Abstract
Simple Summary
Knowledge of genetic alterations in gallbladder cancer (GBC) continues to increase. This systematic review provides an overview of frequently occurring genetic alterations in GBC and describes their possible therapeutic implications. We detected three frequently (>5%) altered genes (ATM, ERBB2 and PIK3CA) for which targeted therapies are available in other cancer types. For solid cancers with microsatellite instability or a high tumor mutational burden pembrolizumab is FDA-approved. Altogether, these five biomarkers might be used in future molecular panels to enable precision medicine for patients with GBC. We found only nine clinical trials evaluating targeted therapies in GBC directed at frequently altered genes (ERBB2, ARID1A, ATM and KRAS). This underlines the challenges to perform such clinical trials in this rare, heterogeneous cancer type and emphasizes the need for multicenter clinical trials.
Abstract
Due to the fast progression in molecular technologies such as next-generation sequencing, knowledge of genetic alterations in gallbladder cancer (GBC) increases. This systematic review provides an overview of frequently occurring genetic alterations occurring in GBC and their possible therapeutic implications. A literature search was performed utilizing PubMed, EMBASE, Cochrane Library, and Web of Science. Only studies reporting genetic alterations in human GBC were included. In total, data were extracted from 62 articles, describing a total of 3893 GBC samples. Frequently detected genetic alterations (>5% in >5 samples across all studies) in GBC for which targeted therapies are available in other cancer types included mutations in ATM, ERBB2, and PIK3CA, and ERBB2 amplifications. High tumor mutational burden (TMB-H) and microsatellite instability (MSI-H) were infrequently observed in GBC (1.7% and 3.5%, respectively). For solid cancers with TMB-H or MSI-H pembrolizumab is FDA-approved and shows an objective response rates of 50% for TMB-H GBC and 41% for MSI-H biliary tract cancer. Only nine clinical trials evaluated targeted therapies in GBC directed at frequently altered genes (ERBB2, ARID1A, ATM, and KRAS). This underlines the challenges to perform such clinical trials in this rare, heterogeneous cancer type and emphasizes the need for multicenter clinical trials.
Keywords: gallbladder cancer, gene mutations, genetic alterations, tumor mutational burden, microsatellite instability, targeted therapy
1. Introduction
Gallbladder cancer (GBC) is a relatively uncommon malignancy, with a worldwide incidence of less than 2 per 100,000 [1]. However, GBC shows a broad geographical and ethnic distribution, with low incidence rates in developed countries and higher incidence rates in South American countries, India, Pakistan, Japan and Korea. Higher incidence rates are also observed among Mexican Americans, Indian Americas, and Eastern Europeans [1,2,3]. Well-established risk factors include age, obesity, female gender, family history, cholelithiasis, and anomalous junction of the pancreatobiliary duct [4,5,6].
At present, radical resection by cholecystectomy with lymphadenectomy of the hepatoduodenal ligament and wedge or segment resection of the liver is the only treatment with curative intent [7]. Unfortunately, most patients present at an advanced stage and are unresectable [8,9]. Even after resection, the five-year survival rate ranges from 18% to 34% [10,11]. Palliative systemic chemotherapy for patients with GBC has shown limited efficacy. In patients with locally advanced or metastatic biliary tract cancer (BTC), including GBC, the ABC-02 trial reported a median overall survival of 11.7 months in the BTC group treated with gemcitabine plus cisplatin and 8.1 months in the BTC group treated with gemcitabine monotherapy [12].
With the rapid developments in next-generation sequencing (NGS), our knowledge of genetic alterations occurring in GBC has increased over the past decade [13]. Consequently, the number of preclinical studies and clinical trials evaluating therapies targeting these genetic alterations is slowly increasing [14]. Several reviews on genetic alterations in biliary tract cancer have been published. However, a systematic review specifically focusing on genetic alterations in GBC and their therapeutic implications is lacking.
2. Materials and Methods
2.1. Literature Search
The protocol for this systematic review was prospectively registered in the PROSPERO registry (CRD42021265246). The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15].
A literature search was performed until 26 March 2021 in PubMed, EMBASE, Cochrane Library, and Web of Science. Keywords or medical subject headings (MeSH) used for the search are provided in Appendix Table A1. Only full-text articles published in English in 2000 or afterwards were selected.
2.2. Study Selection
Citations were deduplicated by using tools in Endnote and Rayyan (rayyan.ai). Subsequently, all duplications were manually verified. Titles and abstracts of all retrieved records were then independently screened for eligibility by two investigators (H.K. and T.J.J.d.B.) using the Rayyan platform for systematic reviews. Discordance was re-evaluated by both investigators and resolved by consensus after discussion. Full papers were obtained for records that were considered potentially eligible by both investigators. In the case of studies with overlapping data, the article reporting the largest cohort was selected.
Only studies were included that identified somatic genetic alterations in human GBC with Polymerase Chain Reaction (PCR) with Sanger sequencing, NGS (targeted, whole-exome, and whole-genome sequencing), MassArray, or SNaPshot. Studies using immunohistochemistry or FISH techniques were excluded. Case reports, retracted articles, and preliminary results were excluded, as well as studies focusing on gene expression, genetic alterations in cell lines, cell-free DNA from serum, and mitochondrial DNA.
This review focused on adenocarcinoma of the gallbladder. Other histologies, i.e., adenosquamous, squamous, neuroendocrine, and sarcomatoid carcinoma, were excluded. In case no histological type was reported, tumors were assumed to represent adenocarcinomas since only 5% of GBC have histology other than adenocarcinoma [16,17].
2.3. Data Extraction
Data extracted included first author, year of publication, study population, sequencing technique, number of samples, and reported genetic alterations and their frequencies. Included alterations were non-synonymous mutations and copy number aberrations (DNA amplifications and deletions). In addition, frequencies of high tumor mutational burden (TMB) and the presence of microsatellite instability (MSI) were extracted.
Only frequently occurring genetic alterations (>5% [18,19] across all studies and in >5 of all included GBC samples) were included. Per genetic alteration, weighted average of reported frequencies was calculated by using the number of samples analyzed in a study as the weight.
A column scatter plot was constructed for all frequently occurring genetic alterations. Bars displayed the minimum and maximum reported frequency. Dots represented the frequency per study. Diamonds represented the weighted averages.
No risk of bias was assessed since no methods are available that could assess confounders such as inter-population diversity and variations in DNA techniques [20,21]. Nevertheless, to facilitate the reader to assess the studies’ quality and risk of bias, details of each included study were displayed in tables.
2.4. Therapeutic Implications
Actionable alterations were identified by comparing the frequently altered genes detected in this study with the actionable genes of the OncoKB database (accessed 21 July 2021) [22]. Only actionable genes with level 1 and 2 therapeutic evidence in solid tumors were analyzed. Level 1 evidence was defined as a U.S. Food and Drug Administration (FDA)-recognized biomarker predictive of response to FDA-approved drugs for a specific indication and level 2 as a standard care biomarker predictive of response to FDA-approved drugs for another indication. Data extracted included targetable alteration, drug, level of evidence, and cancer type.
Second, clinicaltrials.gov and clinicaltrialsregister.eu were examined for clinical trials targeting frequently occurring genetic alterations detected in this study in patients with GBC or BTC (accessed 26 July 2021).
3. Results
3.1. Literature Search and Study Selection
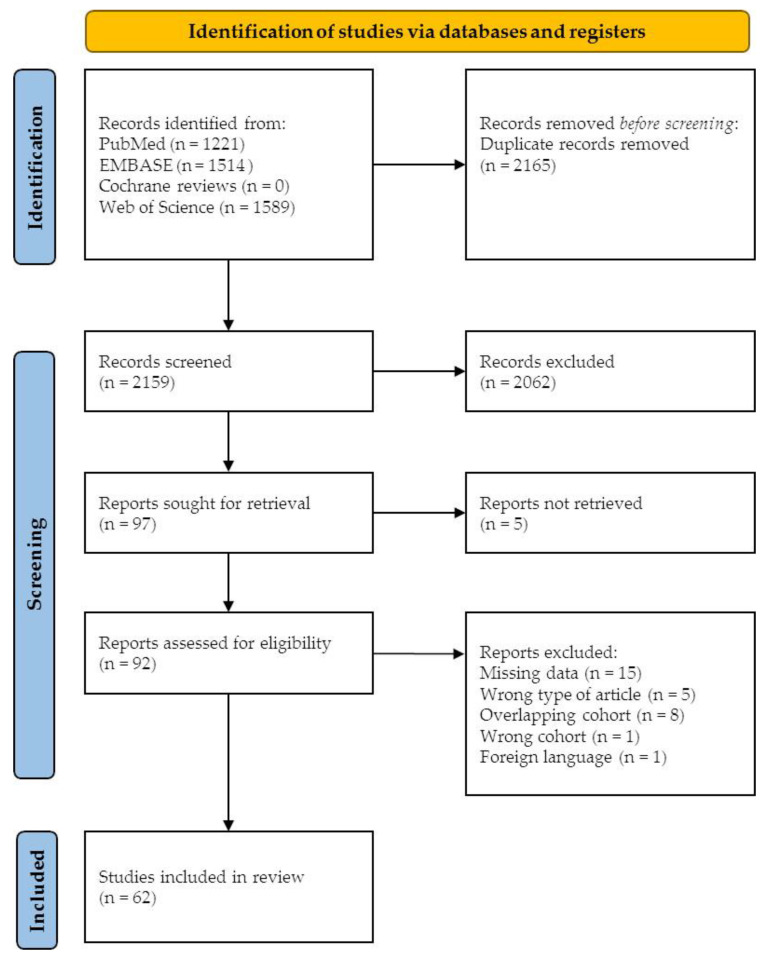
In total, 4324 records were retrieved from all databases. After removing duplicate records, 2159 records were screened for eligibility based on title and abstract (Figure 1). A total of 92 articles underwent full-text reading. Out of 92 articles, 30 were excluded due to: no data for adenocarcinoma of the gallbladder available (N = 12), overlapping cohorts (N = 8), the wrong type of article (N = 5), no genetic alteration frequency data available (N = 3), wrong study population (N = 1), and not written in English (N = 1). A total of 62 articles were included for final data extraction, describing a total of 3893 GBC samples from individual patients [18,19,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82].
Figure 1.
Identification of studies via databases.
3.2. Most Frequently Mutated Genes
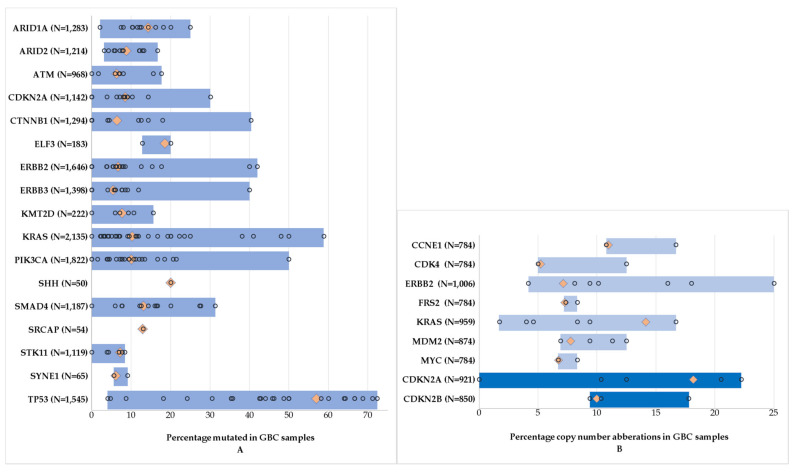
Figure 2A represents all frequently occurring gene mutations in GBC. Appendix Table A2 shows the results for all frequent gene mutations. TP53 was the most frequently mutated gene in GBC (weighted average 57%), followed by SHH (weighted average 20%), ELF3 (weighted average 19%), ARID1A (weighted average 14%), and SMAD4 (weighted average 13%).
Figure 2.
Frequently occurring genetic alterations (>5% across all studies and in >5 of all included samples) in gallbladder cancer. Numbers represent the total number of samples tested for this gene. Blue bars represent the minimum and maximum reported alteration frequencies. Dots represent the mutation frequency per study. Orange diamonds represent the weighted average of all frequencies. (A) Gene mutations. (B) Gene amplifications (light blue) and gene deletions (dark blue).
3.3. Amplifications and Deletions
Ten studies reported frequencies of copy number aberrations occurring in GBC (Figure 2B). Frequently occurring copy number alterations included amplifications of the oncogenes CCNE1, CDK4, ERBB2, FRS2, KRAS, MDM2, and MYC (Table 1). Deletions were observed in CDKN2A and CDKN2B.
Table 1.
Copy number aberrations in gallbladder cancer.
| Gene | WA | N | Frequency | Methods | Histology | Population | Author | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CCNE1 | 11.0% | 82/760 | 11% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 4/24 | 17% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| CDK4 | 5.2% | 38/760 | 5% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 3/24 | 13% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| CDKN2A | 18.2% | 156/760 | 21% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 6/58 | 10% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 4/32 | 13% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 0/25 | 0% | targeted exome sequencing | N.A. | Korea | Chae | 2019 | [79] | ||
| 10/45 | 22% | real-time PCR | AC | Japan | Tadokoro | 2007 | [83] | ||
| CDKN2B | 10.0% | 135/760 | 18% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 6/58 | 10% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 3/32 | 9% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| ERBB2 | 7.1% | 77/760 | 10% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 1/24 | 4% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 1/4 | 25% | TS | AC | Korea | Yoo | 2016 | [37] | ||
| 9/111 | 8% | NGS | N.A. | America | Mondaca | 2019 | [38] | ||
| 9/50 | 18% | NGS | N.A. | India | Patel | 2020 | [39] | ||
| 3/32 | 9% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 4/25 | 16% | targeted exome sequencing | N.A. | Korea | Chae | 2019 | [79] | ||
| FRS2 | 7.3% | 55/760 | 7% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 2/24 | 8% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| KRAS | 14.1% | 35/760 | 5% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 2/24 | 8% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 10/60 | 17% | PCR + DS | AC | Taiwan | Huang | 2017 | [67] | ||
| 1/58 | 2% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 3/32 | 9% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 1/25 | 4% | targeted exome sequencing | N.A. | Korea | Chae | 2019 | [79] | ||
| MDM2 | 7.8% | 86/760 | 11% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 3/24 | 13% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 4/58 | 7% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 3/32 | 9% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| MYC | 6.8% | 51/760 | 7% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
| 2/24 | 8% | NGS | N.A. | America | Okamura | 2021 | [27] |
WA: weighted average (calculated by using the number of samples analyzed in a study as the weight); N.A.: not available; AC: adenocarcinoma; NGS: next-generation sequencing; PCR: polymerase chain reaction; DS: direct sequencing; TS: targeted sequencing.
3.4. Tumor Mutational Burden (TMB)
The reported frequencies of TMB ranged between 2.6 and 7.03 mutations/Megabase (Mb) in GBC samples (Table 2). Among 864 patients with known TMB status, 15 patients (1.7%) had high TMB (TMB-H). However, the threshold to define TMB-H varied between studies.
Table 2.
Tumor mutational burden in gallbladder cancer.
| Author | Origin | Histology | N | TMB (Mut/Mb [Range]) | TMB-H Definition | TMB-H | Ref. |
|---|---|---|---|---|---|---|---|
| Patel | India | N.A. | 43 | 5 (1–14) | - | - | [39] |
| Weinberg | America | N.A | 104 | - | ≥17 mut/Mb | 6/104 (5.8%) | [59] |
| Li | China | N.A. | 12 | 7.03 | - | - | [47] |
| Abdel-Wahab | America | N.A. | 760 | 2.6 (0–403) | ≥19.5 mut/Mb | 9/760 (1.2%) | [19] |
TMB: tumor mutational burden; TMB-H: high tumor mutational burden; mut: mutations; Mb: megabase.
3.5. Microsatellite Instability (MSI)
Table 3 outlines all studies (N = 13) reporting on MSI status in 1162 patients with GBC. This table shows that a high diversity of marker panels and MSI definitions were used. A broad range of MSI-high frequencies were reported, although most studies reported a low incidence. The weighted average for MSI-high frequency was 3.5%.
Table 3.
Microsatellite instability in gallbladder cancer.
| Author | Origin | Histology | MSI Markers | MSI Definition | MSI | Ref. |
|---|---|---|---|---|---|---|
| Nagai | Japan | N.A. | D2S97, D6S477, D8S339, D9S131, D10S197, D17S796, D18S36, TP53 (17p12), DCC (18q21), APC (5q21) |
Shifts in ≥30% of markers | 7/17 (41%) | [24] |
| Kim | Korea | N.A. | 3p12-22 (D3S1274, D3S4103, D3S1766) 5q11-23 (D5S107, D5S409, IRF1) 8p22-23 (D8S254, D8S261) 9p22 (IFNA, D9S126, D9S104) 13q13-14 (D13S118 and D13S133) 17p11-13 (D17S786, D17S796,TP53) 18q12-21 (D18S34) |
Shifts in ≥1 marker | 3/15 (20%) | [31] |
| Nagahashi | Japan, Hungary | AC | NCI: BAT-25, BAT-26, D2S123, D5S346, D17S250 | Shifts in ≥2 markers | 9/34 (27%) | [35] |
| Patel | India | N.A. | Genome-wide analysis of 95 loci | N.A. | 0/43 (0%) | [39] |
| Abdel-Wahab | U.S. | N.A. | 114 loci | Shifts in ≥2 markers | 3/551 (1%) | [19] |
| Wistuba | Chile | AC | 81 loci on 3p, 8p, 9q and 22q | Shifts in ≥1 marker | 6/12 (50%) | [43] |
| Pandey | Chile, Korea, India | AC | Exome-wide analysis | MSI score > 0.35 | 3/152 (2%) | [46] |
| Li | China | N.A. | NGS | N.A. | 0/12 (0%) | [47] |
| Rashid | China | AC | NCI (BAT-25, BAT-26, D2S123, D5S346, D17S250) and TGFβRII | Shifts in ≥40% of D2S123, D5S346, D17S25, or alteration of BAT-25, BAT-26 or TGFβRII | 2/64 (3%) | [49] |
| Goeppert | Germany | AC | BAT25, BAT26, and CAT25 | Shifts in ≥2 markers | 1/69 (1%) | [52] |
| Yoshida | Japan | AC | p53, APC, DCC, NM23-H1, D2S123, D3S1029, D5S107, D17S261, D18S34 | Shifts in ≥33% of markers | 0/30 (0%) | [54] |
| Roa | Chile | AC | NCI: BAT25, BAT26, D2S123, D5S346, D17S250 and BAT40, D3S1067, D3S1286, D3S1262, D3S1478, D12S1638, D12S347, D16S265 |
Shifts in >30% of markers | 6/59 (10%) | [55] |
| Weinberg | U.S. | N.A. | Targeted NGS over 7000 loci | N.A. | 1/104 (1%) | [59] |
MSI: microsatellite instability; N.A.: not available; AC: adenocarcinoma; NCI: National Cancer Institute recommendation; NGS: next-generation sequencing.
3.6. Possible Therapeutic Implications
3.6.1. Targetable Alterations in Other Malignancies
Currently, no FDA-approved therapies targeting gene mutations or copy number aberrations are available for GBC. However, several targeted therapies directed at actionable alterations that were also frequently observed in GBC are FDA-approved in other cancers (Table 4). These alterations include mutations in ATM, PIK3CA and ERBB2, and amplifications in ERBB2. For solid cancers with MSI-H or TMB-H, pembrolizumab is approved by the FDA.
Table 4.
FDA-approved drugs targeting genetic alterations in other malignancies.
| Target | Level | Malignancy | Agent | ORR | Ref. |
|---|---|---|---|---|---|
| ATM ¥ | 1 | Prostate cancer | Olaparib |
BRCA1, BRCA2, or ATM: 28/84 (33%) |
[84] |
| ERBB2 * | 1 | Esophagogastric cancer | Pembrolizumab + trastuzumab + chemotherapy | 32/35 (91%) | [85] |
| Trastuzumab + chemotherapy | 139/294 (47%) | [86] | |||
| Trastuzumab deruxtecan | 61/119 (51%) | [87] | |||
| ERBB2 * | 1 | Breast cancer | Ado-trastuzumab emtansine | 173/397 (44%) | [88] |
| Lapatinib + letrozole | 31/111 (28%) | [89] | |||
| Lapatinib + capecitabine | 36/163 (22%) | [90] | |||
| Margetuximab + chemotherapy | 67/266 (25%) | [91] | |||
| Neratinib | 34/117 (29%) | [92] | |||
| Trastuzumab | 30/114 (26%) | [93] | |||
| Trastuzumab + pertuzumab + chemotherapy | 275/343 (80%) | [94] | |||
| Trastuzumab + tucatinib + capecitabine | 138/340 (41%) | [95] | |||
| Trastuzumab deruxtecan | 112/184 (61%) | [96] | |||
| ERBB2 * | 2 | Colorectal cancer | Lapatinib + trastuzumab | 9/32 (28%) | [97] |
| Trastuzumab + pertuzumab | 18/57 (32%) | [98] | |||
| Trastuzumab deruxtecan | 24/53 (45%) | [99] | |||
| ERBB2 * | 2 | Uterine serous carcinoma |
Trastuzumab + carboplatin-taxol | 4/9 (44%) | [100] |
| ERBB2 ¥ | 2 | NSCLC | Ado-trastuzumab emtansine | 8/18 (44%) | [101] |
| Trastuzumab deruxtecan | 50/91 (55%) | [102] | |||
| PIK3CA ¥ | 1 | Breast cancer | Alpelisib + fulvestrant | 21/121 (17% | [103] |
| TMB-H | 1 | Solid tumors | Pembrolizumab | 30/102 (29%) | [104] |
| MSI-H | 1 | Solid tumors | Pembrolizumab | 59/149 (40%) | [105] |
| MSI-H | 1 | Colorectal cancer | Nivolumab | 23/74 (31%) | [106] |
| Ipilumab + nivolumab | 65/119 (55%) | [107] |
¥: mutation; *: amplification. ORR: objective response rate; NSCLC: non-small cell lung cancer; MSI-H: high microsatellite instability; TMB-H: high tumor mutational burden.
3.6.2. Clinical Trials
Table 5 presents all clinical trials that include GBC patients harboring frequently occurring genetic alterations. No completed studies were identified; all studies are currently recruiting participants.
Table 5.
Ongoing clinical trials targeting genetic alterations in GBC.
| Target | Phase | Agent | Country | Trial ID |
|---|---|---|---|---|
|
ERBB2 signal pathway components |
2 | FORFIRINOX + (cetuximab, trastuzumab, gefitinib, lapatinib, everolimus, sorafenib, or crizotinib) |
China | NCT03768375 |
|
ERBB2 signal pathway components |
2 | GEMOX + afatinib | China | NCT04183712 |
|
ERBB2 overexpression/ amplification |
2 | Trastuzumab + pertuzumab | U.S. | NCT02091141 |
|
ERBB2 overexpression/ amplification |
1,2 | Tucatinib + trastuzumab + (FOLFOX or CAPOX) |
U.S. | NCT04430738 |
|
ERBB2 overexpression/ amplification or mutations |
2, basket | Tucatinib + trastuzumab | U.S., Japan, Belgium | NCT04579380 |
| ERBB2 amplification | 2 | Zanidatamab | U.S., Canada, Chile, China, France, Italy, Korea, Spain, U.K. | NCT04466891 |
| KRAS (or NRAS) mutation | 1 | ELI-002 immunotherapy | U.S. | NCT04853017 |
| DNA repair gene mutations (including ARID1A, ATM, and others) | 2 | Olaparib | U.S. | NCT04042831 |
| TMB ≥ 10 mutations/Mb | 2 | Atezolizumab | U.S. | NCT02091141 |
U.S.: United States; U.K.: United Kingdom; TMB: tumor mutational burden.
Most studies evaluate targeted therapies directed at ERBB2 alterations and related signal pathway components. One trial assesses the safety and efficacy of ELI-002 immunotherapy, a novel amphiphile therapeutic vaccine targeting KRAS-driven cancers, for patients with KRAS or NRAS mutations (G12D or G12R) in various solid tumor types including GBC (NCT04853017). Moreover, a phase 2 trial analyzes the efficacy and toxicity of olaparib in patients with metastatic BTC with an DNA repair gene mutation, including ARID1A and ATM (NCT04042831). The NCT02091141 trial evaluates six treatment regimens based on molecular testing in patients with advanced solid tumors, including BTC. For example, patients with tumors demonstrating elevated TMB (≥10 mutations/Mb) will receive atezolizumab.
4. Discussion
In this systematic review of 62 articles assessing genetic alterations in GBC, 3893 GBC samples were analyzed. Frequently occurring genetic alterations included mutations in 17 genes and amplifications/deletions in nine genes. Since no targeted therapy is currently available for GBC, frequent genetic alterations detected in this study were matched to actionable genetic alterations in other solid tumors. Only mutations in ATM, ERBB2 and PIK3CA and amplifications in ERBB2 are currently targetable with FDA-approved drugs in other solid tumors. Therefore, these alterations are potential targets in GBC and might be included in future molecular testing panels for personalized treatment decisions.
In GBC, the prevalence of TMB-H was low. Previous studies have shown that TMB-H is correlated with clinical response to immunotherapy in several tumors, including BTC, and that patients with TMB-H tumors can benefit from immune checkpoint inhibitors such as pembrolizumab [108,109,110]. In patients with GBC receiving immunotherapy, a higher objective response rate was observed for TMB-high tumors compared to TMB-low tumors (50% vs. 25%) [47]. Pembrolizumab has been FDA-approved for patients with TMB-H advanced solid cancers.
Another biomarker associated with response to immune checkpoint inhibitors is MSI, which was observed in 3.5% of patients in the present study. For patients with MSI-H cancers, pembrolizumab is also approved by the FDA, as well as by the Pharmaceuticals and Medical Devices Agency (PMDA, Japan). The European Medicines Agency (EMA, European Union) and the National Medical Products Administration (NMPA, China) have not yet given approval for this agent in patients with MSI-H (or TMB-H). In patients with cholangiocarcinoma who received pembrolizumab, an objective response rate of 41% was seen [111]. Therefore, although only beneficial in a small proportion of patients, both TMB-H and MSI-H are interesting biomarkers in precision medicine for GBC.
A broad range was observed in genetic alteration frequencies in GBC across all studies. In part, this could be attributed to differences in sequencing technologies and assessed gene regions. For example, NGS has a slightly lower sensitivity for mutation detection compared to some other technologies like real-time PCR testing, although detecting low-frequency genetic alterations through (ultra-) deep sequencing with a higher coverage can partly overcome this issue [112,113,114]. Moreover, geographical differences in mutation patterns might be present, as previous studies have shown [35,46,73]. However, no one-to-one regional comparisons of genetic alteration frequencies could be made due to different sequencing techniques used throughout all studies. Substantial differences were also observed regarding the frequency of MSI. A large variation in marker panels and MSI definitions, and some missing MSI definitions, complicated comparison of all MSI frequencies. Although a weighted average of MSI frequency was calculated to minimalize the influence of sample size on the average frequency, our results should be taken with caution due to the heterogeneity in MSI panels and definitions. Usage of the National Cancer Institute (NCI) panel of microsatellites and definitions in future research would facilitate MSI comparison among studies [115].
Since some genetic alterations might impede the efficacy of therapies targeting other genetic alterations, co-existing alterations might be an important determinant for therapeutic sensitivity and resistance. For example, patients with solid stage IV cancer types harboring amplification in the MDM2 family or EGFR aberrations who received anti-PD1/PDL1 immunotherapy showed increased tumor growth [116]. Unfortunately, co-existing alterations could not be assessed in this study due to differences in sequencing techniques and missing data in some studies.
Only nine clinical trials which evaluated targeted therapies directed at frequently occurring genetic alterations in GBC were identified. Though we assessed only trials targeting these genetic alterations specifically, these limited results underline that GBC remains a relatively under-investigated cancer type regarding therapeutic agents. Unfortunately, initiation of clinical trials including patients with GBC is logistically challenging since GBC is a rare cancer type in most Western countries. The large inter-tumor heterogeneity of GBC poses another challenge for targeted therapies. However, basket trials, a clinical trial that tests agents in different cancer types with the same genetic alteration, could provide a solution to this challenge in future studies.
This study has several limitations. First, histological types and subtypes of GBC samples could not always be retrieved. It is important to report histological (sub)types to be able to determine whether differences in genetic alterations are associated with different histology. Second, a publication bias might exist. Therefore, actual overall alteration frequencies might be over- or underestimated.
5. Conclusions
In conclusion, GBC has a diverse mutational landscape. Frequently occurring genetic alterations in GBC that are actionable in other solid tumors are rare, including mutations in ATM, ERBB2, PIK3CA and amplifications in ERBB2. For all solid tumors with MSI-high or TMB-high, including GBC, the immune checkpoint inhibitor pembrolizumab is FDA-approved. Few clinical trials targeting the frequently altered genes in GBC are performed, emphasizing the need for multicenter clinical basket trials.
Appendix A
Table A1.
Keywords and subject headings used in the literature search.
| Database | |||
|---|---|---|---|
| PubMed | EMBASE | Cochrane Reviews | Web of Science |
| (“Gallbladder Neoplasms”[Mesh] OR (gallbladder[tiab] AND (neoplasm * [tiab] OR cancer * [tiab] OR lesion * [tiab] OR tumor* [tiab] OR tumour * [tiab] OR carcinom * [tiab] OR malignan * [tiab]))) AND (“Mutation”[Mesh] OR “Epigenesis, Genetic”[Mesh] OR “Genome”[Mesh] OR molecular[tiab] OR gene[tiab] OR genes[tiab] OR genetic * [tiab] OR genom * [tiab] OR sequenc * [tiab] OR mutation*[tiab] OR exome * [tiab]) | (‘gallbladder cancer’/exp OR (gallbladder AND (neoplasm * OR cancer* OR tumor * OR tumour * OR carcinom * OR malignan *)):ab,ti,kw) AND (‘gene mutation’/exp OR ‘gene sequence’/exp OR (exome * OR molecular OR gene OR genes OR genetic * OR genom * OR sequenc * OR mutation *):ab,ti,kw) | (gallbladder AND (neoplasm * OR cancer * OR tumor * OR tumour * OR carcinom * OR malignan *)) AND (molecular OR gene OR genes OR genetic * OR genom * OR sequenc * OR mutation * OR exome *) | (gallbladder AND (neoplasm * OR cancer * OR tumor * OR tumour * OR carcinom * OR malignan *)) AND (exome * OR molecular OR gene OR genes OR genetic * OR genom * OR sequenc * OR mutation *) |
*: show keywords in a search strategy.
Table A2.
Studies analyzing frequently mutated genes in gallbladder cancer.
| Gene | WA | N | Frequency | Methods | Histology | Population | Author | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ARID1A | 14.3% | 1/5 | 20% | WES | AC | Japan | Akita | 2019 | [48] |
| 2/14 | 14% | TS | N.A. | China | Li | 2017 | [44] | ||
| 2/47 | 4% | WES | AC | China | Yang | 2021 | [56] | ||
| 2/25 | 8% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 3/24 | 13% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 3/26 | 12% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 4/54 | 7% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 4/39 | 10% | WES/WGS | N.A. | Japan | Ebata | 2021 | [57] | ||
| 15/144 | 10% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 7/58 | 12% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 8/32 | 25% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 10/55 | 18% | NGS | N.A. | America | Javle | 2016 | [23] | ||
| 123/760 | 16% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| ARID2 | 8.8% | 1/32 | 3% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] |
| 1/17 | 6% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 1/14 | 7% | TS | N.A. | China | Li | 2017 | [44] | ||
| 1/24 | 4% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 3/25 | 12% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 3/54 | 6% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 3/39 | 8% | WES/WGS | N.A. | Japan | Ebata | 2021 | [57] | ||
| 19/144 | 13% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 2/12 | 17% | NGS | N.A. | China | Li | 2020 | [47] | ||
| 7/58 | 12% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 8/47 | 17% | WES | AC | China | Yang | 2021 | [56] | ||
| 61/760 | 8% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| ATM | 6.3% | 0/4 | 0% | TS | AC | Korea | Yoo | 2016 | [37] |
| 1/14 | 7% | NGS | N.A. | Japan | Noguchi | 2017 | [32] | ||
| 1/58 | 2% | NGS | AC | America | Maynard | 2020 | [41] | ||
| 2/25 | 8% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 5/32 | 16% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 3/17 | 18% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 4/58 | 7% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 45/760 | 6% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| CDKN2A | 8.5% | 0/24 | 0% | NGS | N.A. | America | Okamura | 2021 | [27] |
| 0/5 | 0% | WES | AC | Japan | Akita | 2019 | [48] | ||
| 0/4 | 0% | TS | AC | Korea | Yoo | 2016 | [37] | ||
| 1/11 | 9% | ultra-deep targeted NGS | N.A. | India | Yadav | 2017 | [77] | ||
| 1/26 | 4% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 1/14 | 7% | NGS | N.A. | Japan | Noguchi | 2017 | [32] | ||
| 13/144 | 9% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 1/12 | 8% | NGS | N.A. | China | Li | 2020 | [47] | ||
| 2/25 | 8% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 2/32 | 6% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 2/14 | 14% | TS | N.A. | China | Li | 2017 | [44] | ||
| 4/13 | 31% | PCR-SSCP + DS | N.A. | Korea | Kim | 2001 | [31] | ||
| 6/58 | 10% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 64/760 | 8% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| CTNNB1 | 6.4% | 0/21 | 0% | SNaPshot | AC | America | Moy | 2015 | [53] |
| 0/68 | 0% | WES + Sanger seq | AC | Japan | Akita | 2019 | [48] | ||
| 0/26 | 0% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 0/4 | 0% | TS | AC | Korea | Yoo | 2016 | [37] | ||
| 0/14 | 0% | NGS | N.A. | Japan | Noguchi | 2017 | [32] | ||
| 0/58 | 0% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 1/25 | 4% | SNaPshot | N.A. | America | Borger | 2012 | [30] | ||
| 2/46 | 4% | mass array + seq | AC | India | Kumari | 2014 | [68] | ||
| 2/14 | 14% | TS | N.A. | China | Li | 2017 | [44] | ||
| 2/17 | 12% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 18/144 | 13% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 9/50 | 18% | PCR-SSCP + seq | AC | India | Dixit | 2020 | [36] | ||
| 19/47 | 40% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 30/760 | 4% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| ELF3 | 18.6% | 29/144 | 20% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] |
| 5/39 | 13% | WES/WGS | N.A. | Japan | Ebata | 2021 | [57] | ||
| ERBB2 | 6.7% | 0/21 | 0% | SNaPshot | AC | America | Moy | 2015 | [53] |
| 0/46 | 0% | mass array | AC | India | Kumari | 2014 | [68] | ||
| 0/4 | 0% | TS | AC | Korea | Yoo | 2016 | [37] | ||
| 0/58 | 0% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 1/26 | 4% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 2/32 | 6% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 1/14 | 7% | TS | N.A. | China | Li | 2017 | [44] | ||
| 2/54 | 4% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 2/5 | 40% | WES | AC | Japan | Akita | 2019 | [48] | ||
| 2/25 | 8% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 3/50 | 6% | NGS | N.A. | India | Patel | 2020 | [39] | ||
| 3/17 | 18% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 3/24 | 13% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 3/39 | 8% | WES/WGS | N.A. | Japan | Ebata | 2021 | [57] | ||
| 4/47 | 9% | WES | AC | China | Yang | 2021 | [56] | ||
| 22/144 | 15% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 6/111 | 5% | NGS | N.A. | America | Mondaca | 2019 | [38] | ||
| 5/12 | 42% | NGS | N.A. | China | Li | 2020 | [47] | ||
| 11/157 | 7% | WES | N.A. | China | Li | 2019 | [18] | ||
| 40/760 | 5% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| ERBB3 | 5.4% | 2/5 | 40% | WES | AC | Japan | Akita | 2019 | [48] |
| 1/11 | 9% | ultra-deep targeted NGS | N.A. | India | Yadav | 2017 | [77] | ||
| 1/17 | 6% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 0/24 | 0% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 0/32 | 0% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 3/39 | 8% | WES/WGS | N.A. | Japan | Ebata | 2021 | [57] | ||
| 4/47 | 9% | WES | AC | China | Yang | 2021 | [56] | ||
| 3/50 | 6% | NGS | N.A. | India | Patel | 2020 | [39] | ||
| 3/54 | 6% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 0/58 | 0% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 17/144 | 12% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 12/157 | 8% | WES | N.A. | China | Li | 2019 | [18] | ||
| 30/760 | 4% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| KMT2D | 7.7% | 0/58 | 0% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] |
| 1/17 | 6% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 1/14 | 7% | TS | N.A. | China | Li | 2017 | [44] | ||
| 5/32 | 16% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 5/54 | 9% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 5/47 | 11% | WES | AC | China | Yang | 2021 | [56] | ||
| KRAS | 10.3% | 1/42 | 2% | nested PCR, PCR-RFLP + DS | AC | Japan, Hungary | Nagahashi | 2008 | [35] |
| 0/27 | 0% | Oncomap | AC | America | Deshpande | 2011 | [66] | ||
| 0/29 | 0% | nested PCR, PCR-RFLP + DS |
AC | Peru | Vidaurre | 2019 | [82] | ||
| 1/35 | 3% | PCR + seq | AC | Bolivia | Asai | 2014 | [42] | ||
| 1/25 | 4% | SNaPshot | N.A. | America | Borger | 2012 | [30] | ||
| 1/46 | 2% | mass array+ seq | AC | India | Kumari | 2014 | [68] | ||
| 1/4 | 25% | TS | AC | Korea | Yoo | 2016 | [37] | ||
| 1/24 | 4% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 1/14 | 7% | TS | N.A. | China | Li | 2017 | [44] | ||
| 2/9 | 22% | PCR + seq | AC | Japan | Shibata | 2008 | [33] | ||
| 2/64 | 3% | Seq | AC | China | Rashid | 2002 | [49] | ||
| 2/29 | 7% | PCR | AC | America | Pai | 2011 | [62] | ||
| 2/60 | 3% | PCR + DS | AC | Taiwan | Huang | 2017 | [67] | ||
| 2/14 | 14% | NGS | N.A. | Japan | Noguchi | 2017 | [32] | ||
| 2/21 | 10% | SNaPshot | AC | America | Moy | 2015 | [53] | ||
| 2/12 | 17% | NGS | N.A. | China | Li | 2020 | [47] | ||
| 2/32 | 6% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 3/15 | 20% | PCR-RFLP + DS | N.A. | Korea | Kim | 2001 | [31] | ||
| 4/35 | 11% | DS | N.A. | Taiwan | Chang | 2013 | [25] | ||
| 4/34 | 12% | PCR | N.A. | Korea | Kim | 2015 | [60] | ||
| 4/68 | 6% | WES and Sanger seq | AC | Japan | Akita | 2019 | [48] | ||
| 6/144 | 4% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 4/58 | 7% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 5/55 | 9% | NGS | N.A. | America | Javle | 2016 | [23] | ||
| 5/26 | 19% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 5/25 | 20% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 5/157 | 3% | WES | N.A. | China | Li | 2019 | [18] | ||
| 6/54 | 11% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 8/21 | 38% | PCR-RFLP | AC | India | Singh | 2004 | [63] | ||
| 8/34 | 24% | Seq | AC | India | Sharma | 2017 | [65] | ||
| 9/81 | 11% | PCR | N.A. | Japan | Tomioka | 2019 | [45] | ||
| 10/17 | 59% | PCR | N.A. | Japan | Nagai | 2002 | [24] | ||
| 10/20 | 50% | PCR + RFLP and DS | N.A. | Korea | Kim | 2000 | [50] | ||
| 12/25 | 48% | PCR-RFLP | AC | India | Shukla | 2020 | [34] | ||
| 16/39 | 41% | PCR-RFLP | N.A. | India | Kazmi | 2013 | [71] | ||
| 72/760 | 9% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| PIK3CA | 10.0% | 0/34 | 0% | PCR | N.A. | Korea | Kim | 2015 | [60] |
| 0/58 | 0% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 1/23 | 4% | PCR+ seq | N.A. | Switzerland | Riener | 2008 | [72] | ||
| 1/68 | 1% | WES + Sanger seq | AC | Japan | Akita | 2019 | [48] | ||
| 1/14 | 7% | TS | N.A. | China | Li | 2017 | [44] | ||
| 1/25 | 4% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 2/46 | 4% | mass array + seq | AC | India | Kumari | 2014 | [68] | ||
| 2/4 | 50% | TS | AC | Korea | Yoo | 2016 | [37] | ||
| 2/21 | 10% | SNaPshot | AC | America | Moy | 2015 | [53] | ||
| 2/24 | 8% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 2/26 | 8% | NGS | N.A | Italy | Simbolo | 2014 | [61] | ||
| 3/27 | 11% | Oncomap | AC | America | Deshpande | 2011 | [66] | ||
| 3/25 | 12% | SNaPshot | N.A. | America | Borger | 2012 | [30] | ||
| 3/14 | 21% | NGS | N.A. | Japan | Noguchi | 2017 | [32] | ||
| 3/21 | 14% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 11/144 | 8% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 2/12 | 17% | NGS | N.A. | China | Li | 2020 | [47] | ||
| 5/47 | 11% | WES | AC | China | Yang | 2021 | [56] | ||
| 6/157 | 4% | WES | N.A. | China | Li | 2019 | [18] | ||
| 7/55 | 13% | NGS | N.A. | America | Javle | 2016 | [23] | ||
| 7/34 | 21% | Seq | AC | India | Sharma | 2017 | [65] | ||
| 8/130 | 6% | TS | N.A. | China | Zhao | 2016 | [78] | ||
| 10/54 | 19% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 102/760 | 13% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| SHH | 20.0% | 10/50 | 20% | PCR + SSCP + seq | AC | India | Dixit | 2017 | [26] |
| SMAD4 | 13.1% | 0/4 | 0% | TS | AC | Korea | Yoo | 2016 | [37] |
| 1/17 | 6% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 1/5 | 20% | WES | AC | Japan | Akita | 2019 | [48] | ||
| 2/12 | 17% | NGS | N.A. | China | Li | 2020 | [47] | ||
| 2/26 | 8% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 2/14 | 14% | NGS | N.A. | Japan | Noguchi | 2017 | [32] | ||
| 3/24 | 13% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 11/144 | 8% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 3/11 | 27% | ultra-deep targeted NGS | N.A. | India | Yadav | 2017 | [77] | ||
| 4/25 | 16% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 10/32 | 33% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 9/55 | 16% | NGS | N.A. | America | Javle | 2016 | [23] | ||
| 16/58 | 28% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 92/760 | 12% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| SRCAP | 13.0% | 7/54 | 13% | NGS | AC | Greece | Papadopoulou | 2018 | [40] |
| STK11 | 7.2% | 0/68 | 0% | WES and Sanger seq | AC | Japan | Akita | 2019 | [48] |
| 1/24 | 4% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 1/26 | 4% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 10/144 | 7% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 3/39 | 8% | WES/WGS | N.A. | Japan | Ebata | 2021 | [57] | ||
| 4/58 | 7% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 64/760 | 8% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] | ||
| SYNE1 | 6.2% | 3/54 | 6% | NGS | AC | Greece | Papadopoulou | 2018 | [40] |
| 1/11 | 9% | ultra-deep targetedNGS | N.A. | India | Yadav | 2017 | [77] | ||
| TP53 | 57.0% | 1/21 | 5% | SNaPshot | AC | America | Moy | 2015 | [53] |
| 1/25 | 4% | SNaPshot | N.A. | America | Borger | 2012 | [30] | ||
| 2/4 | 50% | TS | AC | Korea | Yoo | 2016 | [37] | ||
| 2/11 | 18% | ultra-deep targeted NGS | N.A. | India | Yadav | 2017 | [77] | ||
| 3/5 | 60% | WES | AC | Japan | Akita | 2019 | [48] | ||
| 4/46 | 9% | mass array + seq | AC | India | Kumari | 2014 | [34] | ||
| 5/14 | 36% | PCR-SSCP + DS | N.A. | Korea | Kim | 2001 | [31] | ||
| 6/17 | 36% | PCR-SSCP + seq | N.A. | Japan | Nagai | 2002 | [24] | ||
| 6/17 | 36% | WES | N.A. | India | Iyer | 2019 | [29] | ||
| 7/14 | 50% | TS | N.A. | China | Li | 2017 | [44] | ||
| 7/29 | 24% | nested PCR, PCR-RFLP + DS |
AC | Peru | Vidaurre | 2019 | [82] | ||
| 8/12 | 67% | NGS | N.A. | China | Li | 2020 | [47] | ||
| 9/14 | 64% | NGS | N.A. | Japan | Noguchi | 2017 | [32] | ||
| 17/40 | 43% | nested PCR +DS | AC | Japan, Hungary | Nagahashi | 2008 | [35] | ||
| 11/25 | 44% | PCR-SSCP | AC | India | Shukla | 2020 | [34] | ||
| 11/24 | 46% | NGS | N.A. | America | Okamura | 2021 | [27] | ||
| 12/26 | 46% | NGS | N.A. | Italy | Simbolo | 2014 | [61] | ||
| 1535 | 43% | PCR + seq | AC | Bolivia | Asai | 2014 | [42] | ||
| 16/25 | 64% | targeted exome sequencing |
N.A. | Korea | Chae | 2019 | [79] | ||
| 22/32 | 69% | targeted NGS | AC | Chile, Japan | Narayan | 2019 | [73] | ||
| 18/59 | 31% | Seq | AC | Austria | Puhalla | 2004 | [69] | ||
| 72/144 | 50% | WES | AC | India, Korea, Chile | Pandey | 2020 | [46] | ||
| 19/39 | 49% | WES/WGS | N.A. | Japan | Ebata | 2021 | [57] | ||
| 20/30 | 67% | PCR + DS | AC | Chile | Moreno | 2005 | [81] | ||
| 23/54 | 43% | NGS | AC | Greece | Papadopoulou | 2018 | [40] | ||
| 32/55 | 58% | NGS | N.A. | America | Javle | 2016 | [23] | ||
| 42/58 | 72% | ultra-deep targeted NGS | AC | China | Lin | 2019 | [70] | ||
| 541/760 | 71% | NGS | N.A. | America | Abdel-Wahab | 2020 | [19] |
WA: weighted average (calculated by using the number of samples analyzed in a study as the weight); N.A.: not available; AC: adenocarcinoma; NGS: next-generation sequencing; PCR: polymerase chain reaction; Seq: sequencing; DS: direct sequencing; WES: whole-exome sequencing; WGS: whole-genome sequencing; SSCP: single-strand conformation polymorphism; RFLP: restriction fragment length polymorphism; TS: targeted sequencing.
Author Contributions
Conceptualization, H.K., M.T.d.B., F.J.H.H., M.W.N., T.J.J.d.B., R.S.v.d.P., R.S.N.F. and P.R.d.R.; methodology, H.K., M.T.d.B., F.J.H.H., M.W.N., T.J.J.d.B. and R.S.N.F.; software, H.K.; formal analysis, H.K.; investigation, H.K. and T.J.J.d.B.; resources, H.K. and T.J.J.d.B.; data curation, H.K.; writing—original draft preparation, H.K.; writing—review and editing, H.K., M.T.d.B., F.J.H.H., M.W.N., T.J.J.d.B., R.S.v.d.P., R.S.N.F. and P.R.d.R.; supervision, M.T.d.B., R.S.N.F., F.J.H.H.; project administration, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stinton L.M., Shaffer E.A. Epidemiology of gallbladder disease: Cholelithiasis and cancer. Gut Liver. 2012;6:172–187. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazcano-Ponce E.C., Miquel J.F., Munoz N., Herrero R., Ferrecio C., Wistuba I.I., Alonso de Ruiz P., Aristi Urista G., Nervi F. Epidemiology and Molecular Pathology of Gallbladder Cancer. CA Cancer J. Clin. 2001;51:349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 3.Are C., Ahmad H., Ravipati A., Croo D., Clarey D., Smith L., Price R.R., Butte J.M., Gupta S., Chaturvedi A., et al. Global epidemiological trends and variations in the burden of gallbladder cancer. J. Surg. Oncol. 2017;115:580–590. doi: 10.1002/jso.24546. [DOI] [PubMed] [Google Scholar]
- 4.Larsson S.C., Wolk A. Obesity and the risk of gallbladder cancer: A meta-analysis. Br. J. Cancer. 2007;96:1457–1461. doi: 10.1038/sj.bjc.6603703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A., Sharma K.L., Gupta A., Yadav A., Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J. Gastroenterol. 2017;23:3978–3998. doi: 10.3748/wjg.v23.i22.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell P.T., Newton C.C., Kitahara C.M., Patel A.V., Hartge P., Koshiol J., McGlynn K.A., Adami H.O., De Gonzalez A.B., Freeman L.E.B., et al. Body size indicators and risk of gallbladder cancer: Pooled analysis of individual-level data from 19 prospective cohort studies. Cancer Epidemiol. Biomarkers Prev. 2017;26:597–606. doi: 10.1158/1055-9965.EPI-16-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aloia T.A., Járufe N., Javle M., Maithel S.K., Roa J.C., Adsay V., Coimbra F.J.F., Jarnagin W.R. Gallbladder Cancer: Expert consensus statement. HPB. 2015;17:681–690. doi: 10.1111/hpb.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra S., Chaturvedi A., Misra N.C., Sharma I.D. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. doi: 10.1016/S1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 9.Duffy A., Capanu M., Abou-Alfa G.K., Huitzil D., Jarnagin W., Fong Y., D’Angelica M., Dematteo R.P., Blumgart L.H., O’Reilly E.M. Gallbladder cancer (GBC): 10-Year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) J. Surg. Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 10.Lau C.S.M., Zywot A., Mahendraraj K., Chamberlain R.S. Gallbladder Carcinoma in the United States: A Population Based Clinical Outcomes Study Involving 22,343 Patients from the Surveillance, Epidemiology, and End Result Database (1973–2013) HPB Surg. 2017;2017:1532835. doi: 10.1155/2017/1532835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohman E.D.S., De Bitter T., Verhoeven R., Van Der Geest L., Hagendoorn J., Mohammad N.H., Daams F., Klümpen H.-J., Van Gulik T., Erdmann J., et al. Trends in Treatment and Survival of Gallbladder Cancer in the Netherlands; Identifying Gaps and Opportunities from a Nation-Wide Cohort. Cancers. 2020;12:918. doi: 10.3390/cancers12040918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valle J., Wasan H., Palmer D.H., Cunningham D., Anthoney A., Maraveyas A., Madhusudan S., Iveson T., Hughes S., Pereira S.P., et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 13.Montalvo-Jave E.E., Rahnemai- Azar A.A., Papaconstantinou D., Deloiza M.E., Tsilimigras D.I., Moris D., Mendoza-Barrera G.E., Weber S.M., Pawlik T.M. Molecular pathways and potential biomarkers in gallbladder cancer: A comprehensive review. Surg. Oncol. 2019;31:83–89. doi: 10.1016/j.suronc.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 14.De Lorenzo S., Garajova I., Stefanini B., Tovoli F. Targeted therapies for gallbladder cancer: An overview of agents in preclinical and clinical development. Expert Opin. Investig. Drugs. 2021;30:759–772. doi: 10.1080/13543784.2021.1928636. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:332–336. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel S., Mukherjee S., Ammannagari N., Pokuri V.K., Kuvshinoff B., Groman A., LeVea C.M., Iyer R. Clinicopathological characteristics and outcomes of rare histologic subtypes of gallbladder cancer over two decades: A population-based study. PLoS ONE. 2018;13:e0198809. doi: 10.1371/journal.pone.0198809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu C., Wang S., Guan Q., Ren X., Ji B., Liu Y. Neuroendocrine tumors of the gallbladder (Review) Oncol. Lett. 2020;19:3381–3388. doi: 10.3892/ol.2020.11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M., Liu F., Zhang F., Zhou W., Jiang X., Yang Y., Qu K., Wang Y., Ma Q., Wang T., et al. Genomic ERBB2 / ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: A whole-exome sequencing analysis. Gut. 2019;68:1024–1033. doi: 10.1136/gutjnl-2018-316039. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Wahab R., Yap T.A., Madison R., Pant S., Cooke M., Wang K., Zhao H., Bekaii-Saab T., Karatas E., Kwong L.N., et al. Genomic profiling reveals high frequency of DNA repair genetic aberrations in gallbladder cancer. Sci. Rep. 2020;10:22087. doi: 10.1038/s41598-020-77939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roos E., Soer E.C., Klompmaker S., Meijer L.L., Besselink M.G., Giovannetti E., Heger M., Kazemier G., Klümpen H.J., Takkenberg R.B., et al. Crossing borders: A systematic review with quantitative analysis of genetic mutations of carcinomas of the biliary tract. Crit. Rev. Oncol. Hematol. 2019;140:8–16. doi: 10.1016/j.critrevonc.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Sohani Z.N., Sarma S., Alyass A., De Souza R.J., Robiou-Du-Pont S., Li A., Mayhew A., Yazdi F., Reddon H., Lamri A., et al. Empirical evaluation of the Q-Genie tool: A protocol for assessment of effectiveness. BMJ Open. 2016;6:e010403. doi: 10.1136/bmjopen-2015-010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarty D., Gao J., Phillips S.M., Kundra R., Zhang H., Wang J., Rudolph J.E., Yaeger R., Soumerai T., Nissan M.H., et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017;1:1–16. doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javle M., Bekaii-Saab T., Jain A., Wang Y., Kelley R.K., Wang K., Kang H.C., Catenacci D., Ali S., Krishnan S., et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer. 2016;122:3838–3847. doi: 10.1002/cncr.30254. [DOI] [PubMed] [Google Scholar]
- 24.Nagai M., Watanabe M., Iwase T., Yamao K., Isaji S. Clinical and genetic analysis of noncancerous and cancerous biliary epithelium in patients with pancreaticobiliary maljunction. World J. Surg. 2002;26:91–98. doi: 10.1007/s00268-001-0187-0. [DOI] [PubMed] [Google Scholar]
- 25.Chang Y.T., Chang M.C., Huang K.W., Tung C.C., Hsu C., Wong J.M. Clinicopathological and prognostic significances of EGFR, KRAS and BRAF mutations in biliary tract carcinomas in Taiwan. J. Gastroenterol. Hepatol. 2014;29:1119–1125. doi: 10.1111/jgh.12505. [DOI] [PubMed] [Google Scholar]
- 26.Dixit R., Pandey M., Tripathi S.K., Dwivedi A.N.D., Shukla V.K. Comparative Analysis of Mutational Profile of Sonic hedgehog Gene in Gallbladder Cancer. Dig. Dis. Sci. 2017;62:708–714. doi: 10.1007/s10620-016-4438-1. [DOI] [PubMed] [Google Scholar]
- 27.Okamura R., Kurzrock R., Mallory R.J., Fanta P.T., Burgoyne A.M., Clary B.M., Kato S., Sicklick J.K. Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int. J. Cancer. 2021;148:702–712. doi: 10.1002/ijc.33230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali A., Mishra P.K., Sharma S., Arora A., Saluja S.S. Effects of PTEN gene alteration in patients with gallbladder cancer. Cancer Genet. 2015;208:587–594. doi: 10.1016/j.cancergen.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Iyer P., Shrikhande S.V., Ranjan M., Joshi A., Gardi N., Prasad R., Dharavath B., Thorat R., Salunkhe S., Sahoo B., et al. ERBB2 and KRAS alterations mediate response to EGFR inhibitors in early stage gallbladder cancer. Int. J. Cancer. 2019;144:2008–2019. doi: 10.1002/ijc.31916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borger D.R., Tanabe K.K., Fan K.C., Lopez H.U., Fantin V.R., Straley K.S., Schenkein D.P., Hezel A.F., Ancukiewicz M., Liebman H.M., et al. Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified Through Broad-Based Tumor Genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y.T., Kim J., Jang Y.H., Lee W.J., Ryu J.K., Park Y.K., Kim S.W., Kim W.H., Yoon Y.B., Kim C.Y. Genetic alterations in gallbladder adenoma, dysplasia and carcinoma. Cancer Lett. 2001;169:59–68. doi: 10.1016/S0304-3835(01)00562-6. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi R., Yamaguchi K., Ikenoue T., Terakado Y., Ohta Y., Yamashita N., Kainuma O., Yokoi S., Maru Y., Nagase H., et al. Genetic alterations in Japanese extrahepatic biliary tract cancer. Oncol. Lett. 2017;14:877–884. doi: 10.3892/ol.2017.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata T., Kokubu A., Gotoh M., Ojima H., Ohta T., Yamamoto M., Hirohashi S. Genetic Alteration of Keap1 Confers Constitutive Nrf2 Activation and Resistance to Chemotherapy in Gallbladder Cancer. Gastroenterology. 2008;135:1358–1368. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 34.Shukla S.K., Singh G., Shahi K.S., Pant P. Genetic Changes of P 53 and Kras in Gallbladder Carcinoma in Kumaon Region of Uttarakhand. J. Gastrointest. Cancer. 2020;51:552–559. doi: 10.1007/s12029-019-00283-0. [DOI] [PubMed] [Google Scholar]
- 35.Nagahashi M., Ajioka Y., Lang I., Szentirmay Z., Kasler M., Nakadaira H., Yokoyama N., Watanabe G., Nishikura K., Wakai T., et al. Genetic changes of p53, K-ras, and microsatellite instability in gallbladder carcinoma in high-incidence areas of Japan and Hungary. World J. Gastroenterol. 2008;14:70–75. doi: 10.3748/wjg.14.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixit R., Pandey M., Tripathi S.K., Dwivedi A.N.D., Shukla V.K. Genetic mutational analysis of β-catenin gene affecting GSK-3β phosphorylation plays a role in gallbladder carcinogenesis: Results from a case control study. Cancer Treat. Res. Commun. 2020;23:100173. doi: 10.1016/j.ctarc.2020.100173. [DOI] [PubMed] [Google Scholar]
- 37.Yoo K.H., Kim N.K.D., Kwon W.I., Lee C., Kim S.Y., Jang J., Ahn J., Kang M., Jang H., Kim S.T., et al. Genomic alterations in biliary tract cancer using targeted sequencing. Transl. Oncol. 2016;9:173–178. doi: 10.1016/j.tranon.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mondaca S., Razavi P., Xu C., Offin M., Myers M., Scaltriti M., Hechtman J.F., Bradley M., O’Reilly E.M., Berger M.F., et al. Genomic Characterization of ERBB2 -Driven Biliary Cancer and a Case of Response to Ado-Trastuzumab Emtansine. JCO Precis. Oncol. 2019;3:1–9. doi: 10.1200/PO.19.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel A., Soneji D., Singh H.P., Kumar M., Bandyopadhyay A., Mathur A., Sharma A., Gahlot G.P.S., MS S., Guleria B., et al. Genomic Landscape and Targeted Treatment of Gallbladder Cancer: Results of a First Ongoing Prospective Study. South Asian J. Cancer. 2020;9:074–079. doi: 10.1055/s-0040-1721180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadopoulou K., Murray S., Manousou K., Tikas I., Dervenis C., Sgouros J., Rontogianni D., Lakis S., Poulios C., Pervana S., et al. Genotyping and mRNA profiling reveal actionable targets in biliary tract cancers. Ann. Oncol. 2017;28:v246. doi: 10.1093/annonc/mdx369.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maynard H., Stadler Z.K., Berger M.F., Solit D.B., Ly M., Lowery M.A., Mandelker D., Zhang L., Jordan E., El Dika I., et al. Germline alterations in patients with biliary tract cancers: A spectrum of significant and previously underappreciated findings. Cancer. 2020;126:1995–2002. doi: 10.1002/cncr.32740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asai T., Loza E., Roig G.V.G., Ajioka Y., Tsuchiya Y., Yamamoto M., Nakamura K. High frequency of TP53 but not K-ras gene mutations in bolivian patients with gallbladder cancer. Asian Pac. J. Cancer Prev. 2014;15:5449–5454. doi: 10.7314/APJCP.2014.15.13.5449. [DOI] [PubMed] [Google Scholar]
- 43.Wistuba I.I., Maitra A., Carrasco R., Tang M., Troncoso P., Minna J.D., Gazdar A.F. High resolution chromosome 3p, 8p, 9q and 22q allelotyping analysis in the pathogenesis of gallbladder carcinoma. Br. J. Cancer. 2002;87:432–440. doi: 10.1038/sj.bjc.6600490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M., Chen L., Qu Y., Sui F., Yang Q., Ji M., Shi B., Chen M., Hou P. Identification of MAP kinase pathways as therapeutic targets in gallbladder carcinoma using targeted parallel sequencing. Oncotarget. 2017;8:36319–36330. doi: 10.18632/oncotarget.16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomioka Y., Sung Y.N., Sawada R., Hong S.M., Akita M., Itoh T., Ajiki T., Fukumoto T., Zen Y. IL-33 overexpression in gallbladder cancers associated with pancreatobiliary maljunction. Histopathology. 2019;75:365–375. doi: 10.1111/his.13863. [DOI] [PubMed] [Google Scholar]
- 46.Pandey A., Stawiski E.W., Durinck S., Gowda H., Goldstein L.D., Barbhuiya M.A., Schröder M.S., Sreenivasamurthy S.K., Kim S.W., Phalke S., et al. Integrated genomic analysis reveals mutated ELF3 as a potential gallbladder cancer vaccine candidate. Nat. Commun. 2020;11:4225. doi: 10.1038/s41467-020-17880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J., Wei Q., Wu X., Sima J., Xu Q., Wu M., Wang F., Mou H., Hu H., Zhao J., et al. Integrative clinical and molecular analysis of advanced biliary tract cancers on immune checkpoint blockade reveals potential markers of response. Clin. Transl. Med. 2020;10:e118. doi: 10.1002/ctm2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akita M., Fujikura K., Ajiki T., Fukumoto T., Otani K., Hirose T., Tominaga M., Itoh T., Zen Y. Intracholecystic Papillary Neoplasms Are Distinct from Papillary Gallbladder Cancers: A Clinicopathologic and Exome-sequencing Study. Am. J. Surg. Pathol. 2019;43:783–791. doi: 10.1097/PAS.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 49.Rashid A., Ueki T., Gao Y.T., Houlihan P.S., Wallace C., Wang B.S., Shen M.C., Deng J., Hsing A.W. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: A population-based study in China. Clin. Cancer Res. 2002;8:3156–3163. [PubMed] [Google Scholar]
- 50.Kim S.W., Her K.H., Jang J.Y., Kim W.H., Kim Y.T., Park Y.H. K-ras oncogene mutation in cancer and precancerous lesions of the gallbladder. J. Surg. Oncol. 2000;75:246–251. doi: 10.1002/1096-9098(200012)75:4<246::AID-JSO4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 51.Hirosawa T., Ishida M., Ishii K., Kanehara K., Kudo K., Ohnuma S., Kamei T., Motoi F., Naitoh T., Selaru F.M., et al. Loss of BAP1 expression is associated with genetic mutation and can predict outcomes in gallbladder cancer. PLoS ONE. 2018;13:e0206643. doi: 10.1371/journal.pone.0206643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goeppert B., Roessler S., Renner M., Loeffler M., Singer S., Rausch M., Albrecht T., Mehrabi A., Vogel M.N., Pathil A., et al. Low frequency of mismatch repair deficiency in gallbladder cancer. Diagn. Pathol. 2019;14:36. doi: 10.1186/s13000-019-0813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moy A.P., Shahid M., Ferrone C.R., Borger D.R., Zhu A.X., Ting D., Deshpande V. Microsatellite instability in gallbladder carcinoma. Virchows Arch. 2015;466:393–402. doi: 10.1007/s00428-015-1720-0. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida T., Sugai T., Habano W., Nakamura S.I., Uesugi N., Funato O., Saito K. Microsatellite instability in gallbladder carcinoma: Two independent genetic pathways of gallbladder carcinogenesis. J. Gastroenterol. 2000;35:768–774. doi: 10.1007/s005350070036. [DOI] [PubMed] [Google Scholar]
- 55.Roa J.C., Roa I., Correa P., Vo Q., Araya J.C., Villaseca M., Guzmán P., Schneider B.G. Microsatellite instability in preneoplastic and neoplastic lesions of the gallbladder. J. Gastroenterol. 2005;40:79–86. doi: 10.1007/s00535-004-1497-4. [DOI] [PubMed] [Google Scholar]
- 56.Yang D., Chen T., Zhan M., Xu S., Yin X., Liu Q., Chen W., Zhang Y., Liu D., Yan J., et al. Modulation of mTOR and epigenetic pathways as therapeutics in gallbladder cancer. Mol. Ther. Oncolytics. 2021;20:59–70. doi: 10.1016/j.omto.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebata N., Fujita M., Sasagawa S., Maejima K., Okawa Y., Hatanaka Y., Mitsuhashi T., Oosawa-tatsuguchi A., Tanaka H., Miyano S., et al. Molecular classification and tumor microenvironment characterization of gallbladder cancer by comprehensive genomic and transcriptomic analysis. Cancers. 2021;13:733. doi: 10.3390/cancers13040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spizzo G., Puccini A., Xiu J., Goldberg R.M., Grothey A., Shields A.F., Arora S.P., Khushmann M., Salem M.E., Battaglin F., et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open. 2020;5:e000682. doi: 10.1136/esmoopen-2020-000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinberg B.A., Xiu J., Lindberg M.R., Shields A.F., Hwang J.J., Poorman K., Salem M.E., Pishvaian M.J., Holcombe R.F., Marshall J.L., et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 2019;10:652–662. doi: 10.21037/jgo.2018.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S.T., Jang K.T., Lee J., Jang H.M., Choi H.J., Jang H.L., Park S.H., Park Y.S., Lim H.Y., Kang W.K., et al. Molecular subgroup analysis of clinical outcomes in a phase 3 study of gemcitabine and oxaliplatin with or without erlotinib in advanced biliary tract cancer. Transl. Oncol. 2015;8:40–46. doi: 10.1016/j.tranon.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simbolo M., Fassan M., Ruzzenente A., Mafficini A., Wood L.D., Corbo V., Melisi D., Malleo G., Vicentini C., Malpeli G., et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5:2839–2852. doi: 10.18632/oncotarget.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pai R.K., Pai R.K., Mojtahed K. Mutations in the RAS/RAF/MAP kinase pathway commonly occur in gallbladder adenomas but are uncommon in gallbladder adenocarcinomas. Appl. Immunohistochem. Mol. Morphol. 2011;19:133–140. doi: 10.1097/PAI.0b013e3181f09179. [DOI] [PubMed] [Google Scholar]
- 63.Singh M.K., Chetri K., Pandey U.B., Kapoor V.K., Mittal B., Choudhuri G. Mutational spectrum of K-ras oncogene among Indian patients with gallbladder cancer. J. Gastroenterol. Hepatol. 2004;19:916–921. doi: 10.1111/j.1440-1746.2004.03355.x. [DOI] [PubMed] [Google Scholar]
- 64.Saetta A.A., Papanastasiou P., Michalopoulos N.V., Gigelou F., Korkolopoulou P., Bei T., Patsouris E. Mutational analysis of BRAF in gallbladder carcinomas in association with K-ras and p53 mutations and microsatellite instability. Virchows Arch. 2004;445:179–182. doi: 10.1007/s00428-004-1046-9. [DOI] [PubMed] [Google Scholar]
- 65.Sharma A., Kumar A., Kumari N., Krishnani N., Rastogi N. Mutational frequency of KRAS, NRAS, IDH2, PIK3CA, and EGFR in North Indian gallbladder cancer patients. Ecancermedicalscience. 2017;11:757. doi: 10.3332/ecancer.2017.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deshpande V., Nduaguba A., Zimmerman S.M., Kehoe S.M., MacConaill L.E., Lauwers G.Y., Ferrone C., Bardeesy N., Zhu A.X., Hezel A.F. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer. 2011;11:60. doi: 10.1186/1471-2407-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang W.C., Tsai C.C., Chan C.C. Mutation analysis and copy number changes of KRAS and BRAF genes in Taiwanese cases of biliary tract cholangiocarcinoma. J. Formos. Med. Assoc. 2017;116:464–468. doi: 10.1016/j.jfma.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 68.Kumari N., Corless C.L., Warrick A., Beadling C., Nelson D., Neff T., Krishnani N., Kapoor V.K. Mutation profiling in gallbladder cancer in Indian population. Indian J. Pathol. Microbiol. 2014;57:9–12. doi: 10.4103/0377-4929.130849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puhalla H., Kandioler D., Ludwig C., Filipits M., Wrba F., Laengle F., Jakesz R., Gruenberger T. p53 Analysis in Gallbladder Cancer: Comparison of Gene Analysis Versus Immunohistochemistry. Anticancer Res. 2004;24:1201–1206. [PubMed] [Google Scholar]
- 70.Lin J., Dong K., Bai Y., Zhao S., Dong Y., Shi J., Shi W., Long J., Yang X., Wang D., et al. Precision oncology for gallbladder cancer: Insights from genetic alterations and clinical practice. Ann. Transl. Med. 2019;7:467. doi: 10.21037/atm.2019.08.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kazmi H.R., Chandra A., Nigam J., Noushif M., Parmar D., Gupta V. Prognostic significance of k-ras Codon 12 mutation in patients with Resected gallbladder cancer. Dig. Surg. 2013;30:233–239. doi: 10.1159/000353133. [DOI] [PubMed] [Google Scholar]
- 72.Riener M.O., Bawohl M., Clavien P.A., Jochum W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosom. Cancer. 2008;47:363–367. doi: 10.1002/gcc.20540. [DOI] [PubMed] [Google Scholar]
- 73.Narayan R.R., Creasy J.M., Goldman D.A., Gönen M., Kandoth C., Kundra R., Solit D.B., Askan G., Klimstra D.S., Basturk O., et al. Regional differences in gallbladder cancer pathogenesis: Insights from a multi-institutional comparison of tumor mutations. Cancer. 2019;125:575–585. doi: 10.1002/cncr.31850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peraldo Neia C., Cavalloni G., Balsamo A., Venesio T., Napoli F., Sassi F., Martin V., Frattini M., Aglietta M., Leone F. Screening for the FIG-ROS1 fusion in biliary tract carcinomas by nested PCR. Genes Chromosom. Cancer. 2014;53:1033–1040. doi: 10.1002/gcc.22212. [DOI] [PubMed] [Google Scholar]
- 75.Leone F., Cavalloni G., Pignochino Y., Sarotto I., Ferraris R., Piacibello W., Venesio T., Capussotti L., Risio M., Aglietta M. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin. Cancer Res. 2006;12:1680–1685. doi: 10.1158/1078-0432.CCR-05-1692. [DOI] [PubMed] [Google Scholar]
- 76.Roa I., Garcia H., Game A., De Toro G., De Aretxabala X., Javle M. Somatic Mutations of PI3K in Early and Advanced Gallbladder Cancer: Additional Options for an Orphan Cancer. J. Mol. Diagn. 2016;18:388–394. doi: 10.1016/j.jmoldx.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Yadav S., DE Sarkar N., Kumari N., Krishnani N., Kumar A., Mittal B. Targeted Gene Sequencing of Gallbladder Carcinoma Identifies High-impact Somatic and Rare Germline Mutations. Cancer Genom. Proteom. 2017;14:495–506. doi: 10.21873/cgp.20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao S., Cao Y., Liu S.B., Wang X.A., Bao R.F., Shu Y.J., Hu Y.P., Zhang Y.J., Jiang L., Zhang F., et al. The E545K mutation of PIK3CA promotes gallbladder carcinoma progression through enhanced binding to EGFR. J. Exp. Clin. Cancer Res. 2016;35 doi: 10.1186/s13046-016-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chae H., Kim D., Yoo C., Kim K.-P., Jeong J.H., Chang H.-M., Lee S.S., Park D.H., Song T.J., Hwang S., et al. Therapeutic relevance of targeted sequencing in management of patients with advanced biliary tract cancer: DNA damage repair gene mutations as a predictive biomarker. Eur. J. Cancer. 2019;120:31–39. doi: 10.1016/j.ejca.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 80.Goldenberg D., Rosenbaum E., Argani P., Wistuba I.I., Sidransky D., Thuluvath P.J., Hidalgo M., Califano J., Maitra A. The V599E BRAF mutation is uncommon in biliary tract cancers. Mod. Pathol. 2004;17:1386–1391. doi: 10.1038/modpathol.3800204. [DOI] [PubMed] [Google Scholar]
- 81.Moreno M., Pimentel F., Gazdar A.F., Wistuba I.I., Miquel J.F. TP53 abnormalities are frequent and early events in the sequential pathogenesis of gallbladder carcinoma. Ann. Hepatol. Off. J. Mex. Assoc. Hepatol. 2005;4:192–199. doi: 10.1016/S1665-2681(19)32065-4. [DOI] [PubMed] [Google Scholar]
- 82.Vidaurre T., Casavilca S., Montenegro P., Gomez H., Calderón M., Navarro J., Aramburu J., Poquioma E., Tsuchiya Y., Asai T., et al. Tumor protein p53 and K-ras gene mutations in Peruvian patients with gallbladder cancer. Asian Pac. J. Cancer Prev. 2019;20:289–294. doi: 10.31557/APJCP.2019.20.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tadokoro H., Shigihara T., Ikeda T., Takase M., Suyama M. Two distinct pathways of p16 gene inactivation in gallbladder cancer. World J. Gastroenterol. 2007;13:6396–6403. doi: 10.3748/wjg.v13.i47.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Bono J., Mateo J., Fizazi K., Saad F., Shore N., Sandhu S., Chi K.N., Sartor O., Agarwal N., Olmos D., et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 85.Janjigian Y.Y., Maron S.B., Chatila W.K., Millang B., Chavan S.S., Alterman C., Chou J.F., Segal M.F., Simmons M.Z., Momtaz P., et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821–831. doi: 10.1016/S1470-2045(20)30169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 87.Shitara K., Bang Y.-J., Iwasa S., Sugimoto N., Ryu M.-H., Sakai D., Chung H.-C., Kawakami H., Yabusaki H., Lee J., et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 88.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., Pegram M., Oh D.-Y., Diéras V., Guardino E., et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnston S., Pippen J., Pivot X., Lichinitser M., Sadeghi S., Dieras V., Gomez H.L., Romieu G., Manikhas A., Kennedy M.J., et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-Positive metastatic breast cancer. J. Clin. Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 90.Geyer C.E., Forster J., Lindquist D., Chan S., Romieu C.G., Pienkowski T., Jagiello-Gruszfeld A., Crown J., Chan A., Kaufman B., et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 91.Rugo H.S., Im S.A., Cardoso F., Cortés J., Curigliano G., Musolino A., Pegram M.D., Wright G.S., Saura C., Escrivá-De-Romaní S., et al. Efficacy of Margetuximab vs Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin M., Bonneterre J., Geyer C.E., Ito Y., Ro J., Lang I., Kim S.B., Germa C., Vermette J., Wang K., et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur. J. Cancer. 2013;49:3763–3772. doi: 10.1016/j.ejca.2013.07.142. [DOI] [PubMed] [Google Scholar]
- 93.Vogel C.L., Cobleigh M.A., Tripathy D., Gutheil J.C., Harris L.N., Fehrenbacher L., Slamon D.J., Murphy M., Novotny W.F., Burchmore M., et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 94.Baselga J., Cortés J., Kim S.-B., Im S.-A., Hegg R., Im Y.-H., Roman L., Pedrini J.L., Pienkowski T., Knott A., et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murthy R.K., Loi S., Okines A., Paplomata E., Hamilton E., Hurvitz S.A., Lin N.U., Borges V., Abramson V., Anders C., et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 96.Modi S., Saura C., Yamashita T., Park Y.H., Kim S.-B., Tamura K., Andre F., Iwata H., Ito Y., Tsurutani J., et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tosi F., Sartore-Bianchi A., Lonardi S., Amatu A., Leone F., Ghezzi S., Martino C., Bencardino K., Bonazzina E., Bergamo F., et al. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin. Colorectal Cancer. 2020;19:256–262.e2. doi: 10.1016/j.clcc.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 98.Meric-Bernstam F., Hurwitz H., Raghav K.P.S., McWilliams R.R., Fakih M., VanderWalde A., Swanton C., Kurzrock R., Burris H., Sweeney C., et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siena S., Di Bartolomeo M., Raghav K., Masuishi T., Loupakis F., Kawakami H., Yamaguchi K., Nishina T., Fakih M., Elez E., et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779–789. doi: 10.1016/S1470-2045(21)00086-3. [DOI] [PubMed] [Google Scholar]
- 100.Fader A.N., Roque D.M., Siegel E., Buza N., Hui P., Abdelghany O., Chambers S.K., Secord A.A., Havrilesky L., O’Malley D.M., et al. Randomized Phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 2018;36:2044–2051. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- 101.Li B.T., Shen R., Buonocore D., Olah Z.T., Ni A., Ginsberg M.S., Ulaner G.A., Offin M., Feldman D., Hembrough T., et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase II basket trial. J. Clin. Oncol. 2018;36:2532–2537. doi: 10.1200/JCO.2018.77.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li B.T., Smit E.F., Goto Y., Nakagawa K., Udagawa H. Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rugo H.S., Lerebours F., Ciruelos E., Drullinsky P., Ruiz-Borrego M., Neven P., Park Y.H., Prat A., Bachelot T., Juric D., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 104.Marabelle A., Fakih M., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K., Chung H.C., Kindler H.L., Lopez-Martin J.A., Miller W.H., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 105.Marcus L., Lemery S.J., Keegan P., Pazdur R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 106.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 108.Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ott P.A., Bang Y.J., Piha-Paul S.A., Abdul Razak A.R., Bennouna J., Soria J.C., Rugo H.S., Cohen R.B., O’Neil B.H., Mehnert J.M., et al. T-cell–inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 2019;37:318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 111.Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., de Jesus-Acosta A., Delord J.P., Geva R., Gottfried M., Penel N., Hansen A.R., et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/ mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh R.R. Next-Generation Sequencing in High-Sensitive Detection of Mutations in Tumors: Challenges, Advances, and Applications. J. Mol. Diagn. 2020;22:994–1007. doi: 10.1016/j.jmoldx.2020.04.213. [DOI] [PubMed] [Google Scholar]
- 113.Ma X., Shao Y., Tian L., Flasch D.A., Mulder H.L., Edmonson M.N., Liu Y., Chen X., Newman S., Nakitandwe J., et al. Analysis of error profiles in deep next-generation sequencing data. Genome Biol. 2019;20 doi: 10.1186/s13059-019-1659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berger M.F., Mardis E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018;15:353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boland C.R., Thibodeau S.N., Hamilton S.R., Sidransky D., Eshleman J.R., Burt R.W., Meltzer S.J., Rodriguez-Bigas M.A., Fodde R., Ranzani G.N., et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 116.Kato S., Goodman A., Walavalkar V., Barkauskas D.A., Sharabi A., Kurzrock R. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin. Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]