Abstract
The potential role of nutritional factors in the development of peripheral arterial disease (PAD) remains poorly understood. We evaluated multiple recommended eating patterns as reflected by predefined diet quality indices in relation to long-term risk of PAD. We included 138 506 U.S. postmenopausal women in the Women’s Health Initiative who had no known PAD at baseline (1993–1998). Four diet quality indices, including alternate Mediterranean diet index, alternate Healthy Eating Index (AHEI)-2010, Dietary Approaches to Stop Hypertension (DASH) diet index, and Healthy Eating Index-2015, were derived using dietary information collected by a validated food frequency questionnaire at baseline. Incident cases of symptomatic PAD in the lower extremities were ascertained and adjudicated through March 2019 via medical record review. During a median 18.6 years of follow-up, 1036 incident PAD cases were identified. After multivariable adjustment, all diet quality scores were significantly and inversely associated with 21% (for AHEI-2010) to 34% (for DASH index) lower risk of PAD when comparing the highest with the lowest quartiles (all P-for-trend values ≤0.010). Among contributing food groups and nutrients, intakes of legumes, dietary fiber, and vegetable protein were associated lower risk of PAD, while intakes of unprocessed red meat, processed meat, and regular soft drinks were associated with higher risk. In a broad sample of U.S. postmenopausal women, adhering to different recommended eating patterns is associated with lower risk of PAD. Our findings suggest that current clinical and public health strategies that recommend healthful eating patterns may also be applicable to PAD prevention.
Keywords: Dietary guidelines, Dietary pattern, Diet quality, Peripheral arterial disease, Women’s Health Initiative
Peripheral arterial disease (PAD) is an important atherosclerotic disorder that most commonly occurs in the lower extremities, with >230 million patients in 2015 globally.1 It is estimated that 7.2% (~8.5 million) of U.S. adults aged 40 years or older are being affected by PAD, with a higher prevalence and larger number of patients in women than in men.2,3 Morbidity, mortality, and health economic costs caused by PAD are comparable to those by coronary heart disease and ischemic stroke,4,5 both of which have been closely related to lifestyle behaviors including dietary habits.6 However, only smoking and a few metabolic conditions (i.e., hypertension, dyslipidemia, and diabetes) have been well defined as risk factors for PAD,4,5 highlighting the need for identifying additional modifiable risk factors.
While clinical and public health guidelines recommend building a healthful eating pattern as an important approach for reducing risk of chronic diseases and improving health conditions,7,8 whether such dietary recommendations are also applicable to PAD prevention remains unknown. To this point, there are only a few prospective studies that have assessed dietary factors in relation to risk of PAD, and most have been focused on individual food groups or nutrients.9–12 Because foods or nutrients are consumed in a mixture of various dietary constituents where they often correlate and may interact with each other, ascertaining the health effect of a single dietary component is often difficult. Dietary pattern approaches can take into account a broad picture of individual dietary components and, as such, the yielded findings may be more practical for nutritional recommendations.13
In the current study, we sought to examine several recommended eating patterns as reflected by different a priori diet quality indices for their relationships with risk of PAD in the Women’s Health Initiative (WHI),14,15 a nationwide cohort of diverse U.S. postmenopausal women. The selected diet quality indices included alternate Mediterranean diet (aMED) index, alternate Healthy Eating Index (AHEI)-2010, Dietary Approaches to Stop Hypertension (DASH) diet index, and Healthy Eating Index (HEI)-2015. Because higher scores of these diet quality indices have been widely associated with lower cardiometabolic risk,16,17 we hypothesized inverse relationships between these dietary indices and risk of PAD.
METHODS
Study Design and Participants
The data that support the findings of this study are available from the corresponding author upon reasonable request. Details of the WHI design and study participants have been reported elsewhere.14 Briefly, between 1993 and 1998, 161 808 postmenopausal women aged 50–79 years were recruited at 40 clinical centers throughout the United States. Menopausal status was confirmed according to self-reported age at which a woman last had any menstrual bleeding, self-reported history of hysterectomy or bilateral oophorectomy, and/or use of menopausal hormone therapy. Participants were either enrolled in an observational study or in one or more of clinical trials that evaluated the health effects of hormone replacement therapy (two trials), low-fat dietary modification, and/or calcium and vitamin D supplementation (Figure S1). At the end of the initial WHI study in 2005, the first (2005–2010) and the second (2010–2020) WHI Extension Studies continued follow-up of all women who consented. The study was approved by the institutional review boards of all participating institutions, and all participants provided written informed consent at initial enrollment and for the extension studies.
For the current analysis, we excluded 19 541 participants who were recruited in the active arm of the Dietary Modification Trial in which a low-fat, high vegetable and fruit dietary intervention was administered. We further excluded 2391 participants with self-reported PAD at baseline, 222 without information on dietary intake, and 608 participants missing follow-up information for incident PAD. The final analytic sample consisted of 138 506 participants (Figure S1).
Dietary Assessment and Calculation of Diet Quality Scores
Information on dietary intake at baseline was collected using a self-administered, semi-quantitative food frequency questionnaire (FFQ).18 This instrument included 122 composite and single food line items, 19 adjustment questions, and 4 summary questions for participants to denote their habitual diets over the past 3 months. The WHI-FFQ nutrient database was derived from Nutrition Data Systems for Research (the University of Minnesota Nutrition Coordinating Center) food and nutrient database.19 Previous validations against dietary recalls and food records showed that the FFQ provided reliable estimates of dietary intake.18 Using the MyPyramid Equivalents Database, version 2.0,20 dietary data in units of MyPyramid equivalents were also constructed by translating frequency of food consumption into standardized food quantities.
Components of the diet quality indices included in our analysis and standards for scoring have been described in detail elsewhere21 and are summarized in Tables S1–S4, with higher scores indicating better diet quality. The 9-point aMED index was adapted to reflect adherence to the Mediterranean diet.22 The AHEI-2010 was established based on extensive epidemiologic findings linking foods and nutrients to chronic disease outcomes.23 The DASH index was developed to characterize the DASH diet,24 an eating pattern initially designed for clinical trials aiming at reducing blood pressure.24 Finally, the HEI-2015 assesses the extent to which an individual’s diet aligns with the 2015–2020 Dietary Guidelines for Americans.25
Outcome Ascertainment
The outcome of interest in our analysis was incident symptomatic PAD at the lower extremities. Participants reported overnight hospitalizations and emergency room visits on medical update forms collected semiannually, and corresponding medical records were scrutinized for the potential outcome of interest.26 Locally and/or centrally trained physician adjudicators classified outcomes on the basis of medical record review. Potential cases of PAD at the lower extremities were confirmed by multiple procedures reported in Table S5. Most (94.2%) of the incident cases of PAD included in the current analysis were confirmed by one or both of the following procedures: 1) surgery, angioplasty, or thrombolysis for PAD; and 2) obstruction or ulcerated plaque (i.e., ≥ 50% of the diameter or ≥75% of the cross-sectional area) demonstrated on ultrasound or angiogram of the iliac arteries or below.
Assessment of Other Covariates
Information on demographic and socioeconomic characteristics, reproductive, medical and family histories, exogenous hormone use, smoking, and alcohol drinking was collected at baseline via self-report. Systolic and diastolic blood pressure, and height and weight were measured by trained staff using standard procedures.15 Body mass index was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Recreational physical activity was measured using a specifically developed questionnaire and data were summarized in metabolic equivalent-hours/week.15 Information on previous diagnosis and treatment of hypertension, hyperlipidemia, or diabetes by a physician was collected via questionnaires. Participants were also instructed to bring prescription medication containers during the baseline screening interview. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, a reported physician’s diagnosis, or use of antihypertensive mediations. Dyslipidemia was defined as a reported physician’s diagnosis or recorded statin use.
Statistical Analysis
Baseline characteristics of participants were described by quartile of each diet quality score. Age-adjusted Pearson partial correlations among the diet quality scores were calculated. Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals (CI) of PAD associated with the examined dietary factors. Each diet quality score was modeled both categorically by quartile and continuously in per standard deviation increment. Person-time of follow-up was counted from the date of enrollment through date of diagnosis of PAD, death or withdrawal from the study, or end of the most recent follow up (March 2019), whichever came first. P values for linear trend were computed by treating medians for each quartile of a dietary score as a continuous variable. Two Cox models were constructed to account for potential confounders. The first model was adjusted for age, race/ethnicity, education, annual family income, and study group. The full model was additionally adjusted for smoking status, pack-years of smoking, alcohol consumption (only for DASH index and HEI-2015), recreational physical activity, total energy intake, use of statins, hyperlipidemia, use of antihypertensive drugs, systolic and diastolic blood pressure, and diabetes. Potential nonlinear relationships between the diet quality scores and risk of PAD were examined using restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles of the distribution.
The associations of the dietary scores with risk of PAD were stratified according to various participants’ demographic characteristics and other known risk factors and the potential interactions were assessed. To test the robustness of the findings, several sensitivity analyses were performed by excluding: 1) participants with implausible total energy intake (>5000 or <600 kcal/day); 2) participants with self-reported coronary heart disease or stroke at baseline; 3) incident PAD cases that were identified within the first 5 years of follow-up; and 4) participants who were not diagnosed with PAD and died from a cardiovascular event during the study follow-up because cardiovascular deaths may have occurred competitively with PAD.
To better understand the proportion of incident cases of PAD in the study sample that may be attributed to different risk factors, we estimated population attributable fraction (PAF) for each lifestyle (low-quality diet, physical inactivity, and current smoking) and metabolic risk factors (diabetes, hypertension, and dyslipidemia) for PAD. On the basis of the fully adjusted Cox model (with mutual adjustment for these risk factors), PARs with 95% CIs were estimated using the punafcc post-estimate command in Stata.
We further explored associations of major food groups and nutrients or food components that contribute to the examined diet quality indices with risk of PAD. In these analyses, all individual dietary components (except for alcohol) were adjusted for total energy intake at 2000 kcal/day.27 Statistical analyses were performed using Stata (version 15.1; StataCorp).
RESULTS
Participant Characteristics
Table 1 presents baseline characteristics of the study participants according to quartiles of the diet quality scores. Overall, higher scores of the 4 diet quality indices were associated with older age, higher levels of education and family income, and healthier lifestyle behaviors including nonsmoking, moderate alcohol drinking, and regular exercise. Participants with higher diet quality scores also were leaner, were less likely to have prevalent hypertension, diabetes, or coronary heart disease or stroke, and were more likely to be in the observational study than the clinical trials. The 4 diet quality indices had moderate-to-strong correlations with each other, ranging from 0.54 between aMED index and HEI-2015 to 0.71 between DASH index and HEI-2015.
Table 1.
Baseline participant characteristics according to quartile of diet quality scores in the Women’s Health Initiative (n = 138 506)
| Variables | aMED | AHEI-2010 | DASH | HEI-2015 | ||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Age, y | 63.0 ± 7.3 | 63.6 ± 7.2 | 62.5 ± 7.2 | 63.8 ± 7.2 | 61.8 ± 7.1 | 64.2 ± 7.3 | 62.2 ± 7.2 | 64.5 ± 7.2 |
| Race/ethnicity, % | ||||||||
| White | 78.2 | 87.2 | 77.5 | 87.5 | 70.7 | 90.1 | 78.0 | 87.0 |
| Black/African-American | 11.1 | 6.6 | 14.0 | 4.8 | 17.4 | 4.2 | 11.8 | 6.5 |
| Hispanic/Latino | 6.3 | 2.0 | 5.2 | 2.3 | 6.8 | 2.0 | 5.7 | 2.4 |
| Other/unknown | 4.4 | 4.2 | 3.3 | 5.5 | 5.1 | 3.7 | 4.6 | 4.1 |
| College degree or above, % | 26.5 | 52.1 | 27.2 | 53.7 | 25.7 | 52.2 | 29.5 | 49.2 |
| Family income ≥$50 000/y, % | 32.3 | 47.4 | 30.4 | 50.9 | 32.1 | 45.7 | 33.6 | 45.5 |
| Smoking status, % | ||||||||
| Never | 51.1 | 51.1 | 53.5 | 47.4 | 49.9 | 51.8 | 48.3 | 53.0 |
| Former | 37.7 | 45.1 | 35.8 | 48.9 | 36.7 | 45.1 | 39.6 | 43.7 |
| Current | 11.2 | 3.8 | 10.7 | 3.7 | 13.5 | 3.1 | 12.1 | 3.3 |
| Alcohol consumption, % | ||||||||
| 0 g/d | 7.4 | 5.7 | 5.6 | 7.8 | 4.4 | 9.8 | 4.2 | 10.3 |
| >0 – <5 g/d | 77.4 | 53.0 | 81.2 | 47.6 | 73.0 | 59.8 | 74.3 | 57.0 |
| 5 – <15 g/d | 4.8 | 30.2 | 4.9 | 30.1 | 13.3 | 18.7 | 13.5 | 19.1 |
| 15 – <25 g/d | 5.4 | 6.8 | 1.6 | 11.8 | 4.7 | 7.3 | 4.5 | 8.1 |
| ≥25 g/d | 5.0 | 4.3 | 6.7 | 2.7 | 4.6 | 4.4 | 3.6 | 5.5 |
| Recreational PA, MET-h/wk | 9.0 ± 11.7 | 16.1 ± 14.8 | 8.3 ± 11.0 | 17.6 ± 15.7 | 7.8 ± 10.7 | 17.1 ± 15.4 | 8.5 ± 11.4 | 16.7 ± 15.1 |
| Body mass index, kg/m2 | 28.7 ± 6.2 | 26.8 ± 5.5 | 29.4 ± 6.4 | 26.3 ± 5.2 | 29.4 ± 6.4 | 26.5 ± 5.3 | 29.5 ± 6.5 | 26.2 ± 5.2 |
| Total energy intake, kcal/d | 1282 ± 598 | 1879 ± 744 | 1718 ± 740 | 1590 ± 642 | 1591 ± 752 | 1649 ± 628 | 1858 ± 937 | 1429 ± 503 |
| Hypertension, % | 41.4 | 35.9 | 43.7 | 33.4 | 42.8 | 35.2 | 40.9 | 37.0 |
| Dyslipidemia, % | 13.6 | 14.9 | 14.3 | 13.9 | 13.2 | 14.9 | 12.5 | 16.5 |
| Diabetes, % | 6.2 | 4.8 | 6.0 | 5.0 | 5.8 | 5.3 | 6.1 | 5.3 |
| CHD or stroke, % | 4.7 | 3.7 | 4.8 | 4.0 | 4.5 | 3.9 | 4.1 | 4.4 |
| Study group, % | ||||||||
| WHI OS | 61.3 | 70.9 | 57.8 | 74.5 | 55.0 | 76.5 | 54.5 | 78.3 |
| WHI CT-control arms | 20.9 | 15.8 | 23.0 | 13.8 | 24.7 | 12.0 | 25.7 | 10.6 |
| WHI CT-active arms | 17.8 | 13.3 | 19.2 | 11.7 | 20.3 | 11.5 | 19.8 | 11.1 |
Data are mean ± standard deviation or % unless otherwise indicated. AHEI indicates alternate Healthy Eating Index; aMED, alternate Mediterranean diet; CHD, coronary heart disease; CT, clinical trials; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; MET, metabolic equivalent; OS, observational study; PA, physical activity; and Q, quartile.
Diet Quality Indices and Incident PAD
During up to 25.1 years of follow-up (median: 18.6 years), 1036 cases of incident PAD were identified. With basic adjustment for age, race/ethnicity, education, family income, and study group, participants in the highest quartiles of the 4 diet quality scores were estimated to have 29% (for AHEI-2010) to 45% (for DASH index) significantly lower risk of PAD as compared with those in the lowest quartiles (all P-for-trend values <0.001) (Table 2).
Table 2.
Association of diet quality indices with risk of peripheral arterial disease in the Women’s Health Initiative (n = 138 506)
| Variables | Quartile for diet quality scores | P-trend | Per SD increment | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| aMED | ||||||
| Median score (range) | 2 (0–2) | 3 (3–3) | 4 (4–5) | 6 (6–9) | ||
| No. of cases | 245 | 188 | 387 | 216 | ||
| No. of person-years | 366 894 | 374 610 | 853 296 | 584 371 | ||
| Model 1 (HR [95% CI]) | 1.00 (referent) | 0.78 (0.65–0.95) | 0.75 (0.64–0.89) | 0.66 (0.55–0.80) | <0.001 | 0.87 (0.81–0.92) |
| Model 2 (HR [95% CI]) | 1.00 (referent) | 0.80 (0.66–0.97) | 0.81 (0.69–0.96) | 0.74 (0.61–0.91) | 0.010 | 0.90 (0.84–0.97) |
| AHEI-2010 | ||||||
| Median score (range) | 40.3 (14.4–44.9) | 48.6 (45.0–51.9) | 55.4 (52.0–59.3) | 64.6 (59.4–94.5) | ||
| No. of cases | 333 | 300 | 199 | 204 | ||
| No. of person-years | 522 183 | 536 668 | 552 878 | 567 443 | ||
| Model 1 (HR [95% CI]) | 1.00 (referent) | 0.92 (0.79–1.08) | 0.65 (0.54–0.78) | 0.71 (0.59–0.85) | <0.001 | 0.84 (0.78–0.89) |
| Model 2 (HR [95% CI]) | 1.00 (referent) | 0.95 (0.81–1.11) | 0.69 (0.58–0.83) | 0.79 (0.65–0.95) | <0.001 | 0.87 (0.81–0.93) |
| DASH | ||||||
| Median score (range) | 18 (8–20) | 23 (21–24) | 26 (25–27) | 30 (28–39) | ||
| No. of cases | 299 | 281 | 216 | 240 | ||
| No. of person-years | 414 361 | 586 711 | 500 037 | 678 063 | ||
| Model 1 (HR [95% CI]) | 1.00 (referent) | 0.70 (0.59–0.82) | 0.65 (0.54–0.78) | 0.55 (0.46–0.66) | <0.001 | 0.79 (0.74–0.85) |
| Model 2 (HR [95% CI]) | 1.00 (referent) | 0.75 (0.64–0.89) | 0.74 (0.62–0.89) | 0.66 (0.55–0.80) | <0.001 | 0.86 (0.80–0.92) |
| HEI-2015 | ||||||
| Median score (range) | 53.0 (20.5–58.6) | 62.8 (58.7–66.3) | 69.8 (66.4–73.3) | 77.6 (73.4–96.7) | ||
| No. of cases | 337 | 257 | 243 | 199 | ||
| No. of person-years | 525 109 | 543 435 | 552 974 | 557 654 | ||
| Model 1 (HR [95% CI]) | 1.00 (referent) | 0.76 (0.65–0.90) | 0.73 (0.61–0.86) | 0.59 (0.49–0.71) | <0.001 | 0.83 (0.78–0.88) |
| Model 2 (HR [95% CI]) | 1.00 (referent) | 0.83 (0.71–0.98) | 0.81 (0.69–0.98) | 0.68 (0.56–0.82) | <0.001 | 0.88 (0.83–0.94) |
AHEI indicates alternate Healthy Eating Index; aMED, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; HEI, Healthy Eating Index; and SD, standard deviation.
Model 1 was adjusted for age (y), race/ethnicity (White, Black/African-American, Hispanic/Latino, other/unknown), education (at most high school, some college, college or above), annual family income (<20 000, 20 000–<50 000, 50 000–<75 000, ≥75 000 USD), and study group (Observational Study, control of Clinical Trials, active arms of Clinical Trials).
Model 2 was adjusted for covariates in the model 1 plus smoking status (never, former, current), pack-years of smoking (for current smokers), alcohol consumption (0, 0–<5, 5–<15, 15–<25, ≥25 g/day; only for DASH index and HEI-2015), recreational physical activity (MET-h/week), total energy intake (kcal/d), body mass index (kg/m2), use of statins (yes, no), reported hyperlipidemia (yes, no), use of antihypertensive drugs (yes, no), systolic and diastolic blood pressure, and reported diabetes (yes, no).
All inverse associations between the diet quality scores and risk of PAD were attenuated only slightly and remained significant after further adjustment for other lifestyle behaviors, body mass index, and metabolic factors associated with PAD including blood pressure, dyslipidemia, and diabetes. The multivariable-adjusted hazard ratios comparing the highest with the lowest quartiles of the diet quality scores were 0.74 (95% CI: 0.61–0.91) for aMED index (P-trend = 0.010), 0.79 (95% CI: 0.65–0.95) for AHEI-2010 (P-trend <0.001), 0.66 (95% CI: 0.55–0.80) for DASH index (P-trend <0.001), and 0.68 (95% CI: 0.56–0.82) for HEI-2015 (P-trend <0.001). Each 1-standard deviation increment in the diet quality scores was significantly associated with 10% (for aMED index) to 14% (for DASH index) lower risk of PAD (Table 2). There was no evidence for nonlinear associations between the examined diet quality scores and risk of PAD (all P values for nonlinearity ≥0.24)(Figure S2).
The inverse relationships between the examined diet quality scores and risk of PAD were broadly consistent across various subgroups defined by participants’ demographic characteristics and other known risk factors (Table S6). For specific dietary scores, there was suggestive evidence for stronger associations among participants aged 65 years or older or among participants without dyslipidemia than the associations in the corresponding opposite subgroups. However, these differences in the associations need to be treated with caution given the marginal significance of the P-for-interaction values (≥0.017) and the multiple sub-analyses performed. In a number of the aforementioned sensitivity analyses, the inverse associations of the diet quality scores with risk of PAD were similar to the associations in the whole study sample (Figure S3).
Population Attributable Fraction
For diet quality (reflected by the DASH score, which was most strongly associated with risk of PAD among the examined dietary scores) and other lifestyle and metabolic risk factors, we estimated PAFs after multivariable adjustment and mutual adjustment for each other. Assuming a causal relationship between the DASH diet and risk of PAD, 9.7% (95% CI: 3.3%−15.7%) of the incident PAD cases in the study sample could be prevented if participants whose DASH score was <25 (median) increased the score to 25 or above (Table 3). Moving the threshold for low-quality diet to the 75th percentile of DASH score lead to a higher PAF (14.1%; 95% CI: 1.7%−25.0%). PAF for physical inactivity (9.7%) was comparable with that for diet, while the highest PAF was observed for hypertension (36.5%), followed by PAFs for current smoking (18.0%), diabetes (12.4%) and dyslipidemia (8.4%) (Table 3). However, caution is need when interpreting the PAF estimates for diabetes and dyslipidemia because of the lack of blood glycemic or lipid traits that define the conditions based on clinical criteria.
Table 3.
Population attributable fractions (PAF) of lifestyle and metabolic risk factors for peripheral arterial disease in the Women’s Health Initiative (n = 138 506)
| Risk factors | Prevalence, % | HR (95% CI) | PAF (95% CI),% |
|---|---|---|---|
| Lower DASH score (below median or <25) | 47.5 | 1.21 (1.06–1.38) | 9.7 (3.3–15.7) |
| Physical inactivity (<7.5 MET-h/week)* | 44.6 | 1.21 (1.06–1.39) | 9.7 (3.5–15.5) |
| Smoking (current vs. never/former) | 6.9 | 4.00 (3.24–4.94) | 18.0 (16.7–19.2) |
| Diabetes† | 5.7 | 3.35 (2.82–3.97) | 12.4 (11.5–13.2) |
| Hypertension† | 38.7 | 2.33 (2.04–2.66) | 36.5 (32.7–40.1) |
| Dyslipidemia† | 14.5 | 1.51 (1.30–1.75) | 8.4 (5.9–10.8) |
DASH, Dietary Approaches to Stop Hypertension; MET, metabolic equivalent.
Where appropriate, results were adjusted for all covariates listed for model 2 of Table 2 (as such, all listed lifestyle and metabolic risk factors were mutually adjusted for each other).
Recreational physical activity of 7.5 MET-h/week approximates moderate-to-vigorous physical activity of 150 min/week (the lowest level recommended by the Physical Activity Guidelines for Americans).
Definitions are as follows: diabetes was defined as a self-reported physician’s diagnosis; hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, a self-reported physician’s diagnosis, or use of antihypertensive mediations; and dyslipidemia was defined as a self-reported physician’s diagnosis or use of statins.
Individual Dietary Components and Incident PAD
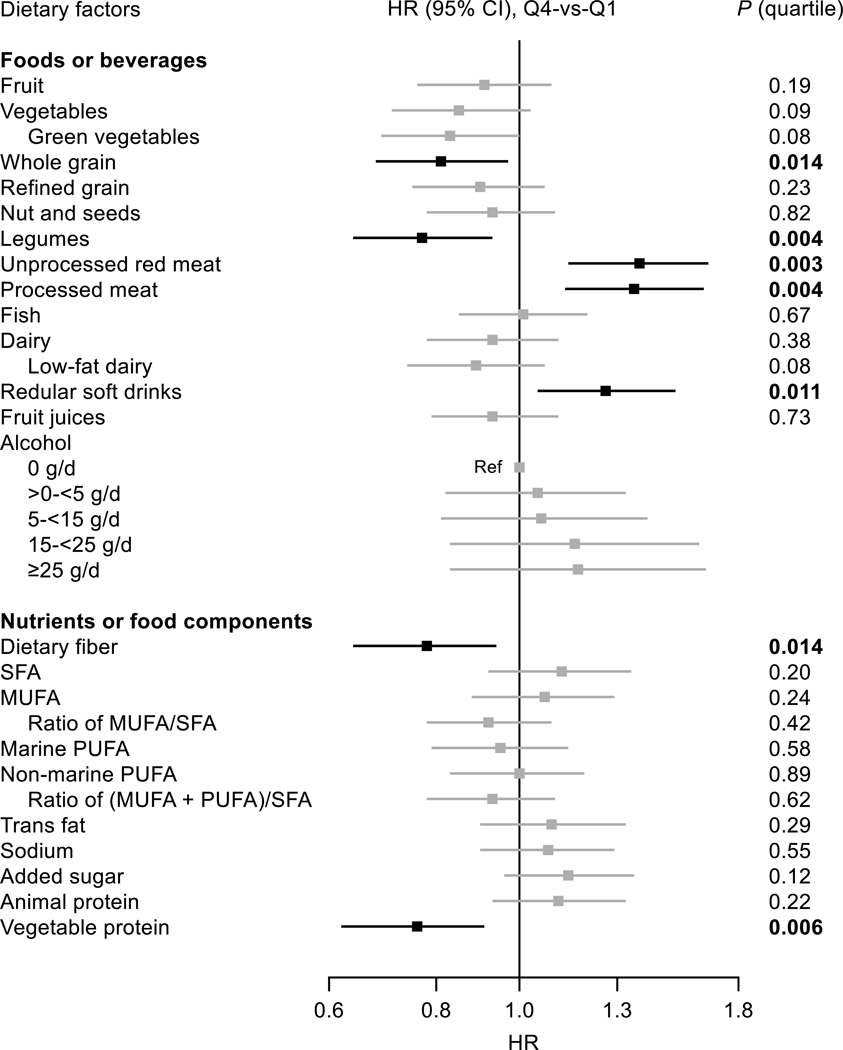
After full adjustment, as presented in Figure and Table S7, intakes of whole grains and legumes both were associated with lower risk of PAD, whereas intakes of unprocessed red meat, processed meat, and regular soft drinks were associated with higher risk (P-trend values across quartile ≤0.014). For several food groups (i.e., fruit, vegetables, nuts, and dairy), significantly lower risk of PAD was observed when comparing the third with the first quartiles of the intakes, but the overall trends of the associations were not significant (Table S7). No associations were observed for intakes of other foods or beverages including alcohol.
Figure. Relationship between food groups or nutrients or food components and risk of peripheral arterial disease in the Women’s Health Initiative (n = 138 506).
Where appropriate, results were adjusted for covariates listed for model 2 of Table 2. P values are P for trend across quartiles of the intakes. MUFA indicates monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; Q, quartile; and SFA, saturated fatty acids.
There were modest correlations (r: −0.22 between whole grains and unprocessed red meat to 0.12 between unprocessed red meat and processed meat) among the aforementioned 5 food groups that were significantly associated with risk of PAD after multivariable adjustment. All except for whole grains (P-trend = 0.12 for whole grains and ≤0.033 for others) remained associated with risk of PAD after mutual adjustment for the other 4 food groups (Table S8).
With respect to dietary nutrients, intakes of dietary fiber (P-trend = 0.014) and vegetable protein (P-trend = 0.006) both were significantly and inversely associated with risk of PAD (Figure and Table S9). Correlation between the 2 nutrients was substantial (r = 0.76) and thus no mutual adjustment was made. Intakes of other nutrients such as sodium or polyunsaturated, saturated or trans fat were not associated with risk of PAD.
DISCUSSION
In this large prospective study of U.S. postmenopausal women, 4 a priori diet quality indices (i.e., aMED index, AHEI-2010, DASH index, and HEI-2015) were significantly and inversely associated with risk of PAD after adjustment for multiple potential confounders including known risk factors for PAD. As compared with participants in the lowest quartiles of the diet quality scores, those in the highest quartiles had 21% (for AHEI-2010) to 34% (for DASH index) lower risk of PAD. Among components of these healthful eating patterns, intakes of legumes, dietary fiber, and vegetable protein were associated lower risk of PAD, while intakes of unprocessed red meat, processed meat, and regular soft drinks were associated with higher risk.
The diet quality indices examined in our study commonly emphasize greater intakes of nutrient- rich and plant-based foods (e.g., whole grains, fruit, and vegetables) and lower consumption of red and processed meat. Adherence to these healthful eating patterns has been widely associated with lower risk of major chronic diseases including atherosclerotic cardiovascular diseases.17,28 Nevertheless, the potential role of overall diet quality in the development of PAD has not been well investigated in epidemiologic studies and the findings are inconsistent. In the U.S. Atherosclerosis Risk in Communities Study,9 no clear associations were found between data-derived dietary patterns and risk of PAD, but the results may be less interpretable than those for a priori dietary patterns. In addition, as compared with our study, there are several other notable differences for that study, such as the use of a FFQ covering a narrow range of dietary intakes (66 items), the inclusion of both sexes with younger age and higher proportion of black individuals, and the identification of PAD according to results of ABI tests.
A higher score of the Mediterranean diet29,30 or AHEI-201031 has been associated with lower odds of PAD in cross-sectional and case-control studies. In a prospective cohort study including Dutch individuals,12 a strong inverse association between the Mediterranean diet and risk of PAD was statistically non-significant after further adjustment for lifestyle confounders. On the other hand, in a dietary intervention trial conducted in Spain that included at-risk individuals,32 adoption of a Mediterranean diet combined with either nuts or extra-virgin olive oil substantially reduced risk of PAD by 50% and 66%, respectively, as compared with a low-fat diet. To the best of our knowledge, no previous studies have examined the longitudinal associations between DASH diet index, AHEI-2010, or HEI-2015 and risk of PAD.
The associations between intakes of individual foods or nutrients and risk of PAD have been evaluated in some cross-sectional studies,33–36 while evidence from prospective studies has been more limited.9–11 Our findings of positive associations between unprocessed red meat and processed meat consumption and risk of PAD are in line with results from the Atherosclerosis Risk in Communities Study.9 In that study, however, there was an unexpected inverse association between sugar-sweetened beverage consumption and risk of PAD, while we observed a positive association between regular soft drinks and risk of PAD.9 Consistent with previous findings, the associations between intakes of several plant foods (e.g., fruit, vegetables, and nuts9,11) and risk of PAD were also not apparent in our study. Notably, the observed associations for these potentially beneficial plant foods were all inverse though statistically non-significant (Table S7). Thus, the combined intakes of all these plant-based foods, as reflected by higher scores of the assessed diet quality indices or by greater nutrient quality (e.g., high dietary fiber10), may still contribute to the inverse associations between the diet quality scores and risk of PAD observed in our study. We did not find any inverse association between moderate alcohol drinking and risk of PAD, which has been reported by some previous studies.9,37,38
Multiple biological mechanisms may underline the observed inverse associations between healthful eating patterns and risk of PAD. In addition to the potentially beneficial impacts on regulating known metabolic risk factors for PAD, such as reductions of diabetes risk and blood pressure and an improvement of lipid profiles,16 a healthful diet may decrease inflammation, oxidative stress, and endothelial dysfunction and thereby prevent or delay the progression of atherosclerosis.39,40
Study Limitations
Several limitations of our study need to be acknowledged. First, dietary information was collected using a self-administered FFQ at baseline, and participants may have changed their dietary habits over the long period of follow-up. In prospective studies, misclassification of baseline exposure would likely to be non-differential and longitudinal changes in exposure would lead to regression dilution bias, both of which may attenuate rather than exaggerate the true risk estimates.41 Second, as dietary sodium was not a focal exposure for the primary aims of the WHI study, the WHI FFQ did not delineate all sources of dietary sodium and was unable to capture discretionary salt use. Thus, the observed lack of association between dietary sodium intake and risk of PAD should be treated with caution. Third, our study only included symptomatic PAD for which the incidence is much lower than asymptomatic PAD. Notwithstanding, both PAD types were previously found to be associated with similarly higher risk of mortality.42 Follow-up of asymptomatic PAD requires periodical reexamination of study participants and thus involves substantial time and expense. As such, it is more feasible for large cohort studies to focus on clinically symptomatic PAD.32,43 Finally, PAD may be sex or race/ethnicity specific in terms of prevalence or progression.4,5 Although we did not observe racial/ethnical differences in the associations between the diet quality scores and risk of PAD, the analyses for non-White participants were based on a relatively small number of PAD cases. Thus, future studies conducted in men and in other race/ethnicity or age groups are still required to confirm our findings for the general population.
PERSPECTIVES
In a large diverse sample of U.S. postmenopausal women, our findings showed that adhering to different recommended eating patterns is associated with lower risk of PAD. Thus, current clinical and public health strategies that recommend healthful eating patterns are potentially applicable to PAD prevention.
Supplementary Material
Novelty and Significance.
What Is New?
The potential role of nutritional factors in the development of peripheral arterial disease (PAD) remains poorly understood.
In a broad sample of U.S. postmenopausal women, adhering to various recommended eating patterns was found to be associated with significantly lower risk of PAD.
Among individual foods/nutrients, intakes of legumes, dietary fiber, and vegetable protein were associated lower risk of PAD, while intakes of unprocessed red meat, processed meat, and regular soft drinks were associated with higher risk.
What Is Relevant?
Our findings suggest that current clinical and public health strategies that recommend healthful eating patterns are potentially applicable to PAD prevention.
Summary
Multiple healthful eating patterns are associated with lower risk of PAD and may be recommended for the primary prevention of PAD.
Acknowledgements
We thank the Women’s Health Initiative investigators, staff, and the trial participants for their outstanding dedication and commitment. A full list of all the investigators who have contributed to Women’s Health Initiative science appears at: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Sources of Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. Dr. Qi is supported by the NHLBI (K01HL129892, R01HL060712, and R01HL140976) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK119268 and R01DK120870), and Dr. Kaplan is supported by the NHLBI (R01-HL146132-01).
Abbreviations
- AHEI
alternate Healthy Eating Index
- aMED
alternate Mediterranean diet
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
food frequency questionnaire
- HEI
Healthy Eating Index
- PAD
Peripheral arterial disease
- PAF
Population attributable fraction
- WHI
Women’s Health Initiative
Footnotes
Disclosures
None.
References
- 1.Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Jelani QU, Petrov M, Martinez SC, Holmvang L, Al-Shaibi K, Alasnag M. Peripheral Arterial Disease in Women: an Overview of Risk Factor Profile, Clinical Features, and Outcomes. Curr Atheroscler Rep. 2018;20:40. doi: 10.1007/s11883-018-0742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 5.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 6.Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA. 2017;317:912–924. doi: 10.1001/jama.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, Lancaster K, Lichtenstein AH, Johnson RK, Thomas RJ, et al. Recommended Dietary Pattern to Achieve Adherence to the American Heart Association/American College of Cardiology (AHA/ACC) Guidelines: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e505–e529. doi: 10.1161/CIR.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 8.DeSalvo KB, Olson R, Casavale KO. Dietary Guidelines for Americans. JAMA. 2016;315:457–458. doi: 10.1001/jama.2015.18396. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie RP, Lutsey PL, Heiss G, Folsom AR, Steffen LM. Dietary intake and peripheral arterial disease incidence in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2017;105:651–659. doi: 10.3945/ajcn.116.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant AT, Hu FB, Spiegelman D, Willett WC, Rimm EB, Ascherio A. Dietary fiber reduces peripheral arterial disease risk in men. J Nutr. 2003;133:3658–3663. doi: 10.1093/jn/133.11.3658. [DOI] [PubMed] [Google Scholar]
- 11.Hung HC, Merchant A, Willett W, Ascherio A, Rosner BA, Rimm E, Joshipura KJ. The association between fruit and vegetable consumption and peripheral arterial disease. Epidemiology. 2003;14:659–665. doi: 10.1097/01.ede.0000086882.59112.9d. [DOI] [PubMed] [Google Scholar]
- 12.Hoevenaar-Blom MP, Nooyens AC, Kromhout D, Spijkerman AM, Beulens JW, van der Schouw YT, Bueno-de-Mesquita B, Verschuren WM. Mediterranean style diet and 12-year incidence of cardiovascular diseases: the EPIC-NL cohort study. PLoS One. 2012;7:e45458. doi: 10.1371/journal.pone.0045458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 14.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu E, Malik VS, Hu FB. Cardiovascular Disease Prevention by Diet Modification: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:914–926. doi: 10.1016/j.jacc.2018.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 19.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 20.Bowman SA, Friday JE, Moshfegh A. (2008). MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004 [Online] Food Surveys Research Group. Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture, Beltsville, MD. Available at: http://www.ars.usda.gov/ba/bhnrc/fsrg. [Google Scholar]
- 21.Haring B, Crandall CJ, Wu C, LeBlanc ES, Shikany JM, Carbone L, Orchard T, Thomas F, Wactawaski-Wende J, Li W, et al. Dietary Patterns and Fractures in Postmenopausal Women: Results From the Women’s Health Initiative. JAMA Intern Med. 2016;176:645–652. doi: 10.1001/jamainternmed.2016.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136:466–472. doi: 10.1093/jn/136.2.466. [DOI] [PubMed] [Google Scholar]
- 23.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 25.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–128. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S; discussion 1229S-1231S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 28.Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, Rexrode KM, Rimm EB, Qi L, Willett WC, et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattioli AV, Coppi F, Migaldi M, Scicchitano P, Ciccone MM, Farinetti A. Relationship between Mediterranean diet and asymptomatic peripheral arterial disease in a population of pre-menopausal women. Nutr Metab Cardiovasc Dis. 2017;27:985–990. doi: 10.1016/j.numecd.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Ciccarone E, Di Castelnuovo A, Salcuni M, Siani A, Giacco A, Donati MB, De Gaetano G, Capani F, Iacoviello L, Gendiabe I. A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with Type 2 diabetes. J Thromb Haemost. 2003;1:1744–1752. doi: 10.1046/j.1538-7836.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- 31.Mattei J, Sotres-Alvarez D, Gellman M, Castaneda SF, Hu FB, Tucker KL, Siega-Riz AM, Kaplan RC. Diet quality, inflammation, and the ankle brachial index in adults with or without cardiometabolic conditions. Clin Nutr. 2018;37:1332–1339. doi: 10.1016/j.clnu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz-Canela M, Estruch R, Corella D, Salas-Salvado J, Martinez-Gonzalez MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA. 2014;311:415–417. doi: 10.1001/jama.2013.280618. [DOI] [PubMed] [Google Scholar]
- 33.Donnan PT, Thomson M, Fowkes FG, Prescott RJ, Housley E. Diet as a risk factor for peripheral arterial disease in the general population: the Edinburgh Artery Study. Am J Clin Nutr. 1993;57:917–921. doi: 10.1093/ajcn/57.6.917. [DOI] [PubMed] [Google Scholar]
- 34.Klipstein-Grobusch K, den Breeijen JH, Grobbee DE, Boeing H, Hofman A, Witteman JC. Dietary antioxidants and peripheral arterial disease : the Rotterdam Study. Am J Epidemiol. 2001;154:145–149. doi: 10.1093/aje/154.2.145. [DOI] [PubMed] [Google Scholar]
- 35.Antonelli-Incalzi R, Pedone C, McDermott MM, Bandinelli S, Miniati B, Lova RM, Lauretani F, Ferrucci L. Association between nutrient intake and peripheral artery disease: results from the InCHIANTI study. Atherosclerosis. 2006;186:200–206. doi: 10.1016/j.atherosclerosis.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naqvi AZ, Davis RB, Mukamal KJ. Nutrient intake and peripheral artery disease in adults: key considerations in cross-sectional studies. Clin Nutr. 2014;33:443–447. doi: 10.1016/j.clnu.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. 2017;356:j909. doi: 10.1136/bmj.j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camargo CA Jr., Stampfer MJ, Glynn RJ, Gaziano JM, Manson JE, Goldhaber SZ, Hennekens CH. Prospective study of moderate alcohol consumption and risk of peripheral arterial disease in US male physicians. Circulation. 1997;95:577–580. doi: 10.1161/01.cir.95.3.577. [DOI] [PubMed] [Google Scholar]
- 39.Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Perez-Caballero AI, Perez-Jimenez F, Lopez-Miranda J. Mediterranean Diet and Cardiovascular Risk: Beyond Traditional Risk Factors. Crit Rev Food Sci Nutr. 2016;56:788–801. doi: 10.1080/10408398.2012.726660. [DOI] [PubMed] [Google Scholar]
- 40.Brostow DP, Hirsch AT, Collins TC, Kurzer MS. The role of nutrition and body composition in peripheral arterial disease. Nat Rev Cardiol. 2012;9:634–643. doi: 10.1038/nrcardio.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. doi: 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]
- 42.Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, Darius H, Burghaus I, Trampisch HJ, German Epidemiological Trial on Ankle Brachial Index Study G. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 43.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.