SUMMARY
Social homeostasis is the ability of individuals to detect the quantity and quality of social contact, compare it to an established set-point in a command center, and adjust the effort expended to seek the optimal social contact expressed via an effector system. Social contact becomes a positive or negative valence stimulus when it is deficient or in excess, respectively. Chronic deficits lead to set-point adaptations such that reintroduction to the previous optimum is experienced as a surplus. Here, we build upon previous models for social homeostasis to include adaptations to lasting changes in environmental conditions, such as with chronic isolation.
INTRODUCTION
How do we adapt when opportunities for social engagement change in a long-lasting manner? Social homeostasis is an adaptive function to regulate behaviors that govern social connection to an optimal level, to avoid a surplus of social contact (wherein competition for resources, space, and mates becomes too fierce) as well as a deficit of social contact (wherein safety, warmth, observational learning, and play are lacking) (Matthews and Tye, 2019).
Drawing from the conceptual framework established by Abraham Maslow for a hierarchy of needs (Maslow, 1943a, 1943b), there are two classes of needs: “being needs” or “self-actualization” and “deficiency needs,” which are reminiscent of homeostatic need states wherein the motivational drive to meet the needs increases when the needs are continually unmet. Even within “deficiency needs,” Maslow conceptualized several tiers: (1) physiological needs that are essential for survival (air, food, water, shelter, clothing, sleep, sex); (2) safety needs that allow an individual a sense of order, predictability, and control (family, police, schools, medical care, legal system, governance structure); (3) love and belongingness needs (love, intimacy, friendship, acceptance); and (4) self-esteem needs that are subcategorized into esteem for oneself and esteem from others (dignity, status, prestige). Importantly, social needs are actually described in all 4 of these tiers. Within the social dimension, Maslow framed sexual contact as the most basic or essential, with a social structure to provide safety, protection, and stability on the next tier, intimate relationships on the next, and finally social rank and influence in the fourth tier. However, Maslow’s evolving framework assumes modern human society, rather than the environments in which our brains have evolved.
A deficit or surplus of social contact can be interpreted as a type of stressor that induces efforts to correct the deviation from the optimum that may trigger both adaptive and pathological responses. A social surplus such as overcrowding may induce psychological stress, social conflict, and aggression (Loo and Ong, 1984), with robustly consistent results in animal studies (for review, see Christian, 1961, 1970). However, human studies on the effects of density and crowding on social behavior have produced inconsistent results, perhaps due to the contribution of variables specific to humans (self-report, social programming, cultural norms) as well as individual variability in social status, gender, culture, and context (Evans et al., 1998; Regoeczi, 2003).
A social deficit can occur with objective or subjective social isolation, social exclusion, or subordination. Social isolation is universally aversive to social species, with behavioral and neuroendocrine changes associated with mental and physical health consequences including depression, shortened lifespan, and increased rates of cancer (Hermes et al., 2009; Ma et al., 2011; Steptoe et al., 2013; Weiss, 1973). Chronic social isolation, particularly during rearing, is well known to be used as a rodent model for schizophrenia (Geyer et al., 1993; Kohn and Clausen, 1955).
In humans, perceived deficits in the objective quantity, or subjective quality, of social contact (“loneliness” [Weiss, 1973]), are correlated with deficits in mental (Cacioppo et al., 2006a) and physical (Hawkley and Cacioppo, 2010; Hawkley et al., 2006) health and higher mortality rates (Berkman and Syme, 1979; Holt-Lunstad et al., 2010; Holwerda et al., 2012; Perissinotto et al., 2012; Steptoe et al., 2013). Perceived loneliness correlates with increased morbidity and mortality with cancer and cardiovascular disease (Hawkley and Cacioppo, 2003). Perceived loneliness is also correlated with the symptom severity in response to viral immune challenges (LeRoy et al., 2017) and inflammatory responses (Balter et al., 2019).
Solitary confinement has been deemed a form of torture (Hresko, 2006; Thoenig, 1972). Yet, the negative valence serves an adaptive purpose—representing a homeostatic need state. We hypothesize that the unpleasant state of loneliness reflects evolutionarily advantageous neuroadaptations that increase motivation to seek social contact (Matthews and Tye, 2019).
With acute social isolation, animals from rodents to humans perform prosocial behaviors (a rebound of social interaction, increased affiliative behaviors) following social isolation for 24 h (Matthews and Tye, 2019; Matthews et al., 2016; Panksepp and Beatty, 1980; Tomova et al., 2019, 2020a). However, with chronic social isolation, flies, rodents, and humans display antisocial behaviors (aggression, avoidance, social anxiety) (Ma et al., 2011; Sciolino et al., 2010; Zelikowsky et al., 2018) that may be manifested in the form of mental health disorders in our modern-day society.
A number of studies have demonstrated that individual differences both across and within species with respect to patterns of social engagement are predetermined by genetic factors (Forkosh et al., 2019; Hoekstra and Coyne, 2007; Lim et al., 2004; Wang et al., 2008); yet, relatively little is known about the neural circuit mechanisms underlying how a given individual adapts and responds to a changing social environment.
With the unprecedented global pandemic of 2020 that induced a government-mandated lockdown in many cities and countries, as well as prolonged “social-distancing,” the urgent need to quarantine dramatically changed the social landscape of almost every human being on the planet. We were pointedly faced with the competing needs of slowing the spread of coronavirus disease 2019 (COVID-19) and maintaining our social needs. Indeed, the uncertainty in how to evaluate these two competing needs has been a challenge that has sparked controversy, has been heavily politicized, and has manifested a wide range of individualized assessments.
Here, we present a conceptual framework toward understanding the dynamic responses and adaptations made in the face of changes in an individual’s social environment. This conceptual framework provides a mechanistic explanation for how the valence of a social stimulus (whether it is evaluated as positive or negative) can change to the same stimulus (reintroduction to a social group) from acute isolation to chronic isolation. We propose that the emotional valence (Tye, 2018) of a social stimulus can be modulated by the internal need state of an individual. Overcrowding changes the perceived valence of social contact, and an isolation-induced reduction in the social homeostatic set-point may make a social stimulus previously perceived as optimal now be interpreted as a surplus. This may explain why chronic social isolation can produce antisocial behaviors, whereas acute social isolation produces prosocial behaviors.
COMPONENTS OF HOMEOSTATIC SYSTEMS
Homeostasis refers to the physiological processes wherein stable states are maintained through compensatory mechanisms and is schematized with receptors, a control center, and effectors. Homeostatic systems are known to exist for a number of physiological needs (Cannon, 1929). For example, in thermoregulation, when an imbalance is detected by temperature-sensing receptors in the skin and brain, signals are sent to a control center which coordinates a response, and signals will be sent to effector systems such as sweat glands to promote cooling in the case of overheating to maintain homeostasis (Tan et al., 2016; Wendt et al., 2007). Motivated behaviors associated with aversive “drive” states are governed by the physiological needs of the individual (hunger or thirst) when in a deficit (Bai et al., 2019; Betley et al., 2013; Oka et al., 2015; Zimmerman et al., 2016). The neural circuit bases of many homeostatic systems (i.e., thermoregulation, osmoregulation, sleep homeostasis, and energy balance) are supported by a rich literature, yet social homeostasis remains uncharted territory ripe for investigation.
NEURAL COMPONENTS OF SOCIAL HOMEOSTASIS
Social homeostasis refers to the ability of an individual to detect the quantity and quality of social contact, compare it to an established set-point in a command center, and adjust the effort expended to seek social contact through an effector system (Figure 1; Table 1; Matthews and Tye, 2019).
Figure 1. Integration of features, rank, and identity at the level of social detection.
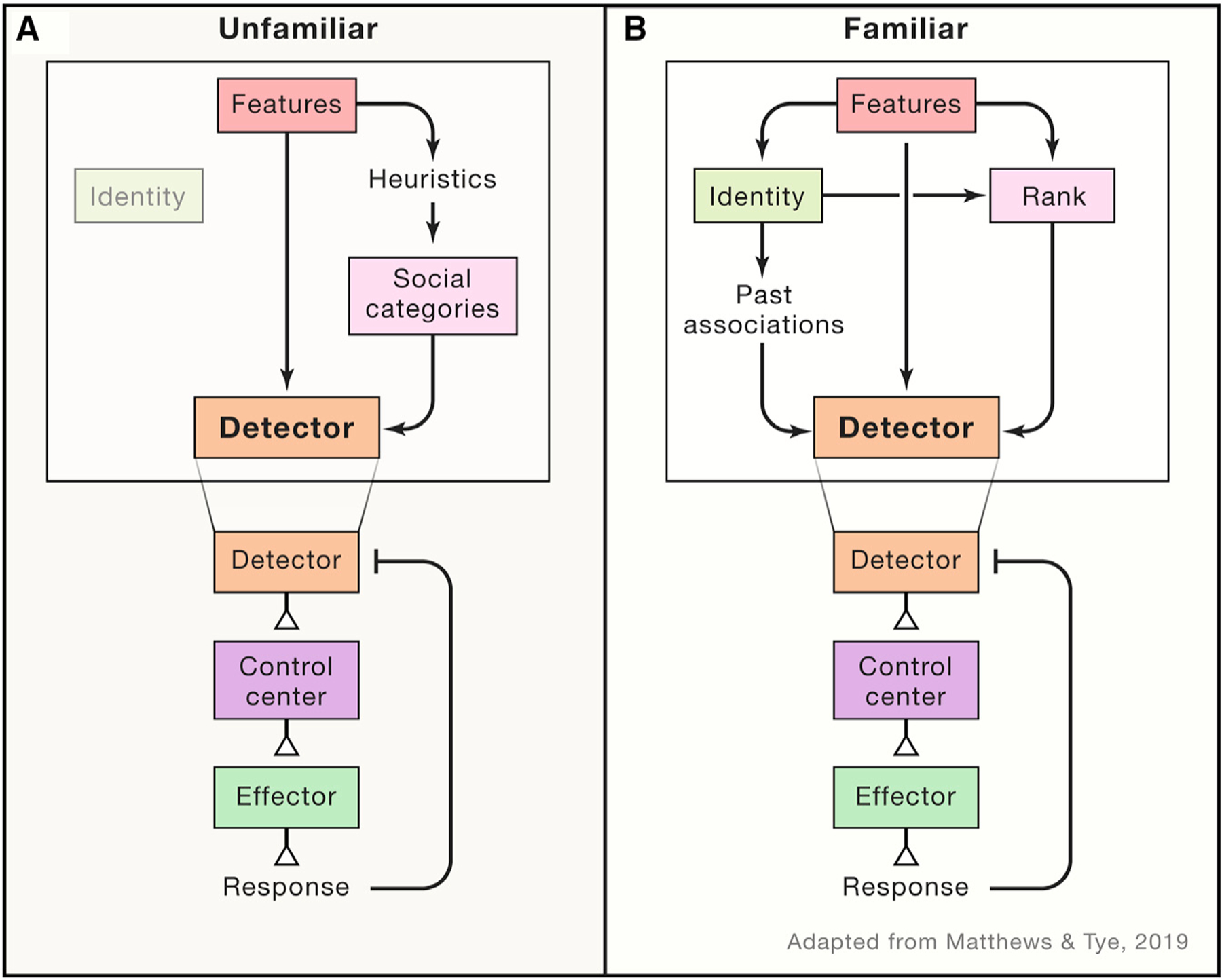
(A and B) Schematic of social information detection from (A) unfamiliar individuals and (B) familiar individuals. The first step of a social homeostatic system is detection, which integrates many social variables such as social features, rank, and identity to determine the overall quality of a social interaction. (A) When an individual first interacts with an unfamiliar conspecific, the individual primarily relies on the social features of the unfamiliar individual, such as age, sex, etc. These social features provide a heuristic to assess a social agent and determine an appropriate behavioral response. (B) When interacting with a familiar individual, information from social features and the learned identity of the other individual feed into the detector node as well as provide information on the relative rank of the individual. The detector integrates all of this information in evaluating social interactions, which is then fed forward to subsequent nodes in the social homeostatic system.
Table 1.
Glossary of terms in the context of social behavior
| Term | Definition |
|---|---|
| Social homeostasis | The ability of individuals to regulate the quantity and quality of social contact and maintain stability within a social structure. |
| Detector | A neural system that senses changes in the quantity of social interactions and quality of an individual’s social environment, integrating factors such as social agent characteristics, relative rank, and identity. |
| Control center | A neural system that compares deviations in social utility to an encoded homeostatic set-point to calculate deficits or surpluses of social interaction. |
| Effector | A neural system that coordinates motivated behavior to resolve deficits and surpluses in social utility (e.g., prosocial affiliative behavior or antisocial aggressive behavior). |
| Social utility | The product of the detected social quantity and quality. The preferred quality of social interactions monotonically increases while the preferred quantity increases to an optimal point and then declines when there is a surplus. |
| Homeostatic set-point | An individual’s ideal level of social utility. |
| Despotic Hierarchy | Social structure in which one animal is dominant over all other individuals, and there is a large resource/power disparity between the dominant and others. |
| Linear Hierarchy | Social structure in which each individual has a ranking, and the linear rankings of the “pecking order” obey the laws of transitivity |
| Egalitarian Hierarchy | Social structure in which resources and power are distributed equally amongst individuals. |
| Prosocial (affiliative) behaviors | Social behaviors that promote group cohesion (friendly/positive gestures), e.g., grooming, touching, hugging. |
| Antisocial behaviors | Social behaviors that hinder group cohesion, e.g., aggression, intimidation, fighting. |
| Loneliness | State of distress or discomfort that results when one perceives a gap between one’s desires for social connection and actual experiences of it. |
| Valence | Positive or negative motivational significance. |
| Acute versus chronic | For the purpose of this Review, acute and chronic social isolation refer to relative timescales, as species with different lifespans, reproductive cycles, metabolisms, etc., will likely also have differing thresholds for acute versus chronic social isolation. |
Detector
The first step within a social homeostatic system is detecting the social environment. The detector node is responsible for sensing deviations in the quality and quantity of social contact (Matthews and Tye, 2019). While the quantity of social interaction is an objective measure, the quality of a social interaction is subjective and is highly dependent on the context of the social interaction—factoring in relative dominance, social hierarchical structure, relationship history, etc. (Shemesh et al., 2013, 2016). We hypothesize that, especially in unfamiliar individuals, the information about the characteristics of the social stimulus (i.e., sex, age, etc.) is weighted more heavily in the assessment of a social interaction and acts as a heuristic (Figure 1A). With familiarization, the learned identity of the individual carries more weight in determining the quality of a specific interaction (Figure 1B).
We conceptualize the overall detected social interaction—which we operationally define as social utility—as the product of both social quantity and quality, where the preferred quality of social interactions monotonically increases while the preferred quantity increases to an optimal point and then declines when there is a surplus (Figure 2). For example, if the quality of social contact is very low, even if there is the optimal or very high quantity the social utility remains low. Additionally, if the quality of social contact is very high, but the quantity is low, the social utility remains low. If the quantity is very high, even if the quality is high, there is a surplus that reduces the social utility. However, if the quality is high and the quantity is optimal, this offers the maximum social utility.
Figure 2. Hypothesized progression of acute and chronic social isolation and crowding.
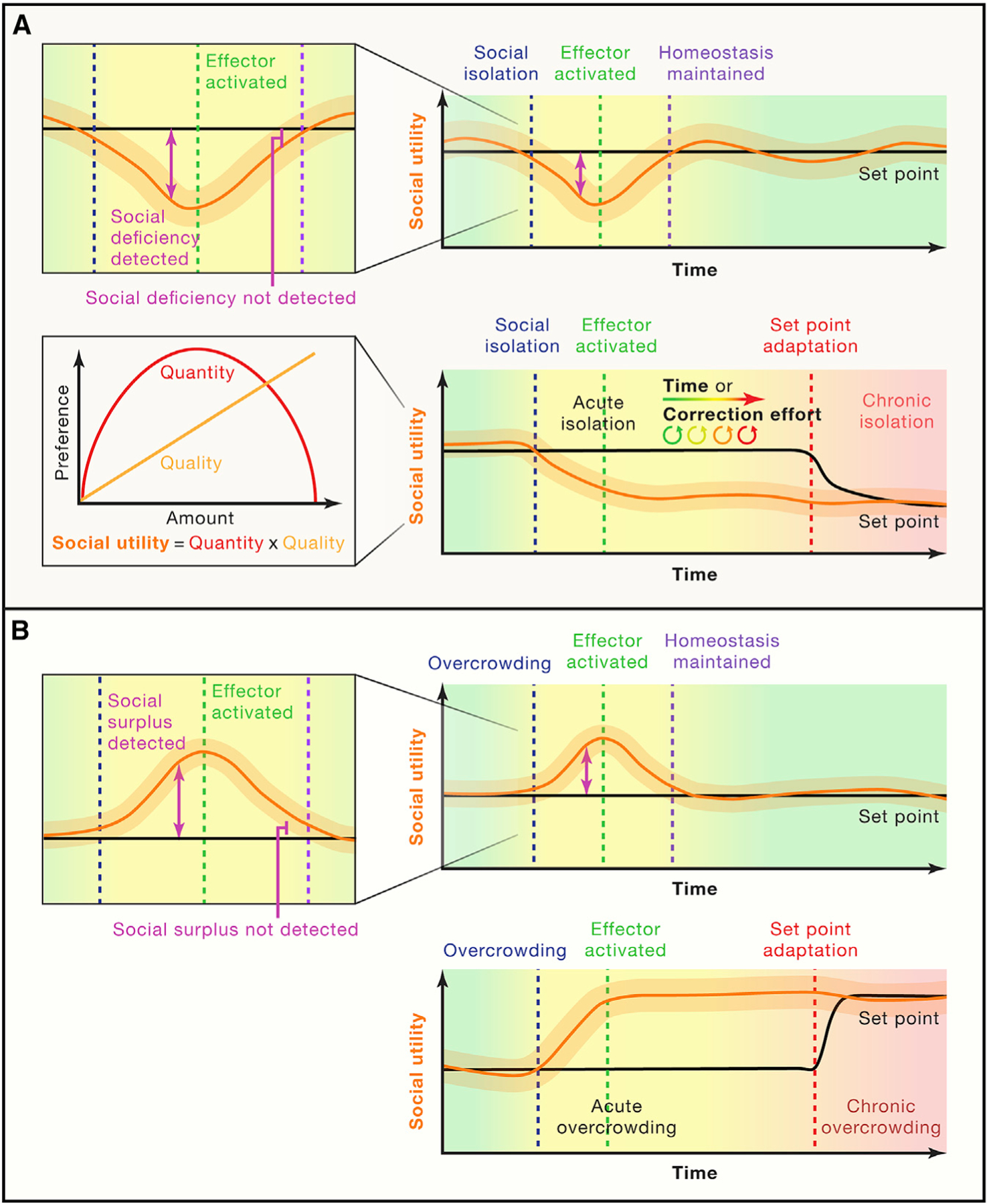
The detector node of the social homeostatic system determines the social utility of a social interaction. The detected social utility is a multiplicative function that integrates both the quantity and quality of social interactions (callout).
(A) When a social utility deficit is detected due to social isolation, the effector system activates and drives motivated behavior in an individual to seek social contact. If the effector system is successful in bringing the detected social utility to the set-point encoded by the control center, homeostasis is ultimately maintained and the effector system inactivates (top). In the case where the effector system fails to bring the detected social utility to the encoded set-point, the individual will experience a transition from acute to chronic social isolation (bottom), indicated by a compensatory set-point adjustment that we hypothesize to occur due to time or repeated correction effort by the effector system.
(B) When a social surplus is detected due to overcrowding, the effector system drives antisocial behavior to rescue the discrepancy. If the effector system is successful in bringing the detected social utility to the set-point encoded by the control center, homeostasis is ultimately maintained and the effector system inactivates (top). In the case where the effector system fails to bring the detected social utility to the encoded set-point, the individual will experience a transition from acute to chronic social overcrowding (bottom).
In the following sections, we examine the factors, as well as the underlying neural circuits and mechanisms, that feed into a detector system.
Features and agent characteristics
An individual’s social environment is often diverse and complex, with features such as age and sex of a conspecific determining the type of social interaction and the behavioral output of that individual (for review, see Chen and Hong, 2018). For example, an individual’s behavior will likely be different for different social stimuli (e.g., a newborn baby, attractive individual of the same age, or an elderly relative). Especially for unfamiliar social stimuli, individuals may rely on sensory cues—such as olfactory, auditory, or visual—as heuristics in determining an appropriate behavioral output in response to a social stimulus (i.e., more affiliative, supportive behavior toward the newborn, and more confident behavior toward the attractive individual). These complex social behaviors and decisions extend to other mammals. For instance, rats will immediately liberate cagemates who are trapped in restrainers, perhaps a demonstration of empathy (Ben-Ami Bartal et al., 2011), and rats are more inclined to help rats that are of the same strain as rats with whom the subject is most familiar (Ben-Ami Bartal et al., 2014), suggesting that rats rely on feature-based heuristics. Additionally, for observational learning, socially derived information about the environment requires the anterior cingulate cortex (ACC)-basolateral amygdala (BLA) circuit (Allsop et al., 2018).
Pheromonal detection occurs in the vomeronasal organ (VNO) and to a lesser extent in the main olfactory epithelium and ultimately leads to innate behaviors owing to hardwired circuitry (for review, see Stowers and Kuo, 2015). For example, chemosensory detection of the major urinary proteins (MUPs) MUP3 and MUP20 in the VNO of male mice induces intermale aggressive behaviors through projections to the accessory olfactory bulb (AOB), which activates hardwired neural circuitry driving aggression (such as the “core aggression circuit,” including the medial amygdala [MeA] and the ventrolateral part of the ventromedial hypothalamus [VMHvl], as described in Lischinsky and Lin, 2020; Hashikawa et al., 2016). By contrast, detection of MUP20 in the VNO of female mice induces attraction toward males (Kaur et al., 2014; Roberts et al., 2010). Meanwhile, sulfated estrogens found in female mouse urine result in male attraction when sensed in the male VNO (Haga-Yamanaka et al., 2014). Additionally, exocrine-gland-secreting peptide 22 (ESP22), a pheromone secreted in the tears of juvenile (2–3 week old) mice, strongly inhibits sexual behavior when detected in the VNO of male mice, whereas abolishing ESP22 in juveniles increases sexual behavior of adult males with juveniles, suggesting that this pheromone is critical for mice to discriminate adults from juveniles (Ferrero et al., 2013). Removal of the VNO in mice, and specifically, removal of transient receptor potential cation channel, subfamily C, member 2 (TRPC2)—a protein integral for chemosensory transduction in the VNO—results in social behavior deficits, including a decrease in intermale aggression, dysfunction in discriminating males and females, and reduction in sexual behavior between males and females (Clancy et al., 1984; Leypold et al., 2002; Stowers et al., 2002). Interestingly, oxytocin (Oxt) signaling in the MeA (downstream of VNO) is necessary for sex discrimination in mice, suggesting further downstream processing is required in the orchestration of detecting agent characteristics (Yao et al., 2017). Altogether, chemosensory cues are necessary for detecting the type of social stimulus presented and determining the appropriate response to that social agent; in a social homeostatic system, detection of social features is critical for determining the quality of a social interaction.
In addition to chemosensory cues, auditory cues offer another sensory modality to social individuals to determine the characteristics of another social agent. Marmosets, for instance, use phee calls to re-unite isolated animals back to their social group (Eliades and Miller, 2017; Moynihan, 1970). Phee calls in male marmosets are markedly different from female phee calls in that they tend to be higher frequency and have greater variability (Norcross and Newman, 1993), providing information about the individual’s sex. Indeed, social context influences the cortical activity in marmosets, as the state of frontal cortex neurons before a vocalization was heard predicted whether the marmoset would respond to a conspecific (Nummela et al., 2017). Free-ranging male baboons use vocalizations—a distinctive “wahoo” call—to indicate their social rank (discussed below), with more frequent and more drawn-out calls indicative of dominance (Fischer et al., 2004). In mice, ultrasonic vocalizations (USVs) provide a mode of communication between individuals. This has been well studied in the context of communication between mothers and pups, where pups—especially those outside the nest—will emit USVs based on changes in their body temperature or in their environment (Ehret, 2005). The acoustic properties of the USVs reflect the pup’s degree of arousal and emotional state, and the USVs elicit a phonotaxic response in the mother to retrieve her pup (Dulac et al., 2014; Kohl and Dulac, 2018; Schiavo et al., 2020). Interestingly, optogenetic activation of oxytocin neurons from the paraventricular nucleus of the hypothalamus (PVN) in the left auditory cortex of naive virgin mice, who initially do not retrieve pups, accelerates pup retrieval behavior (Marlin et al., 2015).
Humans and non-human primates—and to a lesser extent, rodents (Chen and Hong, 2018)—also rely on vision to perceive agent characteristics. The human fusiform face area has long been known to respond to images of faces, showing increased fMRI signals when a subject is exposed to photos of faces and not inanimate objects or other body parts (Kanwisher et al., 1997). Through fMRI in conjunction with single-unit recordings, studies in the inferotemporal cortex of macaques show that these neurons respond selectively to faces and can differentiate faces by tuning to facial geometrical features (Freiwald et al., 2009; Tsao et al., 2006). Additionally, female macaques can discriminate between the photos of male and female monkeys, with neurons in the orbitofrontal cortex responding to macaque photos in a sex-specific manner, suggesting that macaques can perform sex discrimination based solely on visual information (Mizuno et al., 2007). Conspecific sex information is also represented in the dorsomedial prefrontal cortex (dmPFC) of mice, and specific re-activation of “male” and “female” neuronal ensembles modulate social preference toward male and female conspecific mice, respectively (Kingsbury et al., 2020). Interestingly, humans with extensive bilateral amygdala damage experience dysfunction in recognizing emotions signaled by facial expression, such as fear and anger, indicating that social and emotional processing of social stimuli may be downstream of sensory regions such as visual cortex (Adolphs et al., 1999).
Identity and rank
From children recognizing their parents when they come to pick them up to emperor penguins finding their partners and young after months of separation (Jouventin et al., 1999), the ability to recognize an individual’s social identity is paramount to evolutionary fitness. Familiarity of a social agent provides context for an interaction, and while some species may prefer interacting with familiar individuals, others, such as mice, prefer to interact with unfamiliar individuals over familiar individuals (Moy et al., 2004). Studies in rodents, non-human primates, and humans show converging evidence that the hippocampus plays a significant role in social memory (for review, see Miller et al., 2016). Neural recordings from mice show stable representations of familiar mice in ventral hippocampal CA1 (vCA1) pyramidal neurons, and optogenetic stimulation of the vCA1 neuronal ensemble encoding a familiar mouse can sufficiently induce social discrimination, with the vCA1-nucleus accumbens (NAc) circuit being critical for social memory (Okuyama et al., 2016). Additionally, dorsal hippocampal CA2 (dCA2) has been implicated in social recognition memory in rodents, non-human primates, and humans (for review, see Dudek et al., 2016). Specifically, silencing dCA2 pyramidal neuronal output (Hitti and Siegelbaum, 2014), and in particular, input to vCA1, disrupts social recognition memory, but not other hippocampal-dependent tasks (Meira et al., 2018). dCA2 receives strong input from the supramammillary nucleus (SuM) (Cui et al., 2013), and optogenetic stimulation of SuM inputs into dCA2 via feedforward inhibition induces a social novelty response (Chen et al., 2020). Moreover, dCA2 selectively expresses vasopressin receptors (Avpr1b) in the hippocampus (Young et al., 2006), and optogenetic activation of vasopressinergic neurons from the PVN enhances social memory and is reversible with pharmacological Avpr1b antagonism (Smith et al., 2016). Taken together, these hypothalamic-hippocampal circuits are critically important in the identification of conspecifics, a necessary step in determining the quality of a social interaction.
The ability to recognize social rank within a hierarchy is essential to minimize conflict and maintain order, and an individual must be able to know its social rank to emit appropriate behaviors, and to evaluate the quality of social interactions from others. Many highly successful species live in social hierarchies, benefitting from the advantages they provide. However, the structure of hierarchies is diverse, spanning parameters from large to small, inherited to earned, linear to flat, and despotic to egalitarian. Social structures can be as small as a pair or as large as a civilization. Hierarchies can be maintained for life (such as in female rhesus monkeys who are born into their rank) (for review, see Sapolsky, 2005) or they can be dynamic, such as mice, who upon removal of the alpha will detect a “power vacuum” within minutes (Williamson et al., 2017). Linear social hierarchies were first observed in domestic fowl who demonstrate a pecking order (Schjelderup-Ebbe, 1922) and are also observed in CD1 mice (So et al., 2015). By contrast, honeybees organize themselves into a flat hierarchy and work democratically to make collective decisions, such as finding a new home (Seeley, 2010). Some species, such as Asian elephants, exhibit an egalitarian hierarchical structure owing to a year-round productive environment that favors dispersal of resources to all individuals, such that resources cannot be monopolized (de Silva et al., 2017). Other species with despotic hierarchies, such as African wild dogs and dwarf mongooses, maintain dominance through aggression over subordinates; in this case, dominant animals show chronically elevated glucocorticoid levels (a proxy for stress) (Creel et al., 1996). Conversely, subordinate baboons and rats (who establish rank in their despotic social hierarchy through intimidation and subordination gestures), show markedly increased glucocorticoid levels (Popova and Naumenko, 1972; Sapolsky, 1990). The heterogeneity in a species’ environment and social structure determines the quality of conspecific interactions and the quantity an individual prefers and is therefore a major factor in detecting one’s social environment.
How is social rank encoded in the central nervous system? In mice, potentiating and depressing glutamatergic signaling in the medial prefrontal cortex (mPFC) increases and decreases relative hierarchical rank, respectively (Wang et al., 2011). Specifically, optogenetically increasing the synaptic strength of the mediodorsal thalamus (MDT)-to-dmPFC circuit results in sustained winning in the tube test, a common assay used to determine dominance between pairs of mice (Zhou et al., 2017). Additionally, the magnitude of dmPFC activity correlation between a pair of mice during a competitive tube test assay predicts their relative dominance relationship (Kingsbury et al., 2019). Furthermore, a recent study reveals that population-level activity in the mPFC predicts social rank and success in a social competition assay (Padilla-Coreano et al., 2020). In particular, mPFC neurons projecting to the lateral hypothalamus (LH) encode social rank, and optogenetic activation of mPFC-LH neurons promotes social dominance. Although firing rate in BLA neurons correlates with the social rank of conspecifics in rhesus macaques (Munuera et al., 2018), BLA-projecting neurons in the mPFC of mice do not encode social rank (Padilla-Coreano et al., 2020). Remarkably, the encoded relative rank of a mouse can determine the magnitude of sociability and place avoidance when stimulating dorsal raphe nucleus (DRN) dopamine neurons (Matthews et al., 2016) and also the number and size of urine marks a mouse makes, an effect reversible through inhibition of GABAergic medial preoptic area neurons (Hou et al., 2016).
Control center
Computing deficits or surpluses
To understand any homeostatic system, we need to be able to measure the signal that is being regulated by the system—and in the case of social homeostasis this signal is not only dynamic but also high-dimensional. For thermoregulation, the signal is body temperature, whereas for osmoregulation, it would be blood osmolality. In these cases, both of these regulated signals are one-dimensional, where an objective measurement can be taken. For social homeostasis, it would be the perceived social environment—which is a high-dimensional state (including factors such as group size, relative rank, hierarchical structure, individual pairwise relationships, and observational learning), where subjective factors including previous experience and preference are paramount. While we postulate certain aspects of the social environment to be objective (e.g., social rank), the perception of other parameters of the social environment may be influenced by individual experiences, innate individual differences, or other contextual factors.
The requisite functions of any homeostatic control center are that (1) it receives input from detector systems, (2) it stores information about the homeostatic set-point, (3) it computes the delta between the received input and the stored set-point, and (4) it sends information about any deviation from the set-point to a downstream effector system (Cannon, 1929).
The social homeostatic control center integrates information about the current state of social engagement with the desired quality/quantity of social contact (homeostatic set-point) to compute deficits or surpluses in social contact. If a deficit or surplus is detected, this deviation from the desired set-point will trigger engagement of the “effector” system to correct the change.
Surpluses of social contact
Maintenance of social homeostasis is critical for health and survival; deviations (in the form of both deficits and surpluses) from the social homeostatic set-point can result in health consequences. For instance, both objective (persons/room) and subjective (perceived excessive social demands and lack of privacy) human overcrowding in the home, even when controlled for socioeconomic variables, is correlated with poor mental health and social relationships within the home (Gove et al., 1979). Baboons demonstrate an increase in salivary cortisol concentrations following both acute (4-day) and chronic (months-long) overcrowding (Pearson et al., 2015). In mice, social crowding (8 mice/cage) increases anxiety-like behavior and corticosterone levels, as well as hypothalamic corticotropin-releasing factor (CRF), agouti-related peptide (AgRP), and neuropeptide Y (NPY) expression, indicating that crowding is stress inducing (Lin et al., 2015).
Aggression also increases with group size in mice (Van Loo et al., 2001), perhaps indicative of a behavioral mechanism to restore social homeostasis (but see Flanigan et al., 2020; Golden et al., 2016). Interestingly, adult mice who are placed in an overcrowded environment (8 mice/standard laboratory mouse cage) show an increase in plasma corticosterone concentration compared with non-crowded control mice both 1 and 7 days, but not 14 days, after crowding, suggesting an acute stress response to social crowding that diminishes with chronic exposure (Peng et al., 1989).
Deficits in social contact
With acute social isolation, animals from rodents to humans have been shown to display prosocial behaviors (a rebound of social interaction, increased affiliative behaviors) following social isolation for 24 h (Matthews and Tye, 2019; Matthews et al., 2016; Panksepp and Beatty, 1980; Tomova et al., 2019, 2020b). With acute isolation, social homeostatic systems are engaged, and animals will increase vocalizations (Ehret, 2005; Norcross and Newman, 1993), experience a surge in blood glucocorticoids/cortisol (Rukstalis and French, 2005; Taylor et al., 2014), and expend energy to seek social contact (Panksepp and Beatty, 1980). However, with chronic social isolation, flies, rodents, and humans have been shown to perform antisocial behaviors (aggression, avoidance, social anxiety) (Ma et al., 2011; Sciolino et al., 2010; Zelikowsky et al., 2018) that may be manifested in the form of mental health disorders in our modern-day society.
We hypothesize that, with sustained effort toward correcting this deficit, animals will eventually switch from an active coping strategy to a passive coping strategy and that the homeostatic set-point will be adjusted to the new baseline of social contact (Figure 2).
Mechanisms orchestration/need competition
The ability to orchestrate competing homeostatic needs and to select one behavioral output that is most adaptive, taking conflicting mechanisms into account, is essential for survival. Importantly, we already know that while separate homeostatic systems may exist for different needs, they may still be interdependent. For example, although separate homeostatic systems exist for hunger and thirst (Augustine et al., 2018; Burton et al., 1976; Sternson, 2013; Zimmerman et al., 2016, 2019), even in a severe energy balance deficit, if an animal is too dehydrated, it will not be able to eat (Bolles, 1961). Similarly, both food deprivation and artificially activating arcuate AgRP neurons—a regulator for appetite—in singly housed male mice shift preference from social interaction with male juvenile and receptive female mice to food consumption in a preference assay (Burnett et al., 2016). Additionally, the intensity of food deprivation determines the magnitude of preference of food over social interaction (Burnett et al., 2019).
Across evolution, social needs exist for any sexually reproductive animal, which includes all mammals. In many cases, social needs can be overshadowed by more urgent threats to our survival. For example, a predatory threat would take precedence over feeding, sleeping, and sex due to the simple calculation that failure to escape the predator would be a greater threat to survival than delaying feeding, sleeping, or sex. Although many social animals can survive in the absence of social contact, the necessity of socialization for reproduction has embedded social needs as essential for species survival, even if not an individual’s survival. Thus, it is adaptive for animals to be able to prioritize social needs over basic survival needs until those deficits become life-threatening. For example, bears hibernating will stay nurturing their offspring until internal physiological needs become potential threats on their own survival (Evans et al., 2016). Similarly, penguins alternate between extended periods of foraging or incubating offspring, wherein higher corticosterone levels drove foraging while higher prolactin levels drove incubation (Spée et al., 2010).
Effector
The effector system in any homeostatic system is primarily responsible for driving motivated behavior and physiological adaptations to maintain homeostasis (Cannon, 1929). For instance, in the case of thermoregulation, when an individual’s internal temperature is higher than the encoded set-point, the effector in this homeostatic system may motivate the individual to head to a shaded area or it may stimulate sweat glands to cool down the body. Requisites for an effector system include that (1) it must receive input from a control center and (2) activation of the effector system must be able to drive behavior or physiological adaptation independent of the individual’s homeostatic need state. The fairly recent advent of neural recording and manipulation technologies has prompted unprecedented insight into the circuits underlying social behavior, such as the bidirectional modulation of social behaviors by BLA-mPFC and BLA-vCA1 (Felix-Ortiz and Tye, 2014; Felix-Ortiz et al., 2016), the influence of neuromodulation on social reward and social behavior (Dölen et al., 2013; Gunaydin et al., 2014), and the neural circuits underlying aggression (Hong et al., 2014; Lin et al., 2011; Lischinsky and Lin, 2020).
For the purpose of this review, we operationally define the “effector system” as any mechanism that produces a behavior for the purpose of rescuing a social deficit or surplus of an individual and maintains social homeostasis.
Prosocial behaviors
When a social connection deficit is computed, the effector system will attempt to resolve the social inadequacy by driving motivated behavior to interact with others. In doing so, the effector must engage the social motivational circuitry. Acute social isolation generally results in an increase in affiliative, prosocial behaviors across many species. Humans, when experiencing social connection deficits, show increased social memory and heightened social attention toward socially relevant cues, suggesting that a “loneliness” state can activate a social hypervigilant state (Gardner et al., 2005; Pickett et al., 2004). Similarly, following acute (3 day) social isolation, rats demonstrate an increase in social interaction and playful behavior (Panksepp and Beatty, 1980).
Many brain regions are recruited in the social motivation circuitry, including the ventral tegmental area (VTA), which sits at the nexus of the social reward circuitry. Specifically, social interaction during a juvenile intruder task in mice increases activity of VTA dopamine neurons, specifically those neurons that project to D1-receptor-expressing medium spiny neurons in the NAc (Gunaydin et al., 2014). Optogenetic activation of the VTA-NAc projection promotes affiliative social behavior. Additionally, optogenetic stimulation of serotonin terminals (projecting from the DRN) in the NAc also increases social interaction time during a juvenile intruder task (Walsh et al., 2018). In rats, social interaction results in dopamine release in the NAc (Robinson et al., 2002), and antagonizing either D1 or D2 receptors in the NAc decreases time spent playing socially (Manduca et al., 2016). Oxytocin neurons—canonically known for their role in affiliative social behavior, pair bonding, and maternal behaviors (Shamay-Tsoory and Abu-Akel, 2016)—in the PVN project to VTA dopamine neurons, and oxytocin release in the VTA gates social reward (Hung et al., 2017). PVN oxytocin neurons show increased activity during social interactions in mice, and optogenetically and chemogenetically exciting and inhibiting PVN oxytocin neurons enhances and dampens social preference, respectively (Anpilov et al., 2020; Resendez et al., 2020).
While VTA dopamine neurons are typically first considered when investigating dopaminergic involvement in social motivation, another midbrain population of dopamine neurons in the DRN exhibits relevance in producing the “negative” (aversive) drive to motivate social behavior (Matthews et al., 2016). Although historically regarded as a caudal extension of VTA dopamine neurons, DRN dopamine neurons project to distinct downstream regions and are functionally different from VTA dopamine neurons (Cho et al., 2017; Dougalis et al., 2012; Groessl et al., 2018; Hasue and Shammah-Lagnado, 2002; Matthews et al., 2016). In addition, the DRN receives input from hypothalamic areas, such as LH and PVNOxt neurons (Roeling et al., 1993, 1994), regions typically associated with integrating information about an individual’s need state. After 24 h of social isolation, glutamatergic input onto DRN dopamine neurons strengthens in adult male mice, and optogenetically activating these neurons drives both sociability and aversion (Matthews et al., 2016). Additionally, DRN dopamine neurons exhibit generally increased activity during social contact with a novel mouse, as demonstrated by bulk neuronal signals recorded through fiber photometry. Optogenetically inhibiting these neurons decreases sociability only when followed by 24 h of social isolation. Taken together, these results suggest that DRN dopamine neurons are recruited to provide social motivation induced by aversive state. This contrasts with the VTA dopamine-NAc circuit, which seems to elicit the “positive” (rewarding) drive to seek social contact. A recent finding in acutely isolated (10 h) humans shows an increase in social craving alongside increased midbrain dopamine neuronal activity when presented with social cues (Tomova et al., 2020b), consistent with the hypothesized role of DRN dopamine neurons as observed in mice. Altogether, it is tempting to speculate that DRN dopamine neurons may be an element of the effector system of the larger social homeostatic system.
Antisocial behaviors
In a manner reminiscent of a social surplus, chronic social isolation has been shown to increase antisocial behaviors upon reintroduction to a social group, namely territorial aggression, across many species, including rodents (Wiberg and Grice, 1963; Malkesman et al., 2006; Wongwitdecha and Marsden, 1996), fish (Gómez-Laplaza and Morgan, 2000; Clayton and Hinde, 1968), and Drosophila (Agrawal et al., 2020; Liu et al., 2011). While aggression can be prosocial in certain circumstances, such as in the case of establishing and maintaining social hierarchy (largely mediated by dopamine-transporter-expressing neurons in the ventral premammillary nucleus [Stagkourakis et al., 2018]), aggressive behaviors used to ward off other social agents may be considered antisocial.
Aggression can be understood as an adaptive strategy to access and secure resources. Once a threat is detected in one’s environment, key neural populations are recruited to drive an aggressive response (for review, see Lischinsky and Lin, 2020). In particular, the posterior dorsal subdivision of the MeA (MeApd) is a central node in controlling antisocial behaviors in rodents; activation of the GABAergic population drives aggressive and attacking behaviors, while activation of the glutamatergic population drives asocial and self-grooming behaviors (Hong et al., 2014). Additionally, activation of the ventrolateral portion of the VMHvl, which receives projections from the MeA (Pardo-Bellver et al., 2012), produces attacking behavior in both male and female mice (Hashikawa et al., 2017; Lee et al., 2014; Lin et al., 2011). Estrogen receptor alpha (Esr1)-expressing neurons in the posterior amygdala (PA) projecting to the VMHvl are active in intermale aggression, and chemogenetic inhibition of this projection reduces intermale attack duration (Yamaguchi et al., 2020). Glutamatergic VMHvl neurons also project to glutamatergic neurons in the lateral periaqueductal gray (lPAG), which then project to musculature in the jaw to initiate biting; attack and social investigation modulate the activity of VMHvl glutamatergic neurons, while only attack modulates lPAG glutamatergic activity, and inactivation of the VMHvlvGlut2-lPAGvGlut2 projection results in reduced aggression (Falkner et al., 2020).
Neuromodulation may be responsible for producing chronic isolation-induced aggression, as evidenced by conserved mechanisms in different species. Cholecystokinin (CCK) is a neuropeptide that is implicated in negative affective states and disease, such as anxiety-like states and panic disorders, in both rodents and in humans (Rehfeld, 2000; Singh et al., 1991). Singly housed male rats that display aggressive behaviors during a resident-intruder task demonstrate increased CCK concentration in the posterior cortex and tegmentum compared with isolated rats who did not display aggressive behaviors (Panksepp et al., 2004). Interestingly, isolated (4 days) Drosophila display downregulation of the neuropeptide drosulfakinin (Dsk), the homolog of the mammalian CCK (Agrawal et al., 2020). Both upregulation and downregulation of Dsk in Drosophila increase isolation-driven aggressive behavior, indicating that Dsk balance in the Drosophila nervous system plays a critical role in the production of isolation-induced antisocial behavior. Intriguingly, overexpression of tachykinin, but not Dsk activation, is sufficient in producing aggressive behaviors in group-housed Drosophila (Asahina et al., 2014), suggesting that separate mechanisms may exist in the Drosophila central nervous system in the generation of aggressive behaviors depending on the social context.
TEMPORAL DYNAMICS OF SOCIAL HOMEOSTASIS (ACUTE VERSUS CHRONIC ISOLATION)
Although we use the terms “acute” and “chronic” to discretize the qualitative phenomenological divergence of consequences from social isolation of different durations, the reference to temporal dynamics is relative rather than absolute. Indeed, the phenomena described related to the diametrically opposed behavioral responses of acute versus chronic social isolation may be mechanistically linked to a parameter other than time, including effort expended, number of rejected attempts, and social consequences to effector-system-activated behaviors.
Because of the increased relevance of the societal impact of social isolation, we focus our discussion on the health consequences of acute and chronic social isolation below.
Consequences of acute isolation
Physiological health—hypervigilance/arousal, stress, immune response
Isolation from the safety of a social group necessitates modifications in strategy to promote survival. While these strategical changes may prove adaptive in the short term, to the detriment of the isolated individual, the unintended consequences of acute social isolation are often maladaptive.
The immune system is a target for functional adaptation that results from social isolation. Upon social isolation, pro-inflammatory interleukin (IL) genes (such as IL-1B and IL-8) are upregulated, while type I interferon genes associated with antiviral response (such as interferon-stimulated genes and interferon γ-inducible protein family genes) along with antibody production genes are downregulated (Cole, 2014; Cole et al., 2007, 2011). It is hypothesized that this transition occurs to protect the individual from the dangers of being alone (i.e., bacterial infections from physical injury) while reducing the need for protection against viral infections that are normally socially transmitted.
Another major target of physiological adaptations in response to isolation is the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis acts as a central stress response system, such that when a stressor is detected, a neuroendocrine response is activated whereby the PVN releases CRF into the bloodstream, which then binds to CRF receptors on the anterior pituitary gland and results in adrenocorticotropic hormone (ACTH) release (Chrousos, 2009; Deussing and Chen, 2018; Henckens et al., 2016). ACTH then binds to receptors on the adrenal gland, which results in the release of cortisol. The HPA axis is regulated through a negative feedback loop, such that sufficient cortisol concentrations in the bloodstream will inhibit the release of CRF from the hypothalamus and ACTH from the pituitary gland (Deussing and Chen, 2018; Ramot et al., 2017). While acute HPA axis activation may seem adaptive in that it provides a short-term physiological reaction to an environmental stressor, accumulation of cortisol spikes is maladaptive and can cause physiological “wear and tear” (see Allostatic load and alternative models), much less will it assist the individual in returning to social homeostasis. Pair-bonded prairie voles, male mice, and marmosets all exhibit increased cortisol levels following 12 h to 5 days of social isolation, suggesting that the HPA axis is sensitive to acute social isolation (Bosch et al., 2009; McNeal et al., 2014; Rukstalis and French, 2005; Sun et al., 2014; Takatsu-Coleman et al., 2013; Taylor et al., 2014). Additionally, acutely isolated (<24 h) female, but not male, mice demonstrate changes in intrinsic properties of PVN-CRF neurons, including an increase in first spike latency and decreased excitability (Senst et al., 2016). Notably, acute (3 h) crowding in adult male rats also results in an increase of plasma corticosterone (Djordjevi et al., 2003), suggesting that there may be an interaction between deviations in social homeostasis and the central stress response.
In addition to the physiological responses to acute social isolation, changes occur in the central nervous system potentially to guide defensive behavior and protect the isolated individual from environmental threats. Perceived loneliness and objective social isolation produce a state of hypervigilance and heightened arousal, likely an evolutionary feature to detect and protect oneself from environmental threats that may arise from isolation (Cacioppo et al., 2006b). While meaningful for the purposes of threat detection, hypervigilance is not adaptive for social homeostasis, as it does not assist in the maintenance of social needs. Interestingly, individuals who identify as lonely attend more quickly to social threats than those who do not (Cacioppo et al., 2016). Increase in hypervigilance may in part be due to isolation-induced HPA axis activation. Additionally, acute social isolation may modify the noradrenergic system. Noradrenaline is broadly known for its role in increasing arousal and vigilance and is produced in the locus coeruleus (Berridge and Waterhouse, 2003; Sara and Bouret, 2012). In adult male rats, 24 h of social isolation upregulates tyrosine hydroxylase—the rate-limiting enzyme in noradrenaline production—transcription in the locus coeruleus, and antagonizing angiotensin II receptor type 1 (AT1) receptors negates the increase in tyrosine hydroxylase (TH) in the locus coeruleus (Saavedra et al., 2006).
Consequences of chronic isolation
Chronic social isolation (which refers to a relative timescale that may vary with species, context, individual, and experience) results in a number of changes, both arguably adaptive and maladaptive, in an individual’s biology and behavior. In this section, we discuss the physiological, mental, and behavioral consequences of chronic social isolation.
Physiological
Dysregulation of the HPA axis due to chronic activation results in allostatic load (McEwen, 1998), which describes the cumulative physiological effects of chronic cortisol elevation. Allostatic load is known to result in elevated risk of cardiovascular disease, immune system dysregulation, and cognitive decline through corticosterone binding in the hippocampus (McEwen, 1998). The direction of HPA axis activity resulting from chronic social isolation is highly variable in social organisms. In humans, young and older adults with small social circles and who identify as lonely generally exhibit more chronic activation of HPA axis than those who do not (Arnetz et al., 1983; Pressman et al., 2005). While transient activation of HPA axis can be acutely adaptive to meet the energy demands necessary for overcoming a stressor in an environment, chronic activation, resulting in allostatic load, can cause many adverse physiological effects (Deussing and Chen, 2018; Lee et al., 2015). Chronic social isolation also increases the risk of developing obesity and type 2 diabetes in mice (Nonogaki et al., 2007) and increases the risk of spontaneous, malignant mammary tumor development in rats (Hermes et al., 2009).
Mental health
Chronic social isolation in both youth and adulthood can result in changes in biological changes in the central nervous system as well as deleterious changes in behavior, often to the detriment of mental health. Although much of this review is focused on the effects of social isolation on adults, there exists extensive literature on the effects of early-life social deprivation on the learning of social reward (Nardou et al., 2019) and also as developmental models for early-life, stress-induced social dysfunction (Haller et al., 2014; Shin et al., 2018) and schizophrenia (Fone and Porkess, 2008; Lapiz et al., 2003) in rodents. Perhaps one of the most well-known studies on early-life social deprivation is that of Harry Harlow, who showed that early social deprivation resulted in social dysfunction later in life (Harlow and Suomi, 1971). A recent study in mice shows that social reward learning occurs through oxytocin-mediated plasticity in the NAc during a critical window of development (peaking at postnatal day 48) (Nardou et al., 2019), suggesting a developmental role in determining the rewarding value of social interactions. Isolation during the post-weaning period also impairs cognitive function, resulting in long-term social memory dysfunction due to downregulation of ephrin type-B receptor 2 (EphB2) in hippocampal CA1 neurons (Wu et al., 2020). Altogether, the mental health consequences of chronic isolation during youth prove severely detrimental and are unique from those observed in chronically isolated adults.
Chronic social isolation in adulthood also results in many adverse effects in the brain and in behavior. Social isolation (both 1 and 7 days) impairs long-term social recognition memory in mice through elevated Ras-related C3 botulinum toxinn substrate 1 (Rac1) activity in the hippocampus (Liu et al., 2018). Rac1 is a GTPase that has been shown to play a role in active forgetting in Drosophila, mice, and rats (Jiang et al., 2016; Liu et al., 2016; Shuai et al., 2010), suggesting a conserved function across species. Additionally, in adult male rats, prolonged social isolation (10–14 weeks) results in sexual behavior deficits, increased anxiety-like behavior, and reduction of transcription factor cyclic AMP (cAMP) receptor element-binding protein (CREB) expression in the shell region of the NAc (Barrot et al., 2005). Notably, these behavioral deficits are rescued through overexpression of CREB in NAc shell. Chronic social isolation also results in anhedonic behaviors, as demonstrated by less sucrose intake and preference, suggesting that chronic social isolation impacts reward processing (Wallace et al., 2009). While overexpression of CREB rescues anxiety-like behavior, it does not rescue anhedonia-related behaviors. Interestingly, chronically isolated (4 weeks) female, but not male, prairie voles also display anhedonia, suggesting that the behavioral effects of chronic social isolation show variability across species and sex (Grippo et al., 2007). Increased resting heart rate, anhedonia, and increased immobility time during forced swim test (indicative of despair observed in depressive-like behaviors) observed in socially isolated female prairie voles are all rescued by subcutaneous injection of oxytocin (Grippo et al., 2009).
Consequences of perceived loneliness (humans)
Loneliness is a ubiquitous condition that most humans have either indirectly or directly experienced, such as through interactions with an isolated elderly relative or through the more recent global lockdowns to contain the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak. In humans, perceived deficits in the objective quantity, or subjective quality of social contact (referred to as “loneliness” in psychology [Weiss, 1973]), is correlated with deficits in mental (Hawkley et al., 2006) and physical (Hawkley and Cacioppo, 2010; Hawkley et al., 2006) health and higher mortality rates (Berkman and Syme, 1979; Holt-Lunstad et al., 2010; Holwerda et al., 2012; Perissinotto et al., 2012; Steptoe et al., 2013). Interestingly, perceived loneliness reportedly increases functional communication within the “default network” (regions including the prefrontal cortex that are typically active during wakeful rest), suggesting that rumination may be used to fill a given social void (Spreng et al., 2020). Perceived loneliness correlates with increased morbidity and mortality with diseases such as cancer and cardiovascular disease (Hawkley and Cacioppo, 2003), and most noteworthy perceived loneliness is correlated with the severity of symptoms reported in response to a viral immune challenge (LeRoy et al., 2017), as well as inflammatory responses (Balter et al., 2019). Moreover, on average, humans perceive lonely individuals as having lower achievement and lower social skills and as being less liked and attractive, with lonely male individuals being more stigmatized than lonely female individuals (Lau and Gruen, 1992). The stigmatization surrounding loneliness may preclude support to those who need it, further reinforcing loneliness.
Triggers/parameters that govern the shift
The neurobiological triggers and parameters that regulate the transition that occurs between acute social isolation (which induces prosocial behavior) and chronic social isolation (which induces antisocial behavior) are largely shrouded in mystery, but we do have a few potential clues. Notably, a major shift occurs such that an active coping strategy (prosocial, affiliative behaviors) turns into a passive coping strategy (antisocial behaviors and reduced effort to seek social contact) prior to reintroduction to a social group. In adult mice, there is an increase in aggressive behaviors that occurs after 48 h, but not 24 h, of social isolation (Lister and Hilakivi, 1988), and aggressive behaviors in socially isolated adult mice gradually increase for the first few weeks and plateau at ~4 weeks of social isolation (Matsumoto et al., 2005). However, in adult rats, there is an increase in affiliative behavior that lasts up to 7 days following social isolation (Niesink and van Ree, 1982), suggesting that the time course for the transition from prosocial to antisocial behavior is heterogeneous and depends on the species of the animal. Siamese fighting fish, for instance, demonstrate a much quicker transition, displaying aggressive behaviors soon after social isolation (as early as 15 min) (Gómez-Laplaza and Morgan, 2000; Clayton and Hinde, 1968).
What mechanism underlies the transition of behaviors that occurs from acute to chronic social isolation? A recent study shows that, in mice, chronic (2 week) social isolation results in brain-wide upregulation of tachykinin 2 (Tac2) and neurokinin B (NkB), which endogenously binds to the G-protein-coupled neurokinin 3 (NK3) receptor (NK3R) (Zelikowsky et al., 2018). Tachykinin activation and silencing in Drosophila have previously been shown to modulate intermale aggressive behaviors (Asahina et al., 2014). Targeted NK3R antagonism specifically in the dorsomedial hypothalamus (DMH) mitigates chronic isolation-induced aggression in mice, whereas NK3R antagonism in central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) mitigates other aspects of chronic isolation-induced behaviors, such as fear expression (Zelikowsky et al., 2018). Interestingly, knocking out Avpr1b in mice reduces chronic (2 week) isolation-induced aggression and is rescued by expressing Avpr1b into dCA2, suggesting that vasopressinergic signaling in dCA2 may be involved in the transition from affiliative to antisocial behaviors resulting from chronic social isolation as well (Pagani et al., 2015).
We postulate that the transition occurs due to either (1) time or (2) correction effort by the social homeostatic effector system (Figure 2A). Perhaps there is a time-keeping component of the neural circuitry that approximates the duration of time to identify that a transient environmental change is actually long-lasting enough to trigger a neuroadaptation. Alternatively, rather than the trigger being dictated by external signals, the transition from acute to chronic isolation behaviors may be triggered by a threshold of effort exerted to correct the surplus or deficit. Future experiments are needed to differentiate between these possibilities.
Considering that many of the systems that drive motivated social behavior are mechanistically performed through neuromodulation, it is plausible that chronic activation of neuromodulator systems to correct for social deficits may ultimately trigger the transition from acute to chronic isolation. Many examples exist of neuromodulator plasticity induced by aversive experiences. For instance, adult male mice who undergo a social defeat paradigm and are susceptible to social subordination exhibit changes in mesolimbic dopamine system, including increased firing rate of VTA dopamine neurons and increased brain-derived neurotrophic factor (BDNF) signaling in the NAc, inducing plasticity in the VTA-NAc circuit that is involved in emotion and reward processing (Krishnan et al., 2007). Additionally, while stimulation of VTA-NAc in adult female mice promotes social behavior (Gunaydin et al., 2014), stimulation of the VTA-NAc circuit following recent or remote stress produces an antisocial effect and increases optically induced dopamine release in the NAc (Wichmann et al., 2017), suggesting that stressors can induce circuit plasticity that ultimately changes behavioral output. Given that acute social isolation increases the activity of DRN dopamine neurons in response to social stimuli (Matthews et al., 2016), it is tempting to speculate that the chronic homeostatic correction effort expended by the DRN dopamine neurons may indeed induce downstream plasticity that is ultimately responsible for the shift from prosocial to antisocial behaviors.
Allostatic load and alternative models
In addition to considering the immediate social challenge (a deficit or surplus), it may be important to consider the history of challenges as well as the duration of the challenge. An important model that may extend a social homeostatic model is the contribution of increasing allostatic load as being the “trigger” to the negative health consequences of social isolation that resemble those of chronic stress-induced disease states linked to allostatic load (e.g., increased depression, increased morbidity, and mortality of cancer and heart disease) (Cacioppo and Hawkley, 2003; Juster et al., 2010; Karamihalev et al., 2020). An allostatic load model may also be relevant when considering the microstructure of homeostatic set-point adaptation (Lee et al., 2015; Sandi and Haller, 2015).
An alternative model would be to consider social challenges as a subset of stressors, and to contribute to general HPA axis, as discussed above. It is yet unclear to what degree stressors accumulate along specific dimensions (where social challenges would be detected separately from other stress challenges) or whether they are aggregated irrespective of the modality or dimension.
OUTLOOK
Amidst a global pandemic with unpredictable lockdowns and varying quarantine guidelines around the world, no other time in recent history has compelled the urgent need to interrogate the neural mechanisms of social homeostasis more than now. Efforts to contain the SARS-CoV-2 outbreak demand urgent need in parallel with further research on the unintended health consequences of social isolation. At present, existing frameworks from other homeostatic systems and recent findings in socially isolated animals allow us an entry point to conceptualize the neural circuits and mechanisms underlying social homeostasis (Figure 3), although much work is left in fully understanding this system. Considering pandemics and other socially disruptive events are likely to occur again, proactive research into the costs of social isolation can mitigate the effects of an uncertain future.
Figure 3. Neural circuits underlying homeostatic nodes.
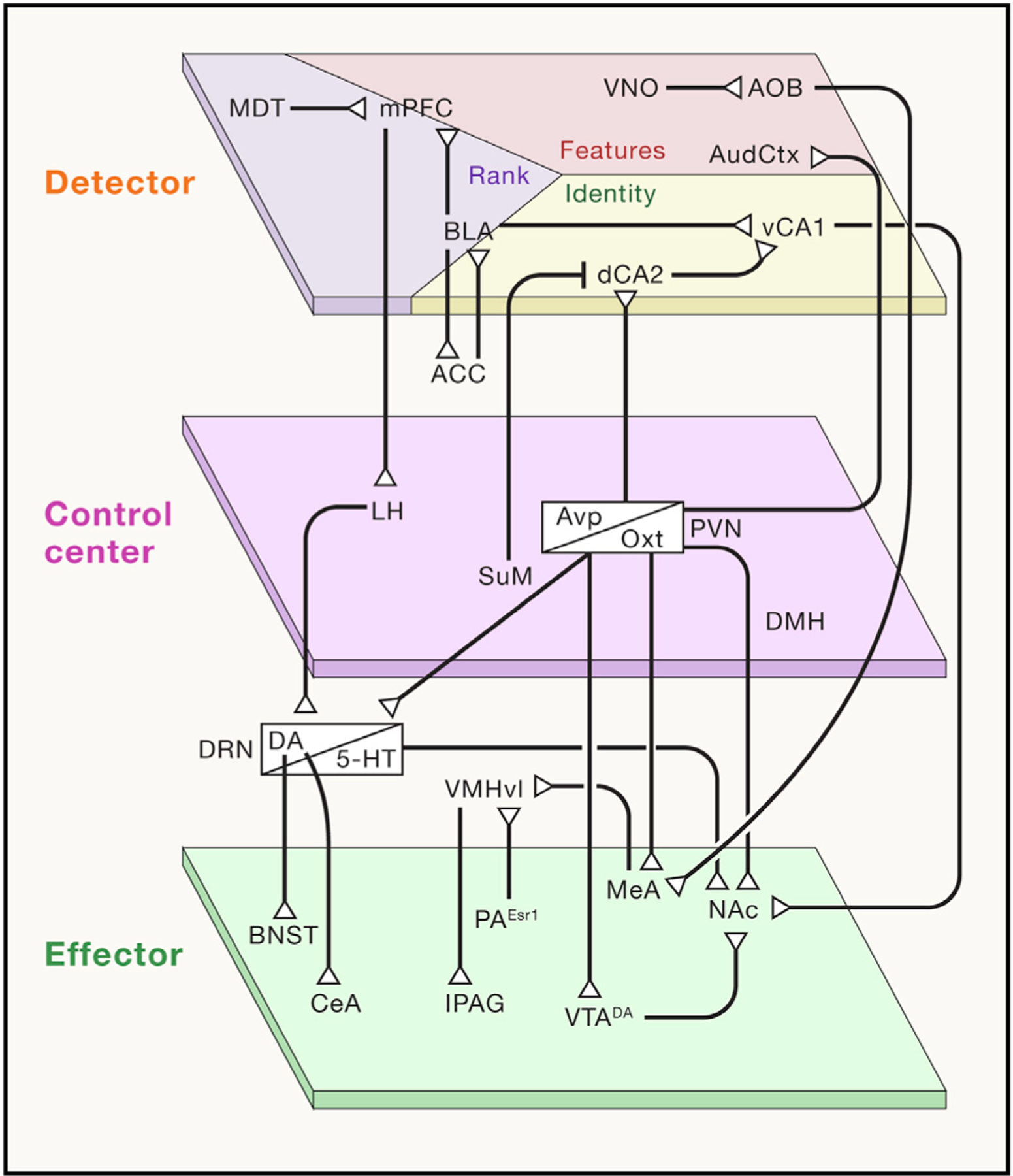
Proposed neural circuits involved in each social homeostatic node. Note: we acknowledge that there are dynamic states that allow flexibility in the positioning of each of the regions and circuits that we speculate to be functionally representing each node of the social homeostatic system. We also acknowledge that there will likely be functional heterogeneity among neurons in any brain region and that many functions are indeed distributed across both local and long-range circuits. ACC, anterior cingulate cortex; AOB, accessory olfactory bulb; AudCtx, auditory cortex; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; dCA2, dorsal hippocampal CA2; DMH, dorsomedial hypothalamus; DRN, dorsal raphe nucleus (DA: dopamine; 5-HT: serotonin); LH, lateral hypothalamus; lPAG, lateral periaqueductal gray; MDT, mediodorsal thalamus; MeA, medial amygdala; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; PA, posterior amygdala (Esr1: estrogen 1 receptor); PVN, paraventricular nucleus of the hypothalamus (Oxt: oxytocin; Avp: vasopressin); SuM, supramammillary nucleus; vCA1, ventral hippocampal CA1; VMHvl, ventrolateral portion of the ventromedial hypothalamus; VNO, vomeronasal organ; VTA, ventral tegmental area (DA).
ACKNOWLEDGMENTS
K.M.T. is the Wylie Vale Chair at Salk Institute for Biological Studies, a New York Stem Cell Foundation-Robertson Investigator, and McKnight Scholar. This work was supported by funding from the JPB Foundation, the PIIF, PNDRF, JFDP, Alfred P. Sloan Foundation, New York Stem Cell Foundation, Klingenstein Foundation, McKnight Foundation, Clayton Foundation, Dolby Family Fund, R01-MH102441 (NIMH), R01-MH115920 (NIMH), RF1-AG047661 (NIA), the NIH Director’s New Innovator Award DP2-DK102256 (NIDDK), and Pioneer Award DP1-AT009925 (NCCIH). C.R.L. is supported by a fellowship from the University of California, San Diego, Neurosciences Graduate Program.
REFERENCES
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, Anderson A, Lee GP, and Damasio AR (1999). Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia 37, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Kao D, Chung P, and Looger LL (2020). The neuropeptide Drosulfakinin regulates social isolation-induced aggression in Drosophila. J. Exp. Biol 223, jeb207407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang C-J, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, et al. (2018). Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell 173, 1329–1342.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anpilov S, Shemesh Y, Eren N, Harony-Nicolas H, Benjamin A, Dine J, Oliveira VEM, Forkosh O, Karamihalev S, Hüttl R-E, et al. (2020). Wireless Optogenetic Stimulation of Oxytocin Neurons in a Semi-natural Setup Dynamically Elevates Both Pro-social and Agonistic Behaviors. Neuron 107, 644–655.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnetz BB, Theorell T, Levi L, Kallner A, and Eneroth P (1983). An experimental study of social isolation of elderly people: psychoendocrine and metabolic effects. Psychosom. Med 45, 395–406. [DOI] [PubMed] [Google Scholar]
- Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, González CR, Eyjólfsdóttir EA, Perona P, and Anderson DJ (2014). Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 156, 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine V, Gokce SK, Lee S, Wang B, Davidson TJ, Reimann F, Gribble F, Deisseroth K, Lois C, and Oka Y (2018). Hierarchical neural architecture underlying thirst regulation. Nature 555, 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, et al. (2019). Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 179, 1129–1143.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balter LJT, Raymond JE, Aldred S, Drayson MT, Veldhuijzen van Zanten JJCS, Higgs S, and Bosch JA (2019). Loneliness in healthy young adults predicts inflammatory responsiveness to a mild immune challenge in vivo. Brain Behav. Immun 82, 298–301. [DOI] [PubMed] [Google Scholar]
- Barrot M, Wallace DL, Bolaños CA, Graham DL, Perrotti LI, Neve RL, Chambliss H, Yin JC, and Nestler EJ (2005). Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 102, 8357–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Decety J, and Mason P (2011). Empathy and pro-social behavior in rats. Science 334, 1427–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, and Mason P (2014). Pro-social behavior in rats is modulated by social experience. eLife 3, e01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, and Syme SL (1979). Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am. J. Epidemiol 109, 186–204. [DOI] [PubMed] [Google Scholar]
- Berridge CW, and Waterhouse BD (2003). The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev 42, 33–84. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZFH, Ritola KD, and Sternson SM (2013). Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC (1961). The interaction of hunger and thirst in the rat. J. Comp. Physiol. Psychol 54, 580–584. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, and Young LJ (2009). The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34, 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Brüning JC, and Krashes MJ (2016). Hunger-Driven Motivational State Competition. Neuron 92, 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Funderburk SC, Navarrete J, Sabol A, Liang-Guallpa J, Desrochers TM, and Krashes MJ (2019). Need-based prioritization of behavior. eLife 8, e44527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MJ, Rolls ET, and Mora F (1976). Effects of hunger on the responses of neurons in the lateral hypothalamus to the sight and taste of food. Exp. Neurol 51, 668–677. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, and Hawkley LC (2003). Social isolation and health, with an emphasis on underlying mechanisms. Perspect. Biol. Med 46 (3, Suppl), S39–S52. [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, and Thisted RA (2006a). Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol. Aging 21, 140–151. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, and Spiegel D (2006b). Loneliness within a nomological net: An evolutionary perspective. J. Res. Pers 40, 1054–1085. [Google Scholar]
- Cacioppo S, Bangee M, Balogh S, Cardenas-Iniguez C, Qualter P, and Cacioppo JT (2016). Loneliness and implicit attention to social threat: A high-performance electrical neuroimaging study. Cogn. Neurosci 7, 138–159. [DOI] [PubMed] [Google Scholar]
- Cannon WB (1929). Organization for Physiological Homeostasis. Physiol. Rev 9, 399–431. [Google Scholar]
- Chen P, and Hong W (2018). Neural Circuit Mechanisms of Social Behavior. Neuron 98, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, He L, Huang AJY, Boehringer R, Robert V, Wintzer ME, Polygalov D, Weitemier AZ, Tao Y, Gu M, et al. (2020). A hypothalamic novelty signal modulates hippocampal memory. Nature 586, 270–274. [DOI] [PubMed] [Google Scholar]
- Cho JR, Treweek JB, Robinson JE, Xiao C, Bremner LR, Greenbaum A, and Gradinaru V (2017). Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron 94, 1205–1219.e8. [DOI] [PubMed] [Google Scholar]
- Christian JJ (1961). Phenomena associated with population density. Proc. Natl. Acad. Sci. USA 47, 428–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JJ (1970). Social subordination, population density, and mammalian evolution. Science 168, 84–90. [DOI] [PubMed] [Google Scholar]
- Chrousos GP (2009). Stress and disorders of the stress system. Nat. Rev. Endocrinol 5, 374–381. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Coquelin A, Macrides F, Gorski RA, and Noble EP (1984). Sexual behavior and aggression in male mice: involvement of the vomeronasal system. J. Neurosci 4, 2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton FL, and Hinde RA (1968). The habituation and recovery of aggressive display in Betta splendens. Behaviour 30, 96–106. [DOI] [PubMed] [Google Scholar]
- Cole SW (2014). Human Social Genomics. PLOS Genetics 10, e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, and Cacioppo JT (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. PNAS 108, 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, and Cacioppo JT (2007). Social regulation of gene expression in human leukocytes. Genome Biol 8, R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S, MarushaCreel N, and Monfort SL (1996). Social stress and dominance. Nature 379, 212. [Google Scholar]
- Cui Z, Gerfen CR, and Young WS 3rd. (2013). Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol 521, 1844–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva S, Schmid V, and Wittemyer G (2017). Fission fusion processes weaken dominance networks of female Asian elephants in a productive habitat. Behav. Ecol 28, 243–252. [Google Scholar]
- Deussing JM, and Chen A (2018). The Corticotropin-Releasing Factor Family: Physiology of the Stress Response. Physiol. Rev 98, 2225–2286. [DOI] [PubMed] [Google Scholar]
- Djordjevi J, Cviji G, and Davidovi V (2003). Different Activation of ACTH and Corticosterone Release in Response to Various Stressors in Rats. Physiol. Res 52, 67–72. [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, and Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougalis AG, Matthews GAC, Bishop MW, Brischoux F, Kobayashi K, and Ungless MA (2012). Functional properties of dopamine neurons and co-expression of vasoactive intestinal polypeptide in the dorsal raphe nucleus and ventro-lateral periaqueductal grey. Eur. J. Neurosci 36, 3322–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Alexander GM, and Farris S (2016). Rediscovering area CA2: unique properties and functions. Nat. Rev. Neurosci 17, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, and Wu Z (2014). Neural control of maternal and paternal behaviors. Science 345, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G (2005). Infant rodent ultrasounds – a gate to the understanding of sound communication. Behav. Genet 35, 19–29. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, and Miller CT (2017). Marmoset vocal communication: Behavior and neurobiology. Dev. Neurobiol 77, 286–299. [DOI] [PubMed] [Google Scholar]
- Evans GW, Lepore SJ, Shejwal BR, and Palsane MN (1998). Chronic residential crowding and children’s well-being: an ecological perspective. Child Dev 69, 1514–1523. [PubMed] [Google Scholar]
- Evans AL, Singh NJ, Friebe A, Arnemo JM, Laske TG, Fröbert O, Swenson JE, and Blanc S (2016). Drivers of hibernation in the brown bear. Front. Zool 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Wei D, Song A, Watsek LW, Chen I, Chen P, Feng JE, and Lin D (2020). Hierarchical Representations of Aggression in a Hypothalamic-Midbrain Circuit. Neuron 106, 637–648.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, and Tye KM (2014). Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci 34, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, and Tye KM (2016). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero DM, Moeller LM, Osakada T, Horio N, Li Q, Roy DS, Cichy A, Spehr M, Touhara K, and Liberles SD (2013). A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature 502, 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Kitchen DM, Seyfarth RM, and Cheney DL (2004). Baboon loud calls advertise male quality: acoustic features and their relation to rank, age, and exhaustion. Behav. Ecol. Sociobiol 56, 140–148. [Google Scholar]
- Flanigan ME, Aleyasin H, Li L, Burnett CJ, Chan KL, LeClair KB, Lucas EK, Matikainen-Ankney B, Durand-de Cuttoli R, Takahashi A, et al. (2020). Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice. Nature Neuroscience 23, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KCF, and Porkess MV (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev 32, 1087–1102. [DOI] [PubMed] [Google Scholar]
- Forkosh O, Karamihalev S, Roeh S, Alon U, Anpilov S, Touma C, Nussbaumer M, Flachskamm C, Kaplick PM, Shemesh Y, and Chen A (2019). Identity domains capture individual differences from across the behavioral repertoire. Nat. Neurosci 22, 2023–2028. [DOI] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY, and Livingstone MS (2009). A face feature space in the macaque temporal lobe. Nat. Neurosci 12, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, Jefferis V, and Knowles M (2005). On the outside looking in: loneliness and social monitoring. Pers. Soc. Psychol. Bull 31, 1549–1560. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, and Robbins TW (1993). Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol. Psychiatry 34, 361–372. [DOI] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, et al. (2016). Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Laplaza LM, and Morgan E (2000). Laboratory studies of the effects of short-term isolation on aggressive behaviour in fish. Mar. Freshwat. Behav. Physiol 33, 63–102. [Google Scholar]
- Gove WR, Hughes M, and Galle OR (1979). Overcrowding in the home: an empirical investigation of its possible pathological consequences. Am. Sociol. Rev 44, 59–80. [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, and Carter CS (2007). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32, 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR 2nd, Porges SW, and Carter CS (2009). Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology 34, 1542–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groessl F, Munsch T, Meis S, Griessner J, Kaczanowska J, Pliota P, Kargl D, Badurek S, Kraitsy K, Rassoulpour A, et al. (2018). Dorsal tegmental dopamine neurons gate associative learning of fear. Nat. Neurosci 21, 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, and Yu CR (2014). Integrated action of pheromone signals in promoting courtship behavior in male mice. eLife 3, e03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Harold G, Sandi C, and Neumann ID (2014). Effects of Adverse Early-Life Events on Aggression and Anti-Social Behaviours in Animals and Humans. Journal of Neuroendocrinology 26, 724–738. [DOI] [PubMed] [Google Scholar]
- Harlow HF, and Suomi SJ (1971). Social recovery by isolation-reared monkeys. Proc. Natl. Acad. Sci. USA 68, 1534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Falkner A, and Lin D (2016). The neural circuits of mating and fighting in male mice. Curr. Opin. Neurobiol 38, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, and Lin D (2017). Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci 20, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasue RH, and Shammah-Lagnado SJ (2002). Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J. Comp. Neurol 454, 15–33. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, and Cacioppo JT (2003). Loneliness and pathways to disease. Brain Behav. Immun 17 (Suppl 1), S98–S105. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, and Cacioppo JT (2010). Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med 40, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, and Cacioppo JT (2006). Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol. Aging 21, 152–164. [DOI] [PubMed] [Google Scholar]
- Henckens MJ, Deussing JM, and Chen A (2016). Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat. Rev. Neurosci 17, 636–651. [DOI] [PubMed] [Google Scholar]
- Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, and McClintock MK (2009). Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc. Natl. Acad. Sci. USA 106, 22393–22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, and Siegelbaum SA (2014). The hippocampal CA2 region is essential for social memory. Nature 508, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra HE, and Coyne JA (2007). The locus of evolution: evo devo and the genetics of adaptation. Evolution 61, 995–1016. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, and Layton JB (2010). Social relationships and mortality risk: a meta-analytic review. PLoS Med 7, e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda TJ, Beekman ATF, Deeg DJH, Stek ML, van Tilburg TG, Visser PJ, Schmand B, Jonker C, and Schoevers RA (2012). Increased risk of mortality associated with social isolation in older men: only when feeling lonely? Results from the Amsterdam Study of the Elderly (AMSTEL). Psychol. Med 42, 843–853. [DOI] [PubMed] [Google Scholar]
- Hong W, Kim D-W, and Anderson DJ (2014). Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158, 1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XH, Hyun M, Taranda J, Huang KW, Todd E, Feng D, Atwater E, Croney D, Zeidel ML, Osten P, and Sabatini BL (2016). Central Control Circuit for Context-Dependent Micturition. Cell 167, 73–86.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko T (2006). In the Cellars of the Hollow Men: Use of Solitary Confinement in U.S. Prisons and Its Implications under International Laws against Torture. Pace Int. Law Rev 18, 1. [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dölen G, and Malenka RC (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mao R, Zhou Q, Yang Y, Cao J, Ding Y, Yang Y, Zhang X, Li L, and Xu L (2016). Inhibition of Rac1 Activity in the Hippocampus Impairs the Forgetting of Contextual Fear Memory. Mol. Neurobiol 53, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Jouventin P, Lengagne T, and Aubin T (1999). Finding One’s Mate in a King Penguin Colony: Efficiency of Acoustic Communication. Behaviour 136, 833–846. [DOI] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, and Lupien SJ (2010). Allostatic load bio-markers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev 35, 2–16. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, and Chun MM (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamihalev S, Brivio E, Flachskamm C, Stoffel R, Schmidt MV, and Chen A (2020). Social dominance mediates behavioral adaptation to chronic stress in a sex-specific manner. eLife 9, e58723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AW, Ackels T, Kuo T-H, Cichy A, Dey S, Hays C, Kateri M, Logan DW, Marton TF, Spehr M, and Stowers L (2014). Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell 157, 676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury L, Huang S, Wang J, Gu K, Golshani P, Wu YE, and Hong W (2019). Correlated Neural Activity and Encoding of Behavior across Brains of Socially Interacting Animals. Cell 178, 429–446.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury L, Huang S, Raam T, Ye LS, Wei D, Hu RK, Ye L, and Hong W (2020). Cortical Representations of Conspecific Sex Shape Social Behavior. Neuron 107, 941–953.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, and Dulac C (2018). Neural control of parental behaviors. Curr. Opin. Neurobiol 49, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn ML, and Clausen JA (1955). Social Isolation and Schizophrenia. Am. Sociol. Rev 20, 265–273. [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Fulford A, Muchimapura S, Mason R, Parker T, and Marsden CA (2003). Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci. Behav. Physiol 33, 13–29. [DOI] [PubMed] [Google Scholar]
- Lau S, and Gruen GE (1992). The Social Stigma of Loneliness: Effect of Target Person’s and Perceiver’s Sex. Pers. Soc. Psychol. Bull 18, 182–189. [Google Scholar]
- Lee H, Kim D-W, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, and Anderson DJ (2014). Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Kim E, and Choi MH (2015). Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep 48, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy AS, Murdock KW, Jaremka LM, Loya A, and Fagundes CP (2017). Loneliness predicts self-reported cold symptoms after a viral challenge. Health Psychol 36, 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, and Axel R (2002). Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. USA 99, 6376–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, and Young LJ (2004). Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, and Anderson DJ (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E-JD, Sun M, Choi EY, Magee D, Stets CW, and During MJ (2015). Social overcrowding as a chronic stress model that increases adiposity in mice. Psychoneuroendocrinology 51, 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischinsky JE, and Lin D (2020). Neural mechanisms of aggression across species. Nat. Neurosci 23, 1317–1328. [DOI] [PubMed] [Google Scholar]
- Lister RG, and Hilakivi LA (1988). The effects of novelty, isolation, light and ethanol on the social behavior of mice. Psychopharmacology (Berl.) 96, 181–187. [DOI] [PubMed] [Google Scholar]
- Liu W, Liang X, Gong J, Yang Z, Zhang Y-H, Zhang J-X, and Rao Y (2011). Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat. Neurosci 14, 896–902. [DOI] [PubMed] [Google Scholar]
- Liu Y, Du S, Lv L, Lei B, Shi W, Tang Y, Wang L, and Zhong Y (2016). Hippocampal Activation of Rac1 Regulates the Forgetting of Object Recognition Memory. Curr. Biol 26, 2351–2357. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lv L, Wang L, and Zhong Y (2018). Social Isolation Induces Rac1-Dependent Forgetting of Social Memory. Cell Rep 25, 288–295.e3. [DOI] [PubMed] [Google Scholar]
- Loo C, and Ong P (1984). Crowding Perceptions, Attitudes, and Consequences among the Chinese. Environ. Behav 16, 55–87. [Google Scholar]
- Ma XC, Jiang D, Jiang WH, Wang F, Jia M, Wu J, Hashimoto K, Dang YH, and Gao CG (2011). Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PLoS ONE 6, e20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Maayan R, Weizman A, and Weller A (2006). Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav. Brain Res 175, 408–414. [DOI] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJ, and Trezza V (2016). Dopaminergic Neurotransmission in the Nucleus Accumbens Modulates Social Play Behavior in Rats. Neuropsychopharmacology 41, 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, and Froemke RC (2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow AH (1943a). A Theory of Human Motivation. Psychol. Rev 50, 370–396. [Google Scholar]
- Maslow AH (1943b). Preface to motivation theory. Psychosom. Med 5, 85–92. [Google Scholar]
- Matsumoto K, Pinna G, Puia G, Guidotti A, and Costa E (2005). Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress 8, 85–93. [DOI] [PubMed] [Google Scholar]
- Matthews GA, and Tye KM (2019). Neural mechanisms of social homeostasis. Ann. N Y Acad. Sci 1457, 5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, et al. (2016). Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 164, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. N. Engl. J. Med 338, 171–179. [DOI] [PubMed] [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, Larocca M, Trahanas DM, and Grippo AJ (2014). Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci 180, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meira T, Leroy F, Buss EW, Oliva A, Park J, and Siegelbaum SA (2018). A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun 9, 4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, and Wang X (2016). Marmosets: A Neuroscientific Model of Human Social Behavior. Neuron 90, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Inoue T, Lukáts B, Sakai K, Sasaki A, and Aou S (2007). Behavioral analysis of visual discrimination of sex in female macaque monkeys. Int. Congr. Ser 1301, 222–225. [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, and Crawley JN (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3, 287–302. [DOI] [PubMed] [Google Scholar]
- Moynihan MH (1970). Some Behavior Patterns of Platyrrhine Monkeys: II. Saguinus Geoffroyi and Some Other Tamarins, Volume 2 (Smithsonian Institution Press; ). [Google Scholar]
- Munuera J, Rigotti M, and Salzman CD (2018). Shared neural coding for social hierarchy and reward value in primate amygdala. Nat. Neurosci 21, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardou R, Lewis EM, Rothhaas R, Xu R, Yang A, Boyden E, and Dölen G (2019). Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 569, 116–120. [DOI] [PubMed] [Google Scholar]
- Niesink RJ, and van Ree JM (1982). Short-term isolation increases social interactions of male rats: a parametric analysis. Physiol. Behav 29, 819–825. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, and Oka Y (2007). Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology 148, 4658–4666. [DOI] [PubMed] [Google Scholar]
- Norcross JL, and Newman JD (1993). Context and gender-specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. Am. J. Primatol 30, 37–54. [DOI] [PubMed] [Google Scholar]
- Nummela SU, Jovanovic V, de la Mothe L, and Miller CT (2017). Social Context-Dependent Activity in Marmoset Frontal Cortex Populations during Natural Conversations. J. Neurosci 37, 7036–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Ye M, and Zuker CS (2015). Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 520, 349–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, and Tonegawa S (2016). Ventral CA1 neurons store social memory. Science 353, 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Coreano N, Batra K, Patarino M, Chen Z, Rock R, Zhang R, Hausmann S, Weddington J, Patel R, Zhang Y, et al. (2020). A cortical-hypothalamic circuit decodes social rank and promotes dominance behavior (In Review) 10.21203/rs.3.rs-94115/v1. [DOI]
- Pagani JH, Zhao M, Cui Z, Avram SK, Caruana DA, Dudek SM, and Young WS (2015). Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry 20, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, and Beatty WW (1980). Social deprivation and play in rats. Behav. Neural Biol 30, 197–206. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J, Beinfeld MC, Kroes RA, and Moskal JR (2004). Regional brain cholecystokinin changes as a function of friendly and aggressive social interactions in rats. Brain Res 1025, 75–84. [DOI] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cádiz-Moretti B, Novejarque A, Martínez-García F, and Lanuza E (2012). Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front. Neuroanat 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Reeder DM, and Judge PG (2015). Crowding increases salivary cortisol but not self-directed behavior in captive baboons. Am. J. Primatol 77, 462–467. [DOI] [PubMed] [Google Scholar]
- Peng X, Lang CM, Drozdowicz CK, and Ohlsson-Wilhelm BM (1989). Effect of cage population density on plasma corticosterone and peripheral lymphocyte populations of laboratory mice. Lab. Anim 23, 302–306. [DOI] [PubMed] [Google Scholar]
- Perissinotto CM, Stijacic Cenzer I, and Covinsky KE (2012). Loneliness in older persons: a predictor of functional decline and death. Arch. Intern. Med 172, 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, Gardner WL, and Knowles M (2004). Getting a cue: the need to belong and enhanced sensitivity to social cues. Pers. Soc. Psychol. Bull 30, 1095–1107. [DOI] [PubMed] [Google Scholar]
- Popova NK, and Naumenko EV (1972). Dominance relations and the pituitary-adrenal system in rats. Anim. Behav 20, 108–111. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, and Treanor JJ (2005). “Loneliness, social network size, and immune response to influenza vaccination in college freshman”: Correction to Pressman et al. (2005). Health Psychol 24, 348. [DOI] [PubMed] [Google Scholar]
- Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, and Chen A (2017). Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat. Neurosci 20, 385–388. [DOI] [PubMed] [Google Scholar]
- Regoeczi WC (2003). When context matters: a multilevel analysis of household and neighbourhood crowding on aggression and withdrawal. J. Environ. Psychol 23, 457–470. [Google Scholar]
- Rehfeld JF (2000). Cholecystokinin and panic disorder–three unsettled questions. Regul. Pept 93, 79–83. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Namboodiri VMK, Otis JM, Eckman LEH, Rodriguez-Romaguera J, Ung RL, Basiri ML, Kosyk O, Rossi MA, Dichter GS, and Stuber GD (2020). Social Stimuli Induce Activation of Oxytocin Neurons Within the Paraventricular Nucleus of the Hypothalamus to Promote Social Behavior in Male Mice. J. Neurosci 40, 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, and Hurst JL (2010). Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol 8, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Heien MLAV, and Wightman RM (2002). Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J. Neurosci 22, 10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeling TAP, Veening JG, Peters JPW, Vermelis MEJ, and Nieuwenhuys R (1993). Efferent connections of the hypothalamic “grooming area” in the rat. Neuroscience 56, 199–225. [DOI] [PubMed] [Google Scholar]
- Roeling TAP, Veening JG, Kruk MR, Peters JPW, Vermelis MEJ, and Nieuwenhuys R (1994). Efferent connections of the hypothalamic “aggression area” in the rat. Neuroscience 59, 1001–1024. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, and French JA (2005). Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm. Behav 47, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, and Sanchez-Lemus E (2006). A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology 31, 1123–1134. [DOI] [PubMed] [Google Scholar]
- Sandi C, and Haller J (2015). Stress and the social brain: behavioural effects and neurobiological mechanisms. Nature Reviews Neuroscience 16, 290–304. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (1990). A. E. Bennett Award paper. Adrenocortical function, social rank, and personality among wild baboons. Biol. Psychiatry 28, 862–878. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2005). The influence of social hierarchy on primate health. Science 308, 648–652. [DOI] [PubMed] [Google Scholar]
- Sara SJ, and Bouret S (2012). Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141. [DOI] [PubMed] [Google Scholar]
- Schiavo JK, Valtcheva S, Bair-Marshall CJ, Song SC, Martin KA, and Froemke RC (2020). Innate and plastic mechanisms for maternal behaviour in auditory cortex. Nature 587, 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjelderup-Ebbe T (1922). Beiträge zur Sozialpsychologie des Haushuhns. [Observation on the social psychology of domestic fowls.]. Zeitschrift Für Psychologie Und Physiologie Der Sinnesorgane. Abt. 1. Z. Psychol. Z. Angew. Psychol 88, 225–252. [Google Scholar]
- Sciolino NR, Bortolato M, Eisenstein SA, Fu J, Oveisi F, Hohmann AG, and Piomelli D (2010). Social isolation and chronic handling alter endocannabinoid signaling and behavioral reactivity to context in adult rats. Neuroscience 168, 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley TD (2010). Honeybee Democracy (Princeton University Press; ). [Google Scholar]
- Senst L, Baimoukhametova D, Sterley T-L, and Bains JS (2016). Sexually dimorphic neuronal responses to social isolation. eLife 5, e18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, and Abu-Akel A (2016). The Social Salience Hypothesis of Oxytocin. Biol. Psychiatry 79, 194–202. [DOI] [PubMed] [Google Scholar]
- Shemesh Y, Sztainberg Y, Forkosh O, Shlapobersky T, Chen A, and Schneidman E (2013). High-order social interactions in groups of mice. eLife 2, e00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh Y, Forkosh O, Mahn M, Anpilov S, Sztainberg Y, Manashirov S, Shlapobersky T, Elliott E, Tabouy L, Ezra G, et al. (2016). Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat. Neurosci 19, 1489–1496. [DOI] [PubMed] [Google Scholar]
- Shin S, Pribiag H, Lilascharoen V, Knowland D, Wang X-Y, and Lim BK (2018). Drd3 Signaling in the Lateral Septum Mediates Early Life Stress-Induced Social Dysfunction. Neuron 97, 195–208.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai Y, Lu B, Hu Y, Wang L, Sun K, and Zhong Y (2010). Forgetting is regulated through Rac activity in Drosophila. Cell 140, 579–589. [DOI] [PubMed] [Google Scholar]
- Singh L, Lewis AS, Field MJ, Hughes J, and Woodruff GN (1991). Evidence for an involvement of the brain cholecystokinin B receptor in anxiety. Proc. Natl. Acad. Sci. USA 88, 1130–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, and Young WS (2016). Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 21, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So N, Franks B, Lim S, and Curley JP (2015). A Social Network Approach Reveals Associations between Mouse Social Dominance and Brain Gene Expression. PLoS ONE 10, e0134509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spée M, Beaulieu M, Dervaux A, Chastel O, Le Maho Y, and Raclot T (2010). Should I stay or should I go? Hormonal control of nest abandonment in a long-lived bird, the Adélie penguin. Horm. Behav 58, 762–768. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Dimas E, Mwilambwe-Tshilobo L, Dagher A, Koellinger P, Nave G, Ong A, Kernbach JM, Wiecki TV, Ge T, et al. (2020). The default network of the human brain is associated with perceived social isolation. Nat. Commun 11, 6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagkourakis S, Spigolon G, Williams P, Protzmann J, Fisone G, and Broberger C (2018). A neural network for intermale aggression to establish social hierarchy. Nat. Neurosci 21, 834–842. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, and Wardle J (2013). Social isolation, loneliness, and all-cause mortality in older men and women. Proc. Natl. Acad. Sci. USA 110, 5797–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM (2013). Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77, 810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, and Kuo T-H (2015). Mammalian pheromones: emerging properties and mechanisms of detection. Curr. Opin. Neurobiol 34, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, and Koentges G (2002). Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295, 1493–1500. [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, and Wang Z (2014). Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res 265, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu-Coleman AL, Patti CL, Zanin KA, Zager A, Carvalho RC, Borçoi AR, Ceccon LMB, Berro LF, Tufik S, Andersen ML, and Frussa-Filho R (2013). Short-term social isolation induces depressive-like behaviour and reinstates the retrieval of an aversive task: mood-congruent memory in male mice? J. Psychiatry Neurosci 38, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Cooke EK, Leib DE, Lin Y-C, Daly GE, Zimmerman CA, and Knight ZA (2016). Warm-Sensitive Neurons that Control Body Temperature. Cell 167, 47–59.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Mustoe AC, and French JA (2014). Behavioral responses to social separation stressor change across development and are dynamically related to HPA activity in marmosets. Am. J. Primatol 76, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenig RH (1972). Solitary Confinement - Punishment within the Letter of the Law, or Psychological Torture. Wis. L. Rev 1972, 223. [Google Scholar]
- Tomova L, Tye K, and Saxe R (2019). The neuroscience of unmet social needs. Soc. Neurosci, 10.1080/17470919.2019.1694580. [DOI] [PubMed] [Google Scholar]
- Tomova L, Wang K, Thompson T, Matthews G, Takahashi A, Tye K, and Saxe R (2020a). The need to connect: Acute social isolation causes neural craving responses similar to hunger. BioRxiv 10.1101/2020.03.25.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova L, Wang KL, Thompson T, Matthews GA, Takahashi A, Tye KM, and Saxe R (2020b). The need to connect: Acute social isolation evokes midbrain craving responses similar to hunger. Nat. Neurosci 23, 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, and Livingstone MS (2006). A cortical region consisting entirely of face-selective cells. Science 311, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM (2018). Neural Circuit Motifs in Valence Processing. Neuron 100, 436–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, and Baumans V (2001). Modulation of aggression in male mice: influence of group size and cage size. Physiol. Behav 72, 675–683. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han M-H, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao J-L, Kirk A, Chakravarty S, Kumar A, et al. (2009). CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci 12, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, Deisseroth K, and Malenka RC (2018). 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dankert H, Perona P, and Anderson DJ (2008). A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc. Natl. Acad. Sci. USA 105, 5657–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, and Hu H (2011). Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693–697. [DOI] [PubMed] [Google Scholar]
- Weiss RS (1973). Loneliness: The Experience of Emotional and Social Isolation (The MIT Press; ). [Google Scholar]
- Wendt D, van Loon LJC, and Lichtenbelt W.D. van M. (2007). Thermoregulation during exercise in the heat: strategies for maintaining health and performance. Sports Med 37, 669–682. [DOI] [PubMed] [Google Scholar]
- Wiberg GS, and Grice HC (1963). Long-term isolation stress in rats. Science 142, 507. [DOI] [PubMed] [Google Scholar]
- Wichmann R, Vander Weele CM, Yosafat AS, Schut EHS, Verharen JPH, Sridharma S, Siciliano CA, Izadmehr EM, Farris KM, Wildes CP, et al. (2017). Acute stress induces long-lasting alterations in the dopaminergic system of female mice. BioRxiv 10.1101/168492. [DOI] [Google Scholar]
- Williamson CM, Romeo RD, and Curley JP (2017). Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Horm. Behav 87, 80–88. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, and Marsden CA (1996). Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav. Brain Res 75, 27–32. [DOI] [PubMed] [Google Scholar]
- Wu X-R, Zhang Y, Liu X-D, Han W-B, Xu N-J, and Sun S (2020). EphB2 mediates social isolation-induced memory forgetting. Transl. Psychiatry 10, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wei D, Song SC, Lim B, Tritsch NX, and Lin D (2020). Posterior amygdala regulates sexual and aggressive behaviors in male mice. Nat. Neurosci 23, 1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Bergan J, Lanjuin A, and Dulac C (2017). Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. eLife 6, e31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, and Palkovits M (2006). The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143, 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hui M, Karigo T, Choe A, Yang B, Blanco MR, Beadle K, Gradinaru V, Deverman BE, and Anderson DJ (2018). The Neuropeptide Tac2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress. Cell 173, 1265–1279.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H, Yang Z, Zhang L, Lin L, Zhan Y, et al. (2017). History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 357, 162–168. [DOI] [PubMed] [Google Scholar]
- Zimmerman CA, Lin Y-C, Leib DE, Guo L, Huey EL, Daly GE, Chen Y, and Knight ZA (2016). Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680–684, advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Huey EL, Ahn JS, Beutler LR, Tan CL, Kosar S, Bai L, Chen Y, Corpuz TV, Madisen L, et al. (2019). A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 568, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]


