Abstract
In assisted reproductive technology treatment, diminished ovarian reserve (DOR) is a condition of utmost clinical and scientific relevance because of its negative influence on patient outcomes. The current methods of infertility treatment may be unsuitable for many women with DOR, which support the need for development of additional approaches to achieve fertility restoration. Various techniques have been tried to improve the quality and increase the quantity of oocytes in DOR patients, including mitochondrial transfer, activation of primordial follicles, in vitro culture of follicles, and regeneration of oocytes from various stem cells. Herein, we review the science behind these experimental reproductive technologies and their potential use to date in clinical studies for infertility treatment in women with DOR.
Keywords: Diminished ovarian reserve, Infertility treatment, Ovarian tissue cryopreservation, Follicle culture, Stem cells
Introduction
Infertile women with diminished ovarian reserve (DOR) are among the most challenging patients to successfully treat with assisted reproductive technology (ART). DOR indicates a reduction in the quantity and/or quality of oocytes such that the ability to reproductive potential is decreased [1–3]. Such patients are clinically defined by low levels of anti-Müllerian hormone (AMH <0.5–1.1 ng/ml), low antral follicle counts (AFCs <5–7), and/or elevated basal follicle-stimulating hormone (FSH) levels among women of reproductive age group. Typically, patients with DOR fulfill the Bologna consensus criteria to characterize patients with poor ovarian responder (POR) due to their reduced ovarian reserve [4]. The prevalence of DOR is reported to vary between 10 and 35%, depending on differences in the definition of DOR [5–7].
Several possible causes for DOR have been identified and include autoimmune diseases, inherited chromosome and genetic disorders, environmental hazards, and iatrogenic causes. However, most cases of DOR are still unexplained [8]. Lately, there has been an increased tendency to delay pregnancy, for social and/or economic reasons, resulting in an increasing number of women seeking infertility treatment at an advanced age. Advanced reproductive age is considered a major cause of DOR. Upon aging, the decline in both ovarian reserve and oocyte competence has been reported [9]. Moreover, as more cancer patients are surviving after gonadotoxic treatments, they are left with, typically irreversible, deleterious effects on ovaries. Consequently, there is an increasing need for methods to restore fertility in all women of ovarian impairment who are seeking reproductive success.
Majority of infertile women with DOR need to undergo ART and generally show lower oocyte yield, lower live birth rates, and higher treatment discontinuation rates than those with normal ovarian reserve [5]. The key problem is that women with DOR have few recruitable follicles despite aggressive gonadotropin stimulation. In the last 30 years, various controlled ovarian stimulation (COS) strategies have been proposed to improve outcome in patients with DOR and include mild ovarian-stimulation protocol which may include low-dose gonadotropins with or without oral agents, luteal phase gonadotropins, flare up agonist protocols, GnRH antagonists, estrogen priming in luteal phase, supplementation with LH, clomiphene citrate and letrozole co-treatment, double stimulation (follicular and luteal phase), and the adjuvant use of baby aspirin, low molecular weight heparin (LMWH), dehydroepiandrosterone (DHEA), or growth hormone (GH) [10, 11]. Unfortunately, because of the heterogeneity of DOR presentation in each patient, there is no specific or successful gold-standard treatment for patients with DOR [12]. Oocyte donation is the last treatment option for many DOR patients due to a lack of follicle growth or good quality oocyte. The pursuit of a healthy genetically linked offspring drives scientists toward investigating ovarian function restoration approaches for these patients. Here, we discuss the present status of restoration of reproductive function by providing both augmented quality of oocytes (Fig. 1) and increased number of oocytes (Fig. 2).
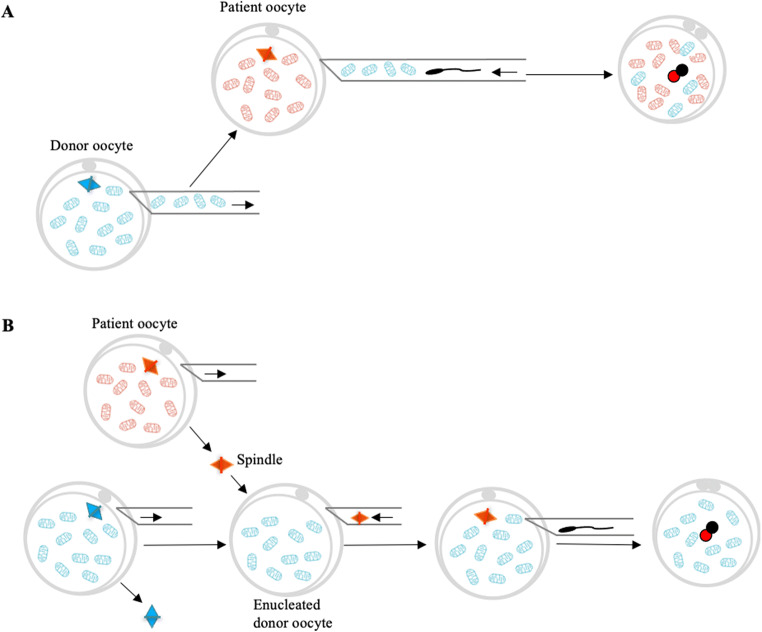
Fig. 1.
Mitochondrial transfer techniques. Ooplasmic transfer involves the transfer of mitochondrial from donor oocyte into patient oocyte (a). Maternal spindle transfer involves the transfer of the patient’s meiotic spindle into the enucleated donor oocyte followed by fertilization (b). Pronuclear transfer involves the transfer of pronuclei from the patient zygote into the enucleated donor zygote (c). Autologous germline mitochondrial energy transfer (AUGMENT) involves the injection of autologous mitochondria into the patient oocyte at the time of ICSI. These mitochondria were isolated from egg precursor cells (EggPCs) present in ovarian cortical tissue
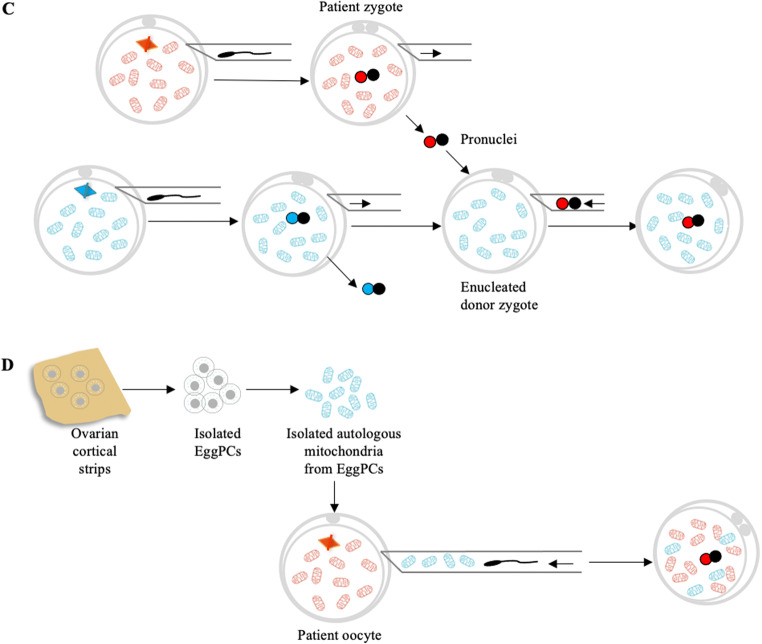
Fig. 2.
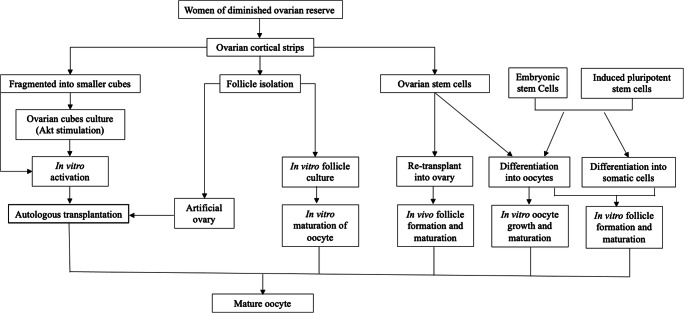
Current technologies and future prospects for increasing oocyte quantity to restore or enhance fertility in women with diminished ovarian reserve (DOR). Fully grown oocytes could be obtained by in vitro activation (IVA) of ovarian cortical strips. Ovarian follicles may be isolated and grown in vitro to obtain mature oocytes. Isolated follicles may be placed inside a three-dimensional scaffold to develop an artificial ovary for later auto-transplantation. Another future prospect lies in the possibility of using oogonial stem cells (OSCs), which can be grown in vitro or re-transplanted following treatment to produce mature oocytes. Differentiating follicle somatic cells and oocytes from embryonic stem cells or induced pluripotent stem cells can be used to assemble follicles de novo for transplantation or in vitro growth and maturation to create mature oocytes
Mitochondrial transfer
Mitochondria play vital roles in mammalian oogenesis and early embryogenesis [13]. Mitochondria provide cellular energy in the form of ATP and participate in several cellular processes, including ion fluxes and management of reduction-oxidation status, which in oocytes is essential for successful meiotic spindle assembly, proper segregation of chromosomes, maturation, fertilization, and preimplantation embryogenesis. Mitochondria contain their own small genome in the form of mitochondrial DNA (mtDNA), which is inherited exclusively from the mother. Defects in mitochondrial function as well as the increased level of mutation and deletion in oocyte mtDNA have deleterious consequences on chromosome segregation and on embryonic development, which could potentially lead to aneuploid embryos and reduced embryo implantation [9, 13–15]. At present, various mitochondrial supplementation techniques have been used to exchange and enhance the integrity, activity, and number of mitochondria in quality compromised oocytes. A scheme of these techniques is shown in Fig. 1.
In the 1990s, ooplasmic transfer was reported in patients who had previously experienced poor oocyte quality and repeated in vitro fertilization (IVF) failures. The procedure essentially involved co-injection of 1–5% young donor ooplasm into patient oocytes during intracytoplasmic sperm injection (ICSI), which led to successful pregnancies with almost 50 live births [16, 17]. However, the procedure was suspended by the United States Food and Drug Administration (FDA) due to the possibility of the heteroplasmy (the coexistence of two mtDNA genomes, linked to the use of mitochondria or ooplasm from a third donor) and chromosomal abnormalities in the subsequent embryos which raised both ethical and genetic questions [18–21].
Nuclear transfer (NT) techniques have been proposed to provide a potential therapeutic option for women who suffer from pathogenic cytoplasm related infertility. Briefly, these technologies involve the transfer of the nuclear genome from patient oocyte or zygote into an enucleated donor oocyte or zygote. The nuclear genome can be transferred from unfertilized oocytes using techniques such as the spindle transfer, polar body transfer, and germinal vesicle transfer, or can be transferred from fertilized oocytes (zygotes) using a technique known as pronuclear transfer [22, 23]. Notably, pronuclear transfer and spindle transfer are the most well studied to date, with clinical application to confirm the potential of these NT techniques to improve embryo quality or to prevent transmission of mtDNA disease [23–26]. However, such mitochondrial manipulations have come under criticism worldwide as these techniques raise the risks of heteroplasmy. NT techniques have therefore been ethically challenged and are currently considered controversial [27].
To solve concerns raised by the use of heterologous donor mitochondria, autologous transfer of mitochondria has attracted much attention as a possible new treatment to revitalize deficient oocytes. This observation was the basis of the so-called autologous germline mitochondrial energy transfer (AUGMENT), launched in 2014 by Ovascience. Briefly, ovarian tissue is processed to obtain the DDX4-positive egg precursor cell (EggPCs) population from a single-cell suspension, which is pelleted by centrifugation to release mitochondria. During ICSI, ~1–2 pL of mitochondrial suspension with sperm was co-injected into oocyte to improve oocyte quality. As a result of this procedure, healthy live births or ongoing pregnancies have been reported [28]. Although initial descriptive studies seemed promising, a recent prospective study could not demonstrate a clear improvement in either embryo development or pregnancy rates [29]. In fact, biased study design, methodological issues, and the lack of information from animal models make it difficult to validate this technology. Furthermore, it is not yet proven whether these germ cell–derived mitochondria actually pass the genetic bottleneck and are suitable to pass on to the next generation [30]. So autologous mitochondria transfer is being proposed but remains in very early stages. The search for an effective solution for oocyte rejuvenation continues.
Activation of primordial follicle
DOR is characterized by a clinically significant reduction in the pool of primordial follicles. Even more significant reductions in follicle numbers are encountered in women with primary ovarian insufficiency (POI; used to be known as premature ovarian failure, or POF). An important point is that with DOR, the ovary contains residual dormant primordial follicles that could theoretically be rescued and grown to increase the final yield of oocytes. Primordial follicle activation dictates individual primordial follicles leave their dormant state and enter into folliculogenesis for the eventual selection of one oocyte for ovulation. Although the molecular autocrine/paracrine mechanisms that control primordial follicle activation remain unknown, it is believed that the local environment (niche) plays a fundamental role. The challenge now focuses on activation and growth of these dormant primordial follicles to generate mature oocytes [31, 32].
To date, studies have attempted to regenerate the damaged ovarian niche by offering an appropriate environment to women with impaired ovarian reserves. The successful use of platelet-rich plasma (PRP) in regenerative medicine has led investigators to study its effect in the DOR treatment [33]. PRP consists of a high concentration of platelets found in plasma obtained after centrifugation of peripherally collected blood [34]. PRP induces accelerated angiogenesis and anabolism, inflammation-control, cell migration, differentiation, and proliferation and has been suggested to promote the development of isolated human primordial and primary follicles to the preantral stage [35, 36]. Clinical studies showed that intra-ovarian autologous PRP infusion increases the ovarian reserve parameters resulting in increased mature oocyte yield, fertilization rate, as well as the formation of good quality embryos. These findings suggest that intra-ovarian injection of autologous PRP might be considered an alternative experimental treatment option in women with DOR [37, 38]. High-quality randomized controlled trials are needed to estimate its efficacy in terms of clinical pregnancy and live birth rate. Also, there is a need to identify an optimum level of serum AMH or another markers of ovarian reserve for the success of intra-ovarian PRP infusion and identify the subpopulation that would get the most benefit from PRP [39].
As the first report of spontaneous pregnancies achieved after bone marrow transplantation in oncologic women with POI [40], many researchers have been attracted by autologous stem cells derived from different tissues. Several mechanisms have been proposed to achieve tissue regeneration by adult stem cell therapy based on the ability of adult stem cells to produce and secrete a variety of cytokines, chemokines, and growth factors, which may be involved in tissue repair [41]. In the context of ovarian tissue, paracrine actions should be evaluated for their ability to activate the preexisting quiescent follicles. Some of these soluble factors are known to promote follicular development, increase ovarian local vascularization, increase follicle and stromal cell proliferation, and reduce cell apoptosis and follicular atresia [42, 43]. Therefore, residual quiescent follicles of aged or damaged ovaries might produce competent oocytes in an adequate ovarian environment [44]. Long-term fertility rescue has been achieved in chemotherapy induced mouse ovaries mimicking aging, POR, or POI after infusion of adult stem cells from different origins as well as several administration techniques, providing scientific evidence to design new alternatives and therapies for humans [45, 46]. Herraiz et al. have investigated the effects of autologous stem cell ovarian transplant (ASCOT) on ovarian reserve in poor responders. In their study, bone marrow derived stem cells (BMDSCs) were mobilized to peripheral blood by granulocyte colony-stimulating factor (G-CSF) treatment and subsequently collected by apheresis. Cells were delivered into the ovarian artery by an intra-arterial catheter. Seventeen women were treated, resulting in increased number of antral follicles and oocytes. Five pregnancies were achieved, three of which were spontaneous, and three healthy babies were born after the stem cell administration [44]. To date, clinical results indicate that stem cell transplantation enhances ovarian function as evidenced by resumed menstruation, regulated hormone levels, and the ability to become pregnant [44, 47, 48]. Despite these promising results, several factors influence the proposed ovarian regenerative therapies. One is the stem cell administration technique. Although animal studies show that direct ovarian infusion is not required, human stem cells have been infused into one or both ovaries by various methods. Further research is needed to determine the most effective approach, although less invasive methods are needed for both stem cell collection and instillation. Stem cell source also appears to be an important factor. Human studies propose BMDSCs, both mesenchymal and hematopoietic, which are feasible candidates to promote ovarian rejuvenation [45]. This is a need to carefully evaluate these results and to identify an optimum stem cell source for clinical use. Currently, there are several ongoing clinical trials in many countries registered in the NIH clinical trial database. In order to accurately analyze the therapeutic effects of stem cells, it is important to recruit the appropriate participants, build a standard for quality control in clinical application, and address legal regulations to ensure safety.
In vitro activation (IVA) is a method for controlling primordial follicle activation using a combination of biochemical factors and mechanical signaling. The Akt pathway, or phosphoinositide-3-kinase (PI3K)-Akt, is a signal transduction pathway that promotes survival, growth, and proliferation in response to oocyte- and granulosa cell-derived factors. Once activated, primordial follicles start growing and advance to primary and secondary stages under the influence of paracrine factors and, later, FSH [49, 50]. By contrast, development of preantral follicles is curbed by the inhibitory Hippo signaling pathway, so disruption of Hippo signaling promotes secretion of downstream growth factors capable of stimulating follicle growth [50–52]. In 2013, Kawamura et al. were the first to combine these two methods to test IVA in a clinical setting. In their study, ovarian tissues were collected from 27 women with POI through laparoscopic surgery and these tissues were cut into strips. After Hippo signaling disruption and Akt stimulation, small fragmented ovarian strips were autotransplanted beneath the serosa of fallopian tubes. Their results showed that follicular growth was observed in 8 of these 27 women (29.6%). Retrieval of mature oocytes occurred in 5 women (18.5%), resulting in a livebirth after embryo transfer [50]. In a follow-up study from the same group, 37 women with POI were treated similarly, resulting in retrieval of mature oocytes from 6 women (16%), and 2 livebirths (5.4%) [53]. In 2016, Zhai et al. reported a live birth in a series of 14 patients with POI after removal of one ovary, 2 days of IVA, and reimplantation beneath the serosa of one of the fallopian tubes [54]. More recently, Kawamura et al. have described a drug-free approach based on Hippo signaling disruption alone by performing partial ovarian cortical removal in women with DOR and autografting back during the same laparoscopic surgery. They treated 11 women with severely DOR. In addition to one spontaneous pregnancy, embryo transfer resulted in one live birth, and two ongoing pregnancies [55]. These data suggest that IVA could offer new treatment potential to women with DOR [56]. However, Dolmans et al. used a xenotransplantation model to investigate the true benefits of IVA and their results were not able to demonstrate any significant benefits of IVA in human ovarian tissue xenografted to SCID mice, and therefore consider it unlikely that IVA improves follicle viability [57]. Lunding et al. did not find any direct effect of the ovarian fragmentation in the number of mature follicles in the biopsied ovary or at the site of transplantation after maximal ovarian stimulation [58]. Moreover, low pregnancy rates and possible carcinogenic effects following exposure to agents used in IVA require special caution [59, 60]. Currently, good evidence for the safe use of IVA in humans are lacking as only few studies on human ovarian tissue have been performed and no randomized controlled trial has been conducted yet. Thus, IVA needs to be thoroughly investigated before drawing any firm conclusions on its clinical value and safety [57, 61]. Finding a reliable and safe method for the synchronous activation of the primordial follicle cohort in vitro still remains challenging. In the future, a better understanding of the mechanisms underlying primordial follicle activation and development of safer physiologic IVA drugs may help to optimize this new technology.
In vitro growth and maturation of oocyte
The development of technologies to grow oocytes from the most abundant primordial follicles to maturity in vitro holds many attractions for clinical practice. Culture systems for in vitro gametogenesis/growth (IVG) need to support all stages of oocyte development from activation of dormant primordial follicles to a stage where oocytes can undergo meiotic maturation and be fertilized. Initial attempts at primordial follicle growth in vitro focused on the growth of isolated follicles [62, 63]. However, isolation and culture of primordial follicles are difficult tasks in humans, due to the small size, the limited connections between the granulosa cells and oocytes, and the complex extracellular matrix environment in which they develop [64]. Due to the fibrous nature of human ovarian cortex where the majority of primordial and primary follicles reside, the most effective way of obtaining a high follicle yield is usually based on a combination of mechanical and enzymatic tissue digestion [65–67]. A major problem facing in vitro culture of isolated primordial follicles is the breakdown of basement membrane material and other intrafollicular components during enzymatic isolation from the stroma. This erosion causes the pre-granulosa cells to round up and detach from the oocyte, which is an irreversible process that produces oocytes incapable of further development [64].
An alternative approach that has consistently proved successful for primordial follicle culture in human is to grow these early stage follicles in situ within fragments of the ovarian cortex until they have developed to a stage at which they can be isolated as granulosa-oocyte complexes for continuing growth. Telfer’s lab has shown that human primordial follicles grow well within mechanically loosened cortical pieces, which contain primordial and primary follicles with the underlying stroma tissue removed, and then can develop to multilaminar preantral (secondary) stages within 6–8 days. Multilaminar preantral follicles grown within human ovarian cortical strips can be isolated and further cultured to antral stage of development if cultured individually within a total culture period of 8–10 days [68]. However, it is assumed that complete follicle development from primordial to the pre-ovulatory stage in human takes up to 8 months and Gougeon calculated the time needed for a follicle to grow from the primary to the pre-ovulatory stage to be 84 days [64]. Whether this altered growth rate in vitro affects subsequent oocyte development is a question that needs to be addressed [69].
Isolated secondary follicles can be cultured in two-dimensional (2D) or three-dimensional (3D) systems. Different from traditional 2D culture on flat tissue culture surface, 3D culture system can use natural (e.g., collagen) and synthetic (e.g., alginate) hydrogels that provide sufficient support to maintain the 3D structure of the follicle [69]. Several reports have found that 3D culture systems are more appropriate than 2D culture systems with regard to maintaining the follicle structure, cell-cell interactions, growth rate, and gene expression patterns associated with normal oocyte development [69, 70]. Secondary human follicles have been able to grow, produce fluid-filled antral cavities, and produce meiotically competent oocytes in 3D culture system [65, 71]. Once follicles reach the antral stage, a further period of growth of the isolated oocyte–granulosa cell complex is required. Finally, cytoplasmic and nuclear maturation is induced in derived oocytes to reach metaphase II [68].
In 2018, Telfer’s group proved that primordial follicles could be grown up to a stage where the oocyte can reinitiate meiosis, which makes the complete in vitro development of oocytes from human tissue a practical and viable prospect. However, a relatively low number of oocytes reached metaphase II with large polar bodies highlights the need for comprehensive testing of human oocytes derived by in vitro growth and maturation [72]. The testing of the health of human oocytes and the embryos they produce is fraught with technical and ethical challenges that must be overcome before in vitro derived human gametes can be used safely to treat patients. Until further extensive research has been conducted, the potential of in vitro oocyte growth and maturation technologies remains to be realized.
Growth and maturation of oocytes in vitro from the primordial follicle stage is a major technical challenge for reproductive science. Although major steps have been taken over the last two decades, improvements and refinements are still needed before in vitro folliculogenesis can be considered for clinical use. The current challenges for follicle culture are numerous and include optimization of culture media and the tailoring of culture environments to match the physiological needs of the cell in vivo; the maintenance of cell–cell communication and signaling during culture; and the evaluation of the epigenetic status, genetic health, and fertility of in vitro derived mature oocytes. It is important to profoundly investigate the molecular and biochemical characterization of follicle development and oocyte maturation in vivo to better define the in vitro environmental conditions. Research also needs to evaluate the metabolic requirements that support acceptable rates of somatic and oocyte growth in vitro and how these demands change as oocyte and follicle development progress [73]. More sophisticated in vitro systems are being designed using the most recent knowledge from the omics, bio-printing technologies, and microfluidics. Continued advances in follicle culture protocols to support the production of high-quality, meiotically and developmentally competent oocytes may one day provide an additional fertility preservation and restoration option for women facing diseases or treatments that threaten their reproductive health [71, 74, 75].
Artificial ovary
Patients undergoing treatment regimens that eradicate their disease, such as cancer, may be left with diminished ovary function, including the inability to undergo puberty, early menopause, and infertility. Ovarian tissue cryopreservation and transplantation after cancer remission is a promising fertility restoration strategy that has already led to more than 130 live births worldwide [76, 77]. Unfortunately, with some types of cancer, there is a risk of reimplanting malignant cells together with the frozen-thawed tissue [78]. The potential presence of malignant cells in cryopreserved ovarian tissue led us to conceive and develop a procedure that avoids the risk of reintroducing malignant cells back to the patient. With a view to offering these women future alternatives allowing them to conceive, in recent years, the scientists have been developing a transplantable artificial ovary. The main objectives of an artificial ovary are to safely transplant isolated primordial and primary follicles, avoiding inadvertent contamination by malignant cells, and to support follicle survival and development after transplantation, restoring ovarian endocrine and reproductive functions [79].
A number of key conditions must be met in order to bioengineer an artificial ovary. The first requirement for the creation of an artificial ovary is isolation of a large number of intact primordial and primary follicles. At the same time, it is essential to prevent any possible malignant cell contamination during follicle pick-up and embedding [67, 80]. It is well known that early follicle survival and growth requires the participation and interaction of the oocyte itself, the granulosa and theca cells, and the surrounding cells and matrix of the ovarian connective tissue. Ovarian stromal cells are involved in activation of primordial follicles and some will be recruited to differentiate into theca cells [81, 82]. Endothelial cells are responsible for vascularization in ovarian tissue, and essential for transportation of paracrine factors, oxygen, and nutrients, as well as metabolic waste removal [83]. Integration of ovarian cells (stromal and endothelial cells) into the artificial ovary could thus serve to better simulate the ovarian micro-environment and potentially improve follicle growth and survival. Ovarian cells could be isolated from ovarian cortex cryopreserved before chemotherapy. In this case, stromal cells would have to undergo malignant cell purging before they could be safely used. An alternative is to take a fresh ovarian biopsy (cortex and/or medulla) just before transplantation of the artificial ovary, once the patient is disease free. These cells isolated from fresh ovarian tissue would then be combined with follicles obtained from cryopreserved cortex and would not require malignant cell purging [84].
To support survival and growth of follicles, the transplantable artificial ovary should have a 3D physical structure for the isolated follicles. The choice of which scaffold structure to use is one of the most challenging and critical elements in the development of an artificial ovary. The proper scaffold would be responsible for maintaining the original structure of follicles, ensuring proper communication between follicles and surrounding ovarian cells, preserving their interaction with the extracellular matrix (ECM), and supplying factors involved in follicular survival and development [79]. Initially, plasma clots were used as the supporting matrix for implantation into immunodeficient mice. Although follicular development to antral stages was obtained, plasma clots were found to have an inconsistent composition and degrade quickly, thereby potentially promoting follicle loss [85]. Therefore, researchers turned to 3D biomaterial based scaffold, including agarose, alginate, gelatin, and fibrin, which have been devised aiming to recreate an environment similar to the natural human ovary [86, 87]. Among all these candidates, fibrin formulations with high concentrations of fibrinogen and thrombin were found to be promising choices as a matrix for the construction of an artificial ovary. This is probably because their ultrastructure and rigidity resemble the human ovarian cortex [88]. A bioengineered ovary can also be generated by the use of a decellularized scaffold in which cells and cellular components are removed. This approach retains the ECM that offers the complex milieu that facilitates the necessary interaction between ovarian follicles and their surroundings to ensure their growth and development [89]. In future years, 3D printing can be used to create a human bioprosthetic ovary. The 3D printed bioprosthetic scaffold can be repopulated with ovarian tissues (either native or stem cells derived) [90, 91].
Isolated follicles in a delivery scaffold need to be finally transplanted to the patients. After transplantation, follow-up and evaluation of graft’s functions are essential for reproductive planning and patient health. Several studies have demonstrated the feasibility of artificial ovary. Dolmans et al. showed that human follicles were able to reach the antral stage after 5 months of xenografting [85]. Paulini et al. demonstrated that a fibrin formulation incorporating fibrinogen and thrombin created an environment that supported follicle and oocyte development. Follicle recovery rates were low (22%), but viability was high (100%) after 7-day xenotransplantation [92]. Pors et al. reported that decellularized human ovarian tissue was able to support survival of human preantral follicles and growth of murine follicles, of which 39% grew to antral stages. The follicular recovery rates after 3-week grafting was low but similar for both human (25%) and murine follicles (21%) [89]. Formation of an ovary-like structure, which is necessary for further follicle development, was also demonstrated in several studies [67, 92].
Although challenging, the current results show that artificial ovary technology open the door to new therapies in the field of fertility preservation and restoration. Ongoing research should now be directed at identifying a viable matrix that could mimic the multiple roles of the native ECM to produce an artificial ovary in order to improve oocyte recovery rates. Clearly, there is still a long way to go toward the development of an artificial human ovary. The technological developments at the interface of reproductive biology, tissue, and organ engineering will foster the development of an effective artificial ovary.
Making new oocytes from stem cells
The starting material for the in vitro growth systems described above is the primordial follicle that has been formed in vivo at birth. There are more futuristic sources for producing in vitro derived oocytes, from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) or from endogenous cells within the ovary that have the potential to become oocytes, often called as germline or oogonial stem cells (OSCs). Creating oocytes from stem cells may have seemed near impossible a decade ago, but advances in the last 10 years have reopened the conversation surrounding the possibilities and challenges of neo-oogenesis.
Both iPSCs and ESCs could be used to generate granulosa cells and oocytes might provide an additional route for fertility restoration. These oocytes and granulosa cells could then be used to build new follicles that could subsequently be transplanted back into the ovary or combined with in vitro maturation and IVF/ICSI protocols to create embryos [93–96]. Currently, iPSCs and ESCs have been differentiated into oocytes, and follicles have grown to maturity culminating in the successful birth of offspring in mice [96]. In humans, Yamashiro et al. reported the differentiation of human iPSCs into cells that resemble oogonia–immature oocytes in the human fetus. These findings establish the germline competence of iPSCs and provide a critical step toward human in vitro gametogenesis [97]. Yang et al. demonstrated that the iPSCs were successfully generated from POI patients’ fibroblasts. The formed iPSCs clones have the same characteristics of human ESCs [98]. However, there are no reports that human ESC or iPSC can be converted into competent oocytes yet [99].
In 2004, a study was published that identified the existence of mitotically active germ cells in postnatal mouse ovaries which were capable of supporting de novo oogenesis and folliculogenesis during adult life [100]. Subsequently, putative human OSCs were isolated from adult human ovaries and these cells showed the ability to spontaneously initiate a differentiation program into oocytes following either culture in vitro or transplantation into ovarian tissue in vivo [101–104]. White et al. reported the isolation of cells from human ovary that were capable of forming oocyte-like structures and those cells became incorporated into follicles under specific in vitro and in vivo conditions [101]. Silvestris et al. demonstrated that OSCs collected from fresh ovarian cortical fragments of non-menopausal and menopausal women are able to differentiate into large haploid oocyte-like cells and enter meiosis under appropriate culture conditions [102]. The concept that women are born with a finite number of eggs has been challenged by the possible existence of OSCs within the adult ovary that may have the potential to form mature oocytes. The existence of OSCs may hold some degree of regenerative potential for the ovary. However, currently, only very few groups have successfully isolated OSCs; others have been unable to identify and isolate such cells from adult mammalian ovaries [105]. A recent study on single-cell analysis of human ovarian cortex has suggested that cells captured by DDX4 antibody may be perivascular cells, rather than the oogonial stem cells [106]. Clearly, the debate regarding the existence of OSCs is far from settled, with compelling evidence supporting both sides [107]. Further research is needed to focus on the interactions between OSCs and the wider ovarian environment. This will provide the greater understanding and context needed to definitively prove the existence, function, and potential usefulness of these elusive cells [108]. Time will tell whether OSCs provide any advantage over other approaches for obtaining germs cells for infertility treatment, such as through the use of iPSCs or ESCs technologies.
Conclusion
The field of fertility preservation and restoration has made tremendous strides over the years in bringing new hopes to infertile women with DOR that they can have genetically related children. Oocyte rejuvenation by mitochondrial supplementation has been achieved at varying success rates following approaches that include heterologous or autologous mitochondrial. Activation of primordial follicle, in vitro culture of follicles and oocytes have been attempted. Regeneration of oocyte-like structures from different stem cells, especially OSCs, is surrounded by controversies and skepticisms. Although there is a very long way to go before the emerging experimental reproductive technologies discussed here may have clinical applications, it seems likely that these novel treatments, coupled with those in other exciting areas of reproductive medicine and bioengineering, may offer hopes for infertility treatment in women with DOR in the future.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zexu Jiao, Email: Zexu.Jiao@UTSouthwestern.edu.
Orhan Bukulmez, Email: Orhan.Bukulmez@UTSouthwestern.edu.
References
- 1.Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J Assist Reprod Genet. 2015;32(12):1709–12. doi: 10.1007/s10815-015-0595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive, M Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2012;98(6):1407–1415. doi: 10.1016/j.fertnstert.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Ferraretti AP, la Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L, on behalf of the ESHRE working group on Poor Ovarian Response Definition ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 5.Oudendijk JF, Yarde F, Eijkemans MJC, Broekmans FJM, Broer SL. The poor responder in IVF: is the prognosis always poor?: a systematic review. Hum Reprod Update. 2012;18(1):1–11. doi: 10.1093/humupd/dmr037. [DOI] [PubMed] [Google Scholar]
- 6.Keay SD, Liversedge NH, Mathur RS, Jenkins JM. Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol. 1997;104(5):521–527. doi: 10.1111/j.1471-0528.1997.tb11525.x. [DOI] [PubMed] [Google Scholar]
- 7.Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35(1):17–23. doi: 10.1007/s10815-017-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis. Detection and clinical relevance. Hum Reprod. 2003;18(6):1137–1139. doi: 10.1093/humrep/deg245. [DOI] [PubMed] [Google Scholar]
- 9.May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L'Hotellier V, Morinière C, Descamps P, Procaccio V, Reynier P. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22(6):725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 10.Patrizio P, Vaiarelli A, Levi Setti PE, Tobler KJ, Shoham G, Leong M, Shoham Z. How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod BioMed Online. 2015;30(6):581–592. doi: 10.1016/j.rbmo.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Blumenfeld Z. What is the best regimen for ovarian stimulation of poor responders in ART/IVF? Front Endocrinol (Lausanne) 2020;11:192. doi: 10.3389/fendo.2020.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, Ubaldi FM. What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol. 2018;30(3):155–162. doi: 10.1097/GCO.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 13.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Van Blerkom J, Davis P, Alexander S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum Reprod. 2001;16(4):719–729. doi: 10.1093/humrep/16.4.719. [DOI] [PubMed] [Google Scholar]
- 15.Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, Yoshimura Y. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30(10):1367–1375. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16(3):513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J, Scott R, Alikani M, Schimmel T, Munné S, Levron J, Wu L, Brenner C, Warner C, Willadsen S. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4(3):269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350(9072):186–187. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- 19.Huang CC, Cheng TC, Chang HH, Chang CC, Chen CI, Liu J, Lee MS. Birth after the injection of sperm and the cytoplasm of tripronucleate zygotes into metaphase II oocytes in patients with repeated implantation failure after assisted fertilization procedures. Fertil Steril. 1999;72(4):702–706. doi: 10.1016/s0015-0282(99)00309-x. [DOI] [PubMed] [Google Scholar]
- 20.Lanzendorf SE, Mayer JF, Toner J, Oehninger S, Saffan DS, Muasher S. Pregnancy following transfer of ooplasm from cryopreserved-thawed donor oocytes into recipient oocytes. Fertil Steril. 1999;71(3):575–577. doi: 10.1016/s0015-0282(98)00504-4. [DOI] [PubMed] [Google Scholar]
- 21.Dale B, Wilding M, Botta G, Rasile M, Marino M, di Matteo L, de Placido G, Izzo A. Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility: case report. Hum Reprod. 2001;16(7):1469–1472. doi: 10.1093/humrep/16.7.1469. [DOI] [PubMed] [Google Scholar]
- 22.Spikings EC, Alderson J, John JCS. Transmission of mitochondrial DNA following assisted reproduction and nuclear transfer. Hum Reprod Update. 2006;12(4):401–415. doi: 10.1093/humupd/dml011. [DOI] [PubMed] [Google Scholar]
- 23.Craven L, Tang MX, Gorman GS, de Sutter P, Heindryckx B. Novel reproductive technologies to prevent mitochondrial disease. Hum Reprod Update. 2017;23(5):501–519. doi: 10.1093/humupd/dmx018. [DOI] [PubMed] [Google Scholar]
- 24.Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF, Taylor RW, Lightowlers RN, Herbert M, Turnbull DM. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465(7294):82–85. doi: 10.1038/nature08958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Zhuang G, Zeng Y, Grifo J, Acosta C, Shu Y, Liu H. Pregnancy derived from human zygote pronuclear transfer in a patient who had arrested embryos after IVF. Reprod BioMed Online. 2016;33(4):529–533. doi: 10.1016/j.rbmo.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Liu H, Luo S, Lu Z, Chávez-Badiola A, Liu Z, Yang M, Merhi Z, Silber SJ, Munné S, Konstantinidis M, Wells D, Tang JJ, Huang T. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod BioMed Online. 2017;34(4):361–368. doi: 10.1016/j.rbmo.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Isasi R, Kleiderman E, Knoppers BM. Genetic technology regulation. Editing policy to fit the genome? Science. 2016;351(6271):337–339. doi: 10.1126/science.aad6778. [DOI] [PubMed] [Google Scholar]
- 28.Oktay K, Baltaci V, Sonmezer M, Turan V, Unsal E, Baltaci A, Aktuna S, Moy F. Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22(12):1612–1617. doi: 10.1177/1933719115612137. [DOI] [PubMed] [Google Scholar]
- 29.Labarta E, de los Santos MJ, Herraiz S, Escribá MJ, Marzal A, Buigues A, Pellicer A. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization-a randomized pilot study. Fertil Steril. 2019;111(1):86–96. doi: 10.1016/j.fertnstert.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Kristensen SG, Pors SE, Andersen CY. Improving oocyte quality by transfer of autologous mitochondria from fully grown oocytes. Hum Reprod. 2017;32(4):725–732. doi: 10.1093/humrep/dex043. [DOI] [PubMed] [Google Scholar]
- 31.Yin O, Cayton K, Segars JH. In vitro activation: a dip into the primordial follicle pool? J Clin Endocrinol Metab. 2016;101(10):3568–3570. doi: 10.1210/jc.2016-2837. [DOI] [PubMed] [Google Scholar]
- 32.Na J, Kim GJ. Recent trends in stem cell therapy for premature ovarian insufficiency and its therapeutic potential: a review. J Ovarian Res. 2020;13(1):74. doi: 10.1186/s13048-020-00671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amable PR, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(6):1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 35.Hosseini L, Shirazi A, Naderi MM, Shams-Esfandabadi N, Borjian Boroujeni S, Sarvari A, Sadeghnia S, Behzadi B, Akhondi MM. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod BioMed Online. 2017;35(4):343–350. doi: 10.1016/j.rbmo.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Sills ES, Wood SH. Autologous activated platelet-rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response. Biosci Rep. 2019:39(6). [DOI] [PMC free article] [PubMed]
- 37.Sfakianoudis K, et al. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. J Clin Med. 2018:8(1). [DOI] [PMC free article] [PubMed]
- 38.Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, Jr, Tiras B, Seli E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY) 2020;12(11):10211–10222. doi: 10.18632/aging.103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panda SR, Sachan S, Hota S. A systematic review evaluating the efficacy of intra-ovarian infusion of autologous platelet-rich plasma in patients with poor ovarian reserve or ovarian insufficiency. Cureus. 2020;12(12):e12037. doi: 10.7759/cureus.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salooja N, Chatterjee R, McMillan A, Kelsey SM, Newland AC, Milligan DW, Franklin IM, Hutchinson RM, Linch DC, Goldstone AH. Successful pregnancies in women following single autotransplant for acute myeloid leukemia with a chemotherapy ablation protocol. Bone Marrow Transplant. 1994;13(4):431–435. [PubMed] [Google Scholar]
- 41.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abedini A, Zamberlam G, Lapointe E, Tourigny C, Boyer A, Paquet M, Hayashi K, Honda H, Kikuchi A, Price C, Boerboom D. WNT5a is required for normal ovarian follicle development and antagonizes gonadotropin responsiveness in granulosa cells by suppressing canonical WNT signaling. FASEB J. 2016;30(4):1534–1547. doi: 10.1096/fj.15-280313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price CA. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J Endocrinol. 2016;228(2):R31–R43. doi: 10.1530/JOE-15-0414. [DOI] [PubMed] [Google Scholar]
- 44.Herraiz S, Romeu M, Buigues A, Martínez S, Díaz-García C, Gómez-Seguí I, Martínez J, Pellicer N, Pellicer A. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril. 2018;110(3):496–505. doi: 10.1016/j.fertnstert.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Herraiz S, Pellicer N, Romeu M, Pellicer A. Treatment potential of bone marrow-derived stem cells in women with diminished ovarian reserves and premature ovarian failure. Curr Opin Obstet Gynecol. 2019;31(3):156–162. doi: 10.1097/GCO.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 46.Herraiz S, Buigues A, Díaz-García C, Romeu M, Martínez S, Gómez-Seguí I, Simón C, Hsueh AJ, Pellicer A. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109(5):908–918. doi: 10.1016/j.fertnstert.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Farquhar C, et al. High-dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database Syst Rev. 2016;5:CD003139. doi: 10.1002/14651858.CD003139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mashayekhi M, Mirzadeh E, Chekini Z, Ahmadi F, Eftekhari-Yazdi P, Vesali S, Madani T, Aghdami N. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: non-randomized clinical trial, phase I, first in human. J Ovarian Res. 2021;14(1):5. doi: 10.1186/s13048-020-00743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJW. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107(22):10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, Sugishita Y, Morimoto Y, Hosoi Y, Yoshioka N, Ishizuka B, Hsueh AJ. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110(43):17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21(8):886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 52.Hsueh AJ, et al. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 54.Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, Hu L, Zhang Y, Wang J, Dai S, Li J, Sun J, Hsueh AJ, Kawamura K, Sun Y. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab. 2016;101(11):4405–4412. doi: 10.1210/jc.2016-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamura K, Ishizuka B, Hsueh AJW. Drug-free in-vitro activation of follicles for infertility treatment in poor ovarian response patients with decreased ovarian reserve. Reprod BioMed Online. 2020;40(2):245–253. doi: 10.1016/j.rbmo.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Lee HN, Chang EM. Primordial follicle activation as new treatment for primary ovarian insufficiency. Clin Exp Reprod Med. 2019;46(2):43–49. doi: 10.5653/cerm.2019.46.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dolmans MM, Cordier F, Amorim CA, Donnez J, Vander Linden C. In vitro activation prior to transplantation of human ovarian tissue: is it truly effective? Front Endocrinol (Lausanne) 2019;10:520. doi: 10.3389/fendo.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lunding SA, Pors SE, Kristensen SG, Landersoe SK, Jeppesen JV, Flachs EM, Pinborg A, Macklon KT, Pedersen AT, Andersen CY, Andersen AN. Biopsying, fragmentation and autotransplantation of fresh ovarian cortical tissue in infertile women with diminished ovarian reserve. Hum Reprod. 2019;34(10):1924–1936. doi: 10.1093/humrep/dez152. [DOI] [PubMed] [Google Scholar]
- 59.Saatcioglu HD, Cuevas I, Castrillon DH. Control of oocyte reawakening by kit. PLoS Genet. 2016;12(8):e1006215. doi: 10.1371/journal.pgen.1006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SY, Ebbert K, Cordeiro MH, Romero MM, Whelan KA, Suarez AA, Woodruff TK, Kurita T. Constitutive activation of PI3K in oocyte induces ovarian granulosa cell tumors. Cancer Res. 2016;76(13):3851–3861. doi: 10.1158/0008-5472.CAN-15-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meirow D, Roness H, Kristensen SG, Andersen CY. Optimizing outcomes from ovarian tissue cryopreservation and transplantation; activation versus preservation. Hum Reprod. 2015;30(11):2453–2456. doi: 10.1093/humrep/dev210. [DOI] [PubMed] [Google Scholar]
- 62.Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 63.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12(5):1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 64.Gosden RG, Mullan J, Picton HM, Yin H, Tan SL. Current perspective on primordial follicle cryopreservation and culture for reproductive medicine. Hum Reprod Update. 2002;8(2):105–110. doi: 10.1093/humupd/8.2.105. [DOI] [PubMed] [Google Scholar]
- 65.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24(10):2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanacker J, Camboni A, Dath C, van Langendonckt A, Dolmans MM, Donnez J, Amorim CA. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil Steril. 2011;96(2):379–383. doi: 10.1016/j.fertnstert.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 67.Chiti MC, Dolmans MM, Hobeika M, Cernogoraz A, Donnez J, Amorim CA. A modified and tailored human follicle isolation procedure improves follicle recovery and survival. J Ovarian Res. 2017;10(1):71. doi: 10.1186/s13048-017-0366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 69.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16(4):395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiao ZX, Woodruff TK. Follicle microenvironment-associated alterations in gene expression in the mouse oocyte and its polar body. Fertil Steril. 2013;99(5):1453–1459. doi: 10.1016/j.fertnstert.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015;5:17323. doi: 10.1038/srep17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod. 2018;24(3):135–142. doi: 10.1093/molehr/gay002. [DOI] [PubMed] [Google Scholar]
- 73.Herta AC, Lolicato F, Smitz JEJ. In vitro follicle culture in the context of IVF. Reproduction. 2018;156(1):F59–F73. doi: 10.1530/REP-18-0173. [DOI] [PubMed] [Google Scholar]
- 74.Anderson RA, Telfer EE. Being a good egg in the 21st century. Br Med Bull. 2018;127(1):83–89. doi: 10.1093/bmb/ldy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Telfer EE. Fertility preservation: progress and prospects for developing human immature oocytes in vitro. Reproduction. 2019;158(5):F45–F54. doi: 10.1530/REP-19-0077. [DOI] [PubMed] [Google Scholar]
- 76.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 77.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 78.Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99(6):1514–1522. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 79.Dolmans MM, Amorim CA. Fertility preservation: construction and use of artificial ovaries. Reproduction. 2019;158(5):F15–F25. doi: 10.1530/REP-18-0536. [DOI] [PubMed] [Google Scholar]
- 80.Soares M, Sahrari K, Amorim CA, Saussoy P, Donnez J, Dolmans MM. Evaluation of a human ovarian follicle isolation technique to obtain disease-free follicle suspensions before safely grafting to cancer patients. Fertil Steril. 2015;104(3):672–680. doi: 10.1016/j.fertnstert.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 81.Orisaka M, Tajima K, Mizutani T, Miyamoto K, Tsang BK, Fukuda S, Yoshida Y, Kotsuji F. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod. 2006;75(5):734–740. doi: 10.1095/biolreprod.105.050344. [DOI] [PubMed] [Google Scholar]
- 82.Tajima K, Orisaka M, Yata H, Goto K, Hosokawa K, Kotsuji F. Role of granulosa and theca cell interactions in ovarian follicular maturation. Microsc Res Tech. 2006;69(6):450–458. doi: 10.1002/jemt.20304. [DOI] [PubMed] [Google Scholar]
- 83.Liu WY, Lin SG, Zhuo RY, Xie YY, Pan W, Lin XF, Shen FX. Xenogeneic decellularized scaffold: a novel platform for ovary regeneration. Tissue Eng Part C Methods. 2017;23(2):61–71. doi: 10.1089/ten.tec.2016.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soares M, Sahrari K, Chiti MC, Amorim CA, Ambroise J, Donnez J, Dolmans MM. The best source of isolated stromal cells for the artificial ovary: medulla or cortex, cryopreserved or fresh? Hum Reprod. 2015;30(7):1589–1598. doi: 10.1093/humrep/dev101. [DOI] [PubMed] [Google Scholar]
- 85.Dolmans MM, Yuan WY, Camboni A, Torre A, Langendonckt AV, Martinez-Madrid B, Donnez J. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod BioMed Online. 2008;16(5):705–711. doi: 10.1016/s1472-6483(10)60485-3. [DOI] [PubMed] [Google Scholar]
- 86.Chiti MC, Donnez J, Amorim CA, Dolmans MM. From isolation of human ovarian follicles to the artificial ovary: tips and tricks. Minerva Ginecol. 2018;70(4):444–455. doi: 10.23736/S0026-4784.18.04231-4. [DOI] [PubMed] [Google Scholar]
- 87.Rios PD, Kniazeva E, Lee HC, Xiao S, Oakes RS, Saito E, Jeruss JS, Shikanov A, Woodruff TK, Shea LD. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng. 2018;115(8):2075–2086. doi: 10.1002/bit.26721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chiti MC, Dolmans MM, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, van Ruymbeke E, Champagne SD, Donnez J, Amorim CA. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet. 2018;35(1):41–48. doi: 10.1007/s10815-017-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pors SE, Ramløse M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY, Kristensen SG. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod. 2019;34(8):1523–1535. doi: 10.1093/humrep/dez077. [DOI] [PubMed] [Google Scholar]
- 90.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 91.Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paulini F, Vilela JMV, Chiti MC, Donnez J, Jadoul P, Dolmans MM, Amorim CA. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod BioMed Online. 2016;33(3):425–432. doi: 10.1016/j.rbmo.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Wang G, Farzaneh M. Mini review; differentiation of human pluripotent stem cells into oocytes. Curr Stem Cell Res Ther. 2020. [DOI] [PubMed]
- 94.Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi K, Saitou M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat Protoc. 2013;8(8):1513–1524. doi: 10.1038/nprot.2013.090. [DOI] [PubMed] [Google Scholar]
- 96.Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303. doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
- 97.Yamashiro C, Sasaki K, Yabuta Y, Kojima Y, Nakamura T, Okamoto I, Yokobayashi S, Murase Y, Ishikura Y, Shirane K, Sasaki H, Yamamoto T, Saitou M. Generation of human oogonia from induced pluripotent stem cells in vitro. Science. 2018;362(6412):356–360. doi: 10.1126/science.aat1674. [DOI] [PubMed] [Google Scholar]
- 98.Yang S, Ding S, He S, He L, Gao K, Peng S, Shuai C. Differentiation of primordial germ cells from premature ovarian insufficiency-derived induced pluripotent stem cells. Stem Cell Res Ther. 2019;10(1):156. doi: 10.1186/s13287-019-1261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarma UC, Findlay JK, Hutt KJ. Oocytes from stem cells. Best Pract Res Clin Obstet Gynaecol. 2019;55:14–22. doi: 10.1016/j.bpobgyn.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 101.White YA, et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silvestris E, Cafforio P, D’Oronzo S, Felici C, Silvestris F, Loverro G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: single-cell isolation and molecular characterization. Hum Reprod. 2018;33(3):464–473. doi: 10.1093/humrep/dex377. [DOI] [PubMed] [Google Scholar]
- 103.Clarkson YL, McLaughlin M, Waterfall M, Dunlop CE, Skehel PA, Anderson RA, Telfer EE. Initial characterisation of adult human ovarian cell populations isolated by DDX4 expression and aldehyde dehydrogenase activity. Sci Rep. 2018;8(1):6953. doi: 10.1038/s41598-018-25116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, Wang J, du M, Teng X, Wu J. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. 2016;6:28218. doi: 10.1038/srep28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A. 2012;109(31):12580–12585. doi: 10.1073/pnas.1206600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S, Hovatta O, Kere J, Lanner F, Damdimopoulou P. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 2020;11(1):1147. doi: 10.1038/s41467-020-14936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grieve KM, McLaughlin M, Dunlop CE, Telfer EE, Anderson RA. The controversial existence and functional potential of oogonial stem cells. Maturitas. 2015;82(3):278–281. doi: 10.1016/j.maturitas.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 108.Horan CJ, Williams SA. Oocyte stem cells: fact or fantasy? Reproduction. 2017;154(1):R23–R35. doi: 10.1530/REP-17-0008. [DOI] [PubMed] [Google Scholar]