Abstract
X-linked inhibitor of apoptosis protein (XIAP) is a key regulator of programmed cell death triggered by various apoptotic triggers. Translation of XIAP is controlled by a 162-nucleotide (nt) internal ribosome entry site (IRES) element located in the 5′ untranslated region of XIAP mRNA. XIAP IRES mediates efficient translation of XIAP under physiological stress and enhances cell protection against serum deprivation and radiation-induced apoptosis. In the present report we describe the assembly of a sequence-specific RNA-protein complex consisting of at least four cytosolic proteins on the XIAP IRES element. We determine that the core binding sequence is approximately 28 nt long and is located 34 nt upstream of the initiation site. Moreover, we identify the La autoantigen as a protein that specifically binds XIAP IRES in vivo and in vitro. The biological relevance of this interaction is further demonstrated by the inhibition of XIAP IRES-mediated translation in the absence of functional La protein. The results suggest an important role for the La protein in the regulation of XIAP expression, possibly by facilitating ribosome recruitment to the XIAP IRES.
Programmed cell death plays a critical role in regulating cell turnover during embryogenesis and in tissue homeostasis as well as viral infections and cancer (56). Recently, we have identified and cloned three mammalian genes encoding inhibitor of apoptosis (IAP) proteins (13, 28, 29). While the IAP genes were initially discovered in baculoviruses, their homologues have since been identified in other viruses, insects, birds, and mammals, suggesting a common evolutionary origin (reviewed in references 27 and 30). The IAP proteins are potent inhibitors of apoptosis in various experimental systems and have recently been shown to bind and inhibit distinct caspases (10, 41, 45, 52) a feature that is postulated to be a primary mode of IAP action in cells.
X-linked IAP (XIAP) is the prototype of mammalian IAP genes. It has been shown that the antiapoptotic function of XIAP is executed, at least in part, by inhibition of caspase 3 and caspase 7, two principal effectors of apoptosis (10, 52). In addition to being a caspase inhibitor in cultured cells, XIAP has been shown recently to inhibit caspase 3 activation in vivo, and this inhibition attenuated ischemic neuronal death in rat brain (57). We have demonstrated recently that expression of XIAP is controlled at the level of translation initiation (18). There is a 162-nucleotide (nt) internal ribosome entry site (IRES) sequence located in the 5′ untranslated region (UTR) of XIAP mRNA, and this sequence is critical for the cap-independent translation of XIAP. The IRES-mediated translation of XIAP mRNA is resistant to the repression of protein synthesis that accompanies cellular stress such as serum deprivation or γ irradiation. Significantly, IRES-mediated translation of XIAP offered enhanced protection against apoptosis induced by serum deprivation, suggesting that modulation of XIAP expression is of potential benefit in cell survival under acute but transient apoptotic conditions (18).
The IRES sequences were initially identified in picornavirus mRNAs (39), where they serve to initiate translation of uncapped viral mRNAs. The 5′ UTR regions of all picornaviruses are long and can mediate translational initiation by directly recruiting and binding ribosomes, allowing cap-independent translation (reviewed in reference 11). In addition, following virus infection, cellular (cap-dependent) protein synthesis is arrested due to cleavage of the translation initiation factor eIF4G by viral proteases (16). IRES-mediated translation remains unaffected, allowing the virus to maintain high levels of viral protein synthesis.
IRES elements are also found in a limited number of cellular mRNAs. To date, the cellular mRNAs which were shown to contain functional IRES elements in their 5′ UTRs include immunoglobulin heavy-chain binding protein (31), Drosophila Antennapedia (38) and Ultrabithorax (58), fibroblast growth factor 2 protein (54), the protooncogene c-myc (37, 50), vascular endothelial growth factor (21, 49), and XIAP (18). Cellular IRES elements have no obvious sequence or structural similarity to picornavirus IRES sequences or among themselves, and no control system for the regulation of IRES-directed translation has been described (5). It is speculated, however, that the presence of an IRES within a cellular mRNA would allow enhanced or continued expression under conditions in which normal, cap-dependent translation is shut off or compromised, such as during heat shock, development, growth arrest, or cell cycle position (43).
Regulation of translation of a typical, capped, eukaryotic mRNA by virtue of modulating the activity of critical translation initiation factors (such as eIF4E and eIF4F) is relatively well characterized (48). However, the translational control of IRES-containing mRNAs is less understood. While the requirement for canonical initiation factors (eIF2,-3,-4A,-4B,-4F, and -4E) seems to be similar for both modes of translation (40), there are at least three additional cellular trans-acting factors that have been implicated in the IRES-mediated translation of viral mRNAs. These auxiliary factors include the La autoantigen (36), the polypyrimidine tract binding protein (PTB) (17), and the poly(C) binding protein (PCBP) (7). With the exception of the PTB, which was shown to interact with the IRES element of vascular endothelial growth factor (21), none of these cellular factors were shown to be involved in the translation of the remaining cellular IRESs examined to date.
In this report we identify a sequence-specific RNA-protein complex which assembles on the 162-nt XIAP IRES element. We demonstrate that the La autoantigen is an essential component of the XIAP IRES ribonucleoprotein (RNP) complex. Furthermore, our data suggest that neither PTB nor PCBP participates in the binding to XIAP IRES. The functional relevance of La binding to the XIAP IRES is demonstrated by the requirement for La for in vitro and in vivo translation that is mediated by the XIAP IRES. The data presented here suggest that the La autoantigen is a central component of the XIAP IRES RNP complex which may facilitate binding of additional cellular factors to the XIAP IRES sequence and is essential for the modulation of XIAP mRNA translation.
MATERIALS AND METHODS
Cell culture and reagents.
Human epitheloid carcinoma cells (HeLa) were cultured in standard conditions in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and antibiotics. Transient DNA transfections were done using Lipofectamine Plus reagent (Gibco) and the protocol provided by the manufacturer. Briefly, cells were seeded at a density of 2.5 × 105 cells/ml in six-well plates and transfected 24 h later in serum-free Opti-MEM medium (Gibco) with 2 μg of DNA and 10 μl of lipid per well. The transfection mixture was replaced 3 h later with fresh DMEM supplemented with 10% FCS. Cells were collected for analysis at 48 h posttransfection. Glutathione S-transferase (GST) fusion constructs of full-length human La protein and the C-terminal dimerization domain (La residues 226 to 348) were a generous gift from N. Sonenberg. Recombinant proteins were expressed in Escherichia coli and purified on glutathione-Sepharose (Pharmacia) as described before (14). Purified proteins were dialyzed against the homogenization buffer (see below) and stored at 4°C in the presence of 5% glycerol. The expression plasmid pCI-mycLa(226,348) was constructed by inserting a PCR-generated dimerization domain of La cDNA into the pCI vector containing the Myc epitope (Invitrogen). The bicistronic vector pβgal/CAT was described previously (18). Bicistronic plasmids with mutated XIAP IRES were constructed by PCR-directed mutagenesis, and their proper orientation and sequence were confirmed by sequencing.
Cell extracts.
The cytosolic extracts (S100) were prepared as previously described (19). Briefly, cells were harvested during the exponential phase by centrifugation from medium at 0°C and washed in ice-cold phosphate-buffered saline (PBS). Cells were resuspended at 5 × 107 cells/ml in homogenization buffer (10 mM Tris-HCl [pH 7.4], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml) and lysed with a Dounce homogenizer with 20 strokes on ice. Nuclei were pelleted by centrifugation at 2,000 × g for 10 min, and the supernatant was adjusted to 150 mM KCl. Following the centrifugation of the cytoplasmic fraction for 60 min at 100,000 × g at 4°C, the supernatant (S100 fraction) was removed and glycerol was added to a final concentration of 5%. The S100 extracts were aliquoted, flash-frozen in a dry ice-ethanol bath, and stored at −80°C.
EMSAs.
For electrophoretic mobility shift assays (EMSAs), DNA templates for synthesis of the XIAP IRES RNA probe were generated by PCR using XIAP IRES-specific primers (probe A, 5′-oligonucleotide 5′-TAATACGACTCACTATAGGGCGAAATTAGAATGTTTCTTAGCGGTC and 3′ oligonucleotide 5′-CTTCTCTGGAAAATAGGAC; probe B, oligonucleotide 5′-TAATACGACTCACTATAGGGCGATTATTCTGCCTGCTTAAATATTAC and 3′ oligonucleotide 5′-CTAAATACTAGAGTTCGACATTAC; probe C, 5′-oligonucleotide 5′-TAATACGACTCACTATAGGGCGAGTCGACAGCTCCTATAACAAAAGTCTG and 3′-oligonucleotide 5′-CTTCTCTGGAAAATAGGAC). The 5′ primers incorporated the T7 promoter sequence (19). Internally labeled RNA probes were synthesized by in vitro transcription with T7 polymerase (MAXISript T7 RNA polymerase kit; Ambion) in the presence of [α-32P]UTP (Amersham). EMSA analysis was carried out as detailed previously (20). Briefly, 15,000 cpm of labeled RNA was mixed with 30 μg of HeLa S100 extract or 3 to 5 μg of purified GST-La protein in a total volume of 15 μl at room temperature for 30 min, followed by the addition of 1 μl of RNase T1 (1 U/μl), and incubated for an additional 10 min at room temperature, and heparin was added to a final concentration of 5 mg/ml. The RNP complex was electrophoresed on a 3% native acrylamide gel at 4°C. Unlabeled competitor RNAs were synthesized using the MEGAScript T7 RNA polymerase kit (Ambion). Competition with the homoribopolymers poly(C), poly(U), poly(G), and poly(A) was carried out by adding 0.1 μg of RNA (Sigma) to the RNP assembly mixture before addition of the target RNA. Mapping of the RNP binding sites by antisense oligonucleotides was carried out as described before (53). Briefly, internally labeled RNA probe was mixed with the antisense oligonucleotides, incubated at 80°C for 10 min, and allowed to cool gradually to room temperature. The EMSAs were performed and analyzed as described above.
UV cross-linking and immunoprecipitation.
RNA-protein complexes for UV cross-linking were prepared as described above. Before adding RNase T1, the samples were transferred into a 96-well dish and irradiated on ice with a 254-nm UV light source at 400,000 μJ/cm2. RNA-protein complexes were then resolved by sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by autoradiography. For the immunoprecipitation, UV-irradiated RNA-protein complexes were diluted in 50 μl of PBS-NP-40 buffer (1× PBS, 2 mM EDTA, 2 mM EGTA, 0.05% NP40), and 5 μl of ascites fluid containing anti-La monoclonal antibody A1 (8) and 20 μl of protein A+G-agarose (Calbiochem) were added. The samples were incubated at room temperature for 30 min and then washed five times in HEPES–NP-40 buffer (15 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40). The immunoprecipitated RNA-protein complexes were released from the agarose beads by boiling in SDS loading buffer (100 mM Tris [pH 6.8], 2.5% SDS, 10% glycerol, 0.025% β-mercaptoethanol, 0.1% bromophenol blue), resolved on an SDS–12.5% PAGE gel, and visualized by autoradiography.
XIAP mRNA was coimmunoprecipitated from whole-cell extracts using a modified method of Seto et al. (46). Briefly, cells from a 35-mm dish were harvested in 1 ml of cold PBS and collected by low-speed centrifugation at 4°C. The cell pellets were resuspended in 100 μl of RNA binding buffer (above) supplemented with 10 U of RNase inhibitor (5 Prime-3 Prime Inc.), and cell extracts were prepared by the freeze-thaw method. To whole-cell extracts, 5 μl of monoclonal anti-La antibody A1 or antiactin antibody (Amersham), 20 μl of protein A+G-agarose beads (Calbiochem), and 5 U of RNase inhibitor were added, and the samples were incubated for 60 min at room temperature. The beads were then washed extensively with RNA binding buffer supplemented with RNase inhibitor. RNA associated with the antibody-antigen complexes was isolated by repeated phenol-chloroform extraction and precipitation with 2 M ammonium acetate and 3 volumes of cold ethanol. RNA was then analyzed by reverse transcription (RT)-PCR using XIAP-specific primers (5′ oligonucleotide, 5′-ATGACTTTTAACAGTTTTGAAGG, and 3′ oligonucleotide, 5′-GCTCGTGCCAGTGTTGATGCTG).
In vitro transcription and translation.
Coupled in vitro transcription and translations (TnT Quick Coupled Transcription/Translation System; Promega) were performed under the conditions recommended by the manufacturer. Each reaction was programmed with 1 μg of purified plasmid DNA of the dicistronic construct pβgal/5′(−162)/CAT (18) and, when indicated, supplemented with purified recombinant GST-La(226–348) or GST protein. In vitro-translated proteins were labeled with l-[35S]methionine (Amersham). Reactions were incubated at 30°C for 90 min and analysed on SDS–10% PAGE. The intensities of the chloramphenicol acetyltransferase (CAT) and β-galactosidase (βGal) bands were determined on a Bio-Rad model GS-670 imaging densitometer.
β-Galactosidase and CAT analysis.
Transiently transfected HeLa cells were harvested in PBS at 48 h posttransfection, and cell extracts were prepared by the freeze-thaw method as described (32). βGal enzymatic activity in cell extracts was determined by the spectrophotometric assay using ONPG (o-nitrophenyl-β-d-galactopyranoside) (32), and the CAT activity was determined by the liquid scintillation method, as described (44).
RESULTS
Sequence-specific RNP complex assembles on the 162-nt XIAP IRES element.
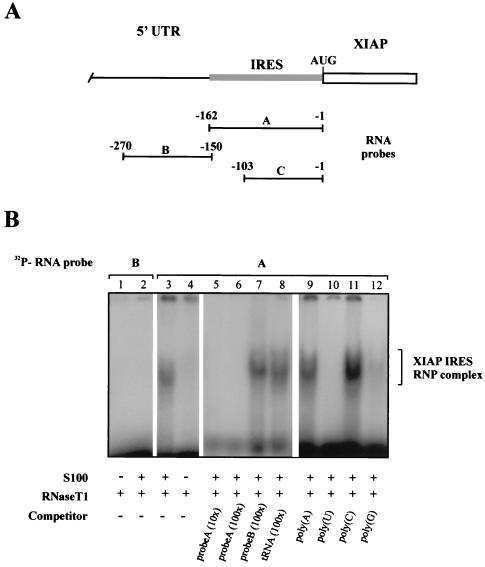
We have shown recently that the 162-nt region of XIAP mRNA immediately upstream of the initiation codon contains a functional IRES element (18). Since the IRES elements are postulated to bind specific cellular factors that recruit ribosomes to the IRES site, we decided to examine the IRES element of XIAP for protein-binding activity. We derived a 162-nt RNA probe spanning the entire IRES element of XIAP (probe A, Fig. 1A) as well as a second, 120-nt probe that lies upstream of the XIAP IRES (probe B, Fig. 1A). Both RNA probes were incubated with the cytosolic S100 extracts from HeLa cells and analyzed on native PAGE. We detected a large RNA-protein complex that formed specifically on the XIAP IRES sequence but did not form on the upstream UTR sequence (Fig. 1B). Incubation of the RNA-protein samples with proteinase K prior to electrophoretic separation completely abolished RNP complex formation (data not shown). These results suggested that a set of cytosolic proteins bind specifically to the IRES element of XIAP. This binding was successfully inhibited with an excess of specific unlabeled competitor (Fig. 1B, lanes 5 and 6), while an excess of nonspecific competitor had no effect on the binding (Fig. 1B, lanes 7 and 8). Furthermore, the formation of the XIAP IRES RNP complex was prevented with an excess of poly(U) (lane 10) and, to a lesser extent, poly(G) (lane 12) homoribopolymers but remained unaffected by the addition of excess of poly(C) (lane 11) or poly(A) (lane 9). These data indicate that a sequence-specific, poly(U)- and poly(G)-sensitive RNP complex assembles on the XIAP IRES element.
FIG. 1.
Sequence-specific RNP complex forms on the XIAP IRES element. (A) Schematic diagram of the position of the different 32P-labeled RNA probes corresponding to the complete IRES element (probe A), shorter IRES element (probe C), and non-IRES upstream 5′ UTR segment (probe B). Relative positions of the 5′ and 3′ ends of each probe are indicated. Numbers indicate nucleotide positions starting from the first nucleotide before the initiation codon AUG. Open rectangle, XIAP coding region; shaded rectangle, XIAP IRES element. (B) RNP complex assembly on the XIAP IRES and non-IRES 32P-labeled RNA probes (detailed in A). Gel mobility shift assays were performed on the indicated RNA segments using S100 extracts from HeLa cells. Lanes 1 and 4, [32P]RNA probes digested with RNase T1; lanes 2 and 3, [32P]RNA probes incubated with S100 extract before RNase T1 digestion; lanes 5 to 12, [32P]RNA probe incubated with S100 extract in the presence of indicated competitors before RNase T1 digestion. The position of the XIAP IRES RNP complex is indicated.
Characterization of the XIAP IRES element.
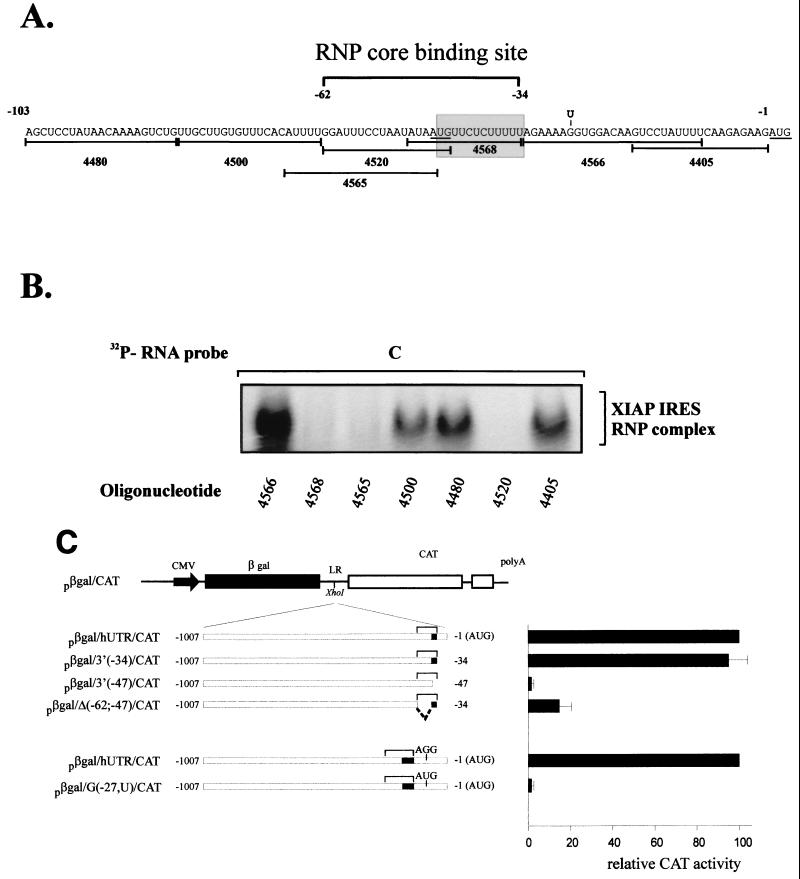
Having shown that the 162-nt XIAP IRES element can specifically recruit cytosolic proteins to form an RNP complex, we wished to determine what sequence of the XIAP IRES is critical for RNP complex formation. We have shown previously that progressive deletions from the 5′ end of the XIAP IRES element still retained substantial IRES activity. Using the EMSA and truncated IRES RNA probes, we determined that the 103-nt segment of XIAP IRES (probe C) can efficiently form an RNP complex indistinguishable from that formed with the larger, 162-nt probe (not shown). The more detailed mapping of the RNP binding site was further delineated by antisense oligonucleotide mapping (53). A 103-nt XIAP IRES probe was annealed with the antisense oligonucleotides spanning the entire region (Fig. 2A) and allowed to cool down. The RNA probe-oligonucleotide hybrids were then incubated with the cytosolic S100 extracts and analyzed on native PAGE. We observed that only three antisense oligonucleotides (4520, 4565, and 4568) were able to disrupt XIAP IRES RNP complex formation, suggesting that this region (−34 to −62) is critical for protein recruitment to the XIAP IRES (Fig. 2B). This region spans a 28-nt segment and overlaps the critical polypyrimidine tract that is essential for XIAP IRES function (18). We have shown previously that the deletion of the polypyrimidine tract [−34 to −47; plasmid pβgal/3(−47)/CAT] resulted in complete loss of XIAP IRES activity in bicistronic constructs (Fig. 2C) (18). We therefore wished to determine if the deletion of the remaining segment of the RNP binding site (−47 to −62) would affect XIAP IRES activity in vivo. We constructed a bicistronic plasmid with the −47 to −62 deletion [pβgal/Δ(−62;−47)/CAT] and tested it for IRES activity. As shown in Fig. 2C, the −47 to −62 deletion drastically reduced XIAP IRES activity. Our data suggest that the ability of the XIAP IRES to form the RNP complex in vitro is paralleled by its ability to support translation of a reporter gene in bicistronic constructs. Furthermore, these and previous experiments (18) suggest that while the 162-nt XIAP IRES is essential for full IRES activity, the −34 to −62 region is likely the core binding segment for RNP complex formation. The sequences outside this region are presumably involved in proper secondary structure and stabilization of protein binding.
FIG. 2.
XIAP IRES RNP core binding sites maps within 28-nt segment of the IRES element. (A) Nucleotide sequence of the 103-nt portion of XIAP IRES (probe C). The positions of oligonucleotides used for binding site mapping are shown under the sequence. The approximate position of the RNP core binding site is indicated by a bracket above the sequence. The polypyrimidine tract is shown in a shaded box. The AUG codons are underlined. The point mutation at position −27 (G to U) which introduces an out-of-frame AUG, is indicated above the sequence. (B) Oligonucleotide mapping of the XIAP IRES RNP core binding site. Indicated oligonucleotides were annealed to the 103-nt [32P]RNA probe (probe C) and the gel mobility shift assays were performed using S100 extracts from HeLa cells. The position of the XIAP IRES RNP complex is indicated. (C) Deletion and mutational analysis of XIAP IRES element. DNA segments corresponding to the indicated regions of the XIAP 5′ UTR were inserted into the XhoI site of the linker region of the plasmid pβgal/CAT. The small solid boxes indicate the position of the polypyrimidine tract. The brackets represent the position of the RNP core binding site. HeLa cells were cotransfected with the indicated plasmids as described in Materials and Methods. The levels of βGal and CAT activity were determined at 48 h posttransfection. Relative CAT activity was calculated by normalizing to βGal activity. Expression of each CAT cistron from the pβgal/hUTR/CAT construct was set at 100%. The bars represent the average ± SD of three independent transfections.
Our data suggest that the XIAP IRES-specific RNP complex forms approximately 34 to 62 nt upstream of the translation initiation start site. Close inspection of the XIAP 5′ UTR sequence disclosed that there are five AUG codons located just upstream of the 162-nt IRES element and three AUG codons within the IRES element, but no AUG is found between the polypyrimidine tract and the authentic initiation site (Fig. 2A). The last AUG before the authentic AUG is located in the middle of the XIAP IRES RNP binding site at position −48, just upstream of the polypyrimidine tract. Analogous to the structure of picornavirus IRES elements, the sequence between the polypyrimidine tract and the initiation codon could serve as an unstructured spacer (23), allowing the loaded ribosome to scan and recognize the initiation codon. Indeed, when we placed an AUG codon at position −27 (out of frame with the reporter gene), this mutation completely abolished translation of the reporter gene in the bicistronic construct [Fig. 2C, plasmid pβgal/G(−27U)/CAT].
La autoantigen is a subunit of XIAP IRES RNP complex.
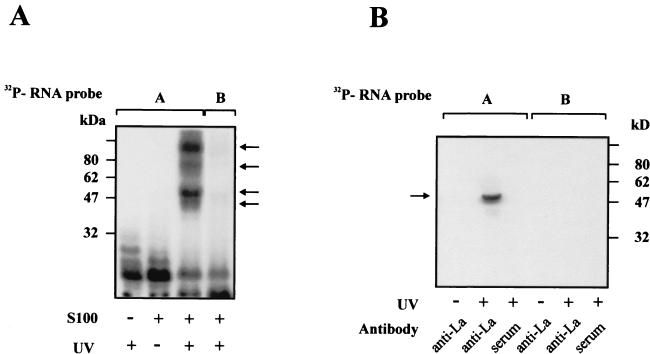
To provide insight into proteins that are present in the XIAP IRES RNP complex, we performed a photoaffinity cross-linking experiment. A 162-nt XIAP IRES probe was cross-linked with the S100 cytosolic HeLa extracts. Following separation on SDS-PAGE, four distinct RNA-protein bands were detected (Fig. 3A, lane 3). The cross-linked complexes have apparent molecular masses of about 45, 50, 75, and 100 kDa. The formation of the cross-linked complexes was prevented if the cytosolic extracts were preincubated with an excess of poly(U) competitor (data not shown). No cross-linked proteins were detected when a non-IRES RNA probe (probe B) was used instead (Fig. 3A, lane 4).
FIG. 3.
La autoantigen is a component of the RNP complex formed on the XIAP IRES element. (A) UV cross-linking of S100 extracts from HeLa cells on [32P]RNA probes (detailed in Fig. 1A). S100 extracts were incubated with the indicated RNA probe, UV irradiated, and separated on an SDS-PAGE gel as described in Materials and Methods. The positions of molecular size markers are indicated on the left; the positions of cross-linked proteins are indicated with arrows on the right. (B) Immunoprecipitation of cross-linked RNP complex with the anti-La antibody. S100 extracts were incubated with the indicated RNA probe, UV cross-linked, and then immunoprecipitated as described in Materials and Methods. The [32P]RNA probes are identified above the lanes. UV cross-linking or the absence of UV cross-linking before immunoprecipitation is indicated below each lane; the identity of the antibody used for the immunoprecipitation is also indicated below each lane.
Picornavirus IRES elements were shown to recruit at least three cytosolic proteins, La, PTB, and PCBP (reviewed in reference 55). One of the major cross-linked proteins that we observed had an apparent molecular mass (50 kDa) close to that of the La autoantigen. We therefore wished to examine whether the La protein is indeed a component of the XIAP IRES RNP complex. The 162-nt IRES probe was mixed with the cytosolic extract, cross-linked, and immunoprecipitated with the anti-La antibody (Fig. 3B). The shorter, non-IRES probe was used as a negative control. As shown in Fig. 3B, the La autoantigen was clearly detectable following the immunoprecipitation of XIAP IRES but not the non-IRES RNA-crosslinked proteins with the anti-La antibody. Furthermore, preincubation of cytosolic extracts with poly(U) prevented the formation and immunoprecipitation of the XIAP IRES-La complex (data not shown).
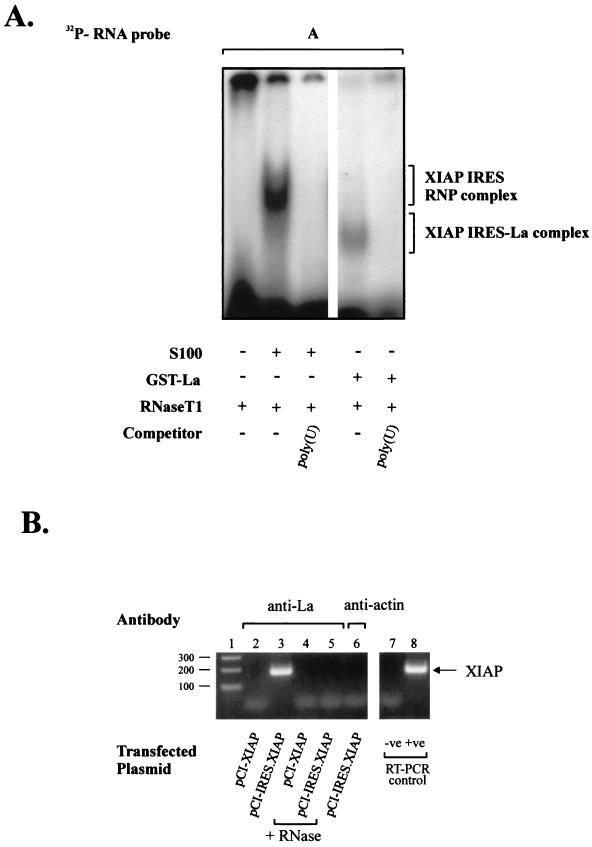
We next wished to determine whether the La autoantigen alone can bind to the XIAP IRES sequence. To this end, both the XIAP IRES and non-IRES RNA probes were incubated with the purified recombinant GST-La fusion protein (Fig. 4A). As shown in this figure, the purified GST-La protein forms a specific RNP complex on the XIAP IRES RNA. This RNP complex is, however, smaller than that formed with the cytosolic S100 extracts. These data indicate that the La autoantigen is a central component of the XIAP IRES RNP complex. The La protein alone is capable of binding the XIAP IRES element, but additional cytoplasmic proteins are recruited to the XIAP IRES to form a large RNP complex.
FIG. 4.
Purified La autoantigen binds XIAP IRES element. Gel mobility shift assays were performed on the [32P]RNA probe A using S100 extracts from HeLa cells (lanes 1 to 3) or purified GST-La protein (lanes 4 and 5) as described in Materials and Methods. Lane 1, [32P]RNA probe digested with RNase T1; lane 2, [32P]RNA probes incubated with S100 extract before RNase T1 digestion; lane 3, [32P]RNA probe incubated with S100 extract in the presence of poly(U) competitor before RNase T1 digestion; lane 4, [32P]RNA probes incubated with purified GST-La protein before RNase T1 digestion; lane 5, [32P]RNA probe incubated with purified GST-La protein in the presence of poly(U) competitor before RNase T1 digestion. The positions of the XIAP IRES RNP complex and the smaller XIAP IRES-La complex are indicated. (B) La autoantigen is associated with XIAP RNA in vivo. Hela cells were transfected with plasmid pCI-XIAP or pCI-IRES.XIAP, and whole-cell extracts were prepared 24 h later as described in Materials and Methods. Following coimmunoprecipitation with the indicated antibodies, the XIAP RNA was detected by RT-PCR analysis. The negative (no template, lane 7) and positive (cDNA, lane 8) controls for RT-PCR are shown on the right. The samples in lanes 4 and 5 were treated with RNase A prior to immunoprecipitation. The molecular sizes of cDNA bands are indicated on the left (in nucleotides).
La protein binds XIAP mRNA in vivo.
The experiments described above demonstrated that the La autoantigen is an essential component of the XIAP IRES RNP complex in vitro. We wished to determine if this interaction also takes place in vivo. We reasoned that if the La autoantigen is part of the in vivo RNP complex that is formed on the XIAP mRNA, then it should be possible to coimmunoprecipitate XIAP mRNA with the anti-La antibody from whole-cell extracts. The endogenous XIAP mRNA is not very abundant in HeLa cells. We therefore enriched XIAP mRNA levels by first transfecting cells with a construct expressing only the XIAP coding region (pCI-XIAP) or a plasmid expressing the XIAP coding region with 1 kb of 5′ UTR that contains the XIAP IRES element (pCI-IRES.XIAP). Following 24 h of recovery after transfection, whole-cell extracts were prepared, and RNA associated with La autoantigen was immunoprecipitated using monoclonal anti-La antibody A1 and analyzed by RT-PCR analysis using XIAP coding region-specific primers. The results of the coimmunoprecipitation experiments are summarized in Fig. 4B. The specific XIAP RT-PCR signal was obtained only if the expression plasmid contained XIAP 5′ UTR sequence (lane 2 versus 3). Furthermore, XIAP RNA was not detected if the cell extracts were treated with RNase prior to immunoprecipitation (lanes 4 and 5) or if we used nonspecific antibody for immunoprecipitation (antiactin, lane 6). These results indicate that the La autoantigen is associated with XIAP RNA in vivo and this interaction is mediated by the 5′ UTR sequence of XIAP RNA.
La protein modulates XIAP translation in vitro and in vivo.
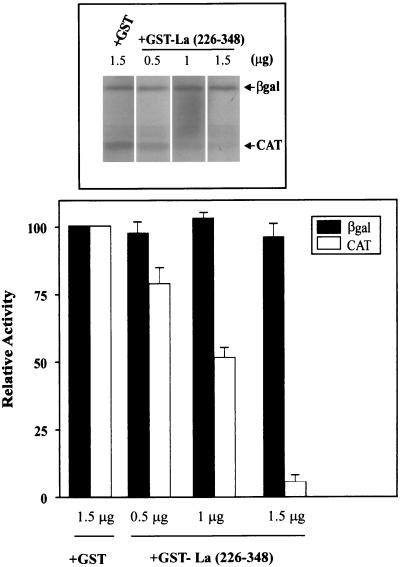
We wished to determine the biological relevance of the binding of La autoantigen to the XIAP IRES. Craig et al. (9) have shown that the La protein forms a dimer under native conditions and that this dimerization is necessary for the RNA binding activity of La. In addition, they identified a C-terminal domain (spanning amino acids 226 to 348) that is responsible for homodimerization. Significantly, the homodimerization domain expressed alone exhibits a dominant negative effect on the activity of the La protein, presumably by sequestering endogenous La protein (9). We used the rabbit reticulocyte lysate (RRL) and the coupled in vitro transcription-translation system programmed with the dicistronic plasmid pβgal/5′(−162)/CAT to assess the effect of the homodimerization domain construct La(226–348) on the translation of the XIAP IRES. Plasmid pβgal/5′(−162)/CAT contains two reporter genes transcribed together on one mRNA that are separated by a 162-nt XIAP IRES element (18). Therefore, the translation of the first reporter gene, βGal, is cap dependent, while the translation of CAT is XIAP IRES dependent and cap independent. We observed that in the rabbit reticulocyte lysate, both βGal and CAT are translated efficiently. However, the addition of the purified GST-La(226–348) protein drastically reduced synthesis of CAT but not the βGal protein (Fig. 5).
FIG. 5.
Dominant negative mutant of La inhibits translation of XIAP IRES. The RRL were programmed with dicistronic plasmid pβgal/5′(−162)/CAT and translated in the absence or presence of various concentration of GST-La(226–348) or GST protein (control; only 1.5 μg shown) as described in Materials and Methods. The top part shows a representative experiment. Translation of each reporter cistron assayed in the control reaction was set at 100%. Bars represent the average ± SD of three independent experiments.
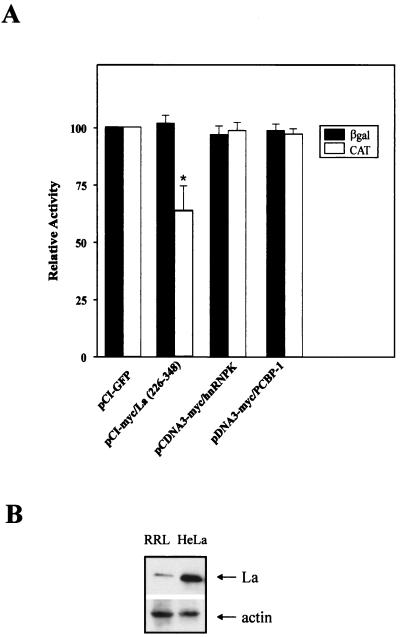
Similar experiments were carried out to elucidate the effect of La(226–348) on the IRES-mediated translation of XIAP in cultured cells. HeLa cells were cotransfected with the dicistronic construct pβgal/5′(−162)/CAT and the expression plasmid pCI-myc/La(226–348) containing the La(226–348) fragment under the control of a cytomegalovirus promoter. Overexpression of La(226–348) but not the control proteins green fluorescent protein (Clontech), heterogeneous nuclear ribonucleoprotein complex K protein (hnRNPK) (34), or PCBP-1 (25) reduced translation of CAT but not the βGal reporter (Fig. 6A). The moderate reduction of the CAT reporter in the transfection experiment when compared to the dramatic reduction of CAT in the in vitro translation experiment is likely due to the larger amount of the endogenous La protein that is present in the cells but not in the RRL (Fig. 6B). These results show that the addition of the dominant negative domain of La specifically abrogates XIAP IRES translation, suggesting that the endogenous La protein participates in the translation of the XIAP IRES containing mRNA in vivo.
FIG. 6.
Effect of expression of dominant negative La fragment on XIAP IRES-mediated translation in vivo. HeLa cells were cotransfected with the dicistronic plasmid pβgal/5′(−162)/CAT together with the plasmid pCI-myc/La(226–348) or with the control plasmid pCI-GFP, pcDNA3-myc/hnRNPK, or pcDNA3-myc/PCBP-1 as described in Materials and Methods. The levels of expression of myc-tagged overexpressed proteins were assessed by Western blot analysis using anti-Myc antibody and found to be comparable (data not shown). The levels of βGal and CAT activity were determined at 48 h posttransfection. Expression of each reporter cistron assayed in the control transfection was set at 100%. Bars represent the average ± SD of three independent transfections (∗, P < 0.05, one-way analysis of variance). (B) Western blot analysis of the levels of La autoantigen in RRL and whole-cell HeLa cell extracts (HeLa).
DISCUSSION
Translation of the intrinsic cellular inhibitor of apoptosis XIAP is controlled by a 162-nt IRES element located just upstream of the initiation site in the XIAP 5′ UTR. The cap-independent, IRES-mediated translation of XIAP seems to be critical for the role of XIAP in apoptosis, because XIAP protein can be synthesized during conditions of cellular stress when the majority of protein synthesis ceases. Indeed, XIAP-mediated cytoprotection is observed in serum starvation and ionizing-radiation apoptotic paradigms, both of which inhibit cap-dependent protein synthesis (18).
In the present study, we set out to characterize the XIAP IRES element and determine the cellular factors which are involved in the translation of the XIAP IRES. We demonstrated that a sequence-specific RNP complex forms on the XIAP IRES. This complex was sensitive to competition with poly(U) and poly(G), but not poly(A) and poly(C) homoribopolymers, indicating that poly(U)- and poly(G)-binding proteins are involved in the formation of the complex. Our results indicate that within the XIAP IRES sequence, the core RNP binding site spans approximately 28 nt and includes the polypyrimidine tract. The deletion of this core binding sequence from the 5′ UTR resulted in the loss of XIAP IRES activity in bicistronic constructs. Furthermore, the placement of an out-of-frame AUG codon between the polypyrimidine tract and the authentic XIAP AUG initiation codon abolished translation of the reporter gene. These results suggest that the XIAP RNP complex functions in the recruitment of ribosomes to the vicinity of the translation initiation site. The ribosome complex then likely scans the remaining 35 to 40 nt and initiates protein synthesis at the first available AUG codon. This situation is similar to that of picornavirus IRES elements (23). However, in stark contrast to viral IRESs, the XIAP IRES element is much shorter (162 versus 450 nt) and the length of the spacer region between the polypyrimidine tract and the initiation AUG codon can vary (18).
We show by UV cross-linking experiments that the XIAP RNP complex consists of at least four cytosolic factors with apparent molecular masses of 45, 50, 75, and 100 kDa. We have identified the 50-kDa protein as the La autoantigen and demonstrate that La protein specifically binds the XIAP IRES sequence. We propose that the La interaction with the XIAP IRES constitutes the core of the XIAP IRES RNP complex. Additional proteins are then recruited to this core complex to form a large RNP complex. This suggestion is supported by the fact that competition with poly(U) homoribopolymer prevents the formation of the XIAP IRES RNP complex completely. Furthermore, the addition of the dominant negative fragment of La inhibited XIAP IRES translation both in vitro and in vivo. These results indicate that the status of the La protein in the cell can potentially modulate expression levels of XIAP.
The identity of the remaining proteins of the XIAP IRES RNP complex remains to be determined. In the translation of viral mRNAs containing IRES, three cytosolic proteins (PCBP, PTB, and La) were shown to be involved (reviewed in reference 55). However, the XIAP IRES RNP complex is not sensitive to poly(C) competition, and we did not observe a 48-kDa protein in UV cross-linking experiments. Furthermore, overexpression of PCBP-1 did not affect the translation of the reporter gene under the translational control of XIAP IRES element. We therefore propose that the PCBP is not involved in the formation of the XIAP IRES RNP complex. Similarly, the absence of a 60-kDa UV cross-linked protein band (the size of PTB protein) suggests that PTB also is not part of the XIAP RNP complex. Our results are consistent with the models proposed by several laboratories that, in addition to the canonical translation factors, specific subsets of IRES elements may require different sets of cytosolic proteins for their translation (5, 35, 55). These auxilary proteins are presumably involved in the assembly of specific RNP complexes that promote folding of the IRES elements in the proper three-dimensional organization. In addition, specific IRES-RNP complex can presumably function as a cap analogue in the sense that it will facilitate binding of the ribosomes to the vicinity of the initiation codon.
The La autoantigen is a member of the RNA recognition motif group of general RNA-binding proteins with great affinity for poly(U)-rich sequences (24). The role of the La protein in RNA metabolism is very diverse. It has been shown to participate in the regulation of initiation and termination of RNA polymerase III transcription (15, 33) and processing of tRNA precursors (12). Furthermore, the La protein was shown to bind the 5′ UTR of several viral mRNAs and to stimulate their translation in RRL (1, 22, 26, 36, 51). It has been proposed but not yet demonstrated that La could also be involved in the translation of cellular mRNAs containing an IRES (6). Recent studies have shown that homozygous deletion of La in Drosophila is lethal during larval development as well as in adult life (4). More importantly, the loss of La correlated with the loss of Ultrabithorax (Ubx), whose translation is mediated by an IRES element. This genetic evidence suggests that La controls translation of Ubx mRNA. We demonstrate here for the first time that La does bind the cellular IRES element of XIAP mRNA in vivo and in vitro and modulates XIAP translation.
Although La is found predominantly in the nucleus of the cells, the La protein has been observed to shuttle between the nucleus and cytoplasm (2, 42, 47). Notably, it has been observed that following poliovirus infection, part of the La protein is truncated by a virus-encoded protease, 3Cpro, and the truncated form of La is redistributed from the nucleus to the cytoplasm (47). The truncated form of La is missing its C-terminal nuclear localization signal but contains the dimerization domain and has been shown to retain activity for stimulation of internal translation initiation of poliovirus in RRL (47). The La protein is also rapidly dephosphorylated and cleaved early during apoptosis triggered by various stimuli (42). The cleavage of the La protein is dependent on the activation of caspases, because the addition of pancaspase inhibitors blocked La cleavage completely (42). While the caspase cleavage site of La is different from the poliovirus 3Cpro cleavage site, the truncated La proteins produced by both cleavage events are very similar. In both cases cleavage removes the C-terminal nuclear localization signal, causing cytoplasmic redistribution of La. Interestingly, UV irradiation was also found to induce translocation of the La protein from the nucleus to the cytoplasm (3). We have reported previously that low-dose ionizing irradiation resulted in the enhanced translation of XIAP and that this translation was mediated by the XIAP IRES element (18). Our data presented here demonstrate that the cellular levels of the La protein may modulate XIAP expression. It is tempting to hypothesize that the observed increase in XIAP translation following ionizing irradiation or other cellular stresses is due to the modification and translocation of the La protein to the cytoplasm. The direct link between these observations, however, remains to be determined.
ACKNOWLEDGMENTS
We thank the members of our laboratory for useful discussion. We are grateful to N. Sonenberg for the generous gift of GST-La and GST-La(226–348) plasmid constructs and to E. Chan for the gift of anti-La A1 antibody.
This work was supported by grants from the Medical Research Council of Canada (MRC), the Canadian Networks of Centers of Excellence (NCE), and the Howard Hughes Medical Institute (HHMI). M.H. is a recipient of an MRC Postdoctoral Fellowship. R.G.K. is a recipient of an MRC Senior Scientist Award, a Fellow of the Royal Society of Canada, and an HHMI International Research Scholar.
REFERENCES
- 1.Ali N, Siddiqui A. The La antigen binds 5′ noncoding region of the hepatitis C virus RNA in the context of the initiator AUG codon and stimulates internal ribosome entry site-mediated translation. Proc Natl Acad Sci USA. 1997;94:2249–2254. doi: 10.1073/pnas.94.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann M, Chang S, Slor H, Kukulies J, Muller W E. Shuttling of the autoantigen La between nucleus and cell surface after uv irradiation of human keratinocytes. Exp Cell Res. 1990;191:171–180. doi: 10.1016/0014-4827(90)90002-r. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann M, Falke D, Schroder H C, Muller W E. Intracellular distribution of the La antigen in CV-1 cells after herpes simplex virus type 1 infection compared with the localization of U small nuclear ribonucleoprotein particles. J Gen Virol. 1989;70:881–891. doi: 10.1099/0022-1317-70-4-881. [DOI] [PubMed] [Google Scholar]
- 4.Bai C, Tolias P P. Genetic analysis of a La homolog in drosophila melanogaster. Nucleic Acids Res. 2000;28:1078–1084. doi: 10.1093/nar/28.5.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belsham G J, Sonenberg N. RNA-protein interactions in regulation of picornavirus RNA translation. Microbiol Rev. 1996;60:499–511. doi: 10.1128/mr.60.3.499-511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belsham G J, Sonenberg N, Svitkin Y V. The role of the La autoantigen in internal initiation. Curr Top Microbiol Immunol. 1995;203:85–98. doi: 10.1007/978-3-642-79663-0_4. [DOI] [PubMed] [Google Scholar]
- 7.Blyn L B, Towner J S, Semler B L, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan E K, Tan E M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987;166:1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig A W, Svitkin Y V, Lee H S, Belsham G J, Sonenberg N. The La autoantigen contains a dimerization domain that is essential for enhancing translation. Mol Cell Biol. 1997;17:163–169. doi: 10.1128/mcb.17.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenfeld E. Initiation of translation by picornavirus RNAs. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 549–573. [Google Scholar]
- 12.Fan H, Goodier J L, Chamberlain J R, Engelke D R, Maraia R J. 5′ processing of tRNA precursors can Be modulated by the human La antigen phosphoprotein. Mol Cell Biol. 1998;18:3201–3211. doi: 10.1128/mcb.18.6.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farahani R, Fong W G, Korneluk R G, MacKenzie A E. Genomic organization and primary characterization of miap-3: the murine homologue of human X-linked IAP. Genomics. 1997;42:514–518. doi: 10.1006/geno.1997.4742. [DOI] [PubMed] [Google Scholar]
- 14.Frangioni J V, Neel B G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb E, Steitz J A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989;8:851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradi A, Svitkin Y V, Imataka H, Sonenberg N. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc Natl Acad Sci USA. 1998;95:11089–11094. doi: 10.1073/pnas.95.19.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellen C U, Witherell G W, Schmid M, Shin S H, Pestova T V, Gil A, Wimmer E. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc Natl Acad Sci USA. 1993;90:7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holcik M, Lefebvre C A, Yeh C, Chow T, Korneluk R G. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- 19.Holcik M, Liebhaber S A. Analysis of mRNP complexes assembled in vitro. In: Richter J, editor. mRNA formation and function. New York, N.Y: Academic Press; 1997. pp. 195–209. [Google Scholar]
- 20.Holcik M, Liebhaber S A. Four highly stable eukaryotic mRNAs assemble 3′ untranslated region RNA-protein complexes sharing cis and trans components. Proc Natl Acad Sci USA. 1997;94:2410–2414. doi: 10.1073/pnas.94.6.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huez I, Creancier L, Audigier S, Gensac M C, Prats A C, Prats H. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol. 1998;18:6178–6190. doi: 10.1128/mcb.18.11.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isoyama T, Kamoshita N, Yasui K, Iwai A, Shiroki K, Toyoda H, Yamada A, Takasaki Y, Nomoto A. Lower concentration of La protein required for internal ribosome entry on hepatitis C virus RNA than on poliovirus RNA. J Gen Virol. 1999;80:2319–2327. doi: 10.1099/0022-1317-80-9-2319. [DOI] [PubMed] [Google Scholar]
- 23.Jackson R J. A comparative view of initiation site selection mechanisms. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 71–112. [Google Scholar]
- 24.Kenan D J, Query C C, Keene J D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991;16:214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- 25.Kiledjian M, Wang X, Liebhaber S A. Identification of two KH domain proteins in the alpha-globin mRNP stability complex. EMBO J. 1995;14:4357–4364. doi: 10.1002/j.1460-2075.1995.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y K, Jang S K. La protein is required for efficient translation driven by encephalomyocarditis virus internal ribosomal entry site. J Gen Virol. 1999;80:3159–3166. doi: 10.1099/0022-1317-80-12-3159. [DOI] [PubMed] [Google Scholar]
- 27.LaCasse E C, Baird S, Korneluk R G, MacKenzie A E. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 28.Liston P, Lefebvre C, Fong W G, Xuan J Y, Korneluk R G. Genomic characterization of the mouse inhibitor of apoptosis protein-1 and 2 genes. Genomics. 1997;46:495–503. doi: 10.1006/geno.1997.5059. [DOI] [PubMed] [Google Scholar]
- 29.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda J E, MacKenzie A, Korneluk R G. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 30.Liston P, Young S S, Mackenzie A E, Korneluk R G. Life and death decisions: the role of the IAPs in modulating programmed cell death. Apoptosis. 1997;2:423–441. doi: 10.1023/a:1026465926478. [DOI] [PubMed] [Google Scholar]
- 31.Macejak D G, Sarnow P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature. 1991;353:90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- 32.MacGregor G R, Nolan G P, Fiering S, Roederer M, Herzenberg L A. Use of E. coli lacZ (β-galactosidase) as a reporter gene. In: Murray E J, Walker J M, editors. Methods in molecular biology. Vol. 7. Clifton, N.J: Humana Press Inc.; 1991. pp. 217–235. [DOI] [PubMed] [Google Scholar]
- 33.Maraia R J. Transcription termination factor La is also an initiation factor for RNA polymerase III. Proc Natl Acad Sci USA. 1996;93:3383–3387. doi: 10.1073/pnas.93.8.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matunis M J, Michael W M, Dreyfuss G. Characterization and primary structure of the poly(C)-binding heterogeneous nuclear ribonucleoprotein complex K protein. Mol Cell Biol. 1992;12:164–171. doi: 10.1128/mcb.12.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBratney S, Chen C Y, Sarnow P. Internal initiation of translation. Curr Opin Cell Biol. 1993;5:961–965. doi: 10.1016/0955-0674(93)90077-4. [DOI] [PubMed] [Google Scholar]
- 36.Meerovitch K, Svitkin Y V, Lee H S, Lejbkowicz F, Kenan D J, Chan E K, Agol V I, Keene J D, Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanbru C, Lafon I, Audigier S, Gensac M C, Vagner S, Huez G, Prats A C. Alternative translation of the proto-oncogene c-myc by an internal ribosome entry site. J Biol Chem. 1997;272:32061–32066. doi: 10.1074/jbc.272.51.32061. [DOI] [PubMed] [Google Scholar]
- 38.Oh S K, Scott M P, Sarnow P. Homeotic gene Antennapedia mRNA contains 5′-noncoding sequences that confer translational initiation by internal ribosome binding. Genes Dev. 1992;6:1643–1653. doi: 10.1101/gad.6.9.1643. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 40.Pestova T V, Hellen C U, Shatsky I N. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol Cell Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy N, Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutjes S A, Utz P J, der Heijden A, Broekhuis C, van Venrooij W J, Pruijn G J. The La (SS-B) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death Differ. 1999;6:976–986. doi: 10.1038/sj.cdd.4400571. [DOI] [PubMed] [Google Scholar]
- 43.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 44.Seed B, Sheen J Y. A simple phase-extraction assay for chloramphenicol acetyltransferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 45.Seshagiri S, Miller L K. Baculovirus inhibitors of apoptosis (IAPs) block activation of Sf- caspase-1. Proc Natl Acad Sci USA. 1997;94:13606–13611. doi: 10.1073/pnas.94.25.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seto A G, Zaug A J, Sobel S G, Wolin S L, Cech T R. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature. 1999;401:177–180. doi: 10.1038/43694. [DOI] [PubMed] [Google Scholar]
- 47.Shiroki K, Isoyama T, Kuge S, Ishii T, Ohmi S, Hata S, Suzuki K, Takasaki Y, Nomoto A. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J Virol. 1999;73:2193–2200. doi: 10.1128/jvi.73.3.2193-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonenberg N. mRNA 5′ cap-binding protein eIF4E and control of cell growth. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 245–269. [Google Scholar]
- 49.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18:3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoneley M, Paulin F E, Le Quesne J P, Chappell S A, Willis A E. c-myc 5′ untranslated region contains an internal ribosome entry segment. Oncogene. 1998;16:423–428. doi: 10.1038/sj.onc.1201763. [DOI] [PubMed] [Google Scholar]
- 51.Svitkin Y V, Pause A, Sonenberg N. La autoantigen alleviates translational repression by the 5′ leader sequence of the human immunodeficiency virus type 1 mRNA. J Virol. 1994;68:7001–7007. doi: 10.1128/jvi.68.11.7001-7007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen G S, Reed J C. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 53.Thomson A M, Rogers J T, Walker C E, Staton J M, Leedman P J. Optimized RNA gel-shift and UV cross-linking assays for characterization of cytoplasmic RNA-protein interactions. Biotechniques. 1999;27:1032–1039. doi: 10.2144/99275rr03. , 1042. [DOI] [PubMed] [Google Scholar]
- 54.Vagner S, Gensac M C, Maret A, Bayard F, Amalric F, Prats H, Prats A C. Alternative translation of human fibroblast growth factor 2 mRNA occurs by internal entry of ribosomes. Mol Cell Biol. 1995;15:35–44. doi: 10.1128/mcb.15.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Velden A W, Thomas A A. The role of the 5′ untranslated region of an mRNA in translation regulation during development. Int J Biochem Cell Biol. 1999;31:87–106. doi: 10.1016/s1357-2725(98)00134-4. [DOI] [PubMed] [Google Scholar]
- 56.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Xu D, Bureau Y, McIntyre D C, Nicholson D W, Liston P, Zhu Y, Fong W G, Crocker S J, Korneluk R G, Robertson G S. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. J Neurosci. 1999;19:5026–5033. doi: 10.1523/JNEUROSCI.19-12-05026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye X, Fong P, Iizuka N, Choate D, Cavener D R. Ultrabithorax and Antennapedia 5′ untranslated regions promote developmentally regulated internal translation initiation. Mol Cell Biol. 1997;17:1714–1721. doi: 10.1128/mcb.17.3.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]