Abstract
Objective:
The goal of this study was to develop rat models of partial body irradiation with bone-marrow sparing (leg-out PBI), to test medical countermeasures (MCM) of both acute radiation syndrome (ARS) and delayed effects of acute radiation exposure (DEARE) under the FDA animal rule.
Methods:
The leg-out PBI models were developed in female and male WAG/RijCmcr rats at doses of 12.5–14.5 Gy. Rats received supportive care consisting of fluids and antibiotics. Gastrointestinal ARS (GI-ARS) was assessed by lethality to day 7 and diarrhea scoring to day 10. Differential blood counts were analyzed between days 1–42 for the natural history of hematopoietic ARS (H-ARS). Lethality and breathing intervals (BI) were measured between days 28–110 to assess delayed injury to the lung (L-DEARE). Kidney injury (K-DEARE) was evaluated by measuring elevation of blood urea nitrogen (BUN) between days 90–180.
Results:
The LD50/30, including both lethality from GI-ARS and H-ARS, for female and male rats is 14.0 Gy and 13.5 Gy respectively, while the LD50/7 for only GI-ARS is 14.3 Gy and 13.6 Gy respectively. The all-cause mortality, including ARS and L-DEARE, through 120 days (LD50/120) is 13.5 Gy and 12.9 Gy respectively. Secondary end points confirmed occurrence of 4 distinct sequelae representing GI, hematopoietic, lung and kidney toxicities after leg-out PBI.
Conclusion:
Adult rat models of leg-out PBI developed showed the acute and long-term sequelae of radiation damage that has been reported in human radiation exposure case studies. Sex-specific differences were observed in the DRR between females and males. These rat models are among the most useful for the development and approval of countermeasures for mitigation of radiation injuries under the FDA animal rule.
Keywords: rat, partial body radiation irradiation, biological indicators, health effects
INTRODUCTION
The aim of the National Institute of Allergy and Infectious Diseases (NIAID) Radiation and Nuclear Countermeasures Program is to develop medical countermeasures (MCM) to treat the acute and delayed effects of radiation that may occur from a nuclear attack or terrorist event (DiCarlo et al. 2008, DiCarlo et al. 2011). Victims of high-dose radiation exposure are likely to experience a range of symptoms to multiple organ systems which manifest at different time points following exposure. In the early days to weeks following exposure, lethality may occur due to gastrointestinal (GI-ARS) or hematopoietic acute radiation syndrome (H-ARS) or combined injury to both systems.
In the months-years following exposure, patients are susceptible to delayed effects of acute radiation exposure (DEARE) to multiple organ systems including the lungs (L-DEARE) and kidneys (K-DEARE). For these reasons, it is critical to develop mitigators for multiple-organ injury (MOI) that successfully rescue victims from injuries due to a radiological or nuclear attack.
It is not ethical to expose human subjects to high-dose radiation; therefore, approval of MCM for ARS and DEARE are conducted using the FDA ‘Animal Rule’ and guidance document (FDA 2002, FDA 2015) that requires established and validated radiation animal models that resemble radiation exposure scenarios consequent to a nuclear attack. In a mass casualty scenario, radiation exposures will likely be heterogeneous, nonuniform and total- or partial-body. The threshold radiation dose for lethal bone marrow injury is below that of the delayed effects of radiation, therefore, to allow dose- and time-dependent survival and assessment of DEARE, a minimal volume of bone marrow must be spared. A partial body irradiation (PBI) rat model with bone-marrow (BM) shielding of only one limb has the potential to define the dose- and time-dependent link between the multiple organ injuries of the ARS and DEARE, providing a more definitive approach to assess MCM efficacy consequent to a terrorist incident.
Several laboratories have established animal models of PBI/BM-sparing or extended uniform total body irradiation (TBI) to assess DEARE (Booth et al. 2015, Unthank et al. 2015, Fish et al. 2016, Chua et al. 2019, Medhora et al. 2019, Fish et al. 2020, Miller et al. 2020, Patterson et al. 2020). Established models and dose response relationships (DRR) are described for non-human primates (NHP), where multiple ARS and DEARE sequelae manifest after PBI with BM-sparing of ~5% (MacVittie et al. 2012, MacVittie et al. 2014, MacVittie et al. 2015) or ~2.5% (Farese et al. 2019). Other, varied exposure protocols have been established using PBI with BM-sparing as well as unilateral, nonuniform TBI to mimic the exposure environment and assess MCM efficacy, albeit focused against the acute H-ARS (Chapel et al. 2003, Drouet et al. 2004, Bertho et al. 2005, Herodin et al. 2007, Drouet et al. 2008). Mouse PBI models with BM shielding have also been developed, although these models have been primarily used to characterize GI-ARS, GI-DEARE and lung injury (Johnston et al. 2011, Booth et al. 2012a, Booth et al. 2012b, Booth et al. 2015). Several teams have used the total body irradiation (TBI) mouse models, over the potentially lethal H-ARS dose range, to assess the incidence of organ-specific DEARE in long-term survivors in control and MCM-treated cohorts (Baker et al. 2009, Chua et al. 2012, Chua et al. 2014, Unthank et al. 2015, Chua et al. 2019, Unthank et al. 2019, Miller et al. 2020, Patterson et al. 2020, Wu et al. 2020).
Some of the most sophisticated PBI models to date have been developed using WAG/RijCmcr rats with shielding to part of one hind limb (~7.5% of BM, Taketa et al. 1970) by leg-out PBI (Fish et al. 2016, Fish et al. 2020). The initial model demonstrated that young adult, female, rats (Fish et al. 2016) manifested GI, lung and renal toxicities, but lethal lung injury was relatively rare and only occurred in <15% rats. Therefore, evaluation of L-DEARE MCMs would require a restrictively large number of animals to observe statistical differences in survival during pneumonitis. Moreover, in male rats, only the 50% lethal dose (LD50) for radiation-nephropathy was described with limited data available for lung and GI toxicities (Fish et al. 2020). Though the BM was shielded in these studies, the natural progression of bone marrow damage was not evaluated, leaving BM sequelae poorly characterized in the leg-out PBI rat model. The goal herein was to optimize and fully assess adult female and male rat models of leg-out PBI, that manifested lethal GI, lung and kidney damage, while also assessing hematopoietic injury after irradiation. The goal required the radiation dose, diet and supportive care to be refined based on previous studies (Fish et al. 2016, Moulder et al. 2019, Fish et al. 2020) and as described in the results herein.
MATERIALS & METHODS
Animal care
All animal protocols were approved by Institutional Animal Care and Use Committees (IACUC) at the Medical College of Wisconsin. WAG/RijCmcr rats bred at MCW were weaned to Teklad 8604 (Envigo, Madison WI) rodent diet along with reverse osmosis hyperchlorinated water. The rats were housed (3–5 per cage) in a 14 h/10 h light/dark cycle, at 22° C with humidity maintained between 30–70%. Based upon direction from the IACUC, rats were designated as moribund and euthanized if they met specified veterinarian’s criteria as described previously (Medhora et al. 2015). The terminal endpoint for such rats was necropsy.
Irradiation
Leg–out PBI rat models:
WAG/RijCmcr female and male rats were unilaterally irradiated, dorsal to ventral, without the use of anesthetics at 11–12 weeks of age. All irradiations were done between 8–10 am. For leg-out PBI, non-anesthetized rats were immobilized in a plastic jig and irradiated using a XRAD 320KV orthovoltage x-ray system (Precision X-Ray, North Branford, Connecticut). The x-ray system was operated at 320 kVp and 13 mAs with a half value layer of 1.4 mm Cu with a dose-rate of 1.69 Gy min−1 for female rats and 1.73 Gy min−1 for males. Doses ranged from 13 −14.5 Gy for female rats and 12.5 −14.5 Gy for males. During irradiation, each rat was confined to a chamber which allows irradiation of 2 rats simultaneously. One hind limb of each rat was carefully externalized from the chamber and shielded with a 0.25-inch lead block. The dose to this leg was approximately 0.15 Gy/Gy (1.9–2.2 Gy over the dose range). The dual-chambered jig was placed on a plane perpendicular to the direction of the beam, with distance from source to the midline of rats set at 61.0 cm for females and 60.8 cm for males. Collimator jaws and dosimetry were used as previously described (Medhora et al. 2014). The irradiation field at midline was large enough to cover both chambers with adequate (at least 2 cm) margins.
Model refinement
Diet and supportive care:
WAG/RijCmcr rats were weaned to Teklad 8604 (Envigo, Madison WI) rodent diet. Two weeks prior to irradiation rats were switched to a moderate antioxidant diet (Teklad 2018 Global 18% protein rodent diet, Envigo, Madison WI); diet details are listed in Table 1. The Teklad 2018 global diet has been used at other institutions for rodent radiation studies (Patterson et al. 2021, Booth et al. 2012a, Booth et al. 2012b, Booth et al. 2015) and is a better reflection of the human diet than Teklad 8604/8904 used previously (Fish et al. 2016, Moulder et al. 2019, Fish et al. 2020).
TABLE 1:
DIET COMPARISON
| Nutrient | T.8904 Teklad Rodent Diet | 2018 Teklad global 18% protein Diet |
|---|---|---|
| Protein (%) | 24.3 | 18.6 |
| Carbohydrate (%) | 40.2 | 44.2 |
| Fat (%) | 4.7 | 6.2 |
| Energy density (kcal/g) | 3.0 | 3.1 |
| Sodium (%) | 0.3 | 0.2 |
| Phosphorus (%)a | 0.7 | 0.4 |
| Selenium (mg/kg) | 0.34 | 0.23 |
| Isoflavones (mg/kg) | 350–550 | 150–250 |
Available (non-phytate)
Supportive care was enhanced from a previous publication (Fish et al. 2016) of a similar model to include bolus hydration by daily subcutaneous injection of saline 40 ml kg−1day−1 from days 2–10 versus days 3–7. This allowed for pelleted stool formation to return to normal. The antibiotic enrofloxacin 10 mg kg−1day−1 was delivered from days 2–14 instead of days 2–28, as neutrophil count was back to nearly normal levels by day 15 after leg-out PBI. Powdered food was provided from days 35 to 70 after irradiation to accommodate tooth loss that resolved by day 70.
Dose response relationship (DRR) for female rats:
139 female rats were given leg-out PBI at doses of 13 Gy (n= 32), 13.5 Gy (n=45), 14 Gy (n=46), 14.25 Gy (n=6) or 14.5 Gy (n=10). An additional 5 rats served as non-irradiated age-matched controls. All rats received supportive care. Rats were followed for morbidity through 150 days. GI-ARS (0–7 days after irradiation), H-ARS (8–30 days after irradiation), L-DEARE (31–120 days after irradiation) and K-DEARE (121–150 days after irradiation) were monitored and confirmed by necropsy. This model was used to determine the DRR, the LD for the different sequelae after leg-out PBI in adult female rats. Female rats were followed for 150 days after PBI. Body weights were recorded weekly. The probit plots estimate the LD for 10, 30, 50, 70 and 90% morbidity with upper and lower 95% confidence intervals (CI).
DRR for male rats:
114 male rats were given leg-out PBI at doses of 12.5 Gy (n=24), 13 Gy (n= 32), 13.5 Gy (n=35), 14 Gy (n=13), or 14.5 Gy (n=10). An additional 8 rats were non-irradiated, age-matched controls. Supportive care was the same as that administered to the female rats. This male model was used to determine the DRR and LD for the different sequelae after leg-out PBI in adult male rats. Since radiation nephropathy manifested later in males, the study duration and observation was extended to 180 days. Body weights were recorded weekly. The probit plots estimate the LD for 10, 30, 50, 70 and 90% rats that were moribund with upper and lower 95% CI.
Secondary endpoints for GI- and H-ARS
The LD50/120 for female and male rats represented the best models to study injuries to multiple organs in each sex, since they elaborated H-ARS as well as lethal GI-ARS, L-DEARE and K-DEARE. Secondary end points were measured in these models to confirm the cause of death in the K-M studies.
To test the progression of GI-ARS at the LD50/120 for the respective sex, 25 female and 32 male rats received 13.5 or 13 Gy leg-out PBI respectively. The rats were evaluated from days 2–10 for GI-ARS by stool scoring: 0=pelleted stool, 1=soft stool and 2=diarrhea. Rats received a score of 2.5 if they were moribund and therefore euthanized, to account for attrition. H-ARS was assessed by complete blood cell counts (CBC). Blood (0.5–1 ml) was harvested from the jugular vein into EDTA-treated tubes, by a trained technician. Samples were obtained at days 1, 3, 5, 7, 9, 12, 15, 22, 30 and 42 after irradiation. CBC were run on a Heska Elements HT5 blood analyzer. The natural history at 13.5 Gy in females and 13 Gy in males was studied because these were the best doses to test MCM.
Secondary endpoints for L-DEARE and K-DEARE
A subset of rats was assessed from the DRR study to measure functional damage in the lung and kidney. Minimally invasive measurements of the breathing interval (BI) or blood urea nitrogen (BUN) were conducted to monitor pulmonary and renal functions respectively.
Breathing interval measurements in rats:
Breathing rates for each rat were measured every two weeks from 28–110 days after exposure as described previously (Medhora et al. 2012a, Medhora et al. 2012b, Medhora et al. 2014). Rats were restrained in a plastic restrainer for 5 minutes for 2 consecutive training days to allow the rats to become acclimated to the apparatus. On the third day, the restrainer was placed in a transparent EMKA plethysmograph (Scireq Scientific Respiratory Equipment Inc., Montreal, QC, Canada) which measured the frequency of pressure changes. Recordings for a maximum of 10 minutes per animal were used. The mean breathing rate for each rat was then calculated from four steady regions of the recording lasting greater than 15 seconds each. The inverse of the breathing rates was calculated to derive the BI or time/breath in seconds. Since higher breathing rates and lower BI indicated more severe lung damage, the BI were set to 0 for animals that died during pneumonitis, to account for attrition (Medhora et al. 2012a, Medhora et al. 2012b).
Measurement of blood urea nitrogen (BUN):
Previous published work has shown that rising BUN levels are superior to histopathology for assessing radiation nephropathy (Moulder et al. 2011). To measure BUN, rats were anesthetized with 3–5% isoflurane for blood draws via the jugular vein conducted by an experienced technician. The BUN was assayed from serum as described previously (Cohen 1994, Medhora et al. 2014) using a urease-nitroprusside colorimetric assay. BUN values were expressed as mg/dl of serum and medians with 95% CI were used for statistical analysis. Irradiated rats with BUN>120mg dl−1 were euthanized and given a value of 120 mg dl−1 to account for attrition, since such rats were previously confirmed to have severe and irreversible renal damage (Moulder et al. 1993a, Moulder et al. 1993b).
Lung histology:
A subset of 13.5 Gy leg-out PBI irradiated female and 13 Gy leg-out PBI male rats that were moribund between 42–70 days (n=6 rats) were randomly selected for histological evaluation of the lungs. These rats typically presented with lung injury at necropsy. The left lung was harvested, fixed and embedded in paraffin. Whole mount lung sections (4 μm thick) were stained with hematoxylin and eosin (H&E) and also stained with tryptase antibody (IMGENEX catalogue #IMG-80250, 1:150) (Gao et al. 2013) and five randomly selected fields (20x) of each lung were evaluated. The foamy macrophages, vascular, and alveolar wall thickness were scored as described previously (Medhora et al. 2014, 2015); injury was measured on a 5-point scale and a mean composite lung injury score was calculated. Mast cells were assessed by positive tryptase staining in five randomly selected fields (20x) of each lung.
Scores from irradiated moribund rats were compared to those from non-irradiated animals (controls). Since no non-irradiated control rats were moribund in this study, lungs from these rats sacrificed at the termination of the study at 150 or 180 days for female and male respectively, were used for comparison (controls). Scoring for all rats (moribund or non-irradiated controls) was performed by operators masked to the treatment groups. Data is presented as means and 95% CI.
Kidney histology:
At morbidity or termination of the study (140–150 days post 13 Gy leg-out PBI for female and 150–180 day for male rats), kidneys were harvested, cut into half and immediately fixed in 10% buffered formalin and processed for paraffin embedding. Kidney sections were stained with Masson’s trichrome stain, and the glomerular fibrosis was scored blinded in coded samples by two investigators (FG and JN) (Medhora et al. 2019). Glomerular fibrosis scoring was as follows: the percent blue staining within each glomerulus was assessed; 0–25% blue staining was given a score of 1, 25–50% blue staining a score of 2, 50–75% blue staining a score of 3 and 75–100% blue staining a score of 4. The number of glomeruli scored in each of the percentage groups was multiplied by the score. The scores were then aggregated to get a composite histologic score. A total of 50 glomeruli were scored for each rat. Higher scores indicated more severe renal fibrosis.
Statistical analyses
Dose-response curves were fit by probit analysis and used to determine 50% lethal doses (LD50). The significance of dose-response trends was assessed by the Mantel extension test; this is a non-parametric test of monotonic trends that does not (unlike probit analysis) assume an exact shape for the dose response. Morbidity data are shown by Kaplan Meier plots and tested for differences between groups by Peto-Peto Wilcoxon tests. Breathing intervals are shown as means. BUN values are medians with 95% confidence intervals. Statistical differences between multiple groups for BUN were calculated by the one-way ANOVA and Dunnett’s multiple comparison tests. For histology scoring, t-test was used for two-group comparisons for scores of lung injury score and mast cell count between morbid rats versus non-irradiated controls of the same sex.
RESULTS
DRR for female rats
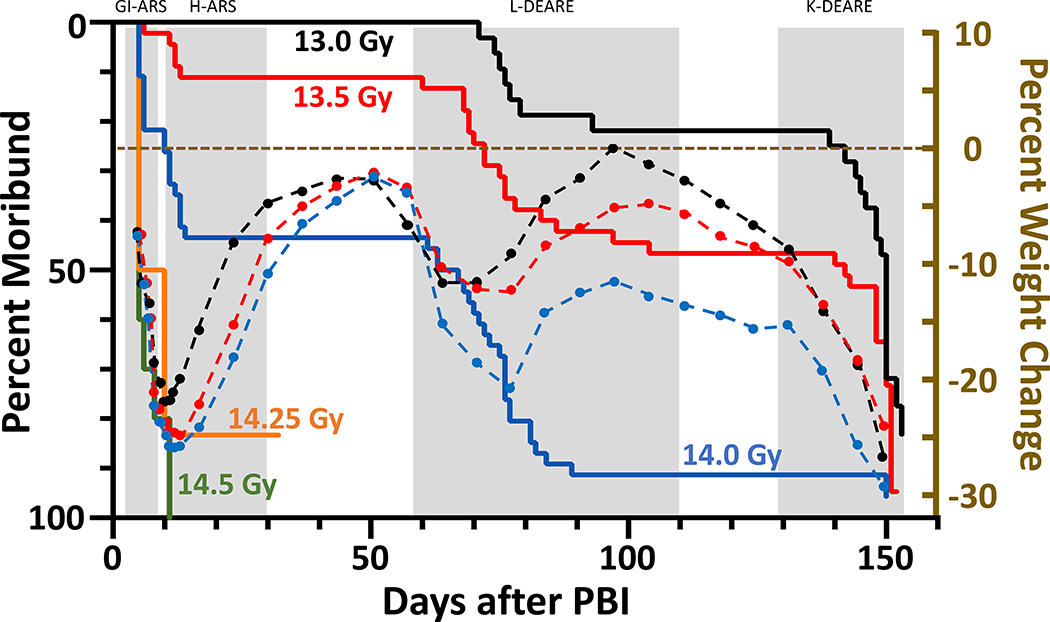
Female WAG/RijCmcr rats were irradiated with a single dose ranging from 13.0–14.5 Gy leg-out PBI as described and provided supportive care. The Kaplan-Meier curves (solid lines in figure 1) show the DRR for organ-specific lethal sequelae in irradiated female rats from days 1–160.
Figure 1.
Kaplan-Meier (KM) survival plots demonstrating dose- and time-dependent lethal sequelae observed after leg-out PBI in female rats. Female rats (11–12-week-old) received PBI at doses ranging from 13.0 to 14.5 Gy. Doses above 14 Gy (green and orange lines) result in > 80% morbidity during ARS. Survivors of the ARS (13 Gy (black line), 13.5 Gy (red line) and 14 Gy (blue line)) showed a dose-dependent survival during pneumonitis but were moribund from radiation nephropathy. At the doses delivered, all rats will develop radiation nephropathy after 120 days. To correlate body weight with organ specific sequelae, superimposed over the KM plot are the time courses (dashed lines) of average percent change in body weight from irradiation for 13.0 (black dots), 13.5 (red dots) and 14 Gy (blue dots). The body weight decreases prior to organ specific lethal sequelae.
ARS:
At the 14.5 Gy dose all animals were moribund during ARS (green line). At doses of 14.25 Gy (orange line) and below (blue, red and black lines) survival through GI- and then H-ARS were observed, survival increased in a dose-dependent manner. The rat surviving at 14.25 Gy was euthanized at 32 days, as the percent survival was too low to study DEARE at this dose.
L-DEARE:
Survivors from the GI- and H-ARS, at doses 14 Gy and below, developed dose- and time-dependent L-DEARE between days 60 to 120. At 14 Gy leg-out PBI, ~90% of rats that survived ARS were moribund by 120 days from L-DEARE. The median (95% CI) survival time for this group was 65 (12–75) days. At 13.5 Gy, ~50% of rats surviving ARS were moribund by 120 days, median survival time 142 days (76–150) and ~22% at 13.0 Gy, median survival time 150 (144–150) days. Pleural effusions were observed in most moribund rats upon necropsy between days 60–120 confirming radiation pneumonitis. Pleural fluid was measured, and volume recorded at necropsy.
K-DEARE:
The dose-dependent renal phase of morbidity followed L-DEARE between 135–160 days when rats exhibited typical signs of radiation-nephropathy (Moulder et al. 2011) with increased levels of blood urea nitrogen (BUN). It should be noted that all female rats at these doses of leg-out PBI will succumb to radiation-nephropathy given enough time beyond 150 days (Medhora et al. 2019).
Body weight (BW) loss:
Superimposed over the Kaplan-Meier curves (figure 1) is the natural history of the average percent change in BW for each group. Body weight decreased in a dose- and time-dependent manner. Interestingly, the start in decrease in BW preceded organ-specific sequelae observed in the Kaplan-Meier plots after leg-out PBI. During ARS, the BW decreased by ~20–25 % at doses from 13–14 Gy. The survivors of ARS then gained weight up to before the onset of L-DEARE, when the percent BW decreased again from 12% to 20%. The rats that survived L-DEARE (13–13.5 Gy leg-out PBI groups) increased in BW until before they started to progress to renal failure, after which the BW dropped precipitously and did not recover.
DRR for male rats
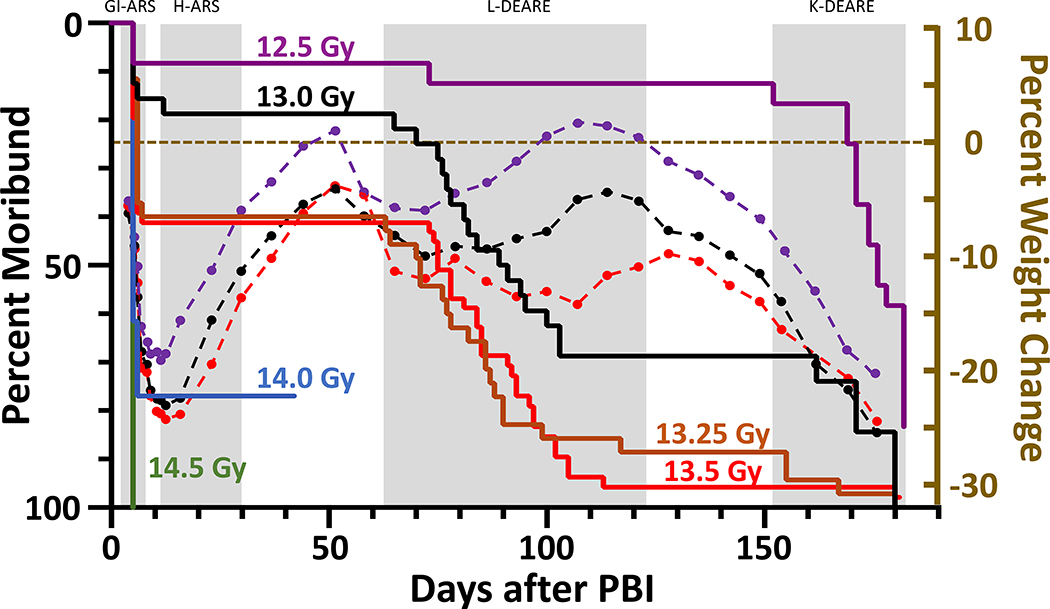
Male WAG/RijCmcr rats were irradiated with single doses ranging from 12.5–14.5 Gy leg-out PBI and given supportive care as described. The Kaplan-Meier curves (figure 2) show the DRR for the sequelae induced by radiation.
Figure 2.
Kaplan-Meier (KM) survival plot demonstrating dose- and time-dependent lethal sequelae observed after leg-out PBI in male rats. Male rats (11–12-week-old) received PBI at doses ranging from 12.5 to 14.5 Gy. Doses 14 Gy and above (blue and green lines) result in > 70% morbidity during ARS. Rats receiving doses at or below 13.5 Gy survived ARS in a dose-dependent manner. Survivor of ARS (12.5 Gy (purple line), 13 Gy (black line), 13.25 Gy (orange line) and 13.5 Gy (red line)) showed a dose-dependent survival during pneumonitis but succumbed to radiation nephropathy. To correlate body weight with organ specific sequela, superimposed over the KM plot are the time courses (dashed lines) of average change in body weight from irradiation for 12.5 (purple dots), 13.0 (black dots) and 13.5 Gy (red dots). The body weight decreases prior to organ specific lethal sequelae.
ARS:
At the 14.5 Gy dose all animals were moribund during ARS (green line). At doses of 14 Gy (blue line) greater than 75% of male rats were moribund to GI, with 1 surviving through BM. This rat was terminated at day 42 after irradiation as the percent survival was too low to study DEARE at this dose. At doses below 14 Gy (red, orange, black and purple lines) significant survival through GI- and H-ARS were observed, and survival increased in a dose-dependent manner.
L-DEARE:
At doses below 14 Gy the male rats that survived ARS proceeded to develop L-DEARE between 60 to 120 days. At doses of 13.25 and 13.5 Gy leg-out PBI the median survival time was 71 (6–86) and 75 (6–85) days respectively. Rats (~90%) surviving ARS were moribund from pneumonitis by 120 days from L-DEARE. At 13.0 Gy ~55% were moribund by 120 days with a median survival time of 90 (76–150) days while only ~10% became moribund at 12.5 Gy, with a median survival time 176 (171–180) days.
K-DEARE:
Male rats succumbed to radiation nephropathy, though the latency period was extended compared to female rats. Eventually all male rats at these doses of leg-out PBI are expected to succumb to radiation-nephropathy.
Body weight (BW) loss:
Superimposed over the Kaplan-Meier curves (figure 2) is the natural history of the average percent change in BW for male rats. The decrease in percent BW was more severe with increasing doses of PBI and similar to females coincided with the start of organ-specific sequelae. During ARS, the BW decreased by ~20–25 % for PBI doses from 12.5 – 13.5 Gy. The survivors of ARS then gained weight up to before the onset of L-DEARE when the percent BW decreased from ~6 to 15%. The rats that survived L-DEARE increased in BW until they progressed to renal failure.
Probit analysis in Female and Male rats
GI- and H-ARS:
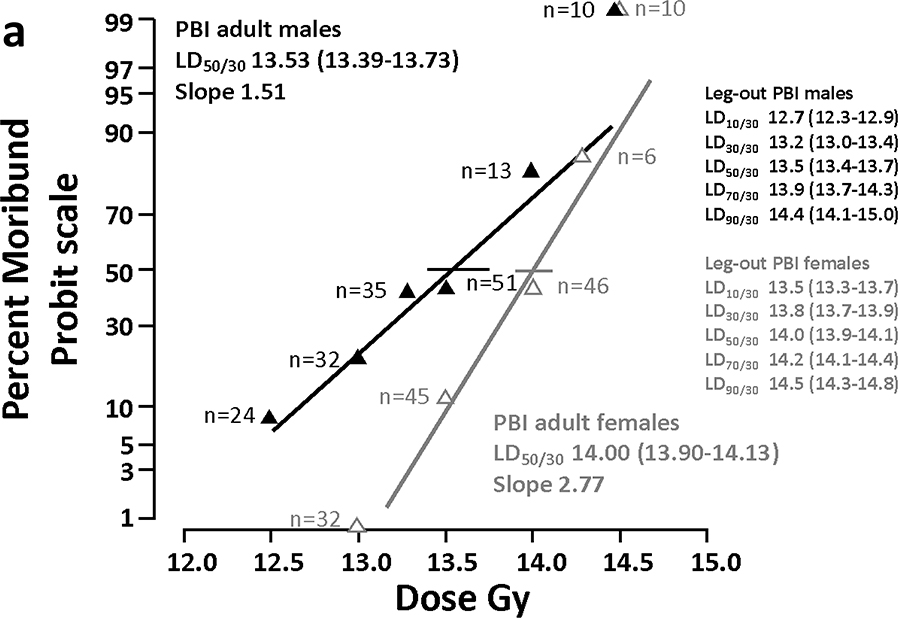
The mortality vs dose probit curves for male and female rats through 30 days (ARS) are shown in figure 3a. The LD50/30 for female rats is 14.00 (13.90–14.13) Gy after leg-out PBI. Male rats are slightly more sensitive with a LD50/30 of 13.53 Gy (13.39–13.73) Gy leg-out PBI. The LD50/30 included rats moribund to both GI-ARS and H-ARS. The LD50/7 for GI-ARS is 14.29 (14.2–14.6) and 13.56 (13.4–13.8) for female and male rats, respectively (not shown in figure 3).
Figure 3a.
Probit Analyses to determine LD50. The left panel shows the dose response relationship (DRR) for female and male WAG/RijCmcr rats for percent moribund rats on a Probit scale, versus leg-out PBI dose (Gy) assessed at 30 days (ARS). The LD50/30 for all-cause mortality through ARS, including GI-ARS and H-ARS for female rats is 14.0 Gy (13.90–14.13) and for male rats is 13.53 Gy (13.39–13.73).
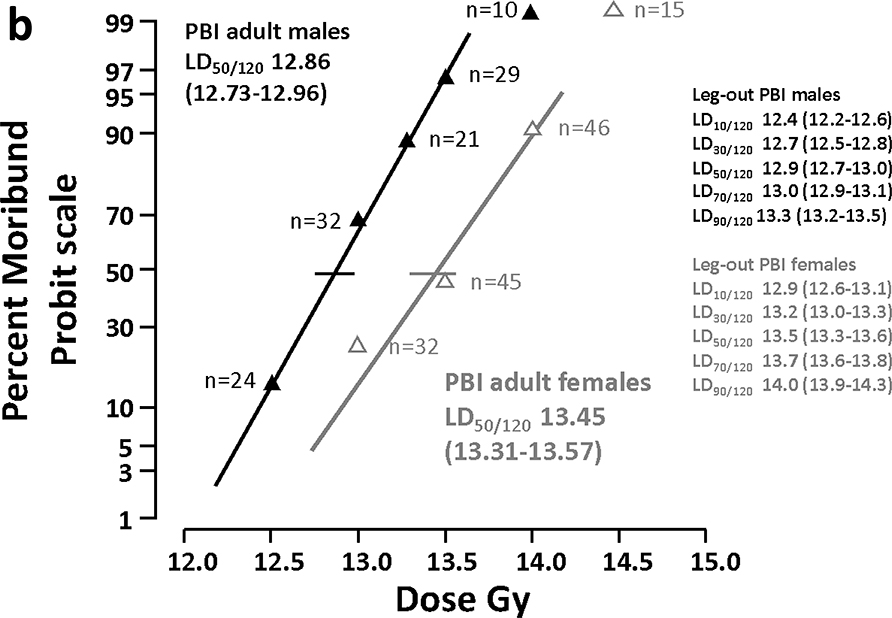
3b. The right panel shows the DRR for female and male WAG/RijCmcr rats assessed at 120 days, including GI-ARS, H-ARS and L-DEARE (see Table 2).
All-cause mortality:
All-cause mortality through 120 days (figure 3b) includes rats moribund for GI-ARS, H-ARS and L-DEARE. The LD50/120 is 13.45 (13.31–13.57) and 12.86 (12.73–12.96) for female and male rats, respectively. Comparing these probit curves for female and male rats the dose modifying factor (DMF) is 1.05 (1.04–1.06). These data show that male rats are more sensitive to radiation induced multiple organ injury (MOI) compared to female rats through 120 day after irradiation. To calculate the LD50 for L-DEARE only, rats moribund between days 31 and 120 were evaluated. The LD50/31–120, the time-interval that corresponds to lethality due to radiation pneumonitis, is 13.53 (13.4–13.7) and 12.96 (12.8–13.1) for female and male rats, respectively (not shown in figure 3). The LD50 values for the different sequelae are listed in Table 2.
TABLE 2:
LD50 Values
| Survival Days (d) after PBI | Sequela | Gender | LD50 (95% CI) Gy |
|---|---|---|---|
| 0–7d | GI-ARS | Female | 14.3 (14.2–14.6) |
| 0–7d | GI-ARS | Male | 13.6 (13.4–13.8) |
| 8–30d | H-ARS | Female | 14.2 (14.0–14.5) |
| 8–30d | H-ARS | Male | NA# |
| 0–30d | All-cause ARS | Female | 14.0 (13.9–14.1) |
| 0–30d | All-cause ARS | Male | 13.5 (13.4–13.7) |
| 30–120d | L-DEARE | Female | 13.5 (13.4–13.7) |
| 30–120d | L-DEARE | Male | 13.0 (12.8–13.1) |
| 0–120d | All-cause through L-DEARE | Female | 13.5 (13.3–13.6) |
| 0–120d | All-cause through L-DEARE | Male | 12.9 (12.7–13.0) |
| 120+d | K-DEARE | Female | NA* |
| 120+d | K-DEARE | Male | NA* |
Did not have sufficient morbidity to calculate the LD50.
Doses used in this study are above the LD50 for radiation nephropathy.
Secondary endpoints, ARS
The DRR provided an optimal dose (LD50/120) of leg-out PBI, to test MCM efficacy against the DEARE. A respective dose of 13.5 Gy leg-out PBI for female rats and 13 Gy leg-out PBI for male rats which represented four sequelae was chosen to measure secondary endpoints and to confirm organ-specific natural history of injury.
GI-ARS, GI scoring:
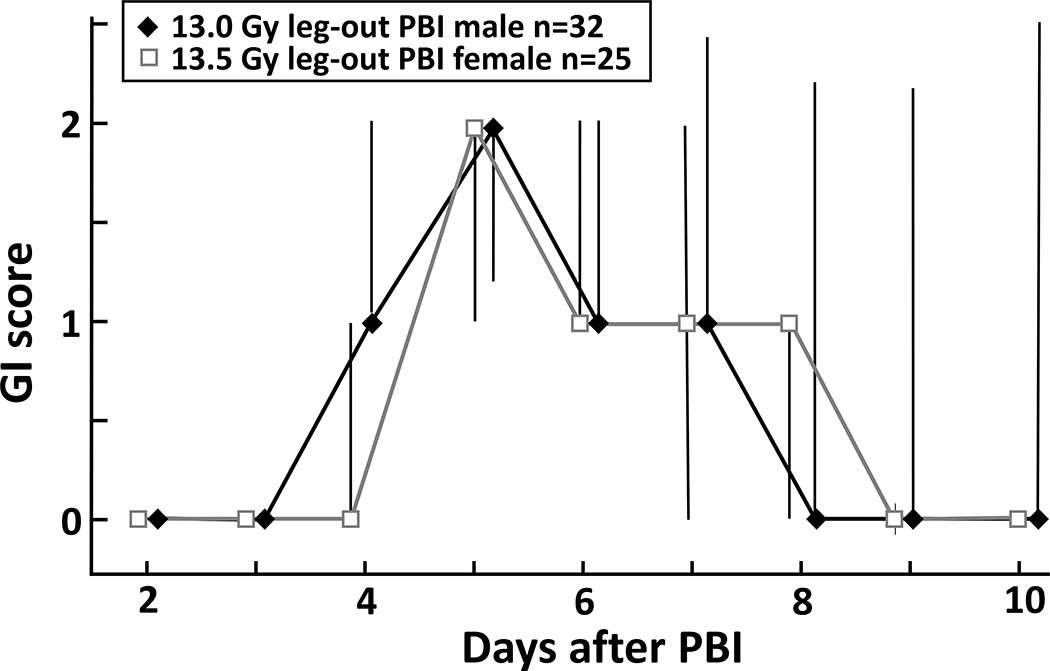
GI scoring, as described in the Methods, was conducted from days 2–10. Both female and male rats reached maximum GI damage (highest scores) on day 5 after irradiation (figure 4). Recovery to normal pelleted stool was seen by day 9 in survivors.
Figure 4.
To evaluate GI-ARS, the LD50/120 was selected as this dose best represents models suitable to test mitigators for L- and K-DEARE. GI injury was scored with a modified scoring system. In both female and male rats, the GI injury scores peak at day 5 after irradiation and return to normal by day 10. Data presented as medians and 20–80 percentiles.
H-ARS, Complete Blood Counts:
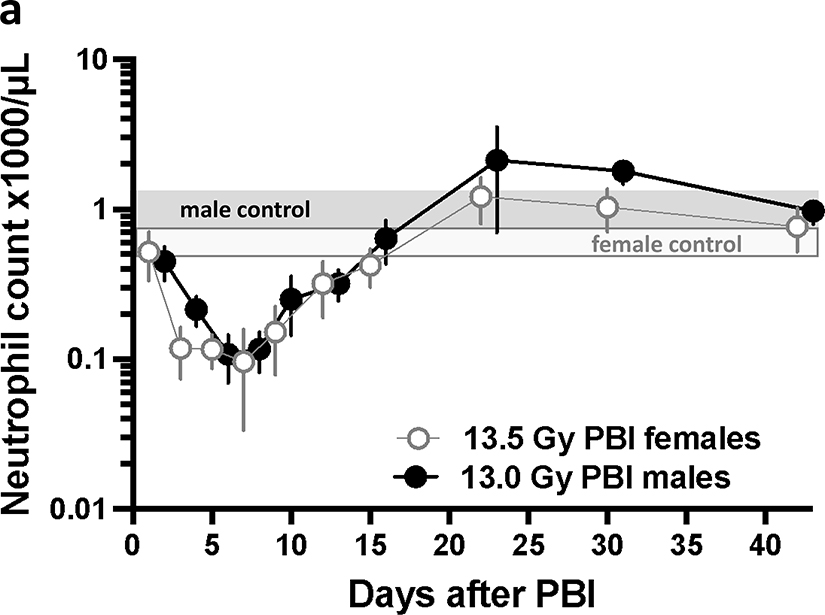
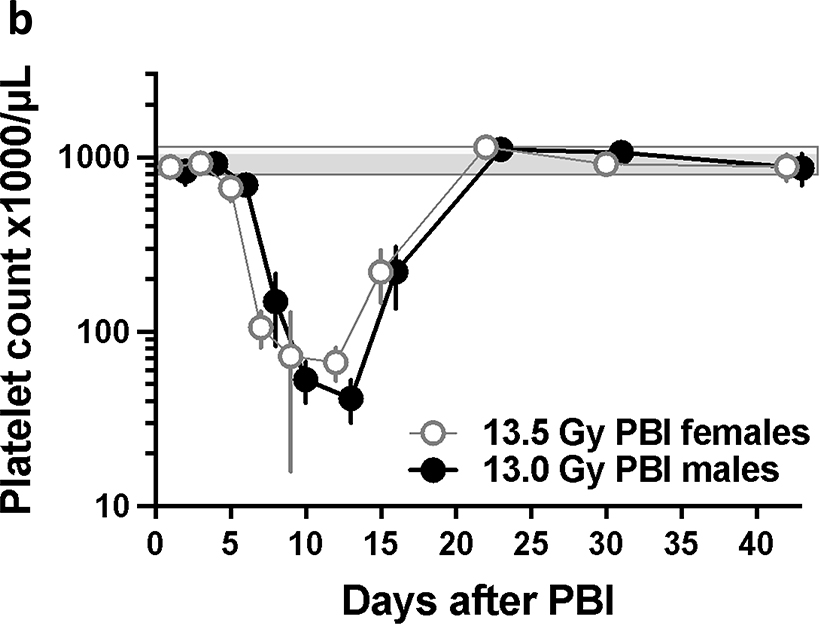
Hematopoietic injury was assessed from complete blood counts (CBC) at multiple time points (see Methods) from 1–42 days after leg-out PBI. Neutrophil counts (figure 5a) reached a nadir by day 7 and recovered by day 22 after irradiation in both female and male rats, while the platelet counts (figure 5b) reached a nadir by day 14 and recovered by day 22. The white blood cell (WBC), red blood cells (RBC), lymphocyte and monocyte count also decreased and recovered by 42 days after irradiation (supplemental figures 1–2). Similarly, the hemoglobin and hematocrit fell and recovered (supplemental figures 3). The mean corpuscular volume (MCV) increased with time after irradiation while the mean platelet volume (MPV) did not change (supplemental figures 4). These results elaborate hematopoietic injury during H-ARS in the rat model of leg-out PBI.
Figure 5a.
Time course of neutrophil counts during H-ARS at the LD50/120. Complete blood counts were assessed from day 1 to 42 after irradiation. The neutrophil count for both female (open gray circles) and male (black circles) rats reached a nadir of ~100 neutrophils/μl, between 5–7 days. The neutrophil counts recover to >1000 neutrophils/μl by day 22 after irradiation. Non-irradiated males have a slightly higher neutrophil concentration (solid grey bar) compared to non-irradiated females (white/gray bar). Data are presented as means and 95% CI for non-irradiated female (white/gray bar) and male (solid grey bar) rats.
5b. Time course of platelet counts during H-ARS at the LD50/120.There was a drop in platelet counts and recovery during H-ARS at the LD50/120. The platelet nadir occurred at 14 days after irradiation with a count of ~35 and 70 platelets ×103/μl for male and females, respectively. The platelet counts recover to >1000 ×103 platelets/μl by day 22 after irradiation. The bars represent the 95% confidence limits for non-irradiated female (white/gray bar) and male (solid grey bar) rats. Data are presented as means and 95% CI.
Secondary endpoints DEARE
L-DEARE, breathing interval (BI):
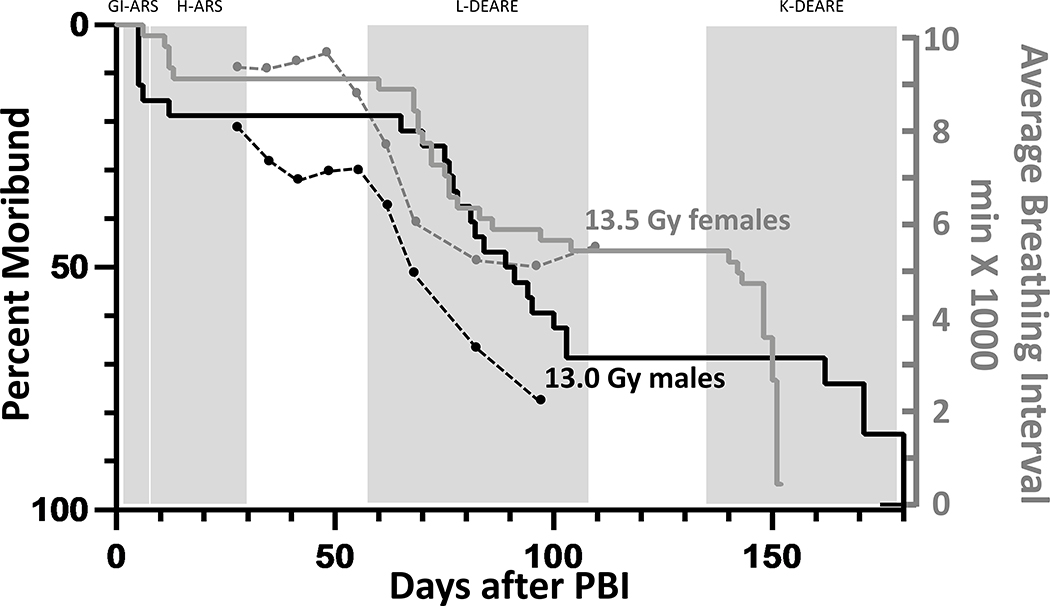
A subset of rats from the DRR study had their breathing rates assessed from day 28 after irradiation to the start of K-DEARE to determine changes in lung function. The average BI (inverse of the breathing rate) is superimposed on the Kaplan-Meier survival curve for both female and male rats (figure 6). There was a decrease in BI first measured at 49 and 35 days in female and male rats, respectively. A decrease in BI is an indicator of radiation pneumonitis.
Figure 6.
Kaplan-Meier (KM) survival plot demonstrating the LD50/120 for all cause mortality for female (gray line) and male (black line) rats after leg-out PBI. To correlate breathing intervals with the onset of lethal lung-DEARE, the KM plot are superimposed with the breathing intervals showing the time course of lethal lung injury (axis on the right). The average breathing intervals were recorded from 28–100 days for males (black dots) and 28–110 days for females (gray dots) in a subset of rats in the DRR study. Note a fall in the breathing interval that precedes lethal radiation pneumonitis. Data are presented as average BI (minutes x1000).
L-DEARE, pleural effusions:
Sixteen of forty female (16/40) rats receiving 13.5 Gy leg-out PBI and surviving the ARS were moribund between 60 and 104 days after irradiation with an average time to morbidity of 76 (70–82) days. Pleural effusions of >1 ml was observed upon necropsy in 14 of 15 (14/15) female rats, 1 rat could not be evaluated.
L-DEARE, absence of lethal radiation nephropathy:
BUN was assessed in these female rats euthanized for pneumonitis to confirm rats did not succumb to renal failure. The average (95% CI) BUN of these rats was 36.3 (30–42) mg/dl confirming the absence of lethal radiation nephropathy (>120 mg/dl). Sixteen of 26 (16/26) male rats receiving 13.0 Gy leg-out PBI and surviving ARS were moribund between 65 and 103 days after irradiation, with an average time to morbidity of 85 (79–91) days. In males, pleural effusions of >3 ml were observed upon necropsy in all rats moribund at this time. The average BUN of these rats was 29.0 (25–33) mg/dl, once again demonstrating an absence of severe radiation nephropathy. Endpoints such as the breathing interval, pleural effusions at necropsy and/or histology have been used to confirm morbidity from lung injury during this period (Medhora et al. 2019).
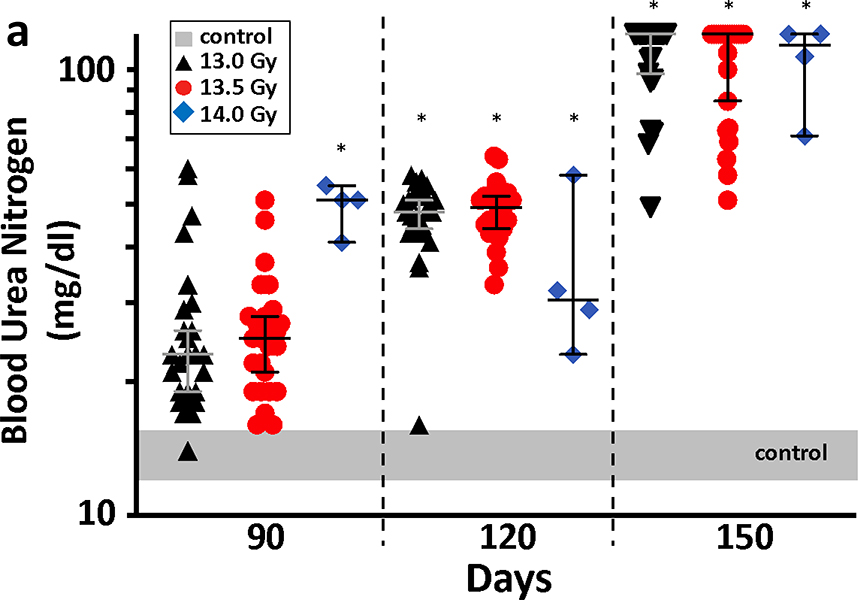
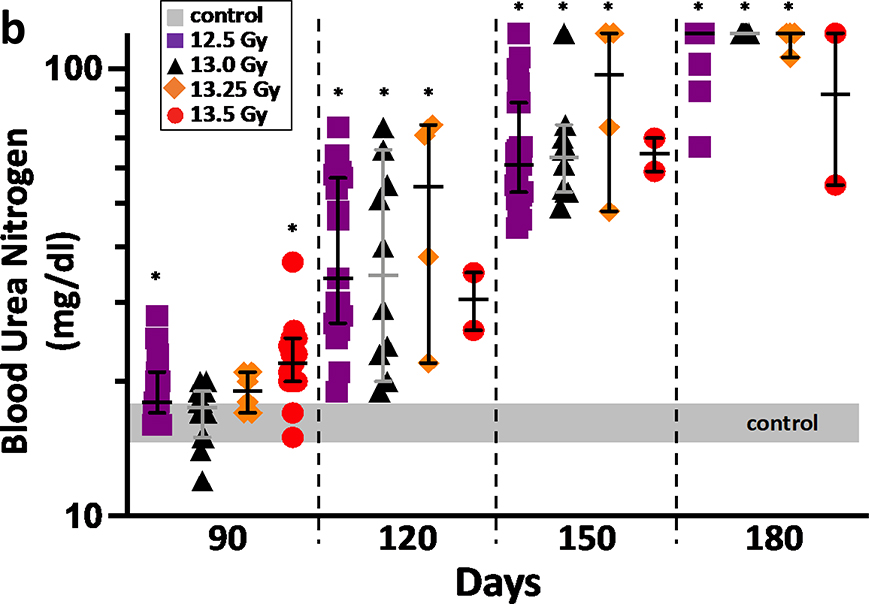
K-DEARE, BUN:
The progression of radiation nephropathy is shown by the BUN at 3–4 times after leg-out PBI (figure 7a and 7b). The increase in BUN is a marker of renal dysfunction (Moulder et al. 2011). All surviving female and male rats, after pneumonitis will develop renal failure (BUN>120 mg/dL) at the radiation doses used here (Medhora et al. 2019). Male rats have a longer latency period and succumb to lethal renal injury at 180 days as compared to 150 days for female rats.
Figure 7a.
Measurement of K-DEARE. Progression of renal injury after leg-out PBI in female WAG/RijCmcr rats is shown in the left panel. Female rats from the DRR study, at doses of 13 Gy (black triangles), 13.5 Gy (red circles) and 14 Gy (blue diamonds) leg-out PBI, had increased BUN level from day 120 as compared to non-irradiated control rats (grey bar). Irradiated female rats at the doses presented, progress to renal failure (defined as a BUN>120 mg/dl) by 150 days after leg-out PBI. Data presented as medians and 95% CI. The asterisk (*) represents p<0.05 versus nonirradiated sex-matched rats.
7b. Progression of renal injury after leg-out PBI in male WAG/RijCmcr rats. Male rats at doses of 12.5 Gy (purple squares), 13 Gy (black triangles), 13.25 Gy (orange diamonds) and 13.5 Gy (red circles) leg-out PBI, had increased BUN levels from day 120 on, compared non-irradiated control rats (grey bar). Note that male rats have a longer latency period for renal injury than female rats, so they were followed to 180 days. Irradiated male rats at the doses presented, progress to renal failure by 180 day after leg-out PBI. Data presented as medians and 95% CI. The asterisk (*) represents p<0.05 versus nonirradiated sex-matched rats.
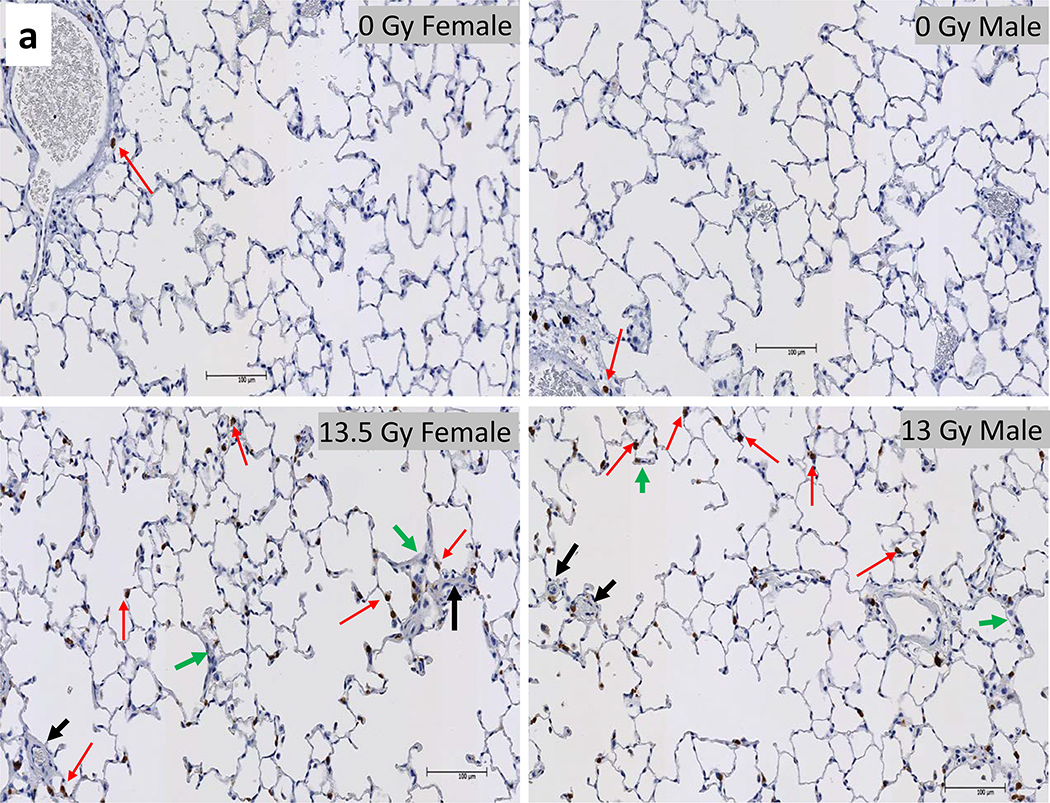
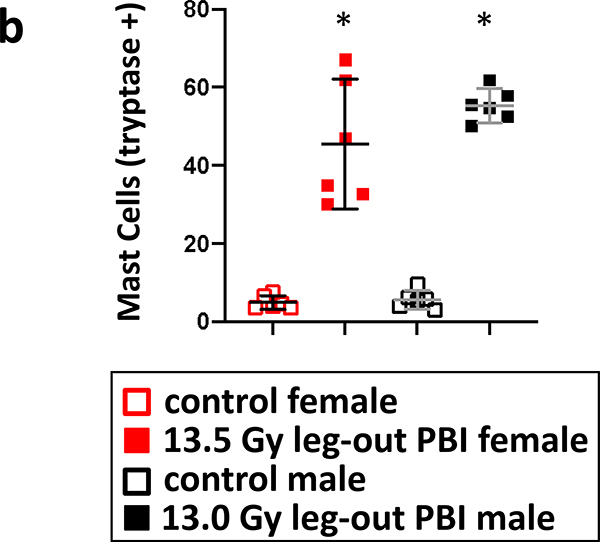
L-DEARE, histology, lung:
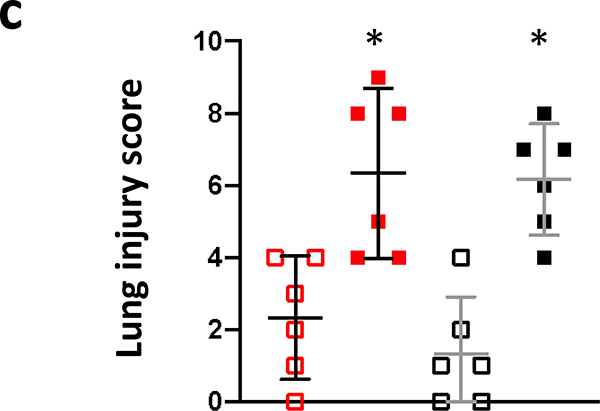
The results of histology scoring of lung sections, from female and male irradiated rats, during pneumonitis (days 60–100 after PBI) are shown in figure 8a. Mast cells (tryptase positive cells; brown stain) were increased in both female (euthanized at 69 days after 13.5 Gy leg-out PBI) and male (euthanized at 89 days after 13.0 Gy leg-out PBI) irradiated rats compared to non-irradiated-sex-matched control rats (euthanized at 150 and 180 days respectively). The quantification (figure 8b) shows the increase of mast cell with the mean and 95% CI, p<0.05. Lung injury scoring was also assessed. Increases in blood vessel thickness (see black arrows), alveolar-wall thickness (green arrows) and an increase in the numbers of foamy macrophages (not shown in these tryptase stained sections) were scored in the lungs. The composite score for these injuries reached statistical significance for both 13.5 Gy PBI female and 13.0 Gy PBI male rats when compared to non-irradiated, sex-matched control rats p<0.05 (figure 8c). Typically, during radiation pneumonitis in WAG/RijCmcr rats (Szabo et al. 2010) there is an increase in tryptase positive mast cells and in lung injury scores.
Figure 8a.
Histology of lung injury during pneumonitis. Irradiated female and male rats that were moribund between days 60–100 were accessed for radiation induced lung injury (RILI). Whole mount lung sections (4 μm thick) were stained with anti-tryptase antibody for mast cells (stained brown, red arrows).
8b. Graphical representation of lung mast cell counts from female and male rats. Lungs from both female and male irradiated rats had increased numbers of mast cells compared to their sex-matched control rats. Nonirradiated control rat lungs were harvested at termination (150-day females and 180-day males) as no rats in these groups were moribund during pneumonitis. Data is presented as means and 95% CI. The asterisk (*) represents p<0.05 versus nonirradiated sex-matched rats.
8c. Lung injury scores. Lung injury was scored as described in Materials and Methods. Individual scores for foamy macrophages, vascular wall thickness and alveolar wall thickness were determined on a 5-point scale as described (Medhora et al. 2014, 2015). A calculated mean composite lung injury score was derived and is shown. Data is presented as means and 95% CI. The asterisk (*) represents p<0.05 versus nonirradiated sex-matched rats.
K-DEARE, histology, kidney:
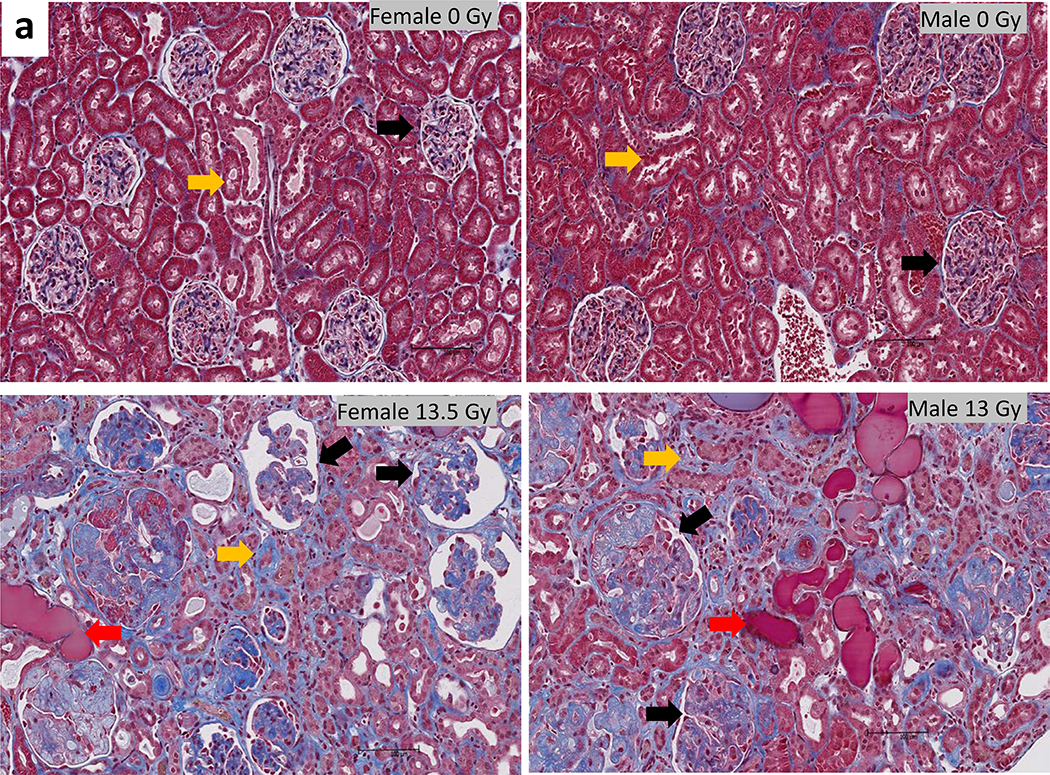
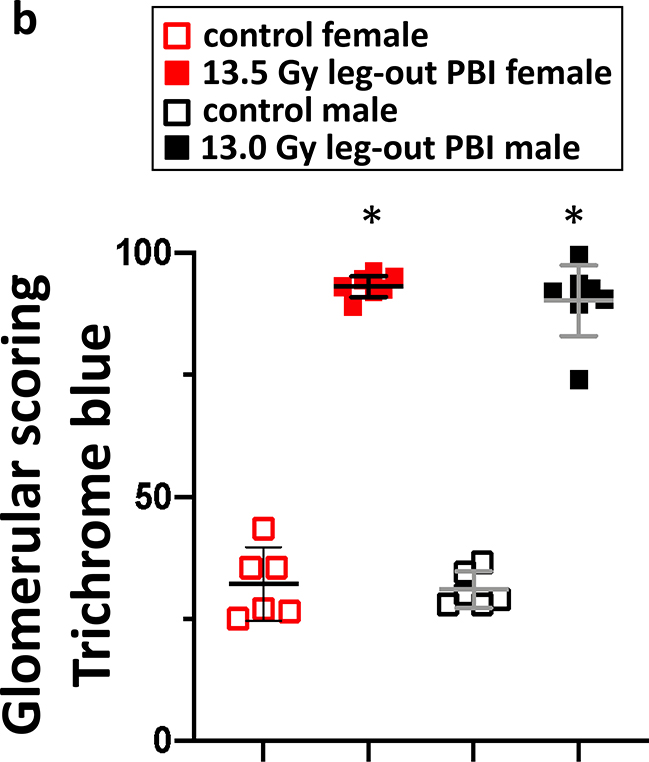
The results of histological scoring of Masson’s trichrome stained kidney sections from female and male rats at 140–180 days are shown in figure 9. Irradiated rats (lower panel) demonstrated marked kidney injury with the presence of microscopic cysts, interstitial fibrosis, tubular proteinaceous casts and glomerular injury in the cortical and medullary regions (Medhora et al. 2019). A composite score for fibrosis (blue staining in the glomeruli) reached statistical significance in both female and male rats as compared to non-irradiated, sex matched controls p<0.05 (figure 9b).
Figure 9a.
Histology of kidney injury and fibrosis during radiation nephropathy. Representative photomicrographs of kidney cortex from control female (upper left panel) and male (upper right panel) rats. Irradiated females at 150 days after 13.5 Gy leg-out PBI (lower left panel) and males at 180 days after 13 Gy leg-out PBI male (lower right panel) rats are shown. Arrows show normal (upper panel) or damaged (lower panel) glomeruli (black), tubules (yellow) and tubular casts (red).
9b. Graphical representation of the results from composite fibrosis scoring of kidneys from female and male rats are shown. Average scores for non-irradiated female and male rats are represented by the open squares, red and black respectively. Irradiated female and male rats are represented by solid red and black squares respectively. Irradiation dramatically increased glomerular fibrosis compared to nonirradiated sex-matched rats. Data presented as means and 95% CI. The asterisk (*) represents p<0.05 versus nonirradiated sex-matched rats.
DISCUSSION
The FDA Animal Rule, animal models:
The goal of this study was to develop animal models of concomitant ARS and DEARE to test efficacy of countermeasures for approval by the FDA animal rule. Specifically, there is a need to develop and test mitigators of radiation-induced pneumonitis in animals undergoing concomitant injury to multiple organs. Human lungs are sensitive to radiation. With supportive care and treatment for the H-ARS, it is likely that victims of radiological incidents will survive the to proceed to develop multiple organ injury (MOI). This DEARE characterized by radiation-induced pneumonitis and nephropathy (Moulder 2014a, Moulder et al. 2014b). There are no mitigators approved by the FDA to treat DEARE, making it imperative to validated animal models that develop radiation-induced MOI, in order to test mitigators of ARS and DEARE.
Recent interaction, presentations and discussions with the FDA, Division of Imaging and Radiation Medicine (DIRM) have initiated renewed emphasis on the use of a PBI/BM-sparing model to assess MCM efficacy against acute radiation-induced lung injury as opposed to the whole thorax lung irradiation (WTLI) model. Furthermore, the primary clinically relevant endpoint for FDA approval, remains focused on a significant decrease in all-cause mortality. In this regard, the statistical design and analysis of the study protocols to determine MCM efficacy to mitigate lethal radiation induced lung injury (RILI) using the PBI/BM-sparing models in the rat, mouse and NHP must account for mortality consequent to GI- and H-ARS, as well as lethal DEARE. This is a potential dilemma for each model that is certainly species and likely sex-dependent relative to the inherent dose- and time-dependent ARS and DEARE mortality. The database herein defined those parameters for the WAG/RijCmcr rat strain. All-cause mortality due to PBI with BM-sparing in the “leg-out” model remains a significant consideration, relative to pivotal study design for FDA approval under the animal rule.
The initial WAG/RijCmcr rat models used young adult (11–12 weeks old), juvenile (5–6 weeks old) female and male rats (Fish et al. 2020); these studies described herein are young adult, 11–12 weeks old rats. Furthermore, in previous studies only a small percentage of female and male rats (~15%) exposed to 13 Gy leg-out PBI developed lethal radiation pneumonitis (Fish et al. 2016) indicating large numbers of animals would be needed to test significant mitigation of lung damage. Using doses higher than 13 Gy would increase early GI toxicity (Fish et al. 2020), leaving fewer rats at risk for radiation pneumonitis. For these and other reasons, the existing rat models needed to be refined.
Model refinement, sex-dependent ARS and DEARE, LD values:
It is well known that antioxidants can alter the radiation sensitivity in animals (Brown et al. 2010, Kennedy et al. 2011, Johnke et al. 2014, Singh et al. 2017). WAG/RijCmcr rats on diets depleted of isoflavones demonstrated increased sensitivity to radiation (Moulder et al. 2019). Therefore, a moderate antioxidant diet, Teklad 2018, that contained an isoflavone content of 150–250 mg/kg was selected to replace Teklad 8604 (containing 350–550 mg isoflavones/kg) used in previous studies (Fish et al. 2016, Fish et al. 2020). A rigorous dose-response relationship (DRR) for survival was conducted in the current study, in male and female rats with Teklad 2018, as shown in figures 1 and 2.
The probit analyses in figure 3a show the LD50/30 to be slightly lower in male (13.5 Gy) than in female rats (14 Gy). This radio-sensitivity of male rats is also evident for mortality due to L-DEARE. The DRR for the rat model estimates the LD50/120 for males to be 12.86 Gy while the LD50/120 for females is 13.45 Gy. The multi-organ sequelae characteristic of the ARS and DEARE may be species-, strain- and sex-dependent. Mortality may also be model dependent, as the evolution of the DEARE occurs in survivors of the ARS. These variables have significant effects on model development. Interestingly, NHP studies report no difference in radiosensitivity between male and female animals though the group sizes are smaller than studies with rats and the diet is different and they have more genetic variability. Recently however, a sex difference in the NHP TBI model (LD50/60) has been reported (Beach et al. 2021). Differences in response to lung injury between males and females was absent in C57L/J mice (Jackson et al. 2014, Jackson et al. 2017) though males had a longer latency period prior to pneumonitis as compared to female mice (Dabjan et al. 2016).
MCM efficacy, all-cause mortality:
At 13.5 Gy, female rats on Teklad 2018 diet, demonstrated ~10% GI-lethality from ARS. From the >90% rats that survived, ~50% succumbed to L-DEARE (figure 1), making the 13.5 Gy dose better suited for MCM testing in female rats than 13 Gy described with Teklad 8604 diet. In male rats, 13 Gy leg-out PBI yielded ~60% lethality during radiation pneumonitis with the Teklad 2018 diet, though ARS lethality was relatively high, between 10–20% (figure 2). With 12.5 Gy, GI injury was below 10%, but lung lethality was very low (<5%) rendering this dose unsuitable for MCM testing for L-DEARE in male rats. Taken together, the DRR studies suggested that the most suitable models for testing MCM to mitigate L-DEARE are 13.5 Gy and 13 Gy for young adult female and male rats respectively and using the Teklad 2018 diet. These doses were chosen to further characterize the models.
The PBI/BM-sparing models allow for the analysis of time- and dose-dependent morbidity and mortality of ARS and DEARE, encompassing GI-, H-ARS, and L-DEARE. The time segments for the GI- and H-ARS mortality (d1–30) for rat are distinct and separated by long latency periods, approximately 50 days for the rat (figure 1 and 2), prior to the incidence of morbidity and mortality attributable to delayed lung injury. Interestingly, K-DEARE is delayed in male rats as compared to the females allowing males to survive longer after radiation (figures 1 and 2). This difference could be attributed to hormones, since the latency in juvenile male WAG/RijCmcr rats is comparable to that of juvenile females (Medhora et al. 2019).
There is an observed sex-dependent difference in mortality in WAG/RijCmcr rat strain obtained after statistical analysis using all-cause mortality at respective LD50/90, and LD50/120 for female and male rats with 80% power to detect p<0.05. In fact, the difference in the LD50 for L-DEARE between females and males will impact MCM study design parameters. Thus, separate efficacy studies with different radiation doses for each sex should be proposed for rats.
Clinical biomarkers:
Interestingly, by superimposing the body weight over the survival plots in figures 1 and 2, it becomes evident that changes in body weight is a simple biomarker to predict the onset and severity of GI, lung and renal injuries after leg-out PBI. At doses of 13, 13.5 and 14 Gy in female rats, and 12.5, 13 and 13.5 Gy in male rats, there was an initial drop in body weight starting from day 2, with the nadir being lower for the higher doses of radiation in both females and males. It is not clear if this nadir also reflects hematopoietic injury since the 2 sequelae, GI and bone marrow toxicities, appear within a few days of each other. Around 42 and 85 days the body weights begin to fall again just before the onset of lethal radiation pneumonitis and nephropathy respectively, with the steepest declines in body weight coinciding with the highest doses of radiation.
Though the primary end point of this study was to assess the time course of survival probability via the Kaplan-Meier analysis, measurements of secondary endpoints are essential to define the natural history and morbidity inherent in each concomitant, organ sequelae. These data confirm and establish the duration of acute, prolonged and delayed radiation effects to better inform MCM efficacy and FDA considerations. Therefore, secondary endpoints for GI, bone marrow, lung and renal toxicities were measured for the optimal 13.5 Gy female and 13 Gy male leg-out PBI models with the Teklad 2018 diet. Between 5–9 days, GI injury as determined by scoring diarrhea, occurring in both female and male rats (figure 4). Though ~7.5% of the bone marrow was spared during radiation, depletion of neutrophils and platelets reached a nadir at ~7 and ~14 days respectively, demonstrating H-ARS toxicity. These and other hematopoietic injuries were not severe enough to cause significant lethality. Decrease in breathing intervals was recorded from 28 days after leg-out PBI. The breathing intervals continued to drop, coincident with lung morbidity. It is known that breathing rate and intervals recover with time after radiation (Zhang et al. 2008, Kma et al. 2012, Medhora et al. 2014, Fish et al. 2016), though breathing intervals during radiation nephropathy remain to be measured in a leg-out PBI model. Renal injury increased exponentially in irradiated female and male rats after 13.5 and 13 Gy leg-out PBI respectively (figures 7a and 7b). These doses of irradiation are well above the threshold for renal damage (Moulder et al. 2011, Fish et al. 2020) so that recovery of kidney function is not possible, and all the rats that survive L-DEARE eventually succumb to radiation nephropathy. It is important to note that renal injury can be effectively mitigated by angiotensin converting enzyme (ACE) inhibitors in the high antioxidant diet model (Moulder et al. 2011, Medhora et al. 2012, Fish et al. 2016). The ACE inhibitor lisinopril is effective in mitigating DEARE in this new model with the moderate antioxidant diet (T-2018, unpublished results).
There are a number of advantages of the WAG/RijCmcr leg-out PBI rat model, most importantly, the occurrence of at least 4 distinct dose-, time- and sex-dependent sequelae. In similar NHP and mouse studies, experiments were terminated at a fixed time usually 180 days, (Booth et al. 2015, Cui et al. 2016, Cohen et al. 2017, MacVittie et al. 2020) and often all the animals were euthanized from severe pneumonitis indicating the dose of radiation may be considerably higher than the threshold for L-DEARE. Secondly, the irradiated WAG/RijCmcr rats developed pleural effusion which are observed in non-human primates (Garofalo et al. 2014, MacVittie et al. 2017, MacVittie et al. 2020) and humans (Zhao et al. 2017) but not in certain strains of mice (Jackson et al. 2010, Jackson et al. 2011). In addition, G-CSF and GM-CSF, which have already been approved for H-ARS by the FDA, can be tested in the PBI/BM-sparing rat models to determine compatibility with new drugs being tested for ARS or different DEARE sequelae. For example, the mitigator lisinopril, an ACE inhibitor, has been shown to attenuate radiation pneumonitis and nephropathy in the WAG/RijCmcr leg-out PBI rat model as poly-pharmacy approach with G-CSF (Fish et al. 2016).
There are also limitations of the WAG/RijCmcr leg-out PBI rat model. For example, the renal injury is more pronounced than reported in comparable NHP (Cohen et al. 2019) and mouse models (Unthank et al. 2015, Unthank et al. 2019). Humans experience radiation nephropathy (Lawton et al. 1991, Cohen et al. 1993), though the severity is not known, since the doses of radiation used in the clinic are restricted to ensure non-lethal toxicities to normal tissues. It is possible that shielding one kidney may increase the lifespan of the rats after leg-out PBI, revealing other DEARE-related injuries in different organs such as heart and brain. Limitations of the current study also include the absence of detailed histological examination of intestinal crypts to accurately record GI toxicity. Measurement of GI scoring was not optimal, as observed by the large error bars in figure 4. Also, long term GI and BM injuries were not measured as reported for mice (Booth et al. 2012a, Booth et al. 2012b, Booth et al. 2015). Future studies are underway with the same models to record injuries to the heart and brain and to determine biomarkers using metabolomics and lipidomic profiles.
In summary, a robust and versatile animal model for testing MCM for acute and delayed radiation injuries is described herein for female and male WAG/RijCmcr rats. The PBI/BM-sparing rat models allow for the analysis of time- and dose-dependent morbidity and mortality of ARS and DEARE, encompassing GI-, H-ARS, L-DEARE and K-DEARE. The time segments for the GI- and H-ARS mortality (d1–30) for rat are distinct and separated by long latency periods, approximately 50d, prior to the incidence of morbidity and mortality attributable to delayed lung injury. Survivors of L-DEARE, after second latency period develop radiation nephropathy (K-DEARE). These rodent models are currently among the best developed to contribute to MCM discovery by the criteria of the FDA Animal Rule.
Supplementary Material
Acknowledgments:
The authors wish to thank the Children’s Research Institute Histology Core Milwaukee, for tissue embedding, sectioning and staining. We also thank Dr. Lanyn Taliaferro at NIAID for the helpful discussions and input. Funding was supported by NIH NIAID contract HHSN272201800012C (BH) IPW, U01AI133594 (MM) MCW and the Department of Radiation Oncology at the Medical College of Wisconsin.
Financial support:
This work was supported by the following grants: NIH/NIAID contract HHSN272201800012C Innovation Pathways, U01s AI133594 & AI107305, and Department of Radiation Oncology, MCW.
Footnotes
Conflicts of Interest and Source of Funding:
The authors declare no conflict of interest.
REFERENCES
- Baker JE, Fish BL, Su J, Haworth ST, Strande JL, Komorowski RA, Migrino RQ, Doppalapudi A, Harmann L, Allen Li X, Hopewell JW, Moulder JE. 10 gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol 85: 1089–100; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach T, Authier S, Javitz HS, Wong K, Bakke J, Gahagen J, Bunin DI, Chang PY. Total body irradiation models in nhps - consideration of animal sex and provision of supportive care to advance model development. Int J Radiat Biol 97: 126–130; 2021. [DOI] [PubMed] [Google Scholar]
- Bertho JM, Prat M, Frick J, Demarquay C, Gaugler MH, Dudoignon N, Clairand I, Chapel A, Gorin NC, Thierry D, Gourmelon P. Application of autologous hematopoietic cell therapy to a nonhuman primate model of heterogeneous high-dose irradiation. Radiat Res 163: 557–70; 2005. [DOI] [PubMed] [Google Scholar]
- Booth C, Tudor G, Tonge N, Shea-Donohue T, MacVittie TJ. Evidence of delayed gastrointestinal syndrome in high-dose irradiated mice. Health Phys 103: 400–10; 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys 103: 383–99; 2012b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C, Tudor GL, Katz BP, MacVittie TJ. The delayed effects of acute radiation syndrome: Evidence of long-term functional changes in the clonogenic cells of the small intestine. Health Phys 109: 399–413; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Kolozsvary A, Liu J, Jenrow KA, Ryu S, Kim JH. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res 173: 462–8; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, Dudoignon N, Germain C, Mazurier C, Aigueperse J, Borneman J, Gorin NC, Gourmelon P, Thierry D. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med 5: 1028–38; 2003. [DOI] [PubMed] [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Joshi M, Tabbey R, Katz BP, MacVittie TJ, Orschell CM. Long-term hematopoietic stem cell damage in a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys 103: 356–66; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Katz BP, Carnathan GW, MacVittie TJ, Lenden K, Orschell CM. Survival efficacy of the pegylated G-CSFs maxy-g34 and neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Phys 106: 21–38; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HL, Plett PA, Fisher A, Sampson CH, Vemula S, Feng H, Sellamuthu R, Wu T, MacVittie TJ, Orschell CM. Lifelong residual bone marrow damage in murine survivors of the hematopoietic acute radiation syndrome (H-ARS): A compilation of studies comprising the indiana university experience. Health Phys 116: 546–557; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EP, Lawton CA, Moulder JE, Becker CG, Ash RC. Clinical course of late-onset bone marrow transplant nephropathy. Nephron 64: 626–35; 1993. [DOI] [PubMed] [Google Scholar]
- Cohen EP. Predictors of mortality in patients undergoing hemodialysis. N Engl J Med 330: 573; author reply 573–4; 1994. [DOI] [PubMed] [Google Scholar]
- Cohen EP, Hankey KG, Bennett AW, Farese AM, Parker GA, MacVittie TJ. Acute and chronic kidney injury in a non-human primate model of partial-body irradiation with bone marrow sparing. Radiat Res 188: 661–671; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EP, Hankey KG, Farese AM, Parker GA, Jones JW, Kane MA, Bennett A, MacVittie TJ. Radiation nephropathy in a nonhuman primate model of partial-body irradiation with minimal bone marrow sparing-part 1: Acute and chronic kidney injury and the influence of neupogen. Health Phys 116: 401–408; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Bennett AW, Zhang P, Barrow KR, Kearney SR, Hankey KG, Taylor-Howell C, Gibbs AM, Smith CP, MacVittie TJ. A non-human primate model of radiation-induced cachexia. Sci Rep 6: 23612; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabjan MB, Buck CM, Jackson IL, Vujaskovic Z, Marples B, Down JD. A survey of changing trends in modelling radiation lung injury in mice: Bringing out the good, the bad, and the uncertain. Lab Invest 96: 936–49; 2016. [DOI] [PubMed] [Google Scholar]
- DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, Ramakrishnan N. Medical countermeasures for radiation combined injury: Radiation with burn, blast, trauma and/or sepsis. Report of an niaid workshop, march 26–27, 2007. Radiat Res 169: 712–21; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. Radiation injury after a nuclear detonation: Medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep 5 Suppl 1: S32–44; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, Thullier P, Lataillade JJ, Herodin F. Single administration of stem cell factor, flt-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: Long-term follow-up of hematopoiesis. Blood 103: 878–85; 2004. [DOI] [PubMed] [Google Scholar]
- Drouet M, Delaunay C, Grenier N, Garrigou P, Mayol JF, Herodin F. Cytokines in combination to treat radiation-induced myelosuppresssion: Evaluation of SCF + glycosylated epo + pegylated G-CSF as an emergency treatment in highly irradiated monkeys. Haematologica 93: 465–6; 2008. [DOI] [PubMed] [Google Scholar]
- Farese AM, Bennett AW, Gibbs AM, Hankey KG, Prado K, Jackson W 3rd, MacVittie TJ. Efficacy of neulasta or neupogen on H-ARS and GI-ARS mortality and hematopoietic recovery in nonhuman primates after 10-Gy irradiation with 2.5% bone marrow sparing. Health Phys 116: 339–353; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Final rule. Fed Regist 67: 37988–98; 2002. [PubMed] [Google Scholar]
- FDA. Guidance for industry: Product development under the animal rule [online]. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/product-development-under-animal-rule. Accessed 02/02/21.
- Fish BL, Gao F, Narayanan J, Bergom C, Jacobs ER, Cohen EP, Moulder JE, Orschell CM, Medhora M. Combined hydration and antibiotics with lisinopril to mitigate acute and delayed high-dose radiation injuries to multiple organs. Health Phys 111: 410–9; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish BL, MacVittie TJ, Szabo A, Moulder JE, Medhora M. Wag/Rijcmcr rat models for injuries to multiple organs by single high dose ionizing radiation: Similarities to nonhuman primates (NHP). Int J Radiat Biol 96: 81–92; 2020. [DOI] [PubMed] [Google Scholar]
- Gao F, Narayanan J, Joneikis C, Fish BL, Szabo A, Moulder JE, Molthen RC, Jacobs ER, Rao RN, Medhora M. Enalapril mitigates focal alveolar lesions, a histological marker of late pulmonary injury by radiation to the lung. Radiat Res 179: 465–74; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo M, Bennett A, Farese AM, Harper J, Ward A, Taylor-Howell C, Cui W, Gibbs A, Lasio G, Jackson W 3rd, MacVittie TJ. The delayed pulmonary syndrome following acute high-dose irradiation: A rhesus macaque model. Health Phys 106: 56–72; 2014. [DOI] [PubMed] [Google Scholar]
- Herodin F, Roy L, Grenier N, Delaunay C, Bauge S, Vaurijoux A, Gregoire E, Martin C, Alonso A, Mayol JF, Drouet M. Antiapoptotic cytokines in combination with pegfilgrastim soon after irradiation mitigates myelosuppression in nonhuman primates exposed to high irradiation dose. Exp Hematol 35: 1172–81; 2007. [DOI] [PubMed] [Google Scholar]
- Jackson IL, Vujaskovic Z, Down JD. Revisiting strain-related differences in radiation sensitivity of the mouse lung: Recognizing and avoiding the confounding effects of pleural effusions. Radiat Res 173: 10–20; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IL, Vujaskovic Z, Down JD. A further comparison of pathologies after thoracic irradiation among different mouse strains: Finding the best preclinical model for evaluating therapies directed against radiation-induced lung damage. Radiat Res 175: 510–18; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IL, Xu PT, Nguyen G, Down JD, Johnson CS, Katz BP, Hadley CC, Vujaskovic Z. Characterization of the dose response relationship for lung injury following acute radiation exposure in three well-established murine strains: Developing an interspecies bridge to link animal models with human lung. Health Phys 106: 48–55; 2014. [DOI] [PubMed] [Google Scholar]
- Jackson IL, Baye F, Goswami CP, Katz BP, Zodda A, Pavlovic R, Gurung G, Winans D, Vujaskovic Z. Gene expression profiles among murine strains segregate with distinct differences in the progression of radiation-induced lung disease. Dis Model Mech 10: 425–437; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnke RM, Sattler JA, Allison RR. Radioprotective agents for radiation therapy: Future trends. Future Oncol 10: 2345–57; 2014. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Manning C, Hernady E, Reed C, Thurston SW, Finkelstein JN, Williams JP. Effect of total body irradiation on late lung effects: Hidden dangers. Int J Radiat Biol 87: 902–13; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AR, Ware JH, Carlton W, Davis JG. Suppression of the later stages of radiation-induced carcinogenesis by antioxidant dietary formulations. Radiat Res 176: 62–70; 2011. [DOI] [PubMed] [Google Scholar]
- Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res 53: 10–7; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton CA, Cohen EP, Barber-Derus SW, Murray KJ, Ash RC, Casper JT, Moulder JE. Late renal dysfunction in adult survivors of bone marrow transplantation. Cancer 67: 2795–800; 1991. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, Shea-Donohue T, Gelfond D, McFarland E, Jackson W 3rd, Lu W, Farese AM. The prolonged gastrointestinal syndrome in rhesus macaques: The relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys 103: 427–53; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett AW, M VC, Farese AM, Higgins A, Hankey KG. Immune cell reconstitution after exposure to potentially lethal doses of radiation in the nonhuman primate. Health Phys 106: 84–96; 2014. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett AW, Farese AM, Taylor-Howell C, Smith CP, Gibbs AM, Prado K, Jackson W 3rd. The effect of radiation dose and variation in neupogen(r) initiation schedule on the mitigation of myelosuppression during the concomitant GI-ARS and H-ARS in a nonhuman primate model of high-dose exposure with marrow sparing. Health Phys 109: 427–39; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Gibbs A, Farese AM, Barrow K, Bennett A, Taylor-Howell C, Kazi A, Prado K, Parker G, Jackson W III. Aeol 10150 mitigates radiation-induced lung injury in the nonhuman primate: Morbidity and mortality are administration schedule-dependent. Radiat Res 187: 298–318; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Parker GA, Bennett AW, Jackson WE, 3rd. Acute radiation-induced lung injury in the non-human primate: A review and comparison of mortality and co-morbidities using models of partial-body irradiation with marginal bone marrow sparing and whole thorax lung irradiation. Health Phys 119: 559–587; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Fish BL, Jacobs ER, Moulder JE, Szabo A. Dose-modifying factor for captopril for mitigation of radiation injury to normal lung. J Radiat Res 53: 633–40; 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Jacobs ER, Moulder JE. Radiation damage to the lung: Mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology 17: 66–71; 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Wu Q, Molthen RC, Jacobs ER, Moulder JE, Fish BL. Model development and use of ace inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res 182: 545–55; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Glisch C, Narayanan J, Sharma A, Harmann LM, Lawlor MW, Snyder LA, Fish BL, Down JD, Moulder JE, Strande JL, Jacobs ER. Whole-thorax irradiation induces hypoxic respiratory failure, pleural effusions and cardiac remodeling. J Radiat Res 56: 248–60; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Gasperetti T, Narayanan J, Khan AH, Jacobs ER, Fish BL. Delayed effects of acute radiation exposure (DEARE) in juvenile and old rats: Mitigation by lisinopril. Health Phys 116: 529–545; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ, Chittajallu S, Sampson C, Fisher A, Unthank JL, Orschell CM. A potential role for excess tissue iron in development of cardiovascular delayed effects of acute radiation exposure. Health Phys 119: 659–665; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP, Fish BL, Hill P. Prophylaxis of bone marrow transplant nephropathy with captopril, an inhibitor of angiotensin-converting enzyme. Radiat Res 136: 404–7; 1993a. [PubMed] [Google Scholar]
- Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ace inhibitors. Int J Radiat Oncol Biol Phys 27: 93–9; 1993b. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res 175: 29–36; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE. 2013 Dade W. Moeller lecture: Medical countermeasures against radiological terrorism. Health Phys 107: 164–71; 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP, Fish BL. Mitigation of experimental radiation nephropathy by renin-equivalent doses of angiotensin converting enzyme inhibitors. Int J Radiat Biol 90: 762–8; 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Fish BL, Cohen EP, Flowers JB, Medhora M. Effects of diet on late radiation injuries in rats. Health Phys 116: 566–570; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AM, Plett PA, Chua HL, Sampson CH, Fisher A, Feng H, Unthank JL, Miller SJ, Katz BP, MacVittie TJ, Orschell CM. Development of a model of the acute and delayed effects of high dose radiation exposure in Jackson diversity outbred mice; comparison to inbred C57BL/6 mice. Health Phys 119: 633–646; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AM, Sellamuthu R, Plett PA, Sampson CH, Chua HL, Fisher A, Vemula S, Feng H, Katz BP, Tudor G, Miller SJ, MacVittie TJ, Booth C, Orschell CM. Establishing pediatric mouse models of the hematopoietic acute radiation syndrome and the delayed effects of acute radiation exposure. Radiat Res; 2021. [DOI] [PubMed] [Google Scholar]
- Singh VK, Hanlon BK, Santiago PT, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part iii. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int J Radiat Biol 93: 885–906; 2017. [DOI] [PubMed] [Google Scholar]
- Szabo S, Ghosh SN, Fish BL, Bodiga S, Tomic R, Kumar G, Morrow NV, Moulder JE, Jacobs ER, Medhora M. Cellular inflammatory infiltrate in pneumonitis induced by a single moderate dose of thoracic x radiation in rats. Radiat Res 173: 545–56; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketa ST, Carsten AL, Cohn SH, Atkins HL, Bond VP. Active bone marrow distribution in the monkey. Life Sci 9: 169–74; 1970. [DOI] [PubMed] [Google Scholar]
- Unthank JL, Miller SJ, Quickery AK, Ferguson EL, Wang M, Sampson CH, Chua HL, DiStasi MR, Feng H, Fisher A, Katz BP, Plett PA, Sandusky GE, Sellamuthu R, Vemula S, Cohen EP, MacVittie TJ, Orschell CM. Delayed effects of acute radiation exposure in a murine model of the H-ARS: Multiple-organ injury consequent to <10 Gy total body irradiation. Health Phys 109: 511–21; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unthank JL, Ortiz M, Trivedi H, Pelus LM, Sampson CH, Sellamuthu R, Fisher A, Chua HL, Plett A, Orschell CM, Cohen EP, Miller SJ. Cardiac and renal delayed effects of acute radiation exposure: Organ differences in vasculopathy, inflammation, senescence and oxidative balance. Radiat Res 191: 383–397; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Plett PA, Chua HL, Jacobsen M, Sandusky GE, MacVittie TJ, Orschell CM. Immune reconstitution and thymic involution in the acute and delayed hematopoietic radiation syndromes. Health Phys 119: 647–658; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Ghosh SN, Zhu D, North PE, Fish BL, Morrow NV, Lowry T, Nanchal R, Jacobs ER, Moulder JE, Medhora M. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: Injury and recovery. Int J Radiat Biol 84: 487–97; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Day RM, Jin JY, Quint L, Williams H, Ferguson C, Yan L, King M, Albsheer A, Matuszak M, Kong FS. Thoracic radiation-induced pleural effusion and risk factors in patients with lung cancer. Oncotarget 8: 97623–97632; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.