Abstract
Purpose:
Study of the relationship between prenatal cocaine exposure (PCE) and executive function (EF) has yielded inconsistent results. The purpose of the current study is to examine whether PCE, biological sex, environmental risk, and their interaction predicted EF in early adolescence.
Methods:
135 12-year-old adolescents (40.7% with PCE), who were followed prospectively from birth, attempted up to 8 Tower of Hanoi (ToH) puzzle trials of increasing complexity. The number of correctly completed puzzles served as the main outcome measure. Survival analysis was used to examine predictors of the number of successfully completed trials.
Results:
As trial difficulty increased, fewer adolescents were able to solve the TOH puzzle. Adolescents from high risk environments and with either prenatal alcohol or prenatal cannabis exposure completed fewer puzzles (p<.05). In addition, a hypothesized 3-way interaction of PCE × sex × environmental risk was found such that cocaine-exposed males with high environmental risk had the worst performance (p<.01).
Conclusions:
The current findings are consistent with prior research indicating that males with PCE may be at particular risk of poorer functioning and highlight the potential importance of examining adolescent’s sex and environmental risk as moderators of PCE effects.
Keywords: executive function, prenatal cocaine exposure, substance use, Tower of Hanoi, environmental risk
1. Introduction
Executive function (EF) empowers people to organize, plan, control, and change behaviors according to circumstances. EF helps them to execute everyday tasks and achieve set goals. It is a necessary component of daily function ranging from optimizing behavior in different and changing environments to facilitating academic achievements. EF comprises a set of four primary skills: working memory, attention control, shifting, and inhibition (Jones et al., 2016). Although there is still disagreement regarding when EF skills reach full maturation, it is generally thought that this process occurs continuously from early childhood to adulthood while undergoing sensitive, vulnerable periods (Ready and Reid, 2019; Thompson and Steinbeis, 2020). EF has been primarily associated with frontal lobe function, especially the prefrontal cortex (PFC), albeit with some parietal involvement (Casey et al., 2000; Guevara et al., 2012; Rottschy et al., 2012; Yuan and Raz, 2014). Deficits in EF are associated with attention-deficit/hyperactivity disorder, autism, depression, schizophrenia, dementia, and traumatic injuries to the brain (Barch, 2006; Demetriou et al., 2018; Foran et al., 2021; Garon et al., 2018; Hervey et al., 2004; Rüsch et al., 2008; Shakehnia et al., 2021; Stuss, 2011; Wagner et al., 2015; Willcutt et al., 2005). EF dysfunction may impact school readiness and achievements, language development, and behaviors leading to increases in violence and initiation of alcohol use and binge drinking (Cruz et al., 2020; McClelland et al., 2013; Morgan, et al., 2019; Nayfeld et al., 2013; Peeters et al., 2015; White et al., 2017). EF deficits are also associated with externalizing problems (Schoemaker et al., 2013), an outcome that youth with prenatal cocaine exposure (PCE) may be at increased risk of exhibiting (Bennett et al., 2013; Min et al., 2018).
According to data from the 2018 National Survey of Drug Use and Health (Substance Abuse and Mental Health Services Administration [SAMHSA], 2019), past month use of illicit drugs by pregnant women was 5.4%, while cocaine use in lifetime among females aged 12 or older was 11.5%, suggesting that the potential for PCE remains high today.
Early effects of PCE have been studied, however, few studies have examined how PCE exposure affects the EF of the offspring of cocaine users. PCE effects at early ages include brain grey matter abnormality (Grewen et al., 2014; Huttenlocher and Dabholkar, 1997), small head size and intrauterine growth retardation (Bateman and Chiriboga, 2000; Bauer et al., 2005; Chiriboga et al., 1999), and neurological and behavioral changes such as abnormalities in movement and tone, difficulties in reactivity, regulation and temperament (Bendersky and Lewis, 1998; Chiriboga et al., 2007, 1999; Eiden et al., 2009; Richardson et al., 2008; Singer et al., 2007). Some physical and brain changes, for instance, smaller structural brain volumes, smaller head circumference, reduced weight and height, have been found to persist into late childhood and beyond (Akyuz et al., 2014; Richardson et al., 2015, 2013). Studies of long term effects of PCE have found increased risk for substance use (Delaney-Black et al., 2011; Min et al., 2014; Minnes et al., 2017; Richardson et al., 2019), risk taking behaviors in males (Allen et al., 2014), early sexual behavior (Min et al., 2015), and externalizing problems (Bennett et al., 2013; Min et al., 2018; Richardson et al., 2011). Recent work indicates that young adults with a history of PCE are at increased risk for conduct disorder and for being arrested (Richardson et al., 2019).
While multiple studies have found PCE to predict externalizing problems, research examining PCE and its association with EF has produced inconsistent results (Buckingham-Howes et al., 2013). Several studies have found PCE to predict poorer EF performance, including Richardson et al. (2015) who found first trimester PCE predicted poorer performance on problem solving and abstract reasoning tasks at age of 10. Minnes et al. (2016), using caregiver ratings on the Behavior Rating Inventory of Executive Function (BRIEF) and child performance on CANTAB Stockings of Cambridge (SOC), found girls with PCE performed worse than non-cocaine exposed (NCE) girls and both groups of boys at age 15 on SOC test, that involves planning and working memory. They also found girls with PCE to have more difficulties in inhibiting behavior and in cognitive flexibility-shifting based on BRIEF at age 12, although showing the greatest improvement by age 15. Several studies have used a Stroop task, a measure of inhibitory control, to assess EF. Bridgett and Mayes (2011) found children with PCE made more errors on a Stroop task at 7 years, with girls of both groups (PCE and NCE) improving their performance faster than boys by 11 years. Furthermore, while no PCE by sex group differences were found on the time it took to complete the Stroop task at 7 years, unexposed children had greater age-related improvements than children with PCE on time taken to complete the task at later visits. Importantly, children with PCE, as well as comparison children without PCE, made more errors at 7 years if they were in a high cumulative risk group that included low maternal education, highlighting the potential impact of environmental risk. Rose-Jackobs et al. (2009), using the Stroop and Rey Osterrieth Complex Figure tasks, found 9.5 and 11-year-old children with heavy PCE exhibited poorer inhibitory control skills, although these deficits were subtle. In contrast to these findings, Betancourt et al. (2011) used a computerized Stroop number task and a spatial working memory task at ages 12, 14 and 17 and found no association between PCE and either inhibitory control or working memory. In a subsample from this same study, Hurt et al. (2008) found no PCE-related differences in either performance or brain activity using fMRI during an n-back working memory task at age 13–14 years. Also using an fMRI task for 21-year-olds, but to assess attention, Willford et al. (2018) found no associations between PCE and task accuracy, speed of processing, or activation in key brain regions associated with the attention networks. Finally, in the current sample, Carmody et al. (2011) found boys with PCE to exhibit more inhibitory control and attention errors on a go/no-go task, and to be less likely to complete the task administered at 6, 9, and 11 years. Collectively, these studies suggest the possibility that PCE negatively impacts EF, but findings are inconsistent and often subtle, perhaps reflecting the unique and often only modest to moderate correlations among measures of EF (e.g.,Testa et al., 2012).
The Tower of Hanoi (ToH) is a commonly used, validated EF task that has not yet been examined as an outcome in the PCE literature. Although considered to be a planning task, the ToH is also thought to involve subgoal creations, recursions, and use of short-term memory for goals and rules, as well as other aspects of working memory, perceptual strategy, and inhibition of prepotent response (Ahonniska et al., 2000; Goel and Grafman, 1995; Zook et al., 2004). The ToH requires participants to move different sized and colored disks among 3 pegs until they are in the goal position. It has been validated in studies of individuals with a variety of EF-related pathologies, including those with schizophrenia, Parkinson’s disease, prefrontal lesions, and intellectual disability (Bustini et al., 1999; Goel and Grafman, 1995; Numminen et al., 2001; Vakil et al., 2014), as well as among typically developing children (Welsh, 1991), and is associated with activation of the prefrontal cortex, the primary region involved in EF (Liang et al., 2016).
Prior research supports that pregnant women who use cocaine are likely to use other substances as well, including alcohol, cannabis, and tobacco (Bendersky et al., 1996). Given that prenatal exposure to alcohol and tobacco has been shown to predict poorer EF (Khoury et al., 2015; Rose-Jacobs et al., 2017), while prenatal cannabis exposure shows mixed results (Sharapova et al., 2018), it is important to consider these substances when examining the association of PCE with future EF.
In order to examine the potential effects of PCE on EF, the role of the environment needs to be considered. Children prenatally exposed to cocaine are more likely to reside in higher risk environments, themselves a risk factor for poor EF (Bendersky et al., 1996; Hurt et al., 2009). Home environment, maternal education and occupation, and family income are each associated with developmental problems and other cognitive outcomes (Biedinger, 2011; Bradley and Corwyn, 2002; Bradley et al., 2000), and lower socioeconomic status has been associated with poor EF (Ardila et al., 2005; Hackman and Farah, 2009; Kishiyama et al., 2008). Considering the potential importance of environmental factors on EF, it is necessary to include environmental risk in analyses of the association between PCE and EF. We used a composite score to evaluate the environment that included multiple variables, including family income, maternal education, home structure, family support and stress, race, instability of child’s environment, and irregularity of child’s schedule. Moreover, in addition to controlling for the effects of environmental risk when examining the association of PCE with developmental outcomes, environmental risk may moderate the potential impact of PCE. Youth with PCE and those who reside in high-risk environments have been found to experience the worst developmental outcomes (Bendersky et al., 2006; Eiden et al., 2014). In addition to examining environmental risk as a moderator, child’s sex may also moderate the effects of PCE, most often such that exposed boys have more adverse outcomes (e.g., externalizing behaviors, increase in marijuanna use, attention difficulties, increase in risk-taking behaviors) than exposed girls, including in the current sample (Allen et al., 2014; Bennett et al., 2013; Kestler et al., 2012; Minnes et al., 2017).
The purpose of the current study is to examine whether PCE, both independently and in conjunction with adolescent’s sex and environmental risk, predicts EF during early adolescence. Given prior research, we hypothesized that: a) PCE would be associated with worse EF; and b) the association between PCE and EF performance on the ToH task would be moderated by high environmental risk and male sex. In testing these hypotheses, the effects of other prenatal substances (alcohol, cigarettes, and cannabis) were covaried given their own potential effect on EF (Mattson et al., 2019; Oh et al., 2020).
1. Methods
2.1. Participants
Participants were from a longitudinal cohort study recruited at birth examining developmental outcomes associated with PCE. Pregnant women from hospital-based prenatal clinics or those newly delivered were approached between February 1993 and December 1995 in Philadelphia, PA and Trenton, NJ. Newborns were excluded from the study if they were born before 32 weeks of gestation, required NICU care or oxygen therapy for more than 24 hours, had congenital abnormalities, were exposed to opiates or phencyclidine in utero, or their mothers were diagnosed with HIV. Participation was voluntary, and incentives were provided at each visit in the form of 30$ vouchers for use at local stores. The study was approved by the Institutional Review Boards of participating institutions, and informed consent was obtained at the time of the study from the child’s legal guardian.
135 adolescents completed the Tower of Hanoi task at age of 12 (M= 12.6 years, SD=0.19). Maternal ethnicity was as follows: 91.1% African American, 6.7% Caucasian, 1.5% Hispanic, and 0.7% Asian. Out of participants seen at the 4 months lab visit, 72 moved out or could not be located, 41 had no ToH data, and 10 were too young to participate, at under 12 years old. Analysis of subjects without data did not differ from subjects originally recruited on prenatal cocaine exposure, sex or environmental risk. Imputation for missing data produced similar findings to the subjects reported.
2.2. Procedure
Recruited children were brought to the lab by their parents. During their first year, they were seen every 4 months, thereafter, every 6 months, excluding 9.5 year visit, which allowed about 30 visits in total. All lab sessions were videotaped through a one-way mirror. Research staff were blind to participant prenatal exposure status.
2.3. Measures
2.3.1. Maternal prenatal substance use
Maternal substance use was obtained by semi-structured interviews within 2 weeks of the infant’s birth. Interviews were administered by substance abuse counselors, or study personnel trained in substance use interview techniques. Amounts were assessed and each scored on a scale based on the number of drinks consumed, cigarettes or cannabis joints smoked, and how many grams of cocaine and in what form it was used per day. PCE was confirmed by analysis of the newborn’s meconium for the presence of benzoylecgonine (cocaine metabolite) using radioimmunoassay followed by gas chromatography/mass spectrometry. Prenatal cocaine exposure was determined based on maternal interview and/or newborn meconium sample analysis. By maternal report 40.7% of mothers used cocaine, 90.2% of whom also used other substances (40.0% used alcohol and cigarettes, 14.5% used alcohol, 12.7% smoked cigarettes, and 25.5% used other combinations of substances, including cannabis), which is consistent with published literature (Richardson et al., 2002). Among mothers who did not use cocaine, 10.0% smoked cigarettes, 7.5% used alcohol and cigarettes, 6.3% used alcohol, and 2.5% used other combinations of alcohol, cigarettes and cannabis. Cocaine use was associated with greater use of cigarettes (r =.54, p=.01) and alcohol (r =.33, p=.01), but not cannabis use (r =.16, p=.075).
2.3.2. Environmental Risk
Environmental risk was assessed using a cumulative risk score. Prior research has found such cumulative risk scores to explain more variance in child and adolescent outcomes than single factor scores (Atzaba-Poria et al., 2004; Bendersky and Lewis, 1994; Deater-Deckard et al., 1998; Sameroff et al., 1987). Moreover, such aggregate variables are more stable than individual ones and there is increased power to detect effects of the environment as errors of measurement decrease when scores are summed and degrees of freedom are preserved (Burchinal et al., 2000; Wachs, 1991). Environmental risk scores were created by converting each individual variable to a z-score at each of time points, and then summing the mean z-scores across each of nine time points (from birth to age 13) with higher numbers indicating greater risk. The score included: family life stress in the past 6 months using the Social Environment Inventory (Orr et al., 1992); maternal social support network size based on the Norbeck Social Support Questionnaire (Norbeck et al., 1981); the irregularity of the child’s schedule (e.g., variability as to when the child woke up, ate, went to sleep during the past week); the instability of the child’s surroundings (e.g., the number of changes in the room in which the child slept, who lived in the house, etc., during the past 6 months) from the Family Chaos Scale; single parenthood (single parent associated with higher risk); maternal education (number of years of education, reverse scored); maternal race (African American associated with higher risk given the potential exposure to racism and structural disadvantage); and public assistance status (whether the family received Aid to Families with Dependent Children [AFDC] or, later, Temporary Assistance to Needy Families [TANF] funding). This cumulative risk score has been used in prior reports from the current study (e.g., Allen et al., 2014; Bendersky et al., 2006; Bennett et al., 2008, 2013; Carmody et al., 2011).
2.3.3. Tower of Hanoi
The ToH puzzle consists of 3 pegs on a wooden board, and 5 different-sized disks that need to be stacked according to a series of rules: 1) a larger disk cannot be placed atop a smaller one; 2) only one disk can be moved at a time; and 3) aside from the disk being moved, all other disks must remain on a peg. To complete each ToH trial, subjects (Ss) must move disks from their initial position to a different final configuration. In addition, a) if at any time a subject began to make an illegal move, the examiner stopped him/her and gave a reminder of the rules while noting that the illegal move did not count as a move; and b) if the Tower was built on the wrong peg, the examiner repeated the rules, and encouraged Ss to move the whole tower, using only legal moves, to the correct peg. The first trial was initiated only after Ss learned all rules and successfully completed a practice trial of a 5-disk, 2 move ToH puzzle after the puzzle solution was demonstrated to the children. The ToH task was stopped if Ss failed to solve 2 consecutive trials, with failure defined as Ss either not solving the puzzle after 100 moves or making no move for a period > 60 sec. Ss had the opportunity to solve each of 8 trials that were administered on the same day and were videotaped. The trials were administered in ascending order of complexity. The optimal number of moves needed to solve a puzzle increased from 10 to 31 over the 8 trials, reflecting the increased difficulty for each subsequent trial. Gradually increasing complexity, which has been used previously (Goel and Grafman, 1995; Emick and Welsh, 2005; Welsh, 1991; Welsh et al., 1999), allowed Ss to learn strategies and provided an opportunity to apply newly learned skills when faced with more complex trials. The number of trials solved correctly served as the primary measure of EF in our study, with correct completions of each trial presented for descriptive purposes.
2.4. Data analysis
Given the change in performance as a function of increased task complexity, a discrete time survival analysis on the number of decreasing Ss who correctly completed trials was used. Latent variable modeling (Raykov et al., 2017) was used to examine the effects of PCE, environmental risk, sex, and their interactions on the adolescent’s ability to complete the tasks across all 8 trials. Different from the traditional Cox regression model for survival analysis, recent advances in latent variable modeling have provided unique analytic advantages to researchers interested in survival time modeling (Muthen and Masyn, 2005, Raykov et al., 2018a, 2018b). Like any other latent variable modeling, discrete time survival analysis via latent variable modeling could offer “the opportunity to account for measurement error” (Raykov et al., 2017), as the latent variable (i.e., propensity for the event) is extracted from multiple indicators (i.e., observations in discrete time points). Additionally, in traditional survival analysis an event is defined as the only appearance of the interested act (e.g., death, high school dropout); in contrast, in latent variable modeling, and in the case of our study, an event (failing of the particular ToH trial, which was coded as “0”) was not defined by one observed wrong response. Rather, Ss needed to demonstrate two consecutive wrong responses from two discrete time points of observation to end the task. Thus, the event history of a subject may include multiple 0s and 1s (with “1” coded as a success on a particular trial), rather than only 0s before ending in two failed trials, which indicates the occurrence of the event. Accordingly, the latent variable modeling was chosen because it allowed the inclusion of correctly and incorrectly solved trials before the event occurrence. The model was estimated using Mplus software version 7 (Muthén and Muthén, 1998–2008).
3. Results
3.1. ToH Trial Completion
Successful ToH trial completion served as our outcome of interest. Table 1 presents the number of subjects who successfully solved trials by sex and by PCE status.
Table 1.
Number of subjects solving trials as a whole, by sex and exposure
| Trials | # of subjects solving trials correctly (n-135) | # of subjects solving trials by sex | # of subjects solving problems by exposure | ||
|---|---|---|---|---|---|
| F (n-61) | M (n-74) | PCE (n-55) | NCE (n-80) | ||
| 1 | 132 | 60 | 72 | 53 | 79 |
| 2 | 131 | 59 | 72 | 53 | 78 |
| 3 | 122 | 56 | 66 | 47 | 75 |
| 4 | 116 | 53 | 63 | 44 | 72 |
| 5 | 116 | 56 | 60 | 43 | 73 |
| 6 | 96 | 46 | 50 | 40 | 56 |
| 7 | 100 | 48 | 52 | 37 | 63 |
| 8 | 98 | 45 | 53 | 36 | 62 |
3.2. EF as a function of PCE, environmental risk, and sex: Survival analysis
Our modeled latent factor, which we called the propensity to succeed in the ToH trials as they become more difficult, was abstracted from 8 indicators (i.e., Ss were assigned a score of 1 or 0 on each of the 8 trials, with 1 indicating success and 0 failure). Table 2 presents estimates for the unique covariate effects on the Propensity to Succeed. Table 3 presents the results of the survival analysis. As shown in the Table 3, there was no main effect for PCE, nor for prenatal cigarette exposure or sex in predicting ToH performance. Environmental risk (p=.038), however, as well as prenatal alcohol exposure (p=.039) and prenatal cannabis exposure (p=.002), were each associated with EF. Specifically, adolescents with greater environmental risk, prenatal alcohol exposure, and prenatal cannabis exposure were each less likely to successfully complete ToH tasks.
Table 2.
Covariance Convergence Matrix on the Propensity to Succeed scores
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | Trial 6 | Trial 7 | Trial 8 | |
|---|---|---|---|---|---|---|---|---|
| Trial 1 | 0.985 | |||||||
| Trial 2 | 0.985 | 1.000 | ||||||
| Trial 3 | 0.985 | 1.000 | 1.000 | |||||
| Trial 4 | 0.985 | 0.993 | 0.993 | 0.993 | ||||
| Trial 5 | 0.978 | 0.993 | 0.993 | 0.985 | 0.993 | |||
| Trial 6 | 0.978 | 0.993 | 0.993 | 0.985 | 0.993 | 0.993 | ||
| Trial 7 | 0.978 | 0.993 | 0.993 | 0.985 | 0.993 | 0.993 | 0.993 | |
| Trial 8 | 0.956 | 0.970 | 0.970 | 0.963 | 0.970 | 0.970 | 0.970 | 0.970 |
Table 3.
Propensity to Succeed on Tower of Hanoi trials as a function of child sex, prenatal substance exposure, and environmental risk: survival analysis
| Estimate | S.E | Est/S.E. | P-value | |
|---|---|---|---|---|
| Sex (0=Female, 1=Male) | −0.342 | 0.449 | −0.763 | 0.446 |
| Environmental risk | −2.219 | 1.071 | −2.072 | 0.038 |
| Prenatal cocaine exposure | −0.044 | 0.621 | −0.070 | 0.944 |
| Prenatal alcohol exposure | −0.178 | 0.086 | −2.063 | 0.039 |
| Prenatal cannabis exposure | −0.249 | 0.078 | −3.174 | 0.002 |
| Prenatal cigarettes exposure | −0.008 | 0.020 | −0.377 | 0.707 |
| Sex x cocaine exposure | −0.402 | 0.711 | −0.566 | 0.572 |
| Sex x Environmental risk | 2.155 | 1.237 | 1.742 | 0.082 |
| Cocaine exposure x Environmental risk | 3.093 | 1.606 | 1.926 | 0.054 |
| Sex x Environmental risk x Cocaine exposure | −5.661 | 1.942 | −2.915 | 0.004 |
There was also support for the hypothesized 3-way interaction between PCE × sex × environmental risk on adolescent’s EF performance (p=.004), indicating that being a male prenatally exposed to cocaine and growing up in a higher risk environment resulted in worse ToH performance. Figures 1a and 1b illustrate the 3-way interaction between exposure × sex × environmental risk. This interaction was independent from the effects of cannabis and alcohol. In addition, there were two non-significant trends for 2-way interactions for environmental risk × sex (p=.082) and environmental risk × PCE (p=.054) in predicting the propensity to succeed. Specifically, males from high-risk environments and adolescents with a history of PCE from a high-risk environment tended to perform worse on the ToH.
Fig. 1a. % of Subjects with low environmental risk* score who completed each trial by exposure status and sex.
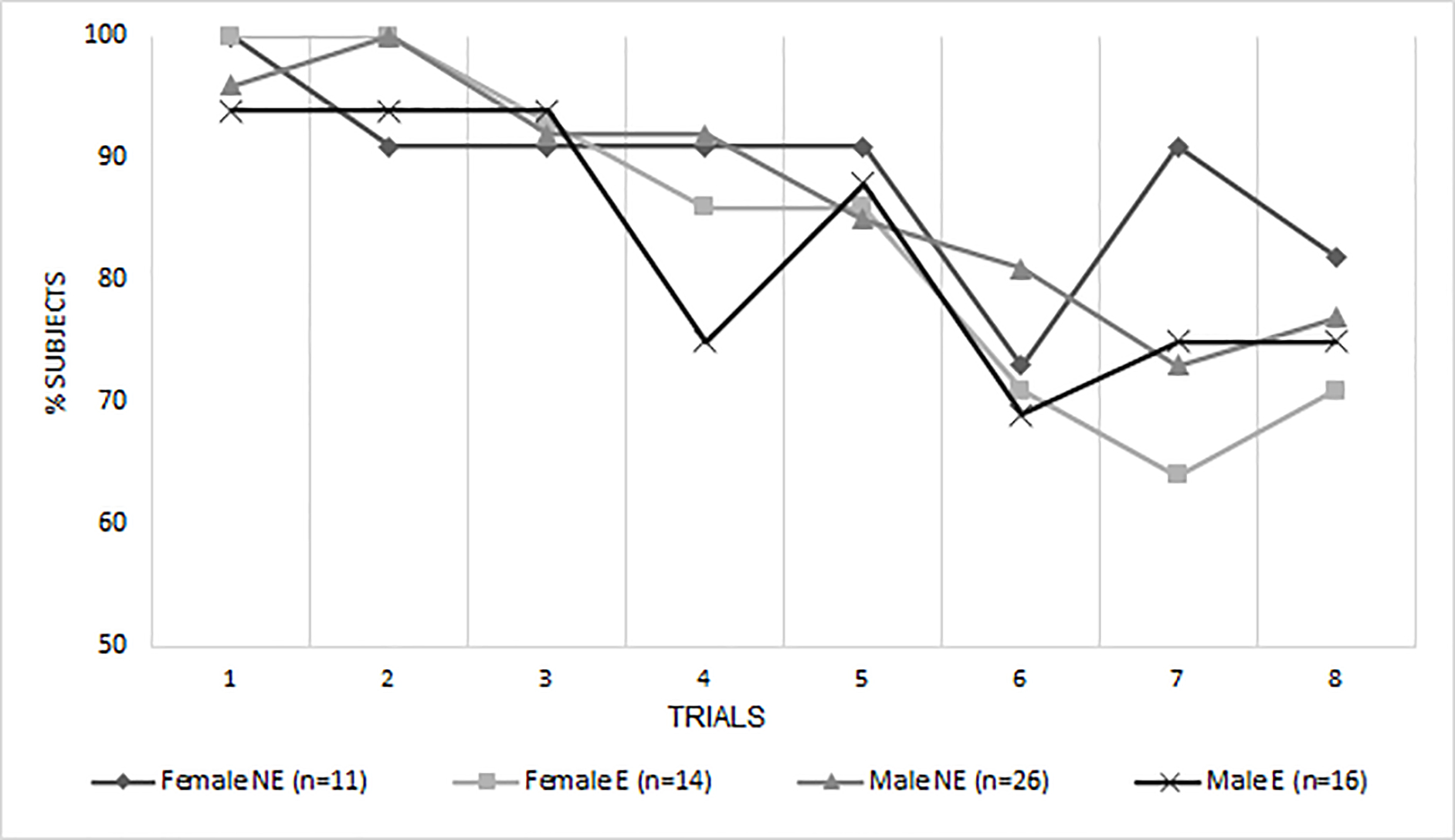
NE = non-exposed to cocaine prenatally, E = exposed to cocaine prenatally.
*For the sake of visual representation ER was divided into high and low risk at the median.
Fig. 1b. % of Subjects with high environmental risk* score who completed each trial by exposure status and sex.
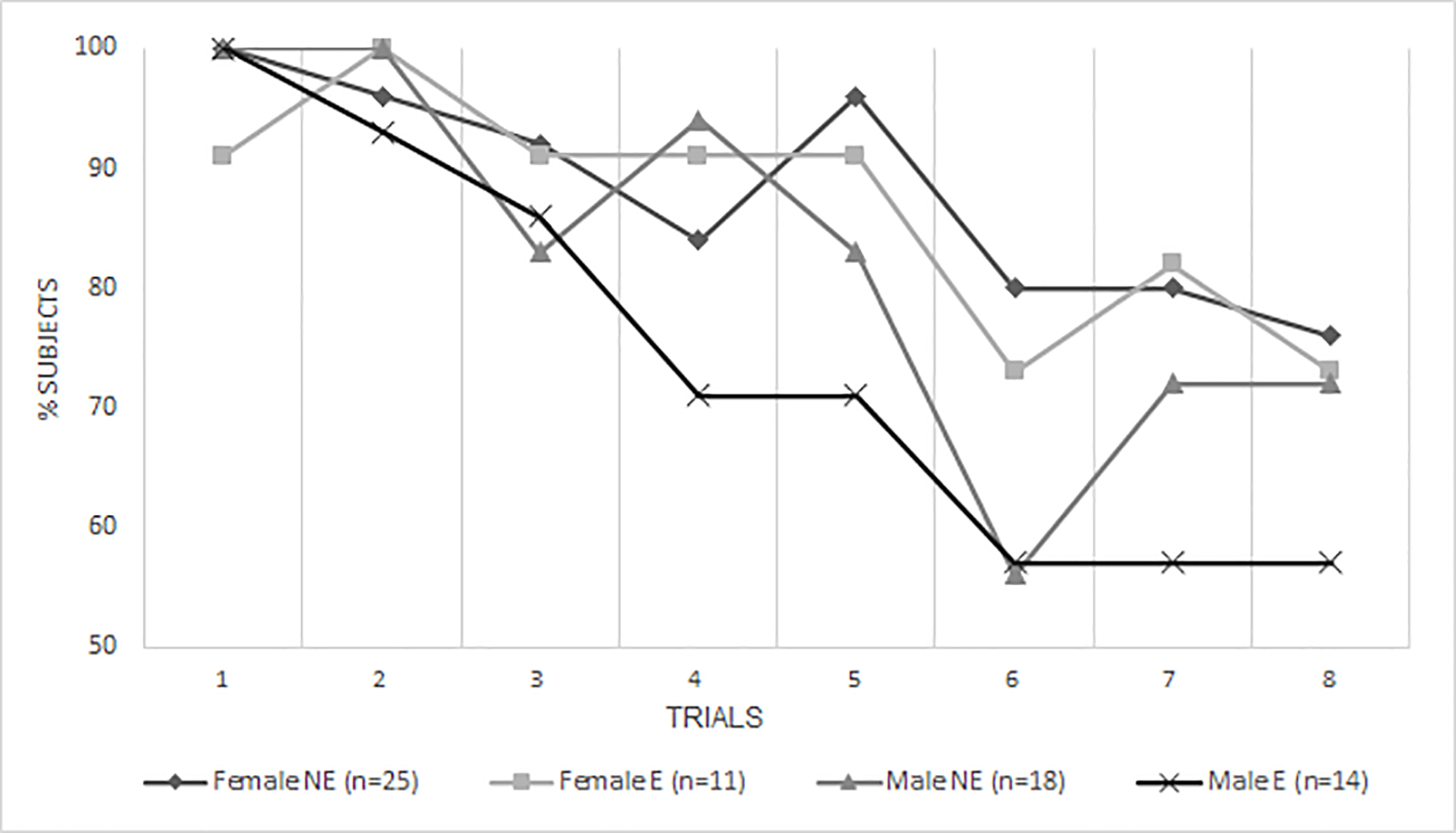
NE = non-exposed to cocaine prenatally, E = exposed to cocaine prenatally.
*For the sake of visual representation ER was divided into high and low risk at the median.
4. Discussion
The purpose of our study was to examine the long-term effects of prenatal cocaine exposure and its interactions with both sex and environmental risk. We found that higher environmental risk was associated with less likelihood of correctly solving ToH tasks. This finding is consistent with research highlighting how environmental factors affect children’s development, behavior, and cognitive abilities (Bendersky and Lewis, 1994; Bennett et al., 2013; Marsh et al., 2020; Ruiz et al., 2016). Due to its importance, the American Academy of Pediatrics advocates for pediatric screening of children and their families for environmental, family, economic, and social conditions (ex., Garner et al., 2012, reaffirmed 2016).
While there are no significant sex differences in executive function in typically developing children (Grissom and Reyes, 2019), our findings of male adolescents with PCE struggling the most with tasks of increasing difficulty are unique in the EF literature and highlight the potential importance of examining moderators of PCE. ToH puzzles, with their escalating complexity, required our Ss to plan, use, and apply newly built knowledge from previously solved trials in order to reach their final goal in subsequent trials. In our sample, males with PCE who had higher environmental risk scores independent of other teratogenic exposures, such as alcohol and cannabis, were more likely to struggle to complete all 8 trials correctly when the demand on their cognitive function was increased due to the escalating complexity of the task. Our sex specific findings of males with PCE demonstrating a worse completion rate differ from findings by Minnes et al. (2016), who found poorer EF performance among girls but not boys with PCE. Our findings, however, are consistent with an earlier report from the current sample in which males with PCE exhibited worse performance on inhibitory control and attention (Carmody et al., 2011).
Prenatal alcohol and cannabis exposure may also affect EF among youth. We found that adolescents who were prenatally exposed to alcohol had worse EF performance, consistent with prior research reporting deficits in some EF domains (Khoury et al., 2015; Kingdon et al., 2016). Long-term effects of cannabis on EF have been less often studied. The National Academies of Sciences, Engineering and Medicine in its report on cannabis effects did not identify clear evidence of prenatal exposure on later child outcomes (NASEM, 2017). A recent review of research publications on outcomes in children 1– 11 years by Sharapova et al. (2018) found varied associations ranging from none to negative and positive between prenatal cannabis exposure and neuropsychological functioning. Smith et al. (2016), using fMRI during four EF tasks in young adults, reported no differences in performance between exposed and non-exposed participants, but did report greater neural activity in the postcentral gyrus, the cuneus, the middle occipital gyrus, and the posterior cingulate depending on EF task among those prenatally exposed to cannabis. Thus, our finding regarding prenatal cannabis exposure affecting successful completion of ToH adds to the growing literature suggesting a possible subtle, long-term effect on EF.
It has been theorized that the ToH puzzle may involve two strategies: one is “goal-recursive”, involving planning and creating subgoals that are held in working memory to reach the end-goal; and the second is perceptual, where making a move brings a person perceptually closer to the final goal. The perceptual strategy is thought to involve inhibitory executive processes because the individual inhibits counterintuitive moves, which are necessary for a more effective and shorter puzzle solution. It is believed that this strategy is used more often because it is less mentally demanding and does not involve holding subgoals in memory (Miyake et al., 2000). Though the extent to which our subjects used each of these strategies is unknown, we can speculate that while overall there were no main effects for PCE, males with PCE from high-risk environments may have had greater difficulty on the ToH because of deficits in their perceptual reasoning or inhibitory control skills (Carmody et al., 2011; Singer et al., 2004; 2008; 2018). These shortfalls could possibly stem from structural differences in the brain due to prenatal cocaine exposure (Lebel et al., 2013; Roussotte et al., 2012).
The current study has several limitations. First, our findings do not necessarily apply to children of different ages, racial, ethnic, or cultural groups. Second, EF is a broad construct and as such our findings do not necessarily generalize to tasks that may assess somewhat distinct aspects of EF. Third, like most studies of PCE, we have examined maternal substance use while pregnant. However, there is some evidence from animal studies that paternal cocaine exposure can also affect the offspring’s cognitive, behavioral, and physical functioning (He et al., 2006; Killinger et al., 2012; White et al., 2016; Yaw et al., 2018), possibly due to epigenetic changes (Vassoler et al., 2013). Future research is needed to examine the relative contributions, if any, of paternal vs. maternal substance use at the time of conception as well as throughout the prenatal and postnatal periods. Fourth, the size of the current sample is modest, limiting our statistical power to examine interaction effects. Fifth, the current findings examined the association between PCE and EF as indicated by the ToH and may not necessarily generalize to other measures of EF. Sixth, while our environmental risk composite score included multiple risk factors, additional risks (e.g., abuse and neglect) that might affect EF were not included.
5. Conclusion
This is the first study to examine PCE as a predictor of EF using the ToH puzzle in adolescents. Our results highlight the potential role of environmental risk on EF, with males with high environmental risk and PCE being most affected, consistent with prior research (Allen et al., 2014; Bendersky et al., 2006; Bennett et al., 2013; Carmody et al., 2011).
The results add important information to our understanding of the complex relationships among a growing child’s environment, prenatal exposures, and the potential vulnerability of male sex. It will be important to replicate these findings and to disseminate this knowledge to the medical, educational, and public health, and policy communities to best direct preventive, educational and supportive measures to this population at greatest risk for EF deficits.
Highlights.
Environmental risk has a potential negative role on adolescents’ executive function
Prenatal alcohol and cannabis exposure may affect executive function
Males with prenatal cocaine exposure from high risk environments had poorer executive function
Acknowledgements
This study was supported by grant USPHS R01-DA07109 from the National Institute on Drug Abuse to Michael Lewis, David S. Bennett, and Dennis P. Carmody. The sponsor agency had no involvement in the study design, collection, analysis, and interpretation of data; writing of this report; or in the decision to submit this report for publication.
Footnotes
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahonniska J, Ahonen T, Aro T, Tolvanen A, Lyytinen H, 2000. Repeated Assessment of the Tower of Hanoi Test: Reliability and Age Effects. Assess. 7(3), 297–310. [DOI] [PubMed] [Google Scholar]
- 2.Akyuz N, Kekatpure M, Liu J, Sheinkopf S, Quinn B, Lala M et al. , 2014. Structural Brain Imaging in Children and Adolescents following Prenatal Cocaine Exposure: Preliminary Longitudinal Findings. Dev. Neurosci. 36, 316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J, Bennett D, Carmody D, Wang Y, Lewis M, 2014. Adolescent risk-taking as a function of prenatal cocaine exposure and biological sex. Neurotoxicol. Teratol. 41, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardila A, Rosselli M, Matute E, Guajardo S, 2005. The Influence of the Parents’ Educational Level on the Development of Executive Functions. J. Dev. Neuropsychol. 1(28), 539–60. [DOI] [PubMed] [Google Scholar]
- 5.Atzaba-Poria N, Pike A, Deater-Deckard K, 2004. Do risk factors for problem behaviour act in a cumulative manner? An examination of ethnic minority and majority children through an ecological perspective. J. Child Psychol. Psychiatr. 45(4), 707–18. [DOI] [PubMed] [Google Scholar]
- 6.Barch D, 2006. What can research on schizophrenia tell us about the cognitive neuroscience of working memory? Neuroscience. 139(1), 73–84. [DOI] [PubMed] [Google Scholar]
- 7.Bateman D, Chiriboga C, 2000. Dose-Response Effect of Cocaine on Newborn Head Circumference. Pediatrics e33, 106(3). [DOI] [PubMed] [Google Scholar]
- 8.Bauer C, Langer J, Shankaran S, Bada H, Lester B, Wright L et al. , 2005. Acute neonatal effects of cocaine exposure during pregnancy. Arch. Pediatr. Adolesc. Med. 159(9), 824–34. [DOI] [PubMed] [Google Scholar]
- 9.Bendersky M, Alessandri S, Gilbert P, Lewis M, 1996. Characteristics of pregnant substance abusers in two cities in the Northeast. Am. J. Drug Alcohol Abuse 22, 349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendersky M, Bennett D, Lewis M, 2006. Aggression at Age 5 as a Function of Prenatal Exposure to Cocaine, Gender, and Environmental Risk. J. Pediatr. Psychol. 31(1), 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendersky M, Lewis M, 1994. Environmental Risk, Biological Risk, and Developmental Outcome. Dev. Psychol. 30(4), 484–94. [Google Scholar]
- 12.Bendersky M, Lewis M, 1998. Developmental Psychology Arousal Modulation in Cocaine-Exposed Infants. Dev. Psychol. 34(3), 555–64. [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett D, Bendersky M, Lewis M, 2008. Children’s Cognitive Ability From 4 to 9 Years Old as a Function of Prenatal Cocaine Exposure, Environmental Risk, and Maternal Verbal Intelligence. Dev. Psychol. 44(4), 919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett D, Marini V, Berzenski S, Carmody D, Lewis M, 2013. Externalizing problems in late childhood as a function of prenatal cocaine exposure and environmental risk. J. Pediatr. Psychol. 38, 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betancourt L, Yang W, Brodsky N, Gallagher P, Malmud E, Giannetta J et al. , 2011. Adolescents with and without gestational cocaine exposure: Longitudinal analysis of inhibitory control, memory and receptive language. Neurotoxicol. Teratol. 33(1), 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biedinger N, 2011. The Influence of Education and Home Environment on the Cognitive Outcomes of Preschool Children in Germany. Child Dev. Res. 2011, 1–10. [Google Scholar]
- 17.Bradley R, Corwyn R, 2002. Socioeconomic status and child development. Annu. Rev. Psychol. 53, 371–99. [DOI] [PubMed] [Google Scholar]
- 18.Bradley R, Corwyn R, Caldwell B, Whiteside-Mansell L, Wasserman G, Mink I, 2000. Measuring the home environments of children in early adolescence. J. Res. Adolesc. 10(3), 247–88. [Google Scholar]
- 19.Bridgett D, Mayes L, 2011. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: The effects of gender and risk and subsequent aggressive behavior. Neurotoxicol. Teratol. 33(1), 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckingham-Howes S, Berger S, Scaletti L, Black M, 2013. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics 131(6), e1917–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burchinal M, Roberts J, Hooper S, Zeisel S, 2000. Cumulative risk and early cognitive development: A comparison of statistical risk models. Dev. Psychol. 36, 793–807. [DOI] [PubMed] [Google Scholar]
- 22.Bustini M, Stratta P, Daneluzzo E, Pollice R, Rossi A, 1999. Tower of Hanoi and WCST performance in schizophrenia: problem-solving capacity and clinical correlates. J. Psychiatr. Res. 33(31), 285–90. [DOI] [PubMed] [Google Scholar]
- 23.Carmody D, Bennett D, Lewis M, 2011. The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicol. Teratol. 33(1), 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey B, Giedd J,Thomas K, 2000. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 54, 241–57. [DOI] [PubMed] [Google Scholar]
- 25.Center for Behavioral Health Statistics and Quality, 2019. Results from the 2018 National Survey on Drug Use and Health: Summary of National Findings (HHS Publication No. PEP19–5068, NSDUH Series H-54). Rockville, MD: Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/ [Google Scholar]
- 26.Chiriboga C, Brust J, Bateman D, Hauser W, 1999. Dose–Response Effect of Fetal Cocaine Exposure on Newborn Neurologic Function. Pediatrics 103(1), 79–85. [DOI] [PubMed] [Google Scholar]
- 27.Chiriboga C, Kuhn L, Wasserman G, 2007. Prenatal cocaine exposures and dose-related cocaine effects on infant tone and behavior. Neurotoxicol. Teratol. 29, 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz A, Castro-Rodrigues A, Barbosa F, 2020. Executive dysfunction, violence and aggression. Aggress. Viol. Behav. 51,101380. [Google Scholar]
- 29.Deater-Deckard K, Dodge K, Bates J, Pettit G, 1998. Multiple risk factors in the development of externalizing behavior problems: Group and individual differences. Dev. Psychopath. 10(3), 469–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaney-Black V, Chiodo L, Hannigan J, Greenwald M, Janisse J, Patterson G et al. , 2011. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol. Teratol. 33(1), 110–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, Sokol RJ, 2004. Prenatal cocaine: quantity of exposure and gender moderation. J. Dev. Behav. Pediatr. 25, 254–63. [DOI] [PubMed] [Google Scholar]
- 32.Demetriou E, Lampit A, Quintana D, Naismith S, Song Y, Pye J et al. , 2018. Autism spectrum disorders: a meta-analysis of executive function. Mol. Psychiatr. 23(5), 1198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eiden R, Godleski S, Colder C, Schuetze P, 2014. Prenatal cocaine exposure: the role of cumulative environmental risk and maternal harshness in the development of child internalizing behavior problems in kindergarten. Neurotoxicol. Teratol. 44, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eiden R, McAuliffe S, Kachadourian L, Coles C, Colder C, Schuetze P, 2009. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicol. Teratol. 31, 60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emick J, Welsh M, 2005. Association between formal operational thought and executive function as measured by the Tower of Hanoi-Revised. Learn. Individ. Differ. 15(3), 177–88. [Google Scholar]
- 36.Fillmore M, Rush C, 2002. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 66, 265–73. [DOI] [PubMed] [Google Scholar]
- 37.Fillmore M, Rush C, Hays L, 2002. Acute effects of oral cocaine on inhibitory control of behavior in humans. Drug Alcohol Depend. 67(2), 157–67. [DOI] [PubMed] [Google Scholar]
- 38.Foran A, Mathias J, Bowden S, 2021. Effectiveness of sorting tests for cognitive decline in older adults with dementia and other common neurodegenerative disorders: A meta-analysis. Neurosci. Biobehav. 120, 442–54. [DOI] [PubMed] [Google Scholar]
- 39.Garner A, Shonkoff J, Siegel B, Dobbins M, Earls M, McGuinn L et al. , 2012. Early Childhood Adversity, Toxic Stress, and the Role of the Pediatrician: Translating Developmental Science Into Lifelong Health. J. Pediatr. 129(1), e224–31. [DOI] [PubMed] [Google Scholar]
- 40.Garon N, Smith I, Bryson S, 2018. Early Executive Dysfunction in ASD: Simple Versus Complex Skills. Autism Res. 11, 318–30. [DOI] [PubMed] [Google Scholar]
- 41.Goel V, Grafman J, 1995. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia 33 (5), 623–42. [DOI] [PubMed] [Google Scholar]
- 42.Grewen K, Burchinal M, Vachet C, Gouttard S, Gilmore J, Lin W et al. , 2014. Prenatal cocaine effects on brain structure in early infancy. NeuroImage 101, 114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grissom N, Reyes T, 2019. Let’s call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacol. 44, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guevara M, Rizo Martínez L, Robles Aguirre F, Hernández González M, 2012. Prefrontal–parietal correlation during performance of the towers of Hanoi task in male children, adolescents and young adults. Dev. Cognit. Neurosci. 2, 129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackman D, Farah M, 2009. Socioeconomic status and the developing brain. Trends Cognit. Sci. 13, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He F, Lidow I, Lidow M, 2006. Consequences of paternal cocaine exposure in mice. Neurotoxicol. Teratol. 28(2), 198–209. [DOI] [PubMed] [Google Scholar]
- 47.Hervey A, Epstein J, Curry J, 2004. Neuropsychology of Adults With Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neuropsychol. 18, 485–503. [DOI] [PubMed] [Google Scholar]
- 48.Hurt H, Betancourt L, Malmud E, Shera D, Giannetta J, Brodsky N et al. , 2009. Children With and Without Gestational Cocaine Exposure: A Neurocognitive Systems Analysis. Neurotoxicol. Teratol. 31(6), 334–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurt H, Giannetta J, Korczykowski M, Hoang A, Tang K, Betancourt L et al. , 2008. Functional magnetic resonance imaging and working memory in adolescents with gestational cocaine exposure. J. Pediatr. 152, 371–77. [DOI] [PubMed] [Google Scholar]
- 50.Huttenlocher P, Dabholkar A, 1997. Regional Differences in Synaptogenesis in Human Cerebral Cortex. J. Comp. Neurol. 387, 167–78. [DOI] [PubMed] [Google Scholar]
- 51.Jones S, Bailey R, Barnes S, Partee A, 2016. Executive function mapping project: Untangling the terms and skills related to executive function and self-regulation in early childhood OPRE report #2016–88. Washington, DC: Office of Planning, Research and Evaluation, Administration for Children and Families, U. S. Department of Health and Human Services. [Google Scholar]
- 52.Kestler L, Bennett D, Carmody D, Lewis M, 2012. Gender-dependent effects of prenatal cocaine exposure. In: Lewis M, Kestler L (Eds.), Gender Differences in Prenatal Substance Exposure. Am. Psychol. Assoc. 11–29. [Google Scholar]
- 53.Khoury J, Milligan K, Girard T, 2015. Executive functioning in children and adolescents prenatally exposed to alcohol: A meta-analytic review. Neuropsychol. Rev. 25(2), 149–70. [DOI] [PubMed] [Google Scholar]
- 54.Killinger C, Robinson S, Stanwood G, 2012. Subtle Biobehavioral Effects Produced by Paternal Cocaine Exposure. Synapse 66(10), 902–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kingdon D, Cardoso C, McGrath J, 2016. Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder – a meta-analysis. J. Child Psychol. Psychiatr. 57(2), 116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kishiyama M, Thomas Boyce W, Jimenez A, Perry L, Knight R, 2008. Socioeconomic Disparities Affect Prefrontal Function in Children. J. Cognit. Neurosci. 21(6), 1106–15. [DOI] [PubMed] [Google Scholar]
- 57.Lebel C, Warner T, Colby J, Soderberg L, Roussotte F, Behnke M et al. , 2013. White matter microstructure abnormalities and executive function in adolescents with prenatal cocaine exposure. Psychiatr. Res.: Neuroimaging. 213(2), 161–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang L, Shewokis P, Getchell N, 2016. Brain Activation in the Prefrontal Cortex during Motor and Cognitive Tasks in Adults. J. Behav. Brain Sci. 6, 463–74. [Google Scholar]
- 59.Marsh S, Dobson R, Maddison R, 2020. The relationship between household chaos and child, parent, and family outcomes: a systematic scoping review. BMC Public Health. 20, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattson S, Bernes G, Doyle L, 2019. Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcoholism: Clin. Exp. Res. 43(6), 1046–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McClelland M, Acock A, Piccinin A, Rhea S, Stallings M, 2013. Relations between preschool attention span-persistence and age 25 educational outcomes. Early Child. Res. Q. 28, 314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Min M, Minnes S, Lang A, Weishampel P, Short E, Yoon S et al. , 2014. Externalizing behavior and substance use related problems at 15 years in prenatally cocaine exposed adolescents. J. Adolesc. 37(3), 269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Min M, Minnes S, Lang A, Yoon S, Singer L, 2015. Effects of prenatal cocaine exposure on early sexual behavior: Gender difference in externalizing behavior as a mediator. Drug Alcohol Depend. 153, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Min M, Minnes S, Park H, Ridenour T, Kim J, Yoon M et al. , 2018. Developmental trajectories of externalizing behavior from ages 4 to 12: Prenatal cocaine exposure and adolescent correlates. Drug Alcohol Depend. 192, 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minnes S, Min M, Kim J, Francis M, Lang A, Wu M et al. , 2017. The association of prenatal cocaine exposure, externalizing behavior and adolescent substance use. Drug Alcohol Depend. 176, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minnes S, Min M, Short E, Wu M, Lang A, Yoon S et al. , 2016. Executive function in children with prenatal cocaine exposure (12–15 years). Neurotoxicol. Teratol. 57, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyake A, Friedman N, Emerson M, Witzki A, Wager T, 2000. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cognit. Psychol. 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- 68.Morgan P, Farkas G, Wang Y, Hillemeier M, Oh Y, Maczuga S, 2019. Executive function deficits in kindergarten predict repeated academic difficulties across elementary school. 46, 20–32. [Google Scholar]
- 69.Muthen B, Masyn K, 2005. Discrete-time survival mixture analysis. J. Educ. Behav. Stat. 30, 27–58. [Google Scholar]
- 70.Muthén L, Muthén B, 1998–2008. Mplus Statistical Analysis With Latent Variables User’s Guide. https://www.statmodel.com/download/usersguide/MplusUserGuideVer_7.pdf
- 71.National Academies of Sciences, Engineering, Medicine. 2017. The Health Effects of Cannabis and Cannabinoids: the Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press (US). ISBN-10: 0–309-45304–6 [PubMed] [Google Scholar]
- 72.Nayfeld I, Fuccillo J, Greenfield D, 2013. Executive functions in early learning: Extending the relationship between executive functions and school readiness to science. Learn. Individ. Differ. 26, 81–8. [Google Scholar]
- 73.Norbeck J, Lindsey A, Carrieri V, 1981. The development of an instrument to measure social support. Nurs. Res. 30(5), 264–69. [PubMed] [Google Scholar]
- 74.Numminen H, Lehto J, Ruoppila I, 2001. Tower of Hanoi and working memory in adult persons with intellectual disability. Res. Dev. Disabil. 22(5), 373–87. [DOI] [PubMed] [Google Scholar]
- 75.Oh K, Xu Y, Terrizzi B, Lanphear B, Chen A, Kalkbrenner A et al. , 2020. Associations between early low-level tobacco smoke exposure and executive function at age 8 years. J. Pediatr. 221, 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orr S, James S, Casper R, 1992. Psychosocial stressors and low birth weight: development of a questionnaire. J. Dev. Behav. Pediatr. 13(5), 343–47. [PubMed] [Google Scholar]
- 77.Peeters M, Janssen T, Monshouwer K, Boendermaker W, Pronk T, Wiers R et al. , 2015. Weaknesses in executive functioning predict the initiating of adolescents’ alcohol use. Dev. Cognit. Neurosci. 16, 139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raykov T, Gorelick P, Zajacova A, Marcoulides G, 2017. On the potential of discrete time survival via latent variable modeling: An application to the study of the vascular depression hypothesis. Struct. Equ. Model.: A Multidiscip. J. 24(6), 926–35. [Google Scholar]
- 79.Raykov T, Gorelick P, Zajacova A, Marcoulides G, 2018a. Discrete time survival analysis via latent variable modeling: A note on lagged depression links to stroke in middle and late life. Struct. Equ. Model.: A Multidiscip. J. 25(1), 115–20. [Google Scholar]
- 80.Raykov T, Zajacova A, Gorelick P, Marcoulides G, 2018b. Using Latent Variable Modeling for Discrete Time Survival Analysis: Examining the Links of Depression to Mortality. Struct. Equ. Model.: A Multidiscip. J. 25(2), 287–93. [Google Scholar]
- 81.Ready D, Reid J, 2019. Children’s executive function development and school socio-economic and racial/ethnic composition. Early Child. Res. Q. 47, 457–71. [Google Scholar]
- 82.Richardson G, De Genna N, Goldschmidt L, Larkby C, Donovan J, 2019. Prenatal cocaine exposure: Direct and indirect associations with 21-year-old offspring substance use and behavior problems. Drug Alcohol Depend. 195,121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richardson G, Goldschmidt L, Larkby C, Day N, 2013. Effects of prenatal cocaine exposure on child behavior and growth at 10 years of age. Neurotoxicol. Teratol. 40, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richardson G, Goldschmidt L, Larkby C, Day N, 2015. Effects of prenatal cocaine exposure on adolescent development. Neurotoxicol. Teratol. 49, 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richardson G, Goldschmidt L, Leech S, Willford J, 2011. Prenatal cocaine exposure: Effects on mother-and teacher-rated behavior problems and growth in school-age children. Neurotoxicol. Teratol. 33, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richardson G, Goldschmidt L, Willford J, 2008. The effects of prenatal cocaine use on infant development. Neurotoxicol. Teratol. 30, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richardson G, Ryan C, Willford J, Day N, Goldschmidt L, 2002. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol. Teratol. 24, 309–320 [DOI] [PubMed] [Google Scholar]
- 88.Rose-Jacobs R, Waber D, Beeghly M, Cabral H, Frank D, 2009. Intrauterine cocaine exposure and executive functioning in middle childhood. Neurotoxicol. Teratol. 31(3), 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rottschy C, Langner R, Dogan I, Reetz K, Laird A, Schulz J et al. , 2012. Modelling neural correlates of working memory: A coordinate-based meta-analysis. NeuroImage. 60(1), 830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roussotte F, Soderberg L, Warner T, Narr K, Lebel C, Behnke M et al. , 2012. Adolescents with prenatal cocaine exposure show subtle alterations in striatal surface morphology and frontal cortical volumes. J. Neurodev. Disord. 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruiz J, Quackenboss J, Tulve N, 2016. Contributions of a Child’s Built, Natural,and Social Environments to Their General Cognitive Ability: A Systematic Scoping Review. PLoS ONE 11(2): e0147741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rüsch N, Tebartz van Elst N, Valerius G, Büchert M, Thiel T, Ebert D et al. , 2008. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr. Res. 99(1–3), 155–163. [DOI] [PubMed] [Google Scholar]
- 93.Sameroff A, Seifer R, Barocas R, Zax M, Greenspan S,1987. Intelligence quotient scores of 4-year-old children: social-environmental risk factors. Pediatr. 79(3), 343–50. [PubMed] [Google Scholar]
- 94.Schoemaker K, Mulder H, Deković M, Matthys W, 2013. Executive Functions in Preschool Children with Externalizing Behavior Problems: A Meta-Analysis. J. Abnorm. Child Psychol. 41, 457–71. [DOI] [PubMed] [Google Scholar]
- 95.Shakehnia F, Amiri S, Ghamarani A, 2021.The comparison of cool and hot executive functions profiles in children with ADHD symptoms and normal children. Asian J. Psychiatr. 55, 1–7, 102483. [DOI] [PubMed] [Google Scholar]
- 96.Sharapova S, Phillips E, Sirocco K, Kaminski J, Leeb R, Rolle I, 2018. Effects of prenatal marijuana exposure on neuropsychological outcomes in children aged 1– 11 years: A systematic review. Paediatr. Perinat. Epidemiol. 32(6), 512–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singer L, Arendt R, Minnes S, Farkas K, Salvator A, 2000. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicol. Teratol. 22, 653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singer L, Min M, Minnes S, Short E, Lewis B, Lang A et al. , 2018. Prenatal and concurrent cocaine, alcohol, marijuana, and tobacco effects on adolescent cognition and attention. Drug Alcohol Depend. 191, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singer L, Minnes S, Short E, Arendt R, Farkas K, Lewis B et al. , 2004. Cognitive Outcomes of Preschool Children With Prenatal Cocaine Exposure. JAMA. 291(20), 2448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singer L, Nelson S, Short E, Min M, Lewis B, Russ S et al. , 2008. Prenatal Cocaine Exposure: Drug and Environmental Effects at 9 Years. J. Pediatr. 153(1), 105–11.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith A, Mioduszewski O, Hatchard T, Byron-Alhassan A, Fall C, Fried P, 2016. Prenatal marijuana exposure impacts executive functioning into young adulthood: An fMRI study. Neurotoxicol. Teratol. 58, 53–9. [DOI] [PubMed] [Google Scholar]
- 102.Stuss D, 2011. Traumatic brain injury: relation to executive dysfunction and the frontal lobes. Curr. Opin. Neurol. 24(6), 584–589. [DOI] [PubMed] [Google Scholar]
- 103.Testa R, Bennett P, Ponsford J, 2012. Factor analysis of nineteen executive function tests in a healthy adult population. Arch. Clin. Neuropsychol. 27(2), 213–224. [DOI] [PubMed] [Google Scholar]
- 104.Thompson A, Steinbeis N, 2020. Sensitive periods in executive function development. Curr. Opin. Behav. Sci. 36, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vakil E, Hassin-Baer S, Karni A, 2014. A deficit in optimizing task solution but robust and well-retained speed and accuracy gains in complex skill acquisition in Parkinson׳s disease: Multi-session training on the Tower of Hanoi Puzzle. Neuropsychol. 57, 12–9. [DOI] [PubMed] [Google Scholar]
- 106.Vassoler F, White S, Schmidt H, Sadri-Vakili G, Pierce R, 2013. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 16(1), 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wachs T, 1991. Environmental considerations in studies with non-extreme groups. In: Wachs T, Plomin R, editors. Conceptualization and measurement of organism-environment interaction. American Psychological Association; Washington, DC. 44–67. [Google Scholar]
- 108.Wagner S, Müller C, Helmreich I, Huss M, Tadić A, 2015. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur. Child Adolesc. Psychiatr. 24, 5–19. [DOI] [PubMed] [Google Scholar]
- 109.Welsh M, 1991. Rule-Guided Behavior and Self-Monitoring On The Tower Of Hanoi Disk-Transfer Task. Cogn. Dev. 6, 59–76. [Google Scholar]
- 110.Welsh M, Hiuzinga M, 2005. Tower of Hanoi disk-transfer task: Influences of strategy knowledge and learning on performance. Learn. Individ. Differ. 15, 283–298. [Google Scholar]
- 111.Welsh M, Satteriee-Cartmell T, Stine M, 1999. Towers of Hanoi and London: Contribution of working memory and inhibition to performance. Brain Cogn. 41, 231–42. [DOI] [PubMed] [Google Scholar]
- 112.White L, Alexander A, Greenfield D, 2017. The relationship between executive functioning and language: Examining vocabulary, syntax, and language learning in preschoolers attending Head Start. J. Exp. Child Psychol. 164, 16–31. [DOI] [PubMed] [Google Scholar]
- 113.White S, Vassoler F, Schmidt H, Pierce R, Wimmer M, 2016. Enhanced anxiety in the male offspring of sires that self- administered cocaine. Addict. Biol. 21(4), 802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Willcutt E, Doyle A, Nigg J, Faraone S, 2005. Validity of the executive function theory of attention-deficit/ hyperactivity disorder: A meta-analytic review. Biol. Psychiatr. 57(11),1136–1346. [DOI] [PubMed] [Google Scholar]
- 115.Willford J, Singhabahu D, Herat A, Richardson G, 2018. An examination of the association between prenatal cocaine exposure and brain activation measures of arousal and attention in young adults: An fMRI study using the Attention Network Task. Neurotoxicol. Teratol. 69, 1–10. [DOI] [PubMed] [Google Scholar]
- 116.Yaw A, Woodruff R, Prosser R, Glass J, 2018. Paternal Cocaine Disrupts Offspring Circadian Clock Function in a Sex-Dependent Manner in Mice. Neurosci. 379, 257–68. [DOI] [PubMed] [Google Scholar]
- 117.Yuan P, Raz N, 2014. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 42, 180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zook N, Davalos D, DeLosh E, Hasker P, 2004. Working memory, inhibition, and fluid intelligence as predictors of performance on Tower of Hanoi and London tasks. Brain Cogn. 56(3), 286–92. [DOI] [PubMed] [Google Scholar]


