Abstract
To better characterize the mechanisms responsible for RNA export from the nucleus, we developed an in vitro assay based on the use of permeabilized HeLa cells. This new assay supports nuclear export of U1 snRNA, tRNA, and mRNA in an energy- and Xenopus extract-dependent manner. U1 snRNA export requires a 5′ monomethylated cap structure, the nuclear export signal receptor CRM1, and the small GTPase Ran. In contrast, mRNA export does not require the participation of CRM1. We show here that NXT1, an NTF2-related protein that binds directly to RanGTP, strongly stimulates export of U1 snRNA, tRNA, and mRNA. The ability of NXT1 to promote export is dependent on its capacity to bind RanGTP. These results support the emerging view that NXT1 is a general export factor, functioning on both CRM1-dependent and CRM1-independent pathways of RNA export.
In eukaryotic cells, molecular exchanges between the nucleus and the cytoplasm occur through the nuclear pore complex (NPC). Members of the karyopherin β family, also known as importins and exportins, specifically interact with cargos and mediate their nuclear import and nuclear export, respectively (33, 52). Nucleocytoplasmic transport also requires the small GTPase Ran (35, 37). The compartmentalization of the RanGDP exchange factor RCC1 in the nucleus (38) and the RanGTPase-activating protein (RanGAP) in the cytoplasm (17) is thought to provide a steep gradient of RanGDP (cytoplasmic)/RanGTP (nuclear) across the nuclear envelope that ensures the directionality of nuclear transport. More precisely, importins bind their import substrates in the absence of RanGTP (in the cytoplasm) and release them upon binding to RanGTP (in the nucleus) (34). In contrast, exportins interact with export cargos in a RanGTP-dependent manner (in the nucleus), and GTP hydrolysis on Ran in the cytoplasm triggers dissociation of exportin-cargo complexes (33).
Nearly all nucleus-encoded RNAs require export to the cytoplasm as part of their maturation process or biosynthetic function. RNAs are transported as ribonucleoprotein (RNP) complexes, and considerable effort has been devoted to understanding the protein- and RNA-based signals that specify export to the cytoplasm. Mature tRNA are directly recognized by a karyopherin β-like export receptor, exportin t or Los1p, in a RanGTP-dependent manner (4, 16, 28, 31). Transport of spliceosomal U snRNAs depends on their 5′ monomethylated cap structure, a cis-acting nuclear export signal (NES) that interacts with a nuclear cap-binding complex (CBC) composed of CBP20 and CBP80 (15, 20). The leucine-rich nuclear export receptor, CRM1 or exportin 1 (12, 39, 48), in cooperation with RanGTP is required for the transport of U snRNAs and CBC to the cytoplasm, but it remains unclear whether CBC, CRM1, and RanGTP represent the minimal machinery responsible for the nuclear export of U snRNAs (11). CRM1 is also responsible for the nuclear export of human immunodeficiency virus (HIV) mRNAs that contain a Rev-responsive element, recognized by the NES-containing HIV Rev protein (9–11, 32).
The mechanisms responsible for mRNA export are unclear, but Ran-dependent pathways involving different soluble proteins likely coexist and converge to the NPC. hnRNP proteins associating with mRNA during or just after transcription could provide NESs. In particular, hnRNP A1 contains an NES and is thought to stimulate mRNA export (18, 36). Similarly, Npl3, a major shuttling hnRNP protein in yeast, is thought to be involved in mRNP export (29). Another factor involved in mRNA export, the yeast Mex67p protein, was identified by its genetic interaction with the nucleoporin Nup85p (46). Mex67p binds directly to mRNA, forms a tight complex with Mtr2p (24), and interacts with Nup85p via Mtr2p (44). The human homolog of Mex67p, TAP, is required for the nuclear export of RNA containing a constitutive transport element found in simple type D retroviruses but could also mediate transport of cellular mRNA (14, 40). In mammalian cells, TAP physically interacts with a 15-kDa protein termed p15 (25) that is 26% identical to NTF2, a RanGDP-binding protein that mediates nuclear import of Ran (42, 47, 49). Coexpression of TAP and p15 partially complements the growth defect of the Mex67p/Mtr2 null mutant, suggesting that these proteins carry out related functions in mammals and yeast, respectively (25). More recently, p15 (referred to as NXT1) has been shown to stimulate nuclear protein export of protein kinase inhibitor in a pathway that relies upon CRM1 as the receptor (5). It was also shown that NXT1 binds specifically to RanGTP and that it colocalizes with the NPC, suggesting it may play a regulatory and/or targeting function in the CRM1 export pathway (5).
To define the roles of individual proteins in the transport of RNA, we set up an assay that reconstitutes RNA export in vitro. We used the assay to show that nuclear export of U1 snRNA and that of tRNA and mRNA proceed through CRM1-dependent and -independent pathways, respectively. Nuclear export of these different classes of RNA relies on NXT1 as a cofactor. Mutations in NXT1 that reduce binding to RanGTP also reduce its ability to stimulate U1 snRNA and tRNA export, indicating that an interaction with RanGTP is required for NXT1-dependent stimulation of RNA export.
MATERIALS AND METHODS
In vitro transcription.
cDNA encoding U3 snRNA (gift of J. P. Bachellerie, Centre National de la Recherche Scientifique, [CNRS], Toulouse, France), was subcloned in pGEM1 in positive orientation relative to the promoter for T7 polymerase. The plasmid was linearized with BamHI. Expression vectors encoding U1ΔSm snRNA, Rab11, and tRNAMet were provided by I. Mattaj (European Molecular Biology Laboratory, Heidelberg, Germany), B. Goud (CNRS, Paris, France), and E. Lund (Madison, Wis.) and linearized with BamH1, HindIII, and MvaI, respectively.
For in vitro transcription, linearized plasmid (3 μg) was incubated for 60 min at 37°C in a 30-μl transcription mixture containing transcription buffer (Promega), 10 mM dithiothreitol (DTT), 0.1 mg of bovine serum albumin (BSA) per ml, 0.8 mM ATP, CTP, and UTP, 0.2 mM GTP, 0.8 mM m7G(5′)ppp(5′)G cap analog except for tRNA (Boehringer Mannheim), 40 U of RNasin (Promega), 50 μCi of [α-32P]GTP (Amersham), and 60 U of T7 polymerase (Promega). DNA was then digested by 4 U of RNase-free DNase I (Promega) for 15 min at 37°C, RNAs were extracted with phenol-chloroform, and unincorporated nucleotides were removed with a Sephadex G-50 column. RNA was precipitated and resuspended in 25 μl of H2O. Fluorescently labeled RNA transcripts were generated by the same procedure but with different concentrations of nucleotides: 1 mM ATP and CTP, 0.25 mM GTP, 1 mM m7G(5′)ppp(5′)G cap analog, 1 mM aminoallyl-UTP–UTP (1:15; Sigma). Resulting RNA was supplemented with 15 mM 5 (and 6)-carboxy-X-rhodamine (succinimidyl ester; Molecular Probes) dissolved in dimethyl formamide (Sigma) and 37.5 mM bicarbonate buffer (pH 9). The labeling reaction was performed at room temperature for 1 h. Unincorporated fluorochromes were removed by gel filtration on a Sephadex G-50 column. RNA was precipitated and resuspended in 20 μl of H2O.
Preparation of Xenopus egg extracts and membranes.
Female Xenopus laevis frogs were purchased from the Elevage de Xenopes du CNRS (Montpellier, France). A 10,000 × g crude extract was prepared from unfertilized Xenopus eggs resuspended in lysis buffer consisting of 250 mM sucrose, 70 mM KCl, 2.5 mM MgCl2, and 20 mM PIPES-KOH (pH 7.5), supplemented with 1 mM DTT, 5 μg of cytochalasin B per ml, 10 μg each of aprotinin, leupeptin, and pepstatin per ml, and 200 μg of 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF; Sigma) per ml. This crude extract was then separated into supernatant, membrane phase, and pellet fraction by ultracentrifugation at 120,000 × g for 60 min at 4°C. The supernatant was recentrifuged at 200,000 × g for 30 min at 4°C, complemented with glycerol 5% (vol/vol), aliquoted, frozen in liquid nitrogen, and stored at −80°C until use (Xenopus extracts). Membranes were collected, diluted in 10 volumes of cold lysis buffer, and centrifuged at 40,000 × g for 30 min at 4°C. Pelleted membranes were resuspended, diluted in 10 volumes of cold lysis buffer, and spun through a cushion of lysis buffer containing 0.5 M sucrose (10,000 × g, 20 min, 4°C). Membranes were recovered in the minimal volume of cold lysis buffer, aliquoted, frozen in liquid nitrogen, and stored at −80°C until use.
Cell permeabilization.
HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and penicillin-streptomycin. Cells were dissociated with cell dissociation solution (Sigma), washed twice in phosphate-buffered saline (PBS), and resuspended at 106 cells/ml in ice-cold piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer containing 50 mM PIPES-KOH (pH 7.4), 50 mM KCl, 5 mM MgCl2, 2 mM EGTA, 1 mM DTT, 1 μg each of aprotinin, leupeptin, and pepstatin per ml, and 200 μg of AEBSF (Sigma) per ml, supplemented with 50 μg of digitonin (Sigma) per ml at 4°C. Digitonin-permeabilized cells were then pelleted, washed twice, finally resuspended at 107 cells/ml in PIPES buffer containing 5% dimethyl sulfoxide, and frozen in 100-μl aliquots at −80°C.
Freshly thawed digitonin-treated HeLa cells (106 cells) were permeabilized with 0.0725% decanoyl-N-methylglucamide (MEGA-10; Sigma) in a final volume of 3 ml PIPES buffer for 6 min at 4°C with occasional gentle mixing. Reaction was stopped by addition of 4% nuclease-free BSA (Sigma) in PIPES buffer. Permeabilized nuclei were pelleted by centrifugation at 210 × g for 5 min and washed twice in 1 ml of PIPES buffer. Pelleted nuclei were resuspended to 107 nuclei/ml and used immediately.
Nuclear envelope repair.
MEGA-10-permeabilized nuclei were repaired by incubation with membranes and extracts prepared from Xenopus eggs. Typically, 10 μl of nuclei (105 nuclei) was mixed with an equal volume of membranes and supplemented with 5.5 μl of Xenopus extracts and an energy-regenerating system (1 mM ATP, 1 mM GTP, 6 mM creatine phosphate, 4.8 U of creatine phosphokinase per ml). The samples were incubated at 20°C for up to 60 min. Routinely, aliquots were taken and assayed for nuclear exclusion of fluorescein isothiocyanate (FITC)-labeled immunoglobulin G.
In vitro nuclear export assay.
Typically, 105 permeabilized nuclei in PIPES buffer were incubated for 5 min at room temperature with 1.5 pmol of labeled RNA, 0.5 mM m7GTP (except for tRNA), and 20 U of RNasin and then repaired as described previously. Repaired nuclei were washed three times in PIPES buffer by centrifugation at 210 × g for 5 min at room temperature and resuspended in 10 μl of PIPES buffer (104 nuclei/μl). Export of labeled RNA was followed under different conditions as described in Results. A standard 20-μl assay was performed in PIPES buffer containing an energy-regenerating system, FITC-labeled BSA coupled to the simian virus 40 large T antigen nuclear localization signal (BSA-NLS-FITC) (20 μg/ml) (13), 10 μl of Xenopus extracts (50%, final concentration), and 105 repaired nuclei. Transport was allowed to proceed for 45 to 90 min at 20°C.
When radiolabeled RNAs were introduced into nuclei, export reactions were separated into nuclear and supernatant fractions by centrifugation at 210 × g for 5 min at room temperature; both fractions were supplemented with 200 μl of homomedium buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 1.5% sodium dodecyl sulfate, 300 mM NaCl, 1.5 mg of proteinase K per ml), incubated for 45 min at 50°C, and extracted twice with phenol-chloroform. RNAs were precipitated, resuspended in 5 μl of H2O, and analyzed on an 8% polyacrylamide–7 M urea gel. When indicated, results were quantified using the Bioprint acquisition system and Bioprofil program (Vilbert Lourmat).
When rhodamine-labeled snRNAs were introduced into nuclei, samples were diluted to 200 μl with 20 mM PIPES-KOH (pH 7.4)–70 mM KCl–2.5 mM MgCl2–250 mM sucrose and fixed by addition of 200 μl of 6% paraformaldehyde–100 mM PIPES-KOH (pH 7.5) for 5 min on ice; nuclei were spun through a 1-ml sucrose cushion (30% [wt/vol] in PIPES buffer) onto polylysine-coated coverslips (1,891 × g, 5 min). Coverslips were briefly washed in water, incubated with DAPI (4′,6-diamidino-2-phenylindole) dye for 1 min, and mounted in PBS containing 50% glycerol. Confocal laser scanning microscopy and fluorescence analysis were performed with a TCS4D confocal microscope based on a DM microscope interfaced with a mixed-gas argon-krypton laser (Leica Laser Technik).
Indirect immunofluorescence.
Digitonin-permeabilized cells (105) or repaired nuclei samples were diluted in 200 μl with 20 mM PIPES-KOH (pH 7.4)–70 mM KCl–2.5 mM MgCl2–250 mM sucrose and fixed by addition of 200 μl of 6% paraformaldehyde–100 mM PIPES-KOH (pH 7.5) for 5 min on ice; nuclei were spun through a 1-ml sucrose cushion (30% [wt/vol] in PIPES buffer) onto polylysine-coated coverslips (1,891 × g, 5 min). Coverslips were briefly washed in PBS and treated with 0.1% Triton X-100 for 5 min. Primary antibodies were applied for 30 min followed by a 30-min incubation with FITC- or Texas red-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Coverslips were then incubated with DAPI dye for 1 min and mounted in PBS containing 50% glycerol. Rabbit polyclonal antibodies against gp210 and Nup84 were a gift from J. C. Courvalin and R. Bastos, respectively. Mouse monoclonal antibody (MAb) to Nup153 was a gift from B. Burke, and MAb 414 was obtained from (BAbCo, Richmond, Calif.).
Electron microscopy.
Cells treated in different conditions were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 h at 4°C. They were washed in the same buffer and then postfixed for 30 min at 4°C with 2% osmic acid (OsO4) in water. They were dehydrated in ethanol before embedding in Epon. Ultrathin sections were counterstained with uranyl acetate and viewed with an electron microscope (CM120TEM; Philips, Eindoven, The Netherlands).
Expression of recombinant proteins.
Expression plasmid for glutathione S-transferase (GST)–Ran was a gift from M. Dasso (National Institutes of Health, Bethesda, Md.), and wild-type Ran (Ran wt) was purified as described previously (8). RanQ69L was expressed and purified essentially as described elsewhere (27), using an expression vector provided by C. Dingwall (Stony Brook, N.Y.). His-tagged NTF2 expression and purification were performed as described elsewhere (37a), using the NTF2 expression vector provided by G. Blobel (The Rockefeller Institute, New York, N.Y.). Expression plasmid for GST-M9 was a gift from G. Dreyfuss (University of Pennsylvania, Philadelphia); GST-M9 and -M9 mutant (GST-M9mut) were purified as described previously (41).
Untagged NXT1 proteins were expressed in Escherichia coli (BL21) and purified by column chromatography as described elsewhere (5). Mutations of the NXT1 open reading frame were generated on the bacterial expression vector by using the QuickChange site-directed mutagenesis system (Stratagene, La Jolla, Calif.) and confirmed by sequencing.
RanGTP binding assay.
Solid-phase binding assays were performed as described elsewhere (5). Ran preloaded with [γ-32P]GTP was incubated in wells containing the indicated recombinant proteins. The bound Ran was eluted and quantitated by scintillation counting. Binding to wells containing BSA alone was subtracted from binding to wells containing NXT1 proteins. Binding assays were performed in duplicate, and the values shown represent the mean ± standard deviation.
RESULTS
Development of an in vitro RNA export assay.
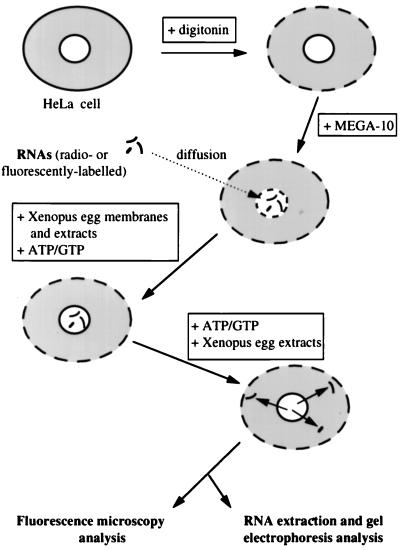
To characterize the molecular mechanisms responsible for nuclear export of RNA, an in vitro assay was developed (Fig. 1). Our assay is based on the use of semipermeabilized cells and was adapted from a previously described cell-free system for DNA replication (30). HeLa cells were first permeabilized with digitonin to remove the endogenous cytosol without affecting the integrity of the nuclear envelope (1). Treatment of the resulting permeabilized cells with the nonionic detergent MEGA-10 resulted in the permeabilization of the nuclear envelope. At this stage, radiolabeled or fluorescently labeled RNAs were introduced into nuclei by diffusion. The nuclear envelope was subsequently resealed upon addition of Xenopus egg membranes in the presence of the nucleotides ATP and GTP and Xenopus egg extracts. After extensive washing, RNA-loaded nuclei were incubated under different conditions, and nuclear export of RNA was analyzed either by direct fluorescence or by RNA extraction and gel electrophoresis.
FIG. 1.
Schematic representation of the in vitro nuclear export system. HeLa cells were permeabilized first with digitonin and then with the nonionic detergent MEGA-10, resulting in permeabilization of the nuclear envelope. At this stage, radiolabeled or fluorescently labeled RNAs were introduced into nuclei by free diffusion. The nuclear envelope was resealed upon addition of Xenopus egg membranes in the presence of the nucleotides ATP and GTP and Xenopus egg extracts. After extensive washing, RNA-loaded nuclei were submitted to different incubation conditions, and nuclear export of RNA was analyzed either by direct fluorescence or by RNA extraction and gel electrophoresis.
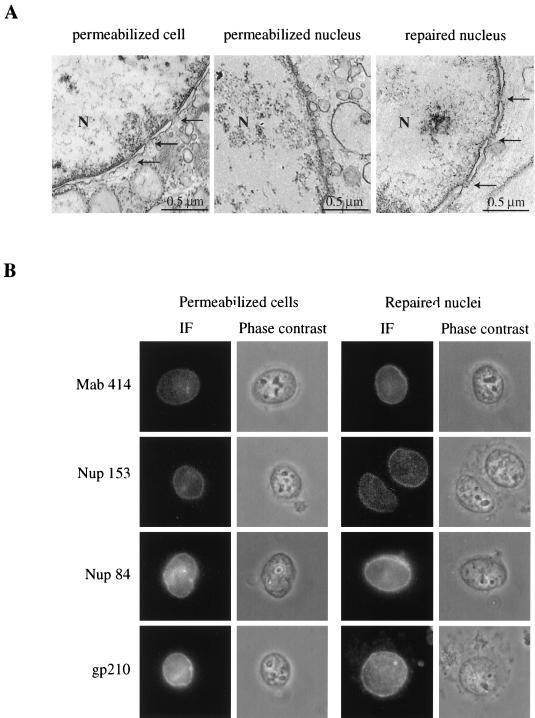
The morphological integrity of the nuclear envelope and NPC was examined at each step by both electron microscopy analysis (Fig. 2A) and indirect immunofluorescence with different antinucleoporin antibodies (Fig. 2B). As previously described, nuclei from digitonin-treated cells displayed an intact nuclear envelope, and rim-like staining was observed using antibodies directed against nucleoporins localized in the transmembrane domain (gp210), at the cytoplasmic face (Nup84), or at the nuclear face (Nup153) of the NPC or recognizing members of the O-linked family of nucleoporins (p62, Nup214, Nup153) (MAb 414). In contrast, the characteristic of the double membrane of the nuclear envelope from MEGA-10-permeabilized nuclei appeared completely disorganized and formed vesicles. Resealing with Xenopus egg membranes restored a normal-looking nuclear envelope with inner and outer membranes and NPC. Immunofluorescence analysis using antinucleoporin antibodies revealed that the normal repertoire of proteins was present at the pore. Moreover, NPC staining observed with the anti-Nup153 antibody which does not recognize the Xenopus Nup153 indicated that NPC of repaired nuclei contained human nucleoporins. However, the presence of Xenopus nucleoporins in NPC of repaired nuclei cannot be formally excluded.
FIG. 2.
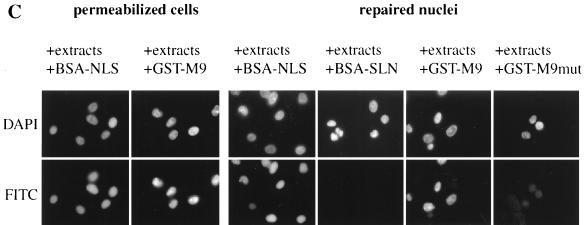
The nuclear envelope is morphologically and functionally intact after permeabilization and resealing. (A) Samples corresponding to each step of the procedure used were fixed with glutaraldehyde and processed for thin sectioning and transmission electron microscopy. N, nucleus. Arrows point to NPCs. (B) Indirect immunofluorescence (IF) labeling of digitonin-permeabilized HeLa cells or repaired nuclei, using antibodies against O-linked nucleoporins (MAb 414), Nup153, Nup84, or gp210. (C) Digitonin-permeabilized HeLa cells or repaired nuclei were incubated for 45 min with 50% Xenopus extracts and FITC-BSA-NLS (20 μg/ml), FITC-BSA-SLN (20 μg/ml), FITC-GST-M9 (50 μg/ml), or FITC-GST-M9mut (50 μg/ml) at 20°C with an energy-regenerating system. After incubation, cells were processed for direct fluorescence.
The functional integrity of the nuclear envelope was analyzed by testing nuclear import of two different cargos, FITC-BSA-NLS and FITC-GST fused to the M9 sequence of hnRNPA1 (GST-M9) (23, 41), which are imported by the importins α/β and transportin-mediated pathways, respectively (Fig. 2C). Nuclei from digitonin-treated cells accumulated BSA-NLS and GST-M9 after incubation at 20°C in the presence of Xenopus egg extracts and energy. In agreement with the drastic morphological change observed after MEGA-10 treatment, permeabilized nuclei were not able to import BSA-NLS (data not shown). In contrast, nuclear import of both BSA-NLS and GST-M9 was restored after nuclear envelope repair. No import was observed using BSA coupled to a reverse NLS peptide (BSA-SLN) or GST fused to a mutant M9 (G274→A). These data indicate that the permeabilization and resealing procedure gave rise to nuclei competent for protein import.
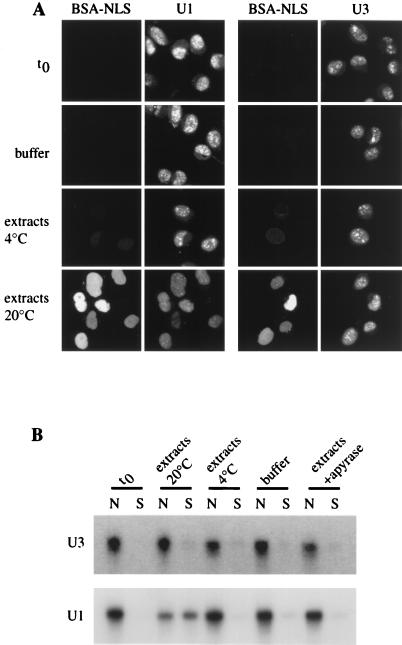
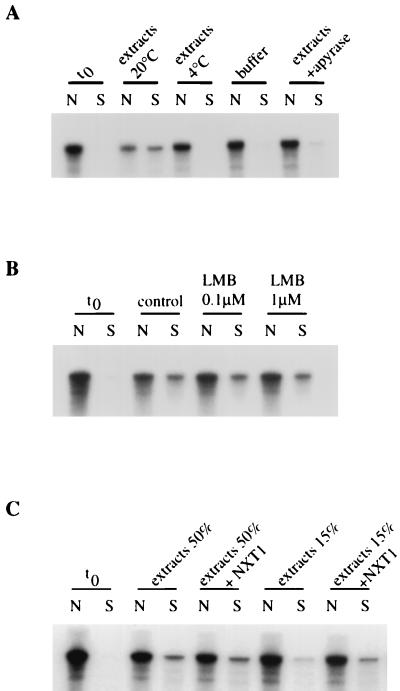
We examined the capacity of the in vitro assay to support nuclear export of U snRNAs. For this purpose, we used both U1ΔSm snRNA (U1), a U1 snRNA mutant lacking the Sm binding site that is therefore unable to be reimported in the nucleus after its export (21, 22), and U3 small nucleolar RNA (U3), an RNA species that is retained in the nucleus in vivo (50). For this purpose, radiolabeled or rhodamine-labeled U1 and U3 were synthesized in vitro and introduced into permeabilized nuclei by free diffusion. Following nuclear envelope repair, the RNA-loaded nuclei were extensively washed to remove RNA background from the cytosol. Nuclei were then incubated for 90 min with BSA-NLS in transport buffer at 20°C or with 50% Xenopus extracts in the presence of an energy-regenerating system at 20 or 4°C or with 50% Xenopus extracts in the presence of apyrase at 20°C. Nuclei were then fixed and observed by direct fluorescence using confocal microscopy (Fig. 3A). Alternatively, incubation reactions were centrifuged to separate nuclei from incubation medium containing exported substrates; RNAs were extracted from both fractions and analyzed by denaturing gel electrophoresis and autoradiography (Fig. 3B). Prior to initiating the transport reaction (time zero [t0]), both U1 and U3 were detected exclusively in the nucleus. RNA appeared mainly localized in nuclear dots, with a small fraction in the nucleoplasm. Incubation of U1- or U3-containing nuclei in the absence of extracts, at 4°C or in the presence of apyrase, allowed neither BSA-NLS nuclear import nor nuclear export of RNAs which remained intact in the nucleus. In contrast, addition of X. laevis egg extracts and ATP at 20°C led to the nuclear accumulation of BSA-NLS and a significant decrease in the nuclear content of rhodamine-labeled U1. RNA extraction and electrophoretic analysis indicated that radiolabeled U1 was exported out of the nucleus in a full-length form (52% ± 8% of loaded RNA was exported). Damage of repaired nuclei appeared after 90 min and prevented export at longer incubation times from being measured. No export of U3 (7% ± 6%) was observed under the same experimental conditions. Thus, our assay reconstitutes nuclear export of U1 in a cytosol- and energy-dependent manner.
FIG. 3.
Nuclear export of U1 and U3 in vitro. Radiolabeled or rhodamine-labeled U1 and U3 were synthesized in vitro and introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were incubated for 90 min with FITC-BSA-NLS and 10% gelatin in transport buffer at 20°C, with 50% Xenopus extracts in the presence of an energy-regenerating system at 20 or 4°C, or with 50% Xenopus extracts in the presence of apyrase at 20°C, as indicated. (A) After incubation under different conditions, nuclei were processed for direct fluorescence and visualized by confocal microscopy. (B) Radiolabeled U1 and U3 were extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by denaturing gel electrophoresis.
U1 nuclear export requires GTP hydrolysis by Ran.
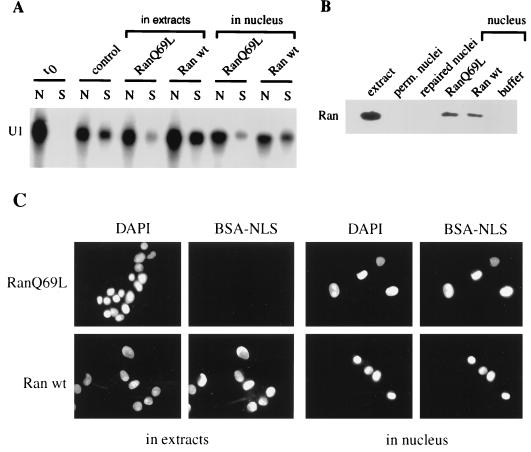
Exportins have been shown to bind their substrates in a RanGTP-dependent manner in the nucleus and release their cargo in the cytoplasm upon GTP hydrolysis (33). However, the translocation step of substrates like NES-containing proteins or tRNA does not require GTP hydrolysis by nuclear Ran (19, 43). To analyze whether the nuclear export of U1 in vitro depends on GTP hydrolysis by Ran, we compared the effects of Ran wt and Ran Q69L, added either in extracts or in nuclei, on both BSA-NLS import and U1 export. Addition of 10 μM Ran Q69L to the extracts resulted in the inhibition of both BSA-NLS import and U1 export (12.8% ± 3%) compared to what observed in the presence of 10 μM Ran wt (46% ± 2%) (Fig. 4A and C, left panel). Addition of the importin β-binding site of importin α in extracts also blocked nuclear import of BSA-NLS as well as U1 export (data not shown). The effect of cytoplasmic RanQ69L on U1 export was therefore likely due to protein import defect rather than an inhibition of U1-CRM1-RanQ69L dissociation after translocation.
FIG. 4.
Nuclear export of U1 in vitro requires GTP hydrolysis by Ran. (A) Effect of cytoplasmic and nuclear RanQ69L on U1 export. To analyze the effect of cytoplasmic RanQ69L, nuclear export of U1 was performed in 50% Xenopus extracts and energy (control) or supplemented with 10 μM RanQ69L (RanQ69L, extracts) or 10 μM Ran wt (Ran wt, extracts). To analyze the effect of nuclear RanQ69L, repaired nuclei containing radiolabeled U1 were incubated with either 28 μM RanQ69L (RanQ69L, nucleus) or 28 μM Ran wt (Ran wt, nucleus). After a 20-min incubation at room temperature, nuclei were washed, resuspended in 50% Xenopus extracts, and processed for nuclear export. After 60 min of incubation, U1 was then extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis. (B) Expression of Ran in Xenopus extracts (extract), in MEGA-10-permeabilized (perm.) nuclei, in repaired nuclei, and in nuclei incubated for 20 min at room temperature with 28 μM RanQ69L, 28 μM Ran wt, or PIPES buffer was analyzed by Western blotting using an anti-Ran MAb. (C) Effect of cytoplasmic and nuclear RanQ69L on protein import. The procedure was the same as for panel A. The import of FITC-BSA-NLS present in the export reaction was followed by direct fluorescence.
To analyze whether U1 nuclear export would require nuclear GTP hydrolysis by Ran, U1-loaded nuclei were repaired and then incubated with 28 μM RanQ69L or Ran wt before being washed and processed for nuclear export reaction. In contrast to permeabilized or repaired nuclei that did not express any Ran protein detectable by Western blotting, nuclei incubated in the presence of Ran wt or mutant retained a significant amount of Ran protein (approximately 5 to 10 μM; Fig. 4B). Nuclear export of U1 was strongly affected in RanQ69L-loaded nuclei (6.5% ± 4%), whereas Ran wt-containing nuclei did not display any defect in U1 export (32% ± 3.1%) (Fig. 4A). These experimental conditions did not affect the ability of nuclei loaded with Ran wt or RanQ69L to import BSA-NLS (Fig. 4C, right panel) indicating that the export inhibition by nuclear RanQ69L did not result from an import defect.
These data are consistent with previous data showing that U1 export in Xenopus oocytes is inhibited by the nuclear injection of RanQ69L and indicate that GTP hydrolysis by Ran is necessary for efficient export of this RNA (19).
5′ monomethylated cap structure and CRM1 are required for U1 export in vitro.
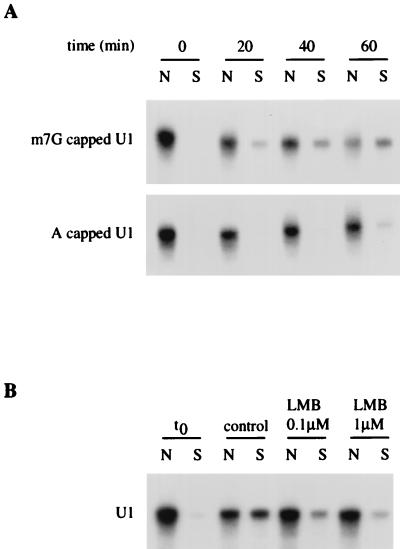
Two specific factors have been shown to efficiently stimulate nuclear export of U1 snRNA: CBC (15, 20), which directly recognizes the monomethylated cap structure m7GpppG of U snRNAs, and CRM1, the receptor for leucine-rich NESs (11). To analyze whether U1 was exported by a cap-dependent pathway in vitro, radiolabeled U1 snRNA capped with a m7GpppG cap or an unmethylated ApppG cap was introduced into permeabilized nuclei, and export was analyzed following nuclear envelope repair (Fig. 5A). After 40 min of incubation, 31% ± 12% of m7GpppG-capped U1 was exported, while only 7% ± 6% of ApppG-capped U1 had left the nucleus during the same period of time. The m7GpppG cap structure therefore strongly increases the export rate of U1 in vitro, as previously shown by microinjection into Xenopus oocyte nuclei (15, 51). This result indicates that interaction between the cap structure and CBC is required for nuclear export of U1 in this reconstitution export assay.
FIG. 5.
Nuclear export of U1 in vitro requires a 5′ monomethylated cap structure and is inhibited by LMB. (A) In vitro nuclear export of U1 is facilitated by the presence of a cap guanosine structure. Radiolabeled U1 containing m7GpppG cap (m7G capped U1) or ApppG cap (A capped U1) was synthesized in vitro and introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were incubated with 50% Xenopus extracts in the presence of an energy-regenerating system at 20°C for different times. U1 was then extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis. (B) In vitro nuclear export of U1 is inhibited by LMB. Radiolabeled U1 was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were resuspended either in 50% Xenopus extracts (control) or in 50% Xenopus extracts pretreated with 0.1 or 1 μM LMB (45 min, room temperature). After 60 min of incubation at 20°C, U1 was extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis.
To determine whether CRM1 is responsible for the nuclear export of U1 in the in vitro system, U1 export was analyzed upon addition of leptomycin B (LMB), a cytotoxin that specifically binds CRM1 and inhibits its export receptor function (11, 39). As shown in Fig. 5B, U1 export was already strongly reduced upon treatment of both nuclei and extracts with 0.1 μM LMB and almost completely inhibited in the presence of 1 μM LMB (13% ± 4% of exported RNA compared to 42% ± 5% in the control), strongly suggesting that U1 is exported by a CRM1-dependent pathway.
NXT1 stimulates nuclear export of U1 in vitro.
NXT1, a mammalian 15-kDa protein related to NTF2, has been recently identified to bind the mRNA export factor TAP (25) and to specifically interact with RanGTP with nanomolar affinity (5). Moreover, NXT1 has been found to facilitate CRM1-dependent nuclear export of both PKI (5) and HIV type 1 Rev (J. M. Holaska and B. M. Paschal, unpublished observations). To determine whether NXT1 promotes CRM1-dependent nuclear export of U1, we supplemented RNA export assay with purified recombinant NXT1. Addition of NXT1 to a transport reaction containing 50% Xenopus extract had no effect (Fig. 6A). Since NXT1 is likely to be present in the Xenopus extract, we performed transport reactions in the presence of a subsaturating level of extract (15%) in the absence and presence of NXT1 protein. NXT1 induced a clear stimulation of U1 export, and the effect was even more pronounced at the higher concentration of NXT1 tested (Fig. 6A). In contrast, NXT1 was unable to induce nuclear export of U3, an RNA restricted to the nucleus (Fig. 6B). Moreover, NXT1 had no effect on nuclear import of BSA-NLS (data not shown). U1 export rate was not affected upon addition of NTF2 (Fig. 6C), indicating that the stimulatory effect of NXT1 on U1 export was specific. Finally, addition of NXT1 to a transport reaction without extracts did not promote any U1 export (Fig. 6C). The NXT1-stimulated export of U1 was inhibited by LMB (data not shown), confirming that NXT1 is involved in an export pathway for which CRM1 is the receptor. We note that LMB also blocks NXT1-stimulated export of PKI in permeabilized cells as well (5).
FIG. 6.
NXT1 facilitates nuclear export of U1 in vitro. (A) Radiolabeled U1 was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were incubated either in 50% Xenopus extracts alone or supplemented with NXT1 (100 μg/ml) or in 15% Xenopus extracts alone or supplemented with NXT1 (100 or 250 μg/ml), as indicated. (B) Radiolabeled U3 was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were incubated in 50% Xenopus extracts or in 15% Xenopus extracts alone or supplemented with NXT1 (250 μg/ml). (C) Radiolabeled U1 was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were incubated either in 50% Xenopus extracts or in 15% Xenopus extracts alone or supplemented with NXT1 (250 μg/ml) or NTF2 (250 μg/ml), as indicated; alternatively, nuclei were incubated with NXT1 (250 μg/ml) in the absence of extracts (NXT1). After 60 min of incubation at 20°C, U1 was extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis.
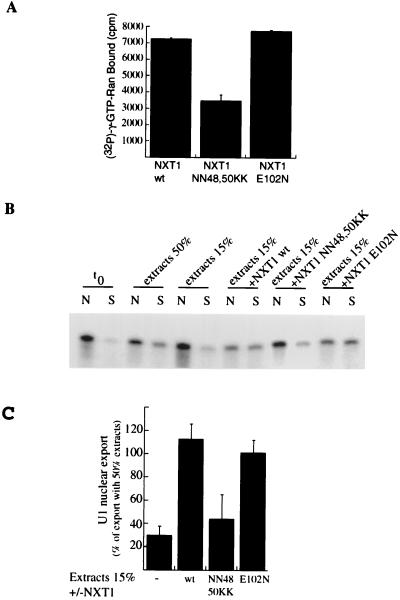
We predicted that NXT1 stimulation of U1 export relies upon its ability to bind RanGTP. To test this, we generated NXT1 mutants by changing residues that are adjacent to its Ran-binding domain. These include two asparagines (N48 and N50) and a glutamate (E102) that are predicted to surround the hydrophobic pocket of NXT1 where RanGTP is thought to bind (5). The interaction of the NN48,50KK mutant with RanGTP was reduced twofold, as measured in a solid-phase binding assay (Fig. 7A). We found that the NXT1 NN48,50KK mutant is 2.5-fold less effective than NXT1 wt in its ability to stimulate U1 export (Fig. 7B). In contrast, the E102N mutation did not affect U1 export or RanGTP binding (Fig. 7). Our results indicate that NXT1 modulates CRM1-dependent export of U1 snRNA through a mechanism that involves RanGTP.
FIG. 7.
The interaction of NXT1 with RanGTP is required to stimulate U1 export. (A) Ran preloaded with [γ-32P]GTP was incubated in wells containing the indicated immobilized NXT1 proteins. NXT1 wt binds RanGTP, as previously demonstrated (5). The NXT1 NN48,50KK mutant is deficient in binding RanGTP; however, this binding is unhindered for the NXT1 E102N mutant. (B) Radiolabeled U1 was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were resuspended either in 50% Xenopus extracts or in 15% Xenopus extracts alone or supplemented with NXT1 wt (250 μg/ml), NXT1 NN48,50KK, or NXT1 E102N. After a 60-min incubation at 20°C, U1 was extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis. (C) Quantification of results from three independent experiments. The value of nuclear export observed at t0 was subtracted from each condition, and results were expressed as a percentage of nuclear export occurring in the presence of 50% extracts.
NXT1 stimulates nuclear export of tRNA and mRNA in vitro.
To determine whether NXT1 function is restricted to the CRM1-dependent nuclear export pathway or is involved more generally in other export routes, we tested the ability of recombinant NXT1 to stimulate tRNA and mRNA export in vitro. For this purpose, tRNAMet (31) and mRNA encoding Rab11 were in vitro transcribed and introduced in permeabilized nuclei, and their export was analyzed in different incubation conditions following nuclear envelope repair. As for U1, the in vitro assay allowed the reconstitution of a cytosol and energy-dependent nuclear export of both tRNA and Rab11 mRNA (Fig. 8A and 9A). In contrast, treatment of nuclei and extracts with LMB did not affect the transport of this mRNA, indicating that Rab11 mRNA was not exported through a CRM1-dependent pathway in vitro (Fig. 9B).
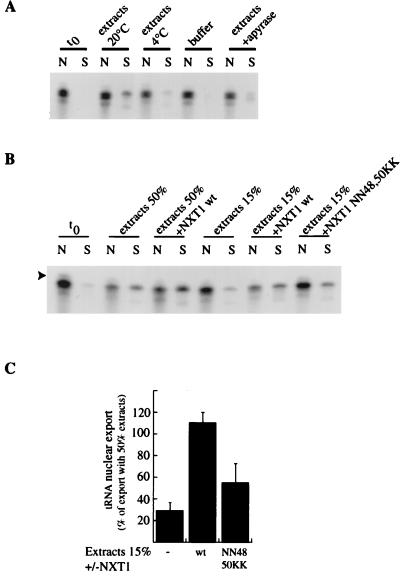
FIG. 8.
NXT1 facilitates nuclear export of tRNA in vitro. (A) Radiolabeled tRNAMet was synthesized in vitro and introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were incubated for 30 min with 10% gelatin in transport buffer at 20°C (buffer), with 50% Xenopus extracts in the presence of an energy-regenerating system at 20 or 4°C, or with 50% Xenopus extracts in the presence of apyrase at 20°C, as indicated. tRNAs were then extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by denaturing gel electrophoresis. (B) Radiolabeled tRNA was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were resuspended either in 50% Xenopus extracts alone or supplemented with NXT1 (100 μg/ml) or in 15% Xenopus extracts alone or supplemented with NXT1 wt (250 μg/ml) or NXT1 NN48,50KK. After a 30-min incubation at 20°C, tRNA was extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis. The arrowhead indicates the band corresponding to tRNA before maturation of its pppGGG-5′ end by RNaseP. (C) Results from three independent experiments were quantified. The value of nuclear export observed at t0 was subtracted from each condition, and results were expressed as percentage of nuclear export occurring in the presence of 50% extracts.
FIG. 9.
NXT1 facilitates nuclear export of mRNA in vitro. (A) Radiolabeled Rab11 mRNA was synthesized in vitro and introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were incubated for 90 min with 10% gelatin in transport buffer at 20°C (buffer), with 50% Xenopus extracts in the presence of an energy-regenerating system at 20 or 4°C, or with 50% Xenopus extracts in the presence of apyrase at 20°C, as indicated. mRNAs were then extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by denaturing gel electrophoresis. (B) Radiolabeled Rab11 mRNA was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were resuspended either in 50% Xenopus extracts (control) or in 50% Xenopus extracts pretreated with 0.1 or 1 μM LMB (45 min, room temperature). After 60 min of incubation at 20°C, mRNAs were extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis. (C) Radiolabeled Rab11 mRNA was introduced by diffusion into permeabilized nuclei. The nuclear envelope was then repaired (t0), and nuclei were resuspended either in 50% Xenopus extracts alone or supplemented with NXT1 (100 μg/ml) or in 15% Xenopus extracts alone or supplemented with NXT1 (250 μg/ml). After 60 min of incubation at 20°C, mRNAs were extracted from nuclear fractions (N) and from incubation buffer (S) and analyzed by gel electrophoresis.
A subsaturating concentration of extracts (15%) induced only a low level of tRNA and Rab11 mRNA export compared to the level observed with 50% extracts (respectively 29% ± 7% and 25% ± 10% of the export observed with 50% extracts). When recombinant NXT1 was added this a subsaturating concentration of extracts, the export level of tRNA and Rab11 mRNA was strongly increased (respectively 110% ± 10% and 77% ± 15% of the export observed with 50% extracts [Fig. 8B, 8C, and 9C). These data show that NXT1 protein is able to stimulate the CRM1-independent export of tRNA and mRNA. In addition, we found that the NXT1 NN48,50KK mutant is twofold less effective than NXT1 wt in its ability to stimulate tRNA export (Fig. 8B and C). These results indicate that the ability of NXT1 to bind RanGTP is required to activate nuclear export of tRNA.
DISCUSSION
We developed an assay that reconstitutes nuclear export of U snRNAs, tRNA, and mRNAs in vitro. Selective permeabilization of the plasma membrane and nuclear envelope of human cells permitted nuclear loading of a homogenous population of in vitro-transcribed RNAs. The ability of Xenopus egg membranes to repair permeabilized human nuclear envelope and restore selective protein import (6) was used to reseal RNA-loaded nuclei and study nuclear export. In agreement with the in vivo observations, not only was U1, tRNA, and Rab11 mRNA nuclear export extract and energy dependent but U1 transport was also strongly stimulated by the 5′ monomethylated cap structure (7, 15, 22). In contrast, U3 was retained into the nucleus both in vitro and in vivo despite its 5′ cap structure, indicating that reconstituted nuclei conserved the selectivity of nuclear export (50). Other reconstitution assays for RNA nuclear export have been previously described. Release of endogenous RNA from isolated mammalian nuclei has been studied previously and found to be not strictly dependent on energy, but in these studies neither integrity of nuclear envelope nor intactness of RNA was carefully controlled (2, 45). More recently, synthetic nuclei assembled in Xenopus egg extracts on paramagnetic DNA-coated beads containing a U1 template were shown to transcribe U1 RNA and support U1 nuclear export in a temperature- and energy-dependent manner. However, the precise nuclear export pathway in this model, and in particular the cap dependence of U1 transport, was not analyzed (3).
Nuclear export of U1 in vitro was inhibited upon addition of either the importin β-binding site of importin α or RanQ69L in the extracts, indicating that it likely requires ongoing NLS-mediated nuclear import or at least nuclear accumulation of essential export factors present in the extracts. U1 export depends on the integrity of its 5′ cap structure, a cis-acting export signal recognized by CBC (20). CBC is a shuttling complex whose NLS-dependent import is ensured by karyopherins α and β. One can assume that an efficient nuclear import of CBC present in the extracts is essential for the cap-dependent export of U1. As reported previously for studies using microinjection in Xenopus oocyte nucleus, U1 was exported by a CRM1-mediated pathway in vitro (11). However, whether CRM1 interacts directly with CBC or through an NES-containing bridging factor remains elusive. Although CRM1 binds NES-containing proteins in a RanGTP-dependent manner, GTP hydrolysis is not required for translocation but rather triggers dissociation of the cargo-CRM1 complex after translocation (26, 43). Surprisingly, data obtained both in vivo and in vitro indicate that expression of RanQ69L in the nucleus inhibits U1 nuclear export (19), suggesting that Ran could be required for an intranuclear step prior export complex assembly. Alternatively, an additional, essential and very limiting export factor could exist such that its GTP hydrolysis-dependent cytoplasmic release from the export complex and subsequent recycling is crucial for an efficient export of U1.
Using the in vitro assay, we show here that the NTF2-related protein NXT1 stimulates the nuclear export of U1 snRNA. NXT1 was found previously to activate the CRM1-dependent nuclear export of leucine-rich NES-containing protein (5) and thus represents a factor involved in both protein and RNA nuclear export routes mediated by CRM1. In addition, our results provide the first direct evidence that NXT1 is also involved in the CRM1-independent export of both tRNA and mRNA. Indeed, the interaction between NXT1 and the mRNA export factor TAP has been clearly established, but the specific role of NXT1 in the TAP-mediated mRNA export pathway remains elusive (25). The ability of NXT1 to interact with RanGTP is clearly required to activate nuclear export. NXT1 could stimulate the formation of or stabilize cargo-containing export complexes either by increasing affinity of export receptors for RanGTP or by binding to both RanGTP and export receptor. This hypothesis is supported by the ability of NXT1 to bind both RanGTP and TAP and by the involvement of Ran in mRNA export (19), although the precise role of Ran in the TAP-mediated mRNA export has not been established. Such a hypothesis would also predict that NXT1 is able to interact with CRM1 and exportin t. Alternatively, it has been shown that NXT1 localizes both in the nucleoplasm and to the NPC and is able to shuttle between nucleus and cytoplasm (5). NXT1 could thus target cargo-export receptor-RanGTP complexes to the NPC or mediate interaction of these export complexes with specific nucleoporins during translocation through the NPC. Although NXT1 has no effect on classical NLS import in vitro (5), the possibility that NXT1 could stimulate RanGTP import and play a role analogous to that of NTF2 in RanGDP import cannot be formally excluded. Future experiments should yield additional clues to the function of NXT1 in nuclear export.
ACKNOWLEDGMENTS
B.O.N. and C.M. contributed equally to this work.
We thank B. Wolff (Novartis) for the generous gift of leptomycin B, I. W. Mattaj, J. P. Bachellerie, B. Goud, and E. Lund for U1ΔSm, U3, rab11, and tRNA constructs, respectively, G. Dreyfuss for GST-M9, J. C. Courvalin for anti-gp210 antibodies, R. Bastos for anti-Nup, and B. Burke for anti-Nup. We are grateful to D. Tenza and G. Raposo for their help in electron microscopy.
This work was supported by grants from the Association de Recherche contre le Cancer and the Ligue contre le Cancer to C.D. and by funds from the American Cancer Society grant RPG98-048-01-CSM to B.M.P. B.O.N. is supported by Rhone Poulenc Rorer.
REFERENCES
- 1.Adam S A, Marr R S, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agutter P S, McArdle H J, McCaldin B. Evidence for involvement of nuclear envelope nucleoside triphosphatase in nucleocytoplasmic translocation of ribonucleoprotein. Nature. 1976;263:165–167. doi: 10.1038/263165a0. [DOI] [PubMed] [Google Scholar]
- 3.Arts G J, Englmeier L, Mattaj I W. Energy- and temperature-dependent in vitro export of RNA from synthetic nuclei. Biol Chem. 1997;378:641–649. doi: 10.1515/bchm.1997.378.7.641. [DOI] [PubMed] [Google Scholar]
- 4.Arts G J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 5.Black B E, Lévesque L, Holaska J M, Wood T C, Paschal B M. Identification of an NTF2-related factor that binds Ran-GTP and regulated nuclear protein export. Mol Cell Biol. 1999;19:8616–8624. doi: 10.1128/mcb.19.12.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coverley D, Downes C S, Romanowski P, Laskey R A. Reversible effects of nuclear membrane permeabilization on DNA replication: evidence for a positive licensing factor. J Cell Biol. 1993;122:985–992. doi: 10.1083/jcb.122.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dargemont C, Kuhn L C. Export of mRNA from microinjected nuclei of Xenopus laevis oocytes. J Cell Biol. 1992;118:1–9. doi: 10.1083/jcb.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasso M, Seki T, Azuma Y, Ohba T, Nishimoto T. A mutant form of the Ran/TC4 protein disrupts nuclear function in Xenopus laevis egg extracts by inhibiting the RCC1 protein, a regulator of chromosome condensation. EMBO J. 1994;13:5732–5744. doi: 10.1002/j.1460-2075.1994.tb06911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 10.Fischer U, Meyer S, Teufel M, Heckel C, Luhrmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 13.Gorlich D, Prehn S, Laskey R A, Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994;79:767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 14.Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber B K, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 15.Hamm J, Mattaj I W. Monomethylated cap structures facilitate RNA export from the nucleus. Cell. 1990;63:109–118. doi: 10.1016/0092-8674(90)90292-m. [DOI] [PubMed] [Google Scholar]
- 16.Hellmuth K, Lau D M, Bischoff F R, Kunzler M, Hurt E, Simos G. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol Cell Biol. 1998;18:6374–6386. doi: 10.1128/mcb.18.11.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopper A K, Traglia H M, Dunst R W. The yeast RNA1 gene product necessary for RNA processing is located in the cytosol and apparently excluded from the nucleus. J Cell Biol. 1990;111:309–321. doi: 10.1083/jcb.111.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izaurralde E, Jarmolowski A, Beisel C, Mattaj I W, Dreyfuss G, Fischer U. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J Cell Biol. 1997;137:27–35. doi: 10.1083/jcb.137.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izaurralde E, Kutay U, von Kobbe C, Mattaj I W, Gorlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izaurralde E, Lewis J, Gamberi C, Jarmolowski A, McGuigan C, Mattaj I W. A cap-binding protein complex mediating U snRNA export. Nature. 1995;376:709–712. doi: 10.1038/376709a0. [DOI] [PubMed] [Google Scholar]
- 21.Izaurralde E, Stepinski J, Darzynkiewicz E, Mattaj I W. A cap binding protein that may mediate nuclear export of RNA polymerase II-transcribed RNAs. J Cell Biol. 1992;118:1287–1295. doi: 10.1083/jcb.118.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarmolowski A, Boelens W C, Izaurralde E, Mattaj I W. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins Y, McEntee M, Weis K, Greene W C. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways. J Cell Biol. 1998;143:875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadowaki T, Hitomi M, Chen S, Tartakoff A M. Nuclear mRNA accumulation causes nucleolar fragmentation in yeast mtr2 mutant. Mol Biol Cell. 1994;5:1253–1263. doi: 10.1091/mbc.5.11.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung J U, Hurt E. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehlenbach R H, Dickmanns A, Kehlenbach A, Guan T, Gerace L. A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. J Cell Biol. 1999;145:645–657. doi: 10.1083/jcb.145.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klebe C, Bischoff F R, Ponstingl H, Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995;34:639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- 28.Kutay U, Lipowsky G, Izaurralde E, Bischoff F R, Schwarzmaier P, Hartmann E, Gorlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee M S, Henry M, Silver P A. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 1996;10:1233–1246. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- 30.Leno G H, Downes C S, Laskey R A. The nuclear membrane prevents replication of human G2 nuclei but not G1 nuclei in Xenopus egg extract. Cell. 1992;69:151–158. doi: 10.1016/0092-8674(92)90126-w. [DOI] [PubMed] [Google Scholar]
- 31.Lund E, Dahlberg J E. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science. 1998;282:2082–2085. doi: 10.1126/science.282.5396.2082. [DOI] [PubMed] [Google Scholar]
- 32.Malim M H, Hauber J, Le S Y, Maizel J V, Cullen B R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 33.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 34.Melchior F, Gerace L. Two-way trafficking with Ran. Trends Cell Biol. 1998;8:175–179. doi: 10.1016/s0962-8924(98)01252-5. [DOI] [PubMed] [Google Scholar]
- 35.Melchior F, Paschal B, Evans J, Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. . (Erratum, 124:217, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michael W M, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 37.Moore M S, Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- 37a.Moroianu J, Hijikata M, Blobel G, Radu A. Mammalian karyopherin alpha 1 beta and alpha 2 beta heterodimers: alpha 1 or alpha 2 subunit binds nuclear localization signal and beta subunit interacts with peptide repeat-containing nucleoporins. Proc Natl Acad Sci USA. 1995;92:6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohtsubo M, Okazaki H, Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989;109:1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 40.Pasquinelli A E, Ernst R K, Lund E, Grimm C, Zapp M L, Rekosh D, Hammarskjold M L, Dahlberg J E. The constitutive transport element (CTE) of Mason-Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J. 1997;16:7500–7510. doi: 10.1093/emboj/16.24.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollard V W, Michael W M, Nakielny S, Siomi M C, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 42.Ribbeck K, Lipowsky G, Kent H M, Stewart M, Gorlich D. NTF2 mediates nuclear import of Ran. EMBO J. 1998;17:6587–6598. doi: 10.1093/emboj/17.22.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards S A, Carey K L, Macara I G. Requirement of guanosine triphosphate-bound ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1844. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- 44.Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, Hurt E. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol. 1998;18:6826–6838. doi: 10.1128/mcb.18.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder H C, Friese U, Bachmann M, Zaubitzer T, Muller W E. Energy requirement and kinetics of transport of poly(A)-free histone mRNA compared to poly(A)-rich mRNA from isolated L-cell nuclei. Eur J Biochem. 1989;181:149–158. doi: 10.1111/j.1432-1033.1989.tb14706.x. [DOI] [PubMed] [Google Scholar]
- 46.Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, Hurt E. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith A, Brownawell A, Macara I G. Nuclear import of Ran is mediated by the transport factor NTF2. Curr Biol. 1998;8:1403–1406. doi: 10.1016/s0960-9822(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 48.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 49.Steggerda S M S, Black B E, Paschal B M. Monoclonal antibodies to NTF2 inhibit nuclear protein import by preventing nuclear translocation of the GTPase Ran. Mol Biol Cell. 2000;11:703–719. doi: 10.1091/mbc.11.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terns M P, Dahlberg J E. Retention and 5′ cap trimethylation of U3 snRNA in the nucleus. Science. 1994;264:959–961. doi: 10.1126/science.8178154. [DOI] [PubMed] [Google Scholar]
- 51.Terns M P, Dahlberg J E, Lund E. Multiple cis-acting signals for export of pre-U1 snRNA from the nucleus. Genes Dev. 1993;7:1898–1908. doi: 10.1101/gad.7.10.1898. [DOI] [PubMed] [Google Scholar]
- 52.Wozniak R W, Rout M P, Aitchison J D. Karyopherins and kissing cousins. Trends Cell Biol. 1998;8:184–188. doi: 10.1016/s0962-8924(98)01248-3. [DOI] [PubMed] [Google Scholar]