confirmed the favourable effect of weight loss on AF.[37–40] Abed et al. randomised 150 patients with AF to either weight management (intervention) or general lifestyle advice (control).[37] At 1 year, the intervention group lost significantly more weight compared with controls, and had fewer AF episodes and symptoms, and less burden. In addition, the LA diameter and interventricular septum wall thickness was significantly reduced compared with the control arm.
In the LEGACY study, Pathak et al. demonstrated that patients who lost and maintained the loss of >10% of their bodyweight over 4 years had sixfold arrhythmia-free likelihood compared with those who lost <3% or gained weight. Moreover, weight fluctuation of >5% had an adverse effect on overall freedom from AF, suggesting the causal relationship between weight and AF.[39] In addition, compared with baseline, patients who lost >10% of bodyweight had significantly decreased left atrial volume indexed for body surface area, interventricular septum thickness, left ventricular end diastolic diameter and E/E’, whereas patients who lost <3% had either an increased or unchanged difference from baseline. It is important to note that while this study targeted weight loss, it was associated with improvements in sleep apnoea, blood pressure and glycaemic control; making it difficult to ascertain the individual impact of each risk factor on both AF reduction and reverse remodelling.
A subanalysis of the same study cohort (REVERSE-AF trial) examined the association between weight loss and progression of AF.[38] When patients were stratified according to the percentage of weight loss (group 1: <3%, group 2: 3–9% and group 3: >10%), 41% progressed from paroxysmal to persistent and 26% from persistent to paroxysmal or no AF in group 1. In group 2, 32% progressed from paroxysmal to persistent and 49% reversed from persistent to paroxysmal or no AF. In group 3, 3% progressed to persistent and 88% reversed from persistent to paroxysmal or no AF (p<0.001). Moreover, increased weight loss was significantly associated with greater AF freedom: 45 (39%) in group 1, 69 (67%) in group 2 and 116 (86%) in group 3 (p<0.001). It is important to remember that the majority of the data on weight loss in AF used lifestyle changes to achieve weight loss, which without constant supervision is often unsustainable or unsuccessful.
As such, the impact of surgical weight loss through bariatric surgery on AF ablation outcomes was recently examined in an observational study by Donnellan et al.[41] They reviewed a cohort of 51 morbidly obese patients (BMI >40 kg/m[2]) who had undergone bariatric surgery prior to AF ablation, and matched them to a cohort of 102 morbidly obese patients without prior bariatric surgery and 102 non obese patients. The three groups had similar LA dimensions on echocardiography at baseline. Bariatric surgery was associated with significant reductions in weight, systolic blood pressure and glycated haemoglobin. During follow-up, the bariatric surgery group had significantly fewer AF recurrences compared with the other two control groups.
The precise mechanism by which weight loss leads to AF reduction is not well defined. Recently, Mahajan et al. studied the impact of weight reduction on reverse remodelling of the atrial electrical and structural substrate.[42] In a cohort of 30 sheep that had sustained obesity induced by a high-calorie diet over 18 months, the cohort was randomised into three groups: sustained obesity, and 15% and 30% weight loss. Sustained obesity was associated with adverse LA structural and electrical remodelling, whereas 30% weight loss was associated with a significant reversal of remodelling, translating into less AF inducibility (Figure 3).
Figure 3: Reversal of Conduction Abnormalities and Inflammation with Weight Reduction.
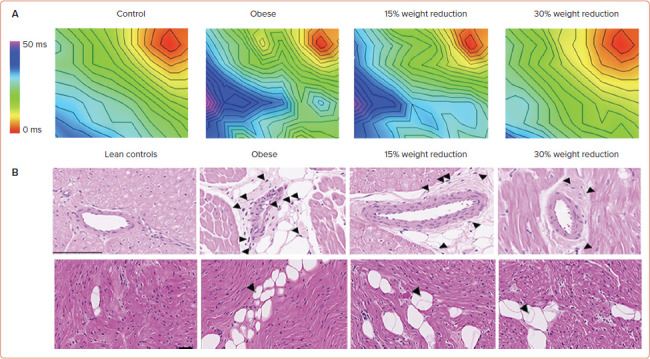
A: Activation maps of the left atrium at 300 ms in the control, obese, 15% weight reduction and 30% weight reduction. The plaque was paced from the corner (red zone). The maps are drawn to the same scale, and 2-ms isochrones are superimposed. B: Haematoxylin–eosin-stained sections showing inflammatory infiltrate in the perivascular space in the left atrial posterior wall. Some of the inflammatory cells are marked by arrowheads. Scale bar: 100 μm. Source: Mahajan et al. 2021.[42] Adapted with permission from Elsevier.
Weight Management in Obese Patients with AF
The compelling body of evidence that supports weight reduction in the management of AF is clear. This has been reflected in scientific statements from both the European Society of Cardiology and the American Heart Association.[43,44] The 2020 European Society of Cardiology guidelines for management of AF proposed the Atrial fibrillation Better Care (ABC) holistic pathway (‘A’ anticoagulation; ‘B’ better symptom management; ‘C’ cardiovascular and comorbidity optimisation) as a simplified approach to care for AF patients across different health disciplines. Intense weight reduction, together with comprehensive management of interacting cardiovascular risk factors, is the cornerstone of the ‘C’ component of the ABC pathway.[44]
However, successful and sustainable weight reduction remains a challenging task to achieve and a number of strategies have been proposed. These strategies stem from the concept of a chronic care model, which has been successful in the management of patients with various chronic illnesses, such as ischaemic heart disease and chronic heart failure. The integrated care approach in which the patient is the primary focus, together with multidisciplinary teams and community support, has been shown to be associated with a reduction in all-cause mortality and cardiovascular hospitalisations in a recent meta-analysis.[45]
Sanders et al. showed that a dedicated risk factor management clinic focusing on all modifiable risk factors through both lifestyle and diet changes can deliver promising results.[39,46] Such clinics have the potential to do this through identifying patient-specific modifiable risk factors, setting individual achievable targets, and working with patients to ensure ongoing motivation and adherence to their goals. Understanding the difficulties associated with achieving and maintaining weight reduction, bariatric surgery has the potential to achieve such results, and has been demonstrated to be associated with both a reduction in the incidence of AF among patients at risk and reversal of AF type among patients with known AF.[47,48]
Conclusion
Obesity and AF have emerged as major global epidemics. Their impact on the healthcare costs and burden on both patients and physicians is evident. There is strong evidence that supports the obesity–AF relationship through multiple and interacting mechanisms, including diastolic dysfunction, local EAT inflammation and infiltration, and systemic inflammation. The current body of evidence strongly supports weight loss as an important pillar in the management of obese patients with AF. Weight reduction together with the management of other risk factors should be delivered using a comprehensive approach to obtain the best results.
Clinical Perspective
Obesity is an independent and important predictor of AF incidence and progression.
Emerging data now support the impact of epicardial adipose tissue on the arrhythmogenic substrate of AF patients, even without elevated BMI.
Weight loss through lifestyle changes or surgery is an important pillar in the management of AF in obese patients.
References
- 1.Caballero B. Humans against obesity: who will win? Adv Nutr. 2019;10((suppl_1)):S4–9. doi: 10.1093/advances/nmy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang YC, McPherson K, Marsh T et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Ball J, Thompson DR, Ski CF et al. Estimating the current and future prevalence of atrial fibrillation in the Australian adult population. Med J Aust. 2015;202:32–5. doi: 10.5694/mja14.00238. [DOI] [PubMed] [Google Scholar]
- 5.Stritzke J, Markus MR, Duderstadt S et al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. 2009;54:1982–9. doi: 10.1016/j.jacc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Foy AJ, Mandrola J, Liu G, Naccarelli GV. Relation of Obesity to New-Onset Atrial Fibrillation and Atrial Flutter in Adults. Am J Cardiol. 2018;121:1072–5. doi: 10.1016/j.amjcard.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–95. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Choi EK, Lee SH et al. Atrial fibrillation risk in metabolically healthy obesity: a nationwide population-based study. Int J Cardiol. 2017;240:221–7. doi: 10.1016/j.ijcard.2017.03.103. [DOI] [PubMed] [Google Scholar]
- 9.Tedrow UB, Conen D, Ridker PM et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women’s Health Study). J Am Coll Cardiol. 2010;55:2319–27. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang TS, Barnes ME, Miyasaka Y et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–33. doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Parise H, Levy D et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 12.Schmieder RE, Messerli FH. Does obesity influence early target organ damage in hypertensive patients? Circulation. 1993;87:1482–8. doi: 10.1161/01.CIR.87.5.1482. [DOI] [PubMed] [Google Scholar]
- 13.Ebong IA, Goff DC Jr., Rodriguez CJ et al. Mechanisms of heart failure in obesity. Obes Res Clin Pract. 2014;8:e540–8. doi: 10.1016/j.orcp.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–93. doi: 10.1016/j.jacc.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Simone G, Palmieri V, Bella JN et al. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–31. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Mehra R, Benjamin EJ, Shahar E et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abed HS, Samuel CS, Lau DH et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Okumura Y, Watanabe I, Nagashima K et al. Effects of a high-fat diet on the electrical properties of porcine atria. J Arrhythm. 2015;31:352–8. doi: 10.1016/j.joa.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Sasano T, Sugiyama K et al. High-fat diet increases vulnerability to atrial arrhythmia by conduction disturbance via miR-27b. J Mol Cell Cardiol. 2016;90:38–46. doi: 10.1016/j.yjmcc.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Mahajan R, Nelson A, Pathak RK et al. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol. 2018;4:1529–40. doi: 10.1016/j.jacep.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Thanassoulis G, Massaro JM, O’Donnell CJ et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345–50. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Chekakie MO, Welles CC, Metoyer R et al. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010;56:784–8. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 23.Wong CX, Abed HS, Molaee P et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745–51. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M, Wang H, Chen J, Zhao L. Epicardial adipose tissue and atrial fibrillation: possible mechanisms, potential therapies, and future directions. Pacing Clin Electrophysiol. 2020;43:133–45. doi: 10.1111/pace.13825. [DOI] [PubMed] [Google Scholar]
- 25.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–71. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 26.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–72. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan R, Lau DH, Brooks AG et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66:1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 28.Venteclef N, Guglielmi V, Balse E et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795–80a. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 29.Chen MC, Chang JP, Liu WH et al. Increased inflammatory cell infiltration in the atrial myocardium of patients with atrial fibrillation. Am J Cardiol. 2008;102:861–5. doi: 10.1016/j.amjcard.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 30.Nalliah CJ, Bell JR, Raaijmakers AJA et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol. 2020;76:1197–211. doi: 10.1016/j.jacc.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Baba S, Jacene HA, Engles JM et al. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–50. doi: 10.2967/jnumed.109.068775. [DOI] [PubMed] [Google Scholar]
- 32.Nerlekar N, Ha FJ, Cheshire C et al. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2018;11:e006973. doi: 10.1161/CIRCIMAGING.117.006973. [DOI] [PubMed] [Google Scholar]
- 33.El Mahdiui M, Simon J, Smit JM et al. Posterior left atrial adipose tissue attenuation assessed by computed tomography and recurrence of atrial fibrillation after catheter ablation. Circ Arrhythm Electrophysiol. 2021;14:e009135. doi: 10.1161/CIRCEP.120.009135. [DOI] [PubMed] [Google Scholar]
- 34.Ciuffo L, Nguyen H, Marques MD et al. Periatrial fat quality predicts atrial fibrillation ablation outcome. Circ Cardiovasc Imaging. 2019;12:e008764. doi: 10.1161/CIRCIMAGING.118.008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandhu RK, Conen D, Tedrow UB et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3:e000916. doi: 10.1161/JAHA.114.000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abed HS, Wittert GA, Leong DP et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–60. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 38.Middeldorp ME, Pathak RK, Meredith M et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–35. doi: 10.1093/europace/euy117. [DOI] [PubMed] [Google Scholar]
- 39.Pathak RK, Middeldorp ME, Meredith M et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–69. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Rienstra M, Hobbelt AH, Alings M et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39:2987–96. doi: 10.1093/eurheartj/ehx739. [DOI] [PubMed] [Google Scholar]
- 41.Donnellan E, Wazni O, Kanj M et al. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol. 2019;12:e007598. doi: 10.1161/CIRCEP.119.007598. [DOI] [PubMed] [Google Scholar]
- 42.Mahajan R, Lau DH, Brooks AG et al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol. 2021;7:630–41. doi: 10.1016/j.jacep.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Chung MK, Eckhardt LL, Chen LY et al. Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e750–e72. doi: 10.1161/CIR.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 44.Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 45.Gallagher C, Elliott AD, Wong CX et al. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart. 2017;103:1947–53. doi: 10.1136/heartjnl-2016-310952. [DOI] [PubMed] [Google Scholar]
- 46.Gallagher C, Fitzgerald JL, Stokes MB et al. Risk factor management in atrial fibrillation: how to deliver a successful clinic. Curr Cardiovasc Risk Rep. 2021;15:9. doi: 10.1007/s12170-021-00671-x. [DOI] [Google Scholar]
- 47.Donnellan E, Wazni OM, Elshazly M et al. Impact of bariatric surgery on atrial fibrillation type. Circ Arrhythm Electrophysiol. 2020;13:e007626. doi: 10.1161/CIRCEP.119.007626. [DOI] [PubMed] [Google Scholar]
- 48.Höskuldsdóttir G, Sattar N, Miftaraj M et al. Potential effects of bariatric surgery on the incidence of heart failure and atrial fibrillation in patients with type 2 diabetes mellitus and obesity and on mortality in patients with preexisting heart failure: a nationwide, matched, observational cohort study. J Am Heart Assoc. 2021;10:e019323. doi: 10.1161/JAHA.120.019323. [DOI] [PMC free article] [PubMed] [Google Scholar]


