Abstract
Cystic fibrosis (CF) is a chronic multisystem disease with manifestations from birth. It involves the entire respiratory system, with increased cough, and recurrent pulmonary infections, and it also leads to intestinal malabsorption, all of which can have an impact on sleep. In this review, we summarize the available literature on the various sleep disturbances in children with CF. Sleep quality and sleep efficiency are often impaired in children with CF. They may be accompanied by symptoms associated with sleep-disordered breathing (SDB), and objective findings such as nocturnal hypoxemia. Importantly, a strong association has been shown between SDB and the severity of lung disease, and some studies have reported a similar association for sleep quality. Further research is needed to better characterize the association of sleep disturbances with respiratory outcomes and the impact of treatment of sleep disorders on pulmonary status in children with CF.
Keywords: Cystic fibrosis, Pediatrics, Sleep
Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that encodes a chloride channel responsible for the regulation of water and electrolyte transport across epithelial cell apical membranes.1,2 The major clinical manifestations of CF are respiratory and gastrointestinal.3 In the respiratory tract there is accumulation of thick mucus, impaired mucociliary clearance, chronic inflammation, and recurrent upper and lower respiratory infections; whereas in the gastrointestinal tract pancreatic insufficiency usually lead to malabsorption and steatorrhea.4
Sleep is an important pillar of general health and normal development in children. Insufficient sleep, sleep disruption, and sleep-disordered breathing (SDB) have far-reaching consequences on the physical and psychological wellbeing of children and adolescents. Sleep disorders and SDB are common, with 1-4% of children suffering from SDB and 5-30% from other sleep problems5,6. Sleep deprivation leads to mood disorders, fatigue, attention deficits, daytime sleepiness and anxiety, as well as impaired decision-making leading to risky behaviors.7-13 Short sleep duration and poor sleep quality are associated with obesity 14,15, metabolic derangements such as insulin resistance14-16, immune dysfunction,17 and cardiovascular alterations.18
A multitude of factors associated with disease activity in CF have the potential to disrupt sleep such as chronic cough, upper- and lower airway inflammation and infection, gastroesophageal reflux, abdominal pain, frequent stooling, and medications effects.19,20 However, controlled studies focusing on sleep in children with CF are scarce, and their results conflicting.
In this review, we summarize the current knowledge on sleep disorders in children with CF and identify knowledge gaps for future research.
This expands on our recent meta-analysis we reviewed on sleep and cystic fibrosis,21 by specifically focusing on children. Since the first publication on sleep in children with cystic fibrosis in 198322 there have been multiple additional studies and reports (see a recent review in this journal23). Thirteen pediatric studies explored PSG findings,22,24-35 three utilized nocturnal oximetry,36-38 and one reported the results of nocturnal noninvasive ventilation.39 One study assessed ENT examination and endoscopy findings,40 another nocturnal cough,41 and one transcutaneous blood gas levels.42 There have been six studies of behavioral aspects of sleep using validated questionnaires.3,43-47 Two publications described case reports,48,49 and there were six reviews21,50-54. Two additional studies assessed sleep in the mothers of children with CF.55,56 One publication was a comment on a questionnaire study.57 In this review we attempt to describe the main findings of these studies and reports.
Sleep Quality in CF
A majority of studies that have included sleep questionnaires reported impaired sleep quality in children with CF21. Overall, both objective and subjective measurements indicate reduced sleep efficiency, defined as the time spent asleep as the percentage of time spent in bed, in children with CF, as compared with healthy children. Decreased sleep efficiency is most often the result of increased awakenings and longer wake-after-sleep-onset (WASO) periods3,26,32,45 , often associated with snoring and symptoms of SDB.26,32,43 Insomnia symptoms are common, manifesting as difficulties in sleep initiation,26,32,43 as well as complaints of daytime sleepiness.26,58 Sleep quality correlates with quality of life in children with CF58. We have shown similar findings in children with CF and children with Primary Ciliary Dyskinesia (PCD).47
Sleep quality and its association with CF lung disease
A significant correlation is often described between the severity of CF lung disease and objective sleep disturbances as well as subjective complaints of sleepiness:
Children with lower FEV1 have longer sleep latencies, more awakenings, and spend more time in WASO, leading to overall lower sleep efficiencies. On the other hand, decreased sleep efficiency and shorter sleep duration are independently associated with lower FEV1.3,45,59 Increased reporting of daytime sleepiness was also found to correlate with a more severe lung disease.45 While limited, these data suggest a bidirectional relationship between sleep quality and pulmonary disease severity in children with CF.
Causes of Poor Sleep Quality in CF
In addition to pulmonary disease severity, there may be multiple other causes for poor sleep in CF. Most of the studies include children with CF and either normal, or mildly impaired, pulmonary functions, assessed during periods of relative stability, and free of acute exacerbations. This would suggest that sleep disruption is not solely the result of gas-exchange abnormalities. It is plausible that sleep is disrupted due to factors related to chronic lung disease such as nocturnal cough, and gastrointestinal symptoms such as gastroesophageal reflux and abdominal pain.3,20 In addition, sleep may be affected by time-consuming treatments such as airway clearance with manual chest physiotherapy, high-frequency chest wall oscillation vest use, and inhaled medications.43 Such treatments, carried out before bedtime, may affect sleep, both from the behavioral and pharmacological aspects. Cystic fibrosis affects the entire respiratory system, causing sinopulmonary disease and impaired mucociliary clearance that can play a role in the pathophysiology of SDB.60,61 This may explain the high prevalence of snoring and mouth breathing on the questionnaires, discussed below.
Cough during sleep is a well-recognized cause of disturbed sleep and poor sleep quality in children with other respiratory conditions as well.62 However, only one study has examined cough objectively using a cough recorder, in children with stable CF.41 The study found that nocturnal cough was more frequent than described for healthy children, and more prevalent in children with more advanced lung disease.
Sleep quality in CF may also be affected by chronic pain, similar to other chronic pediatric conditions, though the exact mechanism of how pain impairs sleep is not clear. Two-thirds of children with CF report recurrent pain episodes. Most of the complaints are related to abdominal pain, but others include musculoskeletal and joint pain.63 Poor sleep leads to a higher perception of pain symptoms.50 The bidirectional model suggests that pain leads to poor sleep that in turn leads to higher pain levels64 while the unidirectional model is based on studies showing poor sleep leading to increased pain but no clear evidence of the other direction.65 Improving sleep quality may diminish pain in children with CF, although this is yet to be demonstrated. A recent review described the prevalence of pain and sleep complaints in children with CF and noted a dearth of studies linking the two.50
It is important to note that acute pulmonary exacerbations are a common cause of morbidity and mortality in CF, but no study thus far has addressed the effect of acute pulmonary exacerbations on sleep quality in children with CF. In adults, it has been shown that acute exacerbations impair sleep and neurobehavioral performance, irrespective of the severity of underlying lung disease.66
Sleep Architecture in CF
Only a handful of studies have looked at sleep architecture, defined as the cyclical distribution of the different sleep stages along the sleep time. This includes N1, N2, N3 (slow-wave sleep) and REM stages and the timing of sleep relative to time in bed. Whereas sleep quality is often disturbed, sleep architecture seems to be relatively preserved in children with CF when compared with healthy controls. Some studies have reported a lower percentage of REM sleep out of total sleep time in children with CF, while others have not (Table 1). In addition, though sleep is disturbed in children with CF, the arousal index as measured by PSG is often within normal limits.
Table 1 –
Polysomnography studies in children with CF.
| Reference | Sample size | Age (years) | Pulmonary Functions | Main findings | |||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | FEV1% | FVC% | ||
| Tepper 198322 | 6 | n/a | 10-16 | n/a | - | 61 (9) | Decreased minute ventilation and tidal volume, and hypoxia during sleep, more pronounced during REM sleep |
| Avital 199124 | 12 | n/a | 7-17 | n/a | 62 (9) | - | Theophylline reduced HR, improved SpO2, and disrupted sleep with lower SE and higher nocturnal wake time. No effect on AHI or PLM |
| Villa 200125 | 19 | 20 | 13.1mos (3-36) | Matched | - | - | Lower mean SpO2 (95.6% vs 96.9%) and SpO2 nadir (85.9% vs 89.1%); no differences in %REM (27% vs 28%). Differences more significant in children with symptoms of airway inflammation. |
| Naqvi 200826 | 24 | 14 | 14.2 (3.8)* | 10.7 (4.4) | - | - | Lower SpO2 nadir (90.3% vs 95.6%); lower SE (75.2% vs. 86.2%) and %REM (12.7% vs 18.3%) |
| Ramos 200927 | 63 | n/a | 2-14 | n/a | - | - | OSA (oAHI≥1) in 55.6% with signs of chronic rhinosinusitis |
| Suratwala 201128 | 25 | 25 | 8-20 | 7-20 | 99 (12) | 92 (14) | Lower mean SpO2 (96.6% vs 97.5%) and SpO2 nadir (92.5% vs 93.8%); no differences in SE (78% vs. 83%) or %REM (19% vs 18%). Nocturnal hypoxemia correlated with impaired glucose tolerance. |
| Spicuzza 201229 | 40 | 18 | 0.5-11 | Matched | 78.6 (4.7) | 81.7 (3.9) | Lower mean SpO2 (94.7% vs 97%); lower SE (80.4% vs. 87.8%) and %REM (11.7% vs 13.1%); higher AHI (7.3/hour vs 0.5/hour) |
| Ramos 201330 | 67 | n/a | 2-14 | n/a | 78.5 (67.0-92.8)† | - | Nocturnal hypoxemia correlated negatively with FEV1, FVC, arousal index and AHI. |
| Paranjape 201531 | 10 | 10 | 9.6 (3.6)† | 9.6 (3.6) | 87.0 (25.7) | - | Children with CF vs snoring age- and BMI-matched controls. Lower mean SpO2 in both REM & NREM, and SpO2 nadir (90% vs 93%); no differences in %REM (19.9% vs 17.5%) or SE (81.6% vs. 81.7%). No difference in SDB parameters on PSG. |
| Silva 201632 | 33 | n/a | 6-18 | n/a | Z score −1.76 (1.6) | - | 87.9% had reported sleep-related complaints. SE, WASO and sleep latency were impaired. REM% was within normal limits. FEV1 was negatively associated with mean nocturnal SpO2. |
| Waters 201633 | 46 | n/a | 8-12 | n/a | 74.6 (18.8) | 87.4 (16.5) | Respiratory parameters altered included increased respiratory rate in slow-wave sleep and mild CO2 retention in REM, both of which were independently associated with FEV1. |
| Isaiah 2019 35 | 35 | n/a | 11.6 (9.5-13.1)** | n/a | 60.7 (53.0-68.5) | - | OSA present in 50%; FEV1 <53% was the best predictor for sleep hypoxemia. |
| Barbosa 202034 | 31 | n/a | 9.6 (7.9–15.1) † | n/a | 68.1 (24.4) | 77.8 (21.4) | OSA present in 32.3%; nocturnal hypoxemia in 29%; OSA and hypoxemia associated with lower FVC and FEV1. |
mean (SE).
median (SD).
mean (95% confidence interval). n/a: not applicable.
Abbreviations: CF: cystic fibrosis; MV: minute ventilation; Vt: tidal volume; REM: rapid eye movement; HR: heart rate; SpO2: oxygen saturation; SE: sleep efficiency as percent of total sleep time; AHI: apnea hypopnea index; PLM: periodic leg movements; %REM: REM as percent of TST; OSA: obstructive sleep apnea; NREM: non REM; FEV1: forced expiratory volume at 1 sec; FVC: Forced Vital Capacity
Sleep-disordered Breathing in CF
Children with CF and their parents often report sleep-disordered breathing (SDB) associated complaints. 26,32,43 The most common complaint is snoring, followed by difficulty breathing during the night, mouth breathing, and pauses in breathing during sleep. Table 1 summarizes pediatric PSG studies, five of which have compared children with CF to healthy controls.25,26,28,29,31 Outcome variables included measures of sleep architecture, gas exchange, apnea-hypopnea (AHI) and/or respiratory-disturbance indices (RDI). Overall, children with CF have lower nighttime SpO2 means and nadirs relative to healthy controls.
The degree to which the prevalence of SDB differs in children with CF from the general population remains unclear. Reports suffer from significant heterogeneity, partly as a result of different respiratory event indices used (AHI versus RDI), and partly due to changes to the scoring criteria guided by the American Academy of Sleep Medicine (AASM) during the years in which the studies were published. Even the cut-offs for the definition of OSA differ between some reports. Uncontrolled studies report a prevalence of OSA in up to 50% of their cohort, though these reports often suffer from referral bias due to selection of symptomatic children who are preferentially referred for PSG. Only one study, which compared 40 children with CF ages 6 months – 11 years to 18 healthy controls, reported a higher AHI in children with CF [7.13 (SD=1.3) vs. 0.5 (0.4), p<0.001].29 In this study, SDB was most severe in children under six years old.29 The other four controlled studies (n=78, control=69) did not identify a significant difference in respiratory event indices.25,26,28,31 Thus, this question remains unresolved and probably requires a more personalized diagnostic approach to understand which clinical characteristics in children with CF predispose them to SDB.
Sleep-Disordered breathing correlation to lung disease severity
Whereas the prevalence of SDB is not clearly increased in children with CF, there seems to be a clear correlation between the severity of CF lung disease, as assessed by FEV1, and the nocturnal oxygen saturation. A direct correlation has been reported between FEV1 and the mean SpO2 ,32,34,37 between FEV1 and the SpO2 nadir,32,33 and between FVC and the mean SpO2.34 In two studies, nocturnal hypoxemia burden (SpO2<90% more than 5% of TST) was associated with lower FEV1.30,34 Finally, Silva et al. identified an association between higher AHI on PSG and lower wake saturations.32 Only two studies have found no association between lung disease severity and nocturnal oxygenation indices: Uyan et al. did not find an association between FEV1 and nocturnal hypoxemia36, although association was observed between nocturnal hypoxemia and a more advanced lung disease on chest CF. Spicuzza et al. reported an association between mean nocturnal SpO2 with wake SpO2, but not with the FEV1.29 In summary, of the studies assessing the relationship between CF lung disease severity in children and nocturnal oxygenation, six reported a significant correlation (n=234) 30,32-35,37 while two found no significant association (n=64).29,36
SDB has a significant impact on the wellbeing and quality of life in children.13 Children with SDB may exhibit aggressiveness, lower social competency, and poorer communication or adaptive skills,12 as well as symptoms of ADHD that improve after adenotonsillectomy10 or after PAP therapy, as is exemplified by the illustrative case presented above. They also tend to have higher resting blood pressure,18 increased systemic inflammation67,68 and impaired glucose metabolism.69 We could find no studies on the specific effects of SDB, in children with CF, on these end-organ or neurobehavioral manifestations. Furthermore, we could find no studies on the effect of treatment of SDB in this population. One notable exception is a study that examined the association between glucose tolerance and nocturnal oxygen saturations. Glucose metabolism is a significant factor in CF disease progression – children with CF and glucose intolerance have worse pulmonary outcomes, and improving glucose status improves lung disease.70 Mirroring the findings from healthy children with SDB, this study described an association between nocturnal hypoxemia and glucose intolerance in children with CF.28 More studies are needed to determine the causal factors for impaired glucose metabolism in CF – nocturnal hypoxemia or early airway obstruction and whether treatment of SDB would improve glucose and pulmonary status.
Noninvasive ventilation
In CF, work of breathing is increased as a result of airway obstruction, mucous plugging, bronchial inflammation and parenchymal destruction. In advanced disease, alveolar hypoventilation becomes increasingly common.71 With the introduction of novel treatments such as CFTR modulators, lung disease progression becomes more gradual for many patients, possibly making the need for non-invasive support less relevant to the pediatric population. Most of the studies on noninvasive ventilation in patients with CF are case series of adults with advanced lung disease. There are several small series in children with the following findings: Caronia et al.39 described a series of nine CF patients with end-stage lung disease, three aged 19-20 years old, awaiting lung transplantation. They started noninvasive bi-level positive airway pressure (BiPAP) treatment after being admitted for respiratory decompensation. All showed improved respiratory status parameters and the authors concluded that this therapy was well tolerated for long-term home use and can provide an extended period of respiratory comfort and stability. Efrati et al.72 also reported a series of 9 patients, two of whom were children age 6 and 17 years old. BiPAP treatment leads to improvement in gas exchange parameters, resolution of morning headaches and improved sleep quality, whereas pulmonary function was not affected. These beneficial effects were reported in the whole cohort but were not observed in the two children sub-group.
Conclusions
The illustrative case described above gives an example of the benefit of addressing sleep disorders in a child with CF. Having reviewed both subjective and objective evidence of frequent sleep disturbances in children with CF, we hope we have emphasized the importance of addressing this domain on routine patient encounters. Children with CF have poor sleep quality with increased cough during sleep, reduced sleep efficiency, increased symptoms of sleep-disordered breathing and objectively lower baseline oxygen saturations. Due to the small samples of the studies to date, it remains unclear whether children with CF have a higher prevalence of OSA when compared to healthy age-matched controls. It seems that there is a clear association between the degree of nocturnal hypoxemia and the severity of CF lung disease. Non-invasive ventilation may be a valuable tool in patients with end-stage lung disease, although the data for children are lacking.
Future directions
Studies looking into screening for and treating sleep disorders in CF are required. Additional research is needed, with larger sample sizes and standardized outcomes, to better define these sleep abnormalities and their relationship with disease severity. It remains to be shown whether novel CFTR modulators have a beneficial effect on sleep quality and SDB prevalence in children with CF, in which case sleep disturbances may serve as a much needed outcome measure in this age group.
Finally, treatment of sleep disturbances needs to be addressed as a potential tool in the arsenal of CF therapy geared towards improving lung function and quality of life in children with CF.
Figure 1:
9 year old girl with CF with her CPAP device (with permission from the family)
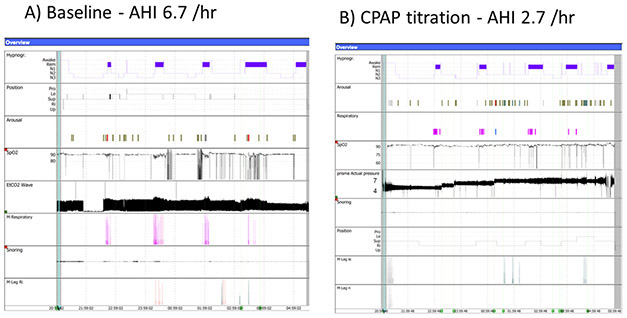
Figure 2:
Comparison of hypnogram before (a) and after (b) initiation of CPAP, demonstrating optimal control of sleep-disordered breathing with a pressure of 7cmH2O.
Illustrative case.
A.T. is a 9-year old girl with cystic fibrosis (CF), homozygous for the delF508 mutation. She presented to the sleep unit due to complaints of snoring, observed apneas, CO2 retention and diurnal hypoxemia. At the time she already had advanced lung disease, with a FEV1 41 percent-predicted, and recurrent admissions for pulmonary exacerbations. Medications included antibiotics, insulin, monteleukast, vitamins and pancreatic enzymes, in addition to daily airway clearance with hypertonic saline and chest physiotherapy. Her family complained of behavioral problems affecting adherence with treatments as well as poor performance at school. She was referred for an overnight polysomnography (PSG) that revealed moderate obstructive sleep apnea (OSA) with an apnea-hypopnea index (AHI) of 6.7, oxygen saturation (SpO2) nadir of 82%, and 30 minutes pulse oximetry saturations below 90%. On otolaryngology evaluation and drug-induced sleep endoscopy (DISE) adenoid and tonsillar tissue obstruction of the airway were ruled out with pharyngomalacia noted as the cause of upper airway obstruction. Subsequently, she was fitted with a continuous positive airway pressure (CPAP) device, with a nasal mask at a pressure of 7 cm H2O after in-lab titration (Figure 1). Repeat PSG showed a significant improvement in OSA and nocturnal hypoxemia with CPAP therapy (Figure 2). Shortly thereafter her family and school reported significant improvements in behavior and academic performance, presumably leading to improved compliance with care. She gained ~2.5kg in weight the following year and required fewer admissions.
Sources of support:
Dr. Gileles-Hillel’s receives research support from the Israel-Science Foundation grant 2779/19.
Dr Forno’s contribution was funded in part by grant HL149693 from the U.S. National Institutes of Health (NIH).
Footnotes
Data from this manuscript has been presented at the 31st Annual Meeting of the Associated Professional Sleep Societies, LLC (APSS), Boston, MA, June 2017.
Data availability statement:
Not applicable – no new data generated
REFERENCES
- 1.Bixler EO, Vgontzas AN, Lin H-M, et al. Sleep Disordered Breathing in Children in a General Population Sample: Prevalence and Risk Factors. Sleep. 2009;32(6):731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerem E, Wilschanski M, Miller NL, et al. Ambulatory quantitative waking and sleeping cough assessment in patients with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. May 2011;10(3):193–200. [DOI] [PubMed] [Google Scholar]
- 3.Amin R, Bean J, Burklow K, Jeffries J. The relationship between sleep disturbance and pulmonary function in stable pediatric cystic fibrosis patients. Chest. Sep 2005;128(3):1357–1363. [DOI] [PubMed] [Google Scholar]
- 4.Ratjen F, Doring G. Cystic fibrosis. Lancet. Feb 22 2003;361(9358):681–689. [DOI] [PubMed] [Google Scholar]
- 5.Fricke-Oerkermann L, Pluck J, Schredl M, et al. Prevalence and course of sleep problems in childhood. Sleep. Oct 2007;30(10):1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. Jun 2009;32(6):731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangwisch JE, Babiss LA, Malaspina D, Turner JB, Zammit GK, Posner K. Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep. Jan 2010;33(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. The effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage. May 1 2013;71:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Short MA, Gradisar M, Lack LC, Wright HR, Dohnt H. The sleep patterns and well-being of Australian adolescents. Journal of adolescence. Feb 2013;36(1):103–110. [DOI] [PubMed] [Google Scholar]
- 10.Sedky K, Bennett DS, Carvalho KS. Attention deficit hyperactivity disorder and sleep disordered breathing in pediatric populations: a meta-analysis. Sleep medicine reviews. Aug 2014;18(4):349–356. [DOI] [PubMed] [Google Scholar]
- 11.Miano S, Paolino MC, Urbano A, et al. Neurocognitive assessment and sleep analysis in children with sleep-disordered breathing. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. Feb 2011;122(2):311–319. [DOI] [PubMed] [Google Scholar]
- 12.Perfect MM, Archbold K, Goodwin JL, Levine-Donnerstein D, Quan SF. Risk of behavioral and adaptive functioning difficulties in youth with previous and current sleep disordered breathing. Sleep. Apr 01 2013;36(4):517–525B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldassari CM, Mitchell RB, Schubert C, Rudnick EF. Pediatric obstructive sleep apnea and quality of life: a meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. Mar 2008;138(3):265–273. [DOI] [PubMed] [Google Scholar]
- 14.Araujo J, Severo M, Ramos E. Sleep duration and adiposity during adolescence. Pediatrics. Nov 2012;130(5):e1146–1154. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JA, Rodriguez D, Schmitz KH, Audrain-McGovern J. Sleep duration and adolescent obesity. Pediatrics. May 2013;131(5):e1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. Oct 2012;35(10):1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatric clinics of North America. Jun 2011;58(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horne RS, Yang JS, Walter LM, et al. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. Jul 2011;128(1):e85–92. [DOI] [PubMed] [Google Scholar]
- 19.Katz ES. Cystic fibrosis and sleep. Clinics in chest medicine. Sep 2014;35(3):495–504. [DOI] [PubMed] [Google Scholar]
- 20.Bouka A, Tiede H, Liebich L, et al. Quality of life in clinically stable adult cystic fibrosis out-patients: associations with daytime sleepiness and sleep quality. Respiratory medicine. Sep 2012;106(9):1244–1249. [DOI] [PubMed] [Google Scholar]
- 21.Reiter J, Gileles-Hillel A, Cohen-Cymberknoh M, et al. Sleep disorders in cystic fibrosis: A systematic review and meta-analysis. Sleep medicine reviews. Jun 2020;51:101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tepper RS, Skatrud JB, Dempsey JA. Ventilation and oxygenation changes during sleep in cystic fibrosis. Chest. Oct 1983;84(4):388–393. [DOI] [PubMed] [Google Scholar]
- 23.Jagpal SK, Jobanputra AM, Ahmed OH, Santiago TV, Ramagopal M. Sleep-disordered breathing in cystic fibrosis. Pediatr Pulmonol. Feb 2021;56 Suppl 1:S23–S31. [DOI] [PubMed] [Google Scholar]
- 24.Avital A, Sanchez I, Holbrow J, Kryger M, Chernick V. Effect of theophylline on lung function tests, sleep quality, and nighttime SaO2 in children with cystic fibrosis. The American review of respiratory disease. Dec 1991;144(6):1245–1249. [DOI] [PubMed] [Google Scholar]
- 25.Villa MP, Pagani J, Lucidi V, Palamides S, Ronchetti R. Nocturnal oximetry in infants with cystic fibrosis. Archives of disease in childhood. Jan 2001;84(1):50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naqvi SK, Sotelo C, Murry L, Simakajornboon N. Sleep architecture in children and adolescents with cystic fibrosis and the association with severity of lung disease. Sleep Breath. Mar 2008;12(1):77–83. [DOI] [PubMed] [Google Scholar]
- 27.Ramos RT, Salles C, Gregorio PB, et al. Evaluation of the upper airway in children and adolescents with cystic fibrosis and obstructive sleep apnea syndrome. Int J Pediatr Otorhinolaryngol. Dec 2009;73(12):1780–1785. [DOI] [PubMed] [Google Scholar]
- 28.Suratwala D, Chan JS, Kelly A, et al. Nocturnal saturation and glucose tolerance in children with cystic fibrosis. Thorax. Jul 2011;66(7):574–578. [DOI] [PubMed] [Google Scholar]
- 29.Spicuzza L, Sciuto C, Leonardi S, La Rosa M. Early occurrence of obstructive sleep apnea in infants and children with cystic fibrosis. Arch Pediatr Adolesc Med. Dec 2012;166(12):1165–1169. [DOI] [PubMed] [Google Scholar]
- 30.Ramos RT, Santana MA, Almeida Pde C, Machado Jr Ade S, Araujo-Filho JB, Salles C. Nocturnal hypoxemia in children and adolescents with cystic fibrosis. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. Nov-Dec 2013;39(6):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paranjape SM, McGinley BM, Braun AT, Schneider H. Polysomnographic Markers in Children With Cystic Fibrosis Lung Disease. Pediatrics. Nov 2015;136(5):920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva AM, Descalco A, Salgueiro M, et al. Respiratory sleep disturbance in children and adolescents with cystic fibrosis. Revista portuguesa de pneumologia. Jul-Aug 2016;22(4):202–208. [DOI] [PubMed] [Google Scholar]
- 33.Waters KA, Lowe A, Cooper P, Vella S, Selvadurai H. A cross-sectional analysis of daytime versus nocturnal polysomnographic respiratory parameters in cystic fibrosis during early adolescence. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. Oct 08 2016. [DOI] [PubMed] [Google Scholar]
- 34.Barbosa RRB, Liberato FMG, de Freitas Coelho P, Vidal PDR, de Carvalho R, Donadio MVF. Sleep-disordered breathing and markers of morbidity in children and adolescents with cystic fibrosis. Pediatr Pulmonol. Aug 2020;55(8):1974–1983. [DOI] [PubMed] [Google Scholar]
- 35.Isaiah A, Daher A, Sharma PB, Naqvi K, Mitchell RB. Predictors of sleep hypoxemia in children with cystic fibrosis. Pediatr Pulmonol. Mar 2019;54(3):273–279. [DOI] [PubMed] [Google Scholar]
- 36.Uyan ZS, Ozdemir N, Ersu R, et al. Factors that correlate with sleep oxygenation in children with cystic fibrosis. Pediatr Pulmonol. Aug 2007;42(8):716–722. [DOI] [PubMed] [Google Scholar]
- 37.van der Giessen L, Bakker M, Joosten K, Hop W, Tiddens H. Nocturnal oxygen saturation in children with stable cystic fibrosis. Pediatr Pulmonol. Nov 2012;47(11):1123–1130. [DOI] [PubMed] [Google Scholar]
- 38.Katsouli G, Polytarchou A, Tsaoussoglou M, Loukou I, Chrousos G, Kaditis AG. Nocturnal oximetry in children with obstructive lung disease or sleep-disordered breathing. Pediatr Pulmonol. May 2019;54(5):551–556. [DOI] [PubMed] [Google Scholar]
- 39.Caronia CG, Silver P, Nimkoff L, Gorvoy J, Quinn C, Sagy M. Use of bilevel positive airway pressure (BIPAP) in end-stage patients with cystic fibrosis awaiting lung transplantation. Clinical pediatrics. Sep 1998;37(9):555–559. [DOI] [PubMed] [Google Scholar]
- 40.Franco LP, Camargos PA, Becker HM, Guimaraes RE. Nasal endoscopic evaluation of children and adolescents with cystic fibrosis. Brazilian journal of otorhinolaryngology. Nov-Dec 2009;75(6):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Giessen L, Loeve M, de Jongste J, Hop W, Tiddens H. Nocturnal cough in children with stable cystic fibrosis. Pediatr Pulmonol. Sep 2009;44(9):859–865. [DOI] [PubMed] [Google Scholar]
- 42.Pradal U, Braggion C, Mastella G. Transcutaneous blood gas analysis during sleep and exercise in cystic fibrosis. Pediatr Pulmonol. 1990;8(3):162–167. [DOI] [PubMed] [Google Scholar]
- 43.Meltzer LJ, Beck SE. Sleep Patterns in Children with Cystic Fibrosis. Children's health care : journal of the Association for the Care of Children's Health. 2012;41(3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward C, Massie J, Glazner J, et al. Problem behaviours and parenting in preschool children with cystic fibrosis. Archives of disease in childhood. May 2009;94(5):341–347. [DOI] [PubMed] [Google Scholar]
- 45.Vandeleur M, Walter LM, Armstrong DS, Robinson P, Nixon GM, Horne RS. How Well Do Children with Cystic Fibrosis Sleep? An Actigraphic and Questionnaire-Based Study. J Pediatr. Mar 2017;182:170–176. [DOI] [PubMed] [Google Scholar]
- 46.Cavanaugh K, Read L, Dreyfus J, Johnson M, McNamara J. Association of poor sleep with behavior and quality of life in children and adolescents with cystic fibrosis. Sleep and Biological Rhythms. 2016;14(2):199–204. [Google Scholar]
- 47.Cohen-Cymberknoh M, Atia O, Gileles-Hillel A, Kerem E, Reiter J. Sleep disorders in patients with primary ciliary dyskinesia, cystic fibrosis with and without pancreatic insufficiency. Respiratory medicine. May 2019;151:96–101. [DOI] [PubMed] [Google Scholar]
- 48.Macdonald KD, McGinley BM, Brown DJ, Sterni LM, Rosenstein BJ, Mogayzel PJ Jr., Primary snoring and growth failure in a patient with cystic fibrosis. Respiratory care. Dec 2009;54(12):1727–1731. [PubMed] [Google Scholar]
- 49.Hayes D Jr., Obstructive sleep apnea syndrome: a potential cause of lower airway obstruction in cystic fibrosis. Sleep Med. Jan 2006;7(1):73–75. [DOI] [PubMed] [Google Scholar]
- 50.Allen JM, Graef DM, Ehrentraut JH, Tynes BL, Crabtree VM. Sleep and Pain in Pediatric Illness: A Conceptual Review. CNS neuroscience & therapeutics. Nov 2016;22(11):880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong D The use of continuous positive airway pressure or non-invasive ventilation as forms of respiratory support in children with cystic fibrosis. Paediatric respiratory reviews. May 2013;14 Suppl 1:19–21. [DOI] [PubMed] [Google Scholar]
- 52.Neelam K, Shekhar AG. Sleep in Pediatric Pulmonary Diseases. Current Respiratory Medicine Reviews. 2009;5(4):220–224. [Google Scholar]
- 53.Splaingard M Sleep Problems in Children with Respiratory Disorders. Sleep medicine clinics.3(4):589–600. [Google Scholar]
- 54.Shakkottai A, O'Brien LM, Nasr SZ, Chervin RD. Sleep disturbances and their impact in pediatric cystic fibrosis. Sleep medicine reviews. Dec 2018;42:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yilmaz O, Sogut A, Gulle S, Can D, Ertan P, Yuksel H. Sleep quality and depression-anxiety in mothers of children with two chronic respiratory diseases: asthma and cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. Nov 2008;7(6):495–500. [DOI] [PubMed] [Google Scholar]
- 56.Vardar-Yagli N, Saglam M, Inal-Ince D, et al. Hospitalization of Children with Cystic Fibrosis Adversely Affects Mothers’ Physical Activity, Sleep Quality, and Psychological Status. Journal of Child and Family Studies. 2017;26(3):800–809. [Google Scholar]
- 57.Bayer JK, Nicholson JM. Problems with sleep, eating, and adherence to therapy are common among children with cystic fibrosis. J Pediatr. Nov 2009;155(5):759–760. [DOI] [PubMed] [Google Scholar]
- 58.Vandeleur M, Walter LM, Armstrong DS, Robinson P, Nixon GM, Horne RSC. Quality of life and mood in children with cystic fibrosis: Associations with sleep quality. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. Nov 2018;17(6):811–820. [DOI] [PubMed] [Google Scholar]
- 59.de Castro-Silva C, de Bruin VM, Cunha GM, Nunes DM, Medeiros CA, de Bruin PF. Melatonin improves sleep and reduces nitrite in the exhaled breath condensate in cystic fibrosis--a randomized, double-blind placebo-controlled study. Journal of pineal research. Jan 2010;48(1):65–71. [DOI] [PubMed] [Google Scholar]
- 60.Cohen-Cymberknoh M, Simanovsky N, Hiller N, Gileles Hillel A, Shoseyov D, Kerem E. Differences in disease expression between primary ciliary dyskinesia and cystic fibrosis with and without pancreatic insufficiency. Chest. Apr 2014;145(4):738–744. [DOI] [PubMed] [Google Scholar]
- 61.Deniz M, Gultekin E, Ciftci Z, et al. Nasal mucociliary clearance in obstructive sleep apnea syndrome patients. Am J Rhinol Allergy. Sep-Oct 2014;28(5):178–180. [DOI] [PubMed] [Google Scholar]
- 62.Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. Sep 2012;130(3):465–471. [DOI] [PubMed] [Google Scholar]
- 63.Sermet-Gaudelus I, De Villartay P, de Dreuzy P, et al. Pain in children and adults with cystic fibrosis: a comparative study. Journal of pain and symptom management. Aug 2009;38(2):281–290. [DOI] [PubMed] [Google Scholar]
- 64.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Seminars in neurology. Mar 2005;25(1):106–116. [DOI] [PubMed] [Google Scholar]
- 65.. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. The journal of pain : official journal of the American Pain Society. Dec 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobbin CJ, Bartlett D, Melehan K, Grunstein RR, Bye PT. The effect of infective exacerbations on sleep and neurobehavioral function in cystic fibrosis. American journal of respiratory and critical care medicine. Jul 01 2005;172(1):99–104. [DOI] [PubMed] [Google Scholar]
- 67.Gileles-Hillel A, Alonso-Alvarez ML, Kheirandish-Gozal L, et al. Inflammatory markers and obstructive sleep apnea in obese children: the NANOS study. Mediators of inflammation. 2014;2014:605280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nachalon Y, Lowenthal N, Greenberg-Dotan S, Goldbart AD. Inflammation and growth in young children with obstructive sleep apnea syndrome before and after adenotonsillectomy. Mediators of inflammation. 2014;2014:146893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koren D, Gozal D, Philby MF, Bhattacharjee R, Kheirandish-Gozal L. Impact of obstructive sleep apnoea on insulin resistance in nonobese and obese children. Eur Respir J. Apr 2016;47(4):1152–1161. [DOI] [PubMed] [Google Scholar]
- 70.Prentice BJ, Chelliah A, Ooi CY, et al. Peak OGTT glucose is associated with lower lung function in young children with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. Mar 2020;19(2):305–309. [DOI] [PubMed] [Google Scholar]
- 71.Fauroux B, Why, when and how to propose noninvasive ventilation in cystic fibrosis? Minerva anestesiologica. Nov 2011;77(11):1108–1114. [PubMed] [Google Scholar]
- 72.Efrati O, Modan-Moses D, Barak A, et al. Long-term non-invasive positive pressure ventilation among cystic fibrosis patients awaiting lung transplantation. The Israel Medical Association journal : IMAJ. Sep 2004;6(9):527–530. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable – no new data generated