Abstract
Transcranial magnetic stimulation (TMS) is increasingly being used to treat posttraumatic stress disorder (PTSD) comorbid with major depressive disorder (MDD). Yet, identifying the most effective stimulation parameters remains an active area of research. We recently reported on the use of 5 Hz TMS to reduce PTSD and MDD symptoms. A recently developed form of TMS, intermittent theta burst stimulation (iTBS), appears noninferior for treating MDD. Because iTBS can be delivered in a fraction of the time, it provides significant logistical advantages; however, evaluations of whether iTBS provides comparable PTSD and MDD symptom reductions are lacking. We performed a retrospective chart review comparing clinical outcomes in veterans with PTSD and MDD who received iTBS (n = 10) with a matched cohort that received 5‐Hz TMS (n = 10). Symptoms were evaluated using self‐reported rating scales at baseline and every five treatments for up to 30 sessions. Both protocols were safe and reduced symptoms, ps < .001, but veterans who received iTBS reported poorer outcomes. These results were observed using mixed‐model analyses, Group x Time interaction: p = .011, and effect sizes, where 5 Hz TMS demonstrated superior PTSD and MDD symptom improvement, ds = 1.81 and 1.51, respectively, versus iTBS, ds = 0.63 and 0.88, respectively. Data from prior controlled trials of iTBS, with increased stimulation exposure, have appeared to provide comparable clinical outcomes compared with 5 Hz TMS. Prospective and controlled comparisons are required; however, the present findings provide important information for clinicians using TMS to treat these commonly comorbid disorders.
抽象
Traditional and Simplified Chinese Abstracts by the Asian Society for Traumatic Stress Studies (AsianSTSS)
簡體及繁體中文撮要由亞洲創傷心理研究學會翻譯
Traditional Chinese
經顱磁刺激治療創傷後壓力症及重度抑鬱症:比較常用的臨床方案
摘要
經顱磁刺激(TMS)被越來越多地用於治療創傷後壓力症(PTSD)合併重度抑鬱症(MDD)。然而, 確定最有效的刺激參數仍然是一個活躍的研究領域。我們最近報導了使用5hz TMS來減少創傷後壓力症及MDD症狀。一種新近發展起來的TMS, 間歇性θ波爆發刺激(iTBS), 在治療重度抑鬱症方面表現良好。由於iTBS可以在一小部分時間內完成, 它提供了顯著的後勤優勢;然而, iTBS是否提供了可比的創傷後壓力症及MDD症狀減少的評估還缺乏。我們進行了一項回顧性的圖表審查, 比較了接受iTBS(n = 10)及接受5Hz TMS(n = 10)的退伍軍人的臨床結果。在基線及每五個療程的30個療程中, 使用自我報告的評級量表對症狀進行評估。兩種方案都是安全的, 症狀減輕, ps < .001, 但接受iTBS的退伍軍人報告的結果較差。這些結果是通過混合模型分析、x組時間交互作用:p = .011及效應量觀察到的, 其中5hz TMS表現出更好的創傷後壓力症及MDD症狀改善, ds分別=1.81及1.51, 與iTBS相比, ds分別= 0.63及0.88。先前的iTBS對照試驗數據顯示, 隨著刺激暴露的增加, 與5hz TMS相比, iTBS的臨床結果具有可比性。需要進行前瞻性及控制性比較;然而, 目前的發現為臨床醫生使用經顱磁刺激治療這些常見的共病提供了重要資訊。
Simplified Chinese
经颅磁刺激治疗创伤后压力症及重度抑郁症:比较常用的临床方案
摘要
经颅磁刺激(TMS)被越来越多地用于治疗创伤后压力症(PTSD)合并重度抑郁症(MDD)。然而, 确定最有效的刺激参数仍然是一个活跃的研究领域。我们最近报导了使用5hz TMS来减少创伤后压力症及MDD症状。一种新近发展起来的TMS, 间歇性θ波爆发刺激(iTBS), 在治疗重度抑郁症方面表现良好。由于iTBS可以在一小部分时间内完成, 它提供了显著的后勤优势;然而, iTBS是否提供了可比的创伤后压力症及MDD症状减少的评估还缺乏。我们进行了一项回顾性的图表审查, 比较了接受iTBS(n = 10)及接受5Hz TMS(n = 10)的退伍军人的临床结果。在基线及每五个疗程的30个疗程中, 使用自我报告的评级量表对症状进行评估。两种方案都是安全的, 症状减轻, ps < .001, 但接受iTBS的退伍军人报告的结果较差。这些结果是通过混合模型分析、x组时间交互作用:p = .011及效应量观察到的, 其中5hz TMS表现出更好的创伤后压力症及MDD症状改善, ds分别=1.81及1.51, 与iTBS相比, ds分别= 0.63及0.88。先前的iTBS对照试验数据显示, 随着刺激暴露的增加, 与5hz TMS相比, iTBS的临床结果具有可比性。需要进行前瞻性及控制性比较;然而, 目前的发现为临床医生使用经颅磁刺激治疗这些常见的共病提供了重要信息。
Resumen
Spanish Abstracts by Asociación Chilena de Estrés Traumático (ACET)
Estimulación magnética transcraneal para el Trastorno de estrés postraumático y depresión mayor: comparación de Protocolos Clínicos de uso común
ESTIMULACIÓN MAGNÉTICA TRANSCRANEAL PARA TEPT Y TDM
La estimulación magnética transcraneal (TMS, por sus siglas en inglés) se usa cada vez más para tratar el trastorno de estrés postraumático (TEPT) comórbido con el trastorno depresivo mayor (TDM). Sin embargo, la identificación de los parámetros de estimulación más efectivos sigue siendo un área activa de investigación. Recientemente informamos sobre el uso de TMS de 5 Hz para reducir los síntomas de TEPT y MDD. Una forma recientemente desarrollada de TMS, la estimulación intermitente theta burst (iTBS, por sus siglas en inglés), parece no ser inferior para el tratamiento de MDD. Dado que iTBS se puede entregar en una fracción del tiempo, proporciona importantes ventajas logísticas; sin embargo, faltan evaluaciones sobre si iTBS proporciona reducciones significativas de síntomas de TEPT y MDD. Realizamos una revisión retrospectiva de gráficos que comparaban los resultados clínicos en veteranos con TEPT y TDM que recibieron iTBS (n = 10) con una cohorte emparejada que recibió TMS de 5 Hz (n = 10). Los síntomas se evaluaron utilizando escalas de calificación auto‐reportadas al inicio y cada cinco sesiones hasta por 30 sesiones. Ambos protocolos fueron seguros y redujeron los síntomas, ps < 0.001, pero los veteranos que recibieron iTBS reportaron peores resultados. Estos resultados se observaron mediante análisis de modelos mixtos, interacción Grupo x Tiempo: p = 0.011 y tamaños del efecto, donde la TMS de 5 Hz demostró una mejoría superior de los síntomas de TEPT y TDM, ds = 1.81 y 1.51, respectivamente, en comparación con iTBS, ds = 0.63 y 0.88, respectivamente. Los datos de ensayos controlados anteriores de iTBS, con una mayor exposición a la estimulación, parecen proporcionar resultados clínicos comparables en contraste con la TMS de 5 Hz. Se requieren comparaciones prospectivas y controladas; sin embargo, los hallazgos presentes brindan información importante para los médicos que usan TMS para tratar estos trastornos comúnmente comórbidos.
Posttraumatic stress disorder (PTSD) remains a highly prevalent psychiatric disorder in U.S. military veterans and individuals worldwide. It is often comorbid with major depressive disorder (MDD) and is associated with a broad range of impairments (Kessler, 2000; Shalev et al., 2017). Although evidence‐based psychotherapy and pharmacotherapy are available for PTSD, treatment resistance, side effects, access, and adherence often limit clinical outcomes (e.g., Watts et al., 2013); thus, novel treatments are clearly needed.
One intervention with increased evidence for PTSD is repetitive transcranial magnetic stimulation (rTMS, hereafter called TMS). Following Faraday's law, TMS uses rapidly fluctuating magnetic fields to induce local neuronal depolarization, which leads to a number of polysynaptic or “downstream” effects that are associated with symptom improvement (Philip, Barredo, Aiken, et al., 2018). For a comprehensive overview of the clinical use of TMS, including important and outstanding questions for the field, see Fitzgerald (2020) and George (2019). Currently, TMS treatment is calibrated against an individual's cortical excitability, and treatments are administered to the dorsolateral prefrontal cortex (DLPFC) daily for up to 6 weeks, with each session taking up to 37.5 min.
Originally cleared by the U.S. Food and Drug Administration (FDA) in 2008 for pharmacoresistant MDD (George et al., 2010; O'Reardon et al., 2007), there is now a large body of evidence from thousands of patients that supports the efficacy of TMS for MDD (e.g., Gaynes et al., 2014). However, not all results have been positive: In one randomized controlled trial (RCT) in a sample of veterans, researchers did not find separation between active and sham stimulation, with poorer outcomes among those with comorbid MDD and PTSD (Yesavage et al., 2018). Yet, the interpretation of this study was complicated by a high placebo response rate, and follow‐up studies have not reliably replicated the association between comorbid PTSD and poorer TMS‐related outcomes (Hernandez et al., 2020). Outside of depression, TMS has also been cleared for use with symptom provocation for obsessive–compulsive disorder (Carmi et al., 2019). Given this data for depression and other indications, TMS is increasingly available in the private sector and as part of a nationwide program to implement TMS at Veterans Affairs (VA) hospitals around the United States, with the first results to be disseminated within the next year.
One important question within the TMS field has been how to best deploy this technology. With a focus on PTSD, several groups have reported the use of different TMS approaches to reduce symptoms in real‐world, naturalistic patient populations (for a review, see Kan et al., 2020), with the understanding that TMS for PTSD, at least in the absence of MDD, currently remains an off‐label clinical use or is used in research settings. Prior approaches that have used noninvasive brain stimulation for PTSD include a broad range of devices and protocols. Although a comprehensive discussion is beyond the scope of the present manuscript, most of these studies have evaluated 1‐ or 10‐Hz TMS, often delivered using a standard figure‐of‐eight coil to stimulate either DLPFC (i.e., left or right) as monotherapy (e.g., Hernandez et al., 2020; Kozel et al., 2019) or in combination with psychotherapy (Fryml et al., 2019; Kozel et al., 2018) or as H‐coil TMS delivered in combination with symptom provocation (Isserles et al., 2013) or other novel TMS devices (e.g., Philip, Aiken, et al., 2019). The findings from both reviews and meta‐analyses indicate efficacy but no clear indication that any singular approach is superior at the current time (Kan et al., 2020).
Over the last few years, a promising new kind of TMS, called theta burst stimulation (TBS), has been developed. In TBS, 50‐Hz bursts are delivered at five per second (i.e., 5 Hz, corresponding to the theta frequency domain in electroencephalography). The TBS intervention was designed as a neurophysiological tool to potentially mimic intrinsic hippocampal rhythms (Huang et al., 2005) wherein intermittent TBS (iTBS) was found to yield durable changes in long‐term potentiation. Although the use of iTBS to this end remains an area of investigation, one immediate advantage is that an entire stimulation session can be delivered very quickly; that is, in as little as 3 min compared to the 37.5 min for standard TMS. Despite the substantial differences in administration time, a large multisite study demonstrated that neuronavigated iTBS had noninferior outcomes compared to neuronavigated 10‐Hz TMS for MDD (Blumberger et al., 2018), which led to FDA clearance of iTBS. The time advantage (i.e., 3 min vs. 37.5 min) provides significant potential to improve access to care. Clear examples of this benefit include reducing the impact of late patients, as a single late patient TMS patient can yield a cascade of delays throughout the day; increased care delivery, as iTBS enables clinicians to provide care to several patients per hour instead of a single patient per hour; optimized personnel utilization; and the ability to deliver many TMS treatments to a single person per day (i.e., an “accelerated” protocol) to potentially hasten positive response (e.g., Cole et al., 2020). Other advantages may be relevant to the COVID‐19 pandemic, as iTBS allows more time for facility cleaning between patients. As a follow‐up evaluation, we tested the use of iTBS in an RCT and found promising results with regard to PTSD and depression, with effects lasting up to 1 year after stimulation (Petrosino et al., 2020; Philip, Barredo, et al., 2019).
In prior reports, we described the use of 5‐Hz TMS for PTSD, with promising clinical (Carpenter et al., 2018; Philip et al., 2016) and neuroimaging (Philip, Barredo, van ‘t Wout‐Frank, et al., 2018) outcomes in approximately 50 patients. Yet, with the recognition that iTBS provided a significant advantage regarding administration time, we started delivering iTBS to all patients at the Providence VA Neuromodulation clinic once the treatment had been cleared by the FDA. In the present study, we evaluated the safety and clinical outcomes among 10 veterans with PTSD and comorbid MDD who received iTBS and compared them with 10 age‐, sex‐, and symptom severity–matched veterans who had previously received 5‐Hz TMS. We hypothesized that iTBS would be safe and comparably effective to our prior TMS approaches.
Method
Participants
We performed a retrospective chart review of the first 10 patients with comorbid PTSD and MDD who received iTBS at the VA Providence Healthcare system, compared to a matched cohort (i.e., age, sex, symptom severity) comprising individuals who received 5‐Hz TMS for the same presentation in the same clinic. All patients received unblinded clinical stimulation. Individuals in the iTBS group received their stimulation from August 2019 to October 2020, and 5‐Hz TMS patients were treated between September 2014 to June 2016. Prior to any stimulation, all patients met the criteria for PTSD and MDD as outlined in the fourth or fifth editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV and DSM‐5, respectively), depending on the treatment date; were not currently participating in research studies; and had been receiving stable treatment (i.e., medication and/or psychotherapy) for at least 6 weeks; concurrent psychiatric treatments were allowed to continue unchanged. Diagnoses were confirmed through a clinical interview as part of a standard clinical screening process by psychiatrists with expertise in PTSD and MDD and corroborated with validated self‐rated scales (see Measures). Written clinical consent was obtained prior to treatment, and the Providence VA Institutional Review Board approved this retrospective chart review. Patient demographic characteristics are described in Table 1. Consistent with patient demographic characteristics at the Providence VA, veterans were commonly male, White, and had various PTSD etiologies.
Table 1.
Demographic Characteristics of the Sample
| TBS cohort | 5 Hz TMS cohort | |||
|---|---|---|---|---|
| Variable | n | % | n | % |
| Male gender | 9 | 90.0 | 8 | 80.0 |
| White race a | 10 | 100.0 | 9 | 90.0 |
| PTSD | ||||
| Combat‐related PTSD | 6 | 60.0 | 8 | 80.0 |
| Other PTSD | 7 | 70.0 | 7 | 70.0 |
| PTSD from military sexual trauma | 1 | 10.0 | 2 | 20.0 |
Note. As participants could report multiple types of trauma exposure, numbers may not equal 100.0%. TBS = theta burst stimulation; TMS = transcranial magnetic stimulation; PTSD = posttraumatic stress disorder.
One participant in the 5‐Hz TMS cohort was Black.
Procedure
Patients were referred to the Neuromodulation Clinic if they demonstrated symptoms of MDD along with comorbid PTSD despite adequate pharmacology, defined as more than 6 weeks of an adequate dose of an antidepressant; were considering electroconvulsive therapy; or were unable to tolerate pharmacotherapy. Screening procedures included standard safety forms to evaluate for absolute contraindications to stimulation, such as seizure disorders or intracranial metal. After an initial consultation and screening visit, veterans returned to the clinic, where their individual motor threshold (MT) was established, defined as the amount of energy required to elicit movement in the resting contralateral hand at least 50% of the time.
After MT determination, the left DLPFC target was defined using standard methods corresponding to individual head measurements (i.e., the “Beam method,” originally described by Beam et al., 2009). Neither neuroimaging nor neuronavigation were utilized, as it remains unclear whether this improves clinical outcomes (e.g., Fitzgerald et al., 2009, Hebel et al., 2021). The MT determination and left DLPFC targeting were identical in both the iTBS and 5‐Hz TMS groups, and for both groups, treatment occurred once every business day for 30 sessions.
In the 5‐Hz TMS group, patients received stimulation using a Neurostar Device (Malvern, PA). Parameters including stimulation at 5 Hz, 4 s on, 12 s off, for 3,000 pulses per session. Total pulses per session were increased to 4,000, with the intertrain interval shortened by 1 s, for the remainder of treatment when patients did not have at least 30% improvement from baseline scores on outcome measures at Treatment 15 (following Carpenter et al., 2018), for a total of approximately 40 min. Individuals in the 5‐Hz TMS group were matched with the iTBS cohort based on age, sex, and baseline PTSD symptom severity.
For iTBS, a Magstim Super Rapid 2+1 system (Magstim; Whitland, UK) was used to deliver iTBS procedures and followed recommendations by Blumberger et al. (2018). Stimulation parameters included 50‐Hz triplet bursts, repeated at 5 Hz, 2 s on and 8 s off, for a total of 600 pulses per session (i.e., a total of approximately 3 min stimulation time). The Neurostar device could not be used because it did not have the capacity to deliver iTBS.
Measures
PTSD Symptoms
For PTSD, we utilized the PTSD Checklist for DSM‐5 (PCL‐5; Weathers et al., 2013), a 20‐item self‐report measure that is used to assess the 20 DSM‐5 PTSD symptoms. Individual items are rated using a Likert scale ranging from 0 to 4, with higher scores indicating more severe symptoms (range: 0–80). The scale has demonstrated excellent internal reliability (Cronbach's α = .94; Weathers et al., 2013). A score of 33 or higher was considered to meet the threshold for clinically significant PTSD symptoms; a score change of at least 10 points was considered to be clinically meaningful.
MDD Symptoms
For MDD, we utilized the Inventory of Depressive Symptomatology‐Self Report (IDS‐SR; Rush et al., 1996). This is a 30‐item rating scale that assesses symptoms of MDD, where each individual item is narratively described by escalating severity and scored on a scale of 0 to 3, for a maximum score of 84. A score less than 15 is considered to meet the threshold for clinical remission, and a score change of more than 10 points was considered clinically meaningful. The IDS‐SR has demonstrated excellent internal reliability (Cronbach's α = .82; Rush et al., 1996). Rating scales were obtained at baseline and at approximately every fifth session until the end of the treatment (i.e., 30 sessions). Safety was assessed as part of normal clinical practice.
Data Analysis
We used linear mixed models for repeated measures over time and participants to analyze the effect of iTBS and 5‐Hz TMS on self‐reported PTSD and MDD symptom severity throughout treatment. This procedure prevents the listwise deletion due to missing data, which was approximately 33% over the entirety of treatment, to provide insight into the effect of improvement over time and describe whether one stimulation procedure potentially yielded faster improvement. Treatment protocol (iTBS, 5‐Hz TMS), time (seven time points, including baseline), and the interaction between treatment protocol and time (i.e., Treatment Protocol x Time) were entered as fixed effects. The PCL‐5 and IDS‐SR were entered as dependent variables in two separate models. All analyses were performed in SPSS (Version 26).
Follow‐up analyses were focused on detecting clinically significant differences between the two groups. Consistent with clinical care, patients could stop treatment early, particularly if they received early benefit or felt no benefit. Therefore, we censored data at the point of last treatment to compare baseline to endpoint responses across the sample. These censored data were used to describe raw PCL‐5 and IDS‐SR score changes (i.e., mean baseline minus endpoint) to inform the clinical utility of mixed‐model observations. As previously indicated, we used a clinically meaningful improvement defined as a symptom score change of more than 10 points for the PCL‐5 (Weathers et al., 2013) and the IDS‐SR (Rush et al., 1996). We calculated effect sizes as Cohen's d and further calculated their 95% confidence intervals. Given the small sample sizes, we also calculated the Reliable Change Index (RCI; Jacobson & Truax, 1991), where RCI values greater than 1.96 indicated reliable results.
Results
Baseline mean symptom scores for both groups were in the moderate range for both PTSD (PCL‐5: M = 39.7, SD = 13.2 for TBS; M = 46.1, SD = 10.9 for 5‐Hz TMS) and MDD (IDS‐SR: M = 45.5 SD = 8.9 For TBS; M = 41.3, SD = 7.3 5 for Hz TMS). The results of t tests comparing between‐group baseline scores were nonsignificant p = .252 for PTSD and p = .265 for MDD. Side effects and safety were consistent with the known profile of TMS, and there were no reported seizures.
For PTSD symptoms, the results of a linear mixed‐model analysis (i.e., comparing PCL‐5 scores over time between iTBS and 5‐Hz TMS groups) indicated a significant main effect of time, F(6, 91.0) = 11.36, p < .001, ηp 2 = .38; a nonsignificant main effect of treatment protocol, F(1, 17.81) = 0.385, p = .543, ηp 2 = .02; and significant Treatment Protocol x Time interaction, F(6, 91.02) = 2.95, p = .011, ηp 2 = .17. Given the significant Treatment Protocol x Time interaction, we performed post hoc tests to provide further insight into these findings. For the 5‐Hz TMS group, the results of a linear mixed‐model analysis with only Time entered as a fixed effect were significant, F(6, 46.03) = 9.85, p <.001. The linear mixed‐model analysis that used the iTBS group also resulted in a significant effect of time, F(6, 44.98) = 2.62, p = .030. These results suggest that although participants in both groups demonstrated reductions, those who received 5‐Hz TMS reported a greater decline in PTSD symptom severity over time compared to those who received iTBS (Figure 1 Panel A).
Figure 1.
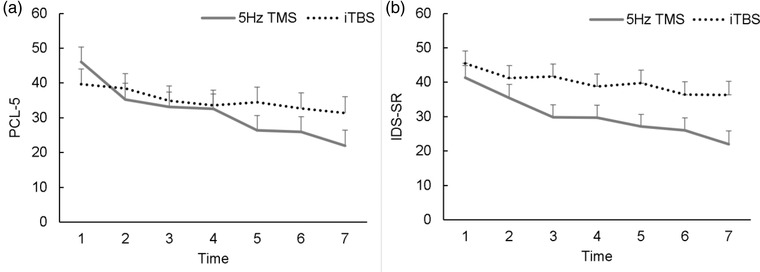
Symptom Trajectories of (A) Posttraumatic Stress Disorder (PTSD) and (B) Major Depressive Disorder (MDD) Symptoms Over Time.
Note. Trajectories of (A) self‐reported PTSD and (B) MDD symptoms evaluated from baseline (Time Point 1) to endpoint (Time Point 7). Each time point represents approximately five treatment sessions. Error bars reflect standard error. Mean scores and the number of observations at each time point are presented in Supplementary Table S1. PCL‐5 = PTSD Checklist for DSM‐5; IDS‐SR: Inventory of Depressive Symptomatology–Self‐Report; TMS = transcranial magnetic stimulation; iTBS = intermittent theta burst.
For MDD symptoms (i.e., IDS‐SR), a linear mixed‐model analysis revealed both a significant main effect of time, F(6, 92.23) = 10.49, p < .001, ηp 2 = .35, and treatment protocol, F(1, 18.02) = 4.73, p = .043, ηp 2 = .22, but no significant Time x Treatment Protocol interaction, F(6, 92.23) = 1.56, p = .170, ηp 2 = .08. This suggests that across treatment groups, veterans reported a reduction in depression symptoms over time, and those who received 5‐Hz TMS reported less severe depressive symptoms than those who received iTBS (Figure 1, Panel B).
When comparing outcomes from baseline to endpoint, several findings emerged, all indicating more favorable clinical outcomes when 5‐Hz TMS was used. Among participants in the 5‐Hz TMS group, there was an average 21.7‐point reduction on the PCL‐5, whereas the average reduction was 14.2 points for veterans who received iTBS. When considering the IDS‐SR, there was an average 18.4‐point drop among participants in the 5‐Hz TMS group, whereas the average reduction was 11.9 among participants who received iTBS. Translated into Cohen's d (95% CI) effect sizes, the 5‐Hz TMS group demonstrated superior effect sizes for PTSD and MDD symptom reduction, d = 1.81, 95% CI [0.68, 2.95] and d = 1.51, 95% CI [0.62, 2.40], respectively), compared to iTBS, d = 0.63, 95% CI [‐0.34, 1.59], and d = 0.88, 95% CI [0.06, 1.69], for PTSD and MDD, respectively. When evaluating reliable change, in the 5‐Hz TMS group, nine out of 10 patients demonstrated reliable change in both PTSD and MDD symptoms, whereas, in the iTBS group, seven of 10 and eight of 10 patients demonstrated reliable PTSD and MDD symptom change, respectively (Table 2).
Table 2.
Reliable Change of Posttraumatic Stress Disorder (PTSD) and Major Depressive Disorder Symptoms from Baseline to Endpoint
| PCL‐5 | IDS‐SR | |||||
|---|---|---|---|---|---|---|
| Participant | Baseline | Endpoint | Reliable change | Baseline | Endpoint | Reliable change |
| iTBS cohort | ||||||
| 1 | 58 | 49 | + | 55 | 55 | – |
| 2 | 30 | 20 | + | 31 | 26 | + |
| 3 | 39 | 26 | + | 48 | 37 | + |
| 4 | 26 | 18 | + | 32 | 20 | + |
| 5 | 50 | 41 | + | 42 | 40 | – |
| 6 | 56 | 48 | + | 58 | 49 | + |
| 7 | 26 | 29 | ‐ | 42 | 30 | + |
| 8 | 63 | 66 | – | 53 | 38 | + |
| 9 | 37 | 18 | + | 51 | 32 | + |
| 10 | 24 | 30 | – | 50 | 46 | + |
| 5‐Hz TMS cohort | ||||||
| 1 | 37 | 21 | + | 33 | 12 | + |
| 2 | 56 | 43 | + | 40 | 29 | + |
| 3 | 52 | 14 | + | 47 | 14 | + |
| 4 | 64 | 7 | + | 40 | 8 | + |
| 5 | 49 | 30 | + | 37 | 14 | + |
| 6 | 27 | 0 | + | 45 | 0 | + |
| 7 | 34 | 24 | + | 56 | 49 | + |
| 8 | 44 | 43 | – | 37 | 45 | – |
| 9 | 48 | 11 | + | 32 | 26 | + |
| 10 | 50 | 32 | + | 46 | 34 | + |
Note. + sign indicates a reliable change index > 1.96. iTBS = intermittent theta burst stimulation; TMS = transcranial magnetic stimulation; PCL‐5 = PTSD checklist for DSM‐ 5; IDS‐SR = Inventory of Depressive Symptoms–Self‐Report.
Discussion
Contrary to our hypothesis, the present findings indicate that although 5‐Hz TMS and iTBS may reduce the symptoms of PTSD and MDD, 5‐Hz TMS yielded superior results, particularly for PTSD symptoms. The results of mixed‐models analyses indicated that patients who received 5‐Hz TMS also achieved quicker PTSD symptom reduction relative to those in the iTBS group. These results, admittedly procured using an unblinded and small sample size, are surprising given the overall inability in the literature to find clear superiority of one kind of stimulation over another when treating PTSD or MDD (e.g., Berlow et al., 2020; Kozel et al., 2019). These results suggest for the first time that specific stimulation parameters may be more important for patients with PTSD.
When evaluating score reductions on measures of PTSD and MDD across the two stimulation profiles, both approaches were shown to reduce symptoms, but 5‐Hz stimulation provided superior outcomes. For both the PCL‐5 and the IDS‐SR, score reductions among participants in the 5‐Hz TMS group were within the range considered to be clinically meaningful, whereas statistical testing and observed effect sizes demonstrated that the magnitude of reductions in patients who received iTBS were lower in both domains. It is important to note that the symptom changes observed in the 5‐Hz group are similar to those observed in prior reports of 5‐Hz TMS in this patient population in both veteran and civilian cohorts (Carpenter et al., 2018; Philip et al., 2016).
Based on the findings that iTBS to the left DLPFC (600 pulses, 120% of MT, approximately 3 min) appeared to be inferior to 5‐Hz TMS to the left DLPFC (3,000–4,000 pulses, 120% of MT, approximately 40 min), we revisited a portion of the outcomes reported by Philip, Barredo, Aiken, et al. (2019), wherein iTBS was delivered to the right DLPFC (1,800 pulses, 80% of MT, approximately 10 min). Specifically, we explored outcomes among participants who received a cumulative stimulation exposure most similar to clinical settings (i.e., the n = 25 participants who received 20 daily sessions over 4 weeks), using the same patient‐rated scales (i.e., the PCL‐5 for PTSD symptoms and the IDS‐SR for MDD symptoms). When comparing baseline to endpoint changes in that group, clinical outcomes were robust, with a Cohen's d for PTSD of 1.83, 95% CI [1.15, 2.51] and a Cohen's d for MDD of 1.09, 95% CI [0.58, 1.60]. This is qualitatively similar to the Cohen's d effect sizes we found for the present study's 5‐Hz group of 1.81, 95% CI [0.68, 2.95], and 1.51, 95% CI [0.62, 2.95] for PTSD and MDD, respectively. We did not perform direct comparisons between groups given the different contexts of their stimulation, namely during the conduct of an RCT or clinical care. Yet, these results may provide important indications that the administration of a higher number of pulses may be required for efficacy in PTSD symptom reduction. Whether there is some optimal role of laterality remains an important question for future investigation.
Of note, there are several other factors that may have led to poorer outcomes in the iTBS group. It is possible that factors related to the novelty of use may have impacted efficacy, although this was delivered by a clinic with significant experience in TMS and novel devices. It is also possible that the first 10 patients to receive iTBS had other clinical features, such as chronicity or further comorbidity of psychiatric illness or underlying medical comorbidities that would predispose the patient to poorer outcomes, although we sought to minimize differences by comparing these participants against a psychiatrically matched cohort.
Limitations of this work are those inherent to small, single‐site clinical reports. It is possible that some bias favoring 5‐Hz TMS occurred with respect to the matched cohort; although individuals were matched by baseline symptom severity, the results of mixed‐model analyses indicated less severe depressive symptoms over time among individuals in the 5‐Hz group. As both devices were not capable of delivering iTBS, it is possible that using different systems contributed to our findings; however, this is unlikely given prior data that has indicated these devices yield identical clinical outcomes (Oliveira‐Maia et al., 2016) as well as our examination of outcomes from our prior RCT, which used the same iTBS device (Philip, Barredo, Aiken, et al., 2019). We did not compare iTBS outcomes with different TMS administrations, such as 10‐Hz TMS, which is the standard approach for MDD without comorbid PTSD (O'Reardon et al., 2007), although prior work has indicated that 5‐Hz TMS is better tolerated and comparably efficacious for MDD (Philip et al., 2015). We also did not specifically control the brain state during stimulation; this is likely a critical area of future research, and prior work has shown that a combination of TMS plus psychotherapy can reduce PTSD symptoms (Fryml et al., 2019; Kozel et al., 2018). Researchers have also explored symptom provocation immediately before stimulation with an H‐coil TMS system. Although the initial results were promising (Isserles et al., 2013), a larger RCT demonstrated negative results, which have been publicly reported but not published in the peer reviewed literature (Brainsway, 2020). Furthermore, in the present study, all patients were receiving stable treatment, and we were not powered to evaluate the effects of particular medications or psychotherapy nor were we able to identify whether the effects were due to TMS alone. For the present chart review, we also relied upon self‐reported rating scales rather than structured clinical interviews; this selection reflects the use of these scales as part of clinical care to minimize patient burden while providing high‐quality and patient‐relevant outcomes.
In conclusion, iTBS, at least as it is currently FDA‐cleared for MDD, does not appear to be as efficacious as 5‐Hz TMS for patients with PTSD and comorbid MDD. However, clinical outcomes appear to be much more similar after delivering a higher number of pulses. Although we recognize that these results require replication in larger samples and controlled trials, ideally with biological assessments to assess the mechanisms underlying observed effects, it appears that higher degrees of cumulative exposure to stimulation may be required to provide optimal outcomes in this patient population
Open Practices Statement
Data for this report were obtained through review of Veterans Hospital Administration (VHA) clinical records, and have therefore not been made available on a permanent third‐party archive; requests for the data should be sent via email to the corresponding author at noah_philip@brown.edu .
Supporting information
Table S1. Rating Scales over time and observed data points – Figures 1A‐B
Department of Veterans Affairs Office of Research Rehabilitation and Development Center for Neurorestoration and Neurotechnology, Providence VA Healthcare System, Providence, Rhode Island, USA
This work was supported in part by the U.S. Department of Veterans Affairs (VA; I01 RX002450, I01 HX002572), the National Institutes of Health (NIH; R01 MH120126, P20 GM130452), and the VA Office of Research Rehabilitation and Development (RR&D) Center for Neurorestoration and Neurotechnology at the Providence VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the NIH. The funders had no role in the design of the study, data analysis, or decision to publish. The authors have no relevant biomedical conflicts of interest to disclose.
The authors wish to thank Dr. Nicole M. Armstrong for her statistical assistance.
References
- Beam, W. , Borckardt, J. J. , Reeves, S. T. , & George, M. S. (2009). An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimulation, 2(1), 50–54. 10.1016/j.brs.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlow, Y. A. , Zandvakili, A. , & Philip, N. S. (2020). Low‐frequency right‐sided and high frequency left‐sided repetitive transcranial magnetic stimulation for depression: The evidence of equivalence. Brain Stimulation, 13(6), 1793–1795. https://doi.org10.1016/j.brs.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberger, D. M. , Vila‐Rodriguez, F. , Thorpe, K. E. , Feffer, K. , Noda, Y. , Giacobbe, P. , Knyahnytska, Y. , Kennedy, S. H. , Lam, R. W. , Daskalakis, Z. J. , & Downar, J. (2018). Effectiveness of theta burst versus high‐frequency repetitive transcranial magnetic stimulation in patients with depression (THREE‐D): A randomized non‐inferiority trial. Lancet, 391(10131), 1683–1692. 10.1016/S0140-6736(18)30295-2 [DOI] [PubMed] [Google Scholar]
- BrainsWay . (2020, February 6). BrainsWay reports results of interim analysis of H7 deep transcranial magnetic stimulation study in post‐traumatic stress disorder [Press release]. https://www.brainsway.com/news_events/brainsway‐reports‐results‐of‐interim‐analysis‐of‐h7‐deep‐transcranial‐magnetic‐stimulation‐study‐in‐post‐traumatic‐stress‐disorder/ [Google Scholar]
- Carmi, L. , Tendler, A. , Bystritsky, A. , Hollander, E. , Blumberger, D. M. , Daskalakis, J. , Ward, H. , Lapidus, K. , Goodman, W. , Casuto, L. , Feifel, D. , Barnea‐Ygael, N. , Roth, Y. , Zangen, A. , & Zohar, J. (2019). Efficacy and safety of deep transcranial magnetic stimulation for obsessive‐compulsive disorder: A prospective multicenter randomized double‐blind placebo‐controlled trial. American Journal of Psychiatry, 176(11), 931–938. 10.1176/appi.ajp.2019.18101180 [DOI] [PubMed] [Google Scholar]
- Carpenter, L. L. , Conelea, C. , Tyrka, A. R. , Welch, E. S. , Greenberg, B. D. , Price, L. H. , Niedzwiecki, M. , Yip, A. G. , Barnes, J. , & Philip, N. S. (2018). 5Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. Journal of Affective Disorders, 235, 414–420. 10.1016/j.jad.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, E. J. , Stimpson, K. H. , Bentzley, B. S. , Gulser, M. , Cherian, K. , Tischler, C. , Nejad, R. , Pankow, H. , Choi, E. , Aaron, H. , Espil, F. M. , Pannu, J. , Xiao, X. , Duvio, D. , Solvason, H. B. , Hawkins, J. , Guerra, A. , Jo, B. , Raj, K. S. , … Williams, N. R. (2020). Stanford Accelerated Intelligent Neuromodulation Therapy for treatment‐resistant depression. The American Journal of Psychiatry, 177(8), 716–726. 10.1176/appi.ajp.2019.19070720 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, P. (2020). An update on the clinical use of repetitive transcranial magnetic stimulation in the treatment of depression. Journal of Affective Disorders, 276, 90–103. 10.1016/j.jad.2020.06.067 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, P. B. , Hoy, K. , McQueen, S. , Maller, J. J. , Herring, S. , Segrave, R. , Bailey, M. , Been, G. , Kulkarni, J. , & Daskalakis, Z. J. (2009). A randomized trial of rTMS targeted with MRI‐based neuro‐navigation in treatment‐resistant depression. Neuropsychopharmacology, 34(5), 1255–1262. 10.1038/npp.2008.233 [DOI] [PubMed] [Google Scholar]
- Fryml, L. D. , Pelic, C. G. , Acierno, R. , Tuerk, P. , Yoder, M. , Borckardt, J. J. , Juneja, N. , Schmidt, M. , Beaver, K. L. , & George, M. S. (2019). Exposure therapy and simultaneous repetitive transcranial magnetic stimulation: A controlled pilot trial for the treatment of posttraumatic stress disorder. The Journal of ECT, 35(1), 53–60. 10.1097/YCT.0000000000000505 [DOI] [PubMed] [Google Scholar]
- Gaynes, B. , Lloyd, S. W. , Lux, L. , Gartlehner, G. , Hansen, R. A. , Brode, S. , Jonas, D. E. , Evans, T. S. , Viswanathan, M. , & Lohr, K. (2014). Repetitive transcranial magnetic stimulation for treatment‐resistant depression: a systematic review and meta‐analysis. Journal of Clinical Psychiatry, 75(5), 477–489. 10.4088/JCP.13r08815 [DOI] [PubMed] [Google Scholar]
- George, M. S. (2019). Whither TMS: A one‐trick pony or the beginning of a neuroscientific revolution? American Journal of Psychiatry, 176(11), 904–910. 10.1176/appi.ajp.2019.19090957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, M. S. , Lisanby, S. H. , Avery, D. , McDonald, W. , Durkalaski, V. , Pavlicova, M. , Anderson, B. , Nahas, Z. , Bulow, P. , Zarkowski, P. , Holtzheimer, P. , Schwartz, T. , & Sackeim, H. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham‐controlled randomized trial. Archives of General Psychiatry, 67(5), 507–516. 10.1001/archgenpsychiatry.2010.46 [DOI] [PubMed] [Google Scholar]
- Hebel, T. , Göllnitz, A. , Schoisswohl, S. , Weber, F. C. , Abdelnaim, M. , Wetter, T. C. , Rupprecht, R. , Langguth, B. , & Schecklmann, M. (2021). A direct comparison of neuronavigated and non‐neuronavigated intermittent theta burst stimulation in the treatment of depression. Brain Stimulation, 14(2), 335–343. 10.1016/j.brs.2021.01.013 [DOI] [PubMed] [Google Scholar]
- Hernandez, M. J. , Reljic, T. , Van Trees, K. , Phillips, S. , Hashimie, J. , Bajor, L. , Yehl, J. , McKenzie, B. C. , Burke, C. , Sullivan, G. A. , Kumar, A. , Sanchez, D. L. , Catalano, G. , & Kozel, F. A. (2020). Impact of comorbid PTSD on outcome of repetitive transcranial magnetic stimulation (TMS) for veterans with depression. Journal of Clinical Psychiatry, 81(4), Article 19m13152. 10.4088/jcp.19m13152 [DOI] [PubMed] [Google Scholar]
- Huang, Y. Z. , Edwards, M. J. , Rounis, E. , Bhatia, K. P. , & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(5), 201–206. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Isserles, M. , Shalev, A. Y. , Roth, Y. , Peri, T. , Kutz, I. , Zlotnick, E. , & Zangen, A. (2013). Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post‐traumatic stress disorder: A pilot study. Brain Stimulation, 6(3), 377–383. 10.1016/j.brs.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Jacobson, N. S. , & Truax, P. (1991). Clinical significance: A statistical approachto defining meaningful change in psychotherapy research. Journalof Consulting and Clinical Psychology, 59(1), 12–19. 10.1037/0022-006X.59.1.12 [DOI] [PubMed] [Google Scholar]
- Kan, R. L. D. , Zhang, B. B. B. , Zhang, J. J. Q. , & Kranz, G. S. (2020). Non‐invasive brain stimulation for posttraumatic stress disorder: a systematic review and meta‐analysis. Translational Psychiatry, 10, Article 168. 10.1038/s41398-020-0851-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel, F. A. , Motes, M. A. , Didehbani, N. , DeLaRosa, B. , Bass, C. , Schraufnagel, C. D. , Jones, P. , Morgan, C. R. , Spence, J. S. , Kraut, M. A. , & Hart, J. (2018). Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial. Journal of Affective Disorders, 229, 506–514. 10.1016/j.jad.2017.12.046 [DOI] [PubMed] [Google Scholar]
- Kozel, F. A. , Van Trees, K. , Larson, V. , Phillips, S. , Hashimie, J. , Gadbois, B. , Johnson, S. , Gallinati, J. , Barrett, B. , Toyinbo, P. , Weisman, M. , Centorino, M. , Gibson, C. , & Catalano, G. (2019). One hertz versus ten hertz repetitive TMS treatment of PTSD: A randomized clinical trial. Psychiatry Research, 273, 153–162. 10.1016/j.psychres.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Kessler, R.C. (2000). Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry, 61(Suppl. 5), 4–12. [PubMed] [Google Scholar]
- Oliveira‐Maia, A. J. , Garcia‐Guarniz, A. L. , Sinanis, A. , Pascual‐Leone, A. , & Press, D. (2016). Comparative efficacy of repetitive transcranial magnetic stimulation for treatment of depression using 2 different stimulation devices: A retrospective open‐label study. The Journal of Clinical Psychiatry, 77(6), 743–744. 10.4088/JCP.15l10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reardon, J. P. , Solvason, H. B. , Janicak, P. G. , Sampson, S. , Isenberg, K. E. , Nahas, Z. , McDonald, W. M. , Avery, D. , Fitzgerald, P. B. , Loo, C. , Demitrack, M. A. , George, M. S. , & Sackeim, H. A. (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208–1216. 10.1016/j.biopsych.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Petrosino, N. J. , van't Wout‐Frank, M. , Aiken, E. , Swearingen, H. R. , Barredo, J. , Zandvakili, A. , & Philip, N. S. (2020). One‐year clinical outcomes following theta burst stimulation for post‐traumatic stress disorder. Neuropsychopharmacology, 45(6), 940–946. 10.1038/s41386-019-0584-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N. S. , Aiken, E. E. , Kelley, M. E. , Burch, W. , Waterman, L. , & Holtzheimer, P. E. (2019). Synchronized transcranial magnetic stimulation for posttraumatic stress disorder and comorbid major depression. Brain Stimulation, 12(5), 1335–1337. 10.1016/j.brs.2019.06.010 [DOI] [PubMed] [Google Scholar]
- Philip, N. S. , Barredo, J. , Aiken, E. , & Carpenter, L. (2018). Neuroimaging mechanisms of therapeutic transcranial magnetic stimulation for major depressive disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(3), 211–222 10.1016/j.bpsc.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N. S. , Barredo, J. , Aiken, E. , Larson, V. , Jones, R. N. , Shea, M. T. , Greenberg, B. D. , & van't Wout‐Frank, M. (2019). Theta‐burst transcranial magnetic stimulation for posttraumatic stress disorder. American Journal of Psychiatry, 176(11), 939–948. 10.1176/appi.ajp.2019.18101160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N. S. , Barredo, J. , van ’t Wout‐Frank, M. , Tyrka, A. R. , Price, L. H. , & Carpenter, L. L. (2018). Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biological Psychiatry, 83(3), 263–272. 10.1016/j.biopsych.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N. S. , Carpenter, S. L. , Ridout, S. J. , Sanchez, G. , Albright, S. E. , Tyrka, A. R. , Prince, L. H. , Carpenter, L. L. (2015). 5Hz Repetitive transcranial magnetic stimulation to left prefrontal cortex for major depression. Journal of Affective Disorders, 186, 13–17. 10.1016/j.jad.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, N. S. , Ridout, S. J. , Albright, S. E. , Sanchez, G. , & Carpenter, L. L. (2016). 5‐Hz Transcranial magnetic stimulation for comorbid posttraumatic stress disorder and major depression. Journal of Traumatic Stress, 29(1), 93–96. 10.1002/jts.22065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush, A. J. , Gullion, C. M. , Basco, M. R. , Jarrett, R. B. , & Trivedi, M. H. (1996). The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine, 26(3), 477–486. 10.1017/s0033291700035558 [DOI] [PubMed] [Google Scholar]
- Shalev, A. , Liberzon, I. , & Marmar, C. (2017). Post‐traumatic stress disorder. New England Journal of Medicine, 376(25), 2459–2469. 10.1056/NEJMra1612499 [DOI] [PubMed] [Google Scholar]
- Watts, B. V. , Schnurr, P. P. , Mayo, L. , Young‐Xu, Y. , Weeks, W. B. , Friedman, M. J. (2013). Meta‐analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry, 74(6), e541–550. 10.4088/jcp.12r08225 [DOI] [PubMed] [Google Scholar]
- Weathers, F. W. , Litz, B. T. , Keane, T. M. , Palmieri, P. A. , Marx, B. P. , & Schnurr, P. P. (2013). The PTSD Checklist for DSM‐5 (PCL‐5). https://www.ptsd.va.gov/professional/assessment/adult‐sr/ptsd‐checklist.asp [Google Scholar]
- Yesavage, J. A. , Fairchild, J. K. , Mi, Z. , Biswas, K. , Davis‐Karim, A. , Phibbs, C. P. Forman, S. D. , Thase, M. , Williams, L. M. , Etkin, A. , O'Hara, R. , Georgette, G. , Beale, T. , Huang, G. D. , Noda, A. , George, M. S. , & VA Cooperative Studies Program Study Team . (2018). Effect of repetitive transcranial magnetic stimulation on treatment‐resistant major depression in US veterans: A randomized clinical trial. JAMA Psychiatry, 75(9), 884–893. 10.1001/jamapsychiatry.2018.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Rating Scales over time and observed data points – Figures 1A‐B


