Abstract
The role of heme oxygenase-1 in resisting oxidative stress and cell protection has always been a hot research topic. With the continuous deepening of research, in addition to directly regulating redox by catalyzing the degradation of heme, HO-1 protein also participates in the gene expression level in a great diversity of methods, thereby initiating cell defense. Particularly the non-canonical nuclear-localized HO-1 and HO-1 protein interactions play the role of a warrior against oxidative stress. Besides, HO-1 may be a promising marker for disease prediction and detection in many clinical trials. Especially for malignant diseases, there may be new advances in the treatment of HO-1 by regulating abnormal ROS and metabolic signaling. The purpose of this review is to systematically sort out and describe several aspects of research to facilitate further detailed mechanism research and clinical application promotion in the future.
Keywords: Heme oxygenase-1, Redox system regulation, Gene expression control, Protein-protein interaction, Disease diagnosis and treatment
Graphical abstract
Highlights
-
•
The different subcellular localizations ofHO-1 implies that it has special functions.
-
•
Nuclear HO-1 plays an indispensable role in gene regulation and other aspects.
-
•
The interactions between HO-1 and others provide the possibility to participate in vital physiological processes.
-
•
HO-1 may become a potential disease assessment marker.
1. Introduction
As early as the late 1960s, Tenhunen et al. found heme oxygenase in the microsomes of rat spleen, kidney, liver, which was a rate-limiting enzyme that can catalyze the degradation of heme into biliverdin Ixα, carbon monoxide (CO), and iron [1,2]. The HO system, along with its catabolism products, is involved in a good supply of crucial physiological functions in the cell. In addition to playing the role of anti-oxidative stress, heme oxygenase also has anti-inflammatory and anti-apoptosis effects [3]. Three heme oxygenase isoforms have been defined: HO-1, HO-2, and HO-3 are HSP32 protein cognates [4]. Among them, HO-1 and HO-2 share 43% amino acid sequence homology in mammalian cells [5]. HO-1, an inducible 32-kDa protein that is up-regulated in response to oxidative stress [6], while HO-2 is a constitutively expressed 36-kDa protein at a high level in some cells and is hardly inducible [7,8]. A third isoform, HO-3, is still considered a pseudogene of the HO-2 gene [9]. The expression of inducible HO-1 can be stimulated by many conditions. The molecular mechanism regulating the HMOX1 gene will also be described and summarized in this article. It also helps to understand the multiple possible ways HO-1 can induce expression under diversified stimuli.
Human HO-1 protein is composed of 288 amino acids, and no signal peptide region has been found in its protein structure, but a hydrophobic fragment containing 22 amino acid residues at the C-terminus is seen as a sequence anchoring endoplasmic reticulum (ER) [10]. HO-1 generally only appears in the ER components. In the face of various stimuli, HO-1 is rapidly induced, and can also be observed in other cellular components [11]. Recently, numerous pieces of evidence indicate that HO-1 may have great physiological functions, which is not related to its enzymatic activity. We started using “non-canonical functions” to describe HO-1 effects in addition to being recognized by the public [12]. Among them, the two most particular aspects are the nuclear localization of HO-1 and the protein-protein interaction. There is a partial overlap in the mechanism of these two parts. In other words, HO-1 not only plays a role through its catalysis of heme and degradation by-products but also participates in antioxidant stress and cell protection through its “non-canonical” functions.
In this paper, we mainly focus that HO-1 is involved in cellular redox regulation through its canonical functions and non-canonical functions. Mostly including the nuclear localization of HO-1, protein-protein interaction, and potential biomarkers of diseases. The possible relationship between these mechanisms and diseases may provide a new potential direction for the treatment, especially tumor disease.
2. Molecular mechanism of HMOX1 regulation
The HO-1 gene (HMOX1) is usually activated under a wide range of stress conditions. The transcriptional control of HMOX1 is determined by inducible regulatory elements located in the 5′ end of the promoter [13]. Under different stimuli, the redox-sensitive transcription factor binds to the stress response element upstream of the promoter and induces HMOX1 expression.
The classic regulation of HMOX1 is mainly the combination of transcription factors and corresponding response elements upstream of the promoter to initiate the large-scale expression of HMOX1. The most important of these is the Nrf2-ARE–HO–1 axis. Under normal circumstances, nuclear factor–erythroid 2–related factor 2 (Nrf2) and its repressor Kelch-like ECH-associated protein 1 (Keap1) combine to form a complex, and the activity of Nrf2 is inhibited and it remains in the cytoplasm. However, when the cell is stimulated by some electrophiles and ROS, it will change the protein conformation of Keap1 and disrupt the Nrf2-Keap1 interaction, thereby releasing Nrf2 and transferring it into the nucleus [14]. Nrf2 binds to small Maf protein forming a heterodimer, and then this dimer can bind to the antioxidant response element (ARE) or Maf recognition elements (MAREs), which sequences are present at the HO-1 promoter [15]. In addition, there is a transcription repressor Bach1 that competes with the small Maf protein to bind and form a heterodimer. This dimer also binds to MARE, thereby inhibiting HO-1 transcription [16]. The activator protein 1 (AP-1) transcription factor as a dimer of Jun and Fos family proteins, bind to enhancers flanking the promoter region of HMOX1 [17]. The induction of HMOX1 expression requires AP-1 activation to respond to some oxidative inducers to initiate cell protection and defense. Of course, in addition to transcription factors, multiple signaling pathways including AKT, MAPK, and STAT3 can all participate in regulating the expression of HMOX1 [18].
In recent years, the regulation of microRNA on HMOX1 has attracted more and more attention. MicroRNA (miRNA), a type of small (about 22 nucleotides) non-coding RNA that can directly bind to the 3′-untranslated region (3′-UTR) of the target, and can be sensitively controlled by mRNA degradation or translation inhibition gene expression [19]. It can be concluded from the existing studies that the gene regulation effect of miRNA on HMOX1 is mainly divided into two ways: direct and indirect. The indirect effect is mostly through targeting HMOX1-related transcription factors (Nrf2/Bach1, etc.), thereby indirectly regulating the expression of HMOX1. For example, miR-143 targets Bach1 to participate in the regulation of HMOX1 expression [20], while miR-140–5p and miR-93 can directly target Nrf2 [21,22]. When miRNA directly targets HMOX1 mRNA, it directly regulates the expression of HMOX1. Some miRNAs participate in regulation through direct and indirect effects at the same time. miR-1254 directly targets HMOX1 3′-UTR through its seed region, and inhibits HMOX1 transcription by the expression of HO-1's transcription activator, transcription factor AP-2α (TFAP2A), through its non-seed region [23]. All in all, the expression of HMOX1 can be involved in multiple ways to ensure the surefire regulation of gene expression in cells, which also increases the difficulty of exploring the occurrence and development of diseases and the prognosis of treatment.
3. Subcellular localization of HO-1
The HO-1 protein was initially identified as being present in the endoplasmic reticulum, and the carboxy-terminal 22 bases in the protein structure were called the endoplasmic reticulum-anchored sequence. HO-1 protein is fixed on the endoplasmic reticulum membrane through this section of TMS, and most of the remaining protein structure faces the cytoplasm, which is convenient for binding to heme. With the further progress of research, more special subcellular locations of HO-1 are gradually revealed [12]. The earliest scientists discovered that HO-1 binds to caveolin (CAV) and locates in the caveolae [10,24]. The caveolae are one of the specialized microdomains on the plasma membrane. The signal protein is located in these lipid-rich areas and is used for vesicle transport and rapid induction of signal cascades in response to external stimuli. CAV is a membrane protein that mainly exists in the caveolae to form a scaffold, where the signal molecules can be assembled to promote rapid cell signal response [25]. Although HO-1 protein binds to caveolin-1 (CAV-1), the enzyme activity is negatively regulated, but this provides a possible basis for the active extracellular transfer of HO-1 protein.
Converso DP et al. demonstrated for the first time that HO-1 protein is localized and expressed in mitochondria and is accompanied by HO activity [26,27]. In another research work, chemical or physiological hypoxia can induce an increase in HO-1 expression and significant translocation to mitochondria. At the same time, deleting the N-terminal ER-targeted domain of HO-1 protein also evidently increased mitochondrial translocation [27]. And surprisingly, the mitochondrial-targeted HO-1 protein is contrary to the well-known cytoprotective effect of ER-associated HO-1, which leads to the loss of heme aa-3 and cytochrome c oxidase (CcO) activity, causing mitochondrial dysfunction and higher levels of ROS.
What attracts attention more is the nuclear transfer of HO-1 protein. In the rat astrocytes [28] and brown adipocytes [29], after stimulation, HO-1 protein appeared the nuclear localization. After hypoxia exposure in neonatal mouse lung tissue [30] and a variety of human malignant tumor cells [5,31], the highly expressed nuclear HO-1 plays a particular role that cannot be ignored. It can be concluded that after the cell is subjected to certain stress stimulation, the carboxyl end of the protein will be cut and removed, and a large number of free truncated proteins appear in the cytoplasm and further translocate into the nucleus. By regulating the level of cell genes in the nucleus, it can resist redox stimulation and protect cells from the threat of death or disease [31]. Due to the peculiar mechanism and function of nuclear HO-1, it will be described separately in detail later.
4. Particular modifications in HO-1 enzyme activity
The biological activity of HO-1 mainly focuses on its enzymatic activity. It is the first rate-limiting enzyme that degrades heme into free iron, carbon monoxide (CO), and biliverdin. CO, under physiological and pathological conditions, plays a role in cell protection and signal transduction, including cell apoptosis, cell proliferation, inflammation, and immune regulation [32,33]. Free iron, which is necessary for the synthesis of ferritin and hemoglobin, plays a role in cell protection [34]. Biliverdin is converted into bilirubin under the action of biliverdin reductase (BVR). Excessively high concentration of bilirubin has great cytotoxicity, but at physiological levels, bilirubin is a good antioxidant [35,36], which is crucial in the pathogenesis and development of cardiovascular diseases [37]. Therefore, HO-1 may play a protective role through its metabolites. It is worth noting that HO-1 enzyme activity has been found in many studies to be affected by certain factors, such as HO-1 integrity. It has been suggested that the truncated HO-1 protein completely loses its activity and thus plays an exceptional role through other mechanisms [31]. However, some experiments have found that HO-1 with C-terminal deletion still has partial enzyme activity rather than complete inactivation [38]. The specific compartmentalization of HO-1 also affects enzyme activity. After being treated with various stimulations (heme, LPS, hypoxia), not only does the HO-1 expression level in the cell increase sharply but also HO-1 was localized in mitochondria [27], caveolin-containing caveolae [24,39], and nuclear components [12]. As mentioned earlier, when HO-1 protein appears in mitochondria and caveolae, it often co-localizes with biliverdin reductase (BVR) and cytochrome P450 reductase (CPR), indicating that its major function may still be enzymatic activity. However, the interaction with CAV-1 makes the activity of HO-1 negatively regulated, and HO-1 in the mitochondria even induces more ROS production. The nuclear-localized HO-1 protein, that is, truncated HO-1, has various changes in its enzymatic activity. But it may also play a more vital role without relying on activity. It shows that the HO-1 protein of different subcellular localization has varying degrees of influence on its cytoprotective function.
5. Nuclear transfer HO-1 participates in gene expression regulation, independent of enzyme activity
Regarding the HO-1 subcellular localization that changes when encountering different stimuli, the nuclear localization shows a more vital function. Plenties of experiments have demonstrated that the HO-1 located in the nucleus is an incomplete and truncated form, and lacks its carboxy-terminal amino acid sequence. Previously, the HO-1 protein in the nucleus after hypoxia was purified and identified. It was pointed out that nuclear HO-1 proteins were truncated and the molecular weight was nicely equivalent to the initial 237 amino acids of the rat HO-1 protein [31]. T Yoshida et al. found that the membrane-anchored end of HO-1 was cleaved from the membrane by a low concentration of trypsin to form a 28 kDa fragment [40]. Also, after being treated with cysteine inhibitor E64d, the nuclear HO-1 was significantly reduced, indicating that the 28 kDa HO-1 protein was obtained by cleavage of the full-length HO-1 protein [31]. Analogously, Boname JM et al. confirm that HO-1 is the direct substrate of SPP [41]. Then, under certain specific stimuli, does the expression and functional activity of SPP change and affect the hydrolysis of HO-1? In HEK293 cells, under hypoxia conditions or when exogenously overexpressed SPP, HO-1 was hydrolyzed and translocated. And it was confirmed that the sites of cleavage of HO-1 by SPP were S275 and F276 [42]. Is the truncated HO-1 completely degraded after SPP cutting, or does it have other effects? These all need to be continuously studied. Moreover, when CPR promotes HO-1 oligomerization to form higher-order complexes, it can prevent the trypsin cleavage and nuclear translocation of HO-1 under hypoxia. It is speculated that the HO-1 binding protein may cover its specific cleavage sites [43].
When the molecular weight of the protein is less than 40 kDa, it can be transferred by diffusion, while active transfer into and out of the nucleus requires the mediation of specific signals, including nuclear localization signal (NLS) and nuclear export signal (NES) [44]. For the majority of nuclear transfer proteins, nuclear localization sequence is crucial, but unfortunately, there is no evidence of a definite NLS in HO-1 currently. However, there is a highly conserved leucine-rich region in the rat HO-1 protein, located at amino acid 207–221, referred to as nuclear shutting sequence (NSS) [31]. A recent study has suggested that several structural sequences at the carboxyl terminus of HO-1 protein are necessary for nuclear transfer, including the PEST domain (Rich in four amino acids, Pro-Glu-Ser-Thr) and NSS [45]. Besides, the cellular localization of alternative splicing of the HMOX1 gene, a 14 kDa form, was different from full-length HO-1 under UV irradiation [46]. So far, the specific mechanism of truncated HO-1 protein transfer into the nucleus remains to be verified by further studies.
The HO-1 truncation and nuclear translocation initiated spontaneously when encountering pathological conditions or external stimuli must play a specific role. In a rat model of spinal cord injury (SCI), the function of the damaged spinal cord was significantly recovered by intrathecal injection of adenovirus vector carrying nuclear HO-1 (Ad-GFP–HO–1CΔ23) into the tenth thoracic vertebra (T10) [47]. It has been confirmed in other experiments that nuclear localization of HO-1 regulates the DNA binding activity of key transcription factors and activates the expression of significant genes in oxidative stress response. Include activating AP-1 [31] and HMOX1 its promoter [48]. However, the mechanism by which nuclear HO-1 enhances the DNA binding activity of transcription factors has not been studied clearly. It was noteworthy that both enzymatically active and inactive HO-1 proteins activated the transcription factors, including AP-1, Brn-3, and CBF [31]. It suggests that the HO-1 protein itself may play a role in cellular signaling,that nuclear localization of HO-1 may be a part of an important signaling pathway to protect cells from oxidative stress.
In the work of Elguero B et al. [49], the association between HO-1 protein and promoter was demonstrated by anti–HO–1 CHIP-qPCR for the first time. HO-1 was significantly enriched at the μPA, MMP9, and PSA proximal promoter regions in testosterone-stimulated LNCaP cells. However, the DNA binding motif of typical transcription factors has not been detected in the HO-1 protein structure, and in other studies, direct recruitment of HO-1 on DNA has not been found [48]. Therefore, nuclear HO-1 is unlikely to regulate the expression level of genes by directly binding them to DNA. Of course, there is further speculation that HO-1 might serve as a transcriptional co-regulator protein to regulate the DNA-binding activities of crucial transcription factors, thereby influencing the expression levels of the corresponding gene. A potential mechanism could involve the binding of HO-1 to a transcription factor or a complex, serving as the non-DNA-binding form, which increases the DNA-binding affinity of transcriptional factors while making no direct contact with the DNA. This hypothetical mechanism has received the support of many experiments. Using H25A mutant HO-1, which has no enzyme activity, increased cell resistance to H2O2 through the up-regulating the expression of catalase and the content of reduced glutathione, and thereby protected cells from oxidative stress-induced by hydrogen peroxide [50]. As previously confirmed, Oxidative stress induced the nuclear localization of 28 kDa HO-1 [51]. This form of nuclear HO-1 protected Nrf2 from GSK3β-mediated proteolytic degradation, stabilizing intracellular Nrf2 protein levels to regulate the transcription of specific downstream antioxidants and metabolic genes [51]. In general, the anti-stress function of nuclear HO-1 may be achieved by regulating the expression of crucial genes in processes such as anti-oxidative stress and oxidative DNA damage repair. Otherwise, this ability to resist oxidative stress does not require the presence of its enzymatic activity. Similarly, in yeast, a simple eukaryotic cell model, the cellular antioxidant protection of the HO-1 homolog Hmx1p also does not require oxygenase activity [52]. The HO-1 yeast homolog mediates resistance to oxidative stress through an adaptive response involving the transcriptional control of antioxidant genes.
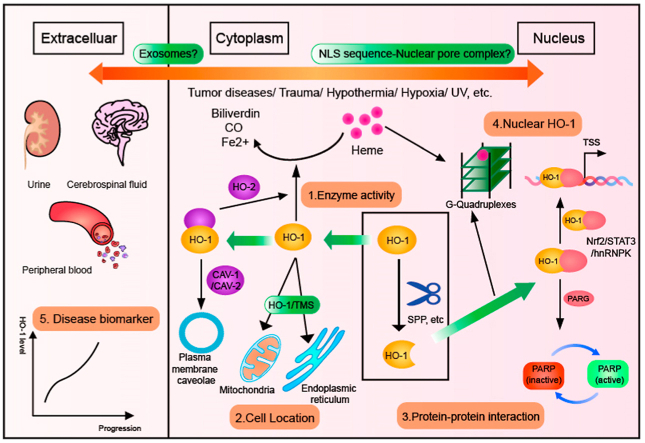
The above possible mechanisms are summarized as followed: the truncated HO-1 is transferred into the nucleus and plays a protective role by regulating the expression level of key genes or participating in the signaling pathway, independent of HO activity (Fig. 1).
Fig. 1.
The nuclear location of HO-1
Under scores of stress stimuli, heme oxygenase-1 initiates certain protein cleavage mechanisms in cells, hydrolyzes, and removes the carboxy-terminal amino acid sequence to form a truncated protein form. The truncated HO-1 dissociates in the cytoplasm and then is transported into the cell under some vital mechanisms. What follows next, nuclear HO-1 responds to the initial emergency stimulus by regulating the expression level of the target gene. That contains a variety of potential new regulatory mechanisms.
6. The heme-binding ability of nuclear HO-1 and the G-quadruplexes
As mentioned earlier, whether nuclear HO-1 has enzyme activity remains a controversial topic [12]. However, the heme-binding capacity retained by nuclear HO-1 may also contribute to the regulation of heme-dependent gene expression in cells [53], which are regulated by the heme response element (HRE) and the mammalian transcriptional repressor Bach1. Studies have found that there is a subtle correlation between heme, HO-1, and G quadruplexes. G-quadruplexes (G4) are non-canonical structures (non-B form) of DNA, formed by guanine-rich sequences and comprise two or more self-stacking G-quartets (made by four guanines held in a plane by Hoogsteen hydrogen bonding) [54]. The G4 structures are thought to affect DNA replication, gene transcription, and translation. In addition, the presence of G4 may cause replication fork stagnation during DNA synthesis resulting in replication stress, a major source of genome instability. One of the G4-stabilizing ligands is heme, a ubiquitous cellular cofactor. Heme binding to G4 can stabilize G4 maintain normal levels of free heme in the nucleus [53]. But numerous G4-heme binding can lead to increased oxidative stress on the DNA and G4 toxicity [55]. When the high-affinity ligand of G4 was used to compete with heme for G4 binding, the isolated heme in the nucleus was released and the expression of downstream target genes was regulated [53]. Alternatively, nuclear HO-1 does not catalyze the degradation of heme, but only binds to heme, thereby isolating heme from G4. It may also cause the stability of G4 to decrease and analyze, and the expression of heme-dependent genes may be affected. Even more remarkably, recent research results confirmed that HO-1 and G4 were co-localized (<40 nm) in the nucleus by a proximity ligation assay (PLA), although this did not indicate a direct binding relationship between the two [55]. What's more, nuclear-localized HO-1 can promote G4 removal. One possibility is that the nuclear HO-1 removes excess heme locally, thereby more effectively destabilizes G4. Another probability is that HO-1 is a part of the G4-resolving complex or facilitates the formation of such a complex. Taken together, this potential mechanism between HO-1 and G4 enriches the function of HO-1 in the nucleus.
7. The protein-protein interaction of HO-1 protein
The protein-protein interaction is a considerable non-canonical function of HO-1 [11]. In recent years, with the development of various technical methods, many HO-1-binding proteins have been detected. The enzymatic proteins involved in important physiological processes and the signal molecules involved in the transmission of intracellular and extracellular signals are the main members. By studying the functions of HO-1-binding proteins and the signal pathways they may be involved in, the new unknown mechanism of HO-1 was predicted and explored.
Multiple experiments previously have confirmed that HO-1 protein does have a binding relationship with a variety of enzyme proteins. Through this protein binding, the localization of HO-1 in the cell and the activity of the enzyme protein are affected, which influences the normal catalytic process. First, both intracellular and extracellular experiments confirmed that HO-1 protein can self-assemble to form oligomers. And in this assembly, the TMS region in the protein structure is necessary [56]. Moreover, this combination of the two TMS regions may affect the interaction between HO-1 protein and other proteins at the same site, such as SPP [41,42]. Except for the HO-1 protein itself, it also has a direct binding relationship with its isoform HO-2 [57]. More strikingly, the relative loss of HO activity was detected when HO-1 and HO-2 were brought together to form a complex. Despite the protective effect of HO-1 at a certain intracellular level, under various stimuli, the HO-1 whose expression level increases rapidly can reverse the protective effect [58]. Therefore, when the cells or tissues co-located with two HO isoforms rapidly induced HO-1 overexpression in disease states, HO-1-HO-2 complex may be formed to keep HO activity within the safe threshold.
Poly (ADP-ribose) polymerase (PARP) is one of the candidated binding partners of nuclear HO-1 detected in vitro [30], which is the major source of poly (ADP-ribose) produced during the cellular response to DNA damage. When cells are subjected to a variety of stresses, they may cause serious damage to the nuclear DNA. Once PARP detects DNA single-strand breaks and then will bind to DNA and begin to synthesize poly (ADP-ribose) chain, which is catalyzed by poly (ADP-ribose) glycohydrolase (PARG) for degradation [59,60]. It is a clear binding between HO-1 and PARG, which may limit PARG-mediated PAR hydrolysis and lead to PAR accumulation [61]. As a result, HO-1 can play a role in PARP-dependent cell death [62]. Owing to PARG's role in DNA damage repair, the authors hypothesized that reduced PARG activity resulted in persistent DNA damage that led to the phenotype of emphysema in the lungs. It was further confirmed that nuclear HO-1 inhibited the repair of lung tissue after hyperoxia injury by inhibiting DNA repair mediated by nuclear HO-1 binding to PARG in alveolar type II cells. Therefore, the binding of HO-1 with other proteins may affect the intracellular distribution and enzyme activity, including the catalytic activity of HO-1 itself and the binding proteins. As previously mentioned, HO-1 localization also affects its enzyme activity.
Recently, substantial studies have found that the HO-1 protein is related to some well-known signal molecules. That implies that HO-1 may become a direct or indirect signal molecule through protein-protein binding, which participates in important signal cascades and regulates various physiological processes in the body.
-
(1)
Wnt/β-Catenin signaling pathway
According to previous studies, hnRNPK interacted with β-catenin [63]. Yang, G., et al. found that HO-1 and hnRNPK can be co-precipitated by immunoprecipitation, but the direct relationship between HO-1 and β-catenin was not detected [30]. However, HO-1 may be involved in the β-catenin signaling pathway by bridging with hnRNPK. In mouse lung tissues, nuclear HO-1 and hnRNPK levels are significantly correlated, supporting the hypothesis that HO-1 is vital to retain hnRNPK in the nucleus. Therefore, enhanced retention of nuclear hnRNPK resulted in an increase in cell cycle-related gene transcription and a decrease in global protein levels [30], which is consistent with the previous conclusion that the cytoplasmic function of hnRNPK was to inhibit protein translation [64]. Thus, the role that the HO-1 protein plays in the Wnt/β-catenin signaling pathway deserves continuous exploration.
-
(2)
Antiviral–IFN–β production
The specific binding of HO-1 to IRF3 was observed, and the combination of HO-1 and IRF3 promoted its phosphorylation and affected the subsequent nuclear translocation of IRF3. In the absence of HO-1, the binding forms of IRF3 (IRF3/P300/CBP coactivators; (IRF-3) 2) were significantly reduced [65]. IRF3 mainly exists in two forms: a monomer, which freely shuttles between cytoplasm and nucleus, with nuclear export as the major form; and a dimer form accumulates in the nucleus due to its C-terminal phosphorylation [66]. Therefore, it can be explained that when the interaction between HO-1 and IRF-3 is lost, IRF-3 mainly exists in the form of a monomer, which leads to a significant decrease in the nuclear accumulation of IRF3. The activation of the transcription factor IRF-3 is necessary for synergistic activation of the IFN-β promoter, which in turn stimulates substantial amounts of IFN-β production [67]. Therefore, the authors hypothesized that early activation of the TRIF-IRF3 and RIG-I-IRF3 pathways requires the involvement of HO-1. Through their specific interactions, it regulates the production of downstream antiviral cytokine IFN-β and the development of an adaptive immune response. It suggests that in addition to its anti-inflammatory effects through enzymatic activity, HO-1 also has other ways to regulate immune responses.
-
(3)
Keap1-Nrf2-ARE antioxidant stress pathway
The interaction between HO-1 protein and Nrf2 protein has been mentioned above. Previously, the association between HO-1 and Nrf2 was limited to the fact that Nrf2 is transferred into the nucleus and can regulate the expression of the antioxidant HO-1. Later, this knowledge was further updated. HO-1 also can regulate Nrf2 proteins by protecting them from GSK3β-mediated ubiquitin-proteasome degradation. It not only further enriches the correlation and regulatory relationship between the Nrf2/HO-1 axis but also helps us to explore the pathogenesis of more diseases.
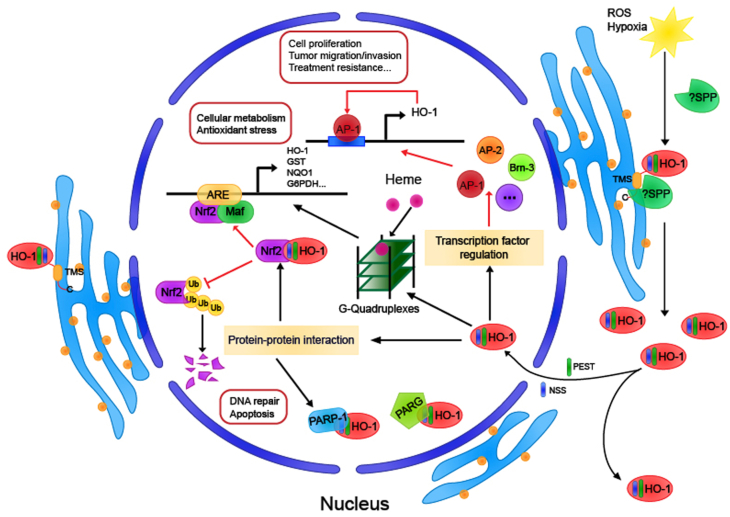
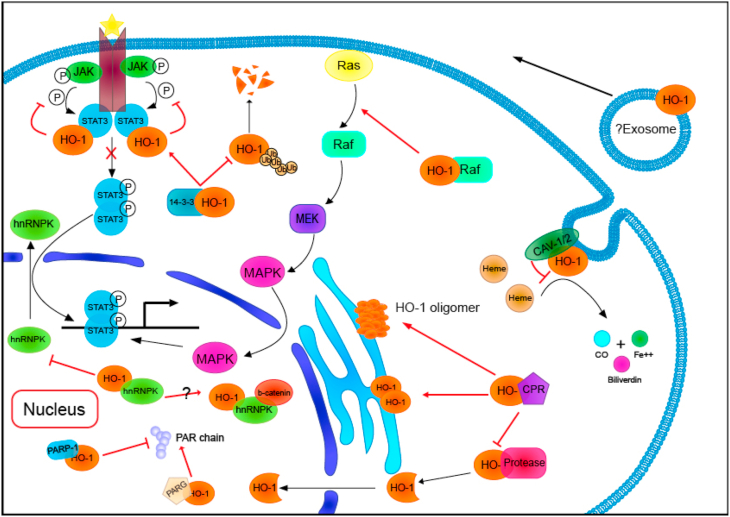
As is known for all, HO-1 protein is a kind of heat shock protein (HSP32). It was predicted by modeling and molecular docking software that HO-1 might be bound to CD91 [68], and the first proposed HSP receptor was the oxidized LDL binding protein CD91/LRP found in antigen-presenting cells and other cell types [69]. CD91 plays a significant role in antigen cross-presentation of HSP, suggesting that HO-1 may have a role in the regulation of immune response as a novel chaperone [70,71], but experimental data have not yet been supported. Potential HO-1 binding proteins, including poly (ADP-Ribose) polymerase-1, were obtained by LC/MS/MS analysis [30], indicating that HO-1 may enrich its effects by binding with proteins, independent of its activity (Fig. 2).
Fig. 2.
The protein-protein interaction of Heme oxygenase-1
Heme oxygenase-1 protein combines with a variety of proteins, not only determines the cellular location of HO-1 but also participates in a variety of intracellular signal transduction pathways. HO-1,heme oxygenase-1; CPR, cytochrome P450 reductase; PARP, poly (ADP-ribose) polymerase; PARG, poly (ADP-ribose) glycohydrolase; Ub, ubiquitin; CO, carbon monoxide; CAV, caveolin.
8. The association between HO-1 and abnormal oxidative stress in cancer diseases
Abnormally elevated levels of oxidative stress (ROS) are widely accepted as one of the hallmarks of cancer [72]. ROS is usually regarded as a toxic substance in cells, and when high ROS cannot be metabolized properly, cells will die or become cancerous. Tumor cells in the tumor microenvironment are overgrown, suggesting they are more resistant to oxidative stress or other harmful factors. Indeed, tumor cells have been found to reprogram their internal metabolization-redox circuits to proliferate and survive [73,74].
Heme oxygenase-1, a vital enzyme in anti-oxidative stress, is often observed to be significantly elevated in tumor diseases [7,75]. Moreover, recent evidence suggests that the “non-canonical” functions of HO-1 also play a non-negligible role in tumor disease. For example, the nuclear localization of the HO-1 protein is associated with the malignant progression of tumor cells independently of its enzymatic activity. In human head and neck squamous cell carcinoma (SCC) cells [5], compared with nonmalignant cells and normal cells, nuclear HO-1 was substantially increased. In addition, the nuclear localization of HO-1 was associated with a histological grade that poorly differentiated tumors showed more nuclear HO-1. Equally, this tumor-promoting effect of nuclear HO-1 also appeared in breast cancer cell lines [75]. It is demonstrated that it is the nuclear translocation of HO-1 that promoted the proliferation and migration/invasion of tumor cells rather than the enzyme activity. Nuclear HO-1 may also be additional post-translational modified, which can lead to the loss of HO activity, although this has not been defined yet [76]. P300/CBP mediated the acetylation of the nuclear t–HO–1 protein. The acetylated HO-1 protein can interact with JunD, enhance the transcriptional activation of AP-1, and promote the growth and migration/invasion of cancer cells.
Besides, the possible role of HO-1 in several classical tumor proliferation-related signaling pathways has also been explored. HO-1, which is highly expressed in melanoma cells, promotes the proliferation of tumor cells, at least partly through the binding of HO-1 with B-Raf. By activating the b-Raf/ERK pathway in melanoma, HO-1 protein increases the expression of cyclin E/CDK2 and promotes the cell cycle progression of melanoma [77]. In prostate cancer cells, HO-1 is directly related to the STAT3 signaling pathway through protein binding. HO-1 and STAT3 bind to each other, thus inhibiting the phosphorylation of STAT3 and subsequent nuclear translocation of pSTAT3 [49]. In other words, it leads to an increase in the cytoplasmic retention of pSTAT3, a significant decrease in the nuclear co-localization of AR and STAT3, leading to a decrease in the expression level of STAT3-targeted genes. These significantly reduced expression genes illustrate that HO-1 was a negative regulator of STAT3 and reduced STAT3 trans-activation in LNCaP cells. That is to say, the STAT3 signaling pathway of prostate cancer cells with high expression of HO-1 was inhibited, and the downstream target genes were reduced. It is also confirmed the conclusion that HO-1 in PCa cells has an anti-tumor effect previously reported [78,79].
However, the role of HO-1 in cancer cells is cell-specific. The up-regulation of HO-1 was associated with cell cycle arrest and death in some malignant tumors, while in other malignant tumors, HO-1 overexpression may be related to tumor progression and survival. For example, in hepatocellular carcinoma, enhanced HO-1 expression promotes the rapid growth and malignant progression of the tumor. In HCC cells, 14-3-3ζ can inhibit its ubiquitination and subsequent proteasome degradation by binding to HO-1 protein, stabilize the HO-1 protein and maintain a high intracellular level [80]. Previous studies have discovered that the activation of transcription factors, including STAT3, increased after overexpression of HO-1 [31]. STAT3 in HCC cells often fails to show negative feedback inhibition in HCC cells and is in a state of continuous activation. Additional detection of the STAT3 pathway showed that the 14-3-3ζ/HO-1 axis promoted STAT3 activation and nuclear metastasis through highly expressed HO-1, thereby regulating the growth and malignant progression of liver cancer [80].
Past studies have shown that tumor cells have been found to reprogram their internal metabolization-redox circuits to proliferate and survive [73,74]. Indeed, one of the hallmarks of cancer cells is increased aerobic glycolysis (Warburg effect). The available evidence indicated that a preferential induction of NQO1 and glucose-6-phosphate dehydrogenase (G6PDH) by Nrf2-HO-1 interaction [51], which favors cell survival by shifting the metabolism to the hexose monophosphate pathway. Even scarier than endless growth, the complex and precise metabolism-redox circuitry in cancer cells may make them more resistant to physical radiotherapy [81]. Meanwhile, HO-1 has been shown to have an enormous impact on the chemotherapy of tumors. During the treatment of chronic myeloid leukemia cells with imatinib, HO-1 nuclear translocation has a protective effect on CML cells, and this protective effect has nothing to do with the by-products of HO-1 catalytic activity [82]. Therefore, nuclear HO-1 may be involved in the mechanism of imatinib resistance in BCR/ABL-positive cells. In the chemotherapy of multiple myeloma (MM), the nuclear translocation of HO-1 was also found to enhance the chemical resistance of bortezomib (BTZ) [83].
In summary, it is increasingly recognized that metabolic and signaling abnormalities in cancer cells limit the effectiveness of traditional tumor therapies. Therefore, treatment regimens targeting high ROS and oxidative stress may be more promising. As mentioned before, in many cases, it is the effect of nuclear HO-1 on the malignant progression and therapeutic tolerance of tumors that provides an additional clinical target for the treatment of malignant tumors.
9. Possible assessment markers for disease
In recent years, studies have found that the presence of HO-1 can be detected in extracellular components, including plasma [84], cerebrospinal fluid [85], and urine [86]. Meanwhile, more data remains us that the appearance of HO-1 protein extracellular localization is more likely to be through a mysterious secretion mechanism, rather than the result of cell necrosis and passive release [86,87]. It may be through the secretion of exosomes or other active mechanisms.
In many cases, the level of extracellular HO-1 is often associated with the severity, progression, and prognosis of the disease, suggesting its potential as a biological marker of disease. It means that HO-1 is conducive to the rapid and specific detection of the disease in the future. In pregnant women with preeclampsia (PE), their serum HO1 levels are increased compared with control normal pregnant women [88], and even the severity of PE is also related to the increase in serum HO-1 levels before and after delivery [89]. In a recent study, hypoxia up-regulated HO-1 expression could result in insufficient STAT3 phosphorylation and impaired JAK-STAT3 signal transduction, which may be the pathogenesis of PE [90]. In experiments using mice to construct an AKI model, the plasma and urine HO-1 levels in mice correspond to the HO-1 gene expression levels in the kidney, throughout the whole process of AKI (including induction, maintenance, and recovery phases). It implies that the HO-1 protein level in the blood and urine of AKI mice is an excellent biomarker of Hmox1 gene activity in the kidney. In addition, a 16 kDa HO-1 protein was detected in the fresh plasma and urine of AKI mice [86]. Although the site, mechanism, and function of HO-1 protein breakage remain to be studied.
Interestingly, accumulated evidence has demonstrated its protective capabilities for the cardiovascular system through its heme degradation activity and non-enzymatic activity [[91], [92], [93]]. It needs to draw attention to the HO-1 protein since peripheral artery disease (PAD) patients show low HO-1 plasma levels, unlike the elevated plasma levels of HO-1 seen in coronary artery disease [94,95]. In other words, HO-1 levels were inversely associated with PAD, and multivariate analysis showed that HO-1 could be an independent predictor of the presence or severity of PAD [96]. HO-1 combats the progression of atherosclerotic diseases since anti-inflammatory, anti-oxidant, and anti-thrombotic properties have been demonstrated [91,97]. The mechanism of nuclear HO-1's involvement in p53-dependent vascular endothelial cell senescence through its interaction with NPM1 has also been discovered [93]. As a result, reduced HO-1 plasma levels demonstrated impairment in the protective properties of HO-1 in PAD patients. Although there was no relationship between HO-1 plasma levels with the progressive stages of PAD [95], plasma HO-1 levels are still a potential therapeutic target and an indicator of the diagnosis and prognosis of PAD.
Furthermore, the level of HO-1 in plasma can also be used as an effective indicator of drug toxicity [98]. Overdose of acetaminophen (APAP) can cause drug-induced hepatotoxicity and may lead to acute liver failure. However, in clinical practice, there is no specific indicator for monitoring drug-induced liver injury. In a study, by detecting the plasma of 6h and 24h after APAP treatment, it was found that plasma HMOX1 showed a sharp increase (about 150 times) [98]. It means that liver injury was detected with a 100% sensitivity to plasma HMOX1. Cross-species analysis of HMOX1 in mice with acute liver injury confirmed that the increase of this protein in plasma was closely related to the severity of liver toxicity and histopathological changes, which suggests that HMOX1 is a potential plasma biomarker of liver injury.
Given the function of HO-1 in tumor diseases, the HO-1 level in extracellular components is also a key research direction for indicating the occurrence and development of tumors. For example, chronic myelogenous leukemia [99]. Studies have found that the level of HO-1 mRNA in the peripheral blood of CML patients is significantly higher than that of healthy people, and there is an apparent linear relationship between the expression of HO-1 and the ratio of original CML cells. This close relationship between the level of HO-1 and the recurrence and progression of CML may make HO-1 protein in peripheral blood a new predictive molecular marker for monitoring the condition of chronic myeloid leukemia (CML).
In addition to the extracellular HO-1 that potentially becomes a disease detection indicator, in recent years, more and more studies have been conducted on the relationship between HMOX1 gene promoter polymorphism and disease. Existing studies have found that there are functional polymorphisms in the promoter region of the HO-1 gene: (1) T (−413) A single nucleotide polymorphism (SNP); (2) guanidine thymidine dinucleotide ([GT]n) repeat polymorphism [100]. Studies on T (−413) A SNPs in the HO-1 promoter region are relatively few and often have conflicting conclusions, while (GT)n polymorphism has been widely studied. The length of (GT)n repeats typically varies between 12 and 45 repeats, presenting a bimodal or trimodal distribution, among which 23 and 30 repeats are the most common. Specifically, shorter (GT)n repeat lengths produce higher basal promoter activity and higher promoter inducibility in response to various stimuli, including oxidative stress [101,102]. However, some studies have shown that these polymorphisms failed to detect any obvious differences in the transcriptional activity of the HO-1 gene, which may be due to the role of other regulatory elements upstream of transcriptional initiation site (TSS) or 5' -untranslated region (UTR) [103]. In continuous research in recent years, the polymorphism of the HO-1 gene promoter has been found to have a certain correlation with a growing number of disease states. In a deal of past clinical work, the association between (GT)n repeat length polymorphism and the risk of various diseases has been reported, including respiratory system diseases [104], malignant tumor [105,106], and others. The epidemiological correlation may be used as a risk index to evaluate the susceptibility and prognosis of the disease, and also be beneficial to the study of disease pathogenesis and potential therapeutic targets. It is worth noting that there are racial differences in the distribution of HO-1 (GT)n repeat length that will affect the judgment, and this should be taken into account when summarizing the experimental data [107,108]. Unfortunately, up to now, the exact molecular mechanism that explains the modulation of HO-1 promoter activity by the (GT)n repeats is unclear. However, this may have no impact that the promoter polymorphism of HO-1 becomes a promising target for disease prevention and treatment in the future.
10. Conclusions and future perspectives
In summary, these years of data show that HO-1 is involved in resisting oxidative stress, and maintaining cell homeostasis is not only dependent on its enzyme activity and degradation products but also includes more unknown mechanisms. In this review, we briefly discuss the following perspectives: (1)HMOX1 gene expression regulation, subcellular localization, and basic functions; (2) The possible mechanism of nuclear-localized HO-1 protein involved in cell protection; (3) HO-1 connects to importance intracellular physiological processes and signaling pathways through protein-protein interactions; (4) The bright prospects of HO-1 in tumor oxidative stress therapy; (5) As a potential detection indicator, HO-1 is associated with disease status to some extent. Strictly speaking, these aspects are often intersected and interrelated. For example, nuclear HO-1 in human lung cancer tissue is acetylated by p300/CBP, which can bind with JunD to enhance AP-1 transcriptional activation, thereby promoting the malignant progression of cancer cells in vivo and in vitro [76]. These are consistent with the clinical findings of differences in the HO-1 acetylation modification between normal lung tissue and lung cancer tissue. At the same time, because this acetylation modification can be removed by classic histone deacetylase (HDAC), acetylation of HO-1 in the tumor cell nucleus may become a screening indicator of whether HDAC inhibitors can be used for anti-cancer therapy [109].
Of course, there are still many questions about the mechanisms of HO-1 in the above aspects, and there are still enormous vacancies for research. Especially in tumor diseases, HO-1 protein may not only play an opposite role in different tumors but also participate in the survival and progression of tumor cells through multiple pathways in the same cell [110]. Understanding the specific functions of HO-1 and identifying the underlying mechanisms by which they affect disease generation or treatment could help to produce targeted therapeutic tools, which is one of the clinical intervention measures with broad prospects for oxidative damage, disease monitoring, and cancer treatment in the future.
Declaration of competing interest
None.
Acknowledgments
This work was supported in part by Sub-project of National Science and Technology Major Project (2018YFA0108603) and National Natural Science Foundation of China (81671255).
References
- 1.Tenhunen R., Marver H.S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. U. S. A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johmura Y., Yamanaka T., Omori S., Wang T.W., Sugiura Y., Matsumoto M., Suzuki N., Kumamoto S., Yamaguchi K., Hatakeyama S., Takami T., Yamaguchi R., Shimizu E., Ikeda K., Okahashi N., Mikawa R., Suematsu M., Arita M., Sugimoto M., Nakayama K.I., Furukawa Y., Imoto S., Nakanishi M. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science. 2021;371:265–270. doi: 10.1126/science.abb5916. [DOI] [PubMed] [Google Scholar]
- 3.Alam J., Igarashi K., Immenschuh S., Shibahara S., Tyrrell R.M. Regulation of heme oxygenase-1 gene transcription: recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase. Antioxidants Redox Signal. 2004;6:924–933. doi: 10.1089/ars.2004.6.924. [DOI] [PubMed] [Google Scholar]
- 4.Cruse I., Maines M.D. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J. Biol. Chem. 1988;263:3348–3353. [PubMed] [Google Scholar]
- 5.Gandini N.A., Fermento M.E., Salomon D.G., Blasco J., Patel V., Gutkind J.S., Molinolo A.A., Facchinetti M.M., Curino A.C. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp. Mol. Pathol. 2012;93:237–245. doi: 10.1016/j.yexmp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Shibahara S. The heme oxygenase dilemma in cellular homeostasis: new insights for the feedback regulation of heme catabolism. Tohoku J. Exp. Med. 2003;200:167–186. doi: 10.1620/tjem.200.167. [DOI] [PubMed] [Google Scholar]
- 7.Loboda A., Jozkowicz A., Dulak J. HO-1/CO system in tumor growth, angiogenesis and metabolism - targeting HO-1 as an anti-tumor therapy. Vasc. Pharmacol. 2015;74:11–22. doi: 10.1016/j.vph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 8.He J.Z., Ho J.J., Gingerich S., Courtman D.W., Marsden P.A., Ward M.E. Enhanced translation of heme oxygenase-2 preserves human endothelial cell viability during hypoxia. J. Biol. Chem. 2010;285:9452–9461. doi: 10.1074/jbc.M109.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scapagnini G., D'Agata V., Calabrese V., Pascale A., Colombrita C., Alkon D., Cavallaro S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002;954:51–59. doi: 10.1016/s0006-8993(02)03338-3. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.P., Wang X., Galbiati F., Ryter S.W., Choi A.M. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. Faseb. J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 11.Vanella L., Barbagallo I., Tibullo D., Forte S., Zappala A., Li Volti G. The non-canonical functions of the heme oxygenases. Oncotarget. 2016;7:69075–69086. doi: 10.18632/oncotarget.11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn L.L., Midwinter R.G., Ni J., Hamid H.A., Parish C.R., Stocker R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxidants Redox Signal. 2014;20:1723–1742. doi: 10.1089/ars.2013.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prawan A., Kundu J.K., Surh Y.J. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxidants Redox Signal. 2005;7:1688–1703. doi: 10.1089/ars.2005.7.1688. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J.S., Surh Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224:171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa K., Sun J., Taketani S., Nakajima O., Nishitani C., Sassa S., Hayashi N., Yamamoto M., Shibahara S., Fujita H., Igarashi K. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam J., Cook J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharmaceut. Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 18.Funes S.C., Rios M., Fernández-Fierro A., Covián C., Bueno S.M., Riedel C.A., Mackern-Oberti J.P., Kalergis A.M. Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020;11:1467. doi: 10.3389/fimmu.2020.01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bushati N., Cohen S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 20.Tamgue O., Gcanga L., Ozturk M., Whitehead L., Pillay S., Jacobs R., Roy S., Schmeier S., Davids M., Medvedeva Y.A., Dheda K., Suzuki H., Brombacher F., Guler R. Differential targeting of c-maf, bach-1, and elmo-1 by microRNA-143 and microRNA-365 promotes the intracellular growth of Mycobacterium tuberculosis in alternatively IL-4/IL-13 activated macrophages. Front. Immunol. 2019;10:421. doi: 10.3389/fimmu.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L., Qi Y., Xu L., Tao X., Han X., Yin L., Peng J. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018;15:284–296. doi: 10.1016/j.redox.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P., Liang X., Lu Y., Zhao X., Liang J. MicroRNA-93 downregulation ameliorates cerebral ischemic injury through the Nrf2/HO-1 defense pathway. Neurochem. Res. 2016;41:2627–2635. doi: 10.1007/s11064-016-1975-0. [DOI] [PubMed] [Google Scholar]
- 23.Pu M., Li C., Qi X., Chen J., Wang Y., Gao L., Miao L., Ren J. MiR-1254 suppresses HO-1 expression through seed region-dependent silencing and non-seed interaction with TFAP2A transcript to attenuate NSCLC growth. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung N.H., Kim H.P., Kim B.R., Cha S.H., Kim G.A., Ha H., Na Y.E., Cha Y.N. Evidence for heme oxygenase-1 association with caveolin-1 and -2 in mouse mesangial cells. IUBMB Life. 2003;55:525–532. doi: 10.1080/15216540310001620968. [DOI] [PubMed] [Google Scholar]
- 25.Parton R.G., Tillu V.A., Collins B.M., Caveolae Curr. Biol. 2018;28:R402–r405. doi: 10.1016/j.cub.2017.11.075. [DOI] [PubMed] [Google Scholar]
- 26.Converso D.P., Taillé C., Carreras M.C., Jaitovich A., Poderoso J.J., Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. Faseb. J. 2006;20:1236–1238. doi: 10.1096/fj.05-4204fje. [DOI] [PubMed] [Google Scholar]
- 27.Bansal S., Biswas G., Avadhani N.G. Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity. Redox Biol. 2014;2:273–283. doi: 10.1016/j.redox.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Volti G., Ientile R., Abraham N.G., Vanella A., Cannavo G., Mazza F., Curro M., Raciti G., Avola R., Campisi A. Immunocytochemical localization and expression of heme oxygenase-1 in primary astroglial cell cultures during differentiation: effect of glutamate. Biochem. Biophys. Res. Commun. 2004;315:517–524. doi: 10.1016/j.bbrc.2004.01.090. [DOI] [PubMed] [Google Scholar]
- 29.Giordano A., Nisoli E., Tonello C., Cancello R., Carruba M.O., Cinti S. Expression and distribution of heme oxygenase-1 and -2 in rat brown adipose tissue: the modulatory role of the noradrenergic system. FEBS Lett. 2000;487:171–175. doi: 10.1016/s0014-5793(00)02217-1. [DOI] [PubMed] [Google Scholar]
- 30.Yang G., Biswasa C., Lin Q.S., La P., Namba F., Zhuang T., Muthu M., Dennery P.A. Heme oxygenase-1 regulates postnatal lung repair after hyperoxia: role of beta-catenin/hnRNPK signaling. Redox Biol. 2013;1:234–243. doi: 10.1016/j.redox.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Q., Weis S., Yang G., Weng Y.H., Helston R., Rish K., Smith A., Bordner J., Polte T., Gaunitz F., Dennery P.A. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 32.Chora A.A., Fontoura P., Cunha A., Pais T.F., Cardoso S., Ho P.P., Lee L.Y., Sobel R.A., Steinman L., Soares M.P. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Invest. 2007;117:438–447. doi: 10.1172/jci28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryter S.W., Choi A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 2016;167:7–34. doi: 10.1016/j.trsl.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozzelino R., Jeney V., Soares M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 35.Stocker R., Glazer A.N., Ames B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. U. S. A. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 37.Maruhashi T., Kihara Y., Higashi Y. Bilirubin and endothelial function. J. Atherosclerosis Thromb. 2019;26:688–696. doi: 10.5551/jat.RV17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuller D.J., Wilks A., Ortiz de Montellano P., Poulos T.L. Crystallization of recombinant human heme oxygenase-1. Protein Sci. 1998;7:1836–1838. doi: 10.1002/pro.5560070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taira J., Sugishima M., Kida Y., Oda E., Noguchi M., Higashimoto Y. Caveolin-1 is a competitive inhibitor of heme oxygenase-1 (HO-1) with heme: identification of a minimum sequence in caveolin-1 for binding to HO-1. Biochemistry. 2011;50:6824–6831. doi: 10.1021/bi200601t. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida T., Ishikawa K., Sato M. Degradation of heme by a soluble peptide of heme oxygenase obtained from rat liver microsomes by mild trypsinization. Eur. J. Biochem. 1991;199:729–733. doi: 10.1111/j.1432-1033.1991.tb16177.x. [DOI] [PubMed] [Google Scholar]
- 41.Boname J.M., Bloor S., Wandel M.P., Nathan J.A., Antrobus R., Dingwell K.S., Thurston T.L., Smith D.L., Smith J.C., Randow F., Lehner P.J. Cleavage by signal peptide peptidase is required for the degradation of selected tail-anchored proteins. J. Cell Biol. 2014;205:847–862. doi: 10.1083/jcb.201312009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu F.F., Yeh C.T., Sun Y.J., Chiang M.T., Lan W.M., Li F.A., Lee W.H., Chau L.Y. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene. 2015;34:2360–2370. doi: 10.1038/onc.2014.166. [DOI] [PubMed] [Google Scholar]
- 43.Linnenbaum M., Busker M., Kraehling J.R., Behrends S. Heme oxygenase isoforms differ in their subcellular trafficking during hypoxia and are differentially modulated by cytochrome P450 reductase. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoneda Y., Hieda M., Nagoshi E., Miyamoto Y. Nucleocytoplasmic protein transport and recycling of Ran. Cell Struct. Funct. 1999;24:425–433. doi: 10.1247/csf.24.425. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer B., Moriishi K., Behrends S. Insights into the mechanism of isoenzyme-specific signal peptide peptidase-mediated translocation of heme oxygenase. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bian C., Zhong M., Nisar M.F., Wu Y., Ouyang M., Bartsch J.W., Zhong J.L. A novel heme oxygenase-1 splice variant, 14kDa HO-1, promotes cell proliferation and increases relative telomere length. Biochem. Biophys. Res. Commun. 2018;500:429–434. doi: 10.1016/j.bbrc.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 47.Bi Y., Chen X., Cao Y., Yu D., Zhao J., Jing Y., Lv G. Nuclear heme oxidase-1 inhibits endoplasmic reticulum stress-mediated apoptosis after spinal cord injury. BioMed Res. Int. 2020;2020:7576063. doi: 10.1155/2020/7576063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Q.S., Weis S., Yang G., Zhuang T., Abate A., Dennery P.A. Catalytic inactive heme oxygenase-1 protein regulates its own expression in oxidative stress. Free Radic. Biol. Med. 2008;44:847–855. doi: 10.1016/j.freeradbiomed.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elguero B., Gueron G., Giudice J., Toscani M.A., de Luca P., Zalazar F., Coluccio-Leskow F., Meiss R., Navone N., de Siervi A., Vazquez E. Unveiling the association of STAT3 and HO-1 in prostate cancer: role beyond heme degradation. Neoplasia. 2012;14:1043–1056. doi: 10.1593/neo.121358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hori R., Kashiba M., Toma T., Yachie A., Goda N., Makino N., Soejima A., Nagasawa T., Nakabayashi K., Suematsu M. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J. Biol. Chem. 2002;277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- 51.Biswas C., Shah N., Muthu M., La P., Fernando A.P., Sengupta S., Yang G., Dennery P.A. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J. Biol. Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collinson E.J., Wimmer-Kleikamp S., Gerega S.K., Yang Y.H., Parish C.R., Dawes I.W., Stocker R. The yeast homolog of heme oxygenase-1 affords cellular antioxidant protection via the transcriptional regulation of known antioxidant genes. J. Biol. Chem. 2011;286:2205–2214. doi: 10.1074/jbc.M110.187062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray L.T., Puig Lombardi E., Verga D., Nicolas A., Teulade-Fichou M.P., Londoño-Vallejo A., Maizels N. G-quadruplexes sequester free heme in living cells. Cell Chem. Biol. 2019;26:1681–1691. doi: 10.1016/j.chembiol.2019.10.003. e5. [DOI] [PubMed] [Google Scholar]
- 54.Davis J.T. G-quartets 40 years later: from 5’-GMP to molecular biology and supramolecular chemistry. Angew Chem. Int. Ed. Engl. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 55.Krzeptowski W., Chudy P., Sokołowski G., Żukowska M., Kusienicka A., Seretny A., Kalita A., Czmoczek A., Gubała J., Baran S., Klóska D., Jeż M., Stępniewski J., Szade K., Szade A., Grochot-Przęczek A., Józkowicz A., Nowak W.N. Proximity ligation assay detection of protein-DNA interactions-is there a link between heme oxygenase-1 and G-quadruplexes? Antioxidants. 2021;10 doi: 10.3390/antiox10010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang H.W., Lee J.R., Chou K.Y., Suen C.S., Hwang M.J., Chen C., Shieh R.C., Chau L.Y. Oligomerization is crucial for the stability and function of heme oxygenase-1 in the endoplasmic reticulum. J. Biol. Chem. 2009;284:22672–22679. doi: 10.1074/jbc.M109.028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weng Y.H., Yang G., Weiss S., Dennery P.A. Interaction between heme oxygenase-1 and -2 proteins. J. Biol. Chem. 2003;278:50999–51005. doi: 10.1074/jbc.M307644200. [DOI] [PubMed] [Google Scholar]
- 58.Suttner D.M., Dennery P.A. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. Faseb. J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 59.Tartier L., Spenlehauer C., Newman H.C., Folkard M., Prise K.M., Michael B.D., Ménissier-de Murcia J., de Murcia G. Local DNA damage by proton microbeam irradiation induces poly(ADP-ribose) synthesis in mammalian cells. Mutagenesis. 2003;18:411–416. doi: 10.1093/mutage/geg015. [DOI] [PubMed] [Google Scholar]
- 60.Okano S., Lan L., Caldecott K.W., Mori T., Yasui A. Spatial and temporal cellular responses to single-strand breaks in human cells. Mol. Cell Biol. 2003;23:3974–3981. doi: 10.1128/mcb.23.11.3974-3981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Namba F., Go H., Murphy J.A., La P., Yang G., Sengupta S., Fernando A.P., Yohannes M., Biswas C., Wehrli S.L., Dennery P.A. Expression level and subcellular localization of heme oxygenase-1 modulates its cytoprotective properties in response to lung injury: a mouse model. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrabi S.A., Kim N.S., Yu S.W., Wang H., Koh D.W., Sasaki M., Klaus J.A., Otsuka T., Zhang Z., Koehler R.C., Hurn P.D., Poirier G.G., Dawson V.L., Dawson T.M. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciarlo M., Benelli R., Barbieri O., Minghelli S., Barboro P., Balbi C., Ferrari N. Regulation of neuroendocrine differentiation by AKT/hnRNPK/AR/β-catenin signaling in prostate cancer cells. Int. J. Cancer. 2012;131:582–590. doi: 10.1002/ijc.26402. [DOI] [PubMed] [Google Scholar]
- 64.Bomsztyk K., van Seuningen I., Suzuki H., Denisenko O., Ostrowski J. Diverse molecular interactions of the hnRNP K protein. FEBS Lett. 1997;403:113–115. doi: 10.1016/s0014-5793(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 65.Tzima S., Victoratos P., Kranidioti K., Alexiou M., Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J. Exp. Med. 2009;206:1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H., Lin C.H., Ma G., Orr M., Baffi M.O., Wathelet M.G. Transcriptional activity of interferon regulatory factor (IRF)-3 depends on multiple protein-protein interactions. Eur. J. Biochem. 2002;269:6142–6151. doi: 10.1046/j.1432-1033.2002.03330.x. [DOI] [PubMed] [Google Scholar]
- 67.Sasai M., Matsumoto M., Seya T. The kinase complex responsible for IRF-3-mediated IFN-beta production in myeloid dendritic cells (mDC) J. Biochem. 2006;139:171–175. doi: 10.1093/jb/mvj025. [DOI] [PubMed] [Google Scholar]
- 68.Li Volti G., Galvano F., Frigiola A., Guccione S., di Giacomo C., Forte S., Tringali G., Caruso M., Adekoya O.A., Gazzolo D. Potential immunoregulatory role of heme oxygenase-1 in human milk: a combined biochemical and molecular modeling approach. J. Nutr. Biochem. 2010;21:865–871. doi: 10.1016/j.jnutbio.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 69.Calderwood S.K., Theriault J., Gray P.J., Gong J. Cell surface receptors for molecular chaperones. Methods. 2007;43:199–206. doi: 10.1016/j.ymeth.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Binder R.J., Han D.K., Srivastava P.K. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 71.Basu S., Binder R.J., Ramalingam T., Srivastava P.K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 72.Zhu J., Xiong Y., Zhang Y., Wen J., Cai N., Cheng K., Liang H., Zhang W. The molecular mechanisms of regulating oxidative stress-induced ferroptosis and therapeutic strategy in tumors. Oxid Med Cell Longev. 2020 doi: 10.1155/2020/8810785. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang K., Jiang J., Lei Y., Zhou S., Wei Y., Huang C. Targeting metabolic-redox circuits for cancer therapy. Trends Biochem. Sci. 2019;44:401–414. doi: 10.1016/j.tibs.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Gandini N.A., Alonso E.N., Fermento M.E., Mascaró M., Abba M.C., Coló G.P., Arévalo J., Ferronato M.J., Guevara J.A., Núñez M., Pichel P., Curino A.C., Facchinetti M.M. Heme oxygenase-1 has an antitumor role in breast cancer. Antioxidants Redox Signal. 2019;30:2030–2049. doi: 10.1089/ars.2018.7554. [DOI] [PubMed] [Google Scholar]
- 76.Hsu F.F., Chiang M.T., Li F.A., Yeh C.T., Lee W.H., Chau L.Y. Acetylation is essential for nuclear heme oxygenase-1-enhanced tumor growth and invasiveness. Oncogene. 2017;36:6805–6814. doi: 10.1038/onc.2017.294. [DOI] [PubMed] [Google Scholar]
- 77.Liu L., Wu Y., Bian C., Nisar M.F., Wang M., Hu X., Diao Q., Nian W., Wang E., Xu W., Zhong J.L. Heme oxygenase 1 facilitates cell proliferation via the B-Raf-ERK signaling pathway in melanoma. Cell Commun. Signal. 2019;17:3. doi: 10.1186/s12964-018-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferrando M., Gueron G., Elguero B., Giudice J., Salles A., Leskow F.C., Jares-Erijman E.A., Colombo L., Meiss R., Navone N., de Siervi A., Vazquez E. Heme oxygenase 1 (HO-1) challenges the angiogenic switch in prostate cancer. Angiogenesis. 2011;14:467–479. doi: 10.1007/s10456-011-9230-4. [DOI] [PubMed] [Google Scholar]
- 79.Gueron G., de Siervi A., Ferrando M., Salierno M., de Luca P., Elguero B., Meiss R., Navone N., Vazquez E.S. Critical role of endogenous heme oxygenase 1 as a tuner of the invasive potential of prostate cancer cells. Mol. Cancer Res. 2009;7:1745–1755. doi: 10.1158/1541-7786.Mcr-08-0325. [DOI] [PubMed] [Google Scholar]
- 80.Song J., Zhang X., Liao Z., Liang H., Chu L., Dong W., Zhang X., Ge Q., Liu Q., Fan P., Zhang Z., Zhang B. 14-3-3zeta inhibits heme oxygenase-1 (HO-1) degradation and promotes hepatocellular carcinoma proliferation: involvement of STAT3 signaling. J. Exp. Clin. Cancer Res. 2019;38:3. doi: 10.1186/s13046-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diehn M., Cho R.W., Lobo N.A., Kalisky T., Dorie M.J., Kulp A.N., Qian D., Lam J.S., Ailles L.E., Wong M., Joshua B., Kaplan M.J., Wapnir I., Dirbas F.M., Somlo G., Garberoglio C., Paz B., Shen J., Lau S.K., Quake S.R., Brown J.M., Weissman I.L., Clarke M.F. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tibullo D., Barbagallo I., Giallongo C., la Cava P., Parrinello N., Vanella L., Stagno F., Palumbo G.A., Li Volti G., di Raimondo F. Nuclear translocation of heme oxygenase-1 confers resistance to imatinib in chronic myeloid leukemia cells. Curr. Pharmaceut. Des. 2013;19:2765–2770. doi: 10.2174/1381612811319150012. [DOI] [PubMed] [Google Scholar]
- 83.Tibullo D., Barbagallo I., Giallongo C., Vanella L., Conticello C., Romano A., Saccone S., Godos J., di Raimondo F., Li Volti G. Heme oxygenase-1 nuclear translocation regulates bortezomibinduced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget. 2016;7:28868–28880. doi: 10.18632/oncotarget.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kishimoto Y., Sasaki K., Saita E., Niki H., Ohmori R., Kondo K., Momiyama Y. Plasma heme oxygenase-1 levels and carotid atherosclerosis. Stroke. 2018;49:2230–2232. doi: 10.1161/strokeaha.118.022256. [DOI] [PubMed] [Google Scholar]
- 85.Casha S., Rice T., Stirling D.P., Silva C., Gnanapavan S., Giovannoni G., Hurlbert R.J., Yong V.W. Cerebrospinal fluid biomarkers in human spinal cord injury from a phase II minocycline trial. J. Neurotrauma. 2018;35:1918–1928. doi: 10.1089/neu.2018.5899. [DOI] [PubMed] [Google Scholar]
- 86.Zager R.A., Johnson A.C., Becker K. Plasma and urinary heme oxygenase-1 in AKI. J. Am. Soc. Nephrol. 2012;23:1048–1057. doi: 10.1681/asn.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Novo G., Cappello F., Rizzo M., Fazio G., Zambuto S., Tortorici E., Marino Gammazza A., Corrao S., Zummo G., de Macario E.C., Macario A.J., Assennato P., Novo S., Li Volti G. Hsp60 and heme oxygenase-1 (Hsp32) in acute myocardial infarction. Transl. Res. 2011;157:285–292. doi: 10.1016/j.trsl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Erdemli H.K., Yıldırımlar P., Alper T.Y., Kocabaş R., Salis O., Bedir A. Increased serum heme oxygenase-1 levels as a diagnostic marker of oxidative stress in preeclampsia. Hypertens. Pregnancy. 2014;33:488–497. doi: 10.3109/10641955.2014.946613. [DOI] [PubMed] [Google Scholar]
- 89.Vitoratos N., Papakonstantinou K., Deliveliotou A., Economou E., Panoulis C., Hassiakos D., Creatsas G.K. Antepartum and postpartum serum heme oxygenase-1 levels in preeclamptic and normotensive pregnant women. Vivo. 2011;25:445–450. [PubMed] [Google Scholar]
- 90.Qu H.M., Qu L.P., Li X.Y., Pan X.Z. Overexpressed HO-1 is associated with reduced STAT3 activation in preeclampsia placenta and inhibits STAT3 phosphorylation in placental JEG-3 cells under hypoxia. Arch. Med. Sci. 2018;14:597–607. doi: 10.5114/aoms.2016.63261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haines D.D., Lekli I., Teissier P., Bak I., Tosaki A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. 2012;204:487–501. doi: 10.1111/j.1748-1716.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 92.Tiwari S., Ndisang J.F. Heme oxygenase system and hypertension: a comprehensive insight. Curr. Pharmaceut. Des. 2014;20:1354–1369. doi: 10.2174/13816128113199990558. [DOI] [PubMed] [Google Scholar]
- 93.Luo W., Li J., Li Z., Lin T., Zhang L., Yang W., Mai Y., Liu R., Chen M., Dai C., Yang H., Lu J., Li H., Guan G., Huang M., Liu P., Li Z. HO-1 nuclear accumulation and interaction with NPM1 protect against stress-induced endothelial senescence independent of its enzymatic activity. Cell Death Dis. 2021;12:738. doi: 10.1038/s41419-021-04035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Signorelli S.S., Marino E., Scuto S., di Raimondo D. Pathophysiology of peripheral arterial disease (PAD): a review on oxidative disorders. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21124393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Signorelli S.S., Li Volsi G., Fiore V., Mangiafico M., Barbagallo I., Parenti R., Rizzo M., Li Volti G. Plasma heme oxygenase-1 is decreased in peripheral artery disease patients. Mol. Med. Rep. 2016;14:3459–3463. doi: 10.3892/mmr.2016.5644. [DOI] [PubMed] [Google Scholar]
- 96.Ishikawa K., Sugawara D., Wang X., Suzuki K., Itabe H., Maruyama Y., Lusis A.J. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ. Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 97.Ishikawa K., Maruyama Y. Heme oxygenase as an intrinsic defense system in vascular wall: implication against atherogenesis. J. Atherosclerosis Thromb. 2001;8:63–70. doi: 10.5551/jat1994.8.63. [DOI] [PubMed] [Google Scholar]
- 98.Gao Y., Cao Z., Yang X., Abdelmegeed M.A., Sun J., Chen S., Beger R.D., Davis K., Salminen W.F., Song B.J., Mendrick D.L., Yu L.R. Proteomic analysis of acetaminophen-induced hepatotoxicity and identification of heme oxygenase 1 as a potential plasma biomarker of liver injury. Proteonomics Clin. Appl. 2017;11 doi: 10.1002/prca.201600123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei S., Wang Y., Chai Q., Fang Q., Zhang Y., Lu Y., Wang J. Over-expression of heme oxygenase-1 in peripheral blood predicts the progression and relapse risk of chronic myeloid leukemia. Chin Med J (Engl). 2014;127:2795–2801. https://www.ncbi.nlm.nih.gov/pubmed/25146616 [PubMed] [Google Scholar]
- 100.Ono K., Goto Y., Takagi S., Baba S., Tago N., Nonogi H., Iwai N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173:315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 101.Chen Y.H., Lin S.J., Lin M.W., Tsai H.L., Kuo S.S., Chen J.W., Charng M.J., Wu T.C., Chen L.C., Ding Y.A., Pan W.H., Jou Y.S., Chau L.Y. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum. Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 102.Sponholz C., Huse K., Kramer M., Giamarellos-Bourboulis E.J., Claus R.A., Kern A., Engel C., Kuhnt E., Kiehntopf M., Routsi C., Mylona V., Tsangaris I., Heinemann S.H., Reinhart K., Platzer M., Bauer M. Gene polymorphisms in the heme degradation pathway and outcome of severe human sepsis. Shock. 2012;38:459–465. doi: 10.1097/SHK.0b013e31826ae951. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka G., Aminuddin F., Akhabir L., He J.Q., Shumansky K., Connett J.E., Anthonisen N.R., Abboud R.T., Paré P.D., Sandford A.J. Effect of heme oxygenase-1 polymorphisms on lung function and gene expression. BMC Med. Genet. 2011;12:117. doi: 10.1186/1471-2350-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Du Y., Zhang H., Xu Y., Ding Y., Chen X., Mei Z., Ding H., Jie Z. Association among genetic polymorphisms of GSTP1, HO-1, and SOD-3 and chronic obstructive pulmonary disease susceptibility. Int. J. Chronic Obstr. Pulm. Dis. 2019;14:2081–2088. doi: 10.2147/copd.S213364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kazemi M., Khosravian F., Sameti A.A., Moafi A., Merasi M.R., Salehi M., Nejati M., Behjati M. Association between (GT)n repeats in heme oxygenase-1 gene promoter and 3-year survival of patients with acute leukemia: a controlled, cross-sectional study. Int. J. Hematol. Oncol. Stem Cell Res. 2018;12:49–56. [PMC free article] [PubMed] [Google Scholar]
- 106.Tang D., Tang W.J., Shi X.L., Li W.P., Zhou H., Lu L.M., Tao L. Association of the microsatellite (GT)n repeat polymorphisms of the HO-1 gene promoter and corresponding serum levels with the risk of laryngeal squamous cell carcinoma. Acta Otolaryngol. 2016;136:806–811. doi: 10.3109/00016489.2016.1157265. [DOI] [PubMed] [Google Scholar]
- 107.Daenen K.E., Martens P., Bammens B. Association of HO-1 (GT)n promoter polymorphism and cardiovascular disease: a reanalysis of the literature. Can. J. Cardiol. 2016;32:160–168. doi: 10.1016/j.cjca.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Gill A.J., Garza R., Ambegaokar S.S., Gelman B.B., Kolson D.L. Heme oxygenase-1 promoter region (GT)n polymorphism associates with increased neuroimmune activation and risk for encephalitis in HIV infection. J. Neuroinflammation. 2018;15:70. doi: 10.1186/s12974-018-1102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soflaei S.S., Momtazi-Borojeni A.A., Majeed M., Derosa G., Maffioli P., Sahebkar A. Curcumin: a natural pan-HDAC inhibitor in cancer. Curr. Pharmaceut. Des. 2018;24:123–129. doi: 10.2174/1381612823666171114165051. [DOI] [PubMed] [Google Scholar]
- 110.Mascaró M., Alonso E.N., Alonso E.G., Lacunza E., Curino A.C., Facchinetti M.M. Nuclear localization of heme oxygenase-1 in pathophysiological conditions: does it explain the dual role in cancer? Antioxidants. 2021;10 doi: 10.3390/antiox10010087. [DOI] [PMC free article] [PubMed] [Google Scholar]