Abstract
Background
In ginseng, there exists a glycolipoprotein complex with a special form of lipid LPAs called Gintonin. The purpose of this study is to show that Gintonin has a therapeutic effect on rheumatoid arthritis through LPA2 receptors.
Methods
Fibroblast-like synoviocytes (FLS) were treated with Gintonin and stimulated with interleukin (IL)-1β. The antioxidant effect of Gintonin was measured using MitoSOX and H2DCFDA experiments. The anti-arthritic efficacy of Gintonin was examined by analyzing the expression levels of inflammatory mediators, phosphorylation of mitogen-activated protein kinase (MAPK) pathways, and translocation of nuclear factor kappa B (NF-κB)/p65 into the nucleus through western blot. Next, after treatment with LPAR2 antagonist, western blot analysis was performed to measure inflammatory mediator expression levels, and NF-κB signaling pathway. Carrageenan/kaolin-induced arthritis rat model was used. Rats were orally administered with Gintonin (25, 50, and 100 mg/kg) every day for 6 days. The knee joint thickness, squeaking score, and weight distribution ratio (WDR) were measured as the behavioral parameters. After sacrifice, H&E staining was performed for histological analysis.
Results
Gintonin significantly inhibited the expression of iNOS, TNF-α, IL-6 and COX-2. Gintonin prevented NF-κB/p65 from moving into the nucleus through the JNK and ERK MAPK phosphorylation in FLS cells. However, pretreatment with an LPA2 antagonist significantly reversed these effects of Gintonin. In the arthritis rat model, Gintonin suppressed all parameters that were measured.
Conclusion
This study suggests that LPA2 receptor plays a key role in mediating the anti-arthritic effects of Gintonin by modulating inflammatory mediators, the MAPK and NF-κB signaling pathways.
Keywords: Gintonin, Kaolin/carrageenan-induced arthritis, Fibroblast-like synoviocytes (FLS), Lysophosphatidic acids (LPAs), Cytokines
Graphical abstract
1. Introduction
Ginseng has a higher concentration of lysophosphatidic acids (LPAs) compared to other edible plants and Chinese herbs [1,2]. In ginseng, there exists a glycolipoprotein complex with a special form of lipid LPAs called Gintonin [3]. Lysophosphatidic acid (LPA) is a metabolic intermediate that is produced by phospholipases in both plants and animals as well as by autotaxins (ATX) in animals. In the plant system, LPA is considered to be a small phospholipid. However, in animals, LPA activates the G-protein coupled LPA receptor and is also considered to act as a growth factor derived from lipids [[4], [5], [6]]. The subtypes of LPA that can be found in Gintonin include LPA C18:2, LPA C18:1, and LPA C16:0. Besides, Gintonin contains ginseng protein and other bioactive lipids such as phosphatidic acids and lysophospholipids. Gintonin is characterized by the fact that lysophosphatidic acid (LPA) C18:2 is found in a relatively higher concentration than other LPA types, and it was observed that LPA C18:2 most strongly suppressed the activity of ATX [7,8].
Rheumatoid arthritis (RA) is one of the autoimmune diseases characterized by chronic inflammation of the synovial membrane (synovitis), which leads to the destruction and deformation of cartilage [9]. Common symptoms of arthritis include pain, edema, stiffness of joints, and fatigue [10]. The cause of rheumatoid arthritis has not been determined, but the onset of RA begins with the induction of an inflammatory immune response to the synovial membrane. Important events in RA are regulated by the complex interaction of pro-inflammatory cytokines, chemokines, and MMPs in synovial fluid and synovial tissue. These suggest that blocking the pro-inflammatory cytokines, chemokines, and MMPs in synovial fluid and synovial tissue would be a measure to treat arthritis [[11], [12], [13], [14], [15]]. LPA receptors are expressed in a wide array of cells in the body which include the cells of the synovial membrane sublining [16]. There have also been reports that the mRNAs of LPA1, LPA2, and LPA3 receptors are expressed in human Fibroblast-like synoviocytes (FLS) [17]. Lysophospholipase D or ATX mRNA has also been observed to be expressed in the FLS of RA patients [18]. ATX is known to be an enzyme that produces the majority of the extracellular LPA and also acts as an autocrine tumor cell motility stimulator [19]. Emerging evidence that may be related to the pathogenesis of RA is the Ca2+ flux. Compared to normal T cells, significant differences in endoplasmic reticulum (ER) Ca2+ concentration in synovial fluid T cells from RA were found [20,21].
Gintonin has higher affinity and selectivity for LPA receptors than other lipid-associated receptors, and also activates these LPA receptors [1,22]. The LPA receptor activated by Gintonin activates the cell membrane signaling system linked to the G protein, releasing free Ca2+ from the calcium storage ER. This increased calcium contributes to various effects on calcium-dependent intracellular, intercellular, and living organism functions [[23], [24], [25]]. Gintonin has also been reported to inhibit ATX [8,26]. Since Gintonin acts as an exogenous LPA receptor-ligand, studies are underway regarding the function of LPA receptors in the nervous system [[27], [28], [29]]. In addition, reports on the relationship between rheumatoid arthritis and LPA continue to be reported [17,30,31]. However, there are no reports of whether Gintonin/LPA2 mediation can also involve in an anti-arthritic effect. Therefore, the purpose of this study is to confirm that Gintonin has a therapeutic effect on rheumatoid arthritis, a type of autoimmune disease, through LPA receptors.
2. Materials and methods
2.1. Reagents
Gintonin was supplied from the Ginsentology Research Laboratory of Konkuk University (Seoul, Korea). All reagents used in cell culture were supplied by WELGENE Inc. (Gyeongsan, Korea). Carrageenan and kaolin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Human IL-1β was supplied by Bio Vision Inc. (Milpitas, CA, USA). H2L5186303 was obtained from R&D systems (Minneapolis, MN, USA). Antibodies for β-actin, phosphorylated- and total-forms of p38, JNK/SAPK, ERK1/2, IKKα, IKKβ, IκBα, and NF-κB/p65 were purchased from Cell Signaling Technology (Danvers, MA, USA) and iNOS, TNF-α, IL-6, and COX-2 were supplied by Santa Cruz Biotechnology (Dallas, TX, USA).
2.2. Cell cultures
Fibroblast-like synoviocytes (FLS), which are primary fibroblast-like cells derived from the synovial tissue of an RA patient, were obtained from Cell Applications, Inc. (San Diego, CA, USA). FLS cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 10 μg/ml streptomycin under a humidified 5% CO2 atmosphere at 37 ºC. FLS cells between 3rd and 5th passages were used and media was changed every day until confluency reached 80%.
2.3. Cell viability
Cell viability was measured using Quanti-Max™ WST-8 cell viability assay kit (BIOMAX Co., Seoul, Korea). Cells were seeded to 2 × 104 cells/well in a 96-well plate. Various concentrations of Gintonin were then added and after 1 hour, treated with IL-1β (10 ng/ml) for 24 hours. To measure cell viability, 10 μl WST-8 was added for 1 h and then read using a microplate reader at 450 nm (Molecular Devices, San Jose, CA, USA).
2.4. Measurement of ROS levels
FLS cells (2.5 × 105 cells/well in a 24-well plate) were prepared by treating the cells under the same conditions as the cell viability assay. The intracellular ROS levels were detected using 7-dichlorodihydrofluorescein diacetate (H2DCFDA, Sigma-Aldrich). In brief, the cells were stained with H2DCFDA solution dissolved at 50 μM in pre-warmed PBS for 30 minutes at 37 ºC and measured at an excitation of 485 nm and emission of 530 nm using a fluorescent reader (BioTek Instruments, Winooski, VT, USA). Mitochondrial ROS production was measured with MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen, Waltham, MA, USA) using the same method in accordance with a previous report [32]. Briefly, MitoSOX working solution (5 μM) dissolved in HBSS/Ca/Mg was added to each well and incubated for 10 minutes at 37 ºC. The fluorescence was monitored with a microplate reader set to 510 nm excitation and 595 nm emission wavelengths.
2.5. Western blot
All cells were lysed using RIPA buffer (ELPIS Biotech Inc., Daejeon, Korea). The proteins were separated using 12% SDS-PAGE, transferred to a PVDF membrane, and were incubated in a chamber at 4 °C with the primary antibodies overnight, and with the secondary antibodies for 1 hour. The membranes were developed using the enhanced chemiluminescence (ECL) detection kits (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.6. Carrageenan/kaolin-induced arthritic rat model
Six-week-old male Sprague-Dawley (SD) rats (200-230 g) were randomly divided into five groups (n = 6); a non-treated normal group (NOR), an arthritis control group by carrageenan/kaolin injection (ART), an arthritis group with Gintonin treatment at 25 mg/kg (ART + GIN25), an arthritis group with Gintonin treatment at 50 mg/kg (ART + GIN50), and an arthritis group with Gintonin treatment at 100 mg/kg (ART + GIN100). The carrageenan/kaolin-induced model is well established as a rheumatoid arthritis model [33]. Referring to a previous study, the rats were injected with 5% carrageenan/kaolin (150 μl) into the left knee joint, inducing arthritis on day 0 [34]. Gintonin (25, 50, and 100 mg/kg) dissolved in saline was administered (P.O.) to treatment groups while normal and arthritis groups received only saline (P.O.) once a day until day 6. The experimental procedures were carried out according to the animal care guidelines of the NIH and the Ewha Womans University Institutional Animal Care and Use Committee.
2.7. Assessment of arthritis symptoms
To evaluate the progress of carrageenan/kaolin-induced arthritis in rats, three different parameters, knee joint thickness, squeaking score, and weight distribution ratio (WDR), were measured 1 hour after treatment every day starting from day 0 until day 6. The thickness of the hind leg knee (left side) was assessed using an electronic digital caliper. The squeaking score was recorded as the average number of vocalizations when each hind leg was bent. The WDR is a measure of the force of both hind legs bearing its weight and this was recorded using an incapacitance meter (UGO-BASIL Biological Research Apparatus). The WDR percentage was calculated as percentage WDR = (weight borne by ipsilateral limb/total weight borne by both limbs) × 100. After Gintonin administration and behavioral tests, rats were sacrificed on day 6. In order to evaluate the effectiveness of Gintonin in the histopathology of the carrageenan/kaolin model, the knee joint tissue obtained from the rats of each group was paraffinized, embedded in paraffin, and sectioned. Subsequently, the formation of pannus and synovitis were evaluated through H&E staining, and scored as described [35]. All parameters were measured by investigators blinded to the experimental groups.
2.8. Paw-pressure test
The nociceptive removal threshold was evaluated using a paw-pressure analgesy meter (Ugo Basile Biological Research Apparatus Co., Comerio-Varese, Italy) in accordance with the Randall-Selitto test protocol provided by previous studies [36,37]. An hour after oral administration of Gintonin (100 mg/kg), 100 μl of 1% carrageenan was injected in the footpad of the rat, causing hyperalgesia. Then, the assessment was conducted three hours later in a blind manner. A gradually increasing force was added to the plantar and dorsal part of the paw of the rat until a withdrawal response resulted. In order to maintain its position over repeated trials, the action points were marked with ink. Six rats were used per group.
2.9. Measurement of cytokine levels in serum
Serum used for cytokine measurement was obtained from the heart of rats. The rats that received Gintonin for each concentration were sacrificed on day 6. The measurement of the levels of TNF-α (Abnova Corp., Taipei, Taiwan), IL-6 (Elabscience biotechnology Inc., Houston, TX, USA), and PGE2 (Abcam Inc., Cambridge, MA, USA) levels were conducted using ELISA kits according to the protocol provided by each supplier.
2.10. Statistical analysis
All experiments were performed at least three times with duplicate samples. Data analyses were carried out using Prism 5.0 (GraphPad Software, San Diego, CA, USA). All data are presented as means ± S.E.M., and statistical comparisons were identified using one-way ANOVA with Tukey's multiple comparison test and two-way ANOVA with Bonferroni's post-hoc testing. P values < 0.05 were considered to indicate statistical significance.
3. Results
3.1. Gintonin reduced the production of ROS.
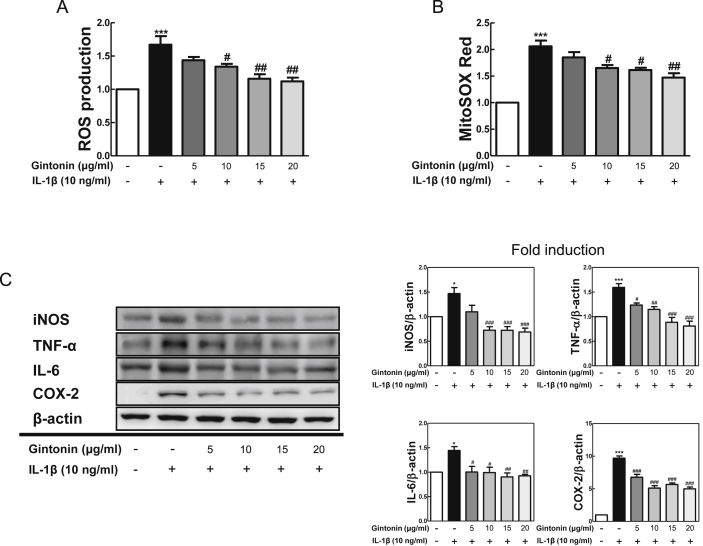
Before starting in vitro experiments in Fibroblast-like synoviocytes (FLS), it was confirmed that there was no cytotoxicity in the presence or absence of Gintonin and IL-1β (10 ng/ml) (data not shown). To determine whether Gintonin affected ROS production in FLS cells, ROS products were measured 24 hours after stimulating the cells with IL-1β (10 ng/ml) following pretreatment with Gintonin (5, 10, 15, and 20 μg/ml) for 1 hour according to each concentration. Both intracellular accumulation of ROS products through H2DCFDA (Fig. 1A) and superoxide production in the mitochondrial matrix through MitoSOX red (Fig. 1B) were significantly reduced by Gintonin.
Fig. 1.
Gintonin inhibited the production of ROS and protein expression of pro-inflammatory mediators in IL-1β-treated FLS cells. FLS cells were pretreated with Gintonin (5, 10, 15, 20 μg/ml) for 1 h, followed by IL-1β (10 ng/ml) for 24 h. (A) H2DCFDA for the measurement of intracellular ROS and (B) MitoSOX red for the detection of mitochondrial ROS production were stained in cells. (C) Western blot analysis using FLS cells pretreated with Gintonin for 1 h and stimulated later with IL-1β (10 ng/ml) for 6 h. The representative results from three independent experiments are shown. Quantification data are presented at the right panel. The data are expressed as means ± S.E.M. (n = 3). ∗p < 0.05, and ∗∗∗p < 0.001 vs. untreated group (None), #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. IL-1β-treated group without Gintonin treatment.
3.2. Gintonin inhibited inflammatory mediator expression.
To verify anti-inflammatory effects in IL-1β-stimulated FLS cells, the protein expressions were measured through western blot. Representative inflammatory mediators, iNOS, TNF-α, IL-6, and COX-2, were evaluated. IL-1β stimulation resulted to protein level increases of each inflammatory mediator, and cells with Gintonin pretreatment decreased the levels of those inflammatory mediators dose-dependently (Fig. 1C). Therefore, these results suggested that Gintonin has an anti-inflammatory effect in FLS cells.
3.3. Gintonin regulated the MAPK signaling pathway in IL-1β-stimulated FLS cells.
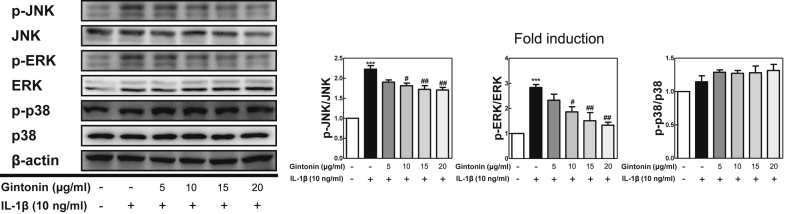
To determine the molecular mechanism behind the suppression of the aforementioned inflammatory mediators induced by Gintonin in FLS cells, the MAPK pathways, which are involved in the upstream signaling inflammatory processes, were investigated using western blot. Western blot analysis was conducted with antibodies against the phosphorylated- and total- forms of extracellular signal regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK), and p38 (Fig. 2). Gintonin significantly reduced the phosphorylation of JNK and ERK MAPKs in IL-1β-stimulated FLS cells. On the other hand, Gintonin was not able to reduce the phosphorylation of p38 MAPK.
Fig. 2.
Gintonin inhibited MAPKs phosphorylation in IL-1β-treated FLS cells. (A) Western blot analysis for MAPK activities using FLS cells pretreated with Gintonin for 1 h and stimulated later with IL-1β (10 ng/ml) for 6 h. The representative results from three independent experiments are shown. Quantification data are presented at the right panel. The data are expressed as means ± S.E.M. (n = 3). ∗∗∗p < 0.001 vs. untreated group (None), #p < 0.05, and ##p < 0.01 vs. IL-1β-treated group without Gintonin treatment.
3.4. Gintonin regulated nuclear translocation of NF-κB/p65 in IL-1β-stimulated FLS cells.
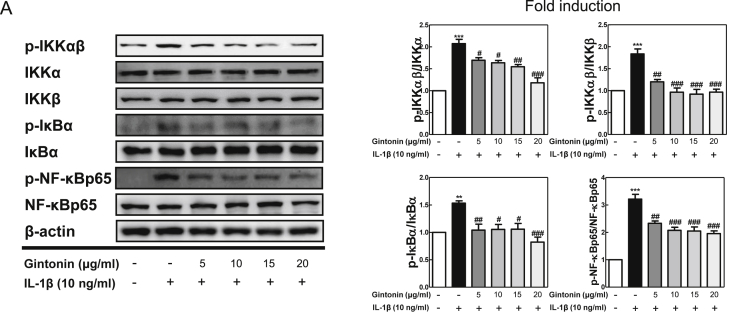
NF-κB signaling pathway is known to be an important upstream modulator for the expression of cytokines and NF-κB pathway activity suppression leads to anti-inflammatory responses of cytokines and chemicals. Thus, the NF-κB signaling pathway was analyzed using western blot (Fig. 3). As expected, the results showed that the expression of phosphorylated IKKαβ, IκBα, and NF-κB/p65 were enhanced in IL-1β-stimulated FLS cells without Gintonin. On the other hand, the pretreatment of Gintonin downregulated these activated protein expressions and therefore suggesting that Gintonin blocked NF-κB/p65 from translocating to the nucleus of IL-1β-stimulated FLS cells.
Fig. 3.
The effect of Gintonin on the NF-κB pathway in IL-1β-treated FLS cells. (A) Western blot analysis for NF-κB pathway activities using FLS cells pretreated with Gintonin for 1 h and stimulated later with IL-1β (10 ng/ml) for 6 h. The representative results from three independent experiments are shown and quantification data are presented at the right panel. The data are expressed as means ± S.E.M. (n = 3). ∗∗p < 0.01 and ∗∗∗p < 0.001 vs. untreated group (None), #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. IL-1β-treated group without Gintonin treatment.
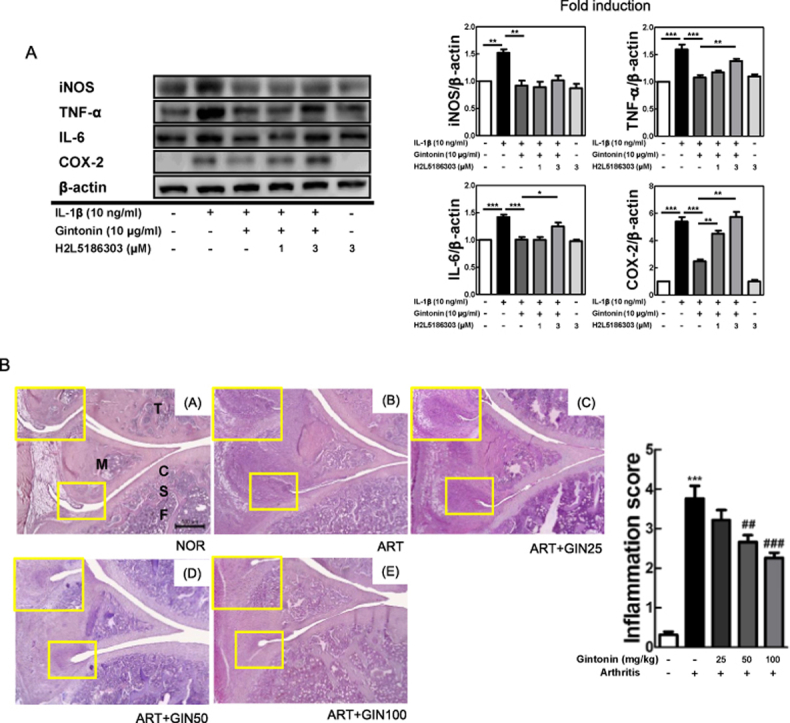
3.5. Gintonin regulated inflammatory mediator expression in IL-1β-stimulated FLS cells via LPA receptor
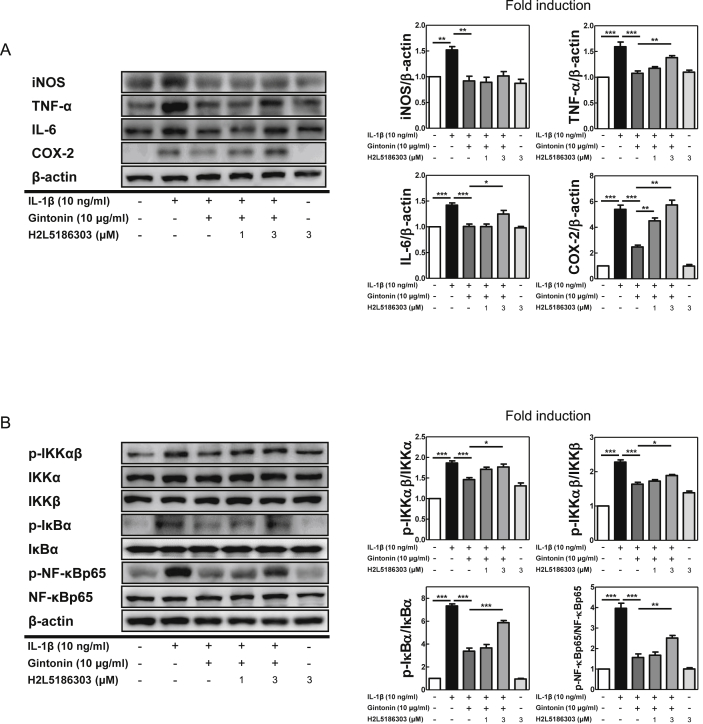
Next, to investigate whether LPA receptors were involved in the anti-inflammatory function of Gintonin, FLS cells were pretreated with H2L5186303 (H2L5), an LPAR2 antagonist, for 1 hour prior to adding Gintonin and then after 1 hour of Gintonin pretreatment, the cells were stimulated with IL-1β for 6 hours. Western blot analysis was then performed. As shown in Fig. 4A, H2L5 at 3 μM significantly reversed the results of Gintonin-mediated suppression of TNF-α, IL-6, and COX-2. The LPAR2 antagonist reversed the downregulation of Gintonin against not only inflammatory factors but also NF-κB signaling pathway. Gintonin-mediated suppression of phosphorylated- IKKαβ, IκBα, and NF-κB/p65 was inhibited by H2L5 at 3μM. These results indicated that the LPAR2 plays an important role in the anti-inflammatory mechanisms of Gintonin in FLS cells.
Fig. 4.
LPAR2 antagonist reverses the effect of Gintonin in IL-1β-stimulated FLS cells. LPAR2 antagonist, H2L5186303 (μM), were pretreated in FLS cells for 1 hour prior to adding Gintonin and then after 1 hour of Gintonin pretreatment, the cells were stimulated with IL-1β for 6 hours. Western blot analysis for (A) inflammatory mediators and (B) NF-κB pathway activities are shown. The representative results from three independent experiments are shown and quantification data are presented at the right panel. The data are expressed as means ± S.E.M. (n = 3). ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
3.6. Gintonin ameliorated arthritis behavioral parameters in the carrageenan/kaolin-induced arthritis model.
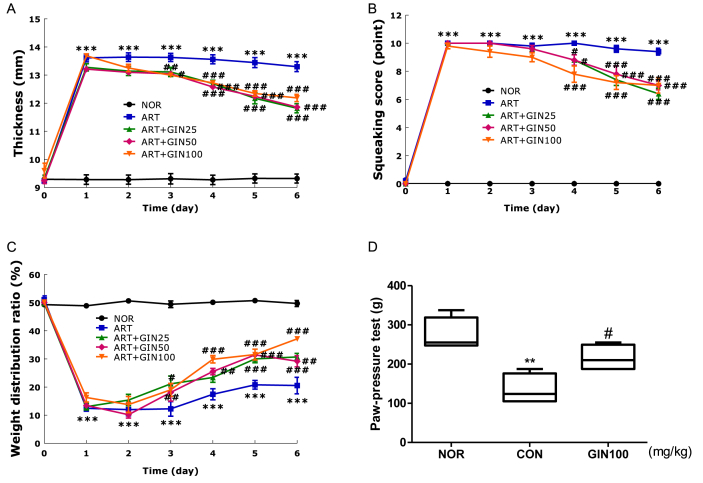
Based on the previous results, the anti-inflammatory effects of Gintonin were identified in FLS cells. This was then followed with experiments related to the effects of Gintonin on arthritis, an inflammatory disease, through in vivo carrageenan/kaolin-induced arthritis model in rats. The measured knee joint thickness, squeaking score, and WDR are indicators of arthritis symptoms in rats. Through the measurement of the knee joint thickness, all groups were indicated to have a severe case of arthritis starting on day 1 (Fig. 5A). Both the ART + GIN50 and ART + GIN100 groups showed a decrease in knee joint thickness from day 3 and the ART + GIN25 group knee joint thickness declined significantly starting from day 4. In the number of vocalizations indicated by the squeaking score (Fig. 5B), all groups had the highest score on day 1, the day after the arthritis was induced, indicating that the arthritis model was well induced. All groups showed a significant drop in the number of vocalizations from day 4. Among them, ART + GIN100 group showed the most significant mitigation. Next, the pain behavior against inflammation in rats was evaluated through WDR (Fig. 5C), a comparison of the distributed weight between the paws of rats after inducing arthritis. The NOR group WDR was normal at 50%. Prior to the injection of carrageenan/kaolin, the values in all groups were not significantly different from the NOR group. However, on the first day after the carrageenan/kaolin injection, there was a significant change in the WDR ratios. Arthritis was markedly reduced, with ART + GIN25 and ART + GIN100 groups having a recovery effect from Day 3, and the ART + GIN50 group from Day 4. All three indicators decreased considerably on the sixth day of treatment in comparison to day 1 when the arthritis symptoms were severe. The extent of this decrease was also statistically significant.
Fig. 5.
Behavioral assessment and anti-analgesic tendency of the carrageenan/kaolin-induced arthritis model and carrageenan-induced paw edema in rats after treating with Gintonin. (A) Thickness, (B) squeaking score (a value of 0 indicates no pain), and (C) weight distribution ratio, indicated the severity of arthritis in rats. (D) Gintonin was administered orally 1 h before intraplantar injection of carrageenan to induce hyperalgesia. Three hours after carrageenan injection, nociceptive thresholds were measured. NOR = non-treated normal group, ART = carrageenan/kaolin-induced arthritis control group, ART + GIN25 = arthritis with Gintonin treatment at 25 mg/kg, ART + GIN50 = arthritis with Gintonin treatment at 50 mg/kg, ART + GIN100 = arthritis with Gintonin treatment at 100 mg/kg. All data are expressed as means ± S.E.M. (n = 6). ∗∗p < 0.01 and ∗∗∗p < 0.001 vs. untreated group (NOR), #p < 0.05, ##p < 0.01, ###p < 0.001 vs. carrageenan/kaolin-induced group without Gintonin treatment (ART) (two-way ANOVA followed by Bonferroni correction) and vs. carrageenan-induced group without Gintonin treatment (CON) (one-way ANOVA followed by Tukey's multiple comparison test).
3.7. Gintonin showed analgesic tendencies in carrageenan-induced paw edema in rats.
Paw-pressure test was performed to assess whether Gintonin has an anti-nociceptive effect (Fig. 5D). The value of the pain withdrawal pressure for three groups was obtained by applying the Randall-Selitto probe. The rats squeaked at the threshold of mechanically added pressure. CON group had a distinctly low pressure tolerance compared to NOR group. The average value of GIN100 group was slightly higher than that of the CON group. The value of GIN100 group was statistically significant, suggesting that Gintonin has a potential analgesic effect.
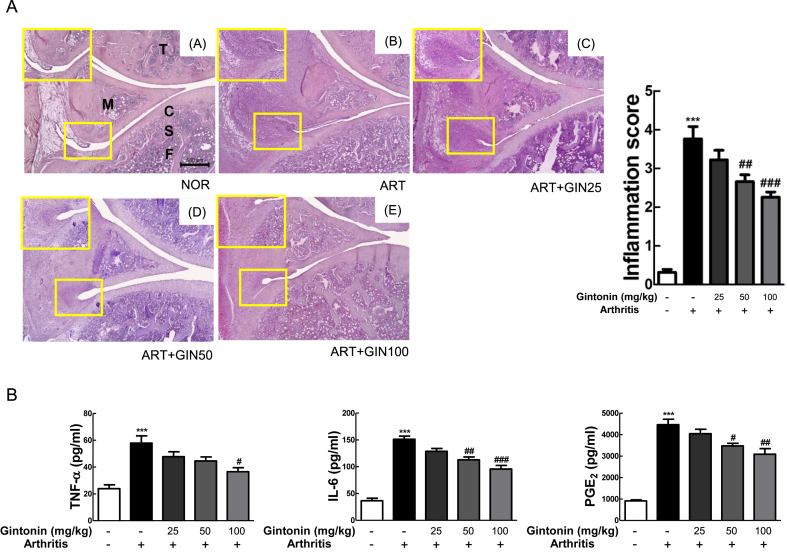
3.8. Gintonin alleviated histological outcome on the knee joints and decreased pro-inflammatory cytokines expression in serum.
The scores were evaluated mainly with a focus on the growth of the pannus and cartilage-pannus junction, number of infiltrated immune cells, and the thickness of the synovial membrane. Gintonin generally showed a dose-dependent anti-arthritic effect compared to the ART group. In the GIN100 treatment group, the development of the pannus decreased significantly enough to be visually identifiable. Thus, the anti-arthritic effects of Gintonin was identified in the in vivo carrageenan/kaolin arthritis rat model.
To explore the levels of inflammatory factors involved in the effects of Gintonin in vivo, TNF-α, IL-6, and PGE2 were determined in rat serum (Fig. 6B). ART group, which did not have any treatment with Gintonin and only had induced arthritis by carrageenan/kaolin, had significantly increased levels of TNF-α, IL-6, and PGE2. As the concentration of Gintonin increased, there was greater inhibition in the levels in each inflammatory factor. Overall, these data showed that Gintonin recovered carrageenan/kaolin-induced arthritis in rats.
Fig. 6.
Gintonin alleviated arthritis based on histopathological evaluation and serum levels. (A) Histological images of H&E staining in knee of rats (×40). The small yellow boxes in the photo are enlarged at the bottom left corner (×100). The extent of inflammation in the knee joint was graded between 0 and 4; 0 = normal (no infiltrate), 1 = minimal inflammation, 2 = mild inflammation, 3 = moderate inflammation, and 4 = severe inflammation. (B) The absolute value of TNF-α, IL-6, and PGE2 in the serum of rats with arthritis were measured using ELISA. NOR = non-treated normal group, ART = carrageenan/kaolin-induced arthritis control group, ART + GIN25 = arthritis with Gintonin treatment at 25 mg/kg, ART + GIN50 = arthritis with Gintonin treatment at 50 mg/kg, ART + GIN100 = arthritis with Gintonin treatment at 100 mg/kg. C: cartilage, F: femur, M: meniscus, S: subchondral bone, T: tibia. The representative results from three independent experiments are shown in the upper panel. The data are expressed as means ± S.E.M. (n = 3). ∗∗∗p < 0.001 vs. untreated group (NOR), #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. carrageenan/kaolin-induced group without Gintonin treatment (ART) (one-way ANOVA followed by Tukey's multiple comparison test).
4. Discussion
LPA is an important phospholipid signaling molecule that binds to seven-transmembrane domain receptors acting through specific G protein-coupled receptors (GPCRs), leading to the regulation of various biological activities such as cell migration, proliferation, apoptosis, differentiation, inflammatory cytokine secretion, and much more [38,39].
In a previous study, we demonstrated that Gintonin contributes to anti-inflammatory and anti-arthritic effects through the polyarthritis mouse model and in vitro experiments. However, it did not provide how the mechanism of these effects by Gintonin works. Gintonin with an exogenous LPA ligand is expected to relieve RA by binding to the LPA receptor present in animal cell membranes. There is a report that among LPA receptors Gintonin has highest affinity with LPA2 followed by LPA5, LPA1, LPA3, and LPA4, consecutively [1]. Thus, we hypothesized that activating LPA2 receptor by Gintomin would have a suppressive effect on the arthritis.
In the first part of this study, the antioxidant and anti-inflammatory effects of Gintonin were verified using IL-1β-stimulated Fibroblast-like synoviocytes (FLS). The treatment of IL-1B induced pro-inflammatory signaling through the MAPK cascade. In the study, Gintonin displayed an anti-arthritic effect by decreasing the nuclear translocation of NF-kB/p65 through the ERK and JNK MAPK signaling pathways. As Gintonin has the highest affinity with LPAR2 out of the LPA receptors, we used the H2L5186303, an LPAR2 antagonist, to confirm whether LPAR2 is involved with Gintonin and its anti-arthritic effect in FLS cell lines. As a result, inhibiting LPAR2 signaling resulted in the reversal of the action of Gintonin. Although distinct sub mechanisms of Gintonin/LPAR2 have not yet been identified, we have fully demonstrated that the LPAR2 pathway plays an important role in the anti-arthritis mechanism of Gintonin in IL-1β-stimulated FLS cells.
We have also showed, not only in in vitro but also in in vivo experiments, the anti-arthritic effect of Gintonin. In the second part of this study, we then carried out the in vivo experiment, using the carrageenan/kaolin-induced arthritis rat model and conducted three behavioral experiments to measure the development of arthritis. In the same way as the results of previous study about the anti-arthritic effect of Gintonin in a polyarthritis mouse model, Gintonin relieved the symptoms of arthritis in the carrageenan/kaolin-induced arthritis rat model, a local arthritis model. The knee thickness was measured to assess the degree of edema caused by arthritis and the squeaking score was performed to evaluate cartilage damage and pain. Weight distribution ratio (WDR) was also used to measure the symptom mitigation of arthritis. In a previous study, the WDR was reported to be a useful and objective indicator for measuring the relief of arthritis. All three concentrations (25, 50, and 100 mg/kg) significantly alleviated the symptoms of arthritis development over six days. We then histologically analyzed the joints of the arthritis model after treatment with Gintonin. The histological analysis of the joints in Gintonin groups showed that the formation of the pannus had been reduced. Through further hyperalgesia experiments, it was found that Gintonin tended to have an anti-nociceptive effect. Gintonin also significantly reduced the production of TNF-α, IL-6, and PGE2, which are pro-inflammatory intermediaries, in serum levels in the arthritis rat model. There are reports of increased levels of TNF-α and IL-6, mainly produced by macrophages, in serum and inflammatory synovial membrane tissues in RA patients. PGE2 also induces and amplifies edema and inflammatory diseases, which can eventually lead to cartilage and bone erosion.
In conclusion, Gintonin alleviates inflammation in synoviocytes and attenuates arthritis in an animal model through the LPAR2. It strongly suggests that Gintonin can be applied as an agent for arthritis therapeutic development.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (MRC, 2010-0029355).
References
- 1.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W., et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee B.H., Choi S.H., Kim H.J., Jung S.W., Kim H.K., Nah S.Y. Plant lysophosphatidic acids: a rich source for bioactive lysophosphatidic acids and their pharmacological Applications. Biol Pharm Bull. 2016;39:156–162. doi: 10.1248/bpb.b15-00575. [DOI] [PubMed] [Google Scholar]
- 3.Pyo M.K., Choi S.H., Shin T.J., Hwang S.H., Lee B.H., Kang J., Kim H.J., Lee S.H., Nah S.Y. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J Ginseng Res. 2011;35:209–218. doi: 10.5142/jgr.2011.35.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi S.H., Kim H.J., Cho H.J., Park S.D., Lee N.E., Hwang S.H., Rhim H., Kim H.C., Cho I.H., Nah S.Y. Gintonin-mediated release of astrocytic vascular endothelial growth factor protects cortical astrocytes from hypoxia-induced cell damages. J Ginseng Res. 2019;43:305–311. doi: 10.1016/j.jgr.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagès C., Simon M.F., Valet P., Saulnier-Blache J.S. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 2001;64:1–10. doi: 10.1016/s0090-6980(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 6.Yung Y.C., Stoddard N.C., Mirendil H., Chun J. Lysophosphatidic Acid signaling in the nervous system. Neuron. 2015;85:669–682. doi: 10.1016/j.neuron.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho H.J., Choi S.H., Kim H.J., Lee B.H., Rhim H., Kim H.C., Hwang S.H., Nah S.Y. Bioactive lipids in gintonin-enriched fraction from ginseng. J Ginseng Res. 2019;43:209–217. doi: 10.1016/j.jgr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang S.H., Lee B.H., Kim H.J., Cho H.J., Shin H.C., Im K.S., Choi S.H., Shin T.J., Lee S.M., Nam S.W., et al. Suppression of metastasis of intravenously-inoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: involvement of autotaxin inhibition. Int J Oncol. 2013;42:317–326. doi: 10.3892/ijo.2012.1709. [DOI] [PubMed] [Google Scholar]
- 9.Guo Q., Wang Y., Xu D., Nossent J., Pavlos N.J., Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D.M., Weinblatt M.E. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 11.Wojdasiewicz P., Poniatowski Ł A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata H., Sakaguchi N., Yoshitomi H., Iwakura Y., Sekikawa K., Azuma Y., Kanai C., Moriizumi E., Nomura T., Nakamura T., et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashizume M., Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis. 2011;2011:765624. doi: 10.1155/2011/765624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey P., Panga V., Raghunathan S. A cytokine signalling network for the regulation of inducible nitric oxide synthase expression in rheumatoid arthritis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuchi H., Shimada W., Nonaka T., Ueshima S., Tanaka S. Significance of serine proteinase and matrix metalloproteinase systems in the destruction of human articular cartilage. Clin Exp Pharmacol Physiol. 1996;23:885–889. doi: 10.1111/j.1440-1681.1996.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 16.Takuwa Y., Takuwa N., Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem. 2002;131:767–771. doi: 10.1093/oxfordjournals.jbchem.a003163. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C., Fernandes M.J., Prestwich G.D., Turgeon M., Di Battista J., Clair T., Poubelle P.E., Bourgoin S.G. Regulation of lysophosphatidic acid receptor expression and function in human synoviocytes: implications for rheumatoid arthritis? Mol Pharmacol. 2008;73:587–600. doi: 10.1124/mol.107.038216. [DOI] [PubMed] [Google Scholar]
- 18.Kehlen A., Lauterbach R., Santos A.N., Thiele K., Kabisch U., Weber E., Riemann D., Langner J. IL-1 beta- and IL-4-induced down-regulation of autotaxin mRNA and PC-1 in fibroblast-like synoviocytes of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 2001;123:147–154. doi: 10.1046/j.1365-2249.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umezu-Goto M., Kishi Y., Taira A., Hama K., Dohmae N., Takio K., Yamori T., Mills G.B., Inoue K., Aoki J., et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong V.K.W., Qiu C., Xu S.-W., Law B.Y.K., Zeng W., Wang H., Michelangeli F., Dias I.R.D.S.R., Qu Y.Q., Chan T.W., et al. Ca2+ signalling plays a role in celastrol-mediated suppression of synovial fibroblasts of rheumatoid arthritis patients and experimental arthritis in rats. 2019;176:2922–2944. doi: 10.1111/bph.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carruthers D.M., Arrol H.P., Bacon P.A., Young S.P. Dysregulated intracellular Ca2+ stores and Ca2+ signaling in synovial fluid T lymphocytes from patients with chronic inflammatory arthritis. 2000;43:1257–1265. doi: 10.1002/1529-0131(200006)43:6<1257::AID-ANR8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 22.Moolenaar W.H. LPA: a novel lipid mediator with diverse biological actions. Trends Cell Biol. 1994;4:213–219. doi: 10.1016/0962-8924(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 23.Choi S.H., Lee B.H., Kim H.J., Jung S.W., Kim H.S., Shin H.C., Lee J.H., Kim H.C., Rhim H., Hwang S.H., et al. Ginseng gintonin activates the human cardiac delayed rectifier K+ channel: involvement of Ca2+/calmodulin binding sites. Mol Cells. 2014;37:656–663. doi: 10.14348/molcells.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.H., Choi S.H., Lee B.H., Hwang S.H., Kim H.J., Rhee J., Chung C., Nah S.Y. Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase α. Neurosci Lett. 2013;548:143–148. doi: 10.1016/j.neulet.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 25.Choi S.H., Lee B.H., Hwang S.H., Kim H.J., Lee S.M., Kim H.C., Rhim H.W., Nah S.Y. Molecular mechanisms of large-conductance ca (2+) -activated potassium channel activation by ginseng gintonin. Evid Based Complement Alternat Med. 2013;2013:323709. doi: 10.1155/2013/323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee B.H., Kim H.K., Jang M., Kim H.J., Choi S.H., Hwang S.H., Kim H.C., Rhim H., Cho I.H., Nah S.Y. Effects of gintonin-enriched fraction in an atopic dermatitis animal model: involvement of autotaxin regulation. Biol Pharm Bull. 2017;40:1063–1070. doi: 10.1248/bpb.b17-00124. [DOI] [PubMed] [Google Scholar]
- 27.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.J., Kwon S.H., Jang C.G., Lee J.H., et al. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 28.Nam S.M., Hwang H., Seo M., Chang B.J., Kim H.J., Choi S.H., Rhim H., Kim H.C., Cho I.H., Nah S.Y. Gintonin attenuates D-galactose-induced hippocampal senescence by improving long-term hippocampal potentiation, neurogenesis, and cognitive functions. Gerontology. 2018;64:562–575. doi: 10.1159/000491113. [DOI] [PubMed] [Google Scholar]
- 29.Hwang S.H., Lee B.H., Choi S.H., Kim H.J., Jung S.W., Kim H.S., Shin H.C., Park H.J., Park K.H., Lee M.K., et al. Gintonin, a novel ginseng-derived lysophosphatidic acid receptor ligand, stimulates neurotransmitter release. Neurosci Lett. 2015;584:356–361. doi: 10.1016/j.neulet.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Orosa B., García S., Martínez P., González A., Gómez-Reino J.J., Conde C. Lysophosphatidic acid receptor inhibition as a new multipronged treatment for rheumatoid arthritis. Ann Rheum Dis. 2014;73:298–305. doi: 10.1136/annrheumdis-2012-202832. [DOI] [PubMed] [Google Scholar]
- 31.Miyabe Y., Miyabe C., Iwai Y., Takayasu A., Fukuda S., Yokoyama W., Nagai J., Jona M., Tokuhara Y., Ohkawa R., et al. Necessity of lysophosphatidic acid receptor 1 for development of arthritis. Arthritis Rheum. 2013;65:2037–2047. doi: 10.1002/art.37991. [DOI] [PubMed] [Google Scholar]
- 32.Wojtala A., Bonora M., Malinska D., Pinton P., Duszynski J., Wieckowski M.R. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 2014;542:243–262. doi: 10.1016/B978-0-12-416618-9.00013-3. [DOI] [PubMed] [Google Scholar]
- 33.Brenn D., Richter F., Schaible H.G. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56:351–359. doi: 10.1002/art.22282. [DOI] [PubMed] [Google Scholar]
- 34.Sur B., Kang S., Kim M., Oh S. Inhibition of carrageenan/kaolin-induced arthritis in rats and of inflammatory cytokine expressions in human IL-1β-stimulated fibroblast-like synoviocytes by a benzylideneacetophenone derivative. Inflammation. 2019;42:928–936. doi: 10.1007/s10753-018-0947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A., Salter D., van den Berg W.B. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Bang J.S., Oh D.H., Choi H.M., Sur B.J., Lim S.J., Kim J.Y., Yang H.I., Yoo M.C., Hahm D.H., Kim K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther. 2009;11:R49. doi: 10.1186/ar2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos-Nogueira E., Redondo Castro E., Mancuso R., Navarro X. Randall-Selitto test: a new approach for the detection of neuropathic pain after spinal cord injury. J Neurotrauma. 2012;29:898–904. doi: 10.1089/neu.2010.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin M.E., Herr D.R., Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130–138. doi: 10.1016/j.prostaglandins.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jja Contos, Ishii I., Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]