Abstract
Objectives
Widespread SARS-CoV-2 testing is critical to identify infected people and implement public health action to interrupt transmission. With SARS-CoV-2 testing supplies and laboratory capacity now widely available in the United States, understanding the spatial heterogeneity of associations between social determinants and the use of SARS-CoV-2 testing is essential to improve testing availability in populations disproportionately affected by SARS-CoV-2.
Methods
We assessed positive and negative results of SARS-CoV-2 molecular tests conducted from February 1 through June 17, 2020, from the Massachusetts Virtual Epidemiologic Network, an integrated web-based surveillance and case management system in Massachusetts. Using geographically weighted regression and Moran’s I spatial autocorrelation tests, we quantified the associations between SARS-CoV-2 testing rates and 11 metrics of the Social Vulnerability Index in all 351 towns in Massachusetts.
Results
Median SARS-CoV-2 testing rates decreased with increasing percentages of residents with limited English proficiency (median relative risk [interquartile range] = 0.96 [0.95-0.99]), residents aged ≥65 (0.97 [0.87-0.98]), residents without health insurance (0.96 [0.95-1.04], and people residing in crowded housing conditions (0.89 [0.80-0.94]). These associations differed spatially across Massachusetts, and localized models improved the explainable variation in SARS-CoV-2 testing rates by 8% to 12%.
Conclusion
Indicators of social vulnerability are associated with variations in SARS-CoV-2 testing rates. Accounting for the spatial heterogeneity in these associations may improve the ability to explain and address the SARS-CoV-2 pandemic at substate levels.
Keywords: COVID-19, social vulnerability, spatial analysis
In 2020, the United States had the highest number of cumulative COVID-19 cases, the disease caused by infection with SARS-CoV-2, of any country. 1 As of May 14, 2021, the Centers for Disease Control and Prevention (CDC) reported 32 643 851 cases of SARS-CoV-2 and 580 837 deaths from SARS-CoV-2 infection in the United States. 2 Early on, geographic hotspots of SARS-CoV-2 infection in the United States were primarily located in metropolitan areas on the West Coast and among states in the Northeast, 3 such as Massachusetts, where the increase in the number of COVID-19 cases was initially driven by a large international meeting. 4 Moreover, major drivers of the rising number of SARS-CoV-2 infections in the United States are asymptomatic and presymptomatic cases or cases with mild symptoms. 5 -8 Therefore, in addition to preventive measures such as social distancing, wearing face masks, and hand washing, widespread testing to identify people who may be unknowingly contributing to the transmission of SARS-CoV-2, 9 regardless of symptom onset, is imperative to slow the COVID-19 pandemic. Consequently, new initiatives, such as the Rapid Acceleration of Diagnostics program developed by the National Institutes of Health, were established with an emphasis on increasing testing capacity across the United States. 10
SARS-CoV-2 testing facilitates the identification of new infections, the follow-up and isolation of infected people, and the quarantine of infected people’s contacts, which are the basic principles for controlling infectious disease pandemics, including the transmission of respiratory infections as in SARS-CoV-2. 11 However, widespread inequities in access to SARS-CoV-2 tests have been identified across the United States, particularly driven by population density and disparities among racial/ethnic minority populations. 12 In contrast, with Massachusetts experiencing one of the first outbreaks of SARS-CoV-2 infection in the United States, 4 Massachusetts public health officials undertook early efforts to implement extensive SARS-CoV-2 testing services across the state to detect people newly infected with SARS-CoV-2. 13 As the availability of SARS-CoV-2 testing supplies and laboratory capacity expands across the United States, it is important to understand the spatial distribution of and determinants associated with SARS-CoV-2 testing.
Data are scarce on the geographic extent and factors facilitating SARS-CoV-2 testing in the United States. Our study had 2 objectives. First, we identified the geographic variations of SARS-CoV-2 testing rates and spatial clusters of high and low SARS-CoV-2 testing rates at the town level in Massachusetts. Second, we assessed the demographic and socioeconomic factors associated with the geographic variation of SARS-CoV-2 testing rates in Massachusetts. Lastly, we explored the spatial autocorrelation between SARS-CoV-2 testing rates and COVID-19 incidence rates in Massachusetts. Findings may inform where to direct testing resources and for which populations to conduct tailored SARS-CoV-2 testing in Massachusetts.
Methods
SARS-CoV-2 Testing Data Sources
The Massachusetts Department of Public Health Bureau of Infectious Disease and Laboratory Sciences (BIDLS) established the Massachusetts Virtual Epidemiologic Network (MAVEN) in 2006 to serve as an integrated, web-based disease surveillance and case management system. 14 BIDLS receives results from state laboratory testing facilities electronically using Health Level 7 messaging standards. 15 Disease events are automatically populated into the MAVEN surveillance system when a case or laboratory report is received and triaged in real time by state and local public health personnel for follow-up. When the COVID-19 pandemic began, MAVEN had already required all tests, including positive and negative results, related to SARS-CoV-2 infection to be reported under a novel coronavirus reporting requirement developed during the SARS outbreak in 2003. We included all molecular tests reported to MAVEN from February 1 through June 17, 2020, in this analysis and calculated SARS-CoV-2 testing rates per 100 000 population in 2018 by city/town using estimated annual population denominators developed by the University of Massachusetts Donahue Institute. 16 We computed the coefficient of variation in the rate of SARS-CoV-2 testing per 100 000 population as the ratio of the SD and mean rate to express the extent of variability in testing rates in relation to the mean testing rate. CDC reviewed this activity and determined it to be conducted consistently with applicable federal law and CDC policy.
SARS-CoV-2 Testing Descriptive Statistics
From February 1 through June 17, 2020, a total of 919 679 molecular SARS-CoV-2 tests were reported for 727 549 Massachusetts residents. Of these tests, 30 733 (4.2%) had incomplete information on city or town of residence, leaving 696 816 (95.8%) people with tests in the analysis. All but 1 town reported at least 1 test, and this town had a population of <100 residents. Of the remaining 350 towns, the testing rates per 100 000 population ranged from <1000 in Leyden to >28 000 in Bedford. The mean testing rate per 100 000 population for Massachusetts was 8066 (SD = 3363). The coefficient of variation was 0.4, indicating great dispersion in the testing rates by city/town.
The Social Vulnerability Index
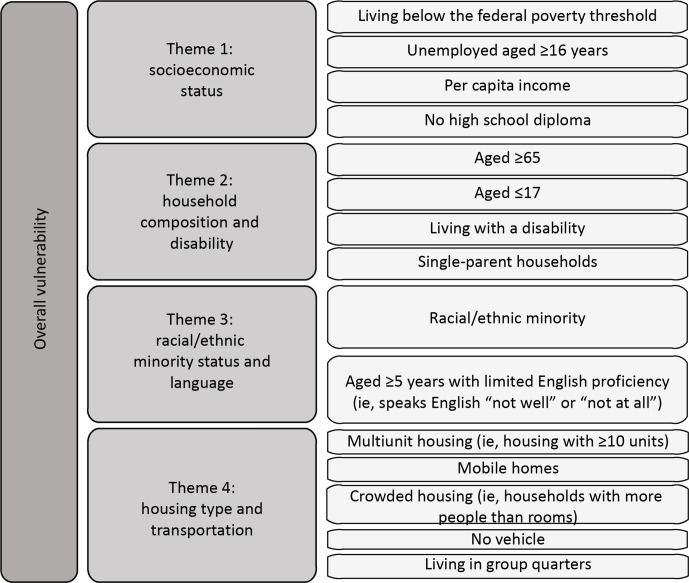
We used the Social Vulnerability Index (SVI) to examine the extent of associations between social vulnerability factors and SARS-CoV-2 testing rates. Developed by the CDC/Agency for Toxic Substances and Disease Registry (ATSDR), the SVI is a database and composite index that models social vulnerability in communities in the United States using 15 census-based indicators that capture data in domains of social vulnerability. It can be used to identify the locations of communities in the United States that are socially vulnerable to hazardous events, such as pandemics. 17,18 Our analysis included SVI data on the following 11 indicators in raw single percentage units: population living below the federal poverty threshold, civilian noninstitutionalized population living with a disability, 19 unemployed population aged ≥16 years, population aged ≥65 years, racial/ethnic minority population, people aged ≥5 years with limited English proficiency (ie, who speak English “not well” or “not at all,” according to US Census Bureau terminology), uninsured people in the civilian noninstitutionalized population, multiunit housing (ie, housing structures with ≥10 units), people living in group quarters, 20 crowded housing (ie, households with more people than rooms), and households without vehicle access. We selected these variables on the basis of existing literature citing social determinants of risk for disaster events 18,21 and the current COVID-19 pandemic. 22 -25 In addition, our analysis included data on the following SVI themes: socioeconomic status, household composition and disability, racial/ethnic minority status and language, and housing type and transportation (Figure 1). For ease of interpretation, we transformed the SVI theme values so that each point increase in testing rates corresponded to a 10% (or 0.1) increase in the theme value.
Figure 1.
Indicators and themes in the Centers for Disease Control and Prevention Social Vulnerability Index (SVI). The following indicators and SVI themes were analyzed: socioeconomic status (living below the federal poverty threshold, unemployed aged ≥16 years), household composition and disability (aged ≥65, living with a disability), racial/ethnic minority status and language (racial/ethnic minority, aged ≥5 years with limited English proficiency [ie, who speak English “not well” or “not at all”]), and housing type and transportation (multiunit housing structures [ie, housing with ≥10 units], crowded housing [ie, households with more people than rooms], no vehicle, living in group quarters). Indicators were selected on the basis of a literature review or were excluded because of multicollinearity. Uninsured in the civilian noninstitutionalized population is not a part of the overall SVI or themes but is included in the SVI database as an adjunct variable. Based on the literature, “uninsured” was included in the analysis. Modified and adapted from Agency for Toxic Substances and Disease Registry. 17
Statistical Analysis
The Geospatial Research, Analysis, and Services Program (GRASP) team at CDC/ATSDR produced an SVI database at the Massachusetts township level by transforming the Massachusetts state-specific SVI database from the census tract level (n = 1475) to the township level (n = 351). Despite having census tracts and counties, Massachusetts uses township as its primary level of operation. For the transformation, we adapted methodology from Hallisey et al. 26 Details on this transformation are available from the authors upon request. To summarize, for one-to-one geographic relationships (ie, one tract to one town), variable values remained the same after transformation. We aggregated SVI variables for many-to-one geographic relationships and disaggregated variables for split census tracts or one-to-many geographic relationships. To estimate count variables, such as the number of people aged ≥65 years, we assigned a weight to each census tract, dependent on the type of geographic relationship, with which to multiply each count. We summed weighted counts for each census tract to estimate town counts. To validate this transformation, we compared GRASP-transformed town population estimates, produced during transformation, with town population estimates from the Donahue Institute. 16 We found agreement between the 2 sets of population estimates. We summed weighted normalized value estimates for each census tract to estimate percentages for each town.
We conducted generalized linear models (GLMs) with Poisson distribution and with robust variance estimation to examine associations at the township level between SARS-CoV-2 testing rates and SVI indicators and themes. To address model multicollinearity, we eliminated variables with variance inflation factors >10. We assessed model fit using the Akaike information criterion. We found significant spatial autocorrelation of residuals in both GLM models with a Poisson distribution using SVI indicators and themes (Moran’s I = 0.14, P < .001 for the individual indicator model and Moran’s I = 0.22, P < .001 for the theme model). For this reason, we used geographically weighted Poisson regression (GWR) with a Poisson distribution to derive estimates for each township to account for the presence of spatial heterogeneity in the relationship between SARS-CoV-2 testing rates and SVI indicators and themes. We used a function from the SPGWR package in R version 4.04 (R Core Team) to identify a bandwidth for the given generalized geographically weighted regression by optimization. We cross-validated bandwidth selection by scoring the root mean square prediction error for the GWR and choosing the bandwidth that minimizes this quantity. In the final model, we selected a bi-square kernel density. In addition, because of the spatial heterogeneity in the GLM’s error and the GWRs having higher adjusted R 2 values than the GLMs (0.87 vs 0.75 in the individual indicator model; 0.76 vs 0.68 in the theme model), the GWR provides a more useful model than the GLM; thus, we present results for the GWR alone.
Geographically weighted Poisson regression models produce exponentiated coefficients, or relative risk (RR). In their base form, coefficients explain the difference in log the ratio between a 1-unit change in the predictor and the outcome. We exponentiated them into RRs so we could explain the effects as the percentage change. Because separate regression equations are obtained for each feature in a GWR, we present the median RR and interquartile range (IQR) across all towns uto describe the magnitude and directionality of the local effect for each independent variable. Lastly, we evaluated clustering between SARS-CoV-2 testing and case rates using bivariate Moran’s I and bivariate geographic mapping. We conducted all analyses in R version 4.04.
Results
Geographically Weighted Regression
In the GWR analysis, using 11 indicators as model covariates, 7 indicators were associated with increases in SARS-CoV-2 testing rates (Table 1). The greatest positive association with SARS-CoV-2 testing rates occurred with increasing percentages of unemployed population aged ≥16 years (median [IQR] RR = 1.05 [0.99-1.12]), population living below the federal poverty threshold (1.05 [0.87-1.07]), civilian noninstitutionalized population with a disability (1.07 [1.05-1.09]), racial/ethnic minority population (1.02 [1.01-1.04]), population residing in multiunit structures (1.05 [1.03-1.10]), population living in group quarters (1.01 [1.01-1.03]), and households without vehicle access (1.04 [1.03-1.16]). The greatest inverse association with SARS-CoV-2 testing rates occurred with increasing percentages of township population residing in crowded housing (0.89 [0.80-0.94]). The IQR for 5 of the 11 indicators was >0.10.
Table 1.
Association of Social Vulnerability Index a indicators of SARS-CoV-2 molecular polymerase chain reaction testing (per 100 000 population) using geographically weighted Poisson regression, Massachusetts, February–June 2020
| Indicator | Relative risk | |
|---|---|---|
| Median (interquartile range) | Range | |
| Unemployed population aged ≥16 years | 1.05 (0.99-1.12) | 0.84-1.21 |
| Population aged ≥65 years | 0.97 (0.87-0.98) | 0.83-1.00 |
| Population living below the federal poverty threshold | 1.05 (0.87-1.07) | 0.84-1.08 |
| Estimate of the civilian noninstitutionalized population living with a disability | 1.07 (1.05-1.09) | 1.01-1.18 |
| Racial/ethnic minority population (ie, all people except non-Hispanic White) | 1.02 (1.01-1.04) | 1.00-1.08 |
| People aged ≥5 years with limited English proficiency (ie, who speak English “not well” or “not at all”) | 0.96 (0.95-0.99) | 0.88-1.44 |
| Uninsured people in the civilian noninstitutionalized population | 0.96 (0.95-1.04) | 0.88-1.16 |
| Multiunit housing (ie, housing structures with ≥10 units) | 1.05 (1.03-1.10) | 1.02-1.36 |
| People living in group quarters | 1.01 (1.01-1.03) | 0.99-1.22 |
| Households without vehicle access | 1.04 (1.03-1.16) | 0.86-1.24 |
| Crowded housing (ie, households with more people than rooms) | 0.89 (0.80-0.94) | 0.58-0.99 |
aDeveloped by the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry, the Social Vulnerability Index is a database and composite index that models social vulnerability in communities in the United States using 15 census-based indicators that capture data in domains of social vulnerability. 17
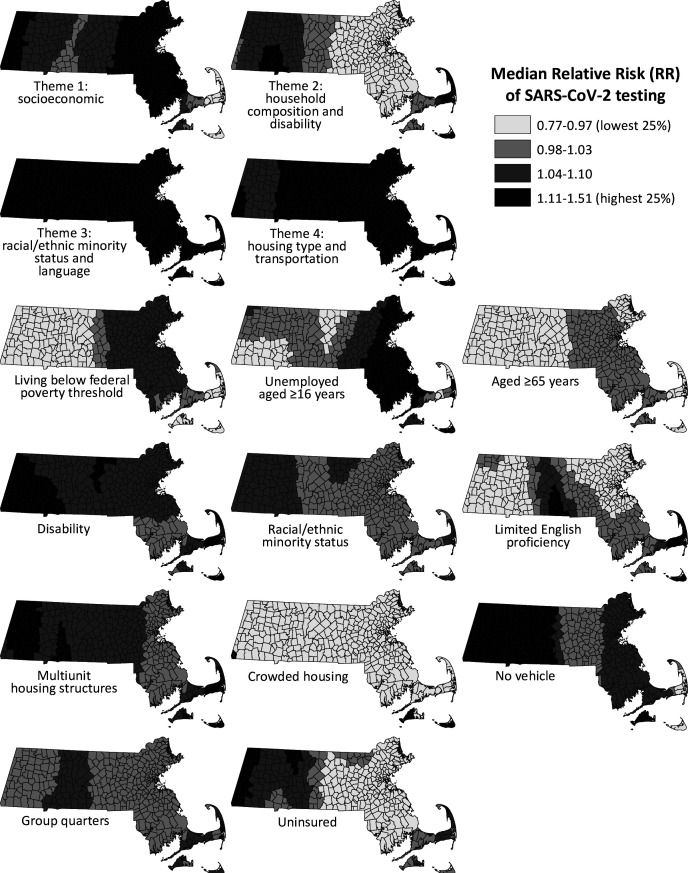
In the GWR analysis using data on SVI themes, 3 of 4 themes had a positive association with SARS-CoV-2 testing rates (Table 2). The rate of testing increased with each 10% increase in social vulnerability by socioeconomic status (median RR [IQR] = 1.12 [1.05-1.14]), racial/ethnic minority status and language (1.23 [1.18-1.38]), and housing type and transportation (1.23 [1.16-1.24]). The rate of testing decreased with each 10% increase in social vulnerability by household composition and disability (0.95 [0.94-1.04]). We illustrate standardized coefficients of the 4 SVI themes and the 11 factors associated with SARS-CoV-2 testing rates by township for Massachusetts to highlight geographic variation in the associations. For example, although the association between rate of testing and SVI Theme 2 (household composition and disability) was protective in eastern Massachusetts, a pocket of townships in southwestern Massachusetts showed a harmful association (Figure 2).
Table 2.
Association of Social Vulnerability Index a themes with rate of SARS-CoV-2 molecular polymerase chain reaction testing (per 100 000 population) using geographically weighted Poisson regression, Massachusetts, February–June 2020
| Theme | Relative risk | |
|---|---|---|
| Median (interquartile range) | Range | |
| Socioeconomic status | 1.12 (1.05-1.14) | 0.77-1.23 |
| Household composition and disability | 0.95 (0.94-1.04) | 0.93-1.18 |
| Racial/ethnic minority status and language | 1.23 (1.18-1.38) | 1.15-1.51 |
| Housing type and transportation | 1.23 (1.16-1.24) | 1.05-1.32 |
aDeveloped by the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry, the Social Vulnerability Index is a database and composite index that models social vulnerability in communities in the United States using 15 census-based indicators that capture data in domains of social vulnerability. 17
Figure 2.
Standardized coefficients of the 4 Social Vulnerability Index (SVI) themes and 11 indicators, by town, Massachusetts, 2018. Values for all 4 themes and indicators were pooled and classified into quartiles. Developed by the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry, the SVI is a database and composite index that models social vulnerability in communities in the United States using 15 census-based indicators that capture data in domains of social vulnerability. 17 Values for all 4 SVI themes and indicators were pooled and then classified into quartiles.
Bivariate Spatial Autocorrelation
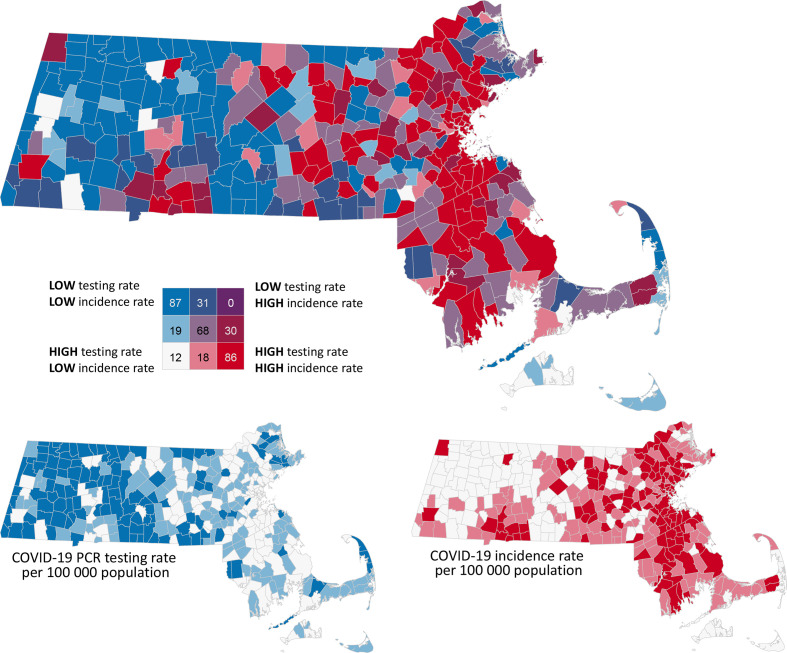
We found significant bivariate spatial autocorrelation between COVID-19 incidence rates and testing rates with a bivariate Moran’s I value of 0.278 (P = .001) as of June 17, 2020. No towns had low testing rates and high incidence rates, although 31 towns had low testing rates and moderate incidence rates, and 30 towns had moderate testing rates and high incidence rates (Figure 3).
Figure 3.
Bivariate geographic distribution between the rate of SARS-CoV-2 molecular polymerase chain reaction (PCR) testing and rate of SARS-CoV-2 incidence, by city/town, Massachusetts, February 1–June 17, 2020. Tertiles classification was used to enable comparison of the distribution of the 2 variables (incidence rate and testing rate). Town counts shown for each category. The distribution of confirmed COVID-19 cases is complex and depends on a combination of interacting factors, including socioeconomic conditions, underlying health, health care access, and testing capacity. Comparing a single variable, COVID-19 testing rate is only part of the story and should be interpreted with caution. Data sources: Massachusetts Department of Public Health data from February–June 17, 2010 14 ; Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry Social Vulnerability Index 2018 for Massachusetts transformed to Massachusetts town level 17 ; and University of Massachusetts Donahue Institute. 16
Discussion
Our findings indicate wide variability in testing rates and associated community factors in a single state (Massachusetts). Factors associated with a person’s residence and social circumstances were shown to be independent risk factors for SARS-CoV-2 in-hospital mortality in a large, population-based study in the United Kingdom, even after accounting for clinical comorbidities. 27 A study in Massachusetts found that affluent communities had higher access to testing resources compared with less affluent communities. 28 In the United States, census tract of residence may explain 70% of variation in individual health outcomes. 29 Understanding the complex social factors associated with access to SARS-CoV-2 testing is a national priority. For example, the National Institutes of Health established funding in June 2020 to improve “reach, access, uptake, and impact for SARS-CoV-2 testing in underserved and/or vulnerable populations.” 30 In addition to providing insight into community factors associated with testing in Massachusetts, our study documents methods that other states or regions can use to identify town- or county-level need for increased SARS-CoV-2 testing.
In presenting GWR models, we illustrate the importance of accounting for spatial heterogeneity in analytic models, especially because the range of estimates from the GWR models indicate that the associations between SVI and testing rates vary considerably among towns. For the individual indicators model, the smaller effect sizes relative to the theme model were expected given the raw single percentage unit of the independent variables compared with the incremental 10% groups used with the theme variables. Highly granular continuous estimators often produce smaller effect sizes than aggregated or dichotomous variables.
Overall, the benefit of GWRs, in addition to having a better model fit than GLMs, is that they allow public health authorities to obtain the separate coefficients, variance, and intercepts for each town, allowing interventions to be tailored for the conditions in each town. With a GWR, it is possible to see how the effects of different covariates vary across Massachusetts. Our results indicate that some towns or counties can even exhibit contrasting effect directions for a single variable, which would not have been identified by a GLM or other global estimate model. Thus, the range in values illustrates the need to examine the unique effect of each covariate in each town as opposed to presenting an overall estimate at the state level.
Although several efforts have been made to understand spatial variation in SARS-CoV-2 infection rates, 31 -33 we believe our study is the first example of using such methods to examine testing variation. 34 Based on results from the GWR models, the testing rates were negatively associated with the percentages of populations with limited English proficiency, aged ≥65, without health insurance, and in households with more people than rooms. These results indicate that additional efforts to expand testing are necessary to address COVID-19 inequities and disparities in these communities, which have been found to be disproportionately affected by SARS-CoV-2. 34
However, towns with higher percentages of racial/ethnic minority residents, people who are unemployed, and residents living in group quarters or multiunit housing had increased relative median testing rates in Massachusetts, which is reassuring considering that elsewhere in the United States, racial/ethnic minority populations have had disproportionately higher rates of SARS-CoV-2 infection and related poor health outcomes. 35 In addition, results from the bivariate Moran’s I analysis and the bivariate choropleth visualization enable public health authorities to identify regions with both low testing rates and high infection rates, which were detected primarily in the central and western regions of Massachusetts. We also identified areas of high testing rates and high infection rates, such as the Boston metropolitan area.
Limitations
This study had several limitations. First, the SVI uses 2018 estimates from the American Community Survey 2014-2018 database; thus, the effect of school closures, social distancing policies, and other interventions to control SARS-CoV-2 transmission was not captured. Second, unmeasured error may have occurred in transforming SVI from county to township level, particularly for disaggregated, or split, census tracts. Fortunately, few census tracts were split: only 24 of 1475 total census tracts were disaggregated. Third, it is unknown which people received testing in each township; thus, each association must be viewed as correlative in nature and representative of population-level relationships.
Conclusions
Ensuring widespread testing for SARS-CoV-2 infection is a primary concern in the United States during the COVID-19 pandemic. Ensuring community access to testing is a priority for public health. Relating variations in testing to various community characteristics is a key component of increasing testing in areas in need. Our results indicate that not only can numerous indicators of social vulnerability be associated with testing rate variation but also that accounting for the spatial heterogeneity in these associations can considerably improve the ability to explain and address the COVID-19 pandemic at substate levels. We suggest that future investigations into SARS-CoV-2 testing or infection rates include spatial components in their analyses to optimize the accuracy of their models and the efficacy of public health interventions.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this research was partially funded by grant T32 ES 07069 from the National Institutes of Health (G. Wilt).
ORCID iDs
Elaine Hallisey, MA https://orcid.org/0000-0002-9733-9611
R. Monina Klevens, DDS, MPH https://orcid.org/0000-0003-2073-5576
References
- 1. World Health Organization . Coronavirus disease (COVID-19) situation report—190. July 28, 2020. Accessed August 5, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200728-covid-19-sitrep-190.pdf?sfvrsn=fec17314_2
- 2. Centers for Disease Control and Prevention . COVID-19 data tracker: United States COVID-19 cases, deaths, and laboratory testing (RT-PCR) by state, territory, and jurisdiction. Updated May 11, 2021. Accessed August 5, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 3. Desjardins MR., Hohl A., Delmelle EM. Rapid surveillance of COVID-19 in the United States using a prospective space–time scan statistic: detecting and evaluating emerging clusters. Appl Geogr. 2020;118:102202. 10.1016/j.apgeog.2020.102202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rimmer A. COVID-19: medical conferences around the world are cancelled after US cases are linked to Massachusetts meeting. BMJ. 2020;368:m1054. 10.1136/bmj.m1054 [DOI] [PubMed] [Google Scholar]
- 5. Gao Z., Xu Y., Sun C. et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54(1):12-16. 10.1016/j.jmii.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huff HV., Singh A. Asymptomatic transmission during the coronavirus disease 2019 pandemic and implications for public health strategies. Clin Infect Dis. 2020;71(10):2752-2756. 10.1093/cid/ciaa654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li R., Pei S., Chen B. et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368(6490):489-493. 10.1126/science.abb3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan J., Liu S., Zhuang L. et al. Transmission and clinical characteristics of asymptomatic patients with SARS-CoV-2 infection. Future Virol. 2020;15(6):373-380. 10.2217/fvl-2020-0087 [DOI] [Google Scholar]
- 9. Schneider EC. Failing the test—the tragic data gap undermining the U.S. pandemic response. N Engl J Med. 2020;383(4):299-302. 10.1056/NEJMp2014836 [DOI] [PubMed] [Google Scholar]
- 10. Tromberg BJ., Schwetz TA., Pérez-Stable EJ. et al. Rapid scaling up of COVID-19 diagnostic testing in the United States—the NIH RADx Initiative. N Engl J Med. 2020;383(11):1071-1077. 10.1056/NEJMsr2022263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holloway R., Rasmussen SA., Zaza S., Cox NJ., Jernigan DB. Updated preparedness and response framework for influenza pandemics. MMWR Recomm Rep. 2014;63(RR-06):1-18. [PubMed] [Google Scholar]
- 12. Rader B., Astley CM., Sy KTL. et al. Geographic access to United States SARS-CoV-2 testing sites highlights healthcare disparities and may bias transmission estimates. J Travel Med. 2020;27(7):taaa076. 10.1093/jtm/taaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker-Polito administration provides update on COVID-19 testing capacity and strategy, PPE procurement [press release] . Office of Governor Charlie Baker and Lt. Governor Karyn Polito, Governor’s Press Office, Department of Public Health, Executive Office of Health and Human Services; May 14, 2020. Accessed July 11, 2020. https://www.mass.gov/news/baker-polito-administration-provides-update-on-covid-19-testing-capacity-and-strategy-ppe
- 14. Troppy S., Haney G., Cocoros N., Cranston K., DeMaria A Jr. Infectious disease surveillance in the 21st century: an integrated web-based surveillance and case management system. Public Health Rep. 2014;129(2):132-138. 10.1177/003335491412900206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. HL7 International . HL7 messaging standard version 2.7. 2011. Accessed August 5, 2020. https://www.hl7.org/implement/standards/product_brief.cfm?product_id=146
- 16. University of Massachusetts Amherst . Massachusetts Population Estimates Program. Accessed July 11, 2020. https://donahue.umass.edu/business-groups/economic-public-policy-research/massachusetts-population-estimates-program/population-projections
- 17. Agency for Toxic Substances and Disease Registry . CDC/ATSDR Social Vulnerability Index. Accessed July 22, 2020. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 18. Flanagan BE., Gregory EW., Hallisey EJ., Heitgerd JL., Lewis B. A social vulnerability index for disaster management. J Homel Secur Emerg Manag. 2011;8(1):article 3. 10.2202/1547-7355.1792 [DOI] [Google Scholar]
- 19. US Census Bureau . How disability data are collected from the American Community Survey. Accessed August 6, 2020. https://www.census.gov/topics/health/disability/guidance/data-collection-acs.html
- 20. American Community Survey . 2018 American Community Survey/Puerto Rico Community Survey group quarters definitions. Accessed August 6, 2020. https://www2.census.gov/programs-surveys/acs/tech_docs/group_definitions/2018GQ_Definitions.pdf
- 21. Flanagan BE., Hallisey EJ., Adams E., Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention’s Social Vulnerability Index. J Environ Health. 2018;80(10):34-36. [PMC free article] [PubMed] [Google Scholar]
- 22. Li X., Xu S., Yu M. et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110-118. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rader B., Nande A., Adlam B. et al. Crowding and the epidemic intensity of COVID-19 transmission. Published May 12, 2020. medRxiv. 10.1101/2020.04.15.20064980 [DOI] [Google Scholar]
- 24. Qiu Y., Chen X., Shi W. Impacts of social and economic factors on the transmission of coronavirus disease 2019 (COVID-19) in China [published online May 9, 2020]. J Popul Econ. 10.1007/s00148-020-00778-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim SJ., Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Educ Behav. 2020;47(4):509-513. 10.1177/1090198120929677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hallisey E., Tai E., Berens A. et al. Transforming geographic scale: a comparison of combined population and areal weighting to other interpolation methods. Int J Health Geogr. 2017;16(1):29. 10.1186/s12942-017-0102-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The OpenSAFELY Collaborative, Williamson E., Walker AJ. et al. OpenSAFELY: factors associated with COVID-19–related hospital death in the linked electronic health records of 17 million adult NHS patients. Published May 7, 2020. medRxiv. 10.1101/2020.05.06.20092999 [DOI] [Google Scholar]
- 28. Dryden-Peterson S., Velásquez GE., Stopka TJ., Davey S., Lockman S., Ojikutu BO. Disparities in SARS-CoV-2 testing in Massachusetts during the COVID-19 pandemic. JAMA Netw Open. 2021;4(2):e2037067. 10.1001/jamanetworkopen.2020.37067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boing AF., Boing AC., Cordes J., Kim R., Subramanian SV. Quantifying and explaining variation in life expectancy at census tract, county, and state levels in the United States. Proc Natl Acad Sci U S A. 2020;117(30):17688-17694. 10.1073/pnas.2003719117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Institutes of Health . NOT-OD-20-121: notice of special interest (NOSI): limited competition for emergency competitive revisions for community-engaged research on COVID-19 testing among underserved and/or vulnerable populations. June 12, 2020. Accessed July 29, 2020. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-20-121.html
- 31. Gatto M., Bertuzzo E., Mari L. et al. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc Natl Acad Sci U S A. 2020;117(19):10484-10491. 10.1073/pnas.2004978117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang D., Choi H., Kim J-H., Choi J. Spatial epidemic dynamics of the COVID-19 outbreak in China. Int J Infect Dis. 2020;94:96-102. 10.1016/j.ijid.2020.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cordes J., Castro MC. Spatial analysis of COVID-19 clusters and contextual factors in New York City. Spat Spatiotemporal Epidemiol. 2020;34:100355. 10.1016/j.sste.2020.100355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karaye IM., Horney JA. The impact of social vulnerability on COVID-19 in the U.S.: an analysis of spatially varying relationships. Am J Prev Med. 2020;59(3):317-325. 10.1016/j.amepre.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. The COVID Tracking Project . The COVID racial data tracker. Accessed August 5, 2020t. https://covidtracking.com/race