Abstract
An important feature of action understanding is that comprehenders segment the perceptual stream into events. Event segmentation dynamically engages a network of brain regions that likely play a role in how events are encoded. Here, in a sample of older adults, we assessed the relationship between changes in brain dynamics during movie watching and event understanding performance. Forty healthy older adults and a comparison sample of twelve younger adults passively viewed short movies of everyday activities while their brain activity was measured with fMRI. Afterward, they segmented the movies into events and performed memory tasks for movie content. The older adults engaged a similar event segmentation network during movie watching as the younger adults. Individual differences analyses revealed that although behavioral measures of event segmentation predicted memory, activity in the segmentation network did not. Intersubject correlation analyses revealed that normative brain dynamics during viewing in the right posterior temporal sulcus and left dorsolateral prefrontal cortex predicted better segmentation performance. These data suggest that these regions play an important role in event understanding, and also that the event segmentation network is preserved in healthy aging.
Neural Dynamics of Event Encoding in Healthy Aging
An observer of an everyday human activity confronts a dynamically changing stream of behavior. For example, a viewer of a cooking show might see the actor perform a new nameable action every few seconds, and interact with a new discrete object almost as frequently. Goal-directed action, like speech, has few overt pauses to serve as guides to temporal structure (Brent, 1999; Newtson, Engquist, & Bois, 1977). Given the cognitive demands of comprehending such a dynamic flow, how does an observer begin to understand what is happening? One adaptive mechanism may be to parse the ongoing stream of activity into meaningful events. The cooking show viewer might segment the program into events corresponding to the actor getting out the ingredients, combining the ingredients, cooking the food, and plating the meal. Individual events can be further broken down into subevents; for example, getting out ingredients might break down into opening the refrigerator, removing the onion, and so on.
According to Event Segmentation Theory (EST; J. M. Zacks, Speer, Swallow, Braver, & Reynolds, 2007), a perceiver segments extended activities such as these into discrete events, and constructs a mental representation of each event in turn. These event models are working memory representations of “what is happening now.” Segmenting and forming an event model is adaptive, because well-structured event models improve perceptual predictions of what will happen next within the ongoing activity. EST proposes that prediction errors are computed in cortical systems that are late in perceptual processing, and that the prediction error signal is used to control event model updating. When prediction error is low, event models’ inputs are gated closed for stability, but when prediction error spikes, midbrain neuromodulatory systems induce an opening of the gates, constituting an up-regulation of attentional and perceptual systems that resets the old model and builds a new one. Once a new model is built, new sets of predictions are brought online and the process begins anew. Thus, the process of segmentation may act to regulate working memory updating, memory encoding, and attentional control during the comprehension of dynamic events.
Behavioral and Neural Correlates of Event Segmentation and Comprehension
Event segmentation can be measured by asking viewers to push a button while watching a movie, under instructions to indicate when one meaningful event ends and another begins (Newtson & Engquist, 1976; J. M. Zacks & Tversky, 2001). This task has demonstrated a high amount of intersubject, and intrasubject, agreement on the location of event boundaries (Speer, Swallow, & Zacks, 2003; J. M. Zacks, Speer, Vettel, & Jacoby, 2006), suggesting a general consensus on the perception of event structure of an activity. Viewer segmentation of behavior tends to be systematic and reliable across observers. People tend to perceive event boundaries when there are changes on one or more of a number of situational dimensions, such as changes in time, characters, causation, goals, etc. (Kurby & Zacks, 2012; Magliano, Miller, & Zwaan, 2001; Magliano, Zwaan, & Graesser, 1999; Speer, Zacks, & Reynolds, 2007; J. M. Zacks, Speer, & Reynolds, 2009; Zwaan & Radvansky, 1998), and when there are particular changes in motion (Schubotz, Korb, Schiffer, Stadler, & von Cramon, 2012; J. M. Zacks, 2004; J. M. Zacks, Kumar, Abrams, & Mehta, 2009). Events are typically perceived to have a hierarchical structure; going to the museum breaks down into entering, obtaining maps, visiting exhibits, and leaving, each of which further break down into constituent events. If viewers are asked to segment the same activity twice, once to identify fine-grained events (i.e., smaller time-scale events) and once to identify coarse-grained events (i.e., larger time-scale events), their fine events form clusters that correspond with the coarse event boundaries (Hard, Tversky, & Lang, 2006; Kurby & Zacks, 2011; J. M. Zacks, Tversky, & Iyer, 2001).
Event segmentation processes are important for comprehension and memory. Memory is typically better for information that occurs at event boundaries (Newtson & Engquist, 1976), and providing signals to event structure during viewing improves memory for the activity (Boltz, 1992; Flores, Bailey, Eisenberg, & Zacks, 2017; Gold, D.A., Zacks, & Flores, 2017; Schwan, Garsoffky, & Hesse, 2000; Schwan, Hesse, & Garsoffky, 1998). Additionally, the perception of a boundary has been shown to modulate the contents of working memory (Radvansky & Copeland, 2010; Speer & Zacks, 2005; Swallow, Zacks, & Abrams, 2009; Zwaan, 1996). Readers tend to lose access to old event information as new event models are brought online at event boundaries (Ezzyat & Davachi, 2011; Swallow et al., 2011, 2009; Zwaan, 1996).
Neuroimaging studies have shown that brain activity in a number of regions is modulated by event structure (Schubotz et al., 2012; Speer et al., 2003, 2007; Whitney et al., 2009; J. M. Zacks, Braver, et al., 2001; J. M. Zacks, Speer, Swallow, & Maley, 2010; J. M. Zacks, Swallow, Vettel, & McAvoy, 2006). In most studies, participants watch, or read about, naturalistic activities in the MRI scanner, and then, in a separate session, segment them into events. Typically, large portions of bilateral occipital-parietal-temporal cortex transiently increase in activity around the points participants later identify as event boundaries, as well as smaller portions of prefrontal cortex (Ezzyat & Davachi, 2011; Speer et al., 2007; J. M. Zacks, Braver, et al., 2001; J. M. Zacks et al., 2010). The posterior activation includes the precuneus, cuneus, extrastriate regions such as the fusiform gyrus, MT+, and the posterior superior sulcus (pSTS) (Kurby & Zacks, 2008; J. M. Zacks et al., 2007). Often, responses in these regions are larger for coarse than fine events, consistent with the possibility that viewers perceive hierarchical structure in events (Speer et al., 2003, 2007; J. M. Zacks, Braver, et al., 2001; J. M. Zacks, Swallow, et al., 2006); more processing is required to update coarse event models (J. M. Zacks, Tversky, et al., 2001). Using a data-driven multi-voxel pattern classification procedure, Baldassano et al. (2017) found a large swath of regions in bilateral occipital/parietal/temporal cortex, both medial and lateral, that show stable activation patterns within an event and transition to new stable patterns as events shift. These regions range from low-level visual regions in the cuneus, with short timescales of activity patterns, to higher-level visual regions such as the angular gyrus and posterior cingulate, with longer timescales. They also found that points of pattern transition in a subset of high-level visual regions were associated with increases in hippocampal activity, suggesting a connection between the perception of an event boundary and memory encoding.
Research is beginning to specify the role of some of these segmentation regions in the process of event segmentation. For example, Zacks et al (2006) found that both MT+ and pSTS modulate their activity at event boundaries, but activity in MT+ was also strongly associated with object movement, whereas pSTS was only weakly so. Additionally, the extent to which different brain regions participate in segmentation depends, in part, on the meaningfulness of the stimuli. Schubotz, Korb, Schiffer, Stadler, and von Cramon (2012) found that MT+ increased in activity for both non-goal-directed actions (e.g., Tai Chi movements) and goal-directed actions, whereas the response in the left superior frontal sulcus was highest for the segmentation of goal-directed actions (as well as the left angular gyrus and bilateral parahippocampal gyrus). During the segmentation of narratives, the pSTS has been shown to respond robustly to meaningful changes in situational aspects of the activity, such as changes in characters and objects, and this response mediates its increase in activity at event boundaries (J. M. Zacks et al., 2010).
The above research suggests that certain brain regions systematically change their processing dynamics as event structure changes. That work has typically found such effects in studies that time-lock analyses to the moment of boundaries (or short windows around them). However, given that events change fluidly across time, it is likely that changes in processing dynamics occur moment-by-moment as continuous activity unfolds. Hasson and colleagues have investigated the continuous dynamics of brain activity during comprehension by measuring the degree to which brain regions in different individuals rise and fall in synchrony while watching the same stimulus (Hasson, Landesman, et al., 2008; Hasson, Furman, Clark, Dudai, & Davachi, 2008; Hasson, Malach, & Heeger, 2010; Hasson, Nir, Levy, Fuhrmann, & Malach, 2004; Hasson, Yang, Vallines, Heeger, & Rubin, 2008). This approach has revealed a large set of regions in posterior cortex, including occipital, parietal, and temporal regions, whose activation dynamics show a high level of intersubject synchrony (Hasson, Landesman, et al., 2008; Hasson et al., 2004; Smith, Levin, & Cutting, 2012). Intersubject synchrony is strongest for compelling narrative sequences, which suggests that such sequences guide viewers’ brains through a consistent sequence of states. The synchrony effect also reflects interpretable coupling of functionally selective brain regions’ activity to the content of a movie. After controlling for anatomically nonselective activity fluctuations, the fusiform face area (Kanwisher & Yovel, 2006) and the parahippocampal place area (Epstein & Kanwisher, 1998) showed selective intersubject coupling, with activity peaks corresponding to the presence of faces in the fusiform face area and places in the parahippocampal place area (Hasson et al., 2004). Work in eye tracking has also shown attentional synchrony during the viewing of dynamic scenes (Loschky, Larson, Magliano, & Smith, 2015; Wang, Freeman, Merriam, Hasson, & Heeger, 2012); viewers tend to look at the same places at the same time. Altogether, these results suggest synchrony in neural processing across individuals as events unfold. In terms of event comprehension, this processing synchrony, measured in changes in brain activity, should be captured by synchrony in how events are segmented.
Aging and Individual Differences in Event Segmentation and Memory
Compared to younger adults, older adults have been shown to have worse and more variable event segmentation and event memory (Bailey, Kurby, Giovannetti, & Zacks, 2013; Kurby & Zacks, 2011; J. M. Zacks, Speer, et al., 2006). Regarding segmentation, older adults show lower agreement with normative event boundaries, and less hierarchically organized segmentation. Normative segmentation appears to be functional for memory encoding: Older adults who have higher agreement tend to also have better memory for the events (J. M. Zacks, Speer, et al., 2006). Further, in an adult sample with a wide age range, this relationship was not accounted for by individual differences in general knowledge, perceptual speed, episodic memory performance, and working memory capacity (Sargent et al., 2013). Better segmentation performance, as measured by segmentation agreement, also has been shown to predict better action performance: Those who segmented events more normatively also performed naturalistic actions (e.g., putting together a child’s school bag) more effectively and produced fewer errors of omission (Bailey, Kurby, et al., 2013). The relationship between event segmentation and memory encoding is associated with neuroanatomical structures important for memory, particularly the medial temporal lobes (MTL). Bailey and colleagues found that MTL volume partially mediated the relationship between segmentation agreement and event memory (Bailey, Zacks, et al., 2013). In short, aging is associated with a decline in event segmentation ability, possibly mediated by changes in the MTL, and individual differences in event segmentation predict older adults’ memory and action performance.
The Current Study
Given that older adults show a wide range of ability in behavioral event segmentation, and that this is predictive of variation in subsequent memory, one possibility is that neural mechanisms of segmentation are influenced by the aging process in a way that affects memory encoding. Alternatively, it could be that it is not ongoing segmentation itself that determines memory, but rather that some other component of the overt task predicts subsequent memory. (For example, in order to do the task one needs to maintain the task set so as to continue to press the button, so individuals who better maintain task set might show better performance on a behavioral segmentation task but not necessarily better ongoing neural event segmentation. Those who can better maintain task set would also be expected to perform better on the memory task, leading to a correlation between the two measures.) If neural mechanisms of ongoing segmentation predict subsequent memory, this could suggest tools for diagnosing memory encoding impairments and strategies to improve memory by improving encoding of ongoing activity. If not, this would suggest that interventions targeting deliberate event segmentation monitoring would be a better target for improving memory.
As mentioned above, viewers of activity tend to have similar neural dynamics during movie understanding (Hasson et al., 2004). However, a recent study discovered age-differences in synchrony, with older adults showing less synchrony with the sample as a whole than younger adults (Campbell et al., 2015), across a number of regions important for language and perception. Further, there were systematic individual differences in synchrony in both younger and older adults; higher synchrony was correlated with better performance on measures of attentional control. Extending these findings, we propose that synchrony during movie watching is also indicative of adaptive event encoding. Therefore, older adults with higher synchrony with a younger adult norm should have better performance in the event understanding measures.
In this study, we tested the hypothesis that normative changes in brain dynamics to changes in event structure are related to better event segmentation and memory for naturalistic activity. Forty older adults and a smaller comparison of 12 younger adults passively watched movies of everyday activities while their brain activity was recorded with fMRI. Then they returned to the lab for measures of event segmentation and memory. We assessed the relation between their behavioral performance on the event understanding tasks and brain activity during viewing. We focused on older adults for two reasons. First, older adults have been shown to have worse event segmentation abilities than younger adults (Kurby & Zacks, 2011; J. M. Zacks, Speer, et al., 2006). This reduced ability may be related to less robust changes in brain response to event boundaries, compared to younger adults. (However, to preview one of the results, we did not observe substantial age differences in segmentation in the current sample.) Second, older adults show systematic and meaningful intersubject differences in event segmentation ability. Such variability has been associated with variability in behavioral markers of event understanding (Bailey, Kurby, et al., 2013; Sargent et al., 2013; J. M. Zacks, Speer, et al., 2006).
We measured two neural correlates of event encoding: transient evoked responses at event boundaries, and synchrony across viewers’ brain activity. We hypothesized that older adults would have reduced transient responses at event boundaries, and that individual differences in these responses would predict individual differences in older adults’ behavioral segmentation and event memory. We predicted that older adults would show reduced neural synchrony, and that individual differences in synchrony with a normative (younger adult) sample also would predict older adults’ behavioral segmentation and event memory. To our surprise, we observed little evidence of age differences in the evoked response to event boundaries, and little evidence that individual differences in evoked responses predicted behavior. However, individual differences in neural synchrony did predict who of the older adults segmented better.
Method
Participants
Forty healthy older adults between the ages of 60 and 80 (M = 69 years, min = 60, max = 79) were recruited from the St. Louis community. The older adults were contacted by phone, at which time they received an initial prescreening inquiring asking about possible presence of ferrous metals in their bodies (e.g., surgical implants). Additionally, older adults were screened out of the study if they indicated that they had (or have) glaucoma, untreated cataracts, legal blindness, a past heart attack or heart surgery, diabetes, untreated high blood pressure, cancer, experienced a loss of consciousness greater than 5 minutes in duration, medication-treated depression or anxiety, schizophrenia, bipolar disorder, engaged in regular excessive drinking, or a neurological disorder. Twelve younger adults (M = 23 years, Min = 19, Max = 28) were recruited from the Washington University Psychology Department participant pool, whose members were mostly current students, and were also screened for the above health concerns. All participants were right-handed and had normal vision, or corrected to normal vision by use of corrective goggles in the scanner. This research was approved by the Human Research Protection Office at Washington University.
Materials
Movies.
Participants viewed five movies depicting an actor engaged in an everyday activity: a man setting up a room for a children’s party (duration 376s), a man sorting and washing laundry (duration 303s), a woman checking out a book in a library (duration 249s), a man planting two window boxes with plants and flowers (duration 354s), and a woman making breakfast in a kitchen (duration 329s). The movies were shot from a wide angle from a fixed head-height perspective using a tripod. The movies did not contain any cuts or dialog. A practice movie was also presented, which depicted a man building a boat out of Duplo blocks (duration 155s). All movies were played without sound during all tasks.
Memory tests.
Memory for the activity in the movies was tested with a recall test, a recognition test, and an order memory test. Recall memory was assessed by asking participants to describe what happened in the movie in the order in which it occurred, with as much detail as possible. The recognition memory test for each movie was a 20-item two-alternative forced choice test. For each movie, twenty distracter items were chosen from movies of the same actor in the same setting. Order memory was tested with a set of 12 visually distinctive images from the movie, printed on 3 × 5 inch cards. Participants’ task was to arrange the cards in the order in which they occurred in the movie.
MRI Scanning
All images were acquired with a Siemens Trio 3T MRI scanner (Siemens, Erlangen Germany). Structural images were obtained with a high-resolution T1-weighted scan (1 mm × 1 mm × 1 mm) using an MPRAGE sequence, and a high-resolution T2 fast-turbo-spin-echo image (1 mm × 1 mm × 4 mm voxels, slice TR = 6150 ms, TE = 88 ms). Functional data were acquired in five funs, one for each movie, using a T2* weighted asymmetric spin-echo echo-planar sequence (TR = 2000 ms, TE = 27 ms) in 35 transverse slices (4.0 mm isotropic voxels) aligned with the anterior and posterior commissures. Prior to analysis, the data were preprocessed to correct for slice-to-slice timing offsets, between and within run movement, to normalize slice-to-slice intensity differences, and to normalize whole brain intensity to a mode of 1000. The functional images were then aligned to the T2 images, which were also aligned with the high-resolution T1 images. Images from both younger and older adults were resampled to fit a standard stereotaxic space (Talairach & Tournoux, 1988) with 3mm isotropic voxels, using the method described in (Buckner et al., 2004).
Procedure
Session 1 – scanning.
In session 1, participants viewed the five movies while their brain activity was recorded in the scanner. The movies were shown in the same order for all participants. For each movie, participants were instructed to watch carefully and to try to remember as much as possible. Twenty frames of fixation preceded the onset and followed the offset of each movie, which allowed us to assess the tonic response to watching the movies. The run durations were as follows: Breakfast = 420 s, Laundry = 394 s, Library = 340 s, Party = 468 s, Window box = 446 s.
Session 2 – behavioral testing.
Participants returned to the lab a mean of 3.4 days later (SD = 2.3, min = 0, max = 14, mode = 2) for behavioral testing. Behavioral testing occurred in two phases: 1) segmentation at one grain and memory tests, 2) segmentation at the other grain. For the segmentation task, participants watched each movie and were instructed to press a button on a button box when, in their opinion, one meaningful unit of activity ended and another began. During one viewing participants identified the smallest meaningful units of activity – fine segmentation, and during another viewing they identified the largest meaningful units – coarse segmentation. The order of segmentation grain was counterbalanced across participants, and the order of movie presentation was the same as the order presented during session 1. After the first viewing of each movie, we tested participants’ recall, recognition, and order memory for the events. (Thus, memory was tested shortly after the second of two presentations, separated by approximately three days.) After the final movie was presented, participants proceeded to the second phase, during which they segmented the movies at the other grain but did not repeat the memory tasks.
Behavioral measures
Segmentation.
From the segmentation data, we calculated measures of unit length and segmentation quality. Fine and coarse unit lengths were computed by dividing the length of the movie by the number of units identified. Segmentation quality was assessed using measures of segmentation agreement and hierarchical alignment. Segmentation agreement reflects the extent to which an individual’s segmentation patterns agree with group norms (Bailey, Zacks, et al., 2013; Bailey, Kurby, et al., 2013; Kurby & Zacks, 2011; Sargent et al., 2013; J. M. Zacks, Speer, et al., 2006). Agreement scores were calculated by first breaking each movie into 1s time bins and then calculating the proportion of participants that indicated an event boundary in each bin, separately by grain and age group. Normative segmentation patterns were formed by averaging across age group to obtain fine and coarse segmentation probability across time. (Averaging removes potential age-group biases in segmentation patterns.) Point-biserial correlations were run between each individual’s segmentation pattern and the norm, which were then rescaled to account for differences in the number of units identified, resulting in a score ranging from zero to one (see Kurby & Zacks, 2011 for details). Hierarchical alignment was assessed in two ways. Continuous alignment reflects the average temporal distance between each coarse boundary and the nearest fine, minus the distance expected by chance (J. M. Zacks, Tversky, et al., 2001). The scores were log-transformed to correct for positive skew (Hard et al., 2006; Kurby & Zacks, 2011). Discrete alignment is computed by dividing the movie into 1 s bins and coding the number of bins that contained both a coarse and fine boundary, minus the number of overlaps expected by chance (J. M. Zacks, Tversky, et al., 2001).
Memory.
To score recall, we coded each movie for the location and identity of action completions as described by the Action Coding System (ACS: Schwartz, Reed, Montgomery, Palmer, & Mayer, 1991). The ACS identifies a 2-level hierarchical goal structure of action sequences by classifying actions as 1st level basic actions (A1 actions), such as “opening a carton of coffee creamer” and “pouring the creamer,” and 2nd level goals which cluster the A1 actions (A2 actions). For example, “put creamer in coffee” clusters the actions of opening the carton and pouring it. The ACS also identifies the central actions that indicate the completion of A2 goals, called crux actions. The crux action of the above example is “pouring the creamer.” For each recall protocol, we scored how many of each type of action was mentioned. We will report analyses only for recall of A1 units, which we will refer to as action recall, because recall of A1 units has been the focus of previous studies of event memory (Bailey, Zacks, et al., 2013; Bailey, Kurby, et al., 2013; Sargent et al., 2013). (Analyses of the other action types were consistent with the results reported here.)
Recognition memory was recorded as the proportion of correct responses, and order memory was assessed as the mean absolute deviation of each placed card from its correct ordinal position.
Results
Analysis of Behavioral Data
Segmentation.
Mean fine and coarse unit lengths and segmentation agreement scores are presented in Table 1, separated by age group. Table 1 also presents the mean alignment scores by age group and results from t-tests comparing younger and older groups on each of these dependent variables. As can be seen in Table 1, participants produced longer units during coarse segmentation than fine, indicating that were able to follow instructions. Numerically, older adults performed worse than younger adults on measures of segmentation agreement and continuous hierarchical alignment; however, the age difference for fine segmentation agreement was the only significant effect.
Table 1.
Segmentation Performance by Age Group (SDs in parentheses) and t-test Results t-test Analysis Results of Segmentation Performance
| Measure | Younger | Older | t | p | d |
|---|---|---|---|---|---|
| Coarse Unit Length | 41.5 (16.4) | 33.6 (12.1) | −1.82 | .076 | −0.55 |
| Fine Unit Length | 14.2 (6.2) | 14.6 (9.5) | 0.14 | .890 | 0.05 |
| Coarse Segmentation Agreement | .56 (.13) | .51 (.12) | −1.40 | .169 | −0.45 |
| Fine Segmentation Agreement | .67 (.07) | .58 (.11) | −2.66 | .010* | −0.97 |
| Continuous Alignment | 0.40 (0.18) | 0.33 (0.25) | −0.95 | .347 | −0.34 |
| Discrete Alignment | 1.50 (1.51) | 1.28 (1.05) | 0.48 | .636 | 0.17 |
| Discrete Alignment | 0.48 | .636 | 0.17 |
Note: Age group served as the independent variable (Younger vs. Older). All t-tests have 50 df.
p < .05.
Memory.
Table 2 presents mean memory scores by age group and results from independent samples t-tests between age group. Older adults performed significantly worse than younger adults on all measures. (Because order memory is scored as an error measure, higher scores are worse.)
Table 2.
Memory Performance by Age Group (SDs in parentheses) and t-test Analysis
| Measure | Younger | Older | t | p | d |
|---|---|---|---|---|---|
| Recognition Accuracy | .87 (.04) | .83 (.06) | −2.16 | .036* | −0.79 |
| Order Memory | 0.32 (0.32) | 0.93 (0.52) | 3.84 | <.001*** | 1.41 |
| Action Recall | .30 (.12) | .22 (.07) | −3.18 | .002** | −0.89 |
Note: Age group served as the independent variable (Younger vs. Older). All t-tests have 50 df.
p < .001,
p < .01,
p < .05.
Relations Between Segmentation and Memory.
Table 3 presents correlations between each measure of segmentation ability (agreement and the two measures of alignment) and memory. As can be seen in Table 3, better segmentation was associated with better memory performance, with segmentation agreement showing the strongest correlations. For illustration, Figure 1 presents the scatterplots of the relations between segmentation agreement and memory.
Table 3.
Correlations between Segmentation Ability and Memory Performance
| Memory Measure | Segmentation Agreement | Continuous Alignment | Discrete Alignment |
|---|---|---|---|
| Recognition Accuracy | .45** | .38* | .03 |
| Order Memory | −.42** | −.37* | −.23 |
| Action Recall | .39* | .19 | .12 |
Note: Older adults only.
p < .001,
p < .01,
p < .05.
Figure 1.
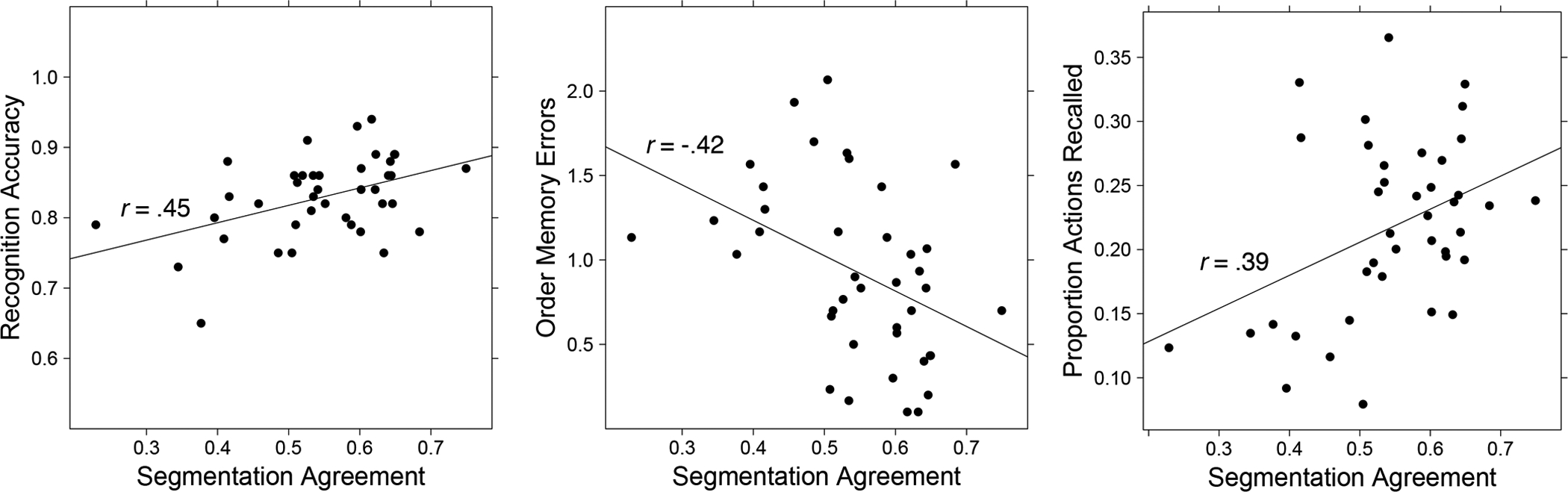
Segmentation agreement predicts recognition memory, order memory, and action recall.
Neuroimaging Data
Whole-brain analysis of the response at event boundaries.
To estimate the brain response at event boundaries, we estimated GLMs using finite impulse responses (Ollinger, Corbetta, & Shulman, 2001). Each individual’s segmentation responses were used to generate regressors coding for the effects on brain activity of proximity to an event boundary. We used a window of 20 s before the boundary to 20 s after the boundary (20 frames total). Responses to coarse and fine boundaries were modeled simultaneously. We also included regressors of no interest to control for scanner drift and run-to-run differences. The regression weights associated with the fine and course segmentation regressors were thus estimates of the timecourse of the response during the window surrounding an event boundary, controlling for the regressors of no interest. For each brain voxel, the estimated timecourse of responses at coarse and fine boundaries for each participant were submitted to a mixed ANOVA with time as the repeated measure and age group as the between subject variable. Voxel-wise maps of resulting F statistics were converted to z statistics and thresholded to include only clusters of two or more voxels with a z value greater than 4.5. This corrects for multiple comparisons by keeping the false positive rate at p = .05 (McAvoy, Ollinger, & Buckner, 2001).
Figure 2a presents the regions showing a main effect of time, and Figure 2b shows weighted average timecourses across all activated voxels. As can be seen in Figure 2, the perception of event boundaries was associated with an increase in activity in large regions in both lateral and medial occipital, parietal, and temporal cortex, as well as smaller regions in the frontal cortex. Figure 2b reveals that this increase in activity is larger for coarse than fine boundaries, and that younger and older adults show similar timecourses around event boundaries.
Figure 2.
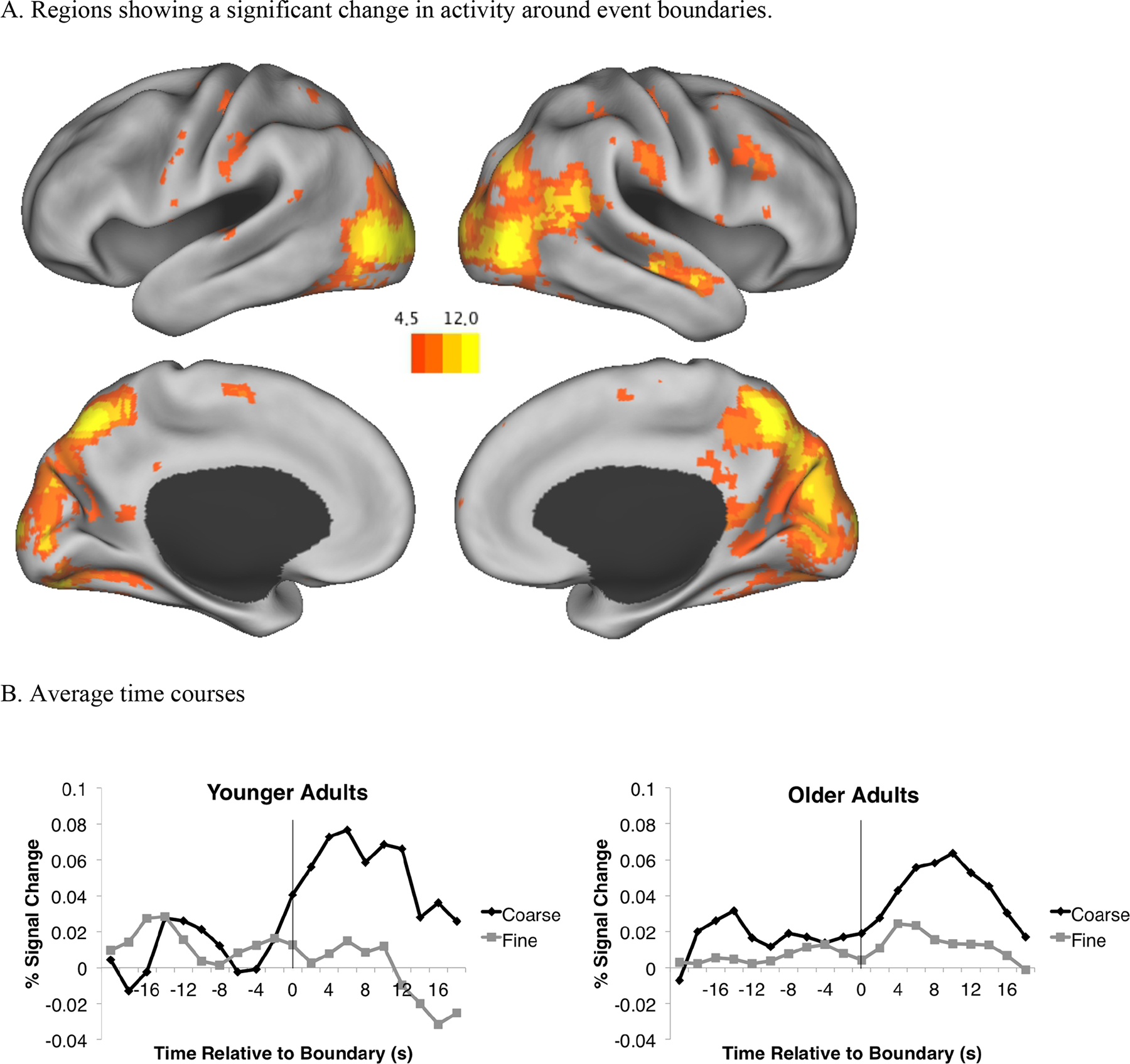
A. Regions showing a main effect of time. B. Average weighted timecourses across all significant voxels, separated by age group. The vertical rules at time zero indicate the location of the event boundary.
In order to better characterize these regions, and to assess interactions among the other variables with time, we used a clustering algorithm to group significant voxels into regions (see Zacks, Speer, Swallow, & Maley, 2010). First, local maxima were identified in the z-maps, among all significant voxels with z scores greater than 6. If two maxima were closer than 30 mm to each other, the maximum with the lowest z value was excluded, and this process iterated until all peaks throughout the map were no closer than 30 mm from each other. Significant voxels were grouped with the closest maximum. The resulting clusters were visually inspected to verify that they produced anatomically plausible units. Table 4 presents descriptive statistics for these 19 clusters. To assess interactions among time, grain, and age, we examined the interaction terms from the ANOVA for these regions. Table 4 lists the results. As can be seen in the table, four regions had a significant grain × time interaction. No regions showed a significant time-by-age interaction, or a time-by-age-by-grain interaction.
Table 4.
Regions showing significant fMRI signal change at event boundaries.
| Atlas coordinate of peak | ||||||||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | Location | Volume (cm3) | Peak z statistic | Grain-by-time interaction | Age-by-time interaction | Age-by-grain-time interaction |
| −58 | −55 | 14 | L. parietal/temporal/occipital junction (BA 39) | 2.46 | 6.10 | -- | -- | -- |
| −54 | −26 | 30 | L. inferior parietal lobule (BA 40) | 5.97 | 6.36 | -- | -- | -- |
| −37 | −77 | 30 | L. superior occipital gyrus (BA 19) | 14.20 | 9.38 | -- | -- | -- |
| −36 | −51 | 60 | L. superior parietal lobule (BA 7) | 0.97 | 6.47 | -- | -- | -- |
| −30 | −74 | −8 | L. lingual gyrus and cuneus (BA 18/19) | 32.51 | 13.62 | -- | -- | -- |
| −30 | −20 | 49 | L. precentral gyrus (BA 4) | 2.43 | 6.54 | -- | -- | -- |
| −8 | −75 | −41 | L. cerebellum | 1.49 | 6.45 | -- | -- | -- |
| −4 | 64 | 20 | Bilateral superior frontal gyrus (BA 10) | 1.46 | 7.21 | -- | -- | -- |
| −3 | −9 | 56 | Bilateral medial frontal gyrus (BA 6) | 2.21 | 6.83 | -- | -- | -- |
| 2 | −79 | 31 | Bilateral lingual gyrus and cuneus (BA 18/19) | 34.37 | 14.22 | ✓ | -- | -- |
| 6 | −48 | 42 | Bilateral precuneus/posterior cingulate gyrus (BA 7) | 15.90 | 8.36 | -- | -- | -- |
| 19 | −88 | 5 | R. lingual gyrus and cuneus (BA 18/19) | 29.78 | 11.04 | ✓ | -- | -- |
| 23 | −57 | −1 | R. lingual gyrus (BA 18) | 18.33 | 12.93 | ✓ | -- | -- |
| 28 | 7 | 3 | R. putamen | 0.76 | 6.34 | -- | -- | -- |
| 36 | −77 | 34 | R. superior occipital gyrus (BA 19) | 11.53 | 9.68 | ✓ | -- | -- |
| 38 | 11 | 44 | R. middle frontal gyrus (BA 9/46) | 6.45 | 7.98 | -- | -- | -- |
| 47 | −26 | 43 | R. postcentral gyrus (BA 2) | 9.75 | 8.47 | -- | -- | -- |
| 51 | −58 | 15 | R. parietal/temporal/occipital junction (BA 39) | 16.34 | 12.37 | -- | -- | -- |
| 55 | −15 | −3 | R. middle temporal gyrus (BA 21) | 6.21 | 8.68 | -- | -- | -- |
Note: Check marks indicate regions that showed a grain-by-time interaction, corrected for multiple comparisons across the 19 regions with the Bonferroni method.
In sum, the evoked-response replicated the finding of robust responses at event boundaries, with larger responses for coarse than fine boundaries (Speer et al., 2007; Whitney et al., 2009; J. M. Zacks, Braver, et al., 2001; J. M. Zacks et al., 2010; J. M. Zacks, Swallow, et al., 2006) These responses did not differ significantly between older and younger adults.
Individual differences in brain response at event boundaries.
We assessed the relation between individual differences in the older adults’ brain response at event boundaries and the behavioral measures of segmentation and memory. First, to obtain a normative brain response to event boundaries in younger adults, we re-ran the ANOVAs within the younger adult group only and identified regions that showed a main effect of time. Using the same thresholding technique as described above, nine regions survived thresholding (See Table 5). (ANOVAs within these regions verified that older and younger adults did not differ in their evoked responses around event boundaries.) We then averaged each younger participant’s BOLD data across the voxels within those nine regions and recomputed the GLM analysis for this single average time series for each participant and averaged the results across participants to obtain a canonical response for each grain. We used these timecourses as assumed response functions and computed new regionwise GLMs for the older adults (including the same regressors of no-interest as above.) From each resulting GLM, we extracted z scores associated with the fine and coarse effect for each older adult participant. These were correlated against segmentation and memory performance.
Table 5.
Regions showing significant fMRI signal change at event boundaries using data from younger adults only.
| Atlas coordinate of peak | ||||||
|---|---|---|---|---|---|---|
| X | Y | Z | Location | Volume (cm3) | Peak z statistic | Grain-by-time interaction |
| −41 | −42 | −27 | L. cerebellum | 0.16 | 5.02 | -- |
| −38 | −63 | −18 | L. cerebellum | 0.16 | 4.95 | -- |
| −17 | −93 | −12 | L. lingual gyrus (BA 18) | 2.43 | 5.73 | -- |
| −8 | −75 | 27 | L. cuneus (BA 18/19) | 0.27 | 5.37 | -- |
| 4 | −66 | 51 | R. precuneus (BA 7) | 0.51 | 5.57 | -- |
| 8 | −90 | 0 | Bilateral lingual gyrus and cuneus (BA 18/19) | 2.46 | 6.54 | ✓ |
| 8 | −78 | 45 | R. precuneus (BA 7) | 0.24 | 5.48 | -- |
| 17 | −75 | 27 | R. cuneus (BA 18/19) | 1.73 | 5.80 | ✓ |
| 44 | −84 | 3 | R. middle occipital gyrus (BA 19) | 4.67 | 7.00 | -- |
Note: Check marks indicate regions that showed a grain-by-time interaction, corrected for multiple comparisons across the 9 regions with the Bonferroni method.
Figure 3a presents scatterplots for the relations among between behavioral responses and fine evoked responses, and 3b presents those relations for coarse evoked responses. As can be seen in the figures, behavior was very weakly correlated with BOLD response magnitude around event boundaries, with none of the correlations approaching statistical significance (smallest p = .461).
Figure 3.
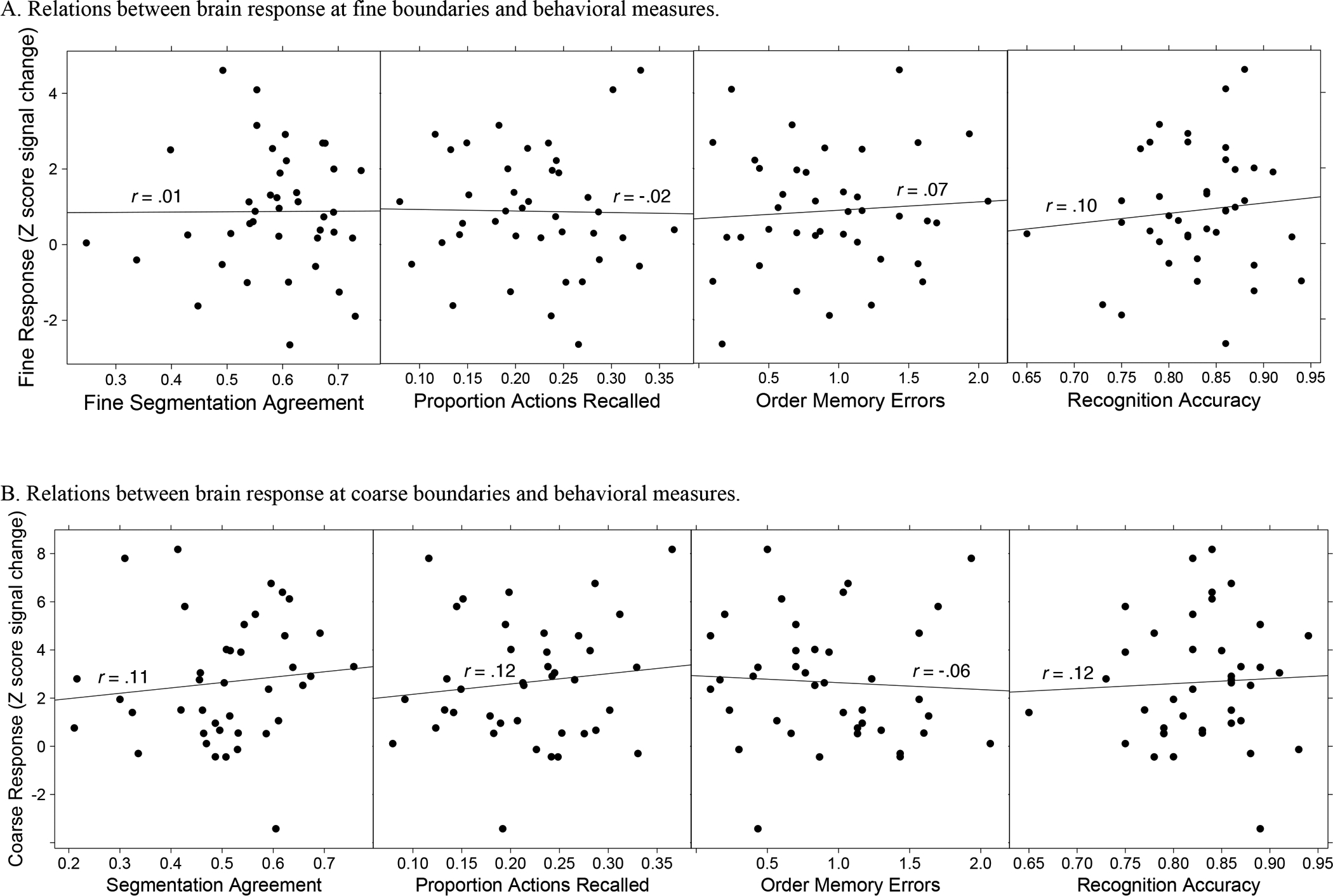
Scatterplots showing the associations among z-scored signal change at event boundaries, segmentation agreement, and memory.
Inter-subject correlation analyses.
We assessed individual differences in the synchronization of the brain response in older adults, across the duration of each movie, to a younger adult norm. We followed procedures described by Hasson et al. (2004) to assess intersubject brain synchronization. The functional data were normalized to a common stereotaxic space and smoothed (12 FWHM). The younger adult data was then averaged across subjects to create a normative pseuodsubject. For each movie, we correlated each voxel of each older adult brain with the corresponding normative voxel, controlling for linear trend, and z-transformed the correlations. The resulting correlations were averaged across movies to obtain a mean correlation z-image for each subject, which were then averaged to produce a grand mean correlation z-image (see Figure 4). Voxel-wise maps of resulting z statistics and were thresholded to include only clusters of 36 or more voxels with a z value greater than 3.5. This corrects for multiple comparisons by keeping the false positive rate at p = .05 (McAvoy et al., 2001). Consistent with Hasson et al. (2004), areas of strong intersubject synchronization included large portions of occipital, partietal, and temporal cortex. This shows that overall, older adults were synchronous with younger adults.
Figure 4.
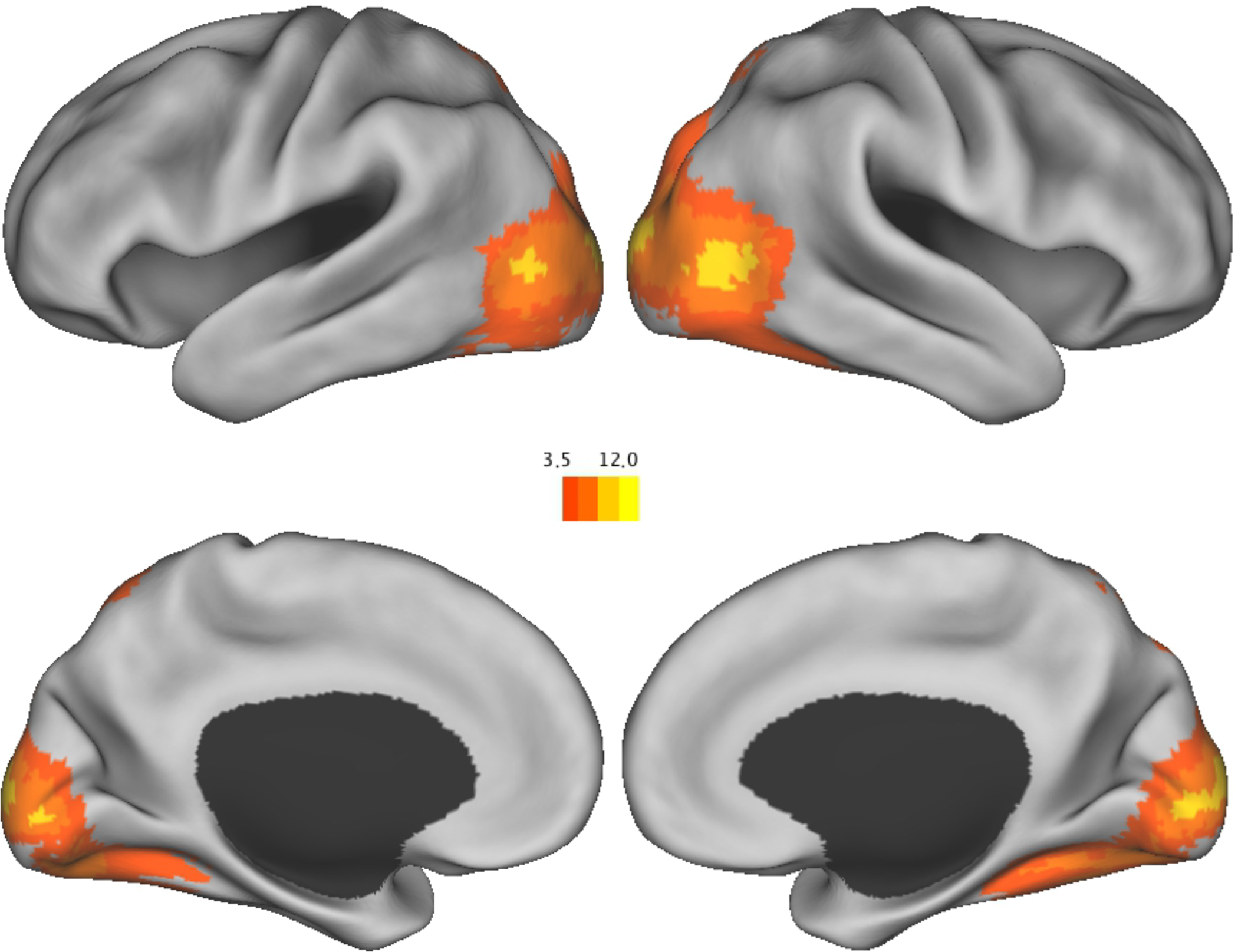
Brain regions showing synchrony between older adults and the younger adult norm.
As described by Hasson et al (2004), the strongest intersubject correlations reflect a non-selective salience signal. We computed new regression models to adjust for this by finding the top two regions that showed the strongest synchrony (Region 1: x = 17, y = −93, z = 4, size = 154 voxels; Region 2: x = −12, y = −94, z = −1, size = 151 voxels) – within visual cortex – then extracting the timecourse of the aggregate of those regions, and including that timecourse as a covariate in the model (and also controlling for linear trend). Figure 5 shows that a region in bilateral temporal-occipital cortex strongly correlated with the younger adult norm, after controlling for the non-selective salience signal. The observed coordinates of this region (Right: x = 47, y = 69, z = 3; Left: x = −45, y = −75, z = 2) are similar to previously reported coordinates of MT+ (Dumoulin et al., 2000; Grossman et al., 2000; Speer et al., 2003).
Figure 5.
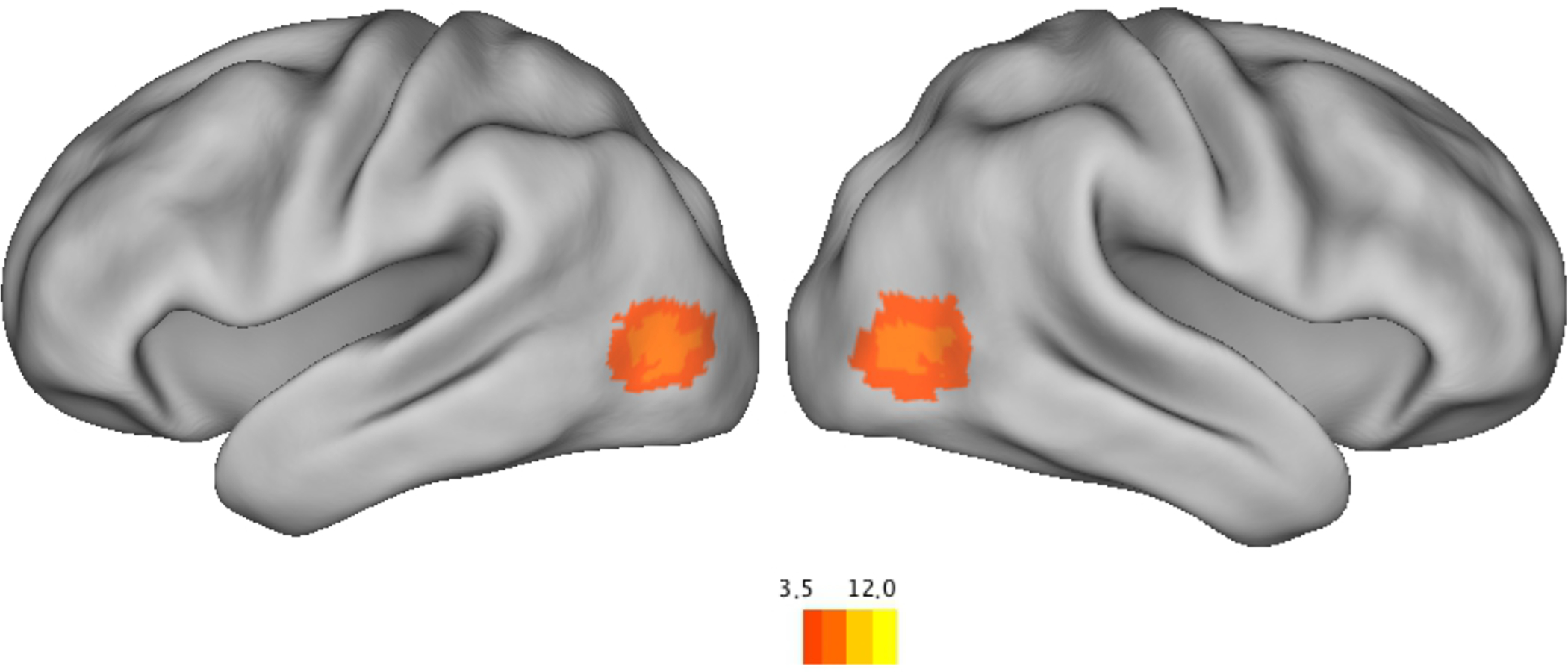
Regions that showed synchrony between older adults and the younger adult norm, after controlling for the large salience synchrony signal.
We then, for each older adult, assessed the relationship between individual differences in voxel synchrony with the younger adult norm and individual differences in the behavioral measures. For each voxel, we correlated that voxel’s synchrony with the younger adult norm (z-transformed correlation) against each of the behavioral measures, z-transformed the correlations, and corrected for multiple comparisons by only including clusters of 36 voxels with a z value greater than 3.5, to retain a p = .05 false positive rate using Monte Carlo simulations (McAvoy et al., 2001). This provided a z-map of the relationship between synchronization of that voxel and the behavioral measure. Higher segmentation agreement scores were related to increased synchrony in the right superior temporal sulcus (pSTS; x = 44, y = −56, z = 21; see Figure 6a). Better hierarchical alignment, measured as discrete segmentation alignment, was related to increased synchrony in the left dorsolateral prefrontal cortex (DLPFC; x = −42, y = 6, z = 29; see Figure 6b). Longer coarse unit lengths were associated with reduced synchrony in the left insular cortex (x = −38, y = 11, z = 1; see Figure 5c). There were no significant relations among synchrony and memory performance.
Figure 6.
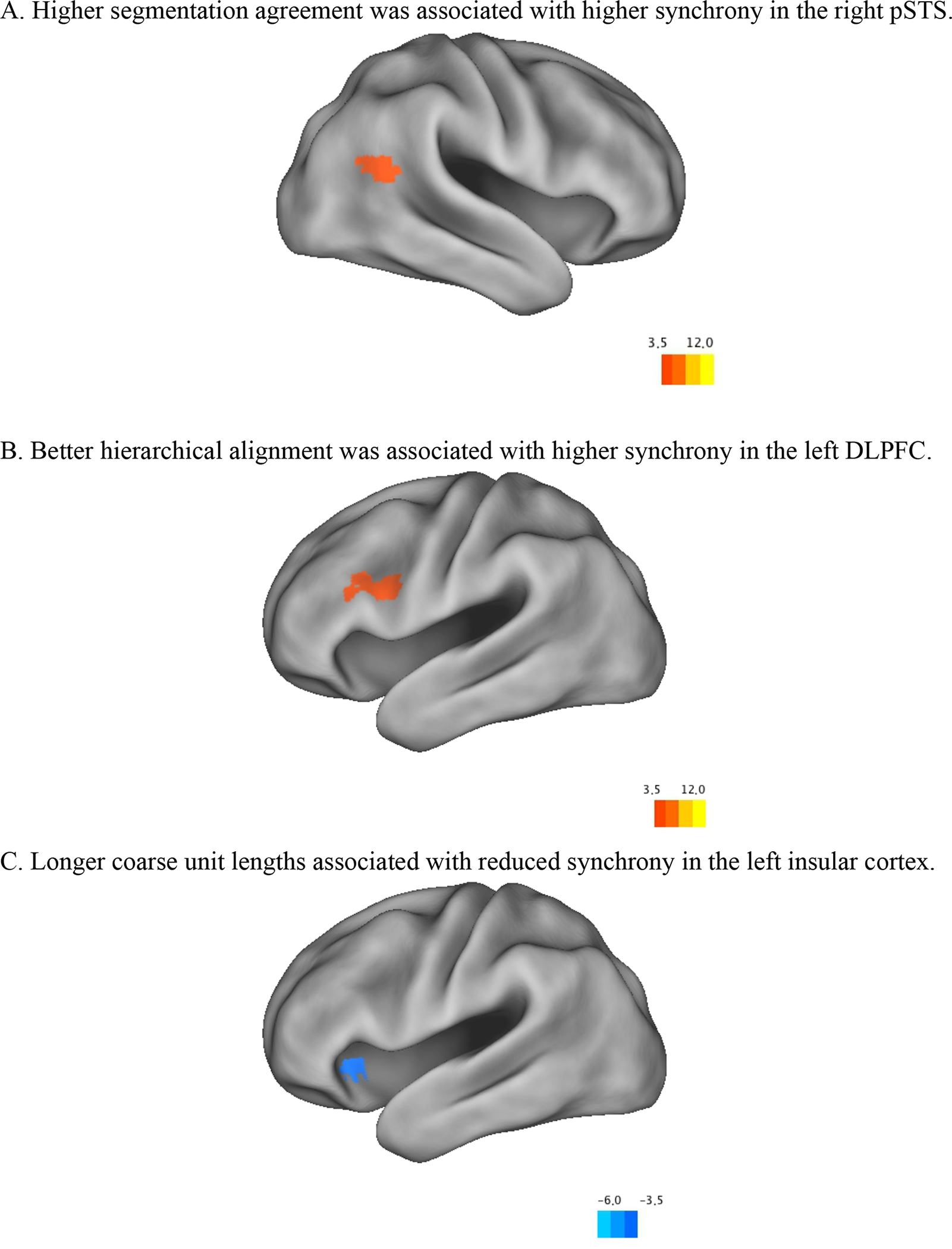
Regions with significant correlations between synchrony and A. segmentation agreement scores, B. hierarchical alignment scores, and C. coarse unit lengths.
Discussion
In a healthy aging sample, we investigated individual differences in event processing and their relation to memory and brain dynamics. Participants first watched movies of everyday activities, then segmented them into events, and then performed memory tests. We tested whether older adults showed normative neural dynamics during event comprehension, and whether the quality of those dynamics is associated with event comprehension quality. Consistent with previous work (Kurby & Zacks, 2011; Sargent et al., 2013; J. M. Zacks, Speer, et al., 2006), we expected event memory performance to vary systematically with segmentation performance. Additionally, we hypothesized that normative neural dynamics of brain regions important for event processing would be associated with better event segmentation performance.
Overall, the older adults showed event comprehension that was similar to the smaller younger adult comparison sample. They had worse fine, but not coarse, segmentation agreement. They also had comparable hierarchical structuring of their segmentation. These results are surprising given previous findings of age-related deficits in event segmentation (Kurby & Zacks, 2011; J. M. Zacks, Speer, et al., 2006; but see Sargent et al., 2013). Zacks, et al. (2006) found that older adults had lower fine and coarse segmentation agreement. Kurby and Zacks (2011) also found age-differences in both fine and coarse segmentation, but also found that younger adults had better hierarchical structuring of their event segmentation behavior. Related work has also shown that adults with reduced cognitive functioning also tend to have worse segmentation performance. Zacks et al. (2006) additionally found that adults with increasing levels of dementia severity produced decreasing levels of segmentation agreement. That finding was replicated by Bailey, Kurby, et al. (2013). Zalla, Labruyere, and Georgieff (2013) found reduced (fine and coarse) segmentation ability in individuals with autism spectrum disorders and individuals with moderate mental retardation. Reduced segmentation ability has also been found among individuals with traumatic brain injuries (J. M. Zacks, Kurby, Landazabal, Krueger, & Grafman, 2016; Zalla, Pradat-Diehl, & Sirigu, 2003) and schizophrenia (Zalla, Verlut, Franck, Puzenat, & Sirigu, 2004). It is notable, however, that a recent study (Sargent et al., 2013) using a large lifespan sample did not find age-related change in event segmentation performance. Why that study showed age-preservation of segmentation ability is unclear, but it may be in part due to sampling issues; they ended up with a large number of high functioning older adults in their sample.
Our results suggest that our sample included older adults with relatively preserved event segmentation ability. Regarding the brain response, we found that, on average, the older adults had a largely canonical response around event boundaries. Both age groups had increased activity in large portions of posterior cortex, including bilateral temporal-occipital-parietal cortex, and right lateral frontal cortex. The older adults had increased activity in statistically the same regions as the younger adults, had statistically similar timecourses, and showed similar differences between fine and coarse event boundaries.
The older adults also showed a substantial amount of neural synchrony with the younger adult comparison sample, in bilateral temporal, occipital, parietal cortex. After factoring out the non-selective salience signal, significant synchrony remained in a region close to bilateral MT+. In previous work, activity in this region has been shown to be driven by non-biological object movement (e.g., Grossman et al., 2000) and also by the perception of event boundaries (Schubotz et al., 2012; J. M. Zacks, Swallow, et al., 2006). Our results suggest that this important event comprehension region operated with similar temporal dynamics between the two age groups. Importantly, older adult neural synchrony with the younger adult norm correlated with segmentation behavior. Better segmentation agreement was associated with higher synchrony in the right pSTS, and better hierarchical alignment in segmentation patterns was associated with higher synchrony in the left DLPFC.
The existence of these associations suggests that the right pSTS and left DLPFC may be important for the maintenance of event models. According to current theories of event cognition (Kurby & Zacks, 2008; Radvansky & Zacks, 2014; J. M. Zacks et al., 2007), once event models are established at event boundaries they must be maintained and updated as events unfold. When a new major event shift occurs, the process begins anew (Bailey & Zacks, 2015; Kurby & Zacks, 2012). An event is a dynamically changing entity and event models must accommodate those changes. Broadly speaking, there are within-event and across-event changes that signal updates to event models (Kurby & Zacks, 2012). Proper management of such changes likely requires adaptive maintenance of event models. Consider, for example, a child making breakfast for herself. Such an activity demands the maintenance of a series of goals to be executed in a certain order, such as getting a bowl, getting the milk, getting the cereal, pouring the cereal, etc. As one moves from one goal to the next, new event models are created. However, within an event, such as getting the bowl, there are changes that indicate that the event is further unfolding, such as opening the cabinet, grasping the bowl, etc. Maintaining a degraded event model will likely make these moment-to-moment updates difficult to conduct, and event comprehension should suffer. The pSTS is a likely candidate to contribute to updating based on changes in biological motion (J. M. Zacks et al., 2007). More normative temporal dynamics of the pSTS during event comprehension are likely associated with better event perception. Additionally, we found that higher left DLPFC synchrony was associated with better hierarchical alignment in segmentation patterns. Better hierarchical structuring likely places higher demands on knowledge structures of events and how they are organized (Bailey, Kurby, et al., 2013; Kurby & Zacks, 2011; J. M. Zacks, Tversky, et al., 2001), which may be subserved by the PFC (Wood & Grafman, 2003). The PFC also has been implicated in the maintenance and updating of action-based goal plans (Miller & Cohen, 2001). Damage to PFC has also been associated with apraxia (Schwartz, 2006; Schwartz et al., 1991), and reduced event segmentation performance (J. M. Zacks et al., 2016; Zalla et al., 2003).1 Lastly, these findings are consistent with claims from Event Segmentation Theory (J. M. Zacks et al., 2007) that PFC serves to maintain event models.
Although we found significant relationships between neural synchrony and event segmentation behavior, we did not find similar relationships between the strength of the brain response around event boundaries and behavioral performance. This is curious given that we replicated previous findings that older adults who segmented more normatively also had better memory for the activities. The increased brain response at event boundaries likely indicates updating processes as one shifts to a new model (Ezzyat & Davachi, 2011). Our data suggest that the older adults in this sample do not have degraded event updating ability, and that event memory quality may not strongly rely on this updating process. That older adults may have preserved event updating mechanisms has been suggested by work showing a lack of age differences in the ability to update situation models in narrative contexts (Radvansky & Dijkstra, 2007). Rather, as the synchrony data indicate, managing the unfolding event and event maintenance may feature more strongly in the quality of event processing. It has been well-documented that older adults may have reduced working memory and attentional control (Balota, Dolan, & Duchek, 2000; Brockmole & Logie, 2013; Hasher & Zacks, 1988; Salthouse & Babcock, 1991; J. M. Zacks & Sargent, 2010; R. T. Zacks & Hasher, 1994), both of which have been implicated in age-related decline in event perception (J. M. Zacks & Sargent, 2010).
Despite the fact that older adults’ behavioral and neural event comprehension was similar to that of younger adults, their memory was consistently worse than that of younger adults. This finding is noteworthy, and is consistent with the broad findings of age-related deficits in episodic memory (Balota et al., 2000; R. T. Zacks, Hasher, & Li, 2000). It is also consistent with the results of Sargent et al. (2013), who observed preserved segmentation but impaired memory in older adults. It is also noteworthy that we did not find relations between synchrony and event memory. A possible interpretation is that those older adults with lower synchrony were encoding events idiosyncratically because they were remembering events to the same extent as those who had higher synchrony. That may be the case, but we think it unlikely. Older adults with lower synchrony also tended to segment worse, which was related to worse memory. This suggests that non-normative event encoding is not idiosyncratic, per se, but rather, maladaptive. It is unclear why we did not observe a direct relationship between memory and synchrony, but it may be the case that our memory tasks were not sensitive to changes in memory encoding that may occur with less-normative changes in brain dynamics in segmentation-related regions.
Overall, in this study, we found that in healthy aging the brain mechanisms engaged in event segmentation remain relatively intact compared to younger adults. Additionally, we replicated previous work that, in an older adult sample, better event segmentation is related to better event memory. Our brain imaging results suggest that normative changes in brain dynamics during event comprehension, in important event processing regions, are related to better event segmentation processing. A possibility is that these dynamics reflect the management of moment-by-moment changes in events, a process that demands an engagement with working memory and attentional control (Campbell et al., 2015). More broadly, the results suggest that event updating may be preserved in healthy aging, but the processes engaged event model maintenance may be sensitive to age-related change in event perception.
Acknowledgments
This project was funded by NIH grant R01 AG031150, PI Jeffrey M. Zacks, and also supported by NIH grant T32 AG000030-31. Some of the results presented in this manuscript were presented as a poster at the 53rd annual meeting of the Psychonomic Society, and at the 2012 meeting of the Cognitive Aging Conference.
Footnotes
In this study, deficits were associated most strongly with lesions to the ventromedial PFC; however, dorsolateral PFC lesions also were associated with reduced segmentation agreement.
References
- Bailey HR, Kurby CA, Giovannetti T, & Zacks JM (2013). Action perception predicts action performance. Neuropsychologia, 51(11), 2294–2304. 10.1016/j.neuropsychologia.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HR, & Zacks JM (2015). Situation model updating in young and older adults: Global versus incremental mechanisms. Psychology and Aging, 30(2), 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey HR, Zacks JM, Hambrick DZ, Zacks RT, Head D, Kurby CA, & Sargent JQ (2013). Medial temporal lobe volume predicts elders’ everyday memory. Psychological Science, 24(7), 1113–1122. 10.1177/0956797612466676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano C, Chen J, Zadbood A, Pillow JW, Hasson U, & Norman KA (2017). Discovering event structure in continuous narrative perception and memory. Neuron, 95(3), 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Dolan PO, & Duchek JM (2000). Memory changes in healthy older adults., 395–409. [Google Scholar]
- Boltz M (1992). Temporal accent structure and the remembering of filmed narratives. Journal of Experimental Psychology: Human Perception and Performance, 18(1), 90–105. 10.1037/0096-1523.18.1.90 [DOI] [PubMed] [Google Scholar]
- Brent MR (1999). Speech segmentation and word discovery: A computational perspective. Trends in Cognitive Sciences, 3(8), 294–301. 10.1016/S1364-6613(99)01350-9 [DOI] [PubMed] [Google Scholar]
- Brockmole JR, & Logie RH (2013). Age-related change in visual working memory: A study of 55,753 participants aged 8–75. Frontiers in Psychology, 4. Retrieved from http://search.proquest.com.ezproxy.gvsu.edu/psycinfo/docview/1399053632/234B9707E94446F3PQ/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, & Snyder AZ (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage, 23(2). 10.1016/j.neuroimage.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Campbell KL, Shafto MA, Wright P, Tsvetanov KA, Geerligs L, Cusack R, … Villis L (2015). Idiosyncratic responding during movie-watching predicted by age differences in attentional control. Neurobiology of Aging, 36(11), 3045–3055. 10.1016/j.neurobiolaging.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CLJ, Le Goualher G, Bruce Pike G, & Evans AC (2000). A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cerebral Cortex (New York, N.Y. : 1991), 10(5), 454–63. 10.1093/cercor/10.5.454 [DOI] [PubMed] [Google Scholar]
- Epstein R, & Kanwisher N (1998). A cortical representation of the local visual environment. Nature, 392(6676), 598–601. 10.1038/33402 [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, & Davachi L (2011). What constitutes an episode in episodic memory? Psychological Science, 22(2), 243–252. 10.1177/0956797610393742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores S, Bailey HR, Eisenberg ML, & Zacks JM (2017). Event segmentation improves event memory up to one month later. Journal of Experimental Psychology: Learning, Memory, and Cognition, 43(8), 1183–1202. 10.1037/xlm0000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DA, Zacks JM, & Flores S (2017). Effects of cues to event segmentation on subsequent memory. Cognitive Research: Principles and Implications, 2. 10.1186/s41235-016-0043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, & Blake R (2000). Brain areas involved in perception of biological motion. Journal of Cognitive Neuroscience, 12, 711–720. [DOI] [PubMed] [Google Scholar]
- Hard BM, Tversky B, & Lang DS (2006). Making Sense of Abstract Events: Building Event Schemas. Memory & Cognition, 34(6), 1221–1235. [DOI] [PubMed] [Google Scholar]
- Hasher L, & Zacks RT (1988). Working memory, comprehension, and aging: A review and a new view. In The psychology of learning and motivation: Advances in research and theory, Vol. 22. (pp. 193–225). Academic Press; (San Diego, CA, US: ). Retrieved from http://search.proquest.com.ezproxy.gvsu.edu/psycinfo/docview/617674532/3664FA80A8147C4PQ/1 [Google Scholar]
- Hasson U, Furman O, Clark D, Dudai Y, & Davachi L (2008). Enhanced intersubject correlations during movie viewing correlate with successful episodic encoding. Neuron, 57(3), 452–62. 10.1016/j.neuron.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Landesman O, Knappmeyer B, Vallines I, Rubin N, & Heeger DJ (2008). Neurocinematics: The Neuroscience of Film. Projections, 2(1), 1–26. 10.3167/proj.2008.020102 [DOI] [Google Scholar]
- Hasson U, Malach R, & Heeger DJ (2010). Reliability of cortical activity during natural stimulation. Trends in Cognitive Sciences, 14(1), 40–8. 10.1016/j.tics.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, & Malach R (2004). Intersubject Synchronization of Cortical Activity During Natural Vision. Science, 303(5664), 1634–1640. [DOI] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, & Rubin N (2008). A hierarchy of temporal receptive windows in human cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(10), 2539–50. 10.1523/JNEUROSCI.5487-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, & Yovel G (2006). The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361(1476), 2109–28. 10.1098/rstb.2006.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurby CA, & Zacks JM (2008). Segmentation in the perception and memory of events. Trends in Cognitive Sciences, 12, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurby CA, & Zacks JM (2011). Age differences in the perception of hierarchical structure in events. Memory & Cognition, 39, 75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurby CA, & Zacks JM (2012). Starting from scratch and building brick by brick in comprehension. Memory & Cognition, 40, 812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschky LC, Larson AM, Magliano JP, & Smith TJ (2015). What Would Jaws Do? The Tyranny of Film and the Relationship between Gaze and Higher-Level Narrative Film Comprehension. PLOS ONE, 10(11), e0142474. 10.1371/journal.pone.0142474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliano JP, Miller J, & Zwaan RA (2001). Indexing space and time in film understanding. Applied Cognitive Psychology, 15, 533–545. [Google Scholar]
- Magliano JP, Zwaan RA, & Graesser AC (1999). The role of situational continuity in narrative understanding. van Oostendorp Herre; Goldman Susan R. (1999). The construction of mental representations during reading. (pp. 219–245). Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers. xiv. [Google Scholar]
- McAvoy M, Ollinger JM, & Buckner RL (2001). Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage, 13, S198. [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Newtson D, & Engquist G (1976). The perceptual organization of ongoing behavior. Journal of Experimental Social Psychology, 12(5), 436–450. 10.1016/0022-1031(76)90076-7 [DOI] [Google Scholar]
- Newtson D, Engquist GA, & Bois J (1977). The objective basis of behavior units. Journal of Personality and Social Psychology, 35(12), 847–862. 10.1037/0022-3514.35.12.847 [DOI] [Google Scholar]
- Ollinger JM, Corbetta M, & Shulman GL (2001). Separating processes within a trial in event-related functional MRI. NeuroImage, 13(1). 10.1006/nimg.2000.0711 [DOI] [PubMed] [Google Scholar]
- Radvansky GA, & Copeland DE (2010). Reading times and the detection of event shift processing. Journal of Experimental Psychology: Learning, Memory, and Cognition, 36(1), 210–216. [DOI] [PubMed] [Google Scholar]
- Radvansky GA, & Dijkstra K (2007). Aging and situation model processing. Psychonomic Bulletin & Review, 14, 1027–1042. [DOI] [PubMed] [Google Scholar]
- Radvansky GA, & Zacks JM (2014). Event cognition Oxford University Press; (New York, NY, US: ). Retrieved from http://search.proquest.com.ezproxy.gvsu.edu/psycinfo/docview/1552598608/30A0026E202A4832PQ/1?accountid=39473 [Google Scholar]
- Salthouse TA, & Babcock RL (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27(5), 763–776. [Google Scholar]
- Sargent JQ, Zacks JM, Hambrick DZ, Zacks RT, Kurby CA, Bailey HR, … Beck TM (2013). Event segmentation ability uniquely predicts event memory. Cognition, 129, 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, Korb FM, Schiffer A-M, Stadler W, & von Cramon DY (2012). The fraction of an action is more than a movement: Neural signatures of event segmentation in fMRI. NeuroImage, 61(4), 1195–1205. [DOI] [PubMed] [Google Scholar]
- Schwan S, Garsoffky B, & Hesse FW (2000). Do film cuts facilitate the perceptual and cognitive organization of activity sequences? Memory & Cognition, 28(2), 214–223. [DOI] [PubMed] [Google Scholar]
- Schwan S, Hesse FW, & Garsoffky B (1998). The relationship between formal filmic means and the segmentation behavior of film viewers. Journal of Broadcasting & Electronic Media, 42(2), 237–249. [Google Scholar]
- Schwartz MF (2006). The cognitive neuropsychology of everyday action and planning. Cognitive Neuropsychology, 23(1), 202–221. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Reed ES, Montgomery M, Palmer C, & Mayer NH (1991). The quantitative description of action disorganisation after brain damage: A case study. Cognitive Neuropsychology, 8(5), 381–414. [Google Scholar]
- Smith TJ, Levin D, & Cutting JE (2012). A window on reality: Perceiving edited moving images. Current Directions in Psychological Science, 21(2), 107–113. 10.1177/0963721412437407 [DOI] [Google Scholar]
- Speer NK, Swallow KM, & Zacks JM (2003). Activation of human motion processing areas during event perception. Cognitive, Affective & Behavioral Neuroscience, 3(4), 335–345. [DOI] [PubMed] [Google Scholar]
- Speer NK, & Zacks JM (2005). Temporal changes as event boundaries: Processing and memory consequences of narrative time shifts. Journal of Memory and Language, 53, 125–140. [Google Scholar]
- Speer NK, Zacks JM, & Reynolds JR (2007). Human brain activity time-locked to narrative event boundaries. Psychological Science, 18, 449–455. [DOI] [PubMed] [Google Scholar]
- Swallow KM, Barch DM, Head D, Maley CJ, Holder D, & Zacks JM (2011). Changes in events alter how people remember recent information. Journal of Cognitive Neuroscience, 23(5), 1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow KM, Zacks JM, & Abrams RA (2009). Event boundaries in perception affect memory encoding and updating. Journal of Experimental Psychology: General, 138, 236–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System, an Approach to Cerebral Imaging. Stuttgart: G. Thieme. [Google Scholar]
- Wang HX, Freeman J, Merriam EP, Hasson U, & Heeger DJ (2012). Temporal eye movement strategies during naturalistic viewing. Journal of Vision, 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Huber W, Klann J, Weis S, Krach S, & Kircher T (2009). Neural correlates of narrative shifts during auditory story comprehension. NeuroImage, 47(1). 10.1016/j.neuroimage.2009.04.037 [DOI] [PubMed] [Google Scholar]
- Wood JN, & Grafman J (2003). Human prefrontal cortex: Processing and representational perspectives. Nature Reviews Neuroscience, 4(2), 139–147. [DOI] [PubMed] [Google Scholar]
- Zacks JM (2004). Using movement and intentions to understand simple events. Cognitive Science, 28(6), 979–1008. [Google Scholar]
- Zacks JM, Braver TS, Sheridan MA, Donaldson DI, Snyder AZ, Ollinger JM, … Raichle ME (2001). Human brain activity time-locked to perceptual event boundaries. Nature Neuroscience, 4, 651–655. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Kumar S, Abrams RA, & Mehta R (2009). Using movement and intentions to understand human activity. Cognition, 112(2), 201–216. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Kurby CA, Landazabal CS, Krueger F, & Grafman J (2016). Effects of penetrating traumatic brain injury on event segmentation and memory. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 74, 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, & Sargent JQ (2010). Event perception: A theory and its application to clinical neuroscience., 53, 253–299. [Google Scholar]
- Zacks JM, Speer NK, & Reynolds JR (2009). Segmentation in reading and film comprehension. Journal of Experimental Psychology: General, 138, 307–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Swallow KM, Braver TS, & Reynolds JR (2007). Event perception: A mind-brain perspective. Psychological Bulletin, 133, 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Swallow KM, & Maley CJ (2010). The brain’s cutting-room floor: Segmentation of narrative cinema. Frontiers in Human Neuroscience, 4, ArtID 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Vettel JM, & Jacoby LL (2006). Event understanding and memory in healthy aging and dementia of the Alzheimer type. Psychology and Aging, 21(3), 466–482. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Swallow KM, Vettel JM, & McAvoy MP (2006). Visual motion and the neural correlates of event perception. Brain Research, 1076(1), 150–162. [DOI] [PubMed] [Google Scholar]
- Zacks JM, & Tversky B (2001). Event structure in perception and conception. Psychological Bulletin, 127(1), 3–21. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Tversky B, & Iyer G (2001). Perceiving, remembering, and communicating structure in events. Journal of Experimental Psychology: General, 130(1), 29–58. [DOI] [PubMed] [Google Scholar]
- Zacks RT, & Hasher L (1994). Directed ignoring: Inhibitory regulation of working memory. In Inhibitory processes in attention, memory, and language. (pp. 241–264). Academic Press; (San Diego, CA, US: ). Retrieved from http://search.proquest.com.ezproxy.gvsu.edu/psycinfo/docview/618559613/F356439D40A04564PQ/1 [Google Scholar]
- Zacks RT, Hasher L, & Li KZH (2000). Human memory. In F. I. M [Ed Craik & A. T [Ed Salthouse (Eds.), 2nd ed. (p. 293–357, Chapter ix, 755 Pages). Lawrence Erlbaum Associates Publishers; (Mahwah, NJ, US: ). Retrieved from https://search-proquestcom/psycinfo/docview/619472687/14478CB451F94D00PQ/1 [Google Scholar]
- Zalla T, Pradat-Diehl P, & Sirigu A (2003). Perception of action boundaries in patients with frontal lobe damage. Neuropsychologia, 41(12), 1619–1627. [DOI] [PubMed] [Google Scholar]
- Zalla T, Verlut I, Franck N, Puzenat D, & Sirigu A (2004). Perception of dynamic action in patients with schizophrenia. Psychiatry Research, 128(1), 39–51. 10.1016/j.psychres.2003.12.026 [DOI] [PubMed] [Google Scholar]
- Zwaan RA (1996). Processing narrative time shifts. Journal of Experimental Psychology: Learning, Memory, and Cognition, 22(5), 1196–1207. [Google Scholar]
- Zwaan RA, & Radvansky GA (1998). Situation models in language comprehension and memory. Psychological Bulletin, 123, 162–185. [DOI] [PubMed] [Google Scholar]


