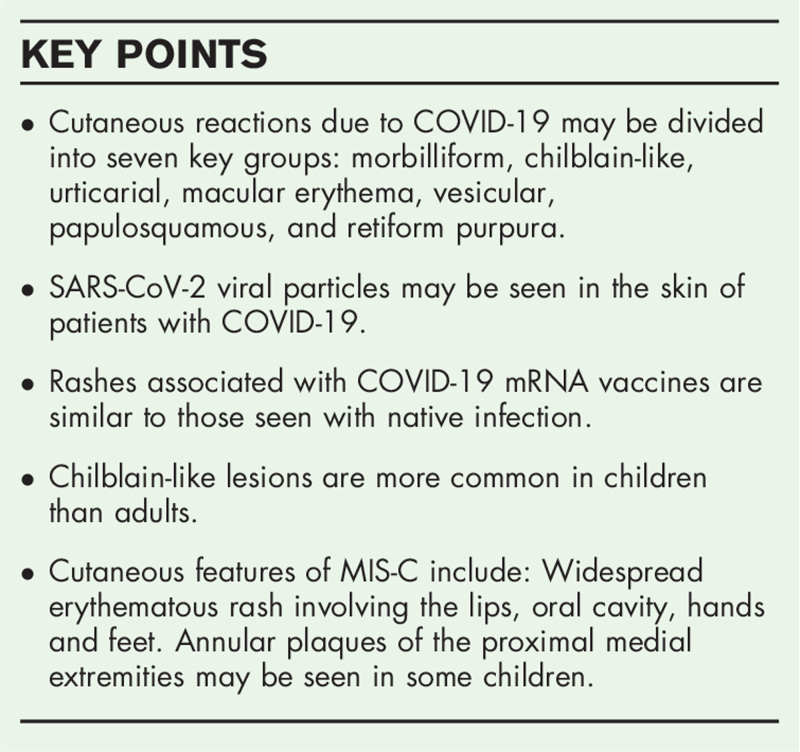
Abstract
Purpose of review
This review examines the global literature regarding rashes encountered in children and adults infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and aims to provide practicing pediatricians with an understanding of the relationship between instances of rashes and coronavirus disease 2019 (COVID-19) in children in order to effectively evaluate and treat patients.
Recent findings
The true incidence of cutaneous reactions in children infected with SARS-CoV-2 is not known. Children's immune systems differ from those of adults and rashes as a manifestation of immune responses, in turn, differ in morphology and distribution. Rarely, children develop a severe multisystem inflammatory syndrome that has overlapping clinical features with Kawasaki disease. In addition, vaccinations produce rashes similar to natural infections. The rashes associated with COVID-19 vaccination are mild and transient, and should not preclude vaccination. Lastly, children who chronically wear masks are more likely to experience flaring of acne around the nose and mouth (’maskne’) and facial conditions such as seborrheic dermatitis.
Summary
There are ongoing worldwide registries, clinical and basic science studies to better understand the burden of skin disease and pathophysiology of rashes seen in patients infected with COVID-19. Robust vaccination programs should be encouraged as a way to contain viral spread among children and the greater population.
Keywords: coronavirus disease-toes, maskne, multisystem inflammatory syndrome in children, pediatric rashes, SARS-CoV-2
INTRODUCTION
In December of 2019, health officials in Wuhan, China [1] began noticing mysterious cases of a pneumonia-like disease. This new viral pathogen was isolated from the lower respiratory tract of infected patients and called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the resulting disease was named coronavirus disease 2019 (COVID-19). In the time since then, the SARS-CoV-2 pathogen has quickly propagated and became a global pandemic. SARS-CoV-2 affects adults and children, with the latter group accounting for approximately 14% of cases. However, the actual disease burden in children is unknown, in large part because of limited testing access and reliability. In addition, children are commonly asymptomatic. According to a joint report of the American Academy of Pediatrics and the Children's Hospital Association (version 4/29/21), children represented 22.4% of new cases, and 0.1–1.9% of all COVID-19 cases in children resulted in hospitalization. This worrisome trend may be due, in part, to the lack of immunization in young children. Unvaccinated infants less than a year old are more likely to develop severe disease.
COVID-19 propagates rapidly through the population because it is highly contagious and spreads via respiratory droplets. Many individuals, especially asymptomatic young children and adolescents, unwittingly spread the virus to more vulnerable older adults and immunosuppressed individuals. These youths are more apt than their older counterparts to engage in socially proximal behavior, creating the perfect opportunity for the virus to disseminate through societies. Therefore, knowledge of any disease-identifying markers is essential to slow down its spread.
At the start of the pandemic, health organizations thought SARS-CoV-2 only impacted the lower respiratory tract. However, now it is clear that multiple organ systems may be affected, including the skin [2▪▪,3▪▪,4,5,6▪▪,7▪▪,8▪,9–12].
Herein, we describe the early reports of rashes related to COVID-19, organize COVID-19 rashes into diagnostic groups, and describe an approach to diagnosing and managing children with COVID-19 rashes. Additionally, we report other dermatological conditions related to the COVID-19 pandemic such as reactions due to the vaccine or mask-wearing.
Box 1.
no caption available
EARLY REPORTS OF CUTANEOUS MANIFESTATIONS IN THE CORONAVIRUS DISEASE 2019 PANDEMIC
Recalcati et al. from Italy reported dermatologic manifestations from COVID-19 infection [13]. In their study, the authors analyzed the skin findings of 88 patients hospitalized for COVID-19. Of these 88 patients, 18 (20.4%) developed dermatological manifestations. This study notes cutaneous findings of erythematous rash (14 cases), urticaria (3 cases), and chickenpox-like vesicles (1 case) [13].
Since the Italian experience with cutaneous manifestations of COVID-19, dermatologists have become more involved in the triage and management of COVID-19 patients worldwide. Galvan Casas et al., from Spain described five principal clinical cutaneous patterns found in their cohort of 375 cases: maculopapular eruption (47%, mean age 61 years), acral erythema (19%, mean age 45 years), urticarial (19%, mean age 53 years), vesicular eruptions (9%, mean age 56 years), and livedo or necrosis (6%, mean age 67 years). In addition, COVID-19 patients developed purpuric flexural lesions, enanthems, and herpes zoster. In adults, vesicular eruptions tended to occur early in the disease course, whereas pseudo-chilblain patterns occurred later. The remainder of documented rashes occurred alongside other symptoms of COVID-19. Patients with acral erythema developed a less severe clinical course than those with other rashes [7▪▪].
These investigators and others have accumulated a vast array of dermatological findings occurring in conjunction with the virus [5,11,14]. However, most systematic review articles describing the cutaneous manifestations of COVID-19 focus on adults and few studies to date have examined children [2▪▪,3▪▪,8▪,15▪▪]. Studies have estimated the percentage of children with rashes resulting from COVID-19 to be between 0.25% [16] and 3% [17], but the actual percentage is unknown. In the US, with the Delta variant being so predominant, the incidence of COVID-19 is skyrocketing in unvaccinated children and adolescents, such that an increase in children presenting to pediatricians will likely be commonplace. Many of these COVID-19 infected children will develop rashes. As dermatologists become more involved in the care of these patients, descriptions and diagnostic evaluations of the rashes will become more precise. A collaborative effort between the American Academy of Dermatology (United States) and the International League of Dermatological Societies (Europe) collates information about COVID-19 patients who develop rashes [18]. This and future international collaborative efforts between epidemiologists, clinicians, and basic scientists will produce a better understanding of the impact of COVID-19 on the skin.
MECHANISTIC INFORMATION OF SARS-COV-2
Mechanistically, the SARS-CoV-2 virus functions by attaching onto an angiotensin-converting enzyme (ACE)-2 receptor via its spike protein. Tissues within the body that express ACE-2 receptors are possible targets onto which the SARS-CoV-2 virus could attach. The SARS-CoV-2 is attached most frequently to the epithelial cells of the lungs (pneumocytes type 2) [19]. The liver, gastrointestinal tract, urinary tract, eye (conjunctiva and cornea), endothelium of blood vessels, epithelial cells of sweat glands, and skin keratinocytes contain ACE-2 receptors. SARS-CoV-2 binding to these receptors produces signs and symptoms in their respective organs. Once inside the cells, SARS-CoV-2 replicates, directly impairs normal cellular function and activates innate immune responses, especially complement (lectin and classical pathways) [20]. Complement activation initiates damaging proinflammatory cytokines (interleukin 1B, interleukin-6, interleukin-8), interferon-gamma, and tumor necrosis factor-alpha [20]. This ‘cytokine storm’ and direct viral injury to endothelial cells triggers catastrophic thrombotic responses in some patients [20]. Patients with diabetes mellitus and cardiovascular disease are more susceptible to severe COVID-19 infection. In the skin, acral cutaneous necrosis and livedo racemosa are signs of more significant coagulopathy in SARS-CoV-2 infected patients [21].
CUTANEOUS REACTIONS TO SARS-COV-2
Cutaneous reactions to COVID-19 are polymorphic, and more than one type of rash can appear in an individual patient (Table 1) [6▪▪,7▪▪,9,22]. Other viral infections, medication reactions, and connective tissue disorders produce similar nonspecific rashes to SARS-CoV-2 [6▪▪,7▪▪,9].
Table 1.
| Morbilliform |
| Chilblain-like/pernio |
| Urticarial |
| Macular erythema |
| Vesicular |
| Acro-cyanosis |
| Acral desquamation |
| Papulosquamous |
| Livedo reticularis-like |
| Erythema multiforme-like |
| Erythroderma |
| Grover-like |
| Retiform purpura |
| Petechial |
| Bullous |
| Papulovesicular |
| Palpable purpura/vasculitis (leukocytoclastic) |
| Dengue-like |
| Pressure injury |
| Erythema nodosum |
| Livedo racemose |
| Miliaria rubra |
| Multisystem inflammatory syndrome in children |
| Acneiform |
| Enanthem |
| Anagen effluvium |
| Erythema elevatum diutinum |
| Photo-distributed |
COVID-19, coronavirus disease 2019.
MACULOPAPULAR/MORBILLIFORM ERUPTIONS
A maculopapular rash is a nonspecific term utilized to describe many cutaneous reaction patterns with macules and papules, and occurs in almost 50% of adults with COVID-19 [7▪▪]. In children, this type of rash presents most commonly on the extremities and heals with exfoliation [23]. In adults, the rash develops commonly on the torso and proximal extremities [6▪▪]. This group likely represents several different cutaneous reaction patterns and some may be due to medications, and not directly virally induced.
CHILBLAIN-LIKE LESIONS
Chilblain-like lesions are one of the most common rashes seen in children with COVID-19 [8▪,21,23]. Chilblain-like lesions typically appear as round macules and papules with ill-defined borders. They are mulberry colored with atrophic centers that may be ulcerated with scale crust. They usually occur on acral sites, including the dorsal and plantar surfaces of the feet, toes (’COVID toes’), ankles, and ears. Chilblain-like lesions may be unilateral or bilateral. Although most do not develop symptoms, a few patients report pruritis, tingling, itchiness, and coldness. The time-lapse from the onset of systemic COVID-19 symptoms to the appearance of cutaneous findings ranges from 0 days to weeks [24–28].
The incidence of pediatric chilblain-like lesions has increased over the past year with the global surge of the COVID-19 virus. Colonna et al. found that from March 26th to April 26th, 2020, the Fondazione IRCCS Ca’ Granda in Milan reported 30 children who developed chilblain-like lesions. These investigators did not anticipate a 10-fold increase from the 3 pediatric patients referred during the same period in 2019 [27,29]. Similarly, investigators from other institutions in Italy and Spain noted spikes in pediatric chilblain-like lesions [7▪▪,30,31]. For many children presenting with chilblain-like lesions, the real time reverse transcriptase polymerase chain reaction (rRT-PCR) test is negative for SARS-CoV-2. Khalili et al. contend that one possible explanation for these negative results stems from the tendency of chilblain-like lesions to appear toward the end of the disease course. Therefore, patients may have cleared a prior SARS-CoV-2 infection, leading to a false negative rRT-PCR[8▪]. Additionally, preanalytical difficulties such as inadequate swab specimen and low viral loads in early and late-stage infections lead to false negative rRT-PCR [32]. Also, novel viral mutations may alter rRT-PCR accuracy [32]. Because children are usually symptom-free and have lower viral loads than adults, COVID-19 serological antibody tests should be combined with rRT-PCR to effectively diagnose COVID-19 in children with chilblain-like lesions [8▪]. Many experts question the accuracy of serum antibody testing. According to Bohn et al., commercial antibody immunoassays have not been peer reviewed and ‘their accuracy, clinical utility, and value remain largely uncharacterized’ [32].
When children present with red-blue toes, clinicians must differentiate classic idiopathic chilblains (pernio) from COVID toes. Classic idiopathic pernio occurs in association with cold and dampness. These external influences produce pericapillary edema and microhemorrhages. In contrast, COVID-toes tend to be asymptomatic and are likely caused by systemic influences [2▪▪]. The histology of chilblains is not specific and isimilar to idiopathic pernio in 70% of cases and in 30% similar to lupus pernio. Thus, a skin biopsy does not help distinguish idiopathic and lupus pernio from COVID-19 associated pernio [33].
Three principal mechanisms may link COVID-19 to chilblain-like lesions: (1) The virus may induce a strong interferon type I response in specific individuals, especially in children, resulting in the microangiopathic changes seen in chilblain-like lesions [34]. Children with type I interferon-related pathologies (Aicardi-Goutieres syndrome, familial chilblain lupus, and hereditary interferonopathies associated with mutations in the TREX1 [three prime repair exonuclease] gene) develop chilblains with cutaneous histological changes similar to chilblain-like lesions from COVID-19. Children with robust COVID-19 interferon responses fare better than those with weaker responses [35]. (2) SARS-CoV-2 induces thrombosis and coagulopathy in a small subset of patients, resulting in the occlusion of small vessels of the heart, lungs, and acral tissues. Microthrombi have been reported in chilblain-like lesions, suggesting that coagulopathy may be necessary for forming chilblain-like lesions [3▪▪,35–37]. (3) Viral proteins invade endothelial cells of patients with chilblain-like lesions. SARS-CoV-2 binds to ACE-2 receptors that are found on endothelial cells and are abundant on pericytes. This binding and direct viral invasion of vascular tissues suggest that blood vessel inflammation and diminished cutaneous perfusion may be operative in causing chilblain-like lesions [37–40].
Freeman et al. report a series of 318 patients from 8 countries with pernio-like lesions (median age 25 years, interquartile range 17–38 years) [41▪]. Pernio developed on feet (87%) more than hands (30%). The most common symptom was pain and burning (70%), followed by pruritus (34%) and cold intolerance (8.7%). Seventeen percent were not symptomatic. Forty-eight percent presented after COVID-19 symptoms and 17% before COVID-19 symptoms. COVID-19 symptoms included cough (39%), fever (39%), headache (30%), shortness of breath (26%), sore throat (22%), and malaise (17%). This study included 93 children and adolescents. Pernio-like lesions were the only manifestation of COVID-19 in more than half of the patients in this study. Seventy-eight received outpatient care, but 5 patients required hospitalization, and 2 died. Seventy-four percent were healthy, and twenty-six percent had comorbid conditions (obstructive lung disease, hypertension, or a rheumatologic disorder) [6▪▪]. It is important to note that some authors suggest other factors account for COVID-toes other than SARS-CoV-2 infection [42]. Additionally, fungal, viral, medication, and cold exposure during the pandemic lockdown cause COVID-toes in many patients [42,43].
Chilblain-like lesions resolve without treatment after 7–10 days (between 5 days and 8 weeks) [8▪]. Some investigators report a median duration of 12 days (interquartile range 7–23 days) [44]. In 7%, pernio-like lesions may last much longer, over 60 days as a sign of ‘long hauler’ or ’long-COVID’ syndrome [44]. Since most children do not show symptoms, no treatment is necessary. Oral analgesics and antihistamines relieve symptoms in symptomatic children [28]. Topical and systemic corticosteroids decrease inflammation and symptoms in children with concurrent erythema multiforme.
URTICARIA (HIVES)
Urticaria is a common cutaneous reaction associated with SARS-CoV-2 infection, accounting for 10–20% of all COVID-19 related rashes [7▪▪,45]. Hives are transient (less than 24 h), pruritic, circumscribed, raised wheals. Many other viruses produce nonspecific urticarial reactions, and most children infected with SARS-CoV-2 are symptom-free. Hence, the diagnosis of SARS-Co-2 infection is challenging based on the presence of urticaria alone. Urticarial reactions last four days (interquartile range 2–10) with a maximum of 28 days [44].
MACULAR ERYTHEMA
A sizable international registry lists macular erythema in 13% of patients. Many factors other than viral infections such as heat and medications cause macular erythema (Table 2) [4,6▪▪]. Many patients experience pruritus and pain.
Table 2.
| Cutaneous Manifestations of COVID-19 virus SARS-CoV-2 | ||||
| Rash Type | Clinical Manifestations | Timing of Rash Onset with Systemic Symptoms and Anatomical Site | Polymerase chain reaction (PCR) positivity, Relative Frequency (RF), Mean Duration (MD), and Prognosis | Treatments |
| Morbilliform/Maculopapular | Cutaneous: erythematous macules and papules, symmetric, pruritic (61%) mild to moderate in severity, asymptomatic (21%) Pain/burning (16%)Systemic: fever (74%), cough (66%), shortness of breath (45%), sore throat (40%), headache (37%), diarrhea, vomiting and nausea (40%) | Before symptoms 7.9%After symptoms 76%Simultaneous 13%No other COVID symptoms 19%Adults – Trunk and proximal extremitiesChildren – Distal extremities | PCR + 84%RF: 22 to 47%MD: 8.6 days (+/- 6.8)Supplemental oxygen 16%Ventilator and or Extracorporeal membrane oxygenation 16%Mortality in 10% | Supportive outpatient care (50–55%), Low to medium strength topical corticosteroids, oral antihistamines and oral corticosteroids for severe cases. |
| Pernio/Chilblain-Like Lesions | Cutaneous:May be asymmetric; asymptomatic (9.7%), pain/burning (71%), pruritus (36%).Edematous pink to purple macules and papules; may be multiple, round and few millimeters to centimetersVesicles, pustules and crusts may form.Lesions on the digits on the feet tend to involve the entire digit with a clear demarcation at the metatarsophalangeal joint.More common in adolescents and young adults.Systemic:Fever and cough (35%), shortness of breath (29%), sore throat (26%), nausea, vomiting and diarrhea (23%), myalgia (32%) | Before symptoms 16%After symptoms 48%Simultaneous 9.7%No other COVID symptoms 19%Feet – 84%Hands – 32% | PCR + 52%RF: 18%MD: 12.7 days (+/- 8)Associated with milder disease Outpatient care in 84% | Most cases do not require treatment.Topical corticosteroids, topical antibiotics for infected open skin. Oral analgesics for pain and antihistamines for itchRarely, oral gabapentin for pain control |
| Urticarial | Cutaneous:Symmetric whealsPruritic (74%), pain/burning (22%), asymptomatic (3.7%)Systemic:Fever (70%), cough (59%) and headache (48%) most common symptoms, diarrhea, nausea, and vomiting (37%), myalgia (33%) | Before symptoms 7.4%After symptoms 67%Simultaneous 22%No other COVID symptoms 3.6%May involve the entire body (15%), trunk (41%), arms and hands (48%), legs and buttock (52%), face (30%)May be localized to face, hands and feet. | PCR 85%RF: 16%MD: 6.8 days (+/- 7.8)The vast majority require outpatient care only [67], may be severe (17.4%) require ventilator and/or extracorporeal membrane oxygenation. 2% mortality. | Oral antihistamines |
| Macular erythema | Cutaneous:Erythematous macules and patchesPruritus (61%), pain and burning (26%) asymptomatic (17%)Systemic:Fever (65%), Cough (52%) shortness of breath, sore throat, nausea, vomiting, diarrhea, malaise (39%) | Before symptoms 8.7%After symptoms 57%Simultaneous 30%No other COVID symptoms 4.3%Back and arm (48%), legs and buttock (44%) abdomen (39%), face and neck (26%) | PCR +83%RF: 13%Outpatient care in 61%, supplemental oxygen in 17% | Oral antihistamines and oral analgesics |
| Vesicular | Cutaneous:Vesicles, may resemble other viral exanthems, usually small and monomorphic, but may be hemorrhagic and largePruritus (72%), pain and burning (50%), asymptomatic (11%)Systemic:Fever (72%), cough (61%), sore throat (50%) | Before symptoms 5.6%After symptoms 72%Simultaneous 22%Abdomen, arms, legs and buttock (44%), hands (39%), face, neck and back (33%).May be diffusely distributed and rarely on the hands and feet. | PCR + 89%RF: 11%MD:10.4 days (+/-9.3)Outpatient care only in 78%Hospitalization in 17%Recover without scarring. | Expectant management. |
| Papulosquamous | Cutaneous:Lesion morphology may be varied resembling spongiotic dermatitis (eczema) and pityriasis roseaPruritus (94%), pain and burning (29%)Comorbid Skin condition:Contact dermatitis (18%), Alopecia areata (12%)SystemicFever (59%), Cough (53%), Sore throat and headache (41%) | Before symptoms 17.6%After symptoms 53%Simultaneous 24%No other COVID symptoms 5.9%Abdomen, back, arms, legs and buttock (65%), chest (47%), face and neck (24%) | PCR +82%RF: 9.9%MD: May last long depending upon the rashPrognosis is excellent with 94% of patients requiring only outpatient care. | Patients do well with treatment of the underlying condition.Topical anti-inflammatory agents |
| Retiform purpura | Cutaneous:Dark red, purple purpura with hemorrhagic blisters and ischemiaAsymptomatic (73%), pain and burning (9.1%)Systemic:Cough and shortness of breath (73%), fever (64%), sore throat and malaise (27%) | Before symptoms (9.1%)After symptoms (91%)Legs and buttock (64%), hands (27%), feet and arms (18%) | PCR +91%RF: 6.4%MD: 9.4 (+/-5.4)Poor prognosis – 91% requiring ventilator or extracorporeal membrane oxygenation | Intensive care support measuresAnticoagulants |
Adapted from [74▪▪].
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
VESICULAR EXANTHEM
SARS-CoV-2 causes a varicella-like papulovesicular rash predominantly in middle-aged women (median age 45.6 ± 20 standard deviation) and children [7▪▪,46]. Mildly pruritic vesicles with hemorrhagic scale crust occur mainly on the trunk with some involvement of the extremities [30,46,47]. On average, the exanthem appears 3 days after the onset of respiratory symptoms and lasts for around 8 days [46]. Generally, it occurs closer to the beginning of COVID-19 infection, compared with chilblain-like lesions, which may arise later in the illness course [48]. Co-infection with herpes simplex virus (HSV-1 and HSV-2), human herpesvirus virus (HHV-6 and HHV-7), parvovirus, and Epstein–Barr virus occurs in some patients [7▪▪,49,50]. PCR on vesicular fluid assists in determining the precise cause of the rash [45,47].
PAPULOSQUAMOUS
This group of rashes likely overlaps with the maculopapular/morbilliform group. Papulosquamous rashes consist of scaly macules and papules that may be localized or confluent and involve the intertriginous areas. Characteristic papules are perifollicular and pruritic [51]. Some patients develop purpura and pseudo-vesicles. The most common morphology in this group is the pityriasis rosea-like rash. Pityriasis rosea-like rashes may be under-reported because many studies catalog papulosquamous rashes as angiomatous and annular [5]. Preexisting papulosquamous rashes such as psoriasis and atopic dermatitis may flare during COVID-19 infection. Rashes similar to punctate purpura, pseudo-vesicular papules on the extremities (especially dorsal hands), erythema elevatum diutinum-like, and targetoid-like erythema multiforme fall into this group of rashes. Papulosquamous eruptions last 20 days (interquartile range 14–28 days), but have been described up to 70 days in patients with ‘long-hauler’ COVID-19 [44].
MULTISYSTEM INFLAMMATORY SYNDROME IN CHILDREN
Rarely, children develop an inflammatory syndrome after the acute infection has resolved, leading to multiple organ failure and shock. Investigators call this condition multisystem inflammatory syndrome in children (MIS-C). Investigators report a 1-year, 30-fold increase of multisystem inflammatory disease in children [52], and 78–100% of the patients have evidence of SARS-CoV-2 infection [53]. Thirty percent of children with MIS-C are rRT-PCR positive for SARS-CoV-2, whereas most are antibody positive, indicating that children with MIS-C are not acutely infected. Exaggerated acquired immune responses triggered by antibodies to SARS-CoV-2 cause MIS-C [54]. Immune mechanisms include the formation of autoantibodies, antibodies or T-cells directed against virally infected cells, immune complexes activating inflammatory cascades, and viral superantigens activating host immune cells [54]. Children with MIS-C have overlapping clinical and laboratory factors with Kawasaki disease, Kawasaki disease shock syndrome, and toxic shock syndrome. These factors are characterized by intense inflammation of multiple organs, including the heart, lungs, brain, kidneys, eyes, and gastrointestinal system. Unlike Kawasaki disease, children with MIS-C develop severe vascular, cardiac, and gastrointestinal symptoms (severe diarrhea occurring in 60% of children) early in the disease course [52]. Fever, myocarditis, pericarditis, cardiogenic shock, and severe diarrhea are hallmarks of MIS-C [8▪,55]. Other distinguishing features of MIS-C include older age of onset (7 years of age or older), an increased incidence in Black, Hispanic or Latino, and Asian and Pacific islanders [56–58], diffuse cardiovascular involvement, and more significant elevation in inflammatory markers [54]. Patients with MIS-C show high troponin and brain natriuretic peptide concentrations, reflecting cardiac injury [54]. The rash involves the lips, oral cavity, hands, and feet [53]. In one small cohort, children with MIS-C developed annular plaques of the proximal medial extremities [59▪]. The widespread erythematous rash is not specific and overlaps with other inflammatory and toxin-mediated disorders (Kawasaki disease, toxic shock syndrome) and infectious diseases (rickettsial infection).
Several biomarkers show promise as prognostic and diagnostic indicators in children with MIS-C. Tang et al. found that most patients had elevated levels of one or more of the following biomarkers: C-reactive protein, procalcitonin, erythrocyte sedimentation rate, ferritin, interleukin-6, and D-dimer [53]. Furthermore, Pouletty et al. elaborated that 86% of their patients had increased ferritin levels and stipulated that it was one of the most critical factors in differentiating between severe and nonsevere cases of MIS-C [60]. An echocardiogram commonly shows myocarditis, decreased left ventricular ejection fraction, pericardial effusion, and coronary artery dilatation [53].
Multidisciplinary team members collaborate to manage and treat this challenging disorder. Patients with MIS-C appear to be more resistant to treatment than classical Kawasaki disease. The main treatments for MIS-C are intravenous immunoglobulins, anticoagulants, inotropic agents, and glucocorticoids. Serum ferritin assists in determining both prognosis and treatment response. Children with higher ferritin levels may require multiple doses of intravenous immunoglobulin [8▪]. Severely ill children may receive systemic corticosteroids, tocilizumab (interleukin -6 antagonist), and anakinra (interleukin-1 receptor antagonist) [52].
ERYTHEMA MULTIFORME-LIKE LESIONS (EM-LIKE LESIONS)
Investigators from Spain report EM-like lesions in children with COVID-19 [38,61,62]. These lesions are pink to red macules, papules, and plaques resembling a target and may have two to three rings. Petechiae and purpura often co-localize with the target lesions. Common locations include forearms, thighs, knees, arms, especially around the elbows and the dorsal aspect of the hands and feet. The SARS-CoV-2 spike protein may be found in the endothelial and epithelial cells of the eccrine glands, suggesting a direct viral cytopathic effect and immune response to these proteins [33,37]. In this report, investigators eliminated other causes of erythema multiforme such as recent vaccinations, Herpes simplex, Mycoplasma pneumonia, and medications. The EM-like lesions resolved after a few days. These lesions improved within 1 to 3 weeks with either topical or oral corticosteroids, or in some cases without any treatment.
RETIFORM PURPURA
Patients may develop a vascular lace-like pattern in their skin. Unlike livedo reticularis, which is transient, mild, and not associated with thrombotic events, retiform purpura is a lace-like purpura that may result in skin necrosis and loss of fingers and toes [7▪▪,63]. Retiform purpura is due to clotting of medium-sized blood vessels and arises in acutely ill patients. COVID-19 viral particles bind to endothelial cells in the skin and subcutaneous fat and activate the complement/coagulation pathways, resulting in clotting and a cytokine storm [64]. COVID-19 patients with retiform purpura have a poor prognosis, with 91% requiring ventilatory support and 10% succumb due to overwhelming respiratory failure and coagulopathy (Table 2) [4,6▪▪].
OTHER RASHES ASSOCIATED WITH CORONAVIRUS DISEASE 2019 INFECTION
Investigators report the presence of thrombocytopenic purpura [65], dengue-like exanthem [66,67], acro-ischemia [68], leukocytoclastic vasculitis [69,70], erythema elevatum diutinum-like [71], and photo-distributed rashes [22] in relation to COVID-19.
APPROACH TO THE DIAGNOSIS AND TREATMENT OF CHILDREN WITH RASH AND SUSPECTED CORONAVIRUS DISEASE 2019
Rashes are one of the most common presenting signs encountered in pediatrics and those described in patients infected with SARS-CoV-2 to date are nonspecific. Moreover, greater than 90% of children with COVID-19 will not be symptomatic or may only have mild symptoms, making the diagnosis of COVID-19 based on skin findings alone even more challenging. More than ever, pediatricians must be acutely aware of the potential and actual COVID-19 exposures in their patients and the epidemiology of COVID-19 within the local community. Almost any rash in a child should raise suspicion of COVID-19, especially acral rashes similar to pernio. Rashes may be subtle, mild, and morphologies may vary. Distributions may differ in children compared with adults. For example, classic pediatric pityriasis rosea, shows an inverse pattern with the rash occurring predominantly on the extremities and in the intertriginous folds. Children with darker skin types develop subtle skin redness, and inflammatory rashes appear as papules and plaques rather than macules and patches. Children may develop mild conjunctivitis and eyelid dermatitis as the only presenting sign [72], and neonates may present with subtle miliaria [73]. Pediatricians should expect to see a broad range of rashes in terms of morphology, distribution and severity in children with COVID-19.
The evaluation of a rash must be careful and systematic. First, common nonpandemic etiologies should be eliminated, such as medications, primary dermatological conditions (e.g., atopic dermatitis and psoriasis), common infections presenting with rash (e.g., viral - molluscum, varicella, herpes; bacterial – staphylococcal and streptococcal infection; and fungal – tinea versicolor). Second, pediatricians should develop a working knowledge of principle cutaneous reaction patterns (Table 2) [4,6▪▪] and recognize uncommon and rare rashes seen in COVID-19 patients (Table 2) [4,6▪▪]. Third, patient histories should include questions about comorbid dermatological conditions such as atopic dermatitis and psoriasis as these may flare during acute COVID-19 infection. Skin biopsy for the diagnosis of COVID-19 may show subtle differences between look-a-like rashes, but in general, it will not confirm the diagnosis of COVID-19. In select children, skin biopsies may be helpful to eliminate other confounding skin conditions, as shown in Fig. 1[3▪▪]. To date, serological antibody testing for immunoglobulin M, immunoglobulin A, and immunoglobulin G has limited diagnostic utility in children with rashes due to COVID-19, and COVID-19 rRT-PCR is the preferred test [2▪▪,3▪▪]. As previously mentioned, biochemical testing has prognostic and diagnostic utility for patients presenting with severe COVID-19 [32]. Clinicians should evaluate select patients for primary or coincident infection and autoimmunity.
FIGURE 1.
Algorithm for diagnosis of COVID-19 in children with a skin eruption [3▪▪]. IHC, immunohistochemistry; LDH, lactate dehydrogenase; PIMS, paediatric inflammatory multisystem syndrome. ∗Fever > 38°C, muscle pain, headaches, asthenia, cough, dyspnoea, nausea/vomiting/diarrhoea and anosmia/agueusia. Source: Andina et al.[3▪▪].
Children with COVID-19 may be on immunosuppressant medications or biologic immune response modifiers, and adjusting these regimens in response to the COVID-19 pandemic should be done in consultation with specialists on a case-by case basis, utilizing recognized guidelines as a reference.
CUTANEOUS REACTIONS TO CORONAVIRUS DISEASE 2019 VACCINES
From December 24th, 2020, to February 14th, 2021, a provider-facing registry collected information on 414 adult patients who reported cutaneous symptoms resulting from either the mRNA COVID-19 vaccine from Moderna (83%) or the Pfizer (17%) [74▪▪]. Many of the rashes such as pernio/chilblains, erythromelalgia, and pityriasis rosea-like exanthem mimicked natural SARS-CoV-2 infection, suggesting that many of the immune responses seen in COVID-19 are due to virally induced immune responses and not direct viral damage (Tables 3 and 4[74▪▪]). Ninety percent of the cutaneous reactions to vaccines occurred in female patients, 78% were white, and 98% were from the United States. Vaccinated patients experienced delayed local reactions, local injection site reactions, urticaria, morbilliform, and erythromelalgia. Most patients (57%) who developed cutaneous reactions to the first dose did not experience similar reactions to the second dose. Of those who developed a reaction to both doses (43%), 28% reported similar reactions to both doses, 28% reported a lesser reaction to the second dose, and 48% reported a more severe reaction to the second dose Table 4[74▪▪]. On average, rashes resolved between 3 and 5 days, and patients reported no serious adverse events. Topical corticosteroids, oral antihistamines and pain-relieving medicines adequately treated most vaccine-related rashes. A few patients required antibiotics for cellulitis. On average, rashes resolved in between 3 and 4 days. The authors conclude that ‘cutaneous reactions to COVID-19 vaccination are generally minor and self-limited and should not discourage vaccination [74▪▪].’ Registry-based study limitations include: (1) they do not capture the total number of patients and do not allow for calculating incidence, (2) providers are more likely to report more severe cases (confirmation bias), (3) rash morphology differs based on provider input, and (4) often there is incomplete follow-up.
Table 3.
Cutaneous reactions to Moderna and Pfizer COVID-19 vaccination [74▪▪]
| Delayed large local reaction |
| Local injection site reactions |
| Swelling |
| Erythema |
| Pain |
| Urticaria |
| Morbilliform |
| Erythromelalgia |
| Flare of existing dermatologic condition |
| Herpes simplex |
| Atopic dermatitis |
| Psoriasis |
| Urticarial vasculitis |
| Eczema |
| Vesicular |
| Pernio/Chilblains |
| Varicella Zoster |
| Angioedema |
| Pityriasis rosea |
| Erythema multiforme |
| Filler reaction |
| Vasculitis |
| Contact dermatitis |
| Reaction in breastfed infant |
| Onset of new dermatologic condition |
| Raynaud's |
| Lichen planus |
| Eczema |
| Petechiae |
| Other reactions |
| Full-body skin pain/burning |
| Hypopigmentation |
| Sweet's-like fixed urticarial plaque |
| Pseudo-vesiculated patches |
| Spongiotic dermatitis |
| Canker sore on tongue |
| Aphthous ulceration on labium |
| Monomorphic popular eruption |
| Eczematous pigmented purpura |
| Spongiotic dermatitis |
COVID-19, coronavirus disease 2019.
Table 4.
Adverse Cutaneous Reactions to the COVID-19 vaccine
| Delayed Large Local Reaction | Local injection site reaction | Urticaria | Morbilliform | Erythromelalgia |
| - 94% Moderna- 93% Female- Median age 47 (range 22–88)- 100% vaccinated arm56% had preceding local site injection reactionsOnset: 1st dose: median 7 days (IQR 7–8) and lasted median of 4 days2nd dose: median 2 days (IQR 1–3) and lasted median of 3 days (IQR 2–5)May be due to excipient polyethylene glycolTreatment: topical steroids, oral antihistamines, and oral analgesics. Oral antibiotics if concern for cellulitis | - 92% Moderna- 94% Female- Median age 44 (range 21–88)- 100% vaccinated armOnset: 0 to 1 days after injection45% larger with second dose | - 57% Moderna- 89% Female- Median age 39 (range 26–69)- Common sites were arms (68%), trunk (57%), and legs (46%)Onset: 2 days after 1st dose (IQR 1–3)Onset: 0 days after 2nd dose75% larger after second dose | - 65% Moderna- 88% Female- Median age 40 (range 22–76)- Common sites were arms (62%), trunk (42%), and legs (27%) | - 77% Moderna- 92% Female- Median age 38 (range 19–83)- Common sites were arms (69%), face (31%), hands (23%), and feet (15%) |
Adapted from [74▪▪].
COVID-19, coronavirus disease 2019; IQR, interquartile range; PCR, polymerase chain reaction.
One patient presented with COVID-like toes on the left foot four days following her first dose of the Pfizer-BioNTech-162b2 vaccine. She received the anticoagulant apixaban and low-dose aspirin. There was no clear distinction between the pseudo-chilblain lesions thought to be caused by SARS-CoV-2 (COVID-toes) and those linked to the vaccine (COVID-like toes). The patient did not receive the second dose of the vaccine [75▪].
On May 12th, 2021, the CDC approved SARS-CoV-2 vaccinations for children 12–15 years old. Although the median age of patients found to have cutaneous reactions to the COVID-19 vaccine is 44 years, children may develop similar responses. Some of the cutaneous reactions seen in these mRNA vaccines are similar to cutaneous reactions seen in other vaccines such as influenza and hepatitis B.
DERMATOLOGICAL CONDITIONS RELATED TO MASK-WEARING
’Maskne’ is a term describing a form of acne mechanica that has recently come to the forefront of dermatology due to the COVID-19 pandemic [76▪]. The skin microbiome must be in balance to have healthy skin. Masks cause follicular occlusion and microbiome dysbiosis. Cutibacterium acnes play a critical role in the pathogenesis of acne, which colonizes the sebaceous follicles. Disruption of the typical skin homeostasis results in the production of strains of C. acnes that activate the innate immune system and drive the skin inflammation seen in acne. Other organisms such as Staphylococcus aureus, fusobacteria, Malassezia furfur, and Demodex mites can produce impetigo, perioral dermatitis, and seborrheic dermatitis, and rosacea, respectively. These conditions are seen more commonly with patients who chronically wear masks [76▪]. Risk factors for ‘maskne’ include tropical climates, increased outdoor activities, seborrhea, and genetic predisposition. Clinically, ‘maskne’ diagnosis can be based upon the following factors: the appearance of acne, worsening of existing acne, within 6 weeks following the onset of mask-wearing, and distinctive acne patterns along the ‘O-zone’ (around the mouth and nose) of the face [75▪,77]. Differential diagnoses include impetigo, perioral dermatitis, seborrheic dermatitis, pityrosporum folliculitis, and acne rosacea [78]. Pediatricians should obtain bacterial and fungal cultures when the diagnosis of acne is in doubt.
Treatment of ‘maskne’ should include topical antibacterial cleansers and moisturizers. The mask may enhance the irritating effects of specific acne medications. Examples include benzoyl peroxide, salicylic acid, α-hydroxy acids, and retinoids. Topical antimicrobials and mild acne cleansers should not produce irritation when utilized in combination with a mask [76▪].
Patients with maskne should pay special attention to the type of mask they wear. Thicker sun screens may flare acne in select patients. In these patients, ultraviolet protective factor 50+ masks may replace sunscreen. Masks should not have metal at the orbitofrontal ridge, as this could cause nickel sensitization. Tighter woven masks with a high thread count incorporating silver, zinc oxide, or copper oxide may benefit patients [76▪].
CONCLUSION
With instances of COVID-19 infection in children continuing to rise in many parts of the world, diagnosis and containment of the virus remains pertinent. Viral containment depends on widespread adaptation of public health measures and strong vaccination policies. Additionally, recognition of the virus’ cutaneous signs can be an effective way to reduce disease burden. The existing literature comprehensively documents these signs in adult patients. However, as our understanding of the virus’ symptoms has developed, it has become clear that children present in a unique and inadequately documented way. As the saying goes, ‘children are not little adults,’ and pediatricians should expect that the cutaneous manifestations due to COVID-19 may differ from those of adults, especially in infants and young children.
This article summarizes and distills the best pediatric information currently available in order to aid pediatricians in the diagnosis and treatment of COVID-19 related skin reactions. Providers should examine the entire cutaneous surface and give particular heed to maculopapular/morbilliform, chilblain-like lesions, urticaria, macular erythema, papulosquamous, erythema multiforme-like lesions, and retiform purpura. Undoubtedly, the vast majority of the rashes seen within a general pediatrics practice will continue to be nonspecific viral exanthems, common bacterial infections, medication reactions and eczema. Nevertheless, the reaction patterns outlined within this paper serve as a critical foundation for the diagnostic evaluation of a child with a rash in the era of COVID-19.
Acknowledgements
Authors would like to thank Paulette Dinulos, MPH (content), Giovanna Boyle, BA (editing), and Johanna Pastoriza, BS (research) for their assistance in manuscript preparation.
Financial support and sponsorship
None
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323:709–710. [DOI] [PubMed] [Google Scholar]
- 2▪▪.Andina D, Belloni-Fortina A, Bodemer C, et al. Skin manifestations of COVID-19 in children: Part 1. Clin Exp Dermatol 2021; 46:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an excellent comprehansive 3 part review of the cutaneous manifestations in children. This is a major work examining COVID-19 associated rashes in children
- 3▪▪.Andina D, Belloni-Fortina A, Bodemer C, et al. Skin manifestations of COVID-19 in children: Part 3. Clin Exp Dermatol 2021; 46:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an excellent comprehansive 3 part review of the cutaneous manifestations in children. This is a major work examining COVID-19 associated rashes in children
- 4.Carrascosa JM, Morillas V, Bielsa I, et al. Cutaneous manifestations in the context of SARS-CoV-2 infection (COVID-19). Actas Dermosifiliogr 2020; 111:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneshgaran G, Dubin DP, Gould DJ. Cutaneous manifestations of COVID-19: an evidence-based review. Am J Clin Dermatol 2020; 21:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪▪.Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol 2020; 83:1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]; This the first major international collaborative effort to evaluate COVID-19 rashes.This important registry is ongoing and should provide useful information in the future
- 7▪▪.Galvan Casas C, Catala A, Carretero Hernandez G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprhensive study from Spain of cutaenous findings seen in COVID-19 pateints These authors attempt to grou COVID-19 rashes into groups
- 8▪.Khalili M, Iranmanesh B, Mohammadi S, et al. Cutaneous and histopathological features of coronavirus disease 2019 in pediatrics: a review article. Dermatol Ther 2021; 34:e14554. [DOI] [PMC free article] [PubMed] [Google Scholar]; A well done analysis of cuteneous findings ant the associated histopathological features seen in children.
- 9.Genovese G, Moltrasio C, Berti E, et al. Skin manifestations associated with COVID-19: current knowledge and future perspectives. Dermatology 2021; 237:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molaee H, Allahyari F, Emadi SN, et al. Cutaneous manifestations related to the COVID-19 pandemic: a review article. Cutan Ocul Toxicol 2021; 40:168–174. [DOI] [PubMed] [Google Scholar]
- 11.Wollina U, Karadag AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther 2020; 33:e13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26:1017–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34:e212–e213. [DOI] [PubMed] [Google Scholar]
- 14.Seirafianpour F, Sodagar S, Pour Mohammad A, et al. Cutaneous manifestations and considerations in COVID-19 pandemic: a systematic review. Dermatol Ther 2020; 33:e13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Andina D, Belloni-Fortina A, Bodemer C, et al. Skin manifestations of COVID-19 in children: Part 2. Clin Exp Dermatol 2021; 46:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an excellent comprehansive 3 part review of the cutaneous manifestations in children. This is a major work examining COVID-19 associated rashes in children
- 16.Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine 2020; 24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parri N, Lenge M, Buonsenso D, et al. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med 2020; 383:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman EE, McMahon DE, Fitzgerald ME, et al. The American Academy of Dermatology COVID-19 registry: crowdsourcing dermatology in the age of COVID-19. J Am Acad Dermatol 2020; 83:509–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 2020; 55:2000607.doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int 2020; 98:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez Gonzalez F, Cortes Correa C, Penaranda Contreras E. Cutaneous manifestations in patients with COVID-19: clinical characteristics and possible pathophysiologic mechanisms. Actas Dermosifiliogr 2021; 112:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacquin-Porretaz C, Ducournau A, Dupond AS, et al. Cutaneous manifestations of COVID-19 in the Franche-Comte region of France: a monocentric study. Ann Dermatol Venereol 2021; 148:124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah S, Akhade K, Ganguly S, et al. Cutaneous manifestations associated with COVID-19 in children: a systematic review. J Family Med Prim Care 2021; 10:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan V, Lind R. Chilblains in COVID-19 infection. Cureus 2020; 12:e9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniaci A, Iannella G, Vicini C, et al. A case of COVID-19 with late-onset rash and transient loss of taste and smell in a 15-year-old boy. Am J Case Rep 2020; 21:e925813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papa A, Salzano AM, Di Dato MT, et al. Images in practice: painful cutaneous vasculitis in a SARS-Cov-2 IgG-positive child. Pain Ther 2020; 9:805–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordoro KM, Reynolds SD, Wattier R, et al. Clustered cases of acral perniosis: clinical features, histopathology, and relationship to COVID-19. Pediatr Dermatol 2020; 37:419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol 2020; 37:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colonna C, Genovese G, Monzani NA, et al. Outbreak of chilblain-like acral lesions in children in the metropolitan area of Milan, Italy, during the COVID-19 pandemic. J Am Acad Dermatol 2020; 83:965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccolo V, Neri I, Filippeschi C, et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020; 34:e291–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastrolonardo M, Romita P, Bonifazi E, et al. The management of the outbreak of acral skin manifestations in asymptomatic children during COVID-19 era. Dermatol Ther 2020; 33:e13617. [DOI] [PubMed] [Google Scholar]
- 32.Bohn MK, Lippi G, Horvath A, et al. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clin Chem Lab Med 2020; 58:1037–1052. [DOI] [PubMed] [Google Scholar]
- 33.El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol 2020; 34:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Ju J, Weyand CM, et al. Age-associated failure to adjust Type I IFN receptor signaling thresholds after T cell activation. J Immunol 2015; 195:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020; 136:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colmenero I, Santonja C, Alonso-Riano M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 2020; 183:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torrelo A, Andina D, Santonja C, et al. Erythema multiforme-like lesions in children and COVID-19. Pediatr Dermatol 2020; 37:442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020; 20:1365–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181:1016–1035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol 2020; 83:486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]; A very large international case series of patients with pernio-like lesions
- 42.Deutsch A, Blasiak R, Keyes A, et al. COVID toes: Phenomenon or epiphenomenon? J Am Acad Dermatol 2020; 83:e347–e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neri I, Virdi A, Corsini I, et al. Major cluster of paediatric ’true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol 2020; 34:2630–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMahon DE, Gallman AE, Hruza GJ, et al. Long COVID in the skin: a registry analysis of COVID-19 dermatological duration. Lancet Infect Dis 2021; 21:313–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morey-Olive M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr 2020; 92:374–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol 2020; 83:280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genovese G, Colonna C, Marzano AV. Varicella-like exanthem associated with COVID-19 in an 8-year-old girl: a diagnostic clue? Pediatr Dermatol 2020; 37:435–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol 2020; 45:872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang K, Wang Y, Zhang H, et al. Cutaneous manifestations of the Coronavirus Disease 2019 (COVID-19): a brief review. Dermatol Ther 2020; 33:e13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahe A, Birckel E, Merklen C, et al. Histology of skin lesions establishes that the vesicular rash associated with COVID-19 is not ’varicella-like’. J Eur Acad Dermatol Venereol 2020; 34:e559–e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gul U. COVID-19 and dermatology. Turk J Med Sci 2020; 50:1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang Y, Li W, Baskota M, et al. Multisystem inflammatory syndrome in children during the coronavirus disease 2019 (COVID-19) pandemic: a systematic review of published case studies. Transl Pediatr 2021; 10:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020; 20:e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 2020; 10:537–540. [DOI] [PubMed] [Google Scholar]
- 56.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Javalkar K, Robson VK, Gaffney L, et al. Socioeconomic and racial and/or ethnic disparities in multisystem inflammatory syndrome. Pediatrics 2021; 147:e2020039933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected With SARS-CoV-2. JAMA Netw Open 2021; 4:e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪.Blatz AM, Oboite M, Chiotos K, et al. Cutaneous findings in SARS-CoV-2-associated multisystem inflammatory disease in children. Open Forum Infect Dis 2021; 8:ofab074. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors studied the cutaneous findings in their patients with MIS-C.
- 60.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020; 79:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Gil MF, Garcia Garcia M, Monte Serrano J, et al. Acral purpuric lesions (erythema multiforme type) associated with thrombotic vasculopathy in a child during the COVID-19 pandemic. J Eur Acad Dermatol Venereol 2020; 34:e443–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colmenero I, Santonja C, Alonso-Riano M, et al. Chilblains and COVID-19: why SARS-CoV-2 endothelial infection is questioned. Reply from the authors. Br J Dermatol 2020; 183:1153–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol 2020; 34:e451–e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magro CM, Mulvey J, Kubiak J, et al. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann Diagn Pathol 2021; 50:151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zulfiqar AA, Lorenzo-Villalba N, Hassler P, et al. Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med 2020; 382:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joob B, Wiwanitkit V. Comment on ‘Chilblains-like lesions in children following suspected COVID-19 infection’. Pediatr Dermatol 2020; 37:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jimenez-Cauhe J, Ortega-Quijano D, Prieto-Barrios M, et al. Reply to ‘COVID-19 can present with a rash and be mistaken for dengue’: Petechial rash in a patient with COVID-19 infection. J Am Acad Dermatol 2020; 83:e141–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Cao W, Xiao M, et al. [Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia]. Zhonghua Xue Ye Xue Za Zhi 2020; 41:E006. [DOI] [PubMed] [Google Scholar]
- 69.Kumar G, Pillai S, Norwick P, et al. Leucocytoclastic vasculitis secondary to COVID-19 infection in a young child. BMJ Case Rep 2021; 14:e242192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camprodon Gomez M, Gonzalez-Cruz C, Ferrer B, et al. Leucocytoclastic vasculitis in a patient with COVID-19 with positive SARS-CoV-2 PCR in skin biopsy. BMJ Case Rep 2020; 13:e238039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catala A, Galvan-Casas C, Carretero-Hernandez G, et al. Maculopapular eruptions associated to COVID-19: a subanalysis of the COVID-Piel study. Dermatol Ther 2020; 33:e14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu P, Liang L, Chen C, et al. A child confirmed COVID-19 with only symptoms of conjunctivitis and eyelid dermatitis. Graefes Arch Clin Exp Ophthalmol 2020; 258:1565–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Y, Peng H, Wang L, et al. Infants born to mothers with a new coronavirus (COVID-19). Front Pediatr 2020; 8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪▪.McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 2021; 85:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study described the cutaneous reactions associated with the mRNA vaccines
- 75▪.Teo WL. Diagnostic and management considerations for ‘maskne’ in the era of COVID-19. J Am Acad Dermatol 2021; 84:520–521. [DOI] [PMC free article] [PubMed] [Google Scholar]; A concise description of acne due to the mechanical pressures of the mask
- 76▪.Teo WL. The ‘Maskne’ microbiome - pathophysiology and therapeutics. Int J Dermatol 2021; 60:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review of acne due to masks, including the mask materials
- 77.Searle T, Ali FR, Al-Niaimi F. Identifying and addressing ‘Maskne’ in clinical practice. Dermatol Ther 2021; 34:e14589. [DOI] [PubMed] [Google Scholar]
- 78.Rudd E, Walsh S. Mask related acne (’maskne’) and other facial dermatoses. BMJ 2021; 373:n1304. [DOI] [PubMed] [Google Scholar]