Abstract
Background:
With improved treatment, individuals with type 1 diabetes are living longer but there is minimal information on the effects of type 1 diabetes on cognitive ability as they become older adults.
Methods:
1,051 participants with type 1 diabetes enrolled in the Diabetes Control and Complications Trial (DCCT) and its follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study completed cognitive assessments: baseline (median age 27 years) and 2, 5, 18, and 32 years later (median age 59). Glycated hemoglobin levels, frequency of severe hypoglycemia, non-glycemic risk factors such as elevated blood pressure, and micro- and macro-vascular complications were assessed repeatedly. We examined the effects of these on measures of memory and psychomotor and mental efficiency.
Findings:
Over 32 years of follow-up, we found substantive declines in memory and psychomotor and mental efficiency. Between years 18 and 32, the decline in psychomotor and mental efficiency was five times larger than the change from baseline to year 18. Independent of the other risk factors and comorbidities, exposure to higher HbA1c levels, more episodes of severe hypoglycemia, and elevated systolic blood pressure were associated with greater decrements in psychomotor and mental efficiency that was most notable by year 32 (p<0·0001). Combined, these three risk factors manifested effects equivalent to an additional 9·4 years of age.
Interpretation:
Cognitive function declines with aging in type 1 diabetes. The association with glycemia and blood pressure levels suggests that better management may preserve cognitive function.
Funding:
United States National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease
INTRODUCTION
Advances in the treatment of type 1 diabetes have led to a dramatic improvement in life expectancy,1 with a corresponding increased susceptibility to aging-related conditions, including cognitive dysfunction and dementia.2 Studies of children and younger adults have reported modest cognitive dysfunction associated with poorer glycemic control and microvascular complications.3,4 Whether and to what extent cognition declines as people with type 1 diabetes age has not been examined longitudinally over an extended period of time.
We address this issue using the deeply-phenotyped cohort from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study with longitudinal cognitive and biomedical data collected over more than 30 years. Individuals were 13 to 39 years old on entry and following an average of 32 years of study, had a median (range) age of 59 (43-75) years. We administered a subset of the original cognitive battery found to be most sensitive to glycemia.5 Our goal was to document the trajectory of cognitive change as this cohort, originally composed of adolescents and younger adults, moved into later adulthood, and to identify key biomedical predictors of decline on measures of memory and psychomotor and mental efficiency. We hypothesized that as our participants enter older adulthood, cognitive functioning would show a steeper decline relative to earlier assessments, which is associated with both glycemic excursions (higher persistent HbA1c levels; episodes of severe hypoglycemia) and micro- and macro-vascular risk factors and complications.
METHODS
Participants
Between 1983 and 1989, 1,441 participants with type 1 diabetes (median age 27 years; range 13-39) were randomly assigned to intensive or conventional diabetes therapy in the DCCT to assess the effects of glycemia on the development and progression of diabetes-related complications.6,7 Participants with a history of hypertension (blood pressure >140/90 mm Hg or receiving medication) or hyperlipidemia (fasting serum cholesterol level ≥3 SDs above age- and sex-specific means) were not eligible to participate. After an average of 6·5 years of follow-up (range 3-9 years), the DCCT was stopped in 1993 after demonstrating the benefits of intensive glycemic therapy. In 1994, 96% of the surviving DCCT cohort enrolled in the ongoing, long-term EDIC observational study of the DCCT cohort.7 Participants originally assigned to conventional therapy were taught intensive therapy, and all returned to their health care providers for ongoing diabetes care. Current HbA1c levels in the intensive and conventional group came together at the beginning of the EDIC follow-up period and remained similar throughout. These analyses included all 1,051 participants who completed cognitive testing at DCCT baseline and during the 2018/2019 assessment (mean follow-up: 32 years) (see flow chart in Figure S1).
Evaluations, Risk Factors, and Coexisting Complications
Potential risk factors for cognitive decline were assessed by standardized methods during DCCT and EDIC.7 Blood pressure was measured quarterly during DCCT and annually during EDIC. Hypertension was defined as systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, documented hypertension, or the use of anti-hypertensive medications. Fasting lipids and albumin excretion rates were measured annually during DCCT and at alternate years during EDIC. Hyperlipidemia was defined as low-density lipoprotein cholesterol of at least 130 mg per deciliter (3·4 mmol per liter) or the use of lipid-lowering medications. Medication history was not obtained during the DCCT, but the use of ACE inhibitors was discouraged, and statins were not widely available or in use during the DCCT.
Diabetes complications were measured and defined as follows. Estimated glomerular filtration rates (eGFR) were calculated from serum creatinine measured annually. Kidney disease was defined as an albumin excretion rate equal to or greater than 30 mg per 24 hours on 2 or more consecutive visits or an eGFR less than 60 ml per minute per 1·73m2. Standardized stereoscopic seven-field fundus photographs were obtained every six months during DCCT, and every fourth year thereafter, staggered from the start of the EDIC follow-up period. Proliferative diabetic retinopathy was defined by neovascularization observed on fundus photograph grading or self-reported and/or confirmed scatter photocoagulation, and clinically-significant macular edema using fundus photography grading or self-reported and/or confirmed focal photocoagulation anti-vascular endothelial growth factor therapy. Neurologic evaluations, nerve conduction studies, and cardiac autonomic testing were conducted periodically. All cardiovascular disease events were adjudicated and classified by a committee masked to treatment group assignment and HbA1c levels.
HbA1c was measured by high performance liquid chromatography quarterly during DCCT and annually during EDIC. Severe hypoglycemia was self-reported and defined as any event leading to coma and/or seizure within the 3-month window prior to each annual DCCT/EDIC visit.5
Cognitive Test Protocol
A comprehensive battery of cognitive tests was administered up to four times during DCCT/EDIC (baseline, years 2, 5, and 18). Test administration, scoring, and quality control procedures have been described in detail.5 To reduce the assessment burden on study participants, the most recent assessment, after 28 to 36 years of follow-up (mean 32 years), included an abbreviated battery that consisted of tests comprising the ‘psychomotor and mental efficiency’ domain that was found to be particularly sensitive to glycemia.5,8 Tests of memory known to be sensitive to aging and mild cognitive impairment were also included.2,9 Three cognitive domains were assessed:
Immediate Memory:
Recall of two stories from the Logical Memory subtest of the Wechsler Memory Scale,10 and recall of the 9 symbols following completion of the Wechsler Adult Intelligence Scale (WAIS) Digit Symbol Substitution Test.11
Delayed Recall:
Recall of the 2 Logical Memory stories after a 10 to 15-minute delay.
Psychomotor and Mental Efficiency:
Verbal Fluency (total correct for letters F, A, and S), WAIS Digit Symbol Substitution Test (total correct in 90-seconds), time to complete Trail Making Part B, and time to complete the Grooved Pegboard with the dominant and non-dominant hands.9 Both Trail Making Part B and Verbal Fluency are also considered to be measures of executive functioning although in an earlier factor analysis of the comprehensive test battery, both tests loaded on the psychomotor and mental efficiency domain.
For each of the test variables at each time point, a standardized z-score was calculated using the mean and standard deviations of the entire cohort from the DCCT baseline evaluation. Within each cognitive domain, the mean z-score was calculated for each individual providing a unit-free measurement of the relative improvement (positive z-score) or deterioration (negative z-score) in performance as compared with the total group at the DCCT baseline assessment.
The Montreal Cognitive Assessment (MoCA)12 was administered only at year 32. This is a widely-used screening instrument designed to assess mild impairment across multiple cognitive domains. Published cut-off scores that distinguish adults with possible mild cognitive impairment from healthy controls range from 25 or less to 17 or less; in these analyses, we used a score of 21 or less.13
Capillary blood glucose levels were monitored immediately prior to cognitive testing to ensure absence of acute hypoglycemia. Participants with blood glucose levels of 70 mg/dl (3·9 mmol/l) or less were provided a snack; cognitive testing commenced when blood glucose values reached 90 mg/dl (5·0 mmol/l) or more. There was no upper-level cut-off for blood glucose.
The research protocol was approved by the institutional review boards at all sites, and all participants provided written informed consent.
Statistical Analysis
Generalized linear mixed models, with a class variable for assessment time, evaluated the association of each summary z-score domain with individual risk factors and complications over time. A comprehensive multivariable regression model was developed for each cognitive domain using backward elimination, where variables significant at p<0·10 were retained at each step. The final multivariable model for each domain retained the selected covariates significant at p<0·05.
In addition to age and sex, models included each risk factor as a time-dependent covariate. Quantitative covariates were characterized by the time-weighted mean of all follow-up values since DCCT baseline up to each visit, weighting each value by the time interval since the last measurement. Categorical time-dependent covariates were defined as any prior report up to each visit.
We estimated the additional number of years of age that would yield the same decrement in psychomotor and mental efficiency as a unit increase in a risk factor (e.g. HbA1c) by taking the ratio of the coefficient (beta) estimate for the risk factor to the coefficient for age from a linear mixed model that included both factors.14
All analyses were performed using SAS software (version 9·4; SAS Institute, Cary, NC).
The funding source had no role in the study design, collection, analysis, interpretation of the data, or in the writing of the report.
RESULTS
The characteristics of the cohort at DCCT baseline and at 32 years of total follow-up, when participants ranged from 43 to 75 years of age with a median of 59 years, are shown in Table 1. We compared the participants who completed the most recent cognitive assessment (n=1,051) with those who did not due to death (n=187), inactive status (n=100) or failure to complete the cognitive protocol (n=103), and found that non-participants were more likely to be in the conventional treatment group, less likely to be college graduates, and had lower full-scale IQ scores, higher HbA1c, and higher lipids at DCCT baseline (Table S1).
Table 1.
Characteristics of Participants at Entry into DCCT and at an average of 32 Years of Follow-up (n = 1,051) *
| Characteristic | Entry into DCCT (1983-1989) |
32 Years Follow-up (2019) |
|---|---|---|
| Intensive treatment assignment in DCCT (%) | 53 | 53 |
| Demographic characteristics | ||
| Age [median (range) years] | 27 (13-39) | 59 (43-75) |
| Female sex (%) | 47 | 47 |
| White race (%)† | 96 | 96 |
| Education (years) | 14 ± 3 | 16 ± 2 |
| Professional or technical occupation (%) | 32 | 55 |
| Verbal IQ‡ | 112 ± 11 | ND |
| Full-scale IQ‡ | 114 ± 10 | ND |
| Medical history | ||
| Duration of diabetes [median (range) years] | 4 (1-15) | 37 (30-51) |
| History of hypertension (%)§ | 3 | 68 |
| History of hyperlipidemia (%)§ | 22 | 76 |
| Current cigarette smoker (%) | 15 | 7 |
| Current occasional or regular alcohol use (%) | 22 | 50 |
| Medication ¶ | ||
| ACE inhibitors or ARB (%) | ND | 43 |
| Lipid-lowering medication (%) | ND | 71 |
| Physical examination | ||
| Body mass index (kg/m2) | 23·3 ± 2·8 | 29·3 ± 5·8 |
| Blood pressure (mm Hg) | ||
| Systolic | 114 ± 12 | 124 ± 15 |
| Diastolic | 72 ± 9 | 69 ± 9 |
| Laboratory values | ||
| Glycosylated hemoglobin A1c (%) | 8·7 ± 1·5 | 7·8 ± 1·2 |
| Plasma lipids (mg/dl)∥ | ||
| Total cholesterol | 175 ± 33 | 171 ± 36 |
| HDL cholesterol | 51 ± 12 | 64 ± 20 |
| LDL cholesterol | 109 ± 29 | 92 ± 30 |
| Triglycerides | 78 ± 41 | 74 ± 53 |
| Complications †† | ||
| eGFR <60 mL/min/1·73 m2 (yes vs. no) | 0 | 13 |
| PDR (yes vs. no) | 0 | 26 |
| Cardiovascular disease (yes vs. no) | 0 | 15 |
Data are means ± SD or percent unless otherwise noted. ND denotes no data, ACE angiotensin-converting enzyme, ARB angiotensin-receptor blocker, HDL high-density lipoprotein, LDL low-density lipoprotein, PDR proliferative diabetic retinopathy, and eGFR estimated glomerular filtration.
Race was self-assigned. “White” denotes white, non-Hispanic.
The normative mean IQ score is 100 ± 15.
Hypertension was defined by a systolic blood pressure of at least 140 mm Hg, a diastolic blood pressure of at least 90 mm Hg, documented hypertension, or the use of antihypertensive medications. Hyperlipidemia was defined by an LDL cholesterol level of at least 130 mg per deciliter or the use of lipid-lowering medications.
Medication history was not obtained during the DCCT, but the use of ACE inhibitors was discouraged, and statins were not widely available or in use during the DCCT.
To convert values for cholesterol to millimoles per liter, multiply by 0·02586. To convert values for triglycerides to millimoles per liter, multiply by 0·1129.
Any report between DCCT baseline and 32 years of follow-up.
During the 32-year follow-up period, a total of 1,608 episodes of severe hypoglycemia leading to coma and/or seizure were reported in 482 participants; 398 reported 1-5 events and 84 reported more than 5 events (Table S2).
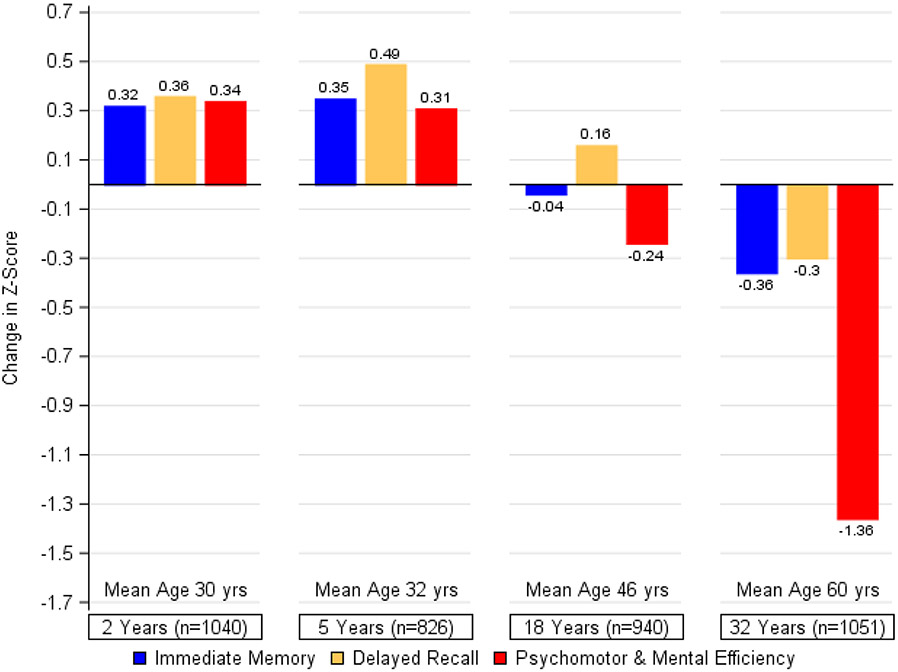
Cognitive performance over 32 years is illustrated in Figure 1. Small improvements on all 3 cognitive domains were evident at the 2- and 5-year assessments. By year 18, psychomotor and mental efficiency showed a modest but statistically significant decrease (change in z-score since baseline = −0·24, p<0·0001), and further decline (more than five-times) was observed from year 18 to year 32 (change in z-score since baseline −0·24 to −1·36, p<0·0001). Smaller declines in immediate and delayed recall were also observed at the most recent assessment (p<0·0001). An examination of differences on individual tests from baseline to year 32 showed substantive declines in psychomotor and mental efficiency ranging from 16% (Digit Symbol) to between 31% and 43% (Trail Making B; Grooved Pegboard). No changes were seen in verbal fluency (Table S3). Smaller changes were seen on the delayed and immediate recall of Logical Memory stories (11%; 13·5%) and memory for the digit-symbol pairs (5%).
Figure 1. Changes in Cognition during DCCT/EDIC.
The bars represent the changes within each of the cognitive domains between testing at DCCT baseline and each follow-up assessment, expressed as changes in z-scores and adjusted for age and sex at DCCT baseline and years of education as a time-dependent covariate. Other than a non-significant difference between years 2 and 5 for immediate memory (p=0·23) and psychomotor efficiency (p=0·49), change was significant over the years within each cognitive domain at p<0·0001.
Consistent with previous analyses at end of DCCT and again after 18 years of combined follow-up,5,15 prior treatment group assignment was not associated with cognitive change (Table 2). For that reason, data for the two treatment groups were pooled for all subsequent analyses.
Table 2.
Association of Traditional Glycemic and Non-Glycemic Risk Factors and Micro- and Macrovascular Complications with Cognitive Domains*
| Immediate Memory | Delayed Recall | Psychomotor and Mental Efficiency | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | t- value |
p-value | β | SE | t- value |
p-value | β | SE | t- value |
p-value | |
| Treatment group (conventional vs. intensive) | 0·027 | 0·041 | 0·65 | 0·52 | −0·010 | 0·054 | −0·19 | 0·85 | 0·028 | 0·052 | 0·53 | 0·59 |
| Demographic Characteristics | ||||||||||||
| Age (per 1 year) | −0·014 | 0·003 | −4·55 | <0·0001 | −0·011 | 0·004 | −2·90 | 0·0039 | −0·041 | 0·004 | −11·48 | <0·0001 |
| Sex (men vs. women) | −0·016 | 0·041 | −0·38 | 0·70 | −0·074 | 0·054 | −1·36 | 0·17 | −0·323 | 0·051 | −6·37 | <0·0001 |
| Education (per 1 year) | 0·034 | 0·006 | 5·23 | <0·0001 | 0·039 | 0·008 | 4·80 | <0·0001 | 0·104 | 0·010 | 10·96 | <0·0001 |
| Risk Factors | ||||||||||||
| Glycemic | ||||||||||||
| HbA1c (per 1 %)† | −0·023 | 0·012 | −1·84 | 0·066 | −0·015 | 0·016 | −0·98 | 0·33 | −0·114 | 0·018 | −6·38 | <0·0001 |
| Severe hypoglycemia‡ | ||||||||||||
| Cumulative (≥1 vs. 0 events) | −0·047 | 0·029 | −1·59 | 0·11 | −0·019 | 0·037 | −0·51 | 0·61 | −0·152 | 0·044 | −3·49 | 0·0006 |
| 1-5 events vs. 0 events | −0·039 | 0·029 | −1·32 | 0·19 | −0·013 | 0·037 | −0·34 | 0·73 | −0·139 | 0·044 | −3·14 | 0·0018 |
| >5 events vs. 0 events | −0·178 | 0·062 | −2·90 | 0·0040 | −0·124 | 0·078 | −1·60 | 0·11 | −0·320 | 0·094 | −3·40 | 0·0007 |
| Recent (≥1 vs. 0 events) | −0·006 | 0·061 | −0·10 | 0·92 | 0·020 | 0·076 | 0·27 | 0·79 | −0·242 | 0·098 | −2·48 | 0·015 |
| Non-glycemic | ||||||||||||
| Body mass index (per 1 kg/m2)† | 0·002 | 0·005 | 0·42 | 0·68 | 0·005 | 0·006 | 0·76 | 0·45 | −0·018 | 0·007 | −2·70 | 0·0071 |
| Blood pressure (per 5 mm Hg)† | ||||||||||||
| Systolic | −0·008 | 0·010 | −0·88 | 0·38 | −0·014 | 0·012 | −1·18 | 0·24 | −0·157 | 0·013 | −11·91 | <0·0001 |
| Diastolic | 0·018 | 0·014 | 1·22 | 0·22 | 0·013 | 0·018 | 0·72 | 0·47 | −0·030 | 0·020 | −1·46 | 0·15 |
| Pulse rate (per 1 bpm)† | −0·001 | 0·002 | −0·32 | 0·75 | 0·002 | 0·002 | 0·85 | 0·39 | −0·012 | 0·003 | −4·42 | <0·0001 |
| Plasma lipids (per 10 mg/dl)† | ||||||||||||
| Total cholesterol | −0·010 | 0·006 | −1·83 | 0·067 | −0·013 | 0·007 | −1·81 | 0·070 | −0·014 | 0·008 | −1·79 | 0·074 |
| HDL cholesterol | 0·008 | 0·012 | 0·65 | 0·51 | 0·001 | 0·015 | 0·06 | 0·95 | 0·063 | 0·017 | 3·77 | 0·0002 |
| LDL cholesterol | −0·005 | 0·006 | −0·95 | 0·34 | −0·009 | 0·007 | −1·32 | 0·19 | −0·008 | 0·008 | −1·00 | 0·32 |
| Triglycerides (log) | −0·259 | 0·317 | −0·82 | 0·41 | −0·704 | 0·403 | −1·75 | 0·080 | −2·254 | 0·467 | −4·83 | <0·0001 |
| Complications | ||||||||||||
| Kidney Disease | ||||||||||||
| Sustained AER ≥30 mg/24 hr (yes vs. no)§ | −0·016 | 0·034 | −0·46 | 0·65 | −0·041 | 0·043 | −0·95 | 0·34 | −0·331 | 0·050 | −6·61 | <0·0001 |
| eGFR <60 mL/min/1·73 m2 (yes vs. no)§ | −0·198 | 0·050 | −3·95 | 0·0001 | −0·162 | 0·063 | −2·57 | 0·011 | −0·866 | 0·080 | −10·88 | <0·0001 |
| Retinopathy | ||||||||||||
| PDR (yes vs. no)§ | −0·012 | 0·035 | −0·34 | 0·74 | −0·035 | 0·044 | −0·80 | 0·42 | −0·561 | 0·054 | −10·34 | <0·0001 |
| CSME (yes vs. no)§ | −0·081 | 0·033 | −2·43 | 0·016 | −0·094 | 0·042 | −2·25 | 0·025 | −0·455 | 0·051 | −8·85 | <0·0001 |
| Neuropathy | ||||||||||||
| Confirmed clinical neuropathy (yes vs. no)§ | −0·041 | 0·035 | −1·16 | 0·25 | −0·121 | 0·045 | −2·71 | 0·0072 | −0·527 | 0·052 | −10·05 | <0·0001 |
| Cardiovascular autonomic neuropathy (yes vs. no)§ | −0·091 | 0·032 | −2·85 | 0·0047 | −0·119 | 0·040 | −2·96 | 0·0033 | −0·476 | 0·049 | −9·74 | <0·0001 |
| Cardiovascular | ||||||||||||
| Cardiovascular disease (yes vs. no)§ | −0·124 | 0·046 | −2·71 | 0·0075 | −0·071 | 0·057 | −1·23 | 0·22 | −0·577 | 0·073 | −7·91 | <0·0001 |
Data are beta coefficients, standard errors, t-values, and p-values from individual generalized linear mixed models evaluating the association of each domain with each covariate of interest, with no adjustment for other factors. With the exception of treatment group, age, and sex, each covariate was entered into the model as a time-dependent covariate. Beta estimates are equal to the difference in means between groups or the slope of the association (e.g. increase or decrease in mean domain score for every 1-unit change in the time-dependent covariate). The signed t-value corresponds to the magnitude and directionality of the association. HDL denotes high-density lipoprotein, LDL low-density lipoprotein, AER albumin excretion rate, eGFR estimated glomerular filtration, PDR proliferative diabetic retinopathy, and CSME clinically-significant macular edema.
Risk factors were characterized by the time-weighted mean of all follow-up values since DCCT baseline up to each visit, weighting each value by the time interval since the last measurement.
Severe hypoglycemia was defined as events leading to coma or seizure documented by self-report for the 3-month period prior to each annual study visit. “Cumulative” refers to the running sum of all events up to each cognitive evaluation. “Recent” refers to the number of events recorded at the time of each cognitive evaluation.
Any report between DCCT baseline and each annual study visit.
Higher HbA1c levels (β±SE −0·114±0·018, p<0·0001) and higher systolic blood pressure (β±SE −0·157±0·013, p<0·0001) were associated with decline on tests of psychomotor and mental efficiency but not immediate or delayed memory (Table 2). A similar relationship was found for other risk factors, including elevated BMI, pulse rate, higher triglyceride level and lower HDL cholesterol. A history of severe hypoglycemia was also associated with a decline in psychomotor and mental efficiency (β±SE −0·152±0·044, p=0·0006) but not memory. This relationship was present when severe hypoglycemia was defined dichotomously (0 vs. 1 or more events). In addition, there was a dose-response relationship, with worsening performance associated with increasing number of severe hypoglycemic events (0, 1-5, more than 5). Micro- and macrovascular complications of diabetes, including kidney disease, retinopathy, clinical neuropathy and cardiovascular autonomic neuropathy, and cardiovascular disease were also associated with decline in both memory and psychomotor and mental efficiency.
Table 3 shows the multivariable modeling of risk factors and complications on cognitive function. For all cognitive domains, fewer years of education and older age were the most significant predictors of decline. After adjusting for all other covariates listed in the Table, higher HbA1c (β±SE −0·088±0·017, p<0.0001), a history of severe hypoglycemia (β±SE −0·178±0·040, p<0.0001), and higher systolic blood pressure (β±SE −0·013±0·003, p<0.0001) were independently associated with a decline in psychomotor and mental efficiency, as were measures of nephropathy, retinopathy, neuropathy, and cardiovascular disease. In addition to fewer years of education and older age, a history of impaired kidney function (β±SE −0·173±0·050, p=0·0008) was associated with a decline in immediate memory while a history of cardiovascular autonomic neuropathy (β±SE −0·101±0·040, p=0·013) was associated with a decline in delayed memory.
Table 3.
Multivariable Models for Cognitive Domains*
| Immediate Memory | Delayed Recall | Psychomotor and Mental Efficiency | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | t-value | p-value | β | SE | t-value | p-value | β | SE | t-value | p-value | |
| Age (per 1 year) | −0·015 | 0·003 | –4·96 | <0·0001 | −0·013 | 0·004 | –3·26 | 0·0011 | −0·039 | 0·003 | −11·70 | <0·0001 |
| Sex (men vs. women) | −0·265 | 0·047 | −5·66 | <0·0001 | ||||||||
| Education (per 1 year) | 0·036 | 0·007 | 5·60 | <0·0001 | 0·042 | 0·008 | 5·05 | <0·0001 | 0·102 | 0·009 | 11·61 | <0·0001 |
| HbA1c (per 1 %)† | −0·088 | 0·017 | −5·30 | <0·0001 | ||||||||
| Severe hypoglycemia (≥1 vs. 0 cumulative events)‡ | −0·178 | 0·040 | −4·50 | <0·0001 | ||||||||
| Systolic blood pressure (per 5 mm Hg)† | −0·013 | 0·003 | −4·98 | <0·0001 | ||||||||
| Pulse rate (per 1 bpm)† | −0·006 | 0·003 | −2·22 | 0·027 | ||||||||
| eGFR <60 mL/min/1·73 m2 (yes vs. no)§ | −0·173 | 0·050 | −3·44 | 0·0008 | −0·241 | 0·057 | −4·25 | <0·0001 | ||||
| PDR (yes vs. no)§ | −0·144 | 0·053 | −2·73 | 0·0067 | ||||||||
| CSME (yes vs. no)§ | −0·430 | 0·076 | −5·64 | <0·0001 | ||||||||
| Confirmed clinical neuropathy (yes vs. no)§ | −0·235 | 0·050 | −4·66 | <0·0001 | ||||||||
| Cardiovascular autonomic neuropathy (yes vs. no)§ | −0·101 | 0·040 | –2·50 | 0·013 | −0·257 | 0·046 | −5·55 | <0·0001 | ||||
| Cardiovascular disease (yes vs. no)§ | −0·293 | 0·069 | −4·22 | <0·0001 | ||||||||
Data are beta coefficients, standard errors, t-values, and p-values from three separate generalized linear mixed models evaluating the association of each domain with all of the risk factors entered into the model together. With the exception of age and sex, each covariate was entered into the model as a time-dependent covariate. Covariates that did not enter into any of the three models were not included in the table. Beta estimates are equal to the difference in means between groups or the slope of the association (e.g. increase or decrease in mean domain score for every 1-unit change in the time-dependent covariate). The signed t-value corresponds to the magnitude and directionality of the association. eGFR denotes estimated glomerular filtration, PDR proliferative diabetic retinopathy, and CSME clinically-significant macular edema.
Risk factors were characterized by the time-weighted mean of all follow-up values since DCCT baseline up to each visit, weighting each value by the time interval since the last measurement.
Severe hypoglycemia was defined as events leading to coma or seizure documented by self-report for the 3-month period prior to each annual study visit. “Cumulative” refers to the running sum of all events up to each cognitive evaluation.
Any report between DCCT baseline and each annual study visit.
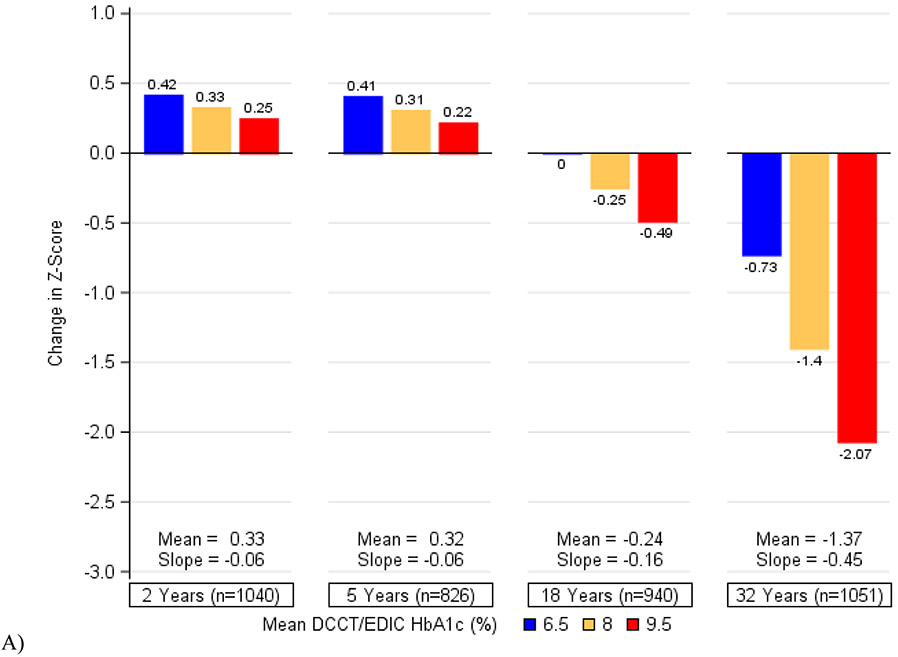
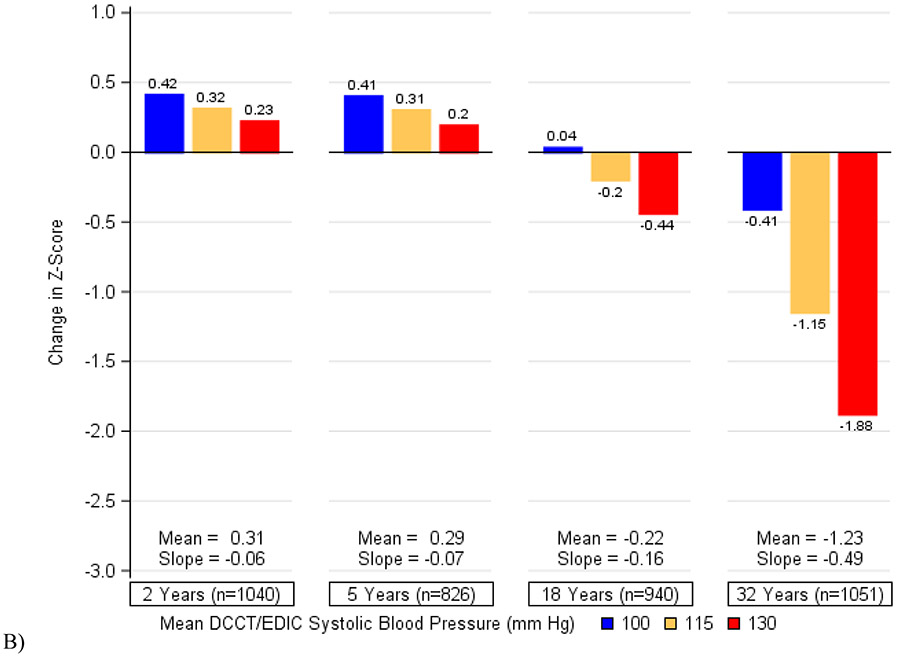
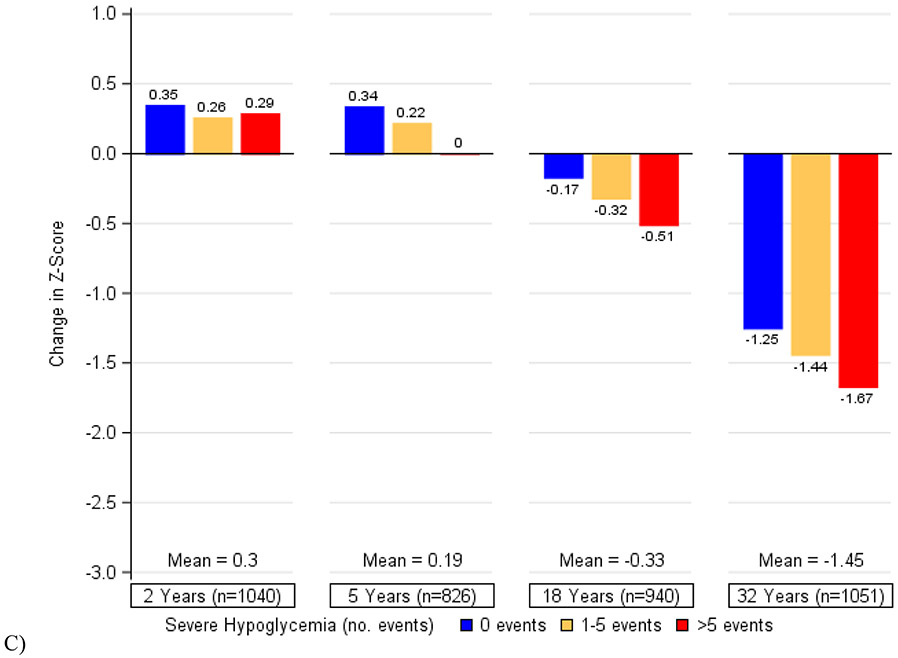
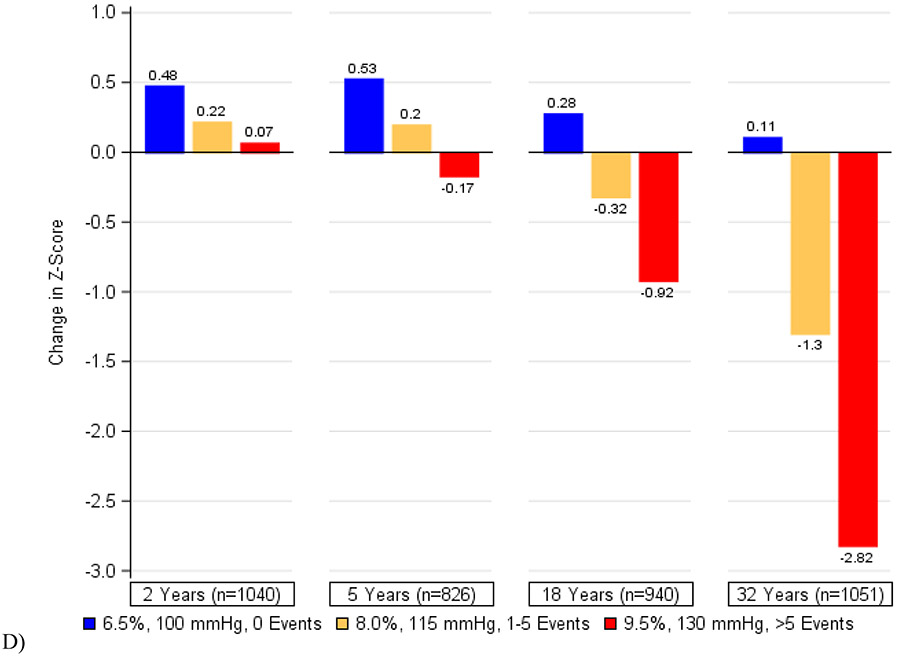
Figure 2 depicts the cumulative associations of (a) HbA1c, (b) systolic blood pressure, (c) a history of severe hypoglycemia, and (d) all three factors combined with decline in psychomotor and mental efficiency from DCCT baseline to year 32. Participants who maintained better control of these risk factors showed the least changes in cognition while those with the worst control exhibited the greatest declines. We found that in this cohort of individuals with type 1 diabetes, a 1-percentage point increase in mean HbA1c (e.g., 7% to 8%) was associated with the same decrement in psychomotor efficiency as an additional 3·3 years of age. Likewise, a 5-mmHg increase in mean systolic blood pressure was associated with the same decrement in psychomotor and mental efficiency as an additional 4·0 years of age and having 1 or more severe hypoglycemic events over the course of the study was associated with the same decrement as an additional 4·6 years of age. The combination of all three factors would have the equivalent effect of an additional 9·4 years of age in this type 1 diabetes population.
Figure 2. Changes in Psychomotor and Mental Efficiency during DCCT/EDIC at Specific Levels of A) Mean DCCT/EDIC HbA1c, B) Mean DCCT/EDIC Systolic Blood Pressure, C) Severe Hypoglycemia (Cumulative), and D) HbA1c, Systolic Blood Pressure, and Severe Hypoglycemia in Aggregate.
The bars represent the model-based estimates of the mean changes in psychomotor and mental efficiency between cognitive testing at DCCT baseline and each follow-up assessment, expressed as changes in z-scores and adjusted for age and sex at DCCT baseline and years of education as a time-dependent covariate. At each study year, the mean changes in z-scores and the slope for the association between HbA1c (or systolic blood pressure) and changes in z-scores are presented. There was a significant interaction between time and HbA1c (p=0·0003) as well as between time and systolic blood pressure (p<0·0001). There was no significant interaction between time and a history of severe hypoglycemia (p=0·45).
Results from the Montreal Cognitive Assessment indicated that the proportion of participants who met our criterion for possible mild cognitive impairment was quite low (Table S3, 5·5%; n=58). Scores ranged between 14 and 30, with a mean of 26·2±2·6, similar to values for the general population (25·6±2·9).16
DISCUSSION
This report provides the first description of cognitive change in adolescents and young adults with type 1 diabetes as they transition into later adulthood, over an average of 32 years. By capitalizing on the exceptionally well-characterized DCCT cohort, we have been able to document the association between diabetes-related exposures, aging, and cognitive decline.
As this group aged, measures of memory and psychomotor and mental efficiency declined significantly. Between follow-up years 18 and 32, the decrease in psychomotor and mental efficiency was approximately five times as large as the change from baseline to 18 years. The presence of micro- and macrovascular complications independently contributed to cognitive decline. After adjusting for these effects, exposure over time to higher HbA1c levels, more episodes of severe hypoglycemia, and elevated systolic blood pressure were associated with greater decrements in cognition that were most notable by an average age of 59. Combined, these three risk factors were associated with cognitive decline equivalent to an additional 9·4 years of age, thereby suggesting premature aging. Conversely, participants with the best glycemic control did not show any decrease in psychomotor and mental efficiency over the observation period.
Although damage to the cerebral vasculature contributes to the development of the cognitive decline seen in our cohort, we cannot rule out non-vascular factors. For example, elevated glutamate levels associated with persistent and acute elevations in blood glucose may adversely affect cognition.17,18 Our participants now display more pronounced decline in cognition as they age. This is consistent with the hypothesis that later adulthood is a period of greater susceptibility to developing neuro-cognitive alterations exacerbated by exposure to diabetes-related risk factors and complications.19 Alternatively, this may represent the accumulation of effects over a longer period of time.
Smaller changes in cognition have been reported previously in this cohort after an average of 6·515 and 185 years of follow-up. Circumscribed cognitive decline has also been associated with microvascular complications and hypoglycemia in two longitudinal studies comparing young and middle-age adults with type 1 diabetes to demographically similar adults without diabetes. Participants in the Pittsburgh Epidemiology of Diabetes Complications study were assessed over seven years with the same tests used in DCCT/EDIC and showed a significant decline, relative to control subjects, that was limited to measures of psychomotor efficiency and was associated with the occurrence of proliferative retinopathy and elevated systolic blood pressure.8 Another study compared diabetic adults with retinopathy to adults without diabetes over a 3.5-year period, and found that executive functioning declined significantly, but other cognitive domains remained essentially unchanged.20 A third study followed older adults with and without type 1 diabetes over a four-year period, and found no significant decline in either group. However, diabetic subjects who experienced one or more episodes of severe hypoglycemia during follow-up showed greater slowing on tests of information processing speed.21
Accelerated rates of cognitive decline have also been reported in large longitudinal studies of older adults with type 2 diabetes followed for periods ranging from 4 to 20 years.22-24 Memory, problem-solving, and psychomotor and mental efficiency were most often affected; these changes were most often associated with higher HbA1c values.22,24
Despite evidence of incipient cognitive decline, DCCT/EDIC participants showed a relatively low rate of possible mild cognitive impairment, as determined by the MoCA (5.5%), when compared to rates in older adults (mean age 68) with type 1 diabetes (11%)13 or to rates from studies in the general population (6·7%).25 Taking a different approach, three recent neuropsychological studies found rates of 11%26, 28%27 and 48%.28 Differences in methodology and in participant characteristics may account for these diverse findings.
Our tests of psychomotor and mental efficiency included measures of executive functioning that required individuals to focus their attention, follow rules, plan a series of actions, execute those actions rapidly, monitor their accuracy, correct errors appropriately, and often hold information in working memory – exactly the skills needed to manage type 1 diabetes and perform complex tasks at work and when driving. Poorer performance on tests like the Grooved Pegboard, Trail Making Part B, and Digit Symbol Substitution has been associated with greater difficulty performing everyday tasks like shopping and meal preparation29 as well as with laboratory-based and on-road measures of driving performance.30 Because we did not obtain direct measures of our participants’ ability to perform activities of daily living, such as driving, we were unable to determine whether this degree of cognitive change has a discernable impact on their everyday lives. Whether rates of cognitive impairment will accelerate as participants grow older remains to be determined.31 Furthermore, changes in one cognitive domain, like psychomotor and mental efficiency, may also presage changes in other domains – like reasoning and memory.32
A history of severe hypoglycemia was not previously associated with significant cognitive decline in this cohort both at the end of the DCCT15 as well as at the 18-year assessment.5,33 However, as our participants enter the period of aging-related neuropathology,19 a history of one or more severe hypoglycemic events appears to be associated with a significant decline in psychomotor and mental efficiency. This possibility is consistent with results from three recent cross-sectional studies of older adults with type 1 diabetes of long-duration.26,28,34 In general, individuals who experienced recent episodes of severe hypoglycemia – in the 12 months prior to assessment, were more likely to perform more poorly on global measures of cognition26, earn lower scores on tests of mental efficiency and nonverbal memory34, and have an increased risk of clinically significant impairment28. In our study and others, as the episodes of severe hypoglycemia increased, cognition declined, and risk of clinically-significant impairment increased.28
Previous research has demonstrated that one or more episodes of ketoacidosis may adversely affect cognition.35-36 However, because of the small number of episodes of ketoacidosis among our participants, we are unable to address those potential effects with a reliable analysis at this time.
Our study has a number of strengths. It is the first to prospectively assess changes in cognition over an extended period from adolescence to older adulthood in a large cohort whose biomedical and psychosocial characteristics have been assessed repeatedly and comprehensively. This design allows for examination of risk factors that change over time and precede the assessments of cognition. Rates of participation have been very high, with 92% of the surviving cohort still being followed.
Limitations include the absence of a comparison group of demographically-similar individuals without diabetes assessed repeatedly over time with the same cognitive measures. Because this assessment included only those cognitive domains previously found to be most sensitive to the effects of aging and glycemia, we are unable to determine the extent to which other cognitive domains may have declined between study years 18 and 32.
Our findings may not generalize to others with diabetes. The DCCT/EDIC participants were highly motivated to monitor their health, were self-selected to participate in a highly demanding clinical trial, highly-educated, and almost entirely Caucasian. A more representative sample of patients from clinical practice might demonstrate greater effects of risk factors on cognition. Finally, we cannot rule out the possibility that cognitive ability affected self-management of diabetes so that worse clinical outcomes were due to cognitive dysfunction, and not the reverse.
In summary, as adults with type 1 diabetes enter their late fifties or early sixties, the magnitude of cognitive decline increases significantly, particularly in individuals with a history of higher HbA1c, severe hypoglycemia, elevated blood pressure, and microvascular complications. Conversely, individuals who are able to maintain metabolic control closer to the non-diabetic range, lower blood pressure, and experience fewer episodes of severe hypoglycemia, have more limited cognitive decline as they age. This study reveals the cognitive path expected for individuals with long-standing type 1 diabetes as they age and brings into clear view the value of better management of glycemia and hypertension to change this course.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We present long-term follow-up data regarding cognitive functioning from the largest cohort of participants with type 1 diabetes (DCCT/EDIC) that has been thoroughly phenotyped for all diabetes complications, for over 30 years. These original and comprehensive analyses were placed in the context of other findings in the literature after extensive searches for from other clinical trials and observational studies evaluating cognitive functioning in MEDLINE, EMBASE, and the Cochrane Library Central Registry of Controlled Trials. Evidence from other cohorts of participants with type 1 diabetes, including the Pittsburgh Epidemiology of Diabetes Complications and the Melbourne Longitudinal cohort studies were carefully considered. However, none of these other cohorts were evaluated in such detail for cognitive functioning repeatedly for more than 30 years in a large number of participants that maintained more than 90% retention.
Added value of this study
This study applied repeated measures of identical cognitive assessments over the entire follow-up period and assessments of potentially modifiable biomedical characteristics in multivariable models to identify independent risk factors for cognitive decline as participants aged. No study of type 1 diabetes has examined these effects over time from adolescence and young adulthood to older adulthood. This valuable information can be used to guide clinicians and patients with type 1 diabetes in their care in order to prevent or delay cognitive impairment as an increasing number of patients with type 1 diabetes live longer.
Implications of all the available evidence
Cognitive functioning in older individuals is a challenging problem in the general population. Understanding the extent to which diabetes and its metabolic changes and complications adds to this risk can have direct impact on daily function. Treatment approaches to modify risk factors can ameliorate the risk of cognitive decline and therefore have a huge impact on patients’ quality of life and health care costs.
ACKNOWLEDGEMENTS
Funding/Support:
The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017, 2017-2022), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA. The funding source had no role in the study design, collection, analysis, interpretation of the data, or in the writing of the report.
Declaration of Interests:
JAL discloses being funded by K24AG045334, as well as consulting for vTv therapeutics, royalties from Springer, and a stipend from Wolters Kluwer. NC discloses personal fees and non-financial support from Eli Lilly, outside the submitted work. None of the other authors have any financial or personal conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893
Guarantor Statement: AMJ, CMR, and BHB are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AMJ had final responsibility for the decision to submit for publication.
Industry Support: Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton, Dickinson and Company (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi-Aventis (Bridgewater, NJ).
Data Sharing: Data collected for the DCCT/EDIC study through June 30th 2017 are available to the public through the NIDDK Repository (https://repository.niddk.nih.gov/studies/edic/). Data collected in the current cycle (July 2017-June 2022) will be available within two years after the end of the funding cycle.
REFERENCES
- 1.Diabetes Control and Complications Trial (DCCT) / Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care 2016; 39(8): 1378–83.27411699 [Google Scholar]
- 2.Oschwald J, Guye S, Liem F, et al. Brain structure and cognitive ability in healthy aging: a review on longitudinal correlated change. Rev Neurosci 2019; 31(1): 1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shalimova A, Graff B, Gasecki D, et al. Cognitive Dysfunction in Type 1 Diabetes Mellitus. J Clin Endocrinol Metab 2019; 104(6): 2239–49. [DOI] [PubMed] [Google Scholar]
- 4.van Duinkerken E, Ryan CM Diabetes mellitus in the young and the old: Effects on cognitive functioning across the life span. Neurobiol Dis 2020; 134: 104608. [DOI] [PubMed] [Google Scholar]
- 5.DCCT/EDIC Research Group. Long term effects of diabetes and its treatment on cognitive function. N Engl J Med 2007; 356: 1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–86. [DOI] [PubMed] [Google Scholar]
- 7.The DCCT/EDIC Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999; 22: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CM, Geckle MO, Orchard TJ Cognitive efficiency declines over time in adults with Type 1 diabetes: Effects of micro- and macrovascular complications. Diabetologia 2003; 46: 940–8. [DOI] [PubMed] [Google Scholar]
- 9.Strauss E, Sherman EMS, Spreen O A Compendium of Neuropsychological Tests. 3 ed. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 10.Wechsler D A standardized memory scale for clinical use. J Psychol 1945; 19: 87–95. [Google Scholar]
- 11.Wechsler D Manual for the Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1955 [Google Scholar]
- 12.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock RS, DuBose SN, Bergenstal RM, et al. Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2016; 39: 603–10. [DOI] [PubMed] [Google Scholar]
- 14.Bebu I, Braffett BH, Schade D, et al. An observational study of the equivalence of age and duration of diabetes to glycemic control relative to the risk of complications in the combined cohorts of the DCCT/EDIC Study. Diabetes Care 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DCCT Research Group. Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial. Ann Intern Med 1996; 124: 379–88. [DOI] [PubMed] [Google Scholar]
- 16.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 2011; 77(13): 1272–5. [DOI] [PubMed] [Google Scholar]
- 17.Lyoo IK, Yoon SJ, Musen G, et al. Altered prefrontal glutamate-glutamine-g-Aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Arch Gen Psychiatry 2009; 66: 879–87. [DOI] [PubMed] [Google Scholar]
- 18.Bolo NR, Jacobson AM, Musen G, Keshavan MS, Simonson DC Acute hyperglycemia increases brain pregenual anterior cingulate cortex glutamate concentrations in type 1 diabetes. Diabetes 2020; 69: 1528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biessels GJ, Deary IJ, Ryan CM Cognition and diabetes: A lifespan perspective. Lancet: Neurology 2008; 7: 184–90. [DOI] [PubMed] [Google Scholar]
- 20.van Duinkerken E, Steenwijk MD, Klein M, et al. Accelerated executive functions decline and gray matter structural changes in middle-aged type 1 diabetes mellitus patients with proliferative retinopathy. J Diabetes 2018; 10(11): 835–46. [DOI] [PubMed] [Google Scholar]
- 21.van Duinkerken E, Brands AMA, Van den Berg E, et al. Cognition in older patients with type 1 diabetes: A longitudinal study. J Am Geriatr Soc 2011; 59: 563–6. [DOI] [PubMed] [Google Scholar]
- 22.Tuligenga RH, Dugravot A, Tabák AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: A post-hoc analysis of the Whitehall II cohort study. Lancet: Diabetes & Endocrinology 2014; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spauwen PJJ, Köhler S, Verhey FRJ, Stehouwer CDA, Van Boxtel MPJ Effects of type 2 diabetes on 12 year cognitive change: Results from the Maastrict Aging Study. Diabetes Care 2013; 36: 1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol 2012; 69(9): 1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90(3): 126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacy ME, Gilsanz P, Eng C, Beeri MS, Karter AJ, Whitmer RA Severe Hypoglycemia and Cognitive Function in Older Adults With Type 1 Diabetes: The Study of Longevity in Diabetes (SOLID). Diabetes Care 2020; 43(3): 541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunley KA, Rosano C, Ryan CM, et al. Clinically relevant cognitive impairment in middle-aged adults with childhood-onset type 1 diabetes. Diabetes Care 2015; 38: 1768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaytor NS, Barbosa-Leiker C, Ryan CM, Germaine LT, Hirsch IB, Weinstock RS Clinically significant cognitive impairment in older adults with type 1 diabetes. J Diabetes Complications 2019; 33: 91–7. [DOI] [PubMed] [Google Scholar]
- 29.Chaytor NS, Riddlesworth TD, Bzdick S, et al. The relationship between neuropsychological assessment, numeracy, and functional status in older adults with type 1 diabetes. Neuropsychol Rehabil 2017; 27(4): 507–21. [DOI] [PubMed] [Google Scholar]
- 30.Wadley VG, Bull TP, Zhang Y, et al. Cognitive processing speed is strongly related to driving skills, financial abilities, and other instrumental activities of daily living in persons with MCI and mild dementia. J Gerontol A Biol Sci Med Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacy ME, Gilsanz P, Karter AJ, Quesenberry CP, Pletcher MJ, Whitmer RA Long-term Glycemic Control and Dementia Risk in Type 1 Diabetes. Diabetes Care 2018; 41(11): 2339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucker-Drob EM, Brandmaier AM, Lindenberger U Coupled cognitive changes in adulthood: A meta-analysis. Psychol Bull 2019; 145(3): 273–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson AM, Ryan CM, Cleary PA, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia 2011; 54: 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan CM, Klein BEK, Lee KE, Cruickshanks KJ, Klein R Associations between recent severe hypoglycemia, retinal vessel diameters, and cognition in adults with type 1 diabetes. J Diabetes Complications 2016; 30: 1513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacy ME, Gilsanz P, Eng CW, Beeri MS, Karter AJ, Whitmer RA Recurrent diabetic ketoacidosis and cognitive function among older adults with type 1 diabetes: findings from the Study of Longevity in Diabetes. BMJ Open Diabetes Research and Care 2020; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aye T, Mazaika PK, Mauras N, et al. Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care 2019; 42: 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.