Abstract
Oral carcinoma represents one of the most common malignancies worldwide. Oral squamous cell carcinomas (OSCCs) account over 90% of all oral malignant tumors and are characterized by high mortality in the advanced stages. Early diagnosis is often a challenge for its ambiguous appearance in early stages. Mucosal infection by the human papillomavirus (HPV) is responsible for a growing number of malignancies, particularly cervical cancer and oropharyngeal carcinomas. In addition, Candida albicans (C. albicans), which is the principal fungi involved in the oral cancer development, may induce carcinogenesis through several mechanisms, mainly promoting inflammation. Medical knowledge and research on adolescent/pediatric patients' management and prevention are in continuous evolution. Besides, microbiota can play an important role in maintaining oral health and therefore all human health. The aim of this review is to evaluate epidemiological and pathophysiological characteristics of the several biochemical pathways involved during HPV and C. albicans infections in pediatric dentistry.
1. Introduction
Oral carcinoma is the fifth most common malignant tumor worldwide and accounts for the majority of head and neck tumors [1, 2]. During the last decades, there has been a progressive increase in proportion of incidence of oral cancer not related to a known etiologic factor, such as the so-called “oral cancer in young,” a relevant disease in nonsmoker nondrinker (NSND) patients [3]. The topic is matter of long standing debate, and adequate study models to analyze this entity are lacking [4].
Squamous cell carcinomas (OSCCs) are the most common type of oral tumor and can occur in larynx and pharynx too, with over 90% of oral cancers and represents 2-3% of all cancers [5]. Besides, OSCCs are more common in people over the age of 50, while some studies report that 1%-6% occur under the age of 40. There is a special preference for men: in fact, the ratio of men to women in Western societies is 2 : 1 [6]. On the other hand, the incidence of injury in young adults has been noted to be increasing worldwide [4, 7]. In recent years, despite advances in diagnosis and oncologic therapy, the 5-year survival rate of oral carcinoma is still 50–60%, with a slight increase in the United States (US) during the last decade (66%) [6].
The current model of oral and pharyngeal carcinoma development suggests a progressive multistep transition from normal mucosa to OSCCs through a series of progressive histological changes (oral epithelial dysplasia) reflecting the accumulation of genetic and epigenetic abnormalities and genetic susceptibility [5, 6].
The evaluation of the literature and surveillance data concerning oral carcinoma is difficult because this neoplasia often is reported associated with other head and neck malignancies, and report of anatomic subsites is often unclear or can create confusion between its localization between oral cavity and oropharynx.
Candida albicans is a commensal fungal species commonly colonizing the human mucosal surfaces [7]. Carriage rates, corresponding to 18.5–40.9% in healthy individuals, are usually higher in individuals with compromised immunity, such as human immunodeficiency virus-positive individuals, diabetes patients, and infants and elder populations [7]. Besides, C. albicans can strongly interact with additional oral microorganisms and enforce noteworthy impact on the virulence of polymicrobial biofilms [7]. Furthermore, it is recognized that coinfection of C. albicans is strongly associated with increasing diseases in pediatric dentistry, such as severe caries in children (S-ECC) [7].
Thus, the purpose of the present paper is to review the current evidence on the role of C. albicans and human papillomavirus (HPV), as well their biomolecular mechanisms, in the pathogenesis of potentially malignant oral disorders (PMODs) and oral carcinoma (Figure 1) in pediatric dentistry.
Figure 1.
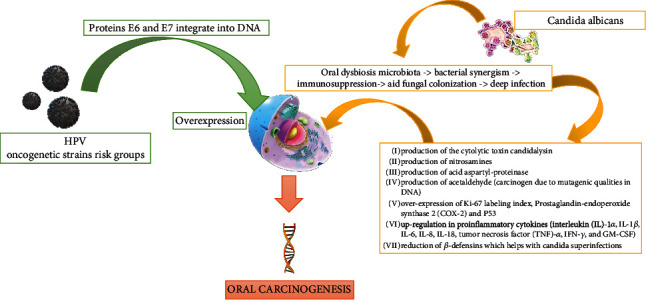
The pathways of carcinogenesis in the oral cavity through the oncogenetic HPV virus strain and Candida albicans. Several hypothetical mechanisms have been proposed for the fungal infection that can induce PMODs and malignant lesions in oral epithelium. The dysbiosis of the microbiota can play an important role with the subsequent alteration of the microbiome. There follows a synergism between “bad” bacteria with Candida albicans and HPV.
2. Risk Factors for Oral Cancer in Pediatric Dentistry
Several risk factors have been involved in the pathogenesis of these lesions, but the mechanisms and the causes of malignant transformations remain unknown. Lifestyle factors, PMODs, modification of the microbiome, systemic sclerosis, genetic disease with dysregulation of DNA metabolism (Zinsser–Engman–Cole syndrome, Fanconi anemia, and Xeroderma pigmentosum), mucosal inflammation and oral mucosa chronic trauma, and hematinic and micronutrient deficiency are the most important causes associated with oral carcinoma [8].
In several Asian countries, the combination of tobacco and/or betel nuts increases the risk for PMODs and oral cancer. In addition, the synergistic effect in consuming large quantities of alcohol and/or tobacco has a high risk associated with consumption and is proportional to the amount of alcohol consumed [9, 10]. Cigarette smoke from combustion releases several carcinogenic chemicals substances such as benzopyrene, dimethylbenzanthracene, nitrosazine, and free radicals [11, 12]. On the other hand, large amounts of alcohol can cause the mitochondrial salivary suppression of aldehyde dehydrogenase ALDH 2 allele gene which leads to high levels of serum acetaldehyde (derived from the ethanol metabolism and turn to acetic acid by ALDH), increasing the risk of carcinogenesis [13, 14].
Virus infections such as HPV (mainly type 16) have been linked to oral carcinomas [15, 16]. Chronic Candida spp. infections also appear to be a major risk factor [17, 18]. The risk by HPV strain infections is involved in a growing percentage of oral cancer, but other infectious agents such as the Candida spp. (more specifically C. albicans) could be involved for their production of endogenous nitrosamines starting from dietary nitrites present in the mouth, particularly in saliva [19, 20].
2.1. The Role of Microbiota Dysbiosis in Candidiasis
The terms of oral microbiome, oral microbiota, or oral microflora are used for the microorganisms present in the human mouth [21]. The mouth is the second largest and diverse microbiota niche after the gut, harboring over 700 species of bacteria. Every person has his or her own microbiome signature [22]. The human microbiota consists of archaeal cells, bacteria, fungi, viruses, and protozoa. Thus, various bacteria of the oral microbiota have a cross talking with C. albicans and HPV by modifying its pathogenicity or the behavior of bacterial population [23].
The over presenting relative phyla, according to the Human Oral Microbiome Database (eHOMD), as reported in Figure 2, are the Streptococcus spp. [24].
Figure 2.
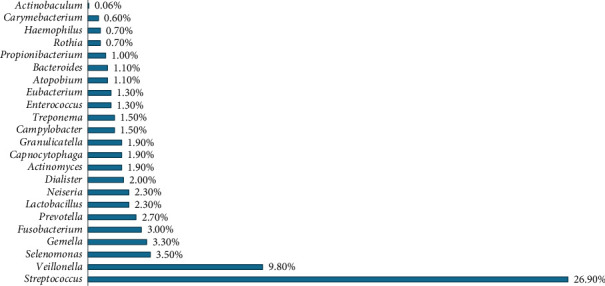
The main relative percentage (%) phyla of oral microbiome reported in eHOMD (source eHOMD, http://www.homd.org/).
The core microbiome is shared to all the persons, while variable microbiome is distinctive for single subject and is due to the lifestyle and to the physiological differences [22]. The mouth has two different types of surfaces on which bacteria can colonize: the hard and the soft tissues, respectively, of the teeth and the oral mucosa [22]. Moreover, the model environment for the development of microorganisms is presented by the oral cavity and related nasopharyngeal regions. In fact, the usual temperature of mouth is about 37°C without significant changes, lead in this way to a stable environment to bacterial survive [23]. In addition, in normal conditions, saliva too has a pH between 6.5 and 7, favorable for most bacteria species [8]. The Streptococcus mitis is one of the first bacteria to colonize the oral cavity, establish dental plaque and, under certain conditions (such as the tooth extraction), may cause systemic infection such as endocarditis [25]. Streptococcus salivarius electively colonizes the oral mucosa and is over presenting in saliva, acting as an opportunistic pathogen that produce polysaccharides in the presence of fructose, increasing in this way salivary pH [26]. Streptococcus mutans (based on the antigens, there are 8 different serotypes), producing bacteriokines and extracellular and intracellular polysaccharides (thus acidifying the oral environment), seems to have a role in the genesis of caries, as well influencing the composition of dental plaque [27]. Vestibular streptococcus produces hydrogen peroxide and urease from which ammonia is produced, with a consequent increases of local pH [28]. On the other hand, Streptococcus anginosus, usually isolated from the mucous membranes, gingival fissure, and dental plaque, do not secrete polysaccharides playing a protective role in caries development [28]. Staphylococci spp. are isolated to a small extent from saliva, gingival cleft of the mucous membranes, and dental plaque (mainly from immunocompromised subjects), with over represented the S. aureus [26]. Lactobacillus species are members of the oral flora in a small percentage (less than 1%) but increase in tooth areas showing caries, also for the characteristic that it could grow in a low pH environment [23, 27]. Several species of potential opportunistic pathogens, such as Propionibacterium and Corynebacterium, have been isolated from the oral environment [28]. Actinomyces spp. are also common members of dental plaque flora [29]. Other pathogens, such as the Porphyromonas gingivalis, Fusobacterium nucleatum, and Treponema denticola, which are anaerobic bacterium Gram-negative (-), can cause periodontitis and are linked to systemic diseases [30, 31]. Archaeal or archaic cells are only an exceedingly small percentage of the oral microbiome with a limited diversity (very few species and phytotypes) that can be adapted as a small minority of organisms in this environment. Methanes have been isolated from the oral cavity and, in fact, in 36% of patients with periodontitis archaeal were detected by in situ fluorescent hybridization [27]. It was found that the archaeal were confined to a subset of human beings and consisted of two different Rinna fililipi within the genus Methanobrevibacter. The archaeal community in periodontal disease was dominated by a Methanobrevibacter oralis type phytologist and a separate subspecies of Methanobrevibacter such as Methanobrevibacter cuticularis, Methanobrevibacter filiformi, Methanobrevibacter ruminantium, and Methanobrevibacter arboriphilius [21, 22]. Fungi are a small part of the oral microbiome. The predominant species is Candida albicans. Other fungal species present in the mouth are Cladosporium, Saccharomycetes, Aspergillus spp. (such as A. Penicillium), Gibberella, Cryptococcus, Fusarium, Rhodotorula, and Schizophyllum [21, 25]. Two species of protozoa were found in physiological flora of the mouth: the Entamoeba gingivalis amoeba and the Trichomonas tenax [21, 25]. The number of these organisms is high in people with poor oral hygiene and gingivitis and was once considered potential pathogens [28]. At present, saprophytes are considered harmless, and the apparent association with the disease is linked to diet, because a poor oral hygiene can allow an increase in the quantities of food intake and bacterial residues, which are the main nutrients for the protozoa [28]. The conditions of dysbiosis can lead to the imbalance of growth and reduction of some microorganisms present in the oral microbiota. Bacteria are not the only microorganisms present in the periodontal area, and there are also several fungi members. Recent studies in patients with periodontal infections found at least 150 species of fungi belonging to the generate Candida albicans, Ascomycota, Basidiomycota, Glomeromycota, and Chytridiomycota [29, 30]. This subsequently damages the periodontal tissue, in turn creating nutrients for the bacteria of the dysbiotic microbiome [31]. It has been noted that Mitis Group Streptococci, Salivarius Group Streptococci, S. gordonii, S. mutans, S. oralis, S. sanguinis, and S. parasanguini can interact with C. albicans. The S. mutans adhering to C. albicans interact through three mechanisms. Firstly, C. ablicans metabolizes carbohydrates and improves the sugar metabolism of S. mutans and S. mitis; secondly, S. mutans can influence the coding of adhesins in C. albicans; finally, the synergism of S. mutans and C. albicans can promote an increase of growth and invasion of mucous tissues in coinfection [32, 33]. The S. salivarius K19 may play a protective role in C. albicans infection because it can inhibit adhesion and fungal filamentation. The S. aureus may be helped by C. albicans in an infection, such as R. dentocariosa, and S. mitis aids fungal colonization [34, 35]. It has been noted that Fusobacterium nucleatum, S. salivarius, A. actinomycetemcomitans, and E. faecalis can have a protective role in a C. albicans infections, because it can inhibit the overall virulence and fungal biofilm formation (Figure 3) [36, 37].
Figure 3.
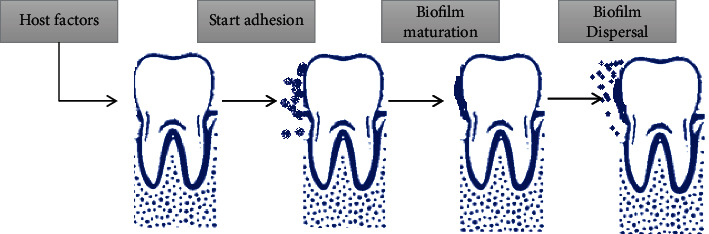
Example stages of biofilm formation process on dental surface (this mechanism occurs throughout the oral cavity). Biofilms are an organized community of microorganisms. Microbes with weak and reversible forces attach first, dental surface. All bacteria that are not immediately removed are rigidly attached to special structures, such as fibrils. Bacteria multiply and offer additional attack sites for other microorganisms. The ability to maintain the consistency of microbes in dental plaque is based on the balance between cooperative and competitive relationships between microorganisms and their host. This microbial homeostasis in combination with host defense prevents the formation of pathogenic microorganism colonization. However, when this community is unbalanced quantitatively and qualitatively, dysbiosis occurs.
Finally, some studies reported several interactions between C. albicans and P. gingivalis that could be used to increase infectivity [38, 39]. In some patients, the inflammatory reaction in candidiasis can be self-limiting, but in other patients, multiple genetic, epigenetic, or external factors (tobacco, alcohol, diet, diabetes, etc.), as well in synergetic crosstalk with oral bacterial, can cause excessive and chronic inflammation, leading to PMODs and alveolar bone injure [40–42].
Moreover recent evidence points out a causal relationship between specific bacterial infections, such as by P. gingivalis, and the development of oral and esophageal carcinoma [43].
2.2. Candidiasis Can Generate Oral Precancerous Conditions?
Major predisposing factors to oral candidiasis could be diabetes, mobile prosthesis, antibiotics, antiblastic or inhaled corticosteroid therapy, xerostomia by radiations, or Sjógren's syndrome and HIV infection, presenting clinical forms as angular cheilitis, mucocutaneous (rare), pseudomembranous, hyperplastic, and erythematous (atrophic) [44, 45]. Moreover, current evidence revealed correlation between Candida infection and malignant transformation of the oral cavity [46, 47]. In fact, several hypothetical mechanisms have been proposed for C. albicans interactions with oral epithelium, leading to PMODs and malignant lesions [48–50]:
Production of the cytolitic toxin candidalysin
Production of nitrosamines
Production of acid aspartyl-proteinase
Production of acetaldehyde (carcinogen inducing mutations in genes)
Overexpression of Ki-67 labeling index, prostaglandin-endoperoxide synthase 2 (COX-2), and p53
Upregulation of proinflammatory cytokines, such as interleukin- (IL-) 1α, IL-1β, IL-6, IL-8, IL-18, tumor necrosis factor- (TNF-) α, interferon gamma (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF)
Reduction of β-defensins which facilitate Candida superinfections
In a prospective cohort study (between 2007 and 2009) on 103 patients, Candida spp. was isolated from 31 (30%) patients with carcinoma and from 33 (32%) patients with PMODs [51]. In another prospective cohort study, it was noted that the presence of Candida infections was related to an increased risk of cancer [52]. In addition, some bacteria such as S. viridans are able to convert ethanol into acetaldehyde for the presence of the alcohol-dehydrogenase enzyme in them [53–55]. The ability to switch among different phenotypic forms has been thought to contribute to C. albicans virulence, and phenotypic switching events in C. albicans can be induced by hydroxyurea [55, 56]. Besides, some chemotherapeutic agents, such as 5-fluorouracil, could reduce the susceptibility of C. albicans to the antifungal drugs [57]. The genotype “A” of C. albicans is more represented in the OSCCs, while the genotype “C” of C. albicans is more represented in the leucoplakia [58, 59]. Candida leukoplakia (CL) lesions are complex to discriminate from non-Candida leukoplakias (NCLs) clinically, but the presence of invading Candida hyphae in the superficial layer of epithelium accompanied by infiltratration of polymorphic neutrophils histologically discriminates CL lesions [60, 61]. Moreover, Candida was isolated by exfoliative cytology and periodic acid–Schiff (PAS) staining of biopsies in a study that analyzed 44 cases with 59.1% presenting oral leucoplakia, showing Candida detection in 62.5% of the cases [62]. Furthermore, in the observed cases with Candida, the DNA alterations were higher [62]. In a retrospective study on 136 patients with oral leukoplakia divided into two groups, it was found that lesions had higher degrees of cell abnormalities (epithelial dysplasia) with Candida coinfection. The first group presenting multiple oral leucoplakia lesions, Candida infection was detected in the 47.9% of the sample (with 28.6% of dysplasia), while in the second group with single oral leucoplakia lesion, Candida infection was detected in the 19.0% of the sample (with 20.0% of dysplasia) [63].
2.3. The Role of Human Papillomavirus (HPV)
HPVs are members of the Papillomaviridae (PV) family, presenting a circular, with supercoiled and double-stranded DNA, and some are considered as oncogenic promoters [64–66]. The virus has a hexahedral symmetry capsid (consisting of pentameric capsomeres) and has two structural proteins, L1 and L2 [67, 68]. Oral HPV is often sexually transmitted, but nonsexual modes of transmission should be considered, including autoinoculation from skin lesion HPV in adolescent and pediatric patients [69, 70]. The HPV infection is commonly is commomly associated to benign lesions (vulgar warts, warts, focal epithelial hyperplasia, squamous cell papilloma, Bowen's papillomatosis), or to cancerous lesions such as squamous cell carcinoma (SCC) [64]. There must be chafing or small lesions for the virus to enter the epithelium, and direct contact with the skin or mucosa is required for virus transmission. The strain of virus determines the different types of lesion that will be developed, as well as the location of the infection. Hence, HPV can be transmitted in many ways through different abrasions, with sexual intercourse, when the newborn passes through an infected genital tract, and from a variety of self-inoculation positions, for example, by scratching the skin [68].
Since HPV does not have a protein or ribosomal synthesis, for its proliferation, it employs the genetic mechanism of the host cell [69, 70]. The virus uses, to direct the metabolic functions of the host cell in its favor, the production of viral messenger RNA, which is produced by transcription of viral genetic material. The genome is divided into three parts. The “E” (early region) which codes for proteins necessary for the viral DNA genome duplication. It includes 7-9 open reading frames (ORFs), coding regions for proteins E1, E2, E4, E5, E6, E7, and E8 (although E5 and E8 ORF are not present in the genomes of all HPV types). The “L” (late region) which encodes the structural proteins of the HPV capsid virus (L1 and L2). Finally, the URR or NCR (upstream regulatory region or noncoding region) comprised between “E” and “L” regions and regulates the function of the viral genome, in addition to four binding sites to E2 proteins and multiple binding sites of the transcription [69–71].
The differences between different strains of the virus are evident when comparing lesions of the same epithelial area. In fact, cell proliferation obtained from the expression of E6 and E7 proteins, due to infection with oncogenic HPV virus strains, facilitates expansion of lesion, with a higher risk of metastasis [71, 72].
2.4. Which Are the Pathways of Oncogenesis in HPV Infection?
The receptor that HPV uses to enter cells is integrin A6 [71]. On one hand, there are many types of HPV such as 16, 18, 31, and 52, and on the other hand, they are characterized by a high potential for malignancy and also have a high risk of metastasis (Table 1) [73, 74].
Table 1.
There are over 450 types of HPV. The HPV's genotypes are divided into 4 groups according to the associate oncogenic risk by IARC/WHO (source from https://monographs.iarc.who.int/agents-classified-by-the-iarc/).
| Group | HPV virus |
|---|---|
| 1 | Carcinogenic to humans: human papillomavirus types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 (the HPV types that have been classified as carcinogenic to humans can differ by an order of magnitude in risk for cervical cancer) |
| 2A | Probably carcinogenic to humans: human papillomavirus type 68 |
| 2B | Possibly carcinogenic to humans: human papillomavirus types 26, 53, 66, 67, 70, 73, and 82; human papillomavirus types 30, 34, 69, 85, and 97 (classified by phylogenetic analogy to the HPV genus alpha types classified in group 1); human papillomavirus types 5 and 8 (in patients with epidermodysplasia verruciformis) |
| 3 | Not classifiable as to its carcinogenicity to humans: human papillomavirus genus beta (except types 5 and 8) and genus gamma; human papillomavirus types 6 and 11 |
The role of the virus on carcinogenesis is due to proteins E6 and E7 that integrate into host DNA. These two proteins could induce the neoplastic growth, since they block the two tumor suppressor proteins, the retinoblastoma protein (pRb) and p53, which regulate the transition from the G1 phase of the cell cycle to S. E7 binds to p53 blocking its ability to interact with the transcription factor E2F [75, 76]. Therefore, cells characterized by E7 overexpression lose the control of the transition from G1 to S phase, which causes continuous cell cycles and therefore cell proliferation without suppression, as well, degradation of the pRb [76].
Levels of p53 in normal cells are exceptionally low. During the E7 overexpression, an increase in p53 was shown for the inhibition of its breakdown in normal cells which is in turn regulated by MDM2 (mediator of DNA damage 2) [77, 78]. E7 also interacts with many other factors; among these, the CDK (cyclin-dependent kinase) inhibits p27 and p21, which induce the dysregulation of cell cycle [78]. E7 forms protein complexes with pRb, p107, and p130 and participates in E6 cleavage of p53 via the E6 complex, E6AP (associated protein E6), and p53 [79, 80]. Dysregulation of pRb phosphorylation-dephosphorylation is an early event. There is a gradual reduction of pRb expression in dysplastic lesions and in neoplastic process [81]. Briefly, the Rb protein pathway could induce carcinogenesis by inactivating cell cycle regulators [82]. Activation of p53 turns on the production of the CD-kinase inhibitor protein, p21WAF1 which contributes to cell cycle regulation by acting on different CD-kinase/cyclin complexes [83]. Thus, failure of p53 has been observed to cause carcinogenesis. This is attributed to the mutation of the genetic locus 17p13. Furthermore, E6 sequentially causes the cleavage of p53 [84]. Oncoprotein E7 causes p16 overexpression. In fact, the gene that codes for the regulatory protein p16 is found in chromosome 9p21, and its mutation is correlated with OSCCs [85, 86] (Figure 4).
Figure 4.
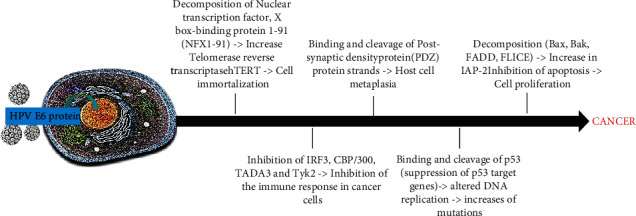
Integration of HPV DNA into the host genome leading to the E6 protein overexpression and to carcinogenic processes.
Besides, E6 inhibits the activity of p73, a type 16 homologous p53. HPV blocks cell apoptosis by inhibiting Bax gene expression in keratinocytes. This results in increased mutations in the DNA of the cells. The apoptosis can be inhibited by E7 binding to the tumor necrosis factor 1 receptor (TNF-R1) [87], originating a variety of bimolecular effects that promote carcinogenesis (Figure 5).
Figure 5.
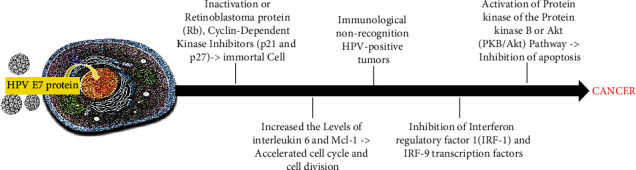
Integration of HPV DNA into the host genome leading to E7 overexpression and to carcinogenic processes.
According to carcinogenesis model proposed by Califano et al., healthy tissues near the lesions show the same pattern of mutations (loss of heterozygosity) of neoplastic cells, since all population come from a single mutant progenitor cell [88]. Another model reports that the progenitor cell of the basal layer acquires a genetic mutation, which it transfers to its daughter cells. The result is the creation of a mass of cells that grows, strains, and also affects nearby tissues, in the development of lesions (clinically, they appear as leukoplakia or erythroplakia). In the final stage, specific clone cells after further mutations acquire a tumoral genotype. In this context, loss of heterozygosity at loci 9p21 and 3p21 appears to increase the risk of malignant recurrence [89, 90]. A mutation in the type of gene duplication has been shown to be involved in oral carcinogenesis [91].
Replication of genetic material in the TERT (telomerase reverse transcriptase) protein coding gene results in overexpression of the TERT human protein (hTERT). Studies conducted on OSCCs have shown an increase in both telomerase activity and hTERT expression [92, 93]. This hTERT expression occurs not only in cancerous cells but also around in the normal epithelium in the early course of carcinogenesis [94]. The spindle assembly checkpoint (SAC) describes the way point to control mitosis, and Cdc20 is a SAC protein able to activate the anaphase promoting complex (APC) which is a key pathway factor and is also responsible for the formation of aneuploidy cells [95–97].
It has been noted that in 70% of oral tumors there is an overexpression of the Cdc20 mRNA [98, 99]. Besides, as further bimolecular mechanisms in the etiology of oral cancer, several studies reported the role of the epidermal growth factor (EGF), the mitogen-activated protein kinase (MAPK) pathway, the PI3K/AKT/mTOR pathway, the signal transducer and activator of transcription (STAT) pathway, TGF (transforming growth factor), NF-κB (nuclear factor κB), and Wnt/β-catenin [100–102]. The ERbB1 gene, responsible for EGF encoding, is found on chromosome 7p12 and has been associated with increased activity in oral cancer [103, 104]. ERbB1 dysregulation in head and neck squamous epithelial neoplasms is attributed more to its overexpression than to the presence of mutations [105, 106]. TGF promotes tumor progression by increasing angiogenesis and reducing sensitivity to the immune system. This action is caused by the tolerance of tumor cells to TGF-induced apoptosis [107, 108]. The TGFβR-II mutation is important, and thus, a reduction of the TGFβR-II/TGFβR-I ratio (suspension/development) is determined, and thus, the protective role of TGF is canceled [109]. A gradual decrease in expression of TGF-β, TGFβR-I, and TGFβR-II was observed in the different stages of carcinogenesis. In cancer cells, there is a reduction in TGFβR-II levels, and this can promote the development and growth of oral cancer, acting as an indicator of differentiation and therefore of aggressive behavior [110]. Activation of the Wnt/β-catenin pathway induces β-catenin release and subsequently cytoplasmic aggregation and its transport to the nucleus, through the interaction with certain genes (with inhibition of apoptosis and increase of cell proliferation) such as COX-2, cyclin D1, and cMyc [105, 111]. Furthermore, this pathway increases the expression of metalloproteinases, which catalyze the basement membrane and the dense structure of the epithelium, favoring infiltration [111]. Lastly, HPV proteins E6 and E7 can regulate the epigenetic mechanisms, as DNA methylation, histone modification, chromatin remodeling, and miRNA production in cell host or viral genes. Therefore, on one hand, E6/E7 induced DNA methylation shut out normal epigenetic processes, and on the other hand, E7 binds and modulates methyltransferase activity [112, 113].
3. Conclusions
Nowadays, several suggestive and consistent correlations between C. albicans and HPV infection in oral cancer progression in adolescent/pediatric patients have been reported worldwide. Candida spp. and in particular C. albicans can produce carcinogens such as nitrosamine or promote the development of oral carcinoma. HPV are involved in the pathogenesis of different types of cancer. In particular, from literature data, it emerges that HPV infection plays an important role in the risk of PMODs of the oral mucosa and consequently in the dysplastic and malignant transformation of these lesions. Based on existing evidence, we can also conclude that, in the composition of the microbiome associated with OSCCs, there are no specific species to implicate in its etiology, of course excluding oncoviruses (i.e., HPV) or fungi (i.e., C. albicans) that are associated with oral cancer. Large multicenter trials are required in order to study the biological behavior and formulate treatment strategies in the management of the same. Perhaps, these findings will produce interest in the possible association among oral microbiota dysbiosis, C. albicans, HPV, and oral cancer and spur controlled prospective clinical studies in this field.
Contributor Information
Andrea Ballini, Email: andrea.ballini@me.com.
Stefania Cantore, Email: stefaniacantore@pec.omceo.bari.it.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declares that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
A.B., I.A.C., and L.S. contributed to the concept and design. M.A., L.B., I.A.C., and A.B. contributed to the acquisition, analysis, and interpretation of data. I.A.C., E.B., and A.B. contributed to the drafting of the manuscript. S.C., E.B., A.P.C., and A.B. contributed to the bibliographic research. M.D.C., S.C., and L.B. contributed to the critical revision of the manuscript for important intellectual content. A.M., R.N., and M.D. contributed to the data interpretation, technical, and material support. L.S., A.B., M.D.C., and L.L.M contributed to the supervision and final approval. All authors have read and agreed to the published version of the manuscript. Lorenzo Lo Muzio, Andrea Ballini, and Stefania Cantore contributed equally as co-first authors. Michele Di Cosola, Luigi Santacroce, and Edoardo Brauner contributed equally as co-last authors.
References
- 1.Rahman Q. B., Iocca O., Kufta K., Shanti R. M. Global burden of head and neck cancer. Oral and Maxillofacial Surgery Clinics of North America . 2020;32(3):367–375. doi: 10.1016/j.coms.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Oral cancer . 2021. https://www.who.int/cancer/prevention/diagnosis-screening/oral-cancer/en .
- 3.Grigolato R., Accorona R., Lombardo G., et al. Oral cancer in non-smoker non-drinker patients. Could comparative pet oncology help to understand risk factors and pathogenesis? Critical Reviews in Oncology/Hematology . 2021;166:p. 103458. doi: 10.1016/j.critrevonc.2021.103458. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. GLOBOCAN . 2020. http://gco.iarc.fr/today/data/factsheets/cancers/1-Lip-oral-cavity-fact-sheet.pdf .
- 5.World Health Organization. WHO Classification Head and Neck Tumours (IARC) http://screening.iarc.fr/doc/BB9.pdf .
- 6.Siriwardena B. S., Tilakaratne A., Amaratunga E. A., Tilakaratne W. M. Demographic, aetiological and survival differences of oral squamous cell carcinoma in the young and the old in Sri Lanka. Oral Oncology . 2006;42(8):831–836. doi: 10.1016/j.oraloncology.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Du Q., Ren B., He J., et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. The ISME Journal . 2021;15(3):894–908. doi: 10.1038/s41396-020-00823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorini L., Bescós Atín C., Thavaraj S., et al. Overview of oral potentially malignant disorders: from risk factors to specific therapies. Cancers . 2021;13(15):p. 3696. doi: 10.3390/cancers13153696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO global report on trends in prevalence of tobacco smoking 2000-2025-third edition 2015 . file:///C:/Users/Operatore3/Downloads/9789240000032-eng.pdf.
- 10.Wang Y., Jia X., Lin P., Geng M., Wang R., Li S. Cancer mortality attributable to cigarette smoking in 2005, 2010 and 2015 in Qingdao, China. PLoS One . 2018;13(9, article e0204221) doi: 10.1371/journal.pone.0204221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspective with updates. Carcinogenesis . 2001;22(12):1903–1930. doi: 10.1093/carcin/22.12.1903. [DOI] [PubMed] [Google Scholar]
- 12.Wyss A., Hashibe M., Chuang S. C., et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. American Journal of Epidemiology . 2013;178(5):679–690. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seitz H. K., Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Reviews Cancer . 2007;7(8):599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J., Ervik M., Lam F., et al. Global Cancer Observatory: Cancer Tomorrow . Lyon, France: International Agency for Research on Cancer; 2018. https://gco.iarc.fr/tomorrow . [Google Scholar]
- 15.García-Martín J. M., Varela-Centelles P., González M., Seoane-Romero J. M., Seoane J., García-Pola M. J. Epidemiology of oral cancer. In: Panta P., editor. Oral Cancer Detection . Cham: Springer; 2019. [Google Scholar]
- 16.Chen X., Zhao Y. Human papillomavirus infection in oral potentially malignant disorders and cancer. Archives of Oral Biology . 2017;83:334–339. doi: 10.1016/j.archoralbio.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Mehanna H., Beech T., Nicholson T., et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck . 2013;35(5):747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 18.Javed F., Al-Kheraif A. A., Kellesarian S. V., Vohra F., Romanos G. E. Oral Candida carriage and species prevalence in denture stomatitis patients with and without diabetes. Journal of Biological Regulators and Homeostatic Agents . 2017;31(2):343–346. [PubMed] [Google Scholar]
- 19.Hashibe M. Risk Factors for Cancer of the Mouth: Tobacco, Betel Quid, and Alcohol. In: Warnakulasuriya S., Greenspan J., editors. Textbook of Oral Cancer. Textbooks in Contemporary Dentistry . Cham: Springer; 2020. [Google Scholar]
- 20.Ballini A., Cosola M. D., Saini R., et al. Comparison of manual nylon bristled versus thermoplastic elastomer toothbrushes in terms of cleaning efficacy and biological potential role on gingival health. Applied Sciences . 2021;11(16):p. 7180. doi: 10.3390/app11167180. [DOI] [Google Scholar]
- 21.Turnbaugh P. J., Ley R. E., Hamady M., Fraser-Liggett C. M., Knight R., Gordon J. I. The human microbiome project. Nature . 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimish Deo P., Deshmukh R. Oral microbiome: unveiling the fundamentals. Journal of oral and maxillofacial pathology: JOMFP . 2019;23(1):122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghannoum M. A., Jurevic R. J., Mukherjee P. K., et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathogens . 2010;6(1, article e1000713) doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. expanded Human Oral Microbiome Database (eHOMD), All Oral genomes . 2021. http://homd.org/index.php?name=GenomeList&link=GenomeList&type=all_oral .
- 25.Chen T., Yu W. H., Izard J., Baranova O. V., Lakshmanan A., Dewhirst F. E. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database . 2010;2010:p. baq013. doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan K., Chen T., Paster B. J. A practical guide to the oral microbiome and its relation to health and disease. Oral Diseases . 2017;23(3):276–286. doi: 10.1111/odi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L., Xu T., Huang G., Jiang S., Gu Y., Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein & Cell . 2018;9(5):488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Wang X., Li H., Ni C., Du Z., Yan F. Human oral microbiota and its modulation for oral health. Biomedicine & Pharmacotherapy . 2018;99:883–893. doi: 10.1016/j.biopha.2018.01.146. [DOI] [PubMed] [Google Scholar]
- 29.Le Bars P., Matamoros S., Montassier E., et al. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Canadian Journal of Microbiology . 2017;63(6):475–492. doi: 10.1139/cjm-2016-0603. [DOI] [PubMed] [Google Scholar]
- 30.Chen J., Domingue J. C., Sears C. L. Microbiota dysbiosis in select human cancers: evidence of association and causality. Seminars in Immunology . 2017;32:25–34. doi: 10.1016/j.smim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori G., Brunetti G., Colucci S., et al. Alteration of activity and survival of osteoblasts obtained from human periodontitis patients: role of TRAIL. Journal of Biological Regulators and Homeostatic Agents . 2007;21(3-4):105–114. [PubMed] [Google Scholar]
- 32.Montelongo-Jauregui D., Lopez-Ribot J. L. Candida interactions with the oral bacterial microbiota. Journal of Fungi . 2018;4(4):p. 122. doi: 10.3390/jof4040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patil S., Rao R. S., Majumdar B., Anil S. Clinical appearance of oral Candida infection and therapeutic strategies. Frontiers in Microbiology . 2015;6:p. 1391. doi: 10.3389/fmicb.2015.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fux C. A., Stoodley P., Hall-Stoodley L., Costerton J. W. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Review of Anti-infective Therapy . 2003;1(4):667–683. doi: 10.1586/14787210.1.4.667. [DOI] [PubMed] [Google Scholar]
- 35.Yang S. F., Huang H. D., Fan W. L., et al. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral Oncology . 2018;77:1–8. doi: 10.1016/j.oraloncology.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Guerrero-Preston R., Godoy-Vitorino F., Jedlicka A., et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget . 2016;7(32):51320–51334. doi: 10.18632/oncotarget.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters B. A., Wu J., Hayes R. B., Ahn J. The oral fungal mycobiome: characteristics and relation to periodontitis in a pilot study. BMC Microbiology . 2017;17(1):p. 157. doi: 10.1186/s12866-017-1064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandra J., Retuerto M., Mukherjee P. K., Ghannoum M. The fungal biome of the oral cavity. Methods in Molecular Biology . 2016;1356:107–135. doi: 10.1007/978-1-4939-3052-4_9. [DOI] [PubMed] [Google Scholar]
- 39.Azzolino D., Passarelli P. C., de Angelis P., Piccirillo G. B., D’Addona A., Cesari M. Poor oral health as a determinant of malnutrition and sarcopenia. Nutrients . 2019;11(12):p. 2898. doi: 10.3390/nu11122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori G., Brunetti G., Colucci S., et al. Osteoblast apoptosis in periodontal disease: role of TNF-related apoptosis-inducing ligand. International Journal of Immunopathology and Pharmacology . 2009;22(1):95–103. doi: 10.1177/039463200902200111. [DOI] [PubMed] [Google Scholar]
- 41.Posa F., Colaianni G., Di Cosola M., et al. The myokine irisin promotes osteogenic differentiation of dental bud-derived MSCs. Biology (Basel) . 2021;10(4):p. 295. doi: 10.3390/biology10040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballini A., Capodiferro S., Toia M., et al. Evidence-based dentistry: what’s new? International Journal of Medical Sciences . 2007;4(3):174–178. doi: 10.7150/ijms.4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Cosola M., Cazzolla A. P., Charitos I. A., Ballini A., Inchingolo F., Santacroce L. Candida albicans and oral carcinogenesis. a brief review. Journal of Fungi . 2021;7(6):p. 476. doi: 10.3390/jof7060476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alnuaimi A. D., Ramdzan A. N., Wiesenfeld D., et al. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Diseases . 2016;22(8):805–814. doi: 10.1111/odi.12565. [DOI] [PubMed] [Google Scholar]
- 45.Chimenos-Küstner E., Font-Costa I., López-López J. Oral cancer risk and molecular markers. Medicina Oral, Patología Oral y Cirugía Bucal . 2004;9(5):381–384. [PubMed] [Google Scholar]
- 46.Pärnänen P., Meurman J. H., Samaranayake L., Virtanen I. Human oral keratinocyte E-cadherin degradation by Candida albicans and Candida glabrata. Journal of Oral Pathology & Medicine . 2010;39(3):275–278. doi: 10.1111/j.1600-0714.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 47.Lovreglio P., Bukvic N., Fustinoni S., et al. Lack of genotoxic effect in workers exposed to very low doses of 1, 3-butadiene. Archives of Toxicology . 2006;80(6):378–381. doi: 10.1007/s00204-005-0046-0. [DOI] [PubMed] [Google Scholar]
- 48.Gainza-Cirauqui M. L., Nieminen M. T., Novak Frazer L., Aguirre-Urizar J. M., Moragues M. D., Rautemaa R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. Journal of Oral Pathology & Medicine . 2013;42(3):243–249. doi: 10.1111/j.1600-0714.2012.01203.x. [DOI] [PubMed] [Google Scholar]
- 49.Cicinelli E., Ballini A., Marinaccio M., et al. Microbiological findings in endometrial specimen: our experience. Archives of Gynecology and Obstetrics . 2012;285(5):1325–1329. doi: 10.1007/s00404-011-2138-9. [DOI] [PubMed] [Google Scholar]
- 50.Casaroto A. R., da Silva R. A., Salmeron S., et al. Candida albicans-cell interactions activate innate immune defense in human palate epithelial primary cells via nitric oxide (NO) and β-defensin 2 (hBD-2) Cell . 2019;8(7):p. 707. doi: 10.3390/cells8070707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galli F., Colella G., Di Onofrio V., Rossiello R., Angelillo I. F., Liguori G. Candida spp. in oral cancer and oral precancerous lesions. The New Microbiologica . 2013;36(3):283–288. [PubMed] [Google Scholar]
- 52.Nørgaard M., Thomsen R. W., Farkas D. K., Mogensen M. F., Sørensen H. T. Candida infection and cancer risk: a Danish nationwide cohort study. European Journal of Internal Medicine . 2013;24(5):451–455. doi: 10.1016/j.ejim.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Meurman J. H., Uittamo J. Oral micro-organisms in the etiology of cancer. Acta Odontologica Scandinavica . 2008;66(6):321–326. doi: 10.1080/00016350802446527. [DOI] [PubMed] [Google Scholar]
- 54.Marttila E., Uittamo J., Rusanen P., Lindqvist C., Salaspuro M., Rautemaa R. Acetaldehyde production and microbial colonization in oral squamous cell carcinoma and oral lichenoid disease. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology . 2013;116(1):61–68. doi: 10.1016/j.oooo.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Pakshir K., Zomorodian K., Karamitalab M., Jafari M., Taraz H., Ebrahimi H. Phospholipase, esterase and hemolytic activities of Candida spp. isolated from onychomycosis and oral lichen planus lesions. Journal de Mycologie Médicale . 2013;23(2):113–118. doi: 10.1016/j.mycmed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Morschhäuser J., Virkola R., Korhonen T. K., Hacker J. Degradation of human subendothelial extracellular matrix by proteinase-secreting Candida albicans. FEMS microbiology letters . 1997;153(2):349–355. doi: 10.1111/j.1574-6968.1997.tb12595.x. [DOI] [PubMed] [Google Scholar]
- 57.Krogh P., Hald B., Holmstrup P. Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis . 1987;8(10):1543–1548. doi: 10.1093/carcin/8.10.1543. [DOI] [PubMed] [Google Scholar]
- 58.Kumar R. S., Ganvir S., Hazarey V. Candida and calcofluor white: study in precancer and cancer. Journal of Oral and Maxillofacial Pathology . 2009;13(1):2–8. doi: 10.4103/0973-029X.44575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdulrahim M. H., McManus B. A., Flint S. R., Coleman D. C. Genotyping Candida albicans from Candida leukoplakia and non-Candida leukoplakia shows no enrichment of multilocus sequence typing clades but enrichment of ABC genotype C in Candida leukoplakia. PLoS One . 2013;8(9, article e73738) doi: 10.1371/journal.pone.0073738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ballini A., Cantore S., Fatone L., et al. Transmission of nonviral sexually transmitted infections and oral sex. The Journal of Sexual Medicine . 2012;9(2):372–384. doi: 10.1111/j.1743-6109.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- 61.Bombeccaria G. P., Spadaria F., Rossia M., Porrinia M., Bosottia M., Giannì A. B. Biology of Candida spp. in potentially malignant disorders and carcinoma of the oral cavity. Dental Cadmos . 2016;84(10):624–634. doi: 10.19256/d.cadmos.10.2016.04. [DOI] [Google Scholar]
- 62.Nakazawa K., Fifita S. F., Kuyama K. The cytological findings of oral inflammatory lesions, lichen planus and leukoplakia coexisted with and without Candida: with special reference to clinical, histopathological, immunohistochemical and flow cytometrical analyses. International Journal of Oral-Medical Sciences . 2007;6(2):81–90. doi: 10.5466/ijoms.6.81. [DOI] [Google Scholar]
- 63.Chiu C. T., Li C. F., Li J. R., et al. Candida invasion and influences in smoking patients with multiple oral leucoplakias--a retrospective study. Mycoses . 2011;54(5):e377–e383. doi: 10.1111/j.1439-0507.2010.01927.x. [DOI] [PubMed] [Google Scholar]
- 64.ICTV 9th report. Papillomaviridae . https://talk.ictvonline.org/ictv-reports/ictv_9th_report/dsdna-viruses-2011/w/dsdna_viruses/121/papillomaviridae .
- 65.Santacroce L., Di Cosola M., Bottalico L., et al. Focus on HPV infection and the molecular mechanisms of oral carcinogenesis. Viruses . 2021;13(4):p. 559. doi: 10.3390/v13040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong H., Shu X., Xu Q., et al. Current Status of Human Papillomavirus-Related Head and Neck Cancer: From Viral Genome to Patient Care. Virologica Sinica . 2021 doi: 10.1007/s12250-021-00413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devaraja K., Aggarwal S., Verma S. S., Gupta S. C. Clinico-pathological peculiarities of human papilloma virus driven head and neck squamous cell carcinoma: a comprehensive update. Life Sciences . 2020;245:p. 117383. doi: 10.1016/j.lfs.2020.117383. [DOI] [PubMed] [Google Scholar]
- 68.Erickson B. K., Alvarez R. D., Huh W. K. Human papillomavirus: what every provider should know. American Journal of Obstetrics and Gynecology . 2013;208(3):169–175. doi: 10.1016/j.ajog.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Z. M., Baker C. C. Papillomavirus genome structure, expression, and post-transcriptional regulation. Frontiers in Bioscience . 2006;11:2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benyo S., Keane A., Warrick J., Choi K. Y. HPV-positive oral papillomas in an adolescent—a diagnostic dilemma. Clinical Case Reports . 2021;9(8, article e04546) doi: 10.1002/ccr3.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.IARC Working group on the evaluation of carcinogenic risks to humans. Human Papillomavirus (HPV) Infection . Lyon (FR): International Agency for Research on Cancer; 2007. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 90.) 1. https://www.ncbi.nlm.nih.gov/books/NBK321770/ [Google Scholar]
- 72.Nair S., Pillai M. R. Human papillomavirus and disease mechanisms: relevance to oral and cervical cancers. Oral Diseases . 2005;11(6):350–359. doi: 10.1111/j.1601-0825.2005.01127.x. [DOI] [PubMed] [Google Scholar]
- 73.Santacroce L., Luperto P., Fiorella M. L., Losacco T. Carcinoma of unknown origin++ with latero-cervical metastasis. Diagnostic problems. Retrospective analysis of 110 cases of latero-cervical tumefaction. Clinical Therapeutics . 2000;151(3):199–201. [PubMed] [Google Scholar]
- 74.Coscia M. F., Monno R., Ballini A., et al. Human papilloma virus (HPV) genotypes prevalence in a region of South Italy (Apulia) Annali dell'Istituto Superiore di Sanità . 2015;51(3):248–251. doi: 10.4415/ANN_15_03_14. [DOI] [PubMed] [Google Scholar]
- 75.Cao J., Zhang Z. Y. Human papillomavirus infection and p53 alteration in oral squamous cell carcinoma. The Chinese Journal of Dental Research . 2000;3(3):44–49. [PubMed] [Google Scholar]
- 76.Charitos I. A., Ballini A., Cantore S., et al. Stem cells: a historical review about biological, religious, and ethical issues. Stem Cells International . 2021;2021:11. doi: 10.1155/2021/9978837.9978837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. WHO/IARC Agents Classified by the IARC Monographs, Volumes 1–128 . https://monographs.iarc.who.int/agents-classified-by-the-iarc/
- 78.Ganguly N., Parihar S. P. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. Journal of Biosciences . 2009;34(1):113–123. doi: 10.1007/s12038-009-0013-7. [DOI] [PubMed] [Google Scholar]
- 79.Pannone G., Nocini P. F., Lo Muzio L., Procaccini M., Pannone G., Santacroce L. Instability of micro-satellite sequences of DNA associated with genetic alterations in head and neck neoplasms. Review of the literature and preliminary results of a research plan. Minerva Stomatologica . 1998;47(11):589–596. [PubMed] [Google Scholar]
- 80.Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science . 1989;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 81.Soni S., Kaur J., Kumar A., et al. Alterations of rb pathway components are frequent events in patients with oral epithelial dysplasia and predict clinical outcome in patients with squamous cell carcinoma. Oncology . 2005;68(4-6):314–325. doi: 10.1159/000086970. [DOI] [PubMed] [Google Scholar]
- 82.Choi S., Myers J. N. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. Journal of Dental Research . 2008;87(1):14–32. doi: 10.1177/154405910808700104. [DOI] [PubMed] [Google Scholar]
- 83.Ralhan R., Agarwal S., Mathur M., Wasylyk B., Srivastava A. Association between polymorphism in p 21 (Waf 1/Cip1) cyclin-dependent kinase inhibitor gene and human oral cancer. Clinical Cancer Research . 2000;6(6):2440–2447. [PubMed] [Google Scholar]
- 84.Westra W. H., Taube J. M., Poeta M. L., Begum S., Sidransky D., Koch W. M. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clinical Cancer Research . 2008;14(2):366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 85.Allen C. T., Lewis J. S., Jr., El-Mofty S. K., Haughey B. H., Nussenbaum B. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope . 2010;120(9):1756–1772. doi: 10.1002/lary.20936. [DOI] [PubMed] [Google Scholar]
- 86.Ohta S., Uemura H., Matsui Y., et al. Alterations of p16 and p14ARF genes and their 9p21 locus in oral squamous cell carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology . 2009;107(1):81–91. doi: 10.1016/j.tripleo.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 87.Filippova M., Song H., Connolly J. L., Dermody T. S., Duerksen-Hughes P. J. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. Journal of Biological Chemistry . 2002;277(24):21730–21739. doi: 10.1074/jbc.M200113200. [DOI] [PubMed] [Google Scholar]
- 88.Califano J., van der Riet P., Westra W., et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Research . 1996;56(11):2488–2492. [PubMed] [Google Scholar]
- 89.Braakhuis B. J., Leemans C. R., Brakenhoff R. H. A genetic progression model of oral cancer: current evidence and clinical implications. Journal of Oral Pathology & Medicine . 2004;33(6):317–322. doi: 10.1111/j.1600-0714.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 90.Uchida K., Oga A., Okafuji M., et al. Molecular cytogenetic analysis of oral squamous cell carcinomas by comparative genomic hybridization, spectral karyotyping, and fluorescence in situ hybridization. Cancer Genetics and Cytogenetics . 2006;167(2):109–116. doi: 10.1016/j.cancergencyto.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Freier K., Pungs S., Flechtenmacher C., et al. Frequent high telomerase reverse transcriptase expression in primary oral squamous cell carcinoma. Journal of Oral Pathology & Medicine . 2007;36(5):267–272. doi: 10.1111/j.1600-0714.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 92.Christensen R., Park N. H., Sapp P., Kang M. K., Park N. H. Elevated expression of hTERT is associated with dysplastic cell transformation during human oral carcinogenesis in situ. Kim HR, Clinical Cancer Research . 2001;7(10):3079–3086. [PubMed] [Google Scholar]
- 93.Musacchio A., Salmon E. D. The spindle-assembly checkpoint in space and time. Nature Reviews Molecular Cell Biology . 2007;8(5):379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 94.Mondal G., Sengupta S., Panda C. K., Gollin S. M., Saunders W. S., Roychoudhury S. Overexpression of Cdc20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis . 2007;28(1):81–92. doi: 10.1093/carcin/bgl100. [DOI] [PubMed] [Google Scholar]
- 95.Roberts P. J., Der C. J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene . 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 96.Richter P., Böhmer F. D., Hindermann W., et al. Analysis of activated EGFR signalling pathways and their relation to laminin-5 gamma2 chain expression in oral squamous cell carcinoma (OSCC) Histochemistry and Cell Biology . 2005;124(2):151–160. doi: 10.1007/s00418-005-0001-4. [DOI] [PubMed] [Google Scholar]
- 97.Segrelles C., Moral M., Lara M. F., et al. Molecular determinants of Akt-induced keratinocyte transformation. Oncogene . 2006;25(8):1174–1185. doi: 10.1038/sj.onc.1209155. [DOI] [PubMed] [Google Scholar]
- 98.Lingen M. W., Xiao W., Schmitt A., et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncology . 2013;49(1):1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Molinolo A. A., Amornphimoltham P., Squarize C. H., Castilho R. M., Patel V., Gutkind J. S. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncology . 2009;45(4-5):324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roh J. L., Cho K. J., Kwon G. Y., et al. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncology . 2009;45(1):63–68. doi: 10.1016/j.oraloncology.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 101.Onishi A., Chen Q., Humtsoe J. O., Kramer R. H. STAT3 signaling is induced by intercellular adhesion in squamous cell carcinoma cells. Experimental Cell Research . 2008;314(2):377–386. doi: 10.1016/j.yexcr.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song J. I., Grandis J. R. STAT signaling in head and neck cancer. Oncogene . 2000;19(21):2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- 103.Sethi G., Sung B., Aggarwal B. B. Nuclear factor-kappa B activation: from bench to bedside. Experimental Biology and Medicine . 2008;233(1):21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 104.Squarize C. H., Castilho R. M., Sriuranpong V., Pinto D. S., Jr., Gutkind J. S. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia . 2006;8(9):733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakanaka C., Sun T. Q., Williams L. T. New steps in the Wnt/beta-catenin signal transduction pathway. Recent Progress in Hormone Research . 2000;55:225–236. [PubMed] [Google Scholar]
- 106.Patmore H. S., Cawkwell L., Stafford N. D., Greenman J. Unraveling the chromosomal aberrations of head and neck squamous cell carcinoma: a review. Annals of Surgical Oncology . 2005;12(10):831–842. doi: 10.1245/ASO.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 107.Sheikh Ali M. A., Gunduz M., Nagatsuka H., et al. Expression and mutation analysis of epidermal growth factor receptor in head and neck squamous cell carcinoma. Cancer Science . 2008;99(8):1589–1594. doi: 10.1111/j.1349-7006.2008.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pring M., Prime S., Parkinson E. K., Paterson I. Dysregulated TGF-beta1-induced Smad signalling occurs as a result of defects in multiple components of the TGF-beta signalling pathway in human head and neck carcinoma cell lines. International Journal of Oncology . 2006;28(5):1279–1285. doi: 10.3892/ijo.28.5.1279. [DOI] [PubMed] [Google Scholar]
- 109.Paterson I. C., Matthews J. B., Huntley S., et al. Decreased expression of TGF-beta cell surface receptors during progression of human oral squamous cell carcinoma. The Journal of Pathology . 2001;193(4):458–467. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH822>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 110.Tian G., Fu Y., Zhang D., Li J., Zhang Z., Yang X. Identification of four key prognostic genes and three potential drugs in human papillomavirus negative head and neck squamous cell carcinoma. Cancer Cell International . 2021;21(1):p. 167. doi: 10.1186/s12935-021-01863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimada T., Nakamura H., Yamashita K., et al. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human oral squamous cell carcinomas: implications for lymph node metastasis. Clinical & Experimental Metastasis . 2000;18(2):179–188. doi: 10.1023/a:1006749501682. [DOI] [PubMed] [Google Scholar]
- 112.Burley M., Roberts S., Parish J. L. Epigenetic regulation of human papillomavirus transcription in the productive virus life cycle. Seminars in Immunopathology . 2020;42(2):159–171. doi: 10.1007/s00281-019-00773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orenuga O. O., Oluwo A., Oluwakuyide R. T., Olawuyi A. B. Recurrent oral squamous papilloma in a pediatric patient: case report and review of the literature. Nigerian journal of clinical practice . 2018;21(12):1674–1677. doi: 10.4103/njcp.njcp_407_17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


