Abstract
Checkpoints, which are integral to the cellular response to DNA damage, coordinate transient cell cycle arrest and the induced expression of DNA repair genes after genotoxic stress. DNA repair ensures cellular survival and genomic stability, utilizing a multipathway network. Here we report evidence that the two systems, DNA damage checkpoint control and DNA repair, are directly connected by demonstrating that the Rad55 double-strand break repair protein of the recombinational repair pathway is a terminal substrate of DNA damage and replication block checkpoints. Rad55p was specifically phosphorylated in response to DNA damage induced by the alkylating agent methyl methanesulfonate, dependent on an active DNA damage checkpoint. Rad55p modification was also observed after gamma ray and UV radiation. The rapid time course of phosphorylation and the recombination defects identified in checkpoint-deficient cells are consistent with a role of the DNA damage checkpoint in activating recombinational repair. Rad55p phosphorylation possibly affects the balance between different competing DNA repair pathways.
The SOS response in Escherichia coli provides the coordination between DNA damage sensing and the cellular responses to DNA damage (reviewed in reference 22). The primary SOS signal, single-stranded DNA (ssDNA), activates RecA in a ternary complex with ATP as a transcriptional regulator (44) and as a DNA repair protein (reviewed in reference 41). The transcriptional induction of the SOS regulon leads to increased expression of certain DNA repair genes (including RecA itself) and also elicits transient cell cycle arrest by the expression of sfiA, a cell division inhibitor (22). The activation of RecA as a repair protein leads to immediate repair of the primary damage that initiated the SOS signal. Although different in mechanism, the DNA damage checkpoints could provide a similar coordination between DNA damage sensing and repair in eukaryotes. First conceptualized as an active cell cycle control system in response to DNA damage in Saccharomyces cerevisiae (29, 89), DNA damage checkpoints were later shown to control also DNA damage-induced gene expression in this organism (3). DNA damage checkpoints and DNA repair serve a common purpose to secure survival and genomic stability after DNA damage. Indirect effects of the DNA damage checkpoints on DNA repair have been discussed before (reviewed in references 18, 85, and 87), but a direct coupling of the DNA damage sensing capabilities of the checkpoint system with DNA damage repair pathways has not been identified yet.
The DNA damage checkpoints in eukaryotes relay a signal in response to DNA damage to transiently delay the entry into the S or M phases, to slow down the ongoing DNA replication, or to arrest in meiotic prophase (reviewed in references 29, 62, and 87). They also elicit DNA damage-induced transcription of many genes, including some coding for DNA repair proteins (87, 93). Moreover, a related DNA replication block checkpoint ensures the dependency of M phase on a completed S phase (reviewed in references 18 and 59). Genetic analysis in S. cerevisiae has identified many components of this regulatory network that control the cell cycle response to DNA damage and/or replication blocks, as well as the DNA damage-regulated gene expression response. These include RAD9, RAD17, RAD24, RAD53, POL2, MEC3, MEC1, and DUN1 (18, 88) (also see Fig. 2A). RAD9, RAD17, RAD24, and MEC3 function in sensing and/or processing of the initial DNA damage (47). RAD9 and POL2 (encoding DNA polymerase ɛ) were identified as G1/G2 and S phase-specific inputs, respectively (57, 58). These sensing branches transduce a signal to Mec1p kinase, a yeast ATM homologue, which in turn controls all checkpoint responses examined to date (69, 90). Mec1p controls the activation of Rad53p kinase (19, 69, 77, 83), which is important in most but not all physiological responses (12, 39). The transcriptional induction after DNA damage is complex and involves Dun1p kinase for some genes, like RNR1-3 (93), but not for others, like RAD51, DDR48, or UB14 (1, 93). Dun1p also acts in one pathway with Rad53p to mediate the G2/M cell cycle arrest, parallel to a pathway acting through Chk1p kinase and the anaphase inhibitor Pds1p (24, 68).
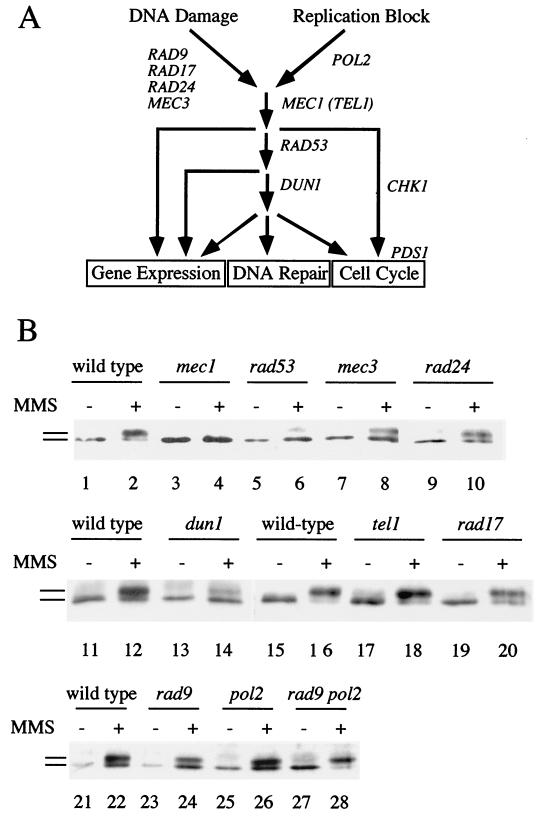
FIG. 2.
Genetic control of Rad55p phosphorylation in response to DNA damage. (A) Overview of the DNA damage and replication block checkpoint in S. cerevisiae and the proposed functions of the genes used in panel B (18, 24, 68). DNA damage in G1 and G2 cells is sensed and/or processed by Rad9p, Rad17p, Rad24p, and Mec3p. Replication blocks are sensed by the DNA polymerase ɛ (Pol2p) (57). Both branches feed into the Mec1p kinase, which controls activation of the Rad53p kinase (69, 76). Rad53p controls some but not all checkpoint responses (12) and leads to the activation of the Dun1p kinase (93). Dun1p kinase is involved in the activation of some DNA damage-inducible genes (93) and, in one pathway with Rad53p, in G2/M cell cycle arrest parallel to a pathway acting through Chk1p kinase and Pds1p (24, 68). As shown here, Dun1p kinase is required for full phosphorylation of Rad55p in response to DNA damage (labeled DNA repair). (B) Rad55p phosphorylation in cycling cells depends on some but not all DNA damage checkpoint functions. Cells (0 or 90 min in 0.1% MMS at 24°C) were analyzed as described in the legend to Fig. 1; wild-type (TWY12; lanes 1 and 2), mec1-1 (TWY308; lanes 3 and 4), rad53 (TWY312; lanes 5 and 6), mec3-1 (TWY316; lanes 7 and 8), rad24-1 (TWY399; lanes 9 and 10), wild-type (Y300; lanes 11, 12, 15, 16, 21, and 22), dun1-Δ100 (Y286; lanes 13 and 14), tel1Δ (WDHY1227; lanes 17 and 18), rad17Δ (WDHY1234; lanes 19 and 20), rad9 (Y438; lanes 23 and 24), pol2-12 (Y439; lanes 25 and 26), and rad9 pol2-12 (Y440; lanes 27 and 28). The wild-type control is shown for each individual experiment. Bars refer to the different forms of Rad55p.
The DNA damage and replication block checkpoints have been evolutionarily conserved, including proteins active in signal sensing and/or processing (Rad9p, Rad17p, Rad24p, and Pol2p) and in signal transduction (Mec1p and Rad53p) (17, 18, 88). The Mec1p kinase, a member of the phosphatidylinositol-like kinase family, also has homologues in Schizosaccharomyces pombe (Rad3p), Drosophila melanogaster (Mei41p), and human (ATM and ATR) (5, 30). Mutations in ATM cause ataxia telangiectasia (AT), a complex human hereditary cancer predisposition syndrome (reviewed in reference 71). Cells from AT patients are highly sensitive to ionizing radiation (IR), a phenotype that is mimicked by the corresponding mutation in the ATM mouse model (71). It is believed that the radiosensitivity of AT cells is caused by the cell cycle checkpoint defect, although it has been suggested that ATM regulates other processes that control survival after DNA damage (71).
DNA double-strand breaks (DSBs), the major genotoxic lesions of IR, and DNA damage caused by the alkylating agent methyl methanesulfonate (MMS) induce the checkpoint-dependent cell cycle arrest (62, 89). In S. cerevisiae, such lesions are preferentially repaired by the evolutionarily conserved RAD52 recombinational repair pathway (reviewed in references 22 and 36). Nonhomologous endjoining (NHEJ) and break-induced replication (BIR) have been identified as alternative pathways (reviewed in references 36 and 61). Three recombinational repair proteins, Rad51p, Rad55p, and Rad57p, exhibit homology to each other and to the paradigmatic bacterial RecA protein (36). Rad51p forms a protein-DNA filament highly similar to that formed by RecA (60) and is active in homology search and strand exchange in vitro (78). Thus, Rad51p performs a central function in the recombinational repair process that may be equivalent to that of RecA in prokaryotes (reviewed in reference 4). However, Rad51p has no regulatory role in the DNA damage checkpoint system like RecA has in the SOS response (3; this study).
RAD55 and RAD57 mutants exhibit essentially identical deficiencies in DNA damage repair, recombination, and meiosis, suggesting that the two proteins have highly similar functions (reviewed in references 23 and 64). In particular, both proteins are essential for DNA damage-induced recombination and important for meiotic recombination but are not required for spontaneous mitotic recombination (23, 46). Both gene deletions are cold sensitive for their DNA repair-related phenotypes, which suggests that Rad55 and Rad57 proteins are involved in a higher-order protein structure acting in DNA repair (23, 46). Such a complex is likely to involve Rad51p, which was found to interact with Rad55p (31, 34). Consistent with this model, Rad55p and Rad57p were shown to form a stable heterodimeric complex which stimulates Rad51p-mediated recombination in vitro by overcoming the inhibitory effect of the ssDNA binding protein Rpa on the formation of the critical Rad51p-ssDNA filament (79).
To determine whether a direct connection exists between a DNA repair pathway and the DNA damage checkpoints, we examined proteins of the RAD52 recombinational repair pathway for DNA damage- and replication block-induced phosphorylation. We observed that Rad55p was phosphorylated in response to DNA damage, dependent on the checkpoint kinases Mec1p, Rad53p, and Dun1p. Analysis of the checkpoint-controlled cell cycle and gene expression responses suggests that Rad55p is a terminal substrate of the DNA damage and replication block checkpoints. Based on our observation that checkpoint deficiency results in a major defect in DNA damage-induced recombination, we propose that the phosphorylation of Rad55p is biologically significant in the activation of recombinational repair in response to DNA damage.
MATERIALS AND METHODS
Strains and plasmids.
The strains used in this study and their relevant genotype are described in Table 1. Full genotypes are available upon request. All experiments have been performed using isogenic strains, except for some experiments (see Fig. 2, 4, and 5), in which the strains were highly related. The null mutations constructed for this study were generated by PCR-mediated deletion and/or substitution of essentially the entire open reading frames by KanMX (84) using appropriate primers.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Relevant genotype | Source and (reference) |
|---|---|---|
| FF18984 | Haploid wild type | F. Fabre |
| WDHY837 | FF18984, but rad55Δ | This study |
| WDHY839 | FF18984, but rad57Δ | This study |
| WDHY994 | FF18984, but rad51Δ | This study |
| WDHY1020 | FF18984, but rad9Δ | This study |
| FF181268 | bar1::LEU2 | F. Fabre |
| FF181270 | FF181268, but rad9::URA3 | F. Fabre |
| WDHY1236 | FF181268, but rad17Δ | This study |
| WDHY1075a | a/α diploid wild type | This study |
| WDHY1082a | WDHY1075, but rad51Δ/rad51Δ | This study |
| WDHY1089a | WDHY1075, but rad55Δ/rad55Δ | This study |
| WDHY1096a | WDHY1075, but rad57Δ/rad57Δ | This study |
| Y300 | Haploid wild type | Allen et al. (3) |
| Y286 | Y300, but dun1-Δ100 | Zhou and Elledge (93) |
| Y438 | Y300, but rad9 | Navas et al. (57) |
| Y439 | Y300, but pol2-12 | Navas et al. (57) |
| Y440 | Y300, but rad9 pol2-12 | Navas et al. (57) |
| WDHY1227 | Y300, but tel1Δ | This study |
| WDHY1234 | Y300, but rad17Δ | This study |
| TWY12b | Haploid wild type | T. Weinert |
| TWY308b | Congenic to TWY12, but mec1-1c | Weinert et al. (90) |
| TWY312b | Congenic to TWY12, but rad53 (mec2-1)c | Weinert et al. (90) |
| TWY316b | Congenic to TWY12, but mec3-1 | Weinert et al. (90) |
| TWY399b | Congenic to TWY12, but rad24-1 | Weinert et al. (90) |
| P7BAB | a/α diploid wild-type leu2-1/leu2-27 his4-4/his4-290 | Kato and Ogawa (37) |
| NR110AB | a/α diploid leu2-1/leu2-27 mec1/mec1 (esr1-1/esr1-1) | Kato and Ogawa (37) |
| WDHY1558 | As P7BAB, but mec1/mec1 (esr1-1/esr1-1) | This study |
Diploid strain derive from haploid strains, including FF18984, that were kindly supplied by F. Fabre.
MEC1/SAD3/ESR1 and RAD53/MEC2/SAD1/SPK1 have been isolated and named several times. In accordance with the generally accepted use (see reference 18) and for the sake of brevity, we use the names MEC1 and RAD53.
FIG. 4.
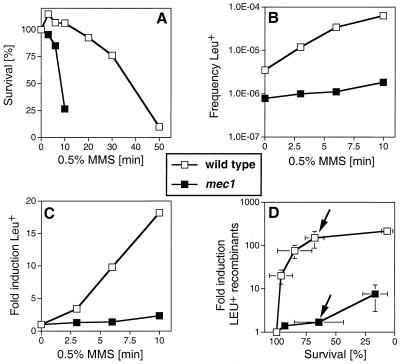
mec1 cells are defective in DNA damage-induced mitotic recombination. (A) Survival of wild-type cells and mec1 cells after acute exposure to 0.5% MMS for the indicated times was measured as described elsewhere (65). (B) Absolute frequencies of Leu+ recombinants per viable cell with respect to MMS dose in wild-type and mec1 cells. (C) Fold induction of Leu+ recombinants with respect to MMS dose. (A to C) Shown is one experiment typical of three performed. The decrease in induced recombination in mec1 cells was seen in every experiment. (D) Induction of Leu+ recombinants per viable cell with respect to survival after MMS exposure in wild-type and mec1 cells. Given are the means of three determinations and standard deviations (error bars). Where no bars appear, the standard deviations were smaller than the symbols used. The wild-type strain was P7BAB, and the mec1 strain was NR110AB. The two arrows in panel D indicate a data point of similar survival between wild-type and mec1 cells (see text).
FIG. 5.
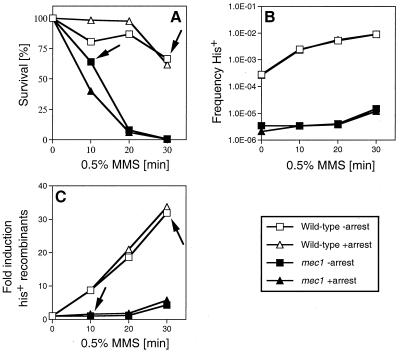
Artificial cell cycle arrest does not rescue the damage-induced recombination defect in mec1 cells. (A) Survival of wild-type cells and mec1 cells after acute exposure to 0.5% MMS for the indicated times was measured as described elsewhere (65). (B) Absolute frequencies of His+ recombinants per viable cell with respect to MMS dose in wild-type and mec1 cells. The spontaneous frequency for His+ recombinants in wild-type cells was 3.35 × 10−4 without arrest and 2.67 × 10−4 with arrest, and the values in mec1 cells were 3.45 × 10−6 without arrest and 2.1 × 10−6 with arrest. (C) Fold induction of His+ recombinants with respect to MMS dose. (A to C) Shown is one experiment typical of three to five performed. The decrease in induced recombination in mec1 cells was seen in every experiment. The wild-type strain was P7BAB, and the mec1 strain WDHY1558. The arrows in panels A and C indicate the effect on induced recombination at comparable survival levels for both strains (see text).
Antibodies, immunoprecipitations, and metabolic labeling.
Purification of Rad51p, Rad52p, Rad54p, Rad55p, and Rad57p as His6 fusion proteins after overexpression in the pT7 system, antibody production in rabbits and rats, and affinity purification of antibodies were done as described for Hrs1p (70). For immunoprecipitations 10 to 15 mg of protein extract was incubated with rabbit antibodies in 50 mM Tris-HCl (pH 7.5)–100 mM NaCl–0.1 mM phenylmethylsulfonyl fluoride–0.2% Triton X-100 for 2 to 3 h at 4°C. Immune complexes were precipitated with protein G-Sepharose for 1 h at 4°C and washed four times in the above buffer. The precipitates were electrophoresed on sodium dodecyl sulfate–9% polyacrylamide gel electrophoresis and transferred to Immobilon-P membrane (Millipore). For protein detection the rat antibodies were used, employing a peroxidase-conjugated rabbit anti-rat second antibody with enhanced chemiluminescence (Amersham) for detection. In dephosphorylation experiments, 1 U of calf intestinal phosphatase (Boehringer Mannheim) was used at 37°C for 1 h. Cells were metabolically labeled with 32P for 2 h as previously described (3) using 1 mCi of [32P]H3PO4 per 108 cells.
Cell cycle experiments.
G1-arrested cells were obtained by addition of α factor to 50 ng/ml for 2 h at 30°C, resulting in quantitative arrest as evidenced by the accumulation of >95% of the population as unbudded cells. In the α-factor the experiment shown in Fig. 4, arrested cells were washed twice and resuspended in prewarmed medium containing pronase (0.02 mg/ml). Fluorescence-activated cell sorter (FACS) analysis was performed with propidium iodide-stained cells as described (56), using a Becton Dickinson FACS Calibur and CELLQUEST software. G2-arrested cells were obtained by treatment with nocodazole (15 μg/ml) for 2 h at 24°C, resulting in quantitative arrest as evidenced by the accumulation of 95% of the population as large budded cells. The presence of the G2 DNA damage checkpoint-induced cell cycle arrest was monitored essentially as described (89, 90). Cells were irradiated on plates with 20 Gy of IR, and arrest was monitored microscopically after 20 to 24 h. Survival was assessed by counting the colonies after 4 days.
Recombination experiments.
All recombination experiments were performed at room temperature because of the temperature sensitivity of mec1 cells. For the experiment shown in Fig. 4, strains P7BAB and NR110AB (leu2 hetero-alleles) were grown to late exponential or early stationary phase by incubation in yeast extract-peptone-dextrose (YPD) for one day and the frequency of recombinants was determined without and with exposure to 0.5% MMS as described (65). Microscopic examination was used to determine that cells resumed budding 4 h after plating. Colonies were counted after 7 days of incubation. An additional experiment (see Fig. 5) was performed with strains P7BAB and WDHY1558 (leu2 and his4 hetero-alleles), which were grown deeper into stationary phase by incubation in YPD for 3 days. The cultures were treated with MMS as described above, and at each MMS dose one aliquot of the culture was withdrawn and held in stationary phase for an additional 6 h by incubation in exhausted medium of the original culture, whereas another aliquot was plated directly. Colonies were counted after 7 days of incubation. Microscopic examination confirmed that cells were in stationary phase (>98% unbudded) and determined that cells resumed budding about 10 h after plating in the presence of the artificial arrest and after 4 h in its absence.
RESULTS
Rad55p is phosphorylated in response to DNA damage and to replication blocks.
To monitor if recombinational repair proteins of the RAD52 group were targets of the DNA damage checkpoint kinases, we analyzed these proteins after DNA damage induction to identify potential electrophoretic shifts that may have been caused by phosphorylation. The status of the S. cerevisiae Rad55 protein in response to DNA damage was analyzed by immunoprecipitation and immunoblotting because the expression of this protein is very low (79) and does not permit detection of the protein by direct immunoblotting of cell extract.
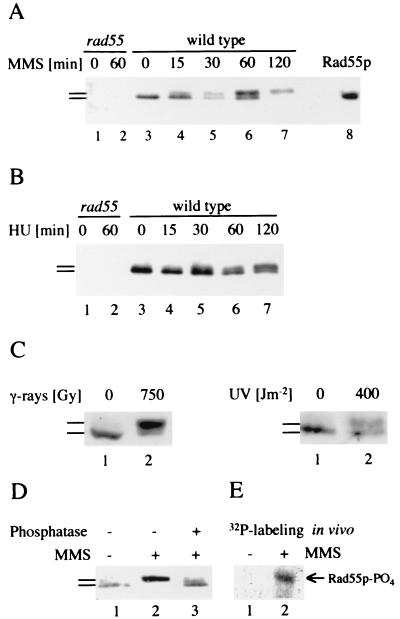
A slower migrating form of Rad55p appeared in a time-dependent manner after induction of DNA damage by MMS. Modified Rad55p was essentially undetectable in wild-type cells that were not genotoxically stressed, whereas an almost quantitative shift to the modified form occurred after 120 min of treatment with MMS (Fig. 1A). Modified Rad55p was detectable at 15 min (Fig. 1A), and additional time course experiments showed that the first evidence of modified Rad55p was as early as 5 to 10 min after the addition of MMS (data not shown). When replication was blocked by hydroxyurea (HU), an inhibitor of ribonucleotide reductase, modified Rad55p also appeared in a time-dependent fashion (Fig. 1B). In all experiments performed (Fig. 1B; also see Fig. 3D and data not shown), MMS produced a more pronounced mobility shift of Rad55p than HU. A similar observation has been made for the DNA damage- and replication block-induced phosphorylation of Rad53p (69). A strong electrophoretic shift of Rad55p was also identified in response to other DNA-damaging agents, UV and gamma rays (Fig. 1C).
FIG. 1.
Phosphorylation of Rad55p in response to DNA damage and replication blocks. (A) Modification of Rad55p in response to DNA damage was analyzed by immunoprecipitation and immunoblotting. Lanes 1 and 2 contain precipitates from rad55Δ cells (WDHY1089) without MMS (lane 1) and after 60 min of exposure to 0.075% MMS (lane 2). Lanes 3 to 7 contain precipitates from wild-type cells (WDHY1075) without MMS (lane 3) and after increasing exposure time (15 to 120 min) to 0.075% MMS (lanes 4–7). In lane 8, extract of wild-type cells overexpressing Rad55p was directly blotted to show the position of the Rad55 protein. In lanes 5 and 7, accidentally less protein was loaded. (B) Modification of Rad55p in response to replication blocks. Cells were treated with HU and the Rad55p status was analyzed as in panel A. Lanes 1 and 2 contain precipitates from rad55Δ cells without HU (lane 1) and after 60 min of exposure to 200 mM HU (lane 2). Lanes 3 to 7 contain precipitates from wild-type cells without HU (lane 3) and after increasing exposure time (15 to 120 min) to 200 mM HU (lanes 4 to 7). (C) Modification of Rad55p in response to UV and gamma radiation. Exponentially growing wild-type cells (FF18984) were irradiated with gamma rays (137Cs source at 8 Gy/min) or UV rays (254 nm) at the indicated doses or mock irradiated. Cell extracts were prepared 1 h postradiation and analyzed for their Rad55p status. (D) Phosphorylation of Rad55p in response to DNA damage. The Rad55p status was analyzed in wild-type cells as in panel A from cells grown in the absence (lane 1) and in the presence (lanes 2 and 3) of MMS (0.075% for 120 min). An immunoprecipitate of a sample from MMS-treated cells was incubated with phosphatase before immunoblot analysis (lane 3). (E) Rad55p is phosphorylated in vivo in response to MMS. Wild-type cells (WDHY1075) were metabolically labeled with 32P in the presence (0.1%) and absence of MMS. Rad55p was immunoprecipitated and analyzed by autoradiography. Immunoblotting confirmed the position of unphosphorylated and phosphorylated Rad55p. Bars refer to the different forms of Rad55p.
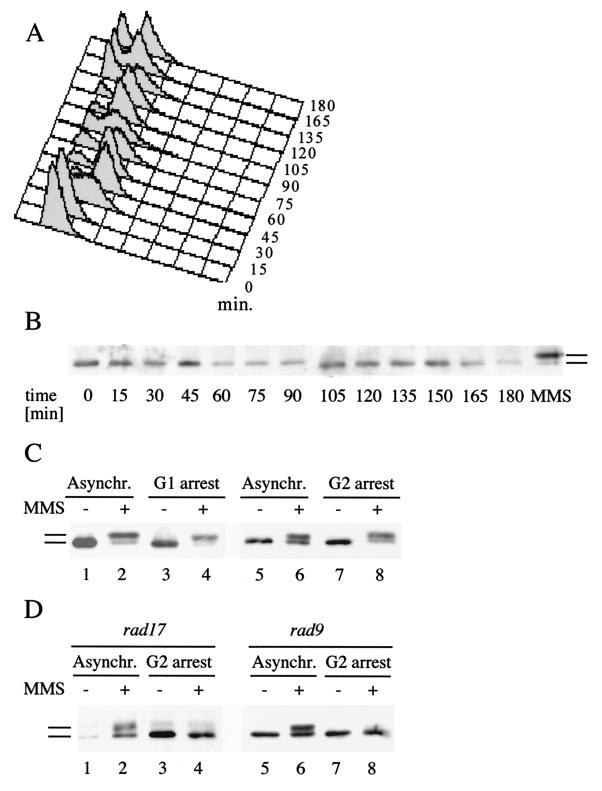
FIG. 3.
Rad55p phosphorylation in response to DNA damage during the cell cycle. (A) FACS analysis of a synchronized cell culture after α-factor arrest and release. At the indicated time intervals, aliquots were withdrawn, stained with propidium iodide, and analyzed by FACS. (B) Rad55p is not detectably phosphorylated during a normal cell cycle. At the same time intervals as in panel A, the Rad55p phosphorylation status was determined as described in the legend to Fig. 1. The rightmost lane (labeled MMS) shows a positive control (2 h with 0.1% MMS), indicating the migration behavior of phosphorylated Rad55p. (C) Rad55p phosphorylation in G1- and G2-arrested cells. Wild-type cells (FF181268) arrested either in G1 by α-factor (lanes 3 and 4) or in G2 by nocodazole (lanes 7 and 8) were treated with 0.1% MMS for 2 h (lanes 4 and 8) or left untreated (lanes 3 and 7). The Rad55p status was analyzed as described in the legend to Fig. 1. As a control, cycling wild-type cells were analyzed before (lanes 1 and 5) and after (lanes 2 and 6) MMS exposure. Asynchr., asynchronous. (D) Rad55p phosphorylation in G2-arrested cells is fully dependent on RAD9 or RAD17. The Rad55p status was analyzed as in panel A in rad9Δ cells (FF181270) left asynchronous (Asynchr.) (lanes 1 and 2) or arrested in G2 by nocodazole (lanes 3 and 4) and in rad17Δ cells (WDHY1236) left asynchronous (lanes 5 and 6) or arrested in G2 by nocodazole (lanes 7 and 8) after treatment with 0.1% MMS (lanes 2, 4, 6, and 8) or without MMS (lanes 1, 3, 5, and 7). Bars refer to the different forms of Rad55p.
To determine whether the electrophoretic mobility shift was a result of phosphorylation, Rad55p was immunoprecipitated from MMS-exposed cells and treated with phosphatase. Treatment with phosphatase almost quantitatively reversed the mobility shift (Fig. 1D). Thus, Rad55p is likely to be posttranslationally modified by phosphorylation in response to DNA damage. This conclusion was corroborated by metabolic labeling of cells with 32P. Rad55p was specifically labeled by 32P during exposure to MMS, whereas in the absence of genotoxic stress, Rad55p was not detectably labeled (Fig. 1E). Close inspection of the blots revealed the appearance of multiple, phosphorylated Rad55p species, suggesting that Rad55p is phosphorylated at more than one amino acid residue (not shown because this subtle feature is lost in reproduction). From these experiments we conclude that Rad55p is phosphorylated in response to replication blocks induced by HU and in response to DNA damage induced by a variety of genotoxic agents.
Rad55p phosphorylation is dependent on checkpoint functions.
The DNA damage checkpoints monitor the genome and use a protein kinase cascade to mediate transient cell cycle arrest and induction of certain gene products in response to DNA damage (Fig. 2A). The Rad55p status was analyzed by immunoprecipitation and immunoblotting in strains with mutations in known checkpoint genes after inducing DNA damage with MMS. Rad55p phosphorylation depended entirely on the central signal transducing kinase Mec1p (Fig. 2B). A small but reproducible amount of residual phosphorylation was detected in rad53 cells. Rad55p phosphorylation was also dependent on Dun1p kinase, but to a lesser extent than observed with Rad53p or Mec1p (Fig. 2B). The TEL1 mutation did not affect Rad55p phosphorylation (Fig. 2B). This is consistent with earlier observations on the role of Tel1p in DNA damage checkpoint control (69). Also, a deletion of the CHK1 gene had no effect on Rad55p phosphorylation (E. Haghnazari and W.-D. Heyer, unpublished result). From these experiments we conclude that DNA damage-induced phosphorylation of Rad55p is dependent on the central checkpoint signal transduction kinases, Mec1p, Rad53p, and Dun1p.
Lesser but significant reduction of Rad55p phosphorylation was observed consistently in rad17, rad24, and mec3 mutants, whereas the deletion of RAD9 and the pol2-12 mutation caused no appreciable reduction (Fig. 2B). These experiments were performed with cycling cultures containing G1, S, and G2 cells. The existence of different DNA damage-sensing and/or -processing branches in the G1/G2 phases and in S phase (18) may explain the reduced dependence of phosphorylation on these proteins (see below).
Parallel sensory branches defined by rad9 and pol2-12 (DNA polymerase ɛ) control the cell cycle and transcriptional response after UV damage (57). Using the rad9 pol2-12 double mutant (kindly supplied by S. J. Elledge), we established that in response to MMS, Rad55p phosphorylation occurred independent of both sensory branches in cycling cells (Fig. 2B). This observation was confirmed in several experiments conducted at the permissive and restrictive temperatures (data not shown). This result may point to the possible existence of an additional sensing branch for Rad55p phosphorylation in response to MMS.
Rad55p phosphorylation in response to DNA damage during the cell cycle.
MMS treatment of cycling cells results in a slowed progression through S phase which is dependent on MEC1, RAD53, RAD9, RAD17, and RAD24 (62, 63) and leads to transient G2/M arrest (18). In case Rad55p is phosphorylated at any stage during a normal cell cycle, phosphorylation of Rad55p after DNA damage may be an indirect consequence of a transient cell cycle arrest. To determine if Rad55p is phosphorylated at any stage during the cell cycle in the absence of DNA damage, we analyzed the Rad55p status in a cell cycle-synchronized cell population (Fig. 3A and B). Cells were synchronized by the release from an α-factor-induced arrest in G1. Progress through the cell cycle was monitored by FACS analysis (Fig. 3A) and microscopic analysis counting budded cells (not shown), demonstrating a homogeneous arrest induced by the α-factor treatment (time, 0 min) and relatively synchronous movement through two cell cycles. At the same time intervals, the Rad55p phosphorylation status was analyzed. The results showed that Rad55p was not detectably phosphorylated in the absence of exogenously induced DNA damage at any time during the cell cycle (Fig. 3B). In particular, at the 30- and 90-min time points, when a substantial portion of the cells were in S phase, no modified Rad55p was detected. These results suggest that Rad55p is specifically phosphorylated in response to DNA damage and replication blocks imposed by HU.
While the previous experiments demonstrated that Rad55p is phosphorylated in response to DNA damage in cycling cells, we wanted to establish whether Rad55p phosphorylation would occur in cells arrested either in G1 or G2. Cells were arrested in G1 with α-factor or in G2 by addition of the microtubule inhibitor nocodazole. Subsequently, the Rad55p status was analyzed for MMS-induced Rad55p phosphorylation. The results from both experiments demonstrated that cells arrested in either G1 or G2 phosphorylated Rad55p in response to DNA damage to a similar extent as did cycling cells (Fig. 3C). Thus, we conclude that Rad55p phosphorylation can occur in the G1 and G2 phases of the cell cycle.
In asynchronous wild-type cells Rad55p phosphorylation was found to be independent of RAD9 and only partially dependent on RAD17 (Fig. 2B). However, experiments in G2-arrested cells demonstrated that Rad55p phosphorylation in response to DNA damage is completely dependent on RAD9 and RAD17 (Fig. 3D). Thus, DNA damage-induced Rad55p phosphorylation is controlled by Rad9p and Rad17p in the G2 phase of the cell cycle.
Intact checkpoint-induced cell cycle arrest and transcriptional induction in rad55Δ.
To determine if Rad55p could act as an additional transducer of the signal in the pathway or, alternatively, if Rad55p is a terminal substrate of the signaling pathway, we analyzed other checkpoint-controlled responses to DNA damage in RAD55 mutant cells. The G2 DNA damage-induced cell cycle arrest is also operative in rad55Δ, rad57Δ, and rad51Δ cells (Table 2). A combined assay of microscopic examination and survival after X-ray exposure of cells expresses the ratio of arrest to lethality as a convenient measure for the function of G2 DNA damage checkpoint (89, 90). As shown in Table 2, the ratio for the wild type approaches 1 (ratio, 0.86), whereas a classical checkpoint mutant (rad9) gives a significantly lower value (ratio, 0.13), consistent with earlier observations (89, 90). The ratios for rad55, rad57, and rad51 cells were very close to, and not significantly different from, the wild-type values (Table 2). Thus, the G2 cell cycle arrest in response to DNA damage is operative in rad55, rad57, and rad51 cells. Although Rad51p is a eukaryotic RecA homologue with respect to its function in the recombination mechanism (4), these data suggest that Rad51p has no apparent regulatory role in the DNA damage checkpoints that would be equivalent to the regulatory function of RecA in the SOS response. In addition, rad55 was found earlier to be proficient for the replication block checkpoint (3).
TABLE 2.
rad55Δ has an intact cell cycle arrest in response to DNA damagea
| Strain | % Lethality | % Arrest | Arrest/lethality |
|---|---|---|---|
| Wild type | 21 | 18 | 0.86 |
| rad9 | 40 | 5 | 0.13 |
| rad51 | 54 | 44 | 0.81 |
| rad55 | 58 | 46 | 0.79 |
| rad57 | 48 | 34 | 0.71 |
The following strains (Table 1) used were: FF18984 (wild-type), WDHY1020 (rad9Δ), WDHY994 (rad51Δ), WDHY837 (rad55Δ), and WDHY839 (rad57Δ). The presence of the G2 DNA damage checkpoint-induced cell cycle arrest was monitored as described in Materials and Methods using an established assay (89, 90). The ratio of percent arrested cells (microcolonies with either a large-budded cell or with two adjacent large-budded cells) to the percent of inviable cells provides a metric of the efficiency of cell cycle arrest. For wild-type cells this metric approaches 1.0 because essentially all cells with unrepairable DNA breaks die in the G2 phase; haploid cells in the G1 or postanaphase stages when irradiated cannot repair the DNA DSBs, arrest in the next G2 phase, and die as large-budded and two adjacent large-budded cells, respectively. Wild-type cells that can repair DSBs (S and G2 phase cells) form large microcolonies that are not counted. In contrast, checkpoint mutants cells that die after irradiation do not usually arrest immediately; rather, they continue to divide for a few generations. Therefore, in checkpoint mutants, the ratio of arrested cells to inviable cells is <<1.0 and typically is <0.3 (see reference 90).
We also monitored whether DNA damage-induced gene expression is operative in rad55Δ cells. Cells were exposed to MMS, and the steady-state levels of specific RNAs were analyzed. The DNA damage-inducible RNR2 and RAD54 mRNAs showed induction after addition of MMS in wild-type cells (3.7- and 9.3-fold compared to an actin standard, respectively; data not shown), which is consistent with earlier observations (1, 3). Likewise, rad55Δ and rad57Δ cells exhibited a similar extent of DNA damage-induced mRNA levels (3.2- and 2.9-fold induction for RNR2 and 6.6- and 7.3-fold induction for RAD54, respectively; data not shown). Thus, in rad55Δ and rad57Δ cells, the major checkpoint functions of cell cycle arrest and induced gene expression are intact. Therefore, neither Rad55p nor Rad57p has an apparent function in these checkpoint-controlled physiological responses.
mec1 cells are defective in DNA damage-induced mitotic recombination.
If Rad55p were a terminal substrate of the DNA damage checkpoints, we speculated that the recombinational repair pathway might be regulated by the checkpoint system, in particular under conditions of genotoxic stress. Homologous recombination in mitotic cells is strongly induced by genotoxic stress caused by DSBs (26) or DNA damage-inducing agents like UV radiation, IR, and MMS (22, 75). As DNA damage-induced Rad55p phosphorylation was eliminated in mec1 cells, we examined if mec1 cells were defective in DNA damage-induced mitotic recombination.
DNA damage-induced intragenic recombination was analyzed in stationary-phase diploid cells. Under these conditions, cells have a G1-equivalent DNA content and recombinational repair using the homolog as a template is the repair pathway preferred over the alternative pathways like NHEJ and BIR (61). First, we established the survival curve after acute exposure to MMS (Fig. 4A). mec1 cells were highly sensitive to acute exposure, consistent with previous observations made with chronic exposure to MMS (37, 90). In the same experiment, we measured the frequency of Leu+ intragenic recombinants between the leu2-1 and leu2-27 alleles (Fig. 4B). The spontaneous recombination frequencies per viable cell were 3.5 × 10−6 in the wild type and 7.8 × 10−7 in mec1, an approximately fivefold reduction in the checkpoint mutant (Fig. 4B). When the recombination data are replotted to analyze the recombinants induced by genotoxic stress (Fig. 4C), a defect in DNA damage-induced recombination is apparent in mec1 cells. For example, at the same MMS dose (10-min exposure to 0.5% MMS), recombination was almost eightfold less induced in mec1 than in wild-type cells (2.3- versus 18.2-fold induction) (Fig. 4C). At this dose the survival of mec1 cells is drastically lower than that of wild-type cells (26.7 versus 106%) (Fig. 4A). The experiment was extended over a larger survival range, to allow plotting the induction of recombinants with respect to survival (Fig. 4D). This may be a physiologically more relevant analysis. At comparable levels of survival (wild type, 68%; mec1, 64%) (arrows in Fig. 4D), wild-type cells induced recombination 150-fold, whereas mec1 cells induced recombination only 1.7-fold (Fig. 4D). This represents an 88-fold reduction for mec1. These results suggest that Mec1p kinase modulates the activity of the recombinational repair pathway.
In order to corroborate this observation and to exclude a locus-specific effect at LEU2, we analyzed DNA damage-induced recombination also at another locus (his4-4 and his4-290 hetero-alleles) (Fig. 5B and C). At the same MMS dose (30 min), the wild type induced recombination at HIS4 eight times more than the mec1 strain (32- versus 4-fold induction). At comparable survival (wild-type, 66%; mec1, 64%), the effect was even more pronounced (32-fold difference) (arrows in Fig. 5A and C). In addition, in this experiment LEU2 was also monitored, showing again the defect in DNA damage-induced recombination in mec1 cells (data not shown) observed previously (Fig. 4). Thus, these data confirmed the observation that mec1 cells are defective in DNA damage-induced recombination.
In response to DNA damage, the checkpoint induces a transient delay at the G1/S border of the cell cycle which is believed to help prevent the replication of damaged DNA (73, 74). To exclude the possibility that the observed defect in DNA damage-induced recombination of mec1 cells was due to a failure of establishing this G1/S delay, we analyzed the times at which cells resumed S phase after plating. In S. cerevisiae, bud appearance signals the onset of S phase (43), an event that can be easily observed with a light microscope. The earliest buds were formed 4 h after plating, indicating that cells remained with a G1-like DNA content for at least this time. A DSB repair event has been shown to last about 1 h in cycling or G1-arrested S. cerevisiae cells (14, 61), leaving the cell ample time for repair. To ascertain this conclusion, cultures were artificially held in arrest for an additional 6 h after exposure to MMS. The earliest buds, signaling the exit from arrest, were seen after 10 h (data not shown). In wild-type and mec1 cells, the artificial arrest showed no significant consequence in survival or in the frequencies of spontaneous and DNA damage-induced recombination at the HIS4 locus (Fig. 5). Concomitant recombination analysis at the LEU2 locus also showed no difference between arrested and nonarrested cells for DNA damage-induced recombination (data not shown). We conclude that the defect in DNA damage-induced recombination in stationary-phase mec1 cells is not due to the failed cell cycle arrest at the G1/S border.
DISCUSSION
Rad55p is a terminal substrate of the DNA damage checkpoints.
Rad55p is phosphorylated specifically in response to DNA damage and replication blocks in a time-dependent manner that is genetically controlled by the DNA damage checkpoints (Fig. 1 to 3). The available evidence strongly suggests that Rad55p is a terminal substrate of the DNA damage checkpoints, as the other responses of the DNA damage checkpoints, cell cycle arrest (Table 2) and DNA damage-induced gene expression, were unaffected in RAD55 mutants. It was previously shown that Rad55p and Rad57p have no role in the S phase checkpoint (3). Here we show that also the G2/M arrest in response to DNA damage is intact in both mutants. Although not all possible cell cycle effects and all DNA damage-inducible genes have been tested, it seems unlikely that Rad55p exerts an active role in the checkpoint system other than being a terminal substrate. The direct kinase(s) responsible for DNA damage-induced Rad55p phosphorylation is not known.
Other substrates of DNA damage checkpoint kinases, Rpa (8) and primase (32, 49), with possible roles in DNA repair have been identified in budding yeast, but it is unclear whether these are terminal substrates of the pathway (45, 49). In higher eukaryotes, Rad51 protein was found to be phosphorylated by c-Abl in vitro and possibly in vivo in response to IR (10, 92). It was proposed that phosphorylation inhibits the DNA repair function of Rad51p (92), but another study (10) reached a different conclusion. The biological significance of these DNA damage-induced phosphorylation events is unclear (reviewed in reference 85).
Possible biological significance of Rad55p phosphorylation: checkpoint modulation of the activity of recombinational DNA damage repair.
Several considerations suggest that Rad55p phosphorylation activates the recombinational repair pathway, but the possibility that it represents an inhibitory or coincidental effect cannot be ruled out presently. Teleologically, DNA damage, like MMS or IR, should activate rather than inhibit recombinational repair, because this pathway represents the primary repair mode of such damage in S. cerevisiae (22, 23). The kinetics of Rad55p phosphorylation in response to DNA damage (less than 15 min) is fast compared to the kinetics of recombinational repair (at least 60 min [14, 26]), which is consistent with an activating role. Moreover, we presented experimental evidence that Mec1p is involved in the activation of recombinational repair in response to DNA damage by directly measuring the biological activity of the recombinational repair pathway in the formation of intragenic recombinants. This observation is consistent with the phenotypes of ATM-deficient chicken DT40 cells, which also suggested a positive role of ATM in the homologous recombination pathway (54, 80). Although at present we cannot exclude the possibility that DNA damage checkpoints have other terminal substrates in the recombinational repair pathway, we suggest that this effect is at least partly mediated by phosphorylation of Rad55p. No evidence for DNA damage-induced phosphorylation was found for other proteins of the RAD52 group (Rad51p, Rad52p, Rad54p, Rad57p [V.I.B. and W.-D.H., unpublished results]), but the absence of an electrophoretic shift does not allow one to rule out this possibility, because not all phosphorylation results in a detectable shift.
Possible effects of DNA damage checkpoints on cellular DNA repair capacity have been discussed before (18, 47, 85, 88), including the checkpoint-controlled relocalization of Ku and SIR proteins in response to some forms of DNA damage (50, 52). Here, we suggest a different mechanism of how the checkpoints could possibly modulate the activity of a major DNA repair pathway by posttranslational modification of the recombinational repair protein Rad55. Rad55p is uniquely positioned to serve as a modulator of this pathway, as it forms a heterodimer with Rad57p that modulates in vitro a very early phase of recombinational repair in the assembly of the Rad51p-ssDNA filament (79), which is a crucial early intermediate in recombinational repair (4).
According to our model, checkpoint deficiency sensitizes cells to DNA damage and replication blocks not only by failing to arrest the cell cycle and failure to induce important proteins but also by a failure to activate and/or optimally recruit the DNA damage repair machinery to the site of damage. Such an interpretation is supported by the failure to fully suppress the DNA damage sensitivity in S. cerevisiae rad9 (89), rad53 (3), and mec1 mutants (this study) as well as the checkpoint rad mutants in S. pombe (2, 67) by an artificial cell cycle arrest. Also in ATM-deficient cells, evidence for cell cycle arrest-independent radiosensitivity (15, 72, 86) has been accumulated (for recent reviews, references 33 and 71). This has been reaffirmed by the epistasis analysis in chicken DT40 cells, suggesting that ATM acts in the homologous repair pathway (54). Cell cycle arrest defect and DNA damage sensitivity are also dissociated in chk1 mutants, which are defective for the DNA damage-induced G2/M arrest but do not exhibit DNA damage sensitivity (68). This suggests that mechanisms other than cell cycle arrest contribute to the increased sensitivity observed in these mutants. One possibility is that this contribution is provided by the induced expression of DNA repair genes (3, 18). RAD54 is one of the most important DSB repair genes and its transcription is induced by DNA damage (13, 23). Ablation of DNA damage-inducible transcription by promoter mutations that retained a low constitutive level did not result in DNA damage sensitivity (13). Thus, it appears that DNA damage-induced transcription of RAD54 makes only a subtle contribution to DNA damage survival. It is also interesting in this context that none of the recombinational repair genes of mammalian cells show DNA damage-induced transcription (36).
Recombination defect in mec1 cells.
In this study we identified a significant reduction in DNA damage-induced intragenic recombination in G1-arrested mec1 cells (Fig. 4 and 5). In wild-type and likely in mec1 cells, intragenic recombinants arise by gene conversion (Fig. 6). We chose G1-arrested cells to study the competition between recombinational repair and alternative pathways (Fig. 6). In cycling or G2 cells the situation is much more complex and potentially difficult to interpret, as partner choice during repair becomes an additional parameter. In G2-arrested cells, recombinational repair uses the sister chromatid as a template preferentially over the homologue (35). Importantly, it is conceivable that the DNA damage checkpoints are involved in this partner choice, because two independent observations suggest that checkpoints control partner choice during meiotic recombination (25, 81). In G1 cells, this complication is eliminated because only the homolog is present as a template.
FIG. 6.
Model of checkpoint regulation of pathway competition in DNA repair. In wild-type cells, several pathways compete for the repair of DNA damage like a DSB (36, 61). In S. cerevisiae, the damage is preferentially repaired by recombinational repair, resulting primarily in gene conversions which are rarely (0 to 20%) associated with a crossing over (for a detailed discussion see reference 61 and references therein). This avoidance of crossing over may be related to the frequent nondisjunction observed for mitotic crossing-over products (11). Under genotoxic stress, Rad55p is essential for the recombinational repair pathway (36, 61). A defect in the checkpoint (mec1) fails to phosphorylate Rad55p in response to DNA damage, which we hypothesize will lead to decreased efficiency in Rad51p filament formation (see reference 79) and a less efficient recombinational repair pathway. Under these conditions, other pathways will contribute more noticeably. BIR results in a genetic outcome that resembles a crossing-over (7, 48, 55, 61). NHEJ will not lead to recombinants (36, 53, 61). Depicted is a diploid cell in G1 with two homologues carrying three heterozygous markers (aA/bB/cC).
The recombination defect in mec1 cells is complex. Kato and Ogawa (37) discovered MEC1 as the meiotic recombination-defective mutant esr1-1. In mitotic mec1 (esr1-1) cells, intergenic recombination was significantly increased (37). In wild-type cells, intergenic recombinants arise by crossing over. However, crossing over can be mimicked by BIR (Fig. 6), an alternative DNA repair pathway identified in cells deficient for recombinational repair (48). We found that spontaneous intragenic recombination (conversion) is reduced in vegetative mec1 cells (Fig. 4 and 5), in contrast to Kato and Ogawa (37) who found no reduction in mec1. This can be explained by a difference in protocol, as in the previous study (37) the mitotic frequency was determined as the 0-h time point of a meiotic time course, at which point some meiotic induction might have occurred (40). However, this explanation appears inadequate to explain the full extent of the up to 59-fold hyperrecombination effect in apparent crossing-over (37). Importantly, the data suggest that different pathways are operative to generate intragenic and intergenic recombinants in mitotic cells which are differently affected by the defect in mec1 cells.
Rad55p acts in the recombinational pathway with Rad51p, and neither protein is involved in the alternative pathways of repair of chromosomal DSBs, NHEJ, and BIR (48, 61) (Fig. 6). Rad51p and Rad55p, however, play a role in one pathway of telomere maintenance in the absence of the telomerase RNA that has been interpreted as occurring by a BIR-type mechanism (42). The recombination defects of the RAD51 mutant resemble that of the MEC1 mutant in several respects. rad51 cells demonstrate a hyporecombination phenotype (with regard to conversion and crossing-over), and intragenic mitotic recombination (conversion) is also severely reduced (64). As in mec1 cells, intergenic mitotic recombination is not reduced but rather elevated (48; J.S.-M. and W.-D.H., unpublished results). This increase in rad51 of apparent crossing-over is most likely due to BIR, which becomes the repair pathway of choice in the absence of recombinational repair (48). The genetic outcome of BIR is equivalent to mitotic, intergenic recombination (Fig. 6), when analyzing a single recombinant chromosome, as done with mec1 (37). Thus, a defect in the Rad51p (Rad55p) pathway, like a defect in MEC1, results in reduced gene conversion but elevated apparent crossing over by shifting the balance from normal recombinational repair in wild-type cells (conversion with low crossing over association) to BIR in mutant cells. This is consistent with our hypothesis that MEC1 modulates the activity of the Rad51p (Rad55p) pathway. This framework of checkpoint control of repair pathway competition and possibly repair template choice can also help rationalize the disparate recombination defects previously observed in checkpoint mutants (20, 21, 82).
The mec1 recombination phenotype observed in G1-arrested cells in this study is unlikely a result of the defect causing delay the cell cycle at the G1/S border in these cells (73, 74). First, microscopic observation showed that the cells did not resume budding for much longer times (4 h) than it takes recombination to repair DNA (1 h) (14). The caveat is that we cannot measure directly the progress of DNA repair of DNA damage caused by MMS. Therefore, we artificially arrested cells in G1 for an additional 6 h, preventing S phase for about 10 h after induction of DNA damage (Fig. 5). The results showed near-identical survival and damage-induced recombination in the absence and presence of the artificial arrest, strongly suggesting that the recombination and survival defects of G1-arrested mec1 cells are cell cycle arrest independent.
Our hypothesis that checkpoints modulate recombinational repair activity may also provide an interpretation for some enigmatic aspects of the cellular defects of MEC1-, mei-41-, and ATM-deficient cells. mec1 and mei-41 are meiotic recombination mutants, and ATM−/− mice show meiotic failure and abnormal chromosome synapsis in meiotic prophase I, but the underlying molecular defects are not understood (27, 30, 37, 91). Meiotic recombination in S. cerevisiae is induced by transient meiosis-specific DSBs delivered by Spo11 protein (6, 38), and the existence of Spo11p homologues in other organisms suggests that this will be a general aspect of meiotic recombination (6, 16, 51). Thus, meiotic recombination resembles DNA damage-induced recombination in mitotic cells. The meiotic recombination defects in mei-41 (30) and mec1 (37) are consistent with a reduced efficiency of the homologous recombination pathway. The reduced number and irregular morphology of recombination nodules in mei-41 oocytes (9) may be interpreted as a lower probability of forming the highly structured recombination protein assemblies because of the failure to optimally recruit Rad55p-like and possibly other proteins as a result of the checkpoint defect. In meiotic recombination, interhomologue interactions are strongly favored over intersister interactions (40, 66), which involves the DNA damage checkpoint (25, 81). A reduction in interhomologue interactions helps explain a meiotic recombination defect (25, 81), and we speculate that partner choice may be enforced by the checkpoint through phosphorylation of critical components of the meiotic recombination pathway.
ACKNOWLEDGMENTS
We thank F. Fabre, D. Schild, T. Weinert, S. Elledge, and H. Ogawa for kindly supplying strains and plasmids; D. Lagarias from the UC Davis FACS facility for her help with FACS analysis; J. Hoeijmakers for helpful comments; and T. Carr and all members of the Heyer laboratory for stimulating discussions and help. We are grateful to S. Hawley, S. Kowalczykowski, J. Nunnari, and K. Shiozaki for their critical reading of the manuscript.
This study was supported in part by a START career development grant and a research grant from the Swiss National Science Foundation to W.-D.H., a Human Frontiers Science Organization grant to W.-D.H., a Russian Foundation for Basic Research grant to V.I.B., a Human Frontier Science Organization postdoctoral fellowship to J.S.K., an International Research Scholar's award from the Howard Hughes Medical Institute to V.I.B. and W.-D.H., a grant from the UC Cancer Research Coordinating Committee, and funds from the University of California, Davis.
REFERENCES
- 1.Aboussekhra A, Vialard J E, Morrison D E, Delatorreruiz M A, Cernakova L, Fabre F, Lowndes N F. A novel role for the budding yeast RAD9 checkpoint gene in DNA damage-dependent transcription. EMBO J. 1996;15:3912–3922. [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Khodairy F, Carr A M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J B, Zhou Z, Siede W, Friedberg E C, Elledge S J. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- 4.Baumann P, West S C. Role of the human RAD51 protein in homologous recombination and double-stranded break repair. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 5.Bentley N J, Holtzman D A, Flaggs G, Keegan K S, DeMaggio A, Ford J C, Hoekstra M, Carr A M. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- 6.Bergerat A, deMassy B, Gadelle D, Varoutas P C, Nicolas A, Forterre P. An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 7.Bosco G, Haber J E. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brush G S, Morrow D M, Hieter P, Kelly T J. The ATM homologue MEC1 is required for phosphorylation of replication protein A in yeast. Proc Natl Acad Sci USA. 1996;93:15075–15080. doi: 10.1073/pnas.93.26.15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter A T C. Recombination nodules and synaptonemal complex in recombination-deficient females of Drosophila melanogaster. Chromosoma. 1979;75:259–292. doi: 10.1007/BF00293472. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Yuan S S F, Liu W, Xu Y, Trujillo K, Song B W, Cong F, Goff S P, Wu Y, Arlinghaus R, Baltimore D, Gasser P J, Park M S, Sung P, Lee E. Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl. J Biol Chem. 1999;274:12748–12752. doi: 10.1074/jbc.274.18.12748. [DOI] [PubMed] [Google Scholar]
- 11.Chua P, Robertson S J. Segregation of recombinant chromatids following mitotic crossing over in yeast. Genetics. 1991;129:359–369. doi: 10.1093/genetics/129.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Fix O, Koshland D. The anaphase inhibitor of Saccharomyces cerevisiae Pds1p is a target of the DNA damage checkpoint pathway. Proc Natl Acad Sci USA. 1997;94:14361–14366. doi: 10.1073/pnas.94.26.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole G M, Mortimer R K. Failure to induce a DNA repair gene, RAD54, in Saccharomyces cerevisiae does not affect DNA repair or recombination phenotypes. Mol Cell Biol. 1989;9:3314–3322. doi: 10.1128/mcb.9.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly B, White C I, Haber J E. Physical monitoring of mating type switching in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:2342–2349. doi: 10.1128/mcb.8.6.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox R, Masson W K, Weichselbaum R R, Nove J, Little J B. The repair of potentially lethal damage in X-irradiated cultures of normal and ataxia-telangiectasia human fibroblasts. Int J Radiat Biol. 1981;39:357–365. doi: 10.1080/09553008114550461. [DOI] [PubMed] [Google Scholar]
- 16.Dernburg A F, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve A M. Meiotic recombination in C-elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell. 1998;94:387–398. doi: 10.1016/s0092-8674(00)81481-6. [DOI] [PubMed] [Google Scholar]
- 17.D'Urso G, Nurse P. Checkpoints in the cell cycle of fission yeast. Curr Opin Genet Dev. 1995;5:12–16. doi: 10.1016/s0959-437x(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 18.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 19.Emili A. MEC1-dependent phosphorylation of Rad9p in response to DNA damage. Mol Cell. 1998;2:183–189. doi: 10.1016/s1097-2765(00)80128-8. [DOI] [PubMed] [Google Scholar]
- 20.Fasullo M, Bennett T, AhChing P, Koudelik J. The Saccharomyces cerevisiae RAD9 checkpoint reduces the DNA damage-associated stimulation of directed translocations. Mol Cell Biol. 1998;18:1190–1200. doi: 10.1128/mcb.18.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fasullo M, Koudelik J, AhChing P, Giallanza P, Cera C. Radiosensitive and mitotic recombination phenotypes of the Saccharomyces cerevisiae dun1 mutant defective in DNA damage-inducible gene expression. Genetics. 1999;152:909–919. doi: 10.1093/genetics/152.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 23.Game J C. DNA double-strand breaks and the RAD50-RAD57 genes in Saccharomyces. Semin Cancer Biol. 1993;4:73–83. [PubMed] [Google Scholar]
- 24.Gardner R, Putnam C W, Weinert T. RAD53, DUN1, and PDS1 define two parallel G2/M checkpoint pathways in budding yeast. EMBO J. 1999;11:3173–3185. doi: 10.1093/emboj/18.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grushcow J M, Holzen T M, Park K J, Weinert T, Lichten M, Bishop D K. Saccharomyces cerevisiae checkpoint genes MEC1, RAD17 and RAD24 are required for normal meiotic recombination partner choice. Genetics. 1999;153:607–620. doi: 10.1093/genetics/153.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haber J E. In vivo biochemistry: physical monitoring of recombination induced by site-specific endonucleases. Bioessays. 1995;17:609–620. doi: 10.1002/bies.950170707. [DOI] [PubMed] [Google Scholar]
- 27.Hari K L, Santerre A, Sekelsky J J, McKim K S, Boyd J B, Hawley R S. The mei-41 gene of D. melanogaster is a structural and functional homolog of the human ataxia telangiectasia gene. Cell. 1995;82:815–821. doi: 10.1016/0092-8674(95)90478-6. [DOI] [PubMed] [Google Scholar]
- 28.Hartwell L H, Mortimer R K, Culotti J, Culotti M. Genetic control of the cell division cycle in yeast. V. genetic analysis of cdc mutants. Genetics. 1973;74:267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartwell L H, Weinert T A. Checkpoints: controls that ensure order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 30.Hawley R S, Friend S H. Strange bedfellows in even stranger places: the role of ATM in meiotic cells, lymphocytes, tumors, and its functional links to p53. Genes Dev. 1996;10:2383–2388. doi: 10.1101/gad.10.19.2383. [DOI] [PubMed] [Google Scholar]
- 31.Hays S L, Firmenich A A, Berg P. Complex formation in yeast double-strand break repair: participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc Natl Acad Sci USA. 1995;92:6925–6929. doi: 10.1073/pnas.92.15.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes A M, Haber J E. Double-strand break repair in yeast requires both leading and lagging strand DNA polymerases. Cell. 1999;96:415–424. doi: 10.1016/s0092-8674(00)80554-1. [DOI] [PubMed] [Google Scholar]
- 33.Jeggo P A, Carr A M, Lehmann A R. Splitting the ATM: distinct repair and checkpoint defects in ataxia-telangiectasia. Trends Genet. 1998;14:312–316. doi: 10.1016/s0168-9525(98)01511-x. [DOI] [PubMed] [Google Scholar]
- 34.Johnson R D, Symington L S. Functional differences and interactions among the putative RecA homologs RAD51, RAD55, and RAD57. Mol Cell Biol. 1995;15:4843–4850. doi: 10.1128/mcb.15.9.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadyk L C, Hartwell L H. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992;132:387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanaar R, Hoeijmakers J H J, van Gent D C. Molecular mechanisms of DNA double-strand break repair. Trends Cell Biol. 1998;8:483–489. doi: 10.1016/s0962-8924(98)01383-x. [DOI] [PubMed] [Google Scholar]
- 37.Kato R, Ogawa H. An essential gene, ESR1, is required for mitotic cell growth, DNA repair and meiotic recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 1994;22:3104–3112. doi: 10.1093/nar/22.15.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keeney S, Giroux C N, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 39.Kiser G L, Weinert T A. Distinct roles of yeast MEC and RAD checkpoint genes in transcriptional induction after DNA damage and implications for function. Mol Biol Cell. 1996;7:703–718. doi: 10.1091/mbc.7.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleckner N. Meiosis: how could it work? Proc Natl Acad Sci USA. 1996;93:8167–8174. doi: 10.1073/pnas.93.16.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le S, Moore J K, Haber J E, Greider C W. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics. 1999;152:143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lew D J, Weinert T, Pringle J R. Cell cycle control in Saccharomyces cerevisiae. In: Pringle J R, Broach J R, Jones E W, editors. The molecular and cellular Biology of the yeast Saccahromyces. Cell cycle and cell Biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 607–695. [Google Scholar]
- 44.Little J W, Edmiston S H, Pacelli L Z, Mount D W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci USA. 1980;77:3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longhese M P, Neecke H, Paciotti V, Lucchini G, Plevani P. The 70 kDa subunit of replication protein A is required for the G1/S and intra-S DNA damage checkpoints in budding yeast. Nucleic Acids Res. 1996;24:3533–3537. doi: 10.1093/nar/24.18.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lovett S T, Mortimer R K. Characterization of null mutants of the RAD55 gene of Saccharomyces cerevisiae: effects of temperature, osmotic strength and mating type. Genetics. 1987;116:547–553. doi: 10.1093/genetics/116.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lydall D, Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995;270:1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- 48.Malkova A, Ivanov E L, Haber J E. Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced DNA replication. Proc Natl Acad Sci USA. 1996;93:7131–7136. doi: 10.1073/pnas.93.14.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marini F, Pellicioli A, Paciotti V, Lucchini G, Plevani P, Stern D F, Foiani M. A role for DNA primase in coupling DNA replication to DNA damage response. EMBO J. 1997;16:639–650. doi: 10.1093/emboj/16.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin S G, Laroche T, Suka N, Grunstein M, Gasser S M. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell. 1999;97:621–633. doi: 10.1016/s0092-8674(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 51.McKim K S, Hagihara A H. mei-W68 in Drosophila melanogaster encodes a Spo11 homolog: evidence that the mechanism for initiating meiotic recombination is conserved. Genes Dev. 1998;12:2932–2942. doi: 10.1101/gad.12.18.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills K D, Sinclair D A, Guarente L. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell. 1999;97:609–620. doi: 10.1016/s0092-8674(00)80772-2. [DOI] [PubMed] [Google Scholar]
- 53.Milne G T, Jin S F, Shannon K B, Weaver D T. Mutations in two Ku homologs define a DNA end-joining repair pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4189–4198. doi: 10.1128/mcb.16.8.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison C, Sonoda E, Takao N, Shinohara A, Yamamoto K, Takeda S. The controlling role of ATM in homologous recombinational repair of DNA damage. EMBO J. 2000;19:463–471. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrow D M, Connelly C, Hieter P. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics. 1997;147:371–382. doi: 10.1093/genetics/147.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nash R, Tokiwa G, Anand S, Erickson K, Futcher A B. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 1988;20:4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navas T A, Sanchez Y, Elledge S J. RAD9 and DNA polymerase epsilon form parallel sensory branches for transducing the DNA damage checkpoint signal in Saccharomyces cerevisiae. Genes Dev. 1996;10:2632–2643. doi: 10.1101/gad.10.20.2632. [DOI] [PubMed] [Google Scholar]
- 58.Navas T A, Zhou Z, Elledge S J. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 59.Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994;80:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 60.Ogawa T, Yu X, Shinohara A, Egelman E H. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science. 1993;259:1896–1899. doi: 10.1126/science.8456314. [DOI] [PubMed] [Google Scholar]
- 61.Paques F, Haber J E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paulovich A G, Hartwell L H. A checkpoint regulates the rate of progression through S phase in S-cerevisiae in response to DNA damage. Cell. 1995;82:841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- 63.Paulovich A G, Margulies R U, Garvik B M, Hartwell L H. RAD9, RAD17, and RAD24 are required for S phase regulation in Saccharomyces cerevisiae in response to DNA damage. Genetics. 1997;145:45–62. doi: 10.1093/genetics/145.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petes T D, Malone R E, Symington L S. Recombination in yeast. In: Broach J R, Jones E, Pringle J, editors. The molecular and cellular biology of the yeast Saccharomyces: genome dynamics, protein synthesis and energetics. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1991. pp. 407–521. [Google Scholar]
- 65.Prakash L, Prakash S. Isolation and characterization of MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977;86:33–55. doi: 10.1093/genetics/86.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roeder G S. Meiotic chromosomes: it takes two to tango. Genes Dev. 1997;11:2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- 67.Rowley R, Subramani S, Young P G. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 1992;11:1335–1342. doi: 10.1002/j.1460-2075.1992.tb05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez Y, Bachant J, Wang H, Hu F H, Liu D, Tetzlaff M, Elledge S J. Control of the DNA damage checkpoint by Chk1 and Rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1171. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez Y, Desany B A, Jones W J, Liu Q H, Wang B, Elledge S J. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- 70.Santos Rosa H, Clever B, Heyer W D, Aguilera A. The yeast HRS1 gene encodes a polyglutamine-rich nuclear protein required for spontaneous and hpr1-induced deletions between direct repeats. Genetics. 1996;142:705–716. doi: 10.1093/genetics/142.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiloh Y. Ataxia-Telangiectasia and the Nijmegen Breakage Syndrome: related disorders but genes apart. Annu Rev Genet. 1997;31:635–662. doi: 10.1146/annurev.genet.31.1.635. [DOI] [PubMed] [Google Scholar]
- 72.Shiloh Y, Tabor E, Becker Y. Repair of potentially lethal and sublethal damage induced by neocarcinostatin in normal and ataxia-telangiectasia skin fibroblasts. Biochem Biophys Res Commun. 1983;110:483–490. doi: 10.1016/0006-291x(83)91175-0. [DOI] [PubMed] [Google Scholar]
- 73.Siede W, Friedberg A S, Dianova I, Friedberg E C. Characterization of G(1) checkpoint control in the yeast Saccharomyces cerevisiae following exposure to DNA-damaging agents. Genetics. 1994;138:271–281. doi: 10.1093/genetics/138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siede W, Friedberg A S, Friedberg E C. RAD9-dependent G1 arrest defines a second checkpoint for damaged DNA in the cell cycle of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1993;90:7985–7989. doi: 10.1073/pnas.90.17.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snow R, Korch C T. Alkylation induced gene conversion in yeast: use in fine structure mapping. Mol Gen Genet. 1970;107:201–208. [Google Scholar]
- 76.Sun Z X, Fay D S, Marini F, Foiani M, Stern D F. Spk1/Rad53 is regulated by Mec1-dependent protein phosphorylation in DNA replication and damage checkpoint pathways. Genes Dev. 1996;10:395–406. doi: 10.1101/gad.10.4.395. [DOI] [PubMed] [Google Scholar]
- 77.Sun Z X, Hsiao J, Fay D S, Stern D F. Rad53 FHA domain associated with phosphorylated Rad9 in the DNA damage checkpoint. Science. 1998;281:272–274. doi: 10.1126/science.281.5374.272. [DOI] [PubMed] [Google Scholar]
- 78.Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science. 1994;265:1241–1243. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
- 79.Sung P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 1997;11:1111–1121. doi: 10.1101/gad.11.9.1111. [DOI] [PubMed] [Google Scholar]
- 80.Takao N, Kato H, Mori R, Morrison C, Sonada E, Sun X, Shimizy H, Yoshioka K, Takeda S, Yamamoto K-I. Disruption of ATM in p53-null cells causes multiple functional abnormalities in cellular response to ionizing radiation. Oncogene. 1999;18:7002–7009. doi: 10.1038/sj.onc.1203172. [DOI] [PubMed] [Google Scholar]
- 81.Thompson D A, Stahl F W. Genetic control of recombination partner preference in yeast meiosis: isolation and characterization of mutants elevated for meiotic unequal sister-chromatid recombination. Genetics. 1999;153:621–641. doi: 10.1093/genetics/153.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vallen E A, Cross F R. Mutations in RAD27 define a potential link between G1 cyclins and DNA replication. Mol Cell Biol. 1995;15:4291–4302. doi: 10.1128/mcb.15.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vialard J E, Gilbert C S, Green C M, Lowndes N F. The budding yeast Rad9 checkpoint protein is subjected to Mec1/Tel1-dependent hyperphosphorylation and interacts with Rad53 after DNA damage. EMBO J. 1998;17:5679–5688. doi: 10.1093/emboj/17.19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 85.Wang J Y J. Cellular responses to DNA damage. Curr Biol. 1998;10:240–247. doi: 10.1016/s0955-0674(98)80146-4. [DOI] [PubMed] [Google Scholar]
- 86.Weichselbaum R R, Nove J, Little J B. Deficient recovery from potentially lethal radiation damage in ataxia telangiectasia and xeroderma pigmentosum. Nature. 1978;271:261–262. doi: 10.1038/271261a0. [DOI] [PubMed] [Google Scholar]
- 87.Weinert T. DNA damage and checkpoint pathways: molecular anatomy and interactions with repair. Cell. 1998;94:555–558. doi: 10.1016/s0092-8674(00)81597-4. [DOI] [PubMed] [Google Scholar]
- 88.Weinert T. DNA damage checkpoints update: getting molecular. Curr Opin Genet Dev. 1998;8:185–193. doi: 10.1016/s0959-437x(98)80140-8. [DOI] [PubMed] [Google Scholar]
- 89.Weinert T A, Hartwell L H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- 90.Weinert T A, Kiser G L, Hartwell L H. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- 91.Xu Y, Ashley T, Brainerd E E, Bronson R T, Meyn M S, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 92.Yuan Z M, Huang Y Y, Ishiko T, Nakada S, Utsugisawa T, Kharbanda S, Wang R, Sung P, Shinohara A, Weichselbaum R, Kufe D. Regulation of Rad51 function by c-Abl in response to DNA damage. J Biol Chem. 1998;273:3799–3802. doi: 10.1074/jbc.273.7.3799. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Z, Elledge S J. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75:1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]