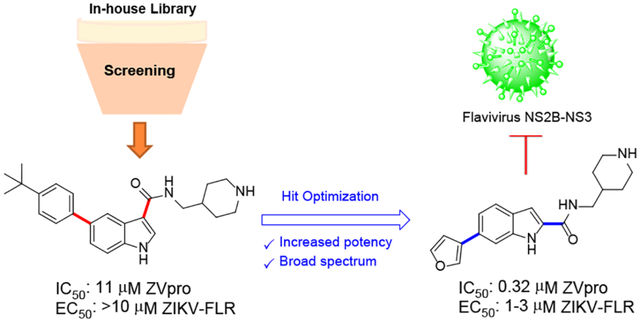
Abstract
Zika virus belongs to the Flavivirus family of RNA viruses, which include other important human pathogens such as dengue and West Nile virus. There are no approved antiviral drugs for these viruses. The highly conserved NS2B-NS3 protease of Flavivirus is essential for the replication of these viruses and it is therefore a drug target. Compound screen followed by medicinal chemistry optimization yielded a novel series of 2,6-disubstituted indole compounds that are potent inhibitors of Zika virus protease (ZVpro) with IC50 values as low as 320 nM. The structure-activity relationships of these and related compounds are discussed. Enzyme kinetics studies show the inhibitor 66 most likely exhibited a non-competitive mode of inhibition. In addition, this series of ZVpro inhibitors also inhibit the NS2B-NS3 protease of dengue and West Nile virus with reduced potencies. The most potent compounds 66 and 67 strongly inhibited Zika virus replication in cells with EC68 values of 1–3 μM. These compounds are novel pharmacological leads for further drug development targeting Zika virus.
Keywords: Zika virus, Flavivirus, NS2B-NS3 protease, structure-activity relationship, antiviral
Graphical Abstract
INTRODUCTION
Zika virus (ZIKV) belongs to the genus Flavivirus of the virus family Flaviviridae, which includes other important human pathogenic viruses such as yellow fever, dengue (DENV), West Nile virus (WNV)1. These viruses are transmitted among humans and other animal hosts by the bite of mosquitoes or another insect (such as ticks). ZIKV was first isolated in 1947 in the Zika Forest of Uganda in Africa2. ZIKV was found to cause only sporadic infection in humans in Africa and Asia3, 4. However, there have been three major ZIKV outbreaks in the Yap Island in the west Pacific Ocean in 2007 with several thousand cases5, 6, in the French Polynesia in the central Pacific Ocean in 2013 with ~28,000 cases7,8–10, and in Brazil in 2015–2016. The last one occurred in a much larger scale and quickly spread across Latin Americas and the southern United States with millions people infected11,12, 13. ZIKV infection causes mild, self-healing symptoms in human, including fever, rashes and conjunctivitis (red eyes). However, it causes a 20-fold increase in the incidence of more serious neurological diseases, including Guillain-Barré syndrome10, 14, 15 and microcephaly (small brain/head) in newborns16, 17,18. More than 4,000 cases of microcephaly with suspected ZIKV involvement were reported in Brazil. Because the prognosis of these infants to have normal brain functions is low and their life expectancy is significantly shorter, this will cause huge negative impacts to these families and the society.
DENV is also a major human pathogen. It infects as many as 400 million people every year, with ~100 million showing symptoms including fever, headache, rash, conjunctivitis and pain in muscle and joints19. However, ~500,000 cases/year develop serious and possibly life-threatening Dengue hemorrhagic fever (DHF) or Dengue shock syndrome (DSS) with symptoms including bleeding, severe vomiting with blood, black stools and drowsiness. ~22,000 people (mostly children) die of DENV per year.
ZIKV and DENV contain a single stranded, positive-sense RNA with ~11,000 nucleotides, which encodes a viral polyprotein of ~3,450 amino acids in length20, 21. The polyprotein is cleaved by the viral NS2B-NS3 protease and several human proteases to produce functional proteins, including, from N- to C-terminus, structural proteins C, prM, E, non-structural NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5. All of these proteins are necessary for the life cycle of the viruses. Given its essential role, the NS2B-NS3 proteases of ZIKV and DENV (ZVpro and DVpro) are promising drug targets20. A number of peptidomimetic compounds have been found to be potent inhibitors of ZVpro22–25 and DVpro20. However, they did not show potent cellular and in vivo antiviral activity, presumably due to poor cell permeability or metabolic stability. Non-peptidic compounds were also found to be inhibitors20, 26–31, but many of these compounds are relatively weak and their mode of inhibition as well as interaction with the protease is unknown. We recently reported a novel series of tri-substituted pyrazine-containing inhibitors of the proteases of ZIKV, DENV and WNV with IC50 values as low as 200 nM32, 33. These compounds also exhibited strong antiviral activities in cells and in a mouse model of ZIKV infection. Here, we report inhibitor discovery, chemical synthesis, structure-activity relationship (SAR) and other biochemical and cellular studies of a new series of 2,6-disubstituted indole-containing inhibitors (compounds 1-73) of the proteases of Zika, dengue and West Nile virus.
RESULTS AND DISCUSSION
Chemical synthesis.
The general methods for synthesis of these indole compounds are shown in Scheme 1A–E. Methyl indole-3-carboxylate 74 was brominated to give compound 75, which was hydrolyzed and subjected to an amide-formation reaction to produce the indole intermediate 76. The target compounds 1 and 67 were obtained by a Suzuki coupling reaction and deprotection of the tert-butyloxycarbonyl (Boc) group (Scheme 1A). Next, treatment of 5- or 6-bromoindole 77 with trifluoroacetic anhydride (TFAA) gave compound 78, which was hydrolyzed to give the corresponding indole-3-carboxylic acid. Amide formation reaction with a 4-amino-1-Boc-piperidine, or 4-aminomethyl-1-Boc-piperidine afforded 5- or 6-bromoindole-3-carboxamide 79, which underwent a Suzuki coupling reaction followed by deprotection to produce compounds 2, 7 and 14-32 (Scheme 1B). Compound 81 was prepared by a nucleophilic substitution reaction of compound 80 with Boc-protected 4-(3-bromopropyl)piperidine. Similar transformations of 81 yielded compound 3 (Scheme 1C). A Suzuki coupling reaction between 5-iodoindole and 4-tert-butylphenylboronic acid gave compound 83, which was reacted with 4-bromobenzyl bromide to afford 84. The target products 4 and 5 were obtained through a Suzuki coupling reaction of compound 84 (Scheme 1D). 5- or 6-bromoindole-carboxylic acid, obtained from its ester 85, was reacted with a 4-amino-1-Boc-piperidine, or 4-aminomethyl-1-Boc-piperidine to give indole-2-carboxamide 86, which underwent similar transformations to give compounds 6, 8 and 33-66 (Scheme 1E).
Scheme 1.
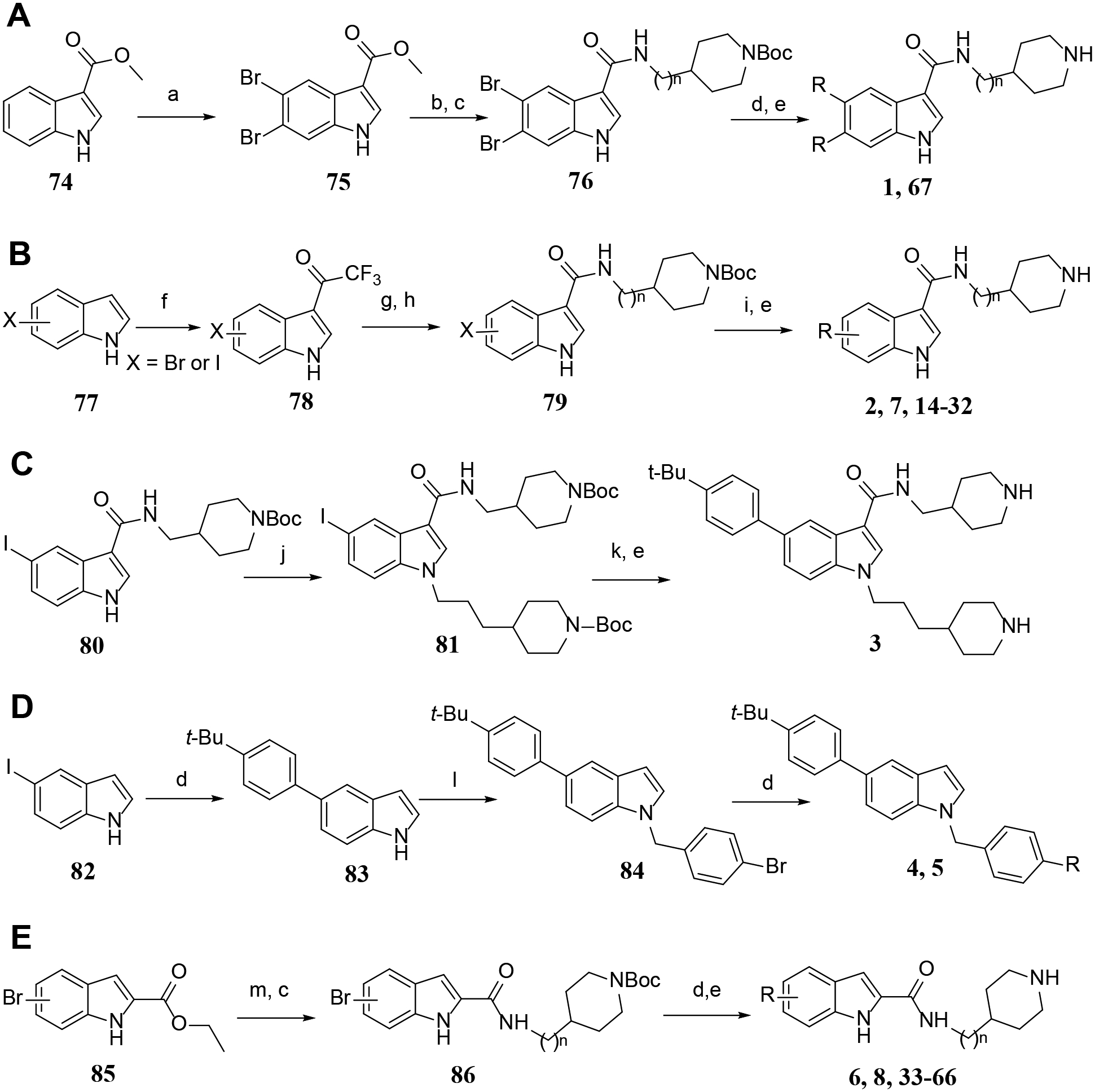
General methods for synthesis of indole compounds 1−8, and 14-67.a
aReagents and conditions: (a) Br2, AcOH, rt, 72 h; (b) NaOH, MeOH-H2O, 50 °C, 12 h; (c) HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate), diisopropylethylamine, DMF, 12 h, 4-amino-1-Boc-piperidine, or 4-aminomethyl-1-Boc-piperidine; (d) Aryl boronic acid or Aryl 4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Pd(PPh3)4, Na2CO3, p-dioxane-H2O, 100 °C; (e) HCl (4 N in p-dioxane), CH2Cl2, 0 °C; (f) TFAA, 0 °C to room temperature, 2 h; (g) NaOH (20% aq.), 80 °C, 2 days; (h) 4-aminomethyl-1-Boc-piperidine, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide (EDC), 1-Hydroxybenzotriazole (HOBt), triethylamine, CH2Cl2; (i) Aryl-boronic acid or Aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Na2CO3, toluene-DMF-EtOH, 80 °C, N2; (j) NaH, 1-Boc-4-(3-bromopropyl)piperidine, DMF, 14 h; (k) (4-(tert-butyl)phenyl)boronic acid, Pd(PPh3)4, Na2CO3, toluene-DMF-EtOH-H2O, 80 °C, 14 h; (l) 4-bromobenzyl bromide, K2CO3, DMF, 16 h; (m) NaOH, THF-H2O, 50 °C, 5 h.
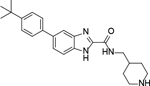
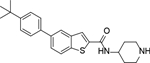
For synthesis of the pyrrolo[2,3-b]pyridine compounds 9 and 70-73 (Scheme 2A), a Friedel-Crafts acylation between 6-bromo-1H-pyrrolo[2,3-b]pyridine 87 and trichloroacetyl chloride gave compound 88 with a 3-trichloroacetyl group, which was hydrolyzed and subjected to an amide-forming and a Suzuki coupling reaction to afford, upon deprotection of the Boc group, the target compounds. 4-Bromobenzene-1,2-diamine 90 was subjected to a Suzuki coupling followed by a cyclization reaction with methyl 2,2,2-trichloroacetimidate to give benzimidazole 91 (Scheme 2B), whose trichloromethyl group was converted the corresponding carboxylic acid by hydrolysis. An amide-formation reaction followed by deprotection of Boc produced compound 10. With a similar route to synthesize compound 1, the benzofuran and benzothiophene analogs 11-13, 68, and 69 were prepared (Scheme 2C).
Scheme 2.
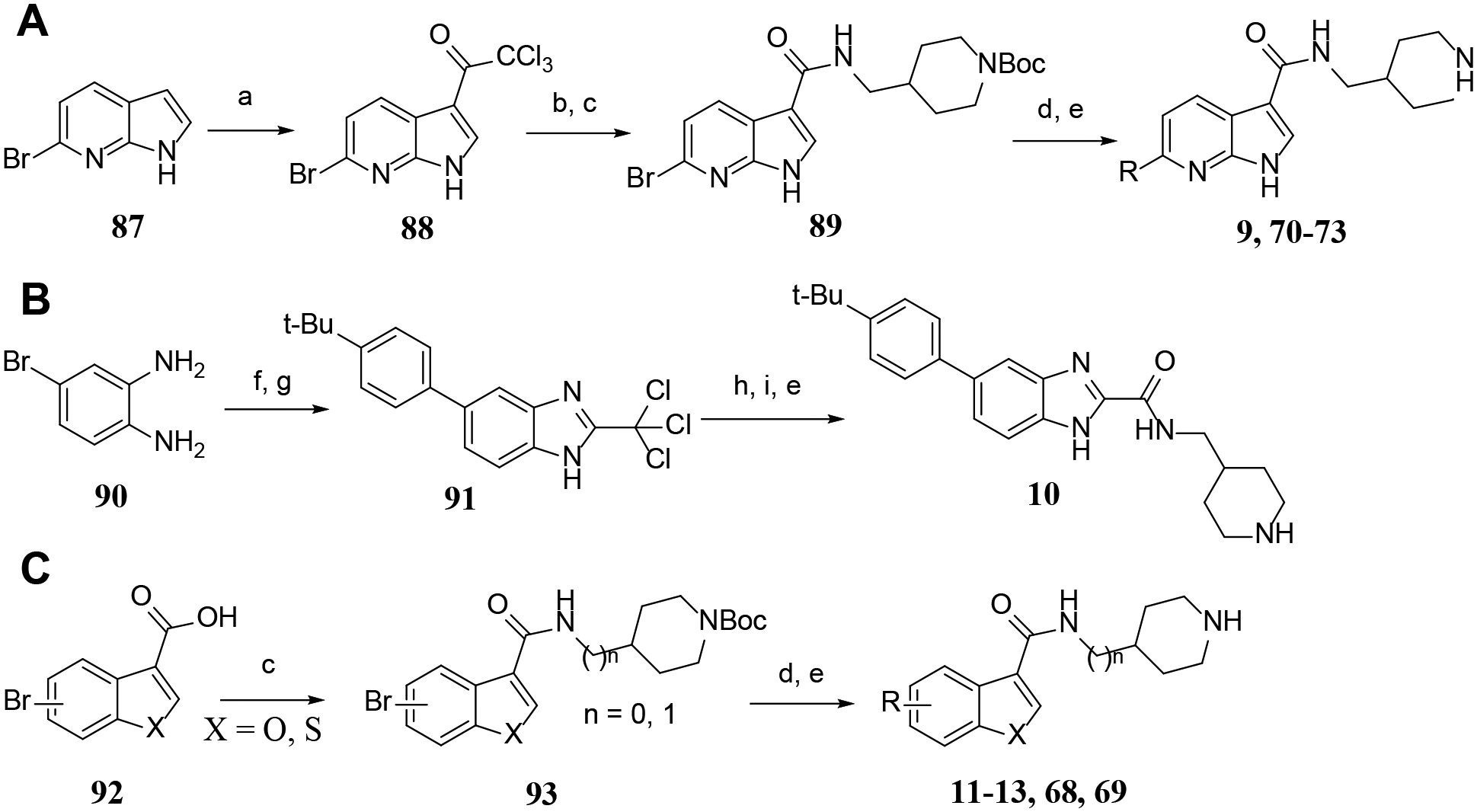
General methods for synthesis of compounds 9-13, and 68-73.a
aReagents and conditions: (a) AlCl3, CH2Cl2, 10 min; then, 2,2,2-trichloroacetyl chloride, 12 h; (b) NaOH (20% aq.), 16 h; (c) 4-aminomethyl-1-Boc-piperidine, HATU, diisopropylethylamine, DMF, 12 h; (d) Aryl-boronic acid or Aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Pd(PPh3)4, Na2CO3, p-dioxane-H2O, 90 °C; (e) HCl (4 N in p-dioxane), CH2Cl2, 0 °C; (f) 4-tert-butylphenylboronic acid, Pd(PPh3)4, Na2CO3, DMF, 90 °C, 12 h; (g) Methyl 2,2,2-trichloroacetimidate, AcOH, 16 h; (h) NaOH (2 N), 12 h; (i) 4-aminomethyl-1-Boc-piperidine, EDC, HOBt, triethylamine, CH2Cl2.
Inhibitor discovery and SAR studies.
A recombinant ZVpro protein, consisting of NS2B (residues 47–95) and NS3 (residues 1–170) of ZIKV connected with a Gly4-Ser-Gly4 linker, was expressed and purified for compound screening32. A fluorescence-based biochemical assay was used and monitored with the excitation wavelength at 360 nm and emission at 460 nm, using benzoyl-norleucine-lysine-lysine-arginine 7-amino-4-methylcoumarine (Bz-Nle-Lys-Lys-Arg-AMC) as the substrate32. ZVpro-mediated hydrolysis of the substrate causes a significant increase of the fluorescence signal, while an inhibitor can reduce it.
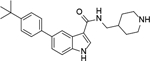
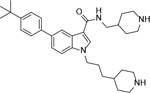
Using this assay, we performed screening of our in-house compound library, which consists of ~1,200 compounds synthesized for medicinal chemistry studies of other protein targets32. Indole-3-carboxamide compounds 1 and 2 with para-tert-butylphenyl substituent were found to be inhibitors of ZVpro with IC50 values of 4.5 and 11 μM (Table 1 and Figure S1). Compound 3 with an additional 1-substituent is less active (IC50 = 14.6 μM) than 2, but compounds 4 and 5 without a 3-carboxamide group are inactive. These data suggest the importance of the 3-carboxamide group for inhibition of ZVpro and a 1-substituent might be disfavored.
Table 1.
Structures and ZVpro inhibitory activities of compounds 1−13 with a para-tert-butylphenyl group.a
| Cpd # | Structure | IC50 (μM) | Cpd # | Structure | IC50 (μM) |
|---|---|---|---|---|---|
| 1 |
|
4.5 | 8 |
|
1.3 |
| 2 |
|
11.0 | 9 |
|
9.1 |
| 3 |
|
14.6 | 10 |
|
3.1 |
| 4 |
|
>50 | 11 |
|
3.2 |
| 5 |
|
>50 | 12 |
|
2.1 |
| 6 |
|
7.0 | 13 |
|
5.1 |
| 7 |
|
5.0 |
Standard errors of all IC50 values are less than 30%.
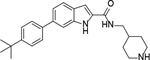
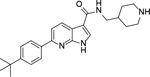
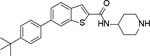
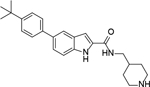
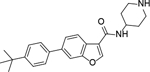
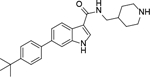
Analogous compounds 6-13 with a tert-butylphenyl group were synthesized for SAR studies. Compound 7 bearing a tert-butylphenyl group inhibited ZVpro with an IC50 of 5.0 μM, exhibiting an improved activity as compared to compound 2 with a 5-substituent. Moving the 3-carboxamide group to the 2-position in compound 8 significantly enhanced the inhibitory activity with an IC50 of 1.3 μM. Comparing the activity of compound 8 with that of 6 also indicates that the 4-tert-butylphenyl group at the 6-position is more favorable. Compounds 9-13 with a different core, i.e., pyrrolo[2,3-b]pyridine, benzimidazole, benzothiophene and benzofuran, exhibited comparable activities with IC50 values of 2.1–9.1 μM.
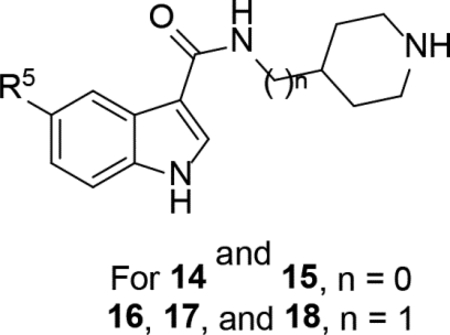
Indole-3-carboxamide compounds 14-18 were synthesized with a variety of aryl group at the 5-position, including more electron-rich or -deficient phenyl groups than the para-tert-butylphenyl in compound 2. However, none of these compounds are active against ZVpro (Table 2).
Table 2.
Structures and ZVpro inhibitory activities of 5-substituted indole-3-carboxamide compounds 14-18.a
| ||
|---|---|---|
| Cpd # | R5 | IC50 (μM) |
| 14 |
|
>50 |
| 15 |
|
>50 |
| 16 |
|
>50 |
| 17 |
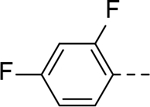
|
>50 |
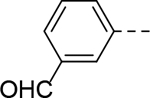
| 18 |
|
>50 |
Standard errors of all IC50 values are less than 30%.
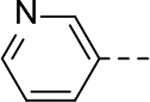
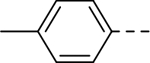
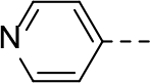
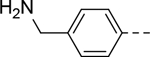
The substituent at the 6-position of the indole-3-carboxamides was next modified (Table 3). Replacement of the tert-butyl group with a smaller group with a different electronic property, such as -OMe, -Me, -F and -CN in compounds 19-24, is disfavored, while changing to a hydrophobic and more bulky group, such as those in compounds 25 and 26 (IC50 = 4.2 and 3.1 μM), retained the inhibitory activity (as compared to compound 7 with an IC50 of 5.0 μM). Except for compound 27 (IC50 = 42 μM) with an additional -Cl substituent, replacing the 6-phenyl ring in compound 7 with a 3- or 4-pyridinyl ring significantly enhanced the inhibitory activity with compounds 28-31 showing IC50 values of 0.39–1.1 μM. Varying the linker length of the 3-carboxamide substituents for compounds 29-31 suggested that one -CH2- (in the most active compound 29) between the amide and the piperidine ring is optimal. Compound 32 (IC50 = 5.7 μM) with a pyrazole ring at the 6-position exhibited a similar activity to compound 7.
Table 3.
Structures and ZVpro inhibitory activities of 6-substituted indole-3-carboxamide compounds 19-32.a
|
|||||
|---|---|---|---|---|---|
| Cpd # | R6 | IC50 (μM) | Cpd # | R6 | IC50 (μM) |
| 19 |
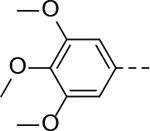
|
>50 | 26 |
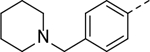
|
3.1 |
| 20 |
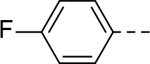
|
>50 | 27 |
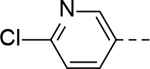
|
42 |
| 21 |
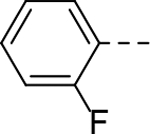
|
>50 | 28 |
|
1.1 |
| 22 |
|
48.9 | 29 |
|
0.39 |
| 23 |
|
>50 | 30 |
|
1.0 |
| 24 |
|
21.8 | 31 |
|
0.63 |
| 25 |
|
4.2 | 32 |
|
5.7 |
Standard errors of all IC50 values are less than 30%.
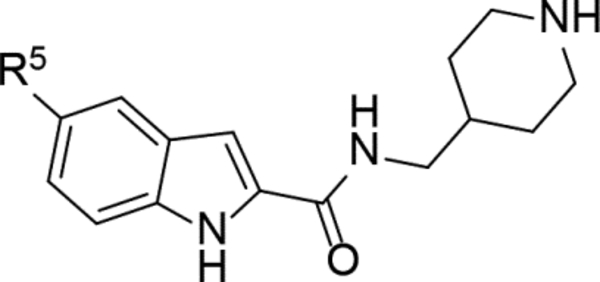
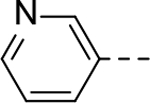
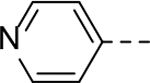
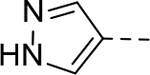
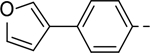
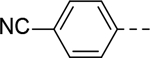
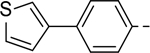
Next, 5-Substituted indole-2-carboxamide compounds 33-46 (Table 4) were synthesized for SAR studies. As compared to compound 6 (IC50 = 7.0 μM), a pyridine ring at the 5-position for compounds 33 and 34 considerably reduces the inhibitory activity, while a more polar pyrazole or para-aminomethylphenyl group at this position in compound 35 and 36 can retain the inhibitory activity. It is of interest that a furan-3-yl or thiophen-3-yl ring in compounds 37 and 38 (IC50 = 0.87 and 1.1 μM) increases the inhibitory potency by ~8-fold. Replacing the furan ring with another cyclic moiety in compounds 39-43 (IC50 = 3.4–33 μM) reduced the activity. Compounds 44-46 with a cyano-substituted phenyl ring at the 5-position exhibited moderate to low activity against ZVpro with IC50 values of 9–40 μM.
Table 4.
Structures and ZVpro inhibitory activities of 5-substituted indole-2-carboxamide compounds 33-46.a
|
|||||
|---|---|---|---|---|---|
| Cpd # | R5 | IC50 (μM) | Cpd # | R5 | IC50 (μM) |
| 33 |
|
>50 | 40 |
|
11.4 |
| 34 |
|
37.0 | 41 |
|
3.4 |
| 35 |
|
6.0 | 42 |
|
33.0 |
| 36 |
|
9.5 | 43 |
|
8.8 |
| 37 |
|
0.87 | 44 |
|
9.2 |
| 38 |
|
1.1 | 45 |
|
40.2 |
| 39 |
|
4.7 | 46 |
|
30.5 |
Standard errors of all IC50 values are less than 30%.
SAR studies based on the indole-2-carboxamide compound 8 (IC50 = 1.3 μM) were performed to optimize its 6-substituent. Compound 47 without a 6-substituent showed a modest activity (IC50 = 24.6 μM), while compound 48 with a 6-phenyl substituent exhibited a ~2.5-fold activity increase with an IC50 of 9.4 μM. Its activity also shows the importance of the para-tert-butyl group in 8 for ZVpro inhibition. While replacing the para-tert-butyl with -F in compound 49 caused a sharply decreased activity, that with a -OCH3 in compound 50 (IC50 = 1.0 μM) slightly enhanced the activity. Compound 51 having a -OCF3 with comparable bulkiness is ~3-fold less active than 50. Therefore, the descending activity for -OCH3 ~ -t-Bu > -OCF3 > -F suggests the importance of an electron-rich phenyl ring at the 6-position. Compounds 52 and 54 bearing a smaller, polar amino- or hydroxy-methyl group exhibited comparable activities to 51, while compound 53 or 55 (IC50 = 3.2 or 45.6 μM) with a more electron-withdrawing or longer group, respectively, is less potent. Compound 56 (IC50 = 1.6 μM) with a more bulky phenoxyphenyl group can retain the inhibitory activity, but compounds 57 and 58 are 4–6-fold less active (than compound 8), presumably due to more rigid bicyclic rings. Compounds 59-66 with several heterocyclic rings were investigated. Pyridine and two other 6-membered rings in compounds 59-63 with IC50 values of 2.2–27 μM are disfavored. 5-membered pyrazine- and furan-containing compounds 64 and 66 (IC50 = 0.99 and 0.32 μM, respectively) were found to possess enhanced inhibitory activities against ZVpro. Thiophene-containing compound 65 (IC50 = 6.4 μM) is significantly less active.
With the finding of potent ZVpro inhibitors such as compound 66, SARs for related compounds 67-73 are discussed. Indole-3-carboxamide compound 67 with two furan-3-yl groups at both 5- and 6-positions showed a comparable inhibitory activity with an IC50 of 0.37 μM. Analogous benzofuran and pyrrolo[2,3-b]pyridine compounds 68−73 showed similar SARs as their corresponding indole compounds (in Table 3), showing the core changes do not significantly affect the inhibitory activity.
Activity against dengue and West Nile virus proteases.
DENV and West Nile virus (WNV) also belong to the Flavivirus family and are important human pathogens. Given the high homology between ZVpro and DENV or WNV proteases32, it is of interest to find whether the identified ZVpro inhibitors inhibit these two enzymes. Recombinant NS2B-NS3 proteases from DENV serotype-2 (DV2pro) and WNV (WVpro) were expressed and purified and similar fluorescence-based biochemical assays developed using our previous methods32. 16 selected ZVpro inhibitors with a wide range of activity against ZVpro were tested for their inhibitory activity against DV2pro and WVpro. As summarized in Table 7, indole-carboxamide based ZVpro inhibitors also inhibit activity of DV2pro and WVpro, but they are general 5->10-fold less potent, particularly for highly potent ZVpro inhibitors such as compounds 66 and 67.
Table 7.
Inhibitory activity IC50 (μM) against Flavivirus proteases ZVpro, DV2pro and WVpro.a
| ZVpro | DV2pro | WVpro | |
|---|---|---|---|
| 66 | 0.32 | 1.6 | 5.7 |
| 67 | 0.37 | 3.1 | 3.7 |
| 72 | 0.45 | 9.2 | 8.6 |
| 64 | 0.99 | 10.3 | 3.5 |
| 30 | 1.0 | 10.0 | 10.5 |
| 50 | 1.0 | 10.6 | 20.1 |
| 8 | 1.3 | 15.0 | 21.8 |
| 56 | 1.6 | 6.5 | 6.6 |
| 26 | 3.1 | 49.7 | 32.0 |
| 51 | 3.2 | 10.7 | 16.0 |
| 7 | 5.0 | 30.4 | 35.1 |
| 32 | 5.7 | 48.3 | 42.0 |
| 40 | 11.4 | 40.2 | 31.0 |
| 24 | 21.8 | 12.0 | 50.5 |
| 45 | 40.2 | >50 | >50 |
| 22 | 48.9 | >50 | >50 |
Standard errors of all IC50 values are less than 30%.
Enzyme kinetics.
Steady-state enzyme kinetic studies for the most potent compound 66 were performed to find its mode of inhibition against ZVpro and DV2pro. Initial velocities were determined in the presence of increasing concentrations of the inhibitor and substrate. IC50 values of compound 66 were then calculated and plotted against the substrate concentrations. As shown in Figure 1A, the IC50 values against DV2pro were found to be almost unchanged when the substrate concentration was increased from 0.5 to 50 μM (~0.05–5×Km) and do not linearly increase according to the Cheng-Prusoff equation (IC50 = Ki + Ki/Km×[S]). These results show compound 66 is not a competitive inhibitor and more likely adopts a non-competitive mode of inhibition. Similarly, the IC50 values of 66 against ZVpro remain almost unchanged when the substrate concentration was increased from 0.5 to 10 μM (~0.03–0.7×Km) (Figure 1B). It is noted that the IC50 values for the highest substrate concentration are slightly higher for both ZVpro and DV2pro, presumably because of the very high initial velocity of the enzyme catalyzed reaction in these conditions. The relatively slow reading speed of our microplate reader could miss the initial velocity and cause larger errors. Another possibility is that the inhibitor might adopt a mixed mode of inhibition at the high substrate concentrations. The results for ZVpro do not exclude a competitive mode of action. However, given the very high similarity between ZVpro and DV2pro in both sequence and structure32, it is postulated that compound 66 inhibits ZVpro with the same mode of action.
Figure 1.
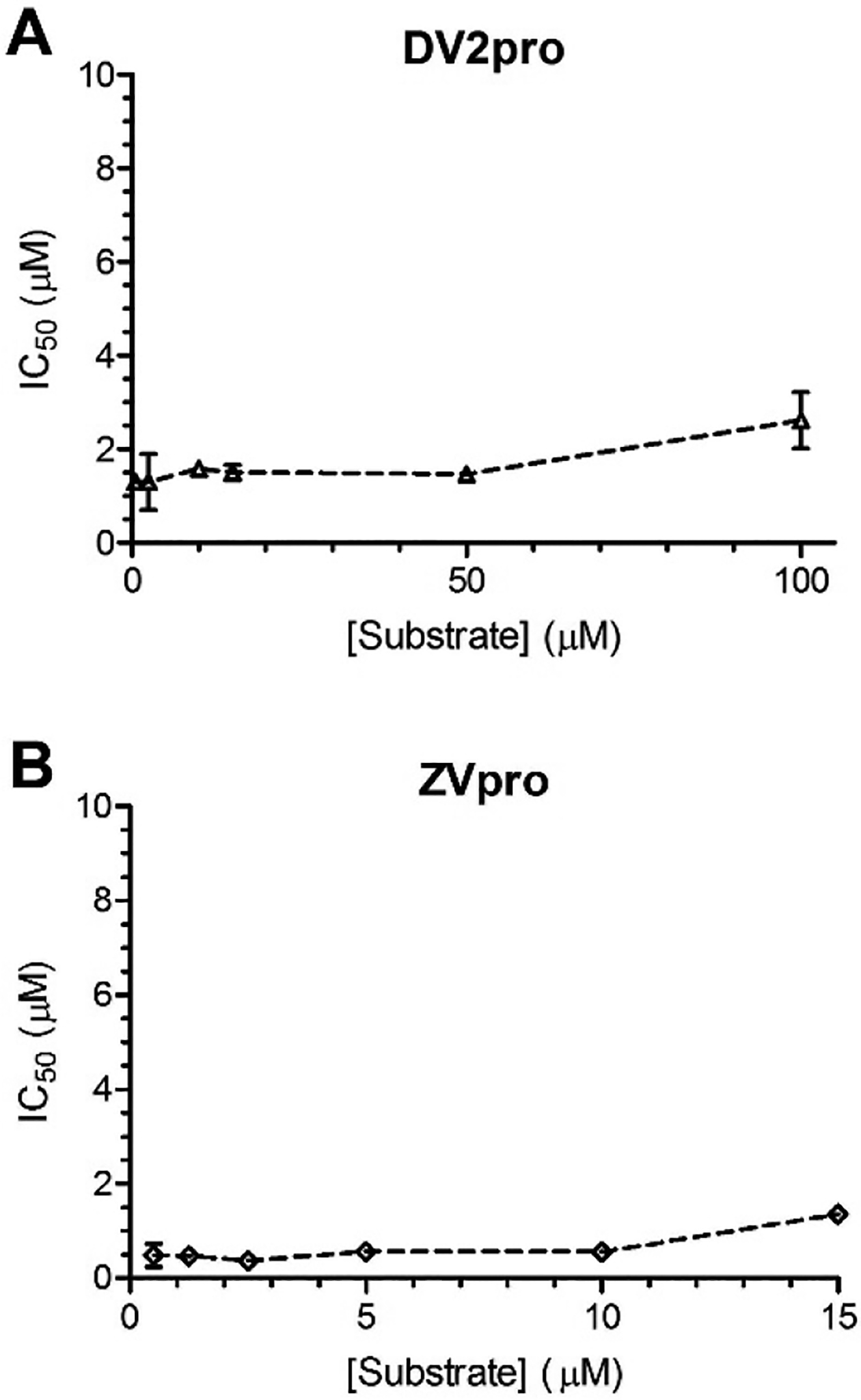
Enzyme kinetics studies of (A) DV2pro and (B) ZVpro with inhibitor 66 at the increasing concentrations of the substrate from 0.5 to 100 μM (~0.05–11×Km for DV2pro and 0.03× to 1×Km for ZVpro). The IC50 values against DV2pro do not linearly increase according to the Cheng-Prusoff equation (IC50 = Ki + Ki/Km×[S]), suggesting compound 66 is not a competitive inhibitor of DV2pro and more likely adopts a non-competitive mode of inhibition.
Anti-ZIKV activity.
Anti-ZIKV activity was evaluated in human U87 glioma cells and monkey Vero cells. ZIKV replicates rapidly in these two cells, but does not cause cytopathic effects (CPE) in U87 cells34. However, ZIKV causes significant CPE and cell lysis (in ~5 days post-infection) in Vero cells which lack interferon-mediated defense35. This feature can be conveniently used to detect ZIKV in the cells. The passage-3 stock of the ZIKV FLR strain, which was isolated from the serum of a patient infected in Colombia in 201536, was used for the assay. 0.01 multiplicity of infection (MOI), as defined by the number of infectious viral particles per cell, of ZIKV was added to a monolayer of cells. Upon virus attachment, cells were washed and incubated with increasing concentrations of a ZVpro inhibitor for 2 days. The supernatant containing newly generated viruses was subjected to an end-point dilution assay to determine the viral titer, which can be used to evaluate the anti-ZIKV activity of the compound32.
Selected ZVpro inhibitors were first tested for their cytotoxicity against U87 cells (using MTT assay) and non-toxic compounds further evaluated for their antiviral activity. As shown in Table 8, all of these compounds did not inhibit growth of U87 cells at 10 μM. The most potent ZVpro inhibitor 66 was able to inhibit ZIKV replication in U87 cells by 68% (i.e., half-log reduction) at 1 μM in two independent experiments. Compound 67 with a comparable enzyme activity exhibited a reduced anti-ZIKV activity with an EC68 of 3 μM. In addition, as compared to compound 66, compounds 72, 30 and 31 with slightly to moderately reduced activity against ZVpro (IC50 = 0.45–1 μM) showed considerably reduced anti-ZIKV activity (EC68 = 10 μM). Compounds 73 and 64 did not inhibit ZIKV replication by 68% at 10 μM. These variable antiviral activities might be due to different cellular permeability of these compounds. Compound 56 with an enzyme IC50 of 1.6 μM exhibited a strong anti-ZIKV activity with an EC68 of 2.5 μM, which might be due to improved cellular uptake or off-target effects. Less potent inhibitors 26, 54, 51 and 25 (IC50 = 3.1–4.2 μM) did not show antiviral activity at 10 μM.
Table 8.
Antiviral EC68 (μM) against ZIKV-FLR in U87 cells.
| ZVpro IC50 (μM) | ZIKV-FLR EC68 (μM) | U87 Cytotoxicity CC50 (μM) | |
|---|---|---|---|
| 66 | 0.32 | 1.0 | >10 |
| 67 | 0.37 | 3.0 | >10 |
| 72 | 0.45 | 10.0 | >10 |
| 73 | 0.60 | >10 | >10 |
| 31 | 0.63 | 10.0 | >10 |
| 64 | 0.99 | >10 | >10 |
| 30 | 1.0 | 10.0 | >10 |
| 56 | 1.6 | 2.5 | >10 |
| 26 | 3.1 | 10.0 | >10 |
| 54 | 3.1 | >10 | >10 |
| 51 | 3.2 | >10 | >10 |
| 25 | 4.2 | >10 | >10 |
The most potent compound 66 also showed dose-dependent anti-ZIKV activity. It was able to inhibit ZIKV (FLR strain) replication in U87 cells by 68%, 90% and 99% (0.5, 1 and 2 log reduction) at the concentration of 1, 3 and 10 μM, respectively, in two independent experiments. These results suggest the potent ZVpro inhibitor 66 is a promising antiviral agent against ZIKV infection.
CONCLUSION
Zika, dengue and other Flavivirus species are important human pathogens. However, except for mosquito controls, there have been no effective antiviral drugs. There is therefore a pressing need to discover and develop novel small-molecule compounds targeting essential Flavivirus proteins such as the viral protease. Compound screening found di-substituted indole compounds 1 and 2 are a novel series of inhibitor of ZVpro. Iterative SAR and medicinal chemistry optimization were performed and found 2,6-disubstituted indole compounds, such as 66, are potent ZVpro inhibitors with IC50 values as low as 320 nM. Changing to another aromatic core did not yield a better inhibitor. Activity optimization for the R3 or R5 group did not give more potent compounds. These ZVpro inhibitors also inhibit activity of homologous proteases of dengue and West Nile virus, but with considerably reduced potencies. The most potent inhibitor 66 exhibited strong antiviral activity against ZIKV with an EC68 value of 1 μM, showing it represents a novel lead for further developing antiviral drugs against Zika and other Flavivirus infections.
Experimental Section
All chemicals for synthesis were purchased from Alfa Aesar (Ward Hill, MA) or Aldrich (Milwaukee, WI). Unless otherwise stated, all solvents and reagents used as received. All reactions were performed using a Teflon-coated magnetic stir bar at the indicated temperature and were conducted under an inert atmosphere when stated. The identity of the synthesized compounds was characterized by 1H and 13C NMR on a Varian (Palo Alto, CA) 400-MR spectrometer and mass spectrometer (Shimadzu LCMS-2020). Chemical shifts were reported in parts per million (ppm, δ) downfield from tetramethylsilane. Proton coupling patterns are described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br). The identity of the potent inhibitors was confirmed with high resolution mass spectra (HRMS) using an Agilent 6550 iFunnel quadrupole-time-of-flight (Q-TOF) mass spectrometer with electrospray ionization (ESI). The purities of the final compounds were determined to be >95% with a Shimadzu Prominence HPLC using a Zorbax C18 (or C8) column (4.6 × 250 mm) monitored by UV at 254 nm.
Chemical synthesis.
1. General procedure for the synthesis of compounds 1 and 67.
To a suspension of methyl 1H-indole-3-carboxylate (74) (4.0 g, 22.8 mmol) in acetic acid (30 mL) was added dropwise liquid Bromine (2.57 mL, 50.2 mmol) under an atmosphere of argon. The reaction stirred in dark for 3 days. The suspension was filtered, washed with hot ethanol, and dried to afford 75 (5 g, 66 %) as a grey solid. Methyl 5,6-dibromo-1H-indole-3-carboxylate (75).
To a solution of methyl 5,6-dibromo-1H-indole-3-carboxylate 75 (4.0 mmol) in MeOH/H2O (3/1,v/v, 16 mL) was added slowly sodium hydroxide solution (20.0 mmol). The resulting mixture was heated at 50 °C for 5 h before it was cooled to room temperature. The solvent was removed in vacuo, and the white slurry was diluted with H2O and acidified by HCl (3 N). It was filtered, washed with H2O, and dried to give acid as a white solid. The crude product was used for the next step without further purification.
To a solution of the crude carboxylic acid (0.4 g, 1.25 mmol) and 4-aminomethyl-1-Boc-piperidine (1.5 mmol), or 4-amino-1-Boc-piperidine (1.5 mmol) in DMF (20 mL) was added N, N-diisopropylethylamine (0.33 mL, 1.88 mmol) and HATU (0.572 g, 1.5 mmol). The mixture was stirred for 12 h before it was quenched with H2O. The mixture was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The solvent was removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 2:1 to 1:1) to afford 76a or 76b as a withe solid.
Tert-butyl 4-((5,6-dibromo-1H-indole-3-carboxamido)methyl)piperidine-1-carboxylate (76b): 1H NMR (400 MHz, CDCl3) δ 11.17 (s, 1H), 8.02 (t, J = 34.8 Hz, 3H), 7.65 (s, 1H), 7.51 (s, 1H), 7.04 (s, 1H), 4.11 – 4.06 (m, 2H), 3.33 (s, 2H), 2.68 (s, 2H), 1.88 – 1.65 (m, 3H), 1.42 (s, 9H), 1.22 – 1.10 (m, 2H).
Compound 76a or 76b (0.1 mmol), aryl boronic acid (0.12 mmol), tetrakis(triphenylphosphine)palladium (5.8 mg, 0.005 mmol), and sodium carbonate (21 mg, 0.2 mmol) in p-dioxane/H2O (5/1, v/v, 6.0 mL) were placed in a sealed tube. The mixture was degassed and heated to 90 °C for 16 h. The reaction was then cooled and quenched with brine (10 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The solvent was removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1:1 to 1:2) to afford the corresponding coupling product.
To a solution of this intermediate (0.08 mmol) in DCM (2 mL) was added dropwise HCl (0.2 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 4 h. The solvent was removed in vacuo to afford the final product 1 or 67 as a hydrochloric salt.
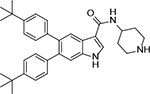
5,6-Bis(4-(tert-butyl)phenyl)-N-(piperidin-4-yl)-1H-indole-3-carboxamide Hydrochloride (1). 1H NMR (400 MHz, DMSO-d6) δ 11.65 (s, 1H), 8.64 (brs, 2H), 8.14 (d, J = 2.8 Hz, 1H), 8.07 (s, 1H), 7.94 (d, J = 7.6 Hz, 1H), 7.36 (s, 1H), 7.20 (d, J = 8.4 Hz, 4H), 7.01 (t, J = 8.4 Hz, 4H), 4.02 (s, 1H), 3.21 (s, 2H), 2.99 (t, J = 11.2 Hz, 2H), 1.97 (d, J = 13.6 Hz, 2H), 1.71 (dd, J = 23.2, 11.6 Hz, 2H), 1.26 – 1.15 (m, 18H); 13C NMR (100 MHz, DMSO-d6) δ 164.0, 148.2, 148.0, 139.7, 139.3, 135.6, 134.5, 133.2, 129.45, 129.38, 129.0, 125.6, 124.45, 124.36, 122.8, 110.4, 92.4, 43.5, 42.4, 34.1, 31.2, 31.1, 28.6. MS (ESI) [M+H]+ 508.3.
5,6-Di(furan-3-yl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (67). 1H NMR (400 MHz, D2O) δ 8.38 (s, 1H), 7.68 (s, 2H), 7.31 (s, 2H), 7.20 (s, 1H), 7.17 (s, 2H), 6.15 (s, 2H), 3.35 (d, J = 11.6 Hz, 2H), 3.18 (s, 2H), 2.85 (t, J = 11.6 Hz, 2H), 1.91 – 1.79 (m, 3H), 1.36 (dd, J = 22.8, 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.5, 142.8, 142.6, 139.7, 139.6, 135.7, 128.8, 126.5, 126.1, 125.6, 125.3, 123.9, 122.4, 112.6, 112.2, 112.0, 110.5, 43.2, 42.9, 34.0, 26.4. MS (ESI) [M+H]+ 390.2.
2. General procedure for the synthesis of compound 2, 7, and 14–32.
To a solution of 5- or 6-halogen substituted indole 77 (3 mmol) in DMF (10 mL) was added TFAA (626 μL, 4.5 mmol) at 0 °C. The resulting mixture was stirred at room temperature for 2 h. Then the reaction was quenched with H2O. A lot of yellow solid precipitated from the solvent. It was filtered to get the intermediate 78, which was used directly for the next step.
The intermediate 78 was added to 20% NaOH solution. The resulting mixture was stirred at 50 °C for 2 days. After the starting material was consumed, the mixture was quenched with 1 N HCl solution. White solid precipitated from the solvent. It was filtered to give the corresponding carboxylic acid which was used for the next step without purification. The preparation of the final compounds 2, 7, and 14–32 followed the same procedure as described for compound 1.
5-(4-(tert-butyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide (2). 1H NMR (400 MHz, DMSO-d6) δ 11.64 – 11.63 (m, 1H), 8.84 (d, J = 12.0 Hz, 1H), 8.54 (d, J = 12.0 Hz, 1H), 8.35 (s, 1H), 8.10–8.01 (m, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.51 – 7.30 (m, 4H), 3.23 (d, J = 12.4 Hz, 2H), 3.15 (t, J = 6.0 Hz, 2H), 2.80 (q, J = 12.0 Hz, 2H), 1.80 (d, J = 12.0 Hz, 3H), 1.36 (d, J = 12.4 Hz, 2H), 1.29 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 165.1, 149.2, 139.3, 136.0, 133.1, 128.9, 127.1, 126.8, 126.0, 121.6, 119.2, 112.6, 111.2, 43.6, 43.3, 34.6, 34.4, 31.6, 26.8. MS (ESI) [M+H]+ 390.3.
6-(4-(tert-butyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride. (7). 1H NMR (400 MHz, DMSO-d6) δ 11.60 (s, 1H), 8.59 (s, 1H), 8.28 (s, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.01 (dd, J = 7.6, 4.4 Hz, 2H), 7.64 – 7.53 (m, 3H), 7.52 – 7.42 (m, 2H), 7.37 (dd, J = 8.4, 1.6 Hz, 1H), 3.25 (d, J = 12.0 Hz, 2H), 3.16 (t, J = 6.0 Hz, 2H), 2.83 (d, J = 12.0 Hz, 2H), 1.81 (d, J = 13.2 Hz, 3H), 1.35–1.29 (m, 11H); 13C NMR (100 MHz, DMSO-d6) δ 165.1, 149.6, 138.7, 137.2, 134.6, 128.7, 126.8, 126.1, 125.8, 121.7, 120.1, 110.9, 109.9, 43.6, 43.4, 34.6, 34.4, 31.6, 26.8. MS (ESI) [M+H]+ 390.3.
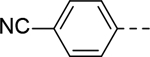
5-(4-cyanophenyl)-N-(piperidin-4-yl)-1H-indole-3-carboxamide Hydrochloride (14). 1H NMR (400 MHz, DMSO-d6) δ 11.82 (s, 1H), 8.94 (s, 2H), 8.47 (s, 1H), 8.21 (d, J = 2.0 Hz, 1H), 8.07 (d, J = 7.2 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 7.83 (d, J = 8.0 Hz, 2H), 7.52 (q, J = 8.0 Hz, 2H), 4.06 (s, 1H), 3.30 (d, J = 12.4 Hz, 2H), 2.99 (s, 2H), 1.99 (d, J = 12.0 Hz, 2H), 1.77 (dd, J = 24.0, 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.5, 146.8, 136.7, 133.2, 131.3, 129.7, 127.9, 127.4, 121.7, 120.3, 119.5, 113.1, 111.2, 109.3, 44.1, 42.6, 28.9. MS (ESI) [M+H]+ 345.4.
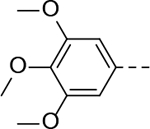
N-(piperidin-4-yl)-5-(3,4,5-trimethoxyphenyl)-1H-indole-3-carboxamide Hydrochloride (15). 1H NMR (400 MHz, DMSO-d6) δ 11.67 (s, 1H), 8.87 (s, 2H), 8.34 (s, 1H), 8.16 (d, J = 4.0 Hz, 1H), 8.00 (d, J = 7.2 Hz, 1H), 7.53 – 7.30 (m, 2H), 6.85 (s, 2H), 4.05 (s, 1H), 3.84 (s, 6H), 3.68 (s, 3H), 3.30 (d, J = 12.0 Hz, 2H), 2.98 (d, J = 8.0 Hz, 2H), 1.98 (d, J = 8.0 Hz, 2H), 1.76 (dd, J = 22.0, 8.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.6, 153.5, 138.2, 136.9, 136.0, 133.6, 129.3, 127.1, 121.9, 119.5, 112.5, 110.9, 104.6, 60.5, 56.4, 44.0, 42.7, 29.0. MS (ESI) [M+H]+ 410.6.
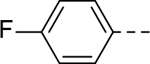
5-(4-fluorophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (16). 1H NMR (400 MHz, DMSO-d6) δ 11.64 (s, 1H), 8.69 (s, 1H), 8.39 (s, 1H), 8.33 (d, J = 1.6 Hz, 1H), 8.07–8.04 (m, 2H), 7.65 – 7.62 (m, 2H), 7.48 (d, J = 12.0 Hz, 1H), 7.39 (d, J = 8.0, 1H), 7.26 (t, J = 8.0 Hz, 2H), 3.29 – 3.19 (m, 2H), 3.16 (d, J = 12.4 Hz, 2H), 2.81 (d, J = 12.0 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.35 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.1, 161.8 (d, J = 242.9 Hz), 138.7, 136.1, 132.3, 129.0, 128.9, 127.1, 121.6, 119.5, 116.0 (d, J = 21.1 Hz), 112.7, 111.3, 43.6, 43.4, 34.4, 26.8. MS (ESI) [M+H]+ 352.1.
5-(2,4-difluorophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (17). 1H NMR (400 MHz, DMSO-d6) δ 11.69 (s, 1H), 8.72 (s, 1H), 8.41 (s, 1H), 8.25 (d, J = 1.6 Hz, 1H), 8.07 (d, J = 4.0 Hz, 2H), 7.56 – 7.48 (m, 2H), 7.34 – 7.24 (m, 2H), 7.18–7.13 (m, 1H), 3.23 (d, J = 12.4 Hz, 2H), 3.14 (t, J = 6.0 Hz, 2H), 2.80 (q, J = 11.6 Hz, 2H), 1.79 (d, J = 12.8 Hz, 3H), 1.34 (q, J = 13.2, 12.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.0, 162.8 (d, J = 12.0 Hz), 160.4 (d, J = 11.7 Hz), 158.2 (d, J = 12.2 Hz), 136.0, 132.5 (dd, J = 9.7, 4.8 Hz), 128.9, 126.9, 126.8, 123.34, 121.9 (d, J = 2.4 Hz), 112.4, 112.4, 112.2, 111.2, 105.0, 104.8, 104.5, 55.3, 43.4, 34.4, 26.8. MS (ESI) [M+H]+ 370.6.
5-(3-formylphenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (18). 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 10.09 (s, 1H), 8.82 (d, J = 12.0 Hz, 1H), 8.53 (d, J = 12.0 Hz, 1H), 8.47 (s, 1H), 8.16 – 8.08 (m, 3H), 8.02 – 7.98 (m, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.67 (t, J = 8.0 Hz, 1H), 7.54 – 7.49(m, 2H), 3.23 (d, J = 16.0 Hz, 2H), 3.16 (t, J = 8.0 Hz, 2H), 2.85 – 2.79 (m, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.37 (d, J = 16.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 193.6, 164.7, 142.6, 136.9, 136.1, 132.8, 131.5, 129.9, 128.8, 127.7, 127.5, 126.9, 121.2, 119.4, 112.6, 111.0, 66.4, 55.0, 43.0, 34.1, 26.5. MS (ESI) [M+H]+ 362.2.
N-(piperidin-4-ylmethyl)-6-(3,4,5-trimethoxyphenyl)-1H-indole-3-carboxamide Hydrochloride. (19). 1H NMR (400 MHz, DMSO-d6) δ 11.63 (d, J = 4.0 Hz, 1H), 8.92 (d, J = 12.0 Hz, 1H), 8.62 (d, J = 12.0 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.11–8.07 (m, 2H), 7.64 (d, J = 1.6 Hz, 1H), 7.40 (dd, J = 8.4, 1.6 Hz, 1H), 6.89 (s, 2H), 3.84 (s, 6H), 3.66 (s, 3H), 3.23 (d, J = 12.0 Hz, 2H), 3.14 (t, J = 6.0 Hz, 2H), 2.87–2.76 (m, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.37 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.1, 153.6, 137.6, 137.1, 134.9, 128.9, 126.0, 121.6, 120.3, 110.9, 110.2, 104.6, 66.8, 60.5, 56.4, 43.3, 34.4, 26.8. MS (ESI) [M+H]+ 424.8.
6-(4-fluorophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (20). 1H NMR (400 MHz, DMSO-d6) δ 11.67 (s, 1H), 8.82 (s, 1H), 8.53 (s, 1H), 8.15 (d, J = 8.4 Hz, 1H), 8.07 (d, J = 2.8 Hz, 2H), 7.68 (dd, J = 8.4, 5.6 Hz, 2H), 7.61 (s, 1H), 7.36 (d, J = 9.6 Hz, 1H), 7.25 (t, J = 8.0 Hz, 2H), 3.23 (d, J = 12.0 Hz, 2H), 3.15 (t, J = 6.0 Hz, 2H), 2.80 (d, J = 11.2 Hz, 2H), 1.81 (d, J = 11.2 Hz, 3H), 1.46 – 1.27 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.0, 161.9 (d, J = 243.2 Hz), 138.1 (d, J = 2.9 Hz), 137.1, 133.6, 129.0 (d, J = 8.0 Hz), 128.9, 125.9, 121.9, 120.1, 116.1 (d, J = 21.2 Hz), 110.9, 110.1, 109.9, 43.6, 43.4, 34.4, 26.8. MS (ESI) [M+H]+ 352.6.
6-(2-fluorophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (21). 1H NMR (400 MHz, DMSO-d6) δ 11.71 (s, 1H), 8.79 (s, 1H), 8.50 (s, 1H), 8.16 (d, J = 8.0 Hz, 1H), 8.09 (d, J = 4.0 Hz, 2H), 7.65 – 7.49 (m, 3H), 7.45 – 7.32 (m, 1H), 7.28 (dd, J = 13.6, 6.0 Hz, 2H), 3.23 (d, J = 12.0 Hz, 2H), 3.16 (t, J = 6.0 Hz, 2H), 2.90 – 2.67 (m, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.36 (q, J = 10.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.0, 159.6 (d, J = 245.1 Hz), 136.6, 132.5 (d, J = 2.7 Hz), 131.9 (d, J = 9.8 Hz), 131.4 (d, J = 3.4 Hz), 129.6 (d, J = 13.0 Hz), 129.3 (d, J = 6.3 Hz), 129.1 (d, J = 3.1 Hz), 129.1, 128.1, 126.5, 126.1, 125.3 (d, J = 3.4 Hz), 121.5, 121.0 (d, J = 70.1 Hz), 116.5 (d, J = 22.8 Hz), 112.6 (d, J = 3.6 Hz), 110.9, 110.5 (d, J = 92.3 Hz), 66.8, 55.3, 34.4, 26.8. MS (ESI) [M+H]+ 352.1.
N-(piperidin-4-ylmethyl)-6-(p-tolyl)-1H-indole-3-carboxamide Hydrochloride (22). 1H NMR (400 MHz, DMSO-d6) δ 11.64 (s, 1H), 8.74 (d, J = 9.6 Hz, 1H), 8.44 (d, J = 12.0 Hz, 1H), 8.12 (d, J = 8.0 Hz, 1H), 8.10 – 7.95 (m, 2H), 7.61 (s, 1H), 7.53 (d, J = 7.6 Hz, 2H), 7.36 (d, J = 8.4 Hz, 1H), 7.23 (d, J = 7.6 Hz, 2H), 3.23 (d, J = 11.6 Hz, 2H), 3.15 (s, 2H), 2.80 (q, J = 12.0 Hz, 2H), 2.30 (s, 3H), 1.80 (d, J = 12.0 Hz, 3H), 1.35 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.2, 138.6, 137.2, 136.4, 134.6, 129.9, 128.8, 126.9, 125.7, 121.7, 119.9, 110.9, 109.8, 66.8, 43.4, 34.4, 26.8, 21.1. MS (ESI) [M+H]+ 348.9.
6-(4-(aminomethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (23).
1H NMR (400 MHz, DMSO-d6) δ 11.77 (s, 1H), 8.98 (s, 1H), 8.69 (s, 1H), 8.47 (s, 3H), 8.20 (d, J = 8.0 Hz, 1H), 8.14 – 8.12 (m, 2H), 7.74 (d, J = 8.0 Hz, 2H), 7.68 (s, 1H), 7.57 (d, J = 8.0 Hz, 2H), 7.44 (dd, J = 8.0, 4.0 Hz, 1H), 4.06 (s, 2H), 3.26 (d, J = 12.0 Hz, 2H), 3.18 (t, J = 6.0 Hz, 2H), 2.83 (q, J = 12.0 Hz, 2H), 1.84 (d, J = 12.0 Hz, 3H), 1.41 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.0, 141.7, 137.2, 133.9, 132.8, 129.9, 129.1, 127.3, 126.3, 121.9, 120.1, 110.9, 110.2, 43.6, 43.3, 42.4, 34.4, 26.8. MS (ESI) [M+H]+ 363.2.
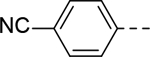
6-(4-cyanophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (24). 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 9.02 (d, J = 8.0 Hz, 1H), 8.73 (d, J = 12.0 Hz, 1H), 8.22 (d, J = 12.0 Hz, 1H), 8.20 – 8.08 (m, 2H), 7.88 (s, 3H), 7.76 (s, 1H), 7.47 (d, J = 8.4 Hz, 1H), 3.23 (d, J = 12.0 Hz, 2H), 3.16 (s, 2H), 2.80 (q, J = 10.4 Hz, 2H), 1.81 (d, J = 11.6 Hz, 3H), 1.48 – 1.24 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.9, 146.2, 137.1, 133.2, 132.4, 129.8, 127.9, 127.1, 122.1, 120.1, 119.5, 111.0, 110.8, 109.5, 43.7, 43.3, 34.4, 26.8. MS (ESI) [M+H]+ 359.5.
6-([1,1’-biphenyl]-4-yl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (25). 1H NMR (400 MHz, DMSO-d6) δ 11.71 (s, 1H), 8.99 (d, J = 11.0 Hz, 1H), 8.70 (d, J = 8.0 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 8.12 (d, J = 2.8 Hz, 2H), 7.88 (s, 1H), 7.73 (d, J = 7.2 Hz, 3H), 7.65 (d, J = 7.6 Hz, 1H), 7.62 – 7.43 (m, 5H), 7.36 (dd, J = 8.0 Hz, 6.0 Hz, 1H), 3.32 – 3.07 (m, 4H), 2.80 (q, J = 12.0 Hz, 2H), 1.81 (d, J = 11.2 Hz, 3H), 1.39 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.1, 142.3, 141.3, 140.8, 137.2, 134.5, 129.9, 129.4, 129.1, 127.9, 127.3, 126.4, 126.2, 125.6, 121.9, 120.3, 110.9, 110.4, 66.8, 43.3, 34.4, 26.8. MS (ESI) [M+H]+ 410.6.
6-(4-(piperidin-1-ylmethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (26). 1H NMR (400 MHz, DMSO-d6) δ 11.83 (s, 2H), 10.86 (s, 2H), 9.11 (d, J = 9.2 Hz, 1H), 8.82 (d, J = 12.0 Hz, 1H), 8.28 – 8.05 (m, 2H), 7.74–7.67 (m, 3H), 7.43 (d, J = 8.4 Hz, 1H), 4.25 – 4.19 (m, 9H), 3.28–3.15 (m, 4H), 2.92 – 2.65 (m, 3H), 1.97 – 1.53 (m, 5H), 1.51 – 1.16 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.0, 142.5, 137.2, 133.7, 132.5, 129.3, 128.5, 127.3, 126.4, 121.9, 120.1, 110.9, 110.3, 58.9, 51.9, 43.7, 43.2, 34.4, 26.8, 22.5, 21.9. MS (ESI) [M+H]+ 431.3.
6-(6-chloropyridin-3-yl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (27). 1H NMR (400 MHz, DMSO-d6) δ 12.09 (s, 1H), 8.81 (d, J = 8.0 Hz, 2H), 8.74 (s, 1H), 8.67 (s, 1H), 8.42 – 8.24 (m, 4H), 8.12 (d, J = 11.2 Hz, 2H), 7.74 (d, J = 8.0 Hz, 1H), 4.03 (s, 1H), 3.30 (d, J = 12.0 Hz, 2H), 2.98 (d, J = 12.0 Hz, 2H), 1.98 (d, J = 11.2 Hz, 3H), 1.88 – 1.63 (m, 3H); 13C NMR (100 MHz, DMSO-d6) δ 164.2, 156.6, 142.6, 136.9, 131.8, 129.4, 128.2, 123.6, 122.5, 120.5, 112.5, 111.0, 44.1, 42.8, 28.9. MS (ESI) [M+H]+ 369.9.
N-(piperidin-4-ylmethyl)-6-(pyridin-3-yl)-1H-indole-3-carboxamide Hydrochloride (28). 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.25 (s, 1H), 8.98 (s, 1H), 8.88 (d, J = 8.4 Hz, 1H), 8.81 (d, J = 4.0 Hz, 1H), 8.71 (d, J = 12.0 Hz, 1H), 8.27 (d, J = 8.4 Hz, 1H), 8.22 (dd, J = 12.0, 4.0 Hz, 2H), 8.08 (dd, J = 8.0, 4.0 Hz, 1H), 7.92 (s, 1H), 7.58 (d, J = 8.4 Hz, 1H), 3.22 (d, J = 12.4 Hz, 2H), 3.15 (t, J = 6.0 Hz, 2H), 2.79 (q, J = 12.0 Hz, 2H), 1.80 (d, J = 12.0 Hz, 3H), 1.38 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.8, 143.2, 140.2, 140.1, 136.9, 130.4, 127.8, 127.7, 127.6, 122.4, 120.1, 111.5, 111.1, 43.7, 43.3, 34.4, 26.8. MS (ESI) [M+H]+ 335.2.
N-(piperidin-4-ylmethyl)-6-(pyridin-4-yl)-1H-indole-3-carboxamide Hydrochloride (29). 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 8.93 (s, 1H), 8.86 (d, J = 8.0 Hz, 2H), 8.67 (s, 1H), 8.42 (d, J = 4.0 Hz, 2H), 8.36 – 8.05 (m, 4H), 7.77 (d, J = 8.0 Hz, 1H), 3.23 (d, J = 11.2 Hz, 2H), 3.17 (s, 2H), 2.80 (d, J = 12.0 Hz, 2H), 1.82 (d, J = 11.6 Hz, 3H), 1.39 (d, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.6, 157.2, 141.9, 136.9, 131.7, 129.5, 127.9, 123.7, 122.5, 120.4, 112.6, 111.3, 66.8, 43.28, 34.4, 26.8. MS (ESI) [M+H]+ 335.7.
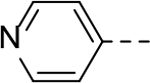
N-(piperidin-4-yl)-6-(pyridin-4-yl)-1H-indole-3-carboxamide Hydrochloride (30). 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 8.99 (s, 2H), 8.85 (d, J = 4.0 Hz, 2H), 8.46 – 8.34 (m, 3H), 8.31 (d, J = 8.4 Hz, 1H), 8.16 (d, J = 8.0 Hz, 1H), 8.10 (s, 1H), 7.75 (d, J = 8.6 Hz, 1H), 4.06 (s, 1H), 3.30 (d, J = 12.4 Hz, 2H), 2.99 (s, 2H), 1.97 (d, J = 11.6 Hz, 2H), 1.79 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.1, 156.5, 142.6, 136.9, 131.9, 129.5, 128.1, 123.6, 122.5, 120.4, 112.5, 111.1, 44.0, 42.6, 28.9. MS (ESI) [M+H]+ 321.6.
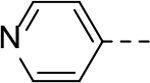
N-(2-(piperidin-4-yl)ethyl)-6-(pyridin-4-yl)-1H-indole-3-carboxamide Hydrochloride (31). 1H NMR (400 MHz, DMSO-d6) δ 11.70 (s, 1H), 8.81 (s, 1H), 8.54 (s, 1H), 8.17 (d, J = 8.0 Hz, 1H), 8.08 (t, J = 5.6 Hz, 2H), 7.72 (d, J = 8.0 Hz, 2H), 7.66 (s, 1H), 7.53 (d, J = 8.0 Hz, 2H), 7.41 (d, J = 8.0 Hz, 1H), 4.03 (d, J = 5.6 Hz, 2H), 3.23 (d, J = 12.0 Hz, 2H), 3.15 (t, J = 6.0 Hz, 2H), 2.81 (q, J = 12.0 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.36 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.4, 156.5, 142.7, 136.9, 131.4, 129.3, 128.1, 123.6, 122.5, 120.3, 112.5, 111.5, 43.6, 36.2, 36.1, 31.3, 28.7. MS (ESI) [M+H]+ 349.4.
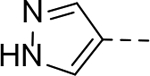
N-(piperidin-4-ylmethyl)-6-(1H-pyrazol-3-yl)-1H-indole-3-carboxamide Hydrochloride (32). 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 8.68 (s, 1H), 8.39 (s, 1H), 8.17 – 7.86 (m, 5H), 7.56 (s, 1H), 7.34 (d, J = 8.0 Hz, 1H), 3.24 (d, J = 12.0 Hz, 2H), 3.14 (t, J = 4.0 Hz, 2H), 2.81 (q, J = 12.0 Hz, 2H), 1.80 (d, J = 11.2 Hz, 3H), 1.39 – 1.29 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 165.1, 137.1, 130.9, 128.1, 127.2, 125.0, 122.6, 121.7, 119.2, 111.1, 108.2, 43.6, 43.4, 34.4, 26.8. MS (ESI) [M+H]+ 324.2.
3. Synthesis of compound 3
To a solution of 80 (483.4 mg, 1 mmol) in DMF (10 mL) were added NaH (84 mg, 2.1 mmol). The resulting mixture was stirred at room temperature for 0.5 h. Then tert-butyl 4-(3-bromopropyl)piperidine-1-carboxylate (336.9 mg, 1.1 mmol) was added to the mixture. The mixture was stirred for 14 h before it was quenched with NH4Cl aq. The mixture was extracted with ethyl acetate (3 × 10 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuum to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate = 3:1) to afford a white solid 81, which was used directly for next step.
The above intermediate 81 (70.9 mg, 0.1 mmol), (4-(tert-butyl)phenyl)boronic acid (21.4 mg, 0.12 mmol), tetrakis(triphenylphosphine)palladium (5.8 mg, 0.005 mmol), and sodium carbonate (21 mg, 0.2 mmol) in Toluene/DMF/EtOH/H2O (5/1/0.8/1 mL) were placed in a sealed tube. The mixture was degassed and heated to 80 °C for 14 h. The reaction was then cooled and quenched with brine (5 mL). The product was extracted with ethyl acetate (3 × 15 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1:1 to 1:2) to afford the corresponding coupling product.
To a solution of this intermediate in DCM (2 mL) was added dropwise HCl (0.2 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 1 h. The volatiles were removed in vacuo to afford an the final product as a hydrochloric salt.
5-(4-(tert-butyl)phenyl)-1-(3-(piperidin-4-yl)propyl)-N-(piperidin-4-ylmethyl)-1H-indole-3-carboxamide Hydrochloride (3). 1H NMR (400 MHz, DMSO-d6) δ 8.89 (s, 2H), 8.65 (s, 2H), 8.38 (d, J = 4.0 Hz, 1H), 8.13 (s, 1H), 8.10 (t, J = 4.0 Hz, 1H), 7.62 – 7.58 (m, 3H), 7.47 (d, J = 8.0 Hz, 3H), 4.22 (t, J = 6.0 Hz, 2H), 3.54 (s, 2H), 3.27 – 3.18 (m, 6H), 2.87–2.76 (m, 4H), 1.85 – 1.73 (m, 8H), 1.41–1.19 (m, 16H); 13C NMR (100 MHz, DMSO-d6) δ 164.8, 149.4, 139.1, 135.9, 133.4, 131.9, 127.4, 126.9, 126.1, 121.6, 119.5, 111.2, 66.8, 43.7, 43.5, 43.3, 34.6, 34.4, 33.0, 32.9, 31.62, 28.7, 27.1, 26.8. MS (ESI) [M+H]+ 515.4.
4. General procedure for the synthesis of 4 and 5.
A mixture of 5-iodoindole 82 (500 mg, 2.06 mmol) 4-tert-Butylphenylboronic acid (403 mg, 2.26 mmol), tetrakis(triphenylphosphine)palladium (119 mg, 0.103 mmol), and sodium carbonate (435 mg, 4.10 mmol) in p-dioxane/H2O (4/1, v/v, 15 mL) were placed in a sealed tube. The mixture was degassed and heated to 80 °C for 16 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuum to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 5:1 to 2:1) to afford 83 (415 mg, 81%).
To a solution of 83 (400 mg, 1.60 mmol) in DMF (6 mL) was added 4-bromobenzyl bromide (480 mg, 1.92 mmol) and potassium carbonate (332 mg, 2.4 mmol). The resulting mixture was stirred for 16 h, quenched with H2O, and extracted with ethyl acetate (3 × 15 mL). the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuum to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 8:1 to 2:1) to afford 84 (612 mg, 91%).
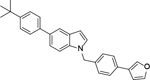
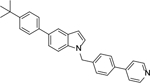
A mixture of 84 (1.0 mmol), furan-3-ylboronic acid or pyridin-4-ylboronic acid (1.2 mmol), tetrakis(triphenylphosphine)palladium (0.10 mmol), and sodium carbonate (2.0 mmol) in p-dioxane/H2O (4/1, v/v, 15 mL) were placed in a sealed tube. The mixture was degassed and heated to 80 °C for 16 h. The reaction was then cooled and quenched with brine (20 mL). The product was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuum to give a crude oil, which was purified by column chromatography to afford 4 or 5.
5-(4-(Tert-butyl)phenyl)-1-(4-(furan-3-yl)benzyl)-1H-indole Hydrochloride (4). 1H NMR (400 MHz, CDCl3) δ 7.85 (s, 1H), 7.69 (s, 1H), 7.58 (d, J = 8.4 Hz, 2H), 7.47 – 7.40 (m, 6H), 7.33 (d, J = 8.4 Hz, 1H), 7.15 (d, J = 8.4 Hz, 3H), 6.66 (s, 1H), 6.60 (d, J = 2.8 Hz, 1H), 5.34 (s, 2H), 1.37 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 149.3, 143.9, 139.7, 138.7, 136.3, 135.9, 133.2, 132.0, 129.4, 128.9, 127.5, 127.1, 126.4, 126.1, 125.7, 121.8, 119.5, 110.0, 108.9, 102.2, 50.2, 34.6, 31.6. MS (ESI) [M+H]+ 406.8.
5-(4-(Tert-butyl)phenyl)-1-(4-(pyridin-4-yl)benzyl)-1H-indole Hydrochloride (5). 1H NMR (400 MHz, CDCl3) δ 8.64 (s, 2H), 7.87 (s, 1H), 7.61 – 7.52 (m, 4H), 7.49 – 7.40 (m, 5H), 7.33 (d, J = 8.4 Hz, 1H), 7.27 – 7.21 (m, 2H), 7.20 – 7.15 (m, 1H), 6.63 (d, J = 2.0 Hz, 1H), 5.40 (s, 2H), 1.37 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 150.3, 149.2, 147.7, 139.5, 138.6, 137.5, 135.7, 133.1, 129.2, 128.8, 127.5, 127.4, 127.0, 125.6, 121.7, 121.5, 119.4, 109.8, 102.3, 49.9, 34.4, 31.4. MS (ESI) [M+H]+ 417.2.
5. General Procedure for the synthesis of compounds 6, 8, and 33−66.
To a solution of 5, or 6-bromoindole (1.8 g, 6.72 mmol) in THF/H2O (12/4 mL) was added slowly sodium hydroxide solution (806.0 mg, 20.2 mmol). The resulting mixture was heated at 50 °C for 5 h before it was cooled to room temperature. The solvent was removed in vacuo, and the white slurry was diluted with H2O and acidified by HCl (3 N). It was filtered, washed with H2O, and dried to give acid as a white solid. The crude product was used for the next step without further purification.
To a solution of the crude acid (1.5 g) and 4-aminomethyl-1-Boc-piperidine (1.47 g, 6. 88 mmol), or 4-amino-1-Boc-piperidine (1.38 g, 6.89 mmol) in DMF (20 mL) was added N, N-diisopropylethylamine (1.84 mL, 12.5 mmol) and HATU (2. 85 g, 7.5 mmol). The mixture was stirred for 12 h before it was quenched with H2O. The mixture was extracted with ethyl acetate (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1.5:1 to 1:1) to afford 86.
Compound 86 (0.2 mmol), aryl boronic acid or aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (0.24 mmol), tetrakis(triphenylphosphine)palladium (11.6 mg, 0.01 mmol), and sodium carbonate (42 mg, 0.4 mmol) in p-dioxane/H2O (5/1, v/v, 6 mL) were placed in a sealed tube. The mixture was degassed and heated to 100 °C for 16 h. The reaction was then cooled and quenched with brine (5 mL). The product was extracted with ethyl acetate (3 × 15 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuum to give a crude oil, which was purified by column chromatography to afford the corresponding coupling product.
To a solution of this intermediate (0.15 mmol) in DCM (3 mL) was added dropwise HCl (0.2 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 6 h. The volatiles were removed in vacuum to afford an oil, which was triturated in diether ether and solidified to give the final product as a hydrochloric salt.
5-(4-(Tert-butyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (6). 1H NMR (400 MHz, DMSO-d6) δ 11.70 (s, 1H), 9.05 (s, 1H), 8.83 – 8.69 (m, 2H), 7.84 (s, 1H), 7.58 (d, J = 8.4 Hz, 2H), 7.52 – 7.40 (m, 4H), 7.21 (s, 1H), 3.30 – 3.16 (m, 4H), 2.82 (dd, J = 21.6, 10.0 Hz, 2H), 1.83 (d, J = 12.8 Hz, 3H), 1.42 (dd, J = 22.8, 10.0 Hz, 2H), 1.31 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 148.8, 138.6, 135.9, 134.0, 132.4, 126.4, 125.6, 124.1, 122.8, 119.1, 112.7, 103.2, 43.2, 42.8, 34.2, 33.8, 31.2, 26.3. MS (ESI) [M+H]+ 390.2.
6-(4-(Tert-butyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (8). 1H NMR (400 MHz, DMSO-d6) δ 8.59 (t, J = 6.0 Hz, 1H), 7.66 – 7.61 (m, 2H), 7.56 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.4 Hz, 1H), 7.11 (s, 1H), 3.27 – 3.17 (m, 4H), 2.82 (t, J = 11.6 Hz, 2H), 1.81 (d, J = 11.6 Hz, 3H), 1.28 (s, 11H); 13C NMR (100 MHz, DMSO-d6) δ 161.3, 149.6, 138.5, 137.1, 135.9, 132.2, 126.6, 126.5, 125.9, 122.1, 119.6, 110.0, 102.7, 43.6, 43.1, 34.4, 33.9, 31.4, 26.4; MS (ESI) [M+H]+ 390.2.
N-(Piperidin-4-ylmethyl)-5-(pyridin-3-yl)-1H-indole-2-carboxamide Hydrochloride (33). 1H NMR (400 MHz, DMSO-d6) δ 11.97 (s, 1H), 9.24 (s, 1H), 9.08 (s, 1H), 8.92 – 8.73 (m, 4H), 8.20 (s, 1H), 8.09 – 7.99 (m, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.59 (d, J = 8.4 Hz, 1H), 7.31 (s, 1H), 3.27 – 3.21 (m, 4H), 2.83 (dd, J = 24.8, 13.1 Hz, 2H), 1.84 (d, J = 12.8 Hz, 3H), 1.43 (dd, J = 23.2, 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.3, 142.4, 140.5, 140.4, 140.2, 137.3, 133.7, 128.2, 127.3, 126.0, 123.0, 121.4, 113.8, 103.9, 44.0, 43.2, 34.2, 26.7. MS (ESI) [M+H]+ 335.8.
N-(Piperidin-4-ylmethyl)-5-(pyridin-4-yl)-1H-indole-2-carboxamide Hydrochloride (34). 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 8.89 – 8.77 (m, 4H), 8.63 – 8.52 (m, 1H), 8.44 (s, 1H), 8.38 (d, J = 5.6 Hz, 2H), 7.87 (d, J = 8.8 Hz, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.34 (s, 1H), 3.26 – 3.23 (m, 4H), 2.84 (dd, J = 20.4, 10.4 Hz, 2H), 1.84 (d, J = 12.8 Hz, 3H), 1.40 (dd, J = 23.2, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 160.8, 156.0, 142.4, 138.1, 133.7, 127.8, 126.0, 122.8, 122.8, 122.5, 113.5, 103.9, 43.6, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 335.2.
N-(Piperidin-4-ylmethyl)-5-(1H-pyrazol-4-yl)-1H-indole-2-carboxamide Hydrochloride (35). 1H NMR (400 MHz, DMSO-d6) δ 11.62 (s, 1H), 9.01 (d, J = 8.4 Hz, 1H), 8.80 – 8.63 (m, 2H), 8.17 (s, 2H), 7.83 (s, 1H), 7.42 (dd, J = 25.6, 8.4 Hz, 2H), 7.12 (s, 1H), 3.29 – 3.13 (m, 4H), 2.80 (dd, J = 22.4, 11.2 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.39 (dd, J = 24.4, 12.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.6, 135.8, 132.6, 130.6, 127.9, 126.0, 124.3, 123.1, 122.4, 118.0, 113.0, 103.1, 43.9, 43.2, 34.2, 26.7. MS (ESI) [M+H]+ 324.4.
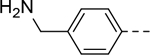
5-(4-(Aminomethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (36).
1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 9.00 (s, 1H), 8.80 – 8.64 (m, 2H), 8.46 (s, 3H), 7.91 (s, 1H), 7.73 (d, J = 8.0 Hz, 2H), 7.56 (d, J = 8.0 Hz, 2H), 7.51 (s, 2H), 7.22 (s, 1H), 4.05 (s, 2H), 3.23 (dd, J = 16.4, 10.0 Hz, 4H), 2.88 – 2.77 (m, 2H), 1.91 – 1.74 (m, 3H), 1.41 (dd, J = 23.6, 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 141.5, 136.1, 132.6, 132.1, 131.5, 129.5, 127.7, 126.8, 122.7, 119.4, 112.8, 103.2, 43.5, 42.8, 41.9, 33.8, 26.3. MS (ESI) [M+H]+ 363.3.
5-(4-(Furan-3-yl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (37). 1H NMR (400 MHz, DMSO-d6) δ 11.70 (s, 1H), 8.87 (s, 1H), 8.71 (s, 1H), 8.58 (s, 1H), 8.23 (s, 1H), 7.92 (s, 1H), 7.76 (s, 1H), 7.73 – 7.62 (m, 4H), 7.57 – 7.47 (m, 2H), 7.21 (s, 1H), 7.01 (s, 1H), 3.26 – 3.19 (m, 4H), 2.91 – 2.78 (m, 2H), 1.84 (d, J = 12.0 Hz, 3H), 1.40 (dd, J = 24.0, 11.6 Hz, 2H). MS (ESI) [M+H]+ 400.2.
N-(Piperidin-4-ylmethyl)-5-(4-(thiophen-3-yl)phenyl)-1H-indole-2-carboxamide Hydrochloride (38). 1H NMR (400 MHz, DMSO-d6) δ 11.65 (s, 1H), 8.73 (s, 1H), 8.65 (s, 1H), 8.41 (s, 1H), 7.91 (s, 1H), 7.89 (s, 1H), 7.77 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 8.4 Hz, 2H), 7.63 (dd, J = 4.8, 2.8 Hz, 1H), 7.58 (d, J = 4.8 Hz, 1H), 7.50 (dd, J = 19.2, 8.4 Hz, 2H), 7.18 (s, 1H), 3.21 (d, J = 7.2 Hz, 4H), 2.82 (dd, J = 22.0, 10.8 Hz, 2H), 1.81 (d, J = 10.8 Hz, 3H), 1.36 (dd, J = 23.2, 10.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.6, 141.6, 140.4, 136.5, 133.6, 132.8, 132.1, 128.1, 127.5, 126.9, 126.5, 123.0, 121.1, 119.5, 113.2, 110.0, 103.4, 43.9, 43.3, 34.2, 26.7. MS (ESI) [M+H]+ 416.2.
5-(4-(Piperidin-1-ylmethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (39). 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 10.60 (s, 1H), 8.97 (d, J = 11.6 Hz, 1H), 8.74 (t, J = 5.6 Hz, 1H), 8.68 (d, J = 10.0 Hz, 1H), 7.94 (s, 1H), 7.76 (d, J = 7.6 Hz, 2H), 7.67 (d, J = 7.6 Hz, 2H), 7.53 (d, J = 4.0 Hz, 2H), 7.23 (s, 1H), 4.27 (d, J = 4.0 Hz, 2H), 3.36 – 3.19 (m, 8H), 2.91 – 2.76 (m, 4H), 1.87 – 1.75 (m, 6H), 1.69 (d, J = 11.6 Hz, 1H), 1.45 – 1.35 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 142.3, 136.2, 132.6, 132.0, 131.3, 129.1, 127.8, 126.8, 122.7, 119.6, 114.9, 112.8, 103.2, 58.6, 51.5, 43.5, 42.8, 33.8, 26.3, 22.2, 21.4. MS (ESI) [M+H]+ 430.3.
5-(4-(Piperidin-1-yl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (40). 1H NMR (400 MHz, DMSO-d6) δ 11.75 (s, 1H), 9.01 (brs, 1H), 8.81 – 8.66 (m, 2H), 7.96 – 7.75 (m, 5H), 7.54 – 7.45 (m, 2H), 7.22 (s, 1H), 3.49 (s, 4H), 3.21 (dd, J = 15.2, 9.2 Hz, 4H), 2.80 (dd, J = 22.4, 11.2 Hz, 2H), 1.98 (s, 3H), 1.81 (d, J = 12.8 Hz, 4H), 1.66 (s, 2H), 1.40 (q, J = 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 136.2, 132.7, 130.7, 127.9, 127.7, 122.7, 121.7, 119.7, 112.9, 111.9, 109.6, 103.3, 43.6, 42.8, 33.8, 26.3, 23.11, 21.1. MS (ESI) [M+H]+ 417.3.
N-(Piperidin-4-ylmethyl)-5-(4-(pyrrolidin-1-yl)phenyl)-1H-indole-2-carboxamide Hydrochloride (41). 1H NMR (400 MHz, DMSO-d6) δ 11.63 (s, 1H), 8.99 (d, J = 9.6 Hz, 1H), 8.69 (t, J = 5.6 Hz, 2H), 7.78 (s, 1H), 7.59 (d, J = 8.4 Hz, 2H), 7.44 (s, 2H), 7.16 (s, 1H), 6.99 (s, 2H), 3.37 (s, 4H), 3.25 – 3.16 (m, 4H), 2.81 (dd, J = 23.2, 12.0 Hz, 2H), 2.01 (s, 4H), 1.81 (d, J = 12.0 Hz, 3H), 1.39 (dd, J = 24.4, 12.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 135.6, 132.3, 132.0, 127.7, 127.4, 127.3, 122.5, 118.4, 112.6, 103.0, 43.5, 42.8, 33.8, 26.3, 24.5. MS (ESI) [M+H]+ 403.2.
5-(4-Morpholinophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (42). 1H NMR (400 MHz, DMSO-d6) δ 11.67 (s, 1H), 8.99 (d, J = 11.2 Hz, 1H), 8.76 – 8.63 (m, 2H), 7.84 (s, 1H), 7.65 (d, J = 8.4 Hz, 2H), 7.46 (s, 2H), 7.35 (d, J = 6.8 Hz, 2H), 7.18 (s, 1H), 3.88 (s, 4H), 3.31 (s, 4H), 3.26 – 3.16 (m, 4H), 2.81 (dd, J = 22.8, 11.6 Hz, 2H), 1.81 (d, J = 12.4 Hz, 3H), 1.39 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 135.9, 132.4, 131.5, 127.7, 127.5, 122.6, 118.9, 117.9, 112.7, 103.1, 65.2, 50.6, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 419.5.
5-(4-(Piperazin-1-yl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (43). 1H NMR (400 MHz, DMSO-d6) δ 11.64 (s, 1H), 9.35 (s, 2H), 9.00 (brs, 1H), 8.71 (d, J = 6.0 Hz, 2H), 7.79 (s, 1H), 7.56 (d, J = 8.8 Hz, 2H), 7.44 (s, 2H), 7.16 (d, J = 2.0 Hz, 1H), 7.05 (d, J = 8.8 Hz, 2H), 3.39 (d, J = 5.2 Hz, 4H), 3.20 (s, 8H), 2.80 (q, J = 11.2 Hz, 2H), 1.81 (d, J = 12.8 Hz, 3H), 1.45 – 1.33 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 148.6, 135.7, 133.2, 132.3, 131.9, 127.7, 127.3, 122.5, 118.4, 116.5, 112.6, 103.1, 45.6, 43.5, 42.8, 42.5, 33.8, 26.3. MS (ESI) [M+H]+ 418.2.
5-(4-Cyanophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (44). 1H NMR (400 MHz, DMSO-d6) δ 11.79 (s, 1H), 8.87 – 8.76 (m, 1H), 8.72 (t, J = 4.8 Hz, 1H), 8.55 – 8.43 (m, 1H), 8.03 (s, 1H), 7.94 – 7.86 (m, 4H), 7.56 (dd, J = 22.2, 8.8 Hz, 2H), 7.24 (s, 1H), 3.23 (d, J = 7.2 Hz, 4H), 2.84 (dd, J = 23.2, 12.0 Hz, 2H), 1.84 (d, J = 12.0 Hz, 3H), 1.45 – 1.34 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.0, 145.9, 136.6, 132.8, 132.7, 130.1, 127.7, 127.4, 122.7, 120.3, 119.1, 113.0, 108.8, 103.3, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 359.6.
5-(4-Cyano-2-methylphenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (45). 1H NMR (400 MHz, DMSO-d6) δ 11.75 (s, 1H), 8.93 (s, 1H), 8.75 – 8.60 (m, 2H), 7.76 (s, 1H), 7.68 (d, J = 8.0 Hz, 1H), 7.58 (s, 1H), 7.48 (d, J = 8.4 Hz, 1H), 7.41 (d, J = 8.0 Hz, 1H), 7.20 – 7.13 (m, 2H), 3.21 (dd, J = 16.0, 10.0 Hz, 4H), 2.81 (dd, J = 22.4, 11.2 Hz, 2H), 2.27 (s, 3H), 1.81 (d, J = 12.4 Hz, 3H), 1.44 – 1.32 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 147.4, 136.9, 135.8, 133.7, 132.6, 131.2, 130.9, 129.6, 127.1, 124.4, 121.6, 119.1, 112.2, 109.3, 103.0, 43.5, 42.8, 33.8, 26.3, 20.1. MS (ESI) [M+H]+ 373.4.
5-(3-Cyanophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (46). 1H NMR (400 MHz, DMSO-d6) δ 11.79 (s, 1H), 9.01 (s, 1H), 8.83 – 8.66 (m, 2H), 8.15 (s, 1H), 8.04 (d, J = 7.6 Hz, 1H), 8.01 (s, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.64 (t, J = 7.6 Hz, 1H), 7.55 (dd, J = 21.2, 8.4 Hz, 2H), 7.24 (s, 1H), 3.23 (dd, J = 14.4, 9.2 Hz, 4H), 2.83 (dd, J = 23.2, 11.2 Hz, 2H), 1.83 (d, J = 12.4 Hz, 3H), 1.42 (dd, J = 22.0, 10.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.0, 142.5, 136.4, 132.8, 131.4, 130.0, 130.04, 130.00, 129.9, 127.7, 122.6, 120.0, 119.0, 113.0, 112.0, 103.3, 43.6, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 359.2.
N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (47). 1H NMR (400 MHz, DMSO-d6) δ 11.69 (s, 1H), 8.69 (s, 1H), 7.65 – 7.53 (m, 2H), 7.16 (d, J = 10.8 Hz, 2H), 3.32 – 3.15 (m, 4H), 2.83 (t, J = 12.0 Hz, 2H), 1.82 (d, J = 12.0 Hz, 3H), 1.36 (dd, J = 24.4, 12.8 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 137.2, 132.5, 126.2, 123.6, 123.1, 116.3, 114.9, 102.9, 43.7, 43.0, 33.9, 26.4. MS (ESI) [M+H]+ 258.5.
6-Phenyl-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (48). 1H NMR (400 MHz, DMSO-d6) δ 11.71 (s, 1H), 8.95 (d, J = 10.4 Hz, 1H), 8.73 – 8.57 (m, 2H), 7.67 (d, J = 8.4 Hz, 1H), 7.64 – 7.58 (m, 3H), 7.44 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.6 Hz, 2H), 7.15 (s, 1H), 3.21 (dd, J = 15.6, 9.6 Hz, 4H), 2.81 (dd, J = 22.0, 11.2 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.48 – 1.30 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 141.2, 137.0, 135.7, 132.4, 128.9, 126.9, 126.8, 126.5, 121.9, 119.3, 110.0, 102.6, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 334.2.
6-(4-Fluorophenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (49). 1H NMR (400 MHz, DMSO-d6) δ 11.69 (s, 1H), 8.64 (s, 2H), 8.33 (s, 1H), 7.72 – 7.64 (m, 3H), 7.61 (s, 1H), 7.36 – 7.25 (m, 3H), 7.16 (s, 1H), 3.25 – 3.19 (m, 4H), 2.85 (dd, J = 21.6, 10.4 Hz, 2H), 1.84 (d, J = 11.2 Hz, 3H), 1.37 (dd, J = 24.4, 12.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 162.8, 161.1, 160.4, 137.79, 137.76, 137.0, 134.7, 132.6, 128.7, 128.6, 126.5, 122.0, 119.3, 115.8, 115.6, 110.1, 102.7, 43.6, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 352.1.
6-(4-Methoxyphenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (50). 1H NMR (400 MHz, DMSO-d6) δ 11.67 (s, 1H), 9.02 (s, 1H), 8.70 (s, 2H), 7.65 (d, J = 8.0 Hz, 1H), 7.61 – 7.53 (m, 3H), 7.30 (d, J = 8.0 Hz, 1H), 7.16 (s, 1H), 7.03 (d, J = 7.2 Hz, 2H), 3.80 (s, 3H), 3.33 – 3.09 (m, 4H), 2.83 (d, J = 9.6 Hz, 2H), 1.83 (d, J = 12.0 Hz, 3H), 1.41 (d, J = 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 158.5, 137.1, 135.4, 133.6, 132.2, 127.8, 126.0, 121.8, 119.1, 114.4, 109.4, 102.6, 55.2, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 364.7.
N-(Piperidin-4-ylmethyl)-6-(4-(trifluoromethoxy)phenyl)-1H-indole-2-carboxamide Hydrochloride (51). 1H NMR (400 MHz, DMSO-d6) δ 11.78 (s, 1H), 9.03 (d, J = 9.6 Hz, 1H), 8.81 – 8.68 (m, 2H), 7.73 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.4 Hz, 1H), 7.62 (s, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.0 Hz, 1H), 7.17 (s, 1H), 3.20 (dd, J = 13.6, 8.4 Hz, 4H), 2.79 (dd, J = 22.0, 11.6 Hz, 2H), 1.80 (d, J = 12.0 Hz, 3H), 1.39 (dd, J = 22.8, 10.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 147.4, 140.6, 136.9, 136.4, 134.1, 132.8, 131.7, 128.6, 126.8, 123.2, 122.1, 121.5, 119.6, 119.3, 118.9, 112.3, 110.3, 102.6, 43.6, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 418.2.
6-(4-(aminomethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (52). 1H NMR (400 MHz, D2O) δ 7.82 – 7.66 (m, 3H), 7.62 (s, 1H), 7.48 (d, J = 7.2 Hz, 2H), 7.37 (d, J = 7.6 Hz, 1H), 7.00 (s, 1H), 4.17 (s, 2H), 3.42 (d, J = 11.6 Hz, 2H), 3.20 (s, 2H), 2.93 (t, J = 12.0 Hz, 2H), 1.93 (d, J = 13.6 Hz, 3H), 1.52 – 1.33 (m, 2H); 13C NMR (100 MHz, D2O) δ 163.3, 141.5, 136.9, 136.0, 131.3, 130.8, 129.3, 127.5, 126.7, 122.4, 119.8, 110.0, 103.8, 43.9, 43.6, 42.6, 33.5, 26.0. MS (ESI) [M+H]+ 363.5.
6-(4-Carbamoylphenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (53). 1H NMR (400 MHz, DMSO-d6) δ 11.77 (s, 1H), 8.92 (d, J = 7.6 Hz, 1H), 8.71 (s, 1H), 8.62 (d, J = 8.0 Hz, 1H), 8.02 (s, 1H), 7.95 (d, J = 8.0 Hz, 2H), 7.78 – 7.60 (m, 4H), 7.39 (d, J = 8.8 Hz, 2H), 7.17 (s, 1H), 3.30 – 3.12 (m, 4H), 2.81 (dd, J = 22.4, 11.2 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.38 (d, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 167.6, 161.1, 143.9, 137.0, 134.6, 132.8, 132.5, 128.2, 126.9, 126.4, 122.1, 119.3, 110.4, 102.6, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 377.4.
6-(4-(Hydroxymethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (54). 1H NMR (400 MHz, DMSO-d6) δ 11.72 (s, 1H), 9.01 (d, J = 11.2 Hz, 1H), 8.76 – 8.68 (m, 2H), 7.71 – 7.63 (m, 3H), 7.61 (d, J = 8.0 Hz, 2H), 7.40 (d, J = 7.6 Hz, 2H), 7.34 (d, J = 8.4 Hz, 1H), 7.18 (s, 1H), 4.54 (s, 2H), 3.28 – 3.21 (m, 4H), 2.83 (dd, J = 22.8, 11.6 Hz, 2H), 1.84 (d, J = 11.6 Hz, 3H), 1.45 – 1.38 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 141.2, 139.6, 137.0, 135.6, 132.4, 129.5, 127.1, 126.4, 121.9, 119.3, 109.9, 102.6, 62.6, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 364.2.
6-(4-(2-Hydroxyethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (55). 1H NMR (400 MHz, DMSO-d6) δ 11.72 (s, 1H), 9.05 (brs, 1H), 8.73 (brs, 1H), 7.67 (d, J = 8.4 Hz, 1H), 7.62 (s, 1H), 7.55 (d, J = 8.0 Hz, 2H), 7.31 (t, J = 8.4 Hz, 4H), 7.18 (s, 1H), 4.71 (t, J = 4.8 Hz, 1H), 3.67 – 3.59 (m, 2H), 3.28 – 3.18 (m, 4H), 2.87 – 2.79 (m, 2H), 2.76 (t, J = 6.8 Hz, 2H), 1.83 (d, J = 12.0 Hz, 3H), 1.42 (dd, J = 26.0, 14.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 138.9, 138.3, 137.1, 135.7, 132.3, 129.5, 126.6, 126.4, 121.9, 119.3, 109.8, 102.6, 62.2, 62.1, 43.4, 42.7, 33.8, 26.3. MS (ESI) [M+H]+ 378.2.
6-(4-Phenoxyphenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (56). 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 9.02 (d, J = 12.4 Hz, 1H), 8.73 (t, J = 6.0 Hz, 2H), 7.73 – 7.56 (m, 4H), 7.47 – 7.37 (m, 2H), 7.34 (dd, J = 8.4, 1.6 Hz, 1H), 7.20 – 7.13 (m, 2H), 7.12 – 6.99 (m, 4H), 3.23 (dd, J = 15.2, 9.2 Hz, 4H), 2.83 (dd, J = 22.0, 11.2 Hz, 2H), 1.84 (d, J = 12.4 Hz, 3H), 1.41 (dd, J = 24.8, 13.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 156.6, 155.9, 137.0, 136.5, 135.0, 132.4, 130.1, 128.3, 126.4, 123.6, 122.0, 119.2, 119.0, 118.8, 109.8, 102.6, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 426.2.
6-(4-(Piperazin-1-yl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (57). 1H NMR (400 MHz, DMSO-d6) δ 11.63 (s, 1H), 9.09 (s, 2H), 8.78 (s, 1H), 8.65 (s, 1H), 8.47 (s, 1H), 7.65 (d, J = 8.4 Hz, 1H), 7.56 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.8 Hz, 1H), 7.14 (s, 1H), 7.09 (d, J = 8.4 Hz, 2H), 3.40–3.10 (m, 12H), 2.84 (dd, J = 22.0, 10.8 Hz, 2H), 1.83 (d, J = 11.2 Hz, 3H), 1.38 (dd, J = 24.4, 13.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.6, 149.3, 137.4, 135.9, 133.2, 132.1, 127.8, 126.4, 122.3, 119.5, 116.9, 109.5, 103.0, 45.8, 43.6, 43.3, 43.0, 34.1, 26.5. MS (ESI) [M+H]+ 418.2.
6-(4-(Piperidin-1-yl)phenyl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (58). 1H NMR (400 MHz, DMSO-d6) δ 11.79 (s, 1H), 9.01 (s, 1H), 8.75 (s, 2H), 7.96 – 7.84 (m, 1H), 7.80 (s, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.66 (s, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.19 (s, 1H), 3.56 (s, 4H), 3.29 – 3.18 (m, 4H), 2.83 (dd, J = 22.0, 12.0 Hz, 2H), 2.17 – 1.75 (m, 7H), 1.75 – 1.58 (m, 2H), 1.41 (dd, J = 24.8, 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 142.4, 141.4, 136.9, 134.3, 132.6, 128.3, 127.0, 122.3, 121.6, 119.5, 110.4, 102.8, 55.6, 43.6, 42.9, 33.9, 26.3, 23.4, 21.2. MS (ESI) [M+H]+ 417.3.
N-(Piperidin-4-yl)-6-(pyridin-4-yl)-1H-indole-2-carboxamide Hydrochloride (59). 1H NMR (400 MHz, DMSO-d6) δ 12.23 (s, 1H), 9.06 (s, 2H), 8.88–8.85 (m, 3H),, 8.34 (s, 2H), 8.01 (s, 1H), 7.86 (d, J = 8.4 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.36 (s, 1H), 4.12 (s, 1H), 3.34 (d, J = 12.4 Hz, 2H), 3.02 (s, 2H), 2.00 (d, J = 11.6 Hz, 2H), 1.86 (dd, J = 23.6, 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 160.4, 156.6, 142.6, 137.1, 135.1, 129.9, 129.7, 123.8, 123.3, 119.7, 112.7, 103.9, 44.6, 42.5, 28.6. MS (ESI) [M+H]+ 321.2.
N-(Piperidin-4-ylmethyl)-6-(pyridin-4-yl)-1H-indole-2-carboxamide Hydrochloride (60). 1H NMR (400 MHz, DMSO-d6) δ 12.15 (s, 1H), 8.98 (brs, 1H), 8.89 (t, J = 6.4 Hz, 1H), 8.84 (d, J = 6.4 Hz, 2H), 8.73 (brs, 1H), 8.30 (d, J = 6.8 Hz, 2H), 7.98 (s, 1H), 7.83 (d, J = 8.4 Hz, 1H), 7.65 (d, J = 8.4 Hz, 1H), 7.25 (s, 1H), 3.22 (d, J = 14.4 Hz, 4H), 2.80 (dd, J = 22.4, 12.0 Hz, 2H), 1.81 (d, J = 13.2 Hz, 3H), 1.46 – 1.33 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 160.7, 156.1, 142.3, 136.6, 134.9, 129.6 129.2, 123.3, 122.8, 119.3, 112.3, 102.9, 43.6, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 335.5.
N-(piperidin-4-ylmethyl)-6-(pyridin-3-yl)-1H-indole-2-carboxamide Hydrochloride (61). 1H NMR (400 MHz, DMSO-d6) δ 12.09 (s, 1H), 9.17 (brs, 2H), 8.93 (brs, 3H), 8.81 (d, J = 7.2 Hz, 1H), 8.08 (s, 1H), 7.82 (d, J = 12.0 Hz, 2H), 7.52 (d, J = 8.0 Hz, 1H), 7.27 (s, 1H), 3.22 (s, 4H), 2.82 (dd, J = 23.2, 12.0 Hz, 2H), 1.84 (d, J = 12.8 Hz, 3H), 1.44 (dd, J = 24.4, 12.4 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 160.8, 142.3, 140.2, 136.7, 133.8, 129.0, 128.0, 122.6, 119.1, 111.3, 102.9, 43.6, 42.7, 33.8, 26.2. MS (ESI) [M+H]+ 335.8.
6-(3,6-Dihydro-2H-pyran-4-yl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (62). 1H NMR (400 MHz, DMSO-d6) δ 11.61 (s, 1H), 8.91 (d, J = 11.6 Hz, 1H), 8.70 – 8.53 (m, 2H), 7.56 (d, J = 8.4 Hz, 1H), 7.41 (s, 1H), 7.22 (d, J = 8.4 Hz, 1H), 7.11 (s, 1H), 6.22 (s, 1H), 4.24 (d, J = 2.4 Hz, 2H), 3.84 (t, J = 5.2 Hz, 2H), 3.31 – 3.10 (m, 6H), 2.83 (dd, J = 22.8, 11.6 Hz, 2H), 1.83 (d, J = 11.2 Hz, 3H), 1.40 (q, J = 14.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.2, 136.7, 135.2, 133.7, 132.1, 126.3, 121.8, 121.3, 117.2, 107.8, 102.6, 65.2, 63.8, 43.5, 42.8, 33.8, 26.9, 26.3. MS (ESI) [M+H]+ 340.4.
N-(Piperidin-4-ylmethyl)-6-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole-2-carboxamide Hydrochloride (63). 1H NMR (400 MHz, DMSO-d6) δ 11.75 (s, 1H), 9.38 (s, 2H), 9.04 (s, 1H), 8.73 (t, J = 6.0 Hz, 2H), 7.59 (d, J = 8.4 Hz, 1H), 7.43 (s, 1H), 7.21 (d, J = 8.4 Hz, 1H), 7.14 (s, 1H), 6.15 (s, 1H), 3.74 (s, 2H), 3.30 (s, 2H), 3.21 (dd, J = 16.8, 10.8 Hz, 4H), 2.82 (dd, J = 22.4, 11.2 Hz, 2H), 2.72 (s, 2H), 1.82 (d, J = 12.4 Hz, 3H), 1.41 (dd, J = 23.2, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.0, 136.5, 134.9, 134.2, 132.5, 126.7, 121.5, 117.2, 115.8, 108.3, 102.7, 43.5, 42.7, 41.5, 33.8, 26.3, 23.5. MS (ESI) [M+H]+ 339.2.
N-(Piperidin-4-ylmethyl)-6-(1H-pyrazol-4-yl)-1H-indole-2-carboxamide Hydrochloride (64). 1H NMR (400 MHz, DMSO-d6) δ 11.72 (s, 1H), 9.16 (d, J = 9.2 Hz, 1H), 8.88 (d, J = 9.2 Hz, 1H), 8.77 (t, J = 5.6 Hz, 1H), 8.32 (s, 2H), 7.60 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.4 Hz, 1H), 7.15 (s, 1H), 3.33 – 3.09 (m, 4H), 2.81 (dd, J = 22.4, 11.2 Hz, 2H), 1.84 – 1.81 (m, 3H), 1.43 (dd, J = 23.2, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.5, 137.4, 132.6, 130.8, 127.3, 126.4, 123.2, 122.4, 119.0, 110.0, 108.9, 103.6, 43.9, 43.2, 34.2, 26.7. MS (ESI) [M+H]+ 340.1.
N-(Piperidin-4-ylmethyl)-6-(thiophen-3-yl)-1H-indole-2-carboxamide Hydrochloride (65). 1H NMR (400 MHz, DMSO-d6) δ 11.66 (s, 1H), 8.85 (s, 1H), 8.66 (t, J = 5.6 Hz, 1H), 8.55 (s, 1H), 7.82 – 7.73 (m, 1H), 7.70 – 7.59 (m, 3H), 7.54 – 7.48 (m, 1H), 7.46 – 7.37 (m, 1H), 7.14 (d, J = 1.2 Hz, 1H), 3.28 – 3.21 (m, 4H), 2.84 (dd, J = 21.6, 11.2 Hz, 2H), 1.85 – 1.82 (m, 3H), 1.40 (dd, J = 23.6, 11.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 142.5, 136.9, 132.3, 130.7, 127.0, 126.33, 126.30, 121.9, 120.0, 119.1, 109.3, 102.7, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 340.1.
6-(Furan-3-yl)-N-(piperidin-4-ylmethyl)-1H-indole-2-carboxamide Hydrochloride (66). 1H NMR (400 MHz, DMSO-d6) δ 11.64 (s, 1H), 8.90 (d, J = 10.4 Hz, 1H), 8.69 – 8.62 (m, 1H), 8.63 – 8.54 (m, 1H), 8.13 (s, 1H), 7.74 (s, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.55 (s, 1H), 7.31 (d, J = 8.4 Hz, 1H), 7.13 (s, 1H), 6.90 (s, 1H), 3.28 – 3.19 (m, 4H), 2.83 (dd, J = 22.4, 10.8 Hz, 2H), 1.84 – 1.81 (m, 3H), 1.40 (dd, J = 23.6, 11.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 144.2, 138.8, 136.9, 132.1, 127.2, 126.7, 126.2, 121.9, 118.6, 108.9, 108.7, 102.8, 43.5, 42.8, 33.8, 26.3. MS (ESI) [M+H]+ 324.2.
6. General Procedure for the synthesis of compounds 9 and 70–73.
To a solution of 6-bromo-1H-pyrrolo[2,3-b]pyridine 87 (591.1 g, 3 mmol) in DCM (10 mL) was added AlCl3 (2 g, 15 mmol) at room temperature. After the resulting mixture was stirred at room temperature for 10 min, 2,2,2-trichloroacetyl chloride (818.2 mg, 4.5 mmol) was added. The mixture was stirred for 12 h. Then it was poured into ice water. A lot of white solid precipitated. It was filtered to get the intermediate 88, which was used directly for the next step.
The above solid 88 was added to 20% NaOH solution. The resulting mixture was stirred at room temperature for 16 h. After the starting material was consumed, the mixture was quenched with 1 M HCl solution. A lot of white solid precipitated from the solvent. It was filtered to get the intermediate, which was used directly for the next step. The preparation of the final compounds followed the same procedure as described for compound 1.
6-(4-(tert-butyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide Hydrochloride (9). 1H NMR (400 MHz, DMSO-d6) δ 12.16 (s, 1H), 8.94 (s, 1H), 8.66 (d, J = 8.0 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 8.24–8.21 (m, 2H), 8.00 (d, J = 8.4 Hz, 2H), 7.72 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 8.4 Hz, 2H), 3.23 (d, J = 12.0 Hz, 2H), 3.16 (t, J = 5.6 Hz, 2H), 2.80 (q, J = 12.0 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.43 – 1.37 (m, 2H), 1.30 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 164.5, 151.4, 150.8, 148.8, 136.9, 130.6, 128.9, 126.7, 125.9, 117.9, 114.2, 109.9, 43.7, 43.3, 34.8, 34.4, 31.5, 26.8. MS (ESI) [M+H]+ 391.5.
N-(piperidin-4-ylmethyl)-6-(4-(trifluoromethyl)phenyl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide Hydrochloride (70). 1H NMR (400 MHz, DMSO-d6) δ 12.29 (s, 1H), 8.90 (s, 1H), 8.62 (s, 1H), 8.53 (d, J = 8.0 Hz, 1H), 8.37 – 8.19 (m, 3H), 7.88–7.82 (m, 3H), 3.24 (d, J = 12.0 Hz, 2H), 3.21 – 3.11 (m, 2H), 2.81 (q, J = 12.0 Hz, 2H), 1.82 (d, J = 12.0 Hz, 3H), 1.38 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 163.9, 148.54, 148.50, 143.2, 130.3, 129.5, 129.0, 128.6, 128.3, 128.0, 127.6, 127.1, 125.8, 125.75, 125.70, 125.62, 125.59, 123.1, 118.4, 114.5, 109.6, 43.2, 42.9, 34.0, 26.4. MS (ESI) [M+H]+ 403.2.
N-(piperidin-4-ylmethyl)-6-(pyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide Hydrochloride (71). 1H NMR (400 MHz, DMSO-d6) δ 12.57 (s, 1H), 8.93 (d, J = 8.0 Hz, 3H), 8.63 (d, J = 8.0 Hz, 4H), 8.48 (d, J = 4.0 Hz, 1H), 8.38 (t, J = 5.6 Hz, 1H), 8.19 (d, J = 8.0 Hz, 1H), 3.23 (d, J = 12.0 Hz, 2H), 3.20 – 3.12 (m, 3H), 2.80 (q, J = 12.0 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.38 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 163.6, 153.5, 148.6, 144.2, 143.1, 131.8, 130.4, 122.8, 120.7, 116.1, 109.9, 43.3, 42.9, 33.9, 26.4. MS (ESI) [M+H]+ 336.4.
6-(4-(piperidin-1-ylmethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide Hydrochloride (72). 1H NMR (400 MHz, DMSO-d6) δ 12.23 (s, 1H), 10.86 (s, 1H), 9.09 (d, J = 12.0 Hz, 1H), 8.80 (d, J = 8.0 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H), 8.32–8.29 (m, 2H), 8.14 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 12.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 2H), 4.27 (d, J = 4.0 Hz, 2H), 3.53 (s, 1H), 3.28–3.14 (m, 6H), 2.87 – 2.74 (m, 4H), 1.82 – 1.64 (m, 8H), 1.44 – 1.30 (m, 3H); 13C NMR (100 MHz, DMSO-d6) δ 164.0, 149.5, 148.4, 140.2, 131.9, 130.2, 129.8, 129.1, 126.6, 118.0, 114.2, 109.6, 66.4, 58.5, 54.9, 43.2, 42.8, 40.2, 34.0, 26.3, 22.1, 21.5. MS (ESI) [M+H]+ 432.3.
6-(4-(morpholinomethyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-pyrrolo[2,3-b]pyridine-3-carboxamide Hydrochloride (73). 1H NMR (400 MHz, DMSO-d6) δ 12.21 (s, 1H), 11.45 (s, 1H), 8.94 (d, J = 12.0 Hz, 1H), 8.65 (d, J = 12.0 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H), 8.26 (s, 2H), 8.16 (d, J = 8.0 Hz, 2H), 7.82 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 2H), 4.36 (d, J = 4.0 Hz, 2H), 3.92 (d, J = 12.0 Hz, 2H), 3.81 (t, J = 12.0 Hz, 2H), 3.25 – 3.07 (m, 8H), 2.80 (q, J = 12.0 Hz, 2H), 1.81 (d, J = 12.0 Hz, 3H), 1.38 (q, J = 12.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 164.0, 149.5, 148.4, 140.4, 132.0, 130.2, 129.2, 129.0, 126.7, 118.0, 114.2, 109.6, 63.1, 58.6, 50.6, 43.2, 42.8, 34.0, 26.4. MS (ESI) [M+H]+ 434.3.
7. Synthesis of compound 10
4-Bromobenzene-1,2-diamine 90 (187 mg, 1 mmol), (4-(tert-butyl)phenyl)boronic acid (214 mg, 1.2 mmol), tetrakis(triphenylphosphine)palladium (58 mg, 0.05 mmol), and sodium carbonate (210 mg, 2 mmol) in DMF/H2O (10/2 mL) were placed in a sealed tube. The mixture was degassed and heated to 90 °C for 12 h. The reaction was then cooled and quenched with brine (5 mL). The product was extracted with ethyl acetate (3 × 15 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to give a yellow solid, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 1:1 to 1:2) to afford the corresponding coupling product.
To a solution of the above coupling product (0.8 mmol) in AcOH (5 mL) were added Methyl 2,2,2-trichloroacetimidate (176.4 mg, 0.96 mmol). The mixture was stirred for 16 h before it was quenched with NaHCO3 aq. The precipitate was filtered and washed with water to afford a yellow solid, which was used directly for next step.
To the above intermediate 91 (0.7 mmol) was added 2 N NaOH (5 mL). The resulting mixture was stirred at room temperature for 12 h before it was quenched with 1M HCl aq. The precipitate was filtered and washed with water to afford a yellow solid, which was used directly for next step.
To a solution of the above intermediate (0.6 mmol) in DCM (5 mL) were added HOBt (81.1 mg, 0.6 mmol) and EDCl (230 mg, 1.2 mmol). The resulting mixture was stirred at room temperature for 0.5 h. Then 4-aminomethyl-1-Boc-piperidine (193 mg, 0.9 mmol) and TEA (0.42 mL, 3 mmol) were added to the mixture. The mixture was stirred for 12 h before it was quenched with H2O. The mixture was extracted with DCM (3 × 30 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The volatiles were removed in vacuo to afford a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate = 2:1) to afford a white solid, which was used directly for next step.
To a solution of this intermediate in DCM (4 mL) was added dropwise HCl (0.4 mL, 4 N in p-dioxane) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 4 h. The volatiles were removed in vacuo to afford the final product as a hydrochloric salt.
5-(4-(tert-butyl)phenyl)-N-(piperidin-4-ylmethyl)-1H-benzo[d]imidazole-2 carboxamide Hydrochloride (10). 1H NMR (400 MHz, DMSO-d6) δ 9.18 (d, J = 4.0 Hz, 1H), 8.88 (s, 1H), 8.60 (s, 1H), 7.79 (s, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.59 (d, J = 8.0 Hz, 3H), 7.47 (d, J = 8.4 Hz, 2H), 3.32 – 3.13 (m, 4H), 2.91 – 2.72 (m, 2H), 2.06 (s, 2H), 1.95 – 1.69 (m, 3H), 1.40 (t, J = 11.6 Hz, 2H), 1.30 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 158.8, 150.1, 146.2, 138.2, 136.8, 127.1, 126.2, 123.6, 44.0, 43.2, 34.7, 33.9, 31.6, 31.1, 26.6. MS (ESI) [M+H]+ 391.6.
8. Synthesis of compound 11, 12, 13 and 68, 69.
To a solution of the corresponding carboxylic acid 92 (4.0 mmol, 1 equiv.) and amine (4.4 mmol, 1.1 equiv.) in DMF (20 mL) were added HATU (4.8 mmol, 1.2 equiv.) and DIPEA (6 mmol, 1.5 equiv.). The reaction was stirred overnight at room temperature. After completion, the mixture was diluted with water (30 mL) and then was extracted with DCM (40 mL). The organic phase was washed with brine three times. The combined organic layers were dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to give amides 93.
Compound 93 (0.2 mmol), aryl boronic acid or aryl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (0.24 mmol), tetrakis(triphenylphosphine)palladium (11.6 mg, 0.01 mmol), and sodium carbonate (42 mg, 0.4 mmol) in p-dioxane/H2O (5/1, v/v, 6 mL) were placed in a sealed tube. The mixture was degassed and heated to 80 °C for overnight. The reaction was then cooled and quenched with brine (10 mL). The mixture was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water and brine and dried over Na2SO4. The solvent were removed in vacuum to give a crude oil, which was purified by column chromatography (silica gel, hexanes: ethyl acetate from 4:1 to 1:1) to afford the corresponding coupling product. Then the Boc-protected intermediate was treated with 0.5 mL HCl (4N in dioxane) in DCM (5 mL) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 6 h. The solvent were removed in vacuum, which was triturated in diether ether and solidified to give the final product as a hydrochloric salt.
5-(4-(Tert-butyl)phenyl)-N-(piperidin-4-yl)benzo[b]thiophene-2-carboxamide Hydrochloride (11). 1H NMR (400 MHz, DMSO-d6) δ 8.87 (d, J = 7.6 Hz, 1H), 8.85 – 8.68 (m, 2H), 8.23 (s, 1H), 8.14 (s, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.73 (d, J = 8.4 Hz, 1H), 7.65 (d, J = 8.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 4.10 – 4.00 (m, 1H), 3.35 – 3.30 (m, 2H), 3.10 – 3.00 (m, 2H), 2.05 – 1.95 (m, 2H), 1.87 – 1.74 (m, 2H), 1.31 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 150.0, 140.5, 139.9, 139.1, 137.3, 137.1, 126.7, 125.8, 125.39, 125.35, 123.3, 122.7, 44.6, 42.3, 34.3, 31.2, 28.2; MS (ESI) [M+H]+ 393.1.
6-(4-(Tert-butyl)phenyl)-N-(piperidin-4-yl)benzo[b]thiophene-2-carboxamide Hydrochloride (12). 1H NMR (400 MHz, DMSO-d6) δ 8.89 – 8.75 (m, 3H), 8.28 (s, 1H), 8.21 (s, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.75 – 7.65 (m, 3H), 7.53 – 7.45 (m, 2H), 4.10 – 4.00 (m, 1H), 3.39 – 3.25 (m, 2H), 3.08 – 2.93 (m, 2H), 2.05 – 1.95 (m, 2H), 1.85 – 1.73 (m, 2H), 1.31 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 161.1, 150.3, 141.2, 140.0, 138.2, 136.8, 126.8, 125.9, 125.5, 124.9, 124.1, 120.3, 44.6, 42.2, 34.3, 31.1, 28.2; MS (ESI) [M+H]+ 393.2.
6-(4-(Tert-butyl)phenyl)-N-(piperidin-4-yl)benzofuran-3-carboxamide Hydrochloride (13). 1H NMR (400 MHz, DMSO-d6) δ 8.90 (s, 1H), 8.69 – 8.50 (m, 3H), 8.09 (d, J = 8.4 Hz, 1H), 7.91 (s, 1H), 7.69 – 7.60 (m, 3H), 7.48 – 7.40 (m, 2H), 3.27 – 3.17 (m, 3H), 2.89 – 2.75 (m, 2H), 1.89 – 1.76 (m, 2H), 1.46 – 1.35 (m, 2H), 1.35 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 162.1, 155.4, 149.9, 147.8, 137.5, 136.9, 126.7, 125.7, 124.3, 122.7, 122.1, 116.9, 109.3, 43.4, 42.8, 34.3, 33.7, 31.1. MS (ESI) [M+H]+ 377.4.
6-(Furan-3-yl)-N-(piperidin-4-yl)benzofuran-3-carboxamide Hydrochloride (68). 1H NMR (400 MHz, DMSO-d6) δ 8.88 (br, 2H), 8.67 (s, 1H), 8.49 (d, J = 7.6 Hz, 1H), 8.26 (s, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.91 (s, 1H), 7.75 (s, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.06 (s, 1H), 4.13 – 4.00 (m, 1H), 3.35 – 3.25 (m, 2H), 3.08 – 2.93 (m, 2H), 2.05 – 1.95 (m, 2H), 1.85 – 1.70 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.5, 155.3, 147.8, 144.4, 139.6, 129.4, 125.7, 124.1, 122.1, 121.7, 116.7, 108.9, 108.3, 43.8, 42.1, 28.2; MS (ESI) [M+H]+ 311.1.
6-(4-Cyanophenyl)-N-(piperidin-4-yl)benzofuran-3-carboxamide Hydrochloride (69). 1H NMR (400 MHz, DMSO-d6) δ 8.89 (br, 2H), 8.77 (s, 1H), 8.57 (d, J = 7.2 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.08 (s, 1H), 7.99 – 7.90 (m, 4H), 7.75 (d, J = 8.4 Hz, 1H), 4.15 – 4.00 (m, 1H), 3.36 – 3.25 (m, 2H), 3.08 – 2.94 (m, 2H), 2.08 – 1.95 (m, 2H), 1.85 – 1.70 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 161.4, 155.3, 148.9, 144.3, 135.5, 132.9, 127.9, 125.8, 123.1, 122.5, 118.9, 116.6, 110.3, 110.0, 43.9, 42.2, 28.2; MS (ESI) [M+H]+ 346.2.
Activity and inhibition assays for Flavivirus proteases.
Expression and purification of Flavivirus proteases were performed using our previous methods32. The biochemical assay for ZVpro was performed using the enzyme (1 nM) and benzoyl-norleucine-lysine-lysine-arginine 7-amino-4-methylcoumarine (Bz-Nle-Lys-Lys-Arg-AMC, 10 μM) as the substrate in a HEPES buffer (20mM, pH 7.3) containing 0.05% Triton X-100. To determine the IC50 values, triplicate samples of a compound with increasing concentrations were pre-incubated with the enzyme for 10 min before adding the substrate in 96-well plate (100 μL final volume). The fluorescence signal (Ex: 360 nm, Em: 460 nm) of each well was monitored every 30s, using a Tecan microplate reader. Data were imported into Prism (version 5.0), and IC50 values from 3 independent experiments with standard deviation were obtained by using a standard dose-response curve fitting in the program. The biochemical assays for DV2pro and WVpro were done similarly.
Cellular antiviral activity testing.
Antiviral activity was evaluated in human U87 glioma following our previous methods32. 2×104 U87 cells/well were seeded into 96-well plates and cultured in DMEM media with 2% FBS. 0.01 MOI (multiplicity of infection) of ZIKV was added to each well of the plates. After 1h, the supernatant was removed and cells were washed with PBS to remove unattached viral particles. Fresh medium (150 μL/well) containing various concentrations of a compound in triplicate were then added to each well. Upon incubation at 37 °C for 2 days, the supernatant of each well was used to determine ZIKV titers. Half-log serial dilution of the viral supernatant (50 μL) was added to a monolayer of Vero cells in quadruplicate in 96-well plates and cultured for 7 days. CPE/cell lysis was determined with microscope followed by MTT assay. TCID50 was calculated based on the highest dilution in which ≥50% (i.e., ≥2 out of the 4 quadruplicate wells) of Vero cells were infected with ZIKV. Compared to controls, the ability for a compound to reduce TCID50 can be determined. The results were from at least 2 independent experiments.
Supplementary Material
Table 5.
Structures and ZVpro inhibitory activities of 6-substituted indole-2-carboxamide compounds 47-66.a
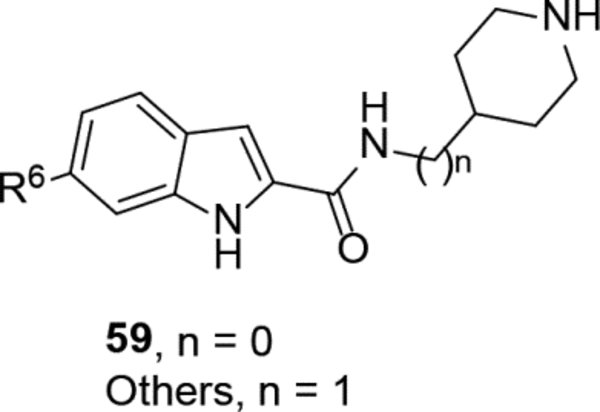
|
|||||
|---|---|---|---|---|---|
| Cpd # | R6 | IC50 (μM) | Cpd # | R6 | IC50 (μM) |
| 47 | H | 24.6 | 57 |
|
5.6 |
| 48 |
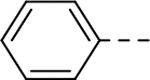
|
9.4 | 58 |
|
7.9 |
| 49 |
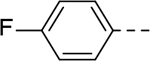
|
14.4 | 59 |
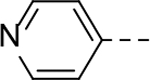
|
9.8 |
| 50 |
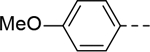
|
1.0 | 60 |
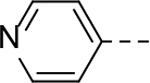
|
19.7 |
| 51 |
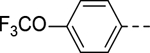
|
3.2 | 61 |
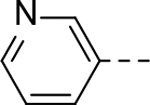
|
27.2 |
| 52 |
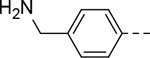
|
3.2 | 62 |
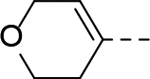
|
2.2 |
| 53 |
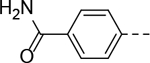
|
7.1 | 63 |
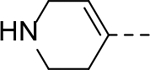
|
21.0 |
| 54 |
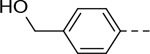
|
3.1 | 64 |
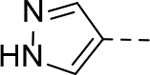
|
0.99 |
| 55 |
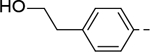
|
45.6 | 65 |
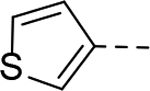
|
6.4 |
| 56 |
|
1.6 | 66 |
|
0.32 |
Standard errors of all IC50 values are less than 30%.
Table 6.
Structures and ZVpro inhibitory activities of compounds 67−73.a
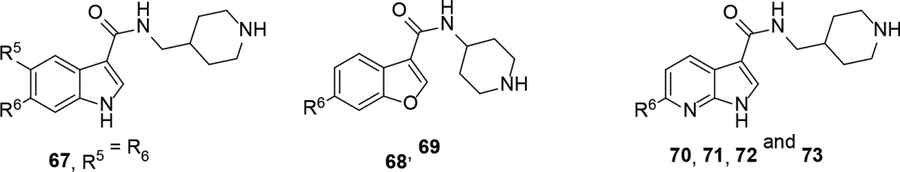
| |||||
|---|---|---|---|---|---|
| Cpd # | R6 | IC50 (μM) | Cpd # | R6 | IC50 (μM) |
| 67 |
|
0.37 | 71 |
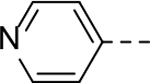
|
1.9 |
| 68 |
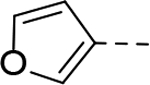
|
2.86 | 72 |
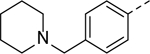
|
0.45 |
| 69 |
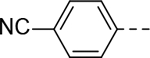
|
>50 | 73 |
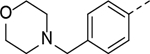
|
0.60 |
| 70 |
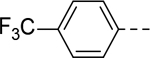
|
18.1 | |||
Standard errors of all IC50 values are less than 30%.
Highlights:
Compound screening identified indole-containing compounds 1 and 2 to be novel inhibitors of Zika virus NS2B-NS3 protease.
Medicinal chemistry optimization gave a significantly more potent inhibitor 66.
Compound 66 exhibited a strong antiviral activity against cellular replication of Zika virus.
Acknowledgement
This work was supported by a grant (W81XWH-18-1-0368) from USAMRAA of the US Department of Defense and grants (RP180177) from Cancer Prevention and Research Institute of Texas to Y.S., NIH grants R01AI099483 and R01AI098715 to R.R.-H.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Pierson TC; Diamond MS The continued threat of emerging flaviviruses. Nat Microbiol 2020, 5, 796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick GW; Kitchen SF; Haddow AJ Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952, 46, 509–520. [DOI] [PubMed] [Google Scholar]
- 3.Yun SI; Lee YM Zika virus: An emerging flavivirus. J Microbiol 2017, 55, 204–219. [DOI] [PubMed] [Google Scholar]
- 4.Medin CL; Rothman AL Zika Virus: The Agent and Its Biology, With Relevance to Pathology. Arch Pathol Lab Med 2017, 141, 33–42. [DOI] [PubMed] [Google Scholar]
- 5.Duffy MR; Chen TH; Hancock WT; Powers AM; Kool JL; Lanciotti RS; Pretrick M; Marfel M; Holzbauer S; Dubray C; Guillaumot L; Griggs A; Bel M; Lambert AJ; Laven J; Kosoy O; Panella A; Biggerstaff BJ; Fischer M; Hayes EB Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009, 360, 2536–2543. [DOI] [PubMed] [Google Scholar]
- 6.Lanciotti RS; Kosoy OL; Laven JJ; Velez JO; Lambert AJ; Johnson AJ; Stanfield SM; Duffy MR Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008, 14, 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao-Lormeau VM; Musso D Emerging arboviruses in the Pacific. Lancet 2014, 384, 1571–1572. [DOI] [PubMed] [Google Scholar]
- 8.Besnard M; Lastere S; Teissier A; Cao-Lormeau V; Musso D Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014, 19. [PubMed] [Google Scholar]
- 9.Musso D; Nilles EJ; Cao-Lormeau VM Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 2014, 20, O595–596. [DOI] [PubMed] [Google Scholar]
- 10.ECDC. Rapid risk assessment: Zika virus infection outbreak, French Polynesia. European Centre for Disease Prevention and Control, Stockholm, Sweden. February 14, 2014. 2014. [Google Scholar]
- 11.WHO. Zika virus outbreaks in the Americas. Wkly. Epidemiol. Rec 2015, 90, 609–610. [PubMed] [Google Scholar]
- 12.PAHO/WHO. Zika - epidemiological update. Pan American Health Organization/World Health Organization, Washington, D.C. January 12. 2017. [Google Scholar]
- 13.CDC. Zika cases reported in the United States. Centers for Disease Control and Prevention, Atlanta, Georgia. January 19, 2017. https://www.cdc.gov/zika/intheus/maps-zika-us.html. 2017. [Google Scholar]
- 14.Oehler E; Watrin L; Larre P; Leparc-Goffart I; Lastere S; Valour F; Baudouin L; Mallet H; Musso D; Ghawche F Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill 2014, 19. [DOI] [PubMed] [Google Scholar]
- 15.Cao-Lormeau VM; Blake A; Mons S; Lastere S; Roche C; Vanhomwegen J; Dub T; Baudouin L; Teissier A; Larre P; Vial AL; Decam C; Choumet V; Halstead SK; Willison HJ; Musset L; Manuguerra JC; Despres P; Fournier E; Mallet HP; Musso D; Fontanet A; Neil J; Ghawche F Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016, 387, 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuler-Faccini L; Ribeiro EM; Feitosa IM; Horovitz DD; Cavalcanti DP; Pessoa A; Doriqui MJ; Neri JI; Neto JM; Wanderley HY; Cernach M; El-Husny AS; Pone MV; Serao CL; Sanseverino MT Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016, 65, 59–62. [DOI] [PubMed] [Google Scholar]
- 17.Victora CG; Schuler-Faccini L; Matijasevich A; Ribeiro E; Pessoa A; Barros FC Microcephaly in Brazil: how to interpret reported numbers? Lancet 2016, 387, 621–624. [DOI] [PubMed] [Google Scholar]
- 18.https://www.cdc.gov/media/releases/2017/s0302-zika-birth-defects.html. In.
- 19.Moreira J; Peixoto TM; Siqueira AM; Lamas CC Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect 2017, 23, 296–305. [DOI] [PubMed] [Google Scholar]
- 20.Nitsche C; Holloway S; Schirmeister T; Klein CD Biochemistry and medicinal chemistry of the dengue virus protease. Chem Rev 2014, 114, 11348–11381. [DOI] [PubMed] [Google Scholar]
- 21.Sangkawibha N; Rojanasuphot S; Ahandrik S; Viriyapongse S; Jatanasen S; Salitul V; Phanthumachinda B; Halstead SB Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol 1984, 120, 653–669. [DOI] [PubMed] [Google Scholar]
- 22.Lei J; Hansen G; Nitsche C; Klein CD; Zhang L; Hilgenfeld R Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science 2016, 353, 503–505. [DOI] [PubMed] [Google Scholar]
- 23.Nitsche C; Zhang L; Weigel LF; Schilz J; Graf D; Bartenschlager R; Hilgenfeld R; Klein CD Peptide-Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural Biology. J Med Chem 2017, 60, 511–516. [DOI] [PubMed] [Google Scholar]
- 24.Morewood R; Nitsche C A biocompatible stapling reaction for in situ generation of constrained peptides. Chemical Science 2021, 12, 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun NJ; Quek JP; Huber S; Kouretova J; Rogge D; Lang-Henkel H; Cheong EZK; Chew BLA; Heine A; Luo D; Steinmetzer T Structure-Based Macrocyclization of Substrate Analogue NS2B-NS3 Protease Inhibitors of Zika, West Nile and Dengue viruses. ChemMedChem 2020, 15, 1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H; Bock S; Snitko M; Berger T; Weidner T; Holloway S; Kanitz M; Diederich WE; Steuber H; Walter C; Hofmann D; Weissbrich B; Spannaus R; Acosta EG; Bartenschlager R; Engels B; Schirmeister T; Bodem J Novel dengue virus NS2B/NS3 protease inhibitors. Antimicrob Agents Chemother 2015, 59, 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coluccia A; Puxeddu M; Nalli M; Wei C-K; Wu Y-H; Mastrangelo E; Elamin T; Tarantino D; Bugert JJ; Schreiner B; Nolte J; Schwarze F; La Regina G; Lee J-C; Silvestri R Discovery of Zika Virus NS2B/NS3 Inhibitors That Prevent Mice from Life-Threatening Infection and Brain Damage. ACS Medicinal Chemistry Letters 2020, 11, 1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millies B; von Hammerstein F; Gellert A; Hammerschmidt S; Barthels F; Goppel U; Immerheiser M; Elgner F; Jung N; Basic M; Kersten C; Kiefer W; Bodem J; Hildt E; Windbergs M; Hellmich UA; Schirmeister T Proline-Based Allosteric Inhibitors of Zika and Dengue Virus NS2B/NS3 Proteases. J Med Chem 2019, 62, 11359–11382. [DOI] [PubMed] [Google Scholar]
- 29.Rassias G; Zogali V; Swarbrick CMD; Ki Chan KW; Chan SA; Gwee CP; Wang S; Kaplanai E; Canko A; Kiousis D; Lescar J; Luo D; Matsoukas M-T; Vasudevan SG Cell-active carbazole derivatives as inhibitors of the zika virus protease. European Journal of Medicinal Chemistry 2019, 180, 536–545. [DOI] [PubMed] [Google Scholar]
- 30.Lee H; Ren J; Nocadello S; Rice AJ; Ojeda I; Light S; Minasov G; Vargas J; Nagarathnam D; Anderson WF; Johnson ME Identification of novel small molecule inhibitors against NS2B/NS3 serine protease from Zika virus. Antiviral Res 2017, 139, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrams RPM; Yasgar A; Teramoto T; Lee MH; Dorjsuren D; Eastman RT; Malik N; Zakharov AV; Li W; Bachani M; Brimacombe K; Steiner JP; Hall MD; Balasubramanian A; Jadhav A; Padmanabhan R; Simeonov A; Nath A Therapeutic candidates for the Zika virus identified by a high-throughput screen for Zika protease inhibitors. Proc Natl Acad Sci U S A 2020, 117, 31365–31375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Y; Huo T; Lin YL; Nie S; Wu F; Hua Y; Wu J; Kneubehl AR; Vogt MB; Rico-Hesse R; Song Y Discovery, X-ray Crystallography and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J Am Chem Soc 2019, 141, 6832–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie S; Yao Y; Wu F; Wu X; Zhao J; Hua Y; Wu J; Huo T; Lin YL; Kneubehl AR; Vogt MB; Ferreon J; Rico-Hesse R; Song Y Synthesis, Structure-Activity Relationships, and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J Med Chem 2021, 64, 2777–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tricarico PM; Caracciolo I; Crovella S; D’Agaro P Zika virus induces inflammasome activation in the glial cell line U87-MG. Biochem Biophys Res Commun 2017, 492, 597–602. [DOI] [PubMed] [Google Scholar]
- 35.Chan JF; Yip CC; Tsang JO; Tee KM; Cai JP; Chik KK; Zhu Z; Chan CC; Choi GK; Sridhar S; Zhang AJ; Lu G; Chiu K; Lo AC; Tsao SW; Kok KH; Jin DY; Chan KH; Yuen KY Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect 2016, 5, e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahon A; Arya RP; Kneubehl AR; Vogt MB; Dailey Garnes NJ; Rico-Hesse R Characterization of a Zika Virus Isolate from Colombia. PLoS Negl Trop Dis 2016, 10, e0005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


