Abstract
The hustle and bustle of the planet Earth have come to a halt thanks to the novel coronavirus. The virus has affected approximately 219 million people globally; taken the lives of 4.55 million patients as of September 2021; and created an ambiance of fear, social distancing, and economic instability. The purpose of this review article is to trace the historical origin and evolution of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). The virus is highly contagious with a unique feature of rapid mutations—the scientific research is paving the way for discoveries regarding novel coronavirus disease (COVID-19) diagnosis, features, prevention, and vaccination. The connections between the coronavirus pandemic and dental practices are essential because COVID-19 is transmitted by aerosols, fomites, and respiratory droplets, which are also produced during dental procedures, putting both the patient and the dentist at risk. The main emphasis of this paper is to highlight the psychological, economic, and social impact of this pandemic on dental practices throughout the world and under what circumstances and guidelines can dental health care be provided. In the current situation of the pandemic, an appropriate screening tool must be established either by using rapid molecular testing or saliva point-of-care technology, which will be effective in identifying as well as isolating the potential contacts and carriers in hopes to contain and mitigate infection. The blessing in disguise is that this virus has united the leaders, scientists, health care providers, and people of all professions from all around the world to fight against a common enemy.
Keywords: SARS-CoV-2, dentistry, dental treatment, coronavirus
1. Introduction
Coronaviruses are a group of viruses causing common cold and flu-like symptoms, infecting both humans and animals [1]. Novel coronavirus disease (COVID-19) is an infectious disorder [1] due to severe acute respiratory syndrome caused by coronavirus type 2 (SARS-CoV-2). The first diagnosed case was in the “wet markets’’ of Wuhan (China) in December 2019 [1]. On 11 March 2020, the disease was announced as a global pandemic [2] by the World Health Organization (WHO). The WHO reports more than 219 million COVID-19 cases globally [3]. SARS-CoV-2 is 88% identical to severe acute respiratory syndrome (SARS-like CoV) derived from bats collected from eastern China in 2018 [4]. It possesses 79% genetic resemblance with SARS-CoV (2003) and 50% with Middle East respiratory syndrome coronavirus (MERS-CoV) [4]. This virus is highly temperature-sensitive with maximum stability at 4 °C, but when the temperature is raised to 70 °C during the incubation period its inactivation time is reduced to 5 min [5,6]. Novel coronavirus (2019-nCoV) can remain stable on inanimate surfaces up to 1.5 week, up to 180 min on printing papers, up to 48 h on clothes and wood, up to 96 h on smooth surfaces such as glass, and may survive for several days on stainless steel, plastics, and the inner or outer surface of surgical masks [5,7]. The purpose of the present review is to emphasize the impact of COVID-19 on dentistry, how the dentist can aid in its diagnosis, and its overall effects on the profession of dentistry worldwide. In addition to this, the article also highlights the role of teledentistry and newly established guidelines for providing dental health care amid the pandemic.
2. History and Evolution Novel Coronavirus
Epidemiological and genetic studies show that SARS-CoV-2 is zoonotic in origin [8]. The first transmission was from animal (bats and pangolins) to human (zoonosis), followed by inter-human transmissions [9]. However, human–animal transmission (anthroponosis) has also been hypothesized [10]. For instance, SARS-CoV-2 infected minks were reported in Sweden, Denmark, United States, Spain, and Netherlands [11]. Domestic dogs and cats of infected persons have also tested positive for SARS-CoV-2 [12]. Dr. Almeida first identified coronavirus at St. Thomas Hospital, London, in 1964, where the virus caused flu-like symptoms in humans [13]. By 1967, scientists discovered similar human and animal viruses and called them due to their crown-like appearances [13]. In 2002, SARS-CoV emerged in southern China, spreading to 28 other countries [14]. More than 8000 people were infected by July 2003, with a mortality rate of 10% [14]. In 2012, MERS-CoV affected 1700 people in Saudi Arabia with a mortality rate of 36% [15,16]. The 2019-nCoV infecting humans was identified with the aid of next-generation sequencing by the end of 2019 [17]. Since its emergence in 2019, SARS-CoV-2 has shown genetic diversity, attributed to a low fidelity viral polymerase and increased recombination frequency. Both of these factors promote a high mutation rate. These genomic mutations of COVID-19 virus have generated variants which have been sorted out in nine clades viz L, V, S, G, GH, GR, GV, GRY, and O. At the time of writing, clades G, GH, GR, and GRY are responsible for the majority of infections while clades L and V, which were responsible for the commencement of pandemic in December 2019, are almost extinct now. Since January 2021 onwards, B.1.1.7, B.1.351, P.1, and the Delta variant (B.1.617.2) derivatives of clade G are the predominant infections. These variants are demonstrating D614G, P323L, and F106F mutations, thus increasing the susceptibility of the virus for dissemination of disease [18]. D614G mutation first appeared in March 2020; B.1.1.7, which was labelled as UK variant, first emerged in September 2020. The South African variant (B.1.351) was first identified in December 2020 while the Brazilian variant or P.1 was first isolated in January 2021. B.1.617.2 was called the double variant and was first identified in December 2020 [19].
3. Specific Virology, Pathophysiology, and Life Cycle of COVID-19
Coronaviridae family possess a single-stranded RNA with the genomic length ranging from 26 to 32 kilobases [1] and can adapt to new environments through mutations causing long-term health effects [20]. The virus demonstrates round, elliptical, or a pleomorphic shape with an approximate diameter of 60–140 nm.
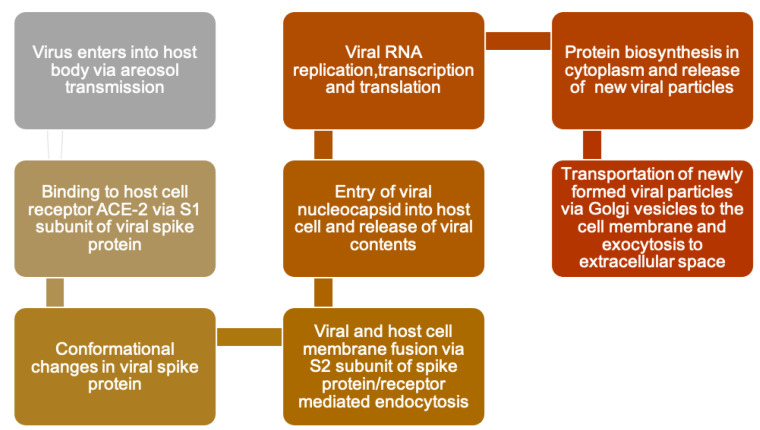
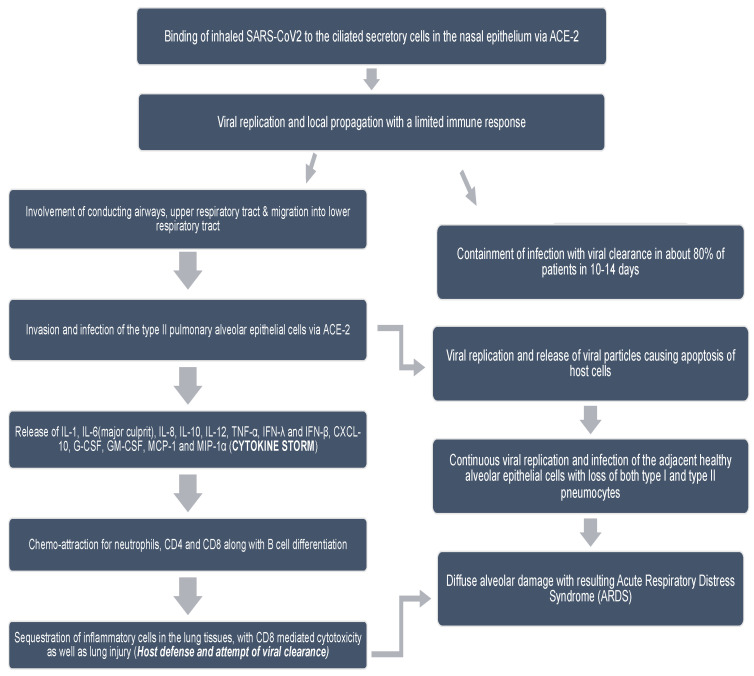
The SARS-CoV-2 life cycle [21], once entered into the human body, is presented in Figure 1. The SARS-CoV-2 pathophysiology, once it has entered the human body through aerosol, is presented in Figure 2. The flow chart describes how the virus replicates inside the human body and how the body reacts to the replication of the virus. Clinical characteristics of COVID-19 are given in Table 1 [22,23,24,25,26]. In serologic studies, 30% of individuals were symptomless [27]. However, the symptomatic patients reported coryza [27]. The re-infection frequency observed with SARS-CoV-2 is high and implies that antibody IgG is not protective [27].
Figure 1.
SARS-CoV-2 life cycle showing virus behaviour inside the body [21].
Figure 2.
COVID-19 pathophysiology demonstrating disease progression from virus entrance in the body to causing acute respiratory distress syndrome (ARDS) [21].
Table 1.
Signs and symptoms of COVID-19.
| Most Common | Less Common | Most Dangerous |
|---|---|---|
| Pyrexia Fatigue Dry cough |
Anosmia and ageusia [22,23] Headache [24] Sore throat Diarrhoea Conjunctivitis Skin rash Fingers and toes discoloration |
Dyspnoea Chest pain/pressure Loss of movement/speech Heart attack Epilepsy [24] Blood coagulation [25] Cerebral infarction [26] Kidney failure Disseminated intravascular coagulation Acute respiratory distress syndrome and multiple organ failure because of cytokine storm |
The virus can infect individuals from all ages irrespective of any gender predilection; however, individuals who are above 60 years of age or those possessing co-morbidities like diabetes, asthma, obesity, ischemic heart disease, cancer, or patients who have undergone organ transplant comprise the high-risk group with almost a 12 times greater chance of fatality with SARS-CoV-2 infection than individuals falling in low risk category [21]. According to a study conducted in Jinyintan and Wuhan pulmonary hospital on 191 adult patients, 48% had comorbid conditions, including high blood pressure (30%), diabetes mellitus (19%), and coronary artery disease (8%) [28]. The New York State Department of Health (NYSDOH) conducted a survey where they analysed the relationship of co-morbidities associated with fatality and the results are presented in Table 2. Similarly, data from the Centre for Disease Control and Prevention (CDC) has shown that Blacks, Hispanics, and Asians are at an increased risk of contracting SARS-CoV-2 infection [21].
Table 2.
Showing relationship of COVID-19 with various comorbid conditions [29].
|
4. Transmission
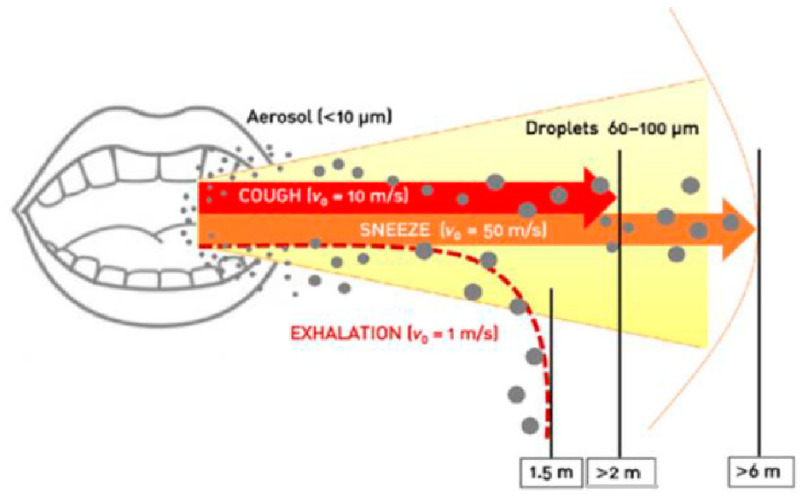
The most common mode of human–human transmission occurs via droplets (coughing, sneezing, talking, and aerosol generating procedures) or blood with smaller droplets traveling a longer distance and larger droplets limited to nearby objects [30]. The effect of droplet size on the distance is shown in Figure 3.
Figure 3.
Showing exhalation distances of aerosols and droplets (Reprinted with permission from Ref. [31]. Copyright 2021 Elsevier.
Furthermore, the studies of conjunctival samples from suspected as well as confirmed 2019-nCoV cases advocates that exposure of eyes may be an efficient way for the virus entrance [32,33]; therefore, there is a need for using protective eye-wear while working in a dental practice and other areas where one may come in contact with potential and confirmed cases.
5. Diagnosis and Diagnostic Methods
The CDC, China, reported the viral genome sequence in the international database banks GenBank and Global Initiative on Sharing All Influenza Data [32,34]. It helped labs to develop a particular real-time polymerase chain reaction (RT-PCR) evaluation for its diagnosis [32]. Other diagnostic tests include molecular tests (digital PCR, next-generation sequencing, microarray analysis, and isothermal nucleic acid amplification) and antibody testing for prior infection [35]. The methods for testing are:
Nasopharyngeal swab.
Oropharyngeal swab.
Blood sampling for antibody detection (immunization).
Expectorated sputum in severe respiratory disease [36].
In terms of sensitivity and specificity of diagnostic methods, sensitivity is the test’s capacity to identify all infected individuals. In contrast, specificity is the test’s ability to detect a particular pathogen [37]. Sensitivity of the RT-PCR varied depending on the specimen type: pharyngeal swab (32%), nasal swab (63%), sputum (72–75%), and bronchoalveolar lavage (93–95%) [38].
5.1. Saliva as a Diagnostic Tool
COVID-19 has also been detected in the saliva of infected patients [33]. There are three suspected pathways for SARS-CoV to be present in saliva:
Lower and upper respiratory tract → exchange of liquid droplets between the oral cavity and respiratory tract → virus in saliva [8,36]
Blood → virus via gingival crevicular fluid (GCF) enters oral cavity→ saliva [39]
SARS-CoV-2 → infection of salivary glands (rhesus macaques) → saliva [40]
Saliva is a cheap, accessible, and non-invasive diagnostic method with minimal risk of transmission and is currently serving as a biomarker for diagnosing and screening different diseases [41,42], including viral, fungal, or bacterial infections; various types of cancers; cardiovascular diseases; and developmental as well as genetic diseases [43,44,45]. It has substantial biomarkers and components, including DNA and RNA, various microorganisms, immunoglobulins, and metabolites [46]. Salivary glands are also a reservoir for the angiotensin-converting enzyme 2 (ACE-2) receptor expression, the functional receptor for this virus [47,48]. Considerably lower expression of ACE-2 receptors has been found in the pharyngeal cells compared to the lower respiratory tract and salivary glands [47].
5.2. Gingival Crevicular Fluid
The GCF is an inflammatory exudate or serum transudate of the pathological or healthy periodontal tissues [49]. It is used for the detection of periodontal diseases, presence of drugs in the periodontal pockets through systematic circulation, and proteomic analysis [50] for isolation and assessment of different viruses (Herpes simplex, Epstein–Barr, and Cytomegalovirus) [51]. GCF can be collected by absorption technique using paper strips/points and can be a non-invasive method for isolating and diagnosing coronavirus and its pathway for entry into the oral cavity [52].
6. SARS-COV-2 Incubation Period in Humans
The mean incubation period after being infected is 5.1 days. During this period, the patient remains asymptomatic. Three weeks is the crucial period for COVID-19 because patients either died after 15 to 22 days or were discharged between 18 to 25 days from the onset of symptoms (Table 3) [28].
Table 3.
Incubation period and onset of symptoms of SARS-CoV-2. Reprinted with permission from Ref. [28]. Copyright 2021 Copyright Elsevier.
| Clinical Features | Population Experiencing (%) | |
|---|---|---|
|
1 to 3 day(s)
Onset of symptoms |
|
80% of patients get these mild symptoms |
|
4 to 9 days
In the lungs |
|
14% of those infected experience these severe symptoms |
|
8 to 15 days
In the blood |
|
5% of those infected need admissions to an intensive care unit |
7. Management of COVID-19
At present, there are no approved and specific therapies for 2019-nCoV. Many immunotherapies and antiviral drugs are under investigation for COVID-19 (Table 4) [53].
Table 4.
Commonly used drugs for the treatment of COVID-19.
| Name of Drug | Potential Role in COVID-19 | Problems/Issues/Remarks |
|---|---|---|
| β-D-N4-hydroxycytidine (NHC) [54] | Ribonucleoside analogue with broad-spectrum antiviral activity (oral route) Effective against Remdesivir-resistant virus, MERS-CoV, SARS-CoV-2, and SARS-CoV in primary HAE cell cultures Reduces virus titres in a dose-dependent manner |
Coronavirus may achieve 2-fold resistance after 30 passages [55]. |
| Interferons (IFN-I and III) [56] | Produces innate immune response in human cells and stimulates IFN-stimulated genes (ISGs) through JAK/STAT pathway, affecting viral replication at all stages of its replicative cycle. Early administration can decrease the viral spread and can produce extended-lasting responses without inflammatory side effects. |
Virus adapts to IFNs by turning over interferon receptors, leading to a diminished response by helper T cells and NK cells. IFNs can produce flu-like symptoms on their own [57,58]. |
| Chloroquine (CQ), Hydroxychloroquine (HCQ) [56] | Inhibits intracellular replication of viral particles. It prevents the interaction between the virus and its receptor, thus blocking its effect. Both drugs are immunomodulatory and downregulate Toll-like receptors, thus suppressing the cytokine storm. |
HCQ is a less toxic derivative of CQ; hence it is favoured in the treatment of COVID-19. Both these drugs produce reactive oxygen species, which can damage host cells. |
| Azithromycin (AZM) [56,59,60] | Inhibits replication of virus in bronchial cells by decreasing the synthesis of adhesion molecules like ICAM-1. Downregulates cytokine production (IL2, 6, 8), maintains alveolar cell integrity and reduces lung fibrosis Acts synergistically with HCQ in reducing viral load It also prevents bacterial co-infection by Prevotella, which can enhance the pathogenicity of SARS-CoV-2 by internalizing it. |
It can cause gastrointestinal upset, nausea, headache, hepatotoxicity, and bacterial resistance. It can prolong QTc interval, ventricular tachycardia, and sudden cardiac arrest by causing intracellular sodium overdose. |
| Tocilizumab [61,62] | Recombinant humanized anti-IL-6 receptor monoclonal antibody, which is a competitive blocker of membrane-bound and soluble IL-6. Potential role in patients presenting with symptoms associated with cytokine storm. |
Compochiaro et al. found no statistically significant survival benefit with a slightly increased propensity towards the development of fungal infections; however, it may reduce the need for ventilatory support in hospitalized COVID-19 patients [63]. |
| Steroids [64,65,66] | Usually administered steroids include methylprednisolone (32 mg/day), dexamethasone (6 mg/day), and hydrocortisone. Dexamethasone is favoured as it causes minimal fluid retention. It may have a role in reducing the tissue injury due to cytokine storm. |
Conflicting body of evidence regarding improvement in survival and decreased hospital stay. May be beneficial but should not be given to all the patients. It can lead towards the development of hyperglycaemia, hypernatremia and mucormycosis and aspergillosis. It can reduce the duration of fever but has no overall effect on the duration of hospitalization. |
| Remdesvir [67,68] | Broad-spectrum antiviral which is an inhibitor of viral RNA-dependent RNA polymerase | Conflicting data on improvement in symptoms with no significant impact on mortality, however, may offer a survival benefit if given early in mild to moderately ill COVID-19 patients. |
| Vitamins | A high dose of vitamin C can prevent cytokine storm in COVID-19 patients, which reduces lung injury and inflammatory damage. |
Vaccination is considered the most effective defence against infectious diseases. The same is true for COVID-19; therefore, over 214 candidate vaccines from different pharmaceutical companies are being developed. The vaccines are broadly classified as: vaccines based on full-length S-protein, protein (RBD-based or S2 based subunit vaccines), inactivated vaccines, live attenuated vaccine, nucleic acid (DNA/mRNA) based vaccines, replicating and non-replicating viral vectors vaccine, and viral-like particle vaccine [69]. COVID-19 vaccines showed promising results by producing specific T cell-mediated immune responses and increased the number of neutralizing antibodies (NAbs) [56]. Different vaccines for COVID-19 available are demonstrated in Table 5. Vaccination priorities include:
-
(1)
Healthcare professionals and inhabitants of long-term care facilities.
-
(2)
Essential workers (such as transportation, food service, finance, and health) and individuals aged 75 years or older.
-
(3)
Individuals aged 65 to 74 years; individuals aged 16 to 64 years with systemic conditions.
Table 5.
Sources, respective companies, and approval status of various COVID-19 vaccines.
| Company | Type | Doses | Route | Efficacy | Storage | Approval/Development | Mechanism of Action |
|---|---|---|---|---|---|---|---|
| Pfizer–BioNTech | Nucleoside modified mRNA (BNT162a1 and BNT162b2) | 2 shots 21 days apart |
I.M inj. | 95% | −70 °C | UK approved | Spike proteins and RBD fragments are introduced into the body producing the desired immune response [71]. |
| Oxford–AstraZeneca | Viral vector (genetically altered nonreplicating chimpanzee adenovirus) | 2 shots 4 to 12 weeks apart |
I.M inj. | 70% | Regular fridge temperature | UK approved | Specifically deliver genes to the target cells thus providing a trigger to cytotoxic T-cells resulting in killing of infected cells [69]. |
| Moderna | Based on lipid nanoparticle-encapsulated mRNA | 2 shots 28 days apart |
I.M inj. | 94.1% | −20 °C | UK approved | Encodes stable form of spike protein of SARS-CoV-2 and educates CD4+ immune cells of the body [72]. |
| Novavax (NVX-CoV2373) | Full-length S (spike) Protein-based | 2 | I.M inj. | Regular fridge temperature | Pending | Promotes migration of leukocytes into lymph nodes thus increasing T-cell, B-cell, and NK cell response [69]. | |
| Janssen (Johnson & Johnson’s) | Viral vector based using adenovirus or pox virus | 1 | I.M inj. | 66.3% | Regular fridge temperature | Pending | DNA of the adenovirus is modified which helps the body to develop humoral and T-cell based cellular immune response against COVID-19 [73]. |
| CoronaVac (Sinopharm/Sinovac) (BBIBP-CorV) | Inactivated virus vaccine | I.M inj. | 79% | Regular fridge temperature | Approved by China, Singapore, Saudi Arabia, and Pakistan | Contains virus has been inactivated through UV light/chemicals and elicits antigen-specific antibody response producing plasma cells, T-cells, and memory B-cells [74]. | |
| CanSino Bioloics (Ad5-nCoV) Convidecia | Non-replicating adenovirus based vaccine | 1 | I.M inj. | 66% to 91% | Regular fridge temperature | Approved by Hungary, China, Mexico, and Pakistan | RBD and spike proteins produce T cell response conferring immunity against virus [69]. |
| Sputnik V | Using two non-replicating adenovirus based vector (Ad26, Ad5) | 2 doses 21 days apart | Undergoing phase 3 trials | Gamaleya Institute, Moscow. | Dose 1 injects Ad26, and in dose 2 Ad5 is given. This produces an enhanced immune response [69]. | ||
| KBP-201 (NCT04473690) | Protein (RBD-based) subunit vaccine | 2 doses 21 days apart | I.M inj. | Currently undergoing phase II trials | - | Pending | RBD in the spike protein binds to ACE-2 receptor producing neutralizing monoclonal antibodies towards SARS-CoV-2 [75]. |
| Covaxin | Inactivated virus vaccine | Currently undergoing trials in India | Same as mentioned above under CoronaVac (sinopharm) | ||||
| BHPIV3/SARS-S | Live attenuated virus vaccine | 1 | I.M. Inj | Currently undergoing phase 2 animal trials | Currently undergoing trials in India and China | Induces production of SARS-CoV neutralizing serum antibodies [69]. | |
| DelNS1-SARS-CoV2-RBD | Live attenuated vaccine with deletion of NS1 influenza strain | 1 | Intra-nasal | Currently undergoing phase 2 animal trials | Modified to include SARS-CoV-2 spike protein and is considered more immunogenic than other LAVs [69]. | ||
| LUNAR-COV19 | Lipid enabled and unlocked nucleomonomer agent-modified RNA (LUNAR) | 1 | Currently undergoing phase 1 and 2 trials | Biospace, Singapore | Entry into host cells and mRNA is translated into protiein, s leading to the production of the immune response against SARS-CoV-2 [69]. |
I.M inj. (Intramuscular injection).
Once the people with priority have been successfully vaccinated, only then the general public will get the opportunity of getting vaccinated [70].
Vaccinations other than parenteral routes are also under development. Following are the examples of some of the vaccines which are under development and do not employ the parenteral route:
hAd5 T-cell (Immunity Bio and NantKwest) [76].
Intranasal COVID-19 vaccine (Ad COVID) [77].
ChAdOx1 nCov-19 inhaled (University of Oxford) [78].
Similarly, already available vaccines like BCG and MMR are being repurposed for developing immunity against COVID-19 as the literature has suggested that it may offer partial immunity against COVID-19 infection [71].
8. Oral Manifestations of COVID-19 Infection
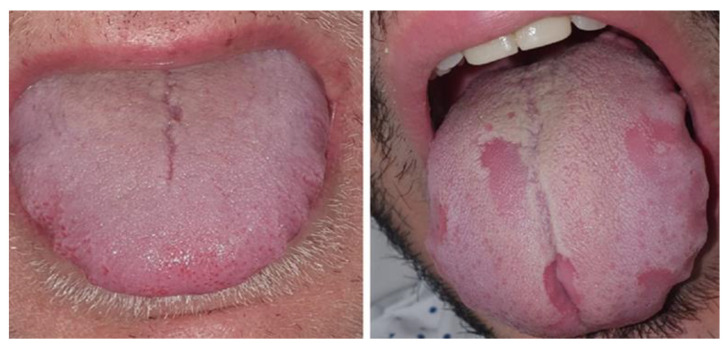
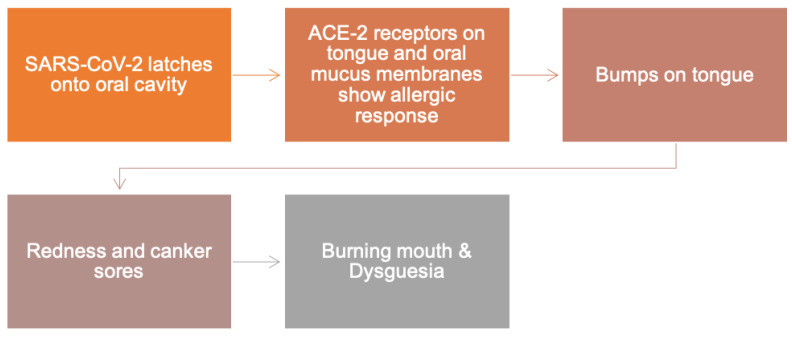
Evaluation of 666 patients at a temporary field hospital in Spain showed that 45% had mucocutaneous symptoms and more than 25% had oral symptoms as follows [79]: The relative frequencies of oral manifestations are 11.5% (lingual papillitis), 6.9% (aphthous stomatitis), 6.6% (glossitis), 5.3% (burning mouth sensation), and 3.9% (patchy depapillation and mucositis) [79]. Other common findings were macroglossia, tongue discoloration/coated tongue [80], and COVID-19 tongue (geographic tongue) (Figure 4) [81,82]. These oral lesions were left undiagnosed in COVID-19 patients because the patients usually wear masks while presenting in hospitals.
Figure 4.
Macroglossia with lateral indentations (left) geographic tongue (right) (Reprinted with permission from Ref. [79]. Copyright 2020 John Wiley and Sons.
Other authors have reported association of COVID-19 with irregular and aphthous like lesions; herpetiform or zosteriform lesions; generalized non-specific ulcerations; erosions on the tongue, palate, and labial mucosa; atrophic and hyperkeratotic patches on the tongue, gingiva, and palate; lesions resembling erythema multiforme; desquamative gingivitis; and angina bullosa-like lesions [83]. Similarly, petechiae, post-inflammatory pigmentation, and vesicular eruptions have also been described. Periodontal manifestations include aggressive necrotizing periodontal disease, which may be due to bacterial co-infection caused by Prevotella intermedia. Two cases with Kawasaki disease and Melkerson–Rosenthal syndrome have also been reported [84]. Halepas et al. has reported oral manifestations of COVID-19 infection in paediatric patients. According to him, red swollen lips were seen in 48.9% cases while 10.6% presented with strawberry tongue and represent multi-system inflammatory syndrome in children [85].
In addition, SARS-CoV-2 is known to affect the salivary glands directly, which can lead to xerostomia and inflammation of the major salivary glands [86]. Dryness of the oral cavity can also be produced by mouth breathing, dehydration, and COVID-19 related medications. Severe halitosis has also been reported in COVID-19 patients [83]. Usually, this xerostomia is self-limiting and transient in nature. However, it can lead to periodontal disease and caries in patients run a protracted course of the disease or who are hospitalized for longer durations. Transneuronal migration of SARS-CoV-2 can lead to neuronal death of cells of the olfactory bulb and the taste buds, which can explain the loss of taste and smell [87]. Release of cytokines as in cytokine storm and acute febrile illness can lead to specific viral and non-specific ulcerations, fissuring, and erythematous eruptions in the oral cavity. The common areas of involvement include lips, tongues, palate, and buccal mucosa [88].
8.1. The Role of ACE-2 in Oral Manifestations of COVID-19
The ACE-2 is most likely to be the cell receptor of the 2019-nCoV [80]. The ACE-2 receptors which bind the SARS-CoV-2 are abundantly found on the surfaces of the oral mucosa, particularly the tongue and masticatory mucosa of the gingiva (Figure 5) [49,80]. These findings elucidate the risk for potentially substantial COVID-19 infectious susceptibility for the oral cavity and dental practices [49,80]. The strong affinity between ACE-2 and COVID-19 S protein proposed that the patients with greater ACE-2 expression are more liable to COVID-19 [89,90]. The cellular serine protease Transmembrane protease, serine 2 also added to the S-protein priming of 2019-nCoV, signifying its potential to comprise a management option [91].
Figure 5.
SARS-CoV-2 mechanism of oral manifestations. Reprinted with permission from Ref. [92]. Copyright 2020 John Wiley and Sons.
8.2. Dentistry Hazards
The highest level of aerosol contamination within 60 cm between the patient’s head and dentist’s right arm has been shown in the literature. Aerosols can remain suspended in the air for 30 min after a dental procedure [93]. SARS-CoV-2 viability is estimated to be 3 h with an adherence capability for one and a half weeks to different surfaces [94].
9. Economic and Emotional Impact on Dentists and Dental Practices
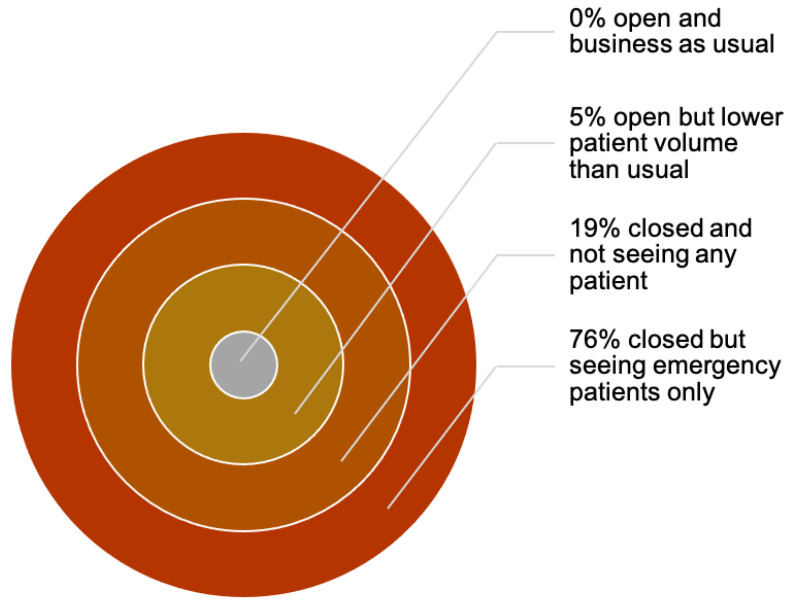
For almost a year now, this pandemic has affected dental care providers both psychologically and economically (Figure 6) [95]. In various countries, dental procedures were banned entirely to prevent viral transmission, thus creating a huge financial implication for dental professionals.
Figure 6.
Economic impact of COVID-19 on dental practices [95].
The infection control during dental healthcare amid the COVID-19 pandemic is demonstrated in Table 6 [96,97,98,99,100,101]. In terms of the psychological impact of COVID-19 on dental personals, Khanagar et al. reported increased mental stress and psychological distress among dentists [102]. Depression, anxiety, fear, and stress have adversely affected dentists across the globe. They experienced fear of being exposed at the workplace and then the possibility of transmitting the infection to the near and dear ones at home [103]. The same was the case with dental students. Suspension of regular classes and closure of their schools took a toll on them as well. They felt anxious and depressed as they believed that they could not learn the skill during online teaching, which will affect their professional career. A study by Hakami et al. has reported that this anxiety and stress was more prevalent among female dentists and dental assistants as compared to their male counterparts [104]. Quarantines, isolations, financial impact, and loss of family members due to COVID-19 put immense pressures on dental surgeons and up to the extent that some dental personnel have expressed suicidal thoughts [105].
Table 6.
Prevention and control of COVID-19 during dental healthcare.
| 1—Teledentistry and Triage Protocols |
|
| 2—Screening Zone |
|
| 3—Waiting Area | |
| 4—Donning Zone | Clean area PPE wearing sequence including the hand disinfection:
|
| 5—Doffing Zone | Dirty area PPE removing sequence:
|
| 6—Dental Surgery Room for Aerosol Generating Procedures |
|
| 7—Procedure Infection Control |
|
| 8—Dental Surgery Disinfection |
|
| 9—Dental Surgery Ventilation |
|
| 10—Dental Equipment Maintenance | Follow the guidelines of IFU for the maintenance of dental unit water-lines, autoclave, compressors, radiography equipment, and suctions [116]. |
A study conducted by Ahmadi et al. in Iran concluded that around 10% of the dentists and their staff members had COVID-19 related symptoms. They have also reported that 63% of the dentists had faced financial problems due to COVID-19 pandemic and 43% reported anxiety and depression with almost 50% of these required consultation with a psychiatrist [106]. Kamran et al. conducted a nationwide survey among dentists in Pakistan and have reported that a significant number of dental practitioners have modified their practices following COVID-19 related guidelines. According to their survey around 70% dentists have installed a physical barrier at their workplace and have tried to maintain 6 feet distance in the waiting area. They also report that 70–80% dentists were using N95 masks and PPE like face shields, gowns, etc., and were avoiding aerosol generating procedures [107]. These studies clearly highlight the economical and emotional toll COVID-19 has on the dental profession.
Disinfectants containing 1000 mg/Chlorine for the walls, floors, and dental operatory disinfection. Alcohol-based sanitizer (75% to 80%) is beneficial against SARS-CoV-2. The suggested disinfectant for SARS-CoV-2 for waste disinfection before disposal is sodium hypochlorite [99]. Povidone-iodine (0.23% to 7%) and hydrogen peroxide (1.5%) are suggested to decrease viral load as a pre-procedural mouth wash [121,122]. Surface disinfectants including ethanol (62–71%), hydrogen peroxide (0.5%), and sodium hypochlorite (0.1%) are also efficient against SARS-CoV-2 [123,124].
10. Categorization of Dental Procedures according to COVID-19 Guidelines
Various dental procedures are categorized as emergencies and non-emergencies according to COVID-19 guidelines. Their details are presented in Table 7 below.
Table 7.
Various emergency and non-emergency dental procedures according to COVID-19 guidelines [125].
| Dental Non-Emergency Procedures | Dental Emergency Procedures |
|---|---|
| New/periodic oral examinations | Uncontrolled bleeding |
| Routine x-rays | Cellulitis/bacterial facial space infection |
| Routine dental cleaning as well as preventive therapies | Severe dental pain (pulpitis) |
| Extraction of asymptomatic teeth | Pericoronitis/3rd molar pain |
| Restorative dental procedures (fillings, crowning) | Dry socket |
| Recall/revisit | Tooth fracture |
| Dento-alveolar trauma | |
| Painful broken filling | |
| Adjustment of ortho-wire damaging gums | |
| Post-surgery treatment |
The transmission can be manageable, i.e., decrease the viral transmission (via close contact or droplets), using the point-of-care technology [126]. Several guidelines have been recommended to prevent and control the disease at various levels of populations. The WHO also advocated recommendations for the decrease in viral load via the disinfection and cleaning [127,128,129]:
Wash hands with alcohol-based soap solution for 20 s.
Wear masks when outside.
Avoid touching face.
Stay 6 feet apart from each other.
Cover your face while coughing or sneezing.
Disinfect the surfaces used repeatedly (doorknobs, tables, and mobile phones).
Avoid crowded areas.
Isolate yourself if sick or at greater risk.
11. Aerosol Generating Procedures
The CDC says that aerosol generating procedures (Table 8 and Figure 7) are the medical procedures that produce greater concentrations of infective respiratory aerosols than sneezing, coughing, breathing, or speaking [130,131].
Table 8.
| 1. Tracheostomy and tracheal intubation procedures | 2. Positive-pressure mechanical ventilation and CPAP |
| 3. Bronchoscopy | 4. Intubation and extubation procedures |
| 5. Surgery, autopsy, or post-mortem procedures with high-speed devices | 6. High frequency oscillatory ventilation |
| 7. Cardiopulmonary resuscitation | 8. High-flow oxygen therapy |
| 9. Sputum induction | 10. Airway suctioning |
| 11. FEES and VFSS | 12. Nebulized or aerosol therapy |
| CPAP (Continuous positive airway pressure); FEES (Fibreoptic endoscopic evaluation of swallowing); VFSS (Video fluoroscopic swallowing study) | |
Figure 7.
Aerosol-generating dental procedures and associated factors [135].
Aerosol-generating procedures are hazardous because they release micro-droplets into the air through spray generating equipment [136]. Severe and potentially life-threatening diseases are spread by droplets and aerosols, including pneumonic plague, tuberculosis, influenza, Legionnaire’s diseases, and SARS infections [137]. These droplets may remain in the air or travel long distances and may lead to inhaled infection (Table 9) [138]. Dental practitioners need to develop an understanding of the following [139,140]:
Risks associated with different modes of transmission (i.e., droplets, aerosols, and fomites).
The sources, nature, kinetics, and the quantity of microbial load in such aerosols.
The efficacy of current and emerging practices in mitigating aerosol-generated microbial load.
Table 9.
Showing different types of aerosols and droplets and their significance [141].
| Droplet Type | Description |
|---|---|
| Splatter droplets | Particle size ≥ 50 µm, briefly airborne, and spread by close contact (typically within 1 m) with the host. |
| Aerosols | Particle size < 50 µm, carry viable pathogens, remain airborne for prolonged period, and spread to distant surfaces. |
| Droplets > 5 µm | Remain in the upper respiratory tract. |
| Droplets ≤ 5 µm | Might be inhaled into the lower respiratory tract. |
| Droplets ≤ 1 µm | Can enter alveoli. |
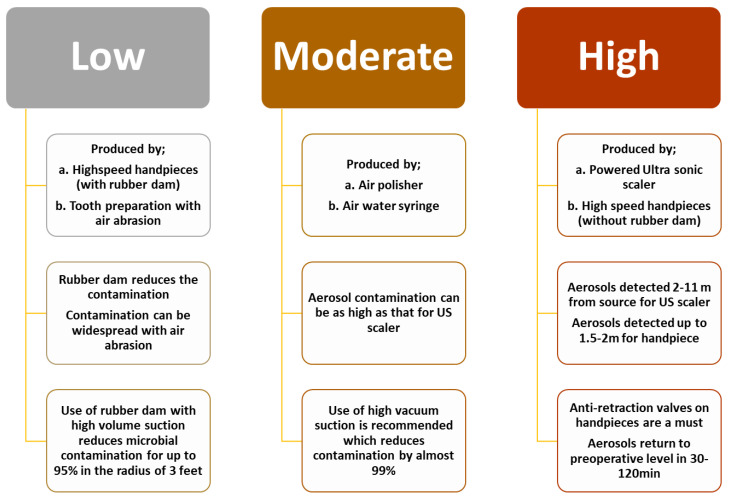
The coolant used with rotary handpieces and powered scalers has a flow rate of 10 to 40 mL per minute [141] which is 5- to 10-fold greater than unstimulated and stimulated saliva. The dilution of salivary or respiratory pathogens occurs in these settings, reducing the overall pathogenic microbial load and the infectivity of such aerosols. The facial pathologies and fractures management are of greater risk because of the viral load in the oral, nasal, and oropharyngeal mucosa. To reduce the risk of potential infections, we have to adopt principles of simplification of surgery, avoid complicated surgical procedures, and limit the operating times [142,143] as well as implement the various protocols listed in Table 10.
Table 10.
Protocols for maxillofacial procedures during COVID-19 pandemic.
| Pre-Procedure Protocols | During Procedure Protocols | Post-Procedure Protocols |
|---|---|---|
|
|
|
| personal protective equipment (PPE); powered air purifying respirator (PAPR) | ||
Cataloguing of suspicious or high-risk patients (history of fever, respiratory problems, travel history, and contact with a COVID-19 patient during the past 14 days) [143].
Repetition of triage [144].
Preoperative testing after 48 h, i.e., two RT-PCR tests 24 h apart with a sensitivity of at least 71% [145] (if both tests are not positive, perform surgery with improved airborne protections) [144].
Accommodation of patient in an isolated ward or room [144].
According to the guidelines for performing oral and maxillofacial surgery during COVID-19 by the Association of Osteosynthesis: Cranio-Maxillofacial (AO CMF):
The therapeutic and surgical procedure for facial fractures as suggested by AO CMF are as follows [144,147,151]:
Scalpel use over monopolar cautery for skin/mucosal incision.
Avoid intra-oral incision, repeated suctioning, and irrigation.
Elective surgery must be delayed for non-critical cancer patients unless it does not affect the prognosis.
Substitution of power saw by a low-speed drill or osteotome.
Application of a low power bipolar cautery for haemostasis.
The following Table 11 [157] highlights the international guidelines for COVID-19 issued by the professional organizations for maxillofacial guidelines [150], the United Kingdom National Health Service [9], the Australian Society for Otolaryngology Head & Neck Surgery, and British Association of Oral and Maxillofacial Surgeons [145].
Table 11.
International guidelines for management of cranio-maxillo-facial trauma [157].
| Urgency for operation | Emergent (require surgical intervention in ≤24 h) | Urgent (require surgical intervention for bone union) |
| Patient presentation | Compromised airway or vision, uncontrolled bleeding, or combined intracranial or upper facial fracture | Facial fracture causing functional or cosmetic deformity including displaced cranio-orbital fractures, orbital dystopia, and naso-orbito-ethmoid fractures |
| COVID-19 screening | RT-PCR or rapid COVID test | RT-PCR or rapid COVID-19 test |
12. Association of Rhino-Cerebral Fungal Infections with COVID-19
Recent literature is showing an association of black fungus (fungal ball) of the lungs and maxillary sinuses with COVID-19 patients. Song et al. have reported an association of COVID-19 with aspergillus flavus, candida albicans, and candida glabrata. The overall incidence of invasive fungal infections along with COVID-19 co-infection was around 5% [158]. Rabaglaiti et al. studied the corelation of invasive mould infection in established COVID-19 patients and found the overall incidence to be around 11% with a mortality of around 30% in these patients [159]. Waizal-Haiat et al. have reported a case of fatal rhino-orbital mucormycosis along with diabetic ketoacidosis in a COVID-19 patient [160]. Mucormycosis, when it affects the maxillary sinus and orbit, usually presents as pain and swelling in the midface region, involving the eyelids and the nasal fold area. There can be associated paraesthesia of the involved infra-orbital nerve. Other signs and symptoms that can point towards rhino-cerebral mucormycosis can be mobile teeth in the maxilla with discharging sinuses; a presentation very similar to chronic osteomyelitis of the maxilla; black, necrosed palate; nasal blockage; and decreased visual acuity from the affected eye. CT scan or MRI is usually the gold standard for diagnosing involvement of the maxillary and or ethmoidal sinuses. There can be an associated epiphora due to blockage of the nasolacrimal apparatus by the fungus [161]. Management usually includes aggressive anti-fungal treatment through surgical debridement, management of the underlying immunosuppressing state, and supportive therapy to improve the nutritional status of the patients. However, despite all these aggressive treatment modalities, the mortality rate is high, and the disease usually carries a poor prognosis [162].
13. Conclusions
Despite scientific advancements, we are still unable to contain the spread of COVID-19, and scientists have yet to develop a definitive treatment for this disease. Currently, there is no evidence that dental healthcare professionals are at a higher risk of airborne viral disease transmission than the general population. Epidemiologic evidence of the prevalence of infections in dental healthcare providers and a comparison to populations as a whole may shine a light on highly protective infection control practices that can be implemented to keep practitioners and patients as safe as possible. Dental professionals should educate patients about the significance of good oral hygiene. Poor oral hygiene is associated with increased plaque deposits and bacterial load, which may lead to bacterial superinfection and risk of complications in COVID-19 patients. Our only preventative measure right now is vaccination and maintenance of cross-infection protocols which can be achieved through proper education of health care workers, patients, and the general public. The COVID-19 pandemic has given us an important message that even the superpowers can collapse and that the most intelligent nations can be startled. Mere weapons cannot defend us and the number of produced medicines cannot suffice our needs. Under the disguise of scientific revolution and industrialization, we have disrupted nature’s equilibrium. A critical question arises: will we realize the importance of our green planet in the post-COVID world?
Author Contributions
Conceptualization, R.T.B. and M.S.Z.; methodology, R.T.B. and O.S.J.; software, F.J.R.-L. and S.M.Q.; validation, R.T.B., M.S.Z., J.G.-G. and F.J.R.-L.; formal analysis, M.S.S. and M.S.Z.; investigation, R.T.B. and O.S.J.; resources, M.S.S., M.S.Z., J.G.-G. and F.J.R.-L.; data curation, R.T.B. and O.S.J.; writing—original draft preparation, R.T.B. and O.S.J.; writing—review and editing, M.S.Z., J.G.-G. and F.J.R.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Su S., Wong G., Shi W., Liu J., Lai A.C., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamid H., Khurshid Z., Adanir N., Zafar M.S., Zohaib S. COVID-19 pandemic and role of human saliva as a testing biofluid in point-of-care technology. Eur. J. Dent. 2020;14((Suppl. 1)):S123–S129. doi: 10.1055/s-0040-1713020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin A.W., Poon L.L. Stability of SARS-CoV-2 in different environmental conditions—Authors’ reply. Lancet Microbe. 2020;1:e146. doi: 10.1016/S2666-5247(20)30095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan Y., Zhang D., Yang P., Poon L.L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saqlain M., Munir M.M., Rehman S.U., Gulzar A., Naz S., Ahmed Z., Tahir A.H., Mashhood M. Knowledge, attitude, practice and perceived barriers among healthcare workers regarding COVID-19: A cross-sectional survey from Pakistan. J. Hosp. Infect. 2020;105:419–423. doi: 10.1016/j.jhin.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020;12:9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He S., Han J., Lichtfouse E. Backward transmission of COVID-19 from humans to animals may propagate reinfections and induce vaccine failure. Environ. Chem. Lett. 2021;19:763–768. doi: 10.1007/s10311-020-01140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oreshkova N., Molenaar R.J., Vreman S., Harders F., Munnink B.B.O., Hakze-van Der Honing R.W., Gerhards N., Tolsma P., Bouwstra R., Sikkema R.S. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill. 2020;25:2001005. doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sit T.H., Brackman C.J., Ip S.M., Tam K.W., Law P.Y., To E.M., Veronica Y., Sims L.D., Tsang D.N., Chu D.K. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyrrell D.A.J., Fielder M. Cold Wars: The Fight against the Common Cold. Oxford University Press; New York, NY, USA: 2002. [Google Scholar]
- 14.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.-H. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 15.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 16.De Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L., Fouchier R.A., Galiano M., Gorbalenya A.E., Memish Z.A. Commentary: Middle east respiratory syndrome coronavirus (mers-cov): Announcement of the coronavirus study group. J. Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan W., Zhao X., Ma X., Wang W., Niu P., Xu W. A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019–2020. China CDC Wkly. 2020;2:61–62. doi: 10.46234/ccdcw2020.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh J., Pandit P., McArthur A.G., Banerjee A., Mossman K. Evolutionary trajectory of SARS-CoV-2 and emerging variants. Virol. J. 2021;18:166. doi: 10.1186/s12985-021-01633-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams H., Hutchinson D., Stone H. Watching Brief: The evolution and impact of COVID-19 variants B. 1.1. 7, B. 1.351, P. 1 and B. 1.617. Glob. Biosecur. 2021;3 doi: 10.31646/gbio.112. [DOI] [Google Scholar]
- 20.Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: Mechanisms of coronavirus cross-species transmission. J. Virol. 2010;84:3134–3146. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khade S.M., Yabaji S.M., Srivastava J. An update on COVID-19: SARS-CoV-2 life cycle, immunopathology, and BCG vaccination. Prep. Biochem. Biotechnol. 2021;51:650–658. doi: 10.1080/10826068.2020.1848869. [DOI] [PubMed] [Google Scholar]
- 22.Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: A systematic review and meta-analysis. Mayo Clin. Proc. 2020;95:1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aragão M.d.F.V.V., Leal M., Cartaxo Filho O., Fonseca T., Valença M. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am. J. Neuroradiol. 2020;41:1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao L., Wang M., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Li Y., Jin H. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z.-M., Fu J.-F., Shu Q., Chen Y.-H., Hua C.-Z., Li F.-B., Lin R., Tang L.-F., Wang T.-L., Wang W. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Clin. Pediatr. 2020;16:240–246. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J. Am. Dent. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hierholzer J.C., Tannock G.A. Laboratory Diagnosis of Infectious Diseases Principles and Practice. Springer; New York, NY, USA: 1988. Coronaviridae: The coronaviruses; pp. 451–483. [Google Scholar]
- 28.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franki R. Comorbidities the Rule in New York’s COVID-19 Deaths. [(accessed on 1 May 2021)]. Available online: https://www.mdedge.com/chestphysician/article/220457/coronavirus-updates/comorbidities-rule-new-yorks-covid-19-deaths.
- 30.Xie X., Li Y., Sun H., Liu L. Exhaled droplets due to talking and coughing. J. R. Soc. Interface. 2009;6:S703–S714. doi: 10.1098/rsif.2009.0388.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Checchi V., Bellini P., Bencivenni D., Consolo U. COVID-19 dentistry-related aspects: A literature overview. Int. Dent. J. 2021;71:21–26. doi: 10.1111/idj.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.To K.K.-W., Tsang O.T.-Y., Yip C.C.-Y., Chan K.-H., Wu T.-C., Chan J.M.-C., Leung W.-S., Chik T.S.-H., Choi C.Y.-C., Kandamby D.H. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020;71:841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Novel Coronavirus—China (Emergencies Preparedness, Response) [(accessed on 1 May 2021)]. Available online: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
- 35.Habibzadeh P., Mofatteh M., Silawi M., Ghavami S., Faghihi M.A. Molecular diagnostic assays for COVID-19: An overview. Crit. Rev. Clin. Lab. Sci. 2021;58:385–398. doi: 10.1080/10408363.2021.1884640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubina R., Dziedzic A. Molecular and serological tests for COVID-19 a comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics. 2020;10:434. doi: 10.3390/diagnostics10060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L., Tu L. Implications of gastrointestinal manifestations of COVID-19. Lancet Gastroenterol. Hepatol. 2020;5:629–630. doi: 10.1016/S2468-1253(20)30132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva-Boghossian C.M., Colombo A.P.V., Tanaka M., Rayo C., Xiao Y., Siqueira W.L. Quantitative proteomic analysis of gingival crevicular fluid in different periodontal conditions. PLoS ONE. 2013;8:e75898. doi: 10.1371/journal.pone.0075898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., Wei Q., Alvarez X., Wang H., Du Y., Zhu H., Jiang H., Zhou J., Lam P., Zhang L. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011;85:4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khurshid Z., Zohaib S., Najeeb S., Zafar M.S., Slowey P.D., Almas K. Human saliva collection devices for proteomics: An update. Int. J. Mol. Sci. 2016;17:846. doi: 10.3390/ijms17060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khurshid Z., Zafar M., Khan E., Mali M., Latif M. Human saliva can be a diagnostic tool for Zika virus detection. J. Infect. Public Health. 2019;12:601–604. doi: 10.1016/j.jiph.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Abdul Rehman S., Khurshid Z., Hussain Niazi F., Naseem M., Al Waddani H., Sahibzada H.A., Sannam Khan R. Role of salivary biomarkers in detection of cardiovascular diseases (CVD) Proteomes. 2017;5:21. doi: 10.3390/proteomes5030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahibzada H.A., Khurshid Z., Khan R.S., Naseem M., Siddique K.M., Mali M., Zafar M.S. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics. 2017;7:21. doi: 10.3390/diagnostics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khurshid Z., Zafar M.S., Khan R.S., Najeeb S., Slowey P.D., Rehman I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018;86:23–70. doi: 10.1016/bs.acc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Khurshid Z., Moin S.F., Khan R.S., Agwan M.A.S., Alwadaani A.H., Zafar M.S. Human salivary protein extraction from RNAPro· SAL™, Pure· SAL™, and passive drooling method. Eur. J. Dent. 2017;11:385–389. doi: 10.4103/ejd.ejd_183_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamming I., Timens W., Bulthuis M., Lely A., Navis G.V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L., Zhao J., Peng J., Li X., Deng X., Geng Z., Shen Z., Guo F., Zhang Q., Jin Y. Detection of 2019-nCoV in saliva and characterization of oral symptoms in COVID-19 patients. Cell Prolif. 2020;53:e12923. doi: 10.1111/cpr.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaikh M.S., Lone M.A., Kabir R., Apu E.H. Periodontal connections to the coronavirus disease 2019: An unexplored novel path? Adv. Hum. Biol. 2020;10:197–198. [Google Scholar]
- 50.Lin P.-H., Yeh S.-K., Huang W.-C., Chen H.-Y., Chen C.-H., Sheu J.-R., Lin C.-T., Huang Y.-K. Research performance of biomarkers from biofluids in periodontal disease publications. J. Dent. Sci. 2015;10:61–67. doi: 10.1016/j.jds.2013.06.007. [DOI] [Google Scholar]
- 51.Grenier G., Gagnon G., Grenier D. Detection of herpetic viruses in gingival crevicular fluid of patients suffering from periodontal diseases: Prevalence and effect of treatment. Oral Microbiol. Immunol. 2009;24:506–509. doi: 10.1111/j.1399-302X.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 52.Majeed Z.N., Philip K., Alabsi A., Pushparajan S., Swaminathan D. Identification of gingival crevicular fluid sampling, analytical methods, and oral biomarkers for the diagnosis and monitoring of periodontal diseases: A systematic review. Dis. Markers. 2016;2016:1804727. doi: 10.1155/2016/1804727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmer C., Corum J., Wee S. Coronavirus Vaccine Tracker. [(accessed on 1 May 2021)]. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 54.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., Stevens L.J. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12:eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agostini M.L., Pruijssers A.J., Chappell J.D., Gribble J., Lu X., Andres E.L., Bluemling G.R., Lockwood M.A., Sheahan T.P., Sims A.C. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 2019;93:e01348-19. doi: 10.1128/JVI.01348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakadia B.M., He F., Souho T., Lamboni L., Ullah M.W., Boni B.O., Ahmed A.A.Q., Mukole B.M., Yang G. Prevention and treatment of COVID-19: Focus on interferons, chloroquine/hydroxychloroquine, azithromycin, and vaccine. Biomed. Pharmacother. 2020;133:111008. doi: 10.1016/j.biopha.2020.111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schreiber G. The role of type I interferons in the pathogenesis and treatment of COVID-19. Front. Immunol. 2020;11:595739. doi: 10.3389/fimmu.2020.595739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdolvahab M.H., Moradi-Kalbolandi S., Zarei M., Bose D., Majidzadeh-A K., Farahmand L. Potential role of interferons in treating COVID-19 patients. Int. Immunopharmacol. 2021;90:107171. doi: 10.1016/j.intimp.2020.107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Echeverría-Esnal D., Martin-Ontiyuelo C., Navarrete-Rouco M.E., De-Antonio Cuscó M., Ferrández O., Horcajada J.P., Grau S. Azithromycin in the treatment of COVID-19: A review. Expert Rev. Anti-Infect. Ther. 2021;19:147–163. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]
- 60.Bleyzac N., Goutelle S., Bourguignon L., Tod M. Azithromycin for COVID-19: More than just an antimicrobial? Clin. Drug Investig. 2020;40:683–686. doi: 10.1007/s40261-020-00933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Q., Lin H., Wei R.-G., Chen N., He F., Zou D.-H., Wei J.-R. Tocilizumab treatment for COVID-19 patients: A systematic review and meta-analysis. Infect. Dis. Poverty. 2021;10:71. doi: 10.1186/s40249-021-00857-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M., Altannir Y., Al-Tannir M., Tleyjeh R., Hassett L. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis. Clin. Microbiol. Infect. 2020;27:215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., Tomelleri A., Baldissera E., Rovere-Querini P., Ruggeri A. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chatterjee K., Wu C.-P., Bhardwaj A., Siuba M. Steroids in COVID-19: An overview. Cleve. Clin. J. Med. 2020;87:715. doi: 10.3949/ccjm.87a.ccc059. [DOI] [PubMed] [Google Scholar]
- 65.Waterer G.W., Rello J. Steroids and COVID-19: We need a precision approach, not one size fits all. Infect. Dis. Ther. 2020;9:701–705. doi: 10.1007/s40121-020-00338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halpin D.M., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: A systematic review and clinical perspective. Eur. Respir. J. 2020;55:2001009. doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madsen L.W. Remdesivir for the Treatment of Covid-19-Final Report. N. Engl. J. Med. 2020;338:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nichols B.E., Jamieson L., Zhang S.R., Rao G.A., Silal S., Pulliam J.R., Sanne I., Meyer-Rath G. The Role of Remdesivir in South Africa: Preventing COVID-19 Deaths Through Increasing Intensive Care Unit Capacity. Clin. Infect. Dis. 2021;72:1642–1644. doi: 10.1093/cid/ciaa937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belete T.M. Review on up-to-date status of candidate vaccines for COVID-19 disease. Infect. Drug Resist. 2021;14:151–161. doi: 10.2147/IDR.S288877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.CDC How CDC Is Making COVID-19 Vaccine Recommendations. [(accessed on 1 May 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations-process.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Frecommendations.html.
- 71.Malik J.A., Mulla A.H., Farooqi T., Pottoo F.H., Anwar S., Rengasamy K.R. Targets and strategies for vaccine development against SARS-CoV-2. Biomed. Pharmacother. 2021;137:111254. doi: 10.1016/j.biopha.2021.111254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nag K., Baray J.C., Khan M.R., Mahmud A., Islam J., Myti S., Ali R., Sarker E.H., Kumar S., Chowdhury M.H. An mRNA-based vaccine candidate against SARS-CoV-2 elicits stable immuno-response with single dose. Vaccine. 2021;39:3745–3755. doi: 10.1016/j.vaccine.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stephenson K.E., Le Gars M., Sadoff J., de Groot A.M., Heerwegh D., Truyers C., Atyeo C., Loos C., Chandrashekar A., McMahan K. Immunogenicity of the Ad26. COV2. S Vaccine for COVID-19. JAMA. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase ½ clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z., Xu W., Xia S., Gu C., Wang X., Wang Q., Zhou J., Wu Y., Cai X., Qu D. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct. Target Ther. 2020;5:282. doi: 10.1038/s41392-020-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.NantKwest ImmunityBio and NantKwest Announce FDA Authorization to Study hAd5 T-Cell COVID-19 Vaccine for Combination of Subcutaneous, Oral and Sublingual Boost to Induce T-Cell, Mucosal, and Antibody Immunity. [(accessed on 1 May 2021)]. Available online: https://www.businesswire.com/news/home/20210211005960/en/ImmunityBio-and-NantKwest-Announce-FDA-Authorization-to-Study-hAd5-T-Cell-COVID-19-Vaccine-for-Combination-of-Subcutaneous-Oral-and-Sublingual-Boost-to-Induce-T-Cell-Mucosal-and-Antibody-Immunity.
- 77.ClinicalTrials.gov Safety and Immunogenicity of AdCOVID in Healthy Adults (COVID-19 Vaccine Study) [(accessed on 1 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04679909.
- 78.O’Hare R. Landmark Coronavirus Study to Trial Inhaled Imperial and Oxford Vaccines. [(accessed on 1 May 2021)]. Available online: https://www.imperial.ac.uk/news/203653/landmark-coronavirus-study-trial-inhaled-imperial/
- 79.Nuno-Gonzalez A., Martin-Carrillo P., Magaletsky K., Martin Rios M., Herranz Mañas C., Artigas Almazan J., García Casasola G., Perez Castro E., Gallego Arenas A., Mayor Ibarguren A. Prevalence of mucocutaneous manifestations in 666 patients with COVID-19 in a field hospital in Spain: Oral and palmoplantar findings. Br. J. Dermatol. 2020;184:184–185. doi: 10.1111/bjd.19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alikhani M., Khalighinejad N., Ghalaiani P., Khaleghi M.A., Askari E., Gorsky M. Immunologic and psychologic parameters associated with geographic tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014;118:68–71. doi: 10.1016/j.oooo.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Hathway R. COVID tongue. Br. Dent. J. 2021;230:114. doi: 10.1038/s41415-021-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farid H., Khan M., Jamal S., Ghafoor R. Oral manifestations of Covid-19-A literature review. Rev. Med. Virol. 2021:e2248. doi: 10.1002/rmv.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iranmanesh B., Khalili M., Amiri R., Zartab H., Aflatoonian M. Oral manifestations of COVID-19 disease: A review article. Dermatol. Ther. 2021;34:e14578. doi: 10.1111/dth.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halepas S., Lee K.C., Myers A., Yoon R.K., Chung W., Peters S.M. Oral manifestations of COVID-2019-related multisystem inflammatory syndrome in children: A review of 47 pediatric patients. J. Am. Dent. Assoc. 2021;152:202–208. doi: 10.1016/j.adaj.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halboub E., Al-Maweri S.A., Alanazi R.H., Qaid N.M., Abdulrab S. Orofacial manifestations of COVID-19: A brief review of the published literature. Braz. Oral Res. 2020;34:e124. doi: 10.1590/1807-3107bor-2020.vol34.0124. [DOI] [PubMed] [Google Scholar]
- 87.Amorim Dos Santos J., Normando A.G.C., Carvalho da Silva R.L., Acevedo A.C., De Luca Canto G., Sugaya N., Santos-Silva A.R., Guerra E.N.S. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J. Dent. Res. 2021;100:141–154. doi: 10.1177/0022034520957289. [DOI] [PubMed] [Google Scholar]
- 88.Farook F.F., Nuzaim M.N.M., Ababneh K.T., Alshammari A., Alkadi L. Covid-19 Pandemic and Challenges of Dentistry: COVID-19 Pandemic: Oral Health Challenges and Recommendations. Eur. J. Dent. 2020;14:S165–S170. doi: 10.1055/s-0040-1718641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guy J., Lambert D., Warner F., Hooper N., Turner A. Membrane-associated zinc peptidase families: Comparing ACE and ACE2. Biochim. Biophys. Acta. 2005;1751:2–8. doi: 10.1016/j.bbapap.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu C., Zheng S., Chen Y., Zheng M. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCoV, in the nasal tissue. MedRxiv. 2020 doi: 10.1101/2020.02.11.20022228. [DOI] [Google Scholar]
- 91.Hoffmann M., Kleine-Weber H., Krüger N., Mueller M.A., Drosten C., Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martín Carreras-Presas C., Amaro Sánchez J., López-Sánchez A.F., Jané-Salas E., Somacarrera Pérez M.L. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2020;27((Suppl. 3)):710–712. doi: 10.1111/odi.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Legnani P., Checchi L., Pelliccioni G., D’Achille C. Atmospheric contamination during dental procedures. Quintessence Int. 1994;25:435–439. [PubMed] [Google Scholar]
- 94.Veena H., Mahantesha S., Joseph P.A., Patil S.R., Patil S.H. Dissemination of aerosol and splatter during ultrasonic scaling: A pilot study. J. Infect. Public Health. 2015;8:260–265. doi: 10.1016/j.jiph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 95.ADA COVID-19 Economic Impacts on Dental Practices by American Dental Association. [(accessed on 1 May 2021)]. Available online: https://www.ada.org/en/science-research/health-policy-institute/covid-19-dentists-economic-impact/survey-results.
- 96.Elkarim I., Abdulla Z., Yahia N., AlQudah A., Ibrahim Y. Basic infection control procedures in dental practice in Khartoum—Sudan. Int. Dent. J. 2004;54:413–417. doi: 10.1111/j.1875-595X.2004.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 97.Loeb M., Dafoe N., Mahony J., John M., Sarabia A., Glavin V., Webby R., Smieja M., Earn D.J., Chong S. Surgical mask vs. N95 respirator for preventing influenza among health care workers: A randomized trial. J. Am. Dent. Assoc. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 98.Singh V., Nagaraja C., Hungund S.A. A study of different modes of disinfection and their effect on bacterial load in dental unit waterlines. Eur. J. Gen. Dent. 2013;2:246–251. doi: 10.4103/2278-9626.115999. [DOI] [Google Scholar]
- 99.Sarfaraz S., Shabbir J., Mudasser M.A., Khurshid Z., Al-Quraini A.A.A., Abbasi M.S., Ratnayake J., Zafar M.S. Knowledge and attitude of dental practitioners related to disinfection during the COVID-19 pandemic. Proc. Healthc. 2020;8:232. doi: 10.3390/healthcare8030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 101.Radonovich L.J., Simberkoff M.S., Bessesen M.T., Brown A.C., Cummings D.A., Gaydos C.A., Los J.G., Krosche A.E., Gibert C.L., Gorse G.J. N95 respirators vs. medical masks for preventing influenza among health care personnel: A randomized clinical trial. J. Am. Dent. Assoc. 2019;322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khanagar S.B., Alfadley A. Psychological Impact of the COVID-19 Pandemic on Dental Interns in Riyadh, Saudi Arabia: A Cross-sectional Survey. Int. J. Clin. Pediatr. Dent. 2020;13:508–512. doi: 10.5005/jp-journals-10005-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed M.A., Jouhar R., Ahmed N., Adnan S., Aftab M., Zafar M.S., Khurshid Z. Fear and practice modifications among dentists to combat novel coronavirus disease (COVID-19) outbreak. Int. J. Environ. Res. Public Health. 2020;17:2821. doi: 10.3390/ijerph17082821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uhlen M., Ansteinsson V., Stangvaltaite-Mouhat L., Korzeniewska L., Skudutyte-Rysstad R., Shabestari M., Mdala I., Hovden E. Psychological impact of the COVID-19 pandemic on dental health personnel in Norway. BMC Health Serv. Res. 2021;21:420. doi: 10.1186/s12913-021-06443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hakami Z., Khanagar S.B., Vishwanathaiah S., Hakami A., Bokhari A.M., Jabali A.H., Alasmari D., Aldrees A.M. Psychological impact of the coronavirus disease 2019 (COVID-19) pandemic on dental students: A nationwide study. J. Dent. Educ. 2021;85:494–503. doi: 10.1002/jdd.12470. [DOI] [PubMed] [Google Scholar]
- 106.Ranka M.S., Ranka S.R. Survey of Mental Health of Dentists in the COVID-19 Pandemic in the UK. J. Int. Soc. Prev. Community Dent. 2021;11:104–108. doi: 10.4103/jispcd.JISPCD_401_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmadi H., Ebrahimi A., Ghorbani F. The impact of COVID-19 pandemic on dental practice in Iran: A questionnaire-based report. BMC Oral Health. 2020;20:354. doi: 10.1186/s12903-020-01341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kamran R., Saba K., Azam S. Impact of COVID-19 on Pakistani dentists: A nationwide cross sectional study. BMC Oral Health. 2021;21:59. doi: 10.1186/s12903-021-01413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niazi M.I.K., Ghafoor S. Teledentistry and COVID-19: Today and Tomorrow. Biomedica. 2020;36:81–83. doi: 10.51441/BioMedica//BioMedica/5-379. [DOI] [Google Scholar]
- 110.Chopra S.S., Sahoo N.K. Protocol for teledentistry during COVID-19 in Armed Forces dental establishments. Med. J. Armed Forces India. 2020;76:356–359. doi: 10.1016/j.mjafi.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan J.A., Ali B., Aslam K., Hasan A., Ali A., Charania A., Phil B. Dental Care During COVID-19 Pandemic: Guidelines for Teaching Hospital OPDs. J. Pak. Dent. Assoc. 2020;29:S43–S52. doi: 10.25301/JPDA.29S.S43. [DOI] [Google Scholar]
- 112.Izzetti R., Nisi M., Gabriele M., Graziani F. COVID-19 transmission in dental practice: Brief review of preventive measures in Italy. J. Dent. Res. 2020;99:1030–1038. doi: 10.1177/0022034520920580. [DOI] [PubMed] [Google Scholar]
- 113.Razmara F., Khayamzadeh M., Shabankare G. Dental practice in the era of COVID-19: A review of literature. J. Fam. Med. Prim. Care. 2021;10:41–47. doi: 10.4103/jfmpc.jfmpc_1008_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhanushali P., Katge F., Deshpande S., Chimata V.K., Shetty S., Pradhan D. COVID-19: Changing trends and its impact on future of dentistry. Int. J. Dent. 2020;2020:8817424. doi: 10.1155/2020/8817424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peditto M., Scapellato S., Marcianò A., Costa P., Oteri G. Dentistry during the COVID-19 epidemic: An Italian workflow for the management of dental practice. Int. J. Environ. Res. Public Health. 2020;17:3325. doi: 10.3390/ijerph17093325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parihar A.V., Sahoo R., Parihar S. Dental practice in Covid times—An overview. Indian J. Prev. Soc. Med. 2020;51:48–60. [Google Scholar]
- 117.Patel M. Infection control in dentistry during COVID–19 pandemic: What has changed? Heliyon. 2020;6:e05402. doi: 10.1016/j.heliyon.2020.e05402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Villani F.A., Aiuto R., Paglia L., Re D. COVID-19 and dentistry: Prevention in dental practice, a literature review. Int. J. Environ. Res. 2020;17:4609. doi: 10.3390/ijerph17124609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi A.H., Guo W., Chng C.K., Chan B.H. Precautions when providing dental care during Coronavirus Disease 2019 (COVID-19) pandemic. Ann. Acad. Med. Singap. 2020;49:312–319. doi: 10.47102/Annals-acadmedsg.2020111. [DOI] [PubMed] [Google Scholar]
- 120.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): Emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020;99:481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parhar H.S., Tasche K., Brody R.M., Weinstein G.S., O’Malley B.W., Jr., Shanti R.M., Newman J.G. Topical preparations to reduce SARS-CoV-2 aerosolization in head and neck mucosal surgery. Head Neck. 2020;42:1268–1272. doi: 10.1002/hed.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kirk-Bayley J., Sunkaraneni S., Challacombe S. The Use of Povidone Iodine Nasal Spray and Mouthwash During the Current COVID-19 Pandemic May Reduce Cross Infection and Protect Healthcare Workers. [(accessed on 1 May 2021)];SSRN. 2020 doi: 10.2139/ssrn.3563092. Available online: https://ssrn.com/abstract=3563092. [DOI] [Google Scholar]
- 123.Hui D.S. Epidemic and emerging coronaviruses (severe acute respiratory syndrome and Middle East respiratory syndrome) Clin. Chest Med. 2017;38:71–86. doi: 10.1016/j.ccm.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kampf G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. J. Infect. Prev. 2020;2:100044. doi: 10.1016/j.infpip.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.ADA Develops Guidance on Dental Emergency, Nonemergency Care: Recommendations Part of Dentists’ Response over COVID-19 Concerns. [(accessed on 1 May 2021)]. Available online: https://www.ada.org/en/publications/ada-news/2020-archive/march/ada-develops-guidance-on-dental-emergency-nonemergency-care.
- 126.Malik Y.S., Kumar N., Sircar S., Kaushik R., Bhat S., Dhama K., Gupta P., Goyal K., Singh M.P., Ghoshal U. Coronavirus disease pandemic (COVID-19): Challenges and a global perspective. Pathogens. 2020;9:519. doi: 10.3390/pathogens9070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.TFSS Practitioners specialized in oral health and coronavirus disease 2019: Professional guidelines from the French society of stomatology, maxillofacial surgery and oral surgery, to form a common front against the infectious risk. J. Stomatol. Oral Maxillofac. Surg. 2020;121:155–158. doi: 10.1016/j.jormas.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin L., Li T. Interpretation of “Guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (Trial version 5)”. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. [DOI] [PubMed] [Google Scholar]
- 129.Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., Sun C., Sylvia S., Rozelle S., Raat H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.RCSLT The Royal College of Speech and Language Therapists Guidance on Reducing the Risk of Transmission and Use of Personal Protective Equipment (PPE) in the Context of COVID-19. [(accessed on 1 May 2021)]. Available online: https://www.rcslt.org/wp-content/uploads/2020/11/RCSLT-guidance-on-reducing-risk-of-transmission-PPE_Jan-2021-update.pdf.
- 131.Speech Pathology Australia Guidance for Service Delivery, clinical procedures and infection control during COVID-19 pandemic. Aerosol Generating Procedures and Exposure to Aerosols. [(accessed on 1 May 2021)]. Available online: https://www.speechpathologyaustralia.org.au/SPAweb/About_us/COVID-19_News_and_Information/COVID-19_-_Guidance_for_Service_Delivery/SPAweb/About_Us/COVID-19/Guidance_for_Service_Delivery.aspx?hkey=fc19a880-e7a8-4246-8631-a474fc43d4ae.
- 132.El-Boghdadly K., Wong D., Owen R., Neuman M., Pocock S., Carlisle J., Johnstone C., Andruszkiewicz P., Baker P., Biccard B. Risks to healthcare workers following tracheal intubation of patients with COVID-19: A prospective international multicentre cohort study. Anaesthesia. 2020;75:1437–1447. doi: 10.1111/anae.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jackson T., Deibert D., Wyatt G., Durand-Moreau Q., Adisesh A., Khunti K., Khunti S., Smith S., Chan X.H.S., Ross L. Classification of aerosol-generating procedures: A rapid systematic review. BMJ Open Respir. Res. 2020;7:e000730. doi: 10.1136/bmjresp-2020-000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS ONE. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Geisinger M. Into the Unknown: Emerging Evidence Regarding Risks of Aerosols in the Dental Office. [(accessed on 1 May 2021)]. Available online: https://orthopracticeus.com/into-the-unknown-emerging-evidence-regarding-risks-of-aerosols-in-the-dental-office/
- 136.Knibbs L.D., Johnson G.R., Kidd T.J., Cheney J., Grimwood K., Kattenbelt J.A., O’Rourke P.K., Ramsay K.A., Sly P.D., Wainwright C.E. Viability of Pseudomonas aeruginosa in cough aerosols generated by persons with cystic fibrosis. Thorax. 2014;69:740–745. doi: 10.1136/thoraxjnl-2014-205213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harrel S.K., Molinari J. Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J. Am. Dent. Assoc. 2004;135:429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hatagishi E., Okamoto M., Ohmiya S., Yano H., Hori T., Saito W., Miki H., Suzuki Y., Saito R., Yamamoto T. Establishment and clinical applications of a portable system for capturing influenza viruses released through coughing. PLoS ONE. 2014;9:e103560. doi: 10.1371/journal.pone.0103560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yan J., Grantham M., Pantelic J., De Mesquita P.J.B., Albert B., Liu F., Ehrman S., Milton D.K., Consortium E. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA. 2018;115:1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wood M.E., Stockwell R.E., Johnson G.R., Ramsay K.A., Sherrard L.J., Jabbour N., Ballard E., O’Rourke P., Kidd T.J., Wainwright C.E. Face masks and cough etiquette reduce the cough aerosol concentration of Pseudomonas aeruginosa in people with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2018;197:348–355. doi: 10.1164/rccm.201707-1457OC. [DOI] [PubMed] [Google Scholar]
- 141.Micik R.E., Miller R.L., Mazzarella M.A., Ryge G. Studies on dental aerobiology: I. Bacterial aerosols generated during dental procedures. J. Dent. Res. 1969;48:49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 142.Gu Y., Co B., Lu J., Yang Y., Ding M., Ma J., Chen Y., Li Q., Tian L. Clinical analysis of 25 oral and maxillofacial emergency patients during the period of COVID-19 epidemic. Chin. J. Oral Maxillofac. Surg. 2020;18:105. [Google Scholar]
- 143.Barca I., Cordaro R., Kallaverja E., Ferragina F., Cristofaro M.G. Management in oral and maxillofacial surgery during the COVID-19 pandemic: Our experience. Br. J. Oral Maxillofac. Surg. 2020;58:687–691. doi: 10.1016/j.bjoms.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang Y., Soh H.Y., Cai Z.G., Peng X., Zhang Y., Guo C.B. Experience of diagnosing and managing patients in oral maxillofacial surgery during the prevention and control period of the new coronavirus pneumonia. Chin. J. Dent. Res. 2020;23:57–62. doi: 10.3290/j.cjdr.a44339. [DOI] [PubMed] [Google Scholar]
- 145.National Health Service, England . Clinical Guide for Management of Patients Requiring Oral and Maxillofacial Surgery during the Coronavirus Pandemic. 2020. [(accessed on 1 May 2021)]. Available online: https://www.baoms.org.uk/_userfiles/pages/files/professionals/covid_19/specialty_guide_omfs_and_coronavirus_v1_20_march.pdf. [Google Scholar]
- 146.Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212:119–123. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eggers M., Koburger-Janssen T., Eickmann M., Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect. Dis. Ther. 2018;7:249–259. doi: 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xue Y., Han B., Pan J., Zhou H., Zhou Q., Zhao J., Chen S., Zhang W., Hu K. Suggestions for how to prevent coronavirus disease 2019 during oral surgeries. Chin. J. Oral Maxillofac. Surg. 2020;18:198–203. [Google Scholar]
- 149.Hsieh T.-Y., Dedhia R.D., Chiao W., Dresner H., Barta R.J., Lyford-Pike S., Hamlar D., Stephan S.J., Schubert W., Hilger P.A. A guide to facial trauma triage and precautions in the COVID-19 pandemic. Facial Plast. Surg. Aesthet. Med. 2020;22:164–169. doi: 10.1089/fpsam.2020.0185. [DOI] [PubMed] [Google Scholar]
- 150.Chinese Society of Oral and Maxillofacial Surgery Expert proposal for the management of oral maxillofacial surgery during the epidemic period of 2019-nCoV. Chin. J. Oral Maxillofac. Surg. 2020;18:97–99. [Google Scholar]
- 151.Ma S., Yuan Z., Peng Y., Luo Q., Song H., Xiang F., Tan J., Zhou J., Li N., Hu G. Recommendations for the regulation of medical practices of burn treatment during the outbreak of the coronavirus disease 2019. Zhonghua Shao Shang Za Zhi. 2020;36:E004. doi: 10.3760/cma.j.cn501120-20200224-00083. [DOI] [PubMed] [Google Scholar]
- 152.Givi B., Schiff B.A., Chinn S.B., Clayburgh D., Iyer N.G., Jalisi S., Moore M.G., Nathan C.-A., Orloff L.A., O’Neill J.P. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol. Head Neck Surg. 2020;146:579–584. doi: 10.1001/jamaoto.2020.0780. [DOI] [PubMed] [Google Scholar]
- 153.Grant M., Buchbinder D., Dodson T.B., Fusetti S., Leung M.Y.Y., Aniceto G.S., Schramm A., Strong E.B., Wolvius E. AO CMF international task force recommendations on best practices for maxillofacial procedures during COVID-19 pandemic. Craniomaxillofac. Trauma Reconstr. 2020;13:151–156. doi: 10.1177/1943387520948826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel Z.M., Fernandez-Miranda J., Hwang P.H., Nayak J.V., Dodd R., Sajjadi H., Jackler R.K. Precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurgery. 2020;87:E66–E67. doi: 10.1093/neuros/nyaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Licina A., Silvers A., Stuart R.L. Use of powered air-purifying respirator (PAPR) by healthcare workers for preventing highly infectious viral diseases—A systematic review of evidence. Syst. Rev. 2020;9:173. doi: 10.1186/s13643-020-01431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Canelli R., Connor C.W., Gonzalez M., Nozari A., Ortega R. Barrier enclosure during endotracheal intubation. N. Engl. J. Med. 2020;382:1957–1958. doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.DeSerres J.J., Al-Shaqsi S.Z., Antonyshyn O.M., Fialkov J.A. Best practice guidelines for the management of acute craniomaxillofacial trauma during the COVID-19 pandemic. [(accessed on 1 May 2021)];J. Craniofac. Surg. 2020 doi: 10.1097/SCS.0000000000006654. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7282404/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Song G., Liang G., Liu W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rabagliati R., Rodríguez N., Núñez C., Huete A., Bravo S., Garcia P. COVID-19–Associated Mold Infection in Critically Ill Patients, Chile. Emerg. Infect. Dis. 2021;27:1454–1456. doi: 10.3201/eid2705.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Waizel-Haiat S., Guerrero-Paz J.A., Sanchez-Hurtado L., Calleja-Alarcon S., Romero-Gutierrez L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021;13:e13163. doi: 10.7759/cureus.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saldanha M., Reddy R., Vincent M.J. Paranasal Mucormycosis in COVID-19 Patient. [(accessed on 1 May 2021)];Indian J. Otolaryngol. Head Neck Surg. 2021 22:1–4. doi: 10.1007/s12070-021-02574-0. Available online: https://link.springer.com/article/10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mekonnen Z.K., Ashraf D.C., Jankowski T., Grob S.R., Vagefi M.R., Kersten R.C., Simko J.P., Winn B.J. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast. Reconstr. Surg. 2021;37:e40–e80. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.