ABSTRACT
Chikungunya virus (CHIKV) and Mayaro virus (MAYV) are closely related members of the Semliki Forest virus antigenic complex classified as belonging to the genus Alphavirus of the family Togaviridae. These viruses cause human disease, with sudden fever and joint inflammation that can persist for long periods. CHIKV is the causative agent of large outbreaks worldwide, and MAYV infection represents a growing public health concern in Latin America, causing sporadic cases and geographically limited outbreaks. Considering the relationship between CHIKV and MAYV, the present study aimed to evaluate if preexisting CHIKV immunity protects against MAYV infection. Immunocompetent C57BL/6 mice were intraperitoneally infected with CHIKV and, 4 weeks later, they were infected with MAYV in their hind paw. We observed that the preexistence of CHIKV immunity conferred partial cross-protection against secondary MAYV infection, reducing disease severity, tissue viral load, and histopathological scores. Interestingly, CHIKV antibodies from humans and mice showed low cross-neutralization to MAYV, but neutralizing activity significantly increased after secondary infection. Furthermore, depletion of adaptive immune cells (CD4+ T, CD8+ T, and CD19+ B cells) did not alter the cross-protection phenotype, suggesting that distinct cell subsets or a combination of adaptive immune cells stimulated by CHIKV are responsible for the partial cross-protection against MAYV. The reduction of proinflammatory cytokines, such as interferon gamma (IFN-γ), in animals secondarily infected by MAYV, suggests a role for innate immunity in cross-protection. Our findings shed light on how preexisting immunity to arthritogenic alphaviruses may affect secondary infection, which may further develop relevant influence in disease outcome and viral transmission.
IMPORTANCE Mosquito-borne viruses have a worldwide impact, especially in tropical climates. Chikungunya virus has been present mostly in developing countries, causing millions of infections, while Mayaro virus, a close relative, has been limited to the Caribbean and tropical regions of Latin America. The potential emergence and spread of Mayaro virus to other high-risk areas have increased the scientific community’s attention to an imminent worldwide epidemic. Here, we designed an experimental protocol of chikungunya and Mayaro virus mouse infection, which develops a measurable and quantifiable disease that allows us to make inferences about potential immunological effects during secondary virus infection. Our results demonstrate that previous chikungunya virus infection is able to reduce the severity of clinical outcomes during secondary Mayaro infection. We provide scientific understanding of immunological features during secondary infection with the closely related virus, thus assisting in better comprehending viral transmission and the pathological outcome of these diseases.
KEYWORDS: chikungunya virus, cross-protection, Mayaro virus, cellular response, humoral response, immune response
INTRODUCTION
Chikungunya virus (CHIKV) and Mayaro virus (MAYV) are both alphaviruses (family Togaviridae) classified as members of the Semliki Forest antigenic complex (1). CHIKV is most often spread to humans by Aedes aegypti or Aedes albopictus mosquitoes during the urban cycle of transmission (2). CHIKV outbreaks have caused millions of cases in tropical and subtropical regions throughout the world (3–8). In contrast, MAYV is endemic to South America and the Caribbean and is maintained through a sylvatic transmission cycle involving Haemagogus mosquitoes and vertebrate hosts, such as small mammals, birds, and nonhuman primates (9). However, in the last decade, MAYV has spread to the central and southeastern regions of Brazil and, more recently, to the Caribbean (10–12), areas where CHIKV circulation is endemic (13). Based on laboratory experiments, MAYV may have a diverse array of vectors, including urban Aedes and Anopheles mosquitoes (14, 15).
CHIKV and MAYV can cause acute febrile illnesses in humans, with headache, rash, arthralgia, myalgia, and arthropathy, which, in some cases, during the late stage, can evolve into a persistent and debilitating chronic disease that can last for several months or years (16–18). Human infections with CHIKV and MAYV can elicit a robust inflammatory immune response, including the development of strong neutralizing antibodies and secretion of proinflammatory immune mediators (19, 20). B and T cell activation is required for viral clearance and protection against secondary CHIKV infection in mice (21). In addition, an innate immune response, as well as induction of type I interferon (IFN), is essential for controlling the acute phase of alphavirus disease (20). However, the profile compositions of immune mediators induced by CHIKV and MAYV infection are distinct and include lower levels of interleukin 10 (IL-10), IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) in MAYV infection (19). In fact, how previous CHIKV immunity may affect a secondary heterologous infection, such as MAYV infection, remains unclear.
A live attenuated CHIKV vaccine candidate has been tested in mice, demonstrating that it provides protective cross-immunity against o’nyong-nyong virus (ONNV), mainly mediated by cross-reactive antibodies (22). Similarly, a potential vaccine candidate has also been developed for CHIKV and MAYV utilizing adenoviral vectors encoding their structural proteins, thus conferring partial cross-protection against heterologous infection by MAYV and CHIKV, respectively (23). Furthermore, antibodies from convalescent CHIKV-infected patients have been reported to demonstrate low cross-neutralization of MAYV in vitro (24). A screening of murine and human monoclonal antibodies against CHIKV identified broadly neutralizing antibodies that were able to cross-protect against MAYV and ONNV infection in vivo (25). Additionally, it is known that previous infection by CHIKV and MAYV is able to confer protection against ONNV infection in rhesus monkeys (26). These studies suggest the existence of conserved epitopes that mediate cross-protection among alphaviruses. In contrast, another study showed that the presence of subneutralizing anti-CHIKV antibodies can increase viral attachment and replication in cell cultures as well as increasing viral loads, joint inflammation, and disease severity in mice (27). Thus, it is still unclear how previous immunity to CHIKV may affect a secondary infection by MAYV.
In the present study, we investigated whether CHIKV immunity in mice protects against MAYV infection by analyzing footpad swelling accompanied by clear histological signs of disease, tissue viral load, inflammatory cell infiltration, and expression of inflammatory mediators. We also assessed cellular and antibody-mediated cross-protection, analyzing the clinical outcomes, viral clearance, and pathogenesis resolution.
RESULTS
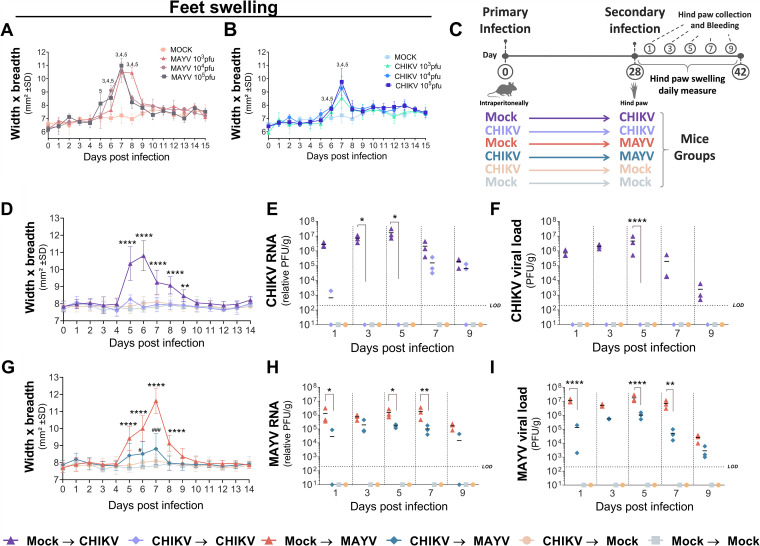
Previous infection with CHIKV reduces footpad swelling and viral load during MAYV infection.
To evaluate the cross-protection immunity between CHIKV and MAYV, we used a C57BL/6 mouse model of acute disease, which develops a clearly visible footpad swelling (25). The magnitude of inflammation with different viral concentrations (ranging from 103 to 105 PFU) was similar for both viruses, with the most severe levels of swelling observed at 7 days postinfection (dpi) (Fig. 1A and B). The animals were intraperitoneally inoculated with 106 PFU of CHIKV, followed by a secondary hind-paw inoculation with 105 PFU of MAYV after 28 days and daily measurements of hind-paw swelling for 14 days (Fig. 1C). Among the mouse control groups, those infected with CHIKV that received a secondary CHIKV hind-paw inoculation did not develop any apparent signs of disease and had reduced viral loads (Fig. 1D to F). Compared to the non-CHIKV-infected mice, animals infected with CHIKV followed by MAYV inoculation exhibited a 1.3-fold area (mm2) reduction in hind-paw swelling, an 18-fold reduction in MAYV viral RNA, and a 136-fold decrease in MAYV viral load in the hind paw at 7 dpi (Fig. 1G to I). Furthermore, MAYV viremia was only detected 1 day after MAYV infection in the previously mock-infected group (average titer of 1,250 PFU/ml). No evidence of disease or tissue viral load was found in the mock-infected control groups. Therefore, our data indicate that a previous infection with CHIKV in a mouse model of MAYV infection causes a decrease in signs of disease based on hind-paw swelling and viral load.
FIG 1.
Previous CHIKV infection during secondary MAYV infection reduces hind-paw swelling and viral load. (A and B) C57BL/6 mice were inoculated in the hind paws with 20 μl of different viral concentrations of CHIKV or MAYV or mock infected. The metatarsal regions of their feet were measured daily over 15 days for (A) MAYV and (B) CHIKV infection (n = 3 per group). (C) Experimental design including CHIKV infection followed by MAYV footpad inoculation. (D to I) C57BL/6 mice were injected intraperitoneally with 100 μl of CHIKV (106 PFU) or mock infected. After 28 days, the mice were injected subcutaneously in the hind paw with 20 μl of 105 PFU of CHIKV or 105 PFU of MAYV or mock infected. (D and G) Perimetatarsal hind-paw swelling (width × breadth) was measured over 14 days after secondary inoculation (n = 6/group). The mice were euthanized at 1, 3, 5, 7, and 9 days after secondary infection, and the viral load of CHIKV or MAYV in the mice feet was determined by (E and H) qRT-PCR and by (F and I) plaque assay (n = 3/group). For panels A and B, statistical analysis was performed by two-way ANOVA with Dunnett’s test. Numbers indicate significant differences (P < 0.05) between MAYV- or CHIKV-infected mice and the mock-infected group. The number “3” represents the mouse group inoculated with 103 PFU of MAYV or CHIKV, “4” represents 104 PFU of MAYV or CHIKV, and “5” represents 105 PFU of MAYV or CHIKV. Data are representative of two independent experiments. For panels D and G, statistical analysis was performed by two-way ANOVA with Tukey’s test. Asterisks indicate significant differences (****, P < 0.0001) between (D) mock-infected–CHIKV and CHIKV-CHIKV or (G) mock-infected–MAYV and CHIKV-MAYV groups. ##, P < 0.001, and ####, P < 0.0001, for differences between (D) CHIKV-CHIKV and CHIKV–mock-infected groups or (G) CHIKV-MAYV or CHIKV–mock-infected groups. For panels E, F, H, and I, statistical analysis was performed by two-way ANOVA with Dunnett’s test. *, P < 0.05, **, P < 0.01, and ****, P < 0.0001 for differences between (E and F) mock-infected–CHIKV and CHIKV-CHIKV groups or (H and I) mock-infected–MAYV and CHIKV-MAYV groups. SD, standard deviation; LOD, limit of detection.
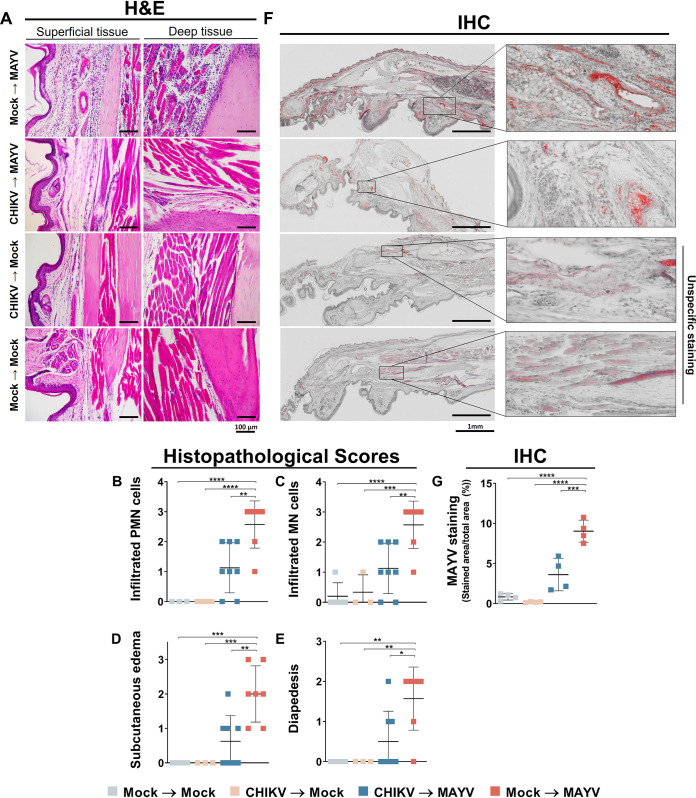
Histopathological signs of inflammation are reduced during secondary MAYV infection.
Mouse hind-paw inflammation was evaluated by histopathological score and immunohistochemical analysis, both performed 7 days after MAYV infection. Mice previously infected with CHIKV followed by MAYV infection showed a reduction in tissue clinical scores, with decreased mononuclear and polymorphonuclear cell infiltration in the dermal region. A reduction in diapedesis events and edema was also observed compared to nonprimary-CHIKV-infected mice (Fig. 2A to E). The footpads of MAYV-infected mice were strongly stained by the anti-MAYV antibody (mean, 9.03%), with infection distributed in distinct tissues (Fig. 2F). Thus, there was a significant decrease (±5.4%) in MAYV footpad distribution area in mice previously infected with CHIKV (mean, 3.61%) (Fig. 2F and G). CHIKV- or mock-infected mice with subsequent mock infection did not show increased immunohistochemistry (IHC) staining for MAYV, indicating the specificity of the antibody used in the assay. Histopathological alterations or increased viral presence were not observed in secondarily mock-infected control groups. In addition, cartilage damage of the ankle was not observed in any group (data not shown). Our data show that previous CHIKV infection reduces histopathological inflammatory scores and viral tissue antigen distribution during secondary MAYV infection in mice.
FIG 2.
Histology and immunohistochemistry of mice during MAYV disease peak. The C57BL/6 mice were mock infected or infected with 106 PFU of CHIKV, and after 28 days, they were mock infected or infected with 105 PFU of MAYV by hind-paw inoculation. Histopathological analyses were performed 7 days after MAYV infection. The inoculated feet were dissected, processed for histological analysis, and stained with H&E or subjected to IHC for viral antigen visualization. (A) Representative external and internal hind-paw images after H&E staining. (B to E) Histopathological scores of each mouse (n = 4 to 8 per group). (F) Representative immunohistochemistry images of hind paws and (G) staining quantification; each point represents an average of 5 to 7 high-power field of view of each animal (n = 4). Statistical analysis was conducted by one-way ANOVA with Sidak’s test. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). H&E, hematoxylin and eosin; IHC, immunohistochemistry; PMN, polymorphonuclear; MN, monomorphonuclear.
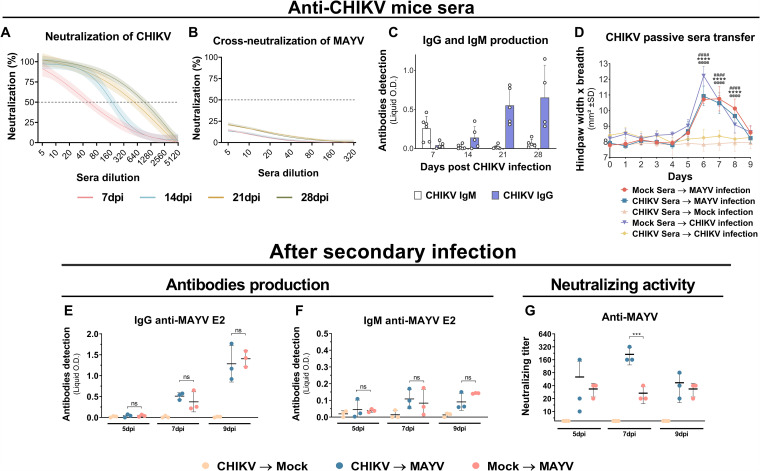
CHIKV antibodies develop limited in vitro and in vivo cross-protection against MAYV.
Mouse sera were collected at 7, 14, 21, and 28 days after CHIKV infection. Increasing neutralization activity against CHIKV was observed at different time points after infection (Fig. 3A). The assay for cross-neutralization to MAYV, with sera from CHIKV-infected mice, revealed low cross-neutralization activity (Fig. 3B). It was also noted that isotype class switching from IgM to IgG antibodies occurred throughout the 28 days after viral infection (Fig. 3C). The passive serum transfer from convalescent CHIKV-infected mice to naive mice, which were subsequently inoculated in the hind paw with CHIKV, did not result in the development of any apparent footpad swelling. However, animals that were infected with MAYV demonstrated an increased footpad swelling, indicating an absence of in vivo cross-protection against MAYV (Fig. 3D). Production of MAYV IgG and IgM antibodies after the secondary MAYV infection did not demonstrate a significant difference between previously mock- or CHIKV-infected mice (Fig. 3E and F). However, significant higher cross-neutralizing activity of MAYV was observed in animals previously infected with CHIKV at 7 dpi (Fig. 3G). These results suggest that CHIKV-infected mice do not promptly produce potent cross-reactive antibodies, yet during secondary infection with MAYV, recall immune response may trigger relevant cross-reactive antibody production.
FIG 3.
Antibody levels and neutralization titers before and after secondary infection and in vivo cross-protection with sera from CHIKV-infected mice. The C57BL/6 mice were intraperitoneally infected with 106 PFU of CHIKV. Sera were taken once a week for 28 days. (A and B) CHIKV-infected mouse serum homologous and heterologous in vitro neutralization of CHIKV (n = 5 per group) or MAYV (n = 5 per group), respectively. Nonlinear regression curves were generated with a maximum number of interactions of 1,000 and 95% confidence intervals. Error bars are delimited by the colors. Dotted line delimitates 50% neutralization. (C) IgG and IgM quantification by indirect ELISA using recombinant CHIKV E2 protein (n = 4 or 5 per group). (D) Hind-paw swelling measurements after in vivo passive sera transfer from mock infection or CHIKV convalescent-phase sera to naive mice with subsequent hind-paw infection by MAYV or CHIKV (n = 5 per group). (E and F) IgG and IgM antibody quantification by indirect ELISA using recombinant MAYV E2 protein and (G) neutralizing activities against MAYV after secondary MAYV infection (n = 3 per group). Data are representative of two independent experiments. Statistical analysis was performed by two-way ANOVA with Tukey’s test. In panel D, significant differences (P < 0.0001) between the CHIKV Sera → Mock Infection group and other groups are indicated by “@@@@” (Mock Sera → CHIKV infection), “****” (CHIKV Sera → MAYV infection), and “####” (Mock Sera → MAYV infection). In panel G, significant differences (P < 0.0001) between the CHIKV → MAYV and Mock → MAYV groups are indicated by “***.” O.D., optical density; SD, standard deviation; ns, not significant different; dpi, days postinfection; PRNT, plaque reduction neutralization test.
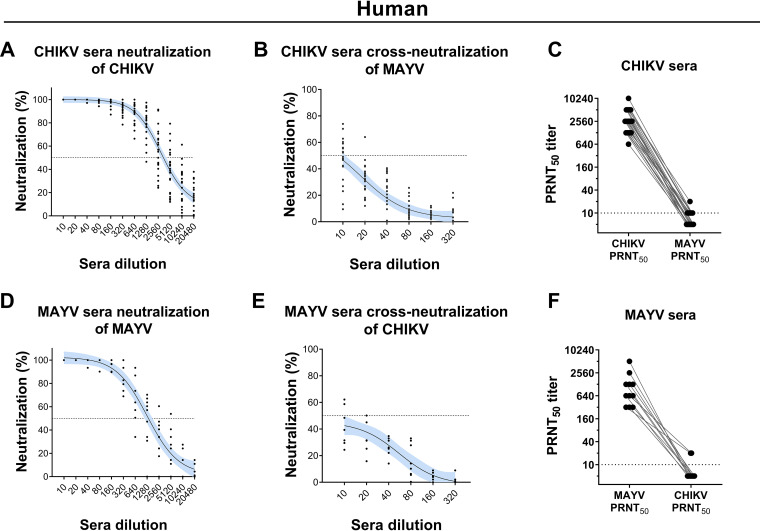
Sera from convalescent human CHIKV and MAYV patients show low in vitro cross-neutralization.
Sera from convalescent CHIKV- and MAYV-infected patients exhibited elevated homologous neutralizing activity by 50% plaque reduction neutralization test (PRNT50), with titers ranging from 320 to 10,240 (Fig. 4A and D). However, low levels of cross-neutralization against heterologous infection were observed, with titers no higher than 20 for most individuals (Fig. 4B, C, E, and F and Table 1). These results suggest that specific neutralizing antibodies to MAYV and CHIKV from humans do not mediate relevant in vitro cross-neutralization activity against CHIKV and MAYV infection.
FIG 4.
Homologous and heterologous neutralization of CHIKV and MAYV by convalescent-phase human sera. Human serum samples were collected from convalescent patients infected with CHIKV (n = 22) or MAYV (n = 8) and evaluated for homologous neutralization of (A) CHIKV and (D) MAYV and heterologous cross-neutralization of (B) MAYV and (E) CHIKV, respectively. PRNT50 of homologous and heterologous neutralization of CHIKV and MAYV were obtained using (C) CHIKV sera and (F) MAYV sera. Nonlinear regression curves were generated with a maximum number of interactions of 1,000 and 95% confidence intervals. Error bars are delimited by the color blue. Dotted line delimitates 50% neutralization.
TABLE 1.
Analysis of MAYV and CHIKV convalescent-phase human serum samples
| Virus | Sample ID | ELISA result for: |
PRNT50 titer |
||||
|---|---|---|---|---|---|---|---|
| CHIKV |
MAYV |
||||||
| IgM | IgG | IgM | IgG | MAYV | CHIKV | ||
| MAYV | 1 | − | − | − | + | 1,280 | <10 |
| 2 | − | + | − | + | 1,280 | <10 | |
| 3 | − | + | − | + | 2,560 | <10 | |
| 4 | − | − | − | + | 640 | <10 | |
| 5 | − | + | − | + | 640 | 20 | |
| 6 | − | + | − | + | 320 | 20 | |
| 7 | − | + | − | + | 1,280 | <10 | |
| 8 | − | − | − | + | 640 | <10 | |
| 9 | − | + | − | + | 5,120 | <10 | |
| 10 | − | + | − | + | 320 | <10 | |
| 11 | − | − | − | + | 320 | <10 | |
| CHIKV | 12 | + | + | − | − | <10 | 1,280 |
| 13 | − | + | − | − | <10 | 2,560 | |
| 14 | + | + | − | − | 20 | 5,120 | |
| 15 | − | + | − | − | 10 | 1,280 | |
| 16 | − | + | − | − | 10 | 5,120 | |
| 17 | − | + | − | − | 10 | 1,280 | |
| 18 | + | + | − | − | 10 | 2,560 | |
| 19 | + | + | − | − | <10 | 1,280 | |
| 20 | + | + | − | − | <10 | 2,560 | |
| 21 | + | + | − | − | 10 | 5,120 | |
| 22 | + | + | − | − | 10 | 2,560 | |
| 23 | + | + | − | − | <10 | 2,560 | |
| 24 | − | + | − | − | 10 | 1,280 | |
| 25 | − | + | − | − | <10 | 640 | |
| 26 | − | + | − | − | 10 | 10,240 | |
| 27 | + | + | − | − | <10 | 1,280 | |
| 28 | − | + | − | − | <10 | 5,120 | |
| 29 | + | + | − | − | <10 | 1,280 | |
| 30 | + | + | − | − | 10 | 2,560 | |
| 31 | + | + | − | − | 10 | 2,560 | |
| 32 | + | + | − | − | <10 | 5,120 | |
| 33 | + | + | − | − | <10 | 5,120 | |
| 34 | + | + | − | − | <10 | 2,560 | |
| 35 | + | + | − | − | <10 | 2,560 | |
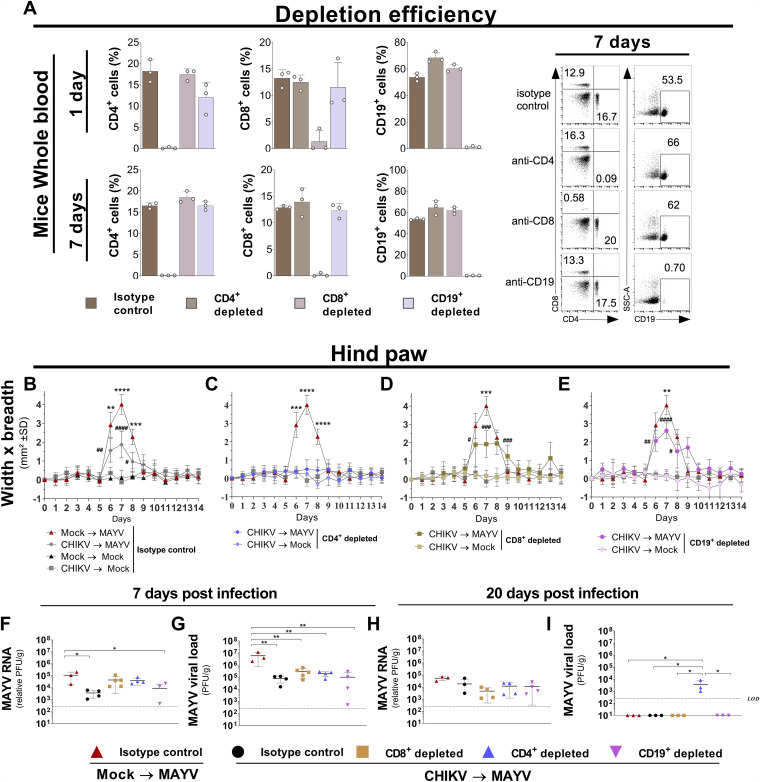
CD4+ T, CD8+ T, and CD19+ B cells do not interfere in CHIKV cross-protection against MAYV.
CD4+ T, CD8+ T, and CD19+ B cells were independently depleted in the CHIKV-infected mice 1 day before MAYV hind-paw inoculation (Fig. 5A). The depletion of CD8+ T and CD19+ B cells did not significantly change the cross-protection pattern of hind-paw swelling, whereas depletion of CD4+ T cells completely abolished the swelling pattern (Fig. 5B to E). Additionally, 7 days after infection, the amounts of MAYV RNA in the hind paw were not altered in mice with CD8+ T and CD4+ T cell depletion compared to the isotype control (Fig. 5F). All cell-depleted mouse groups showed significantly reduced viral loads compared to nondepleted naive MAYV-infected mice (Fig. 5G). Furthermore, all mouse groups presented similar amounts of MAYV RNA 20 days after infection. Notably, infectious MAYV was found only in CD4+ T cell-depleted mice (Fig. 5H and I). Collectively, these results show that individual depletion of CD4+ T, CD8+ T, and CD19+ B cells does not abrogate the cross-protection phenotype.
FIG 5.
Evaluation of CD4+ and CD8+ T and B cells in cross-protection against MAYV. C57BL/6 mice were infected with CHIKV or mock infected, and after 28 days, the animals were infected in the hind paw with MAYV or mock infected. One day prior to infection, CD4+, CD8+, or CD19+ cells were independently depleted from the C57BL/6 mice. (A) Depletion efficiency was evaluated by collecting whole blood at 1 and 7 days after intraperitoneal injection of the specific depletion antibodies: anti-CD4 (GK1.5), anti-CD8 (2.43), and anti-CD19 (1D3) or an IgG isotype control. (B to E) Perimetatarsal hind-paw swelling (width × breadth) was measured for 14 days (n = 4 per group). The amount of MAYV in each mouse hind paw was determined by viral load (PFU) and viral RNA quantification by RT-qPCR on days (F and G) 7 (peak of disease) and (H and I) 20 (disease recovery) (n = 3 to 5 per group). Statistical analysis was performed by two-way ANOVA with Tukey’s test. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001) between the Mock → MAYV (isotype control) and CHIKV → MAYV (depleted) groups; in panels B to E, pound signs indicate significant differences (#, P < 0.05; ##, P < 0.01; ###, P < 0.001; #####, P < 0.0001) between CHIKV → MAYV (depleted) and CHIKV → Mock (depleted) groups. The compared groups are indicated in panels F to I. LOD, limit of detection; SD, standard deviation.
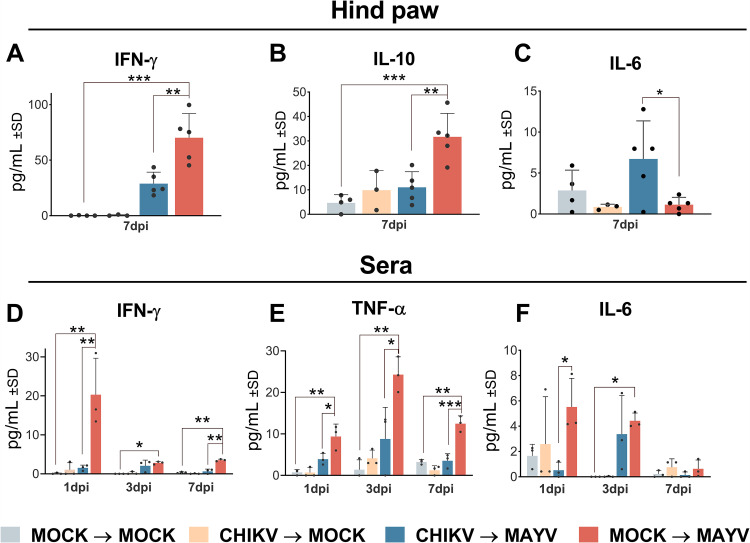
Previous CHIKV infection reduces inflammatory mediator levels during MAYV infection.
It is well known that both CHIKV and MAYV infection elicit strong immune responses (28). The levels of Th1/Th2/Th17 cytokines in the mouse hind paws and serum were evaluated at 1, 3, and 7 days after MAYV infection. In the hind paw, at 7 days postinfection, 2.4- and 2.8-fold reductions were observed in IFN-γ and IL-10 levels, respectively (Fig. 6A and B). In the sera, we also noted 13.3- and 5.5-fold decreases in IFN-γ levels at 1 and 7 days, respectively, and a 2.4- to 3.5-fold reduction in TNF-α levels at 1, 3, and 7 days post-MAYV infection compared to the non-previously CHIKV-infected group (Fig. 6D and E). Interestingly, the levels of IL-6 were 5.9-fold higher in the hind paws at 7 days post-MAYV infection in mice previously infected with CHIKV, whereas in sera, they were 10-fold higher in MAYV-infected naive mice during the initial days after infection (Fig. 6C and F). No significant differences in tumor necrosis factor alpha (TNF-α), IL-17A, IL-4, and IL-2 levels were observed in the hind paw, or in IL-17A serum levels after MAYV infection (data not shown). IL-10, IL-4, and IL-2 levels were below the detection limit in the mouse sera. These results indicate that a primary CHIKV infection reduces the levels of IFN- γ and IL-10 in the hind paw during a secondary MAYV infection while increasing IL-6 at 7 days postinfection and reducing the levels of TNF-α, IFN-γ, and IL-6 in mouse sera during the subsequent days after infection.
FIG 6.
Previous CHIKV infection reduced inflammatory cytokine levels in the hind paw and serum of MAYV-infected mice. The C57BL/6 mice (n = 3 to 5 per group) were mock or CHIKV infected and, after 28 days, inoculated in the hind paw with MAYV or mock infected. (A to C) The animals’ hind paws were collected at 7 days after secondary MAYV infection, and the cytokines were quantified in the hind-paw homogenate’s supernatant. (D to F) Sera were collected at 1, 3, and 7 days after secondary MAYV infection, and the cytokines were quantified. Data are representative of two independent experiments. Statistical analysis was performed by two-way ANOVA with Sidak’s test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. IFN-γ, interferon gamma; IL-10, interleukin 10; TNF-α, tumor necrosis factor alpha; dpi, day postinfection.
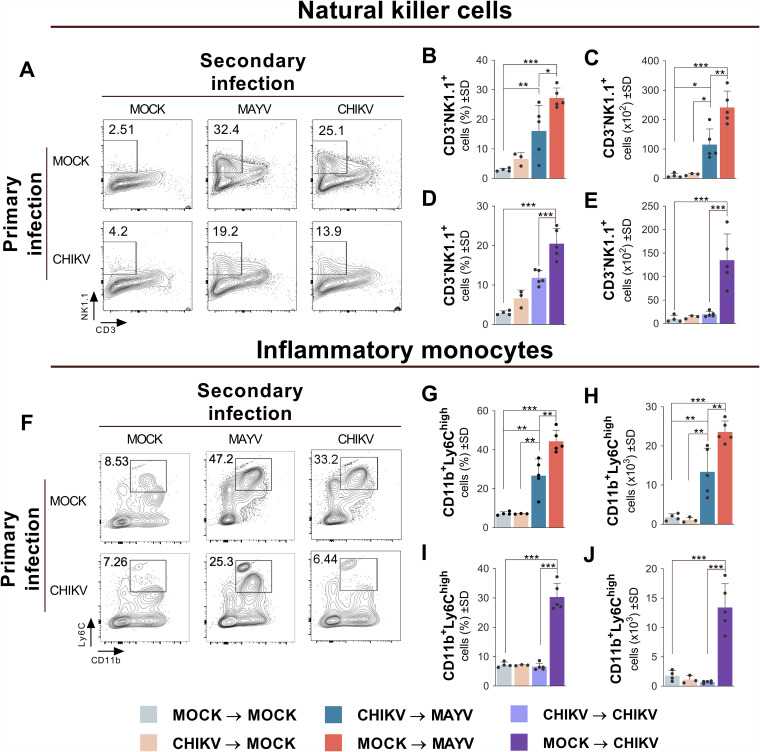
Previous CHIKV infection reduces NK cell and inflammatory monocyte recruitment during MAYV infection.
Considering the important contribution of hematopoietic and myeloid cells during alphavirus infection (29), the recruitment profile of specific cellular subsets in the hind paw of mice previously infected with CHIKV was analyzed at 7 days after MAYV infection. An average recruitment reduction of 1.7-fold was observed in NK cells (CD3-NK1.1+) and inflammatory monocytes (CD11b+ Ly6C+) in the hind paw compared to naive mice secondarily infected with MAYV (Fig. 7B, C, G, and H). Similarly, 1.73- and 4.6-fold reductions in NK cell and inflammatory monocyte recruitment, respectively, were verified in the hind paws of mice primarily and secondarily infected with homologous CHIKV compared to naive mice secondarily infected with CHIKV (Fig. 7D, E, I, and J). No significant recruitment of other cellular subsets was observed in the hind paws or ankles of secondarily MAYV-infected mouse groups with or without previous CHIKV infection (data not shown). Our results show that, during the peak of the MAYV disease, mice previously infected with CHIKV demonstrated a decreased recruitment of NK cell and inflammatory monocyte subsets at the site of infection.
FIG 7.
Tissue infiltration of NK cells and inflammatory monocytes is reduced during MAYV infection in mice previously infected with CHIKV. The C57BL/6 mice were mock or CHIKV infected, and after 28 days, mice were mock, CHIKV, or MAYV infected in the hind paw (n = 3 to 5 per group). After 7 days, mice were euthanized, and their feet removed and processed for cell immunophenotyping analysis. Representative contour plots showing the frequency of (A) NK cells (CD3-NK1.1+) and (B) inflammatory monocytes (CD11b+ Ly6Chigh) in the hind paws of each experimental group. The frequencies (B, D, G, and I) and absolute numbers (C, E, H, and J) of both cell subtypes are shown. Data are representative of two independent experiments. Statistical analysis was performed by one-way ANOVA with Tukey’s test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
In the present study, we report that the immune response after CHIKV infection partially cross-protects mice infected with MAYV, reducing disease severity based on partial reduction of hind-paw swelling. Our results corroborate other studies that show a partial decrease in footpad swelling after MAYV infection in immunocompetent mice previously exposed to CHIKV (30). Furthermore, we observed a reduction of viremia and tissue viral load in the feet after the secondary infection, indicating the existence of a previous cross-protective immune response able to develop an early viral control. Transposing this fact to humans, the development of viremia and tissue viral load may represent an evolutionary barrier to the urban adaptation of MAYV transmission through Aedes mosquitoes, as well as the clinical severity correlation with CHIKV disease (15, 31). A reduction in MAYV viral load in mice preexposed to CHIKV may have further implications for viral transmission and human disease severity in areas of cocirculation of both viruses.
The histopathological analysis of CHIKV infection in mouse feet showed a locally generalized infiltrate of mononuclear cells, subcutaneous edema, and large foci of cellular infiltrate in muscle tissue (32). In this study, MAYV inoculation in the mouse footpad increased tissue infiltration of mononuclear and polymorphonuclear cells, accompanied by elevated diapedesis events and edema development. We emphasize that all these histological inflammatory phenomena were reduced in animals previously exposed to CHIKV. Changes in cartilage composition in the different mouse groups were not observed, suggesting that this model is not suitable for evaluation of cartilage damage, probably because analysis took place after only a short period of infection.
Monoclonal antibodies that neutralize CHIKV have shown potent cross-neutralization activity against multiple alphaviruses, including MAYV, suggesting the existence of conserved epitopes in the genus (25). However, it has been demonstrated that CHIKV infection elicits low cross-neutralizing polyclonal antibodies against MAYV and fails to cross-protect against MAYV infection after in vivo serum transfer (30). In the present study, mice infected with CHIKV produced high levels of CHIKV-neutralizing antibodies but poor cross-neutralization or cross-protection against MAYV in vitro and in vivo infection, respectively. Furthermore, we observed low cross-neutralizing activity against MAYV infection with sera from convalescent CHIKV-infected patients. However, after secondary MAYV infection, we observed the development of significantly higher cross-neutralizing antibodies in animals previously infected by CHIKV. These results indicate the existence of low levels of cross-reactive antibodies after primary infection, which are induced during secondary infection and develop significant activity during an early immune response. Our results are in accordance with a previous study that described low MAYV cross-neutralization levels from CHIKV-infected patient antibodies (24). Collectively, our findings suggest that primary infection with CHIKV does not promptly induce efficient cross-reactive antibodies. However, secondary infection by MAYV may significantly trigger the production of cross-reactive antibodies, which develop higher neutralizing activities and play a relevant role during early immune response.
The engagement of adaptive immune response in the early stages of infection by arthritogenic alphaviruses, especially those involving T and B cells, is essential for viral clearance and protection (21). Previous studies have shown that CHIKV infection in immunocompetent mice leads to persistent viral RNA detection in their feet and demonstrated that although CD4+ T cells are essential for the development of acute CHIKV disease, they are not directly essential for virus replication control (21, 33). In addition, it has been demonstrated that CD4+ and CD8+ T cells from CHIKV-immunized mice are able to cross-respond against ex vivo stimulation to MAYV antigen (30). In our study, mice infected with CHIKV and with depleted CD8+ T cells did not present changes in the pattern of hind-paw swelling or MAYV RNA amounts and viral load. However, when CD4+ T cells were depleted, the animals did not develop any apparent hind-paw swelling and exhibited persistent MAYV viral load at 20 dpi. Furthermore, it has been demonstrated that the lack of mature B cells leads to an increase in viral load and prolonged foot swelling in mice during CHIKV infection (34). However, we did not observe any apparent changes in disease outcome and tissue viral load development in CD19+ B cell-depleted mice during MAYV heterologous infection, suggesting that this cell subset may not promptly affect replication control during secondary infection. Nevertheless, we did not observe a complete phenotypical reversion of viral load and hind-paw swelling in mice with CD4+ T, CD8+ T, or CD19+ B cell depletion during acute disease. However, we should not overlook the facts that we did not observe 100% cell depletion and that the cell population from blood may not totally reflect the cell profile from mice feet tissue. In addition, it is possible that the individual depletion of these cells may result in the compensatory activity of other cell subsets that cross-protect against MAYV. Moreover, we should not overlook the involvement of the memory-like phenotypes of other cell subsets, which may develop important roles during cross-protection.
High levels of pro-inflammatory mediators (e.g., IFN-γ and TNF-α) have been associated with vascular leakage and edema during CHIKV infection (35). An initial increase in IFN-γ expression, accompanied by long-lasting TNF-α production, was identified in the blood of mice, monkeys, and humans during CHIKV infection (32, 36, 37). It has been suggested that initial viral replication may induce IFN-γ production through NK cell activation (38). According to Nakaya et al., the lack of IFN-γ production in IFN−/− animals does not interfere with CHIKV viral load but reduces footpad swelling, indicating its distinctive role in inducing the inflammatory disease (39). In contrast, a different study showed that IFN-γ is crucial for CHIKV viral control but is irrelevant to footpad swelling disease outcome (33). In the present study, peaks in IFN-γ production were observed in the sera and hind paws at 1 and 7 days after MAYV infection, respectively, which were substantially reduced in mice previously infected with CHIKV. Our results suggest that cross-protection immunity induced by CHIKV infection decreased the expression of IFN-γ, correlating with the reduction in viral detection and footpad swelling.
IFN-γ production is a potent enhancer of TNF expression by monocytes/macrophages during the immune response (40). The early production of TNF-α during alphavirus infection has been attributed to infiltrating and tissue-resident macrophages (36, 41). Accordingly, in CHIKV-infected mice, we observed a decrease in TNF-α blood levels after MAYV infection. Low TNF-α production could be correlated with a reduction in infiltrating inflammatory monocytes in the footpads and consequent reduced clinical scores. The lower levels of IFN-γ and TNF-α during secondary MAYV infection indicate the existence of a cross-protection mechanism able to attenuate the viral inflammatory response, which further results in the reduction of viral load, inflammation, and disease scores.
Herein, we show that IL-10 levels in the hind paws were reduced during the peak of MAYV disease in mice previously infected with CHIKV. Accordingly, human cohort studies have shown that the levels of circulating IL-10 during acute CHIKV infection are increased (37, 42). However, IL-10 is an anti-inflammatory cytokine, and its production may appear contradictory considering that CHIKV and MAYV are causative agents of inflammatory diseases (43, 44). Alternatively, it has been suggested that the pro-inflammatory response might occur earlier in infection and is later downregulated by an anti-inflammatory counterresponse with type II cytokine production, including IL-10 (42). Furthermore, we observed increased levels of IL-6 in the hind paws and reduced levels in the sera of mice previously infected with CHIKV during MAYV disease. Increased levels of IL-6 production were observed in patients during acute phase of CHIKV infection and remained elevated during a short period after recovery (37). Although increased IL-6 levels are linked to exacerbation of clinical outcomes, previous evidences have also demonstrated that IL-6 is able to directly suppress viral replication in vitro (45). However, inappropriate consequences in viral infections have also been associated with increased IL-6 production (46). In the present study, increased levels of IL-6 were observed in the mouse feet during acute disease, which appears contradictory to reduced local inflammation. Therefore, different immunological scenarios might be associated with IL-10 and IL-6 production during viral infections, and further work is necessary to elucidate their exact roles.
Mouse hind-paw infection with CHIKV induces the expression of cytokines and chemokines responsible for recruiting NK cells, macrophages, inflammatory monocytes, and CD8+ and CD4+ T cells (32, 47). During CHIKV infection, NK cells develop prominent roles in the control and destruction of infected cells (48). Human infection by CHIKV leads to high NK cell levels in the blood during the early acute phase of the disease (49). Accordingly, we found reduced levels of NK cells (CD3-NK1.1+) and histological scores in the hind paws of mice preexposed to CHIKV and subsequently infected with MAYV, suggesting a previous development of cross-immunity, since it was previously described during acute CHIKV infection (50). Therefore, it is conceivable that cross-protection against MAYV could include the development of “memory-like” NK cells during preexposure to CHIKV (51). Furthermore, pathogenic and protective roles have been described for monocytes and monocyte-derived cells during alphavirus infection in mice (52, 53). Depletion of monocytes or neutralization of their proinflammatory factors during Ross River virus infection reduces disease severity, inflammatory infiltrates, and tissue damage (54). In the present study, previous CHIKV infection decreased inflammatory monocyte (CD11b+ Ly6Chigh) recruitment in the hind paws of mice after MAYV infection, a fact that further correlates with reduced histological scores and footpad swelling. Interestingly, we observed the development of subpopulations within Ly6Chigh and CD11b+ cells gating in CHIKV convalescent mice, which apparently increase after secondary infection. We considered this population as inflammatory monocytes according to our standard definition, but further studies may better elucidate the heterogeneity of monocyte development after alphavirus infection. These results suggest that monocytes develop important and relevant roles during secondary alphavirus infection.
In sum, we demonstrated that the preexistence of CHIKV immunity confers partial cross-protection against secondary MAYV infection. This partial cross-protection reduced tissue viral load and histopathological scores during MAYV disease. Interestingly, CHIKV antibodies from humans and mice showed low cross-neutralization against MAYV infection. However, after secondary infection, increased titers of cross-neutralizing antibodies were observed in mice. Also, the results observed after the depletion of adaptive immune cells indicate that distinct cell subsets or a combination of adaptive immune cells may be relevant during cross-protection against secondary MAYV infection. The observed reduction in proinflammatory cytokines, NK cells, and monocytes cells during MAYV infection of mice previously infected with CHIKV suggests a potential role of innate immunity in cross-protection. Our findings shed light on how preexisting immunity to arthritogenic alphaviruses may affect a secondary infection. Nonetheless, further studies are needed to better elucidate the immunological mechanisms involved.
MATERIALS AND METHODS
Ethics statement.
All procedures involving animal care followed ethical principles of animal research and were approved by the local ethical committee on animal experimentation (Comissão de Ética em Experimentação Animal [CETEA]) at the Ribeirão Preto Medical School, according to protocol 182/2020. Experiments involving human samples were approved by the local ethics committee in research (CEP) of the Ribeirão Preto Medical School (protocol 2017/2.206.200), which followed the guidelines of the National Research Ethics Commission (CONEP).
Viruses.
The virus strains CHIKV S27 African and MAYV TRVL 4675 were used in this study. For the experiments involving mice, viral stocks were produced in suckling mouse brain, homogenized in phosphate-buffered saline (PBS) solution, clarified by centrifugation at 5,500 × g to remove debris, filtered through a 0.22-μm syringe filter, placed in aliquots, and kept in a freezer at −80°C. PBS diluent was used for mock infection. In the in vitro assays, viral stocks were produced in Vero cells (ATCC CCL-81), which were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 2% heat-inactivated fetal bovine serum (FBS), and the supernatant was collected after 2 to 3 dpi. Cell debris were removed by centrifugation at 5,500 × g, followed by supernatant filtration using a 0.22-μm syringe filter, and aliquots were maintained in a freezer at −80°C. The viral stocks were titrated by plaque assay using Vero cells (55).
Mice.
C57BL/6 (JAX 000664) mice were used in the present study. The animals were kept and bred under specific-pathogen-free conditions at the animal facilities of the Ribeirão Preto Medical School, University of São Paulo. All in vivo experiments were conducted with 6- to 8-week-old female mice.
Serum samples from CHIKV- and MAYV-infected humans.
IgG-positive serum samples for CHIKV were collected from patients in the city of Ribeirão Preto after local outbreaks of the virus. IgG positive serum samples for MAYV were collected in 2015 from patients in the city of Sinop in the Brazilian Amazon region, where MAYV circulation is endemic. Serum samples from healthy subjects with no history of previous arbovirus infection were collected and used as negative controls.
Mouse infection, footpad swelling measurement, and blood collection.
For immunization, 6- to 8-week-old female C57BL/6 mice were intraperitoneally (i.p.) inoculated with 106 PFU of CHIKV at a final volume of 100 μl. In order to measure antibody production and neutralization activity, blood from the CHIKV-immunized mice was collected by facial bleeding on days 7, 14, 21, and 28 after infection. Twenty-eight days after the first infection, the animals were infected with 105 PFU of CHIKV or MAYV by hind-paw inoculation into the ventral side of each foot, toward the ankle, at a final volume of 20 μl. Mock-immunized mice were inoculated with conditioned medium and PBS as a diluent. Using a digital caliper, the hind-paw swelling outcome was measured considering the width and breadth of the perimetatarsal region of the hind paw for 14 days postinfection. Paw tissue and blood were collected at 1, 3, 5, 7, and 9 days after secondary infection for viral quantification and histopathological and cytokine analyses.
ELISA and plaque reduction neutralization assay.
The infected mice were bled at the indicated time points; the blood was centrifuged at 13,000 × g, and serum was collected for analysis. Sera from humans and the CHIKV-infected mice were submitted to a previously in-house-developed indirect enzyme-linked immunosorbent assay (ELISA) for CHIKV- or MAYV-specific IgG and IgM detection using recombinant envelope protein 2 (56, 57). The samples were also subjected to the plaque reduction neutralization test (PRNT50) for CHIKV and MAYV, as previously described (57).
Quantitation of viral loads by plaque assay and RT-qPCR.
The mice were euthanized and intracardially perfused with PBS at the indicated times after viral infection. Their feet were removed, weighed, and homogenized in sterile PBS (1:5 [wt/vol]) using TissueLyser II (Qiagen, USA) with a 5-mm stainless steel bead (Qiagen, USA). The samples were then processed for tissue disruption for 10 min at 30 Hz and centrifuged for 5 min at 10,000 × g, and the supernatant was collected and stored at −80°C. For viral quantification by plaque assay, the tissue-homogenized supernatant was inoculated onto a Vero cell monolayer seeded on 24-well plates, as previously described (55). As for viral RNA quantification, the same homogenized supernatant samples were processed using the QIAamp viral RNA minikit (Qiagen, USA) according to the manufacturer’s protocol. Reverse transcription-quantitative PCR (RT-qPCR) was performed using TaqMan Fast Virus one-step master mix (Applied Biosystems, USA) according to the manufacturer’s recommendations. The primers and probe were designed for amplification of a 120-bp amplicon from the MAYV NsP4 gene region (forward, 5′-TACCATGTCAGATATGCTAAGCCTCGG-3′; reverse, ‘5-TCTGTGCCGGTGATGCAAAGACTTAGCAGCGC-3′; probe, 5′-FAM [6-carboxyfluorescein]-CGCCACTGTAGGGTAGTTGCG-BHQ1 [black hole quencher 1]-3′). Meanwhile, the primers and probe for CHIKV amplified a 124-bp amplicon from the NsP3 viral gene region (forward, 5′-CGACGGATGCAGACGTGGTC-3′; reverse, 5′-ACATCGCAGTCTATGGAGATGTGC-3′; probe, 5′-HEX [6-carboxy-2,4,4,5,7,7-hexachlorofluorescein]-TGCGGACCCAAGTGGAGCTGCTGGA-BHQ1-3′). The reactions were carried out using a StepOnePlus real-time PCR system (Applied Biosystems, USA). Standard curves were generated using extracted RNA from titrated virus stocks. Each sample was analyzed in duplicate, and the results were normalized to the relative amount of virus per gram of tissue.
Histopathological analysis.
After 7 days of MAYV hind-paw inoculation, the mice were euthanized and intracardially perfused with 4% formalin solution, and their hind paws were collected and fixed for 2 days in 4% formalin solution. Subsequently, the hind paws were incubated in 10% (wt/vol) EDTA solution for 3 weeks with gentle agitation at 4°C for decalcification. Next, they were longitudinally cut, and the metatarsal region was separated from the ankle region. Tissue samples were dehydrated in increasing concentrations of ethanol, cleared in xylene, embedded in paraffin, and sectioned at a thickness of 5 μm. The metatarsal region and ankle sections were stained with Harris’s hematoxylin and eosin (H&E) for histopathological analysis. The ankle specimen sections were stained with safranin-O for cartilage destruction visualization (58). The 5-μm sections were graded by two pathologists in a blind fashion according to the modified score parameters: (1) polymorphonuclear cell infiltration, (2) monomorphonuclear cell infiltration, (3) subcutaneous edema, and (4) diapedesis events. Each parameter was scaled as none (0), mild (1), moderate (2), or severe (3) (59). Image acquisition was performed using an upright Nikon Eclipse E800 microscope (Nikon Corporation, Japan).
Immunohistochemistry.
The formalin-fixed, paraffin-embedded (FFPE) decalcified hind-paw tissue sections were subjected to antigen retrieval by trypsin–0.25% EDTA treatment (Sigma-Aldrich, USA) for 15 min at 37°C. The sections were incubated with an in-house polyclonal mouse anti-MAYV antibody for 1 h after a blocking step with SuperBlock blocking buffer (Thermo Fisher Scientific, USA). Primary antibodies were detected with a secondary biotinylated horse anti-mouse IgG antibody (Vector Laboratories, USA) and revealed using the AEC kit (Vector Laboratories, USA). Counterstaining with Harris’s hematoxylin was also performed to improve visualization and analysis. The stained sections were scanned with a bright-field ScanScope VS120 microscope (Olympus Life Sciences, Japan) in bright field at ×400 magnification. Virtually scanned slides (four animals per group) were sectioned into 12 fields per slide using the ImageJ software. MAYV staining (red staining) was enhanced with the Adobe Photoshop software, using black and white adjustment tools. Quantitation of total tissue area and the IHC stained area (μm2) from each field was carried out using the ImageJ software. The results obtained are shown as a percentage ratio between the IHC-stained area and total tissue area (mean of 12 fields per animal).
Passive CHIKV serum transfer.
The C57BL/6 mice (n = 6) were intraperitoneally infected with 106 PFU of CHIKV. After 4 weeks, their sera were collected and pooled. Thereafter, 200 μl of the serum pool was i.p. injected into naive C57BL/6 mice (n = 6) 1 day prior to infection with MAYV or CHIKV.
Depletion of CD4+ T, CD8+ T, and CD19+ B lymphocytes of mice.
In order to deplete the CD4+ T, CD8+ T, and CD19+ B cells, the mice were i.p. injected with 500 μg of anti-CD4 antibody (GK1.5) (Bioxcell, USA), anti-CD8 antibody (2.43) (Bioxcell, USA), or anti-CD19 antibody (1D3) (Bioxcell, USA) 1 day before infection. The animals in the control groups were injected with 500 μg of IgG isotype control antibody (Bioxcell, USA). The antibodies were diluted in PBS at a final inoculum volume of 200 μl. For efficacy control, whole blood from depleted animals was collected at 1 and 7 days after depletion. The red blood cells were lysed, and the remaining cells were stained with mouse anti-CD4–peridinin chlorophyll protein (PerCP) (BioLegend, USA), anti-CD8–fluorescein isothiocyanate (FITC) (BioLegend, USA), and anti-CD19–phycoerythrin (PE) (BioLegend, USA). Cell acquisition was performed on a BD FACSCanto II flow cytometer (BD Biosciences, USA) and analyzed using FlowJo software (Tree Star, USA).
Cytokine quantification.
Cytokine protein levels in mouse sera and hind-paw homogenate were determined at the indicated time points after infection using the cytometric bead array (CBA) mouse Th1/Th2/Th17 cytokine kit (BD Biosciences, USA) according to the manufacturer’s instructions. Samples were acquired on a BD FACSCanto II flow cytometer (BD Biosciences, USA) and analyzed using the FCAP Array software version 3.0 (BD Biosciences, USA).
Immunophenotyping of the hind paws.
The mouse groups were euthanized after 7 days of hind-paw infection; their feet were removed and the metatarsal region separated from the ankle. The bone marrow of ankle specimens was washed with sterile PBS for hematopoietic cell removal. For tissue dissociation, the specimens were minced and incubated with 100 U of collagenase VIII (Invitrogen, USA) for 2 h at 37°C and passed through a 70-μm mesh cell strainer. The cells were counted and added to 96-well U-bottom plates, after which they were blocked with Fc Block (BD Biosciences, USA) and stained for flow cytometry analysis. The antibodies used were anti-CD3ε–PerCP (clone 145-2C11) (BioLegend, USA), anti-CD19–allophycocyanin (APC) (clone 6D5) (BioLegend, USA), anti-NK1.1–FITC (clone PK136) (BioLegend, USA), anti-CD45–APC (clone 30-F11) (BD Biosciences), anti-CD11b–FITC (clone M1/70) (BioLegend, USA), anti-Ly6G–PerCP (clone 1A8) (BioLegend, USA), and anti-Ly6C–PE (clone HK1.4) (BioLegend, USA). Samples were acquired on a BD FACSCanto II flow cytometer (BD Biosciences, USA) and analyzed with FlowJo software (Tree Star, USA).
Statistical analyses.
The data obtained were plotted and analyzed using GraphPad Prism 8.0.2 software (GraphPad, USA). For multiple group comparisons, one-way or two-way analysis of variance (ANOVA) was used, coupled with multiple correction tests (indicated in the figure legends).
ACKNOWLEDGMENTS
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant 2019/27333-6 and scholarships 2018/09383-3 and 2017/13981-0.
Contributor Information
Marcilio Jorge Fumagalli, Email: marcilio_jorge@hotmail.com.
Mark T. Heise, University of North Carolina at Chapel Hill
REFERENCES
- 1.Karabatsos N. 1975. Antigenic relationships of group A arboviruses by plaque reduction neutralization testing. Am J Trop Med Hyg 24:527–532. 10.4269/ajtmh.1975.24.527. [DOI] [PubMed] [Google Scholar]
- 2.Weaver SC, Chen R, Diallo M. 2020. Chikungunya virus: role of vectors in emergence from enzootic cycles. Annu Rev Entomol 65:313–332. 10.1146/annurev-ento-011019-025207. [DOI] [PubMed] [Google Scholar]
- 3.Kariuki Njenga M, Nderitu L, Ledermann JP, Ndirangu A, Logue CH, Kelly CH, Sang R, Sergon K, Breiman R, Powers AM. 2008. Tracking epidemic Chikungunya virus into the Indian Ocean from East Africa. J Gen Virol 89:2754–2760. 10.1099/vir.0.2008/005413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R, Djauzi Wandra T, Master J, Kosasih H, Hartati S, Beckett C, Sedyaningsih ER, Beecham HJ, III, Corwin AL. 2005. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg 99:128–141. 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Mavalankar D, Shastri P, Raman P. 2007. Chikungunya epidemic in India: a major public-health disaster. Lancet Infect Dis 7:306–307. 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 6.Nunes MR, Faria NR, de Vasconcelos JM, Golding N, Kraemer MU, de Oliveira LF, Azevedo Rdo S, da Silva DE, da Silva EV, da Silva SP, Carvalho VL, Coelho GE, Cruz AC, Rodrigues SG, Vianez JL, Jr, Nunes BT, Cardoso JF, Tesh RB, Hay SI, Pybus OG, Vasconcelos PF. 2015. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med 13:102. 10.1186/s12916-015-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Bortel W, Dorleans F, Rosine J, Blateau A, Rousset D, Matheus S, Leparc-Goffart I, Flusin O, Prat C, Cesaire R, Najioullah F, Ardillon V, Balleydier E, Carvalho L, Lemaitre A, Noel H, Servas V, Six C, Zurbaran M, Leon L, Guinard A, van den Kerkhof J, Henry M, Fanoy E, Braks M, Reimerink J, Swaan C, Georges R, Brooks L, Freedman J, Sudre B, Zeller H. 2014. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill 19:20759. 10.2807/1560-7917.es2014.19.13.20759. [DOI] [PubMed] [Google Scholar]
- 8.Watson R. 2007. Europe witnesses first local transmission of chikungunya fever in Italy. BMJ 335:532–533. 10.1136/bmj.39332.708738.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasconcelos PF, Travassos da Rosa AP, Rodrigues SG, Travassos da Rosa ES, Degallier N, Travassos da Rosa JF. 2001. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad Saude Publica 17(Suppl):155–164. 10.1590/s0102-311x2001000700025. [DOI] [PubMed] [Google Scholar]
- 10.Blohm G, Elbadry MA, Mavian C, Stephenson C, Loeb J, White S, Telisma T, Chavannes S, Beau De Rochar VM, Salemi M, Lednicky JA, Morris JG, Jr.. 2019. Mayaro as a Caribbean traveler: evidence for multiple introductions and transmission of the virus into Haiti. Int J Infect Dis 87:151–153. 10.1016/j.ijid.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunini S, Franca DDS, Silva JB, Silva LN, Silva FPA, Spadoni M, Rezza G. 2017. High frequency of Mayaro virus IgM among febrile patients, central Brazil. Emerg Infect Dis 23:1025–1026. 10.3201/eid2306.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuchi N, Heinen LB, Santos MA, Pereira FC, Slhessarenko RD. 2014. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem Inst Oswaldo Cruz 109:820–823. 10.1590/0074-0276140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yactayo S, Staples JE, Millot V, Cibrelus L, Ramon-Pardo P. 2016. Epidemiology of Chikungunya in the Americas. J Infect Dis 214:S441–S445. 10.1093/infdis/jiw390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brustolin M, Pujhari S, Henderson CA, Rasgon JL. 2018. Anopheles mosquitoes may drive invasion and transmission of Mayaro virus across geographically diverse regions. PLoS Negl Trop Dis 12:e0006895. 10.1371/journal.pntd.0006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long KC, Ziegler SA, Thangamani S, Hausser NL, Kochel TJ, Higgs S, Tesh RB. 2011. Experimental transmission of Mayaro virus by Aedes aegypti. Am J Trop Med Hyg 85:750–757. 10.4269/ajtmh.2011.11-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiredo ML, Figueiredo LT. 2014. Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev Soc Bras Med Trop 47:677–683. 10.1590/0037-8682-0246-2014. [DOI] [PubMed] [Google Scholar]
- 17.Halsey ES, Siles C, Guevara C, Vilcarromero S, Jhonston EJ, Ramal C, Aguilar PV, Ampuero JS. 2013. Mayaro virus infection, Amazon Basin region, Peru, 2010–2013. Emerg Infect Dis 19:1839–1842. 10.3201/eid1911.130777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, Brouqui P, Hance P, Kraemer P, Ali Mohamed A, de Lamballerie X, Charrel R, Tolou H. 2007. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore, MD) 86:123–137. 10.1097/MD/0b013e31806010a5. [DOI] [PubMed] [Google Scholar]
- 19.Santiago FW, Halsey ES, Siles C, Vilcarromero S, Guevara C, Silvas JA, Ramal C, Ampuero JS, Aguilar PV. 2015. Long-term arthralgia after Mayaro virus infection correlates with sustained pro-inflammatory cytokine response. PLoS Negl Trop Dis 9:e0004104. 10.1371/journal.pntd.0004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. 2011. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis 204:115–123. 10.1093/infdis/jiq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. 2013. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J Virol 87:13878–13888. 10.1128/JVI.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partidos CD, Paykel J, Weger J, Borland EM, Powers AM, Seymour R, Weaver SC, Stinchcomb DT, Osorio JE. 2012. Cross-protective immunity against o'nyong-nyong virus afforded by a novel recombinant chikungunya vaccine. Vaccine 30:4638–4643. 10.1016/j.vaccine.2012.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroon Campos R, Preciado-Llanes L, Azar SR, Kim YC, Brandon O, Lopez-Camacho C, Reyes-Sandoval A, Rossi SL. 2020. Adenoviral-vectored Mayaro and Chikungunya virus vaccine candidates afford partial cross-protection from lethal challenge in A129 mouse model. Front Immunol 11:591885. 10.3389/fimmu.2020.591885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins KA, Gregory MK, Valdez SM, Sprague TR, Encinales L, Pacheco N, Cure C, Porras-Ramirez A, Rico-Mendoza A, Chang A, Pitt ML, Nasar F. 2019. Neutralizing antibodies from convalescent Chikungunya virus patients can cross-neutralize Mayaro and Una viruses. Am J Trop Med Hyg 100:1541–1544. 10.4269/ajtmh.18-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox JM, Long F, Edeling MA, Lin H, van Duijl-Richter MKS, Fong RH, Kahle KM, Smit JM, Jin J, Simmons G, Doranz BJ, Crowe JE, Jr, Fremont DH, Rossmann MG, Diamond MS. 2015. Broadly neutralizing alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell 163:1095–1107. 10.1016/j.cell.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binn LN, Harrison VR, Randall R. 1967. Patterns of viremia and antibody observed in rhesus monkeys inoculated with chikungunya and other serologically related group A arboviruses. Am J Trop Med Hyg 16:782–785. 10.4269/ajtmh.1967.16.782. [DOI] [PubMed] [Google Scholar]
- 27.Lum FM, Couderc T, Chia BS, Ong RY, Her Z, Chow A, Leo YS, Kam YW, Renia L, Lecuit M, Ng LFP. 2018. Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity. Sci Rep 8:1860. 10.1038/s41598-018-20305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzere BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debre P, Autran B, Gasque P. 2010. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol 184:5914–5927. 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 29.Fox JM, Diamond MS. 2016. Immune-mediated protection and pathogenesis of chikungunya virus. J Immunol 197:4210–4218. 10.4049/jimmunol.1601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb EM, Azar SR, Haller SL, Langsjoen RM, Cuthbert CE, Ramjag AT, Luo H, Plante K, Wang T, Simmons G, Carrington CVF, Weaver SC, Rossi SL, Auguste AJ. 2019. Effects of Chikungunya virus immunity on Mayaro virus disease and epidemic potential. Sci Rep 9:20399. 10.1038/s41598-019-56551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waggoner JJ, Gresh L, Vargas MJ, Ballesteros G, Tellez Y, Soda KJ, Sahoo MK, Nunez A, Balmaseda A, Harris E, Pinsky BA. 2016. Viremia and clinical presentation in Nicaraguan patients infected with Zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 63:1584–1590. 10.1093/cid/ciw589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. 2010. Chikungunya virus arthritis in adult wild-type mice. J Virol 84:8021–8032. 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teo TH, Lum FM, Claser C, Lulla V, Lulla A, Merits A, Renia L, Ng LF. 2013. A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J Immunol 190:259–269. 10.4049/jimmunol.1202177. [DOI] [PubMed] [Google Scholar]
- 34.Lum FM, Teo TH, Lee WW, Kam YW, Renia L, Ng LF. 2013. An essential role of antibodies in the control of Chikungunya virus infection. J Immunol 190:6295–6302. 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudd PA, Wilson J, Gardner J, Larcher T, Babarit C, Le TT, Anraku I, Kumagai Y, Loo YM, Gale M, Jr, Akira S, Khromykh AA, Suhrbier A. 2012. Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock. J Virol 86:9888–9898. 10.1128/JVI.00956-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. 2010. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest 120:894–906. 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venugopalan A, Ghorpade RP, Chopra A. 2014. Cytokines in acute chikungunya. PLoS One 9:e111305. 10.1371/journal.pone.0111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alsharifi M, Lobigs M, Simon MM, Kersten A, Muller K, Koskinen A, Lee E, Mullbacher A. 2006. NK cell-mediated immunopathology during an acute viral infection of the CNS. Eur J Immunol 36:887–896. 10.1002/eji.200535342. [DOI] [PubMed] [Google Scholar]
- 39.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. 2012. Gene profiling of Chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum 64:3553–3563. 10.1002/art.34631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bundschuh DS, Barsig J, Hartung T, Randow F, Docke WD, Volk HD, Wendel A. 1997. Granulocyte-macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol 158:2862–2871. [PubMed] [Google Scholar]
- 41.Kumar S, Jaffar-Bandjee MC, Giry C, Connen de Kerillis L, Merits A, Gasque P, Hoarau JJ. 2012. Mouse macrophage innate immune response to Chikungunya virus infection. Virol J 9:313. 10.1186/1743-422X-9-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng LF, Chow A, Sun YJ, Kwek DJ, Lim PL, Dimatatac F, Ng LC, Ooi EE, Choo KH, Her Z, Kourilsky P, Leo YS. 2009. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS One 4:e4261. 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Castro-Jorge LA, de Carvalho RVH, Klein TM, Hiroki CH, Lopes AH, Guimaraes RM, Fumagalli MJ, Floriano VG, Agostinho MR, Slhessarenko RD, Ramalho FS, Cunha TM, Cunha FQ, da Fonseca BAL, Zamboni DS. 2019. The NLRP3 inflammasome is involved with the pathogenesis of Mayaro virus. PLoS Pathog 15:e1007934. 10.1371/journal.ppat.1007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. 2007. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7:319–327. 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 45.Kuo TM, Hu CP, Chen YL, Hong MH, Jeng KS, Liang CC, Chen ML, Chang C. 2009. HBV replication is significantly reduced by IL-6. J Biomed Sci 16:41. 10.1186/1423-0127-16-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. 2019. The role of interleukin 6 during viral infections. Front Microbiol 10:1057. 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, Heise MT. 2011. A mouse model of chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am J Pathol 178:32–40. 10.1016/j.ajpath.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petitdemange C, Becquart P, Wauquier N, Beziat V, Debre P, Leroy EM, Vieillard V. 2011. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog 7:e1002268. 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thanapati S, Das R, Tripathy AS. 2015. Phenotypic and functional analyses of NK and NKT-like populations during the early stages of chikungunya infection. Front Microbiol 6:895. 10.3389/fmicb.2015.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teo TH, Her Z, Tan JJ, Lum FM, Lee WW, Chan YH, Ong RY, Kam YW, Leparc-Goffart I, Gallian P, Renia L, de Lamballerie X, Ng LF. 2015. Caribbean and La Reunion Chikungunya virus isolates differ in their capacity to induce proinflammatory Th1 and NK cell responses and acute joint pathology. J Virol 89:7955–7969. 10.1128/JVI.00909-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun JC, Lanier LL. 2018. Is there natural killer cell memory and can it be harnessed by vaccination? NK cell memory and immunization strategies against infectious diseases and cancer. Cold Spring Harb Perspect Biol 10:a029538. 10.1101/cshperspect.a029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haist KC, Burrack KS, Davenport BJ, Morrison TE. 2017. Inflammatory monocytes mediate control of acute alphavirus infection in mice. PLoS Pathog 13:e1006748. 10.1371/journal.ppat.1006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lidbury BA, Simeonovic C, Maxwell GE, Marshall ID, Hapel AJ. 2000. Macrophage-induced muscle pathology results in morbidity and mortality for Ross River virus-infected mice. J Infect Dis 181:27–34. 10.1086/315164. [DOI] [PubMed] [Google Scholar]
- 54.Lidbury BA, Rulli NE, Suhrbier A, Smith PN, McColl SR, Cunningham AL, Tarkowski A, van Rooijen N, Fraser RJ, Mahalingam S. 2008. Macrophage-derived proinflammatory factors contribute to the development of arthritis and myositis after infection with an arthrogenic alphavirus. J Infect Dis 197:1585–1593. 10.1086/587841. [DOI] [PubMed] [Google Scholar]
- 55.Juarez D, Long KC, Aguilar P, Kochel TJ, Halsey ES. 2013. Assessment of plaque assay methods for alphaviruses. J Virol Methods 187:185–189. 10.1016/j.jviromet.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 56.Fumagalli MJ, de Souza WM, Esposito DLA, Silva A, Romeiro MF, Martinez EZ, da Fonseca BAL, Figueiredo LTM. 2018. Enzyme-linked immunosorbent assay using recombinant envelope protein 2 antigen for diagnosis of Chikungunya virus. Virol J 15:112. 10.1186/s12985-018-1028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fumagalli MJ, de Souza WM, Romeiro MF, de Souza Costa MC, Slhessarenko RD, Figueiredo LTM. 2019. Development of an enzyme-linked immunosorbent assay to detect antibodies targeting recombinant envelope protein 2 of Mayaro virus. J Clin Microbiol 57:e01892-18. 10.1128/JCM.01892-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bancroft JD, Stevens A. 1990. Theory and practice of histological techniques, 3rd ed Churchill Livingstone, Edinburgh, United Kingdom. [Google Scholar]
- 59.Ierna M, Kerr A, Scales H, Berge K, Griinari M. 2010. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet Disord 11:136. 10.1186/1471-2474-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]