Dear Editor,
The coronavirus disease 2019 (COVID-19) pandemic is a global public health emergency that requires the immediate implementation of control measures. COVID-19 vaccines help reduce the risk of COVID-19 and protect against severe illness, even among people infected after being vaccinated.1 Although large-scale investigations have shown favorable safety profiles regarding COVID-19 vaccines,2 the long-term safety and rare adverse reactions are unclear. Interstitial pneumonitis has been reported as a rare complication of vaccination against other microorganisms.3, 4, 5 However, it was not reported in the initial COVID-19 vaccine trial.2 We herein report three cases of interstitial pneumonitis that occurred after COVID-19 vaccination (Pfizer).
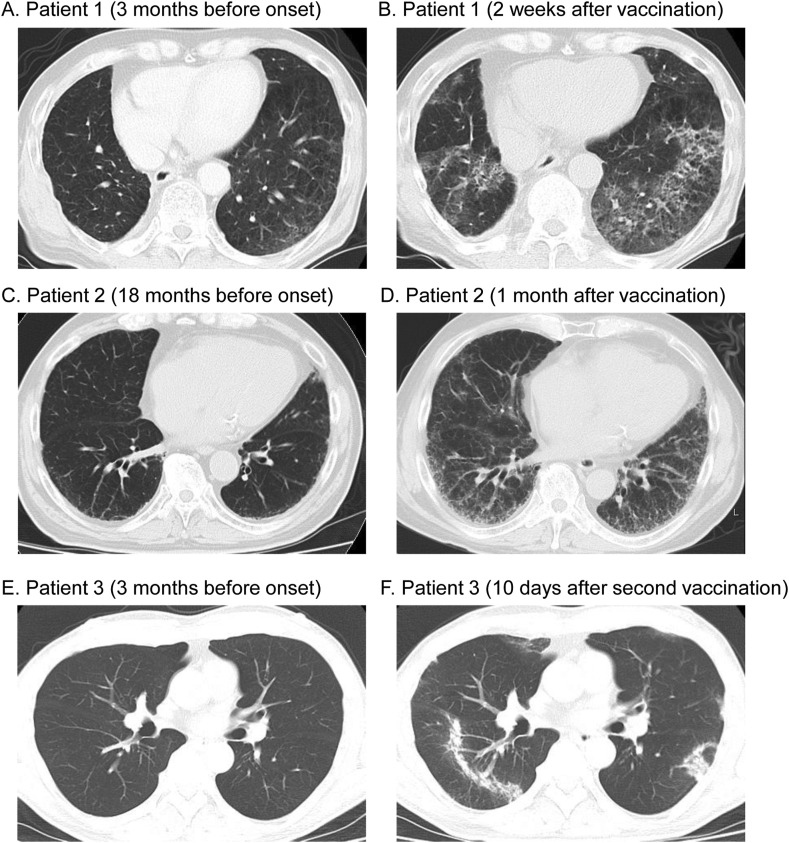
Patient 1: A 66-year-old ex-smoking man visited our hospital for regular follow-up after resection of lung cancer. Four years earlier, he had been diagnosed with lung cancer coexisting with emphysema and smoking-related interstitial lung disease, which was histologically diagnosed as desquamative interstitial pneumonia (DIP) (Fig. 1 A). The patient had received the first dose of a COVID-19 vaccine 15 days before presentation. He had a prolonged fever and fatigue from the day after vaccination. He had not had any contact with anyone known to have COVID-19 and was not taking any other medications. Chest computed tomography (CT) showed diffuse and patchy ground-glass opacities and irregular reticulation with relative subpleural sparing in the lower lobes (Fig. 1B). Real-time fluorescence polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2) nucleic acid was negative. Bronchoalveolar lavage (BAL) demonstrated lymphocytosis (42.3%) and the absence of pigmented smoker's macrophages. BAL fluid cultures were negative for bacteria, fungi, and mycobacteria. Further clinical characteristics are shown in Table 1 . Because a temporal relationship between vaccination and the clinical symptoms was suspected, the patient decided to skip his second COVID-19 vaccination. The interstitial abnormalities spontaneously resolved one month later without any treatment (Supplementary Fig. 1).
Fig. 1.
(A) Chest computed tomography (CT) three months before the onset in Patient 1 showing emphysema and ground-glass opacities in the lower lobes, which was histologically diagnosed as desquamative interstitial pneumonia. (B) Chest CT two weeks after vaccination in Patient 1, showing diffuse and patchy ground-glass opacities and irregular reticulation with relative subpleural sparing in the lower lobes. (C) Chest CT 18 months before the onset in Patient 2, showing mild reticulation in the lower lobes. (D) Chest CT one month after vaccination in Patient 2, showing diffuse ground-glass opacities and reticular shadows in subpleural predominance. (E) Chest CT three months before the onset in Patient 3, showing no abnormalities. (F) Chest CT 10 days after the second vaccination in Patient 3, showing patchy bilateral air-space consolidation and linear opacities with subpleural and peribronchial distribution.
Table 1.
Characteristics of the three patients at presentation and clinical outcomes.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age/Gender | 66/Male | 85/Male | 62/Male |
| Smoking status | Ex-smoker | Ex-smoker | Non-smoker |
| Underlying ILD | Yes (DIP) | Yes (unknown) | No |
| Onset since given vaccine | 1 day after first vaccination | 3–5 days after first vaccination | 2 days after second vaccination |
| Symptoms | Fever, fatigue | Dyspnea | Fever |
| RT-PCR test for SARS-CoV-2 nucleic acid | Negative | Negative | Negative |
| Autoantibodies for CVD | Negative | Negative | MPO-ANCA (+) |
| Serological tests | |||
| KL-6, U/mL (normal range <500 U/mL) | 1306 | 4084 | 297 |
| SP-D, ng/mL (normal range <110 ng/mL) | 376.4 | 675.5 | 189.0 |
| BAL findings | |||
| Macrophages, % | 55 | 30.7 | – |
| Lymphocytes, % | 42.3 | 62.7 | – |
| Neutrophils, % | 1.7 | 0 | – |
| Eosinophils, % | 1 | 6.7 | – |
| CD4/CD8 | 1.3 | 6.6 | – |
| Treatment for pneumonitis | None | Methylprednisolone, predonisolone | Predonisolone |
| Serological tests 3 months after the onset | |||
| KL-6, U/mL (normal range <500 U/mL) | 529 | 1550 | – |
| SP-D, ng/mL (normal range <110 ng/mL) | 163.3 | 200.3 | – |
| Outcome | Improved | Improved | Improved |
DIP, desquamative interstitial pneumonia; RT-PCR, real-time fluorescence polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome-Coronavirus-2; CVD, collagen vascular disease; MPO-ANCA, myeloperoxidase-anti-neutrophil cytoplasmic antibody; KL-6, Krebs von den Lungen 6; SP-D, surfactant protein D; BAL, bronchoalveolar lavage
Patient 2: An 85-year-old ex-smoking man presented with exertional dyspnea. Chest CT performed 18 months previously had shown mild reticulation in the lower lobes (Fig. 1C). Four days earlier, he had received the first dose of the COVID-19 vaccine and developed prolonged chest pain and shortness of breath afterward. He was suspected of having acute coronary syndrome and underwent percutaneous coronary intervention but experienced no improvement. He received the second dose of the vaccine 21 days after the first vaccination, and his symptoms worsened. Chest CT revealed diffuse ground-glass opacities and reticular shadows with subpleural predominance (Fig. 1D). He had not had any contact with anyone known to have COVID-19 and was not taking any new medications. RT-PCR test for SARS-CoV-2 nucleic acid was negative. The findings of the BAL fluid revealed 62.7% lymphocytes and 6.6% eosinophils. No microorganisms or malignant cells were detected in the BAL fluid. Further clinical characteristics are shown in Table 1. Because the patient had respiratory failure on exertion, he was started on intravenous methylprednisolone at 1000 mg daily for 3 days, followed by oral prednisolone at 60 mg (1.0 mg/kg/day) daily. His symptoms and radiographic findings gradually resolved (Supplementary Fig. 1). Although the patient still needed home oxygen therapy, prednisolone was subsequently tapered.
Patient 3: A 62-year-old non-smoking man presented with a fever and fatigue. Chest CT performed three months previously showed no abnormalities (Fig. 1E). One month previously, the patient had received the first dose of the COVID-19 vaccine. He did not experience any side effects after vaccination. Ten days before presentation, he had received the second dose. After the second vaccination, the patient had a prolonged fever. He had not had any contact with anyone known to have COVID-19 and was not taking any other medications. Chest CT revealed patchy bilateral air-space consolidation and linear opacities with a subpleural and peribronchial distribution (Fig. 1F). RT-PCR for SARS-CoV-2 nucleic acid was negative. Further clinical characteristics are shown in Table 1. Although the patient showed a positive myeloperoxidase-anti-neutrophil cytoplasmic antibody (MPO-ANCA), there were no symptoms of ANCA-associated vasculitis, including skin rash or nephritis. Because a temporal relationship between vaccination and clinical symptoms was suspected, the patient was started on oral prednisolone at 20 mg daily. His symptoms and radiological findings were markedly resolved (Supplementary Fig. 1). Prednisolone was then subsequently tapered.
All three patients met the proposed criteria of drug-related pneumonitis6 including newly identified pulmonary parenchymal opacities on chest CT with a bilateral non-segmental distribution, a temporal association of presentation with the administration of the vaccine, and the exclusion of other likely causes. All patients suffered from an unusual “prolonged” fever, fatigue, or dyspnea after vaccination (4–15 days). CT findings were variable, including ground-glass opacities and reticulation with subpleural sparing or subpleural predominance, and air-space consolidation with linear opacities (Figs. 1B, D, and F). The BAL cell differential counts showed lymphocytosis with mild eosinophilia, suggesting an immune-mediated mechanism. Of note, all three patients showed improvement by avoiding secondary vaccination or with the administration of corticosteroid therapy.
COVID-19 vaccines have been shown to be safe in large-scale trials.2 Local or systemic minor acute reactions happen within a day or two of getting the vaccine and usually go away within a few days.7 Several severe adverse events, including anaphylaxis, myocarditis, and thrombocytopenia, have been reported.8 However, no cases of interstitial pneumonitis were reported in the clinical trial.2 Thus far, only one case of interstitial pneumonitis after COVID-19 vaccination has been reported from South Korea: the patient was an 86-year-old man with multiple comorbidities.9 Because clinical trials for vaccines have largely enrolled younger, relatively healthy adults, it is important to evaluate the safety of these vaccines in all age populations, especially older individuals with severe underlying disease. Although risk factors remain unknown, preexisting interstitial lung disease is associated with an increased likelihood of drug-induced pneumonitis.6 In addition, the frequency of drug-induced pneumonitis is higher in Japan than in other countries.10 Further studies are needed to elucidate the incidence, risk factors, clinical course, treatment strategies, and long-term outcomes after COVID-19 vaccination.
In summary, to our knowledge, this is the first case series of interstitial pneumonitis after COVID-19 vaccination. Physicians should keep in mind that this vaccine has the potential to cause interstitial pneumonitis. Knowledge of the spectrum of manifestations of interstitial pneumonitis may assist clinicians in managing this rare but potentially important adverse event.
Footnotes
Peer review under responsibility of Japanese Society of Allergology.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.alit.2021.10.003.
Conflict of interest
The authors have no conflict of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Centers for Disease Control and Prevention (CDC) Coronavirus Disease 2019 (COVID-19) Vaccine Effectiveness. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/how-they-work.html Available at:
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe S., Waseda Y., Takato H., Inuzuka K., Katayama N., Kasahara K., et al. Influenza vaccine-induced interstitial lung disease. Eur Respir J. 2013;41:474–477. doi: 10.1183/09031936.00146912. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y., Kazebayashi Y., Hirai N., Sasaki T., Ohsaki Y. Interstitial lung disease associated with human papillomavirus vaccination. Respir Med Case Rep. 2015;16:15–17. doi: 10.1016/j.rmcr.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu G., Amano R., Nakamura I., Wada A., Kitagawa M., Toru S. Disseminated Bacillus Calmette-Guérin (BCG) infection and acute exacerbation of interstitial pneumonitis: an autopsy case report and literature review. BMC Infect Dis. 2020;20:708. doi: 10.1186/s12879-020-05396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johkoh T., Lee K.S., Nishino M., Travis W.D., Ryu J.H., Lee H.Y., et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Chest. 2021;159:1107–1125. doi: 10.1016/j.chest.2020.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Coronavirus Disease 2019 (COVID-19) Vaccines. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html Available at:
- 8.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J.Y., Kim J.H., Lee I.J., Kim H.I., Park S., Hwang Y.I., et al. COVID-19 vaccine-related interstitial lung disease: a case study. Thorax. 2021 doi: 10.1136/thoraxjnl-2021-217609. [DOI] [PubMed] [Google Scholar]
- 10.Skeoch S., Weatherley N., Swift A.J., Oldroyd A., Johns C., Hayton C., et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7:356. doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.