Abstract
Brazil has a long history of research with rhizobia and plant growth–promoting rhizobacteria (PGPR). Currently, the use of bio-based products in Brazil, containing microorganisms that are effective in promoting plant growth through various mechanisms, is already a consolidated reality for the cultivation of several crops of agricultural interest. This is due to the excellent results obtained over many years of research, which contributed to reinforce the use of rhizobia and PGPR by farmers. The high quality of the products offered, containing elite strains, allows the reduction and prevention in the use of mineral fertilization, contributing to low-cost and sustainable agriculture. Currently, research has turned its efforts in the search for new products that further increase the efficiency of those already available on the market and for new formulations or inoculation strategies that contribute to greater productivity and efficiency of these products. In this review, the history of biological products for main crops of agricultural interest and the new biotechnologies and research available in the agricultural market are discussed.
Keywords: Rhizobia, PGPR, Inoculant, Azospirillum, Bradyrhizobium
Introduction
It is estimated that, in the year 2050, the world population will reach the mark of 10 billion people, which makes greater agricultural production necessary to make the population’s food security feasible [1]. Currently, Brazil occupies the fifth place among the countries that have the largest territorial extension focused on agriculture, with about 7.8% of its territory used for agricultural practices [2]. In Brazil, the agribusiness participation in the Brazilian GDP (Gross Domestic Product) was 26.6% in 2020, within this amount the agricultural sector contributes with 70%, with grain production being the main one [3]. In order to guarantee high productivity in the agricultural sector, chemical fertilizers are widely used by agricultural producers, which increases the final cost of production by up to 30% [4]. In addition, improper handling of chemical fertilizers can negatively affect the environment and the soil. Nitrogen fertilizers, for example, are associated with soil biota acidification and imbalance, pollution of groundwater by nitrates, and increased release of gases that participate in the greenhouse effect, such as nitrous oxide (N2O), combined with ozone layer depletion and global warming [5–7].
The set of economic and environmental implications that have arisen with the intensive use of fertilizers guides the search for solutions that enable a productive and, at the same time, sustainable agriculture [8, 9]. The use of biological products, which contain microorganisms that promote plant growth, called inoculants, in some countries also called biofertilizers, is one of the main strands in the search for better use efficiency, or complete replacement, of mineral fertilizers.
In Brazil, microbial inoculants are defined as a product that contains microorganisms that are beneficial to plant growth [10] which are equivalent to the biofertilizers internationally [5, 11]. The main inoculants produced and commercialized in Brazil are currently formulated with bacteria called rhizobia, which comprise a group of species that establish a symbiotic relationship with plants of the Fabaceae family. This interaction has been known for over 120 years and it forms structures called nodules, where the biological nitrogen fixation (BNF) occurs [11]. The use of these bacteria in agriculture can supply, totally or partially, the nitrogen needs of several leguminous plants.
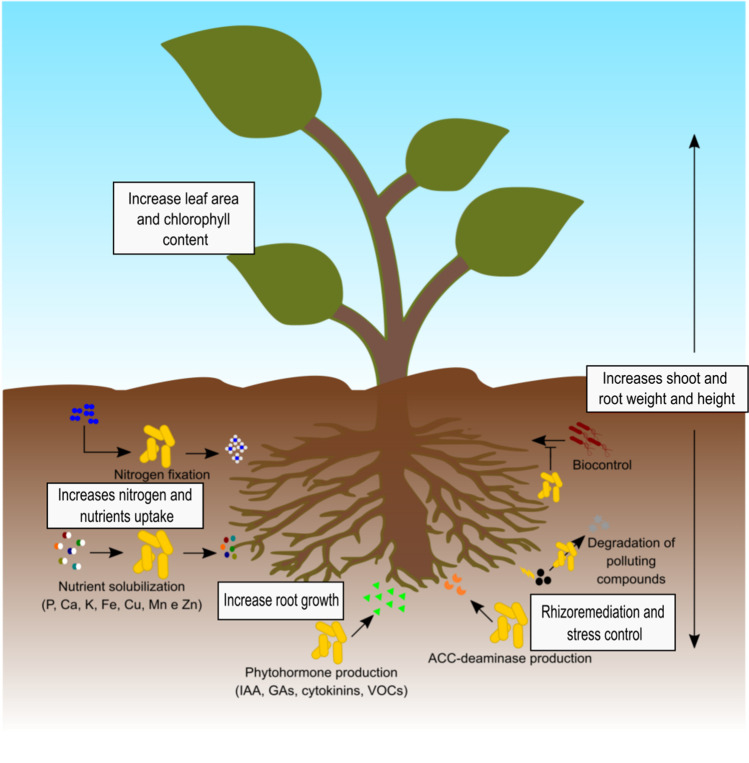
There are other nitrogen fixing (diazotrophic) bacteria that are free-living or associated with plants, without the formation of nodules, also provide nitrogen to the plant, but less efficiently. Bacteria of the genera Azospirillum, Pseudomonas, Bacillus, Azotobacter among others are called plant growth–promoting rhizobacteria (PGPR) and are used in inoculant formulations to directly assist plant growth by improving the acquisition of nutrients by the plant through various mechanisms, such as mineralization and solubilization, BNF, and synthesis of growth regulators (e.g., indole acetic acid, cytokinin), which act directly on root, increasing the root-soil contact surface, allowing greater absorption of water and nutrients and volatile organic compounds (VOC) which has an antifungal activity [12]. Indirectly, the PGPRs or the products of their metabolism can act as biological control agents and induce systemic resistance. Also, the production of the ACC deaminase enzyme acts controlling plant stress, decreasing the ethylene levels by cleaving its precursor and reducing its production, providing resistance to crops under abiotic stress conditions, acting on hormonal metabolic pathways in the plant [12–14] (Fig. 1).
Fig. 1.
Direct and indirect mechanisms for promoting plant growth by PGPR. In the rectangles, the benefits to plants of plant-PGPR interaction
In general, studies with growth-promoting microorganisms focus on isolating and characterizing new PGPR [15–20] and evaluate the performance of these microorganisms in plant development in order to produce new formulations [21–23]. In addition, technologies that improve the use of microorganisms in agricultural processes have been studied [24] such as the combined use of two or more microorganisms [25–30], use of secondary metabolism molecules [31, 32], and new inoculant formulations, which aim at the greater effectiveness of these products. The objective of this review is to present a brief history of the use of inoculants in Brazil, focusing on the main products for crops of agricultural interest, and present new researches and technologies that have been developed to increase the quality and efficiency of inoculants already available.
Bacteria of agricultural interest: history and use in Brazil
The first studies in Brazil with rhizobia of agricultural interest took place in the state of Rio Grande do Sul to select strains for clover (Trifolium spp.) and alfalfa (Medicago sativa) crops intended for livestock production [33]. In the early 1960s, when soybean (Glycine max (L.) Merr) production started its expansion in Brazil [33], the industry of rhizobia-based products also followed the same trend.
A decisive milestone for the production of commercial inoculants was the research which prioritized the search for strains chosen in the inoculation of soybeans and other crops of agricultural interest [34]. Brazil has a long history of research with inoculants and it is impossible not to mention the fundamental role that great researchers such as Johanna Döbereiner and João Ruy Jardim Freire played in this scenario [34, 35]. According to Döbereiner [34], the soybean crop has become a highlight in the Brazilian agricultural panorama, with the use of nitrogen fertilization being entirely dispensed, due to the joint work of microbiologists and breeders. Another decisive factor to guarantee the production and wide acceptance of commercial inoculants in Brazil was the creation of a legislation that establishes criteria with regard to specifications, guarantees, registration, packaging, and labeling of the inputs intended for agricultural use; in addition, it lists the authorized and recommended microorganisms for the production of the inoculants [36]. The first law which defined the commercial standards for this type of product appeared in 1980, and since then has been updated in several occasions. According to the most recent update, about 118 strains of rhizobia are authorized for the production of inoculants for legumes and 12 PGPRs recommended for other crops such as rice, wheat, corn, and eucalyptus [10].
The companies that produce inoculants in Brazil must accomplish a series of requirements with regard to concentration, purity, shelf life guarantee, and absence of biological contaminants in the products. Nowadays, the production of crops of agricultural interest such as soybeans, common beans, corn, wheat, and sugar cane, including brachiaria pastures, has biological products that are recommended for inoculation with wide acceptance.
The case of soybean in Brazil
Soybean (Glycine max (L.) Merr.) is a leguminous plant of the Fabaceae family, originally from East Asia used as a staple food in the eastern civilization. Only in the 1960s soybean becomes economically important in the Brazilian scenario, developing a larger area of cultivation and investment in technologies that resulted in yield increase [37]. The expansion in the agricultural area for soybean cultivation started from 6.8 million hectares in the 1970s to 38.26 million hectares in 2021 [2]. Currently, soybean production is among the most profitable agricultural activities in Brazil, which is the largest producer and exporter in the world. The BNF has always been a priority in the soybean planting system in the country. Since the entrance of this crop in Brazil, research has been focused on the identification and selection of strains of Bradyrhizobium with symbiotic superiority. However, Brazilian soils, at the beginning of soybean cultivation in the country, were free of Bradyrhizobium and due to the absence of the natural diversity of these microorganisms the first inoculants used were imported from other countries. Subsequently, the search for variant genotypes with high capacity for N2 fixation and more adapted to the Brazilian soils and environmental conditions began using techniques for re-isolating rhizobia from soybean nodules [38].
The research with rhizobia capable of nodulating soybean began in the 1940s with the selection of strains, tests with imported inoculants, and attempts at re-isolation [36]. However, it was only in the 1950s that the selection of effective strains in soybean nodulation and the production of inoculants were actually carried out [38]. The pioneering work of the researchers Johanna Döbereiner and Jardim Freire in the search for more competitive and effective strains for this crop was essential for the independence of nitrogen fertilizers in soybean cultivation. The work of these researchers was also decisive for the soybean breeding programs to be carried out in the absence of nitrogen fertilizers, considering only the symbiosis of this plant with the rhizobia [36]. Due to the intense search for superior rhizobia genotypes, Brazil is currently one of the largest producers of inoculants in the world and the consumption of this technology has a high acceptance by great part of the farmers [38–40]. The expansion of soybean cultivation to the Cerrado in the 1980s started with the selection of elite rhizobia strains capable of meeting plant’s demand for nitrogen in low humidity conditions and in acidic soils. The first strains selected for soybean inoculation in the Brazilian Cerrado were Bradyrhizobium elkanii SEMIA 587 and SEMIA 5019 (= 29w) [41]. These strains possessed a high capacity to fix nitrogen, had a good competitive ability, and were able to nodulate a wide variety of soybean cultivars [41]. The inoculation success with these strains in the 1980s among other advances in crop production system contributed to increase the agricultural frontier in the country and, as a result, soybean became the most important agricultural crop in Brazil [40].
Peres et al. [42] presented the strains of B. japonicum SEMIA 5079 (= CPAC 15) and B. diazoefficiens SEMIA 5080 (= CPAC 7), as highly competitive and with high nitrogen-fixing potential [38, 39, 43]. The inoculation of soybeans with SEMIA 5079 and SEMIA 5080 showed gains in productivity 8.8% higher than those presented using the strains of B. elkanii SEMIA 5019 and SEMIA 587 [40]. In 2019, a consumption of 70 million doses of soybean inoculants was estimated, used in more than 90% of the total planted area in Brazil [44]. Soybean inoculation allows an average yield of 3.5 tons of grains ha−1, without the need of nitrogen fertilizers [45]. On the other hand, only 15% of the total area used for planting soybeans in the USA are inoculated [46, 47]. This is due to the low cost of nitrogen fertilizers and low incentives in the use and development of biological-based technologies in that country [36].
Considering the reduction in the total spending on nitrogen fertilization for the crop and the wide acceptance of the technology by producers, an annual savings of US$15 billion in Brazil is calculated, allied to a significant reduction in the emission of greenhouse gases and groundwater contamination [44]. The calculation of savings in nitrogen is carried out as described by Hungria and Mendes [48], which considers the size of the total planted area in the country, the price of the nitrogen (urea), and the average yield per hectare. Furthermore, this calculation also considers a 50% nitrogen use efficiency and the estimate of the total N exported by the crop. Assuming that the use of nitrogen in soybean cultivation is unnecessary, it is possible to assume that the calculated amount that would be spent on urea is saved with the use of rhizobia in soybean inoculation.
Between 2009 and 2018, the use of inoculants for soybean has increased to 221% [49], used both domestically and for export to countries in South America and Africa, with the vast majority containing the elite strains SEMIA 5079 and SEMIA 5080 [38]. Currently, new ways of inoculation and improvement of the formulation have been studied to improve its efficiency, and in return, increase the demand for this type of product in agriculture.
Common bean
Common bean (Phaseolus vulgaris L.) is a legume from the Fabaceae family grown in different regions of the world. Brazil is one of the largest producers and consumers of this legume, which is widely consumed in South America and Africa. Currently, about 2.9 million hectares in Brazil are used for the common beans cultivation, in three annual harvests, reaching a production of 3 million tons. However, the average yield of each crop is 1000 kg ha−1, well below its productive capacity [2]. Environmental stresses such as drought and low nutrient supply are limiting factors for the development of the plant and, common causes, for the common beans low productivity [27, 50].
Common bean is considered a host for the rhizobia with low specificity, or promiscuous, capable to form nodules with different genera and species of bacteria of the alpha-proteobacteria group (mainly Rhizobium) and beta-proteobacteria (as Paraburkholderia nodosa, P. tuberum, P. sabiae, and Cupriavidus necator). The most common bacterial species capable of nodulating common bean belong to the genus Rhizobium, such as R. tropici [51], R. etli [52], R. freirei [53], R. leucaenae [54], and R. paranaense [55], among others. In Brazil, there are three bacteria of the genus Rhizobium authorized for commercialization in inoculant formulations and recommended for common beans cultivation, the R. tropici SEMIA 4077 (= CIAT 899) and SEMIA 4088 (= H12) and R. freirei SEMIA 4080 (= PRF 81). These bacteria were selected mainly for having greater genetic stability and high tolerance to environmental stress conditions [56, 57].
Due to a series of factors that affect the efficiency of BNF in common beans and the great demand for nitrogen by the crop, the results in productivity can be variable, being necessary, in some cases, a supplementation with nitrogen fertilizers to reach high production levels [58, 59]. The main reported factors involve early senescence of the nodules [60], genotype-specific interaction between bacteria and host plant [61, 62], and abiotic factors, mainly water deficit [63], soil fertility, and temperature [64]. The presence of highly competitive native rhizobia capable of nodulating the common bean (but with a low fixative capacity) is one of the main causes of the low efficiency of BNF in this crop [65, 66] and can affect inoculation responses with elite strains [67].
In the state of Paraná, inoculation of common beans with R. tropici CIAT 899 and R. freirei PRF81 resulted in productivity above 2.500 kg ha−1 without the addition of N fertilizer [56, 57]. In the Midwest region, common bean production may still depend on complementary doses of N fertilizers, along with inoculation to achieve high levels of productivity. According to Pelegrin et al. [68], common bean inoculation was equivalent to a fertilization of 80 kg of N ha−1 in the state of Mato Grosso do Sul. In addition, when 20 kg of N ha−1 was added in the seeding, associated with the inoculation with R. tropici CIAT 899, a yield of 3.339 kg of grains ha−1 was obtained, equivalent to a fertilization with 160 kg of N ha−1 [68]. Depending on the cultivar, the productivity of beans can vary from 840 to 2.741 kg ha−1 of grains, when inoculated with elite strains recommended for the crop [61, 62].
Based on the promising results of the use of commercial inoculants in common bean crops and, mainly, in the possibility of reducing production costs, the sale of this biological input increased by 85% between 2009 and 2013 [49], and only in 2018, approximately 280 thousand doses of inoculant for beans were sold in peat and liquid formulations [49]. Strategies, such as co-inoculation with Azospirillum brasilense [69] and Bradyrhizobium spp. [26], nitrogen fertilization associated with elite strain inoculation [68], and the search for new elite bacteria [70], are the subject of studies for common bean.
Grasses
The cultivation of cereals started thousands years ago and, until the present moment, represents the basis of the world nutrition. Among the main crops, wheat, corn, and rice stand out as important cereals for human and animal food [71]. Cultivated pastures are also an economical and viable agricultural practice for cattle feed; in addition, it is an important practice for the recovery of degraded areas [72]. The cultivation of grasses is dependent on mineral fertilization, especially with nitrogen, to achieve high yields. In Brazil, there is a long history of research with PGPR associated with grasses, especially for pioneering work conducted by Dr. Johanna Döbereiner, which aimed at reducing mineral fertilization and guaranteeing the maximum production of crops with agricultural sustainability [73–75]. Among the most studied microorganisms, bacteria of the Azospirillum genus stand out as PGPRs for a wide variety of host plants, many of great economic importance, such as corn and wheat [75].
Bacteria from the Azospirillum genus are alpha-proteobacteria, which can be free-living or associated with the rhizospheric region and/or endophytically in the colonization of more than one hundred hosts [76]. This bacterial genus was first described by Tarand [77], with A. brasilense and A. lipoferum species. Currently, a total of 22 species have been described, isolated mainly from the soil, with worldwide distribution[78].
The first studies carried out with this bacteria aimed to assess its ability to fix atmospheric nitrogen and reduce the use of mineral N, especially in grasses [73, 79]. Its first species was described by Beijerinck in 1925 and it was called Spirillum lipoferum, but it was only in 1978 that it was discovered that this bacterium has the ability to fix atmospheric nitrogen, having its scientific name changed to Azospirillum lipoferum [77]. Shortly after, Tien et al. [80] reported the production of several growth regulators by Azospirillum, such as auxins, gibberellins, and cytokinins. It is now known that the benefits of inoculation with this bacterium go beyond BNF, which reinforces the theory of Bashan and Levanoy [81] about the “additive hypothesis,” that considers the involvement of multiple mechanisms of action in the association of Azospirillum with the host. The hypothesis suggests that the mechanisms operate simultaneously or in association, with the contribution of one of the mechanisms being less effective when evaluated separately.
The inoculation with the A. brasilense species benefits the plant in several ways, such as the induction of systemic resistance, inducing the synthesis of a variety of secondary metabolites by the host [82]. Furthermore, the association among plants and A. brasilense can provide protection from abiotic stress conditions, such as salt and oxidative stress [83, 84] and the production of growth regulators by bacteria results in morphological changes in the roots, promoting greater root growth and resulting in better absorption of nutrients and water [80, 85–89]. In the case of seed inoculation, the growth regulators produced by Azospirillum in the product act in “seed primming” effect, that is, after inoculation, the number of viable bacterial cells is drastically reduced; however, the concentration of growth regulators remains promoting plant growth [78].
Several studies point out the beneficial effects of inoculation with A. brasilense in grasses of agronomic interest such as corn (Zea mays L.), wheat (Triticum aestivum L.) [75, 90, 91], rice (Oryza sativa) [88], and brachiaria (Urochloa spp.) [72]. Hungria et al. [75] evaluated the inoculation of A. brasilense Abv5 and Abv6 in wheat and corn by conducting 17 experiments carried out in different stations located in nine areas with different edaphoclimatic conditions. There was an increase in nutrients concentration in the grains and a greater root mass in the inoculated plants, with an increase of 24–30% and 13–18% in corn and wheat productivity, respectively. Díaz-Zorita and Fernández-Canigia [92] found similar results in 297 experiments conducted in Argentina with wheat, using A. brasilense Az-39, and positive responses were observed in 70% of the experimental areas, with an 8% gain in grain yield. Okon and Labandera-Gonzalez [86] in experiments carried out in several countries with A. brasilense also reported a 5–30% increase in grain yield in 70% of the evaluated areas.
In Brazil, the inoculant commercialization containing the strains of A. brasilense Abv5 and Abv6 has been carried out since 2009, for wheat, rice, corn [75] and, more recently, for brachiaria [72]. In 2018, more than nine million doses of inoculants with A. brasilense were commercialized, accounting for approximately 10% of the total inoculants sold in Brazil, indicating the great acceptance of this bioproduct by producers [49].
The Nitrospirillum amazonense species, isolated from sugar cane and other grasses such as corn, sorghum, and rice, is widely distributed in the Brazilian territory [93]. Possibly, its wide occurrence in the Brazilian soils is associated with high adaptability to acidic soils, a very common characteristic in most of the country’s soils. [94, 95]. Recently a public–private partnership between the Brazilian Agricultural Research Corporation (EMBRAPA) and BASF company announced a new inoculant that has this bacterial species in its formulation and it is recommended for the cultivation of sugar cane. BASF is commercializing this inoculant associated with other products of insecticidal and fungicidal action, with the name of “Muneo Bio” Kit.
Even though inoculation with beneficial bacteria such as rhizobia, Azospirillum spp., Bacillus spp., and Pseudomonas spp. has gained more space in the agricultural market, the use of bioproducts still accounts for a small share of the fertilizers and pesticides in the world, far below what is necessary for greater productive sustainability [96]. New technologies are needed to make these products more effective in the field, less costly, and more attractive to producers. Currently, several studies are being carried out with regard to new formulations of beneficial bacteria and the use of secondary metabolites in combinations with them.
Phosphate solubilization
Phosphorus is one of the most limiting elements for plant growth and decisive for agricultural crops productivity. Tropical soils used in agriculture are generally acidic, with the presence of phosphates, especially iron phosphate (FePO4) and aluminum phosphate (AlPO4), that are unavailable to plant metabolism [97]. The lack of this nutrient is supplied with the application of phosphate fertilizers. High doses should be applied to the soil, due to its low efficiency, which makes the use of phosphate fertilization costly to the producer, especially in Brazil, which has a high dependence on the import of this input [98].
Phosphate-solubilizing microorganisms (PSM) convert the sparingly soluble phosphate to its soluble form, or assimilable, to plant metabolism through the production of compounds capable of breaking the phosphate bond with its chelating agent. Among the main forms of action of these microorganisms are the production of organic acids, siderophores, protons, and CO2 [98–100]. Several microorganisms, including bacteria and fungi, have the ability to solubilize phosphates, such as Aspergillus, Penicillium, Bacillus, Pseudomonas, and Paraburkholderia. Many studies have focused on the prospecting of PSM for the production of inoculants [99].
Several microorganisms are marketed as PSM in the world, one of the main is the Penicillium bilaiae fungus, commercialized in Canada in a formulation called JumpStart XL® (Bayer), recommended for use in various cultures. In field, the use of this product increased productivity and P content and reduced phosphate application in wheat, in addition to contributing to greater phosphate absorption in other crops [101]. Currently, many researches have focused on the combined use of PSM with other microorganisms such as rhizobia [102] and mycorrhizal.
Oliveira et al. [103] described 45 PSM isolated from the corn rhizosphere grown in an area of the Brazilian Cerrado with low phosphate concentration in the soil; the authors report that the isolates Bacillus sp. (B17), Burkholderia sp. (B5), and Streptomyces platensis (A4) were the most efficient in solubilizing calcium phosphate Ca3(PO4)2, promising for the formulation of an inoculant. Ribeiro et al. [97] found that strains of Bacillus B1923, B2084, and B2088 promoted foliar and root growth and accumulation of macronutrients in millet, independently, varying according to the inoculated strain and source of phosphate present. These microorganisms have other characteristics in promoting growth, such as the production of growth regulators and siderophores. The isolates B2084 and B2088 produced gluconic acid in vitro and it is recognized by the literature as one of the most efficient mechanisms in the solubilization of phosphate by bacteria.
These studies contributed to the development of the first commercial inoculant based on phosphate-solubilizing bacteria in Brazil, composed of Bacillus megaterium CNPMS B119 and B. subtilis CNPMS B2084, BiomaPhos® (Bioma), the result of research by the Brazilian Agricultural Research Corporation (Embrapa) with the company Bioma [104]. Paiva et al. [104] show that the combined use of these two strains was effective in increasing corn productivity. The authors carried out several experiments, in three harvests, in different locations in Brazil and the average results of productivity gain with inoculation were 8.9%, resulting in average yield of 10 bags of corn grains per hectare. The use of this product in the cultivation of soybeans was also effective in increasing productivity, resulting in an average increase of 5 bags per hectare [101]. Currently, research with these same strains continues to be carried out with other cultures.
The production of phosphate fertilizers is carried out from non-renewable sources, generating environmental impacts. Furthermore, Brazil is still dependent on imports of this product, although there is an enormous reserve of phosphorus that cannot be assimilated in the soil. The development of inoculants based on PSM is extremely important to reuse the phosphate stock fixed in the soil, chelated in clay or metals (Al, Fe, and Ca, especially) and reduce the use of phosphate fertilizers.
Co-inoculation
The combined use of different PGPRs that work through different mechanisms of action promoting plant growth is a strategy that has been widely explored in the inoculation of different cultures [69, 105]. The combination of two or more microorganisms has already been used in agriculture, such as the combination of rhizobia with phosphate-solubilizing bacteria [106], PGPRs with another PGPR that acts as a pathogen biocontrol agent; or bacteria that have the same mechanisms of action in promoting growth, but with differences in tolerance to abiotic conditions of the medium or specificity to the plant’s genotype [8, 107].
In Brazil, there has been success in the combined use of rhizobia strains recommended for soybeans and common beans with A. brasilense Abv5 and Abv6 [30, 69]. The inoculation of rhizobia with A. brasilense increased the productivity in 16.1% and 19.6%, in soybean and common beans, respectively, when compared with the controls inoculated only with rhizobia [69]. The combined use of rhizobia and A. brasilense benefits the plant in increasing the number of nodules [108, 109], greater tolerance to environmental stress conditions [83], and it is an economically profitable practice for the producer. Galindo et al. [25] demonstrated that the inoculant based on A. brasilense represents only 1.1% of the total operational cost of soybean cultivation; however, productivity gains are approximately 10 bags of grains per hectare compared to cultivation with only Bradyrhizobium spp.
The co-inoculation of rhizobia and A. brasilense AbV5 and AbV6 is a practice recommended for soybean and common beans producers in Brazil and, currently, co-inoculation is already a reality for most farmers. Between 2015 and 2018, there was an increase of 220% in the commercialization of inoculants composed by A. brasilense [49]. Although the increase in the commercialization of this product is due to grasses cultivation, a large part of the sales is destined to the combined use of inoculants recommended for legumes [36]. Currently, some inoculant companies in Brazil already offer for sale a single product containing Bradyrhizobium spp and A. brasilense for soybean inoculation.
The combined use of five strains of PGPR is recommended for sugarcane in Brazil. This formulation is composed by Gluconacetobacter diazotrophicus (BR 11,281), Herbaspirillum seropedicae (BR 11,335), Herbaspirillum rubrisubalbicans (BR 11,504), Nitrospirillum amazonense (BR 11,145), and Paraburkholderia tropica (BR 11,366). All bacteria present in the formulation are diazotrophic and phytohormone-producing, and isolated from sugar cane [110]. Co-inoculation with the five strains is recommended in order to reduce the difference in response among distinct sugarcane cultivars [111].
Co-inoculation offers several benefits to the plant, such as an increase in root area, which enables a greater use of mineral fertilization and water absorption, ensuring protection from water stress conditions, and provides legumes with greater root surface for rhizobia infection, increasing nodules formation [25]. It is the target of several studies that pursuit to deepen the knowledge about bacteria that can be the object of new formulations, compatibility of different strains, and use in different cultures.
New inoculant formulations
Molecular inoculants: addition of secondary metabolism molecules in formulations already consolidated on the market
Nodulation factors (Nod factors)
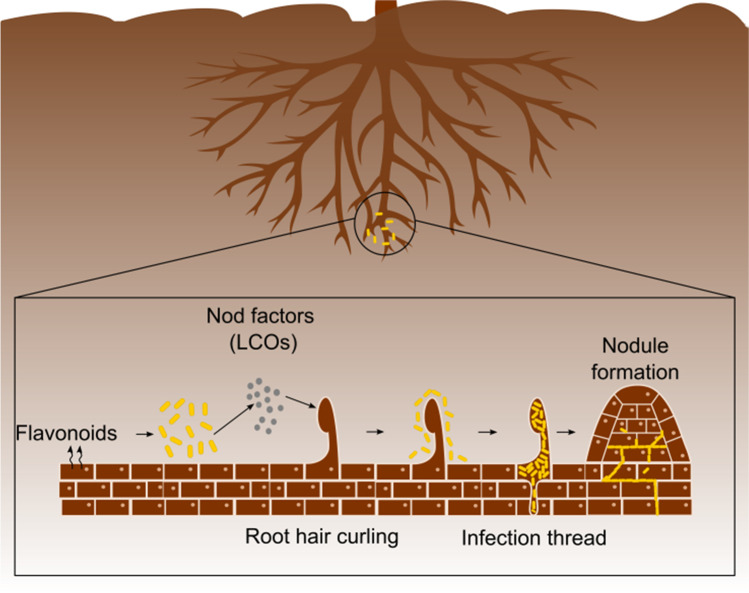
The process of forming active nodules in leguminous plants is extremely complex and depends on the communication between the host plant and the rhizobia through signal molecules. The formation of functional nodules is initiated by the production of phenolic compounds by the host, such as flavonoids, which act as an inducing molecule [112, 113]. These compounds, in addition to acting as chemotactic agents for rhizobia, activate the transcription of nodulation genes (nod genes) and promote the production of signal molecules by rhizobia called lipochitooligosaccharides (LCOs), or nodulation factors (NF), essential for the specificity of host-symbiotic communication [12, 114, 115]. Nodulation factors play a crucial role in the formation of active nodules and in submicromolar concentrations, among 10−9 and 10−12 M [116], they induce physiological and morphological changes in the host such as alteration of the ions flow, resulting in the root hair curling and formation of the infection cord that allows the rhizobia to enter the cells. In addition, nodulation factors promote the nodular primordium formation, which after the rhizobia infection and differentiation form the active nodule (Fig. 2) [8, 113, 116–119].
Fig. 2.
Colonization of rhizobia and nodule formation. Flavonoids are produced by the host and act as a signal molecule for the production of nodulation factors by rhizobia. The perception of nodulation factors by the plant induces the root hair curling, facilitating the entry of bacteria in the cortical zone of plant tissue. Invaginations in the region of the hair bending form the infection cord that leads the bacteria to the region where the nodule will be formed. The mitogenic action of nodulation factors stimulates the proliferation of cortical cells in the plant and the nodular primordium formation or emerging nodule. Rhizobia colonize the emerging nodule, forming the symbiosome, and differ in to bacteroids at the N2 fixation stage
Nodulation factors act in several physiological processes in the host, in addition to those involved with nodulation, such as the formation of lateral roots [120]. Furthermore, LCOs activate the expression of genes involved in the plant cell cycle, stimulating cell division, not only in leguminous plants and, because of this, they stimulate germination, seedling growth, and root growth in several non-target hosts, when applied in seeds [121, 122]. They are also able to promote an increase in leaf area, increase in photosynthetic rate, and total dry weight when foliar is inoculated [115]. Souleimanov et al. [123] observed that in submicromolar concentrations (10−7 and 10−9 M), the purified LCO of B. japonicum 532C increased the germination rate of maize, rice, and soybean, and promoted greater biomass accumulation, supporting the theory of the hormone-like action of these molecules proposed by Fisher and Long [124].
Some studies also report that exudates from non-leguminous plants act as inducers of the genes responsible (nod genes) for nodulation in rhizobia [115]. Lian et al. [125] observed the production of LCOs in a culture of B. japonicum after the addition of corn, soybean, and wheat root extracts. The corn root extract induced the production of LCOs by the bacteria in high concentrations. The response to exudates from non-leguminous plants by rhizobia suggests that, in addition to acting in symbiosis with leguminous plants, these bacteria act as PGPRs and that nodulation factors have hormone-like action, such as stimulation of formation of lateral roots, allowing greater assimilation of nutrients and water by the host [115].
In leguminous plants, it was observed that the treatment with LCO extracted from R. leguminosarum bv. viciae GR09 in pea (Pisum sativum) and vetch (Vicia villosa) seeds increased germination, biomass, and nodulation efficiency [122]. Similar results were obtained in Medicago truncatula treated with LCO of S. meliloti via seed [117].
Nodulation factors combined with the use of host-specific rhizobia are beneficial to leguminous growth. The purified LCOs, in the absence of rhizobia, are sufficient to induce the root hair curling, cell division, and the formation of nodule-like structures [126]. López-Lara et al. [127] added purified LCO from Rhizobium sp. GRH2 in Phaseolus sp. and Acacia spp. and observed the formation and deformation of root hair.
Several studies have been carried out with the exogenous application of LCOs in legumes and an increase in the nodules number and nitrogen concentration in the leaf as well as greater expansion of the root area are reported. [117, 122]. In non-leguminous plants, tolerance to high temperature is reported [116]. The use of LCO with rhizobia increases symbiotic competitiveness and can benefit the recruitment of soil rhizobia to increase nodulation efficiency [122].
Studies carried out in Brazil showed that soybean inoculation with strains of Bradyrhizobium spp. added with secondary metabolites, containing LCOs, extracted from B. diazoefficiens USDA 110 increased grain yield by 4.8% compared to treatment inoculated only with the inoculant recommended for this crop [31]. In corn, the addition of R. tropici CIAT 899 metabolites to the A. brasilense inoculant was evaluated and there was an increase of 11.4% in productivity [31]. The application of A. brasilense enriched with R. tropici CIAT 899 LCOs showed an increase in corn productivity in five, out of a total of six, experiments conducted compared to the non-inoculated treatment [32].
There are still few companies that produce bioformulations containing secondary metabolites, such as LCOs. Among the products available, there are those that carry the purified molecule, which can be inoculated via foliar or seed and bioformulations composed of the molecule and the bacteria (rhizobia or PGPRs) recommended for the crop of interest [128]. Products that use this biotechnology are marketed by multinational companies and recommended for both leguminous plants such as soybeans, peanuts, and alfalfa and for non-leguminous plants such as maize and wheat.
Since 2011, a product called Ratchet® (Monsanto) based on LCO, recommended for foliar use in soybean and corn, has been commercialized in the USA, which act by stimulating photosynthesis, sugar production, increasing plant growth, and resulting in better harvest performance. In experiments conducted between 2008 and 2010, an increase of, approximately, 4 to 5 bags of grains per hectare in maize and 1 to 2 bags of soybean grains was evaluated with the inoculation of the commercial LCO via foliar, according to the manufacturer. Another product in the same line, TagTeam LCO® (NexusBioAg), commercialized in the USA, combines R. leguminosarum and purified LCO, and it is recommended for the cultivation of lentils (Lens culinaris) and peas (Pisum sativum). Also in the USA, the LCO Promoter Technology® (Novozymes), which carries purified nodulation factors, is commercialized. The formulation called Optimize ST® (NexusBioAg), commercialized in Canada, carries LCO with B. japonicum in a single product, ensuring increased nodule formation, nutrient absorption, and increased harvest production. In addition to rhizobia, LCOs are commercialized in bioformulations with other microorganisms that promote plant growth such as Penicillium bilaiae, in a product called JumpStart LCO® (Novozymes) (Canada). P. bilaiae is a natural occurring fungus in several types of soils and has been studied for many years due to the high production of important compounds for phosphate solubilization. Despite its potential, the use of this technology in Brazil is still incipient. Further studies are needed on crop responses in the field, since work with these molecules is still very restricted to controlled conditions.
Molecules involved in biofilm formation
Biofilms are structures formed by cellular aggregates coated with an array of extracellular polysaccharides (EPS), proteins, and lipids. The EPS is the main structural component of biofilms, being responsible for the architecture, stability, and organization of the cellular agglomerate in micro-colonies. The structural components of biofilm provide resistance to desiccation, passive absorption of nutrients from the adhesion region, act as a carbon source and resistance to biotic stress conditions (protection against antimicrobial compounds and toxins) and abiotic (changes in pH and variations in temperature) [129]. In the soil, the formation of bacterial biofilm ensures the protection of both pathogenic and non-pathogenic bacteria to elevated temperatures, nutritional and water limitations in microenvironments, in addition to enabling the adhesion of these cells to various surfaces, such as the rhizosphere [129–131] (Fig. 3).
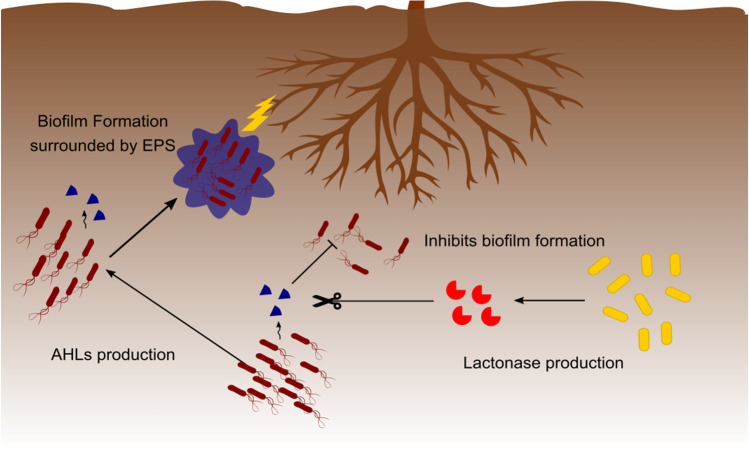
Fig. 3.
Biofilm formation by soil bacteria and action of lactonase enzymes. Soil bacteria control cell density by molecular signals called autoinducers. N-acyl homoserine lactone (AHL) is the main cell–cell communication molecule studied, and acts directly in the control of cell density and regulates the expression of genes involved in the biofilm formation (formed by exopolysaccharides—EPS) and virulence. Some bacterial genera, especially involved in biological control, produce enzymes called lactonases that act by breaking the lactone ring and preventing signaling via AHL. The lactonase enzymes action is related to the attenuation of the pathogenic bacteria virulence and the reduction of bacterial biofilms formation, both by pathogenic bacteria and plant growth promoters
The molecules involved in biofilm formation, such as EPS, have elucidated effects on the structure of bacterial micro-colonies and it is currently known that host plants recognize these molecular patterns and exhibit physiological responses [132–135]. Although they have physiological effects on plants and are crucial for the colonization of rhizobia and other PGPRs, there are still no formulations available on the market that have bacteria-bacteria communication molecules in their composition. The use of this type of molecule in formulations has a promising potential in increasing the rhizobia colonization on the root surface and improving the inoculant colonization efficiency.
Quorum-sensing (QS) is defined as an intra and/or inter-specific mechanism for regulating the density of the microbial population, mediated by molecules called autoinducers that act in cell–cell communication. This communication system between bacteria acts in the coordination of bacteria cells and has already been reported for many species of Gram-negative bacteria [136]. The main self-inducing molecule produced by this group of bacteria is N-acyl homoserine lactone (AHL) [137]. They are a group of molecules with low molecular weight and diffusible by the cell membrane that act in the expression of specific genes in response to environmental changes [129, 130, 133, 138, 139]. Among the phenotypic responses to the action of the QS, the most studied and relevant are the genes expression involved in virulence, biofilm formation, production of exopolysaccharides (EPS), colonization, and symbiosis [137, 140–143].
The QS process in rhizobia is crucial for the surface polysaccharides production, adaptation to the stationary phase, the symbiosome development, and nodulation efficiency, since it is associated with the biofilm and micro-colonies formation, which are crucial steps for the bacterial colonization in the rhizospheric region [131]. Pérez-Montaño et al. [141] showed that in the presence of flavonoids, the biofilm structure of S. fredii SMH12 changes from monolayer to micro-colony and then the effective colonization of soybean cv. Osumi, which is a crucial stage for the nodule formation. Pérez-Montaño et al. [132] observed that the total molecule production involved in the QS in S. fredii SMH12, R. etli ISP42, and R. sullae IS123 is dependent on the type of flavonoid that is used.
The production of AHL and consequently the biofilm formation in the rhizosphere are crucial for the colonization of several microorganisms, in addition to rhizobia. It is now known that plants recognize a wide variety of AHL-type molecules and these modify the root architecture. Ortíz-Castro et al. [135] evaluated the biological activity of AHL in root development, using molecules with an acyl chain ranging from 4 to 14 carbons and observed a reduction in the primary root, lateral root. and root hair growth in Arabidopsis thaliana, especially when applied to the N-decanoyl-HL (C10-HL). This study suggests that plant growth–promoting bacteria produce molecules of type C8 to C12-HL, and these molecules are those that have the greatest biological activity in root development. These results coincide with studies of Pérez-Montaño et al. [132], that when evaluating the molecules produced by S. fredii, R. sullae, and R. etli, identified that in all cases there was the production of C8-HL. These results suggest that rhizobia and other growth-promoting bacteria possibly have the same molecular pattern, which is recognized by the host plant and acts by stimulating root development as a strategy to reinforce interactions with the bacterial partner [135, 143].
Microbial carriers and bacteria bioencapsulation
The encapsulation, or immobilization, of microorganisms comprises an alternative technology that aims to protect the microbial cells in the soil and promote their gradual release [144–146]. The polymeric matrices can be composed of alginates, clay, agar, pectin, chitosan, polyacrylamide, and gum, such as xanthan gum, which have different rates of degradation. Once encapsulated, cells are protected in a matrix permeable to water and nutrients that protects microorganisms from mechanical damage and environmental stress [147, 148]. The slow and gradual degradation of the material releases the microorganisms continuously in the environment, allowing the inoculum to remain in the soil for a longer time [11, 145, 149–151]. The advantages associated with the use of PGPR encapsulation, according to conventional formulations, are in increasing the effectiveness of inoculants, in the controlled and gradual release of bacteria, and in reducing the toxic effects of agrochemicals in seeds and in soils. These products are biodegradable and non-polluting and provide physical protection for the inoculum increasing its shelf life [11].
Among the matrices used, sodium alginate is the most common for agronomic uses [11, 144, 146]. The preparation of these matrices is done by adding the sodium alginate in the same solution as the inoculum, and through the addition of a calcium chloride solution, alginate particles are formed. These are washed and later lyophilized or prepared in liquid emulsions for stabilization in microcapsules [148, 151].
Several methodologies are used to define particle size, shape, and texture that will vary from the type of microorganism studied to the application method. Macroencapsulation are particles that vary in size from a few millimeters to centimeters but offer little contact with the seed. Microencapsulation, in turn, are particles of size ranging from 10 to 100 µm and offer greater contact between the inoculum and the seed [148]. The review by John et al. [151] details several technologies used in the production of these particles.
The PGPR encapsulation was first proposed by Bashan [152] and, in spite of all the aforementioned benefits, it still has no applications in the field and there is no large-scale production. One of the main reasons for this is the difficulty in maintaining a completely sterile and contaminant-free environment; cell mortality in the lyophilization stage, with a significant reduction in cell concentration; in addition to a production high cost superior to peat and liquid formulations [151, 153]. In this sense, research has sought to circumvent these obstacles with the search for alternatives that reduce the bottlenecks that still exist for the diffusion of this technology. Kadmiri et al. [146] showed that the use of hybrid polymeric matrices composed of calcium alginate of two types of clay was efficient in preserving the concentration of P. fluorescens Ms-01 and A. brasilense DSM1690 (Ab) cells for 3 months at room temperature and when added to a saline solution, biocapsules released the inoculum for 15 days, revealing a slow and gradual release capacity. In addition, the authors found that the inoculation of these particles in wheat significantly increased the root and biomass area and the accumulation of nitrogen in the roots, regarding the uninoculated control. Young et al. [154] observed that the viability of PGPR B. subtilis CC-pg 104 cells encapsulated in a matrix of sodium alginate and humic acid was not altered after lyophilization during 5 months storage at room temperature. This study also showed that the bacteria encapsulation increased the persistence of the bacteria by 104 CFU (cm of root −1) in the rhizosphere and by 10 times the number of CFU cm of root −1 in the rhizoplane compared to free cell inoculation. On an industrial scale, Strobel et al. [149] studied a spray drying method that combines the use of high temperatures and cell dehydration for the production of calcium alginate microparticles containing PGPR Methylobacterium radiotolerans. It was observed that M. radiotolerans cells maintained their viability after the process in a concentration of 1010 CFU g−1 of lyophilizate, but there was a decline in the bacterial population after 1 year of storage. The authors reinforce that the methodology used is applicable at an industrial level for the inoculant production for seeds or foliar application. These studies show that the use of bacterial encapsulation for agronomic applications is close to becoming a reality for producers. The encapsulation techniques improvement has allowed a reduction in production costs, as well as an increase in the microorganism viability, which can bring significant promises both for use in microbial inoculant and biopesticide formulations.
Seed pre-inoculation technology
Inoculant production involves, in addition to contaminant-free microbial growth, the use of a carrier formulation that should provide favorable conditions for maintaining the microorganism viability and cell concentration for as long as possible. The desirable characteristics in a carrier are as follows: do not have toxic substances to the microorganism; be easily sterilizable; have an adequate and buffered pH; allow the microorganism initial growth; and ensure cell viability and concentration for as long as possible [155]. The choice of a suitable carrier is crucial for the production of a microbial inoculant, since any factor that acts by reducing the rhizobia cells concentration, consequently, reduces the BNF efficiency [156].
For decades, peat has been the main carrier used in several inoculants. This material is rich in organic matter, which acts as a nutritional reserve for microorganisms and protects cells from osmotic stress conditions. This carrier ensures that the product final formulation maintains cell concentration and viability and is free from contaminants, in accordance with the Brazilian legislation [156]. Peat inoculation should be performed using an adhesive agent that will allow the product to contact the seed, in Brazil, a 10% sucrose solution is commonly used [36, 156]. However, inoculation with peat in large production areas consumes a long period at sowing and requires specialized machinery. In addition, peat is a non-renewable product in nature and can generate irreversible environmental impacts [155]. In this sense, new formulations are demanded by the market, being the most accepted currently the liquid formulation.
Liquid formulations are produced from the bacterial culture medium with stabilizing agents such as mineral and organic oils and cell protectors and their use is less laborious and, generally, with the same quality as peat formulations [11]. Alternatively, these formulations allow the use of new inoculation techniques such as the foliar inoculation or in furrow, which can be an advantage, as in cases of remedying the inoculation, if it has not been done correctly via seed or to avoid contact of the inoculant with agrochemicals treated seeds [24, 155, 157, 158]. Due to its great usefulness, it currently comprises 80% of the total doses of inoculants sold in Brazil [44].
Despite advances both in the inoculant formulation and in the application techniques, there are still some important issues to be addressed. The low viability of the microorganism after inoculation in the seed makes it necessary that the inoculation occurs within a period of up to 24 h before sowing, both in the products use with peat carriers and liquids [157]. The short period since inoculation until sowing might reduce the process quality and delay the work in the field and it demands a larger number of people for the process. Furthermore, inoculation carried out in the planting area itself requires specialized machinery, which can be a decisive factor in adhering to the use of technology and if performed incorrectly reduces its efficiency [159].
Pre-sowing seed inoculation has emerged as an alternative technique to the practice of inoculation, which consists in the seed’s previous inoculation that can occur days and even weeks before sowing [157]. The benefits associated with the pre-inoculation technique include a reduction in the possibility of inoculation errors, which can result in a poor microorganism distribution in the seeds and, consequently, a reduction in the technique efficiency. The availability of pre-inoculated seeds reduces one of the steps in the sowing process and guarantees the efficiency of inoculation, these gains can increase the producers search for this technology [159].
In an experiment conducted in four different agricultural areas in Brazil, Hungria et al. [44] found that pre-inoculated seeds 15 days before planting did not show statistical differences in productivity and nitrogen accumulation in the grain compared to seeds inoculated at the time of planting. The authors show that the treatment’s average productivity with pre-inoculation was 89% higher than the non-inoculated and non-fertilized control, pointing out that the use of this technique guaranteed the efficiency of the BNF. However, the study was carried out on seeds not treated with agrochemicals. Much of the totality of seeds used in large plantations in Brazil are treated with fungicides and chemical additives that are toxic agents to microorganisms and can drastically reduce their population in the seed [160]. A long period of exposure of the inoculum with the chemical agent reduces the number of cells capable of forming nodules and decreases the efficiency of BNF [156, 157, 161]. Campo et al. [160] found that 2 h after inoculation of Bradyrhizobium sp. in soybeans previously treated with fungicides, 62% of the initial cell population was no longer viable, and after 24 h only 5% remained viable.
Alternatively, several studies have been conducted in search of cell protectors that are biopolymers that maintain optimal water activity for the rhizobia survival in the cell and reduce the contact of the bacteria with the seed pesticides [162]. Neto et al. [163] suggest that the use of additives as cell protectors is efficient in the pre-inoculation of Bradyrhizobium sp. in soybean up to 45 days before sowing. Sandini et al. [164] show that seeds pre-treated with insecticides and fungicides and inoculant added to a cell protector maintain cell viability without compromising BNF (estimated by the number of nodules and nitrogen in the aerial part of the plant) for more than 71 days in storage. According to Araujo et al. [158], seed pre-inoculation is feasible if associated with the use of cell protectors. According to the results found, the use of cell protectors increased nodulation, grain yield, and plant development in seeds inoculated 30 days before planting compared to treated seeds inoculated at the time of sowing without the presence of cell protectors [158].
One of the first products in the Brazilian market for seed pre-inoculation was the Biagro NGTM (Bayer). This product was developed in a partnership between Embrapa and Bayer, and since 2013 it has been available on the market. However, the manufacturer does not recommend it for using in seeds treated with pesticides and guarantees the cell viability for up to 15 days. In the Brazilian market, there are companies that already offer seed pre-inoculation technology combined with the treatment with pesticides.
Bayer markets the CTS 500® and, according to the manufacturer, is compatible with the main nematicides, insecticides, and fungicides used in soybean seeds, with cell viability up to 60 days. Granouro® (Basf) is a kit composed of B. elkanii SEMIA 587 and SEMIA 5019, an adhesive and a protective agent that is applied during industrial seed treatment and guarantees cell viability without compromising the nitrogen supply up to 45 days from seed application. In the 2019/2020 crop season, the Rizoliq LLI product, from Rizobacter, was used in more than one million hectares of soybean. This inoculant has an osmoprotective agent that protects cells from Bradyrhizobium spp. SEMIA 5079 and SEMIA 5080 for up to 60 days, according to the manufacturer. All of these products are commercialized in Brazil.
Conclusions and perspectives
For more than 50 years, inoculants containing rhizobia have been marketed in Brazil and, more recently, products containing other microorganisms have shown benefits and gained acceptance from farmers. Currently, bio-based products have been more studied as for the interest in a more sustainable agriculture and for the gains in productivity that these products offer. As reported in this review, we have a scenario of biotechnological innovations aimed at the development of new products or agricultural processes that search to facilitate the management of the farmer and ensure greater efficiency and, consequently, greater crop productivity. Therefore, a promising agricultural scenario is expected in the coming years and decades with the launch of biotechnological innovations.
We are currently living global emergencies such as agricultural areas desertification and climate change that can alter the entire methods of food production in the world. In that regard, there is an urgent search for biological solutions that can mitigate the effects that these environmental impacts may have on food production. The search for biological solutions in agriculture is a matter of food security, and in this sense, new products and technologies must be developed and the use of microbiological products must be a reference in the Brazilian and global agricultural market in the coming years.
Author contribution
Conceptualization: Catharine Abreu Bomfim; writing — original draft preparation: Catharine Abreu; writing — critically review and editing: Lucas Gabriel Ferreira Coelho, Helson Mario Martins do Vale, Ieda de Carvalho Mendes, Manuel Megías, Francisco Javier Ollero, Fábio Bueno dos Reis Junior.
Funding
This research was funded by Ministerio de Economía y Competitividad of the Spanish government (project AGL2016-77163-R), INCT-Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq 465133/2014–2, Fundação Araucária-STI 043/2019, CAPES), and the authors C.A.B and L.G.F.C were funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES)—finance code 001.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Luc F.M. Rouws
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Catharine Abreu Bomfim, Email: catharineabreu@gmail.com.
Lucas Gabriel Ferreira Coelho, Email: lucasferrerc@gmail.com.
Helson Mario Martins do Vale, Email: helson@unb.br, Email: helson@unb.br.
Ieda de Carvalho Mendes, Email: ieda.mendes@embrapa.br.
Manuel Megías, Email: megiasg@us.es.
Francisco Javier Ollero, Email: fjom@us.es.
Fábio Bueno dos Reis Junior, Email: fabio.reis@embrapa.br.
References
- 1.FAO (2020) Food security and nutrition in the world. doi:10.4060/ca9692en
- 2.Brasil (2020) Acomp. safra bras. grãos, Safra 2019/20 - Décimo segundo levantamento, Brasília, p. 1–68, ISSN 2318–6852
- 3.Brasil (2021) PIB do agronegócio alcança participação de 26,6% no PIB brasileiro em 2020. CEPEA (Centro de Estudos avançados em economia aplicada)
- 4.Olivares FL, Busato JG, De PAM, Lima S, Aguiar NO, Canellas LP. Plant growth promoting bacteria and humic substances : crop promotion and mechanisms of action. Chem Biol Technol Agric. 2017;4:30. doi: 10.1186/s40538-017-0112-x. [DOI] [Google Scholar]
- 5.Adesemoye AO, Kloepper JW. Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol. 2009;85:1–12. doi: 10.1007/s00253-009-2196-0. [DOI] [PubMed] [Google Scholar]
- 6.Calvo P, Watts DB, Kloepper JW, Torbert HA. Effect of microbial-based inoculants on nutrient concentrations and early root morphology of corn (Zea mays) J Plant Nutr Soil Sci. 2016;000:1–15. doi: 10.1002/jpln.201500616. [DOI] [Google Scholar]
- 7.Vejan P, Abdullah R, Khadiran T, Ismail S, Boyce AN. Role of plant growth promoting rhizobacteria in agricultural sustainability - a review. Molecules. 2016;21:1–17. doi: 10.3390/molecules21050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Montaño F, Alías-Villegas C, Bellogín RA, et al. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res. 2014;169:325–336. doi: 10.1016/j.micres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Etesami H, Maheshwari DK. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol Environ Saf. 2018;156:225–246. doi: 10.1016/j.ecoenv.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Brasil (2011) Instrução Normativa SDA No 13, de 24 de março de 2011. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-sda-13-de-24-03-2011-inoculantes.pdf
- 11.Bashan Y, de Bashan LE, Prabhu S, Hernandez J-P. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives. Plant Soil. 2014;1886:1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- 12.Rosier A, Medeiros FHV, Bais HP. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil. 2018;428:35–55. doi: 10.1007/s11104-018-3679-5. [DOI] [Google Scholar]
- 13.Ahemad M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ - Sci. 2014;26:1–20. doi: 10.1016/j.jksus.2013.05.001. [DOI] [Google Scholar]
- 14.Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Maurya BR, Raghuwanshi R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.) Biocatal Agric Biotechnol. 2014;3(4):121–128. doi: 10.1016/j.bcab.2014.08.003. [DOI] [Google Scholar]
- 16.Pathak D, Lone R, Khan S, Koul KK. Isolation, screening and molecular characterization of free-living bacteria of potato (Solanum tuberosum L.) and their interplay impact on growth and production of potato plant under mycorrhizal association. Sci Hortic. 2019;252:388–397. doi: 10.1016/j.scienta.2019.02.072. [DOI] [Google Scholar]
- 17.Kumar Meena R, Kumar Singh R, Pal Singh N, Kumari Meena S, Singh Meena V. Isolation of low temperature surviving plant growth - promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal Agric Biotechnol. 2015;4(4):806–811. doi: 10.1016/j.bcab.2015.08.006. [DOI] [Google Scholar]
- 18.Kumari P, Meena M, Upadhyay RS. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean) Biocatal Agric Biotechnol. 2018;16:155–162. doi: 10.1016/j.bcab.2018.07.029. [DOI] [Google Scholar]
- 19.Goswami D, Dhandhukia P, Patel P, Thakker JN. Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis. Microbiol Res Journal. 2014;169:66–75. doi: 10.1016/j.micres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Sun P, Zhang Y, Jin C, Guan C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ Exp Bot. 2020;174:104–123. doi: 10.1016/j.envexpbot.2020.104023. [DOI] [Google Scholar]
- 21.Grobelak A, Napora A, Kacprzak M. Using plant growth-promoting rhizobacteria (PGPR) to improve plant growth. Ecol Eng. 2015;84:22–28. doi: 10.1016/j.ecoleng.2015.07.019. [DOI] [Google Scholar]
- 22.Li H, Qiu Y, Yao T, Ma Y, Zhang H, Yang X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020;199:104577. doi: 10.1016/j.still.2020.104577. [DOI] [Google Scholar]
- 23.Kour D, Rana KL, Kaur T, et al. Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal Agric Biotechnol. 2020;23:101501. doi: 10.1016/j.bcab.2020.101501. [DOI] [Google Scholar]
- 24.Fukami J, Nogueira MA, Araujo RS, Hungria M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express. 2016;6(1):1–13. doi: 10.1186/s13568-015-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galindo FS, Filho MCMT, Buzetti S, Ludkiewicz MGZ, Rosa PAL, Tritapepe CA. Technical and economic viability of co-inoculation with Azospirillum brasilense in soybean cultivars in the Cerrado. Rev Bras Eng Agrícola e Ambient. 2018;22(1):51–56. doi: 10.1590/1807-1929/agriambi.v22n1p51-56. [DOI] [Google Scholar]
- 26.De CRH, Jesus C, Favero VO, Straliotto R, Paulo A. The co-inoculation of Rhizobium and Bradyrhizobium increases the early nodulation and development of common beans. J Soil Sci Plant. 2020 doi: 10.1007/s42729-020-00171-8. [DOI] [Google Scholar]
- 27.de Souza JEB, Ferreira EPB. Improving sustainability of common bean production systems by co-inoculating rhizobia and azospirilla. Agric Ecosyst Environ. 2017;237:250–257. doi: 10.1016/j.agee.2016.12.040. [DOI] [Google Scholar]
- 28.Braccini AL, Caiubi L. Effects of associated co-inoculation of Bradyrhizobium japonicum with Azospirillum brasilense on soybean yield and growth. Afr J Agric Res. 2017;12:6–11. doi: 10.5897/AJAR2016.11711. [DOI] [Google Scholar]
- 29.Sánchez AC, Gutiérrez RT, Santana RC, et al. Effects of co-inoculation of native Rhizobium and Pseudomonas strains on growth parameters and yield of two contrasting Phaseolus vulgaris L. genotypes under Cuban soil conditions. Eur J Soil Biol. 2014;62:105–112. doi: 10.1016/j.ejsobi.2014.03.004. [DOI] [Google Scholar]
- 30.Hungria M, Nogueira MA, Araujo RS. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: a new biotechnological tool to improve yield and sustainability. Am J Plant Sci. 2015;6:811–817. doi: 10.4236/ajps.2015.66087. [DOI] [Google Scholar]
- 31.Marks BB, Megías M, Nogueira MA, Hungria M. Biotechnological potential of rhizobial metabolites to enhance the performance of Bradyrhizobium spp. and Azospirillum brasilense inoculants with soybean and maize. AMB Express. 2013;3(21):1–10. doi: 10.1186/2191-0855-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks BB, Megías M, Ollero FJ, Nogueira MA, Araujo RS, Hungria M. Maize growth promotion by inoculation with Azospirillum brasilense and metabolites of Rhizobium tropici enriched on lipo-chitooligosaccharides (LCOs) AMB Express. 2015;5:1–11. doi: 10.1186/s13568-015-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araújo SC de (2013) A evolução da produção de inoculantes no Brasil. Associação Nacional dos Produtores e Importadores de Importadores de Inoculantes (ANPII). http://www.anpii.org.br/a-evolucao-da-producao-de-inoculantes-no-brasil/. Accessed 21 Feb 2021
- 34.Döbbereiner J, Duque FF. Contribuição da pesquisa em fixação biológica de nitrogênio para o desenvolvimento do Brasil. R Econ Rural. 1980;18(3):447–460. [Google Scholar]
- 35.Freire JRJ, de Vernetti FJ. A pesquisa com soja, a seleção de rizóbios e a produção de inoculantes no Brasil. Pesqui Agropecuária Gaúcha. 1997;5:117–121. [Google Scholar]
- 36.Santos MS, Nogueira MA, Hungria M. Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express. 2019;9(205):1–22. doi: 10.1186/s13568-019-0932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks BB (2013) Ação de metabólitos secundários e de inoculantes microbianos na promoção do crescimento de soja (Glycine max (L.) Merr.) e milho (Zea mays L.). Dissertation, Federal University of Paraná
- 38.Siqueira AF, Ormeño-Orrillo E, Souza RC, et al. Comparative genomics of Bradyrhizobium japonicum CPAC 15 and Bradyrhizobium diazoefficiens CPAC 7: elite model strains for understanding symbiotic performance with soybean. BMC Genomics. 2014;15(1):1–20. doi: 10.1186/1471-2164-15-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendes IC, Hungria M, Vargas MAT. Establishment of Bradyrhizobium japonicum and B. elkanii strains in a Brazilian Cerrado oxisol. Biol Fertil Soils. 2004;40(1):28–35. doi: 10.1007/s00374-004-0739-1. [DOI] [Google Scholar]
- 40.Vargas MAT, de Mendes IC, Suhet AR, Peres JRR. Duas novas estirpes de rizóbio para a inoculação da soja. Comunicado Técnico. Embrapa. 2001;061:4–7. [Google Scholar]
- 41.Peres JRR, Vidor C. Seleção de estirpes de Rhizobium japonicum e competitividade por sítios de infecção nodular em cultivares de soja. Agron sulriograndense. 1980;16:205–219. [Google Scholar]
- 42.Peres JRR, Mendes IC, Suhet AR, Vargas MAT. Eficiência e competitividade de estirpes de rizóbios para a soja em solos do Cerrado. Rev Bras Ciência do Solo. 1993;17:357–363. [Google Scholar]
- 43.Mendes IC, Vargas MAT, Hungria M. Estabelecimento de estirpes de Bradyrhizobium japonicum/B. elkanii e seus efeitos na reinoculação da soja em solos de cerrado. Documentos Embrapa Cerrados. 2000;20:1–18. [Google Scholar]
- 44.Hungria M, Nogueira MA, Campos LJM, Menna P, Brandi F, Ramos YG. Seed pre-inoculation with Bradyrhizobium as time-optimizing option for large-scale soybean cropping systems. Agron J. 2020;112(6):5222–5236. doi: 10.1002/agj2.20392. [DOI] [Google Scholar]
- 45.de Barbosa LP, Costa PF, Ribeiro PRA, Rufini M, Guimarães AA, de Moreira FMS. Symbiotic efficiency and genotypic characterization of variants of Bradyrhizobium spp. in commercial inoculants for soybeans. Rev Bras Cienc do Solo. 2017;41:1–15. doi: 10.1590/18069657rbcs20160572. [DOI] [Google Scholar]
- 46.Graham PH, Hungria M, Tlusty B. Breeding for better nitrogen fixation in grain legumes: where do the rhizobia fit in? Crop Manag. 2004;3(1):1–6. doi: 10.1094/cm-2004-0301-02-rv. [DOI] [Google Scholar]
- 47.Chang W-S, Lee H-I, Hungria M (2015) Soybean production in the americas. In: Lugtenberg BJJ (ed) Principles of plant-microbe interactions: microbes for sustainable agriculture, edn. Springer International Publishing Switzerland, pp 393–400. 10.1007/978-3-319-08575-3
- 48.Hungria M, Mendes IC (2015) Nitrogen fixation with soybean: the perfect symbiosis? In: De Bruijn F (ed) Biological nitrogen fixation, edn John Wiley & Sons; pp 1005–1019. 10.1002/9781119053095.ch99
- 49.ANPII (2018). Estatísticas. http://www.anpii.org.br/estatisticas. Accessed 15 Feb 2021
- 50.Melo P, Olivares FL, Médici LO, Neto AT, Dobbss LB, Canellas LP (2017) Mixed rhizobia and Herbaspirillum seropedicae inoculations with humic acid‑like substances improve water stress recovery in common beans. Chem Biol Technol Agric. 1-9. 10.1186/s40538-017-0090-z
- 51.Martínez-Romero E, Segovia L, Mercante FM, Franco AA, Graham P, Pardo M. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol. 1991;41(3):417–426. doi: 10.1099/00207713-41-3-417. [DOI] [PubMed] [Google Scholar]
- 52.Segovia L, Young JPW, Martinez-Romero E. Reclassification of American Rhizobium leguminosarum biovar phaseoli type I strains as Rhizobium etli sp. nov. Int J Syst Bacteriol. 1993;43(2):374–377. doi: 10.1099/00207713-43-2-374. [DOI] [PubMed] [Google Scholar]
- 53.Dall’ Agnol RF, Ribeiro RA, Ormeño-Orrillo E, et al. Rhizobium freirei sp. nov., a symbiont of Phaseolus vulgaris that is very effective at fixing nitrogen. Int J Syst Evol Microbiol. 2013;63:4167–4173. doi: 10.1099/ijs.0.052928-0. [DOI] [PubMed] [Google Scholar]
- 54.Ormeño-Orrillo E, Gomes DF, del Cerro P, et al. (2016) Genome of Rhizobium leucaenae strains CFN 299 T and CPAO 29.8: searching for genes related to a successful symbiotic performance under stressful conditions. BMC Genomics 17(1):1–15. 10.1186/s12864-016-2859-z [DOI] [PMC free article] [PubMed]
- 55.Dall Agnol RF, Ribeiro RA, Delamuta J, et al. Rhizobium paranaense sp. nov., an effective N2-fixing symbiont of common bean (Phaseolus vulgaris L.) with broad geographical distribution in Brazil. Int J Syst Evol Microbiol. 2014;64:3222–3229. doi: 10.1099/ijs.0.064543-0. [DOI] [PubMed] [Google Scholar]
- 56.Hungria M, Andrade DDS, Chueire LMDO, Probanza A, Guttierrez-Mañero FJ, Megías M. Isolation and characterization of new efficient and competitive bean (Phaseolus vulgaris L.) rhizobia from Brazil. Soil Biol Biochem. 2000;32(11–12):1515–1528. doi: 10.1016/S0038-0717(00)00063-8. [DOI] [Google Scholar]
- 57.Hungria M, Campo RJ, Mendes IC. Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium tropici strains. Biol Fertil Soils. 2003;39(2):88–93. doi: 10.1007/s00374-003-0682-6. [DOI] [Google Scholar]
- 58.Barros RLN, de Oliveira LB, Magalhães WB, Médici LO, Pimentel C. Interaction of biological nitrogen fixation with sowing nitrogen fertilization on common bean in the two seasons of cultivation in Brazil. J Plant Nutr. 2018;41(6):774–781. doi: 10.1080/01904167.2018.1426016. [DOI] [Google Scholar]
- 59.Barros RLN, De Oliveira LB, De Magalhães WB, Pimentel C. Growth and yield of common bean as affected by seed inoculation with rhizobium and nitrogen fertilization. Exp Agric. 2018;54(1):16–30. doi: 10.1017/S001447971600065X. [DOI] [Google Scholar]
- 60.Luqueño FF, Victoria DE, Munive A, Chee LC, Covarrubias LMS. Nodule senescence and biomass components in common bean cultivars. Rev Fitotec Mex. 2008;31(3):195–201. [Google Scholar]
- 61.Andraus MP, Cardoso AA, Ferreira EPB. Differences in nodulation and grain yield on common bean cultivars with different growth cycles. Commun Soil Sci Plant Anal. 2016;47(9):1148–1161. doi: 10.1080/00103624.2016.1166376. [DOI] [Google Scholar]
- 62.Pereira HS, Melo LC, de Faria LC, et al. Linhagens elite de feijoeiro-comum cultivadas com fertilização nitrogenada e inoculação com Rhizobium tropici. Cienc Rural. 2015;45(12):2168–2173. doi: 10.1590/0103-8478cr20141135. [DOI] [Google Scholar]
- 63.Gerosa Ramos ML, Parsons R, Sprent JI, James EK. Effect of water stress on nitrogen fixation and nodule structure of common bean. Pesqui Agropecu Bras. 2003;38(3):339–347. doi: 10.1590/s0100-204x2003000300002. [DOI] [Google Scholar]
- 64.Hungria M, Franco AA. Effects of high temperature on nodulation and nitrogen fixation by Phaseolus vulgaris L. Plant Soil. 1993;149(1):95–102. doi: 10.1007/BF00010766. [DOI] [Google Scholar]
- 65.Michiels J, Dombrecht B, Vermeiren N, Xi C, Luyten E, Vanderleyden J. Phaseolus vulgaris is a non-selective host for nodulation. FEMS Microbiol Ecol. 1998;26(3):193–205. doi: 10.1016/S0168-6496(98)00035-X. [DOI] [Google Scholar]
- 66.Cardoso JD, Hungria M, Andrade DS. Polyphasic approach for the characterization of rhizobial symbionts effective in fixing N 2 with common bean (Phaseolus vulgaris L.) Appl Microbiol Biotechnol. 2012;93(5):2035–49. doi: 10.1007/s00253-011-3708-2. [DOI] [PubMed] [Google Scholar]
- 67.Vargas MAT, Mendes IC, Hungria M. Response of field-grown bean (Phaseolus vulgaris l.) to Rhizobium inoculation and nitrogen fertilization in two cerrados soils. Biol Fertil Soils. 2000;32(3):228–233. doi: 10.1007/s003740000240. [DOI] [Google Scholar]
- 68.Pelegrin R, Mercante FM, Otsubo IMN, Otsubo AA. Resposta da cultura do feijoeiro à adubação nitrogenada e à inoculação com rizóbio. Rev Bras Ciência do Solo. 2009;33(1):219–226. doi: 10.1590/S0100-06832009000100023. [DOI] [Google Scholar]
- 69.Hungria M, Antonio M, Araujo RS. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: strategies to improve sustainability. Biol Fertil Soils. 2013;49:791–801. doi: 10.1007/s00374-012-0771-5. [DOI] [Google Scholar]
- 70.Mercante FM, Otsubo AA, Brito OR. New native rhizobia strains for inoculation of common bean in the Brazilian savanna. Rev Bras Cienc do Solo. 2017;41:1–11. doi: 10.1590/18069657rbcs2015012. [DOI] [Google Scholar]
- 71.Taylor P, Dobbelaere S, Vanderleyden J, Okon Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci. 2003;22(2):107–149. doi: 10.1080/713610853. [DOI] [Google Scholar]
- 72.Hungria M, Nogueira MA, Araujo RS. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: an environment-friendly component in the reclamation of degraded pastures in the tropics. Agric Ecosyst Environ. 2016;221:125–131. doi: 10.1016/j.agee.2016.01.024. [DOI] [Google Scholar]
- 73.Day JM, Dobereiner J. Physiological aspects of N2-fixation by a Spirillum from Digitaria roots. Soil Biol Biochem. 1975;8:45–50. doi: 10.1016/0038-0717(76)90020-1. [DOI] [Google Scholar]
- 74.Fukami J, Cerezini P, Hungria M. Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Express. 2018;73(8):1–12. doi: 10.1186/s13568-018-0608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hungria M, Campo RJ, Souza EM, Pedrosa FO. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil. 2010;331(1):413–425. doi: 10.1007/s11104-009-0262-0. [DOI] [Google Scholar]
- 76.Cassán F, Diaz-Zorita M. Azospirillum sp. in current agriculture: from the laboratory to the field. Soil Biol Biochem. 2016;103:117–130. doi: 10.1016/j.soilbio.2016.08.020. [DOI] [Google Scholar]
- 77.Tarrand JJ, Krieg NR, Dobereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azosporillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb., nov. and Azospirillum brasilense sp. nov. Can Jounal Microbiol. 1978;24:967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- 78.Cassán F, Coniglio A, López G, et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol Fertil Soils. 2020;56(4):461–479. doi: 10.1007/s00374-020-01463-y. [DOI] [Google Scholar]
- 79.Okon Y. Azospirillum as a potential for agriculture inoculant. Trends Biotechnol. 1985;3(9):223–228. doi: 10.1016/0167-7799(85)90012-5. [DOI] [Google Scholar]
- 80.Tien TM, Gaskins MH, Hubbell D (1979) Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol. 37(5):1016–1024. doi: 0099–2240/79/05–1016/09$02.00/0 [DOI] [PMC free article] [PubMed]
- 81.Bashan Y, Levanony H. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can J Microbiol. 1990;36(9):591–608. doi: 10.1139/m90-10. [DOI] [Google Scholar]
- 82.Fukami J, Ollero FJ, de la Osa C, et al. Antioxidant activity and induction of mechanisms of resistance to stresses related to the inoculation with Azospirillum brasilense. Arch Microbiol. 2018;45:328–339. doi: 10.1007/s00203-018-1535-x. [DOI] [PubMed] [Google Scholar]
- 83.Fukami J, de la Osa C, Ollero FJ, Megías M, Hungría M. Co-inoculation of maize with Azospirillum brasilense and Rhizobium tropici as a strategy to mitigate salinity stress. Funct Plant Biol. 2018;45:328–339. doi: 10.1071/FP17167. [DOI] [PubMed] [Google Scholar]
- 84.Fukami J, Ollero FJ, Megías M, Hungria M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express. 2017;7(1):1–13. doi: 10.1186/s13568-017-0453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fallik E, Okon Y, Epstein E, Goldman A, Fischer M. Identification and quantification of IAA and IBA in Azospirillum brasilense-inoculated maize roots. Soil Biol Biochem. 1989;21(1):147–153. doi: 10.1016/0038-0717(89)90024-2. [DOI] [Google Scholar]
- 86.Okon Y, Labandera-Gonzalez CA. Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol Biochem. 1994;26(12):1591–1601. doi: 10.1016/0038-0717(94)90311-5. [DOI] [Google Scholar]
- 87.Bashan Y, Holguin G, de Bashan LE. Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003) Can J Microbiol. 2004;50(8):521–577. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- 88.Chamam A, Sanguin H, Bellvert F, et al. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa association. Phytochemistry. 2013;87:65–77. doi: 10.1016/j.phytochem.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 89.Cassán F, Vanderleyden J, Spaepen S. Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul. 2014;33(2):440–459. doi: 10.1007/s00344-013-9362-4. [DOI] [Google Scholar]
- 90.Ferreira AS, Pires RR, Rabelo PG, Oliveira RC, Luz JMQ, Brito CH. Implications of Azospirillum brasilense inoculation and nutrient addition on maize in soils of the Brazilian Cerrado under greenhouse and field conditions. Appl Soil Ecol. 2013;72:103–108. doi: 10.1016/j.apsoil.2013.05.020. [DOI] [Google Scholar]
- 91.Kazi N, Deaker R, Wilson N, Muhammad K, Trethowan R. The response of wheat genotypes to inoculation with Azospirillum brasilense in the field. F Crop Res. 2016;196:368–378. doi: 10.1016/j.fcr.2016.07.012. [DOI] [Google Scholar]
- 92.Díaz-Zorita M, Fernández-Canigia MV. Field performance of a liquid formulation of Azospirillum brasilense on dryland wheat productivity. Eur J Soil Biol. 2009;45(1):3–11. doi: 10.1016/j.ejsobi.2008.07.001. [DOI] [Google Scholar]
- 93.Lin SY, Hameed A, Shen FT, et al. (2014) Description of Niveispirillum fermenti gen. nov., sp. nov., isolated from a fermentor in Taiwan, transfer of Azospirillum irakense (1989) as Niveispirillum irakense comb. nov., and reclassification of Azospirillum amazonense (1983) as Nitrospirillum amazonense gen. nov. Antonie Van Leeuwenhoek 105(6):1149–1162. 10.1007/s10482-014-0176-6 [DOI] [PubMed]
- 94.Reis Junior FB, Silva M, dos Teixeira KR, S, Urquiaga S, Reis VM, Identificação de isolados de Azospirillum amazonense associados a Brachiaria spp., em diferentes épocas e condições de cultivo e produção de fitormônio pela bactéria. Rev Bras Cienc do Solo. 2004;28:103–113. doi: 10.1590/S0100-06832004000100011. [DOI] [Google Scholar]
- 95.Rodrigues EP, Rodrigues LS, de Oliveira ALM, et al. Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.) Plant Soil. 2008;302:249–261. doi: 10.1007/s11104-007-9476-1. [DOI] [Google Scholar]
- 96.Figueiredo MVB, Bonifacio A, Rodrigues AC, de Araujo FF, Stamford NP (2016). Beneficial microorganisms: current challenge to increase crop performance. In: Arora NK, Mehnaz S, Balestrini R, (eds) Bioformulations: for sustainable agriculture. Springer. 10.1007/978-81-322-2779-3
- 97.Ribeiro VP, Marriel IE, de Sousa SM, et al. Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Brazilian J Microbiol. 2018;49:40–46. doi: 10.1016/j.bjm.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28(4):1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 99.Alori ET, Glick BR, Babalola OO. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol. 2017;8:1–8. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richardson AE, Lynch JP, Ryan PR, et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil. 2011;349:121–156. doi: 10.1007/s11104-011-0950-4. [DOI] [Google Scholar]
- 101.Leggett M, Cross J, Hnatowich G, Holloway G (2007) Challenges in commercializing a phosphate-solubilizing microorganism: Penicillium bilaiae, a case history. In: First international meeting on microbial phosphate solubilization. Springer, pp 215–222
- 102.Suresh BM, Khot GG, Patil NP, Patil PP, Shewale SA. Co-inoculation effect of Rhizobium leguminosarum and phosphate solubilizing fungi on growth, yield and nutrient uptake in ground nut (Arachis hypogaea) Pharma Innov J. 2021;10(1):472–476. [Google Scholar]
- 103.Oliveira CA, Alves VMC, Marriel IE, et al. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem. 2009;41(9):1782–1787. doi: 10.1016/j.soilbio.2008.01.01. [DOI] [Google Scholar]
- 104.Paiva CAO, Marriel IE, Gomes EA, et al. (2020) Recomendação agronômica de cepas de Bacillus subtilis (CNPMS B2084) e Bacillus megaterium (CNPMS B119) na cultura do milho. Circular Técnica Embrapa
- 105.Chaparro JM, Sheflin AM, Manter DK, Vivanco JM. Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils. 2012;48:489–499. doi: 10.1007/s00374-012-0691-4. [DOI] [Google Scholar]
- 106.Korir H, Mungai NW, Thuita M, Hamba Y, Masso C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front Plant Sci. 2017;8:1–10. doi: 10.3389/fpls.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Compant S, Samad A, Faist H, Sessitsch A. A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res. 2019;19:29–37. doi: 10.1016/j.jare.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rondina AB., Sanzovo AWS, Guimarães GS, Wendling JR, Nogueira MA, Hungria M (2020) Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol Fertil Soils. 10.1007/s00374-020-01453-0
- 109.Cassán F, Perrig D, Sgroy V, Masciarelli O, Penna C, Luna V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L) and soybean (Glycine max L) Eur J Soil Biol. 2009;45(1):28–35. doi: 10.1016/j.ejsobi.2008.08.005. [DOI] [Google Scholar]
- 110.Reis VM, Baldani JI, Urquiaga S (2009) Recomendação de uma mistura de estirpes de cinco bactérias fixadoras de nitrogênio para inoculação de cana-de-açúcar: Gluconacetobacter diazotrophicus (BR 11281), Herbaspirillum seropedicae (BR 11335), Herbaspirillum rubrisubalbicans (BR 11504), Azospirillum amazonense (BR 11145) e Burkholderia tropica (BR 11366). Circular Técnica. Embrapa
- 111.de Oliveira ALM, Canuto EL, Urquiaga S, Reis VM, Baldani JI. Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil. 2006;284:23–32. doi: 10.1007/s11104-006-0025-0. [DOI] [Google Scholar]
- 112.Hungria M, Stacey G. Molecular signals exchanged between host plants and rhizobia: basic aspects and potential application in agriculture. Soil Biol Biochem. 1997;29(5–6):819–830. doi: 10.1016/S0038-0717(96)00239-8. [DOI] [Google Scholar]
- 113.Oldroyd GED. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol. 2013;11(4):252–263. doi: 10.1038/nrmicro2990. [DOI] [PubMed] [Google Scholar]
- 114.Miller JB, Oldroyd GED (2012) The role of diffusible signals in the establishment of rhizobial and mycorrhizal symbioses. In: Perotto S and Baluska F (eds) Signaling and communication in plants. Springer, Berlin, pp 1–30. 10.1007/978-3-642-20966-6
- 115.Khan W, Prithiviraj B, Smith DL. Nod factor [Nod Bj V (C18:1, MeFuc)] and lumichrome enhance photosynthesis and growth of corn and soybean. J Plant Physiol. 2008;165(13):1342–1351. doi: 10.1016/j.jplph.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 116.Schwinghamer T, Souleimanov A, Dutilleul P, Smith D. The plant growth regulator lipo-chitooligosaccharide (LCO) enhances the germination of canola (Brassica napus [L.]) J Plant Growth Regul. 2015;34:183–195. doi: 10.1007/s00344-014-9456-7. [DOI] [Google Scholar]
- 117.Macchiavelli RE, Brelles-Mariño G. Nod factor-treated Medicago truncatula roots and seeds show an increased number of nodules when inoculated with a limiting population of Sinorhizobium meliloti. J Exp Bot. 2004;55(408):2635–2640. doi: 10.1093/jxb/erh261. [DOI] [PubMed] [Google Scholar]
- 118.Gourion B, Berrabah F, Ratet P, Stacey G. Rhizobium-legume symbioses: the crucial role of plant immunity. Trends Plant Sci. 2015;20(3):186–194. doi: 10.1016/j.tplants.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Kaschuk G, Hungria M (2017) Diversity and importance of diazotrophic bacteria to agricultural sustainability in the tropics. In: Azevedo JL, Quecine M. (eds) Diversity and benefits of microorganisms from the tropics, Springer International Publishing, pp 269–292. 10.1007/978-3-319-55804-2
- 120.Oláh B, Brière C, Bécard G, Dénarié J, Gough C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J. 2005;44(2):195–207. doi: 10.1111/j.1365-313X.2005.02522.x. [DOI] [PubMed] [Google Scholar]
- 121.Prithiviraj B, Zhou X, Souleimanov A, Kahn WM, Smith DL, Khan WM. A host-specific bacteria-to-plant signal molecule (Nod factor) enhances germination and early growth of diverse crop plants. Planta. 2002;216(3):437–445. doi: 10.1007/s00425-002-0928-9. [DOI] [PubMed] [Google Scholar]
- 122.Kidaj D, Wielbo J, Skorupska A. Nod factors stimulate seed germination and promote growth and nodulation of pea and vetch under competitive conditions. Microbiol Res. 2012;167(3):144–150. doi: 10.1016/j.micres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 123.Souleimanov A, Prithiviraj B, Smith D. The major Nod factor of Bradyrhizobium japonicum promotes early growth of soybean and corn. J Exp Bot. 2002;53(376):1929–1934. doi: 10.1093/jxb/erf034. [DOI] [PubMed] [Google Scholar]
- 124.Fisher RF, Long SR. Rhizobium-plant signal exchange. Nature. 1992;357:655–660. doi: 10.1038/357655a00. [DOI] [PubMed] [Google Scholar]
- 125.Lian B, Souleimanov A, Zhou X, Smith DL. In vitro induction of lipo-chitooligosaccharide production in Bradyrhizobium japonicum cultures by root extracts from non-leguminous plants. Microbiol Res. 2002;157(3):157–160. doi: 10.1078/0944-5013-00145. [DOI] [PubMed] [Google Scholar]
- 126.Skorupska A, Wielbo J, Kidaj D, Marek-kozaczuk M (2010) Enhancing Rhizobium–legume symbiosis using signaling factors. In: Khan MS (eds) Microbes for legume improvement, Springer-Verlag, pp 27–54. 10.1007/978-3-211-99753-6
- 127.López-Lara IM, van Der Drift KMGM, van Brussel AAN, et al. Induction of nodule primordia on Phaseolus and Acacia by lipo-chitinoligosaccharide nodulation signals from broad-host-range Rhizobium strain GRH2. Plant Mol Biol. 1995;29(3):465–477. doi: 10.1007/BF00020978. [DOI] [PubMed] [Google Scholar]
- 128.Morel M., Cagide C, Castro-Sowinski S (2016) The contribuition of secondary metabolites in the success of bioformulation. In: Arora NK, Mehnaz S, Balestrini R, (eds) Bioformulations: for sustainable agriculture. Springer India, pp 235- 250. 10.1007/978-81-322-2779-3
- 129.Flemming H, Wingender J, Szewzyk U, Steinberg P, Rice SA. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–575. doi: 10.1038/nrmicro.2016.94132. [DOI] [PubMed] [Google Scholar]
- 130.Loh J, Pierson EEA, Pierson LS, et al. Quorum sensing in plant-associated bacteria. Curr Opin Plant Biol. 2002;5(4):285–290. doi: 10.1016/S1369-5266(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 131.Pérez-Montaño F, Jiménez-Guerrero I, Sánchez-Matamoros RC, et al. Rice and bean AHL-mimic quorum-sensing signals specifically interfere with the capacity to form biofilms by plant-associated bacteria. Res Microbiol. 2013;164:749–760. doi: 10.1016/j.resmic.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 132.Pérez-Montaño F, Guasch-Vidal B, González-Barroso S, et al. Nodulation-gene-inducing flavonoids increase overall production of autoinducers and expression of N -acyl homoserine lactone synthesis genes in rhizobia. Res Microbiol. 2011;162:715–723. doi: 10.1016/j.resmic.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev. 2001;25(4):365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 134.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density- responsive transcriptional regulators. J Bacteriol. 1994;176(2):269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ortíz-Castro R, Martínez-Trujillo M, López-Bucio J. N-acyl-L-homoserine lactones: a class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant, Cell Environ. 2008;31(10):1497–1509. doi: 10.1111/j.1365-3040.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- 136.Miller M., Bassler B (2001) Quorum sensing in bacteria. Annu Rev Microbiol.55:165-199. 0066-4227/01/1001-0165$14.00 [DOI] [PubMed]
- 137.Hartmann A, Rothballer M, Hense BA, Schröder P. Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front Plant Sci. 2014;5:1–4. doi: 10.3389/fpls.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dong Y, Zhang L. Quorum sensing and quorum-quenching enzymes. J Microbiol. 2005;43:101–109. [PubMed] [Google Scholar]
- 139.dos Fagotti D SL, Abrantes JLF, Cerezini P, et al. (2018) Quorum sensing communication: Bradyrhizobium-Azospirillum interaction via N-acyl-homoserine lactones in the promotion of soybean symbiosis. J Basic Microbiol. 2018:1-16. 10.1002/jobm.201800324 [DOI] [PubMed]
- 140.Bauer WD, Mathesius U. Plant responses to bacterial quorum sensing signals. Curr Opin Biotechnol. 2004;7:429–433. doi: 10.1016/j.pbi.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 141.Pérez-Montaño F, Jiménez-Guerrero I, Del Cerro P, et al. The symbiotic biofilm of Sinorhizobium fredii SMH12, necessary for successful colonization and symbiosis of Glycine max cv Osumi, is regulated by quorum sensing systems and inducing flavonoids via NodD1. PLoS ONE. 2014;9(8):1–11. doi: 10.1371/journal.pone.0105901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fukami J, Abrantes JLF, del Cerro P, et al. Revealing strategies of quorum sensing in Azospirillum brasilense strains Ab-V5 and Ab-V6. Arch Microbiol. 2017;200(1):47–56. doi: 10.1007/s00203-017-1422-x. [DOI] [PubMed] [Google Scholar]
- 143.Ortiz-Castro R, López-Bucio J. Phytostimulation and root architectural responses to quorum-sensing signals and related molecules from rhizobacteria. Plant Sci. 2019;284:135–142. doi: 10.1016/j.plantsci.2019.04.0100. [DOI] [PubMed] [Google Scholar]
- 144.Panichikkal J, Prathap G, Nair RA, Krishnankutty RE. Evaluation of plant probiotic performance of Pseudomonas sp. encapsulated in alginate supplemented with salicylic acid and zinc oxide nanoparticles. Int J Biol Macromol. 2021;166:138–143. doi: 10.1016/j.ijbiomac.2020.10.110. [DOI] [PubMed] [Google Scholar]
- 145.Schoebitz M, López M, Roldán A. Bioencapsulation of microbial inoculants for better soil – plant fertilization. Agron Sustain Dev. 2013;33:751–765. doi: 10.1007/s13593-013-0142-0. [DOI] [Google Scholar]
- 146.Kadmiri IM, El Mernissi N, Azaroual SE, Mekhzoum MEM, Qaiss AEK, Bouhfid R. Bioformulation of microbial fertilizer based on clay and alginate encapsulation. Curr Microbiol. 2021;78(1):86–94. doi: 10.1007/s00284-020-02262-2. [DOI] [PubMed] [Google Scholar]
- 147.He Y, Wu Z, Tu L, Han Y, Zhang G, Li C. Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. Appl Clay Sci. 2015;109:68–78. doi: 10.1016/j.clay.2015.02.001. [DOI] [Google Scholar]
- 148.Herrmann L, Lesueur D. Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol. 2013;97(20):8859–8873. doi: 10.1007/s00253-013-5228-. [DOI] [PubMed] [Google Scholar]
- 149.Strobel SA, Allen K, Roberts C, Jimenez D, Scher HB, Jeoh T. Industrially-scalable microencapsulation of plant beneficial bacteria in dry cross-linked alginate matrix. Ind Biotechnol. 2018;14(3):138–147. doi: 10.1089/ind.2017.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vejan P, Abdullah R, Khadiran T, Ismail S. Encapsulation of Bacillus salmalaya 139SI using double coating biopolymer technique. Lett Appl Microbiol. 2019;68(1):56–63. doi: 10.1111/lam.13088. [DOI] [PubMed] [Google Scholar]
- 151.John RP, Tyagi RD, Brar SK, Surampalli RY, Prévost D. Bioencapsulation of microbial cells for targeted agricultural delivery. Crit Rev Biotechnol. 2011;31(3):211–226. doi: 10.3109/07388551.2010.513327. [DOI] [PubMed] [Google Scholar]
- 152.Bashan Y. Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl Environ Microbiol. 1986;51(5):1089–1098. doi: 10.1128/aem.51.5.1089-1098.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vassilev N, Vassileva M, Martos V, et al. Formulation of microbial inoculants by encapsulation in natural polysaccharides: focus on beneficial properties of carrier additives and derivatives. Front Plant Sci. 2020;11:1–9. doi: 10.3389/fpls.2020.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Young C, Rekha PD, Lai W, Arun AB. Encapsulation of plant growth-promoting bacteria in alginate beads enriched with humic acid. Biotechnol Bioeng. 2006;95(1):76–83. doi: 10.1002/bit.209577. [DOI] [PubMed] [Google Scholar]
- 155.Hungria M, Loureiro MF, Mendes IC, Campo RJ, Graham PH (2005) Inoculant preparation, production and application. In:D. Werner and W. E. Newton (eds) Nitrogen fixation in agriculture, forestry, ecology, and the environment. Springer, Netherlands, pp 223–253. 10.1007/1-4020-3544-6_11
- 156.Hungria M, Marco AN, Ricardo AS. Alternative methods of soybean inoculation to overcome adverse conditions at sowing. African J Agric Res. 2015;10(23):2329–2338. doi: 10.5897/ajar2014.8687. [DOI] [Google Scholar]
- 157.Zilli JE, Campo RJ, Hungria M. Eficácia da inoculação de Bradyrhizobium em pré-semeadura da soja. Pesqui Agropecu Bras. 2010;45(3):335–337. doi: 10.1590/S0100-204X2010000300015. [DOI] [Google Scholar]
- 158.Araujo RS, Da CSP, Souchie EL, et al. Preinoculation of soybean seeds treated with agrichemicals up to 30 days before sowing: technological innovation for large-scale agriculture. Int J Microbiol. 2017;2017:1–17. doi: 10.1155/2017/5914786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stecca JDL, Martin TN, Dall’Coll Lúcio A, Deak EA, Fipke GM, Bruning LA (2019) Inoculation of soybean seeds coated with osmoprotector in diferents soil pH’s. Acta Sci - Agron. 41(1):1-9. 10.4025/actasciagron.v41i1.39482
- 160.Campo RJ, Araujo RS, Hungria M. Nitrogen fixation with the soybean crop in Brazil: compatibility between seed treatment with fungicides and bradyrhizobial inoculants. Symbiosis. 2009;48(1–3):154–163. doi: 10.1007/BF03179994. [DOI] [Google Scholar]
- 161.Anghinoni FBG, Braccini AL, Scapim CA, et al. (2017) Pre-inoculation with Bradyrhizobium spp. in industrially treated soybean seeds. Agric Sci. 8(07):582–590. 10.4236/as.2017.87044
- 162.Callaghan MO. Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl Microbiol Biotechnol. 2016;100:5729–5746. doi: 10.1007/s00253-016-7590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Neto MR, Jakoby ICMC, De Medeiros Buso PH, Bermudez M, Diaz-Zorita M, Souchie EL. Anticipated inoculation of soybean seeds treated with agrochemicals under Brazilian production conditions. Asian J Microbiol Biotechnol Environ Sci. 2018;20(1):188–199. [Google Scholar]
- 164.Sandini I, Belani RB, Falbo MK, Pacentchuk F, Huzar-Novakowiski J. Pre-inoculation of soybean seeds: effects on survival of Bradyrhizobium elkanii, nodulation and crop yield. African J Agric Res. 2018;13(47):2680–2690. doi: 10.5897/ajar2018.13591. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.