Abstract
Background
The gut microbial dysbiosis and gender differences in the pathogenesis of acne vulgaris have long been postulated respectively. However, there was no data about a gender-related discrepancy in gut microbiota and microbial metabolism in acne.
Objective
This study aimed at identifying the underlying gender-related difference in gut microbiota and metabolism in acne vulgaris.
Methods
Fecal samples were collected from 43 acne patients and 43 age and gender-matched controls. Gut microbiota was analyzed by sequencing the V3-V4 region of 16SrDNA gene and microbial metabolites were quantitatively detected using gas chromatography time-of-flight mass spectrometry.
Results
Compared with healthy controls, the men had a lower abundance of 18 microbes such as Butyricicoccus, Clostridium sensu stricto, Faecalibaculum, Bacillus, Lactococcus, Blautia, Clostridiales, Lachnospiracea incertae sedis, Ruminococcus at genus level. However, the female patients only showed increased Clostridium sensu stricto and declined Oscillibacter and Odoribacterin. Additionally, the disordered metabolism of fatty acids was identified in male patients, while the dysbiosis of amino acids metabolism in female ones.
Conclusion
The disorder of gut microbiota and metabolism in acne vulgaris was gender-specific, which supported the potential role of gender difference in the pathogenesis of this disease.
Keywords: Acne vulgaris, Gastrointestinal microbiome, Gender differences, Metabolites
INTRODUCTION
Acne vulgaris is a common inflammatory dermatosis of the pilosebaceous units, affecting 85% of adolescents and young adults aged between 12 to 25 years1. Although acne prevalence among adolescents is comparable across gender, with a man to woman ratio of about 1/1.1~1.25 in Asians, acne is much more common in adult women than in adult men and it can be more severe in men than in women2,3. Recently, there has been an increasing interest in the gender differences both in the pathogenesis and treatment of acne vulgaris. The facial sebaceous glands in Asian skin have been detected by three-dimensional ultrasound microscopy in a study, which observed cauliflower-shaped sebaceous glands in men while more cylindrical and smaller sebaceous glands in women than the young men4. CAG polymorphism in the androgen receptor gene of female acne patients was more associated with nodulocystic acne, comparing with it in man cases5. Even, the serum metabolomic profile in patients with acne vulgaris was gender specific6. In clinical practice, hormone-based treatment has been always suggested to women patients rather than to men cases. Additionally, increasing studies have reported the gender-related difference in responses to the treatment of acne vulgaris7,8.
Recently, it has been recognized that gut microbiota dysbiosis was not just a marker but also contributed to disease pathology9,10. Our previous study reported the existence of a gut microbial dysbiosis in patients with acne vulgaris, which was characterized by less microbial diversity and decreased Firmicutes/Bacteroidetes ratio11. In addition, Yan et al.12 found a decrease in Lactobacillus, Bifidobacterium, Butyricicoccus, Coprobacillus, and Allobaculum in acne patients compared with controls, which provided a new understanding of the link between acne and the alteration of gut flora. However, it remains unclear whether the dysbiosis of gut microbiota and its associated metabolism in patients with acne vulgaris were gender-specific.
In this study, we aim to investigate the discrepancies of gut microbes and associated metabolites between men and women acne patients, which may help explain the gender-related pathogenesis and provide potential therapeutic targets of acne vulgaris.
MATERIALS AND METHODS
Sample collection
All participants were middle-school or college students in Luzhou City, Sichuan province between August 2016 and May 2017, including 43 subjects with acne vulgaris and 43 age- and gender-matched healthy controls, which were described in our previous studies11. The severity of acne was determined according to the Japanese Acne Study Group criteria13, which was defined as mild (S1), moderate (S2), severe (S3), and very severe (S4) according to the number of open and closed comedones, papules, pustules, cysts and nodules on half of the face. The participants were divided further into four different groups according to gender: defined as woman acne set (FAS), woman control set (FCS), man acne set (MAS), and man control set (MCS). The participants had not used antibiotics, glucocorticoids, immunosuppressive drugs, or herbal medicines within the past 6 months and did not present with other dermatoses, obesity, infections, tumors, mental diseases, immunodeficiency, or any other systemic disorders. Fresh fecal samples were collected in a sterile container and frozen within 30 minutes at −80℃ until they were processed. All participants provided written informed consent for the use of data and samples for scientific purposes. This study was approved by the Ethical Committees of the Affiliated Hospital of Southwest Medical University (KY2019139).
16S amplicon preparation, sequencing, processing, and analysis
Microbial DNA was extracted from fecal samples using the QIAamp DNA stool Mini Kit (Qiagen Ltd., Strasse, Germany) following the manufacturer's instruction. Amplification and sequencing of the V3-V4 16S rDNA gene region was performed as described previously1. The raw 16S data were processed by USEARCH to form operational taxonomic units (OTUs) at a 3% dissimilarity level. Bacterial taxonomy assignment was performed using the RDP database and classifier (http://rdp.cme.msu.edu). Measures of α-diversity (Simpson diversity index, Shannon diversity index) among groups were calculated based on the rarefied OTU counts. Principle component analysis (PCA) was performed on OTU in order to explore the natural distribution of the four group samples. Statistical analyses of the differences in gut microbiota among the four groups were performed by Wilcoxon test and Kruskal-Wallis test using R3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). All p-values reported are two-sided, and p<0.05 was considered to be statistically significant. We also applied the Benjamin and Hochberg false discovery rate test (FDR) or calculated the 95% confidence intervals (CI) if the FDR q value was >0.1.
Sample preparation for metabolites
Targeted quantitative analysis of 118 gut microbiome metabolites, including short-chain fatty acids (SCFAs), amino acids, carboxylic acids, benzoic acid derivatives, phenols, and indoles, was performed using the MicrobioMET platform (Metabo-Profile, Shanghai, China) with previously published methods14,15. Briefly, the frozen fecal samples were kept cool on a salt-ice bath, and approximately 50 mg of feces was homogenized with 300 µl of NaOH (1 M) solution followed by 200 µl of cold methanol using a homogenizer (BB24; Next, Advance Inc., Averill Park, NY, USA). The supernatant from the extractions was combined and capped in an autosampler vial. The derivatization with methyl chloroformate and injection was performed with a robotic Multi-Purpose Sampler (MPS2) with dual heads (Gerstel, Muehlheim, Germany).
Instrumentation for metabolite measurements
An Agilent 7890B gas chromatograph coupled with a GC-TOFMS system (Pegasus HT; Leco Corp., St. Joseph, MO, USA) operated in electron ionization (EI) mode was used to quantitate microbial metabolites in this project. A Rxi-5 ms capillary column (30 m×250 µm i.d., 0.25-µm film thickness; Restek Corporation, Bellefonte, PA, USA) was used for metabolite separation. The temperature program was set at 45℃ for 1 minute, increased to 260℃ at 20℃/min, reached 320℃ at 40℃/min, and remained at 320℃ for 2 minutes. Helium was used as the carrier gas at a constant flow rate of 1.0 ml/min. The temperature of the injection and transfer interface were both set to 270℃. The measurements were made using electron impact ionization (70 eV) in the full scan mode (m/z 50-500). Instrument optimization was performed as needed.
Metabolomic data analysis
The raw data generated by GC-TOFMS were processed using ADAP software16 for automatic baseline denoising, smoothing, peak picking, and peak signal alignment. Before the statistical analysis, we applied the Shapiro-Wilk test to examine the distribution of each continuous variable, including clinical characteristics, microbiomes, and microbial metabolomes. As a result, over 90% of the variables deviated from normal distribution; thus, non-parametric tests were used in this study. The Kruskal-Wallis test was used to compare the differences in metabolites among the four groups, including FAS, FCS, MAS, and MCS. Next, we used the Mann-Whitney U test to compare the difference of each metabolite between two sample sets, such as MAS and MCS groups. Variables with p-values smaller than 0.05 were considered to be statistically significant. In addition, we calculated Spearman's rank correlation coefficients to measure the relationships between metabolome and microbiome, which were further visualized using heat map analysis to indicate their positive or negative correlations.
RESULTS
Study cohorts
A total of 86 participants were divided into four sub-groups according to health state and their gender, their demographic information of the participants is summarized in Table 1. Gender and age between patients and healthy controls were matched. There was no difference in body mass index (p=0.225) among the four groups in this study. Women with acne vulgaris were more elderly than the men cases (p=0.02), but all of them were less than 25 years old and could be diagnosed as adolescent acne. Significantly, the men had more serious acne, compared with women (p<0.001). However, we have found no significant difference in bacterial diversity and structures among patient subgroups with different severity11.
Table 1. Summary of demographic information of study participants.
| MAS | FAS | MCS | FCS | p-value | |
|---|---|---|---|---|---|
| Number | 26 | 17 | 26 | 17 | - |
| Age (yr) | 19.31±2.45 | 21.00±2.98 | 19.31±2.45 | 21.00±2.98 | 0.015 |
| BMI (kg/cm2) | 21.08±1.78 | 19.70±1.51 | 21.41±5.24 | 20.16±2.05 | 0.225 |
| Disease severity (S1, S2, S3, S4) | 2, 9, 7, 8 | 10, 3, 2, 2 | - | - | <0.001 |
Values are presented as mean±standard error of mean. p-value was calculated from the Kruskal-Wallis test. BMI: body mass index, MAS: man acne set, FAS: woman acne set, MCS: man control set, FCS: woman control set.
Gender-based comparison in diversity and structure of gut microbiota between patients with healthy controls
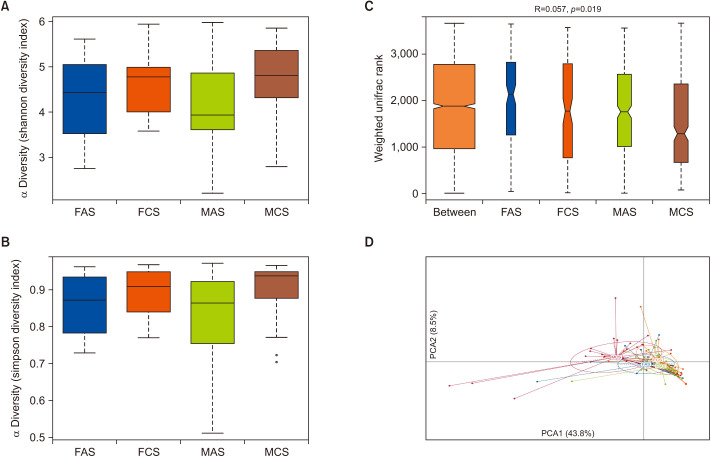
A total of 3,989,761 clean sequencing reads that passed the Q20 filtering were obtained from the 86 fecal samples, and the average length of base pairs was 416. Compared with healthy controls, microbial diversity was significantly decreased in MAS, as calculated by the Shannon diversity index (p=0.048) and Simpson diversity index (p=0.047; Fig. 1A, B). However, no significant difference in microbial diversity was observed between FAS patients and the controls. ANOSIM analysis indicated the significance of clustering samples among the four groups (R=0.057, p=0.019) (Fig. 1C). PCA scores plot of four groups based on OTUs indicated that MCS was the most different group from the other three groups, and MAS and MCS were separated while FAS and FCS groups were close to each other (Fig. 1D). Multi-response permutation procedure analysis indicated a significant difference among each set (p=0.044, data not shown).
Fig. 1. Comparisons of different diversity indices and microbiota structure in four samples. Participants were separated into four groups as the MAS, FAS, and their control sets (MCS, FCS). (A) Shannon diversity index (p=0.048) among four groups. (B) Simpson diversity index (p=0.047) among four group. (C) The difference among and within groups was assessed by ANOSIM analysis. (D) PCoA plot with different relative abundances of OTU among four groups. FAS: woman acne set, FCS: woman control set, MAS: man acne set, MCS: man control set.
Gender-specific bacterial taxa differences between patients with acne vulgaris and healthy controls
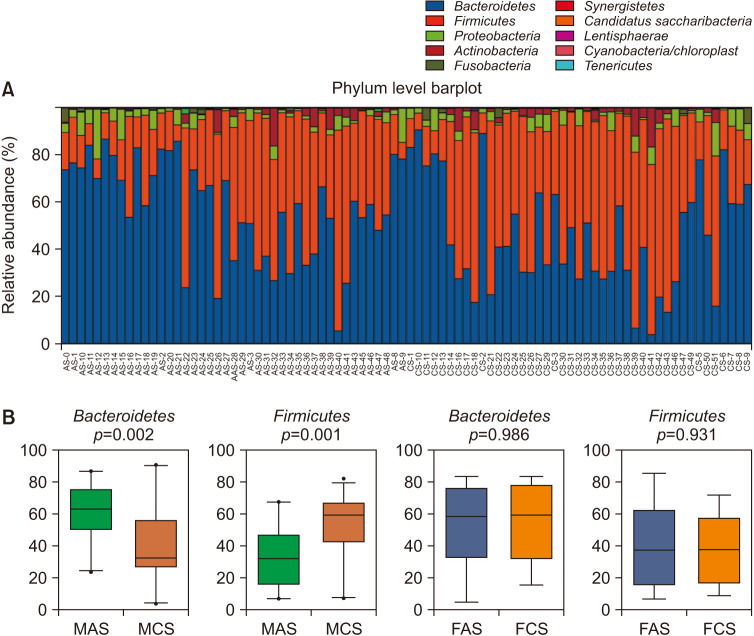
The majority of OTUs were assigned to four main phyla: Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria, which were also the most abundant ones in all of the subjects (Fig. 2A). The abundance of Firmicutes (p=0.001) and Bacteroidetes (p=0.002) were significantly increased and decreased in the MAS group compared to MCS respectively. However, the difference was not found between FAS and FCS groups (Fig. 2B). Then the gender-based comparison for patients and healthy people were further analyzed by Mann-Whitney test. When compared with healthy man, men with acne vulgaris had significantly lower abundance of such 18 genus as Lysinibacillus, Paenibacillus, Aerococcus, Alkaliphilus, Carnobacterium, Lactococcus, Oceanobacillus, Bacillus, Blautia, Butyricicoccus, Gemmiger, Lachnospiracea_incertae_sedis, Exiguobacterium, Pseudomonas, Enterococcus, Faecalibacterium, Bilophila, and Ruminococcus. However, the women just had increased Clostridium sensu stricto and declined Oscillibacter and Odoribacterin (Supplementary Fig. 1). Although the q-value >0.1 was calculated for all above genus, the 95% CIs of mean abundance difference of those taxa never spanned 0 (Supplementary Table 1).
Fig. 2. The majority of microbes at phyla level in all samples, and the gender-based difference of taxa at phyla level in patients with acne vulgaris. (A) The majority of OTU were assigned to four main phyla, Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria. (B) The comparison of the relative abundance of Firmicutes and Bacteroidetes between acne patients and their gender-matched controls. FAS: woman acne set, FCS: woman control set, MAS: man acne set, MCS: man control set.
Targeted analysis of gut microbial metabolites associated with gender in patients with acne vulgaris
Using time-of-flight mass spectrometry (GC-TOFMS) metabolomics platform, a total of 118 gut microbial metabolites were identified in fecal samples, including amino acids, fatty acids, carboxylic acids, hydroxylic acids, and phenolic acids, benzoyl and phenyl derivatives, and indoles. Multivariate orthogonal partial least squares discriminant analysis models were applied and the scores plots showed the overall differences of metabolic profiles between two groups (Supplementary Fig. 2). Then, we applied Mann-Whitney U test and identified 14 differential metabolites between the MCS and FCS groups, including increased 2-phenylglycine, D-2-hydroxyglutaric acid, glyceric acid, Glycine, L-alanine, L-alpha-aminobutyric acid, L-cystine, L-histidine, L-methionine, L-serine, myristic acid, ornithine, putrescine and decreased 3-hydroxyisovaleric acid in man (Supplementary Table 2). In addition, we identified 14 differential metabolites between the MAS and MCS groups and 13 ones between FAS and FCS (Table 2). Men had an increased level of 3-methylindole, alpha-linolenic acid, glyceric acid, glyceric acid, L-asparagine, L-tryptophan, linoleic acid, N-acetyltryptophan, oxoglutaric acid, phenyllactic acid, purine, stearic acid, succinic acid, and only one decreased valeric acid. The concentration of serum 2-phenylglycine, 4-hydroxyphenylpyruvic acid, glycine, L-alanine, L-histidine, L-leucine, L-methionine, L-serine, L-tryptophan, L-valine, methylsuccinic acid, N-acetyltryptophan, ornithine was higher in women than them in healthy controls. We can see, the women and men patients had completely different changes of metabolites while comparing with controls. The men tended to have disordered metabolism of fatty acids, but the women's cases had dysbiosis of metabolites from amino acids.
Table 2. Gender-based difference of metabolites between patients and healthy controls.
| Metabolites | Group | p-value | Z-value | ||
|---|---|---|---|---|---|
| Acne set | Control set | ||||
| Man | |||||
| 3-Methylindole | 0.63±0.94 | 0.33±1.15 | 0.029 | –2.18 | |
| Alpha-linolenic acid | 39.44±48.74 | 35.93±20.90 | 0.046 | –2.00 | |
| Glyceric acid | 516.53±663.92 | 174.41±298.02 | 0.039 | –2.07 | |
| Hydroxypropionic acid | 25.61±39.27 | 17.23±32.45 | 0.039 | –2.07 | |
| L-asparagine | 19.07±32.05 | 17.35±57.91 | 0.037 | –2.09 | |
| L-tryptophan | 11.58±16.71 | 6.74±13.70 | 0.042 | –2.03 | |
| Linoleic acid | 124.66±120.85 | 62.47±52.79 | 0.022 | –2.29 | |
| N-acetyltryptophan | 21.14±31.97 | 12.00±26.23 | 0.044 | –2.01 | |
| Oxoglutaric acid | 48.06±57.30 | 17.49±29.14 | 0.013 | –2.47 | |
| Phenyllactic acid | 0.42±0.42 | 0.18±0.16 | 0.019 | –2.35 | |
| Purine | 1.62±2.38 | 1.07±1.45 | 0.021 | –2.31 | |
| Stearic acid | 11.07±7.10 | 7.92±6.26 | 0.035 | –2.11 | |
| Succinic acid | 28.89±31.97 | 13.84±18.99 | 0.046 | –2.00 | |
| Valeric acid | 4,506.14±1,841.31 | 5,499.25±1,502.47 | 0.017 | –2.38 | |
| Woman | |||||
| 2-Phenylglycine | 2.73±2.66 | 0.75±0.60 | 0.018 | –2.36 | |
| 4-Hydroxyphenylpyruvic acid | 1.77±1.42 | 0.91±0.32 | 0.007 | –2.70 | |
| Glycine | 5.51±3.43 | 2.77±2.01 | 0.008 | –2.67 | |
| L-alanine | 2.76±1.72 | 1.39±1.00 | 0.008 | –2.67 | |
| L-histidine | 6.78±3.82 | 3.80±3.36 | 0.015 | –2.43 | |
| L-leucine | 33.83±24.89 | 20.01±14.88 | 0.048 | –1.98 | |
| L-methionine | 74.60±53.23 | 38.71±28.07 | 0.022 | –2.29 | |
| L-serine | 12.90±7.88 | 8.22±6.89 | 0.031 | –2.15 | |
| L-tryptophan | 5.97±6.05 | 3.16±2.72 | 0.024 | –2.26 | |
| L-valine | 27.12±24.65 | 13.29±10.00 | 0.034 | –2.12 | |
| Methylsuccinic acid | 5.31±16.59 | 0.70±0.46 | 0.044 | –2.02 | |
| N-acetyltryptophan | 9.97±10.87 | 5.26±4.93 | 0.024 | –2.26 | |
| Ornithine | 3.21±10.37 | 0.34±1.13 | 0.031 | –2.15 | |
Values are presented as mean±standard error of mean.
Integrated analysis of gut microbiota and metabolites
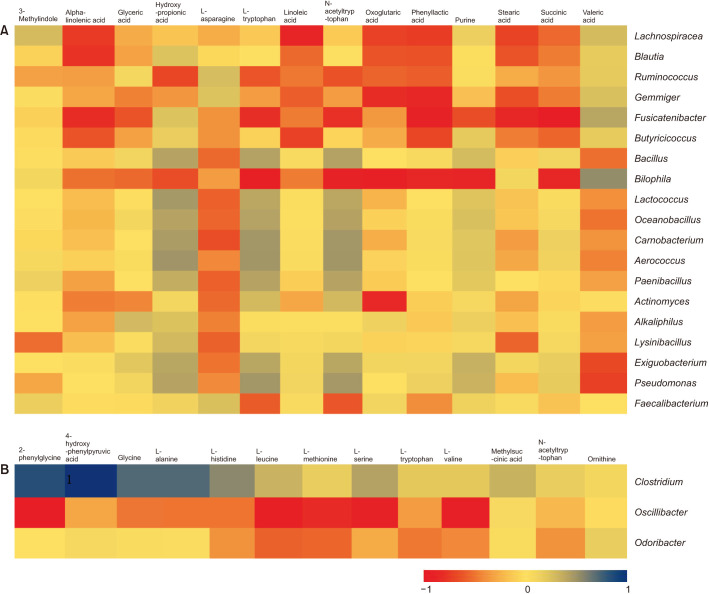
Next, we performed the Spearman correlation analysis between differential microbes and metabolites identified by comparing gender-based patients with their controls. Their relative positive or negative relationships were visualized using heat maps (Fig. 3). Most interestingly, we observed that the metabolites of linoleic acid, alpha-Linolenic acid, oxoglutaric acid, phenylacetic acid, stearic acid, succinic acid were negatively associated with such microbes as Lachnospiracea, Blautia, Ruminococcus, Gemmiger, Fusicatenibacter, and Butyricicoccus in men with acne. For women, the metabolites of 2-phenylglycine, 4-hydroxyphenylpyruvic acid, glycine, L-alanine were positively related to Clostridium sensu stricto and 2-phenylglycine, L-leucine, L-methionine, L-serine, L-valine was negatively correlated with declined Oscillibacter.
Fig. 3. Correlation between gut microbial taxa and metabolites was assessed by Person correlation test and displayed as a heat map. (A) For men patients; (B) for women patients.
DISCUSSION
Acne is the most common skin disease, as well as a cardinal component of many systemic diseases or syndromes17. Although it clearly develops from an interplay of environmental factors and genetic predisposition, the exact cause of acne remains elusive. It was increasingly believed that the gut microbiota could be involved in the pathogenic process of acne11,12. In addition, the previous data have indicated that disease-associated differences in gut microbiota composition, functions, and ecological networks were markedly influenced by gender18,19,20,21,22,23. In this study, the decreased diversity of gut microbiota was only found in the men acne samples but not in women, which also occurs in other inflammatory skin diseases24,25. It was shown in previous studies that people with low microbial diversity are characterized by a more pronounced inflammatory phenotype26. For acne vulgaris, the men tend to suffer more severe lesion3, which could be associated with the low bacterial richness.
Besides, the lower abundance of anti-inflammation-related bacteria such as Butyricicoccus, Bacillus, Lactococcus, Blautia, Clostridium sensu stricto, Faecalibaculum, Clostridiales, Lachnospiracea_incertae_sedis, and Ruminococcus also linked to the greater inflammation in men with acne3. Butyricicoccus generates butyrate, which provides energy to cells and prevents mucosal barrier damage and inflammation27. The Clostridium sensu stricto, Faecalibaculum are both SCFAs-producing bacteria28, which plays an important role in the connection between gut and skin microbiota29. SCFAs were shown to have a profound antimicrobial effect against methicillin-resistant Staphylococcus aureus, consequently contributing to shaping the skin microbiota, which may influence cutaneous immunity30. Propionibacterium acne and Staphylococcus epidermidis are examples of skin commensals enduring wide SCFA shifts. In addition, the decreased Clostridiales, Lachnospiracea incertae sedis, and Ruminococcus were also found in men with een reported to be less abundant in immune and inflammatory diseases11,31. Furthermore, the man patient group showed several decreased genera taxa belonging to Bacilli class, such as Lactococcus, Carnobacterium, Aerococcus, Lysinibacillus, Bacillus, and Oceanobacillus. Bacilli is known to produce a vast array of antimicrobial compounds, such as 3-hydroxypropionaldehyde and common probiotics32,33. Recent studies reported the declined abundance of beneficial bacteria, Lactococcus, Bacillus, Clostridium in correlation with the inhibition of the mTOR pathway, the key signaling for promoting the proliferation and secretion of sebaceous glands34,35. Finally, our study found the significantly rising long-chain saturated fatty acids, Alpha-linolenic acid, linoleic acid, stearic acid, while the declining SCFA valeric acid in men. Linoleic acid, an important omega-6 fatty acid, has been reported to be a pro-inflammatory substance and even aggravate the severity of acne vulgaris36,37. A previous study showed that a large number of these unsaturated fatty acids, alpha-linolenic acid, linoleic acid, were parallel to the down-regulation of the PI3K/Akt/mTOR pathway38. However, further studies should be performed to identify how the disorder of gut microbiota and its associated metabolites contribute to the pathogenesis of man acne through modulating mTOR signaling.
A cohort study including 4,7111 patients with acne vulagris found that woman gender was independently and jointly associated with major depression and suicide39. In this study, the increased microbes of Clostridium sensu stricto and declined Oscillibacter and Odoribacterin were identified in women acne patients, which also has been reported to be associated with psychological stress40. In the aspect of metabolism, microbial metabolites from aminoadipic acids, such as L-alanine, L-histidine, L-leucine, L-methionine, L-serine, L-tryptophan, L-valine, were increased in the FAS group. The dysbiosis of aminoadipic acid metabolism has been reported to be associated with an increased risk of stress-related psychiatric illnesses41. This study also found the positive correlation of L-leucine, L-methionine, L-serine, L-valine with the relative abundance of Oscillibacter, a depression-associated bacteria42. In 1930, Stokes and Pillsbury postulated that psychological stress causes intestinal microbes to produce neurotransmitters that cross the intestinal mucosa to enter the bloodstream, resulting in systemic inflammation16. The theory of the microbiota-gut-brain axis may further support the mechanism of gut microbiota underlying the pathogenesis of acne vulgaris in women.
In conclusion, this study demonstrated for the first time that men and women acne vulgaris patients have significantly different dysbiosis of gut microbiota and associated metabolites. However, there were some limitations of this study. Firstly, we detected 145 gut microbiome metabolites but did not perform micro metabolomics analysis; however, the measurement of these products was quantitative. Additionally, the sample size of adolescent acne was limited. But in this study, all participants had not been treated with antibiotics for at least 6 months and were age- and gender-matched, which could consolidate the result. Lastly, the study was descriptive and the causal relationship of gut microbiota and acne was not shown.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This study was funded by the youth fund of the major special project for social development of Sichuan Science and Technology Department (granted number: 2019YFS0253), the Luzhou Science and Technology Project of the Office of Science and Technology and Talent Work of Luzhou (granted number: 2017-S-40), and Scientific research project of Health Commission of Sichuan Province (grant no. 18PJ414). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-33-531-s001.pdf.
Gender-based difference of gut microbiota at genus level
Difference of metabolites between men and women controls
Gender-specific bacterial taxa differences between acne patients and healthy controls. Box plots with relative abundance of the different microbial taxa at genus level. Median values with the 95% confidence interval (of the median) are presented. FAS: woman acne set, FCS: woman control set, MAS: man acne set, MCS: man control set.
Multivariate orthogonal partial least squares discriminant analysis models were applied and the scores plots showed the overall differences of metabolic profiles between two groups. FAS: woman acne set, FCS: woman control set, MAS: man acne set, MCS: man control set.
References
- 1.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 2.Kubota Y, Shirahige Y, Nakai K, Katsuura J, Moriue T, Yoneda K. Community-based epidemiological study of psychosocial effects of acne in Japanese adolescents. J Dermatol. 2010;37:617–622. doi: 10.1111/j.1346-8138.2010.00855.x. [DOI] [PubMed] [Google Scholar]
- 3.Cook-Bolden FE, Gold MH, Guenin E. Tazarotene 0.045% lotion for the once-daily treatment of moderate-to-severe acne vulgaris in adult males. J Drugs Dermatol. 2020;19:78–85. doi: 10.36849/JDD.2020.3979. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara T, Nakagawa N, Shimizu N, Hirai N, Saijo Y, Sakai S. Gender-and age-related differences in facial sebaceous glands in Asian skin, as observed by non-invasive analysis using three-dimensional ultrasound microscopy. Skin Res Technol. 2019;25:347–354. doi: 10.1111/srt.12657. [DOI] [PubMed] [Google Scholar]
- 5.Demirkan S, Sayın DB, Gündüz Ö. CAG polymorphism in the androgen receptor gene in women may be associated with nodulocystic acne. Postepy Dermatol Alergol. 2019;36:173–176. doi: 10.5114/ada.2019.84592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MH, Ha IJ, Kim E, Kim K. Integrated targeted serum metabolomic profile and its association with gender, age, disease severity, and pattern identification in acne. PLoS One. 2020;15:e0228074. doi: 10.1371/journal.pone.0228074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lain E, Day D, Harper J, Guenin E. Tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: impact of gender and race on efficacy and safety. J Drugs Dermatol. 2019;18:1128–1138. [PubMed] [Google Scholar]
- 8.Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381–384. [PubMed] [Google Scholar]
- 9.Vojvodic A, Peric-Hajzler Z, Matovic D, Vojvodic P, Vlaskovic-Jovicevic T, Sijan G, et al. Gut microbiota and the alteration of immune balance in skin diseases: from Nutraceuticals to fecal transplantation. Open Access Maced J Med Sci. 2019;7:3034–3038. doi: 10.3889/oamjms.2019.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YB, Byun EJ, Kim HS. Potential role of the microbiome in acne: a comprehensive review. J Clin Med. 2019;8:987. doi: 10.3390/jcm8070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Y, Wang H, Zhou J, Mou Y, Wang G, Xiong X. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venereol. 2018;98:783–790. doi: 10.2340/00015555-2968. [DOI] [PubMed] [Google Scholar]
- 12.Yan HM, Zhao HJ, Guo DY, Zhu PQ, Zhang CL, Jiang W. Gut microbiota alterations in moderate to severe acne vulgaris patients. J Dermatol. 2018;45:1166–1171. doi: 10.1111/1346-8138.14586. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi N, Akamatsu H, Kawashima M. Establishment of grading criteria for acne severity. J Dermatol. 2008;35:255–260. doi: 10.1111/j.1346-8138.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Ni Y, Su M, Li H, Dong F, Chen W, et al. High throughput and quantitative measurement of microbial metabolome by gas chromatography/mass spectrometry using automated alkyl chloroformate derivatization. Anal Chem. 2017;89:5565–5577. doi: 10.1021/acs.analchem.7b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni Y, Qiu Y, Jiang W, Suttlemyre K, Su M, Zhang W, et al. ADAP-GC 2.0: deconvolution of coeluting metabolites from GC/TOF-MS data for metabolomics studies. Anal Chem. 2012;84:6619–6629. doi: 10.1021/ac300898h. [DOI] [PubMed] [Google Scholar]
- 16.Forsythe P, Kunze W, Bienenstock J. Moody microbes or fecal phrenology: what do we know about the microbiota-gut-brain axis? BMC Med. 2016;14:58. doi: 10.1186/s12916-016-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zouboulis CC. Acne as a chronic systemic disease. Clin Dermatol. 2014;32:389–396. doi: 10.1016/j.clindermatol.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Santos-Marcos JA, Haro C, Vega-Rojas A, Alcala-Diaz JF, Molina-Abril H, Leon-Acuña A, et al. Sex differences in the gut microbiota as potential determinants of gender predisposition to disease. Mol Nutr Food Res. 2019;63:e1800870. doi: 10.1002/mnfr.201800870. [DOI] [PubMed] [Google Scholar]
- 19.Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, Quintana-Navarro GM, Garcia-Carpintero S, Malagon MM, et al. Influence of gender and menopausal status on gut microbiota. Maturitas. 2018;116:43–53. doi: 10.1016/j.maturitas.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Zhang M, Xue J, Huang J, Zhuang R, Zhou X, et al. Body mass index differences in the gut microbiota are gender specific. Front Microbiol. 2018;9:1250. doi: 10.3389/fmicb.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, et al. The impact of gut microbiota on gender-specific differences in immunity. Front Immunol. 2017;8:754. doi: 10.3389/fimmu.2017.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 24.Morales P, Fujio S, Navarrete P, Ugalde JA, Magne F, Carrasco-Pozo C, et al. Impact of dietary lipids on colonic function and microbiota: an experimental approach involving orlistat-induced fat malabsorption in human volunteers. Clin Transl Gastroenterol. 2016;7:e161. doi: 10.1038/ctg.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consolandi C, Turroni S, Emmi G, Severgnini M, Fiori J, Peano C, et al. Behçet's syndrome patients exhibit specific microbiome signature. Autoimmun Rev. 2015;14:269–276. doi: 10.1016/j.autrev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Boesmans L, Valles-Colomer M, Wang J, Eeckhaut V, Falony G, Ducatelle R, et al. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of Butyricicoccus pullicaecorum to healthy volunteers. mSystems. 2018;3:e00094-18. doi: 10.1128/mSystems.00094-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JW, Fang B, Pang GF, Zhang M, Ren FZ. Age-and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic Biochem Physiol. 2019;159:68–79. doi: 10.1016/j.pestbp.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Salem I, Ramser A, Isham N, Ghannoum MA. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459. doi: 10.3389/fmicb.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz A, Bruhs A, Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. 2017;137:855–864. doi: 10.1016/j.jid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Mao J, Zhou L, Xiong X, Deng Y. The imbalance of gut microbiota and its correlation with plasma inflammatory cytokines in pemphigus vulgaris patients. Scand J Immunol. 2019;90:e12799. doi: 10.1111/sji.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilinskaya ON, Ulyanova VV, Yarullina DR, Gataullin IG. Secretome of intestinal Bacilli: a natural guard against pathologies. Front Microbiol. 2017;8:1666. doi: 10.3389/fmicb.2017.01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise C, Novitsky L, Tsopmo A, Avis TJ. Production and antimicrobial activity of 3-hydroxypropionaldehyde from Bacillus subtilis strain CU12. J Chem Ecol. 2012;38:1521–1527. doi: 10.1007/s10886-012-0219-2. [DOI] [PubMed] [Google Scholar]
- 34.Jung MJ, Lee J, Shin NR, Kim MS, Hyun DW, Yun JH, et al. Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci Rep. 2016;6:30887. doi: 10.1038/srep30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan Y, Zhang Y, Dong H, Wang Y, Zhang J. Effects of dietary poly-β-hydroxybutyrate (PHB) on microbiota composition and the mTOR signaling pathway in the intestines of litopenaeus vannamei. J Microbiol. 2017;55:946–954. doi: 10.1007/s12275-017-7273-y. [DOI] [PubMed] [Google Scholar]
- 36.Ozdarska K, Osucha K, Savitskyi S, Malejczyk J, Galus R. [Diet in pathogenesis of acne vulgaris] Pol Merkur Lekarski. 2017;43:186–189. Polish. [PubMed] [Google Scholar]
- 37.Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18:2645. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C, Qiu S, Liu P, Ge Y, Gao X. Rhizoma Amorphophalli inhibits TNBC cell proliferation, migration, invasion and metastasis through the PI3K/Akt/mTOR pathway. J Ethnopharmacol. 2018;211:89–100. doi: 10.1016/j.jep.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Yang YC, Tu HP, Hong CH, Chang WC, Fu HC, Ho JC, et al. Female gender and acne disease are jointly and independently associated with the risk of major depression and suicide: a national population-based study. Biomed Res Int. 2014;2014:504279. doi: 10.1155/2014/504279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geng S, Yang L, Cheng F, Zhang Z, Li J, Liu W, et al. Gut microbiota are associated with psychological stress-induced defections in intestinal and blood-brain barriers. Front Microbiol. 2020;10:3067. doi: 10.3389/fmicb.2019.03067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. 2018;15:36–59. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chi L, Khan I, Lin Z, Zhang J, Lee MYS, Leong W, et al. Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine. 2020;67:153157. doi: 10.1016/j.phymed.2019.153157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gender-based difference of gut microbiota at genus level
Difference of metabolites between men and women controls
Gender-specific bacterial taxa differences between acne patients and healthy controls. Box plots with relative abundance of the different microbial taxa at genus level. Median values with the 95% confidence interval (of the median) are presented. FAS: woman acne set, FCS: woman control set, MAS: man acne set, MCS: man control set.
Multivariate orthogonal partial least squares discriminant analysis models were applied and the scores plots showed the overall differences of metabolic profiles between two groups. FAS: woman acne set, FCS: woman control set, MAS: man acne set, MCS: man control set.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.