Abstract
Background
Although clinical studies have evaluated dexmedetomidine as a strategy to improve noninvasive ventilation (NIV) comfort and tolerance in patients with acute respiratory failure (ARF), their results have not been summarized.
Research Question
Does dexmedetomidine, when compared with another sedative or placebo, reduce the risk of delirium, mortality, need for intubation and mechanical ventilation, or ICU length of stay (LOS) in adults with ARF initiated on NIV in the ICU?
Study Design and Methods
We electronically searched MEDLINE, EMBASE, and the Cochrane Library from inception through July 31, 2020, for randomized clinical trials (RCTs). We calculated pooled relative risks (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes with the corresponding 95% CIs using a random-effect model.
Results
Twelve RCTs were included in our final analysis (n = 738 patients). The use of dexmedetomidine, compared with other sedation strategies or placebo, reduced the risk of intubation (RR, 0.54; 95% CI, 0.41-0.71; moderate certainty), delirium (RR, 0.34; 95% CI, 0.22-0.54; moderate certainty), and ICU LOS (MD, –2.40 days; 95% CI, –3.51 to –1.29 days; low certainty). Use of dexmedetomidine was associated with an increased risk of bradycardia (RR, 2.80; 95% CI, 1.92-4.07; moderate certainty) and hypotension (RR, 1.98; 95% CI, 1.32-2.98; moderate certainty).
Interpretation
Compared with any sedation strategy or placebo, dexmedetomidine reduced the risk of delirium and the need for mechanical ventilation while increasing the risk of bradycardia and hypotension. The results are limited by imprecision, and further large RCTs are needed.
Trial Registry
PROSPERO; No.: 175086; URL: www.crd.york.ac.uk/prospero/
Key Words: dexmedetomidine, noninvasive ventilation, positive pressure ventilation, respiratory failure, sedation
Abbreviations: ARR, absolute risk reduction; HR, heart rate; LOS, length of stay; MD, mean difference; NIV, noninvasive ventilation; RCT, randomized clinical trial; RR, relative risk
Noninvasive ventilation (NIV) is a life-saving intervention commonly used in the ICU. It is effective in the management of hypercapnic respiratory failure resulting from COPD exacerbations, hypoxemic respiratory failure resulting from cardiogenic pulmonary edema, mild to moderate acute respiratory failure in immunocompromised patients, and other diseases.1 Not only is NIV cheaper and less invasive than endotracheal intubation and mechanical ventilation, it also is associated with a shorter ICU length of stay (LOS), reduced risk of pneumonia, and lower mortality.2, 3, 4, 5, 6
Unfortunately, patients often find NIV intolerable because of the interface and required high driving respiratory pressures.7 Gastric insufflation can cause nausea and bloating, whereas the facemask has been associated with discomfort, claustrophobia, and skin breakdown.8,9 For a multitude of reasons, more than one-third of patients being treated with NIV will become delirious and agitated.10 Alternative interfaces to a facemask, such as a helmet, can improve tolerability in some patients,9 but are not available widely. NIV intolerance often results in NIV failure and requires escalation to endotracheal intubation and mechanical ventilation.5,11 Although commonly used sedatives such as propofol can improve compliance with NIV, they also can cause profound sedation and respiratory depression, limiting adjuncts to improve NIV tolerability, leaving physicians with no option but to intubate and mechanically ventilate the patient.12
Dexmedetomidine is a pharmacologic agent with emerging uses in the clinical setting.13 It is a selective α2-adrenergic agonist with mild sedative and analgesic properties and seems to lack many of the side effects of the γ-aminobutyric acid receptor agonists (eg, benzodiazepines). Theoretically, dexmedetomidine allows for lighter sedation without causing respiratory depression, prevents delirium, and may improve compliance with NIV.14, 15, 16
No systematic review or meta-analysis has examined the use of dexmedetomidine in NIV compared with other strategies. In addition, guidelines lack recommendations on how to sedate patients undergoing NIV safely, if it should be done at all.17 Hence, our objective was to conduct a systematic review and meta-analysis to determine the efficacy and safety of dexmedetomidine use compared with other sedation strategies or placebo for critically ill adults with acute respiratory failure receiving NIV in the ICU.
Methods
Protocol Registration
This protocol was registered on PROSPERO (https://www.crd.york.ac.uk/prospero/; Identifier: 175086).
Study Selection
We included randomized clinical trials (RCT). Pseudorandomized or quasirandomized and nonrandomized trials were excluded. The study population included adults (age ≥ 18 years) admitted to the ICU with acute respiratory failure who were supported with NIV. We excluded studies that focused on patients who were managed chronically with NIV at home and were admitted to the ICU with acute respiratory failure or that exclusively enrolled patients who were supported with NIV for postextubation acute respiratory failure. Studies that focused on treating acute alcohol withdrawal with dexmedetomidine also were excluded, because this topic was reviewed recently.18 We included studies in which the intervention group received dexmedetomidine in the ICU (at any dose, initiation day, route, frequency, formulation, continuous or intermittent administration, and for any duration of time) and the control group received any other form of pharmacologic sedation (including, but not limited to, propofol, antipsychotics, opioids, or benzodiazepines at any dose, initiation day, route, frequency, formulation, continuous or intermittent administration, and for any duration of time) or placebo. Eligible studies reported on at least one of the following outcomes: invasive mechanical ventilation; delirium at any time during the ICU stay; mortality at 30 days and at the longest reported follow-up; ICU LOS; duration of NIV; pneumonia acquired in hospital (as defined by the study authors); adverse events including any of the following: hypertension (ie, mean arterial pressure > 120 mm Hg), hypotension (ie, mean arterial pressure < 65 mm Hg), clinically important hypotension (defined as hypotension requiring intervention), bradycardia (ie, defined as a heart rate [HR] of < 60 beats/min), severe bradycardia (ie, defined as an HR of < 40 beats/min), and clinically important bradycardia (defined as bradycardia requiring intervention).
Electronic Search Strategy
We electronically searched EMBASE, MEDLINE, and the Cochrane Central Register of Controlled Trials from inception through July 31, 2020 (e-Appendix 1, e-Tables 1-3). We did not restrict the search strategies by publication status or language. To create the search strategy, one of the authors (K. L.) cross-referenced recently completed systematic reviews and meta-analyses that investigated individual elements of the Population/Intervention/Control/Outcome (PICO) question.19, 20, 21, 22 A Canadian Agency for Drugs & Technologies in Health database search strategy was used to identify RCTs.23 We also searched ongoing trials in ClincialTrials.gov and the World Health Organization International Clinical Trials Registry Platform through July 31, 2020 (e-Tables 4, 5). One of the authors (J. P.) searched Google Scholar. Content experts were contacted to ensure that no relevant studies were missing. All relevant documents were uploaded into Covidence systematic review software (Veritas Health Innovation), where duplicates were deleted.
Data Collection and Analysis
Two reviewers (K. L. and J. P.) screened titles and abstracts, independently and in duplicate, to identify potentially eligible studies for review and evaluated the full texts of eligible studies. Reviewers also screened the reference lists of review articles and other systematic reviews and meta-analyses for additional studies. Disagreements between reviewers were resolved through discussion, followed by arbitration by a third reviewer (W. A.) if necessary. Independently and in duplicate, two reviewers (K. L. and J. P.) used a predesigned and piloted data abstraction form to extract data from the included studies.
Risk of Bias
Two reviewers (J. P. and K. L.) independently assessed studies for risk of bias using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2).24 We assessed risk of bias for each study that informed each outcome in the domains of randomization process, deviations from intended interventions (effect of assignment to intervention), missing outcome data, measurement of the outcome, and selection of reported results. The overall risk of bias for each trial objective was categorized as low if the risk of bias was low in all domains, as some concern if the risk of bias was deemed to have some concern in at least one domain and with no high risk of bias in any domain, or as high if the risk of bias was high in at least one domain per the Revised Cochrane risk-of-bias tool.24 We resolved disagreements by discussion and consensus.
Measurement of Treatment Effect
Analysis was conducted using RevMan software (Review Manager version 5.3 software, The Nordic Cochrane Centre, The Cochrane Collaboration). We used the DerSimonian and Laird random-effects model to pool the weighted effect of estimates across all studies.25 The Mantel-Haenszel method was used to estimate study weights. Pooled relative risks (RRs) were calculated for dichotomous outcomes and mean differences (MDs) were calculated for continuous outcomes with a corresponding 95% CI. We converted continuous outcomes reported as medians and interquartile ranges to means and SDs to permit meta-analysis.26 Funnel plots were inspected to assess for publication bias if 10 or more studies existed for that outcome.27
Unit of Analysis
When a study included more than two arms, each arm was reported. For all main outcomes, only one pairwise comparison was conducted so the same groups of participants were included only once in the meta-analysis. For cross-over trials, data were extracted from the first phase to avoid the potential of carry-over effects.
Missing Data
Four study authors were contacted for missing or unclear information and one responded. If the author did not respond, the available data were analyzed and the potential impact of missing data on results was reported in the risk of bias section.
Heterogeneity and Subgroup Analysis
Statistical heterogeneity was assessed using the χ 2 and I2 statistics. A χ 2 P value of < .1 or an I2 value of > 50% qualified as significant heterogeneity.28 Heterogeneity between studies was explored through performing predefined subgroup analyses to investigate whether certain baseline factors influenced treatment effects. These subgroups included: (1) the class of agents with which dexmedetomidine was compared, (2) the presence of agitation or delirium at the time of randomization, (3) age (older than 65 years vs younger than 65 years), and (4) the dose of dexmedetomidine (≤ 0.7 μg/kg/h vs > 0.7 μg/kg/h).
Sensitivity Analysis
We conducted a prespecified sensitivity analysis restricted to studies without concerns for risk of bias. We hypothesized that the treatment effect would be smaller after excluding studies with some or high concerns of bias.
Assessing the Certainty of Evidence
We evaluated the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation approach for each major outcome.29 Two reviewers (K. L. and D. C.) assessed the certainty of the evidence as very low, low, moderate, or high using the Grading of Recommendations Assessment, Development and Evaluation criteria (risk of bias, inconsistency indirectness, imprecision, and publication bias) independently and in duplicate. Very low and low certainty ratings indicate that the true effect is probably or may be, respectively, markedly different from the estimated effect.30 A moderate certainty means that the true effect is probably close to the estimated effect. High certainty indicates a lot of confidence that the true effect is similar to the estimated effect.30
GRADEpro software (McMaster University) was used to prepare the summary of findings table.31 Justification of all decisions are presented in the footnotes. The minimal important differences can be found in the summary of findings table footnotes. All values were based on clinical judgements post hoc.
Results
Screening
The electronic search resulted in a total of 1,388 citations (Fig 1). Of those, 27 underwent full-text review after title and abstract screening was completed, and 15 studies were excluded (e-Table 6). Twelve studies met the inclusion criteria and underwent quantitative analysis.32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Four studies were published as abstracts,35,36,38,41 and the remainder were published as full articles.32, 33, 34,37,39,42,43 One of the abstracts was an interim report of an ongoing study.35
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart. ICTRP = International Clinical Trials Registry Platform; WHO = World Health Organization.
Characteristics of Included Studies
Overall, 12 eligible RCTs enrolled a total of 738 patients with a mean age of 61.5 ± 6.8 years (Table 1).32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 On average, 36.1% of participants were women, and the mean Acute Physiologic Assessment and Chronic Health Evaluation II score was 19 ± 3.3. Four studies enrolled patients with agitation or delirium at baseline.34,36,41,43 Fifty percent of the studies exclusively enrolled medical patients,33, 34, 35,38,42,43 two studies exclusively enrolled surgical patients,37,39 three studies enrolled a mix of medical and surgical patients,32,40,41 and one study did not report the patient population.36 For the intervention, four studies gave a loading dose of dexmedetomidine,32, 33, 34,43 two studies did not report if one was administered,36,41 and six studies did not use a loading dose.35,37, 38, 39, 40,42 Of the 10 studies that reported dosing, most studies allowed dexmedetomidine dosing that ranged from 0.2 to 0.7 μg/kg/h.32,33,38,40,42,43 One study administered dexmedetomidine only in a narrow dosing range of 0.5 to 0.7 μg/kg/h,34 whereas three others allowed administration at wide dosing ranges of 0.4 to 1.4 μg/kg/h,35 0.7 to 2 μg/kg/h,37 and 0.2 to 1.3 μg/kg/h.39 Six studies compared dexmedetomidine with placebo,32,38, 39, 40, 41, 42 two studies compared dexmedetomidine with haloperidol,32,36 three studies compared dexmedetomidine with midazolam,33,34,43 one study compared dexmedetomidine with propofol,37 and one study compared dexmedetomidine with sedation according to the ICU treating team.35 One study had three arms (dexmedetomidine, placebo, and midazolam).32
Table 1.
Characteristics of Included Studies
| Source | Patient Characteristics | Inclusion Criteria | Intervention | Median Duration of Dexmedetomidine Infusion | Control Group | Primary Outcome | Definition of NIV Failure | Funding Source |
|---|---|---|---|---|---|---|---|---|
| Abdelgalel et al32 (2016), Egypt (n = 90) | Mean age, 50.4 ± 8.4 y Female sex, 25.6% Mean APACHE II score, 16.8 Medical, mixed Surgical, mixed |
Age > 18 y, ASA III or IV, needing NIV for ARF secondary to AECOPD, acute hypoxemic cardiogenic pulmonary edema, or postoperative respiratory failure | Dexmedetomidine loading dose of 1.0 μg/kg IV over 10 min then 0.2-0.7 μg/kg/h IV infusion | NR | Haldol loading dose of 2.5 mg IV over 10 min then continuous infusion of 0.5-2 mg/h or saline 10 ml IV bolus followed by 2-8 mL/h | Incidence of delirium | Patient declined or did not tolerate NIV, pH < 7.2, P/F < 150, systolic BP < 70 mm Hg, cardiac ischemia, ventricular arrhythmia, failure to protect airway because of seizures or vomiting | NR |
| Allam33 (2016), Saudi Arabia (n = 200) | Mean age, NR Female sex, 33.0% Mean APACHE II score, NR Medical, 100% Surgical, 0% |
Age > 18 y, ARF, RR > 35/min, SpO2 < 80%, pH < 7.2, PO2 < 60 mm Hg, HR > 120 beats/min | Dexmedetomidine loading dose of 1 mg/kg IV then infusion 0.2-0.7 mg/kg/h IV to achieve RASS score –2 to 3 | NR | Midazolam loading dose 0.05 mg/kg IV then 0.05-0.1 mg/kg/h IV to achieve RASS score 2-3 | NR | Persistent hypoxemia (SpO2 < 80%), hemodynamically unstable (30% decrease in mean HR or BP), increased tracheal secretions | None |
| Baturova and Levikov36 (2014), Russia (n = 31) | Mean age, NR Female sex, NR Mean APACHE II score, NR Medical, NR Surgical, NR |
ARF in whom NIV was difficult because of agitation | Dexmedetomidine dose not recorded | NR | Haloperidol | NR | NR | NR |
| Bialka et al37 (2018), Poland (n = 38) | Mean age, NR Female sex, 47.4% Mean APACHE II score, NR Medical, 0% Surgical, 100% |
Age 18-70 y, immediately after elective thoracic surgery, ASA I-III, BMI 19-30 kg/m2 | Dexmedetomidine 0.7-2.0 μg/kg/h IV infusion, no load | 6 h | Propofol 1-4 mg/kg/h IV infusion | Hemodynamic effects of dexmedetomidine | NR | None |
| Bielka et al38 (2018), Ukraine (n = 29) | Mean age, NR Female sex, NR Mean APACHE II score, NR Medical, 100% Surgical, 0% |
Adults with ARDS resulting from community acquired pneumonia with a P/F 150-300 mm Hg | Dexmedetomidine 0.2-0.7 μg/kg/h to a RASS score 0 to –2, without bolus | Maximum 72 h or until NIV was stopped for ≥ 2 h, or until intubation | Placebo | NR | NR | NR |
| Deletombe et al39 (2019), France (n = 19) | Median age, 56 y ± 28.1 y Female sex, 10.5% Mean APACHE II score, NR Medical, 0% Surgical, 100% |
Adults critically ill with blunt chest trauma and a Thorax Trauma Severity Score of > 6, required NIV, and an arterial catheter | Dexmedetomidine started at 0.7 μg/kg/h, maximum dose 1.3 μg/kg/h, titrated to RASS score 0 to –3; no loading dose was given | For the duration of NIV for a maximum of 8 h | Placebo | Duration of the NIV session | RASS score > +2, grabbing/pulling NIV mask off, request to stop NIV, indication for IMV | Foundation Apicil and Baxter Healthcare Corporation |
| Devlin et al40 (2014), United States (n = 33) | Mean age, 65 ± 11.5 y Female sex, 48.5% Mean APACHE II score, 15.5 Medical, mixed Surgical, mixed |
Adults admitted to the ICU with ARF managed with NIV for ≤ 8 h | Dexmedetomidine 0.2-0.7 μg/kg/h IV infusion without bolus | 2 h after NIV mask removal, intubation, or 72 h maximum | Placebo | Tolerance of NIV | NIV failure was defined as need for intubation or death while NIV was applied | Hospira, but they had no influence in the study design, conduct, analysis, or in the preparation of the article |
| Domin et al41 (2018), Ukraine (n = 67) | Mean age, 64.4 ± 19.7 y Female, NR Mean APACHE II score, NR Medical, mixed Surgical, mixed |
GCS > 13, agitation or lack of cooperation (RASS score > +2), requiring NIV for ARF (Pao2 < 80 mm Hg or SpO2 < 90% or room air or Paco2 > 50 mm Hg) | Dexmedetomidine dose not recorded | 72 h | Placebo | Intubation rate | NR | NR |
| Hou et al42 (2018), China (n = 80) | Mean age, NR Female sex, NR Mean APACHE II score, NR Medical, 100% Surgical, 0% |
AECOPD, Pao2 < 60 mm Hg, Paco2 > 50 mm Hg, or pulmonary encephalopathy | Dexmedetomidine 0.2-0.7 μg/kg/h IV infusion without bolus | Until NIV was discontinued | Placebo | NR | Malignant arrhythmias, severe hypoxemia, CO2 retention, fainting | NR |
| Huang et al43 (2012), China (n = 62) | Mean age, 64.5 ± 7.75 y Female sex, 58.1% Mean APACHE II score, 22 Medical, 100% Surgical, 0% |
Age > 18 y, with acute cardiogenic edema and NIV failure secondary to patient declining because of discomfort, claustrophobia, or marked agitation | Dexmedetomidine optional loading dose of 1 μg/kg then maintenance infusion at 0.2-0.7 μg/kg/h | Until mechanical ventilation was discontinued | Midazolam optional loading dose of 0.05 mg/kg IV then maintenance infusion 0.05-0.1 mg/kg/h IV | Percentage converting to ETT intubation and invasive mechanical ventilation | Indication for intubation was P/F < 150 mm Hg, condition requiring airway protection, secretion, increase in Paco2 with accompanying pH < 7.2, hemodynamic instability, systolic BP < 70 mm Hg, ischemia, ventricular arrhythmias, patient declining because of persistent interface intolerance | NR |
| Senoglu et al34 (2010), Turkey (n = 40) | Median age, 59 y ± 13 y Female sex, 52.5% Mean APACHE II score, 21.5 Medical, 100% Surgical, 0% |
Age > 18 y with ARF secondary to AECOPD and uncooperative, defined as RSS of 1 or RSAS score ≥ 1 | Dexmedetomidine loading dose of 1 μg/kg IV over 10 min then infusion of 0.5-0.7 μg/kg/h | 24 h | Midazolam loading dose of 0.05 mg/kg IV over 10 min then maintenance infusion of 0.1-0.2 mg/kg/h IV | Sedation scores | NIV failure was defined as need for intubation or death related to NIV | NR |
| Vallejo de la Cueva et al35 (2019), Spain (n = 49) | Mean age, 70.9 ± 9.9 y Female sex, 35.2% Mean APACHE II score, NR Medical, 100% Surgical, 0% |
Age > 18 y admitted with ARF secondary to AECOPD, pneumonia, pulmonary edema, or high risk after extubation | Dexmedetomidine 0.4-1.4 μg/kg/h IV infusion, no loading dose | NR | No drug or boluses of midazolam 0.03-0.05 mg/kg IV or propofol 0.25 mg/kg IV or morphine 3-5 mg IV or fentanyl 50-100 μg IV or remifentanil infusion of 6 μg/kg/h IV | Percentage converting to ETT | SpO2 < 80%, P/F < 150 mm Hg, seizures, poor secretion management, hypercapnia and pH < 7.2, hypotension as per systolic BP < 80 mm Hg despite vasoactive drugs, ischemia on ECG, ventricular arrhythmia | Orion Pharma for supply of dexmedetomidine |
AECOPD = acute exacerbation of COPD; APACHE = Acute Physiologic Assessment and Chronic Health Evaluation; ARF = acute respiratory failure; ASA = American Society of Anaesthesiology physical status classification system; ETT = endotracheal tube; GCS = Glasgow Coma Scale; HR = heart rate; NIV = noninvasive ventilation; NR = not recorded; P/F = Pao2 to Fio2 ratio; RASS = Richmond Agitation-Sedation Scale; RR = relative risk; RSAS = Riker Sedation-Agitation Scale; RSS = Ramsay Sedation Score.
Risk of Bias
Only two studies were at low risk of bias across all domains and all outcomes (e-Table 7).31,39 Seven studies warranted some concern for bias because of a lack of discussion about the randomization process and allocation concealment.33,35,37,38,41, 42, 43 There was high concern arose regarding deviation from the assigned intervention and missing outcomes in one open-label study published as an abstract that lacked a discussion about intention-to-treat analysis and completion of outcomes.35
Intubation and Mechanical Ventilation
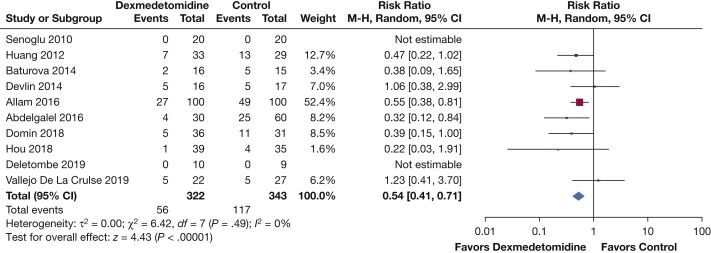
Ten RCTs (n = 665 patients) reported on NIV failure and need for endotracheal intubation and mechanical ventilation.32, 33, 34, 35, 36,39, 40, 41, 42, 43 The use of dexmedetomidine reduced the risk of intubation and mechanical ventilation when compared with any other sedative or placebo (RR, 0.54; 95% CI, 0.41-0.71; I2 = 0%; moderate certainty) (Fig 2, e-Table 8). This translates into an absolute risk reduction (ARR) of 16% (95% CI, 20%-10%). The subgroup analysis found that dexmedetomidine reduced the risk of intubation and mechanical ventilation compared with placebo, benzodiazepines, and haloperidol, but not when compared with sedatives according to the ICU treating team; however, the test of interaction suggested no significant subgroup effect (P = .37; I2 = 5.6%) (e-Fig 1). A state of agitation or delirium at enrollment, or lack thereof, did not seem to modify the effect of dexmedetomidine reducing the need for intubation and mechanical ventilation because no significant subgroup interaction was found (P = .26; I2 = 22.7%) (e-Fig 2). Dexmedetomidine reduced the risk of intubation even when doses of ≤ 0.7 μg/kg/h were administered (RR, 0.53; 95% CI, 0.39-0.72) (e-Fig 3). In none of the studies did patients exclusively receive a dose of > 0.7 μg/kg/h, and therefore, no test for interaction could be assessed. The effect was preserved when the high risk of bias studies were excluded, although this left only one study that contributed to the pooled effect (RR, 0.32; 95% CI, 0.12-0.84) (e-Fig 4, e-Table 9). The reasons for intubation were not reported consistently, but are summarized in e-Figure 5.
Figure 2.
Forest plot showing endotracheal intubation and invasive mechanical ventilation. df = degrees of freedom; M-H= Mantel-Haenszel.
Delirium
The pooled estimate of seven RCTs (n = 537 patients) showed that dexmedetomidine reduced the risk of delirium when compared with other sedation strategies (RR, 0.34; 95% CI, 0.22-0.54; I2 = 0%; moderate certainty) (Fig 3A).32,33,35,38,40,42,43 The ARR of delirium with the administration of dexmedetomidine was 18% (95% CI, 21%-13%). No statistically significant subgroup interaction was found (P = .98; I2 = 0%) based on the type of sedation strategy used in comparison with dexmedetomidine (e-Fig 6). In addition, the risk of delirium was reduced significantly regardless of whether agitation or delirium was present at enrollment, because no significant subgroup interaction was found (P = .94; I2 = 0%) (e-Fig 7). A subgroup analysis by dose of dexmedetomidine revealed that the effect was significant when doses of ≤ 0.7 μg/kg/h were administered (RR, 0.33; 95% CI, 0.21-0.54) (e-Fig 8). No studies exclusively used a dose of > 0.7 μg/kg/h, and therefore, no test for interaction could be assessed for dexmedetomidine dosing strategies. In the sensitivity analysis, only one study was considered low for risk of bias, but the delirium-reducing effect was preserved (RR, 0.26; 95% CI, 0.09-0.80) (e-Fig 9, e-Table 10).
Figure 3.
A, Forest plot showing risk of delirium. B, Forest plot showing ICU LOS (days). C, Forest plot showing mortality. D, Forest plot showing bradycardia. E, Forest plot showing hypotension. df = degrees of freedom; LOS = length of stay; M-H= Mantel-Haenszel.
ICU LOS
Six studies reported ICU LOS (n = 375 patients).32,35,40, 41, 42, 43 Dexmedetomidine shortened the ICU LOS compared with other strategies (MD, –2.40 days; 95% CI, –3.51 to –1.29 days; I2 = 83%; low certainty) (Fig 3B). No significant subgroup interaction was found based on the control sedation strategy (P = .43; I2 = 0%) (e-Fig 10) or the presence or absence of delirium at enrollment (P = .99; I2 = 0%) (e-Fig 11); however, for both cases, substantial unexplained heterogeneity was found (ie, placebo, I2 = 90%; agitation or delirium absent at enrollment, I2 = 85%). A subgroup analysis by dose of dexmedetomidine revealed that doses of ≤ 0.7 μg/kg/h shortened the ICU LOS (MD, –2.31 days; 95% CI, –3.89 to –0.74 days) (e-Fig 12). No studies exclusively used a dose of > 0.7 μg/kg/h, and therefore, no test for interaction could be assessed for dexmedetomidine dosing strategies. Only one of the six studies that reported ICU LOS was rated to be low risk of bias, but the effect was preserved (MD, –3.70 days; 95% CI, –4.02 to –3.38 days) (e-Fig 13 and e-Table 11).
Mortality
Seven RCTs (n = 541 patients) reported on mortality (at longest follow-up).32, 33, 34, 35,40,41,43 The pooled estimates were limited by imprecision, suggesting both appreciable benefit and harm (RR, 0.61; 95% CI, 0.26-1.40; I2 = 37%; low certainty) (Fig 3C). The ARR of mortality with the administration of dexmedetomidine was 5% (95% CI, 9% less-5% more). Likewise, no significant subgroup interaction was found (P = .52; I2 = 0%) based on the sedation strategy of the control group (e-Fig 14) or on the presence or absence of agitation or delirium at enrollment (P = .86; I2 = 0%) (e-Fig 15). Dexmedetomidine doses of ≤ 0.7 μg/kg/h also did not reduce mortality reliably (RR, 0.49; 95% CI, 0.14-1.72) (e-Fig 16). No studies exclusively used a dose of > 0.7 μg/kg/h, and therefore, no test for interaction could be assessed for dexmedetomidine dosing strategies. The point estimate was not preserved when studies with only low risk of bias were pooled (RR, 1.09; 95% CI, 0.30-4.02) (e-Fig 17, e-Table 12). It was not possible to perform a meta-analysis of mortality at 30 days because only two studies reported it, and both studies had no events reported in either arm at 30 days.34,40
Duration of NIV
The pooled estimates across six RCTs (n = 307 patients) showed no difference in duration of NIV with dexmedetomidine compared with other strategies (MD, –9.56 h; 95% CI, –23.16 to 4.04 h; I2 = 96%; very low certainty) (e-Fig 18).32,35,39,40,42,43 Subgroup analysis of sedation strategy used compared with dexmedetomidine showed a significant interaction (P < .00001; I2 = 95.1%), suggesting that dexmedetomidine reduced the duration of NIV compared with benzodiazepines (MD, –35.90 h; 95% CI, –42.69 to –29.11 h), but not when compared with placebo (MD, –6.10 h; 95% CI, –22.56 to 10.36 h), antipsychotics (MD, –4.40 h; 95% CI, –9.08 to 0.28 h) or sedation according to the ICU treating team (MD, –3.00 h; 95% CI, –12.97 to 6.97 h) (e-Fig 19). Also a significant subgroup effect was found (P < .0001; I2 = 94.6%) when those with agitation or delirium at enrollment (MD, –31.18 h; 95% CI, –41.07 to –21.28 h) were compared with those without agitation or delirium (MD, 0.07 h; 95% CI, –10.21 to 10.36 h) (e-Fig 20), suggesting a larger effect in patients with baseline agitation or delirium. No effect was found when dexmedetomidine doses of ≤ 0.7 μg/kg/h were pooled (MD, –14.67 h; 95% CI, –31.39 to 2.05 h) (e-Fig 21). A subgroup interaction for dose of dexmedetomidine could not be assessed because no studies exclusively used a dose of > 0.7 μg/kg/h. Also, no reduction in NIV duration was found when high risk of bias studies were excluded (MD, –4.17 h; 95% CI, –17.76 to 9.42 h) (e-Fig 22, e-Table 13).
Pneumonia
Five studies (n = 468 patients) reported on the risk of developing pneumonia.32,33,35,41,43 The pooled estimate showed that dexmedetomidine reduces the risk of pneumonia compared with other sedation strategies (RR, 0.30; 95% CI, 0.17-0.52; I2 = 0%; moderate certainty) (e-Fig 23). In absolute terms, dexmedetomidine was associated with an ARR of 16% (95% CI, 11%-19%). Subgroup analysis of comparator agent showed a significant effect for placebo, benzodiazepines, and antipsychotics; however, the test of interaction suggested no significant subgroup effect (P = .93; I2 = 0%) (e-Fig 24). Subgroup analysis of agitation or delirium existing at enrollment vs not existing at enrollment both were significant, and no significant subgroup interaction was found (P = .76; I2 = 0%) (e-Fig 25). The risk of pneumonia was reduced when dexmedetomidine doses of ≤ 0.7 μg/kg/h were administered (RR, 0.28; 95% CI, 0.16-0.49) (e-Fig 26), but no studies exclusively used a dose of > 0.7 μg/kg/h, and therefore, subgroup interactions could not be assessed. The effect was preserved when a sensitivity analysis including only low risk of bias studies was performed (RR, 0.21; 95% CI, 0.05-0.84) (e-Fig 27, e-Table 14).
Bradycardia
Seven RCTs (n = 501 patients) reported on bradycardia (Fig 3D). The risk of bradycardia increased with dexmedetomidine use (RR, 2.80; 95% CI, 1.92-4.07; I2 = 0%; moderate certainty).32,33,35,37,38,40,43 In absolute terms, this was an absolute risk increase of 19% (95% CI, 10%-32%). A subgroup analysis by dose of dexmedetomidine revealed that the effect was persistent when doses of ≤ 0.7 μg/kg/h were administered (RR, 2.94; 95% CI, 1.97-4.39) (e-Fig 28). Only one study used a dose of > 0.7 μg/kg/h; however, no events were found in either group. The increased risk of bradycardia was preserved when we analyzed the two studies32,40 with low risk of bias (RR, 5.33; 95% CI, 1.52-18.66) (e-Table 15, e-Fig 29). Four studies (n = 401 patients) found that dexmedetomidine results in little to no difference in the risk of requiring intervention for bradycardia (RR, 1.30; 95% CI, 0.67-2.50; I2 = 0%; low certainty) (e-Fig 30).32,33,35,43 One study reported on severe bradycardia (defined as a HR < 40 beats/min) and found no difference between the two arms (RR, 6.71; 95% CI, 0.31-147.37).35 Another study reported that one of 19 patients in the dexmedetomidine group experienced an HR of < 40 beats/min, but did not describe the occurrence in the comparison group.39
Hypotension
Data on hypotension were available from seven studies (n = 494 patients).32,33,35,36,38,40,43 The administration of dexmedetomidine increased the risk of hypotension (RR, 1.98; 95% CI, 1.32-2.98; I2 = 11%; moderate certainty) (Fig 3E). This equated to an absolute risk increase of 14% (95% CI, 5%-29%). A subgroup analysis by dose of dexmedetomidine revealed that the effect was not preserved when doses of ≤ 0.7 μg/kg/h were administered (RR, 1.77; 95% CI, 0.87-3.60) (e-Fig 31). No studies exclusively used a dose of > 0.7 μg/kg/h. When five studies with some concern for bias or high risk of bias (e-Table 16) were eliminated,33,35,36,38,43 two studies remained,32,40 and the effect was not preserved (RR, 1.33; 95% 0.41-4.37) (e-Fig 32). Four studies with 383 patients provided data on interventions for hypotension.32,33,36,43 When the data were pooled, the evidence suggested that dexmedetomidine results in little to no difference in requiring intervention for hypotension (RR, 1.45; 95% CI, 0.86-2.46; low certainty) (e-Fig 33).
Post Hoc Analyses
Post hoc sensitivity analyses were conducted. Sensitivity analyses excluding abstracts did not alter the results for all outcomes except hypotension (although the point estimate was preserved) (e-Table 17).
Discussion
Although NIV is a life-saving intervention, its true therapeutic potential can be diminished by patient noncompliance. In this systematic review and meta-analysis of 12 RCTs (n = 738 patients), we found that use of dexmedetomidine in patients with acute respiratory failure being supported with NIV in the ICU reduces the risk of intubation, lowers the chance of delirium, and shortens the ICU LOS. Although use of dexmedetomidine was not associated with a mortality benefit, the number of study patients who died in the ICU (or within 30 days) was low. Even in large trials of critically ill mechanically ventilated adults in whom mortality rates are far greater, dexmedetomidine use has not been associated with a mortality reduction.17
The use of dexmedetomidine in this population is not without safety concerns; the risks of both bradycardia and hypotension were large. However, there was no increased need for interventions to treat the bradycardia (from 2 fewer to 9 more per 100 patients) or hypotension (from 1 fewer to 14 more per 100 patients) when dexmedetomidine was administered. Multiple other studies have examined adverse events associated with dexmedetomidine use. In a recently published international RCT 4,000 mechanically ventilated patients in the ICU were randomized either to usual care or dexmedetomidine. Significantly more patients in the dexmedetomidine group experienced bradycardia (5.1% vs 0.5%; P < .001) and hypotension (2.7% vs 0.5%; P < .0001),44 mirroring our findings and indicating that dexmedetomidine is likely a safe medication to use in patients without contraindications and those at low risk of experiencing adverse events, including cardiovascular adverse effects.
To our knowledge, this is the first systematic review and meta-analysis of RCTs on this topic; however, many of the outcomes have been studied in other populations. Dexmedetomidine has been studied extensively in patients admitted to the ICU who have undergone endotracheal intubation and mechanical ventilation. For instance, a recent systematic review and meta-analysis reported a reduction in the risk of delirium compared with other sedative regimes (RR, 0.63; 95% CI, 0.46-0.86).45 One perhaps can draw a parallel to the “Dexmedetomidine to Lessen ICU Agitation” study, which randomized 74 adults for whom extubation was considered inappropriate because of severe delirium. They found that dexmedetomidine shortened the time to extubation and accelerate recovery from delirium.46 This effect also was seen in a study by Djaiani et al,47 in which dexmedetomidine significantly reduced the incidence of postoperative delirium compared with propofol in cardiac surgery patients who were older than 60 years and were not delirious or agitated before surgery (odds ratio, 0.46; 95% CI, 0.23-0.92).
The strength of this systematic review and meta-analysis is that it is the first review of this novel topic and addressed the clinically important area of sedating patients undergoing NIV. We also undertook a rigorous methodologic process with publication of the protocol to which we adhered, an extensive search of the literature, and duplication of all aspects of the review.
However, our report has some limitations. First, we were unable to complete some of our prespecified outcomes, including hypertension, as well as subgroup analyses by age and by dose of dexmedetomidine. This is because little primary research exists on this topic and a lack of patient-level data. Therefore, as research grows in this area, we may be able to assess whether dexmedetomidine shows an effect modification by age and whether an optimal dose exists, both areas where future research may be directed. The lack of patient data also may temper our subgroup analyses. For example, no overall significant subgroup interaction was found when dexmedetomidine was used in patients with and without agitation or delirium at enrollment, although dexmedetomidine did reduce the need for intubation in those with agitation or delirium at enrollment (RR, 0.41; 95% CI, 0.24-0.70), but not in those without agitation or delirium at enrollment (RR, 0.63; 95% CI, 0.38-1.03). It is possible that the analysis may not have been able to detect subgroup differences because of the low number of trials and participants contributed to each individual subgroup.48 Second, we were unable to examine funnel plots to detect publication bias given the small number of studies pooled for each outcome. We attempted to reduce publication bias through searching many databases, clinical trial registries, and Google scholar, and imposed no language restrictions. Third, we included multiple abstracts, resulting in a relatively small patient population (n = 738 patients). It is therefore conceivable that we may have spuriously overestimated (type I error) or underestimated (type II error) the statistical significance of our findings. A trial sequential analysis adjusts the threshold for statistical significance through expansion or contraction of the CI, thereby reducing the type I and II errors.49 This was not in our plan a priori, and therefore was not carried out, but should be considered for updated meta-analyses on the same topic. Future trials are needed to improve the precision of the estimates, to examine the most appropriate dose, and potentially to explore the effect on subgroups (ie, those with pre-existing delirium or agitation and elderly patients). Subgroup analysis by cause of acute respiratory failure was not performed (as was not specified a priori); however, this may be of interest in future work. Subgroup analysis by cause of acute respiratory failure would not have been possible to perform because of the lack of data; for example, only one study exclusively reported on acute exacerbation of COPD.34
Conclusions
In conclusion, compared with any sedation strategy or placebo, dexmedetomidine reduced the risk of delirium and need for intubation and mechanical ventilation; however, the benefits of dexmedetomidine should be weighed against the probable undesirable effects of hypotension and bradycardia. Future trials are needed to improve the precision of the estimates, to explore the effect on subgroups, and to examine the most appropriate dose.
Acknowledgments
Author contributions: K. L. guarantees the content of the manuscript, including the data and analysis. K. L., J. P., J. W. D., and W. A. contributed to the study conception and design. Data collection was carried out by K. L. and J. P. Material preparation and analysis were performed by K. L., J. P., D. C., and M. H. M. The first draft of the manuscript was written by K. L., J. P., D. C., and W. A. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Financial/nonfinancial disclosures: None declared.
Other contributions: The authors thank Karin Dearness, MLIS, for her continuous support and wisdom in all things related to search strategies.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Rochwerg B., Brochard L., Elliott M.W., et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 2.Girou E., Brun-Buisson C., Taillé S., Lemaire F., Brochard L. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290(22):2985–2991. doi: 10.1001/jama.290.22.2985. [DOI] [PubMed] [Google Scholar]
- 3.Girou E., Schortgen F., Delclaux C., et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284(18):2361–2367. doi: 10.1001/jama.284.18.2361. [DOI] [PubMed] [Google Scholar]
- 4.Plant P.K., Owen J.L., Parrott S., Elliott M.W. Cost effectiveness of ward based non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326(7386):956. doi: 10.1136/bmj.326.7396.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonelli M., Conti G., Moro M., et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27(11):1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 6.Keenan S.P., Gregor J., Sibbald W.J., Cook D., Gafni A. Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28(6):2094–2102. doi: 10.1097/00003246-200006000-00072. [DOI] [PubMed] [Google Scholar]
- 7.L’Her E., Deye N., Lellouche F., et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med. 2005;172(9):1112–1118. doi: 10.1164/rccm.200402-226OC. [DOI] [PubMed] [Google Scholar]
- 8.Hasan M., Naveed M., Burhan H., Smyth C. Patients’ perspective of acute non-invasive ventilation (NIV) for decompensated type 2 respiratory failure (T2RF) Eur Respir J. 2011;38(suppl 55):3792. [Google Scholar]
- 9.Patel B.K., Wolfe K.S., Pohlman A.S., Hall J.B., Kress J.P. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome a randomized clinical trial. JAMA. 2016;315(22):2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlesworth M., Elliott M.W., Holmes J.D. Noninvasive positive pressure ventilation for acute respiratory failure in delirious patients: understudied, underreported, or underappreciated? A systematic review and meta-analysis. Lung. 2012;190(6):597–603. doi: 10.1007/s00408-012-9403-y. [DOI] [PubMed] [Google Scholar]
- 11.Carlucci A., Richard J.-C., Wysocki M., Lepage E., Brochard L. Noninvasive versus conventional mechanical ventilation. Am J Respir Crit Care Med. 2001;163(4):874–880. doi: 10.1164/ajrccm.163.4.2006027. [DOI] [PubMed] [Google Scholar]
- 12.Conti G., Antonelli M., Navalesi P., et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28(12):1701–1707. doi: 10.1007/s00134-002-1478-0. [DOI] [PubMed] [Google Scholar]
- 13.Collet M.O., Caballero J., Sonneville R., et al. Prevalence and risk factors related to haloperidol use for delirium in adult intensive care patients: the multinational AID-ICU inception cohort study. Intensive Care Med. 2018;44(7):1081–1089. doi: 10.1007/s00134-018-5204-y. [DOI] [PubMed] [Google Scholar]
- 14.Pandharipande P.P., Sanders R.D., Girard T.D., et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakob S.M., Ruokonen E., Grounds R.M., et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation. JAMA. 2012;307(11):1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulou C., Kondili E., Diamantaki E., et al. Effects of dexmedetomidine on sleep quality in critically Ill patients: a pilot study. Anesthesiology. 2014;121(4):801–807. doi: 10.1097/ALN.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 17.Devlin J.W., Skrobik Y., Gélinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 18.Yavarovich E.R., Bintvihok M., McCarty J.C., Breeze J.L., Lacamera P. Association between dexmedetomidine use for the treatment of alcohol withdrawal syndrome and intensive care unit length of stay. J Intensive Care. 2019;7(1):49. doi: 10.1186/s40560-019-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X.P., Zhang X.C., Hu S.L., et al. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45(7):e727–e733. doi: 10.1097/CCM.0000000000002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H.-W., Sun X.-M., Shi Z.-H., et al. Effect of high-flow nasal cannula oxygen therapy versus conventional oxygen therapy and noninvasive ventilation on reintubation rate in adult patients after extubation: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care Med. 2018;33(11):609–623. doi: 10.1177/0885066617705118. [DOI] [PubMed] [Google Scholar]
- 21.Yin S., Hong J., Sha T., et al. Efficacy and tolerability of sufentanil, dexmedetomidine, or ketamine added to propofol-based sedation for gastrointestinal endoscopy in elderly patients: a prospective, randomized, controlled trial. Clin Ther. 2019;41(9):1864–1877. doi: 10.1016/j.clinthera.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Berbenetz N., Wang Y., Brown J., et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2019;4(4):CD005351. doi: 10.1002/14651858.CD005351.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canadian Agency for Drugs & Technologies in Health Strings attached: CADTH’s database search filters. Canadian Agency for Drugs & Technologies in Health website. https://www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#rand
- 24.Sterne J.A.C., Savovic J., Page M.J., et al. ROB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;4(pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Cochrane Collaboration Cochrane Handbook Section 7.7.3.5. Medians and interquartile ranges. The Cochrane Collaboration website. https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges.htm
- 27.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.BMJ Best Practice What is GRADE? BMJ Best Practice website. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/
- 31.McMaster University Guideline development tool. GRADEpro website. https://gdt.gradepro.org/app/#projects/p_1 _kimberley_lewis_medportal_ca_0_85ef76f6-eae3-43b4-b217-155e5f96d0a2/evidence-syntheses/71268ddd-830a-44b8-8420-e0e8f3dfc6a3/quality-of-evidence
- 32.Abdelgalel E.F. Dexmedetomidine versus haloperidol for prevention of delirium during non-invasive mechanical ventilation. Egypt J Anaesth. 2016;32(4):473–481. [Google Scholar]
- 33.Allam M.I.M. Dexmedetomidine versus midazolam for sedation of critically ill patients on noninvasive mechanical ventilation. Ain-Shams J Anaesthesiol. 2016;9(2):178–185. [Google Scholar]
- 34.Senoglu N., Oksuz H., Dogan Z., Yildiz H., Demirkiran H., Ekerbicer H. Sedation during noninvasive mechanical ventilation with dexmedetomidine or midazolam: a randomized, double-blind, prospective study. Curr Ther Res Clin Exp. 2010;71(3):141–153. doi: 10.1016/j.curtheres.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallejo de la Cueva A., Quintano Rodero A., Perez Francisco I., et al. Intermediate analysis: dexmedetomidine versus standard clinical practice during non invasive mechanical ventilation (DEX-SCP-NIV) Intensive Care Med Exp. 2019;7(suppl 3):000145. [Google Scholar]
- 36.Baturova V., Levikov D. Effectiveness of dexmedetomidine in agitated patients receiving non-invasive ventilation for acute hypoxemic respiratory failure: a pilot study. Eur Respir J. 2014;44(suppl 58):2963. [Google Scholar]
- 37.Bialka S., Copik M., Pryzbyla M., et al. Effect of dexmedetomidine or propofol sedation on haemodynamic stability of patients after thoracic surgery. Anaesthesiol Intensive Ther. 2018;50(5):359–366. doi: 10.5603/AIT.a2018.0046. [DOI] [PubMed] [Google Scholar]
- 38.Bielka K., Kuchyn I. Dexmedetomidine for sedation during noninvasive ventilation in acute respiratory syndrome patients. Eur Respir J. 2018;52(suppl 62) [Google Scholar]
- 39.Deletombe B., Trouve-Buisson T., Godon A., et al. Dexmedetomidine to facilitate non-invasive ventilation after blunt chest trauma: a randomised, double-blind, crossover, placebo-controlled pilot study. Anaesth Crit Care Pain Med. 2019;38(5):477–483. doi: 10.1016/j.accpm.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Devlin J.W., Al-Qadheeb N.S., Chi A., et al. Efficacy and safety of early dexmedetomidine during noninvasive ventilation for patients with acute respiratory failure: a randomized, double-blind, placebo-controlled pilot study. Chest. 2014;145(6):1204–1212. doi: 10.1378/chest.13-1448. [DOI] [PubMed] [Google Scholar]
- 41.Domin I., Dubrov S., Glumcher F., Slavuta H. Sedation with dexmedetomidine is safe, increasing success rate of noninvasive positive pressure ventilation. Eur J Anaesthesiol. 2018 doi: 10.26226/MORRESSIER.5AEB0ACB07B0D6001A79A968. [DOI] [Google Scholar]
- 42.Hou H., Tang Y., Qu X., Hua S. Clinical effect of dexmedetomidine combined with non-invasive positive pressure ventilation in treatment of patients with AECOPD complicated with pulmonary encephalopathy and evaluation on its safety. J Jilin Univ Med Ed. 2018;44(5):1014–1019. [Google Scholar]
- 43.Huang Z., Chen Y.S., Yang Z.L., Liu J.Y. Dexmedetomidine versus midazolam for the sedation of patients with non-invasive ventilation failure. Intern Med. 2012;51(17):2299–2305. doi: 10.2169/internalmedicine.51.7810. [DOI] [PubMed] [Google Scholar]
- 44.Shehabi Y., Howe B.D., Bellomo R., et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380(26):2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 45.Flükiger J., Hollinger A., Speich B., et al. Dexmedetomidine in prevention and treatment of postoperative and intensive care unit delirium: a systematic review and meta-analysis. Ann Intensive Care. 2018;8(1):92. doi: 10.1186/s13613-018-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reade M.C., Eastwood G.M., Bellomo R., et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315(14):1460–1468. doi: 10.1001/jama.2016.2707. [DOI] [PubMed] [Google Scholar]
- 47.Djaiani G., Silverton N., Fedorko L., et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–368. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 48.Richardson M., Garner P., Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epi Glob Health. 2019;7:192–198. [Google Scholar]
- 49.Wetterslev J., Jakobsen J.C., Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39. doi: 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.